Abstract
Objective:
To characterize neurologic manifestations in post-hospitalization Neuro-PASC (PNP) and non-hospitalized Neuro-PASC (NNP) patients.
Methods:
Prospective study of the first 100 consecutive PNP and 500 NNP patients evaluated at a Neuro-COVID-19 clinic between 5/2020 and 8/2021.
Results:
PNP were older than NNP patients (mean 53.9 vs 44.9 y; p < 0.0001) with a higher prevalence of pre-existing comorbidities. An average 6.8 months from onset, the main neurologic symptoms were “brain fog” (81.2%), headache (70.3%), and dizziness (49.5%) with only anosmia, dysgeusia and myalgias being more frequent in the NNP compared to the PNP group (59 vs 39%, 57.6 vs 39% and 50.4 vs 33%, all p < 0.003). Moreover, 85.8% of patients experienced fatigue. PNP more frequently had an abnormal neurologic exam than NNP patients (62.2 vs 37%, p < 0.0001). Both groups had impaired quality of life in cognitive, fatigue, sleep, anxiety, and depression domains. PNP patients performed worse on processing speed, attention, and working memory tasks than NNP patients (T-score 41.5 vs 55, 42.5 vs 47 and 45.5 vs 49, all p < 0.001) and a US normative population. NNP patients had lower results in attention task only. Subjective impression of cognitive ability correlated with cognitive test results in NNP but not in PNP patients.
Interpretation:
PNP and NNP patients both experience persistent neurologic symptoms affecting their quality of life. However, they harbor significant differences in demographics, comorbidities, neurologic symptoms and findings, as well as pattern of cognitive dysfunction. Such differences suggest distinct etiologies of Neuro-PASC in these populations warranting targeted interventions.
Introduction
As of February 10, 2023, over 102 million people in the United States have developed confirmed infection with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and more than 1.1 million have died from coronavirus disease 2019 (COVID-19).1 Although COVID-19 was initially identified primarily as a respiratory disease, neurologic manifestations have been reported in up to 82% patients hospitalized with severe COVID-19 pneumonia which may linger in the post-acute phase.2-7 While most infected individuals have mild and transient respiratory symptoms and never require hospitalization for pneumonia and hypoxemia,8,9 some develop lingering neurologic, pulmonary, cardiac, and gastrointestinal symptoms.10 This persistent multi-system dysfunction, occurring in patients with both severe and mild COVID-19 constitute the “long COVID” syndrome, also called “post-acute sequelae of SARS-COV-2 infection” (PASC).11-13
Although neurologic symptoms of PASC (Neuro-PASC) may persist for more than a year in previously hospitalized7,14 and non-hospitalized patients alike,15,16 little is known about the differences and similarities in neurologic manifestations experienced by these 2 distinct populations. We therefore sought to evaluate prospectively the neurologic symptoms, cognitive dysfunction, and quality of life in post-hospitalization Neuro-PASC (PNP) and non-hospitalized Neuro-PASC (NNP) patients.
Methods
Patients
We prospectively evaluated the first consecutive 100 PNP and 500 NNP patients who were SARS CoV-2 + at the Neuro-COVID-19 clinic of Northwestern Memorial Hospital, in Chicago, Illinois, between May of 2020 and August 2021. The first 50 SARS-CoV-2-positive patients were previously reported.15 The opening of the clinic was announced on a webpage as customary at our institution for all new clinics,17 without further advertising. Patients were able to schedule an appointment without physician referral for in-person or televisits based on their preference on a first-come-first-served basis, regardless of their geographic location in the United States. Since long COVID was a new syndrome that was not yet defined, we accepted patients complaining of any type of neurologic manifestations associated with SARS-COV-2 infection. Our only exclusion criteria were absence of any neurologic symptoms (e.g., patients complaining only of shortness of breath after COVID-19).
Patients were included in this study if they had clinical manifestations of COVID-19 compatible with the Infectious Diseases Society of America (IDSA) guidelines, confirmed by positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) or rapid antigen positive test of nasopharyngeal swab, and/or positive SARS-Cov-2 antibody testing prior to COVID-19 vaccination, and had persistent neurological symptoms for at least 6 weeks from onset. This definition is more stringent than that of the CDC that that was formulated after the opening of the Neuro-COVID-clinic, and only requires symptoms lasting more than 4 weeks.18 Our patients also fit the subsequent WHO criteria of long COVID as well as the PASC criteria from NIH.19,20 All laboratory, radiologic, and electrophysiological assessments were performed as part of routine clinical care. This study received prior approval by Northwestern University institutional review board (STU00212583).
Procedures
All patients were evaluated by a board-certified attending neurologist, at times assisted by a neuroimmunology fellow (G.S.P., E.L.G.), nurse practitioners, and neurology residents. Patients were seen both in person (53.3%) and in video-conference televisit (46.7%), the latter included patients from 31 US states. Medical records of all patients were obtained and reviewed ahead of the scheduled office visit. Diagnostic testing was recorded after direct review of test results in the patients’ records. Both video televisit and in-person appointments lasted 1 hour and were equivalent in terms of medical history through the use of a standardized Neuro-COVID-19 Epic template with survey of medical history, neurological and other symptoms, test results, and mental function exam. The rest of the neurologic exam (cranial nerves, movements, coordination, and gait) was performed in more limited manner in patients seen in video-conference televisits than in-person.Direct exam of strength, reflexes, and sensations could not be performed in televisit patients. Patient reported quality of life in cognition and fatigue domains was assessed using the validated Patient Reported Outcome Measurement Information System (PROMIS).21,22 PROMIS measures of sleep disturbance, anxiety and depression were added on January 1, 2021. Finally, patient reported their subjective impression of % recovery compared to their baseline prior to their COVID-19 disease and SARS-COV-2 diagnosis.
A cognitive function evaluation with the National Institutes of Health (NIH) toolbox was completed for all the patients seen in person, as well as those who were seen via telemedicine but lived in the greater Chicago area and were able to come to our center within a week from their televisit. The NIH toolbox v2.1 included assessments of processing speed (pattern comparison processing speedtest); attention (inhibitory control and attention test); executive function (dimensional change card sort test); and working memory (list sorting working test).23-26 Both PROMIS and NIH toolbox results are expressed as T-scores, with a score of 50 representing the normative mean/median of the US reference population with a standard deviation of 10. NIH Toolbox results are controlled for age, sex, education, race, and ethnicity. Lower cognition T-scores indicate worse performance while higher fatigue, sleep disturbance, anxiety, and depression T-scores indicate greater severity.
Statistical Analysis
Data were summarized as number of patients (frequency), mean (standard deviation) for normally distributed variables, and median (interquartile range [IQR]) for non-normally distributed variables. Group differences were assessed using Fisher’s exact test, unpaired t-test, and Wilcoxon rank-sum test. Correlations between variables were assessed with Pearson’s or Spearman’s correlation tests, as appropriate. To determine if results of PROMIS and NIH Toolbox domains differed from expected, patient group T-scores were compared to the demographic-matched normative US population median of 50 using 1-sample Wilcoxon signed-rank tests. Twosided p < 0.05 was considered significant and all analyses were performed in GraphPad Prism version 9.0.0. Study data were collected and managed using Redcap electronic data capture tools.
To illustrate the relative phenotypic similarities and differences between patients we performed a principal component analysis (PCA) using the 5 patient-reported PROMIS quality of life domains as well as a PCA using the 4 objectively measured NIH toolbox cognitive tests. PCA was performed using R (version 4.2.1) and RStudio (2022.07.2 + 576). PCA results were presented as a 2-dimensional graph of principal component 1 (PC1) versus principal component 2 (PC2) since graphical representation of these 2 principal components reflects the majority of variance in the observations. In this graphical representation, the distance between individual patients on the PC1 axis, followed by distance between individual patients on the PC2 axis, is proportionate to the patients’ phenotypic differences. Furthermore, we color-coded individual patients by whether they were or were not previously hospitalized for COVID-19 and generated 95% confidence interval ellipses encompassing these groups. To facilitate phenotypic interpretation of the principal components, we calculated Pearson correlation coefficients between each PROMIS or NIH toolbox domain and the corresponding PC1 and PC2. As a further exploratory analysis to consider whether patients evaluated by televisits differed phenotypically from patients examined in- person, we generated the same PROMIS and NIH toolbox PCA graphs but instead grouped by whether patients were evaluated by televisits.
Results
Patient Demographics and Comorbidities
Of the 100 PNP included in this study, the mean age was 53.9 years, 58% were female, 62% were White, 18% Black, 3% Asian, and 19% were Hispanic. Conversely, the mean age of the NNP group was younger, 44.9 years, 65.8% were women, and the race/ethnic background was significantly different with 77% White, 8.2% Black, 3.8% Asian, and 12.2% Hispanic. All 600 patients were confirmed SARS-CoV-2 positive either by RT-PCR or serology, and 33% PNP and 44.8% NNP patients had received SARS-CoV-2 vaccination (p = 0.09). All but 1 patient were infected prior to vaccination (Table 1). The prevalence of comorbidities varied between the 2 groups. PNP more frequently than NNP patients had hypertension (39% vs 15.4%; p < 0.0001), dyslipidemia (22% vs 12.8%; p = 0.03), diabetes type 2 (21% vs 4.2%; p < 0.0001), lung (16% vs 4.2%; p < 0.0001), and cardiovascular diseases (10% vs 2.2%; p = 0.0008). However, NNP were more likely than PNP patients to suffer from depression/anxiety prior to COVID-19 (40% vs 9%: p < 0.0001). Demographics and comorbidities are shown in Table 2.
TABLE 1.
Study Subjects’ Demographics in Post-hospitalization and Non-hospitalized Neuro-PASC Patients
| Overall | Post-hospitalization Neuro-PASC (PNP) |
Non-hospitalized Neuro-PASC (NNP) |
p-Value | |
|---|---|---|---|---|
| n | 600 | 100 | 500 | |
| Age, years (mean (1 SD)) | 46.4 (14.0) | 53.9 (14.7) | 44.9 (13.4) | <0.0001 |
| Gender | 0.14 | |||
| Male, n (%) | 213 (35) | 42 (42) | 171 (34) | |
| Female, n (%) | 387 (65) | 58 (58) | 329 (65.8) | |
| Race, n (%) | 0.004 | |||
| White | 447 (74.5) | 62 (62) | 385 (77.0) | |
| Black or African American | 59 (9.8) | 18 (18) | 41 (8.2) | |
| Asian | 22 (3.7) | 3 (3) | 19 (3.8) | |
| American Indian/Alaskan Native | 2 (0.3) | 1 (1) | 1 (0.2) | |
| Native Hawaiian/Other Pacific Islander | 2 (0.3) | 1 (1) | 1 (0.2) | |
| Other | 47 (7.8) | 10 (10) | 37 (7.4) | |
| Multiracial | 6 (1.0) | 3 (3) | 3 (0.6) | |
| Not specified | 15 (2.5) | 2 (2) | 13 (2.6) | |
| Ethnicity, n (%) | 0.11 | |||
| Not Hispanic or Latino | 504 (84.0) | 80 (80) | 424 (84.8) | |
| Hispanic or Latino | 80 (13.3) | 19 (19) | 61 (12.2) | |
| Not specified | 16 (2.7) | 1 (1) | 15 (3) | |
| Visit type, n (%) | 0.51 | |||
| In person | 320 (53.3) | 50 (50) | 270 (54) | |
| Televisit | 280 (46.7) | 50 (50) | 230 (46) | |
| SARS-CoV-2 RT-PCR, n (%) | 0.02 | |||
| Positive | 532 (88.7) | 96 (96) | 436 (87.2) | |
| Negative | 39 (6.5) | 4(4) | 35 (7) | |
| Not Performed | 29 (4.8) | 0 (0) | 29 (5.8) | |
| SARS-CoV-2 serology, n (%) | 0.04 | |||
| Positive | 266 (44.3) | 54 (54) | 212 (42.4) | |
| Negative | 37 (6.2) | 2 (2) | 35 (7) | |
| Not Performed | 297 (49.5) | 44 (44) | 253 (50.6) | |
| Positive RT-PCR and serology, n (%) | 198 (33) | 50 (50) | 148 (29.6) | 0.0001 |
| Either RT-PCR or serology positive, n (%) | 600 (100) | 100 (100) | 500 (100) | 1 |
| SARS-CoV-2 vaccination, n (%) | 0.09 | |||
| Yes | 257 (42.8) | 33 (33) | 224 (44.8) | |
| No | 309 (51.5) | 61 (61) | 248 (49.6) | |
| Unknown | 34 (5.7) | 6 (6) | 28 (5.6) |
TABLE 2.
Study Subjects’ Comorbidities in Post-hospitalization and Non-hospitalized Neuro-PASC Patients
| Overall | Post-hospitalization Neuro-PASC (PNP) |
Non-hospitalized Neuro-PASC (NNP) |
p-Value | |
|---|---|---|---|---|
| n | 600 | 100 | 500 | |
| Any pre-existing comorbidity n (%) | ||||
| Depression/anxiety | 209 (34.8) | 9 (9) | 200 (40) | <0.0001 |
| Hypertension | 116 (19.3) | 39 (39) | 77 (15.4) | <0.0001 |
| Dyslipidemia | 86 (14.3) | 22 (22) | 64 (12.8) | 0.03 |
| Autoimmune diseasea | 70 (11.6) | 14 (14) | 56 (11.2) | 0.4 |
| Headache | 46 (7.7) | 4 (4) | 42 (8.4) | 0.15 |
| Type 2 Diabetes | 42 (7) | 21 (21) | 21 (4.2) | <0.0001 |
| Cancer | 41 (6.8) | 11 (11) | 30 (6) | 0.08 |
| Other Endocrine disordersb | 40 (6.6) | 9 (9) | 31 (6.2) | 0.28 |
| Insomnia | 38 (6.3) | 2 (2) | 36 (7.2) | 0.07 |
| Lung Diseasec | 37 (6.2) | 16 (16) | 21 (4.2) | <0.0001 |
| Gastrointestinal diseased | 29 (4.8) | 1 (1) | 28 (5.6) | 0.07 |
| Neuropsychiatric diseasee | 24 (4) | 5 (5) | 19 (3.8) | 0.58 |
| Cardiovascular diseasef | 21 (3.5) | 10 (10) | 11 (2.2) | 0.0008 |
| Traumatic brain injury | 19 (3.2) | 4 (4) | 15 (3) | 0.54 |
| Peripheral vascular disease | 12 (2) | 5 (5) | 7 (1.4) | 0.03 |
| Dysautonomia | 8 (1.3) | 2 (2) | 6 (1.2) | 0.63 |
| Chronic kidney disease | 7 (1.2) | 2 (2) | 5 (1.0) | 0.33 |
| Cerebrovascular disease | 6 (1) | 4 (4) | 2 (0.4) | 0.008 |
| Neuromuscular diseaseg | 6 (1) | 4 (4) | 0 (0) | 0.005 |
| Organ transplant | 1 (0.1) | 1 (1) | 0 (0) | 0.17 |
| Otherh | 70 (11.6) | 17 (17) | 53 (10.6) | 0.09 |
PNP: Hashimoto thyroiditis (5), rheumatoid arthritis (3), Multiple Sclerosis (2), connective tissue disease (2), systemic lupus erythematosus (2), diabetes type 1 (1), Bechet’s (1), ulcerative colitis (1), giant cell arteritis (1), psoriatic arthritis (1). NNP: Hashimoto thyroiditis (15), rheumatoid arthritis (11), Sjogren (4), Crohn’s disease (3), eczema (3) multiple sclerosis (2), systemic lupus erythematosus (2), celiac disease (3), psoriasis (2), Graves’ disease (2), ulcerative colitis (1), sarcoidosis (1), Henoch Schonlein purpura (1), diffuse scleroderma (1), multifocal motor neuropathy (1), eosinophilic esophagitis (1), Raynaud’s (1).
PNP: Hypothyroidism (6), adrenal insufficiency (2), PCOS (1). NNP: Hypothyroidism (18), PCOS (7), osteoporosis (3), thyroid cysts (1), primary hyperparathyroidism (1), adrenal insufficiency (1).
PNP: COPD/emphysema (4), interstitial lung disease (6), asthma (4), obstructive sleep apnea (2). NNP: Interstitial lung disease (20), asthma (19), obstructive sleep apnea (4), COPD/emphysema (1).
PNP: Primary sclerosing cholangitis (1). NNP: GERD (18), inflammatory bowel disease (6), polycystic liver disease (1), fatty liver (1), duodenal atresia (1), colitis (1).
PNP: Prior alcohol use disorder (2), fibromyalgia (1), HSV meningoencephalitis (1), congenital hydrocephalus (1). NNP: Fibromyalgia (6), benign paroxysmal positional vertigo (BPPV) (2), attention deficit hyperactivity disorder (2), essential tremor (1), tinnitus (1), Tourette’s syndrome (1), narcolepsy (1), bipolar disease (1), spinal stenosis (1), neurogenic bladder (1), autonomic dysreflexia (1), Chiari malformation type 1 (1).
PNP: Congestive heart failure (5), coronary artery disease (4), atrial fibrillation (1), cardiomyopathy (1). NNP: coronary artery disease (6), arrhythmia other than atrial fibrillation (2), atrial fibrillation (1), mitral valve prolapse (1), myocarditis (1).
PNP: Muscular dystrophy (1), cervical compression (1), lumbar spinal stenosis (1). NNP: None.
PNP: Cigarette smoking (14), essential thrombocytopenia (1), MGUS (1), myelodysplastic syndrome (1). NNP: Osteoarthritis (7), seasonal allergies (7), postural orthostatic tachycardia syndrome (POTS) (5), anemia (5), syphilis (3), gout (3), osteopenia (3), endometriosis (2), thrombocytopenia (2), glaucoma (2), Ehlers Danlos (1), infertility (1), urticaria (1), deep venous thrombosis (1), sinusitis (1), thrombocytopenia (1), nephrolithiasis (1), costochondritis (1), pernicious anemia (1), infectious mononucleosis (1), shingles (1), erectile dysfunction (1), protein S deficiency (1), sickle cell trait (1).
Frequency of Neurologic Symptoms and Signs Attributed to COVID 19.
Patients were evaluated in our clinic on average 6.8 months after symptom onset. The total average subjective impression of recovery compared to pre-COVID-19 baseline was 59.8% overall, with no statistically significant difference between the PNP and NNP groups (55.7% vs 60.6%, p = 0.07). The median number of neurologic symptoms attributed to COVID-19 was 7, and 91% reported more than 4 neurological symptoms with no differences between the 2 groups. Overall, the 10 most common neurological symptoms included non-specific cognitive complaints, which patients referred to as “brain fog” (81.2%), headache (70.3%), anosmia (55.7%), dysgeusia (54.5%), dizziness (49.5%), myalgia (47.5%), numbness/tingling (42.2%), pain other than chest (40.5%), tinnitus (28.7%), and blurred vision (26%). NNP patients more frequently reported anosmia (59% vs 39%; p = 0.0003), dysgeusia (57.6% vs 39%; p = 0.0009), and myalgias (50.4 vs 33%; p = 0.002) compared to the PNP group. Seizures, movement disorders, ischemic stroke, meningitis, or polyradiculitis were rare in both groups. None of our patients presented with Guillain-Barre syndrome/acute inflammatory demyelinating polyneuropathy (AIDP), or acute disseminated encephalomyelitis (ADEM).
The most common non-neurologic symptoms were fatigue (85.8%), depression/anxiety (69.3%), insomnia (57.0%), shortness of breath (48.3%), self-reported variation of heart rate and blood pressure which was documented as dysautonomia (34.0%), chest pain (29.7%), and gastrointestinal symptoms (nausea, vomiting, diarrhea; 27.0%). Of those, only shortness of breath (72% vs 43.6%; p < 0.0001) and chest pain (41% vs 27.4%; p = 0.008) were significantly more frequent in the PNP group. We performed a complete neurologic physical exam on the 320 patients who came to the clinic in-person and a limited exam for the 280 patients who were seen via televisits. We found that PNP more frequently than NNP patients had an abnormal neurologic exam (62.2% vs 37.0%; p < 0.0001), short-term memory deficit (37.8% vs 21.1%; p = 0.0007), attention deficit (22.4% vs 9.5%; p = 0.0008), sensory dysfunction (18.4% vs 6.2%; p = 0.0004), gait dysfunction (16.3% vs 3.7%; p < 0.0001), and motor dysfunction (13.3% vs 2.4%; p < 0.0001). The neurologic symptoms and signs are shown in Table 3.
TABLE 3.
Neurologic Symptoms and Signs Attributed to Long COVID in Post-hospitalization and Non-hospitalized Neuro-PASC Patients
| Overall | Post-hospitalization Neuro-PASC (PNP) |
Non-hospitalized Neuro-PASC (NNP) |
p-Value | |
|---|---|---|---|---|
| Time from symptom onset to clinic visit (month, mean (1 SD)) | 6.8 (3.69) | 7.3 (3.9) | 6.7 (3.6) | 0.14 |
| Subjective impression of recovery compared to pre-COVID-19 baseline (mean % (1 SD)) | 59.8 (23.4) | 55.7 (23.7) | 60.6 (23.4) | 0.07 |
| n = 92 | n = 475 | |||
| No. of neurologic manifestations/symptoms attributed to COVID-19 (median [IQR]) | 7 [5–10] | 7 [5–9] | 7 [5–10] | 0.38 |
| Neurologic symptom n (%) | ||||
| ≥4 | 543 (91) | 89 (89) | 454 (90.8) | 0.58 |
| Brain fog | 487 (81.2) | 86 (86) | 401 (80.2) | 0.21 |
| Headache | 422 (70.3) | 63 (63) | 359 (71.8) | 0.09 |
| Anosmia | 334 (55.7) | 39 (39) | 295 (59) | 0.0003 |
| Dysgeusia | 327 (54.5) | 39 (39) | 288 (57.6) | 0.0009 |
| Dizziness | 297 (49.5) | 49 (49) | 248 (49.6) | 1 |
| Myalgia | 285 (47.5) | 33 (33) | 252 (50.4) | 0.002 |
| Numbness/tingling | 253 (42.2) | 51 (51) | 202 (40.4) | 0.06 |
| Pain other than chest | 243 (40.5) | 39 (39) | 204 (40.8) | 0.82 |
| Tinnitush | 172 (28.7) | 29 (29) | 143 (28.6) | 1 |
| Blurred vision | 156 (26.0) | 23 (23) | 133 (26.6) | 0.53 |
| Seizure | 8 (1.3) | 3 (3) | 5 (1) | 0.13 |
| Movement disordera | 7 (1.2) | 1 (1) | 6 (1.2) | 1 |
| Ischemic Stroke | 7 (1.2) | 2 (2) | 5 (1) | 0.33 |
| Encephalitis | 2 (0.3) | 1 (1) | 1 (0.2) | 0.31 |
| Focal motor deficitb | 1 (0.2) | 0 (0) | 1 (0.2) | 1 |
| Focal sensory deficit | 1 (0.2) | 1 (1) | 0 (0) | 0.17 |
| Hemorrhagic stroke | 1 (0.2) | 0 (0) | 1 (0.2) | 1 |
| Meningitis | 1 (0.2) | 1 (1) | 0 (0) | 0.17 |
| Polyradiculitisc | 1 (0.2) | 1 (1) | 0 (0) | 0.17 |
| Other symptom n (%) | ||||
| Fatigue | 515 (85.8) | 84 (84) | 431 (86.2) | 0.53 |
| Depression/Anxiety | 416 (69.3) | 69 (69) | 347 (69.4) | 1 |
| Insomnia | 342 (57.0) | 60 (60) | 282 (56.4) | 0.58 |
| Shortness of breath | 290 (48.3) | 72 (72) | 218 (43.6) | <0.0001 |
| Dysautonomiad,i | 204 (34.0) | 35 (35) | 169 (33.8) | 0.82 |
| Chest pain | 178 (29.7) | 41 (41) | 137 (27.4) | 0.008 |
| GI symptomse | 162 (27.0) | 21 (21) | 141 (28.2) | 0.17 |
| Sign n/n tested (%) | ||||
| Abnormal exam | 233/563 (41.4) | 61/98 (62.2) | 172/465 (37.0) | <0.0001 |
| Short-term memory deficit | 135/563 (24.0) | 37/98 (37.8) | 98/465 (21.1) | 0.0007 |
| Attention deficit | 66/563 (11.7) | 22/98 (22.4) | 44/465 (9.5) | 0.0008 |
| Sensory dysfunctionf | 47/563 (8.3) | 18/98 (18.4) | 29/465 (6.2) | 0.0004 |
| Gait dysfunction | 33/563 (5.9) | 16/98 (16.3) | 17/465 (3.7) | <0.0001 |
| Motor dysfunction | 24/563 (4.3) | 13/98 (13.3) | 11/465 (2.4) | <0.0001 |
| Cranial nerve dysfunctiong | 20/563 (3.6) | 5/98 (5.1) | 15/465 (3.2) | 0.37 |
| Movement disorder | 11/563 (2.0) | 3/98 (3.1) | 8/465 (1.7) | 0.42 |
| Cerebellar dysfunction | 5/563 (0.9) | 3/98 (3.1) | 2/465 (0.4) | 0.04 |
PNP: Self-reported abnormal movement (3). NNP: Twitches and jerks (2), functional movement disorder (1), non-specified (4).
PNP: None. NNP: History of right arm and leg numbness with weakness due to a right medullary demyelinating plaque, later determined to be due to multiple sclerosis (1).
PNP: Lumbosacral radiculitis (1). NNP: None.
PNP: Self-reported variation of heart rate (10), POTS (3), variation of blood pressure (1), orthostatic hypotension (0), other non-defined attributed to variation of heart rate and blood pressure. NNP: Self-reported variation of heart rate (39), variation of blood pressure (19), POTS (16) orthostatic hypotension (9).
PNP: Nausea (8), vomiting (2), diarrhea (14), gastroparesis (0). NNP: Nausea (47), vomiting (13), diarrhea (0), gastroparesis (0).
Evaluated for in person visits only.
PNP: Decreased visual acuity, mild (2), right VII nerve weakness (1), obscuration of disc margins (1), sustained end gaze nystagmus (1). NNP: Decreased visual acuity ranging from mild to severe (4), facial nerve paralysis (3), nystagmus, non-specified (2), baseline optic neuropathy due to NAION (1), left afferent pupillary defect and pale optic nerve (1), hearing decreased in right side (2) with Weber lateralizing to the right (1), right hypertropia with left supraduction (1).
1NNP patient reported auditory hallucinations, hearing music/melody for a few seconds 2 to 3 times a week.
1 NNP patient reported fluctuations in body temperature.
Radiological, Electrophysiological, and Laboratory Testing.
We reviewed diagnostic testing done prior and at the time of the visit (Table 4). There were no significant differences in the prevalence of abnormalities found on computed tomography (CT) of the brain, magnetic resonance imaging (MRI) of the brain and spinal cord, MR vessel wall imaging, electromyography (EMG), electroencephalogram (EEG) and cerebrospinal fluid (CSF) analysis, and tilt table test between PNP and NNP patients. Of note, non-specific white matter lesions were seen on the MRI brain in both groups. Conversely, except for the antinuclear antibody (ANA test), markers of inflammation include erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), D-dimer, and ferritin were all more frequently abnormal in the PNP group.
TABLE 4.
Diagnostic Testing in Post-hospitalization and Non-hospitalized Neuro PASC Patients
| Overall | Post-hospitalization Neuro PASC (PNP) |
Non-hospitalized N euro PASC (NNP) |
p-Value | |
|---|---|---|---|---|
| n abnormal/n tested (%) | ||||
| Brain CTa | 16/74 (21.6) | 7/21 (33.3) | 9/53 (17.0) | 0.21 |
| Brain MRIb | 78/181 (43.1) | 15/34 (44.1) | 63/147 (42.9) | 1 |
| MR vessel wall imagingc | 2/14 (14.3) | 2/5 (40.0) | 0/9 (0) | 0.11 |
| Spine MRId | 38/51 (74.5) | 6/8 (75.0) | 32/43 (74.4) | 1 |
| EMGe | 10/23 (43.5) | 5/6 (83.3) | 5/17 (29.4) | 0.05 |
| EEGf | 6/13 (46.2) | 1/3 (33.3) | 5/10 (50.0) | 1 |
| CSF analysisg | 1/9 (11.1) | 0/1 (0) | 1/8 (12.5) | 1 |
| Tilt table testh | 2/3 (66.7) | 0 (0) | 2/3 (66.7) | 1 |
| ANA ≥ 1:160 | 12/135 (8.9) | 2/23 (8.7) | 10/112 (8.9) | 1 |
| ESR | 81/218 (37.2) | 35/51 (68.6) | 46/167 (27.5) | <0.0001 |
| CRP | 93/247 (37.7) | 64/78 (82.1) | 29/169 (17.2) | <0.0001 |
| D-dimer | 67/197 (34.0) | 49/74 (66.2) | 18/123 (14.6) | <0.0001 |
| Ferritin | 68/170 (40.0) | 54/77 (70.1) | 14/93 (15.1) | <0.0001 |
PNP: Nonspecific white matter disease (4), age indeterminate lacunar infarct (1), diffuse atrophy after cardiac arrest (1). NNP: Nonspecific white matter disease (1), Mild irregularities of intracranial vessels on CTA angiogram (2), multifocal irregularity of vertebral arteries suggestive of fibromuscular dysplasia (2), incidental macular calcifications along the globes (1), remote right cerebellar stroke (1).
PNP: Nonspecific white matter disease (10), volume loss in frontoparietal cortical regions and caudate nuclei (1), mild atrophy, generalized (1) and localized in frontal and temporal lobes (1), multiple areas of restricted diffusion involving the right MCA territory and right frontal and parietal lobes, compatible with multifocal acute infarcts, possibly due to embolic phenomena (1), residual temporal lobe cystic encephalomalacia (1), optic nerve, chiasm and tracts diffusely decreased in caliber with T2 hyperintense signal in left optic nerve (1), temporal lobe T2 hyperintensities (1), mild to moderate narrowing of superior sagittal sinus, chronic nonocclusive thrombus not excluded. NNP: Nonspecific white matter disease (19), arachnoid cyst (4), chronic demyelinating plaque in brainstem (3), developmental venous anomaly (3), chronic demyelinating plaques in optic tracts (3), chronic cavitary lesion in corpus callosum, chronic focal leptomeningeal enhancement in occipital lobe (1), T2/FLAIR hyperintensity in olfactory bulbs (2), meningioma (2), small outpouching intracranial aneurysms (2), 2 mm cyst in right auditory canal (1), baseline ocular cavernous hemangioma (1), partial occlusion of superior sagittal sinus (1), Schwannoma (1).
PNP: Minimal vessel wall enhancement right internal carotid artery (1), small outpouching small aneurysm (1). NNP: None.
PNP: Degenerative changes of the spine (6). NNP: Multifocal short segment myelitis (1), degenerative changes of the spine (31).
PNP: Ulnar mononeuropathy (2), polyneuropathy (1), C8-T1 radiculopathy (1), critical illness neuropathy (1). NNP: Mononeuropathy at the wrist (2), sensory polyneuropathy (1), axonal polyneuropathy (1), L5-S1radiculopathy (1).
PNP: Bilateral temporal dysfunction and mild encephalopathy (1). NNP: Nonspecific generalized slowing (2), alpha-delta sleep (1), intermittent, brief spike wave discharges in frontal region, left greater than right (1), TRDA pattern (1).
PNP: No abnormal findings. NNP: Elevated proteins in 1 patient.
PNP: No tilt test was done. NNP: Results suggestive of POTS (2).
Quality of Life Measures and Standardized Cognitive Tests.
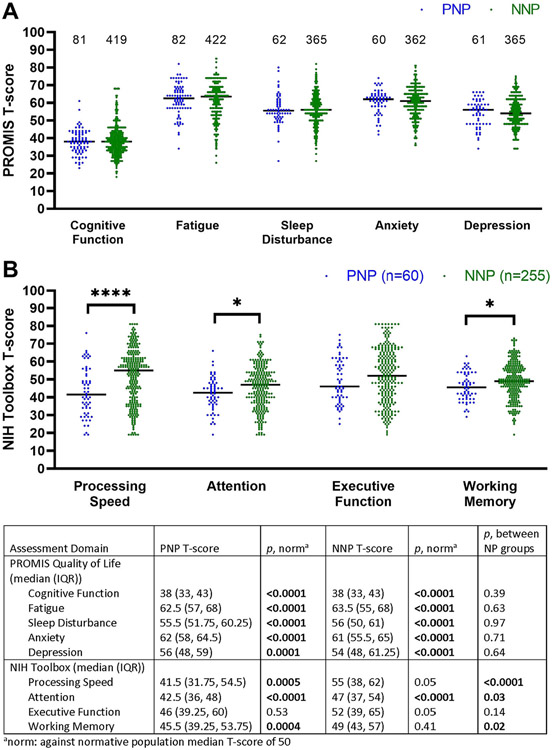
We analyzed the impact of COVID-19 on the quality of life of PNP and NNP patients with the PROMIS measures and tested their cognitive function with the NIH toolbox tests, both reported as T scores. The results are displayed in Fig 1. Both the PNP and NNP patients demonstrated significant altered quality of life in domains of cognition, fatigue, sleep, anxiety, and depression compared to the US normative population, with no statistically significant difference between the 2 groups with the median T scores indicating moderate impairment. The tablet-based NIH toolbox cognitive test could be administered to in-person patients only. However, 16/50 (32%) PNP and 49/230 (21%) NNP patients evaluated initially in televisits who lived in Illinois or neighboring states came to our clinic within a week to perform the NIH toolbox cognitive tests. Altogether, the PNP patients had significantly worse performance on NIH toolbox in processing speed, attention and working memory compared to the NNP group and a demographic-matched US normative population. The NNP patients had significantly lower results in attention task only compared to a demographic-matched US normative population.
FIGURE 1:
Quality of life and cognitive results in PNP and NNP. Both PNP and NNP groups exhibited impaired quality of life in cognitive, fatigue, sleep, anxiety, and depression domains. PNP patients had worse performance on NIH toolbox in processing speed, attention, and working memory as compared to NNP patients and to a US normative population. NNP patients had lower results in attention task only compared to a US normative population.
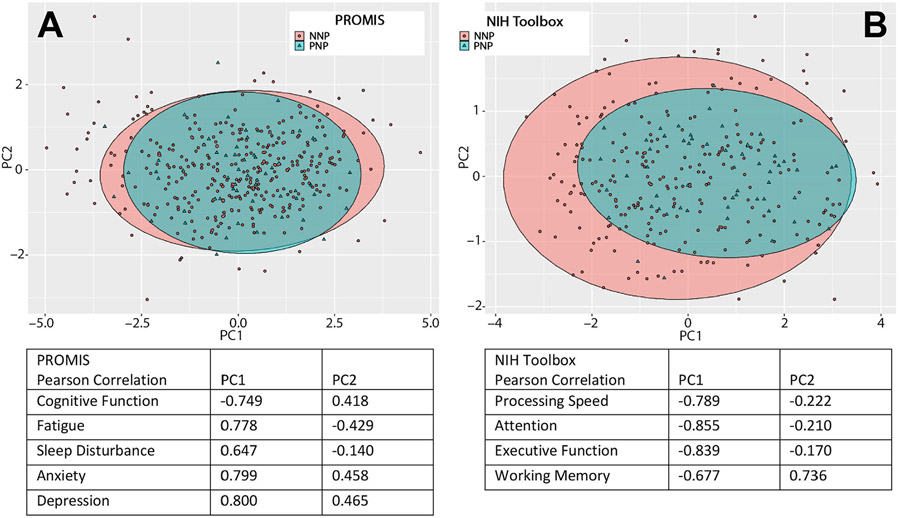
We further analyzed similarities and differences between PNP and NNP groups using principal component analyses (PCA). Fig 2A shows the PCA using the 5 patient-reported PROMIS quality of life domains. For the PROMIS domains, PC1 accounted for 57% of the variance in the observations and PC2 accounted for 16%, for a total of 73% of the variance represented by the PC1 versus PC2 graph. For the PROMIS domains, Pearson correlations demonstrated that increases in PC1 corresponded to worsening T-scores in each PROMIS domain while increases in PC2 corresponded to worse anxiety and depression T-scores and improved T-scores in cognitive function, fatigue, and sleep disturbance. Ninety-five percent confidence ellipses demonstrated that NNP and PNP groups have largely similar PROMIS phenotypes. However, the phenotype distribution appeared somewhat broader for the NNP group; primarily, a small portion of NNP patients have larger PC1 values than the PNP group and a similar size portion of NNP patients having smaller PC1 values than the PNP group.
FIGURE 2:
Principal component analyses of quality of life and cognition in PNP and NNP patients. Principal component analyses for (A) PROMIS quality of life measures and (B) NIH toolbox cognitive tests in PNP (green triangles) and NNP (red dots) patients. Ellipses representing the 95% confidence interval encompassing PNP (green) and NNP (red) groups are shown. Pearson correlation tables indicate that patient experiencing the worst quality of life on (A) all PROMIS domains, and who have the worst results on (B) all NIH toolbox cognitive tests, are located on the right distal part of the plots on the PC1 axis whereas those with (A) best quality of life and (B) best cognitive results are located on the left on the PC1 axis.
Figure 2B shows the PCA using the 4 objective NIH toolbox cognitive tests. For the NIH toolbox tests, PC1 accounted for 63% of the variance in the observations and PC2 accounted for 17%, for a total of 80% of the variance represented by the PC1 versus PC2 graph. For the NIH toolbox tests, Pearson correlations demonstrated that increases in PC1 corresponded to worsening T-scores in each NIH toolbox domain while increases in PC2 corresponded to worse processing speed, attention, and executive function T-scores but improved working memory T-scores. Ninety-five percent confidence ellipses demonstrated substantial phenotype overlap between NNP and PNP groups; however, PC1 in particular suggests that the NNP group is skewed to include less severe phenotypes than the PNP group. Finally, PCA comparing PROMIS and NIH Toolbox results of patients evaluated in-person with those seen in televisit showed overlapping ellipses, demonstrating that these 2 groups were largely identical (Figure S1).
Assessment of Subjective Recovery to Pre-Covid-19 Baseline and Correlations of Quality of Life and Cognitive Measures.
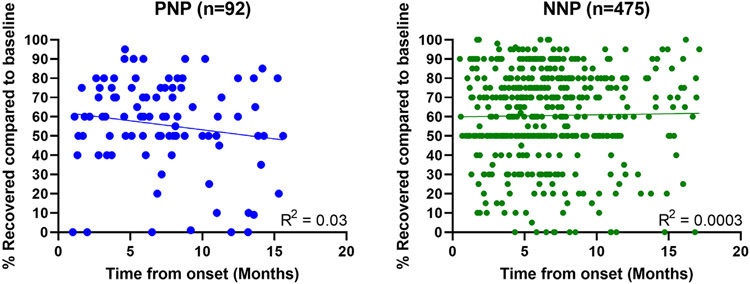
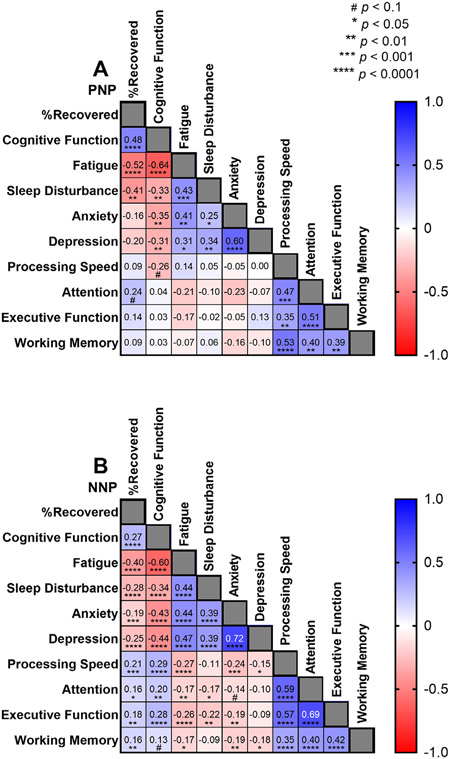
We analyzed the subjective impression of % recovery compared to pre-COVID-19 baseline in PNP and NNP patients at the time of the visit. Time from symptom onset was not associated with the subjective impression of recovery in either group (Fig 3; PNP Pearson’s R2 = 0.003, NNP Pearson’s R2 = 0.001). Finally, we correlated the subjective impression of recovery, PROMIS QoL measures, and NIH Toolbox cognitive test results (Fig 4). In both groups, QoL PROMIS measures were significantly correlated with each other, and so were NIH Toolbox cognitive tests. However, while the NNP group showed significant associations between % recovered, and subjective impression of cognitive function and fatigue with cognitive test results (Fig 4B), this was not the case in for PNP patients (Fig 4A).
FIGURE 3:
Recovery to pre COVID baseline in PNP and NNP. Subjective impression of recovery compared to pre-Covid-19 baseline for PNP (A) and NNP (B). The patients were asked to grade their recovery at the time of their visit, assuming a pre-COVID-19 baseline of 100%. Each person is represented by a single time point, and R2 values demonstrate no significant relationship between time from onset and percent recovery in both PNP and NNP.
FIGURE 4:
Correlation coefficients between subjective impression of recovery, quality of life PROMIS measures and NIH toolbox cognitive tests. (A) In PNP patients, subjective impression of % recovery compared to pre-COVID-19 baseline correlated moderately with PROMIS Qol measures of cognition, fatigue, and sleep disturbance. PROMIS cognition showed a strong negative correlation with fatigue, and PROMIS fatigue correlated moderately with sleep disturbance and anxiety. There was a trend for negative correlation between PROMIS cognition and processing speed. There was no correlation between the subjective impression of cognitive function and NIH toolbox measurements in attention, executive function and working memory. This suggests impaired insight of objective cognitive function difficulties. (B) In NNP patients, subjective impression of % recovery compared to pre-COVID-19 baseline correlated moderately with PROMIS Qol measures of cognition, fatigue, and sleep disturbance. QoL PROMIS measures correlated with each other as well as NIH toolbox cognitive tests. The worse the impression of cognitive function, the worse processing speed, attention, executive function and working memory, which was statistically significant in all domains.
Discussion
Varying terminology has been used to describe post-COVID conditions. “Long COVID” is a patient-created term that appeared in the spring of 2020, which has been modified as “post-COVID conditions” by the Center for Disease Control (CDC) and the World Health Organization (WHO). Long COVID is described as a wide range of new, returning or ongoing symptoms lasting more than 4 weeks after infection with SARS-CoV-2 that cannot be explained by an alternative diagnosis.18,19 In February 2021, the NIH introduced the name of “PASC”, encompassing a constellation of symptoms persisting long past the time of recovery of the initial stages of COVID-19.20
However, neither the CDC, WHO, nor NIH definitions differentiate patients based on acute symptom severity. This has been very detrimental to the Neuro-PASC field. Indeed, older people with multiple comorbidities who have cognitive problems after mechanical ventilation for severe COVID-19 pneumonia complicated by anoxia, cytokine storm, intravascular clotting, and multi-organ failure, and previously healthy young individuals with brain fog after transient sore throat and cough from mild SARS-CoV-2 infection are both considered to have Neuro-PASC. Accordingly, most publications have lumped these 2 very distinct populations together, causing decreased rigor in the field.27,28.
Investigators in the United Kingdom, Italy, and Spain have already highlighted differences in post-hospitalization and non-hospitalized PASC patients. However, our data are novel since those studies were not focused on Neuro-PASC and did not include quality of life or objective cognitive measures.29-31 We propose the terminology of PNP and NNP in SARS-CoV-2+ individuals with neurologic manifestations of PASC, in an effort to better stratify this heterogenous population of patients.32 Indeed, our data show that PNP and NNP groups have distinct demographics and comorbidities. In our study, the PNP are a decade older than NNP patients, they are more ethnically diverse and have a higher prevalence of pre-existing hypertension, dyslipidemia, lung, and cardiovascular disease. This is consistent with ample literature on demographics, comorbidities and neurologic manifestations in hospitalized COVID-19 patients.2,4-6,33 Conversely, NNP patients had a higher prevalence of depression/anxiety prior to COVID-19. These findings are consistent with our studies of neurologic manifestations in acutely hospitalized COVID-19 patients4 and non-hospitalized long haulers.15,16 In addition, PNP and NNP patients have distinct symptomatology, neurologic examination findings, and laboratory test results. While NNP more frequently present with anosmia, dysgeusia, and myalgia, PNP have a higher prevalence of symptoms of shortness of breath and chest pain and are more likely to have an abnormal neurologic physical examination and laboratory markers of inflammation. Finally, PNP had different pattern of cognitive dysfunction than NNP patients. PNP performed significantly worse than NNP in tasks of processing speed, attention and working memory than NNP patients and a US normative population, whereas NNP had worse results on test of attention only, compared to what would be expected based on the demographic features. However, both groups of patients equally complained of decreased quality of life in domains of cognition, fatigue, sleep disturbance, anxiety, and depression. Differences in the composite phenotype of cognitive dysfunction and similarities in the composite phenotype of quality of life disruption between PNP and NNP groups were further demonstrated by PCA. Finally time from onset was not associated with subjective impression of recovery in either group.
The demographic, comorbidities, clinical, and cognitive differences highlighted in our study suggest distinct etiologies of Neuro-PASC in PNP and NNP patients, warranting targeted interventions. During hospitalization for the acute phase of COVID-19, it is possible that PNP suffered diffuse brain damage, which could have been caused by a combination of hypoxemia, cytokine storm, multi-organ failure, or encephalopathy which may not result in specific findings on brain CT or MRI. This is consistent with the broad cognitive dysfunction harbored by these patients. Accordingly, PNP seemed to have also lost insight in their cognitive dysfunction. This is of importance since failure to recognize deficits in post-hospitalization groups may be a source of bias, in view of their decreased insight in their cognitive deficits.
Conversely, the female predominance of NNP patients, as well as ongoing research, suggest an autoimmune etiology of Neuro-PASC in this population, perhaps triggered by viral persistence.34-37 Indeed, women are more likely than men to develop autoimmune diseases.38-40 In addition, the higher prevalence of depression/anxiety before COVID-19 suggest a neuropsychiatric vulnerability to developing long COVID and is consistent with the findings of a recently published prospective study, in which preexisting psychological distress was found to be associated with risk of developing post-COVID-19 conditions and decreased likelihood of full recovery.41 However, this does not imply that long COVID is a psychosomatic disease since 60% of NNP patients never had pre-existing mental health issues, but highlights further heterogeneity in this population warranting multiple therapeutic approaches.
Alternatively, it is also possible that some PNP and NNP patients could be affected by the same pathogenic mechanisms, but NNP patients more often experience lighter versions of those mechanisms that result in milder clinical phenotypes.
Our study has limitations. As is the case for any study on any disease performed in an ambulatory setting, it is based on self-selected individuals who sought care at our Neuro-COVID-19 clinic. This is similar to any studies on any disease performed in hospital setting, based on self-selected individuals seeking inpatient care, or to internet surveys, where participants self-select based on their access to technology and other socio-economic factors.42-44 However, our protocol of providing in-person or televisits to patients from the entire United States without physician referral was deliberately designed to improve access and avoid referral bias. Therefore, our patients are representative of the US Neuro-PASC population that is seeking care at post-COVID clinics. Since approximately half of the patients enrolled from both groups came from televisits, this restricted features of the neurologic exam and NIH Toolbox assessment, which is performed on a tablet. Nevertheless, patients living in Illinois and surrounding states had the opportunity to come in-person after their televisit and complete the cognitive tests. Of note, analyses of age, PROMIS quality of life measures, NIH toolbox cognitive tests and % recovery compared to pre-COVID-19 baseline showed no significant differences in either PNP or NNP group between those surveyed initially via televisit versus in-person. Our study did not have contemporaneous control groups and relied on the normative population of the NIH Toolbox cognitive tests.23-26,45 The NIH Toolbox was created under the auspices of the “NIH blueprint for Neurosciences Research” using rigorous methodologies to allow for targeted, accurate comparisons for any research study participant group against the US population from ages 3 to 85, using a sample of 4,859 participants representative of the US population based on gender, race/ethnicity, and socioeconomic status around the country.46 Therefore, the NIH toolbox has been used in hundreds of publications on a wide range of neurologic diseases over more than a decade.47 The normative data of the NIH toolbox tests was also critical to the success of our study due to the limitation of human subject testing for research outside of patient-care associated with the pandemic, which would have precluded testing of contemporaneous control groups. Furthermore, there was an unequal number of study participants in both groups. This reflects the population of the clinic during the period of observation, which was constituted of 17% PNP and 83% NNP patients. Consequently, our study in underpowered to detect sex and ethnic differences between the PNP and NNP groups. Our statistical analyses do not adjust for multiple comparisons. This is due to the exploratory nature of this first-of-its-kind study aiming at guiding further investigations, knowing that those adjustments may increase the type II error for those associations that are not null.48,49 Finally, since the population of the clinic was ambulatory, it may not include the most affected PNP patients requiring intensive home care or skilled nursing facilities.
Conclusions
Long COVID/PASC continues to occur despite vaccination and boosters, and the Government Accountability Office (GAO) estimated that up to 23 million Americans were affected in March 2022, pushing 1 million people out of work.50 Neurologic manifestations of PASC are very debilitating, and the president of the American Academy of Neurology stated in July 2022 that long COVID was the third leading neurologic condition in the United States.51 Accordingly, the need for dedicated training in Neuro-PASC care is evidenced by the high demand of televisits from patients coming from many US states where they do not have access to Neuro-infectious diseases specialists. As the number of hospitalized individuals with COVID-19 continues to decrease, Neuro-PASC will affect predominantly the younger group of non-hospitalized patients. The loss of productivity associated with lingering cognitive dysfunction experienced by people in their prime will undoubtebly have a substantial economic impact.
Differences between PNP and NNP patients characterized in our study suggest that distinct pathogenic mechanisms may be at play in those 2 groups, emphasizing the need to evaluate these populations separately. Of concern, a large treatment trial of PASC with Nirmatrelvir/Ritonavir (Paxlovid) which is being organized by the NIH RECOVER initiative, does not include outcome measures based on severity of acute COVID-19.52 Although much progress has been made in the symptomatic management of Neuro-PASC,32 further research is urgently needed to elucidate the root cause of Long COVID, delineate risk factors and biomarkers of disease activity, and devise targeted therapeutic interventions for this debilitating syndrome.10,53-55
Supplementary Material
Acknowledgments
EML was supported in part by NIH/NIA grant K23AG078705. This study was supported in part by a gift from Mr. and Mrs. Michael Ferro.
Footnotes
Potential Conflicts of Interest
The authors do not have conflicts of interest.
Data Availability Statement
Data will be deposited in the COVID-19 Neuro Databank after publication.
References
- 1.Johns Hopkins University & Medicine. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopins University & Medicine. https://coronavirus.jhu.edu/map.html. Accessed September 21, 2022.
- 2.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19. Neurology 2020;95:e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol 2020;7:2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frontera J, Mainali S, Fink EL, et al. Global consortium study of neurological dysfunction in COVID-19 (GCS-NeuroCOVID): study design and rationale. Neurocrit Care 2020;33:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 2021;96:e575–e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frontera JA, Yang D, Lewis A, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci 2021;426:117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergquist SH, Partin C, Roberts DL, et al. Non-hospitalized adults with COVID-19 differ noticeably from hospitalized adults in their demographic, clinical, and social characteristics. SN Compr Clin Med 2020;2:1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 10.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 2020;20:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancet T. Facing up to long COVID. Lancet 2020;396:1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIH announces research opportunities to study “Long COVID”. National Institutes of Health https://www.ninr.nih.gov/newsandinformation/newsandnotes/pasc-initiative 2021. Accessed September 21, 2022.
- 14.Becker JH, Lin JJ, Doernberg M, et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open 2021;4:e2130645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 "long haulers". Ann Clin Transl Neurol 2021;8:1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali ST, Kang AK, Patel TR, et al. Evolution of neurologic symptoms in non-hospitalized COVID-19 "long haulers". Ann Clin Transl Neurol 2022;9:950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinic adresses neurological effects of COVID-19. Available at: https://www.nm.org/healthbeat/covid-19/advances-in-care/clinic-addresses-neurological-effects-of-covid-19.
- 18.Long COVID or Post-COVID Conditions. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
- 19.WHO COVID-19 Case definition. 2020. Available at: https://apps.who.int/iris/handle/10665/337834.
- 20.NIH announces research opportunities to study "Long COVID". Available at: https://www.ninr.nih.gov/newsandinformation/newsandnotes/pasc-initiative.
- 21.Lai JS, Wagner LI, Jacobsen PB, Cella D. Self-reported cognitive concerns and abilities: two sides of one coin? Psychooncology 2014;23:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil 2011;92:S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gershon RC, Wagster MV, Hendrie HC, et al. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80:S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH toolbox. Neurology 2013;80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton RK, Akshoomoff N, Tulsky D, et al. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J Int Neuropsychol Soc 2014;20:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gershon RC, Cella D, Fox NA, et al. Assessment of neurological and behavioural function: the NIH toolbox. Lancet Neurol 2010;9:138–139. [DOI] [PubMed] [Google Scholar]
- 27.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of Postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021;4:e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heightman M, Prashar J, Hillman TE, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ Open Respir Res 2021;8:e001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021;27:1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Gonzalez A, Araujo-Ameijeiras A, Fernandez-Villar A, et al. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci Rep 2022;12:3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham EL, Koralnik IJ, Liotta EM. Therapeutic approaches to the neurologic manifestations of COVID-19. Neurotherapeutics 2022;19:1435–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frontera JA, Yang D, Medicherla C, et al. Trajectories of neurologic recovery 12 months after hospitalization for COVID-19: a prospective longitudinal study. Neurology 2022;99:e33–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020;5:434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Parker J, Smits S, et al. Persistent viral shedding of SARS-CoV-2 in faeces—a rapid review. Colorectal Dis 2020;22:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natarajan A, Zlitni S, Brooks EF, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Medicine 2022;3:371–387 e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis 2023;8:e487–e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duquette P, Pleines J, Girard M, et al. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci 1992;19:466–471. [PubMed] [Google Scholar]
- 39.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum 2010;62:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 2007;56:2092–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Quan L, Chavarro JE, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post-COVID-19 conditions. JAMA Psychiatry 2022;79:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenacre ZA. The importance of selection bias in internet surveys. Open J Stat 2016;6:397–404. [Google Scholar]
- 43.Henderson M, Page L. Appraising the evidence: what is selection bias? Evid Based Ment Health 2007;10:67–68. [DOI] [PubMed] [Google Scholar]
- 44.Schaurer I, Weiss B. Investigating selection bias of online surveys on coronavirus-related behavioral outcomes. Surv Res Methods 2020;14:103–108. [Google Scholar]
- 45.Carlozzi NE, Tulsky DS, Wolf TJ, et al. Construct validity of the NIH toolbox cognition battery in individuals with stroke. Rehabil Psychol 2017;62:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NIH Toolbox: Measure Development and Research. Available at: https://www.healthmeasures.net/explore-measurement-systems/nih-toolbox/measure-development-and-research.
- 47.NIH Toolbox Publications per year. Available at: https://www.healthmeasures.net/explore-measurement-systems/nih-toolbox/measure-development-and-research/publications-by-year/197-nih-toolbox-publications-by-year. [Google Scholar]
- 48.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 49.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998;316:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Science & Tech Spotlight: Long COVID. 2022. [Google Scholar]
- 51.Avitzur O. American Academy of Neurology, 2022. President’s column. Available at: https://www.aan.com/AAN-Resources/Details/about-the-aan/board-of-directors/presidents-column/july-2022/. Accessed September 21, 2022. [Google Scholar]
- 52.SARS CoV-2 Viral Persistence Study (PASC)-Study of Long COVID-1 (PASC). ClinicalTrials.gov # NCT05595369. Available at: https://clinicaltrials.gov/ct2/show/NCT05595369.
- 53.Visvabharathy L, Hanson B, Orban Z, et al. Neuro-COVID longhaulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv 2022. [Google Scholar]
- 54.Hanson BA, Visvabharathy L, Ali ST, et al. Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with Postacute sequelae of SARS-CoV-2 infection. Neurology 2022;9:e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visvabharathy L, Orban ZS, Koralnik IJ. Case report: treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence. Front Med 2022;9:1003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be deposited in the COVID-19 Neuro Databank after publication.