Key Clinical Message
A 30‐year‐old woman arrived at our hospital with polymorphic ventricular tachycardia and hypothermia. Later, it was found that the cause was an overdose of caffeine exceeding the lethal dose. Although it is common for toxidrome caused by caffeine intoxication to produce hyperthermia, here we report a case of hypothermia.
Keywords: caffeine intoxication, hypothermia, sympathomimetic, toxidrome
In treating acute caffeine intoxication, the toxidrome is very useful for identifying the causative agent. Caffeine intoxication usually increases body temperature owing to its sympathomimetic effects, but hypothermia may also occur depending on the patient's condition.
1. INTRODUCTION
One of the most widely consumed psychoactive substances in the world, caffeine is readily available in coffee, energy drinks, and over‐the‐counter, such as cold medicines.
Caffeine intoxication occurs in humans after ingesting 1–3 g of caffeine and at a blood concentration of 25 μg/mL or higher. Lethal doses are 5–50 g orally, 1 with a blood concentration of 80–100 μg/mL 2 , 3 or higher.
The liver metabolizes 70%–80% of caffeine to paraxanthine (80%), theobromine (11%), and theophylline (5%). 4 Its pharmacological effects are dose‐dependent, and overdose can cause refractory cardiac arrhythmia, blood pressure changes, respiratory urgency, nausea and vomiting, and seizures.
A survey conducted by the Japanese Society of Clinical Toxicology, an organization of clinical addiction specialists, based on data from 38 medical facilities from April 2011 to March 2016, reported 101 emergency room visits due to single‐use caffeine poisoning. 5
In Japan, caffeine‐related deaths have increased since 2015, when a man in his 20s was reported to have died from caffeine intake because of consuming energy drinks and Stimulant drugs for a long period.
According to the 38th annual report by the American Association of Addiction Control Centers, in 2020, there were 1527 reported single exposures from energy drinks containing caffeine and 2996 reported single exposures from caffeine as a street drug. 6
Herein, we aim to report a case of cardiopulmonary arrest due to caffeine intoxication with hypothermia that was successfully treated with multidisciplinary therapy after resuscitation.
2. CASE PRESENTATION
A 30‐year‐old woman with a history of borderline personality disorder contacted a friend 3 h before her visit to report that she was distressed. When the friend visited her home, she was vomiting repeatedly and requested emergency medical services (EMS) before she lost consciousness. When the EMS arrived, the patient was in cardiac arrest. An automated external defibrillator was indicated, defibrillation was performed, and 3 min later, her heartbeat resumed. The patient's body temperature, measured by the EMS team, was 32°C, which was concerningly low, and the patient was transported to our hospital. The duration of the cardiac arrest was unknown but appeared to have been brief.
The findings on arrival were as follows: respiratory rate, 22/min; SpO2, 99% (with 10 L oxygen); blood pressure, 102/66 mmHg; heart rate, 166/min; Glasgow Coma Scale score, 3 points (E1V1M1); pupillary diameter 3 mm/3 mm; light reflex, +/+; and body temperature, 32.6°C (superficial temperature). Physical examination revealed no cold sweats, markedly lean body mass (BMI of 16.4 on admission), and numerous self‐inflicted scars on the left forearm.
After admission, the patient continued to develop ventricular tachycardia (Figure 1), although her heartbeat had resumed. As the patient was expected to be moderately hypothermic (32.4°C surface temperature), we started warming her up with warming fluids. Although we considered hypothermia a possible cause of cardiopulmonary arrest associated with fatal arrhythmia, we suspected poisoning rather than hypothermia, given the patient's marked emaciation and self‐inflicted wounds. However, since it was difficult to identify the causative agent at this point, we asked a friend to check the drugs that were present in her house. We found an empty box of over‐the‐counter caffeine tablets, which led to the diagnosis of caffeine intoxication.
FIGURE 1.
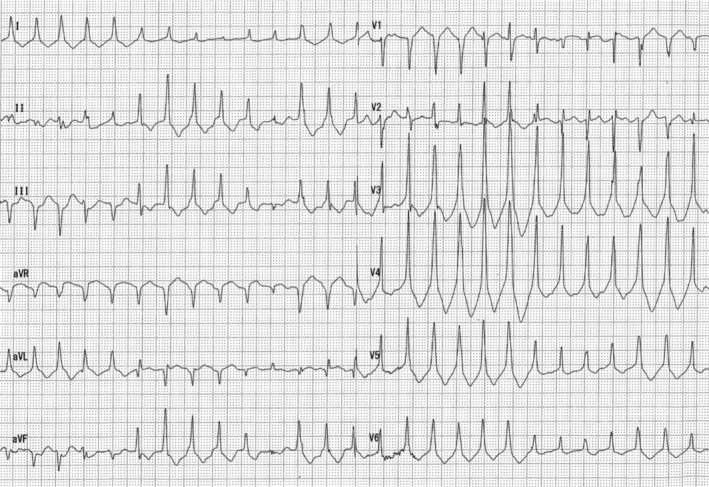
Electrocardiogram at the time of hospital arrival multiple ventricular tachycardias are observed.
3. INVESTIGATIONS
Laboratory findings at admission (Table 1) included elevated white blood cell count and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and creatine kinase (CK) levels. CK‐MB levels were only mildly elevated. High‐sensitivity troponin levels were not elevated. Electrolyte levels revealed the presence of hypokalemia. Arterial blood gas analysis results were as follows: pH, 7.060; PaO2, 634.0 mmHg; PaCO2, 20.8 mmHg; HCO3, 5.8 mmol/L; base excess, −22.9 mmol/L; lactate, 16.92 mmol/dL; metabolic acidosis; and hyperlactatemia. Electrocardiography revealed continuous ventricular tachycardia of 130–160/min. Whole‐body computed tomography showed no significant findings.
TABLE 1.
Laboratory data at the time of hospital arrival.
| CBC | ||
| WBC | 16,570 | /μL |
| RBC | 404 × 104 | /μL |
| Hb | 13.1 | g/dL |
| Hct | 43.0 | % |
| Plt | 23.6 × 104 | /μL |
| Coagulation system | ||
| PT | 48.5 | % |
| PT‐INR | 1.46 | |
| APTT | 27.8 | sec |
| Fib | 222 | mg/dL |
| D‐dimer | 1.2 | μg/mL |
| Biochemical parameters | ||
| TP | 7.3 | g/dL |
| Alb | 4.5 | g/dL |
| T‐Bil | 0.3 | mg/dL |
| AST | 87 | U/L |
| ALT | 80 | U/L |
| LDH | 322 | U/L |
| ALP | 121 | U/L |
| AMY | 142 | U/L |
| CK | 231 | U/L |
| CK‐MB | 4.6 | ng/mL |
| hsTrI | 22.4 | pg/mL |
| BNP | 29.1 | pg/mL |
| BUN | 9.0 | mg/dL |
| Cre | 0.80 | mg/dL |
| Na | 144 | mEq/L |
| K | 2.5 | mEq/L |
| Cl | 103 | mEq/L |
| Ca | 2.5 | mg/dL |
| P | 6.9 | mg/dL |
| NH3 | 400 | μg/mL |
| Glu | 308 | mg/dL |
| CRP | <0.04 | mg/dL |
| OSM | 316 | mOSM/kg |
| ABG | ||
| pH | 7.060 | |
| pCO2 | 20.8 | mmHg |
| pO2 | 634.0 | mmHg |
| HCO3 − | 5.8 | mmol/L |
| BE | −22.9 | mmol/L |
| Lac | 16.92 | mmol/L |
The Department of Toxicology, Division of Basic Medical Pharmacy, Showa University School of Pharmacy, analyzed the caffeine components of the tablets. The analysis was performed by adding 250 mg of ammonium sulphate, 500 ng of an internal standard (1,2,3‐benzotriazole), and 200 μL of 0.1% acetic acid to 200 μL of serum, followed by extraction with 2 mL of chloroform: isopropanol (85:15). The mixture was stirred for 10 min and then centrifuged again. After stirring for 10 min, the mixture was centrifuged, and the lower layer was collected. After removing the solvent under a nitrogen stream, the residue was dissolved in 200 μL of 10 mM phosphate buffer (pH 6.8), filtered, and subjected to high‐performance liquid chromatography analysis. Calibration curves were also prepared by adding each standard to human standard serum and extracting them in the same manner as described above. The caffeine blood concentrations are shown in Table 2.
TABLE 2.
Changes in the blood levels of caffeine and its metabolites over time.
| Elapsed time from visit [h] | 0 | 7 | 12 | 24 | 48 | 69 | 75 | |
|---|---|---|---|---|---|---|---|---|
| Blood concentration[μg/m L] | Caffeine | 384.96 | 245.99 | 87.23 | 55.20 | 7.51 | 1.72 | 1.24 |
| Paraxanthine | 6.48 | 15.13 | 11.18 | 8.09 | 2.47 | 0.99 | 0.98 | |
| Theobromine | 1.32 | 2.55 | 2.37 | 1.49 | 0.56 | 0.29 | 0.30 | |
| Theophylline | 0.50 | 1.12 | 0.91 | 0.86 | 0.22 | 0.07 | 0.07 | |
Note: Blood tests performed more than 40 h after initiating CHD show that the patient's blood levels are below the toxic range for the first time.
4. TREATMENT
The patient was scheduled for blood purification therapy 1–2 h after her arrival with an estimated caffeine intake of 14.4 g. She was unresponsive to repeated defibrillation and medication. She was judged to have developed refractory ventricular fibrillation (VF), and the decision was made to introduce veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) in the cardiology department. The patient transitioned intermittently from ventricular tachycardia to VF until VA‐ECMO was introduced and was also administered 11 defibrillations and 300 mg and 150 mg intravenous amiodarone. Further, 1% lidocaine was administered, which was immediately stopped, as the response was short‐lived.
VA‐ECMO was started 3–4 h after the patient arrived at the hospital. At the same time, aortic balloon pumping (IABP) was started at 1:2 because of difficulty maintaining circulation, and continuous hemodialysis (CHD) was started after admission to the intensive care unit. Although the patient had hypothermia, she was also treated with temperature control therapy after the occurrence of cardiopulmonary arrest associated with fatal arrhythmia.
The post‐hospitalization course involving targeted temperature management was started when VA‐ECMO was introduced. Since the patient was in cardiopulmonary arrest with hypothermia, the temperature was set at 35°C. The patient's body temperature was controlled for 24 h so as not to exceed 36°C. CHD was performed using a dialysis membrane (EXCELFLO® AEF‐10; Asahi Kasei Medical Corporation, Tokyo, Japan) with a blood flow rate of 100 mL/min and a dialysate flow rate of 800 mL/h. The patient was resuscitated and managed for hypothermia for 24 h, and recuperation started on the second day. VA‐ECMO was withdrawn on the fourth day, and CHD was terminated on the same day. Regarding medication, although IABP‐assisted circulation was used, the patient still had difficulty maintaining blood pressure; therefore, noradrenaline, which had been administered since the third hour of admission, continued to be used.
Finally, noradrenaline was discontinued 7 h after weaning from IABP. After VA‐ECMO was introduced, the patient continued to have tachycardia; therefore, ONOACT® was initiated 11 h after admission and was used until 30 h after admission, when it was discontinued.
5. OUTCOME AND FOLLOW‐UP
Sedation was stopped after the patient returned to the hospital on the second day. Although communication was confirmed, ventilator management was continued until the 11th day owing to weight control after thermotherapy. Midazolam was used for sedation during ventilator use owing to unstable circulation, and fentanyl was used for analgesia. After psychiatric consultation, the patient was discharged on the 18th day of illness.
6. DISCUSSION
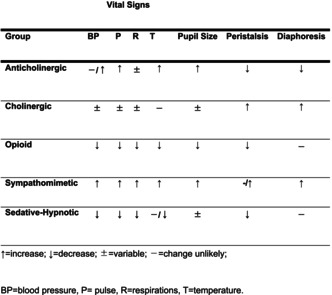
When treating intoxication, a toxidrome 7 is used to identify the causative drug at the time of treatment, which is estimated by combining vital signs, physical findings, symptoms, blood tests, and electrocardiogram. Toxidromes are divided into five categories (Table 3): anticholinergic, cholinergic, sedative‐hypnotic, opioid, and sympathomimetic. The effects of sympathomimetic drugs include elevated blood pressure, heart rate, and body temperature as vital signs and dilated pupils as physical findings, which are characteristic of caffeine intoxication.
TABLE 3.
Categories of toxidrome. 15
| Vital Signs | |||||||
|---|---|---|---|---|---|---|---|
| Group | BP | P | R | T | Pupil Size | Peristalsis | Diaphoresis |
| Anticholinergic | −/↑ | ↑ | ± | ↑ | ↑ | ↓ | ↓ |
| Cholinergic | ± | ± | ± | ‐ | ± | ↑ | ↑ |
| Opioid | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ‐ |
| Sympathomimetic | ↑ | ↑ | ↑ | ↑ | ↑ | −/↑ | ↑ |
| Sedative‐Hypnotic | ↓ | ↓ | ↓ | −/↓ | ± | ↓ | ‐ |
Abbreviations: −, change unlikely; ±, variable; ↑, increase; ↓, decrease; BP, blood pressure; P, pulse; R, respirations; T, temperature.
In this case, ventricular tachycardia was sustained after resuscitation associated with VF immediately after the patient presented to the hospital. Although there were findings of sympathetic predominance, hypothermia was also present, making it difficult to identify the drug using the usual toxidrome.
There have been case reports of poisoning in which symptoms were not typical, involving ingesting alprazolam, sodium valproate, and fenpyroximate. This was difficult to diagnose because of the lack of specific symptoms owing to drug interactions. 8 As a toxidrome, sedative‐hypnotic drugs such as benzodiazepines, barbiturates, and alcohol, as well as opioid drug intoxication, depress body temperature by suppressing the thermoregulatory center. 7 In this case, no alcohol was found at the scene, and there was no recent history of hospital visits; therefore, there seemed to be no concomitant use of CNS‐depressing drugs such as benzodiazepines, barbiturates, or opioids.
According to data from the Japan Meteorological Agency for that day, the average temperature in Yokohama was 29.5°C (26.8–34.4°C), and it is unlikely that the hypothermia was due to environmental factors, as there was no excessive air conditioning in the room. Since the time of cardiac arrest seems short, decreased body temperature due to cardiac arrest is unlikely, and accidental hypothermia associated with drug intoxication is possible.
Accidental hypothermia is classified into three categories: impaired thermoregulation, decreased heat production, and increased heat loss. 9 Caffeine is classified as a central nervous system stimulant. It has psychostimulant effects and, thus, affects cognitive function. 10 There are no reports of impaired thermoregulatory centers that cause hypothermia. The body temperature changes usually associated with caffeine intoxication are due to sympathetic effects: peripheral blood vessels constrict, which decreases heat release from the skin, increases heat production by fat cells, and increases heat production by muscle spasms, increasing body temperature. 11
However, the patient also had anorexia nervosa, with a height of 143 cm, a weight of 33.5 kg, and a BMI of 16.4, suggesting that the decrease in fat cells and muscle mass necessary for heat production prevented an increase in body temperature. It is unlikely that hypothermia would result from decreased heat production alone, and a mechanism of increased heat loss may have occurred as well.
There are few reports of hypothermia associated with caffeine intoxication, including a 47‐year‐old man who died of caffeine intoxication 12 and a 27‐year‐old woman who had repeated VF and developed hypothermia after hospitalization. 13 There was no discussion regarding the cause of hypothermia in both cases, and they were not reported as caffeine‐associated hypothermia. However, it was reported that both had sweating in common.
There are no reported cases of hypothermia caused by sweating itself. It is known that sympathetic nerve action suppresses heat dissipation by constricting peripheral blood vessels and heat dissipation by sweating from the sweat glands. 14 This suggests that persistent perspiration from the sweat glands due to sympathetic nerve action may decrease body temperature because of predominant heat dissipation.
Although there is a risk of hypothermia in circulatory disturbances associated with refractory VF and anorexia nervosa, 9 we believe that hypothermia may also occur in caffeine intoxication, which is generally considered a temperature increase because of continuous heat release from the sweat glands associated with sympathetic effects (Figure 2).
FIGURE 2.
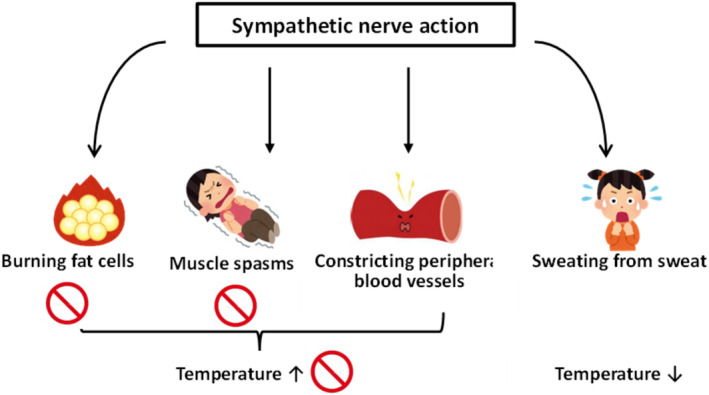
Body temperature change due to sympathetic nerve action.
In conclusion, we encountered a case of cardiopulmonary arrest due to caffeine intoxication with hypothermia that was successfully treated with multidisciplinary therapy after resuscitation. Although hypothermia due to sympathomimetic caffeine intoxication is an atypical toxidrome, hypothermia due to predominant heat dissipation should be considered if the patient's condition suggests they may be incapable of heat production.
AUTHOR CONTRIBUTIONS
Takanori Ohno: Writing – original draft. Shino Katsuki: Supervision. Kazuyuki Miyamoto: Supervision. Asuka Kaizaki‐Mitsumoto: Investigation. Masashi Kanazawa: Writing – review and editing. Toshitaka Ito: Writing – review and editing. Munetaka Hayashi: Supervision.
FUNDING INFORMATION
No funding was received to assist with the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICS STATEMENT
This research work does not contain human subject research material, as it is an individual anonymized case report. IRB approval was not applied for as it is not required for individual case reports.
CONSENT
Written informed consent was obtained from the patient's mother to publish this case report because the patient re‐attempted suicide and is not alive.
ACKNOWLEDGMENTS
The authors thank the patient's family members. We would like to thank Editage (www.editage.com) for English language editing.
Ohno T, Katsuki S, Miyamoto K, et al. A case of caffeine intoxication resulting in hypothermia. Clin Case Rep. 2023;11:e8235. doi: 10.1002/ccr3.8235
DATA AVAILABILITY STATEMENT
Data will be made available on request.
REFERENCES
- 1. Kamijo Y, Soma K. Clinical Toxicology. 1st ed. IGAKUSHOIN; 2009:130‐133. [Google Scholar]
- 2. Cappelletti S, Piacentino D, Fineschi V, Frati P, Cipolloni L, Aromatario M. Caffeine‐related deaths: manner of deaths and categories at risk. Nutrients. 2018;10(5):611. doi: 10.3390/nu10050611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray A, Traylor J, eds. Caffeine toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2022:2022. [Google Scholar]
- 4. Elbokl M, Randall I, Lok C. Severe caffeine intoxication treated with hemodialysis: a case report. Kidney Med. 2021;3(2):299‐302. doi: 10.1016/j.xkme.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamijo Y, Takai M, Fujita Y, Usui K. A retrospective study on the epidemiological and clinical features of emergency patients with large or massive consumption of caffeinated supplements or energy drinks in Japan. Intern Med. 2018;57(15):2141‐2146. doi: 10.2169/internalmedicine.0333-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gummin DD, Mowry JB, Beuhler MC, et al. 2020 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 38th annual report. Clin Toxicol. 2021;59(12):1282‐1501. doi: 10.1080/15563650.2021.1989785 [DOI] [PubMed] [Google Scholar]
- 7. Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479‐498. [DOI] [PubMed] [Google Scholar]
- 8. Kheirkhah S, Bahmani K. A rare case of fenpyroximate poisoning with presenting anticholinesterase poisoning signs. Clin Case Rep. 2020;8(12):2406‐2408. doi: 10.1002/ccr3.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paal P, Pasquier M, Darocha T, et al. Accidental hypothermia: 2021 update. Int J Environ Res Public Health. 2022;19(1):501. doi: 10.3390/ijerph19010501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brian F, Lawrence Z, Brian LM. The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus. 2021;13(5):e15032. doi: 10.7759/cureus.15032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mills EM, Weaver KL, Abramson E, Pfeiffer M, Sprague JE. Influence of dietary fats on ecstasy‐induced hyperthermia. Br J Pharmacol. 2007;151(7):1103‐1108. doi: 10.1038/sj.bjp.0707312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujihara J, Yasuda T, Kimura‐Kataoka K. Two fatal cases of caffeine poisoning and a review of the literatur. Shimane J Med Sci. 2017;34:55‐59. [Google Scholar]
- 13. Bioh G, Gallagher MM, Prasad U. Survival of a highly toxic dose of caffeine. BMJ Case Rep. 2013;2013:bcr2012007454. doi: 10.1136/bcr-2012-007454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu Y, Converse C, Lyons MC, Hsu WH. Neural control of sweat secretion: a review. BDJ. 2017;178(6):1246‐1256. doi: 10.1111/bjd.15808 Epub 2018 Apr 25. [DOI] [PubMed] [Google Scholar]
- 15. Nelson SW, Goldfrank LR, Hoffman RS, et al. Goldfrank's Toxicologic Emergencies. 11th ed. McGraw‐Hill; 2018:29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


