Abstract
Colorectal cancer (CRC) is a common and preventable cancer. CRC screening is underutilized, particularly within medically underserved communities. Most interventions aimed at increasing CRC screening are delivered through primary care clinics. Pharmacies are more accessible than traditional primary care settings and may be ideally suited for delivering CRC screening and increasing access. Fecal immunochemical test is an at-home, stool-based CRC screening test that could be distributed through pharmacies. The purpose of our study was to assess patient perspectives on receiving fecal immunochemical test-based CRC screening through pharmacies. We conducted semi-structured interviews with participants residing in North Carolina and Washington. Interviews explored acceptability and intervention design preferences for a pharmacy-based CRC screening program. The interview guide was informed by Andersen’s Healthcare Utilization Model and the Theoretical Domains Framework. Interviews were conducted at the University of North Carolina at Chapel Hill and Fred Hutchinson Cancer Research Center, audio-recorded, and transcribed. Patients perceived a pharmacy-based CRC screening program to be highly acceptable, citing factors such as ease of pharmacy access and avoiding co-pays for an office visit. Some concerns about privacy and coordination with patients’ primary care provider tempered acceptability. Trust and positive relationships with providers and pharmacists as well as seamless care across the CRC screening continuum also were viewed as important. Patients viewed pharmacy-based CRC screening as an acceptable option for CRC screening. To improve programmatic success, it will be important to ensure privacy, determine how communication between the pharmacy and the patient’s provider will take place, and establish closed-loop care, particularly for patients with abnormal results.
Keywords: colorectal cancer, screening, pharmacy, FIT, uninsured
Patients are open to the idea of receiving colorectal cancer screening through their pharmacy, leading to possibilities for developing pharmacy-based programs to increase screening access.
Graphical Abstract
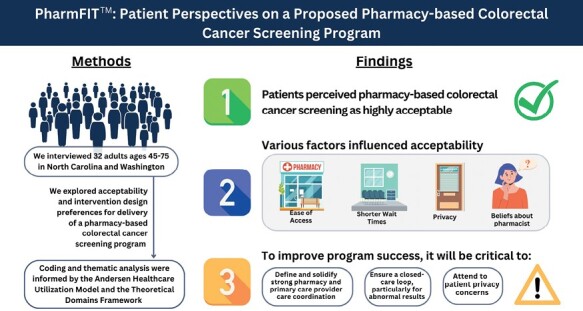
Implications.
Practice: Patients are open to receiving stool-based colorectal cancer screening through pharmacies primarily for its ease of access, while also desiring assurances of privacy and coordination with their primary care provider.
Policy: Effective and desirable pharmacy-based colorectal cancer screening programs will need to ensure patient privacy and establish a medical neighborhood with strong linkages with primary care providers, particularly to ensure appropriate follow-up of abnormal screening results.
Research: Future implementation research should consider patients’ needs and expectations when designing and implementing pharmacy-based colorectal cancer screenings that would impact adoption and use of this cancer prevention service.
Introduction
Colorectal cancer (CRC) is the third most common cancer, accounting for a projected 52, 580 deaths in men and women in the USA in 2021 [1]. CRC screening is effective at detecting cancer early, when it is more treatable, or preventing cancer from ever developing [2]. With some variation, all CRC screening modalities illustrate notable reduction of CRC incidence and mortality [3]. Compared with no CRC screening, CRC screening substantially increases life expectancy and reduces lifetime numbers of CRC cases and deaths [4]. Recognizing screening effectiveness, both the United States Preventive Services Task Force and the American Cancer Society recommend screening for all adults aged 45–75 [2].
The Healthy People (HP) 2030 objective for use of cancer screening tests includes increasing the proportion of men and women 50–75 years of age to be screened for CRC, with a target of 74.4% [5]. In 2016 and 2018, respectively, 20% of eligible adults in the USA had never been screened and 28% were not up to date with screening in 2020 [6]. To promote awareness and action, the National Colorectal Cancer Roundtable launched an “80% in Every Community” initiative in collaboration with local clinics, health plans, employers, and local governments to achieve an 80% CRC screening rate among all eligible adults in the USA [7]. The campaign focuses on reducing disparities in CRC mortality by addressing multilevel barriers in communities with suboptimal screening rates.
Maintaining regular CRC screening substantially reduces risk of death and poor health outcomes from CRC [8]. Unfortunately, low screening rates persist and are particularly pronounced within medically underserved communities [9]. While screening rates have increased in these communities over the past two decades, socioeconomic and geographic disparities persist [10]. For example, a cross-sectional study of Medicare enrollees found a dose–response relationship between education or income and CRC screening: the less education or the lower income of a person, the lower their screening rates [9].
One recommended CRC screening modality is the fecal immunochemical test (FIT) [2]. FIT identifies blood in the stool, which can be caused by pre-cancerous growths (polyps) in the colon and rectum, and is recommended for patients at average risk for CRC (i.e. persons without a family history of CRC, and without a personal history of CRC, polyps, or inflammatory bowel diseases) [2]. If blood is present, a follow-up colonoscopy is required to identify the reason for the presence of blood. Compared with colonoscopy, this test is relatively inexpensive, can be completed at home by collecting a minute stool specimen, does not require a prescription or bowel preparation, is low risk, and can be sent via mail directly to a medical lab for processing. FIT is also significantly less expensive compared with DNA stool tests (i.e. Cologuard®). These aspects may provide an advantage for communities where lack of health insurance and poor access to primary care and other health care resources are barriers to screening.
Community pharmacies (retail pharmacies located in the communities in which they serve) have been shown to be one of the most accessible sources of health care in the USA [11]. Due to easy access, proximity, and the frequency of picking up prescription refills, community members visit pharmacies two to three times more frequently than their primary care provider’s (PCP) office [12]. Over time, community pharmacies have broadened their scope of practice beyond dispensing medication by incorporating primary care and preventive services into their services, and continue to do so [13]. In the context of their expanding role, community pharmacies may offer a new and more accessible avenue for distributing CRC screening tests, thus increasing CRC screening rates.
Despite the promise of expanded community pharmacy services, there are patient-level barriers such as concerns about pharmacist training and ability to provide services outside the traditional scope, privacy and confidentiality in the pharmacy setting, and cost of services [14–16]. Other factors might support expansion, including patient compliance with pharmacist recommendations and perceiving community pharmacies as more accessible and convenient than a primary care visit [17–19].
Although expanded community pharmacy services have been explored in other contexts [20, 21], we are unaware of any study published to date evaluating patient perceptions of pharmacy-based CRC screening. To address this gap, this study sought to explore patient acceptability of the use of community pharmacies as a non-traditional distribution site for FIT kits. We were particularly interested in learning how patients currently use pharmacy services, their thoughts about receiving a FIT kit from a pharmacist (including FIT eligibility assessment), their preferences for notification of results, and their vision of their PCP’s role in a pharmacy-based CRC screening program. For the purposes of this study, we refer to the hypothetical program as “PharmFIT™.”
Method
Design
This study represents one aspect of a larger formative, observational study exploring patient, provider, and pharmacist perceptions of a PharmFIT™ program. The parent study includes interviews with patients, pharmacists, and PCPs, and national surveys of patients and pharmacists regarding acceptability and feasibility of pharmacy-based CRC screening.
The investigation described here focuses on the patient perspective. We used a qualitative descriptive design to explore patient perspectives regarding CRC screening via community pharmacies. Qualitative descriptive designs focus on describing participant responses with minimal analyst interpretation (i.e. staying close to the data) [22]; this design is well suited to our study aim of learning what people think of a hypothetical pharmacy-based CRC screening program.
Guiding constructs
Constructs from Andersen’s Healthcare Utilization Model [23], the Theoretical Domains Framework (TDF) [24], and the Consolidated Framework for Implementation Research (CFIR) [25] guided data collection (by informing interview question development) and analysis (in codebook development and by informing interpretation). Andersen’s model is used to assess factors that influence utilization of health care services and is particularly useful for understanding how and why certain services are used [23]. TDF has been largely used to inform intervention development by identifying barriers and facilitators that influence individuals’ behaviors, in this case, the behavior of utilizing pharmacy-based CRC screening [24]. We used all constructs from the Andersen Health Utilization model (e.g. enabling resources, personal health practices, use of health services) and many of the Theoretical Domains Framework constructs, including: knowledge; intentions; goals; memory, attention, and decision processes (specifically, the decision processes aspect); environmental context and resources; and emotion. We selected one CFIR construct, “relative advantage,” defined as “stakeholders' perception of the advantage of implementing the intervention versus an alternative solution" [25]. We selected this construct for its suitability in framing and eliciting participant preferences for one form of CRC screening over another.
Participants
Eligible participants were between the ages of 45 and 75 residing in both rural and urban geographic locations in North Carolina (NC) and Washington (WA). The NC catchment area included Orange County, NC, a primarily urban county with about 1/3 of the population residing in rural parts of the county, located in central NC [26]. The catchment area for WA included King, Kitsap, Snohomish, and Pierce counties. All are within western WA and are predominantly urban or semi-urban with outlying rural communities [27].
Recruitment
Participants were recruited using convenience sampling via social media advertising, email communication, and clinic-based recruitment. No participants were previously known to recruiters or interviewers. The approach varied by site: the research team in NC used a university-based general listserv and the team in WA used a combination of in-person recruitment at clinics/health fairs and social media advertisements.
Materials and procedures
The interview guide was developed by the full study team, including health services researchers, implementation scientists, behavioral scientists, pharmacists, and a PCP. The guide was cognitively tested with a convenience sample of two participants meeting the CRC screening age criterion (ages 45–75). Topics included: prior experiences with and knowledge of CRC screening; current pharmacy access and use; attitudes and beliefs about home-based CRC screening; and perspectives on receiving CRC screening through a pharmacy. The interview guide covered the basic steps of FIT-based CRC screening, including that pharmacy-based screening would entail a pharmacist determining the appropriateness of screening using FIT (Supplementary Material 1, Interview Guide). When asking opinions about CRC screening in a pharmacy setting, interviewers defined the process as: talking to a pharmacist about whether FIT screening is “right for you” (i.e. assessing eligibility for FIT-based screening); getting a FIT from the pharmacist; and receiving either negative or positive results, including the need for a follow-up colonoscopy with a positive result.
Study staff emailed an IRB-approved consent form to participants several days prior to the scheduled interview. Participants provided verbal consent at the time of the interview and completed a brief demographic questionnaire at the conclusion of the interview.
Interviews were with one interviewer and one participant and were arranged via telephone or email and conducted either in person or via telephone depending on patient preference. To ensure a shared understanding of the CRC screening process using a FIT kit, interviewers read a standardized introduction prior to asking questions about CRC screening. Interviews were conducted by trained study staff with many years’ experience, audio-recorded, and transcribed. North Carolina interviews were conducted by M.W. and J.R., and Washington interviews were conducted by D.L.A., P.S., and D.v.R.
Data collection and analysis
We conducted a total of 32 in-depth interviews with participants from August 2019 to November 2019, including 12 from NC and 20 from WA. At the time of the interview, participants were asked to report basic characteristics of age, gender, race, ethnicity, education level, and CRC screening history (Table 1). Interviews averaged 24 min in length.
Table 1.
Participant demographics and CRC screening history
| Participant characteristics | North Carolina (N = 12) n (% or range) |
Washington (N = 20) n (% or range) |
|---|---|---|
| Average age in years, SD (range)a | 61 years, SD = 8 (50, 73) | a 62 years, SD = 9 (46, 75) |
| Female | 6 (50%) | 16 (80%) |
| Male | 6 (50%) | 4 (20%) |
| Race | ||
| White | 8 (67%) | 17 (85%) |
| Black or African American | 3 (25%) | 1 (5%) |
| Other or more than one | 0 (0%) | 2 (10%) |
| Not reported | 1 (8%) | 0 (0%) |
| Ethnicity | ||
| Hispanic | 0 (0%) | 1 (5%) |
| Non-Hispanic | 10 (83%) | 19 (95%) |
| Not reported | 2 (17%) | 0 (0%) |
| Education level | ||
| <High school | 0 (0%) | 0 (0%) |
| High school or GED | 0 (0%) | 1 (5%) |
| Some college | 0 (0%) | 5 (25%) |
| College or >4-year college graduate | 11 (92%) | 13 (65%) |
| Not reported | 1 (8%) | 1 (5%) |
| Aware of home-based CRC screening tests | 12 (100%) | 19 (95%) |
| Prior CRC screeningb | ||
| Never screened | 1 (8%) | 3 (15%) |
| Colonoscopy or flexible sigmoidoscopy | 10 (83%) | 13 (65%) |
| FIT or FOBT | 4 (33%) | 10 (50%) |
| >1 screening method | 3 (14%) | 5 (25%) |
FOBT, fecal occult blood test; GED, general equivalency diploma; SD, standard deviation.
aAge sample size for Washington participants is n = 19.
bSome participants utilized more than one screening test.
The study team analyzed interviews using directed content analysis, in which we developed codes a priori based on theory and our previous work as well from our read of the transcripts [28]. We began with a rapid turnaround analysis of the transcripts [29]. The team first developed topical domains drawn from the interview guide and created a rapid summary form that listed the domains coupled with their corresponding interview questions. Using the summary form as a guide, team members then abstracted the same transcript, mapping interview content to the topical domains listed on the form. The team met to review the summaries to reach consensus regarding the appropriate level of detail for future abstractions. Remaining transcripts were divided among the team members for summarizing. The final step involved combining the summaries into a matrix depicting each transcript (in a row) by interview guide topical domains (in columns). Matrices were used to explore themes and patterns across the data.
Following rapid analysis, transcripts were coded using a codebook constructed from the interview guide and rapid summaries (Supplementary Material 2, Codebook). Coders (D.L.A. and A.I.) performed multiple rounds of consensus coding, in which the same transcript was reviewed and coded by each coder to ensure consistent code application. Each transcript was then independently coded by one member of the coding team and reviewed by another team member who documented any discrepancies in code application. Discrepancies were resolved through group discussion with the larger team. Code co-occurrence tables and queries were used to identify themes and constructs. Analyses included repeated reading of transcripts, memo-writing to explore emerging ideas and themes [30], and group discussions of findings. ATLAS.ti version 8 was used to support data analysis and management. Study method and results are reported following the Consolidated criteria for reporting qualitative research (COREQ) framework [31]. Throughout the manuscript, participant quotations are italicized and identified by their state of residency (NC or WA) and a unique identifier number. Some quotations included in the manuscript were edited for length and clarity.
Results
Patient characteristics and pharmacy use
Table 1 depicts the sample characteristics stratified by location (NC or WA). The sample was primarily urban, White, and highly educated. Most interviewees reported prior experience with CRC screening, primarily colonoscopy but also stool-based tests such as FIT or Cologuard® (Table 1).
Nearly all respondents identified chain pharmacies (e.g. CVS, Walgreens, Walmart) as their primary pharmacy, with a few using local, independent pharmacies. Respondents reported living within a few miles, or about a 10-min drive, from their pharmacy; a notable exception included one WA participant residing 30 min from the nearest pharmacy. All participants visited pharmacies at least annually and typically monthly or more. Most mentioned proximity to home or work as the main reason for choosing a pharmacy, while others identified cost as a factor, either switching from a higher- to a lower-cost pharmacy or utilizing a particular pharmacy based on insurance coverage for a specific prescription.
Few respondents reported discussing health topics with their pharmacist beyond medication use and/or prescription side effects. Health topics that were discussed mainly included vaccines (e.g. flu, shingles), but also advice on over-the-counter medications and personal care such as nutrition or advice about how to treat insect bites. Experiences with receiving pharmacy-based medical services and discussing non-prescription health topics were largely positive or neutral.
FIT awareness and knowledge
All but two participants said they were aware of home stool-based testing, with many indicating their awareness stemmed from television commercials with the “dancing box” (presumably Cologuard®), and/or from their experience with completing home-based stool testing for CRC screening. In fact, FIT appealed to many largely because of ease and convenience, avoiding colonoscopy and its associated bowel preparation, and for the lower cost of FIT compared with colonoscopy.
Although aware of the existence of FITs and home-based stool testing, many participants asked questions about FIT screening or made comments indicating incomplete understanding. Participants inquired about matters such as: how FIT eligibility is determined; details about how to use FIT kits; FIT effectiveness and false positive rates; cost and insurance coverage; and whether a prescription would be necessary. Examples of misconceptions about FIT-based screening included lack of clarity about: what a FIT is (equating FIT with Cologuard®); how to use the FIT (assuming a FIT sample would include a full stool rather than a very small specimen); and appropriate use of FIT (expressing a desire to couple FIT with colonoscopy for added “peace of mind”).
PharmFIT™ acceptability
Nearly all participants expressed their willingness to participate in a pharmacy-based CRC screening program. This was true regardless of previous experiences with CRC screening. Interviewees said they would be interested in such a program primarily for its convenience and ease of access.
It’s a lot easier for me to get to see a pharmacist than it is to see a physician – getting a simple prescription from my doctor takes multiple calls before I can actually get them to call the pharmacy with the prescription. Whereas I can walk to a pharmacy and right there and then I can speak to somebody.—NC 001
PharmFIT™ also appealed to participants because it meant not having to visit the PCP’s office, which they said necessitated a co-payment for the visit and sometimes involved having to schedule appointments weeks or months in advance.
You know, (for a PharmFIT™ program) you show up, you go to the counter, you get handed the thing, you pay for it, and you go. That would be super convenient, as opposed to going to a primary care physician, where you’re then cranking out the medical insurance, and paying your copay, and doing all that to end up with a take-home kit.—NC 014
Eligibility determination and FIT kit distribution
When asked how they feel about a pharmacist talking to them about CRC screening, their eligibility for FIT-based screening, and receiving a kit from a pharmacist (versus a PCP), a few participants wanted to be assured of the pharmacist’s training in CRC screening. Most, however, said they were comfortable with the idea and trusted the pharmacist, with the assumption that they have training, knowledge, and skills by virtue of their pharmacy degree.
I wouldn’t have a problem with that. I mean, I’m figuring that they’re a professional. They should know what they’re talking about, so, yeah, I wouldn’t have a problem with it.—NC 001
When queried about preferences for what CRC screening and FIT kit information they would like and how they would like to receive that information, participants expressed a desire for clear, basic instructions, provided on a one-page handout for them to take home for later reference.A couple respondents preferred a link to a video they could access online. Preference for mode of receiving reminders for the annual test varied greatly, with no clear preferences for reminders via email, text, or electronic health record patient portal.
(I would like) just nice, clear, concise bullet steps, because it’s actually a simple process. But when you look at all the material they give you, you think it’s much more complicated than it is.—WA 008
FIT results notification and follow-up of abnormal results
When asked from whom they would want to hear test results—the pharmacist or the PCP—several participants reported not having a preference either way. Most participants, however, indicated a clear preference for hearing results from their PCP—this was especially true with abnormal findings. While participants said they would trust their pharmacist as much as their doctor to relay results, they preferred hearing results from their doctor given their established patient–provider relationship, or for reasons such as trusting their provider with colonoscopy referral and follow-up, needing a PCP referral for insurance purposes, or concerns about limited privacy in a pharmacy.
Because I have a doctor, and I have a good relationship with her, I’d prefer to hear (test results) from her. I guess if the pharmacist was able to answer some questions and was medically trained to do so, then I think I would be okay with that, knowing that I could have a follow-up conversation with my doctor.—WA 004
Others preferred to hear results from the PCP to prevent miscommunication or missteps, because CRC screening has “too many pieces to the puzzle” and including the pharmacist would have “too many people involved.” Similarly, hearing results from a pharmacist wasn’t a problem per se, except that it might be unnecessary given the desire/need to talk to their PCP anyway, and therefore it would create more effort for the individual.
I think that the way things work right now, if I didn’t do it through my doctor, then it’s my responsibility (to report results). It’d be great if the pharmacist did it. The way it is now, I don’t know that that loop exists today. I would love (a closed-care loop) because that leaves less for me to do.—WA 014
Commonly, participants suggested that the pharmacist communicate results to their PCP for follow-up, sometimes likening the process to mammograms as an example for how results can be reported, with the radiology department representing the proposed pharmacy role (i.e. radiology reports results to PCP and to patient).
I would think it would be better if the pharmacy reported (results) to my doctor just as when I get a mammogram, they report it to my doctor.—WA 004
If the pharmacist initiates the process, participants expected that the pharmacist should be able to provide a list of colonoscopy referrals and that those referrals would be covered by the patient’s insurance and be geographically close to where the patient resides. Participants raised concerns about those without insurance, expecting that any necessary follow-up would ensure linkage to affordable colonoscopy.
I’m comfortable just having the pharmacist refer me to my doctor for follow-up testing, but because they’re the ones handing it out, I also think that they should have an ability to refer me on to where I might get testing, particularly for patients who do not have insurance. I would hope that you haven’t just given someone news about a big, expensive test and left them hanging with that.—WA 004
Concerns about a PharmFIT™ program
Despite wide appeal, a few participants indicated that they would not use a program like PharmFIT™—not because it was pharmacy based, but because of the screening modality. These participants said they preferred colonoscopy over FIT screening because direct visualization of the colon is better than FIT at detecting problems, while others expressed a desire to learn more about how such a program would work before fully committing to the idea. Some expressed concern that the pharmaceutical industry might be driving pharmacy promotion of CRC screening. Even those enthusiastic about a PharmFIT™ program had some recommendations related to privacy, coordination with their PCP, and follow-up of abnormal results.
Privacy was a common concern of several participants. Participants understood that they would need to speak to a pharmacist to determine their eligibility and to receive and learn about how to use the FIT kit, and expressed worry about the lack of privacy at the pharmacy counter. Many did not trust that others would not overhear a conversation.
(A con is) probably just, you know, the privacy issue. I mean, I don’t think anyone wants to get a, you know, ‘Mr. Jones, your FIT kit is ready at counter five,’ broadcast over the store’s intercom.—WA 010
Among those who were hesitant, participants expressed some concerns about health information privacy and the nature of the discussion, finding stool tests embarrassing for others to know about. They wanted bags that would hide the contents from others, including discreet mailers for mailing back samples.
(I prefer) if the stuff that I walk out with would be in a discreet bag, you know, I don’t want my whole neighborhood knowin ‘what I’m doin’.—WA 024
Participants were open to pharmacists participating in delivering CRC screening but wanted it in coordination with their PCP, primarily because of established relationships with and trust in their PCP, PCP knowledge of the patient’s health history, and because they believed PCP involvement would decrease miscommunication. Several said they would want to talk with their doctor before completing a FIT through the pharmacy.
I would think I would have that [CRC screening] be a first conversation with my doctor, and if it could be done through a pharmacy, no problem. But I’d wanna know that that’s part of my overall general care, it isn’t just done when I’m picking up my headache medicine or something.—WA 014
Responses varied regarding the desired level of pharmacist involvement. Sometimes participants referred to the pharmacist as a “middleman,” preferring that the pharmacist role be limited to FIT kit distribution and education (how to do the kit) but not eligibility assessment or results communication. Others were fine with pharmacist involvement at all levels so long as the pharmacist was trained and sensitive to the whole screening process, not “just handing out kits.” Those who were comfortable hearing results from pharmacists still wanted their PCP involved.
(Pharmacists) don’t have a knowledge base of the whole health history of the individual patient. So, I’d be concerned if they tried to give out any advice other than the training that they got for giving out the kit and returning it.—NC 004
I see the pharmacist as the middleman. Hands off the kit or mails the kit – lets my doctor know I have a kit. But the original conversation was between me and my doctor and I’d like the follow-up conversation between me and my doctor.—WA 024
Following discussion of what a PharmFIT™ program might look like, participants were asked to identify any pros and/or cons associated with a pharmacy-based FIT screening program. The most frequently mentioned proswere convenience and ease of access, in part due to pharmacists being easier to reach in the event of questions and because, unlike PCP offices, no appointment would be necessary. Cons were more varied and frequently prefaced with “It’s only a con if…”—i.e. participants seemed to feel that the con was a drawback only if a particular condition was unmet (e.g. only if the PCP is not involved, only if the pharmacist is untrained, only if the pharmacist is unavailable to answer questions). If the condition was satisfied, then PharmFIT™ would be acceptable. Many people stated that they saw no cons to a PharmFIT™ program.
Discussion
Acceptability and preferences
Nearly all participants in our study found the idea of pharmacy-based CRC screening to be highly acceptable, primarily for reasons of convenience, ease of access, and because visiting a pharmacy precluded the need for scheduling a primary care office visit with its associated co-pay. Our findings echo a study by Steckowych et al., in which focus group participants aged 50 and older were presented with three scenarios describing pharmacy services beyond dispensing medication (administration of non-immunization injectables, refills for chronic medications, and pharmacist diabetes management) and asked to reflect on barriers and facilitators to their use of these hypothetical expanded services [32]. Consistent with our population, the focus group participants emphasized convenience, timeliness (getting in sooner than with a doctor), and acceptability as important when considering expanded pharmacy services. They also expressed concerns about potential increased costs associated with a pharmacy-based screening program, in contrast to our population which emphasized the benefit of receiving a FIT test without the accompanying insurance co-pay necessary for a visit to their doctor’s office.
Unlike previous literature on patient discomfort with stool tests [33], the participants we interviewed expressed no such concerns; regardless of previous experience with at-home CRC screening tests, they were open to completing a stool-based test at home and could readily see the advantages of a PharmFIT™ program.
Preferences for communication about a PharmFIT™ program varied greatly (such as preferences for learning about a PharmFIT™ program, communication about CRC screening and how to do the kit, communication about results, and results follow-up). Variation in responses suggests that a variety of methods for communication should be used and patients should be given options to choose their preferred mode.
Frequent mention of elements such as simple instructions included in a private bag with the FIT kit, the option to return the FIT via mail or drobox if done discreetly, and the ability to call in with questions suggest that these elements would be important to include in a pharmacy-based CRC screening program (Supplementary Material 3, Proposed Design Elements of a PharmFIT™ Intervention from the Patient Perspective).
The primacy of privacy
Although most participants supported the idea of a PharmFIT™ program, they wanted assurance of privacy. Indeed, privacy was raised as a primary concern for nearly all participants. Our findings echo others who also identified patient concerns about privacy [16, 32]. Le and Braunack-Mayer acknowledge that pharmacy settings are often not private, which can be especially problematic with stigmatized conditions such as opioid dependency [34]. Discomfort or embarrassment about the nature of CRC screening (i.e. stool-based) [33] adds a layer of privacy worries for participants. This might be especially true for residents of small communities, where anonymity is challenging. Thus, our findings suggest that any discussion of personal health information can be concerning in the pharmacy setting and that private spaces should be designated or created for screening services [35]. Physical alterations of the pharmacy might be needed to provide private consult spaces [36].
The expectation of care coordination
Participants emphasized the importance of coordinated communication between the pharmacist and their PCP, primarily around results communication but also related to pharmacists having knowledge of the patient’s health history. Importantly, coordinated care and collaboration with other health professions is also an expectation of the field of pharmacy and one of the key components of the pharmacist’s patient care process [37], suggesting that any effort to develop and sustain CRC screening care coordination between pharmacists and PCPs would be strongly endorsed by pharmacists.
Lack of care coordination could be a major barrier for patient participation in pharmacy-based screening. Participants in other studies raised similar concerns about lack of coordination [16, 32, 38]. For example, a qualitative study assessing the community pharmacist role (largely around dispensing and drug interactions) uncovered insufficient communication between pharmacists and PCPs as a barrier to community pharmacist counseling [38].
In the context of pharmacy-based CRC screening, coordinated care will likely require, at a minimum, a willingness of all parties to work toward the goal of coordinated care and the creation of specific agreements around communication and attention to trust. For example, Bradley et al. propose a conceptual model for coordination between general practitioners and community pharmacists which highlights the importance of trust between pharmacists and health care providers [39]. Qualitative work by McKeirnan and Garrelts MacLean assessed provider and community pharmacist willingness to work together focused on minor ailments in rural communities [40]. Their findings support the use of pharmacists to treat minor ailments when using a signed collaborative drug therapy agreement that includes standards for communication between pharmacists and physicians. Others have recommended use of a care manager to ensure seamless coordination [41]. Electronic care plans have also shown promise in facilitating pharmacist–PCP communication [42].
Participants’ clear desire for health care provider involvement in pharmacy-based CRC screening coupled with their concerns about whether solid communication between pharmacies and primary care is in place emphasizes the critical importance of robust coordinated care. Addressing coordinated care will likely challenge electronic health record systems, as current communications rely on fax and ascertaining CRC screening eligibility might require access to basic patient information. This, in turn, will also raise issues of health information privacy that will need addressing.
The role of trust
Trust is essential to quality health care and a prerequisite to patient adherence and satisfaction [43, 44]. Unsurprisingly, in our study trust emerged as an underlying element across the patient interviews, infusing virtually all aspects of participants’ reflections on what they think and feel about receiving CRC screening through a community pharmacy. Respondent thoughts about PharmFIT™ were underpinned by questions of trust in such things as pharmacist training to provide CRC screening, FIT as a valid screening test, and CRC screening as valid in a pharmacy and not part of a pharmaceutical industry sales pitch. Participants also needed to trust that their doctor believed CRC screening to be necessary and appropriate for the individual at that time and through the pharmacy venue, that the pharmacist and PCP will communicate effectively, and the health care system will function as intended to ensure seamless, robust communication and coordination.
Questions of trust suggest that introducing new pharmacy services may initially be met with some level of resistance. Participants in our study indicated trust in the pharmacist by virtue of their professional capacity and training but noted trust in their provider because of an existing relationship with them. Pharmacists may find it challenging to develop similar relationships in part because pharmacy settings do not typically support pharmacist–patient relationships beyond medication dispensing [45]. Pharmacy-based CRC screening programs can be seen as a way of building relationships that maintain a pharmacist–patient relationship but will require a reimagining and reconfiguring of pharmacy settings and structures to promote trust. In the interest of building on the patient–PCP relationship and ensuring care coordination, CRC screening in pharmacies may also require bringing the pharmacist into the patient care team more formally as part of a collaborative care model [46], e.g. through signed collaborative agreements developed in the context of care coordination [40, 47].
Trust is bidirectional—for patients to trust pharmacy-based CRC screening, health care providers, programs, and systems will need to be trustworthy. One way of promoting trustworthiness is through communication that is “credible, clear, and complete” [48]—in the context of PharmFIT™ this might include, e.g., providing evidence of FIT validity, demonstrating pharmacist knowledge about CRC screening, and making explicit the linkages between the pharmacist and patient’s PCP.
Consistent with some of our participants questioning pharmacists’ financial motivations in promoting CRC screening, authors Gregory and Austin note that lack of trust in pharmacies is due at least in part to the pharmacies as places of retail (versus strictly health care settings) [49]. Their qualitative study explored how trust is built between pharmacists and patients—they identified four trust-promoting factors that echoed our findings and underscore their importance when thinking about fostering trust for new services such as PharmFIT™. These factors include accessibility (ready availability of pharmacists to answer questions); affability (pharmacist friendliness, interpersonal chemistry); acknowledgment (listening and explaining things clearly); and respect (honoring privacy and confidentiality) [49]. Of these, the themes of accessibility, acknowledgment, and respect were particularly evident throughout our interviews.
Misunderstandings about FIT screening
Finally, our findings suggest a need for clarity around FIT screening. Lack of awareness may be due in part to PCP reliance on colonoscopy as the primary method of screening, suggesting they are less likely to emphasize FIT screening in clinical practice [50]. Awareness of home CRC screening related to Cologuard® television ads presents an opportunity for increasing awareness about CRC in general, and perhaps increases acceptability of home-based stool testing, but it also leads to conflating FIT with Cologuard®. Patient education about FIT screening and how it compares to other CRC screening tests would be important to include with a PharmFIT™ program. For example, patient materials could include illustrated instructions for completing the FIT as well as written FAQs (Frequently Asked Questions) that address common misconceptions. Many FIT screening patient education materials already exist, but less common is information comparing FIT to Cologuard®.
Limitations
Our study has some important limitations. Because this formative work was meant to inform development of a PharmFIT™ program, we utilized convenience sampling. Thus, although the intent and promise of this work was to seek ways to improve access to CRC screening for populations who are medically underserved, the resulting sample was homogenous, consisting primarily of persons with high levels of education and those in urban settings. We are unable to speak to the acceptability of pharmacy-based FIT screening or design preferences among lesser-educated and more rural populations. Similarly, we are unable to speak to equity around geography, race, health care insurance status, or whether a patient has an established PCP. Still, even in our sample of high-resource populations, participants viewed pharmacy-based CRC screening as a welcome alternative to clinic-based distribution primarily for ease of access and avoiding co-pays, both of which might be relevant for rural and/or low-resource populations. Further, we tested the hypothesis that these findings will translate to rural populations, expanding this work by conducting a national survey with a diverse patient population and developing pilot PharmFIT™ programs in rural settings with the intent of shedding light on acceptability and reach in diverse populations. We found that rural participants had frequent use of and positive experiences with pharmacies, and willingness to use a pharmacy-based CRC screening program [51]. Future research should continue to explore preferences and issues of equity in lower-income, rural, and/or low-resource populations, including access to follow-up colonoscopy.
Conclusions
Patients perceived a pharmacy-based CRC screening program as highly acceptable, citing convenience and reduced costs (no co-pay) as advantages over traditional primary care clinic-based screening. Patients indicated trust in pharmacists’ capabilities to provide CRC screening but also desired PCP involvement in the process, especially regarding abnormal test results. Patient willingness to participate in a PharmFIT™ program was tempered by concerns about privacy and fractured communication between the pharmacist and their PCP. Pharmacy-based programs will need to devote attention to ensuring privacy throughout the entire screening process and building robust systems of communication and closed-loop patient care for patients to trust and utilize pharmacy-based CRC screening programs. Supplementary Material 3 provides suggested components to consider based on participant feedback. Next steps for our project include combining what we have learned from patient interviews with findings from surveys and interviews with pharmacists and PCPs. The collective learnings will inform pilot interventions in a small number of pharmacies in North Carolina and Washington.
Supplementary Material
Acknowledgments
The authors wish to thank Caroline Luther for assistance with the literature review, Ben Chilampath for assistance with environmental scans and data reporting, and Drs. Ben Urick and Macary Marciniak for expert guidance and critical review.
Contributor Information
Renée M Ferrari, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Gillings School of Global Public Health, Maternal and Child Health Department, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Dana L Atkins, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Mary Wangen, Center for Health Promotion and Disease Prevention, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Catherine L Rohweder, Center for Health Promotion and Disease Prevention, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Austin R Waters, Gillings School of Global Public Health, Health Policy and Management Department, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sara Correa, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Jennifer Richmond, Department of Social Sciences and Health Policy, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Dillon van Rensburg, Office of Community Outreach and Engagement, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Annika Ittes, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Olufeyisayo Odebunmi, Gillings School of Global Public Health, Health Policy and Management Department, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Rachel B Issaka, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Center, Seattle, WA, USA; Division of Gastroenterology, University of Washington School of Medicine, Seattle, WA, USA.
Rachel Ceballos, Division of Public Health Sciences, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Parth D Shah, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Stephanie B Wheeler, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Gillings School of Global Public Health, Health Policy and Management Department, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Alison T Brenner, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Center for Health Promotion and Disease Prevention, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of General Medicine and Clinical Epidemiology, The University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Conflict of Interest
Annika Ittes is employed by and owns stock in Genentech, Inc., as of May 2022. Rachel Ceballos received pilot funding for a project titled “A Multilevel Approach to Improving Colorectal Cancer Screening in Rural Communities of the FH/UW Catchment Area.” Stephanie Wheeler received grant money paid to her institution from The Pfizer Foundation, through December 2022. All other authors declare no conflict of interest.
Funding
This study is supported by the Centers for Disease Control and Prevention (CDC) of the United States Department of Health and Human Services (HHS) cooperative agreement #U48 DP006400 (MPI: Leeman/Brenner/Wheeler) and a donation by the Safeway Foundation to the Fred Hutchinson Cancer Center (PI: Shah). Dr Richmond is supported by grant number T32HS026122 from the Agency for Healthcare Research and Quality and a Loan Repayment Award from the National Cancer Institute (L60CA264691). The manuscript contents are those of the authors and do not necessarily represent the official views of, nor an endorsement by Safeway, the Fred Hutchinson Cancer Center, the University of North Carolina, the Centers for Disease Control/Department of Health and Human Services, or the United States government.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Welfare of Animals
This article does not contain any studies with animals performed by any of the authors.
Transparency Statements
(1) Study registration: This study was not formally registered. (2) Analytic plan preregistration: The analysis plan was not formally preregistered. (3) Analytic code availability: There is not analytic code associated with this study. (4) Materials availability: Materials used to conduct the study are not publically available.
Data Availability
Deidentified data from this study are not currently available in a public archive. Deidentified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author.
References
- 1. Siegel RL, Miller KD, Fuchs HEet al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Davidson KW, Barry MJ, Mangione CMet al.; US Preventive Services Task Force. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2021;325:1965–1977. 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 3. Lin JS, Perdue LA, Henrikson NBet al. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA 2021;325:1978–1998. 10.1001/jama.2021.4417 [DOI] [PubMed] [Google Scholar]
- 4. Knudsen AB, Rutter CM, Peterse EFPet al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA 2021;325:1998–2011. 10.1001/jama.2021.5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Increase the Proportion of Adults Who Get Screened for Colorectal Cancer—C-07—Healthy People 2030. health.gov. https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-adults-who-get-screened-colorectal-cancer-c-07 (29 September 2022, date last accessed). [Google Scholar]
- 6. Use of Colorectal Cancer Screening Tests. CDC. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm (29 September 2022, date last accessed). [Google Scholar]
- 7. 80% in Every Community . National Colorectal Cancer Roundtable. https://nccrt.org/80-in-every-community/ (30 September 2022, date last accessed). [Google Scholar]
- 8. Edwards BK, Ward E, Kohler BAet al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–573. 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doubeni CA, Laiyemo AO, Reed Get al. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev 2009;18:2170–2175. 10.1158/1055-9965.EPI-09-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doubeni CA, Laiyemo AO, Major JMet al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012;118:3636–3644. 10.1002/cncr.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berenbrok LA, Tang S, Gabriel Net al. Access to community pharmacies: a nationwide geographic information systems cross-sectional analysis. J Am Pharm Assoc (2003) 2022;62:1816–1822. 10.1016/j.japh.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 12. Berenbrok LA, Gabriel N, Coley KCet al. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among Medicare beneficiaries. JAMA Netw Open 2020;3:e209132. 10.1001/jamanetworkopen.2020.9132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goode J-V, Owen J, Page Aet al. Community-based pharmacy practice innovation and the role of the community-based pharmacist practitioner in the United States. Pharmacy (Basel) 2019;7:106. 10.3390/pharmacy7030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brewer A, Hanna C, Eckmann Let al. Patient awareness, willingness, and barriers to point-of-care hepatitis C screening in community pharmacy. J Am Pharm Assoc (2003) 2018;58:S69–S72.e1. 10.1016/j.japh.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 15. Mospan CM, Gillette C, Wilson JA.. Patient and prescriber perceptions of depression screening within a community pharmacy setting. J Am Pharm Assoc (2003) 2020;60:S15–S22. 10.1016/j.japh.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 16. Westrick SC, Hohmann LA, McFarland SJet al. Parental acceptance of human papillomavirus vaccinations and community pharmacies as vaccination settings: a qualitative study in Alabama. Papillomavirus Res 2017;3:24–29. 10.1016/j.pvr.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steltenpohl EA, Barry BK, Coley KCet al. Point-of-care testing in community pharmacies: keys to success from Pennsylvania pharmacists. J Pharm Pract 2017;31:629–635. 10.1177/0897190017735243 [DOI] [PubMed] [Google Scholar]
- 18. Cook DM, Moulton PV, Sacks TMet al. Self-reported responses to medication therapy management services for older adults: analysis of a 5-year program. Res Social Adm Pharm 2012;8:217–227. 10.1016/j.sapharm.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 19. Rickles NM, Skelton JB, Davis Jet al. Cognitive memory screening and referral program in community pharmacies in the United States. Int J Clin Pharm 2014;36:360–367. 10.1007/s11096-013-9904-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bright D, Worley M, Porter BL.. Patient perceptions of pharmacogenomic testing in the community pharmacy setting. Res Social Adm Pharm 2021;17:744–749. 10.1016/j.sapharm.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 21. Garrett DG, Martin LA.. The Asheville Project: participants’ perceptions of factors contributing to the success of a patient self-management diabetes program. J Am Pharm Assoc (Wash) 2003;43:185–190. 10.1331/108658003321480722 [DOI] [PubMed] [Google Scholar]
- 22. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health 2000;23:334–340. [DOI] [PubMed] [Google Scholar]
- 23. Babitsch B, Gohl D, von Lengerke T.. Re-revisiting Andersen’s behavioral model of health services use: a systematic review of studies from 1998–2011. Psychosoc Med 2012;9:Doc11. 10.3205/psm000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atkins L, Francis J, Islam Ret al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damschroder LJ, Aron DC, Keith REet al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Population, Estimate. Population & Growth, Orange County NC, May 2022. Access NC community demographics. https://accessnc.nccommerce.com/DemoGraphicsReports/pdfs/countyProfile/NC/37135.pdf (30 September 2022, date last accessed).
- 27. Defining Rural Population. HRSA. https://www.hrsa.gov/rural-health/about-us/what-is-rural (26 October 2022, date last accessed). [Google Scholar]
- 28. Hsieh H-F, Shannon SE.. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 29. Hamilton A. QUERI Implementation Research Group Cyberseminar—Rapid Qualitative Analysis: Updates/Developments . U.S. Department of Veterans Affairs Health Services Research & Development. 29 September 2020. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=3846 (15 February 2023, date last accessed). [Google Scholar]
- 30. Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. Nurse Res 2006;13:84. 10.7748/nr.13.4.84.s427702218 [DOI] [Google Scholar]
- 31. Tong A, Sainsbury P, Craig J.. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–357. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 32. Steckowych K, Smith M, Spiggle Set al. Building the case: changing consumer perceptions of the value of expanded community pharmacist services. J Pharm Pract 2019;32:637–647. 10.1177/0897190018771521 [DOI] [PubMed] [Google Scholar]
- 33. Gordon NP, Green BB.. Factors associated with use and non-use of the Fecal Immunochemical Test (FIT) kit for colorectal cancer screening in response to a 2012 outreach screening program: a survey study. BMC Public Health 2015;15:546. 10.1186/s12889-015-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le P-P, Braunack-Mayer A.. Perspectives on privacy in the pharmacy: the views of opioid substitution treatment clients. Res Social Adm Pharm 2019;15:1021–1026. 10.1016/j.sapharm.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 35. Mohiuddin AK. Pharmacist-patient relationship: commitment to care. Biomed J Sci Tech Res 2019;21:15588–15589. [Google Scholar]
- 36. Hattingh HL, Emmerton L, Ng Cheong Tin Pet al. Utilization of community pharmacy space to enhance privacy: a qualitative study. Health Expect 2016;19:1098–1110. 10.1111/hex.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Pharmacists’ Patient Care Process . JCPP. https://jcpp.net/patient-care-process/ (30 September 2022, date last accessed). [Google Scholar]
- 38. Tarn DM, Paterniti DA, Wenger NSet al. Older patient, physician and pharmacist perspectives about community pharmacists’ roles. Int J Pharm Pract 2012;20:285–293. 10.1111/j.2042-7174.2012.00202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bradley F, Ashcroft DM, Noyce PR.. Integration and differentiation: a conceptual model of general practitioner and community pharmacist collaboration. Res Social Adm Pharm 2012;8:36–46. 10.1016/j.sapharm.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 40. McKeirnan KC, Garrelts MacLean L.. Pharmacist, physician, and patient opinions of pharmacist-treated minor ailments and conditions. J Am Pharm Assoc (2003) 2018;58:599–607. 10.1016/j.japh.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 41. Turner K, Weinberger M, Renfro Cet al. The role of network ties to support implementation of a community pharmacy enhanced services network. Res Social Adm Pharm 2019;15:1118–1125. 10.1016/j.sapharm.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 42. Jindal N, Clifton C, Trahms Ket al. Community-based pharmacy use of the pharmacist eCare plan: a retrospective review. J Am Pharm Assoc (2003) 2021;61:S161–S166. 10.1016/j.japh.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 43. Khullar D. Building trust in health care—why, where, and how. JAMA 2019;322:507–509. 10.1001/jama.2019.4892 [DOI] [PubMed] [Google Scholar]
- 44. Goold SD. Trust, distrust and trustworthiness. J Gen Intern Med 2002;17:79–81. 10.1046/j.1525-1497.2002.11132.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haddad AM. Reflections on the pharmacist-patient covenant. Am J Pharm Educ 2018;82:6806. 10.5688/ajpe6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mey A, Knox K, Kelly Fet al. Trust and safe spaces: mental health consumers’ and carers’ relationships with community pharmacy staff. Patient 2013;6:281–289. 10.1007/s40271-013-0032-1 [DOI] [PubMed] [Google Scholar]
- 47. Choe HM, Lin AT, Kobernik Ket al. Michigan pharmacists transforming care and quality: developing a statewide collaborative of physician organizations and pharmacists to improve quality of care and reduce costs. J Manag Care Spec Pharm 2018;24:373–378. 10.18553/jmcp.2018.24.4.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castaldo S, Grosso M, Mallarini Eet al. The missing path to gain customers loyalty in pharmacy retail: the role of the store in developing satisfaction and trust. Res Social Adm Pharm 2016;12:699–712. 10.1016/j.sapharm.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 49. Gregory PA, Austin Z.. How do patients develop trust in community pharmacists? Res Social Adm Pharm 2021;17:911–920. 10.1016/j.sapharm.2020.07.023 [DOI] [PubMed] [Google Scholar]
- 50. Heidenreich S, Finney Rutten LJ, Miller-Wilson L-Aet al. Colorectal cancer screening preferences among physicians and individuals at average risk: a discrete choice experiment. Cancer Med 2022;11:3156–3167. 10.1002/cam4.4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brenner AT, Waters AR, Wangen Met al. Patient preferences for the design of a pharmacy-based colorectal cancer screening program. Cancer Causes Control 2023;19:1–14. 10.1007/s10552-023-01687-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from this study are not currently available in a public archive. Deidentified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author.


