Abstract
The present study clarified changes in physiological sensitivities of cultured Nieuwkoop and Faber stage 57 Xenopus laevis tadpole-organ-heart exposed to thyroxine (T4) using acetylcholine (ACh), norepinephrine (NE) and atropine. For preliminary life span and the chemical tests, 60% minimum essential medium (MEM), two types of modified Hank's balanced salt-solution-culture-media (MHBSS-CM) I and II containing relatively lower concentrations of amino acids and collagen were prepared. In preliminary lifespan-test of cultured tadpole hearts, the hearts maintained in 60% MEM was 50 days on average, whereas that of the tadpole-hearts in MHBSS-CMs was extended by 109 days on average, showing superior effectiveness of MHBSS-CMs. 4 min-stimulation by 5 × 10−9 M T4 tended to increase the tadpole heartbeat. 10−9 M ACh decreased the tadpole heartbeat. Frog-heart at 2–4 weeks after metamorphosis completion and tadpole heart treated with 5 × 10−10 M T4 for 45 h also responded to 10−9 M ACh, and low-resting hearts were restored to the control level with the competitive muscarinic antagonist 10−8 M atropine, whereas excessive exposure of 10−5 M atropine to T4-treated tadpole heart did not increase heartbeat in spite of the increased frog heartbeat over the control. 10−14 —10−12 M NE increase the tadpole heartbeat in a concentration-dependent manner, however, 10−12 M NE did not act to stimulate adrenergic receptors on both T4-treated tadpole- and the frog-hearts. These results suggest that T4 induces the desensitization of atropine-sensitive muscarinic and adrenergic receptors in organ-cultured tadpole-heart.
Keywords: Acetylcholine, Frog, Norepinephrine, Organ-cultured heart, Tadpole, Thyroxine hormone (T4), Xenopus laevis
Highlights
-
•
Preliminary life span test to evaluate performance of the culture media MHBSS-CM I and II supplemented with a lower concentration of amino acids, type I and III collagen indicates that X. laevis st. 57 tadpole organ-hearts under spontaneous beating condition is kept longer than 60% MEM.
-
•
Cultured anuran tadpole organ hearts do not lose high adrenergic (α1 and β1) and cholinergic sensitivities.
-
•
T4-induced two responses in the in vitro tadpole hearts; 1) adrenergic receptor activation by short time stimulation of 5 × 10−9 M T4, and 2) adrenergic and atropine-sensitive muscarinic receptor-desensitization by 5 × 10−10 T4 treatment for long time.
1. Introduction
In vitro amphibian organ heart with spontaneous beating is useful for conducting physiological, pharmacological and toxicological research. Advantages of isolated heart model are as follows 1) actions to various chemical substances are capable of being assessed without affecting nervous and hormonal systems connected with heart, 2) electrical activities of the pacemaker tissue and cardiac muscles are examined more easily than living animal heart, and 3) various cardiac disease conditions could be simulated (Olejnickova et al., 2015). As an example of in vitro cardiac function-assessment, myofiber sheet made from iPS cells is well known to investigate in vitro human myofiber muscle physiology and drug discovery (Takahashi et al., 2018), suggesting that the information obtained from the data of the myofiber sheet is partially limited. While reaction of in vitro three-dimensional anuran organ heart, composed of sinus-venosus including pacemaker tissue, two arterials and one ventricle, is observable even though the survival time of organ heart is short (Del Castillo and Katz, 1955; Hernández et al., 1987; Ju and Allen, 1998). It is extremely difficult to maintain in vitro spontaneously beating anuran organ heart even for a short time. Because a culture system for isolated organ heart does not been developed despite being the research-advantages. In some examples for physiological and pharmacological studies with isolated anuran organ heart, the frog-Ringer-solution for maintaining the frog hearts contains no antioxidant substance for protection against tissue ischemia producing reactive oxygen species (Zhou et al., 2018), other than supplemental oxygen for the experiment time (Loewi, 1921; Loewi and Navratil, 1926; Aceves and Erlij, 1967; Buckley and Jordan, 1970). In other words, improvement of culture conditions should become possible to improve isolated heart viability and function, and to attain stability of heartbeats for longer time. As an example of one possibility, embryonic Cynops pyrrhogaster heart, the embryonic hearts have been shown to pulse spontaneously for long-term culture with medium L-15 plus 10% fetal bovine serum (FBS) although the shape of embryonic hearts has been lost (Uehara et al., 1989; Taguchi et al., 1989). L-15 contains vitamin B1 thiamine and B6 folic acid, that function as antioxidant defenses, and thereby has a potential to survive ischemia occurred in isolated heart tissue for a long-term (Okai et al., 2007; Hsu et al., 2015). Antioxidant effect of the vitamins on embryonic heart tissues is obvious based on their research results (Uehara et al., 1989; Taguchi et al., 1989).
Electric pulses generated in pacemaker tissue trigger cardiomyocyte contraction during heartbeats (Hutter and Trautwein, 1955, 1956; Aceves and Erlij, 1967; Buckley and Jordan, 1970; Olejnickova et al., 2015). Frequency and amplitude of the heartbeats are controlled by natural neuroactive substances including the representative neurotransmitters ACh and NE (Loewi, 1921; Loewi and Navratil, 1926; Hutter and Trautwein, 1955, 1956; Aceves and Erlij, 1967; Buckley and Jordan, 1970). Exogenous ACh suppresses tadpole and frog heartbeats through muscarinic receptor activation, blocked by atropine (Loewi, 1921; Loewi and Navratil, 1926; Hartzell, 1980; Burggren and Doyle, 1986). Exogenous NE increases the heart rate of the frog through adrenergic α1 and β1 receptor activation (Aceves and Erlij, 1967; Buckley and Jordan, 1970; Stene-Larsen and Helle, 1978; Jensen et al., 2011). Responses of frog hearts to ACh and NE are common in the embryonic human heart tissues (Chang and Cumming, 1972). This is a great benefit for using in vitro anuran heart. In addition, there are advantages of frog prolificacy in the context of securing materials (Slooff and Baerselman, 1980; Kashiwagi et al., 2010), and tolerance of surgical procedures (Burggren and Warburton, 2007).
Endogenous and exogenous thyroid hormone (TH) has shown to induce anuran metamorphic change including morphological and physiological tissue reorganization (Dodd and Dodd, 1976; Kashiwagi, 1995; Hanada et al., 1997). Acute morphological and physiological change from aquatic life to terrestrial life is unavoidable adaptation to relatively higher gravity during metamorphosis (Duellman and Trueb, 1986; Kashiwagi et al., 2000; Wright and Turko, 2016). Especially, gill extinction and lung development in metamorphic tadpole are a crucial process to change the way of respiration, with accompanied biochemical metamorphosis of hemoglobin included in erythrocytes circulated by tadpole heart (Hamada et al., 1966; Dodd and Dodd, 1976; Duellman and Trueb, 1986). Regarding TH action on the tadpole heart, in vivo Rana altemporaria tadpole heart has been reported to increase the heartbeat-number by the addition of exogenous thyroxine to the water in water bath (Ruthsatz et al., 2020). In the effects of TH on adrenergic and cholinergic receptors, TH has reduced nicotinic receptors in SH-SY5Y neuroblastoma cells, followed by receptor-desensitization (Puia and Ravazzini, 2020), and also increased β-adrenergic responsive receptor number and sensitivity in rat myocardium (Williams et al., 1977). While TH remains unknown how anuran adrenergic and cholinergic receptors change functionally.
The aim of the present study is to elucidate the mechanism of changes in T4-induced neurophysiological sensitivities of X. laevis tadpole heart maintained in developed culture media.
2. Materials and methods
2.1. Chemicals
Penicillin-streptomycin solution was purchased from Invitrogen Co. Ltd., San Diego, California, USA. Sulfathiazole, Minimum Essential Medium (MEM) and N-[2-hydroxyethyl]piperazine-N’-[2-ethanesulfonic acid] (HEPES) were purchased from Sigma-Aldrich, Inc. (St. Louis, Mo, USA). 50 × MEM amino acids solution and 100 × MEM vitamin solution were purchased from Gibco. FBS, ACh and NE were also purchased from Sigma-Aldrich, Inc. (St. Louis, Mo, USA). 0.5% levobupivacaine hydrochloride was purchased from AstraZeneca PLC. Vitamin A acetate, vitamin E and vitamin C were purchased from Sigma-Aldrich, Inc. (St. Louis, Mo, USA). Sirius red was purchased from Muto Pure Chemicals CO. LTD. Vitamin-A acetate, -E and linoleic acid were solubilized in ethanol, and used. Human chorionic gonadotropin (hCG) was purchased from ASKA Animal Health Co., Ltd.
2.2. Extraction of collagen, fetal calf serum and blood pigment
Frozen chicken hearts for extraction of collagen and blood pigment were purchased from the distributor N.G.S. CO. Ltd.
2.2.1. Collagen extraction
Commercially available collagen solution has no known solvent for dissolving collagen. For that reason, we needed to extract collagen from chicken heart by ourselves. A frozen chicken heart cut into slices was immersed in 0.125 M sodium hydroxide (NaOH) for 5 days, and in turn treated with 10 mM Tris-HCl buffered solution (pH 8.0) containing 0.8% Triton X-100 and 1 mM sodium ethylenediaminetetraacetate at room temperature for 1 day. After the treatment with the Triton X-100 solution, one piece of chicken heart slices was picked up, and stained with picrosirius red stain solution in order to confirm the presence of collagen. Slices identified as collagen type I and III were transferred to 9 cm diameter plastic Petri dish plate with high-purity water and then washed for at least 5 times. Collagen was dissolved in 2 M NaOH. Protein concentration of collagen was estimated using Bradford method (1976) and stored at 4 °C until use.
2.2.2. FBS protein extraction
Serum protein was extracted from FBS by salt out method. 1 mL of 0.5 M hydrochloric acid (HCl) and 3 mL of saturated sodium chloride (NaCl) solution was poured into 5 mL FBS. FBS became clouded immediately. Clouded FBS was centrifugated at 715 g for 10 min. Supernatant was discarded, and then 5 mL of 0.5 M NaOH was poured into precipitant. After dissolution of the precipitant, solution of the precipitant was stored at 4 °C until use. Concentration of the precipitant dissolved in 0.5 M NaOH was estimated using Bradford method (1976).
2.2.3. Extraction of blood pigment
For in vitro tissue respiration of anuran heart, chicken blood pigment was purified. 8 g (wet weight) blood clot was collected from frozen chicken hearts. Blood clot was ground in mortar for 2–3 min, and then 25 mL of 2.5 M NaOH was poured into mortar. 25 mL of dissolved blood clot solution was dispensed to 5 tubes. Blood solution was centrifugated at 715 g for 10 min. Supernatant was transferred to another tube, and debris was discarded, and then washed with the following processes. Operation 1) 5.5 mL of 2.5 M HCl was poured into 5 mL of supernatant. 2) After 1 min, suspended blood pigment was centrifugated at 715 g for 10 min, and then precipitated. 3) 5.5 mL of 2.5 M NaOH was poured into each tube and dissolved. Operation 1)-3) was repeated for at least 3 times until the blood pigment solution became clean. Concentration of blood-pigment-protein dissolved in 2.5 M NaOH was estimated using Bradford method (1976), and stored at −20 °C until use.
2.3. Animals
Animals were treated according to the guideline “Regulations for animal experiments and related activities at Hiroshima University” that was established for the care and use of experimental animals by Hiroshima University (Permit number: G-16-4). All surgery was carried out under 0.5% levobupivacaine hydrochloride anesthesia, and thereby we made effort to reduce suffering of tadpoles and frogs.
Xenopus (X.) laevis tadpoles and frogs from two to four weeks after metamorphosis completion were used in the present study. Adult X. laevis frogs were derived from standard strains maintained by the Hiroshima University Amphibian Research Center. Mating was induced by injecting hCG into the dorsal lymph sac (males 60 U, females 250 U). Matings were conducted by separating individual male-female-pairs into divided water tanks containing 7–10 cm of chlorine free tap water at 20 °C for 10–12 h. Tadpoles were maintained at 20–22 °C, fed SERA Micron (Sera Heinsberg, Germany) and Tetramin (Spectrum Brand Japan & Spectrum Brand, Inc.), and staged according to Nieuwkoop and Faber (NF) (1956).
2.4. Culture medium preparation for organ culture and chemical testing
Culture medium preparation: Prepared media were MEM, MHBSS-CM I and II. NF stage 57 tadpoles and frogs were used in the present study. Used animals were sterilized with 0.025% sodium sulfathiazole at 22 °C for 2 h before removal of hearts. All animals were anesthetized by injecting 5–10 μL of 0.5% levobupivacaine hydrochloride solution into abdominal cavity. After examining that animals were deeply anesthetized, we dissected and isolated hearts of the animals. The size of isolated hearts was approximately 1 mm in vertical length, and then transferred into the glass Petri dish (35 mm diameter) with each 1 mL of the culture media 60% MEM, MHBSS-CM I and II. MHBSS-CM I and II were modified by optional reduction of NaCl to induce more stable anuran heart, based on the composition of anuran leukocyte culture medium (Hanada, 2011, 2012). The components of each medium are as below. Medium (1) MEM: The medium was used for both long term culture and chemical testing. 60% MEM contained 0.04% fetal bovine serum, 3% albumin, 10−8 M vitamin E (VE), 10−8 M vitamin A acetate (VA), 5 × 10−5 M ascorbic acid, 0.45 mg/mL chicken cardiac muscle lysate by 2.5 M NaOH, 21.3 μg/dL blood pigment derived from chicken-clotted-blood, 0.025% sulfathiazole, 260 Units/mL penicillin, 260 μg/mL streptomycin and 10 mM HEPES. (2) MHBSS-CM I: The medium was used for long term culture. It was composed of 2.8 g/L NaCl, 0.11 g/L calcium chloride (CaCl2), 0.026 g/L magnesium chloride hexahydrate (MgCl2 6H2O), 0.14 g/L potassium chloride (KCl), 5 mM HEPES, 44/1000-diluted 100 × MEM vitamin solution, 1/1000-diluted 50 × MEM amino acids solution, 2 × 10−4 M L-glutamine, 0.02% sulfathiazole, 180 Units/mL penicillin, 180 μg/mL streptomycin, 1.3% albumin, 850 μg/dL blood pigment derived from chicken-clotted-blood, 10−7 M VE, 10−7 M VA, 2.5 × 10−5 M ascorbic acid (VC), 890 μg/dL alkali solution of FBS protein, 10−5 μL/mL linoleic acid, 0.1 μL/mL DMSO, 2.5 μg/mL Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin (Mn(III)TMpyP) and 0.025 U/mL catalase. (3) MHBSS-CM II was used for both long term culture. MHBSS-CM II: The medium was used for long term culture and pharmacological testing. It was composed of 2.8 g/L NaCl, 0.11 g/L CaCl2, 0.026 g/L MgCl2 6H2O, 0.14 g/L KCl, 5 mM HEPES, 44/1000-diluted 100 × MEM vitamin solution, 2.5/1000000-diluted 50 × MEM amino acids solution, 5 × 10−7 M L-glutamine, 436 μg/dL collagen (average concentration), 0.02% sulfathiazole, 180 U/mL penicillin, 180 μg/mL streptomycin, 1.3% albumin, 850 μg/dL chicken blood pigment, 10−7 M VE, 10−7 M VA, 2.5 × 10−5 M VC, 890 μg/dL alkali solution of FBS protein, 10−5 μL/mL linoleic acid, 0.1 μL/mL DMSO, 2.5 μg/mL Mn(III)TMpyP, 0.025 U/mL catalase.
PH of all culture media was adjusted to 7.2 by adding 1 M NaOH or 1 M HCl. Subsequently culture media were sterilized by syringe with cellulose membrane filter (pore size 0.45 μm). After 1 h, isolated hearts were further moved to a new Petri dish containing fresh culture medium to prevent contamination by various bacteria and mold. Culture media were changed to the fresh one every tenth day. Before experiments, organ-cultured heart with morphological change or no spontaneous heartbeats was excluded. All tadpole- and frog-hearts within 53 days after the culture initiation were used for the pharmacological tests.
ACh, NE, atropine and T4 experiments were performed using 0.5 mL of MHBSS-CM II.
2.5. T4 treatment for 45 h (long time treatment)
T4 treatment was performed in 0.5 mL of MHBSS-CM II. Four st.57 tadpole hearts in day 5–18 after the culture initiation were treated with 5 × 10−10 M T4 for 45 h. After T4-treatment, these hearts were returned to Petri dish containing 1 mL of fresh MHBSS-CM II, and then continued to culture for 4–5 more days until use. In this way, T4-treated hearts were prepared for ACh and NE experiment.
2.6. Experimental methods of cultured heart exposed to T4, ACh, NE and atropine
For all experiments excluding T4-short-stimulation experiment (n = 6), number of hearts used in each experiment was n = 4, and 24 well plastic cell culture plate tray was used. Experiments were carried out with one heart per 0.5 mL of MHBSS-CM II containing T4, ACh, NE and atropine at room temperature (22–23 °C).
2.7. Criteria for counting heartbeat
Observation of tadpole heart contraction/relaxation was carried out, according to Lajmanovich et al. (2019) and Peltzer et al. (2019) with a slightly modified observation method. First effect of ACh on cultured tadpole and frog heart appears in their ventricles (Lajmanovich et al., 2019; Peltzer et al., 2019). The ventricular paralysis (ventricular arrhythmia) occurred at 15 min after initiation of ACh treatment was monitored and counted as heartbeat-suppression marker. And also, sequential movements of sinus venosus including pacemaker tissue, cardiac atriums and ventricle were monitored and counted as heartbeats on atropine-, NE- and T4-stimulated hearts.
2.8. Statistical analysis
Statistical analysis for Fig. 1, Fig. 2, Fig. 4, Fig. 6 was performed with R (R 3.6.3 for Mac OS ver. 10.15.7 and R i386 4.0.2 for Windows OS 10). Data for each Fig. were tested for normality using Kolmogorov-Smirnov, and for homogeneity of variance using Levene's test. Significance of differences between control and treated groups were evaluated by one-way analysis of variance (ANOVA) following a Dunnett's post-hoc test. Results are expressed as mean ± SEM. As no normal values for culture days have been posited and are not discernible in the present study, data were assessed by a non-parametric Kruskal-Wallis test following a Dunn's Multiple Comparison test. P-Values below 0.05 were considered statistically significant.
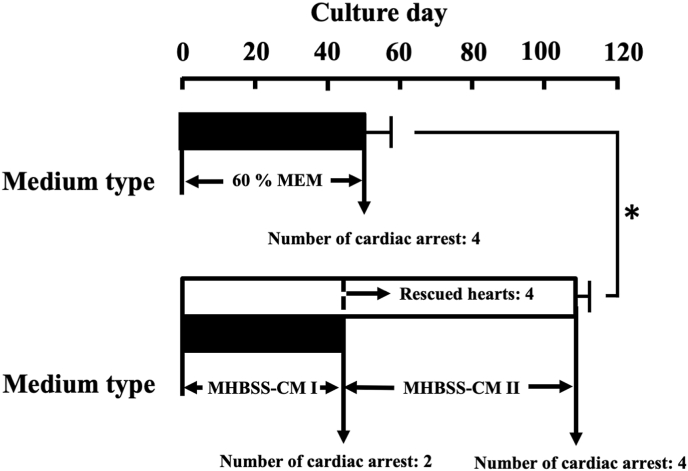
Fig. 1.
Culture days of st. 57 X. laevis heart in 60% MEM and MHBSS-CM I and II. This experiment was carried out using 4 hearts in 60% MEM and 6 hearts in MHBSS-CM I-II. Values given represent the mean value ± standard error. *Significantly greater (P < 0.05) than the corresponding value for 60% MEM. Long-term culture of spontaneously beating tadpole-hearts. Each medium composition of MHBSS-CM I and II was mentioned in the section of ‘Materials and Methods’.
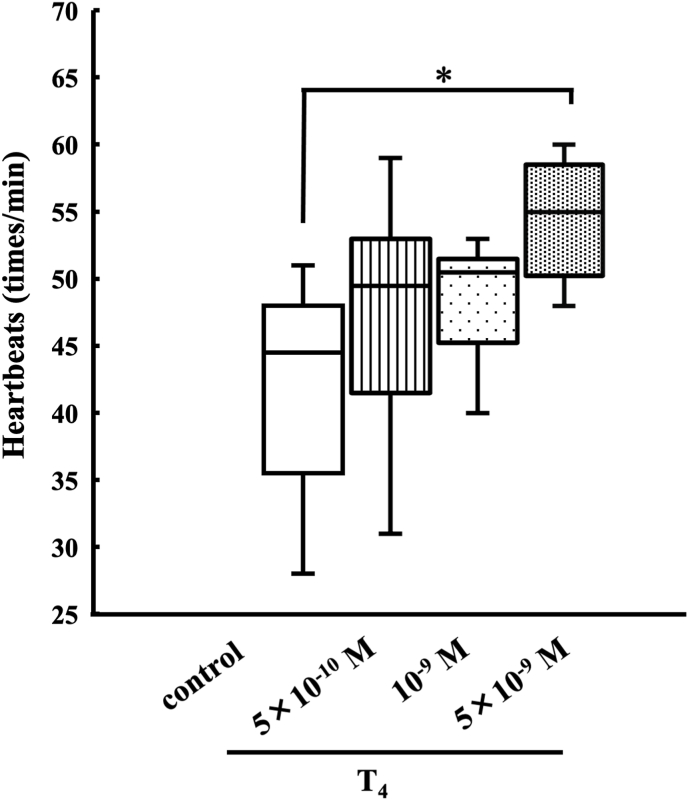
Fig. 2.
Effect of T4 on st. 57 tadpole heart. Box length indicates the interquartile range or IQR (25th to 75th percentile), and the horizontal line shows the median. The whiskers show distance 1.5 times IQR. This experiment was carried out using 6 hearts. *Significantly greater (P < 0.05) than the corresponding control value.
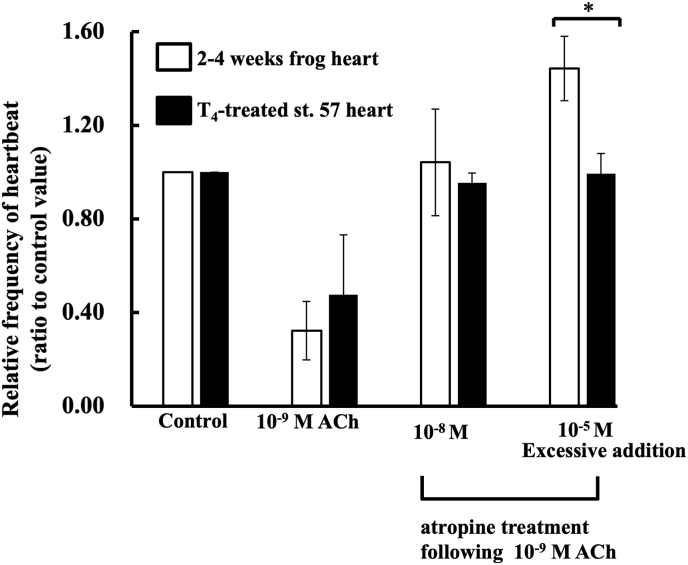
Fig. 4.
Effects of ACh and atropine on T4-treated tadpole- and 2–4 weeks frog-hearts. This experiment was carried out using four hearts. Values given represent the mean value ± standard error. Atropine treatments were carried out at 2 min (10−8 M atropine) and 4 min (10−5 M atropine) after ACh treatment. Each value was measured by number of heartbeats times per minute, and represented by ratio given to the control value. Experiment was carried out using 4 hearts. *Significantly less (P < 0.05) than the corresponding value for the rate for the frog heartbeats.
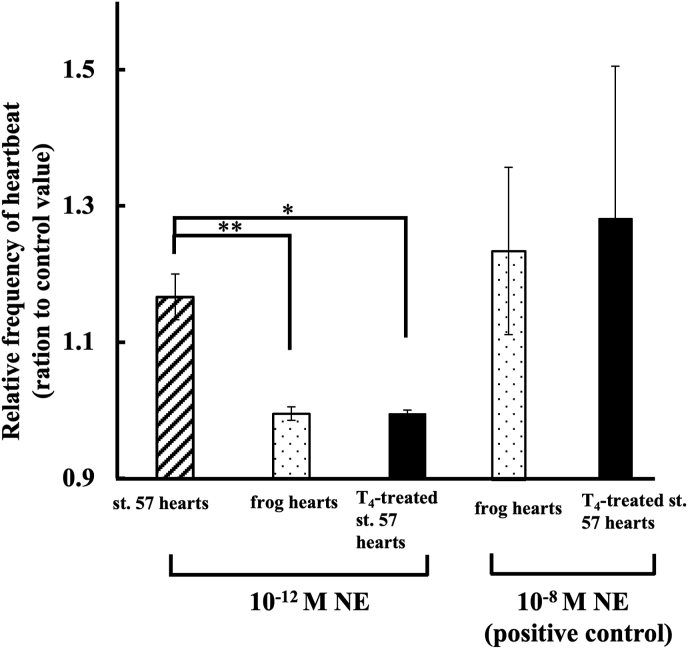
Fig. 6.
Effect of NE on T4-treated st. 57- and 2–4 weeks frog-hearts after metamorphosis completion. Values given represent the mean value ± standard error. Each value was measured by number of heartbeats times per minute, and represented by ratio given to the control value. Experiment was carried out using 4 hearts. *Significantly less (P < 0.05) than the corresponding values for the ratio given to the value from st. 57 hearts. *Significantly less (P < 0.05) than the corresponding values for the ratio given to the value from st. 57 hearts (control). **Significantly less (P < 0.01) than the corresponding values for the ratio given to the value from st. 57 hearts (control).
3. Results
3.1. Difference in heart-beating days between 60% MEM and two-successively-used MHBSS-CM I and II
Fig. 1 shows the effect of 60% MEM (n = 4) and two successively used MHBSS-CM I and II on isolated tadpole hearts. Beating days of tadpole hearts maintained in 60% MEM were average 50 ± 7.5 days. Beats of 2 hearts (2 hearts out of 6 hearts) in MHBSS-CM I stopped by day 44 after the initiation of culture due to relatively excess amino acids and lack of collagen, although excluded in Fig. 1. For those reasons, culture medium to keep the other 4 hearts alive were changed from MHBSS-CM I to MHBSS-CM II that were prepared by further amino acids-reduction and supplementation with collagen. Afterwards, heartbeats of the survived 4 hearts were kept by average 109 ± 3.75 days (n = 4). Heart-beating days of the survived 4 hearts in MHBSS-CM I and II are significantly longer (P < 0.001) than those of 60% MEM (n = 4).
3.2. Effect of T4 on heartbeats of st. 57 hearts in MHBSS-CM II
Fig. 2 shows the effect of T4 on st. 57 hearts in MHBSS-CM II. Box plot in the control has relatively wider spread from 36 times/min to 48 times/min accompanying the maximum (51 times/min) and minimum (28 times/min) values, and the median in the control indicates 45 times/min. 5 × 10−9 M T4 showed a significant increase in heartbeats compared to the control group (P < 0.05).
3.3. Effect of ACh on st. 57 hearts in 60% MEM
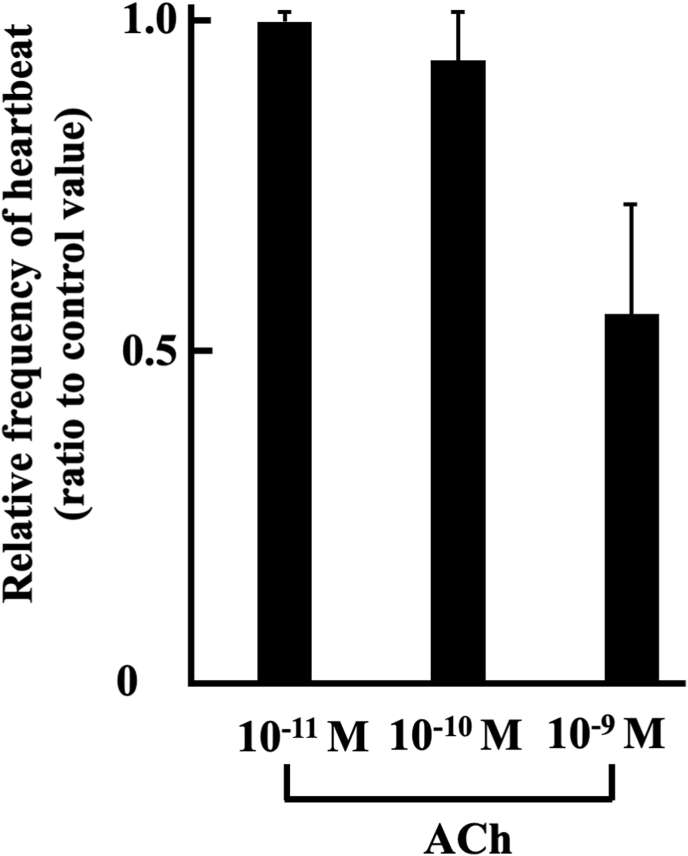
Fig. 3 shows the effect of ACh on st. 57 hearts under the 60% MEM culture conditions. In initial step of the experiment, heartbeats of the hearts in culture medium without ACh were recorded on video movie for 1 min. In second, the hearts were transferred to each culture medium containing 10−11―10−9 M ACh, and then heartbeats were recorded on video movie during 1 min from 15 to 16 min after the initiation of ACh treatment. 15 min of the ACh pretreatment were necessary reaction time, because propagation of ACh into heart tissue was sluggish. Analysis was conducted based on the data obtained from the video movies. 10−11―10−9 M ACh-induced frequencies are as follows; 10−11 M (n = 4, 0.98 ± 0.019), 10−10 M (n = 4, 0.93 ± 0.084) and 10−9 M (n = 4, 0.55 ± 0.019). The frequencies tend to decrease by ACh.
Fig. 3.
ACh-decreased heartbeats frequency of organ-cultured st. 57 hearts. Each value was measured by number of heartbeats times per minute, and represented by ratio to the control value. Experiment was carried out using 4 hearts. Values given represent the mean value ± standard error.
3.4. Effect of ACh on T4-treated st. 57- and 2–4 weeks frog-hearts in MHBSS-CM II
Fig. 4 shows that the effect of ACh and atropine on 2–4 weeks frog- and T4-treated st. 57- hearts in MHBSS-CM II. Experimental conditions were the same as mentioned in Fig. 3. 10−8 M and 10−5 M (excessive addition) atropine treatments were conducted after 2 min and 4 min of 10−9 M ACh treatment, respectively. There was no significant difference between T4-treated st. 57- (n = 4, 0.48 ± 0.255) and 2–4 weeks frog-hearts (n = 4, 0.32 ± 0.124). Relative frequency of heartbeats of both T4-treated st. 57- (n = 4, 0.95 ± 0.042) and 2–4 weeks frog-hearts (n = 4, 1.04 ± 0.228) were recovered by 10−8 M atropine treatment. T4-treated st. 57-hearts (n = 4, 0.99 ± 0.088) were not more reacted to10−5 M atropine similar to 2–4 weeks frog hearts (n = 4, 1.44 ± 0.138), however. Significant difference (P < 0.05) between 2 and 4 weeks frog- and T4-treated st. 57- hearts was found in 10−5 M atropine treatment (excessive addition).
3.5. Effect of NE on st. 57 hearts in 60% MEM
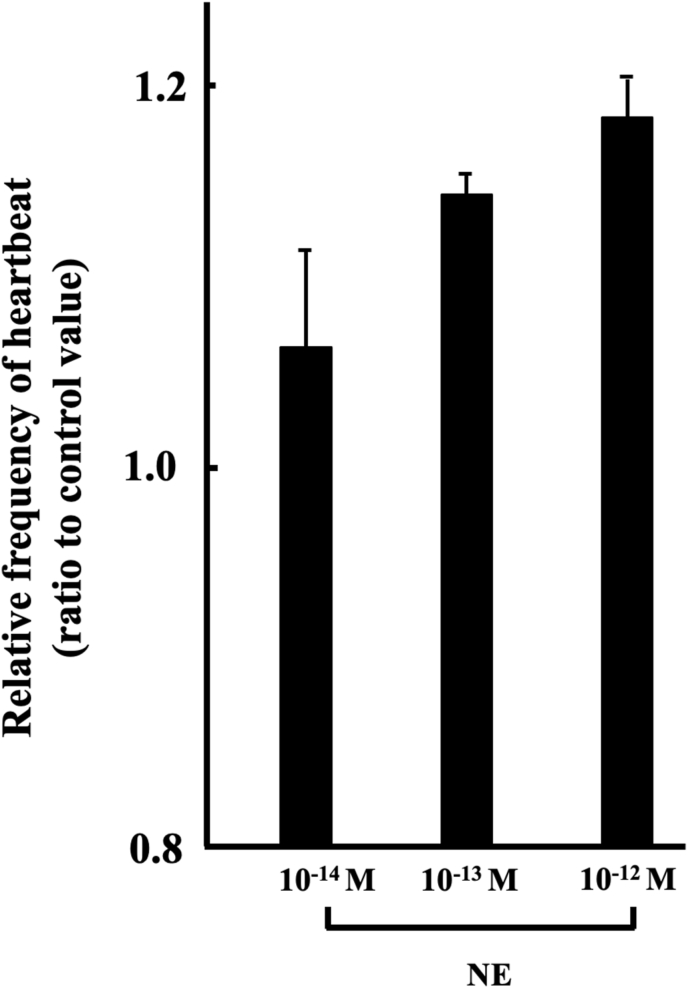
Fig. 5 shows that the effect of NE on spontaneous heartbeats of cultured hearts. Experimental conditions were the same as mentioned as below. In initial step, the heartbeats of the hearts in 60% MEM without NE were recorded on video movie for 1 min. In second step, the hearts were transferred to culture medium containing 10−14―10−12 M NE. Heartbeats were recorded on video movie during 1 min from 3 to 4 min after the initiation of NE treatment. 3 min after start of NE treatment was necessary reaction time. Analysis was conducted based on the data obtained from the video movies. 10−14―10−12 M NE-induced frequencies are as follows; 10−14 M (n = 4, 1.06 ± 0.050), 10−13 M (n = 4, 1.14 ± 0.011) and 10−12 M (n = 4, 1.18 ± 0.028). The heartbeat frequency in 60% MEM were increased by 10−14―10−12 M NE, as shown in Fig. 5.
Fig. 5.
Effect of NE on in vitro st. 57 hearts in 60% MEM. Each value was measured by number of heartbeats times per minute, and represented by ratio given to the control value. Experiment was carried out using 4 hearts. Values given represent the mean value ± standard error.
3.6. Difference between T4-treated st.57- and 2–4 weeks frog-heartbeats with 10−12M NE treatment
Fig. 6 shows that the effect of NE on spontaneous heartbeats of T4-treated and 2–4 weeks frog hearts. Experimental conditions were the same as mentioned in Fig. 5. Spontaneous heartbeat frequency of st. 57 hearts were increased by 10−12 M NE (n = 4, 1.16 ± 0.034) as sown in Fig. 5. The frog-hearts (n = 4, 0.99 ± 0.010) were significantly less (P < 0.01) than the corresponding control value. Similarly, T4-treated hearts (n = 4, 1.00 ± 0.005) were significantly less (P < 0.01) than the corresponding control value. Both the frog-(n = 4, 1.24 ± 0.123) and T4-treated hearts (n = 4, 1.29 ± 0.225) reacted to 10−8 M NE (excessive addition).
4. Discussion
T4-induced changes in cholinergic and adrenergic sensitivities were elucidated by using the beating tadpole organ-heart that was technologically improved in the present study.
Some reports have shown the possibility to keep the human fetal heart-tissue and the embryonic organ heart of newt stable under the culture conditions for long-term (Chang and Cumming, 1972; Uehara et al., 1989; Taguchi et al., 1989). Also, continuous use of MHBSS-CM I and II maintained X. laevis tadpole heart longer than 60% MEM, although 2 of 6 hearts was lost by the middle term of the culture (data not shown). Especially, switching to MHBSS-CM II containing lower concentration of amino acids and collagen played a crucial role to extend the period of the survived 4 heart-lives. These results indicate that relatively high concentrated amino acids shorten lives of in vitro hearts, alleviated by addition of collagen to the culture medium.
Studies concerning pharmacological effects on heartbeats of spontaneously beating anuran hearts in oxygenated frog-Ringer-solution have shown cholinergic and adrenergic sensitivities (Loewi, 1921, 1999; Navratil, 1926; Hutter and Trautwein, 1956; Aceves and Erlij, 1967; Buckley and Jordan, 1970; Stene-Larsen and Helle, 1978). In receptor activation by thyroid hormones, pharmacological analysis has indicated that thyroid hormones increase β-adrenergic receptor number and sensitivity in rat myocardium, and stimulate in vivo baboon heartbeats through adrenergic receptor β2 rather than β1 activation (Williams et al., 1977; Hoit et al., 1997). The present study also shows that lower concentration of T4 increases the number of tadpole heartbeats, suggesting that T4 induces cardiac pulsation via adrenergic receptor activation.
Chang and Cumming (1972) have reported that spontaneous heartbeats of human-embryonic-heart-tissues maintained in Connaught's H597 containing 15% FBS is suppressed by transferring the heart tissue to the culture medium containing ACh. Further they have shown that spontaneous heartbeats in the human-heart-tissues are not suppressed until ACh concentration reaches 10−4 g/ml (6.8 × 10−4 M), indicating the existence of threshold value in the cultured human-embryonic-heart-tissues (Chang and Cumming, 1972). In the present study, the value of ACh-threshold in st. 57 hearts was 10−10 M, based on the results that spontaneous heartbeat rate of st. 57 hearts exposed to 10−10—10−9 M ACh decreased. These results show that st. 57 heart retains in vitro high sensitivity to ACh, and the existence of threshold of sensitivity to ACh. In a change in developmental sensitivity to ACh, Burggren and Doyle (1986) have reported that cholinergic sensitivity of the in vivo Rana catesbeiana tadpole hearts decreases after metamorphosis completion. In the present study, the difference between 2 and 4 weeks frog- and T4-treated st. 57 hearts was not examined in cholinergic receptor, and 2–4 weeks frog- and T4-treated st. 57-hearts through muscarinic receptors were temporally activated by ACh, and then inactivated via addition of 10−8 M atropine. However, excessive addition of 10−5 M atropine to T4-treated tadpole hearts did not increase the tadpole heartbeats more than that value of 10−8 M atropine, regardless of the increase of frog heartbeat-frequency. Thus, sensitivity of atropine-sensitive muscarinic receptors is suggested to be strongly desensitized by the excessive T4 treatment (T4 treatment for 45 h).
Regarding developmental sensitivity to NE, in situ hearts of NF st. 40―53 X. laevis tadpoles have been observed to react to 10−11―10−10 M epinephrine (EPN) (Jacobsson and Fritsche, 1999), despite catecholamine-producing cells are confirmed in the tadpole kidney (Jacobsson and Fritsche, 2002), showing that the juvenile tadpole does not have adrenergic sensitivity to low concentrations of EPN. In addition, 5 × 10−4 g/mL (3 × 10−3 M) NE was observed to increase spontaneous heartbeats of frog atriums including sinus venosus (Aceves and Erlij, 1967), and also 10−8―10−4 M NE increases tension response in isolated atriums derived from R. esculenta, R. pipiens and R. temporaria frog (Stene-Larsen and Helle, 1978). Thus, it has been thought that the tadpoles and the frog hearts require relatively high concentration of adrenergic agents, so far. In the present study, heartbeats of st. 57 hearts exposed to 10−13―10−12 M NE increased, supporting the conclusion that even isolated st. 57 hearts have high sensitivity to NE. On the other hand, T4-treated st. 57 hearts did not respond to 10−12 M NE, similar to those of the frog hearts. Further both of the frog- and T4-treated st. 57-hearts were activated by 10−8 M NE, indicating that adrenergic sensitivity decreases. These results that suggest that excessive T4 treatment disturbs the function of adrenergic receptors, leading to adrenergic desensitization.
In conclusion, culture media containing low concentrations of amino acids and collagen can keep in vitro spontaneously beating organ-heart for long term. Using 60% MEM and the culture media, the present study reveals that one pharmacological action by short-time-stimulation of 5 × 10−9 M T4 to in vitro st. 57 heart is adrenergic action, while st. 57 heart treated with T4 for long time induces atropine-sensitive-cholinergic and adrenergic receptor-desensitization.
Our future work is to evaluate test chemical substances with regard to induction of reactive oxygen species using MBSS-CMs.
Funding statement
The present study received no financial support for the research.
CRediT authorship contribution statement
Hideki Hanada: Conceptualization, Methodology, Investigation, Data curation, Writing-review. Fumihiro Morishita: Conceptualization, Formal analysis, Writing-review. Seigo Sanoh: Formal analysis, Writing-review. Keiko Kashiwagi: Formal analysis, Writing-review, Laboratory animal supply. Akihiko Kashiwagi: Conceptualization, Formal analysis, Writing-review.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
Acknowledgements
Tadpoles and juvenniles of X. laevis and X. tropicalis, that were used to develop the culture medium, was provided by Hiroshima University Amphibian Research Center with support in part by the National Bio-Resource Project of the AMED, Japan. We are also grateful to Interuniversity Bio-Backup Project for Basic Biology for allowing us to use laboratory instrument.
Data availability
Data will be made available on request.
References
- Aceves J., Erlij D. Effects of norepinephrine on tissues of the frog heart atrium poisoned by tetrodotoxin. Nature. 1967;215:1178–1179. doi: 10.1038/2151178b0. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckley G.A., Jordan C.C. Temperature modulation of α- and ß-adrenoceptors in the isolated frog heart. Br. J. Pharmacol. 1970;38:394–398. [PMC free article] [PubMed] [Google Scholar]
- Burggren W., Doyle M. The action of acetylcholine upon heartbeats changes markedly with development in bullfrogs. J. Exp. Zool. 1986;240:137–140. doi: 10.1002/jez.1402400117. [DOI] [PubMed] [Google Scholar]
- Burggren W.W., Warburton S. Amphibians as animal models for laboratory research in physiology. ILAR J. 2007;48:260–269. doi: 10.1093/ilar.48.3.260. [DOI] [PubMed] [Google Scholar]
- Del Castillo J., Katz B. Production of membrane potential changes in the frog's heart by inhibitory nerve impulses. Nature. 1955;175:1035. doi: 10.1038/1751035a0. [DOI] [PubMed] [Google Scholar]
- Chang T.D., Cumming G.R. Chronotropic responses of human heart tissue cultures. Circ. Res. 1972;30:628–633. doi: 10.1161/01.res.30.6.628. [DOI] [PubMed] [Google Scholar]
- Dodd M.H.I., Dodd J.M. In: Lofts B., editor. vol. 3. Academic Press; New York, San Francisco, London: 1976. The biology of metamorphosis; pp. 467–599. (Physiology of the Amphibia). [Google Scholar]
- Duellman W.E., Trueb L. McGraw-Hill Book Company; New York, St. Louis, San Francisco, Auckland, Bogotá, Guatemala, Hamburg, Johannesburg, Lisbon, London, Madrid, Mexico, Montreal, New Delhi, Panama, Paris, San Juan, São Paulo, Singapore, Sydney, Tokyo, Toronto: 1986. Biology of Amphibians. [Google Scholar]
- Hamada K., Sakai Y., Tsushima K., Shukuya R. Biochemical metamorphosis of hemoglobin in Rana catesbeiana. III. Molecular change of hemoglobin during spontaneous metamorphosis. J. Biochem. 1966;60:37–41. doi: 10.1093/oxfordjournals.jbchem.a128396. [DOI] [PubMed] [Google Scholar]
- Hanada H. Dl-α-tocopherol enhances the herbicide 1,1’-dimetyl-4,4’-bipyridium dichloride (paraquat, PQ) genotoxicity in cultured anuran leukocytes. Hereditas. 2011;148:118–124. doi: 10.1111/j.1601-5223.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- Hanada H. Phenolic antioxidant 2,6-di-tert-butyl-p-cresol (vitamin E synthetic analogue) does not inhibit 1,1′-dimetyl-4,4′-bipyridium dichloride (paraquat)-induced structural chromosomal damage in cultured leukocytes of the dark-spotted-frog Pelophylax (Rana) nigromaculatus. Hereditas. 2012;149:173–177. doi: 10.1111/j.1601-5223.2012.02260.x. [DOI] [PubMed] [Google Scholar]
- Hanada H., Kashiwagi A., Takehara Y., Kanno T., Yabuki M., Sasaki J., Inoue M., Utsumi K. Do reactive oxygen species underlie the mechanism of apoptosis in the tadpole tail? Free Radic. Biol. Med. 1997;23:294–301. doi: 10.1016/s0891-5849(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Hartzell H.C. Distribution of muscarinic acetylcholine receptors and presynaptic nerve terminals in amphibian heart. J. Cell Biol. 1980;86:6–20. doi: 10.1083/jcb.86.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández D., Guerrero S., Morales M. Electrophysiological characteristics of cardiac pacemaker cells of the frog Caudiverbera caudiverbera. Comp. Biochem. Physiol. 1987;87A:649–656. doi: 10.1016/0300-9629(87)90377-x. [DOI] [PubMed] [Google Scholar]
- Hoit B.D., Khoury S.F., Shao Y., Gabel M., Liggett S.B., Walsh R.A. Effects of thyroid hormone on cardiac ß-adrenergic responsiveness in conscious baboons. Circulation. 1997;96:592–598. doi: 10.1161/01.cir.96.2.592. [DOI] [PubMed] [Google Scholar]
- Hsu C.C., Cheng C.H., Hsu C.L., Lee W.J., Huang S.C., Huang Y.C. Role of vitamin B6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food Nutr. Res. 2015;59 doi: 10.3402/fnr.v59.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O.F., Trautwein W. Effect of vagal stimulation on the sinus venosus of the frog's heart. Nature. 1955;176:512–513. doi: 10.1038/176512a0. [DOI] [PubMed] [Google Scholar]
- Hutter O.F., Trautwein W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J. Gen. Physiol. 1956;39:715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson A., Fritsche R. Development of adrenergic and cholinergic cardiac control in larvae of the African clawed frog Xenopus laevis. Physiol. Biochem. Zool. 1999;72:328–338. doi: 10.1086/316669. [DOI] [PubMed] [Google Scholar]
- Jensen B.C., O'Connell T.D., Simpson P.C. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J. Mol. Cell. Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.K., Allen D.G. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J. Physiol. 1998;508:153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A. Peroxisomal enzyme activity changes in the tail of anuran tadpoles during metamorphosis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995;111:483–489. doi: 10.1016/0305-0491(95)00021-y. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Hanada H., Yabuki M., Utsumi K. Reactive oxygen species and the mechanism for tail apoptosis in Rana rugosa and Rana japonica. Recent Res. Devel. Comparative Biochem. Physiol. 2000;1:51–66. [Google Scholar]
- Kashiwagi K., Kashiwagi A., Kurabayashi A., Hanada H., Nakajima K., Okada M., Takase M., Yaoita Y. Xenopus tropicalis: an ideal experimental animal in Amphibia. Exp. Anim. 2010;59:395–405. doi: 10.1538/expanim.59.395. [DOI] [PubMed] [Google Scholar]
- Lajmanovich R.C., Peltzer P.M., Martinuzzi C., Attademo A.M., Bassó A., Colussi C. Insecticide pyriproxyfen (Dragón®) damage biotransformation, thyroid hormones, heart rate, and swimming performance of Odontophyrynus americanus tadpoles. Chemosphere. 2019;220:714–722. doi: 10.1016/j.chemosphere.2018.12.181. [DOI] [PubMed] [Google Scholar]
- Loewi O. Über humorale übertragbarkeit der Herznervenwirkung. I. Mitteilung. Pflügers Arch. Ges. Physiol. 1921;189:239–242. [Google Scholar]
- Loewi O., Navratil E. Über humorale Übertragbarkeit der Herznervenwirkung. X. Mitteilung. Über das Schicksal des Vagusstoffes. Pflügers Arch. Ges. Physiol. 1926;214:678–688. [Google Scholar]
- Nieuwkoop P.D., Faber J. North Holland; Amsterdam: 1956. Normal Tables ofXenopus Laevis(Daudin): a Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. [Google Scholar]
- Okai Y., Higashi-Okai K., Sato E.F., Konaka R., Inoue M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J. Clin. Biochem. Nutr. 2007;40:42–48. doi: 10.3164/jcbn.40.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnickova V., Novakova M., Provaznik I. Isolated heart models: cardiovascular system studies and technological advances. Med. Biol. Eng. Comput. 2015;53:669–678. doi: 10.1007/s11517-015-1270-2. [DOI] [PubMed] [Google Scholar]
- Peltzer P.M., Lajmanovich R.C., Martinuzzi C., Attademo A.M., Curi L.M., Sandoval M.T. Biotoxicity of diclofenac on two larval anuran amphibians: assessment of development, growth, cardiac function and rhythm, behavior and antioxidant system. Sci. Total Environ. 2019;683:624–637. doi: 10.1016/j.scitotenv.2019.05.275. [DOI] [PubMed] [Google Scholar]
- Puia G., Ravazzini F. Thyroid hormones reduce nicotinic receptor mediated currents in SH-SY5Y neuroblastoma cells. Pharmacol. Rep. 2020;72:1766–1771. doi: 10.1007/s43440-020-00170-7. [DOI] [PubMed] [Google Scholar]
- Ruthsatz K., Dausmann K.H., Paesler K., Babos P., Sabatino N.M., Peck M.A., Glos J. Shifts in sensitivity of amphibian metamorphosis to endocrine disruption: the common frog (Rana temporaria) as a case study. Conserv. Physiol. 2020;8 doi: 10.1093/conphys/coaa100.eCollection2020. coaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooff W., Baerselman R. Comparison of the usefulness of the mexican axolotl (Ambystoma mexicanum) and the clawed toad (Xenopus laevis) in toxicological bioassays. Bull. Environ. Contam. Toxicol. 1980;24:439–443. [Google Scholar]
- Stene-Larsen G., Helle K.B. Cardiac β2-adrenocepter in the frog. Comp. Biochem. Physiol. C Comp. Pharmacol. 1978;60:165–173. doi: 10.1016/0306-4492(78)90090-4. [DOI] [PubMed] [Google Scholar]
- Taguchi M., Uehara M., Asashima M., Pfeiffer C.J. Development of the heartbeats during normal ontogeny and during long-term organ culture of hearts of the newt, Cynops pyrrhogaster. Cell Differ. Dev. 1989;27:95–102. doi: 10.1016/0922-3371(89)90739-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Shimizu T., Okano T. Engineered human contractile myofiber sheets as a platform for studies of skeletal muscle physiology. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara M., Taguchi M., Asashima M., Pfeiffer C.J. Developmental physiology of cardiac contraction in the Japanese newt in vivo and in vitro. J. Mol. Cell. Cardiol. 1989;21:709–718. doi: 10.1016/0022-2828(89)90612-3. [DOI] [PubMed] [Google Scholar]
- Williams L.T., Lefkowitz R.J., Watanabe A.M., Hathaway D.R., Besch H.R., Jr. Thyroid hormone regulation of β-adrenergic receptor number. J. Biol. Chem. 1977;252:2787–2789. [PubMed] [Google Scholar]
- Wright P.A., Turko A.J. Amphibious fishes: evolution and phenotypic plasticity. J. Exp. Biol. 2016;219:2245–2259. doi: 10.1242/jeb.126649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.