1. Introduction
The specific anatomy, physiology, and health needs of women have long been overlooked and understudied, with the male body and biology centered as the ‘baseline’ in biomedical research (Merone et al., 2022), (Mirin, 2021). Despite an increased focus on female sex-specific diseases and physiology in recent years, there is still a limited understanding of vaginal physiology and anatomy, leading to fields with many open questions about basic function, growth, and remodeling as it relates to physiologic processes and disease etiology and progression (Woitowich et al., 2020), (Arnegard et al., 2020).
The vagina is a fibromuscular canal connecting the uterine cervix to the vulva, with a unique range of functional roles in arousal and intercourse, fetal delivery, and the passage of menstrual fluid. The vagina is tubular, gently curved, and approximately 6–10 cm in length as it ascends from the vulva to the cervix (Barnhart et al., 2006), (Luo et al., 2016). Baseline anatomic characteristics, such as length and width, vary dramatically from person to person (Barnhart et al., 2006), (Luo et al., 2016).
The vagina begins to form in the female embryo around the 8th week of gestation, when the paramesonephric (or Müllerian) ducts contact the urogenital sinus. The Müllerian ducts then fuse, creating a structure that forms the superior portion of the vaginal canal, while the urogenital sinus expands into the vaginal plate (Schoenwolf et al., 2015). By the 22nd week of gestation, a complete vaginal structure is present, derived primarily from the vaginal plate (Schoenwolf et al., 2015), (Robboy et al., 2017).
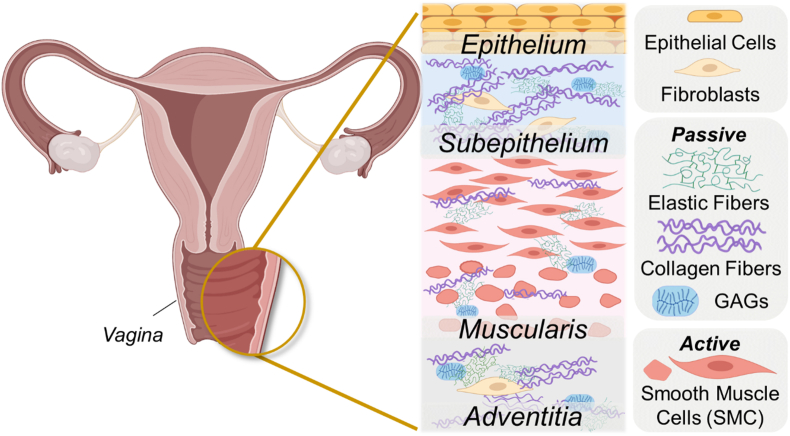
The vagina is composed of four layers: the epithelium, subepithelium, muscularis, and adventitia. The epithelium is composed of epithelial cells and serves as a protective barrier against infections and pathogens. The vaginal epithelium (part of the mucosa) has a crucial immune role that contributes to pelvic floor health and plays a role in successful reproductive processes such as fertilization (Amabebe and Anumba, 2018), (Di Simone et al., 2020). The stratified squamous epithelial cells provide a physical barrier, and the commensal colonization of the vaginal mucus by microbiota defends against viral, bacterial, and fungal pathogens to maintain vaginal health (Amabebe and Anumba, 2018).
The mechanical properties of the vagina are imbued by microstructural components (such as elastic fibers, collagen fibers, and smooth muscle cells) of the subepithelium and muscularis. These layers, which are deep to the epithelium and mucosa, dictate the elasticity, distensibility, and contractility that are critical for vaginal function (Fig. 1). The subepithelium is a dense connective tissue that dictates the passive properties of the organ, primarily through components of the extracellular matrix, such as collagen and elastic fibers. The active behavior of the vagina is governed by the muscularis, which comprises two layers of smooth muscle cells oriented in the circumferential and axial directions. The outermost adventitia is a loose connective tissue layer laden with blood vessels, lymphatic vessels, and nerves (Łaniewski et al., 2018).
Fig. 1.
This diagram shows the layers and microstructural components of the vagina. The composition and organization of these components (smooth muscle cells, elastic fibers, and collagen fibers) determine the mechanical behavior of the organ. Some figure components created in Biorender.com.
Innervation differs among anatomic regions: the proximal vagina is innervated primarily by the uterovaginal nerve plexus, whereas the distal vagina receives somatic innervation from the pudendal nerve (Łaniewski et al., 2018). The organ is also highly vascular and receives blood from the vaginal and uterine arteries (both branches of the internal iliac arteries). That vasculature is a critical component of the remodeling that occurs during arousal and sexual intercourse, which involves dilation, lengthening, and contractions as well as an increase in lubrication. During pregnancy, the vagina experiences dramatic changes in microbiome, hormones, and blood flow, in addition to a change in the loading environment that culminates in fetal delivery (Nuriel-Ohayon et al., 2016). To accommodate the width of the fetal head and shoulders, the vagina undergoes extreme geometric changes. The human fetal head is similar in size to the pelvic outlet, requiring a carefully orchestrated process of fetal rotation and progression through the birth canal.
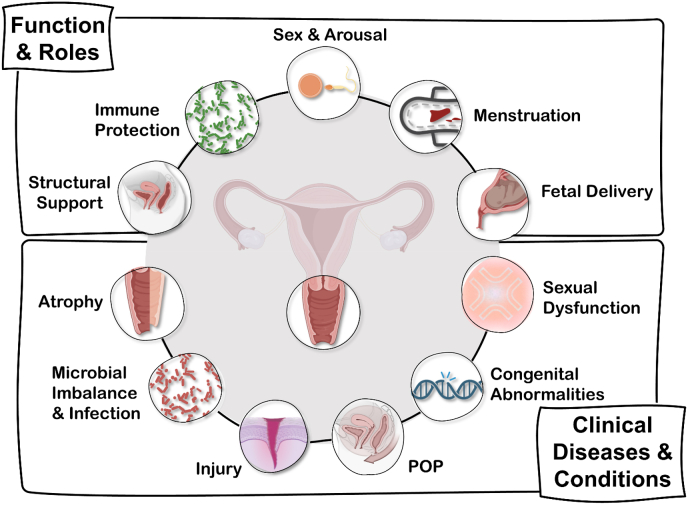
In addition to remodeling to accommodate and respond to various physiologic events, the vagina is subject to complex diseases and disorders (Fig. 2).
Fig. 2.
This chart is a visual overview of the various functions and roles the vagina plays throughout life, as well as the clinical diseases and conditions that drive the need for innovation through bioengineering approaches. Some figure components created using Biorender.com.
The vaginal microbiota is dominated by Lactobacillus species, and disturbances in this microenvironment can lead to vaginitis, preterm labor, and an increased risk of contracting sexually transmitted diseases (Chen et al., 2021). Bacterial vaginosis (BV) results from a dysbiosis of the vaginal microbiome and presents as vaginal discharge, which results from the presence of many microbes with proinflammatory features (Coudray and Madhivanan, 2020). This imbalance is linked to an increased risk of contracting and transmitting sexually transmitted infections such as herpes, HIV, chlamydia, and gonorrhea (Lewis et al., 2017). Currently, there are no data correlating the vaginal microbiome with vaginal biomechanics. This is an area worth exploring as it may allow for the identification of early microbiome changes that can affect tissue stability.
The most common cause of vaginal injury in women of reproductive age is associated with vaginal delivery. As a result of the passage of the fetus or assistive procedures, injury or tearing of the vagina can occur, ranging from superficial cuts to deep lacerations into the perineal muscles and anal sphincter. These injuries can lead to both acute and chronic complications, such as bleeding, infection, fistula formation, urinary and fecal incontinence, and sexual dysfunction (ACOG Practice Bulletin No). Vaginal delivery is also associated with an increased risk of pelvic organ prolapse (POP) (Vergeldt et al., 2015).
POP is the result of loss of connective tissue support in the pelvic floor and is defined as herniation of the pelvic organs to or beyond the vaginal walls (DeLancey, 2016). The current understanding of prolapse pathophysiology is poor, but it is a multifactorial process. The contributing factors that result in the development of prolapse can be divided into two broad categories: trauma – such as that caused by pregnancy, delivery, obesity, or chronic cough; and connective tissue deficiencies – such as age and mutations in extracellular matrix remodeling.
Abnormal extracellular matrix metabolism in the pelvic floor connective tissue is one of the predominant pathogenic mechanisms of POP. The main molecular changes that result in deterioration of the vaginal extracellular matrix include increased expression of matrix metalloproteinases (MMPs), increased elastases, and decreased expression of Fibulin-5 (FBLN5) and Lysyl Oxidase (LOXL1) (Deng et al., 2021). Biopsies from pelvic floor tissues of women with POP show increased expression of MMP2 and MMP9. Increased elastase in prolapsed tissue causes rupture of the elastic fiber, resulting in degradation of extracellular matrix structural integrity.
Furthermore, the vagina is extremely sensitive to the hormonal environment. The gradual loss of hormones that occurs during menopause can result in a variety of vaginal changes that manifest as genitourinary syndrome of menopause (GSM) (Nappi et al., 2019). GSM symptoms include vaginal atrophy, dryness, sexual dysfunction, irritation, and pathologic changes in the vaginal microenvironment. Sexual dysfunction secondary to dyspareunia (painful intercourse) is most commonly related to GSM (Streicher, 2023). Other physiologic causes of sexual dysfunction include high-tone pelvic floor, clitoral adhesions, and/or vaginal adhesions. High tone pelvic floor is due to involuntary contraction of the pelvic floor muscles, causing undue tension on the vagina. This disorder is understudied and often misdiagnosed, resulting in women suffering for years before an appropriate diagnosis and treatment plan. Vaginal adhesions are the result of immune disorders such as graft-vs-host disease, lichen sclerosus, and lichen planus. In these cases, agglutination of the tissue causes pain, bleeding, and potential malignant transformation (Burrows et al., 2008).
Although both POP and GSM are associated with aging, some vaginal conditions are the result of congenital anomalies. A closed or absent vagina (atresia or agenesis) usually occurs as part of a broader group of congenital abnormalities, such as Mayer–Rokitansky–Kuster–Hauser syndrome (MRKH). Vaginal agenesis related to MRKH may be treated nonsurgically using a series of dilators or through surgical creation of a neovagina (Herlin et al., 2020). Although recent vaginoplasty techniques using tissue engineering approaches show promise, traditional surgical approaches include the use of grafts from the labia majora, bowel, peritoneum, or skin to form a neovaginal structure (Herlin et al., 2020).
While guided by epidemiologic and clinical narratives, fundamental gaps exist in the mechanistic understanding of vaginal physiology and pathophysiology. For example, what factors determine whether a patient develops pelvic organ prolapse or GSM? What is the mechanism of disease and disease progression? How do other factors, such as age, hormones, microbial environments, or immune and inflammatory responses affect that mechanism?
In trying to understand the etiology and progression of a single disease, questions are raised that require expertise in microbiology, immunology, clinical care, biomechanics, and other fields. Modeling the vagina in a way that is useful for developing treatment strategies also requires expertise in mathematics and constitutive modeling, computer-aided design, and benchtop devices such as culture and microphysiologic systems.
In various healthy or diseased states, bioengineering and clinical collaboration facilitates the innovative study of vaginal anatomy and physiology through integrative, cross-disciplinary approaches. The evolution of novel scientific approaches and the reapplication of long-standing research practices formed the basis of our current understanding of the vagina, resulting in a combination of multiscale in vitro work, in vivo models, advances in materials science, computational models, and clinical treatment development. This review is not intended to be comprehensive but instead presents a broad survey of current and future bioengineering approaches in the field of vaginal research.
2. 2-D culture
Because the in vitro environment can be strictly regulated, cell culture studies are useful for investigating fundamental cellular processes in normal and pathological states. Variables such as temperature, pH, media, and nutrient concentration can be adjusted and controlled according to the experiment. For example, the vagina is sensitive to environmental changes, including fluctuations in hormone levels, such as androgens (Farage and Maibach, 2006).
Maseroli et al. isolated vaginal smooth muscle cells (vSMCs) from rat and postmenopausal human vaginas and demonstrated that vSMCs play a (non species-specific) role in the inflammatory response in the vagina (Maseroli et al., 2020). Human vSMCs expressed toll-like receptors and secreted proinflammatory markers when induced with lipopolysaccharide, a molecule found on the surface of pathogens. Furthermore, Cellai et al. reported that human vSMCs were responsive to dehydroepiandrosterone (DHEA), an androgen precursor. Understanding cell responsiveness to hormones may better elucidate possible therapeutic options, such as local administration of DHEA to treat symptoms of GSM (Cellai et al., 2021). Although 2-D culture is a helpful starting point when investigating cellular biology, it does not recapitulate the 3-D environment in which cells reside in vivo.
3. 3-D culture/organoids
While 2-D systems involve a single layer of cells growing in a dish, 3-D systems support cell growth that more closely mimics the native tissue environment. For example, using an air–liquid interface culture, Zhu et al. engineered the vaginal epithelium from isolated primary human normal vaginal epithelial cells (HNVEC) (Zhu et al., 2017). Unlike previous 2-D culture efforts, the epithelial model was stratified, and the 3-D structure had similar expression of biomarkers commonly found in human vaginal epithelia in vivo. To verify that the cultured model behaved pathologically when exposed to a virus, the authors introduced herpes simplex virus type 2 (HSV-2) – one of the most common sexually transmitted viruses – into the system (Whitley and Roizman, 2001). Within the engineered 3-D system, HSV-2 proliferated and replicated, gradually destroying the epithelial cell layers and cell-cell tight junctions from the apical to basal side as it would in vivo. This study focused primarily on the validation of a normal epithelium model and an HSV-2-infected model; however, this technology could be leveraged to investigate other mechanisms of viral infection or drug discovery.
3-D culture systems are a newer approach to investigate wound healing in the vagina. Shafaat et al. (2023) created a 3-D tissue-engineered vaginal model to evaluate the effect of estradiol-17ꞵ (E2) on wound healing (Shafaat et al., 2022). Decellularized sheep vaginal matrices were seeded with a co-culture of vaginal epithelial cells and fibroblasts. The presence of E2 improved wound healing, as demonstrated by improved stratification and re-epithelialization of the epithelial layer. In addition, E2 enhanced cellular proliferation and metabolic activity, while also downregulating α-SMA, which reduced fibrotic tissue formation. These studies are critical for understanding how estrogen affects vaginal wound healing because most pelvic floor operations occur in postmenopausal patients with lower estrogen levels (Wu et al., 2014).
4. Microphysiologic systems (MPS) and organ-on-a-chip
Other in vitro technology on the rise are organ-on-a-chip approaches, which are platforms designed to mimic organ physiology in a highly controlled setting using microfluidic chips. Mahajan et al. developed a microfluidic model of the vaginal mucosa to investigate the interactions between the vaginal microbiome and the underlying vaginal tissue (Mahajan et al., 2022). Primary human vaginal epithelial cells were seeded within the device in the apical channel on an extracellular matrix-coated porous membrane. On the lower surface of the membrane, the second channel was seeded with primary human uterine fibroblasts. Through this design, the research group modeled a differentiated vaginal epithelium, which was validated by multiple layers of stratified squamous epithelial cells and tissue-specific markers such as cytokeratin 5, 14, 13, and 15.
The authors then evaluated microbial interactions by introducing L. crispatus, a bacterial species considered optimal for a healthy vaginal microbiota, and non-optimal Gardnerella vaginalis, which is associated with bacterial vaginosis (Mahajan et al., 2022). Vaginal chips cultured with L. crispatus maintained a physiologically appropriate low pH, epithelial cell viability, and downregulated inflammatory cytokines. Chips co-cultured with G. vaginalis and other anaerobes found in the pathological vaginal microbiota showed increased secretion of inflammatory cytokines and an increase in pH. These organ-on-a-chip systems may be applied to understand the interactions between the vaginal microbiome and vaginal tissue properties. In addition, these systems may be used to evaluate the efficacy and safety of new clinical treatments for various vaginal pathologies.
5. Animal models
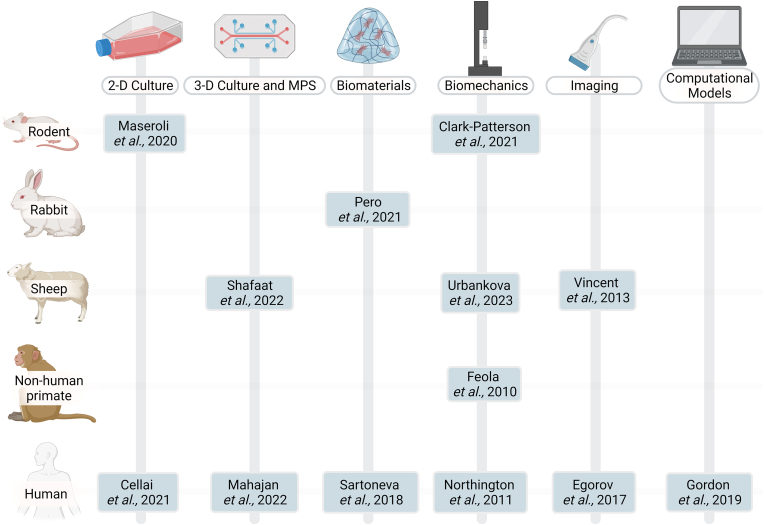
Animal models – including rodents, rabbits, sheep, and non-human primates – enable examination of physiology, disease progression, and potential vaginal treatment methods as part of a multi-system organism. Although no single animal model is a comprehensive analog for human tissue, each model offers benefits for investigating vaginal (patho)physiology (Fig. 3).
Fig. 3.
This table provides at-a-glance references for the intersection of models (rodent, rabbit, sheep, NHP, human) and bioengineering approaches (2-D culture, 3-D culture, MPS, biomaterials, biomechanics, imaging, and computational models. This is not an exhaustive table-additional discussion of recent work can be found in the respective sections. Figure created using Biorender.com.
5.1. Rodents
Rodents are the most commonly used animal models in biomedical research (Hickman et al., 2017). From a practical perspective, they are easy to acquire, handle, and inexpensive to maintain. Like humans, mice and rats have separate urethral and vaginal openings and a single cervix, but unlike humans, they have a bicornate uterine body that can support large (>5 pups) litters.
Rodents are also favored as a model system because of their well-characterized reproductive (estrous) cycle. The rodent estrous cycle lasts 3–5 days and spans four different stages: proestrus, estrus, metestrus, and diestrus (Ajayi and Akhigbe, 2020). During this cycle, dramatic changes occur in the thickness, composition, and function of the vaginal epithelial layer. Identifying the estrous cycle stage in mice may be done in various ways, including visual assessment or vaginal cytology (Ajayi and Akhigbe, 2020), (Caligioni, 2009).
Knockout (KO) strains are used to target the role of specific genes such as LOXL1 and FBLN5. These genetically modified mice enable the investigation of how the disruption of elastic fiber homeostasis contributes to pelvic floor disorders such as POP (Drewes et al., 2007; Lee et al., 2008; Clark-Patterson et al., 2021). In addition, a range of vaginal functions are investigated in rodents, including sexual arousal and postmenopausal sexual dysfunction. In rats, nitric oxide (NO)-mediated behaviors in the endothelium, epithelium, vasculature, and musculature are modulated by changes in the primary sex hormones estrogen and testosterone (Cellai et al., 2022; Berman et al., 1998; Kim et al., 2004). In addition, rodent KO models serve as a tool to investigate the development of postmenopausal vaginal atrophy by focusing on the mediation of epithelial cell proliferation, particularly in the context of hormone-dependent signaling and activation processes (Wan et al., 2022).
However, while rodents are a useful alternative to human samples, they are quadrupeds and therefore have different support structures that are critical to the vagina. For example, the levator ani muscles, which are thought to be involved in POP risk in humans, largely support the tail of the rodent, not the vaginal wall (Moalli et al., 2005). Investigations that focus on connective tissue remodeling can leverage anatomic and physiologic differences in alternative animal models to compliment vaginal research in rodents.
5.2. Rabbits
The rabbit is an intermediate model, in both physical scale and practical cost, between rodents and large animal models such as sheep. Rabbits are a common animal model for biocompatibility and pharmacology assessment, in part because the epithelial layer is much thinner in rabbits than in humans, meaning there is a lower threshold for irritation or other adverse inflammatory responses to topical substances (Costin et al., 2011). Therefore, rabbits are frequently used for irritation testing for intravaginal microbicides, spermicides, antifungals, and contraceptives, as well as for assessment of potential treatments for vaginal atrophy (Rochani et al., 2022; Xia et al., 2020; Barberini et al., 1992; Chollet et al., 2019). The rabbit shows promise as a model for studying female sexual function and arousal by investigating contractility and distension of the vaginal wall and its support structures in addition to blood flow and responsiveness to stimuli (Cruz et al., 2002; Kim et al., 2002; Oh et al., 2003). In addition, rabbit models have been used to assess the local complications of biological and synthetic meshes for vaginal and pelvic floor reconstructive surgery (Peró et al., 2021). The increase in size (relative to rodents) is beneficial because meshes can be used to evaluate the impact of implant location on host reactions and material outcomes in both abdominal and vaginal defects, as opposed to rodent models, where scale limits possible mesh placements (Peró et al., 2021; Pero et al., 2022; Knight et al., 2020).
The rabbit has some limitations as a model for the human vagina that should be considered; notably, major differences in gross anatomical structure. The rabbit vagina is long, especially relative to body size, and it has distinct regional components and different support structures than the human.
5.3. Large animal models (sheep)
Sheep are a preferred large animal alternative to non-human primates for studying vaginal pathologies because they spontaneously develop POP. Although this occurs most frequently during pregnancy as opposed to postmenopausal (as found in humans), the factors for vaginal prolapse mirror those of humans: age, number of previous pregnancies, changes in vaginal mechanics, and an increase in intra-abdominal pressure (McLean, 1956). Primiparous (first pregnancy) and ovariectomized sheep also show morphological and functional changes in the vagina that are analogous to changes seen in primiparous and postmenopausal women, such as changes in epithelium, size, stiffness, contractility, and composition (Isali et al., 2022), (Urbankova et al., 2019).
While rabbits and rodents are often used to study the local biocompatibility of grafts for pelvic reconstruction, the anatomic scale and similarities in physiology make ewes a useful tool for the evaluation of large-scale mesh implantation and other pelvic floor reconstructive approaches (Isali et al., 2022), (Shapiro et al., 2021; Diedrich et al., 2022; Hympánová et al., 2020). The similarities between human and ovine reproductive structures that make it a preferred model for meshes, surgical intervention, and biomechanics testing also make it a valuable model for preclinical testing of vaginal drug delivery mechanisms and biocompatibility of various intravaginal drugs and devices (Zhu et al., 2021), (Pyles et al., 2021). Additional recent work has used sheep in a range of disease applications, such as the evaluation and treatment of vaginal atrophy or as a disease model for vesicovaginal fistulas (Mackova et al., 2021), (Maljaars et al., 2022).
5.4. Non-human primates
Non-human primates (NHP) have the highest ethical and practical burden of any animal model but provide unique insights into the anatomy and physiology of the vagina. Rhesus macaques are often used as high-fidelity animal models of the human vagina across various research topics. Among a complex array of physiologic and anatomic similarities, they are transiently bipedal, develop POP spontaneously, and have a large fetal head size relative to pelvic outlet, a combination of factors not captured by lower animal models (Otto et al., 2002). These models enable studies to advance fundamental understanding of the vagina, including the potential microstructural and mechanical impacts of parity (Feola et al., 2010). In addition, morphologic and functional changes resulting from mesh implantation in non-human primates help identify the failure mechanisms of POP mesh surgery (Shaffer et al., 2019), (Knight et al., 2022). Stem cell-driven approaches to vaginal repair and remodeling have also shown success in an ovariectomized NHP model (Zhang, 2021).
Recent work in macaques also targeted the vaginal microbiome, with the goal of developing a more accurate mimic of the human microenvironment to better understand its role in reproductive health, disease transmission, fertility, and pregnancy (Langner et al.). To date, this has only been partially successful. A human-like microbiome (dominated by Lactobacilli) was temporarily induced in the rhesus macaque, which has a diverse native microenvironment (Langner et al.; Daggett et al., 2017; Chen et al., 2018). They hold potential as a model organism for microbial imbalances and infection because of the shared bacteria responsible for diseases such as bacterial vaginosis (Langner et al.; Daggett et al., 2017; Chen et al., 2018).
Considering practical, ethical, and scientific factors, animal models can be invaluable tools for investigating fundamental questions about the vagina, from microbiome and irritation models to organ-scale biomechanics.
6. Biomechanical properties
The vagina undergoes significant remodeling throughout a person's lifetime, including puberty, monthly menstruation, childbirth, postpartum healing, aging, and menopause. Vaginal biomechanical function, including the response to physical stressors, adapts due to this remodeling. To quantify the mechanical function of the vagina, both animal models and human tissue samples are utilized in mechanical testing setups. In these biomechanical tests, either a force or deformation is applied to the vaginal tissue, and the corresponding mechanical response (deformation or reaction force, respectively) is measured. Examples of these experiments include planar tensile testing, biaxial extension-inflation, and creep testing.
Previous investigations used uniaxial and biaxial tensile testing, biaxial shear lap protocols, and biaxial extension-inflation testing methods to characterize the mechanical properties of the vagina in mice (Akintunde et al., 2019; Capone et al., 2019; Clark et al., 2019; Robison et al., 2017), rats (Moalli et al., 2005), (Basha et al., 2009), (Feola et al., 2011), sheep (Rubod et al., 2012) and non-human primates (Feola et al., 2010), (Feola et al., 2011). Although these animal models provide a basic scientific understanding of the mechanical properties of the vagina, there are potential discrepancies between animal models and human-derived samples. For example, sheep (Rubod et al., 2012), rat (Skoczylas et al., 2013), and mouse (Clark et al., 2019) tissues demonstrated regional variations in their mechanical properties; the postmenopausal human vagina, however, was not observed to have region-dependent mechanical properties (Feola et al., 2013a).
In addition, vSMCs are of interest from a biomechanical perspective because of their contractile nature and the structural integrity they provide to the vaginal wall. In particular, smooth muscle contractile function, as it relates to pelvic organ prolapse, was investigated in both mouse and human samples (Clark et al., 2019), (Northington et al., 2011). In humans, vaginal samples with prolapse had a thinner muscularis along the longitudinal strip, as compared to patients without prolapse. Interestingly, patients with prolapse exhibited an increased contractile response to the general agonist potassium chloride (Northington et al., 2011). Although this was surprising, the prolapse samples did not contract in response to phenylephrine, which may suggest differences in vaginal cholinergic and adrenergic receptors are associated with prolapse development. Indeed, immunofluorescence techniques observed a decrease in adrenergic receptor density in vaginal samples with prolapse (Northington et al., 2011).
Quantifying the mechanical properties of the vagina may lead to a better understanding of the physical demands placed on the reproductive system in various healthy or diseased states. These functional characterizations are critical for the development of effective therapeutics for pelvic floor diseases or rehabilitation regimes for life events such as postpartum healing.
7. Imaging
The use of various imaging modalities in the treatment and diagnosis of vaginal pathophysiologies highlights the value of a bioengineering-driven approach to integrating open research questions and immediate clinical needs. Following serological testing, diagnosis of MKRH is commonly determined based on physical examination, which can identify anomalies and indicate the need for medical imaging. Magnetic resonance imaging (MRI) of the pelvis is considered to be the most accurate and effective nonoperative visualization technique for detecting discrepancies within the reproductive structure (Herlin et al., 2020), (Cooper et al., 2023). A combination of various MRI scans is the accepted process for MKRH analysis to generate complementary contrast from both tissue lipids (T1-weighted) and fluids (T2-weighted) within the region of interest, allowing visualization of the entire reproductive tract (Cooper et al., 2023; Jaiswal et al., 2023; Kawahara and Nagata, 2021). In instances where MRI is unavailable, transperineal or transabdominal ultrasonography (US) is conducted to establish the existence and condition of the cervix, vaginal canal, and uterus (Herlin et al., 2020), (Rogers and Merideth, 2015).
The vaginal microbiota are instrumental in the equilibrium of the microenvironment and the strength of the immune response to foreign organisms and materials (Chen et al., 2021). Flora imbalance and increased microbial diversity are conducive to diseases such as BV, which exacerbate the microenvironment and permit the entry of higher-risk pathogens (Chen et al., 2021). The decline of the native Lactobacillus microbe due to various internal or external factors correlates with the subsequent decrease in antimicrobial production, fostering an environment conducive to bacterial overpopulation (Chen et al., 2021). Individuals with BV are at increased risk of contracting HIV and subsequently, acquired immunodeficiency syndrome (AIDS) (Chen et al., 2021). Interest in proactive protection of the vaginal microenvironment gave rise to microbicidal gels and a novel imaging technique termed Fourier-domain multiplexed low-coherence interferometry (mLCI) for assessment of microbicidal treatments (Drake et al., 2011). The device utilizes fiber optic interferometers condensed into a polycarbonate probe in conjunction with a fluorometric imaging system to quantify the thickness and dilution of the microbicidal gel over the vaginal epithelium (Drake et al., 2011). Drake et al. used the mLCI technique to analyze the dilution of gel within the vaginal canal and found that the thickness remained constant; however, the fluorimetry signal, representing the concentration of the gel, decreased as a function of time (Drake et al., 2013). The application of mLCI imaging technology to the study of STIs invokes questions regarding how advancements in this technique could increase the understanding of (patho)physiology and interventions for the reproductive tract in the future.
Imaging techniques can assess not only treatment efficacy but also investigate the physiologic factors that contribute to disease transmission. Epithelial thickness during the menstrual cycle affects both the effectiveness of intravaginal drug delivery and the risk of STI transmission (Vincent et al., 2013). To understand changes within the vaginal epithelium in vivo, optical coherence tomography (OCT) can be employed. OCT is a noninvasive imaging technique in which high-resolution cross-sectional images are acquired (Aumann et al., 2019). Vincent et al. published an investigation in 2013 involving OCT probe measurements of the thickening and thinning of the vaginal epithelium in sheep models (Aumann et al., 2019), (Vincent et al., 2013). The capacity for longitudinal quantification of epithelial changes in sheep models illustrates the potential impact of OCT technology on future gynecological research. More recent work developed and translated this approach in pilot imaging studies using an OCT catheter system for in vivo intravaginal tissue imaging in the human vaginal canal to monitor tissue microstructure. These studies evaluate both postmenopausal and healthy premenopausal patients undergoing experimental CO2 laser treatment to mitigate symptoms related to GSM (Miao et al., 2022). Although ultrasound is an effective method for visualizing reproductive structures, it is unable to resolve stratified layers of vaginal tissue, including the epithelium, limiting its applicability for GSM (Miao et al., 2022). The intravaginal OCT endoscope is minimally invasive and can produce 3-D structures of the vaginal microanatomy with depth resolution, allowing quantification of anatomical properties (Miao et al., 2022).
Changes in the mechanical properties of tissue associated with pelvic floor disorders are traditionally assessed in clinic through bimanual palpation. Quantifiable information and mapping of the biomechanincal properties of the vagina could provide valuable information to both clinicans and patients for both initial diagnosis and evaluation of treatment response. The recently FDA-approved tactile imaging (TI) device (the vaginal tactile imager, or VTI) provides a more objective measure of the mechanical and functional properties of the tissue (Egorov et al., 2017).
To evaluate and quantify the specific biomechanical properties of pelvic tissue in women before delivery, a modified VTI device, the antepartum tactile imager (ATI), was developed in 2020 (Brandt et al., 2020). This preliminary probe-based design uses six degrees of freedom and 128 sensors on a double convex head to simulate delivery and obtain 3-D images with particular emphasis on the perineum and pubic symphysis (Brandt et al., 2020). An improved version of the VTI, dubbed the vaginal tactile ultrasound imager (TIUSv), was introduced in 2021 to evaluate individuals with POP conditions and those with normal pelvic floor function (Egorov et al., 2021). The use of tactile imaging to quantify biomechanical properties in patients with POP is a noteworthy medical advancement in the imaging and clinical assessment of pelvic floor disorders. The current standard for analysis of the pelvic floor is an MRI or US following a physical examination from the clinician; however, these imaging modalities are strictly structural and lack the ability (of TI) to quantify biomechanical properties (Fitzgerald and Richter, 2020). Alternative imaging devices, such as the Vaginal Biomechanic Analyzer (VBA), are also used in clinical settings for initial assesment of vaginal laxity and evaluation of treatment effectiveness (Zimmern et al., 2021).
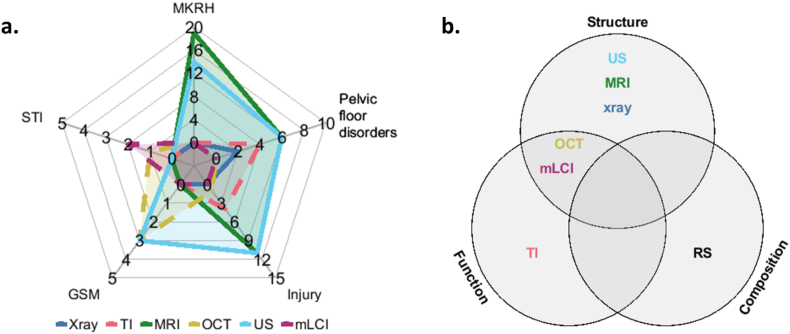
Imaging techniques ideally serve as noninvasive methods to visualize and quantify changes associated with physiologic conditions, disease progression, or response to treatment. Different modalities span a vast range of resolution, scale, speed, cost, and accessibility. Specifically for vaginal imaging, conventional imaging techniques (US, MRI, X-ray) primarily provide structural contrast. Modalities that translated from other fields (OCT) or developed specifically for vaginal and pelvic floor conditions (TI, mLCI) can provide additional functional information to further characterize tissue and disease status (Fig. 4). Compositional changes at the cellular level precede structural and functional manifestations and indicate yet another level of imaging contrast that may be critical for understanding vaginal conditions.
Fig. 4.
This visual representation of literature is intended to highlight unmet needs and opportunities for investigation of vaginal (patho)physiology using imaging approaches. Part (a.) displays a radar chart of the discussed vaginal conditions and the respective imaging modalities for each. The solid lines indicate clinically applied technologies, while the dashed lines represent investigational techniques. The radii are representative of the reviewed literature and relevant citations. Part (b.) exhibits a Venn diagram relating to the results of the aforementioned imaging types.
One tool that may be able to achieve label-free compositional analysis of vaginal tissues is Raman spectroscopy. Previously investigated in both mice and humans, this approach demonstrated the potential to detect changes in tissue composition in the uterine cervix during the course of pregnancy and labor that correlate with alterations in tissue remodeling and biomechanics that are essential for vaginal delivery (O'Brien et al., 2017), (Masson et al., 2022). The confirmation of the efficacy of biochemical imaging of the cervix offers a unique translational opportunity into the realm of inadequately understood vaginal diseases. Investigational imaging tools that capture biochemical information may offer avenues for preventative medicine by assisting surgical planning, clinical therapy monitoring, and achieving a more complete understanding of the underlying disease mechanisms.
8. Computational models used in labor and delivery
Engineers and scientists use imaging techniques such as ultrasound and MRI to formulate mathematical and computational modeling tools to visualize the evolving geometry of the pelvic floor. For example, statistical shape modeling determined the variability between medical professionals visually discerning vaginal anatomical changes using MRI techniques (Easley et al., 2015). This is particularly relevant as the field moves toward improving the assessment of therapies available for women undergoing vaginal disorders, such as POP (Couri et al., 2012; Deprest et al., 2022; Word et al., 2009). However, interobserver repeatability when reading MRI results varied greatly, which makes it difficult to draw reliable quantitative conclusions about anatomical changes (Hodroff et al., 2002), (Kruger et al., 2008). Employing similar statistical shape modeling methods may serve as a useful tool to assess vaginal remodeling during life events, such as gestation, or pelvic floor pathologies, such as prolapse.
In addition, longitudinal (time-dependent) modeling and finite element analysis may be employed alongside computer-aided design (CAD) software to render complex biomechanical models of the female pelvic floor. Phenomena such as pregnancy or pelvic organ prolapse are associated with significant vaginal growth and remodeling. These events are challenging to study in vivo, but computational modeling efforts offer a potential method for investigating the biomechanical mechanisms of growth and remodeling. Along these lines, recent work developed a 3-D finite element model of the anterior vaginal wall to investigate anatomical changes associated with the development of pelvic organ prolapse (Gordon et al., 2019). Future work employing similar computational models may offer a unique opportunity to improve our understanding of vaginal growth and remodeling while also highlighting clinical directions where in-depth mechanobiological characterization of underlying cellular and molecular processes is needed.
9. Medical devices
Common medical devices utilized in the female reproductive system include those used in the management of menstruation, pelvic organ prolapse meshes, and pessary repairs. A pessary is an intravaginal device used to support the vaginal wall during POP or to alleviate symptoms associated with urinary incontinence. Pessaries are one of the most common devices used in the clinic due to the ability to simultaneously treat symptoms of prolapse and incontinence, as up to 80% of patients with stress urinary incontinence have some development of prolapse (Groutz et al., 2010). In a previous clinical study, vaginal pessary use had 90.7% efficacy and a high level of treatment satisfaction (Zeiger et al., 2022). In an additional study, while over 70% of participants were satisfied with their pessary use, more than half experienced mild complications, such as vaginal erosion (Meriwether et al., 2015).
Pessaries have several clear benefits, including wide acceptance by insurance providers and increasing incorporation of biofeedback information, but not all patients are fit for pessary use. This is because of complications ranging from the severity of presenting incontinence or prolapse, pelvic infection, or an allergy to the product materials (Zeiger et al., 2022), (Ray et al., 2021). In such instances, indwelling surgical devices such as pelvic floor meshes were historically used to treat prolapse.
At one point in time, more than 60 implant types were temporarily found on the US market for pelvic organ prolapse and urinary incontinence (Ray et al., 2021). Over the last decade, however, several clinical studies indicated the unsafe nature and risk associated with various mesh implants (Diwadkar et al., 2009; Feola et al., 2013b; Jean-Charles et al., 2010; Kim and Jeon, 2020; Liang et al., 2013), which caused the FDA to recategorize surgical meshes intended for pelvic organ prolapse repair from class II to class III medical devices in 2016 (Sassani et al., 2020). This reclassification to the agency's most strict safety category required additional clinical studies. As a result, all mesh manufacturers, except Boston Scientific and Coloplast, stopped marketing and distributing mesh implants for pelvic organ prolapse (Sassani et al., 2020). In 2019, the FDA issued a landmark order that the remaining manufacturers stop selling and distributing their products immediately because reasonable assurances of safety and effectiveness were not being met (Junge et al., 2002).
Given the widely known controversy and lack of safety for patients, there is an active need to develop biocompatible pelvic floor mesh materials. Such materials are often non-absorbable and will need to remain permanently in the body to provide continuous support to the pelvic floor. Most biocompatible materials that are currently being investigated, however, are absorbable materials derived from animal tissues, such as the intestinal tract or skin (Junge et al., 2002), (Maurer et al., 2014). Future directions for pelvic floor medical device development may include the use of specialized biomaterials, as described below.
10. Biomaterials
Biomaterial and tissue engineering approaches seek to use both synthetic and natural materials to design therapeutic options for various vaginal pathologies (Henckes et al., 2019), (Gudde et al., 2022). Combinations of biocompatible polymers, cells, and cell culture conditions are the subject of ongoing and future research, which attempts to capture and emulate the relevant features and nuances of native tissue. Materials, such as poly-l-lactide-co-ϵ-caprolactone (PCL), were seeded with human vaginal epithelial and stromal cells for in vitro assessment of biocompatibility for vaginal reconstruction materials (Sartoneva et al., 2018). The elastic modulus of the scaffold was within a physiologically relevant range, and although the cells remained healthy and proliferative in separate cultures, conditions were not ideal for stromal cells in the co-culture medium (Henckes et al., 2019), (Gudde et al., 2022). Animal tissue can also serve as a material for vaginal reconstruction because they are native biological materials and have high bioactivity and similar ECM components. For example, the porcine vagina was used to create a cytocompatible acellular matrix with a retained basement membrane (Greco et al., 2018). Engineered tissue models and alternative development methods can be used to create materials that can be highly tailored to specific application-based requirements in biophysical properties such as hydration rate or mechanical strength. Shafaat et al. (2022) tissue-engineered a vaginal tissue model by seeding primary vaginal epithelial cells and vaginal fibroblasts in a decellularized sheep vaginal extracellular matrix (Shafaat et al., 2022). This approach successfully mimicked select physiological attributes of native human tissue, including estrogen (estradiol-17ꞵ) dose-dependent changes in epithelial thickness and cellular proliferation.
Furthermore, alternative manufacturing techniques can be leveraged to create novel mesh properties. Vashaghian et al. demonstrated the feasibility of electrospun polymer nanofibrous matrices for the repair of POP by seeding meshes with human vaginal fibroblasts from both prolapsed and healthy tissues and evaluating the properties of the mesh and cell behavior (Vashaghian et al., 2017). The technique was also used by Mangǵr et al., who created a mesh capable of releasing estrogen, which resulted in increased stimulation of new blood vessel formation and ECM production (Mangır et al., 2019). These tissue engineering and biomaterials applications offer a unique opportunity to use the principles of materials science and manufacturing to attune synthetic and natural materials to recapitulate native vaginal tissue.
11. Conclusion
Rapid intensification of focus and research interest in the vagina has driven considerable progress in our fundamental understanding of the organ. This has been facilitated by a collaborative bioengineering approach that incorporates cell biology, materials science, mathematical modeling, mechanics, and clinical knowledge to create a more comprehensive understanding of the organ.
Ongoing and future research in tissue engineering, biomechanics, imaging, computational modeling, and material science offers promising opportunities for better understanding the relationship between microstructural composition and functional behavior as well as the process of growth, remodeling, repair, and maintenance across major life events, including pregnancy, fetal delivery, postpartum recovery, and menopause. This information will be critical for developing effective therapeutics for patients who give birth, for managing various pathologies, and for developing future treatment options for women with vaginas. There are also unique opportunities centered around the de novo creation of vaginas for people without them, such as those with genetic agenesis (MRKH syndrome) or transgender women.
The development of materials for surgical repair of pelvic floor disorders has multidisciplinary implications. The vagina has complex material properties that interact with a variety of biologic and mechanical stimuli, providing an interesting platform for the development of biomimetic materials for use in robotics applications or in other organs. Previous findings and experimental approaches used to study organs with similar function, geometry, or microstructure as the vagina (such as the esophagus, gastrointestinal tract, or vasculature) shape the field of vaginal research, and information flow can become reciprocal as knowledge of the vaginal pathophysiology expands.
Furthermore, given the very public failure of previous pelvic meshes, successful deployment of these techniques is an opportunity to restore public and patient faith in the scientific and medical community.
The gaps in our fundamental knowledge about the vagina cannot be fully attributed to the challenges presented by its pathophysiologic complexity. It is also a byproduct of the long-standing acceptance of scientific and social ignorance surrounding female reproductive anatomy and, by extension, diseases of that system. Given the scientific and societal nuance surrounding the vagina— with topics ranging from maternal-fetal health to sexual pleasure— this tissue, and all tissues of the female pelvic floor, is deserving of a greater research focus as well as more awareness that there is a high burden of safety and efficacy for any scientific knowledge or discovery that impacts clinical care choices.
The most effective and efficient way to move forward in building a relevant and rigorous body of information on vaginal physiology and pathophysiology is not only to grow the field of study but also to harness the potential of combined interdisciplinary teams of engineers, scientists, and clinicians. Continued scientific interest, research, and advocacy are required to piece together the complex multiscale puzzle that is the next generation of fundamental scientific breakthroughs in our understanding of the vagina and the impact it will have on future clinical treatments for related diseases and disorders.
CRediT authorship contribution statement
Lily M. Buchanan: Conceptualization, Writing – original draft, Writing – review & editing. Mari J.E. Domingo: Conceptualization, Writing – original draft, Writing – review & editing. Shelby E. White: Conceptualization, Writing – original draft, Writing – review & editing. Triniti N. Vanoven: Writing – original draft, Writing – review & editing. Niyousha Karbasion: Writing – review & editing. Matthew R. Bersi: Writing – review & editing. Isaac J. Pence: Writing – original draft, Writing – review & editing, Supervision. Maria Florian-Rodriguez: Writing – original draft, Writing – review & editing. Kristin S. Miller: Conceptualization, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Lily M. Buchanan, Email: Lily.Buchanan@utdallas.edu.
Mari J.E. Domingo, Email: mdomingo1@tulane.edu.
Shelby E. White, Email: swhite18@tulane.edu.
Triniti N. Vanoven, Email: Triniti.Vanoven@utdallas.edu.
Niyousha Karbasion, Email: k.niyousha@wustl.edu.
Matthew R. Bersi, Email: mbersi@wustl.edu.
Isaac J. Pence, Email: Isaac.Pence@utsouthwestern.edu.
Maria Florian-Rodriguez, Email: Maria.Florian-Rodriguez@UTSouthwestern.edu.
Kristin S. Miller, Email: Kristin.Miller@utdallas.edu.
Data availability
No data was used for the research described in the article.
References
- Ajayi A.F., Akhigbe R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil. Res. Pract. 2020;6:5. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintunde A.R., Robison K.M., Capone D.J., Desrosiers L., Knoepp L.R., Miller K.S. Effects of elastase digestion on the murine vaginal wall biaxial mechanical response. J. Biomech. Eng. 2019;141(2) doi: 10.1115/1.4042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabebe E., Anumba D.O.C. The vaginal microenvironment: the physiologic role of lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnegard M.E., Whitten L.A., Hunter C., Clayton J.A. Sex as a biological variable: a 5-year progress report and call to action. J. Womens Health. 2020;29(6):858–864. doi: 10.1089/jwh.2019.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann S., Donner S., Fischer J., Müller F. In: High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Bille J.F., editor. Springer; 2019. Optical coherence tomography (OCT): principle and technical realization.http://www.ncbi.nlm.nih.gov/books/NBK554044/ (Cham (CH)). [Online]. Available: [PubMed] [Google Scholar]
- Barberini F., De Santis F., Correr S., Motta P.M. The mucosa of the rabbit vagina: a proposed experimental model for correlated morphofunctional studies in humans. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992;44(3):221–227. doi: 10.1016/0028-2243(92)90103-6. [DOI] [PubMed] [Google Scholar]
- Barnhart K.T., et al. Baseline dimensions of the human vagina. Hum. Reprod. 2006;21(6):1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- Basha M., Labelle E.F., Northington G.M., Wang T., Wein A.J., Chacko S. Functional significance of muscarinic receptor expression within the proximal and distal rat vagina. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(5):R1486–R1493. doi: 10.1152/ajpregu.90516.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.R., McCarthy M.M., Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998;51(4):650–656. doi: 10.1016/S0090-4295(97)00683-3. [DOI] [PubMed] [Google Scholar]
- Brandt J.S., Rosen T., Van Raalte H., Kurtenos V., Egorov V. Characterization of perineum elasticity and pubic bone-perineal critical distance with a novel tactile probe: results of an intraobserver reproducibility study. Open J. Obstet. Gynecol. 2020;10(4):493–503. doi: 10.4236/ojog.2020.1040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows L.J., Shaw H.A., Goldstein A.T. The vulvar dermatoses. J. Sex. Med. 2008;5(2):276–283. doi: 10.1111/j.1743-6109.2007.00703.x. [DOI] [PubMed] [Google Scholar]
- Caligioni C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Protein Sci. 2009;48(1) doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone D.J., et al. Evaluating residual strain throughout the murine female reproductive system. J. Biomech. 2019;82:299–306. doi: 10.1016/j.jbiomech.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellai I., et al. Insight on the intracrinology of menopause: androgen production within the human vagina. Endocrinology. 2021;162(2):bqaa219. doi: 10.1210/endocr/bqaa219. [DOI] [PubMed] [Google Scholar]
- Cellai I., et al. Testosterone positively regulates vagina NO-induced relaxation: an experimental study in rats. J. Endocrinol. Invest. 2022;45(6):1161–1172. doi: 10.1007/s40618-022-01743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., et al. Diversity of macaque microbiota compared to the human counterparts. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-33950-6. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lu Y., Chen T., Li R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet J., Mermelstein F., Rocamboli S.C., Friend D.R. Vaginal tamoxifen for treatment of vulvar and vaginal atrophy: pharmacokinetics and local tolerance in a rabbit model over 28 days. Int. J. Pharm. 2019;570 doi: 10.1016/j.ijpharm.2019.118691. [DOI] [PubMed] [Google Scholar]
- Clark G.L., et al. Smooth muscle regional contribution to vaginal wall function. Interface Focus. 2019;9(4) doi: 10.1098/rsfs.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Patterson G.L., Roy S., Desrosiers L., Knoepp L.R., Sen A., Miller K.S. Role of fibulin-5 insufficiency and prolapse progression on murine vaginal biomechanical function. Sci. Rep. 2021;11:20956. doi: 10.1038/s41598-021-00351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N., et al. preprint; Preprints: 2023. Magnetic Resonance Imaging (MRI) and Clinical Features of Mayer-Rokitansky-Küster–Hauser (MRKH) Syndrome: a 10-year Review from a Dedicated Specialist Centre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costin G.-E., Raabe H.A., Priston R., Evans E., Curren R.D. Vaginal irritation models: the current status of available alternative and in vitro tests. Altern. Lab. Anim. 2011;39(4):317–337. doi: 10.1177/026119291103900403. [DOI] [PubMed] [Google Scholar]
- Coudray M.S., Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couri B.M., Lenis A.T., Borazjani A., Paraiso M.F.R., Damaser M.S. Animal models of female pelvic organ prolapse: lessons learned. Expet Rev. Obstet. Gynecol. 2012;7(3):249–260. doi: 10.1586/eog.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Y., Hudson R., Pacheco P., Lucio R.A., Martínez-Gómez M. Anatomical and physiological characteristics of perineal muscles in the female rabbit. Physiol. Behav. 2002;75(1):33–40. doi: 10.1016/S0031-9384(01)00638-2. [DOI] [PubMed] [Google Scholar]
- Daggett G.J., et al. Comparison of the vaginal environment in rhesus and cynomolgus macaques pre- and post-lactobacillus colonization. J. Med. Primatol. 2017;46(5):232–238. doi: 10.1111/jmp.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLancey J.O.L. What's new in the functional anatomy of pelvic organ prolapse? Curr. Opin. Obstet. Gynecol. 2016;28(5):420–429. doi: 10.1097/GCO.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.-M., Dai F.-F., Yuan M.-Q., Yang D.-Y., Zheng Y.-J., Cheng Y.-X. “Advances in molecular mechanisms of pelvic organ prolapse. Exp. Ther. Med. 2021;22(3):1–7. doi: 10.3892/etm.2021.10442. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprest J.A., et al. International Urogynecological Consultation (IUC): pathophysiology of pelvic organ prolapse (POP) Int Urogynecol J. 2022;33(7):1699–1710. doi: 10.1007/s00192-022-05081-0. [DOI] [PubMed] [Google Scholar]
- Di Simone N., et al. Recent insights on the maternal microbiota: impact on pregnancy outcomes. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.528202. https://www.frontiersin.org/articles/10.3389/fimmu.2020.528202 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich C.M., et al. Evaluation of the short-term host response and biomechanics of an absorbable poly-4-hydroxybutyrate scaffold in a sheep model following vaginal implantation. BJOG An Int. J. Obstet. Gynaecol. 2022;129(7):1039–1049. doi: 10.1111/1471-0528.17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar G.B., Barber M.D., Feiner B., Maher C., Jelovsek J.E. Complication and reoperation rates after apical vaginal prolapse surgical repair : a systematic review. Obstet. Gynecol. N. Y. 1953. 2009;113(2):367–373. doi: 10.1097/AOG.0b013e318195888d. [DOI] [PubMed] [Google Scholar]
- Drake T.K., et al. Design and validation of a multiplexed low coherence interferometry instrument for in vivo clinical measurement of microbicide gel thickness distribution. Biomed. Opt Express. 2011;2(10):2850–2858. doi: 10.1364/BOE.2.002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T.K., Shah T., Peters J.J., Wax A., Katz D.F. Measuring dilution of microbicide gels with optical imaging. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes P.G., et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am. J. Pathol. 2007;170(2):578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley D.C., Menon P.G., Moalli P.A., Abramowitch S.D. IMECE2015, Volume 3: Biomedical and Biotechnology Engineering. 2015. Inter-observer variability of vaginal wall segmentation from MRI: a statistical shape analysis approach. [DOI] [Google Scholar]
- Egorov V., et al. Quantitative assessment and interpretation of vaginal conditions. Sex. Med. 2017;6(1):39–48. doi: 10.1016/j.esxm.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov V., van Raalte H., Shobeiri S.A. Tactile and ultrasound image fusion for functional assessment of the female pelvic floor. Open J. Obstet. Gynecol. 2021;11(6):674–688. doi: 10.4236/ojog.2021.116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M.A., Maibach H.I. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006;273(4):195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- Feola A., Abramowitch S., Jones K., Stein S., Moalli P. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am. J. Obstet. Gynecol. 2010;203(6):595.e1–595.e8. doi: 10.1016/j.ajog.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola A., Moalli P., Alperin M., Duerr R., Gandley R.E., Abramowitch S.D. Impact of pregnancy and vaginal delivery on the passive and active mechanics of the rat vagina. Ann. Biomed. Eng. 2011;39(1):549–558. doi: 10.1007/s10439-010-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola A., Duerr R., Moalli P., Abramowitch S. Changes in the rheological behavior of the vagina in women with pelvic organ prolapse. Int. Urogynecology J. 2013;24(7):1221–1227. doi: 10.1007/s00192-012-2002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola A., et al. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG An Int. J. Obstet. Gynaecol. 2013;120(2):224–232. doi: 10.1111/1471-0528.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J., Richter L.A. The role of MRI in the diagnosis of pelvic floor disorders. Curr. Urol. Rep. 2020;21(7):26. doi: 10.1007/s11934-020-00981-4. [DOI] [PubMed] [Google Scholar]
- Gordon M.T., DeLancey J.O.L., Renfroe A., Battles A., Chen L. Development of anatomically based customizable three-dimensional finite-element model of pelvic floor support system: POP-SIM1.0. Interface Focus. 2019;9(4) doi: 10.1098/rsfs.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco K.V., Jones L.G., Obiri-Yeboa I., Ansari T. Creation of an acellular vaginal matrix for potential vaginal augmentation and cloacal repair. J. Pediatr. Adolesc. Gynecol. 2018;31(5):473–479. doi: 10.1016/j.jpag.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Groutz A., Levin I., Gold R., Pauzner D., Lessing J.B., Gordon D. ‘Inside-out’ transobturator tension-free vaginal tape for management of occult stress urinary incontinence in women undergoing pelvic organ prolapse repair. Urology. 2010;76(6):1358–1361. doi: 10.1016/j.urology.2010.04.070. [DOI] [PubMed] [Google Scholar]
- Gudde A.N., van Velthoven M.J.J., Roovers J.-P.W.R., Kouwer P.H.J., Guler Z. Polyisocyanides as a substrate to trigger vaginal fibroblast functioning in an in vitro model for prolapse repair. Biomater. Adv. 2022;141 doi: 10.1016/j.bioadv.2022.213104. [DOI] [PubMed] [Google Scholar]
- Henckes N.A.C., et al. Tissue-engineered solution containing cells and biomaterials—an in vitro study: a perspective as a novel therapeutic application. Int. J. Artif. Organs. 2019;42(6):307–314. doi: 10.1177/0391398819833383. [DOI] [PubMed] [Google Scholar]
- Herlin M.K., Petersen M.B., Brännström M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: a comprehensive update. Orphanet J. Rare Dis. 2020;15:214. doi: 10.1186/s13023-020-01491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman D.L., Johnson J., Vemulapalli T.H., Crisler J.R., Shepherd R. Commonly used animal models. Princ. Anim. Res. Grad. Undergrad. Stud. 2017:117–175. doi: 10.1016/B978-0-12-802151-4.00007-4. [DOI] [Google Scholar]
- Hodroff M.A., Stolpen A.H., Denson M.A., Bolinger L., Kreder K.J. Dynamic magnetic resonance imaging of the female pelvis: the relationsip with the pelvic organ prolapse quantification staging system. J. Urol. 2002;167(3):1353–1355. doi: 10.1016/S0022-5347(05)65298-6. [DOI] [PubMed] [Google Scholar]
- Hympánová L., et al. Assessment of electrospun and ultra-lightweight polypropylene meshes in the sheep model for vaginal surgery. Eur. Urol. Focus. 2020;6(1):190–198. doi: 10.1016/j.euf.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Isali Ilaha, et al. Comparison of morphological and histological characteristics of human and sheep: sheep as a potential model for testing midurethral slings in vivo. Urol. Int. 2022:1–7. doi: 10.1159/000522138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Prasad U., Gupta R., Kumari M. Magnetic resonance imaging in patients of Mayer–Rokitansky–Kuster–Hauser (MRKH) syndrome presenting with primary amenorrhoea: a prospective study. Egypt. J. Radiol. Nucl. Med. 2023;54(1):69. doi: 10.1186/s43055-023-01015-y. [DOI] [Google Scholar]
- Jean-Charles C., Rubod C., Brieu M., Boukerrou M., Fasel J., Cosson M. Biomechanical properties of prolapsed or non-prolapsed vaginal tissue: impact on genital prolapse surgery. Int. Urogynecology J. 2010;21(12):1535–1538. doi: 10.1007/s00192-010-1208-z. [DOI] [PubMed] [Google Scholar]
- Junge K., et al. Influence of mesh materials on collagen deposition in a rat model. J. Investig. Surg. Off. J. Acad. Surg. Res. 2002;15(6):319–328. doi: 10.1080/08941930290086137. [DOI] [PubMed] [Google Scholar]
- Kawahara D., Nagata Y. T1-weighted and T2-weighted MRI image synthesis with convolutional generative adversarial networks. Rep. Pract. Oncol. Radiother. J. Gt. Cancer Cent. Poznan Pol. Soc. Radiat. Oncol. 2021;26(1):35–42. doi: 10.5603/RPOR.a2021.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.Y., Jeon M.J. Risk factors for vaginal mesh erosion after sacrocolpopexy in Korean women. PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0228566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.N., Min K., Huang Y., Goldstein I., Traish A.M. Biochemical and functional characterization of alpha-adrenergic receptors in the rabbit vagina. Life Sci. 2002;71(24):2909–2920. doi: 10.1016/S0024-3205(02)02162-8. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Jeong S.-J., Munarriz R., Kim N.N., Goldstein I., Traish A.M. An in vivo rat model to investigate female vaginal arousal response. J. Urol. 2004;171(3):1357–1361. doi: 10.1097/01.ju.0000109868.19569.d7. [DOI] [PubMed] [Google Scholar]
- Knight K.M., Artsen A.M., Routzong M.R., King G.E., Abramowitch S.D., Moalli P.A. New Zealand white rabbit: a novel model for prolapse mesh implantation via a lumbar colpopexy. Int. Urogynecology J. 2020;31(1):91–99. doi: 10.1007/s00192-019-04071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K.M., King G.E., Palcsey S.L., Suda A., Liang R., Moalli P.A. Mesh deformation: a mechanism underlying polypropylene prolapse mesh complications in vivo. Acta Biomater. 2022;148:323–335. doi: 10.1016/j.actbio.2022.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J.A., Heap S.W., Murphy B.A., Dietz H.P. Pelvic floor function in nulliparous women using three-dimensional ultrasound and magnetic resonance imaging. Obstet. Gynecol. N. Y. 1953. 2008;111(3):631–638. doi: 10.1097/AOG.0b013e3181655dc2. [DOI] [PubMed] [Google Scholar]
- C. A. Langner, A. M. Ortiz, J. K. Flynn, H. Kendall, L. A. Lagenaur, and J. M. Brenchley, “The vaginal microbiome of nonhuman primates can Be only transiently altered to become lactobacillus dominant without reducing inflammation,” Microbiol. Spectr., vol. 9, no. 3, pp. e01074-21, doi: 10.1128/Spectrum.01074-21. [DOI] [PMC free article] [PubMed]
- Łaniewski P., Herbst-Kralovetz M. In: Encyclopedia of Reproduction. second ed. Skinner M.K., editor. Academic Press; Oxford: 2018. Vagina; pp. 353–359. [DOI] [Google Scholar]
- Lee U.J., et al. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am. J. Physiol. Ren. Physiol. 2008;295(2):F545–F555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- Lewis F.M.T., Bernstein K.T., Aral S.O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 2017;129(4):643–654. doi: 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R., et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG An Int. J. Obstet. Gynaecol. 2013;120(2):233–243. doi: 10.1111/1471-0528.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Betschart C., Ashton-Miller J.A., DeLancey J.O.L. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int. Urogynecology J. 2016;27(7):1087–1095. doi: 10.1007/s00192-016-2949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackova K., et al. Vaginal Er:YAG laser application in the menopausal Ewe model: a randomised estrogen and sham-controlled trial. BJOG An Int. J. Obstet. Gynaecol. 2021;128(6):1087–1096. doi: 10.1111/1471-0528.16558. [DOI] [PubMed] [Google Scholar]
- Mahajan G., et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome. 2022;10:201. doi: 10.1186/s40168-022-01400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljaars L.P., et al. Validation of an ovine vesicovaginal fistula model. Int. Urogynecology J. 2022;33(11):3185–3193. doi: 10.1007/s00192-022-05342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangır N., Hillary C.J., Chapple C.R., MacNeil S. Oestradiol-releasing biodegradable mesh stimulates collagen production and angiogenesis: an approach to improving biomaterial integration in pelvic floor repair. Eur. Urol. Focus. 2019;5(2):280–289. doi: 10.1016/j.euf.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Maseroli E., et al. Anti-inflammatory effects of androgens in the human vagina. J. Mol. Endocrinol. 2020;65(3):109–124. doi: 10.1530/JME-20-0147. [DOI] [PubMed] [Google Scholar]
- Masson L.E., et al. In vivo Raman spectroscopy monitors cervical change during labor. Am. J. Obstet. Gynecol. 2022;227(2):275.e1–275.e14. doi: 10.1016/j.ajog.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M.M., Röhrnbauer B., Feola A., Deprest J., Mazza E. Mechanical biocompatibility of prosthetic meshes: a comprehensive protocol for mechanical characterization. J. Mech. Behav. Biomed. Mater. 2014;40:42–58. doi: 10.1016/j.jmbbm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- McLean J.W. Vaginal prolapse in sheep. N. Z. Vet. J. 1956;4(2):38–55. doi: 10.1080/00480169.1956.33219. [DOI] [Google Scholar]
- Meriwether K.V., Rogers R.G., Craig E., Peterson S.D., Gutman R.E., Iglesia C.B. The effect of Trimosan© gel on pessary-associated bacterial vaginosis: a multi-center, randomized, controlled trial. Am. J. Obstet. Gynecol. 2015;213(5):729.e1–729.e9. doi: 10.1016/j.ajog.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merone L., Tsey K., Russell D., Nagle C. Sex inequalities in medical research: a systematic scoping review of the literature. Womens Health Rep. 2022;3(1):49–59. doi: 10.1089/whr.2021.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., et al. Optical coherence tomography evaluation of vaginal epithelial thickness during CO2 laser treatment: a pilot study. J. Biophot. 2022;15(11) doi: 10.1002/jbio.202200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirin A.A. Gender disparity in the funding of diseases by the U.S. National institutes of health. J. Womens Health. 2021;30(7):956–963. doi: 10.1089/jwh.2020.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalli P., et al. A rat model to study the structural properties of the vagina and its supportive tissues. Am. J. Obstet. Gynecol. 2005;192(1):80–88. doi: 10.1016/j.ajog.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Nappi R.E., et al. Addressing vulvovaginal atrophy (VVA)/Genitourinary syndrome of menopause (GSM) for healthy aging in women. Front. Endocrinol. 2019;10:561. doi: 10.3389/fendo.2019.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington G.M., Basha M., Arya L.A., Wein A.J., Chacko S. Contractile response of human anterior vaginal muscularis in women with and without pelvic organ prolapse. Reprod. Sci. 2011;18(3):296–303. doi: 10.1177/1933719110392054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel-Ohayon M., Neuman H., Koren O. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01031. https://www.frontiersin.org/articles/10.3389/fmicb.2016.01031 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.-J., Sk H., Sw K., Kim S., Paick J.-S. Histological and functional aspects of different regions of the rabbit vagina. Int. J. Impot. Res. 2003;15(2):142–150. doi: 10.1038/sj.ijir.3900986. [DOI] [PubMed] [Google Scholar]
- Otto L.N., Slayden O.D., Clark A.L., Brenner R.M. The rhesus macaque as an animal model for pelvic organ prolapse. Am. J. Obstet. Gynecol. 2002;186(3):416–421. doi: 10.1067/mob.2002.121723. [DOI] [PubMed] [Google Scholar]
- O'Brien C.M., et al. In vivo Raman spectral analysis of impaired cervical remodeling in a mouse model of delayed parturition. Sci. Rep. 2017;7(1):6835. doi: 10.1038/s41598-017-07047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peró M., et al. Rabbit as an animal model for the study of biological grafts in pelvic floor dysfunctions. Sci. Rep. 2021;11(1):10545. doi: 10.1038/s41598-021-89698-z. 10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero M., et al. Comparison of a human acellular dermal matrix and a polypropylene mesh for pelvic floor reconstruction: a randomized trial study in a rabbit model. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-22190-4. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles R.B., et al. Characterization of the ovine vaginal microbiome and inflammation patterns as an improved testing model of human vaginal irritation. Front. Reprod. Health. 2021;3 doi: 10.3389/frph.2021.714829. https://www.frontiersin.org/articles/10.3389/frph.2021.714829 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Clifton M.M., Koo K. Inaccuracies in news media reporting about the 2019 US food and drug administration ban on transvaginal mesh for pelvic organ prolapse repair. Urology. 2021;150:194–200. doi: 10.1016/j.urology.2020.05.009. [DOI] [PubMed] [Google Scholar]
- Robboy S.J., Kurita T., Baskin L., Cunha G.R. New insights into human female reproductive tract development. Differ. Res. Biol. Divers. 2017;97:9–22. doi: 10.1016/j.diff.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison K.M., Conway C.K., Desrosiers L., Knoepp L.R., Miller K.S. Biaxial mechanical assessment of the murine vaginal wall using extension–inflation testing. J. Biomech. Eng. 2017;139(10):1045041–1045048. doi: 10.1115/1.4037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochani A., et al. Development and preclinical investigation of physically cross-linked and pH-sensitive polymeric gels as potential vaginal contraceptives. Polymers. 2022;14(9) doi: 10.3390/polym14091728. Art. no. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers B.C., Merideth K.L. Sonographic detection of mayer-rokitansky-küster-hauser syndrome. J. Diagn. Med. Sonogr. 2015;31(2):103–108. doi: 10.1177/8756479314557279. [DOI] [Google Scholar]
- Rubod C., et al. Biomechanical properties of human pelvic organs. Urology. 2012;79(4) doi: 10.1016/j.urology.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Sartoneva R., et al. Porous poly-l-lactide-co-ϵ-caprolactone scaffold: a novel biomaterial for vaginal tissue engineering. R. Soc. Open Sci. 2018;5(8) doi: 10.1098/rsos.180811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassani J.C., Artsen A.M., Moalli P.A., Bradley M.S. Temporal trends of urogynecologic mesh reports to the U.S. Food and drug administration. Obstet. Gynecol. 2020;135(5):1084–1090. doi: 10.1097/AOG.0000000000003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf G.C., Bleyl S.B., Brauer P.R., Francis-West P.H. fifth ed. United States: Elsevier; 2015. Development of the Reproductive System; pp. 394–428. [DOI] [Google Scholar]
- Shafaat S., Mangir N., Chapple C., MacNeil S., Hearnden V. A physiologically relevant, estradiol-17β [E2]-responsive in vitro tissue-engineered model of the vaginal epithelium for vaginal tissue research. Neurourol. Urodyn. 2022;41(4):905–917. doi: 10.1002/nau.24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer R.M., Liang R., Knight K., Carter-Brooks C.M., Abramowitch S., Moalli P.A. Impact of polypropylene prolapse mesh on vaginal smooth muscle in rhesus macaque. Am. J. Obstet. Gynecol. 2019;221(4):330.e1–330.e9. doi: 10.1016/j.ajog.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K.K., et al. Comparison of 2 single incision slings on the vagina in an ovine model. Am. J. Obstet. Gynecol. 2021;224(1) doi: 10.1016/j.ajog.2020.07.005. 78.e1-78.e7. [DOI] [PubMed] [Google Scholar]
- Skoczylas L.C., et al. Regional differences in rat vaginal smooth muscle contractility and morphology. Reprod. Sci. 2013;20(4):382–390. doi: 10.1177/1933719112472733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher L.F. Diagnosis, causes, and treatment of dyspareunia in postmenopausal women. Menopause N. Y. N. 2023;30(6):635–649. doi: 10.1097/GME.0000000000002179. [DOI] [PubMed] [Google Scholar]
- Urbankova I., et al. First delivery and ovariectomy affect biomechanical and structural properties of the vagina in the ovine model. Int. Urogynecology J. 2019;30(3):455–464. doi: 10.1007/s00192-017-3535-9. [DOI] [PubMed] [Google Scholar]
- Vashaghian M., et al. Toward a new generation of pelvic floor implants with electrospun nanofibrous matrices: a feasibility study. Neurourol. Urodyn. 2017;36(3):565–573. doi: 10.1002/nau.22969. [DOI] [PubMed] [Google Scholar]
- Vergeldt T.F.M., Weemhoff M., IntHout J., Kluivers K.B. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int. Urogynecology J. 2015;26(11):1559–1573. doi: 10.1007/s00192-015-2695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K.L., Vargas G., Wei J., Bourne N., Motamedi M. Monitoring vaginal epithelial thickness changes noninvasively in sheep using optical coherence tomography. Am. J. Obstet. Gynecol. 2013;208(4):282. doi: 10.1016/j.ajog.2013.01.025. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., et al. mTORC1 signaling pathway integrates estrogen and growth factor to coordinate vaginal epithelial cells proliferation and differentiation. Cell Death Dis. 2022;13(10):862. doi: 10.1038/s41419-022-05293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- Woitowich N.C., Beery A., Woodruff T. A 10-year follow-up study of sex inclusion in the biological sciences. Elife. 2020;9 doi: 10.7554/eLife.56344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word R.A., Pathi S., Schaffer J.I. Pathophysiology of pelvic organ prolapse. Obstet. Gynecol. Clin. N. Am. 2009;36(3):521–539. doi: 10.1016/j.ogc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Wu J.M., Matthews C.A., Conover M.M., Pate V., Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet. Gynecol. 2014;123(6):1201. doi: 10.1097/AOG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., et al. dl-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: a potential non-surfactant spermicide for contraception. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110104. [DOI] [PubMed] [Google Scholar]
- Zeiger B.B., da Silva Carramão S., Del Roy C.A., da Silva T.T., Hwang S.M., Auge A.P.F. Vaginal pessary in advanced pelvic organ prolapse: impact on quality of life. Int. Urogynecology J. 2022;33(7):2013–2020. doi: 10.1007/s00192-021-05002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. Mesenchymal stem cell transplantation for vaginal repair in an ovariectomized rhesus macaque model. Stem Cell Res. Ther. 2021;12(1):406. doi: 10.1186/s13287-021-02488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., et al. Ex vivo 2D and 3D HSV-2 infection model using human normal vaginal epithelial cells. Oncotarget. 2017;8(9):15267–15282. doi: 10.18632/oncotarget.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., et al. Surrogate post-coital testing for contraceptive efficacy against human sperm activity in the ovine vaginal model†. Biol. Reprod. 2021;104(2):317–324. doi: 10.1093/biolre/ioaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern P.E., Goueli R., Souders C., Patel P.V., Lassiter D., Gardetto B. Second generation vaginal biomechanics analyzer to visualize and measure suction deflection of the anterior vaginal wall to reliably determine vaginal laxity: a feasibility study. ics.org. 2021 https://www.ics.org/2021/abstract/103 [Online]. Available: [Google Scholar]
- ACOG practice Bulletin No.“ACOG practice Bulletin No. 198: prevention and management of obstetric lacerations at vaginal delivery.” Accessed: May 20, 2023. [Online]. Available: https://oce.ovid.com/article/00006250-201809000-00066?relatedarticle=y.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.