Abstract
Substitution of threonine or serine for the evolutionary conserved intramembrane proline P347 of the Bacillus subtilis multidrug transporter Bmr significantly increases the toxin-effluxing activity of Bmr without affecting its abundance in the cell. In cocultivation experiments, we demonstrate that although the mutant T347 Bmr is advantageous to cells growing in the presence of a toxin, the wild-type P347 Bmr is advantageous under the conditions of nutritional limitation. This may explain why Bmr has evolved the way it did, that is, with proline at position 347. These observations provide a basis for speculating that the evolution of Bmr has been determined by its presently unidentified natural function rather than by its ability to expel diverse toxins from the cell.
Bacteria, like eukaryotes, express multiple membrane transport proteins with unusually low substrate specificity, so-called multidrug transporters. These proteins, although not significantly different from their substrate-specific homologs, recognize, by an unknown mechanism, structurally diverse toxic compounds and pump them out of cells, thus protecting cells from the action of toxins (reviewed in references 7 and 8). It is not clear whether the efflux of diverse toxins is the primary physiological function of multidrug transporters or, alternatively, is merely a fortuitous side effect of the unrecognized, more specific functions of these proteins, which may involve the transport of specific cellular molecules (5).
The subject of this study, the multidrug transporter Bmr of Bacillus subtilis, promotes the efflux of dissimilar drugs in exchange for external hydrogen ions (6). On the basis of sequence homology, Bmr belongs to the evolutionary group of toxin-extruding antiporter proteins with 12 transmembrane domains (8, 9). In addition to Bmr, this group includes closely related bacterial multidrug transporters such as Blt of B. subtilis and NorA of Staphylococcus aureus, substrate-specific bacterial transporters such as tetracycline efflux transporters of various bacteria, mammalian monoamine transporters, whose undisputed function is to transport neurotransmitter molecules but which are capable of interacting with a variety of toxins (9), and a large number of uncharacterized eukaryotic and prokaryotic transporters revealed in the course of genome sequencing projects (8). These transporters share several conserved sequence motifs that are presumed to be essential for their function (8).
Here we demonstrate that the substitution of threonine or serine for the highly conserved proline residue (P347) located in the middle of transmembrane domain XI of Bmr increases the toxin-effluxing activity of this transporter instead of destroying its function, as could be expected. These results prompted us to question whether the efflux of toxins has been the determining factor in the evolution of Bmr.
MATERIALS AND METHODS
Bacterial strains.
B. subtilis BD170 (thr-5 trpC2) was obtained from the Bacillus Genetics Stock Center, Ohio State University. Strain BD170-VB, which contains the bmr gene under the control of the PvegII promoter, the bmrR gene disrupted by the chloramphenicol resistance genetic determinant (cat), and the blt gene disrupted by the erythromycin resistance genetic determinant (emr), is described in reference 4. The same reference describes its derivative BD170-VB(V143), which contains the bmr gene modified to encode the Bmr transporter with the F143V substitution. Strains BD170/bmr::cat, blt::emr, and BD170/blt::emr are described in reference 2. Transformation-competent Escherichia coli JM109 was from Promega. Luria-Bertani (LB) medium was used for cultivating all strains.
Construction of plasmids.
DNA coding for TetC (tetracycline-specific efflux transporter of class C) was obtained by PCR using plasmid pBR322 (Sigma) as a template. Substitution of threonine or serine for P352 was performed by PCR-based site-directed mutagenesis and verified by direct sequencing of the entire gene. PCR fragments containing either the wild-type or mutated promoterless tetC gene were cloned between the KpnI and EcoRI sites of the plasmid pBluescript SK+ (Stratagene).
Mutagenesis of B. subtilis.
B. subtilis BD170-VB or its mutant variant carrying the F143V substitution in Bmr was mutagenized by ethyl methanesulfonate (Sigma) as previously described (4) and selected on LB plates containing either 10 μg of ethidium bromide and 1 μg of reserpine per ml or 15 μg of ethidium bromide per ml, respectively.
The P347S substitution in strain BD170-VB was created by using site-directed mutagenesis according to the technique described in reference 4. For substituting threonine for P347 in strain BD170, a PCR product containing the mutation and encompassing the entire bmr and bmrR genes was generated and used to transform the BD170/bmr::cat strain. Clones selected on an LB plate containing 3 μg of ethidium bromide per ml were analyzed further for the loss of resistance to chloramphenicol. All substitutions were confirmed by direct local sequencing (ca. 150 bp) with the fmol PCR-based sequencing system (Promega). Several clones obtained for each substitution demonstrated indistinguishable drug resistance characteristics, thus demonstrating that the observed effects were due to the substitution itself rather than to additional mutations that potentially could occur in the PCR products used for the mutagenesis.
FLAG modification of Bmr and immunoblotting.
We generated PCR products which encompassed the entire 5-kb bmr locus of strain BD170-VB (with PvegII promoter upstream of bmr and cat inserted into the bmrR gene) or of its Bmr mutants. In addition, a DNA fragment encoding FLAG epitope (DYKDDDDK) was inserted in frame immediately upstream of the last three triplets of the bmr gene in these PCR products. These products were used to transform strain BD170/blt::erm. Clones were selected for chloramphenicol resistance (5 μg/ml) and either tetraphenylphosphonium resistance (50 μg/ml, for the F143V mutant) or ethidium bromide resistance (10 μg/ml, for all other variants). The transfer of the mutations and the FLAG epitope-encoding DNA was confirmed by sequencing.
Cells were grown to an optical density at 600 nm (OD600) of 0.5, collected by centrifugation, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 6 M urea, and loaded on two sodium dodecyl sulfate–12% polyacrylamide gels. One gel was stained with Coomassie blue; another gel was analyzed by immunoblotting after the transfer of proteins to nitrocellulose. Immunodetection of FLAG-containing proteins was accomplished by using a monoclonal anti-FLAG M2 antibody (Kodak) at a 1:1,000 dilution and horseradish peroxidase-conjugated goat anti-mouse antibody (Sigma) at a 1:1,000 dilution. To reduce background, 5% dry fat-free milk in phosphate-buffered saline was used in all incubations. Peroxidase activity was detected by the ECL (enhanced chemiluminescence) Western blotting detection system (Amersham).
Determination of bacterial sensitivity to drugs.
Cells at a logarithmic stage of growth (OD600 of 0.4 to 0.8) were diluted to an OD600 of 0.005 in LB medium. Drugs at 10 different concentrations were added to 1-ml aliquots of cell suspension. After incubation in a 37°C shaker for 4 to 5 h, at which point the control culture without added drugs reached OD600 of approximately 1.0, the optical densities of all the cultures were measured. The IC50s (50% inhibitory concentrations) were determined from the obtained OD600-drug concentration graphs.
Cocultivation experiments.
Strains BD170 and the P347T Bmr variant of BD170 were grown separately in LB medium to OD600 of 0.4 to 0.6, diluted to an OD600 of 0.20 in LB medium, and mixed to obtain a 1:1 ratio. Five microliters of this mixture was added to 5 ml of each of following media: LB, 1% tryptone medium (1% Difco tryptone, 1% NaCl, 50 μg of threonine per ml, 50 μg of tryptophan per ml), and 0.1% tryptone medium (0.1% Difco tryptone, 1% NaCl, 50 μg of threonine per ml, 50 μg of tryptophan per ml) with or without addition of ethidium bromide (0.2 μg/ml) or reserpine (5 μg/ml). Every 12 h, 5 μl of each culture was transferred to 5 ml of the corresponding fresh medium. After 10 such transfers, total DNA from each culture was isolated, and a PCR product encompassing the bmr gene was sequenced.
RESULTS
Selection of the P347T and P347S Bmr mutations.
Recently we described a procedure for selecting mutant variants of Bmr with reduced sensitivity to its inhibitor, the plant alkaloid reserpine (4). This procedure uses a specially created B. subtilis strain, BD170-VB, which overexpresses Bmr due to the presence of a strong promoter PvegII inserted immediately upstream of the chromosomal bmr gene instead of the natural bmr promoter. Additionally, the gene of the bmr transcriptional regulator BmrR (1) and the gene of the second B. subtilis multidrug transporter Blt (2) are disrupted in this strain. To obtain mutant Bmr variants, BD170-VB cells are mutagenized with ethyl methanesulfonate and selected on plates containing a toxic Bmr substrate, e.g., ethidium bromide, in the presence of reserpine. The absolute majority of the obtained clones contained amino acid substitutions of Bmr residue F143, V286, or F306, which led to a dramatic loss of reserpine sensitivity of Bmr (4).
One of the obtained clones, which has not been described previously, was unusual in that it demonstrated approximately a twofold increase in the level of resistance to dissimilar Bmr substrates, i.e., ethidium, norfloxacin, and acriflavine (Table 1), without significant changes in the sensitivity of Bmr to inhibition by reserpine (not shown). Apparently, the increase in the level of ethidium resistance was sufficient for this clone to survive on a plate containing ethidium and reserpine. Sequencing of the entire bmr gene in this clone revealed a single mutation at the DNA level leading to the substitution of threonine for the Bmr residue P347 (proline-encoding triplet CCT changed to ACT).
TABLE 1.
IC50s of toxic Bmr substrates for BD170-VB cells expressing the wild-type and mutant variants of Bmr
| Strain | IC50 (μg/ml)
|
||
|---|---|---|---|
| Ethidium | Norfloxacin | Acriflavine | |
| Wild type | 12 | 1.0 | 20 |
| P347T | 25 | 2.0 | 40 |
| F143V | 3 | 0.15 | 4 |
| F143V P347S | 15 | 0.5 | 12 |
| P347S | 25 | 2.0 | 40 |
Another substitution of P347 was subsequently selected in a different experiment. BD170-VB cells expressing the F143V mutant variant of Bmr, which provides very little resistance to ethidium, norfloxacin, and acriflavine (4), were mutagenized and selected for compensatory mutations restoring the ability of the transporter to protect cells from ethidium toxicity. As the F143V substitution had been artificially created by changing two nucleotides within the F143 codon, the direct reversal to the wild-type sequence was nearly impossible. Three of the obtained clones contained the P347S substitution (CCT→TCT) in addition to the F143V mutation. As Table 1 demonstrates, cells expressing the F143V P347S variant of Bmr displayed approximately three- to five-times-higher levels of resistance to ethidium, norfloxacin, or acriflavine than the cells expressing the F143V variant of Bmr. Transfer of the P347S Bmr mutation alone into the BD170-VB cells resulted in a strain indistinguishable in its properties from the strain carrying the P347T mutation of Bmr; that is, either substitution of P347 caused approximately a twofold increase in the bacterial resistance to toxic Bmr substrates.
The P347T and P347S substitutions increase the drug transport activity of Bmr.
The stimulatory effect of the P347T or P347S substitution on Bmr-mediated drug resistance can be explained either by the increase in the amount of the Bmr protein in the cell (e.g., due to its increased half-life) or by the increase in its activity. To discern between these possibilities, we genetically modified the cytoplasmically located hydrophilic C termini of the wild-type and mutant Bmr transporters, expressed by the BD170-VB cells. The C termini of the modified transporters contained the FLAG epitope recognizable by the M2 monoclonal antibody (3). The FLAG-modified Bmr variants did not differ from the corresponding unmodified Bmr variants in the ability to provide drug resistance (data not shown).
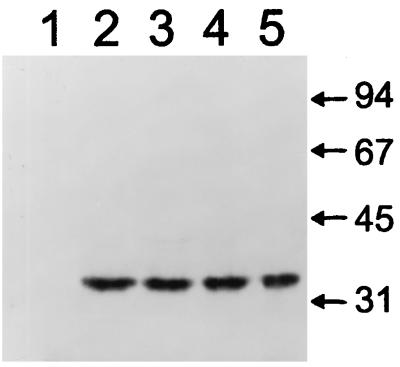
Figure 1 demonstrates the results of immunoblotting of the FLAG-modified Bmr variants with M2 monoclonal antibody. No significant effects of either the P347T or P347S substitution on the amounts of Bmr in the cell could be detected (Fig. 1). This result strongly suggested that the obtained substitutions of P347 stimulate the Bmr-mediated drug resistance by increasing the transport activity of Bmr rather than its amount in the cell.
FIG. 1.
Immunoblotting of FLAG-containing proteins in BD170-VB B. subtilis cells overexpressing different Bmr variants. Bmr was either unmodified (lane 1), FLAG modified (lane 2), FLAG-modified P347T mutant (lane 3), FLAG-modified F143V mutant (lane 4), or FLAG-modified F143V P347S double mutant (lane 5). Forty micrograms of total cellular protein was loaded in each lane. The apparent molecular mass of Bmr is 35 kDa, which is lower than the theoretically predicted 42 kDa, likely due to the high hydrophobicity of this protein. Sizes are indicated in kilodaltons.
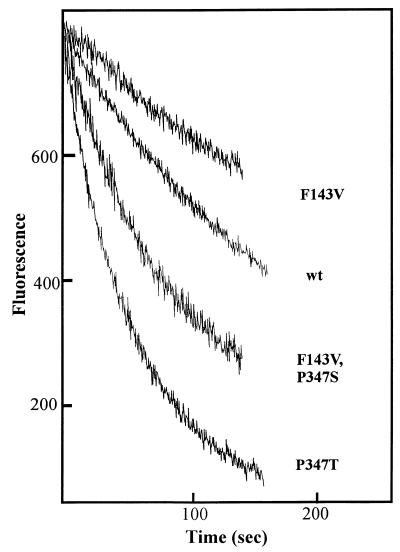
To verify that these substitutions do indeed increase the drug transport activity of Bmr, we determined the rates of ethidium bromide efflux from BD170-VB cells expressing different FLAG-modified variants of Bmr. Cells were loaded with ethidium in the presence of the Bmr inhibitor reserpine and then resuspended in a drug-free medium. The release of ethidium from the cells was detected by monitoring the decline of ethidium fluorescence in the cell suspension (the fluorescence of ethidium released from the cells and no longer associated with nucleic acids or proteins diminishes dramatically). Figure 2 demonstrates that the P347T substitution significantly stimulates the efflux of ethidium. The cells expressing the F143V variant of Bmr effluxed ethidium much more slowly than the cells expressing wild-type Bmr, which correlated with their lower resistance. However, the additional P347S substitution significantly increased the ethidium efflux rate (Fig. 2).
FIG. 2.
Fluorimetric detection of ethidium efflux from BD170-VB B. subtilis cells overexpressing different variants of FLAG-modified Bmr. Cells loaded with ethidium bromide in the presence of reserpine were placed into drug-free medium, and their fluorescence (λex = 530 nm; λem = 600 nm) was monitored as described previously (4). Fluorescence is proportional to the amount of ethidium remaining in the cells. wt, wild type.
Why has P347 been selected in the course of Bmr evolution?
The finding that either the P347T or P347S substitution in Bmr makes it a more efficient multidrug transporter appears paradoxical from the evolutionary standpoint. Indeed, what is the reason for proline to be present at position 347 of Bmr if threonine or serine at this position apparently would make the transporter more effective?
In model evolution experiments, we demonstrated that the variant of Bmr with threonine at position 347 does indeed have a fitness advantage over the wild-type transporter if the presence of toxins becomes a significant factor in the environment of B. subtilis. A strain of B. subtilis which differed from the wild-type strain BD170 only by the P347T substitution in Bmr was constructed for these experiments. As expected, the T347 strain demonstrated approximately a two- to three-fold-higher resistance to drug substrates of Bmr than the wild-type P347 strain (Table 2).
TABLE 2.
IC50s of toxic substrates of Bmr for BD170 cells expressing the wild-type and P347T mutant Bmr
| Strain | IC50 (μg/ml)
|
||||
|---|---|---|---|---|---|
| Norfloxacin | Ethidium | Tetraphenylphosphonium | Rhodamine | Acriflavine | |
| Wild type | 0.1 | 1 | 3 | 0.2 | 3 |
| P347T | 0.2 | 3 | 9 | 0.4 | 6 |
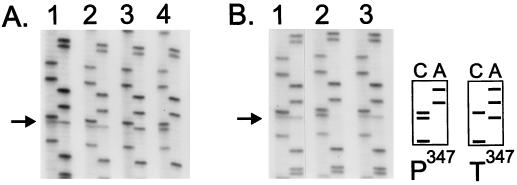
The logarithmically growing P347 and T347 cells were mixed at a ratio of 1:1 and cocultivated for approximately 100 generations. The ratio of the wild-type and mutant bmr sequences in the DNA prepared from the resulting bacterial population was then determined. This ratio remained unchanged if the cocultivation was performed in a relatively rich medium, either LB (not shown) or a medium containing 1% tryptone and 1% NaCl (Fig. 3A, lane 2). However, the mutant T347 cells completely dominated (Fig. 3A, lane 3) if the cocultivation was performed in the presence of as little as 0.2 μg of the toxic Bmr substrate ethidium bromide per ml (5% of the MIC of ethidium for the wild-type cells).
FIG. 3.
Relative fitness of wild-type B. subtilis cells expressing either the wild-type P347 or the mutant T347 variant of Bmr. The cells were mixed at a 1:1 ratio and then cocultivated in different media for approximately 100 generations. DNA, isolated from the original mixture of cells or from the cells which underwent cocultivation, was used as a template to amplify the bmr gene by PCR. Shown are the sequences of the resulting PCR products (in each lane pair, cytosine at left and adenine at right). The position of the nucleotide that is different in the two strains is indicated by an arrow. As shown schematically, cytosine at this position corresponds to the wild-type sequence (triplet CCT encoding proline), whereas adenine at this position corresponds to the mutated sequence (triplet ACT encoding threonine). Results of two independent experiments are shown in panels A and B. (A) Lane 1, original mixture; lane 2, cocultivation in a nutrient-rich medium (1% tryptone, 1% NaCl); lane 3, cocultivation in the same rich medium containing the toxic Bmr substrate ethidium bromide (0.2 μg/ml); lane 4, cocultivation in a poor medium (0.1% tryptone, 1% NaCl). (B) Lane 1, original mixture; lane 2, cocultivation in a poor medium (0.1% tryptone, 1% NaCl); lane 3, cocultivation in the same poor medium containing reserpine (5 μg/ml).
Since normally Bmr contains P347, one would assume that under certain environmental conditions this variant should have fitness advantage over the T347 variant. In fact, we found this to be the case in conditions of a limited supply of nutrients. In a poor medium (0.1% tryptone, 1% NaCl), the wild-type P347 cells repeatedly outgrew the mutant T347 cells (Fig. 3A, lane 4, and Fig. 3B, lane 2). Importantly, selection of the wild-type P347 variant over the mutant T347 variant depended on the function of Bmr. If cocultivation in a poor medium was performed in the presence of the Bmr transport inhibitor reserpine, neither of the strains gained selective advantage (Fig. 3B, lane 3).
The P352 substitutions in TetC equivalent to the P347 substitutions in Bmr.
Proline at position 347 of Bmr is highly conserved among its homologs and is included into the conserved motif G (GXXXGP) in the middle of transmembrane domain XI (8). One of these homologs is TetC, which is encoded by the E. coli plasmid pBR322. We genetically substituted either threonine or serine for P352 of TetC, which is equivalent to P347 of Bmr. The promoterless wild-type and the mutated tetC genes were then recloned into the pBluescript plasmid vector under the control of the lac promoter. As expected, the E. coli cells harboring the plasmid containing the wild-type tetC demonstrated resistance to tetracycline, which was dependent on the presence of isopropyl-1-β-d-galactoside (IPTG), the inducer of the lac promoter (tetracycline IC50s = 7.5 and 50 μg/ml in the absence and presence of IPTG, respectively). In contrast, cells harboring the empty vector, or vector carrying either the P352T or P352S mutant tetC, showed no tetracycline resistance (IC50 = 1 μg/ml regardless of the presence of IPTG). This result demonstrates that at least some transporters similar to Bmr require the presence of proline in the middle of transmembrane domain XI to be functional. It also underscores the unusual character of our finding that a substitution of this conserved proline in Bmr leads to an increase in its toxin-effluxing activity.
DISCUSSION
Our results demonstrate that the P347T or P347S substitution in the Bmr molecule makes it a more potent multidrug transporter. The similar properties of the T347 and S347 Bmr variants are not surprising considering the structural similarity of threonine and serine. The observed increase in the transporter activity, although easily detectable in both drug resistance and ethidium efflux experiments, is not particularly strong: approximately two- to threefold if the substitution occurs in the wild-type Bmr and three- to fivefold if it occurs in the transporter weakened by the F143V mutation. Experiments with the FLAG-modified Bmr variants indicate that this increase in Bmr activity results not from the increase in the abundance of Bmr but, most likely, from the increase in the activity of each individual transporter molecule.
Although the effect of the P347 substitutions is relatively modest, we were able to find only one publication describing a similar finding: a twofold increase in the transport affinity of lactose permease as a result of the S67A substitution (12). It is additionally unusual that a substitution improving transporter function affects an intramembrane proline residue. Prolines in transmembrane domains of membrane proteins are thought to be of particular importance since they induce kinks in the transmembrane α-helices and provide the basis for conformational transitions due to cis-trans isomerization (15). Unlike the substitution described here, the previously described substitutions of intramembrane prolines were either detrimental to the function of membrane proteins or, at best, neutral (10, 11, 14, 15).
The evolutionary aspect of the finding described here is the most paradoxical. Indeed, why would evolution have preserved a residue which makes a transporter somewhat less efficient than it can potentially be? As our model evolution experiments demonstrate, the presence of threonine, instead of proline, at position 347 of Bmr would have benefited bacteria growing in the presence of toxic Bmr substrates, even if the toxins were present in the bacterial environment at a relatively small concentration. Other results of these model experiments explain, however, why Bmr has evolved the way it did, that is, with proline being present at position 347. Under the conditions of nutritional limitation, which are likely to be common for B. subtilis in nature, the P347 variant of Bmr is superior to the T347 variant. Evidently, in the course of the evolution of Bmr, this selective pressure has outweighed the selective pressure imposed by environmental toxins.
One mechanistic explanation of this result postulates that due to its low specificity, Bmr promotes the efflux of not only toxins but also of some natural cellular molecules. If the transporter is too active, this may become detrimental for the cells experiencing nutritional limitation. In other words, according to this hypothesis, the role of proline at position 347 of Bmr is to limit the activity of the transporter and prevent it from becoming damaging to the cell.
This hypothesis can hardly explain, however, another our finding. Since P347 is conserved in evolution, this proline residue should be expected to play a similar activity-limiting role in homologous transporters. Contrary to this prediction, we found that in at least one of the Bmr homologs, the tetracycline transporter TetC, the proline corresponding to P347 of Bmr (P352) is absolutely required for the transporter function. To account for this observation, we would like to propose an alternative explanation for the results presented here. This alternative hypothesis postulates that similar to TetC, which requires proline at position 352 to be functional, Bmr requires proline at position 347 to perform its natural, presently unidentified specific transport function, which aids cells experiencing nutritional limitation. The Bmr-mediated efflux of toxins, according to this hypothesis, is merely a fortuitous side effect of the true function of Bmr and therefore has never been a determining factor in the evolution of this transporter. This hypothesis explains all of the results presented here and is in agreement with recent findings that the mammalian multidrug transporter P-glycoprotein may have evolved to transport cellular phospholipids (13) and that the Bmr homolog, multidrug transporter Blt of B. subtilis, may have evolved to promote the outward transport of spermidine (16). We fully realize, however, that this hypothesis will remain purely speculative until the hypothetical specific natural function of Bmr is identified.
ACKNOWLEDGMENTS
We are grateful to A. S. Mankin and P. N. Markham for helpful discussions and critical reading of the manuscript.
This work was supported by NIH grant GM49819.
REFERENCES
- 1.Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. [PubMed] [Google Scholar]
- 2.Ahmed M, Lyass L, Markham P N, Taylor S S, Vazquez-Laslop N, Neyfakh A A. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J Bacteriol. 1995;177:3904–3910. doi: 10.1128/jb.177.14.3904-3910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopp T P, Prickett K S, Price V, Libby R T, March C J, Ceretti P, Urdal D L, Conlon P J. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology. 1988;6:1205–1210. [Google Scholar]
- 4.Klyachko K A, Schuldiner S, Neyfakh A A. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter Bmr. J Bacteriol. 1997;179:2189–2193. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neyfakh A A. Natural functions of bacterial multidrug transporters. Trends Microbiol. 1997;5:309–313. doi: 10.1016/S0966-842X(97)01064-0. [DOI] [PubMed] [Google Scholar]
- 6.Neyfakh A A, Bidnenko V E, Chen L B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard D N, Travis S M, Ishihara H, Welsh M J. Contribution of proline residues in the membrane-spanning domains of cystic fibrosis transmembrane conductance regulator to chloride channel function. J Biol Chem. 1996;271:14995–15001. doi: 10.1074/jbc.271.25.14995. [DOI] [PubMed] [Google Scholar]
- 11.Tamori Y, Hashiramoto M, Clark A E, Mori H, Muraoka A, Kadowaki T, Holman G D, Kasuga M. Substitution at Pro385 of GLUT1 perturbs the glucose transport function by reducing conformational flexibility. J Biol Chem. 1994;269:2982–2986. [PubMed] [Google Scholar]
- 12.Tsen S D, Lai S C, Pang C P, Lee J I, Wilson T H. Chemostat selection of an Escherichia coli mutant containing permease with enhanced lactose affinity. Biochem Biophys Res Commun. 1996;224:351–357. doi: 10.1006/bbrc.1996.1032. [DOI] [PubMed] [Google Scholar]
- 13.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 14.Wellner M, Monden I, Mueckler M M, Keller K. Functional consequences of proline mutations in the putative transmembrane segments 6 and 10 of the glucose transporter GLUT1. Eur J Biochem. 1995;227:454–458. doi: 10.1111/j.1432-1033.1995.tb20409.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams K A, Deber C M. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991;30:8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- 16.Woolridge D P, Vazquez-Laslop N, Markham P N, Chevalier M S, Gerner E W, Neyfakh A A. Efflux of the natural polyamine spermidine facilitated by the Bacillus subtilis multidrug transporter Blt. J Biol Chem. 1997;272:8864–8866. doi: 10.1074/jbc.272.14.8864. [DOI] [PubMed] [Google Scholar]