Abstract
The self-templating nature of prions plays a central role in prion pathogenesis and is associated with infectivity and transmissibility. Since propagation of proteopathic seeds has now been acknowledged a principal pathogenic process in many types of dementia, more insight into the molecular mechanism of prion replication is vital to delineate specific and common disease pathways. By employing highly discriminatory anti-PrP antibodies and conversion-tolerant PrP chimera, we here report that de novo PrP conversion and formation of fibril-like PrP aggregates are distinct in mechanistic and kinetic terms. De novo PrP conversion occurs within minutes after infection at two subcellular locations, while fibril-like PrP aggregates are formed exclusively at the plasma membrane, hours after infection. Phenotypically distinct pools of abnormal PrP at perinuclear sites and the plasma membrane show differences in N-terminal processing, aggregation state and fibril formation and are linked by exocytic transport via synaptic and large-dense core vesicles.
Subject terms: Prion diseases, Molecular neuroscience, Protein aggregation
In this work, the authors investigated the cellular mode of prion propagation. The report that proteopathic seeds of abnormal PrP are N-terminally truncated and detected within minutes after infection. These seeds reach the plasma membrane by regulated secretory pathways where phenotypically distinct fibril-like PrP aggregates are formed with a lag of 24 h after infection.
Introduction
The “protein only hypothesis”1 proposes that an abnormal conformer of PrP causes self-templating protein aggregation by converting host-encoded cellular prion protein (PrPc)2–4, but the cellular mechanism of conversion has remained elusive. A major roadblock for the study of self-templating protein aggregation in prion diseases is the lack of anti-PrP antibodies that discriminate PrPc from abnormal PrP, here termed disease-associated PrP (PrPd). Since the vast majority of anti-PrP antibodies characterised to date are non-discriminatory or “pan” antibodies5, reliance on the enzymatic or chemical removal of PrPc has greatly limited progress in the investigation of PrP conversion.
Pivotal questions concerning the biology of PrP conversion have remained a matter of controversy. Firstly, the cellular site of prion replication remains unsolved and putative sites include the endocytic recycling pathway6,7, the endosomal-lysosomal pathway8–10, the Golgi apparatus11,12 and the plasma membrane13,14. Secondly, the kinetics of de novo PrP conversion, a comparatively unexplored question in prion biology, is a matter of debate. Evidence that PrP converts within minutes after infection13 has been disputed by others15, who claim that proteinase K-resistant PrP (PrPSc) is not detectable before 24 h after infection, raising the question whether distinct oligomeric states of abnormal PrP aggregates may explain these inconsistencies. Thirdly, early studies of prion-infected mice showed evidence of “prion-amyloid filaments” in the extracellular space beneath the ependyma16 and recent studies characterised ex vivo isolated prion rods17,18, but the formation and elongation of prion fibrils has not been recapitulated in neuronal cells. A recent study reported the detection of lipid raft-associated amyloid strings and webs of PrP, but the molecular mode of amyloid formation is unknown14.
To address these current challenges, we identified highly discriminatory anti-PrP antibodies and conversion-tolerant, tagged Prnp chimera to examine aggregation phenomena in freshly and persistently prion-infected neuronal cells.
We provide evidence that (i) PrP conversion occurs within minutes after infection at two distinct cellular sites and precedes the formation of abnormal PrP conformers, (ii) fibril-like PrPd aggregates are formed and elongated at the plasma membrane, while PrPd fibrils are absent intracellularly, (iii) the prion infectious state is dependent on functional dynamins and Cdc42, (iv) PrPd segregates into synaptic and large-dense core vesicles of the regulated secretory pathway.
Results
Phenotypically distinct PrPd aggregates in prion-infected cells identified by monospecific anti-PrP antibodies
To identify highly validated anti-PrP antibodies, we characterised 18 frequently used monoclonal antibodies (mAbs) with respect to their target-specificity and propensity to discriminate PrPd from PrPc (Fig. 1A). We deleted Prnp in N2a cells to unequivocally demonstrate that mAbs are target-specific as shown for example for 5B2 and 6D11 (Fig. 1B). Seven mAbs were target-specific, also termed monospecific19, while 4 mAbs showed moderate and 7 mAbs showed significant off-target effects (Supplementary Fig. 1A and Supplementary Table 1). Validated mAbs used in this study recognise distinct PrP regions which include the N-terminus (5B2, 8B4), the octapeptide repeat region (Saf32), charge cluster 2 (6D11), α-helix 1 (ICSM 18) and α-helix 2 (8H4; Fig. 1A).
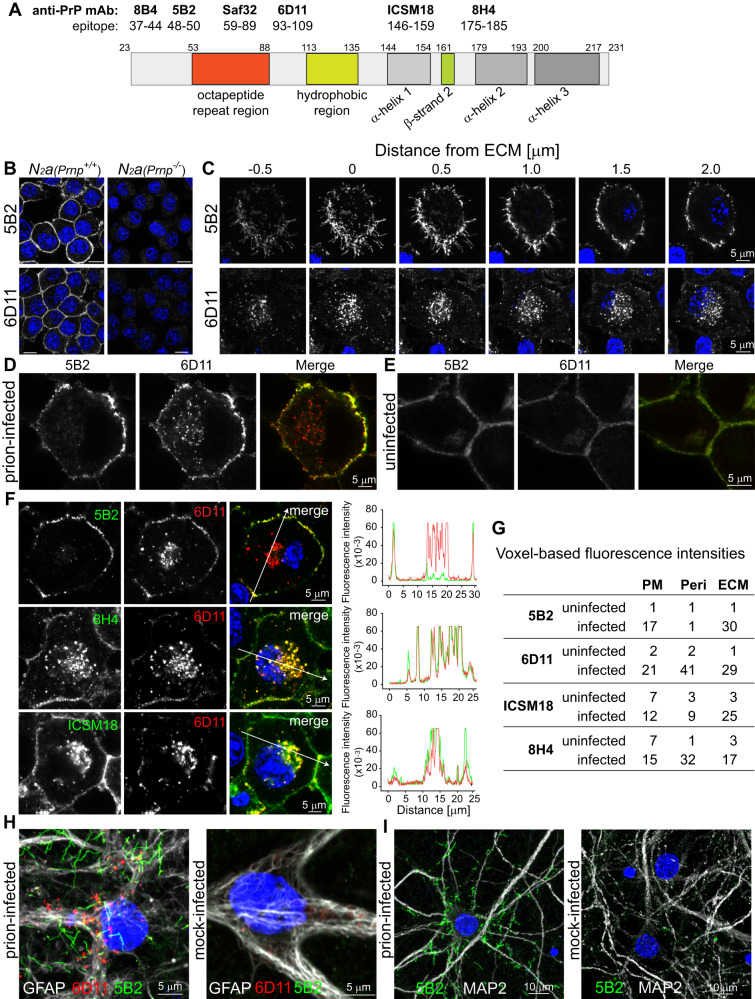
Fig. 1. Identification of phenotypically distinct PrPd aggregates in prion-infected cells using monospecific anti-PrP antibodies.
A Schematic diagram of validated anti-PrP monoclonal antibodies (mAbs) and their putative epitopes on the context of mouse PrP domains76. B Exemplary data for anti-PrP mAb validation using N2a (Prnp-/-) wild-type (Prnp+/+) cells. For the full data set of anti-PrP mAbs tested see Supplementary Fig. 1A. Scale bars correspond to 10 µm. C Serial confocal sections of prion-infected S7 cells, labelled with anti-PrP mAbs 5B2 and 6D11, respectively. Cells were grown for extended cultures times, i.e. 6 days (see “Methods” section, “extended TC protocol”). D, E Co-labelling of prion-infected (D) and uninfected (E) S7 cells with 5B2 and 6D11. F Detection of perinuclear PrPd by core, but not by N-terminal anti-PrP mAbs in prion-infected S7 cells. Arrows in merged images depict orientation and placement of fluorescence intensity profiles, shown at the right hand side of image panels. For complete data set with all monospecific mAbs see Supplementary Fig. 2. G MAb-dependent differences in the detection of PrPd versus PrPc, mapped as normalised voxel-based intensities in cellular loci where PrPd deposits (PM plasma membrane, Peri perinuclear region, ECM extracellular matrix). For data see Supplementary Table 2. H Glial fibrillary acidic protein (GFAP)-positive astrocytes in cultures of primary neuronal cells from embryonic e17 mouse brains, infected with 10−5 dilutions of the prion strain RML (10% brain homogenate, w/v) and uninfected CD1 (10% brain homogenate, w/v, mock), triple-labelled with 5B2, 6D11 and anti-GFAP. I Primary hippocampal neuronal cultures from embryonic e17 mouse brains were isolated, cultured for 6 days and incubated with 1 µM AraC. The following day, cells were infected with a 10−5 dilution of RML (10%, w/v) or uninfected CD1 (10%, w/v, mock-infected) brain homogenate, respectively. Three weeks after infection, cells were fixed and double-labelled with 5B2 and anti-Map2. Source data are provided as a Source Data file.
Unexpectedly, monospecific mAbs showed phenotypic differences in single-labelled prion-infected cells of the same culture (Supplementary Fig. 1B-E). Fibril-like PrPd aggregates were detected with 5B2 at the extracellular matrix (ECM) in infected cells (Supplementary Fig. 1B), while 6D11 labelling revealed a punctuate staining predominantly at perinuclear regions (Supplementary Fig. 1C). ICSM18 detected PrPd aggregates at the ECM (Supplementary Fig. 1D) and 8H4 labelled aggregates were reminiscent of the punctate 6D11 phenotype (Supplementary Fig. 1E). This finding prompted us to investigate mAb-dependent differences of PrPd phenotypes in more detail.
Fibril-like PrPd aggregates are tethered to the plasma membrane and extend to the ECM
Unless otherwise specified, all labelling experiments were conducted under denaturing conditions, i.e. in presence of guanidinium thiocyanate (GTC), where the majority of anti-PrP mAbs detect abnormal PrP5,20,21. Prolonged cultures ( ≥ 6 days) of prion-infected S7 cells displayed extensive fibril-like 5B2-positive PrPd aggregates that are tethered to the plasma membrane and extend to the ECM (Fig. 1C, upper panel). To normalise the distance of focal planes in z-direction, we used in-focus detection of the ECM-resident protein focal adhesion kinase (FAK), arbitrarily denoted “zero µm”, as focal reference level as elaborated in “Methods” section and Supplementary Fig. 1F. Serial sections from the ECM to the focal plane of the nucleus demonstrate that 5B2 labelled PrPd at the plasma membrane, while the cytosolic face of the cell remained void with no 5B2-positive PrPd detected intracellularly. In contrast, 6D11 labelled punctate PrPd aggregates at perinuclear sites, at the plasma membrane and extracellularly (Fig. 1C, lower panel). Double-labelling experiments with 5B2 and 6D11 corroborate these phenotypic differences. Whilst both antibodies colabelled PrPd aggregates at the plasma membrane, 5B2 failed to detect perinuclear PrPd (Fig. 1D). In uninfected control cells, both antibodies weakly labelled the plasma membrane (Fig. 1E). In contrast to 5B2, mAbs against α-helix 1 (ICSM 18) and α-helix 2 (8H4) colabelled 6D11-positive PrPd aggregates at perinuclear sites as depicted in fluorescence intensity profiles (Fig. 1F). Double-labelled images for all selected monospecific mAbs are shown in Supplementary Fig. 2. While failure to label perinuclear PrPd with 5B2 is confirmed with the N-terminal mAb 8B4, all other mAbs labelled perinuclear PrPd aggregates.
To identify anti-PrP mAbs that best discriminate PrPd from PrPc, we quantified the relative fluorescence intensities of mAbs in prion-infected and uninfected cells at all cellular compartments where PrPd is detected, i.e. the plasma membrane, perinuclear regions and the ECM. Voxel-based fluorescence intensities in Fig. 1G are normalised and provide a quantitative scale for their discriminatory propensities at the specified subcellular locations (see Supplementary Table 2 for data). Notably, at the plasma membrane, only 5B2 and 6D11 showed 10-fold or higher fluorescence intensities in infected versus uninfected cells, while a more intense PrPc label with ICSM18, Saf32 and 8H4 limits their use to detect PrPd at the plasma membrane. All mAbs, except 8H4, were sufficiently discriminatory to call PrPd at the ECM, while low differential fluorescence intensities for ICSM18 limit its use in detecting PrPd at perinuclear regions.
Phenotypic differences between intracellular and extracellular PrPd aggregates, detected with 5B2 and 6D11, respectively are not limited to mouse neuroblastoma cell lines, as shown in Fig. 1H, I. Primary neuronal cultures from embryonic e17 mouse brains, infected with RML prions showed 5B2-positive long fibril-like PrPd aggregates at the cell periphery of GFAP-positive astrocytes with 6D11-positive PrPd puncta at perinuclear sites at three weeks post infection (Fig. 1H), thus corroborating the phenotypic features of the mAb pair in neuroblastoma cells. When glial proliferation in primary neuronal cultures was inhibited with 1 µM cytosine arabinoside (AraC), followed by infection with RML or uninfected CD1 control homogenates, Map2-positive neurons with fibril-like PrPd aggregates were visible upon RML infection (Fig. 1I), demonstrating that this fibrillar PrPd phenotype is prevalent in neurons as well.
Elongation, average length and structural features of fibril-like PrPd aggregates
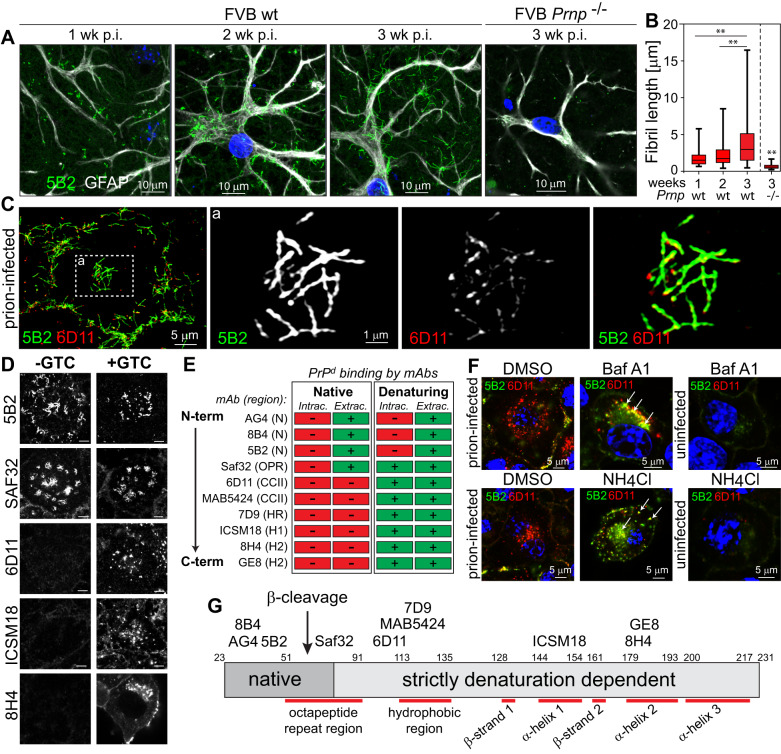
To characterise the time-dependence of PrPd fibril elongation, we infected primary neuronal cultures from brains of FVB wild-type (wt) and prion-replication deficient FVB Prnp-/- mice with the mouse RML prions. In cultures from FVB wt mouse brains, fibril length continuously and significantly increased over three weeks, at mean fibril elongation rates of 190 nm per day, reaching lengths of up to 15 µm, while no fibrils were detected at the periphery of astrocytes from brains of FVB Prnp-/- mice (Fig. 2A, B).
Fig. 2. Elongation and structural features of fibril-like PrPd aggregates and crypticity of PrPd epitopes.
A Time-dependent growth of 5B2-positive PrPd fibrils in primary astrocytes of FVB-wt (Prnp+/+), but not in those of FVB-ko (Prnp -/-) mice, following infection with RML. B Lengths of fibril-like PrPd aggregates in FVB-wt versus FVB-ko mice. Significance levels for time-dependent fibril growth in FVB-wt (Prnp+/+) mice and for FVB-wt versus FVB-ko were assessed from 2 independent experiments with at least 30 replicates per group using Kruskal–Wallis test with Dunn’s multiple comparisons with **p < 0.001. The statistical difference in rod length between FVB-wt versus FVB-ko mice at 3 weeks was determined by unpaired Mann–Whitney U Test; ** denotes a p-value < 0.001. For definition of boxplot elements see “Methods” section. C SIM images of fibril-like PrPd aggregates at the ECM of prion-infected S7 cells, cultured according to “standard TC protocol” in “Methods” section, labelled with 5B2 and 6D11 after guanidinium thiocyanate (GTC) treatment. Magnified area (a) denoted by dashed box. D Identification of cryptic epitopes in PrPd-bearing cells. Prion-infected S7 cells were incubated with anti-PrP mAbs in absence and presence of GTC. For complete image data set see Supplementary Fig. 4. Scale bars are 5 µm. E Summary of anti-PrP mAb binding to PrPd aggregates under native (-GTC) and denaturing (+GTC) conditions with corresponding PrP domains (for abbreviations of PrP regions see Supplementary Fig. 4). F Detection of intracellular 5B2-positive PrPd aggregates following a 16 h incubation with 6 nM bafilomycin A1 (BafA1) and 16 mM ammonium chloride (NH4Cl), respectively. Arrows denote intracellular 5B2-positive PrPd aggregates. G Model depicting cryptic, exposed and putative PrP-cleavage sites, respectively (modified from Rouvinski et al.14). Source data are provided as a Source Data file.
To investigate the phenotype of fibril-like PrPd at subdiffraction resolution, we co-labelled PrPd at the ECM of prion-infected S7 cells with 5B2 and 6D11 and imaged labelled cells using structured illumination microscopy (SIM, Fig. 2C). Notably, extended segments of PrPd fibrils did not show co-labelling under denaturing conditions. Instead, 5B2 immuno-positivity prevailed with 6D11-positive puncta along the lengths and at ends of fibrils. Where 6D11 puncta were detected in close proximity to 5B2-positive fibrils, areas of colocalisation were apparent (see insets in Fig. 2C). This result is consistent with the observed punctate and fibril-like phenotype of PrPd aggregates in single-labelled prion-infected cells (Fig. 1C) and suggests (i) that different aggregation states may co-exist during PrPd fibril formation and (ii) that the charge cluster 2 region where 6D11 binds may be cryptic in highly ordered fibrillar aggregates.
Identification of conformational and truncated PrPd variants in prion-infected cells
While it is well documented that some anti-PrP mAbs that recognise PrPc under native conditions fail to detect PrPd aggregates in prion-infected cells, unless cells are treated with denaturing agents after fixation5,20,21, this phenomenon has not been broadly investigated with panels of validated anti-PrP mAbs. Our initial results show that prion-infected cells, labelled with the monospecific core mAbs 6D11, ICSM18 and 8H4 in absence of GTC are phenotypically indistinguishable from uninfected cells (Supplementary Fig. 3), suggesting that those PrP epitopes are cryptic under native conditions. This notion however does not explain why 5B2 fails to detect intracellular PrPd under denaturing conditions (Fig. 1C, D, F).
We thus examined monospecific anti-PrP mAbs under native and denaturing conditions (Fig. 2D) at all sites of PrPd deposits (Supplementary Fig. 4). The binding characteristics of all investigated mAbs are summarised in Fig. 2E. In keeping with the binding characteristics of 5B2 in Fig. 1, all N-terminal mAbs detected fibril-like PrPd at extracellular sites (ECM and plasma membrane), but not intracellularly. MAbs that bind to the “core” PrP region (90-231)22 failed to detect PrPd in prion-infected cells under native conditions, while PrPd aggregates were readily detected following denaturation. Saf32, an antibody that has multiple binding sites at the octapeptide repeat region23 showed notable propensities, in that it detected extracellular fibril-like PrPd under native conditions, like N-terminal mAbs and intracellular PrPd under denaturing conditions, unlike N-terminal mAbs, suggesting that extracellular and intracellular PrPd species are conformationally distinct at the octapeptide repeat region (Fig. 2E and Supplementary Fig. 4). Alternatively, inherent conformational differences may exist between N-proximal and C-proximal octapeptide repeats that render C-proximal octapeptide repeats cryptic and invariant to limited proteolysis. Notably, a considerable body of evidence shows that PrP is cleaved at the level of the OPR, giving rise to the C2 fragment24,25.
To investigate whether failure to detect intracellular PrPd with N-terminal mAbs is due to proteolytic processing, we tested whether an increase in the endosomal/lysosomal pH may restore intracellular PrPd labelling by 5B2. Activation of endosomal/lysosomal proteinases is tightly regulated by the intraorganellar pH26 and bafilomycin A1 (BafA1), an inhibitor of V-ATPases prevents luminal acidification27. Notably, dissipation of the luminal pH with BafA1 and the lysosomotropic agent NH4Cl gave rise to intracellular 5B2-positive aggregates in prion-infected cells (Fig. 2F), suggesting that truncation of full-length (FL)-PrPd is blocked upon inhibition of pH-dependent proteinases, thus excluding crypticity as cause for the failure to detect intracellular FL-PrPd. Data on cryptic epitopes and the putative β-cleavage site of PrP are summarised in Fig. 2G.
In summary, we provide evidence that PrPd undergoes proteolytic processing in a pH-sensitive manner, while core mAbs fail to detect abnormal PrP under native conditions due to crypticity of their corresponding binding sites. In contrast, elongating PrPd fibrils at the plasma membrane can be labelled with N-terminal mAbs under native conditions in agreement with Rouvinski et al.14. These results suggest existence of two distinct aggregated PrPd species in prion-infected cells, FL-PrPd which is associated with fibril elongation at the plasma membrane and a truncated (TR-) PrPd type at perinuclear sites. Evidence that these two PrPd types are conformationally distinct at the level of the octapeptide repeat region (OPR) is suggested by the binding propensities of the monospecific anti-PrP mAb Saf32 which labels FL-PrPd at the plasma membrane under native conditions, but TR-PrPd at perinuclear sites under denaturing conditions.
Asynchronous changes in intra- and extracellular PrPd pools after cell dissociation
Dynamic shifts in prion steady-state levels have been reported after cell dissociation of persistently infected cells28; an initial rapid drop of PrPSc, measured as a proxy for prion levels was followed by gradual recovery of PrPSc levels28. We used this experimental paradigm to further characterise the relationship between intra- and extracellular PrPd pools. Confluent layers of chronically infected S7 cells were resuspended and replated into fresh medium (Supplementary Fig. 5A). Following cell trituration, long 5B2-positive PrPd fibrils remained attached to the substrate (Supplementary Fig. 5B). Attempts to immuno-isolate PrPd fibrils from decellularised ECM (dECM) following stringent lysis with RIPA buffer failed (Supplementary Fig. 5C). Under these conditions, 5B2-positive fibrils were still visible at the dECM (Supplementary Fig. 5D). Unexpectedly, when cells were resuspended and replated according to Supplementary Fig. 5A, 5B2-positive PrPd aggregates were absent in cells within the first two days after replating, while punctate 6D11-positive PrPd aggregates were detected at the ECM and at perinuclear sites (Supplementary Fig. 5E). 5B2-positive PrPd aggregates at the plasma membrane were first detected at day three, after which a rapid rise of 5B2- and co-labelled PrPd aggregates was evident (Supplementary Fig. 5E, F). This result suggests that cell dissociation leads to a quantitative clearance of FL-PrPd and that fibril growth at the plasma membrane commences after a considerable lag time following cell dissociation. To provide further evidence that FL-PrPd is cleaved under these conditions, we exploited our previous observation that dissipation of the luminal pH by BafA1 blocked truncation of FL-PrPd in persistently infected cells (Fig. 2F). Notably, BafA1 treatment of infected S7 cells led to (i) detection of abundant intracellular 5B2-positive FL-PrPd at 2 days after BafA1 treatment (Supplementary Fig. 5G) and (ii) a significant rise in 5B2 fluorescence intensity in prion-infected, compared to uninfected cells (Supplementary Fig. 5H). Prion propagation in S7 cells is associated with proteolytic processing of PrP, giving rise to truncated PrP conformers as evident in cell lysates after deglycosylation with PNGase (Supplementary Fig. 5I); bands, representing full-length (FL-) and truncated (TR-) PrP are indicated. As evident on Western blots of cell lysates, BafA1 treatment of infected (Supplementary Fig. 6A), but not of uninfected cells (Supplementary Fig. 6C) led to higher levels of FL-PrP. Proteinase K (PK) digestion of cell lysates from infected cells gave rise to PK-resistant PrP (PrPSc), while PrPc in cell lysates from uninfected cells was fully digested (band 5 in Supplementary Fig. 6A). Notably, BafA1 treatment of infected cells resulted in higher levels of PrPSc, when compared to mock-treated cells. Since endolysosomes have been proposed as a potential site where abnormal PrP is N-proximally truncated8, we enriched lysosomes of BafA1 and vehicle-treated cells by immunoprecipitation (Supplementary Fig. 6D). A band shift from TR-PrP to FL-PrP upon BafA1 treatment is apparent in Lamp1-enriched lysosomes (Supplementary Fig 6D). In summary, we provide evidence that cell dissociation leads to asynchronous changes in extracellular and intracellular PrPd pools. The slow kinetics in PrPd fibril formation is unexpected and suggests that considerable cellular or energetic barriers have to be overcome for PrPd fibrils to form.
Formation of PrPd fibrils at the plasma membrane is associated with synaptic vesicle trafficking and sensitive to cholesterol-lowering
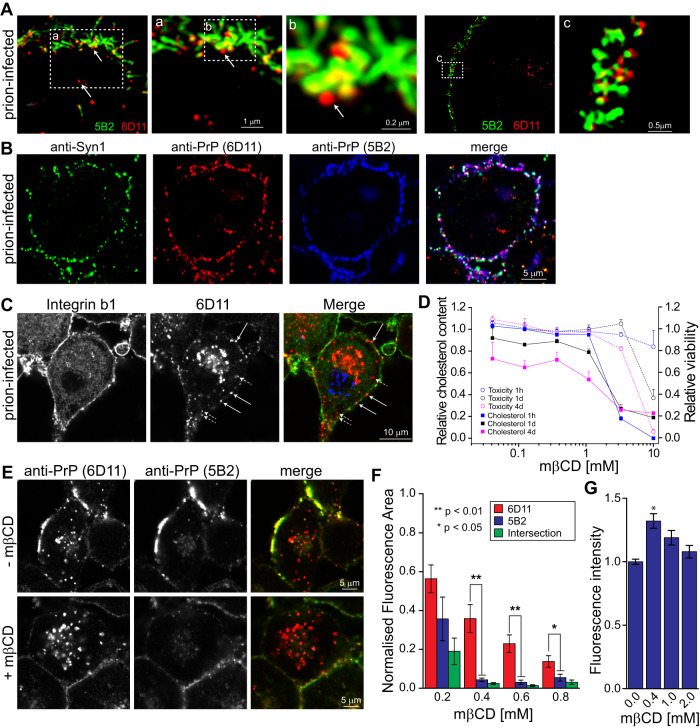
We next examined whether the antibody pair 5B2/6D11 may provide further insight into the mode of fibril formation at the plasma membrane. At subdiffraction resolution, 6D11-positive puncta can be detected at close proximity to 5B2-positive fibrils, suggesting that TR-PrPd aggregates are recruited to membrane areas where PrPd fibril form (Fig. 3A). A pivotal question is thus whether TR-PrPd reaches the plasma membrane by passive diffusion or by vesicular transport. Triple labelling of prion-infected cells with Synapsin 1 (Syn1), a marker for synaptic vesicles and anti-PrP mAbs 6D11/5B2 provides evidence that PrPd may reach the plasma membrane by vesicular transport (Fig. 3B). Single-, double- and triple-labelled puncta can be detected at focal planes above the nucleus and underscore the heterogeneity of overlapping distribution patterns. Double-labelling of PrPd and integrin β1, a membrane spanning protein which favourably visualises plasma membrane boundaries demonstrates that PrPd is detected proximal to (short hatched arrows) and at the level of (long straight arrows) the plasma membrane (Fig. 3C), suggesting that PrPd may dock and fuse with the plasma membrane.
Fig. 3. The formation of PrPd fibrils at the plasma membrane is associated with vesicular trafficking and is sensitive to cholesterol-lowering.
A SIM images of prion-infected S7 cells, cultured according to “standard TC protocol” in “Methods” section, double-labelled with 5B2 and 6D11 at the level of the plasma membrane. Magnified areas a, b and c are denoted by dashed boxes. Arrows denote punctate 6D11-positive PrPd in juxtaposition with 5B2-positive PrPd fibrils. Estimated lateral resolution SIM: 100 nM. B Triple-labelling of prion-infected S7 cells with anti-Syn1, 6D11 and 5B2. For quantification of colocalisation, see Fig. 4G. C Prion-infected S7 cells, labelled with anti-integrin β1 and 6D11 show PrPd aggregates proximal to (short hatched arrows) and at the level of (long straight arrows) the plasma membrane. D Dose-response relationship of mβCD on cholesterol-lowering and cell viability in prion-infected S7 cells at the time points and mβCD concentrations specified. E Colabelling of PrPd with 6D11 and 5B2 at the plasma membrane in presence and absence of 0.4 mM mβCD at 16 h following mβCD addition. F Quantification of mβCD effects on total fluorescence above threshold and colabelling of PrPd with 6D11 in red, 5B2 in blue and 6D11/5B2 intersection in green at 16 h after addition of specified inhibitor concentrations. Data is normalised to vehicle control (1.0) and represents mean and SEM. Statistical significance was determined by ANOVA. G Dose-dependent effects of mβCD on PrPc levels at the plasma membrane during a 16 h-incubation as specified in “Methods section” “Quantifying relative PrPd fluorescence intensities”. Mean values and SEM of three independent experiments are shown. Statistical significance was assessed by ANOVA with Bonferroni multiple comparison test with at least 148 images per condition analysed. * denotes p-value < 0.01. Source data are provided as a Source Data file.
Since lowering cholesterol impairs the docking and fusion of transport vesicles with the plasma membrane29,30, we incubated cells with the cholesterol-lowering agent methyl-β cyclodextrin (mβCD) in a dose-dependent manner to reduce cellular cholesterol. While cellular cholesterol levels decreased by 30-40% under these conditions, cytotoxic effects, measured by a sensitive ATP assay, were absent below 1 mM mβCD (Fig. 3D). Notably, at 0.4 mM mβCD, the fluorescence intensities of 6D11 and 5B2 decreased considerably at the plasma membrane, while 6D11-positive PrPd aggregates were still detected intracellularly at perinuclear sites (Fig. 3E). Quantitative analysis of fluorescence areas confirmed a significant reduction of 5B2 fluorescence and 6D11/5B2 colabelling at a concentration range between 0.4 mM and 0.8 mM mβCD (Fig. 3F). To exclude the possibility that mβCD treatment leads to an extraction of PrPc under the experimental conditions of Fig. 3E, F, we treated uninfected cells with mβCD concentrations of up to 2 mM (Fig. 3G), confirming that PrPc levels are not reduced at mβCD concentrations below 2 mM. A slight increase in PrPc surface levels between 0.4 and 1 mM mβCD may be due to a higher cell surface retention of PrPc.In summary, the drop of 6D11/5B2 colabelling following the lowering of cellular cholesterol levels suggests (i) that intra- and extracellular pools of PrPd can be uncoupled and (ii) that blocking of PrPd transport to the plasma membrane inhibits the formation of FL-PrPd at the plasma membrane. Alternatively to inhibition of vesicle transport, a perturbation of lipid rafts by mβCD could account for the reduced 5B2/6D11 colocalisation at the plasma membrane. Lipid rafts are thought to play an important role in the formation and propagation of prions31–33.
PrPd is segregated into vesicles of the regulated secretory pathway
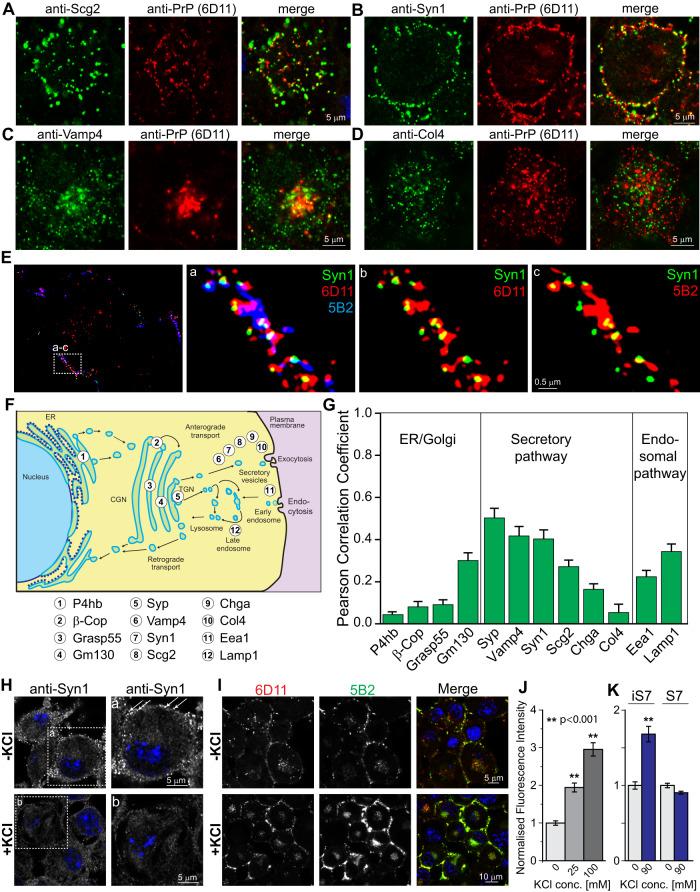
Experimental evidence that PrPd colocalises with a marker for synaptic vesicles (Fig. 3B) prompted us to further investigate the contribution of other secretory pathways to PrPd trafficking. Perinuclear PrPd colocalises with protein markers of large dense-core (Secretogranin 2 (Scg2)), synaptic (Syn1) and immature (Vesicle-associated membrane protein 4 (Vamp4)) vesicles (Fig. 4A–C), providing evidence that PrPd egresses cells by regulated exocytosis. In contrast, no colocalisation between PrPd and Collagen 4 (Col4), an extracellular matrix protein that is constitutively secreted, was observed (Fig. 4D). To map the route of PrPd aggregates from perinuclear sites to the plasma membrane, we determined the degree of 6D11 colocalisation, expressed as Pearson correlation coefficient, with markers of the ER (P4hb), Golgi network (β-Cop, Grasp55, Gm130), secretory vesicles (Syp, Vamp4, Syn1, Scg2, Chga, Col4) and endosomal/lysosomal markers (Eea1, Lamp1) (Fig. 4F, G). Representative images for all studied marker proteins, co-labelled with 6D11 can be found in Supplementary Fig. 7. This result confirms a role of the vesicular secretory pathways and the endosomal/lysosomal system in PrPd trafficking. To further examine whether intracellular TR-PrPd segregates into regulated exocytosis, we triggered membrane depolarisation with potassium chloride (KCl). Depolarisation led to a fast depletion of Syn1-positive synaptic vesicles (Fig. 4H) and a significant increase in PrPd aggregates at the plasma membrane (Fig. 4I, J), confirming that PrPd reaches the plasma membrane by regulated exocytosis. In contrast, in uninfected S7 cells, KCl-evoked depolarisation did not lead to an increase in PrPc levels at the plasma membrane (Fig. 4K).
Fig. 4. PrPd is segregated into exocytotic vesicles of the regulated secretory pathway.
A–D Persistently prion-infected S7 cells were co-labelled with 6D11 and vesicular markers (A) Scg2, (B) Syn1 and (C) Vamp4 as well as with constitutively secreted Col4 (D). E SIM image of plasma membrane, triple-labelled with Syn1, 6D11 and 5B2. Magnified areas (a–c) are denoted by a dashed box in the first image. F Diagram of marker proteins used to map trafficking routes of 6D11-positive PrPd. G Levels of colocalisation between 6D11 and organelle/vesicular markers, expressed as Person correlation coefficients. Data from two independent experiments with at least 10 per protein are shown. For representative images and gene names we refer to Supplementary Fig. 7. H Neuronal depolarisation with 25 mM KCl led to rapid depletion of Syn1-positive vesicles in S7 cells. Cells were fixed at 5 min after incubation with KCl. Arrows in magnified areas denote synaptic vesicles. I Increased levels of co-labelled PrPd aggregates at the plasma membrane, following KCl-evoked depolarisation. Cells were fixed at 5 min after incubation with KCl. J Quantitative changes of co-labelled PrPd after depolarisation in dependence of the KCl concentration normalised to untreated cells from three independent experiments with at least 36 replicates per condition. For statistical analysis, ANOVA with Bonferroni correction for multiple comparisons was conducted. K Quantitative changes in surface PrP levels after KCl-evoked depolarisation in uninfected S7 versus prion-infected iS7 cells. Following fixation, cells were stained with anti-PrP antibody 8H4. Data from three independent experiments with at least 48 images per experiment were analysed. Data represent mean values ± SEM. Statistical significance was evaluated by Student’s t-test (p < 0.01). Source data are provided as a Source Data file.
PrPd internalisation is dependent on functional dynamin and Cdc42
We next investigated whether perturbation of endocytosis affects the prion steady-state level of persistently infected cells. While evidence suggests that PrPc is internalised via clathrin-dependent endocytosis, mediated by its N-terminal domain34,35, the mode of PrPd endocytosis remains obscure. We here targeted known regulators of clathrin-dependent endocytosis (CME) and clathrin-independent endocytosis (CIE) pathways, using pharmacological inhibitors and RNA interference. While CME is considered the main endocytic pathway for “housekeeping functions” in cells, a growing number of fast, non-canonical pathways, classified as CIE, has been characterised and include Cdc42-, RhoA-, Arf6-regulated pathways36,37.
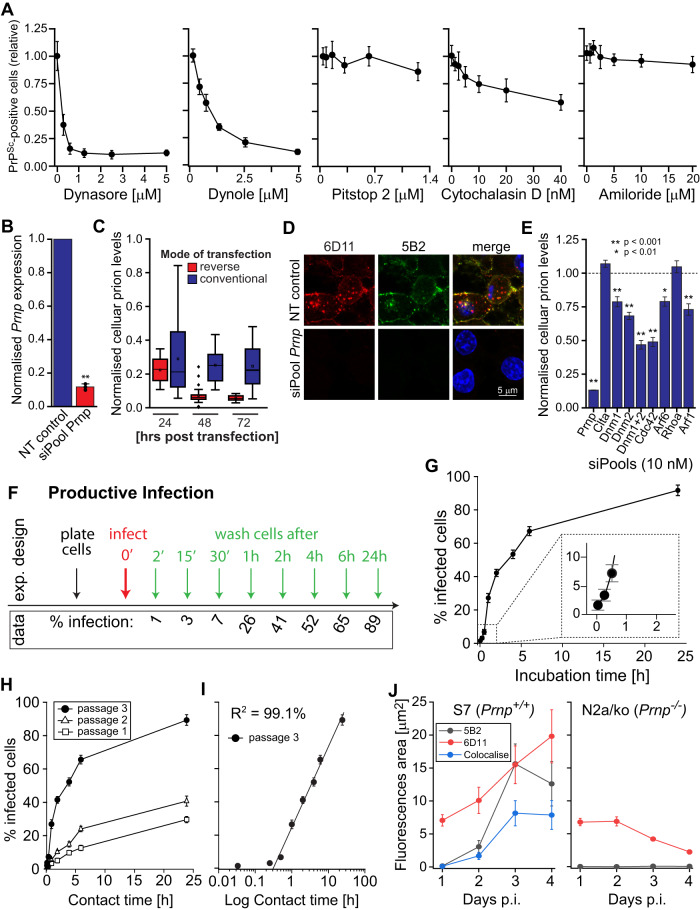
Absence of toxicity was carefully monitored for all inhibitor studies and toxic thresholds are reported in Supplementary Table 3. Two pharmacological dynamin inhibitors, dynasore38 and dynole39 showed a strong dose-dependent decrease of cellular prion steady-state levels by as much as 90% over three days, as determined by Scrapie Cell assay (SCA, Fig. 5A). However, the clathrin inhibitor Pitstop 240 did not affect prion steady-state levels. Further, the macropinocytosis inhibitor Amiloride41 and the actin polymerisation inhibitor Cytochalasin D showed moderate effects on prion steady-state levels (Fig. 5A). Two frequently used inhibitors, the CIE inhibitor EGA and Vacuolin which induces vacuole formation by homotypic fusion of endosomes and lysosomes42, reduced prion steady-state levels significantly by 60% and 80%, respectively (Supplementary Table 4), but the molecular targets of either inhibitor remains unknown.
Fig. 5. Identification of PrPd internalisation pathways and the minimum contact time for productive cell infection.
A Persistently prion-infected S7 cells were incubated with endocytosis inhibitors for 3 days and changes in prion levels were determined. Mean values ± SEM of at least three independent experiments are shown (n = 12). For toxic threshold levels, SCA data and significance levels see Supplementary Tables 3 and 4. B Gene silencing of Prnp in S7 cells using pooled siRNA, determined by RT-qPCR; mean values + SD of three independent experiments shown (p < 0.01). C Time-dependent effect of Prnp silencing on prion levels following conventional (blue bars) and reverse (red bars) transfection of cells from two independent experiments. For definition of boxplot elements see “Methods” section. D Effect of Prnp knockdown on PrPd aggregates in chronically infected cells following reverse transfection with siPools against Prnp and a NT control. E Silencing of gene targets associated with endocytosis in persistently infected S7 cells and its effect on prion levels. For knockdown efficacies and gene names see Supplementary Table 5. F Schematic for assessing the minimum contact time for productive prion infection. S7 cells were plated out in parallel and infected with clarified RML homogenates (see “Methods” section “Minimum contact time for productive infection”) at a 10−4 dilution (v/v, red arrow), followed by gentle washing of cells with PBS at the specified time points (green arrows) to remove inoculum. Cells were grown to confluence and the proportion of infected cells determined. G Proportion of prion-infected S7 cells, determined by SCA, plotted against contact times after infection with RML. Inset: magnified area of the proportion of infected cells at early time points, 2′, 15′ and 1 h. H Relationship between contact time and proportion of infected cells following infection with RML during subsequent cell passages. I Replotted data from (G) as a semi-logarithmic (linear-log) graph. J Prion replication-permissive (S7) and -refractory (N2a/ko) cells were infected with prion-infected exosomes and the formation of 6D11-, 5B2-positive and double-positive PrPd determined. Data represent average values ± SD of three independent experiments. Source data are provided as a Source Data file.
To corroborate a role of CIE pathways in the maintenance of cellular prion steady-state levels, we next targeted key regulators of endocytic pathways using RNA interference. To effectively silence gene expression, we used pools of 30 custom-designed siRNAs (siPools)43 per gene target. We first tested the use of siPools by silencing Prnp, since a loss of Prnp expression directly compromises prion propagation. Knockdown of Prnp leads to a 86% decrease in Prnp expression levels, measured by qPCR, when compared to a non-targeting control (NT control, Fig. 5B). To verify that transient Prnp knockdown persists over 3 days of culture, we next examined the time-dependence of gene knockdown, following conventional versus reverse transfection of cells. Reverse transfection, where siPools are directly mixed with suspended cells leads to a fast and sustained knockdown of Prnp levels over the duration of 72 h, while conventional transfection, where siPools are added to adherent cells 16 h after plating leads to higher variance and lower knockdown efficacies (Fig. 5C). That RNA silencing of Prnp by reverse transfection leads to efficient curing of prion-infected cells is corroborated by labelling cells with discriminatory anti-PrP mAbs (Fig. 5D). Following proof of concept for the use of siPools in modulating prion steady-state levels, we next targeted key regulatory genes involved in endocytosis. Levels of knockdown for individual gene targets are reported in Supplementary Table 5. Despite a 98% knockdown of gene expression, clathrin a (Clta) loss-of-function did not affect prion steady-state levels (Fig. 5E). Knockdown of the dynamin isoforms Dnm1 and Dnm2 led to a decrease of prion steady-state levels by 20-30% in single knockdown experiments, while double-knockdown resulted in a 50% decrease in prion steady-state levels (Fig. 5E). A highly efficient knockdown of cell division cycle 42 (Cdc42) by 97% led to a 50% drop of cellular prion titres, suggested that Cdc42-mediated CIE plays an important role in maintaining prion steady-state levels. Conversely, depletion of ADP ribosylation factor 6 (Arf6), Ras homologue family member A (Rhoa), Arf1 and Caveolin 1 (Cav1) did not affect prion steady-state levels (Fig. 5E). In summary, we provide evidence that prion propagation in N2a cells is dependent on functional dynamin and Cdc42, but independent of clathrin.
Evaluating the minimum contact time required for productive infection
Due to the lack of PrPd-specific anti-PrP mAbs, the kinetics of de novo PrP conversion is one of the most unexplored questions. While Goold et al., who expressed myc-tagged PrP to distinguish between PrPc and PrPSc suggested that de novo conversion commences within minutes after infection13, Yamasaki et al., who used a PrPSc-preferring mAb suggested that PrPSc is formed within days after infection44.
To investigate discordances in the kinetics of PrPd formation, we first determined the minimum contact time for prions with cells required for productive infection to occur, henceforth termed “contact time”. Productive infection is here defined as an infectious event that leads to persistent prion infection. We thus monitored incremental changes of the cell infectious state over several passages after infection in relation to the initial contact time using the SCA. As depicted in the schematic of Fig. 5F we infected S7 cells with the mouse-adapted prion strain RML on a time-scale between 2 min and 24 h in parallel, followed by gentle washing of cells to remove the inoculum. Cells were then grown to confluence and passaged three times before the proportion of infected cells in the total cell population was determined. The relationship between contact time and the percentage of infected cells, following two weeks of cell culture is depicted in Fig. 5G and the corresponding mean percent infection rates were included in the lower panel of Fig. 5F. This confirms that a contact time of 2 min suffices to infect neuronal cells, while the mean contact time required for infecting 50% of the cell population, defined as PI50 (PI for productive infection) is 4 h. Furthermore, a 2- to 4-fold increase in the percentage of infected cells between subsequent cell passages confirms stable propagation of prions (Fig. 5H). When plotted as a linear-log graph, a highly correlated linear relationship between the logarithm of contact time and the percentage of infected cells is apparent from 30 min to 24 h, while a minor cell population undergoing fast infection after 2 min of contact time is in the non-linear region of the plot (Fig. 5I).
We next addressed the problem of residual inoculum. Crude brain homogenates from mice at disease end stage, typically used for infections, are a non-physiological source of infectivity and may present unspecific effects. We thus tested alternative prion sources. Exosomes are biological nanoparticles that are formed from late endosomes by inward budding into multivesicular bodies45,46 and are constitutively shed into the cell supernatant by prion-infected cells47. When compared to RML, infectious exosomes showed a 10-fold lower immuno-positive background (residual inoculum) following infection (Supplementary Fig. 8A) and lower toxicity at high infectious doses (Supplementary Fig. 8B). Following infection of replication-incompetent Prnp-/- cells with exosomes from chronically infected S7 cells, 6D11-positive spots were detected intracellularly 1, 2 and 3 days after infection (Supplementary Fig. 8C). Remarkably, 5B2-positive FL-PrPd was not detected in susceptible S7 cells prior to 2 day after infection (Fig. 5J and Supplementary Fig. 8C), indicating that the formation of fibril-like PrPd at the plasma membrane significantly lags behind infection.
Prion infection leads to PrP conversion at two distinct cellular locations and precedes the formation of FL-PrPd
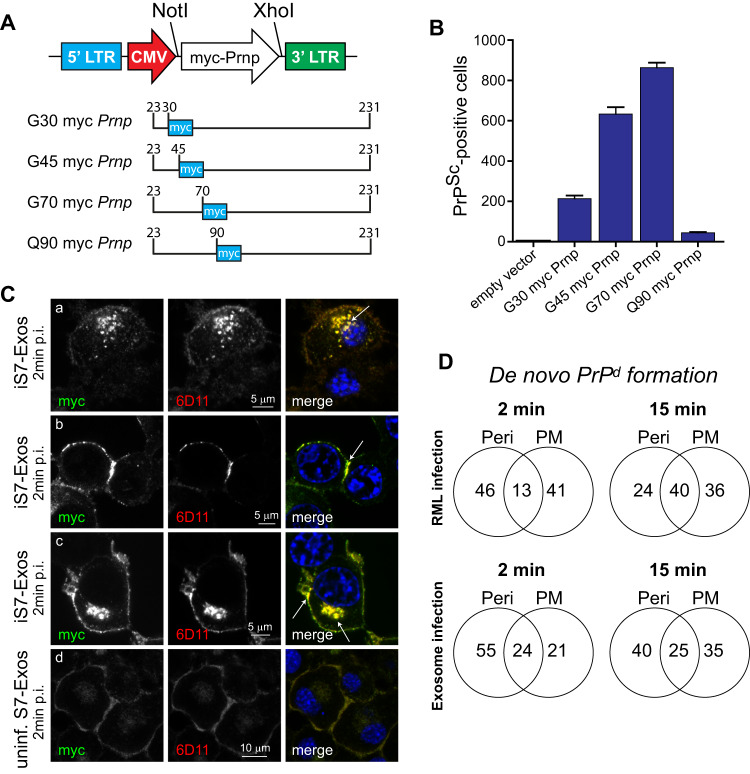
While a contact time of 2 min suffices to infect neuronal cells (Fig. 5F–I), early events after prion infection cannot be resolved using anti-PrP mAbs. We therefore generated a cell model expressing myc-tagged versions of PrP to distinguish newly formed abnormal PrP from inoculum. Myc-tagged Prnp chimera were expressed in PK1-10 cells where endogenous Prnp is stably silenced with a small hairpin RNA targeting the 3’-UTR of Prnp13. Our data on truncation and crypticity of PrP epitopes (Fig. 2D–G) provides guiding information for a rational design of N-terminally tagged PrP variants and suggests that the OPR meets critical prerequisites for monitoring de novo PrP conversion. To test this hypothesis, we generated the myc-PrP fusion proteins Gly30-myc PrP (henceforth G30 PrP), G45 PrP, G70 PrP (OPR) and Q90 PrP (CC2) as depicted in Fig. 6A and characterised the tagged fusion proteins. Susceptibility to prion infection of myc-Prnp expressing cells, inferred from the number of PrPSc-positive cells following infection with RML prions, increased in the order G30 PrP, G45 PrP to G70 PrP, while Q90 PrP was refractory to infection (Fig. 6B). Notably, failure in detecting intracellular myc-tagged PrPd in persistently infected G30 and G45, but not in G70 PrP-expressing cells under denaturing conditions confirms that PrPd is N-terminally truncated prior to the third octapeptide repeat (Supplementary Figs. 8D and 9A, B). Remarkably, the myc tag is rendered cryptic in G70 PrPd which can be unambiguously shown in persistently infected G70 PrP-expressing cells treated in absence and presence of GTC (Supplementary Fig. 8E). While myc-tagged PrPd can be distinguished from PrPc in uninfected cells by fluorescence intensity and the granularity of PrPd aggregates, respectively (Supplementary Fig. 8D), we colabelled cells with the discriminatory anti-PrP mAb 6D11 and anti-myc to unambiguously detect infected cells (Fig. 6C, D and Fig. 7A, B).
Fig. 6. Prion infection leads to PrP conversion at two distinct cellular locations.
A Cloning of N-terminal versions of myc-tagged Prnp into the retroviral vector pLNCX2. The position of insertion of the myc tag is depicted. Prnp chimera were expressed in PK1-10 Si8 Prnp double-knockdown cells (see “Methods” section). B Comparative susceptibilities of myc-tagged Prnp expressing cells after infection with a 10−4 dilution of RML prions (titre: 108.4 LD50 units/g). An empty vector confirms that cells transcriptionally silenced with hairpins against the 3’UTR of Prnp are refractory to prion infection. Data represents one experiment with 12 repeats. C G70 PrP-expressing cells, fixed at 2 min following infection with infected exosomes were colabelled with AF488-conjugated anti-myc and the discriminatory 6D11 mAbs. Detection of myc PrPd at distinct cellular locations is depicted by arrows in merged images: perinuclear (A), plasma membrane (B), perinuclear and plasma membrane (C) and mock-infected control cells (D). D Identifying the cellular sites of de novo conversion. Detection of de novo converted PrP in G70 PrP expressing cells at 2 min and 15 min after infection with RML and exosomes at perinuclear sites (Peri), the plasma membrane (PM), or both sites as represented by Venn diagrams in percent of total. At least 50 myc PrPd positive cells were analysed per condition by three investigators. Data represent the percentage occurrence of PrP conversion at the specified cellular loci. Source data are provided as a Source Data file.
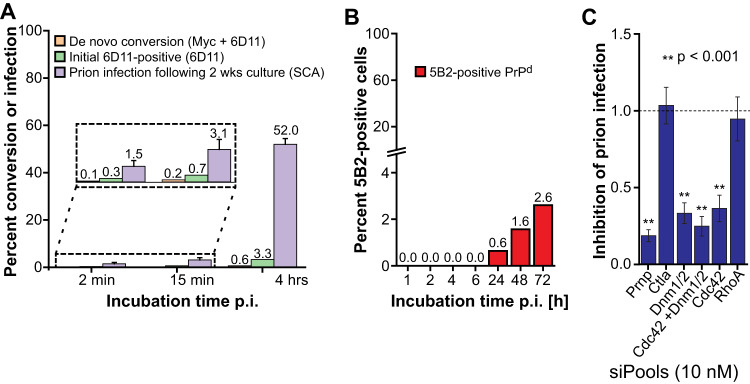
Fig. 7. De novo PrP conversion precedes the formation of FL-PrPd and is inhibited by transcriptional silencing of Cdc42 and dynamins.
A Quantitative analysis of contact times against the proportion of cells with evident de novo PrP conversion (Myc + 6D11) and initially infected (6D11) cells, respectively. G70 PrP-expressing cells were infected with RML for the specified times (post infection: p.i.), gently washed to remove the inoculum, fixed and labelled. For comparison, the proportion of infected cells after two weeks of tissue culture following exposure with prions at the specified contact times, data reblotted from Fig. 5G, are shown (violet bars). Mock-infected background-corrected data from 81 frames of about 60 cells per frame were scored as specified in “Methods” section. B Relationship between cell contact times after RML infection and the detection of 5B2-positive FL-PrPd. G70 PrP-expressing cells were infected with RML brain homogenate at a 10−4 dilution for the time periods specified above. Cells were subsequently fixed and labelled with 5B2 and 6D11, followed by fluorescence conjugated secondary antibodies. As above, the number of double-positive cells was scored. C Inhibition of prion infection by gene silencing of dynamins and Cdc42. G70 myc Prnp-expressing cells were transfected with siPools against the specified targets, followed by infection with exosomes. Levels of prion infection were determined from 4 independent experiments with at least 24 replicates per gene target by SCA and expressed as mean values ± SEM, relative to cells transfected with non-targeting control (1.0 ± 0.013). For statistical analysis Kruskal–Wallis test with Dunn’s multiple comparisons was conducted (**p < 0.001). For gene names see Supplementary Table 5. Source data are provided as a Source Data file.
Next, we interrogated de novo PrP conversion in G70 Prnp-expressing cells, infected with RML or exosomes, according to the experimental design in Fig. 5F. Notably, infection of cells with exosomes gave rise to myc-tagged PrPd in two distinct subcellular sites, the plasma membrane and perinuclear sites as early as 2 min after infection (Fig. 6C). We next identified the sites of PrP conversion at 2 and 15 min after infection. Irrespective of the source of inoculum, myc PrPd aggregates were detected in perinuclear regions (Peri), at the plasma membrane (PM) or in either location as depicted by Venn diagrams in Fig. 6D. All experiments were scored independently by three investigators and data is summarised in Supplementary Table 6. This suggests that abnormal PrP is formed rapidly after infection at two distinct cellular locations, the plasma membrane and an unidentified location at perinuclear sites of neuronal cells.
We next determined the proportion of phenotypic cells at early time points after infection with RML prions (Fig. 7A). Cells bearing de novo converted PrP (6D11/myc double-labelled) account for less than 0.2% of the total cell population at 2 and 15 min and less than 1% at 4 h after infection. For comparison, we reblotted the number of prion-infected cells following 2 weeks of tissue culture, representing perpetuating prion propagation after infection (Fig. 5G). An up to 80-fold increase in the number of prion-infected cells at 2 weeks, compared to the initial myc PrPd-bearing cells suggests that an unexpectedly low number of cells becomes infected initially, while prion replication and cell-to-cell transmission account for the rapid spread of prions.
To address the discrepancy between the fast kinetics of PrP conversion and the delayed formation of FL-PrPd, we infected G70 PrP-expressing cells according to Fig. 7A and labelled them with the discriminatory anti-PrP mAb pair 5B2/6D11 (Fig. 7B). Notably, 5B2-positive cells could not be detected before 24 h after infection, suggesting that de novo PrP conversion precedes FL-PrPd fibril formation at the plasma membrane.
The low frequency of de novo PrP converting cells (Fig. 7A) greatly limits investigation of the underpinning mechanism of PrP conversion. However, since prion steady-state levels in persistently infected cells are highly dependent on functional dynamins and Cdc42, we further explored whether infection of cells can be inhibited by transcriptional silencing of dynamin and Cdc42, respectively (Fig. 7C). In fact, single knockdown of Cdc42, double-knockdown of Dnm1 and Dnm2 (Dnm1/2) and triple-knockdown (Dnm1/2/Cdc42) significantly blocks infection, suggesting that internalisation of the infectious seed is critical in establishing prion infection.
Discussion
The self-perpetuating replication of prions is a central tenet of prion pathogenesis and represents a prototypic non-nucleic acid-based mechanism of infection and heritability. While long believed to be unique to prions, self-assembly and aggregation of proteopathic seeds is now considered a common trait of amyloids in many neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease. It is therefore pivotal to identify common and specific disease pathways to assess potential risk and therapeutic strategies alike. This study has advanced our knowledge on the molecular underpinning of prion propagation in several ways.
Built on our initial observation of distinct PrPd phenotypes in neuronal cells and primary astrocytes, we provide evidence that extracellular fibril-like FL-PrPd is exclusively formed and elongated at the plasma membrane. In contrast, proteolytically truncated TR-PrPd aggregates reside at perinuclear sites and segregate into the regulated exocytotic pathway, where they dock and fuse with the plasma membrane, thus providing evidence for a directional transport of PrPd seeds, opposed to passive diffusion. We further provide evidence that FL-PrPd gives rise to distinct aggregation states, where the charge cluster 2 region remains cryptic under denaturing conditions over extended segments, suggesting that amorphous and fibrillar aggregates may co-exist during fibril formation at the plasma membrane. Notably, N-terminally truncated TR-PrPd at perinuclear sites fails to form fibrillar aggregates which suggests that the PrP N-terminus might be necessary for fibril formation. This is in agreement with the notion that the N-terminus mediates higher-order aggregation processes48–50. Owing to the lack of fibril formation, TR-PrPd may represent amorphous aggregates or oligomers.
That the prion state of cells is dependent on functional dynamins and Cdc42 implies that reuptake of PrPd aggregates in prion-infected cells is critical to maintain prion propagation. While PrPc is believed to be internalised via CME35, we provide evidence that PrPd reuptake is mediated by CIE. This notion is associated with another important aspect of PrPd turnover, proteolytic processing. In keeping with the observation that FL-PrPd is absent intracellularly, unless cells are treated with BafA1, proteolytic processing of FL-PrPd following reuptake may result in fibril fragmentation, a cellular process that is considered critical to maintain prion replication. In contrast, prion propagation in yeast is believed to be strongly dependent on chaperon activity51,52.
We provide evidence that PrP conversion occurs within minutes after infection at two distinct cellular locations, the plasma membrane and an unidentified perinuclear site. This result accommodates opposing views on the primary sites of PrP conversion6,9,13, but also supports the notion of a fast rate of PrP conversion13. We think that labelling of cells under non-denaturing condition may explain why intracellular PrP conversion was not observed in the Goold study13. Our study provides two further important aspects of seeded PrPd aggregation.
Firstly, despite the fast rate of PrP conversion, formation of FL-PrPd aggregates was not detected prior to 24 h after infection. This suggests (i) that the formation of truncated PrP species is energetically favoured and (ii) that the infectious seeds that trigger the fast formation of TR-myc PrPd at both cellular conversion sites are N-terminally truncated. Notably, non-fibrillar amorphous oligomers are thought to represent the most infectious seeds for prion propagation53,54, although their state of proteolytic processing remains unknown. While the presence of truncated versions of PrPSc and PrPc in prion diseases has been widely reported55–59, this study provides insight into the formation and the molecular relationship between truncated and full-length versions of PrPd. In extension to current models of PrPd formation (for review see ref. 60), we propose that the conformational transition from PrP seeds to fibrils is favoured by neutral pH conditions and can be blocked by retaining PrPd seeds at an acidic luminal pH.
Secondly, the frequency of de novo PrP conversion is much lower than previously suggested13, as verified by correlative imaging and prion titre output methods for various contact times. While de novo conversion was observed at a frequency of only 0.1%, 0.2% and 0.6% for a contact time of 2 min, 15 min and 4 h, respectively, prion titres increased up to 80-fold when cells were serially passaged for 2 weeks. This suggests that cell-to-cell transmission is a crucial cellular mechanism that underpins prion propagation.
We provide evidence that PrPd segregates into synaptic and large-dense core vesicles of the regulated exocytosis pathway. Notably, peptide hormones and neuropeptides are thought to be sorted into immature secretory granules by virtue of protein aggregation61–64, suggesting that prions may be sorted into the secretory pathway by default. Importantly, the self-assembly of functional amyloids is reversible and more information on the reversibility of the amyloid-like aggregation state of peptide hormones and neuropeptides62 will be critical to understand proteopathic seeds. Our finding that PrPd aggregates are detected in synaptic vesicles may further provide an important link between prion propagation and pathogenesis, since synaptic dysfunction is considered an early pathogenic process in neurodegeneration65.
Methods
Antibodies and reagents
The antibodies used in this study were summarised as follows (clone, company, catalogue number). Unless otherwise stated, antibodies were used at a 1:1,000 dilution. Mouse monoclonal anti-PrP (8H4, Sigma, P0110), rat monoclonal Lamp1 (1D4B, Santa Cruz Biotechnology (SCB), sc-19992), mouse monoclonal anti-PrP (5B2, SCB, sc-47730, used at a 1:500 dilution), mouse monoclonal anti-PrP (AH6, SCB, sc-69896), mouse monoclonal anti-PrP (8B4, SCB, sc-47729), mouse monoclonal anti-Syp (7.2, Synaptic Systems, 101 011), rabbit polyclonal anti-Vamp4 (Synaptic Systems, 136 002), rabbit polyclonal anti-Chga (Synaptic Systems, 259 003), rabbit polyclonal anti-phospho FAK (Tyr925, Cell Signaling Technology, 3284), rabbit polyclonal anti-beta Cop (Thermo Fisher Scientific, PA1-061), rabbit polyclonal anti-Scg2 (Abcam, ab12241), mouse monoclonal anti-PrP (7D9, Abcam, ab14219), rabbit polyclonal anti Col4 (AbD Serotech, 2150-1470), rat monoclonal anti-CD29 (9EG7, BD Biosciences, 553715), mouse monoclonal anti-GM130 (35, BD Biosciences, 610822), mouse monoclonal anti-PrP (6D11, BioLegend, 808002), mouse monoclonal anti-PrP (Saf32, Cayman, 189720), rabbit monoclonal anti-PDI (C81H6, Cell Signaling Technology, 3501 S), rabbit monoclonal anti-Eea1 (C45B10, Cell Signaling Technology, 3288), rabbit polyclonal anti-Lc3a (Cell Signaling Technology, 4599), rabbit polyclonal anti-GFAP (DAKO, Z0334, used at a 1:10,000 dilution), Mouse monoclonal anti-PrP (3F4, Merck, MAB1562), mouse monoclonal anti-PrP (MAB5424, Merck, MAB5424), mouse monoclonal anti-myc, AF 488-conjugated (9E10, Merck, 16-308), rabbit polyclonal anti-Syp1 (Millipore, AB1543), mouse monoclonal anti-Snap25 (SP14, Millipore, MAB331), rabbit polyclonal anti-Gorasp2 (Grasp55) (Proteintech, 10598-1-AP), mouse monoclonal anti-PrP (AG4, TSE Resources Centre, TSE RC, RC 059), mouse monoclonal anti-PrP (GE8, TSE Resources Centre, TSE RC, RC 061), ICSM18 and ICSM35 (UCL Institute of Prion Diseases), chicken polyclonal anti-Map2 (Abcam, ab5392). The reagents in this study were summarised as follows (company, catalogue number): Dynasore (Cambridge Bioscience, 1900-25), Pitstop 2 (Generon, AOB3600-25), Bafilomycin A1 (Insight Biotechnology, BIV-1829-250), Dynole (Insight Biotechnology, sc-362731), Cytochalasin D (Cambridge Bioscience, B1176-5), Cell Titre Glo luminescent cell viability assay (Promega, G7571), Lipofectamine RNAimax transfection reagent (Thermo Fisher Scientific, 13778150), Pierce LDH cytotoxicity assay (Thermo Fisher Scientific, C20301), Amplex Red assay (Thermo Fisher Scientific, A12216), One Shot TOP10 chemically competent E.Coli (Thermo Fisher Scientific, C404010).
Cell lines and tissue culture
Neuro2a (N2a, CCL-131) cells were purchased from American Type Culture Collection (ATCC). Prion-infected and uninfected mouse neuroblastoma N2a-derived cell lines were maintained in OptiMEM, containing 10% foetal calf serum (FBS) and 1% penicillin/streptomycin (OFCS). The highly prion-susceptible cell line S7 was derived from N2aPK1 cells as previously reported in Marbiah et al.66 and was split twice weekly for cell maintenance. Extended TC protocol: Where S7 cells were grown for extended culture times (Fig. 1C), the serum concentration was reduced by 50%, while the culture medium was changed daily from 3 days of culture. Under these conditions, the build-up of fibril-like PrPd aggregates at the plasma membrane is observed from three days onwards. Standard TC protocol: Daily replacement of ½ the conditioned medium with fresh OFCS from 3 days of culture was alternatively used for extended tissue culture times where specified (Figs. 2C and 3A).
Primary neuronal cultures
Primary cortico-hippocampal cultures were prepared from embryonic e17 FVB mouse brains essentially as described previously67. These cultures contain GFAP-positive prion-susceptible astrocytes which, when infected with RML prions, form 5B2-positive PrPd fibrils (Fig. 1H). To culture neuronal monocultures (Fig. 1I), primary hippocampal cultures were treated with 1 µM AraC at 6 days after isolation. Following infection with RML prions, PrPd fibrils were observed after three weeks (Fig. 1I). Primary neuronal cultures were maintained in Neurobasal medium, supplemented with 2% (v/v) B27, 0.25% (v/v) GlutaMAX and 1% penicillin/streptomycin (Pen/Strep). Where primary neuronal cultures were maintained up to four weeks, half the medium was replaced with fresh medium twice a week. Primary hippocampal cultures were conducted essentially as reported in Ramsberger et al.68.
Safety and ethical declaration
All experimental procedures involving mouse-adapted prion strains were carried out in microbiological containment level 2 facilities with strict adherence to safety protocols and guidelines. All procedures involving animals were performed under approval and license granted by the UK Home Office (Animals (Scientific Procedures) Act 1986), project license number 70/9022 in compliance with UCL institutional guidelines and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (www.nc3rs.org.uk/ARRIVE/). FVB mice were purchased from Envigo Ltd (London, UK) and were housed under specific pathogen-free conditions in a temperature and light-controlled environment (12 h light/dark cycles) and had free access to food and water.
Prion infection of cultured cells
Crude brain homogenates (10% w/v) were prepared essentially as described by Hill et al.69. To improve homogeneity, samples were ribolysed as reported previously70. Briefly, crude brain homogenates were transferred to 2 ml microtubes (Sarstedt Ltd., Leicester, UK), containing zirconium beads, Protease Inhibitor Cocktail Set I (Pierce, Leicester, UK) and 25 U benzonase (Novagen, Madison, WI) using a Ribolyser (Hybaid, Cambridge, UK) at maximum speed for two cycles of 45 seconds. To infect prion-permissive cells, aliquots of 5 × 104 S7 cells/ml OFCS were plated into wells of 96-well plates. Sixteen hours later, cells were infected with serially 1:10 diluted ribolysed RML brain homogenates. Cells were typically infected with 10−4 or serial 1:10 dilutions of 10% brain homogenates (w/v) from prion-diseased mice at clinical disease with a titre of 108.4 LD50 units/g. Following infection, cells were grown to confluence and split three times 1:8 to dilute out PrPSc-positive inoculum. At each split after reaching confluence, the proportion of PrPSc-positive cells was determined by the Scrapie cell assay (SCA) as specified in “Quantification of prion infection rates”. If not otherwise specified, persistently prion-infected S7 cells were used in this manuscript. Prion-permissive S7 or myc-tagged S7 cells were freshly infected in Figs. 5F–J and 6C–D, 7A–C and Supplementary Fig. 8A–D.
Minimum contact time for productive infection
To determine the minimum contact time for productive infection (Fig. 5F–I), prion-susceptible S7 cells were infected with clarified RML brain homogenates to reduce the amount of residual immune-positive inoculum, detected as background in the SCA and diluted out by serial cell passages71. RML brain homogenates were clarified by centrifugation at 300 × g for 10 min. To monitor the amount of residual inoculum in clarified RML homogenates, prion replication-refractory Prnp-/- cells (see “Generation of Prnp knockout cells using Crispr-Cas9” below) were plated out in parallel experiments and infected with clarified RML brain homogenate alongside with S7 cells. Residual inoculum, detected in Prnp-/- cells was less than 10% of the total signal in prion-susceptible cells after the first passage and less than 1% after the second passage, confirming that residual inoculum in clarified RML homogenates is rapidly diluted out during sequential passaging of cells. To assess the minimum contact time for productive infection, aliquots of 5 × 104 S7 cells/ml OFCS were plated into wells of 96-well plates and infected with a 1 × 10−4 dilution of clarified RML brain homogenate the following day. After varying time points after infection, ranging from 2 min to 24 h (Fig. 5F), cells were carefully washed with PBS to remove the inoculum, grown to confluency and the number of infected cells was determined in subsequent cell passaged (Fig. 5 G, H).
Quantification of prion infection rates
The number of prion-infected cells following infection with prion-containing media was determined using the SCA as described previously71. The SCA is based on the microscopic detection of proteinase K-resistant PrP (PrPSc) in prion-permissive cells in an automated manner using a Biomek FX liquid handling robot. Suspended cells (approximately 25,000 cells) were transferred onto ELISPOT (Multi Screen Immobilon-P, Millipore, UK) plates. ELISPOT plates were vacuum-drained and dried at 50 °C prior to storage at 4 °C until further processing. Where prion titres in persistently infected cells were determined, suspended cells were diluted in PBS 1:10 and approximately 2000 cells per well were transferred onto ELISPOT plates. The number of cells was determined by Trypan blue as specified below. The revelation and quantification of PrPSc-positive cells was done essentially as described in Schmidt et al.72 using a Bioreader 5000-Eß system (BioSys, Karben, Germany). Changes in the prion steady-state levels of persistently infected cells following incubation with small inhibitory molecules or siRNA were also quantified by SCA. Where the percentage of infected cells on a population level was quantified in subsequent passages (Fig. 5G–I), the total number of cells was determined in parallel experiments by transferring ~1000 cells per ml onto ELISPOT plates. Subsequently, plates were stained with Trypan blue in cell lysis buffer (50 mM Tris HCl, 150 mM NaCl, 0.5% (w/v) sodium deoxycholate and 0.5% (v/v) Triton-X 100, pH 8.0) and counted with the Bioreader 5000-Eß to determine the total number of transferred cells.
We further used crude exosome fractions as a source of prion infectivity. To isolate exosomes, chronically infected S7 cells were grown to confluence in 15-cm Petri dishes. After removing the growth medium, cell layers were overlaid with fresh medium and conditioned medium was collected after 24 h for 4 consecutive days. Cell supernatants were stored at 4 °C until further processing. Combined supernatants were cleared from whole cells and cell debris by centrifugations at 300 x g for 5 min and at 4500 x g for 10 min, respectively. Clarified supernatants were then centrifuged at 32,000 x rpm for 2 h in a Beckman ultracentrifuge, equipped with SW 32 Ti swinging-bucket rotors (Beckman Coulter UK, High Wycombe, UK). Supernatants were discarded and pellets resuspended in residual medium. Concentrated exosome-containing suspensions were aliquoted and kept at −80C until use. Infectious titres of the exosome concentrates were determined using the SCA, essentially described previously71. Typically, one litre of cell supernatant from prion-infected S7 cells yielded 2-3 millilitres of concentrated exosomal pellet with an infectious titre of 107.88 Tissue culture infectious units (TCIU)/ml.
Cell curing assay
Effects of small molecule inhibitors on the prion steady-state levels were determined by the SCA while cytotoxicity was carefully monitored. Stock solutions of inhibitors were prepared in DMSO unless specified otherwise in the manufacturer’s guidelines. Since 1:1,000 dilutions of DMSO did not affect the cell doubling rates and the prion steady-state levels of S7 cells, the concentration of DMSO-soluble inhibitor stock solutions was adjusted to allow dilutions of at least 1:1,000 or higher. To determine effects of small molecule inhibitors on prion steady-state levels, 300 µl aliquots of chronically infected S7 cells were plated into wells of 96-well plates at a concentration of 5 × 104 cells per ml OFCS. After 16 h, cells were incubated with serially diluted inhibitors. After 3 days, cells were resuspended, diluted 1:10 in PBS and 100 μl of the cell suspension was transferred onto ELISPOT plates (Cat#. MSIPN4550, Merck Millipore Ltd, Tullagreen, Ireland) and prion titres were determined. Data in Supplementary Table 4 represent mean values of at least three independent experiments and are normalised to vehicle (DMSO)-treated cells.
Determination of cell viability and cytotoxic thresholds
Cytotoxicity was assessed by measuring LDH release (Pierce LDH cytotoxicity assay kit, Thermo Fisher Scientific) and ATP levels by Luminescent Cell Viability Assay (Cell Titre Glo, Promega). To take experimental variation into account, cytotoxic effects were scored positive, when a threshold of 10% was exceeded, when compared to mock controls. Since cytotoxicity of small molecule inhibitors is highly correlated with the cell density, we determined cytotoxic thresholds of inhibitors for distinct cell densities (see Supplementary Table 3). In the ATP assay, luminescence values of inhibitor-treated cells were normalised with those of mock-treated cells and changes in ATP levels were recorded. In the LDH assay, the levels of toxicity were calculated as below,
where unspecific LDH activity represents absorbance values of cells treated with 10% water (v/v) and maximal LDH represents absorbance values following cell lysis.
To determine the effects of methyl-β-cyclodextrin on cell viability, cholesterol and PrPd levels, chronically infected S7 cells were plated into 96-well plates (Cat#: 3505,Scientific Laboratory Supplies Ltd, Nottingham, UK) at a concentration of 5 × 104 cells per ml OFCS. After 16 h, cells were incubated with methyl-β-cyclodextrin (Cat#: Sc-215379, Santa Cruz Biotechnology, Heidelberg, Germany), dissolved in culture media without FBS, at concentrations specified in Fig. 3D. To determine the cellular cholesterol levels, cell layers were washed with PBS and lysed in 100 μl TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0) per well. Aliquots of 50 μl lysates were transferred into black 96-well microplates (Cat#: 655077, Greiner Bio One Ltd, Gloucestershire, UK) and cholesterol levels where determined using the Amplex red assay (Cat#: A12216, Thermo Fisher Scientific, Loughborough, UK) according to the manufacturer’s specification. While cholesterol levels were not normalised for cell numbers in Fig. 3D, the effects of cholesterol lowering on the level of colabelling between 5B2 and 6D11 (Fig. 3E, F) were determined for non-toxic mβCD concentrations ( < 1 mM) only. Cell viabilities were analysed by CellTiter-Glo luminescent cell viability assay (Cat#: G7571, Promega, Southampton, UK).
Processing of cells for imaging
For immunolabelling, cells were processed as specified previously66. Briefly, cells were fixed with 3.7% formalin (Thermo Fisher Scientific, Loughborough, UK) in PBS for 12 min. Unless otherwise specified, cells were washed with chilled acetone for 1 min. After rehydration with PBS, cells were incubated with 3.5 M guanidine thiocyanate (GTC, Melford) for 10 min. Cells were washed at least five times with PBS prior to labelling with primary antibodies, diluted into Superblock (Thermo Fisher Scientific)/PBS (1:4), supplemented with 10% Pen/Strep and incubated at 4 °C overnight. After washing with PBS, cells were labelled with highly cross-adsorbed fluorescence-conjugated secondary antibodies (AffiniPure, Jackson ImmunoResearch) and incubated at 4 °C overnight. Cells were imaged after a final wash with PBS.
Image acquisition using laser-scanning microscopy
Images were acquired with a Zeiss LSM710 laser-scanning microscope, equipped with a ×63 objective (1.4 oil, Plan‐Apochromat) using immersion oil Immersol 518 F (Carl Zeiss, Cambridge, UK). Cells were fixed and treated as described above. Cells were labelled with fluorescence-conjugated secondary antibodies (AffiniPure, Jackson ImmunoResearch) and Alexa Fluor 488 and Rhodamine Red-X fluorescence was measured using a 460-540 nm and 565–640 nm bandpass filters, respectively, following excitation with an argon laser at 488 nm and a diode-pumped solid state laser at 561 nm, respectively. To unambiguously distinguish PrPd deposits at distinct cellular sites, i.e. the plasma membrane, perinuclear regions and the extracellular matrix (ECM), we conducted serial z-stacks and normalised the focal planes using ECM-resident focal adhesion kinase (FAK). Notably, in-focus detection of FAK, labelled with an anti-FAK antibody (Tyr925, Cell Signaling Technology, 3284) coincides with in-focus detection of PrP and was arbitrarily denoted “zero μm” as a focal reference level with an estimated standard deviation of ± 0.2 μm. At least 3 independent experiments were conducted for all immunofluorescence experiments and representative images are shown.
Structural illumination microscopy
To image PrPd fibrils at the plasma membrane and ECM, cells were grown for extended culture times (5-7 days) with daily medium changes from day 4. Cells were processed as specified in “Processing of cells for imaging” and labelled with anti-PrP antibodies 5B2 and 6D11, followed by highly cross-adsorbed secondary antibodies, anti-mouse IgG1-Rhodamine and anti-mouse IgG2a-Alexa Fluor 488 (AffiniPure, Jackson ImmunoResearch), respectively. Images were acquired by Structured Illumination Microscopy (SIM) using a Zeiss ELYRA PS.1 microscope with Plan-Apochromat DIC M27 63X objective and 1.4 immersion oil. Images were acquired using 5 phase shifts and 3 grid rotations for the 561 nm and 488 nm lasers at a 5-10% output. Images were acquired using a sCMOS (pco.edge sCMOS) camera. Channels were aligned using a slide with multi-coloured beads (100 nm, TetraSpeck microspheres T7279, Thermo Fisher Scientific) with the same image acquisition settings. After SIM reconstruction and channel alignment, images were concatenated into a single image stack. Images were processed with ZEN black version 11.0.2 software (Zeiss).
Quantifying relative PrPd fluorescence intensities
Chronically prion-infected and uninfected cells were seeded into 8-well chamber slides and grown to confluence prior to processing as described in “Processing of cells for imaging”. Quantitative analysis of PrPd aggregates at perinuclear and extracellular sites was conducted following double-labelling of cells with anti-PrP antibodies 5B2 and 6D11 overnight at 4 °C. After washing with PBS, cells were labelled with highly cross-adsorbed isotype-specific fluorescence-conjugated secondary antibodies (AffiniPure, Jackson ImmunoResearch) for 12-24 h. Randomly selected fields with 20 to 40 cells were analysed from at least three replicate experiments using Volocity. To quantify fluorescence intensities of PrPd aggregates, threshold levels of PrPc fluorescence were determined in uninfected cells and used as baseline levels. Intersection denotes colabelled areas where pixels are detected in more than one fluorescence channel. To determine levels of colocalisation of 6D11-positive PrPd with intracellular markers at perinuclear sites, stacks of 10-15 images in z-direction above the basement membrane were recorded with a step size of 0.5 µm and levels of colocalisation between PrPd and organelle markers analysed using imaging software Volocity. Data of at least 20 single cells were analysed using the Volocity cropping function. Cells were double-labelled with primaries for 24 h and washed with PBS. Cells were subsequently labelled with highly cross-adsorbed fluorescence-conjugated secondary antibodies (AffiniPure, Jackson ImmunoResearch) for 24 h. Levels of colocalisation were determined by Pearson’s correlation with Costes threshold correction73 according to the specification of Perkin Elmer, the manufacturer of Volocity. To examine whether mβCD extracts PrPc from the plasma membrane, we treated confluent layers of uninfected cells with 0.4 to 2 mM mβCD for 16 h at 37 °C. Cells where then fixed and labelled with anti-PrP mAb 8H4, followed by AF488-conjugated secondary antibody. Prior to imaging, cells were permeabilised with 0.04% TX-100 and labelled with DAPI for 15 min to reveal nuclei. Changes in fluorescence intensities of total PrPc were determined as described above.
Validating the specificity of anti-PrP antibodies
To validate the target specificity of anti-PrP antibodies, we generated Prnp knockout cells. Briefly, the Prnp open reading frame (ORF) in N2a cells was deleted by CRISPR-Cas9 according to Ran et al.74. Single guide RNAs (sgRNAs) were selected using the web-based “sgRNA designer” from the Broad Institute and cloned into the Addgene vector pX459 (Ref: 62988) as specified below. Out of four sgRNAs tested for their efficacy of Prnp knockdown, sgRNA-PrnP−69, which recognises the complementary Prnp sequence 53-ATGTCGGCCTCTGCAAAAAG-72 and enables a Cas9-mediated double-strand break at position 69 was found to be superior. To synthesise sgRNA-PrnP−69, two oligonucleotides (fw-5′-CACCGATGTCGGCCTCTGCAAAAAG-3′ and rev-5′-AAACCTTTTTGCAGAGGCCGACATC−3′), flanked by the NGG PAM sequence with overhangs (underlined) were annealed and cloned into the BpI-digested vector pX459 using T4-polynucleotide kinase ligase (New England Biolabs, Hitchin, UK) according to the specification of the manufacturer. To generate Prnp knockout cells, pX459/gRNA-PrnP−69 was transfected into N2a cells using Lipofectamine LTX/Plus (Thermo Fisher Scientific) as recommended by the manufacturer and cells were selected with 2 μg puromycin per ml of medium.
Cloning of Myc-tagged PrP variants
Double stranded DNA molecules of the full-length mouse Prnp gene (Genbank NP_001265185.1) with Myc tag (aa’s EQKLISEEDL), inserted at positions p.Gly45, p.Gly70 and p.Glu90 were synthesised by GeneArt (Thermo Fisher Scientific, UK). Corresponding Prnp fragments (10 ng) were Gibson assembled by overlap extension using GeneArt Gibson Assembly HiFi MM (Thermo Fisher Scientific, Regensburg, Germany) into the linearised pLNXC2 vector (25 ng) (Addgene, Teddington, UK). Vector and inserts at a 1:1 molar ratio were incubated for 1 h on a thermocycler (PCRmax, East Sussex, UK), transformed at 37 °C for 1 h in One Shot TOP10 chemically competent E.Coli cells (Thermo Fisher Scientific, UK) and then plated onto LB agar plates, containing 50 µg/ml ampicillin (Sigma, UK). After overnight culture, positive plasmids were digested with restriction enzymes and analysed by gel electrophoresis to confirm that the insert is in the correct position prior to Sanger sequencing (Eurofins Genomics, Ebersberg, Germany). Mouse Prnp, tagged at Gly30 with myc, containing Not1 and Xho1 restriction sites was obtained from GeneArt Life Technology. Cloning into the linearised pLNXC2 vector was done as described above. Transformation of competent JS4 cells was carried out in presence of ampicillin overnight. Colonies were screened by PCR to identify those with inserts. Plasmid DNA was extracted and digested and the sequence of Gly30-myc Prnp was confirmed by Sanger sequencing.
For retroviral production, HEK-293 derivative Phoenix cells were seeded at a concentration of 1 × 106 cells per 10 cm plate in producer cell growth media (DMEM, 10% FCS, 1% Penicillin-Streptomycin, 1% Glutamine), 24 h before transfection with 3 µg Prnp Myc positive constructs and 12 µl Fugene HD (Promega, UK) as per manufacturer’s instructions. After 24 h the medium was replaced with fresh growth medium and supernatants were collected at 2 days after transfection. After filtration of supernatants through 0.45 µm filters (Acrodisc Syringe filters, Cat# 4219, PALL Europe, Portsmouth, UK) and supplementation of polybrene (8 µg/ml), PK1-10 Si8 double-knockdown cells13 were transduced and incubated at 37 °C for 4–5 h before replacing with complete media (Optimem, 10% FCS, 1% Penicillin-Streptomycin). In the next split the cells were cultured under subsequent selection markers G418 (200 µg/ml, ThermoFisher, UK) and Puromycin (2 µg/ml, Invitrogen, UK) and passaged at least 5 times in presence of antibiotics. A control with empty pLNXC2 vector was set up in parallel experiments to monitor the efficacy of antibiotic selection.
Assessing prion susceptibility of myc-tagged Prnp
To investigate the permissiveness of myc-Prnp-expressing cell lines at position p.Gly30, p.Gly45, p.Gly70 and p.Glu90 to mouse-adapted prion strains, cells were plated out in 96-well culture plates at a concentration of 1 × 105 cells/ml OFCS. The next day, the culture medium was replaced with 300 µl OFCS, containing 10−4 dilutions of RML (10%, w/v) and CD1 (10%, w/v) for mock infections and left until cells were sub-confluent to undergo a split. At passages 3 and 4, respectively, 100 µl of a 1:10 dilution of cells, resuspended in PBS were transferred onto wells of 96-well Elispot plates (PerkinElmer, UK) to determine the number of PrPSc-infected cells using Scrapie Cell Assay, essentially as described previously71.
Infection and labelling of myc PrP-expressing cells
For immunofluorescence-based detection of myc-tagged PrPd aggregates, 500 µl aliquots of 5 × 104 cells per ml OFCS were seeded into 8-well chamber slides (Thermo Fisher Scientific, UK). After 16 h, cells were infected with prion-containing media, i.e. RML-prion and uninfected CD1 homogenates or exosome-enriched cell supernatants from persistently infected or uninfected S7 cells. Twenty four hours following cell plating, the culture medium was replaced with 500 µl OFCS, supplemented with a 1 × 10−4 dilution of 10% brain homogenates (RML and CD1 mock) or 30 µl exosomes per ml medium. To examine de novo PrP conversion following prion infection, myc PrP-expressing cells were grown for 3 days prior to infection with brain homogenates or exosome-enriched supernatants as above. Prion-containing solutions were removed by washing with PBS after various time points as specified in Figs. 6 and 7 and cells were fixed with 4% formalin for 15 min at room temperature. Cells were then incubated with ice cold acetone for 1 min, followed by denaturation with 3.5 M GTC for 10 min at room temperature. The cells were incubated with anti-PrP mAb 6D11 (1:10,000 BioLegend) and directly conjugated mAb AF488 anti-myc 9E10 (1:1000 Millipore, UK) in Superblock/PBS antibody diluent containing 5% FBS and left overnight at 4 °C. AffiniPure Rhodamine Red goat anti-mouse IgG2a subclass secondary antibody was used at a 1:1,000 dilution overnight at 4 °C. All images were captured on a Zeiss LSM 700 confocal microscope, equipped with a 63 × oil objective at the same acquisition settings.
Identification of de novo PrP conversion sites
To investigate the cellular sites of de novo PrP conversion in G70 PrP-expressing cells, threshold levels for the detection of myc-tagged PrPc were first determined using mock-infected cells and PMT gains were adjusted to exclude background fluorescence. The same settings were then applied to examine the sites of de novo PrP conversion in freshly prion-infected cells at the time points specified in Fig. 6C, D. All cells were co-labelled with the discriminatory mAb 6D11 to increase the confidence of scoring de novo infected cells. Only double-labelled cells were scored as shown in representative images (Fig. 6C). All experiments were scored independently by three investigators and at least 50 myc-PrPd positive cells were analysed for each condition.
Quantifying infected myc Prnp cells
To determine the proportion of infected G70 PrP-expressing cells at early time points after prion infection, three separate automated scans of 9 × 9 tiles with approximately 40 cells per tile were set up for every condition with a 5% overlap among tiles. Background levels were adjusted as specified above. For cell phenotypic analysis, cells double-positive for anti-myc and 6D11 were manually scored based on fluorescence intensity using Zen black 2.3SP1. The total number of cells was determined by scoring DAPI-positive nuclei using Volocity software. To determine the percentage of 5B2-positive cells (Fig. 7B), tile scans of 9 × 9 tiles were acquired and cells were scored based on the detection of anti-5B2-positive PrPd at the plasma membrane.
Transcriptional gene silencing
Transient knockdown of gene expression was conducted by reverse transfection of cells using siRNA pools (siTOOLs Biotech GmbH, Martinsried, Germany) which contain up to 30 siRNAs to achieve maximal transcript coverage and highly efficient gene knockdown. Briefly, siRNA pools were reconstituted with nuclease-free water to a stock concentration of 10 µM. Two µl siPool and 6 µl RNAimax transfection reagent were added to 42 µl FBS-free OPTIMEM and incubated to form a complex. After 5 min, 950 µl OFCS were added to the RNA complex and 1 ml of 1.33 × 105 cells (reverse transfection) was added for a final concentration of 6.7 × 105 cells/ml.
Quantifying gene knockdown levels
We used real-time quantitative PCR (RT-qPCR) to determine the level of gene expression in cells, following transcriptional silencing. Briefly, total RNA was isolated 72 hrs after transfection of cells with siPools and a non-targeting control using Direct-zol RNA Mini-prep kit (Cambridge Bioscience, Cambridge, UK). Genomic DNA was removed during RNA isolation as per manufacturer’s instructions. RNA purity and concentration was measured spectrophotometrically (Nanodrop 2000, Thermo Fisher Scientific, USA). Total RNA (500 ng) was used to synthesise the first strand complementary DNA (cDNA) using QuantiTect Reverse Transcription kit (Qiagen, Manchester, UK). All real-time qPCR reactions were carried out with QuantStudio 12 K Flex (Applied Biosystems, Cheshire, UK) with the following cycling parameters: 50 °C, 2 mins; 94 °C, 15 min; 40 cycles at 94 °C, 15 s; 60 °C, 1 min. Samples were set up in triplicates in 10 μl reactions containing QuantiTect SYBR Green (Qiagen), 1x QuantiTect customised Primer Assay (lyophilised in TE, pH 8.0), 25 ng of cDNA made to a final volume with ddH20. Gapdh and Actb were used as endogenous controls for normalising target gene expression levels. Data acquisition of the fluorescent signal was performed at the end of the run to assess the expression of mRNA by evaluating threshold cycle (CT) values. Double delta CT calculations were measured as logarithm and then converted to fold change of 100, after untreated control levels were subtracted from target levels.
Cell lysis and proteinase K digestion
Uninfected and prion-infected S7 cells were grown to confluency in 6-well plates and treated with 0.5 mM BafA1 or DMSO (0.05%) for 1 h. Cells were washed with OFCS and further incubated for 16 h. Cells were then lysed in RIPA buffer (50 mM Tris, 150 mM sodium chloride, 0.5% sodium deoxycholate, 1% Triton (v/v), pH 7.4) on ice for 15 min. Lysates (100 µg total protein) were incubated with 10 µg/ml proteinase K (PK, Merck, 539480) for 30 min at 37 °C. Protein digestion was stopped with 10 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) for 10 min on ice. Lysates were mixed with NuPAGE LDS sample buffer (4X) (Thermo Fisher Scientific, NP0007) and boiled for 10 min.
Western blotting
Fifty micrograms of total protein or protein marker SeeBlue2 (Invitrogen) were separated on 12% Bis-tris gels (NuPage, Thermo Fisher Scientific, UK) at 200 V for 90 min. Proteins were then transferred onto methanol-activated PVDF membranes (Immobilon-P, Millipore) at 15 V overnight. Blots were incubated in blocking buffer (5% skimmed milk powder (Sigma Aldrich), 0.05% Tween-20, PBS) for 1 h at room temperature. Blots were then incubated with 0.15 µg anti-PrP antibody ICSM35 (D-Gen Ltd, MRC Prion Unit at UCL, London, UK) per ml blocking buffer for 1 h. After washing, blots were incubated with AP-conjugated anti-mouse IgG (Fab) antibody (A2179, Sigma Aldrich) for 1 h at room temperature. After at least five washes in PBST, blots were incubated twice in activation buffer (20 mM Tris, 1 mM MgCl2, pH 9.8) for 5 min. Blots were incubated with Tropix CDP-Star chemiluminescent substrate (CDP-Star, Thermo Fisher Scientific, T2147) and revealed on Amersham Hyperfilm ECL (Cytiva, 28906836). To control for equal loading, blots were stripped with Restore Plus Western blot stripping buffer (Thermo Fisher Scientific, 46430) and reprobed with anti-mouse actin antibody (Sigma Aldrich, A5441).
Immunoprecipitation
Prion-infected S7 cells were grown to confluency on 15 cm dishes for 5 days with half medium changes on days three and four. Cells were washed with PBS, 10 ml sterile distilled water was added and cells were incubated for 15 min at room temperature, followed by thorough resuspension under microscopic control. The cells were discarded and 1.5 ml ice-cold RIPA buffer was added to the decellularised ECM, followed by vigorous resuspension. The lysate was collected and centrifuged at 1000 × g for 5 min at 4 °C. In parallel experiments confluent dishes of prion-infected S7 cells were washed with PBS and lysed with 1.5 ml of RIPA buffer for 15 min. All lysates were centrifuged for 5 min at 1000 × g at 4 °C. For immunoprecipitation, 1 µg anti-PrP antibody ICM18 (D-Gen Ltd, MRC Prion Unit at UCL, London, UK) and 50 µl µMACS Protein G Microbeads (Miltenyi Biotech, 130-071-101) were added to 300 µl lysates. Following incubation on ice for 30 min, immune complexes were separated magnetically on µ columns essentially as specified by the manufacturer Miltenyi Biotech. The immunoprecipitates were eluted from µ columns with boiling 1 x LDS sample buffer. Western blots were conducted as described in “Western blot”. Following blocking in 5% milk for 1 h, membranes were briefly washed twice in PBST and incubated with biotin-conjugated ICM35 (D-Gen). After washing, membranes were incubated with avidin/biotin ABC complex (Vectastain ABC-HRP, Vector Laboratories, Peterborough, UK). All antibodies were incubated in 3% BSA/PBST for 1 h and revealed with ECL substrate SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, 34579).
Lysosome isolation
Lysosomes were isolated by Lamp1 immunoprecipitation according to Stahl-Meyer et al.75 with minor modifications. Briefly, uninfected and prion-infected S7 cells were grown to confluency in 15 cm dishes and treated with 0.5 mM BafA1 or DMSO (0.05%) for 1 h. Cells were washed with OFCS and further incubated for 16 h. Cells were then washed with cold PBS, resuspended in 10 ml PBS and centrifuged at 500 x g for 5 min at 4 °C. Cell pellets were resuspended in isolation buffer (10 mM HEPES, 70 mM sucrose, 210 mM mannitol, pH 7.5) and centrifuged at 1000 x g for 5 min at 4 °C. This wash step was repeated once more. Cells were then lysed in 700 µl isolation buffer, supplemented with 2.5 U Benzonase/ml, 0.5 mM Tris(2-carboxyethyl)phosphine hydrochloride and 7 µl Halt Protease and phosphatase inhibitor (Cat# 78440, Thermo Fisher Scientific) by syringing the cells 15 times through a 25-gauge needle and centrifuged at 1500 x g for 10 min at 4 °C. The supernatant was carefully collected and centrifuged again at 1500 x g for 10 min at 4 °C. The protein concentration of post-nuclear supernatants was determined by Pierce BCA assay (Cat#: 23225, Thermo Fisher Scientific). Equal amounts of protein were incubated with 3 µl polyclonal Lamp1 antibody (ab24170, Abcam), pre-bound on 50 µl Miltenyi µMACS protein G beads (Cat# 130-071-101, Miltenyi Biotech) and shaken overnight at 4 °C. MACS columns were rinsed with isolation buffer and mounted onto a magnetic separator. The immuno-precipitate was captured on MACS columns, washed with isolation buffer and eluted with pre-heated SDS sample buffer. For on-column PK digestion, enriched lysosomes were incubated with 10 µg PK/ml PBS. MACS columns were sealed with parafilm and digested for 30 min at 37 °C. Protein was eluted with pre-heated SDS sample buffer.
Statistics
Data was analysed using SPSS, Graphpad Instat 3.10 and Microsoft Excel. The Kolmogorov-Smirnov test was applied to test whether experimental data were normally distributed. Normally distributed data was analysed by parametric tests, including Student’s t-test and Analysis of variance (ANOVA) followed by post-hoc Bonferroni test. Unless otherwise stated, statistical tests were two-sided. In case of small sample sizes ( < 20) or data that did not fit the normal distribution, Kruskal–Wallis and Mann–Whitney U test were employed, followed by the Dunn’s multiple range test. Unless otherwise stated, a p-value < 0.01 was considered statistically significant. Where technical replicates were pooled across independent experiments, data was normalised within each data set. Boxplot elements in Figs. 2B and 5C are defined as following: The line within the box represents the median, the bounds of the box represent the interquartile range, and the whiskers represent the minimum and maximum values no more than 1.5 times the interquartile range. Where the minima and maxima lie outside the whiskers they are represented as dots.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This study was funded by the UK Medical Research Council (MRC) and by the UK Biotechnology and Biological Sciences Research Council (BBSRC); S. A. T. was supported by The Francis Crick Institute which receives its core funding from Cancer Research UK (FC001187), the UK Medical Research Council (FC001187). This research was funded in whole, or in part, by the Wellcome Trust (FC001187). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. We are grateful to Ms Parineeta Arora and Prof Parmjit Jat for providing PK1-10 Si8 double-knockdown cells and a Gly30-myc Prnp expression clone. We thank Christian Schmidt, Parvin Ahmed and George Thirlway for technical assistance with Automated Scrapie Cell Assays and the staff of the MRC Prion Unit at UCL Biological Services Facility for animal care and technical assistance. We are grateful to Prof John Collinge for helpful discussion.
Author contributions
Conceptualisation, P.C.K. and S.A.T.; Methodology, J.M.R., M.P., H.H., A.B. and P.C.K.; Validation, J.M.R., M.P., H.H. and P.C.K.; Investigation, J.M.R, M.P., H.H. and P.C.K; Resources, J.M.R., M.P., P.C.K.; Writing – original draft, P.C.K.; Writing – review & editing, S.A.T., J.M.R., M.P. and P.C.K.; Visualisation, P.C.K.; Project administration, P.C.K.; supervision, P.C.K.; Funding acquisition, P.C.K.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Data supporting the findings of this study are presented within the article and supplementary information and are available from the corresponding author upon request. The data underlying Figs. 1G, 2B, 3D, F, G, 4G, J–K, 5A–C, E, G–J, 6B and 7A–C and Supplementary Figs. 5C, F, H–O and 7A–B are provided in the Source Data file. The composition of siPools and primer sequences of qPCR assay is proprietary information by siTOOLs Biotech GmbH and Qiagen, respectively. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Juan Manuel Ribes, Mitali P. Patel.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43961-1.
References
- 1.Griffith JS. Self replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Scrapie prions. Annu Rev. Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner, S. B. Molecular biology and genetics of prion diseases. Cold Spring Harb. Symp. Quant. Biol.61, 473–493 (1996). [PubMed]
- 4.Weissmann C, et al. The role of PrP in pathogenesis of experimental scrapie. Cold Spring Harb. Symp. Quant. Biol. 1996;61:511–522. doi: 10.1101/SQB.1996.061.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi M, et al. Generation of monoclonal antibody that distinguishes PrPSc from PrPC and neutralizes prion infectivity. Virology. 2009;394:200–207. doi: 10.1016/j.virol.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Marijanovic, Z., Caputo, A., Campana, V. & Zurzolo, C. Identification of an Intracellular Site of Prion Conversion. PLoS Pathog.5, e1000426 (2009). [DOI] [PMC free article] [PubMed]
- 7.Yim, Y. I. et al. The multivesicular body is the major internal site of prion conversion. J. Cell Sci.128, 1434–1443 (2015). [DOI] [PMC free article] [PubMed]
- 8.Caughey B, Raymond GJ, Ernst D, Race RE. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 1991;65:6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borchelt DR, Taraboulos A, Prusiner SB. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 1992;267:16188–16199. doi: 10.1016/S0021-9258(18)41985-0. [DOI] [PubMed] [Google Scholar]
- 10.Magalhaes AC, et al. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barmada SJ, Harris DA. Visualization of prion infection in transgenic mice expressing green fluorescent protein-tagged prion protein. J. Neurosci. 2005;25:5824–5832. doi: 10.1523/JNEUROSCI.1192-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dron, M., Dandoy-Dron, F., Salamat, M. K. & Laude, H. Proteasome inhibitors promote the sequestration of PrPSc into aggresomes within the cytosol of prion-infected CAD neuronal cells. J. Gen. Virol. 90, 2050–2060 (2009). [DOI] [PubMed]
- 13.Goold R, et al. Rapid cell-surface prion protein conversion revealed using a novel cell system. Nat. Commun. 2011;2:281. doi: 10.1038/ncomms1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouvinski A, et al. Live imaging of prions reveals nascent PrPSc in cell-surface, raft-associated amyloid strings and webs. J. Cell Biol. 2014;204:423–441. doi: 10.1083/jcb.201308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki T, et al. Characterization of intracellular localization of PrP(Sc) in prion-infected cells using a mAb that recognizes the region consisting of aa 119-127 of mouse PrP. J. Gen. Virol. 2012;93:668–680. doi: 10.1099/vir.0.037101-0. [DOI] [PubMed] [Google Scholar]
- 16.DeArmond SJ, et al. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985;41:221–235. doi: 10.1016/0092-8674(85)90076-5. [DOI] [PubMed] [Google Scholar]
- 17.Wenborn A, et al. A novel and rapid method for obtaining high titre intact prion strains from mammalian brain. Sci. Rep. 2015;5:10062. doi: 10.1038/srep10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry, C. et al. Ex vivo mammalian prions are formed of paired double helical prion protein fibrils. Open Biol.6, 160035 (2016). [DOI] [PMC free article] [PubMed]
- 19.Bradbury ARM, et al. When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. MAbs. 2018;10:539–546. doi: 10.1080/19420862.2018.1445456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taraboulos A, Serban D, Prusiner SB. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 1990;110:2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veith, N. M., Plattner, H., Stuermer, C. A., Schulz-Schaeffer, W. J. & Burkle, A. Immunolocalisation of PrP(Sc) in scrapie-infected N2a mouse neuroblastoma cells by light and electron microscopy. Eur. J. Cell Biol. 88, 45–63 (2008). [DOI] [PubMed]
- 22.Serban D, Taraboulos A, DeArmond SJ, Prusiner SB. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990;40:110–117. doi: 10.1212/WNL.40.1.110. [DOI] [PubMed] [Google Scholar]
- 23.Yin, S. et al. Prion proteins with insertion mutations have altered N-terminal conformation, increased ligand-binding activity and are more susceptible to oxidative attack. J. Biol. Chem. 281, 10698–10705 (2006). [DOI] [PubMed]
- 24.Mange A, et al. Alpha- and beta- cleavages of the amino-terminus of the cellular prion protein. Biol. Cell. 2004;96:125–132. doi: 10.1016/j.biolcel.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Lewis V, et al. Increased proportions of C1 truncated prion protein protect against cellular M1000 prion infection. J. Neuropathol. Exp. Neurol. 2009;68:1125–1135. doi: 10.1097/NEN.0b013e3181b96981. [DOI] [PubMed] [Google Scholar]
- 26.Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol. Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- 27.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl Acad. Sci. USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghaemmaghami S, et al. Cell division modulates prion accumulation in cultured cells. Proc. Natl Acad. Sci. USA. 2007;104:17971–17976. doi: 10.1073/pnas.0708372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man WK, et al. The docking of synaptic vesicles on the presynaptic membrane induced by a-synuclein is modulated by lipid composition. Nat. Commun. 2021;12:927. doi: 10.1038/s41467-021-21027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Xue R, Ong WY, Chen P. Roles of cholesterol in vesicle fusion and motion. Biophys. J. 2009;97:1371–1380. doi: 10.1016/j.bpj.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis V, Hooper NM. The role of lipid rafts in prion protein biology. Front. Biosci. 2011;16:151–168. doi: 10.2741/3681. [DOI] [PubMed] [Google Scholar]
- 32.Marshall, K. E. et al. PrP knockout cells expressing transmembrane PrP resist prion infection. J. Virol.91, e01686-16 (2017). [DOI] [PMC free article] [PubMed]
- 33.Kobayashi, A. et al. Ganglioside synthase knock-out reduces prion disease incubation time in mouse models. Am. J. Pathol. 189, 677–686 (2019). [DOI] [PubMed]
- 34.Shyng S-L, Moulder KL, Lesko A, Harris DA. The N-terminal domain of a glycolipid-anchored prion protein is essential for its endocytosis via clathrin-coated pits. J. Biol. Chem. 1995;270:14793–14800. doi: 10.1074/jbc.270.24.14793. [DOI] [PubMed] [Google Scholar]
- 35.Sunyach C, et al. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 2003;22:3591–3601. doi: 10.1093/emboj/cdg344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe S, Boucrot E. Fast and ultrafast endocytosis. Curr. Opin. Cell Biol. 2017;47:64–71. doi: 10.1016/j.ceb.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 38.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Hill TA, et al. Inhibition of dynamin mediated endocytosis by the dynoles–synthesis and functional activity of a family of indoles. J. Med. Chem. 2009;52:3762–3773. doi: 10.1021/jm900036m. [DOI] [PubMed] [Google Scholar]
- 40.Declercq J, Meulemans S, Plets E, Creemers JW. Internalization of proprotein convertase PC7 from plasma membrane is mediated by a novel motif. J. Biol. Chem. 2012;287:9052–9060. doi: 10.1074/jbc.M111.306407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grinstein S, Rotin D, Mason MJ. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim. Biophys. Acta. 1989;988:73–97. doi: 10.1016/0304-4157(89)90004-X. [DOI] [PubMed] [Google Scholar]
- 42.Cerny J, et al. The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2004;5:883–888. doi: 10.1038/sj.embor.7400243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannus M, et al. siPools: highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res. 2014;42:8049–8061. doi: 10.1093/nar/gku480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki T, Baron GS, Suzuki A, Hasebe R, Horiuchi M. Characterization of intracellular dynamics of inoculated PrP-res and newly generated PrP(Sc) during early stage prion infection in Neuro2a cells. Virology. 2014;450-451:324–335. doi: 10.1016/j.virol.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 46.Couzin J. Cell biology - the ins and outs of exosomes. Science. 2005;308:1862–1863. doi: 10.1126/science.308.5730.1862. [DOI] [PubMed] [Google Scholar]
- 47.Fevrier B, et al. Cells release prions in association with exosomes. Proc. Natl Acad. Sci. USA. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahn R. The octapeptide repeats in mammalian prion protein constitute a pH-dependent folding and aggregation site. J. Mol. Biol. 2003;334:477–488. doi: 10.1016/j.jmb.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 49.Frankenfield KN, Powers ET, Kelly JW. Influence of the N-terminal domain on the aggregation properties of the prion protein. Protein Sci. 2005;14:2154–2166. doi: 10.1110/ps.051434005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevitt CR, et al. N-terminal domain of prion protein directs its oligomeric association. J. Biol. chem. 2014;289:25497–25508. doi: 10.1074/jbc.M114.566588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chernoff YO, Lindquist SL, Ono B, Inge Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 52.Shorter J, Lindquist S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science. 2004;304:1793–1797. doi: 10.1126/science.1098007. [DOI] [PubMed] [Google Scholar]
- 53.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanni I, et al. Isolation of infectious, non-fibrillar and oligomeric prions from a genetic prion disease. Brain. 2020;143:1512–1524. doi: 10.1093/brain/awaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris DA, et al. Processing of a cellular prion protein: identification of N- and C-terminal cleavage sites. Biochemistry. 1993;32:1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- 56.Chen SG, et al. Truncated forms of the human prion protein in normal brain and in prion diseases. J. Biol. Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 57.Zanusso, G. et al. Identification of distinct N-terminal truncated forms of prion protein in different Creutzfeldt-Jakob disease subtypes. J. Biol. Chem.279, 38936–42 (2004). [DOI] [PubMed]
- 58.Laffont-Proust I, et al. Truncated PrP(c) in mammalian brain: interspecies variation and location in membrane rafts. Biol. Chem. 2006;387:297–300. doi: 10.1515/BC.2006.039. [DOI] [PubMed] [Google Scholar]
- 59.Parchi, P., Strammiello, R., Giese, A. & Kretzschmar H. Phenotypic variability of sporadic human prion disease and its molecular basis: past, present, and future. Acta Neuropathol. 121, 91–112 (2011). [DOI] [PubMed]
- 60.Horiuchi M, Caughey B. Prion protein interconversions and the transmissible spongiform encephalopathies. Struct. Fold. Des. 1999;7:R231–R240. doi: 10.1016/S0969-2126(00)80049-0. [DOI] [PubMed] [Google Scholar]
- 61.Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr. Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 62.Maji SK, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nespovitaya N, et al. Dynamic assembly and disassembly of functional beta-endorphin amyloid fibrils. J. Am. Chem. Soc. 2016;138:846–856. doi: 10.1021/jacs.5b08694. [DOI] [PubMed] [Google Scholar]
- 64.Jacob RS, et al. Amyloid formation of growth hormone in presence of zinc: relevance to its storage in secretory granules. Sci. Rep. 2016;6:23370. doi: 10.1038/srep23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mallucci G. R. Prion neurodegeneration: Starts and stops at the synapse. Prion3 (2009). [DOI] [PMC free article] [PubMed]
- 66.Marbiah, M. M. et al. Identification of a gene regulatory network associated with prion replication. EMBO J.33, 1527–47 (2014). [DOI] [PMC free article] [PubMed]
- 67.Philiastides, A. et al. A new cell model for investigating prion strain selection and adaptation. Viruses10.3390/v11100888 (2019). [DOI] [PMC free article] [PubMed]
- 68.Ramberger M, et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain. 2020;143:1731–1745. doi: 10.1093/brain/awaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill AF, et al. Molecular classification of sporadic Creutzfeldt-Jakob disease. Brain. 2003;126:1333–1346. doi: 10.1093/brain/awg125. [DOI] [PubMed] [Google Scholar]
- 70.Castro-Seoane, R. et al. Plasmacytoid dendritic cells sequester high prion titres at early stages of prion infection. Plos Pathog.8, e1002538 (2012). [DOI] [PMC free article] [PubMed]
- 71.Klohn P, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl Acad. Sci. USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt, C. et al. A systematic investigation of production of synthetic prions from recombinant prion protein. Open Biol5, 150165 (2015). [DOI] [PMC free article] [PubMed]
- 73.Costes SV, et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stahl-Meyer, J., Holland, L. K. K., Liu, B., Maeda, K. & Jäättelä M. Lysosomal changes in mitosis. Cells11, 875 (2022). [DOI] [PMC free article] [PubMed]
- 76.Riek R, et al. NMR structure of the mouse prion protein domain PrP (121-231) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are presented within the article and supplementary information and are available from the corresponding author upon request. The data underlying Figs. 1G, 2B, 3D, F, G, 4G, J–K, 5A–C, E, G–J, 6B and 7A–C and Supplementary Figs. 5C, F, H–O and 7A–B are provided in the Source Data file. The composition of siPools and primer sequences of qPCR assay is proprietary information by siTOOLs Biotech GmbH and Qiagen, respectively. Source data are provided with this paper.