Abstract
Background
Amphotericin B is the gold standard treatment for severe mycoses. A new orally delivered, less-toxic formulation of amphotericin has been developed.
Methods
In our randomized clinical trial, we tested oral lipid nanocrystal (LNC) amphotericin B (MAT2203, Matinas Biopharma) vs intravenous (IV) amphotericin for human immunodeficiency virus–associated cryptococcal meningitis in 4 sequential cohorts. Two pilot cohorts assessed safety and tolerability (n = 10 each), and 2 cohorts assessed efficacy with/without 2 IV loading doses (n = 40 each). The experimental arm received 1.8 g/d oral LNC amphotericin through 2 weeks with 100 mg/kg/d flucytosine, then 1.2 g/d LNC amphotericin through 6 weeks. The randomized control arm (n = 41) received 7 days of IV amphotericin with flucytosine, then 7 days of fluconazole 1200 mg/d. The primary end point was cerebrospinal fluid (CSF) early fungicidal activity (EFA).
Results
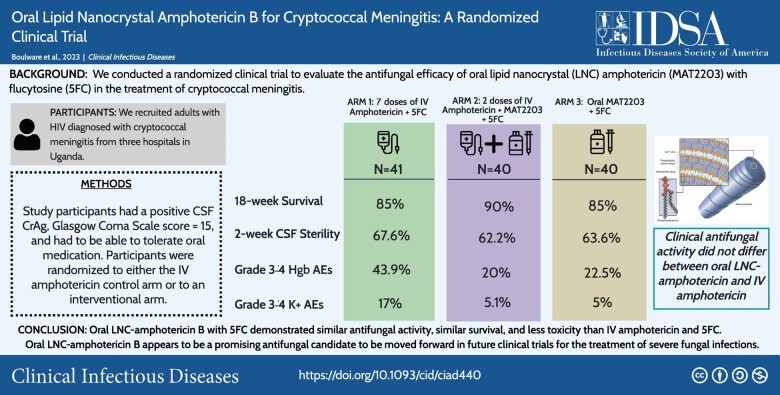
We randomized 80 participants to oral LNC amphotericin + flucytosine with (n = 40) and without (n = 40) 2 IV loading doses and 41 control participants to IV amphotericin + flucytosine. Mean EFA was 0.40 log10 colony-forming units (CFU)/mL/d for all-oral LNC amphotericin, 0.42 log10 Cryptococcus CFU/mL/d for oral LNC amphotericin with IV loading doses, and 0.46 log10 CFU/mL/d for IV amphotericin controls. LNC amphotericin groups achieved 2-week CSF sterility in 63% (44 of 70) vs 68% (23 of 34) of controls. The 18-week survival was 85% (34 of 40) with all-oral LNC amphotericin, 90% (36 of 40) with oral LNC amphotericin given IV loading doses, and 85% (35 of 41) with IV amphotericin.
Grade 3–4 laboratory adverse events occurred less frequently in LNC amphotericin groups (41%) than the IV amphotericin group (61%, P = .05), particularly for anemia (21% vs 44%; P = .01) and potassium (5% vs 17%; P = .04).
Conclusions
This new oral amphotericin B LNC formulation appears promising for cryptococcal meningitis with antifungal activity, similar survival, and less toxicity than IV amphotericin.
Clinical Trials Registration
Keywords: cryptococcal meningitis, HIV, amphotericin B, randomized controlled trial, AIDS-related opportunistic infection
A new oral amphotericin B lipid nanocrystal formulation appears promising for cryptococcal meningitis with similar quantitative antifungal activity in human CSF, similar 18-week survival, and less toxicity than intravenous amphotericin B in a randomized phase 2 trial.
Graphical Abstract
Graphical Abstract.
Human immunodeficiency virus (HIV)–associated cryptococcal disease causes 15%–19% of AIDS-related mortality globally [1, 2]. The standard induction antifungal therapy for cryptococcal meningitis is intravenous (IV) amphotericin B plus flucytosine for 1–2 weeks or liposomal amphotericin at 10 mg/kg once [3].
However, IV amphotericin is associated with severe and potentially life-threatening toxicities such as kidney impairment, anemia, thrombophlebitis, and electrolyte abnormalities [4, 5]. One week of IV amphotericin was associated with frequent grade ≥3 adverse events (AEs), including kidney injury (6%), anemia (40%), and hypokalemia (7%) even with potassium supplementation [4]. Administering IV amphotericin is logistically challenging, requiring hospitalization and intensive laboratory monitoring.
A novel lipid nanocrystal (LNC) oral amphotericin B (MAT2203; Matinas Biopharma) has been developed as an alternative to IV-administered amphotericin. The LNC has 3 components: amphotericin B, calcium, and phosphatidylserine, which is a natural phospholipid derived from soy. When the LNC is administered, target cells (eg, macrophages) engulf and transport LNCs to sites of infection. The LNC structure protects against degradation in unfavorable environments (eg, acidic stomach pH) while allowing targeted intracellular delivery into macrophages and reticuloendothelial cells. Once the LNC drug is captured intracellularly, the low intracellular calcium concentration triggers nanocrystals to open, releasing the drug inside the cell. Because LNC amphotericin is locked up inside the solid nanocrystal particle or in target cells, the body is protected from amphotericin toxicity.
Lu et al demonstrated that orally administered LNC amphotericin colocalized to the brain within macrophages of Cryptococcus-infected mice by the third day of treatment [6]. Further, in murine cryptococcal meningoencephalitis, LNC amphotericin combined with flucytosine had efficacy equivalent to parenteral injected amphotericin B with flucytosine and without untoward toxicity [6]. A phase 1 trial found good tolerability and safety of LNC amphotericin. The most common AEs were dose-dependent nausea and mild abdominal pain [7].
We conducted an open-label, phase 2, randomized trial to evaluate the antifungal efficacy of oral LNC amphotericin in HIV-associated cryptococcal meningitis. We quantified the antifungal activity in humans at the site of infection using serial quantitative cerebrospinal fluid (CSF) cultures to calculate the rate of CSF Cryptococcus yeast clearance, termed “early fungicidal activity” (EFA) [8–10]. EFA has been used in multiple phase 2 trials over the past 2 decades to provide early supportive information on regimens that have been validated in phase 3 trials.
METHODS
Trial Design
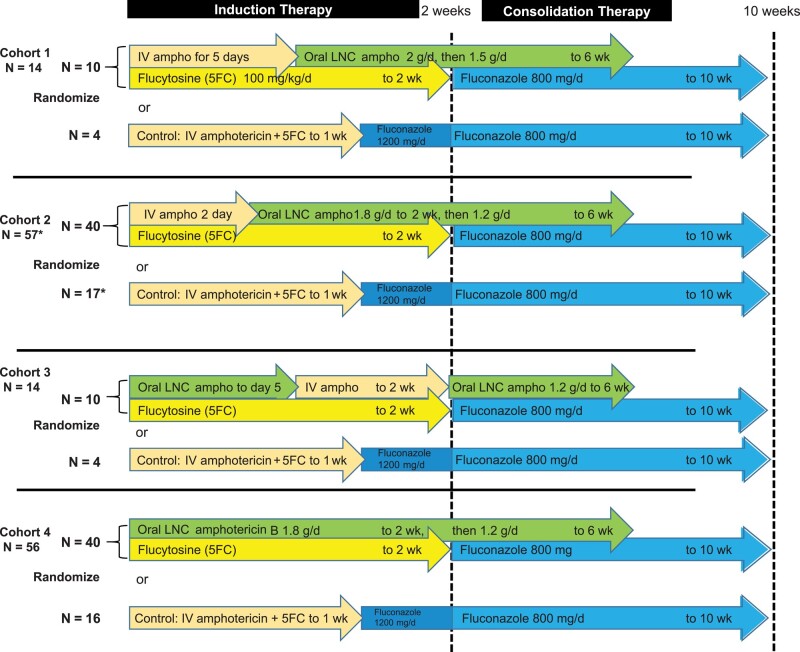
We conducted a randomized clinical trial to evaluate oral LNC amphotericin (MAT2203; Matinas BioPharma, Bedminster, NJ) combined with flucytosine compared with IV amphotericin B for induction therapy, combined with flucytosine. There were 4 sequential cohorts (Figure 1). Each cohort sequentially decreased the IV amphotericin duration and increased the oral LNC amphotericin duration using the maximum tolerated dose from our phase 1 trial [7]. In each cohort, we randomized participants 2.5-to-1 to an interventional arm or IV amphotericin control. IV amphotericin randomized controls were pooled across the 4 cohorts as 1 control group.
Figure 1.
Oral LNC amphotericin B randomized trial design. Cohort 1 was a pilot assessing tolerability and toxicity of oral lipid nanocrystal (LNC) amphotericin B (ampho) in an ill population with advanced human immunodeficiency virus disease and cryptococcal meningitis. After Cohort 1, investigators reduced the LNC amphotericin B dose from 333 mg per unit dose to 300 mg per unit dose with induction therapy at a 1.8 g daily total dose and consolidation therapy from week 2 to week 6 at a 1.2 g daily total dose. Cohort 3 was a safety pilot assessing an initial all-oral regimen with scheduled IV amphotericin B given from day 6 to day 14. Cohort 2 and cohort 4 assessed efficacy of a 6-week oral LNC amphotericin B regimen with or without 2 IV amphotericin B loading doses, respectively. Randomized controls were pooled across all 4 cohorts, receiving IV liposomal amphotericin B 3 mg/kg (n = 22) or IV amphotericin B deoxycholate 1 mg/kg (n = 19). Flucytosine was administered at 100 mg/kg/d in 4 divided doses. This 4-stage trial was designed to maximize participant safety using a new experimental therapy for a 100% fatal infection. *Randomization was stratified by first vs second episode of cryptococcosis, resulting in cohort 2 enrolling 1 more participant randomized to control (n = 57 in total). Abbreviations: 5FC, flucytosine; Ampho, amphotericin B; IV, intravenous; LNC, lipid nanocrystal.
Study Participants and Setting
We enrolled Ugandan adults with HIV and cryptococcal meningitis, as diagnosed by a positive CSF cryptococcal antigen lateral flow assay (IMMY, Norman, OK) [11]. We excluded participants who received >2 doses of IV amphotericin, were unable to take enteral medications, had altered mental status with a Glasgow coma scale score <15, were unlikely to attend clinic visits, were pregnant, were breastfeeding, were receiving chemotherapy, were receiving corticosteroids, had paradoxical immune reconstitution inflammatory syndrome, or had initiated HIV therapy less than 2 weeks previously. Participants with second-episode cryptococcosis (ie, culture-positive relapse) were eligible, based on similar survival outcomes as first-episode cryptococcosis [12]. Participants provided written informed consent.
We recruited participants at 3 hospitals in Uganda: Kiruddu National Referral Hospital and Mulago National Specialized Hospital in Kampala and Mbarara Regional Referral Hospital in Mbarara.
Intervention
Experimental group participants received oral LNC amphotericin and flucytosine 100 mg/kg/d. The LNC amphotericin dose was 2 g/d (333 mg × 6 doses) in cohort 1 through 2 weeks followed by 1.5 g/d through 6 weeks. Due to some gastrointestinal intolerance in cohort 1, we reduced the induction daily dose to 1.8 g/d (300 mg × 6 doses) and reduced the consolidation daily dose to 1.2 g/d (300 mg × 4 doses) from day 15 to week 6. This change was proposed to and approved by the study’s data and safety monitoring board. At 300-mg doses, gastrointestinal side effects were minimal; above 300 mg [7], side effects increased in frequency, with only 2 of 10 participants in cohort 1 tolerating all 333-mg doses over 10 days.
Randomized controls received IV liposomal amphotericin 3 mg/kg/d (AmBisome, Gilead Sciences, Foster City, CA) or IV amphotericin B deoxycholate 1.0 mg/kg/d plus flucytosine 100 mg/kg/d in 4 divided doses for 7 days, then fluconazole 1200 mg/d through 14 days, per 2018 World Health Organization cryptococcal guidelines [13]. Liposomal amphotericin B was reconstituted in 500 mL of 5% dextrose, filtered, and administered over 2 hours. Liposomal amphotericin became available in 2021. Amphotericin B deoxycholate was reconstituted in 500 mL of 5% dextrose and administered over 4 hours. Participants received 1 L of normal saline before any IV amphotericin plus 1 additional liter of intravenous fluid (5% dextrose or normal saline) after IV amphotericin. Participants received potassium and magnesium supplements daily when receiving IV amphotericin followed by 2 additional days [5].
Participants were hospitalized for a minimum of 12 days. After discharge, all participants received fluconazole 800 mg/d from day 15 to 10 weeks and 200 mg/d thereafter. We followed participants in clinic every 2 weeks through 10 weeks and thereafter every 4 weeks through 18 weeks. If participants missed clinic appointments, investigators performed follow-up either by telephone or in-person home visits. ART was initiated or switched at 6-week clinic visits in accordance with best practices [3, 14].
Safety-monitoring blood tests were routinely performed during the first 2 weeks and repeated at weeks 4 and 6. Lumbar punctures for quantitative CSF cultures were performed at diagnosis and days 3, 7, and 14 [15]. Participants with ongoing symptoms of increased intracranial pressure received additional therapeutic lumbar punctures. Participants with ongoing CSF culture growth at week 2 were offered repeat lumbar punctures at 4 weeks to document CSF sterility.
Outcomes
The primary trial end point was the EFA rate of CSF Cryptococcus clearance quantified by the change of log10 Cryptococcus colony-forming units (CFU)/mL CSF/d as measured by serial quantitative CSF fungal cultures over approximately 2 weeks [15].
Secondary end points included incidence of grade 3–5 laboratory AEs; incidence of clinical grade 3–5 AEs or serious AEs; tolerability, defined as the cumulative proportion of induction therapy doses received; 18-week survival time; 18-week hospital-free survival time among 2-week survivors; and additional IV amphotericin administered.
Randomization
Participants were randomized in a 2.5-to-1 ratio to either intervention or control groups, using a computer-generated, permuted block randomization with unequal size blocks of 2 or 4. The study pharmacist in Kampala kept the randomization sequence. Randomization was stratified by first-episode vs second-episode cryptococcal meningitis, resulting in n = 41 controls being enrolled.
Sample Size
This study was planned to enroll 140 participants. Cohorts 1 and 3 were designed as pilot safety cohorts with 10 interventional participants and 4 randomized controls. Cohorts 2 and 4 were designed to evaluate efficacy against previously established antifungal regimens by estimating the mean EFA.
An EFA benchmark of 0.20 log10 CFU/mL/d was selected based on previous studies, where EFAs below this threshold have increased mortality [16]. Assuming the EFA standard deviation is ±0.20 [17, 18] and the mean of EFA ≥0.28 log10 CFU/mL/d, a minimal sample size of 32 was required for a 95% confidence interval (CI) with lower bound >0.2 log10 CFU/mL/d, using 1-sample t distribution. Assuming 80% of participants would contribute data by having nonsterile baseline cultures, we chose 40-participant sample size cohorts to provide the statistical evidence that the mean EFA is >0.2 log10 CFU/mL/d.
Statistical Methods
The primary end point of CSF EFA was calculated for participants with at least 2 CSF cultures in the first 18 days. CSF culture time points were log10 transformed for analysis. Participants with sterile baseline CSF cultures did not contribute data. The primary analysis for EFA is based on simple linear regression models, as this approach has been reported in multiple cohorts [8–10, 16–18]. A simple linear regression model was fitted for each individual, using all feasible data points and excluding data points after CSF cultures were sterilized. Slopes from simple linear regression were considered the EFA estimates for each participant for statistical inference. As a sensitivity analysis, we used a linear mixed-effects regression model with random intercepts and random slopes for individual participants to account for the intrasubject correlation induced by repeated measures over time.
To analyze time-to-event end points, such as mortality and hospitalization-free survival, we utilized Kaplan–Meier estimates. We summarized categorical secondary end points with counts and proportions using the Fisher exact test for statistical inference. We summarized continuous end points using mean with 95% CIs or median (interquartile range [IQR]), as appropriate, and statistical inference using t test or Kruskal–Wallis test, respectively. We conducted analyses using SAS version 9.3 (SAS Institute, Cary, NC) and R version 3.6.0.
RESULTS
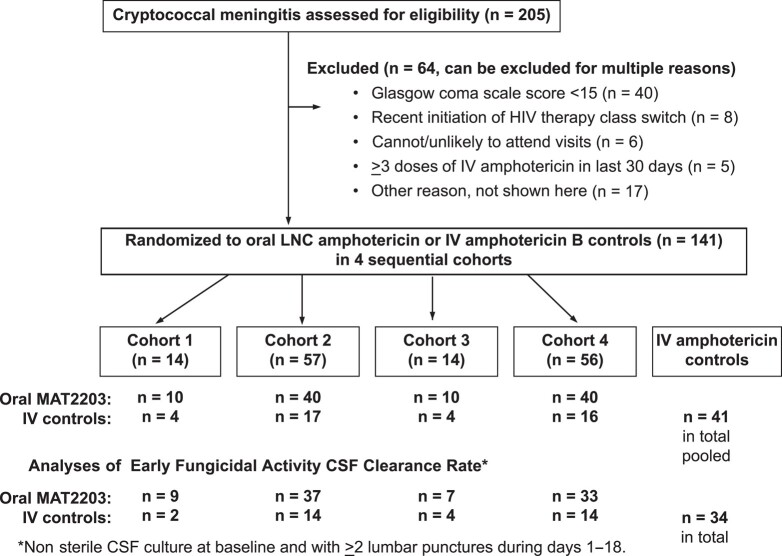
From 21 July 2020 through 12 October 2022, we screened 205 participants with cryptococcal meningitis. Of these participants, 141 met eligibility criteria and were randomized to LNC amphotericin (n = 100) or standard-of-care IV amphotericin (n = 41) across 4 cohorts (Figure 2). Follow-up was completed on 15 February 2023. Overall, the median age was 36 years (IQR, 31 to 42), 50% (70 of 141) were women, 67% (94 of 141) were ART–naive, and the median CD4 count was 30 cells/μL (IQR, 10 to 85). The median CSF quantitative culture was 6600 Cryptococcus CFU/mL (IQR, 65 to 75 860) with 85% (120 of 141) of participants having nonsterile CSF at baseline and surviving to contribute EFA data. Table 1 presents demographic and baseline CSF data by randomized group. Cohort 1 (n = 10) and cohort 3 (n = 10) were pilot safety cohorts, with data provided in the Supplementary Material.
Figure 2.
CONSORT (Consolidated Standards of Reporting Trials) diagram. Randomization occurred in a 2.5:1 ratio in 4 sequential cohorts, stratified by first (n = 139) vs second (n = 2) episode of cryptococcal meningitis. The randomized IV amphotericin B controls were pooled across the 4 cohorts with n = 41 in total as 1 pooled overall control group for comparison. Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IV, intravenous; LNC, lipid nanocrystal; MAT2203, lipid nanocrystal amphotericin B (Matinas Biopharma).
Table 1.
Demographics and Baseline Cerebrospinal Fluid Data of Randomized Trial Participants With Cryptococcal Meningitis
| Demographic | N | Oral LNC Amphotericin B With 2 IV Loading Doses | N | All-Oral LNC Amphotericin B | N | IV Amphotericin B Controls |
|---|---|---|---|---|---|---|
| Age, y | 40 | 35 [30–40] | 40 | 37 [32–43] | 41 | 39 [33–43] |
| Female | 40 | 20 (50%) | 40 | 18 (45%) | 41 | 21 (51%) |
| Weight, kg | 40 | 52 [45–58.5] | 40 | 50.5 [45–55] | 41 | 53 [46–58] |
| Receiving HIV therapy | 40 | 10 (25%) | 40 | 18 (45%) | 41 | 15 (37%) |
| Currently on tuberculosis therapy | 40 | 5 (12.5%) | 40 | 6 (15%) | 41 | 8 (19.5%) |
| Second episode cryptococcal meningitis | 40 | 0 (0%) | 40 | 0 (0%) | 41 | 2 (4.9%) |
| CD4 count, cells/μL | 37 | 25 [15–75] | 38 | 25 [9–75] | 39 | 27 [9–100] |
| Hemoglobin, g/dL | 40 | 11.5 [9.6–13.5] | 40 | 11.7 [10.1–13.5] | 40 | 11.8 [10.2–12.9] |
| Sodium, mmol/L | 40 | 135 [132–137] | 39 | 133 [128–137] | 40 | 134 [132–137] |
| Potassium, mmol/L | 40 | 4.3 [3.8–4.7] | 39 | 4.2 [3.8–4.4] | 40 | 4.4 [4.0–4.8] |
| Creatinine, mg/dL | 40 | 0.69 [0.55–0.84] | 39 | 0.83 [0.68–0.94] | 40 | 0.76 [0.63–0.90] |
| CSF opening pressure, cm H2O | 38 | 20 [12–36] | 40 | 13 [6–27] | 38 | 21 [12–30] |
| Cryptococcus log10 colony-forming units/mL CSF culture | 40 | 4.61 [1.62–5.11] | 40 | 3.58 [1.78–4.75] | 41 | 3.73 [1.85–4.79] |
| Sterile CSF culture | 40 | 3 (7.5%) | 40 | 7 (17.5%) | 41 | 7 (17%) |
Values are median [interquartile range] or N (%). Additional baseline demographics and laboratory values are provided in Supplementary Table 1.
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IV, intravenous; LNC, lipid nanocrystal.
Antifungal Activity
The primary end point was the CSF Cryptococcus clearance rate measured by longitudinal quantitative CSF cultures (Table 2). The cohort 2 oral LNC amphotericin given after 2 IV amphotericin loading doses had a mean EFA of 0.423 log10 Cryptococcus CFU/mL CSF/d (95% CI: .294 to .551). The IV amphotericin control group had a mean EFA of 0.455 (95% CI: .359 to .551) log10 CFU/mL/d. The mean EFA difference between groups was −0.032 (95% CI: −.196 to .131) log10 CFU/mL/d. Therefore, no clinical or statistical evidence indicated any difference in antifungal activity between the cohort 2 LNC amphotericin regimen and IV amphotericin.
Table 2.
Early Fungicidal Activity Comparison of Oral Lipid Nanocrystal Amphotericin vs Intravenous Amphotericin Controls
| CSF Clearance and Sterility Characteristic | Oral LNC Amphotericin B With 2 IV Loading Doses, Cohort 2 |
All-Oral LNC Amphotericin B, Cohort 4 |
IV Amphotericin B, Controls Cohorts 1–4 |
|---|---|---|---|
| Number randomized | 40 | 40 | 41 |
| Number contributing to EFA and sterility analyses | 37 | 33 | 34 |
| EFA by general linear modelsa | |||
| Maximum | 2.352 | 4.161 | 1.234 |
| 75th percentile | 0.518 | 0.379 | 0.645 |
| Median | 0.324 | 0.249 | 0.366 |
| 25th percentile | 0.216 | 0.195 | 0.260 |
| Minimum | 0.000 | 0.017 | 0.066 |
| EFA mean (95% CI) | 0.423 (.294 to .551) |
0.402 (.165 to .640) |
0.455 (.359 to .551) |
| EFA difference, mean (95% CI) | −0.032 (−.196 to .131) |
−0.053 (−.317 to .211) |
Reference |
| EFA mean (95% CI) outliers imputedb | 0.423 (.294 to .551) |
0.348 (.211 to .484) |
0.455 (.359 to .551) |
| EFA by mixed model slope (95% CI)a | 0.328 (.270 to .386) |
0.253 (.193 to .312) |
0.374 (.310 to .438) |
| CSF sterile at 2 wk, N (%) | 23 (62.2%) | 21 (63.6%) | 23 (67.6%) |
| CSF sterile ever, N (%) | 36 (97.3%) | 30 (90.9%) | 28 (82.4%) |
| Additional IV amphotericin B received during induction therapy | 1 | 0 | 0 |
| CSF culture positive at >28 dc | 4 (10%) | 5 (12.5%) | 2 (4.9%) |
| CSF culture positive >28 d or death | 7 (17.5%) | 11 (27.5%) | 8 (19.5%) |
Participants contributing data had nonsterile CSF at baseline and at least 2 lumbar punctures during study days 1–18. EFA unit is in log10 Cryptococcus colony-forming units (CFU) per milliliter CSF per day. Supplementary Table 2 provides data for cohorts 1 and 3. Supplementary Figure 1 provides individual participant-level EFA plot, and Supplementary Figure 2 provides summary mean EFA (95% CI) by cohort and for historical oral regimens.
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; EFA, early fungicidal activity; IV, intravenous; LNC, lipid nanocrystal.
aMixed regression model: log10 CSF ∼ arm + day + arm * day, with random intercepts and random slopes for day with patients as the random effect.
bEFA of participant 110 807 (cohort 4) has EFA = 4.161 log10 CFU/mL/d is imputed as 2.352 (cohort second highest EFA) as a sensitivity analysis.
c Supplementary Table 4 provides quantitative culture data for all positive CSF cultures beyond day 28.
The all-oral LNC amphotericin regimen cohort 4 had an overall mean EFA of 0.402 (95% CI: .165 to .640) log10CFU/mL/d. The mean EFA difference between the all-oral regimen and IV amphotericin was −0.053 (95% CI: −.317, .211) log10 CFU/mL/d. However, more variability was present in CSF clearance with the all-oral regimen (EFA range, 0.02 to 4.161 log10 CFU/mL/d). Among the all-oral regimen, those with CSF pleocytosis (≥5 white cells/μL CSF) had a 2-fold faster rate of mean CSF clearance (EFA = 0.564 vs 0.250 log10 CFU/mL/d; Supplementary Table 3). The max 4.161 EFA reflected a rapid clearance of 14 500 Cryptococcus/mL yeasts to CSF sterility in 1 day. When imputing this outlier value as the penultimate cohort maximum of 2.352 log10 CFU/mL/d, the mean EFA of the all-oral regimen was 0.348 (95% CI: .211 to .484) log10 CFU/mL/d.
Among participants with positive CSF cultures at baseline, the incidence of CSF culture sterility was similar by 2 weeks. Approximately 62% (23 of 37) of cohort 2 participants achieved CSF sterility in the first 2 weeks compared with 64% (21 of 33) who received the all-oral LNC amphotericin (cohort 4) and 68% (23 of 34) who received IV amphotericin.
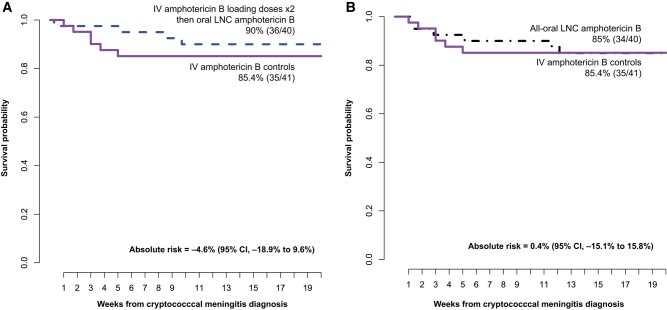
Survival
We followed participants for 18-week survival (Figure 3). The IV amphotericin control group had 85% (35 of 41) survival, similar to the all-oral LNC amphotericin 85% (34 of 40) survival. The survival with oral LNC amphotericin with 2 IV loading doses was 90% (36 of 40), with an absolute risk difference in mortality of −4.6% (95% CI: −18.9% to 9.6%) compared with the IV amphotericin control group. Notably, only 1 participant in the IV loading dose oral LNC amphotericin group died in the first 30 days, dying on day 2 before receiving LNC amphotericin.
Figure 3.
Survival through 18 weeks with oral LNC amphotericin B (MAT2203; Matinas Biopharma) vs IV amphotericin B with (A) and without (B) 2 IV amphotericin loading doses. The standard-of-care control arm received liposomal amphotericin B (n = 22) at 3 mg/kg/d or amphotericin B deoxycholate (n = 19) 1 mg/kg/d for 7 days with flucytosine followed by fluconazole 1200 mg/d through day 14. Oral LNC amphotericin B regimens were given with oral flucytosine 100 mg/kg daily for 2 weeks. After 2 weeks, all participants received fluconazole 800 mg/d to week 10, then secondary prophylaxis with fluconazole 200 mg/d. Absolute risk difference calculated with Wald 95% confidence intervals. Abbreviations: CI, confidence interval; IV, intravenous; LNC, lipid nanocrystal.
Toxicity
Laboratory toxicities were substantially lower with 6 weeks of oral LNC amphotericin than 1 week of IV amphotericin (Table 3). Grade ≥3 potassium AEs occurred in 5% (4 of 80) of oral LNC amphotericin groups vs 17% (7 of 40) with IV amphotericin (P = .04). Grade ≥3 anemia occurred in 21% (17 of 80) of the LNC amphotericin groups vs 43.9% (18 of 41) with IV amphotericin (P = .01). Among participants who entered the trial with a normal hemoglobin level, only 4.2% (2 of 48) developed grade ≥3 anemia with LNC amphotericin and flucytosine compared with 38% (11 of 29) who received IV amphotericin (Supplementary Table 6A). Kidney toxicity with 6 weeks of LNC amphotericin was infrequent. With IV amphotericin, 43% (17 of 40) of controls had grade 1 or greater creatinine increase compared with 23% (9 of 39) with oral LNC amphotericin given 2 IV amphotericin loading doses and only 13% (5 of 39) with the all-oral amphotericin regimen (Supplementary Table 6B). Clinical AEs (41% LNC amphotericin vs 56% controls) and serious AEs (26% LNC amphotericin vs 27% controls) did not statistically differ; most were HIV-related or cryptococcosis-related.
Table 3.
Grade 3 and Higher Adverse Events and Relatedness
| AE | Oral LNC Amphotericin B With 2 IV Loading Doses | All-Oral LNC Amphotericin B | IV Amphotericin B Controls | P Value | |
|---|---|---|---|---|---|
| Number randomized | 40 | 40 | 41 | ||
| Participants with grade ≥3 clinical AEs excluding SAEs | 16 (40%) | 17 (42.5%) | 23 (56.1%) | .13 | |
| Total number of grade ≥3 clinical AEs excluding SAEs | 27 | 25 | 33 | ||
| AE relatedness to amphotericina | 2 | 2 | 2 | ||
| SAEsb | |||||
| Participants with ≥1 SAE | 10 | 11 | 11 | .99 | |
| Total number of unique SAEs | 11 | 15 | 11 | ||
| Laboratory AEs, grade ≥3 N randomized with data |
40 | 40 | 41 | ||
| Participants with | Grade 3 | 14 (35%) | 8 (20%) | 19 (46.3%) | .04 |
| Grade 4 | 2 (5%) | 9 (22.5%) | 6 (14.6%) | ||
| Total number AEsc | Grade 3 | 21 | 17 | 33 | |
| Grade 4 | 7 | 11 | 18 | ||
| N randomized with data | 39 | 39 to 40 | 40 | ||
| Hemoglobin | Grade 3 | 7 (17.5%) | 4 (10%) | 14 (34.1%) | .01 |
| Grade 4 | 1 (2.5%) | 5 (12.5%) | 4 (9.8%) | ||
| Creatinine | Grade 3 | 3 (7.5%) | 1 (2.6%) | 2 (4.9%) | .99 |
| Grade 4 | 1 (2.4%) | 0 (0%) | 1 (2.4%) | ||
| Potassium | Grade 3 | 2 (5.1%) | 2 (5%) | 6 (14.6%) | .04 |
| Grade 4 | 0 (0%) | 0 (0%) | 1 (2.4%) | ||
| Sodium | Grade 3 | 2 (5.1%) | 6 (15.4%) | 2 (4.9%) | .40 |
| Grade 4 | 0 (0%) | 1 (2.6%) | 5 (12.2%) | ||
P values via the Fisher exact test compare LNC amphotericin pooled cohorts vs IV amphotericin controls for grade ≥3 AE vs not.
Abbreviations: AE, adverse event; IV, intravenous; LNC, lipid nanocrystal; SAE, serious adverse event.
aClassified as definite/probable for relatedness in the opinion of the investigator toward amphotericin B. All AEs related to LNC amphotericin were classified as probable. Supplementary Table 8 lists AE diagnoses by cohort.
bNo SAEs were related to study drug or antifungal medicines. Supplementary Table 9 lists SAEs by diagnosis, and Supplementary Table 10 lists SAEs by category.
c Supplementary Table 5 provides additional data for less-common laboratory AEs, and Supplementary Tables 6A–6D provide shift tables of the change from baseline by AE grade.
The tolerability of LNC amphotericin was excellent (Supplementary Table 7). Only 1 participant had early LNC amphotericin discontinuation before 6 weeks. Only 1 all-oral regimen participant received additional IV amphotericin during induction.
DISCUSSION
In this randomized trial, we demonstrated similar CSF antifungal activity and 18-week survival with a novel, oral LNC formulation of amphotericin B when combined with flucytosine. The measured antifungal activity in CSF at the site of infection was similar to that of the standard 7-day course of IV amphotericin with flucytosine. When 2 IV loading doses of amphotericin B were given followed by oral LNC amphotericin, the 18-week survival was 90% with only 1 death in the first 30 days and no persons lost to follow-up or censored.
These impressive results using 2 IV loading doses then oral LNC amphotericin were not due to cohort 2 being a “healthy cohort” as the median CD4 count was 25 cells/μL, median Cryptococcus culture was 40 750 CFU/mL CSF, and the majority were ART-naive. These demographics and fungal burden were similar to those for the subset of 231 Ugandan participants enrolled in the Ambition-cm single-dose 10 mg/kg liposomal amphotericin trial with a Glasgow coma score of 15 [4] who had a median CD4 count of 23 cells/μL and median CSF culture of 31 500 CFU/mL. Among these Ugandan Ambition-cm participants, survival outcomes were comparable with 85% (197 of 231) 30-day survival and 79% (182 of 231) 16-week survival [4]. The improvement in survival is in marked contrast to our 2001–2002 Kambugu et al prospective cohort, where with 2 weeks of IV amphotericin B deoxycholate, 51% mortality occurred in those with a Glasgow coma score of 15 [19] and where amphotericin-induced nephrotoxicity and hypokalemia likely contributed to mortality [5].
The toxicity associated with LNC amphotericin formulation was better than IV formulations. In this trial, the majority (22 of 41) of controls received IV liposomal amphotericin B. Clinical AEs did not differ, being mostly attributable to cryptococcosis or HIV/AIDS. However, laboratory AEs were significantly less frequent within the oral LNC amphotericin cohorts, particularly for anemia and hypokalemia, both strongly associated with mortality [5, 20].
An interesting observation was that although the all-oral regimen had 85% 18-week survival, which was similar to that for IV amphotericin controls, more variability occurred in Cryptococcus CSF clearance, both interparticipant (greater than expected) and intraparticipant over time (unexpected). The variability observed with the all-oral regimen may be due to CSF pleocytosis, as oral LNC amphotericin is taken up by monocytes that then traffic into the central nervous system [6]. Although small sample sizes preclude definitive conclusions, those with CSF pleocytosis had 2-fold higher mean rates of fungal clearance. The all-oral EFA did not meet our prespecified criteria of having a lower 95% CI that was greater than 0.2 log10 CFU/mL/d as one participant had an EFA of 4.161 log10 CFU/mL/d (10-fold better than the cohort mean), which greatly increased the standard deviation and thereby the 95% CI width. When the 10-fold better EFA participant was excluded, the lower 95% CI was >0.2 log10 CFU/mL/d, meeting our prespecified criteria. Given the variability in CSF pleocytosis, which is somewhat unique to cryptococcal meningitis, we believe the IV loading dose(s) is a wise strategy for which there is precedence with antimicrobial therapy for bacterial infections.
The uptake of LNC amphotericin into macrophages, which is where the facultative intracellular yeasts of Cryptococcus reside, has a distinct benefit of creating high intracellular amphotericin levels and low extracellular amphotericin levels. Thus, the degree of systemic toxicity was lower than IV amphotericin. Another advantage of LNC amphotericin could be that the macrophages would have yeast-killing activity, regardless of type-1 T-helper cell (Th1) classical activation vs Th2 polarization. Alternatively, activated Th2 macrophages generate inflammation and fibrosis yet do not kill yeast [21]. With LNC amphotericin present, such alternatively activated macrophages may well retain fungicidal activity. This should be an area of further investigation that uses experimental models [21].
Beyond the observed clinical efficacy and lower toxicity, other potential LNC amphotericin benefits may exist. First, electrolyte monitoring and supplementation appear to be unnecessary, hence, enabling outpatient therapy, where the capacity to perform extensive laboratory monitoring is limited. Second, not requiring IV access for the longer term decreases the risk of thrombophlebitis and bacteremia [22, 23]. Third, LNC amphotericin is stable at room temperature, making it easily administered in outpatient settings.
This study has limitations. As combination LNC amphotericin with flucytosine therapy was given, the relative contribution of each is unknown; however, flucytosine alone is ineffective with rapid development of resistance [24]. This trial recruited adults with HIV without altered mental status, as the investigational product was an oral formulation, hence, appropriate for those able to swallow oral medications. While this LNC formulation could theoretically be administered via nasogastric tube, such patients are likely better served initially by IV medicines. The wide 95% CI of the all-oral LNC amphotericin arm (EFA, 0.171 to 0.646 log10 CFU/mL/d) has imprecision due to the greater interperson variability observed.
In conclusion, the LNC amphotericin oral formulation had similar efficacy as traditional IV amphotericin B but was less toxic. Herein, we demonstrated similar antifungal activity at the site of infection in humans and noninferior 18-week survival outcomes when 2 IV loading doses were given. We consider cryptococcal meningitis as a proof-of-concept fungal infection model. If LNC amphotericin with flucytosine can treat a 100% fatal fungal infection of the brain in severely immunocompromised persons with HIV, treatment of other fungal infections should be possible. This requires future clinical trials to confirm.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
David R Boulware, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Mucunguzi Atukunda, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Enock Kagimu, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Abdu K Musubire, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Andrew Akampurira, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Lillian Tugume, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Kenneth Ssebambulidde, Infectious Diseases Institute, Makerere University, Kampala, Uganda; Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
John Kasibante, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Laura Nsangi, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Timothy Mugabi, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Jane Gakuru, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Sarah Kimuda, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Derrick Kasozi, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Suzan Namombwe, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Isaac Turyasingura, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Morris K Rutakingirwa, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Edward Mpoza, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Enos Kigozi, Department of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Conrad Muzoora, Department of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Jayne Ellis, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Caleb P Skipper, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Theresa Matkovits, Matinas Biopharma Nanotechnologies, Bedminster, New Jersey, USA.
Peter R Williamson, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Darlisha A Williams, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Ann Fieberg, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
Kathy H Hullsiek, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
Mahsa Abassi, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Biyue Dai, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
David B Meya, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA; Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Notes
Acknowledgments. We dedicate this article to the memory of Dr Edmund Tramont who was a proponent of oral, nontoxic amphotericin B and had a vision as to how this novel formulation could revolutionize antifungal treatment during a pivotal time of emerging antimicrobial resistance. We thank the data and safety monitoring board members Drs Tihana Bicanic, Thomas Harrison, Yunus Moosa, Peter Pappas, and Bozena Morawski for their oversight and thoughtful input. We thank Matinas Biopharma for supplying the lipid nanocrystal amphotericin, external study monitoring, and funding for food for participants during the coronavirus disease 2019 lockdowns.
Disclaimer. The study funder (National Institutes of Health [NIH]) had no role in study design and all conduct of the study. The pharmaceutical sponsor had input into trial design with US Food and Drug Administration review but did not have input on data analysis, data interpretation, writing, or decision to publish. The corresponding author had full access to all data and final responsibility. Regulatory approval was obtained from Ugandan and US institutions.
Regulatory approvals and registrations include. Mulago Hospital Research Ethics Committee 1195; University of Minnesota STUDY00000139; Uganda National Council of Science & Technology HS 2351; Uganda National Drug Authority 854/NDA/DPS/08/2018; US Food and Drug Administration IND 72807; Clinicaltrials.gov: NCT04031833.
Collaborators. Asmus Tukundane, Jane F. Ndyetukira, Cynthia Ahimbisibwe, Alisat Sadiq, Florence Kugonza, Shifah Nabbaale, Tadeo Kiiza, Alice Namudde, Tony Luggya, Richard Kwizera, Michael Okirwoth, Dora Babirye, Catherine Nanteza, Suzan Mulwana, Rhona Muyise, John Kisembo, Andrew Luswata, Carol Namujju, Eva Laker, Lydia Nankungu, Jesca Asienzo, Stewart Walukaga, Minda Liu, Nicole Engen, Abduljewad Wele, Ananta Bangdiwala, Thomas C. McHale, Susan Johansson, Frank Calamusa, Haran Schlamm, Jenel Cobb, Irene Rwomushana, Mable Kabahubya, Edwin Nuwagira, Michael Ssemusu, James Mwesigye, Joan Rukundo, and Samuel Jjunju.
Financial support. This study was funded by the NIH (R01NS086312). The research was supported by the National Institute of Neurologic Diseases and Stroke and the Fogarty International Center (R01NS086312, K23NS122601, D43TW009345), the National Institute of Allergy and Infectious Diseases (T32AI055433), and the National Center for Advancing Translational Sciences (KL2TR002492 and UL1TR002494). P. R. W. is supported by the Intramural Research Program of the National Institute of Allergy and Immunology of the NIH (AI001123 and AI001124). T. M. reports support for this work from Matinas BioPharma (full-time employee).
References
- 1. Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022; 22:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. Available at: https://www.who.int/publications/i/item/9789240052178. Accessed 27 June 2022. [PubMed]
- 4. Jarvis JN, Lawrence DS, Meya DB, et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 2022; 386:1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahr NC, Rolfes MA, Musubire A, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource-limited settings. Open Forum Infect Dis 2014; 1:ofu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu R, Hollingsworth C, Qiu J, et al. Efficacy of oral encochleated amphotericin B in a mouse model of cryptococcal meningoencephalitis. mBio 2019; 10:e00724-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skipper CP, Atukunda M, Stadelman A, et al. Phase I EnACT trial of the safety and tolerability of a novel oral formulation of amphotericin B. Antimicrob Agents Chemother 2020; 64:e00838-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 2009; 49:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis 2008; 47:123–30. [DOI] [PubMed] [Google Scholar]
- 10. Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 2004; 363:1764–7. [DOI] [PubMed] [Google Scholar]
- 11. Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 2014; 20:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bahr NC, Skipper CP, Huppler-Hullsiek K, et al. Recurrence of symptoms following cryptococcal meningitis—characterizing a diagnostic conundrum with multiple etiologies. Clin Infect Dis 2022; 76:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. Available at: https://apps.who.int/iris/bitstream/handle/10665/260399/9789241550277-eng.pdf. Accessed 25 October 2018. [PubMed]
- 14. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dyal J, Akampurira A, Rhein J, et al. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pullen MF, Hullsiek KH, Rhein J, et al. Cerebrospinal fluid early fungicidal activity as a surrogate endpoint for cryptococcal meningitis survival in clinical trials. Clin Infect Dis 2020; 71:e45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhein J, Huppler Hullsiek K, Tugume L, et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 2019; 19:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 2008; 46:1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tugume L, Morawski BM, Abassi M, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med 2017; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stenzel W, Muller U, Kohler G, et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol 2009; 174:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajasingham R, Williams D, Meya DB, Meintjes G, Boulware DR, Scriven J. Nosocomial drug-resistant bacteremia in 2 cohorts with cryptococcal meningitis, Africa. Emerg Infect Dis 2014; 20:722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahimbisibwe C, Kwizera R, Ndyetukira JF, et al. Management of amphotericin-induced phlebitis among HIV patients with cryptococcal meningitis in a resource-limited setting: a prospective cohort study. BMC Infect Dis 2019; 19:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perfect JR, Durack DT. Treatment of experimental cryptococcal meningitis with amphotericin B, 5-fluorocytosine, and ketoconazole. J Infect Dis 1982; 146:429–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.