Abstract
Background
Pericoronary adipose tissue (PCAT) may influence plaque development through inflammatory mechanisms. We assessed PCAT density, as a measure of pericoronary inflammation, in relationship to coronary plaque among people with human immunodeficiency virus (HIV [PWH]) and to a matched control population.
Methods
In this baseline analysis of 727 participants of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) Mechanistic Substudy, we related computed tomography–derived PCAT density to presence and extent (Leaman score) of coronary artery disease (CAD), noncalcified plaque, coronary artery calcium (CAC), and vulnerable plaque features using multivariable logistic regression analyses. We further compared the PCAT density between PWH and age, sex, body mass index, CAC score, and statin use–matched controls from the community-based Framingham Heart Study (N = 464), adjusting for relevant clinical covariates.
Results
Among 727 REPRIEVE participants (age 50.8 ± 5.8 years; 83.6% [608/727] male), PCAT density was higher in those with (vs without) coronary plaque, noncalcified plaque, CAC >0, vulnerable plaque, and high CAD burden (Leaman score >5) (P < .001 for each comparison). PCAT density related to prevalent coronary plaque (adjusted odds ratio [per 10 HU]: 1.44; 95% confidence interval, 1.22–1.70; P < .001), adjusted for clinical cardiovascular risk factors, body mass index, and systemic immune/inflammatory biomarkers. Similarly, PCAT density related to CAC >0, noncalcified plaque, vulnerable plaque, and Leaman score >5 (all P ≤ .002). PCAT density was greater among REPRIEVE participants versus Framingham Heart Study (−88.2 ± 0.5 HU versus −90.6 ± 0.4 HU; P < .001).
Conclusions
Among PWH in REPRIEVE, a large primary cardiovascular disease prevention cohort, increased PCAT density independently associated with prevalence and severity of coronary plaque, linking increased coronary inflammation to CAD in PWH.
Keywords: pericoronary adipose tissue, HIV, coronary plaque, coronary artery disease, inflammation
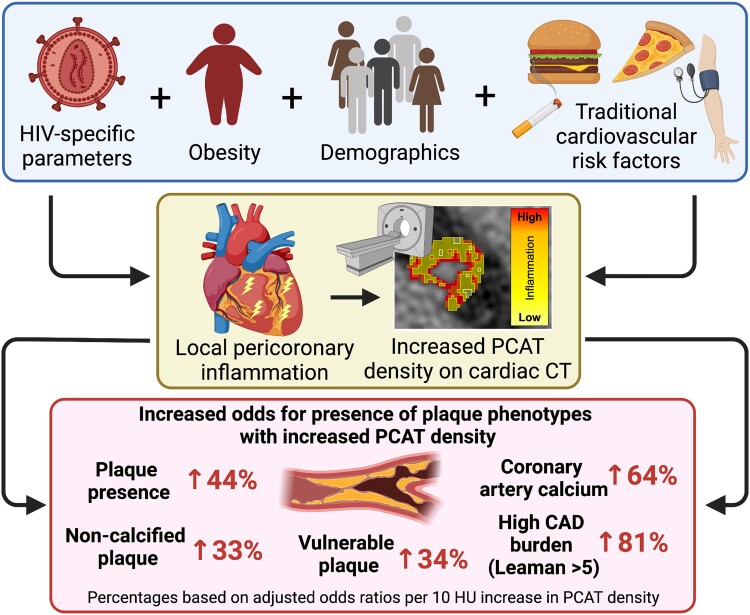
Among people with human immunodeficiency virus, increased pericoronary adipose tissue density, an index of coronary inflammation, was independently associated with prevalence and severity of coronary plaque. This suggests a link between coronary inflammation and coronary artery disease in people with human immunodeficiency virus.
Graphical Abstract
Graphical Abstract.
People with human immunodeficiency virus (HIV [PWH]) have a significantly higher prevalence of coronary artery disease (CAD) compared with the general population [1–3]. Although traditional risk factors explain only a portion of the risk increase [4], other factors, including immune activation and inflammation [5–12], are thought to contribute. The composition of pericoronary adipose tissue (PCAT) surrounding the coronary arteries can be used as an index of vascular inflammation [13]. The composition of PCAT is assessed by its density, measured in Hounsfield units (HU) on computed tomography (CT). Epidemiologic studies have associated increased PCAT density with more than 2 times the elevated risk of all-cause and cardiac mortality in patients with stable chest pain [14] and the general population with high-risk coronary artery plaque morphology and acute coronary syndrome [15, 16]. A recent meta-analysis demonstrated a pooled hazard ratio for major adverse cardiovascular events (MACEs) of 3.29 (1.88–5.76) in the general population [17]. However, it is unknown whether increased PCAT density relates to coronary plaque or is increased in PWH compared with the general population.
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) was designed to assess the effects of a primary prevention strategy in PWH at low-to-moderate traditional atherosclerotic cardiovascular disease (ASCVD) risk [18, 19]. REPRIEVE has recently been closed for a robust efficacy signal (35% reduction in MACE in daily pitavastatin vs placebo) [20]. Here, leveraged REPRIEVE mechanistic substudy baseline data assessed PCAT density in relationship to detailed coronary plaque indices, traditional risk and critical pathways of inflammation, and immune activation for the first time in PWH [8]. Furthermore, we compared PCAT density between PWH and matched controls from the community-based Framingham Heart Study (FHS).
METHODS
Study Population and Clinical Characteristics
REPRIEVE enrolled 7769 women and men (age 40–75 years) with low-to-moderate traditional ASCVD risk [19, 21], receiving antiretroviral therapy, asymptomatic without prior clinical cardiovascular disease, and not receiving statins [21]. Participants with active systemic infections and serious non-HIV illnesses were excluded. Thirty-one U.S. sites (mainly from the AIDS Clinical Trials Group) coenrolled 805 participants in the REPRIEVE Mechanistic Substudy (A5333s) between May 2015 and February 2018 [18]. For full exclusion criteria, see Supplementary Text 1.
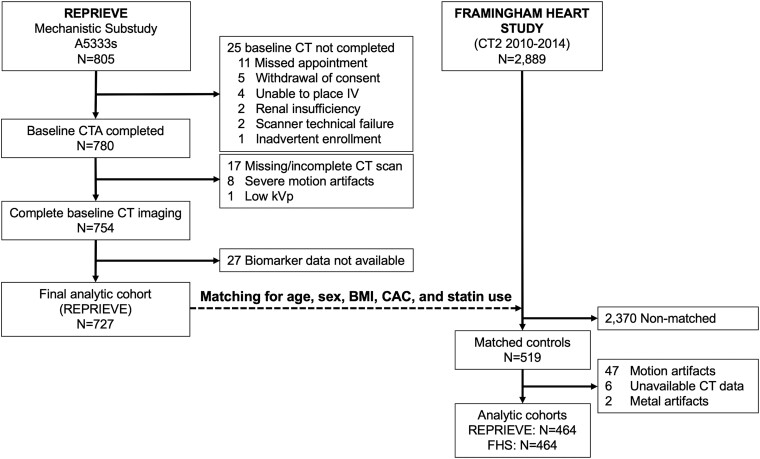
Analogous data on PCAT density was assessed in a control group of asymptomatic participants in the multidetector CT substudy of the Offspring, Third Generation, and Omni cohorts of the community-based FHS [22] not on statin therapy. Control subjects were matched 1:1 without replacement in advance before PCAT measurements for age, sex, body mass index (BMI), coronary artery calcium (CAC) score, and statin use. The matching criteria included age ±2 years, sex 1:1, BMI ±2 kg/m2, and CAC in the same category (0, 1–10, 11–100, 101–400, or >400) (Figure 1).
Figure 1.
Consort diagram. ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAC, coronary artery calcium; CT, computed tomography; CTA, CT angiography; FHS, Framingham Heart Study.
The institutional review boards of the Massachusetts General Hospital (REPRIEVE) and the Boston University Medical Center (FHS) approved the study protocols. All research sites obtained institutional review board/ethics committee approval and written informed consent from the participants.
CT Image Acquisition
All CT scans in REPRIEVE were acquired on ≥64-slice CT scanners using standard protocols, including acquisition of a standardized electrocardiogram (ECG)-synchronized noncontrast CAC scoring scan and subsequent contrast-enhanced coronary CT angiography [18]. FHS cardiac CT scans consisted exclusively of ECG-synchronized noncontrast CAC scoring performed as in REPRIEVE using standardized acquisition parameters (120 kVp, ECG synchronization, 2.5- to 3-mm slice thickness, and field of view <250 mm) [22].
Assessment of Presence, Extent, and Composition of CAD on Cardiac CT
A central core laboratory quantified CAC on noncontrast CT images using standard methods in both REPRIEVE and FHS [23]. In REPRIEVE, contrast-enhanced coronary CT angiography scans were available and assessed for the presence, extent, and composition of atherosclerotic plaques using the standard 18-segment SCCT model [24]. Plaques were categorized by stenosis degree (0%, 1%–49%, 50%–69%, 70%–99%, and 100%) [24], composition (including noncalcified plaque), and assessed for vulnerability based on low attenuation (plaque with density <30 HU), positive remodeling (>1.1 remodeling index), and napkin ring sign (low central density core and ring-like peripheral hyperdensity) [8, 25]. The Leaman score, based on the number of affected segments, plaque location, stenosis degree, and plaque composition, provided information on coronary plaque burden [26].
Measurement of Pericoronary Adipose Tissue Density
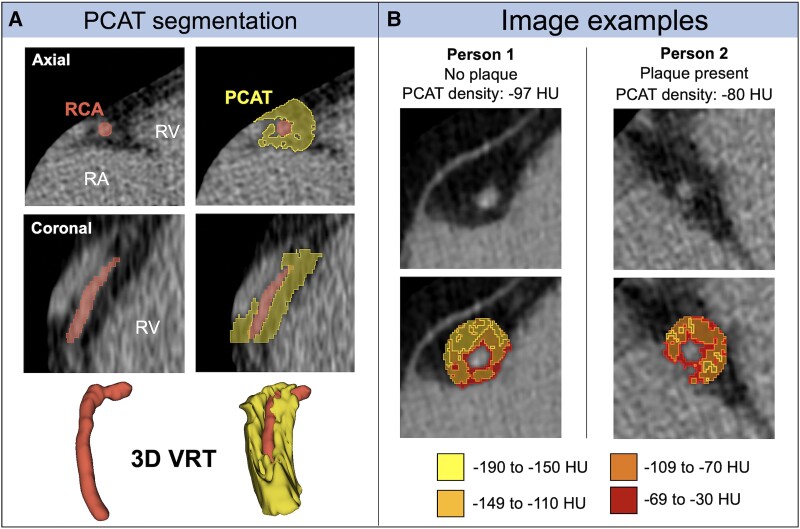
PCAT density was determined similarly in both REPRIEVE and FHS participants in a representative area around the proximal 4 cm of the right coronary artery (RCA). PCAT is part of the epicardial adipose tissue that directly surrounds the coronary arteries. Supplementary Text 2 provides detailed descriptions of the measurement methods. Figure 2 shows representative examples.
Figure 2.
Pericoronary adipose tissue on noncontrast cardiac computed tomography. A, Segmentation of PCAT surrounding the right coronary artery to measure PCAT density. B, Examples of patients with and without CAD and correspondingly higher PCAT density in the patient with CAD. 3D VRT, 3-dimensional volume rendering technique; CAD, coronary artery disease; HU, Hounsfield unit; PCAT, pericoronary adipose tissue; RA, right atrium; RCA, right coronary artery; RV, right ventricle; VRT, volume-rendering technique.
Plasma Biomarkers of Immune Activation and Inflammation
Monocyte chemoattractant protein-1, high-sensitivity interleukin-6, lipoprotein-associated phospholipase 2, oxidized low-density lipoprotein, and high-sensitivity C-reactive protein were assessed as previously published [8] and included in the multivariate modeling.
Statistical Methods
Continuous variables were presented as mean ± standard deviation or medians (25th–75th percentiles) and categorical variables as absolute and relative frequencies. PCAT density comparisons were performed using the Kruskal–Wallis test and logistic regression modeling. Odds ratios (ORs) were calculated per 10 HU higher PCAT density, ∼25% of the measured range of PCAT density. Adjustments were performed for: ASCVD risk score, BMI, and substance use (model 1); model 1 + systemic inflammatory and immune activation biomarkers (model 2); or model 2 with individual cardiovascular risk factors instead of ASCVD risk score, including age, sex, race, and smoking (model 3). Biomarkers were log2-transformed and then divided by 0.32 to give effects per 25% increase of biomarker value. A sensitivity analysis included HIV-related indices in the modeling. A supplementary analysis was conducted for PWH without plaque in the proximal- or mid-RCA, assessing PCAT density's relation to plaque elsewhere.
Finally, a matched-cohort analysis compared PCAT density between REPRIEVE and FHS participants using a paired t test and general least squares random effects models adjusting for smoking and race. Statistical analyses were performed using Stata version 17.1 (StataCorp), and 2-sided P values <.05 were considered significant. The sample size was based on available data.
RESULTS
REPRIEVE Study Population
A total of 727 REPRIEVE Mechanistic Substudy participants had CT imaging suitable for PCAT and plaque assessment and provided immune/inflammatory biomarker data (Figure 1). Demographic characteristics, including substance use, are shown in Table 1 and footnote. Waist circumference cutoffs are per World Health Organization guidelines [27]. Viral load was suppressed in the great majority of participants.
Table 1.
Detailed Baseline Demographics, Risk Factors, Comorbidities, and Other Descriptors in all REPRIEVE Participants and Stratified by PCAT Density
| Demographics | n/N (%), Mean ± SD, or Median (25%–75%) | PCAT Density–HU Median (25%–75%) | P Value |
|---|---|---|---|
| Age, y | 50.8 ± 5.8 | … | |
| Age categories, y | .998 | ||
| 40–44 | 102 (14.0) | −88.8 (−95.9 to −80.3) | |
| 45–49 | 208 (28.6) | −88.8 (−95.9 to −80.3) | |
| 50–54 | 228 (31.4) | −88.6 (−94.2 to −82.4) | |
| 55–59 | 135 (18.6) | −89.1 (−94.6 to −82.0) | |
| ≥60 | 54 (7.4) | −88.5 (−94.6 to −79.8) | |
| Sex | .026 | ||
| Female | 119 (16.4) | −91.0 (−97.2 to −83.6) | |
| Male | 608 (83.6) | −88.2 (−94.6 to −80.9) | |
| Race | … | … | <.001 |
| White | 388 (53.4) | −89.5 (−95.7 to −82.7) | |
| Black or African American | 257 (35.4) | −86.4 (−92.8 to −78.6) | |
| Asian | 10 (1.4) | −89.8 (−91.5 to −85.0) | |
| Other | 72 (9.9) | −90.3 (−96.7 to −82.6) | |
| Ethnicity | .004 | ||
| Hispanic or Latino | 176 (24.2) | −90.7 (−96.0 to −84.8) | |
| Not Hispanic or Latino | 541 (74.4) | −87.8 (−94.6 to −80.4) | |
| Unknown | 10 (1.4) | −91.4 (−94.2 to −78.7) | |
| Behavioral risk factors | |||
| Smoking status | .003 | ||
| Current | 176 (24.2) | −86.7 (−92.8 to −78.4) | |
| Former | 227 (31.3) | −88.9 (−95.3 to −82.0) | |
| Never | 323 (44.5) | −89.8 (−95.9 to −81.2) | |
| Substance usea | <.001 | ||
| Current | 16 (2.2) | −80.2 (−86.3 to −75.7) | |
| Former | 357 (49.2) | −87.1 (−93.0 to −79.9) | |
| Never | 352 (48.6) | −91.2 (−97.0 to −83.5) | |
| Cardiovascular risk factors | |||
| Family history of premature disease | .529 | ||
| Yes | 164 (22.6) | −89.8 (−95.5 to −82.5) | |
| No | 540 (74.5) | −88.4 (−94.8 to −81.0) | |
| Unknown | 21 (2.9) | −88.2 (−95.3 to −80.5) | |
| Prior statin use | .586 | ||
| Yes | 57 (7.8) | −88.3 (−92.6 to −80.9) | |
| No | 670 (92.2) | −88.9 (−95.3 to −81.2) | |
| History of hypertension | .861 | ||
| Yes | 228 (31.4) | −88.0 (−95.3 to −81.6) | |
| No | 499 (68.6) | −88.9 (−94.8 to −81.1) | |
| Median ASCVD risk score, % | 4.5 (2.6–6.8) | … | |
| ASCVD risk categories, % | .613 | ||
| <2.5 | 168 (23.1) | −89.4 (−95.2 to −81.7) | |
| 2.5–4.9 | 238 (32.7) | −89.0 (−95.2 to −80.8) | |
| 5.0–7.4 | 177 (24.4) | −88.3 (−95.8 to −81.6) | |
| 7.5–9.9 | 98 (13.5) | −87.9 (−94.6 to −81.6) | |
| ≥10.0 | 46 (6.3) | −87.3 (−92.4 to −79.7) | |
| Metabolic risk factors and orthometric measures | |||
| BMI, kg/m2 | 27.3 ± 4.4 | … | |
| BMI categories, kg/m2 | .001 | ||
| <18.5 | 8 (1.1) | −79.0 (−99.1 to −74.1) | |
| 18.5–24.9 | 238 (32.7) | −86.6 (−92.3 to −79.0) | |
| 25–29.9 | 295 (40.6) | −90.0 (−95.8 to −82.0) | |
| 30–34.9 | 142 (19.5) | −90.1 (−95.7 to −84.0) | |
| ≥35 | 44 (6.1) | −89.3 (−95.8 to −83.2) | |
| Waist circumference, cm | 95.6 ± 11.9 | … | |
| Waist circumference categories | <.001 | ||
| High (male: ≥102 cm; female ≥88 cm) | 220 (31.9) | −90.9 (−95.5 to −84.2) | |
| Normal (male: <102 cm; female <88 cm) | 469 (68.1) | −87.7 (−94.3 to −79.9) | |
| HIV parameters | |||
| HIV RNA, copies/mL | .039 | ||
| <LLQ | 633 (88.2) | −89.2 (−95.3 to −81.6) | |
| LLQ, <400 | 70 (9.8) | −86.8 (−91.9 to −79.6) | |
| ≥400 | 15 (2.1) | −83.0 (−92.3 to −76.4) | |
| Total ART use | .742 | ||
| <5 y | 117 (16.1) | −89.6 (−95.2 to −79.7) | |
| 5–9.9 y | 190 (26.1) | −89.1 (−94.8 to −82.0) | |
| ≥10 y | 420 (57.8) | −88.3 (−94.9 to −81.2) | |
| ART regimen by class | .991 | ||
| NRTI + INSTI | 325 (44.7) | −88.6 (−95.3 to −81.6) | |
| NRTI + NNRTI | 190 (26.1) | −89.0 (−95.8 to −81.2) | |
| NRTI + PI | 121 (16.6) | −89.4 (−93.9 to −80.8) | |
| NRTI-sparing | 21 (2.9) | −86.6 (−95.1 to −82.8) | |
| Other NRTI-containing | 70 (9.6) | −88.3 (−94.2 to −80.8) | |
| Abacavir exposure | .834 | ||
| Yes | 246 (33.9) | −88.7 (−95.1 to −81.6) | |
| No | 479 (66.1) | −88.8 (−94.8 to −81.1) | |
| CD4, cells/mm3 | .779 | ||
| <350 | 110 (15.1) | −88.3 (−94.8 to −80.5) | |
| 350–499 | 145 (19.9) | −89.0 (−95.2 to −81.9) | |
| ≥500 | 472 (64.9) | −88.8 (−95.0 to −81.2) | |
| Nadir CD4, cells/mm3 | .186 | ||
| <50 | 157 (21.6) | −88.9 (−96.3 to −81.8) | |
| 50–199 | 212 (29.2) | −89.3 (−95.7 to −82.9) | |
| 200–349 | 196 (27.0) | −88.6 (−94.3 to −80.4) | |
| ≥350 | 140 (19.3) | −88.7 (−93.4 to −81.2) | |
| Unknown | 22 (3.0) | −84.5 (−90.9 to −75.3) |
aSubstance use among PWH was classified as current (2.2%) or former (49%) (IV drug [current: 0%, former: 11.2%], cocaine [current: 1.2%, former: 45.8%], or methamphetamine [current: 1.1%, former: 19.7%]. P values were obtained using a nonparametric Kruskal–Wallis test.
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; HIV, human immunodeficiency virus; HU, Hounsfield unit; INSTI, integrase strand transfer inhibitors; LLQ, lower level of quantification; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PCAT, pericoronary adipose tissue; PI, protease inhibitor; REPRIEVE, Randomized Trial to Prevent Vascular Events in HIV.
PCAT Density in Relation to Demographic, Traditional Cardiovascular Risk Factors, and Anthropometric Measures
Among the 727 analyzed participants, the mean PCAT density was −88.0 ± 10.5 HU. Male sex, Black race, current smoking, and current or former substance use were associated with higher PCAT density. In contrast, higher BMI and waist circumference were associated with lower PCAT density (Table 1).
Relationship of PCAT Density to HIV Parameters
PCAT density was not associated with the duration of HIV infection or the length or type of antiretroviral therapy. Although HIV RNA was less than the lower level of quantification in 88% of REPRIEVE participants, higher PCAT density was marginally associated with a greater percentage of quantifiable HIV RNA (P = .039) (Table 1).
PCAT Density and CAD in People With HIV
Comparison of PCAT Density By Plaque Phenotype in PWH
Higher PCAT density was seen among those with coronary plaque, increasing stenosis degree and CAC, noncalcified plaques, vulnerable plaques, and increasing Leaman score (all P < .001; Table 2 and Figure 3). For example, PCAT density was −87.0 (−93.9 to −79.6) versus −90.4 (−96.3 to −83.6) HU in those with and without plaque, respectively (P < .001). See Figure 2B for representative examples.
Table 2.
CT-Derived CAD Indices in all Participants and by PCAT Density
| n/N (%) | PCAT Density, HU Median (25%–75%) | P Value | |
|---|---|---|---|
| Participants with any plaque | <.001 | ||
| No | 371 (51.0) | −90.4 (−96.3 to −83.6) | |
| Yes | 356 (49.0) | −87.0 (−93.9 to −79.6) | |
| Number of noncalcified segments | <.001 | ||
| 0 segments with noncalcified plaque | 436 (60.0) | −90.1 (−95.7 to −82.5) | |
| 1–2 segments with noncalcified plaque | 234 (32.2) | −87.5 (−94.1 to −80.1) | |
| ≥3 segments with noncalcified plaque | 57 (7.8) | −84.0 (−90.6 to −78.9) | |
| Stenosis | <.001 | ||
| No (stenosis 0%) | 371/715 (51.9) | −90.4 (−96.3 to −83.6) | |
| Mild (stenosis 1%–49%) | 319/715 (44.6) | −87.2 (−94.0 to −79.4) | |
| Moderate (stenosis 50%–69%) | 16/715 (2.2) | −83.5 (−88.9 to −79.9) | |
| Severe (stenosis ≥70% or ≥50% left main) | 9/715 (1.3) | −84.0 (−91.7 to −79.0) | |
| CAC score | <.001 | ||
| CAC = 0 | 452/698 (64.8) | −90.2 (−96.2 to −83.5) | |
| CAC = 1–100 | 173/698 (24.8) | −85.3 (−92.9 to −78.7) | |
| CAC = 101–400 | 61/698 (8.7) | −84.3 (−92.7 to −77.8) | |
| CAC >400 | 12/698 (1.7) | −83.9 (−89.5 to −79.9) | |
| Vulnerable plaque features | |||
| Any vulnerable plaque | <.001 | ||
| No | 563 (77.4) | −89.5 (−95.7 to −81.9) | |
| Yes | 164 (22.6) | −85.3 (−92.8 to −79.2) | |
| Vulnerable plaque category | |||
| Positive remodeling | <.001 | ||
| No | 568 (78.1) | −89.4 (−95.6 to −81.9) | |
| Yes | 159 (21.9) | −85.5 (−92.9 to −79.0) | |
| Low-attenuation plaque | .530 | ||
| No | 683 (94.0) | −88.9 (−95.2 to −81.1) | |
| Yes | 44 (6.1) | −86.6 (−94.2 to −82.5) | |
| Napkin ring sign | .342 | ||
| No | 704 (96.8) | −88.6 (−94.8 to −81.1) | |
| Yes | 23 (3.2) | −89.6 (−98.7 to −83.9) | |
| Leaman score | <.001 | ||
| 0 | 371/715 (51.9) | −90.4 (−96.3 to −83.6) | |
| >0–5 | 230/715 (32.2) | −88.3 (−95.1 to −80.8) | |
| >5 | 114/715 (15.9) | −83.9 (−90.7 to −77.9) |
Leaman score reflects coronary artery disease burden based on the number of segments with plaque, plaque location, stenosis degree, and plaque morphology. P values were obtained using a nonparametric Kruskal–Wallis test.
Abbreviations: CAC, coronary artery calcium; CT, computed tomography; HU, Hounsfield unit; PCAT, pericoronary adipose tissue.
Figure 3.
PCAT density differences by CT findings. Higher PCAT density in REPRIEVE participants with coronary plaques, noncalcified plaques, coronary calcium score >0, vulnerable plaques, and increased coronary artery disease burden (Leaman score >5). Each boxplot displays the median (solid horizontal line), interquartile range (box), and 1.5 interquartile ranges of the upper and lower quartiles (whiskers). Leaman score reflects coronary artery disease burden based on the number of segments with plaque, plaque location, stenosis degree, and plaque composition. P values were obtained using a nonparametric Kruskal–Wallis test. CAC, coronary artery calcium; CT, computed tomography; HU, Hounsfield unit; PCAT, pericoronary adipose tissue; REPRIEVE, Randomized Trial to Prevent Vascular Events in HIV.
In modeling, increased PCAT density was significantly associated with presence of coronary artery plaque (OR = 1.30; 95% confidence interval [CI]: 1.13–1.50; P < .001; OR per 10 HU increase in PCAT density). This association remained significant after adjustment for ASCVD risk score, BMI, substance use (model 1: adjusted OR [aOR] = 1.26; 95% CI: 1.09–1.47; P = .002), and model 1 + systemic inflammatory biomarkers (model 2: aOR = 1.38; 95% CI: 1.18–1.62; P < .001). Similar results were seen after correcting for individual cardiovascular risk factors instead of ASCVD risk score (Table 3). Supplementary Table 1 provides results per 1 standard deviation difference in PCAT density. Similarly, PCAT density was significantly associated with noncalcified plaque, any CAC, vulnerable plaque, and Leaman score >5 (Supplementary Tables 2–5). In fully adjusted models, the aORs were 1.33; 95% CI: 1.13–1.57; P = .001 for noncalcified plaque; 1.64, 95% CI: 1.37–1.97; P < .001 for CAC > 0; 1.34, 95% CI: 1.11–1.62; P = .002 for vulnerable plaque; and 1.81, 95% CI: 1.44–2.28; P < .001 for Leaman score >5).
Table 3.
Uni- and Multivariable Regression Results Comorbidities Versus Presence of Plaque
| Any Coronary Plaque | Univariable | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95%CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| PCAT density (per 10 HU) | 1.30 | 1.13–1.50 | <.001 | 1.26 | 1.09–1.47 | .002 | 1.38 | 1.18–1.62 | <.001 | 1.44 | 1.22–1.70 | <.001 |
| Age, y | … | … | … | … | … | … | 1.10 | 1.07–1.13 | <.001 | |||
| Sex (male) | … | … | … | … | … | … | 2.18 | 1.34–3.56 | .002 | |||
| Race | ||||||||||||
| White | … | … | … | … | … | … | ref. | … | ||||
| Black or African American | … | … | … | … | … | … | 0.69 | .46–1.03 | .066 | |||
| Asian | … | … | … | … | … | … | 1.61 | .39–6.72 | .513 | |||
| Other | … | … | … | … | … | … | 0.98 | .56–1.70 | .941 | |||
| ASCVD, % | … | … | 1.16 | 1.10–1.22 | <.001 | 1.14 | 1.08–1.21 | <.001 | … | … | ||
| BMI, kg/m2 | … | … | 1.01 | .97–1.04 | .619 | 1.01 | .97–1.04 | .772 | 1.02 | .98–1.06 | .272 | |
| Substance abuse | … | … | 1.26 | .93–1.72 | .134 | 1.20 | .87–1.65 | .265 | 1.23 | .87–1.72 | .237 | |
| Smoking (current) | … | … | … | … | … | … | 1.27 | .84–1.92 | .259 | |||
| MCP-1, pg/mL | … | … | … | … | 1.09 | .99–1.20 | .066 | 1.06 | .96–1.17 | .259 | ||
| IL-6, pg/mL | … | … | … | … | 1.05 | 1.00–1.11 | .048 | 1.06 | 1.00–1.12 | .041 | ||
| LpPLA2, ng/mL | … | … | … | … | 1.19 | 1.10–1.28 | <.001 | 1.15 | 1.06–1.25 | .001 | ||
| oxLDL, mU/L | … | … | … | … | 1.09 | .99–1.20 | .090 | 1.13 | 1.02–1.25 | .021 | ||
| hsCRPm mg/L | … | … | … | … | 1.02 | .98–1.07 | .342 | 1.03 | .99–1.08 | .179 | ||
P values were obtained with logistic regression modelling.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; HU, Hounsfield unit; IL-6, interleukin 6; LpPLA2, lipoprotein-associated phospholipase A2; MCP-1, monocyte chemoattractant protein 1; OR, odds ratio; oxLDL, oxidized low-density lipoprotein; PCAT, pericoronary adipose tissue.
Supplementary Analysis
Using an identical technique to ours, Takahashi et al demonstrated MACE in 14.2% versus 5.6% in those with higher PCAT density ≥ −93.55 HU [28]. We analyzed the data in our study using this clinically relevant cutoff from the literature. Using this cutoff, 70% of the PWH versus 60% in the FHS in the current study (P = .01) demonstrated pericoronary inflammation. In REPRIEVE, of the 70% above the threshold, 52% had any plaque versus 42% in the 30% of the population below the threshold (P = .01).
In addition, 17% of the REPRIEVE population demonstrated coronary artery plaque in the proximal, mid, or both RCA segments where PCAT density was measured. In a supplementary analysis limited to the 83% of participants without proximal- or mid-RCA plaque, PCAT density around the proximal RCA was similarly associated with coronary artery plaque in other coronary segments (aOR = 1.39; 95% CI: 1.15–1.69; P = .001; Supplementary Table 6).
Finally, adjustment for HIV-specific parameters, including antiretroviral therapy duration, CD4 cell count, HIV-RNA, and abacavir exposure did not affect any of the relationships between PCAT density and CT-derived CAD characteristics (aORs = 1.31–1.78; P ≤ .005; Supplementary Table 7).
PCAT Density in PWH Versus Controls
Overall, 464 REPRIEVE subjects were matched for age, sex, BMI, statin use, and CAC with corresponding FHS participants (Figure 1). Supplementary Table 8 details the characteristics of the REPRIEVE and FHS groups. PCAT density was significantly, but modestly, higher in REPRIEVE compared with matched controls in the FHS (PCAT density: −88.2 ± 0.5 HU vs −90.6 ± 0.4 HU; P < .001). The difference in PCAT density remained similar in magnitude and significant in random-effects modeling, further controlling for smoking and race (coefficient: 1.86 HU; 95% CI: .44–3.28; P = .010; Supplementary Table 9). As in REPRIEVE, increased PCAT density related to the presence of CAC in the FHS participants with a generally similar OR (OR = 1.58 per 10 HU, 95% CI: 1.34–1.82; P < .001).
DISCUSSION
The current study leveraged the large REPRIEVE cohort to analyze PCAT density, a novel imaging marker of local coronary inflammation, and its association with coronary artery plaque in PWH. Moreover, we compared PCAT density in PWH with matched controls from the community-based FHS to investigate HIV's relationship with increased coronary inflammation. This study revealed 2 main findings. First, PCAT density was strongly associated with critical CT indices of subclinical CAD in PWH, independent of cardiovascular risk factors, body composition, and immune activation biomarkers. Second, we observed significantly higher coronary inflammation in REPRIEVE than in an age-, sex-, BMI-, statin use–, and CAC-matched FHS population.
PCAT Density as a Novel Marker of Vascular Inflammation
PCAT is an important fat depot with a unique location. PCAT surrounds coronary arteries without a connective tissue barrier, making it susceptible to inflammatory mediators released by inflamed vessel walls [29]. Moreover, PCAT contains immune cells such as macrophages and T lymphocytes [30] and is thought to have paracrine functionality. The less differentiated smaller PCAT adipocytes have a secretory profile favoring local production of pro-inflammatory cytokines (eg, interleukin-6 [IL-6], IL-8, monocyte chemoattractant protein 1) and less adiponectin, potentially contributing to vascular wall inflammation [31]. Indeed, ex vivo studies have demonstrated that vessel-derived inflammatory cytokines (eg, tumor necrosis factor-alpha, IL-6, interferon-gamma) inhibit human preadipocyte differentiation and lower adipocyte lipid content [13]. This effect leads to a higher connective tissue-to-lipid volume ratio, regional fibrosis, and increased PCAT density on CT. In contrast, obesity leads to a higher adipocyte lipid content, a lower connective tissue-to-lipid ratio, and lower PCAT density on CT [13], reflected in our study by a lower PCAT density in those with higher BMI and waist circumference (Table 1). Therefore, we adjusted our multivariable analyses for BMI and showed that PCAT density is associated with CAD characteristics independent of obesity. Moreover, elevated 18F-sodium fluoride uptake on positron emission tomography has been correlated with increased subcutaneous adipose tissue density in animal studies (rho = 0.69, P < .001) in which adipocyte size is driven by the infiltration of activated macrophages and insulin resistance [13]. Elevated 18F-sodium fluoride uptake has been linked to increased lesion-specific PCAT density in high-risk plaques, suggesting a role of coronary inflammation in the development of CAD [32].
Increased PCAT Density is Strongly Related to Coronary Plaque in PWH
In REPRIEVE, the prevalence of coronary artery plaques was relatively high (49%) despite an overall low to moderate ASCVD risk of 4.5% [8]. This important observation aligns with prior investigations in PWH [11, 33] and indicates that low-clinical-risk individuals may develop CAD through unique pathophysiological pathways. In this regard, increased arterial inflammation may promote CAD among PWH. Prior data demonstrated increased arterial inflammation using (technetium-99 m–tilmanocept single photon emission computed tomography/CT) and 18F-2-deoxy-glucose positron emission tomography among virologically controlled PWH [5, 12]. However, these studies assessed aortic radiotracer uptake without assessing coronary artery inflammation. We now show that PCAT density, as an index of local coronary inflammation, relates not only to presence of plaque but to critical plaque features in this population, including vulnerable plaque and plaque burden (ie, Leaman score), known to relate to clinical disease [26]. This finding was robust, and a 10-HU difference in pericoronary fat density was associated with a 44% higher risk of coronary plaque, independent of clinical ASCVD risk and inflammatory indices. To provide perspective, a 1-percentage point difference in ASCVD risk score, representing approximately 25% of the median risk score of 4.5% in REPRIEVE, was associated with a 14% higher risk of coronary plaque in this model. Providing further clinical context, Takahashi et al demonstrated MACE in 14.2% versus 5.6% in those with PCAT density ≥ −93.55 HU. A higher percentage of PWH in REPRIEVE met this clinically relevant cutoff than in the FHS.
Of note, our results were in line with data from the Cardiovascular RISk Prediction using Computed Tomography study, revealing that perivascular inflammation around the proximal segments of the major coronary arteries without detectable CAD was associated with high-risk plaque features elsewhere [14].
Relationship of PCAT Density to CAD is Independent of Systemic Inflammation
Interestingly, PCAT density's association with CAD was independent of systemic inflammatory and immune activation indices, highlighting the important paracrine nature of pericoronary inflammation with respect to plaque as suggested in prior studies [13, 17]. Moreover, the relationship of PCAT to plaque remained significant controlling for HIV-related indices. Indeed, assessing PCAT density allows us to evaluate an index of local coronary inflammation in juxtaposition to the coronary artery wall, which may influence plaque independent of systemic inflammatory indices.
Comparison of PWH to FHS Controls
We compared PCAT density in PWH with FHS controls for the first time, showing a modestly higher PCAT density in PWH compared with age, sex, BMI, CAC, and statin use–matched controls. This observation remained significant in a model further adjusting for relevant covariates not used in the matching. The magnitude of this difference in PCAT density between FHS and controls was about two-thirds the difference between those with and without plaque among PWH in REPRIEVE. Furthermore, the magnitude of the relationship between PCAT density and CAD, at least as assessed by CAC, available in both studies, is generally similar.
Optimal Imaging of Pericoronary Adipose Tissue Density
Although there are patented artificial intelligence–enhanced algorithms (eg, CaRi-HEART algorithm [34] fat attenuation index) [13, 14], we chose to assess PCAT density using the noncontrast highly standardized (consistent 120 kVp and slice thickness) coronary artery calcium scans available from both studies [35] to minimize the effect of technical confounding and harmonize the measurement across studies. This method successfully showed a robust association of PCAT density with CAD in both REPRIEVE and FHS and has been used successfully by other groups assessing pericoronary inflammation [28, 35]. Furthermore, our data suggest that assessing PCAT density from low-cost, low-radiation, and noncontrast calcium scoring, recommended for primary prevention, may be a valuable strategy to identify those at increased risk for CAD in PWH with low traditional cardiovascular risk.
Strengths and Limitations
To the best of our knowledge, this is the first study investigating pericoronary inflammation in PWH. We assessed PCAT density in a large, well-phenotyped primary prevention cohort of PWH and compared it with data in a well-known community-based cohort, controlling for traditional risk factors and key systemic inflammatory indices. The REPRIEVE population was recruited from ACTG sites across the United States and represents the HIV population here in terms of race, gender, and substance use [36]. The current study is cross-sectional, limiting causal conclusions between PCAT and CAD. In our comparison to controls from the FHS, we matched key covariates and controlled for differences in the study populations. There may be other differences in CT technique that may influence our results, but we used a uniform method to assess PCAT from standardized noncontrast images across both studies to limit any differences. Future studies with other more racially diverse control populations are needed to confirm these results. We hypothesized that there might be a difference in the relationship of PCAT density to plaque characteristics, though this relationship was similar for CAC in both REPRIEVE and FHS. Future studies should investigate the relationship between PCAT density and increased hard clinical endpoints and compare the relationship of PCAT to other CAD indices among PWH and control populations.
CONCLUSIONS
Among PWH living in the United States at low-to-moderate cardiovascular risk, PCAT density was associated with a higher prevalence of CAD, CAC, vulnerable plaque, and overall plaque burden. This relationship remained significant adjusting for cardiovascular risk factors, anthropometric measures, and systemic inflammatory indices. Moreover, PCAT density was higher among PWH in REPRIEVE compared with FHS controls, suggesting higher vascular inflammation in PWH. PCAT density may thus represent a novel marker of vascular and subclinical coronary pathology. Future studies should assess the mechanisms of increased perivascular inflammation in PWH and how strategies to affect PCAT density may affect plaque and future events. Early identification of CAD risk markers such as PCAT density may help to optimize ASCVD preventive strategies among PWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Borek Foldyna, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Thomas Mayrhofer, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA; Department of Health Economics, School of Business Studies, Stralsund University of Applied Sciences, Stralsund, Germany.
Markella V Zanni, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Asya Lyass, Department of Mathematics and Statistics, Boston University, Boston, Massachusetts, USA.
Radhika Barve, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Julia Karady, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Sara McCallum, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Tricia H Burdo, Department of Microbiology, Immunology, and Inflammation and Center for NeuroVirology and Gene Editing, Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, USA.
Kathleen V Fitch, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Kayla Paradis, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Evelynne S Fulda, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Marissa R Diggs, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Gerald S Bloomfield, Department of Medicine, Duke Global Health Institute and Duke Clinical Research Institute, Duke University, Durham, North Carolina, USA.
Carlos D Malvestutto, Division of Infectious Diseases, Ohio State University Medical Center, Columbus, Ohio, USA.
Carl J Fichtenbaum, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Judith A Aberg, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Judith S Currier, Division of Infectious Diseases, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California, USA.
Heather J Ribaudo, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Udo Hoffmann, Innovative Imaging Consulting LLC, Waltham, Massachusetts, USA.
Michael T Lu, Department of Radiology, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Pamela S Douglas, Department of Medicine (Cardiology), Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Steven K Grinspoon, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Notes
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. In addition, we thank the following: the AIDS Clinical Trial Group (ACTG) for clinical site support; ACTG Clinical Trials Specialists for regulatory support; the data management center, Frontier Science Foundation for data support; and the Center for Biostatistics in AIDS Research for statistical support. The parent trial is registered at ClinicalTrials.gov under: NCT03070223
Financial support. This work was supported by National Institutes of Health (NIH) grants U01HL123336 to the REPRIEVE Clinical Coordinating Center and U01HL123339 to the REPRIEVE Data Coordinating Center, as well as funding from Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. The National Institute of Allergy and Infectious Diseases (NIAID) supported this study through grants UM1 AI068636, which supports the AIDS Clinical Trials Group (ACTG) Leadership and Operations Center; and UM1 AI106701, which supports the ACTG Laboratory Center. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute or the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tawakol A, Lo J, Zanni M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr 2014; 66:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zanni MV, Toribio M, Wilks MQ, et al. Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis 2017; 215:1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann U, Lu MT, Foldyna B, et al. Assessment of coronary artery disease with computed tomography angiography and inflammatory and immune activation biomarkers among adults with HIV eligible for primary cardiovascular prevention. JAMA Netw Open 2021; 4:e2114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a U.S. health care system. J Acquir Immune Defic Syndr 2010; 55:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS Lond Engl 2013; 27:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toribio M, Wilks MQ, Hedgire S, et al. Increased macrophage-specific arterial infiltration relates to noncalcified plaque and systemic immune activation in people with human immunodeficiency virus. J Infect Dis 2022; 226:1823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017; 9:eaal2658. [DOI] [PubMed] [Google Scholar]
- 14. Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018; 392:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goeller M, Achenbach S, Cadet S, et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol 2018; 3:858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuneman JH, van Rosendael SE, van der Bijl P, et al. Pericoronary adipose tissue attenuation in patients with acute coronary syndrome versus stable coronary artery disease. Circ Cardiovasc Imaging 2023; 16:e014672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sagris M, Antonopoulos AS, Simantiris S, et al. Pericoronary fat attenuation index—a new imaging biomarker and its diagnostic and prognostic utility: a systematic review and meta-analysis. Eur Heart J—Cardiovasc Imaging 2022; 23:e526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann U, Lu MT, Olalere D, et al. Rationale and design of the mechanistic substudy of the randomized trial to prevent vascular events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daily statin reduces the risk of cardiovascular disease in people living with HIV, large NIH study finds. 2023. Available at: https://www.nih.gov/news-events/news-releases/daily-statin-reduces-risk-cardiovascular-disease-people-living-hiv-large-nih-study-finds. Accessed 14 June 2023.
- 21. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol 2008; 102:1136–1141.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15:827–32. [DOI] [PubMed] [Google Scholar]
- 24. Leipsic J, Abbara S, Achenbach S, et al. SCCT Guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014; 8:342–58. [DOI] [PubMed] [Google Scholar]
- 25. Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014; 64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonçalves P dA, Garcia-Garcia HM, Dores H, et al. Coronary computed tomography angiography-adapted Leaman score as a tool to noninvasively quantify total coronary atherosclerotic burden. Int J Cardiovasc Imaging 2013; 29:1575–84. [DOI] [PubMed] [Google Scholar]
- 27. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–421. [PubMed] [Google Scholar]
- 28. Takahashi D, Fujimoto S, Nozaki YO, et al. Usefulness of new method to quantify pericoronary adipose tissue on ECG-gated non-contrast chest CT scan. J Am Coll Cardiol 2022; 79:1236.35361345 [Google Scholar]
- 29. Lin A, Dey D, Wong DTL, Nerlekar N. Perivascular adipose tissue and coronary atherosclerosis: from biology to imaging phenotyping. Curr Atheroscler Rep 2019; 21:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y-J, Lu C, Su L-Y, Sharma AM, Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 2007; 151:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes. Circ Res 2009; 104:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacek K, Damini D, Sebastien C, et al. Peri-coronary adipose tissue density is associated with 18F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging 2019; 12:2000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antoniades C, Antonopoulos A, Neubauer S, Channon K. Method for characterisation of perivascular tissue. 2020; Available at:https://patents.google.com/patent/US10695023B2/en. Accessed 2 November 2021.
- 35. Almeida S, Pelter M, Shaikh K, et al. Feasibility of measuring pericoronary fat from precontrast scans: effect of iodinated contrast on pericoronary fat attenuation. J Cardiovasc Comput Tomogr 2020; 14:490–4. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. HIV Surveillance Report. Diagnoses of HIV infection in the Unites States and dependent areas 2020. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-33/index.html. Accessed 13 February 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.