Introduction
Global attention to the threat and mitigation of Antimicrobial resistance (AMR) has been increasing with more research and funding dedicated to understanding the problem and the optimal tools to mitigate it. Although the World Health Organization (WHO)'s 2015 Global Action Plan on AMR [1] helped trigger a series of government led initiatives to develop AMR National Action Plans (NAPs), in Low and Middle Income Countries settings, competing priorities for limited resources and even the publication of NAPs may not facilitate a robust in-country response to the challenge. More work is needed to support tailoring NAPs, and the proposed interventions to the specific contexts and needs of countries, together with identifying methods to make NAPs actionable locally [2].
Antimicrobial Stewardship (AMS) programs promote rational use of antimicrobials to help reduce the risk of AMR, and are key approaches specified in NAPs developed by countries. Lifting and shifting AMS interventions from one setting or country to another can be ineffective, especially with the AMS approach only just starting to ‘surface’ in some LMICs. The word stewardship does not translate easily to many other languages, in that it may not foster an intellectual understanding of its meaning in the context in which it is being promoted. As such, there is still a lack of understanding of AMS not just because of the incongruity of the term, but also because of the work needed to catch up developmentally to address the burden of disease and the escalating issue of AMR.
Here, we share perceptions of challenges to implementing AMS interventions from the perspective of the co-authors who originate from or have worked in the following LMICs across multiple continents (Africa, Asia and Europe): Lebanon, Cambodia, Uzbekistan, Moldova, Armenia, Georgia, Kenya, Tanzania, Rwanda, Zambia, Ghana, Nigeria, Uganda, Mozambique and Malawi. We highlight some of the available open-access AMS resources that have been co-developed by healthcare workers across multiple countries including LMICs.
Context matters
AMS preparedness between LMICs varies greatly. In some, the building blocks are not yet in place, with the foundations to support stewardship needing to be addressed first, while others have systems that allow AMS integration into current practices. Testing of AMS tools across several countries has demonstrated the need for a better contextual understanding to support implementation of country-specific, practical, and effective tools. Since implementation depends on functional health systems, a whole-systems approach is required. The term LMIC itself groups a wide range of countries that may have very different characteristics, challenges and capacity, [3] and has the potential to overgeneralize understanding of how AMS tools for LMICs can be lifted and shifted, even within the LMIC setting. Developing a true understanding of behaviour around AMS should be at the forefront of decision making to avoid inadvertent harm. While external experts may have an important supporting role, local experts and frontline stewards are crucial to developing and tailoring AMS tools based on the specific country and health facility needs.
Challenges to implementation of AMS programmes and tools
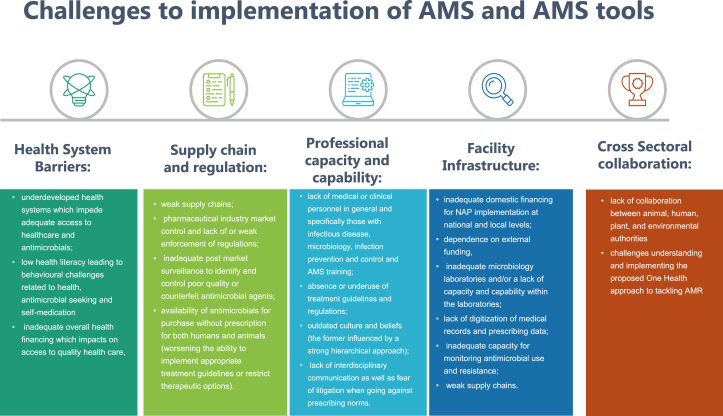
Several barriers to implementation of AMS in LMICs have been identified by the authors. A simplistic approach is to correlate the spread of AMR with the two main factors: overuse of antimicrobials in humans, animals, and the environment, and increased transmission of multidrug-resistant organisms due to inadequate infection prevention and control (IPC). Tackling these contributing factors is complicated by a number of overlapping barriers organised into the following themes: health system, supply chain and regulation, professional capacity and capability, health facilities infrastructure, and cross-sectoral collaborations (Figure 1).
Figure 1.
Challenges to implementation of AMS and AMS tools.
One explicitly challenging barrier often deterring the capabilities of governments, as well as international organisations, is the presence of political strife and conflict and the associated difficulties pertinent to physical and mental trauma. Conflict leads to destabilisation of health systems, healthcare worker (HCW) migration and forceful displacement of populations internally and externally. This creates pockets of vulnerable populations with different needs requiring different AMS approaches during direct and long-term crisis management. Thirty-five million refugees are scattered mostly in the eastern Mediterranean region and sub-Saharan Africa followed by south Asia and Europe and central Asia [4]. In general, the fragility of states impedes the capacity to provide basic health services and mitigate the impact of infections and AMR. Although many governments have been attempting to improve access to healthcare through insurance schemes, over 92% of people in low-income countries and 73% in middle-income countries remain uninsured [5]. Resources diverge from healthcare to basic need provision where AMS is often brushed aside. In such settings, even use of available AMS and IPC tools becomes challenging. Conflict and AMR seem to be associated through not only the socio-economic impact, but also through the abundance of toxic heavy metals which may contribute to heavy-metal resistance as in Acinetobacter baumannii [6] for example. Global interventions targeting AMR need to pay special attention to conflict resolution.
Reframing the AMS approach
Well-coordinated, integrated and context-specific regional approaches should be considered in the development of global resources and tools aimed at implementing AMS in LMICs. Data scarcity, on which low-cost interventions should be prioritised in LMICs (especially if facing political instability) is a barrier to tool applicability particularly in settings of competing health priorities. Tailored rather than generalised approaches will be required to manage country-specific challenges. Questions to be addressed are: How can these public health emergencies be triaged? How can IPC, water, sanitation and hygiene (WASH) and AMS tools be integrated into current workflows rather than creating siloed initiatives that potentially become “new projects”? It is crucial to reframe our approach and consider the overall impact of resource-poor health systems, and work towards achieving sustainable development goals which touch on a wide range of determinants of AMR.
To tackle some of the major practical barriers to AMS implementation at national level and at the frontline, we argue that international and national organisations should acknowledge the importance of including pharmacy and nursing leadership in AMS alongside physicians in policy and practice settings. Current AMS recommendations by various institutions tend to name physicians as AMS leaders due to their medical training and possibly due to existing leadership hierarchies within the medical field, both at the frontline as well as for international health organisations. However, many countries do not provide dedicated AMS training for healthcare professionals. Multidisciplinary dedicated training in AMS for pharmacists, nurses, and physicians would be ideal. If not available, finding interested HCWs supported by guidelines and action roadmaps can guide the strengths of the multidisciplinary team (MDT) members to implement practical AMS interventions.
AMS team key members should include local HCWs who have recently or currently work at the bedside.
Acknowledgment of the importance of bottom-up AMS initiatives in LMICs by professional, governmental and international organisations is needed. Written AMS recommendations by global organisations such as the WHO is vital, for example promoting subnational expert participation in national groups and government policy processes as being valuable to support change.
Capacity building is needed, targeting ground-level multidisciplinary HCWs. Development of comprehensive, freely-accessible, tailored educational resources (and formal education, where deemed necessary) is needed for pharmacists, physicians, microbiologists, and all HCWs crucial to the team. High-level policy and project management training is also needed. A list of some currently available free resources co-produced by colleagues across both high and low/middle income countries are included in Table II. Multiple-country collaborations and bidirectional learning across different income settings is important for building and strengthening capacity for developing and implementing AMS interventions. Examples of international collaborative AMS efforts have recently been provided by Ashiru-Oredope et al. [7].
Table II.
Open access Antimicrobial Stewardship Tools. Co-developed by colleagues in more than one country especially those within resource limited settings
| AMS section | Tool and description | Created/led by | Access |
|---|---|---|---|
| Education and programme development | 1. Regional Guidance Document for the Development of Antimicrobial Stewardship Programs 1st Edition; July 26 2023 2. AMS Training curriculum for Schools of Pharmacy 3. Open AMR/AMS/IPC E-Learning modules |
1. East Central Southern Africa Health Community 2. University of Nairobi, School of Pharmacy; MOH Kenya 3. WHO |
https://ecsahc.org/document/antimicrobial-stewardship-guidance-document/ https://pharmacy.uonbi.ac.ke/index.php/event/antimicrobial-stewardship-training https://openwho.org/courses/practical-toolkit-for-AMS https://openwho.org/courses/IPC-AMR-EN https://openwho.org/courses/AMR-competency https://openwho.org/courses/IPC-leadership-EN https://openwho.org/courses/IPC-INTRO-EN |
| Free training available elsewhere | 4. IPC, AMS and Patient Safety 5. The Fleming Fund knowledge and resources 6. British Society for Antimicrobial Chemotherapy Massive Open Online Course 7. BSAC MOOC dedicated to Middle East |
4. Ministry of Health (MOH) Kenya 5. Fleming Fund 6. BSAC 7. BSAC E-learning Academy |
https://elearning.health.go.ke/course/index.php?categoryid=24 https://www.flemingfund.org/knowledge-resources/ https://bsac.org.uk/stewardship-surveillance/massive-open-online-course-on-antimicrobial-stewardship/ https://www.futurelearn.com/courses/antimicrobial-stewardship-for-the-middle-east |
| Surveillance/Audit tools | 8. Global Point Prevalence Survey - free web-based tool 9. World Health Organization Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals. Version 1.1 1 February 2019 - Technical Document 10. WHO AMS Assessment Checklist for National and Facility level preparedness |
8. Global PPS 9. WHO 10. WHO |
https://www.global-pps.com/project/ https://www.who.int/publications/i/item/WHO-EMP-IAU-2018.01 https://siapsprogram.org/wp-content/uploads/2012/12/12-096-AMR-Hospital-Indicator-Manual.English.final-11.13.12.pdf |
| AMR awareness campaigns | 11. World Health Organisation Campaigns: The World AMR Awareness Week | 11. WHO | https://www.who.int/campaigns/world-antimicrobial-awareness-week#:∼:text=World%20AMR%20Awareness%20Week%20 |
| Innovative | 12. The AMS Game 13. AMS Explainer Videos for healthcare workers and the public |
12, 13. Commonwealth Partnerships for Antimicrobial Stewardship Programme, (CwPAMS) |
https://www.amsgame.com/ https://www.youtube.com/playlist?list=PL9qDtywmdsRBhwyc0XFUz5204Z1vQCrWd |
| Repositories/observatories | 14. BSAC Global AMS partnership hub | 14. BSAC | https://bsac.org.uk/bsac-launches-global-antimicrobial-stewardship-partnership-hub/ |
| International professional bodies/networks | 15. International Society of Antimicrobial Chemotherapy A-Z List of societies. Free Membership | 15. ISAC | https://www.isac.world/member-societies/members-list |
| Treatment Guidelines | 16. Africa Centres for Disease Control (CDC) 17. WHO AWaRe Antibiotic Book |
16. Africa CDC 17. WHO |
https://africacdc.org/programme/surveillance-disease-intelligence/antimicrobial-resistance-control/ https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2022.02 |
| Policy tools | 18. WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities | 18. WHO | https://openwho.org/courses/policy-guidance-on-AMS# |
| Open access journals with helpful AMS content | 19. Antibiotics MDPI 20. JAC-Antimicrobial Resistance 21. Journal of Antimicrobial Chemotherapy 22. Journal of Infection and Public Health 23. Antimicrobial Stewardship & Healthcare Epidemiology |
19. MDPI 20. JAC-AMR 21. JAC 22. JIPH 23. ASHE |
https://www.mdpi.com/journal/antibiotics https://academic.oup.com/jacamr https://academic.oup.com/jac https://www.sciencedirect.com/journal/journal-of-infection-and-public-health https://www.cambridge.org/core/journals/antimicrobial-stewardship-and-healthcare-epidemiology |
| Diagnostic stewardship | 24. Diagnostic stewardship A guide to implementation in antimicrobial resistance surveillance sites (2016) | 24. WHO | https://apps.who.int/iris/bitstream/handle/10665/251553/WHO-DGO-AMR-2016.3-eng.pdf |
| IPC | 25. Infection Prevention and Control Training-MOH KenyaWHO IPC Surveys: 26. Infection prevention and Control assessment framework at the facility level 27. WHO Global Survey on Minimum Requirements for Infection Prevention and Control programmes at the National level WHO IPC Guidance: 28. WHO Infection Prevention and Control: Guidance to Action Tools: Respiratory and Hand Hygiene; Personal Protective Equipment; Environmental Cleaning, Waste and Linen Management 29. Guides and tools for improvement in Hand Hygiene 30. WHO Infection Prevention and Control Implementation tools |
25. MoH Kenya 26-30. WHO |
IPC Training-MOH Kenya https://elearning.health.go.ke/course/index.php?categoryid=24 https://www.who.int/publications/i/item/WHO-HIS-SDS-2018.9 https://www.who.int/news-room/articles-detail/who-global-survey-on-minimum-requirements-for-infection-prevention-and-control-programmes-at-the-national-level#:∼:text=The%20IPCAT%2DMR%20is%20a,HQ%2C%20regional%20or%20country%20offices. https://www.who.int/europe/publications/i/item/9789289055437 https://www.who.int/teams/integrated-health-services/infection-prevention-control/hand-hygiene/implementation-tools https://www.who.int/teams/integrated-health-services/infection-prevention-control/hand-hygiene/monitoring-tool https://www.who.int/teams/integrated-health-services/infection-prevention-control 30 https://www.who.int/health-topics/infection-prevention-and-control#tab=tab_1 |
Funding of projects and research initiated by frontline stewards who develop and implement practical stewardship tools is needed. This is particularly important in the setting of data scarcity, as many in LMICs may not showcase their work through peer-reviewed publication and may not have the required visibility or platform to connect. The new AMR exchange community allows those in the field to post comments and questions. This helps, at least partially, to bridge networking gaps and, depending on the setting, allows experiences to be shared and re-purposed.
We have discussed some of the changes needed to create realistic environments that foster AMS and implementation of AMS tools. Beyond direct program policy difficulties, patient behaviour has been cited as a significant determinant of antimicrobial use (AMU) in the community setting through self-medication. Although well described that education alone has a limited impact on changing behaviour, it is still an important base for the implementation of interventions. Most current interventions focus on raising patient awareness, which is important. Not enough work is done, however, on promoting early school-level education on systems-thinking, hygiene, AMR, and One Health. Tools available through the global e-Bug maybe useful as starting points for adaptation [8]. Working with ministries of education can be a solution in some countries. In addition, many LMIC school systems depend on accreditation bodies, and coordination with such bodies to include these issues may help build creative communities, empower local initiatives, and possibly improve the sense of accountability, political and social justice [9]. This could help improve health in general in addition to assisting efforts to combat AMR, as we have seen with the impact of education on individual lifetime carbon emissions [10].
Changing approaches to AMS policy and practice is challenging, more so in makeshift temporary health systems. It is therefore particularly important that AMR strategies attempt to address the basic healthcare needs of forcibly displaced populations, focusing on improving living conditions to reduce risk of disease and subsequently, AMR [11]. A significant proportion of healthcare in these settings is delivered through aid, which may not address AMR and AMS initiatives. One example of successful non-government organization (NGO) AMR efforts includes the Doctors Without Borders [12] development of an AMR department and publication of freely available tools to the countries they serve. Similar work should be encouraged, promoted and published through peer-reviewed journals to increase visibility and reproducibility of such interventions.
In all settings, a recently available easily achieved intervention is the implementation of the AWaRe antibiotic book [13]. By having an easy-to-read format and available as a mobile phone application, it can be used at the end-user level of all backgrounds. It does not need significant governmental support to roll out in terms of policy, since the book focuses on community acquired infections that generally do not need to be tailored for local resistance patterns. Governments, however, would need to ensure that antimicrobials from the book, which are from the Essential Medicines list, are available.
Learning from infection prevention & control interventions for LMICs
Whilst AMS interventions, which are designed to optimise rational AMU, can decrease the rate of AMR development and acquisition, acquisition of infections and the spread of AMR can also be prevented through effective IPC practice, important for reducing antimicrobial use. The World Health Organisation Global Report on Infection Prevention and Control [14] outlines that IPC is at the heart of many health priorities such as health emergencies, International Health Regulations, patient and health worker safety, AMR action plans, sepsis prevention, WASH and integrated people centred care. Therefore, a strong focus on strengthening IPC would serve to strengthen other health priorities.
Partnership working between professions, such as between nurse-physician-pharmacist, across both IPC and AMS, will enhance both patient safety and the patient experience (Table I). The nurse's near-patient vigilance gives a unique opportunity to support a robust approach to infection detection, monitoring and treatment evaluation.
Table I.
Identified themes that can influence infection prevention, management and prescribing practices at the frontline
| Theme | Areas identified that can be impacted at the frontline |
|---|---|
| IPC practice and capacity | Working to influence the IPC agenda, seeking an IPC infrastructure that can be led and/or supported by nurses together with other disciplines |
| Avoidance of lifting and shifting of policies and practices from HICs | Seeking to develop relevant and feasible ways of working for LMIC settings by influencing development of tailored policies and practices for the setting |
| Culture and belief | Influencing health-seeking behaviour through awareness-raising and education in the community and the healthcare setting |
| Inter- and intra-disciplinary relationships and collaboration | Promoting partnership working between nurse-pharmacist-physician and within disciplines |
| Surveillance capacities | Establishing and strengthening the role of the nurse in surveillance activities from community/ward-level upwards |
| Education of healthcare workers | Lobbying to expand education and training from an early stage to strengthen nurse/pharmacist leadership and professional development |
| Bottom-up approaches | Working at community, ward and clinic level to develop both partnership-working for nurse-pharmacist-physician and strengthen the influence of the nurse's ‘near-patient’ role |
| Nurses and pharmacists being and seeking champions | Identifying healthcare professionals who can champion the role of AMS |
| WHO AWaRe Guidelines (Book) | Maintaining awareness amongst professionals; championing and promoting compliance; reviewing practice and antimicrobial availability as part of a team |
| Strengthening systems | Lobbying government for system improvement to facilitate effective IPC and AMS including allocation of funds e.g., for digitization of patient records and prescribing |
Conclusion
Assessing the success of adaptation of interventions into LMIC settings, particularly in relation to AMS, is subjective. Measuring what success looks like, more so with AMS, depends on the starting point in any given country. Financial constraints and current system structures can be seen as prohibitive. With almost ten years since the WHO adopted the GAP in 2015, AMS has only just started to emerge beyond small sound bites for some LMICs. In one country, the starting point has been to prove the worth of the system by undertaking a national pilot study, introducing AMS onto key units within a number of national hospitals, focusing on areas including surgery, intensive care, nephrology and endocrinology. This has yet to be completed due to interruption by COVID-19 and the impact of war. Addressing issues such as health infrastructure and availability (and control) of antimicrobials entering a given country is key if the AWaRe system is to be used effectively. As the term ‘stewardship’ does not translate well into many languages, it can be seen as confusing and daunting. Small steps provide a starting point, promoting a national champion for example and engaging clinicians and other practitioners both nationally and locally.
The barriers to implementing AMS in LMICs are many. To understand AMR and its determinants, delving into health inequities and identifying politics that impact health decisions is indivisible. To tackle AMR, a wholesome move towards education, capacity-building, and reframing the problem and its mitigating factors is necessary. Inclusivity in policy development and implementation by end users in LMICs is also key to fostering creative context-specific interventions, while more funding for implementation and qualitative behavioural science research is needed. Whilst global collaborations are valuable, it is especially important that tools and interventions are co-developed and context-specific. The freely-available resources are important to share with all frontliners, and advocacy for participation by all relevant healthcare workers will help optimise approaches to AMS in LMICs.
Acknowledgements
We acknowledge Mrs Karen Shaw who provided initial insights into the manuscript.
References
- 1.World Health Organization . World Health Organization; Geneva: 2015. Global action plan on antimicrobial resistance [Internet]https://apps.who.int/iris/handle/10665/193736 [cited 2023 May 2]. 28 p. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Charani E., Mendelson M., Pallett S.J.C., Ahmad R., Mpundu M., Mbamalu O., et al. An analysis of existing national action plans for antimicrobial resistance—gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Global Health. 2023 Feb doi: 10.1016/S2214-109X(23)00019-0. S2214109X23000190. [DOI] [PubMed] [Google Scholar]
- 3.Lencucha R., Neupane S. The use, misuse and overuse of the ‘low-income and middle-income countries’ category. BMJ Glob Health. 2022 Jun;7(6) doi: 10.1136/bmjgh-2022-009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations Refugee Agency. Refugee Data Finder [Internet]. [cited 2023 Jul 15]. Available from: https://www.unhcr.org/refugee-statistics/download/?url=9b1rSC.
- 5.Hooley B., Afriyie D.O., Fink G., Tediosi F. Health insurance coverage in low-income and middle-income countries: progress made to date and related changes in private and public health expenditure. BMJ Glob Health. 2022 May;7(5) doi: 10.1136/bmjgh-2022-008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazzi W., Abou Fayad A.G., Nasser A., Haraoui L.P., Dewachi O., Abou-Sitta G., et al. Heavy Metal Toxicity in Armed Conflicts Potentiates AMR in A. baumannii by Selecting for Antibiotic and Heavy Metal Co-resistance Mechanisms. Front Microbiol. 2020 Feb 3;11:68. doi: 10.3389/fmicb.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashiru-Oredope D., Langford B.J., Bonaconsa C., Nampoothiri V., Charani E., Goff D.A. Global collaborations in antimicrobial stewardship: All hands on deck. Antimicrob Steward Healthc Epidemiol. 2023 Apr 5;3(1):e66. doi: 10.1017/ash.2023.122. PMID: 37113199; PMCID: PMC10127241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Health Security Agency. e-Bug [Internet]. Available from: https://www.e-bug.eu/.
- 9.Spiteri J. In: Handbook of Human and Planetary Health. Climate Change Management. Leal Filho W., editor. Springer; Cham: 2022. The Untapped Potential of Early Childhood Education for Planetary Health: A Narrative Review. [DOI] [Google Scholar]
- 10.Cordero E.C., Centeno D., Todd A.M. The role of climate change education on individual lifetime carbon emissions. Pausata FSR. PLoS One. 2020 Feb 4;15(2) doi: 10.1371/journal.pone.0206266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capturing the evidence on access to essential antibiotics in refugee and migrant populations. World Health Organization; Geneva: 2022. [PubMed] [Google Scholar]
- 12.MSF Doctors without Boarders. Antibiotic Resistance. https://www.doctorswithoutborders.org/what-we-do/medical-issues/antibiotic-resistance.
- 13.World Health Organization . 2022. The WHO AWaRe (access, watch, reserve) antibiotic book. Geneva.https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2022.02 Available from: [Google Scholar]
- 14.World Health Organization . WHO; 2022. Global Report on Infection Prevention and Control. [Google Scholar]