Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a complex behavioral disorder characterized by hyperactivity, impulsivity, inattention, and deficits in working memory and time perception. While animal models have advanced our neurobiological understanding of this condition, there are limited and inconsistent data on working and elapsed time memory function. Inflammatory signaling has been identified as a key factor in memory and cognitive impairments, but its role in ADHD remains unclear. Additionally, the disproportionate investigation of male subjects in ADHD research has contributed to a poor understanding of the disorder in females. This study sought to investigate the potential connections between memory, neuroimmunology, and ADHD in both male and female animals. Specifically, we utilized the spontaneously hypertensive rat (SHR), one of the most extensively studied animal models of ADHD. Compared to their control, the Wistar-Kyoto (WKY) rat, male SHR are reported to exhibit several behavioral phenotypes associated with ADHD, including hyperactivity, impulsivity, and poor sustained attention, along with impairments in learning and memory. As the hippocampus is a key brain region for learning and memory, we examined the behavior of male and female SHR and WKY rats in two hippocampal-dependent memory tasks. Our findings revealed that SHR have delay-dependent working memory deficits that were similar to, albeit less severe than, those seen in hippocampal-lesioned rats. We also observed impairments in elapsed time processing in female SHR, particularly in the discrimination of longer time durations. To investigate the impact of inflammatory signaling on memory in these rats, we analyzed the levels of several cytokines in the dorsal and ventral hippocampus of SHR and WKY. Although we found some sex and genotype differences, concentrations were generally similar between groups. Taken together, our results indicate that SHR exhibit deficits in spatial working memory and memory for elapsed time, as well as some differences in hippocampal cytokine concentrations. These findings contribute to a better understanding of the neurobiological basis of ADHD in both sexes and may inform future research aimed at developing effective treatments for the disorder. Nonetheless, the potential mediating role of neuroinflammation in the memory symptomatology of SHR requires further investigation.
Keywords: Spontaneously hypertensive rat, ADHD, Working memory, Memory for elapsed time, Hippocampus, Cytokines
Highlights
-
•
Both male and female Spontaneously Hypertensive Rats (SHR) exhibit working memory deficits in a delayed alternation task.
-
•
Working memory deficits in SHR are comparable to those observed in hippocampal-lesioned rats, albeit less severe, particularly under conditions of high working memory load.
-
•
Only female SHR exhibited impaired performance in discriminating long time intervals (i.e., 20 s), while their ability to discriminate short time intervals (i.e., 10 s) was relatively unaffected.
-
•
Limited sex and genotype differences in hippocampal levels of IL-1ɑ, IL-1β, IL-4, IL-6, IL-10, IL-18, and TNF-ɑ were found among SHR and WKY rats.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder broadly characterized by symptoms of inattention, impulsivity, and hyperactivity which impair social, academic and/or occupational functioning (American Psychiatric Association, 2013). According to the Centers for Disease Control, 2022, an estimated 6 million American children aged 3–17 years have been diagnosed with ADHD, accounting for nearly 10% of all children in this age range in the United States (2022). Often persisting into adulthood (Sontag et al., 2013), this disorder is associated with a variety of negative outcomes, including impaired social function (Bunford et al., 2015), academic underachievement across all educational levels (Daley and Birchwood, 2010), poorer employment outcomes (Shaw et al., 2012), and financial burden both individually (Altszuler et al., 2016) and societally (Pelham et al., 2007). Although ADHD is most often associated with inattention and hyperactivity, deficits in both working memory and memory for elapsed time have also been measured in people with ADHD (Smith et al., 2002; Martinussen et al., 2005; Raiker et al., 2012; Lee and Yang, 2019; Mukherjee et al., 2021).
Broadly, working memory is a limited capacity cognitive function involved with the internal representation of information necessary for conscious thought, decision-making, and overt behavior during an activity (Martinussen et al., 2005; Baddeley, 2007; Campez et al., 2020). Relatedly, time perception can be defined as the subjective experience of time tied to the ability to arrange one's life in temporal order (Weissenberger et al., 2021). Importantly, impairments in working memory and time perception have been suggested to be the key underlying force behind other symptoms of ADHD, like inattention (Orban et al., 2018; Fried et al., 2019), underscoring the need to identify the neural mechanisms underlying the links between working memory deficits and ADHD-associated behavioral traits.
The hippocampus is a key brain region known to contribute to these functions, both through its subregion-specific functioning and connections to other regions of interest including the prefrontal cortex and medial entorhinal cortex (mEC) (Preston and Eichenbaum, 2013; Tsao et al., 2018). In rats, hippocampal lesions have been previously shown to cause delay-dependent deficits in both working memory and memory for elapsed time using delayed alternation (DA) and time duration discrimination (TDD) tasks, respectively (Ainge et al., 2007; Sabariego et al., 2019, 2021; Vo et al., 2021). While these lesion studies demonstrate the central role of the hippocampus in modulating working memory and memory for time, it remains unknown whether these observed deficits in ADHD are hippocampal-dependent.
Animal models can provide mechanistic insight into conditions that cannot be easily obtained from human studies. The spontaneously hypertensive rat (SHR) is the most widely investigated animal model of ADHD studied to date. Derived from the Wistar-Kyoto (WKY) rat (Okamoto and Aoki, 1963), the SHR displays many of the major behavioral characteristics of ADHD, as well as some genetic and brain structure similarities to people with ADHD. More specifically, SHR are inattentive, hyperactive, and impulsive, especially over time when reinforcers are infrequent (see Sagvolden et al., 2009 for review).
However, past studies examining memory function in SHR have yielded mixed results and have also consistently under-studied female animals (Sontag et al., 2013; and see Wyss et al., 1992; Mori et al., 1995; Nakamura-Palacios et al., 1996; Ferguson et al., 2007; Hernandez et al., 2003; Mook et al., 1993), despite evidence that ADHD manifests differently in males and females and may be associated with distinct comorbidities in each sex (Young et al., 2020). If consistent with ADHD symptomatology in humans, SHR would be expected to have deficits in their working memory and time processing (Smith et al., 2002; Martinussen et al., 2005; Raiker et al., 2012; Lee and Yang, 2019; Mukherjee et al., 2021).
To investigate these potential deficits in working memory and time perception among SHR, in this study, we utilized two hippocampal-dependent behavioral tasks, a DA and TDD task. Additionally, we used a light-dark box (LDB) test to evaluate whether underlying differences in anxiety might confound interpretations of memory performance (Rafaela et al., 2012). Because the behavior of hippocampal-lesioned rats had not been previously examined under a 60-s delay condition in the DA task, we also collected data from hippocampal- and sham-lesioned animals in the DA task, which allowed us to make comparisons between hippocampal-lesioned animals and SHR. Finally, to explore the potential mechanisms underlying the hypothesized hippocampal-dependent memory dysfunction in SHR, we investigated the role of neuroinflammation in the hippocampus.
Inflammation in the hippocampus can cause long-lasting memory impairments through altered cytokine signaling (Yirmiya and Goshen, 2011; Marin and Kipnis, 2013; Donzis and Tronson, 2014). Elevated concentrations of IL-6 and IL-10 have been observed in the serum of children with ADHD (Donfrancesco et al., 2021) and pro-inflammatory cytokine levels have been found to positively correlate with severity of ADHD symptoms (Oades et al., 2010). Drtilkova et al., 2008 have even found a significant difference in the allele and genotype frequency of polymorphisms affecting IL-6 and TNF-ɑ transmission in children with ADHD.
In SHR, past studies have only assessed cytokine levels in male animals and never in the hippocampus. In their assessment of serum cytokine concentrations, Kozłowska et al. (2019) found elevated levels of a variety of proinflammatory cytokines in 5-week-old but not 10-week-old SHR. These similarities in peripheral cytokine elevations in humans and rodent models underscore the validity of the SHR rodent model to interrogate plausible neuroimmune mechanisms underlying memory performance in ADHD. In our study, we assessed hippocampal concentrations of two anti- and five pro-inflammatory cytokines previously studied in the context of memory and/or ADHD (delRey et al., 2013; Donzis and Tronson, 2014; Kozłowska et al., 2019; Xiao et al., 2021): IL-1ɑ, IL-1β, IL-4, IL-6, IL-10, IL-18, and TNF-ɑ.
To summarize, we aimed to investigate several questions related to memory deficits and cytokine levels in the hippocampi of both male and female SHR and WKY rats. First, we assessed whether SHR exhibit any impairments in working memory and time perception and completed an exploratory assessment of anxiety levels in SHR and WKY rats. Second, we compared the performance of SHR and WKY rats with that of hippocampal- and sham-lesioned rats in a spatial working memory task. Finally, we studied cytokine levels in the hippocampi of SHR and WKY rats to determine whether inflammatory-mediated dysfunction in this brain region critical for memory could underlie observed deficits. We hypothesized that SHR would exhibit deficits in working memory and time perception as well as elevated pro-inflammatory cytokine concentrations, as compared to WKY rats in both sexes.
2. Materials and methods
2.1. Subjects
All experimental procedures were approved by the Institutional Animal Care and Use Committees at Mount Holyoke College and the University of San Diego. In total, 70 experimentally-naïve rats were used: Long-Evans, n = 12 (12 females); SHR, n = 30 (15 females; 15 males); and WKY rats, n = 28 (13 females; 15 males). All rats were acquired from Charles River Laboratories (Long-Evans strain code: 006; SHR strain code: 007; WKY strain code: 008). Rats were housed individually on a reversed 12-h light/dark cycle (at Mount Holyoke College) and on a conventional 12-h light/dark cycle (at the University of San Diego).
Rats tested at Mount Holyoke College completed the LDB test (n = 23) and DA task (n = 24), while rats tested at the University of San Diego completed the TDD task (n = 22) (Table 1). All rats were food restricted and maintained at ∼85% of their ad libitum weight while running the DA and the TDD tasks. The order in which animals ran the tasks was counterbalanced between cohorts. Rats were approximately 9–11 weeks at the start of behavioral testing. After completing behavioral testing, all rats were sacrificed at approximately 12–16 weeks of age at Mount Holyoke College and at 25–44 weeks old at the University of San Diego.
Table 1.
Behavioral tasks and experimental manipulations. Cohort 1 (n = 12) consisted of female Long-Evans rats, half of which were hippocampal-lesioned. This cohort completed the delayed alternation (DA) task. Cohorts 2, 3 and 4 consisted of equal numbers of SHR and WKY rats of both sexes. Cohort 2 (n = 24) and Cohort 4 (n = 12) completed the light-dark box (LDB) test. Cohort 2 also completed the DA task. Cohort 3 (n = 22) completed the time duration discrimination (TDD) task. All cohorts were food deprived for ∼6 weeks and sacrificed after behavioral testing. Cohorts 2, 3 and 4 underwent brain extraction and cytokine analysis via a Luminex immunoassay.
| DA | TDD | LDB | Luminex | |
|---|---|---|---|---|
| COHORT 1 | ✓ | |||
| COHORT 2 | ✓ | ✓ | ✓ | |
| COHORT 3 | ✓ | ✓ | ||
| COHORT 4 | ✓ | ✓ |
2.2. Surgical procedures
Twelve female Long-Evans rats underwent brain surgery, six received hippocampal-lesions and six received sham-lesions. All surgery was performed using aseptic procedures. Anesthesia was maintained throughout surgery with isoflurane gas (0.8–2.0% isoflurane delivered in O2 at 1 L/min). The animal was positioned in a Kopf stereotaxic instrument and the incisor bar was adjusted until Bregma was level with Lambda. The bone overlying the target site was removed using a high-speed drill. Bilateral excitotoxic hippocampal-lesions were produced by local microinjections of ibotenic acid (IBO, Fisher Scientific). IBO was dissolved in 0.01 M phosphate-buffered saline to provide a solution with a concentration of 10 mg/mL before being infused at a rate of approximately 0.1 μL/min using a 10 μL Hamilton syringe mounted on a stereotaxic frame and held with a Kopf model 5000 microinjector. The syringe needle was lowered to the target and left in place for 1 min before the start of the infusion. After the infusion was completed, the syringe needle was left in place for 2 min to reduce the spread of IBO up the needle tract. IBO was injected into 18 sites (total volume 0.51 μL) within each hippocampus (all coordinates are in millimeters and relative to Bregma): anteroposterior (AP) −2.4, mediolateral (ML) ± 1.0, dorsoventral (DV) −3.5; AP −3.2, ML ± 1.4, DV −3.1, −2.3; AP −3.2, ML ± 3.0, DV −2.7; AP −4.0, ML ± 2.5, DV −2.8, −1.8; AP −4.0, ML ± 3.7, DV −2.7; AP −4.8, ML ± 4.9, DV −7.2, −6.4; AP −4.8, ML ± 4.3, DV −7.7, −7.1, −3.5; AP −5.4, ML ± 4.2, DV −4.4, −3.9; AP −5.4, ML ± 5.0, DV −6.6, −5.9, −5.2, −4.5. The procedure for sham animals was the same as for animals with hippocampal lesions, except that burr holes were not drilled and IBO was not injected. After completion of each lesion, the wound was closed, and the animal was allowed to recover from anesthesia on a water-circulating heating pad. Behavioral testing began two weeks after surgery.
2.3. Behavioral testing
For tasks performed at Mount Holyoke College, behavioral testing occurred in the dark phase of the light-dark cycle. The TDD task, which took place at the University of San Diego, was run during the light phase. All behavioral assessments were conducted in a dimly lit room. Behavioral apparatuses were cleaned with 70% ethanol between rats and animal order was counterbalanced. Animals were handled by all experimenters regularly during the week before behavioral tasks began.
2.3.1. Working memory: delayed alternation (DA) task
The DA task is a hippocampal-dependent working memory task (Ainge et al., 2007), which requires rats to alternate between left and right sides of a figure- 8 maze in order to receive a food reward (Cocoa Pebbles™) (Fig. 1a). Here, we adapted the task to include a longer (60-s) delay condition. For detailed methods on the procedure used for this task, see Hoxha and Sabariego (2020). In short, delays of varying lengths (no delay, 10 s, and 60 s) were used to assess spatial working memory performance at varying working memory loads (delays), with longer delays correlating to higher working memory loads. The task consisted of three phases after habituation. For all phases, the task began by placing the rat at the base of the central stem of the apparatus, facing the choice arms. Rats were trained every day for ∼ four weeks.
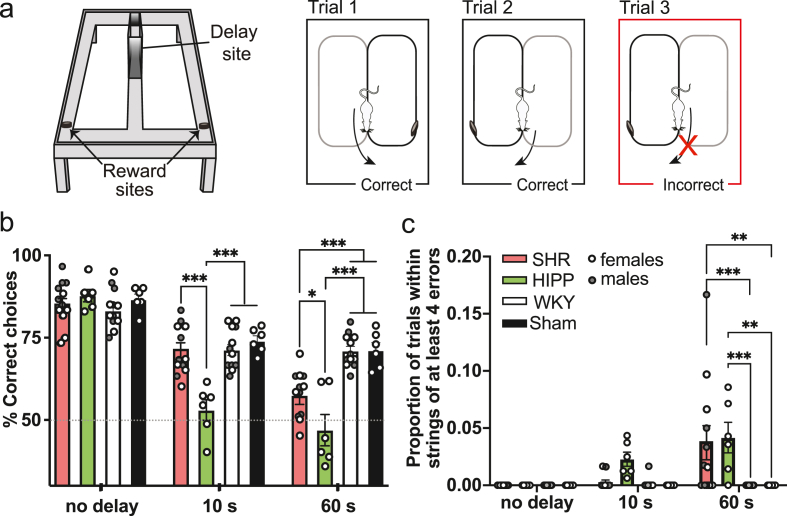
Fig. 1.
SHR exhibit similar working memory deficits to hippocampal-lesioned rats, particularly in long delay trials of the DA task. Tukey's multiple comparison test: *p < 0.05, ***p < 0.001. (a) Experimental apparatus and paradigm for the delayed alternation task. This task was run on an elevated figure-eight maze. Animals received a food reward for correctly alternating between left and right arms of the maze. (b) Average percentage of correct choices for female and male SHR (n = 12), WKY (n = 12), hippocampal-lesioned (n = 6), and sham-lesioned (n = 6) rats in each delay condition (no delay, 10 s, and 60 s). All groups performed similarly in the 0-s delay condition, but hippocampal-lesioned rats performed significantly worse than sham-lesioned, SHR, and WKY rats in the 10-s delay trials. In the 60-s delay condition, hippocampus-lesioned rats once again performed worse than all groups, but SHR also performed worse than sham-lesioned and WKY rats. (c) Average proportion of trials with strings of four or more errors for SHR, WKY, hippocampal lesion, and sham-lesioned rats in each delay condition (no delay, 10 s, and 60 s). No groups made error strings of four or more errors in the no delay condition. In the 10-s delay condition, there was a trend for hippocampal-lesioned rats to make more errors than the other groups. In the 60-s delay condition, SHR and hippocampus-lesioned rats performed similarly, with both SHR and hippocampus-lesioned rats making a higher number of error strings of four or more errors than WKY and sham-lesioned rats.
In phase one, barriers were manually placed at the choice point to force the rat to alternate between right and left choice arms. A food reward (one Cocoa Pebbles™) was delivered at the end of the choice arm, just around the corner and out-of-sight. The first phase continued until the rats ran consistently, reliably ate the food rewards, and did not turn around or go in the wrong direction—usually for five days. Training sessions consisted of 30 trials or 20 min, whichever came first. In phase two, the rats were free to choose between the two arms of the maze, but only received a food reward for alternating trials. Once achieving 85% accuracy in the 30 trials on two out of three consecutive days, rats moved on to the final phase. In phase three, delays were introduced such that in some trials, animals had to wait 10 or 60 s at the delay site before being free to choose between the two arms of the maze. Delays were imposed using manual opaque plastic barriers at the base of the center arm. This phase was run for six days with each session consisting of 10 trials of each delay length, and 10 trials without any delay between trials (i.e. 0-s delay), totaling to 30 trials. The accuracy of the rats’ performance in this phase was then analyzed and compared between sexes, delay lengths, and genotypes. Lower percent accuracy was interpreted as worse working memory function.
2.3.2. Memory for elapsed time: time duration discrimination (TDD) task
The TDD task is a hippocampal-dependent task designed to assess a rat's ability to discriminate between different lengths of elapsed time (Sabariego et al., 2021; Tenney et al., 2021). For detailed methods on the procedure used for this task, see Tenney et al. (2021). In short, rats were trained to turn to one side of a figure-8-maze (e.g., left) after a 10-s delay and to the other side (e.g., right) after a 20-s delay, as depicted in Fig. 2a. A tone (2000 Hz, 70 dB) was played throughout the timed delay to increase the salience of the delay length. When the rats discriminated between the two delay lengths correctly, they received a food reward (one Cocoa Pebbles™) at the end of the choice arm, just around the corner and out-of-sight. Throughout all phases, the reward was placed during the delay, while the rat was in the delay box, so as not to cue the animal to its location.
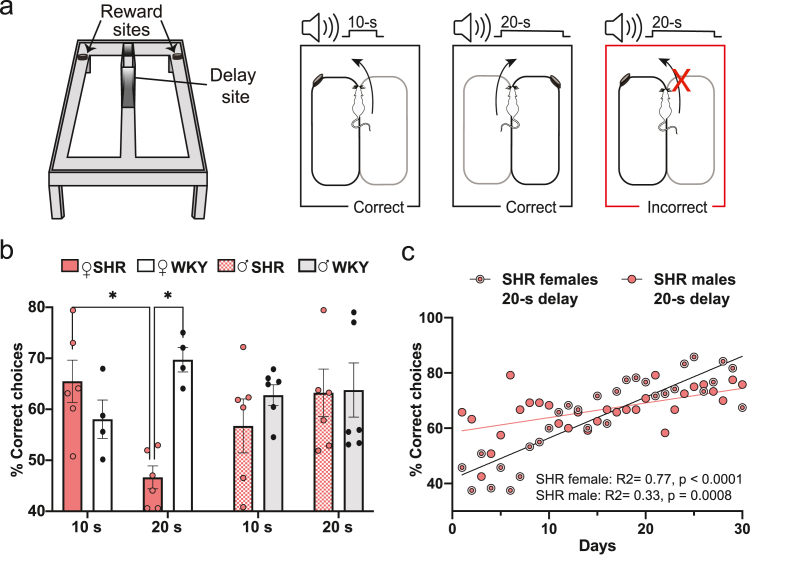
Fig. 2.
Memory for elapsed time initially impaired in female SHR in the TDD task. Tukey's multiple comparison test: *p < 0.05. (a) Experimental apparatus and paradigm for the TDD task. This task was run on an elevated figure-eight maze. Animals received a food reward for turning left after a 10-s delay and for turning right after a 20-s delay. (b) Average percentage of correct choices during the first 10 days of testing for each sex, genotype, and time duration. SHR females (n = 6) performed significantly worse in the 20-s versus 10-s delay condition. Relative to WKY females (n = 4), SHR females made significantly more errors during 20-s duration trials. No genotype differences were found between SHR (n = 6) and WKY (n = 6) males. (c) Average percentage of correct choices made by SHR females and males in the 20-s delay trials across all 30 days of the experiment. Although both sexes improved in task accuracy across the training period, the regression slopes were significantly different between sexes. Females began the training period performing worse than males, but ultimately achieved similar performance by the end of the testing period.
After habituation, the task consisted of two phases, training and testing. For all phases, the task began by placing the rat at the base of the central stem of the apparatus, facing the delay box and choice arms, and consisted of 40 trials or 30 min, whichever came first. During training, when the delay ended, the rats were directed to turn the proper direction with manual plastic barriers. Rats moved on to the testing phase once they ran consistently, reliably ate the food rewards, and did not turn around or go the wrong direction for two consecutive days. In the testing phase, when the delay ended, rats had free choice between the left and right arms of the maze, but only received a food reward for making correct turn choices. This phase was run for 30 days. Memory for elapsed time was assessed by calculating the percentage of correctly completed trials at each delay duration.
2.3.3. Anxiety-like behavior: light-dark box (LDB) test
The LDB test is a common behavioral measure used to assess rats' unconditioned anxiety response by putting rats’ innate light aversion and spontaneous exploratory behavior into conflict (Miller et al., 2011). Here, rats were placed in the light compartment of a two-compartment chamber apparatus facing away from the exit into the dark chamber and allowed to freely explore for 10 min (Fig. 3a). The dimensions of the light compartment were 30.48 cm × 21 cm x 8.25 cm. The dimensions of the dark compartment were 42.55 cm × 21 cm x 21 cm. Three behavioral measures were recorded: (1) the latency for the rat to enter the dark compartment with all four paws after being placed in the light compartment, (2) the latency for the rat to re-enter the light compartment with all four paws after first entering the dark compartment, and (3) the total time spent in the light vs dark compartments. Time spent in each compartment was automatically measured (by Med Associates Inc. Med-PC software) while the latencies for the rat to enter the dark compartment and then re-enter the light compartment were recorded by hand by two experimenters using stop watches, then averaged between experimenters. Shorter latencies to leave the light compartment, longer latencies to re-enter the light compartment, and less overall time spent in the light compartment were interpreted as increased anxiety-like behavior.
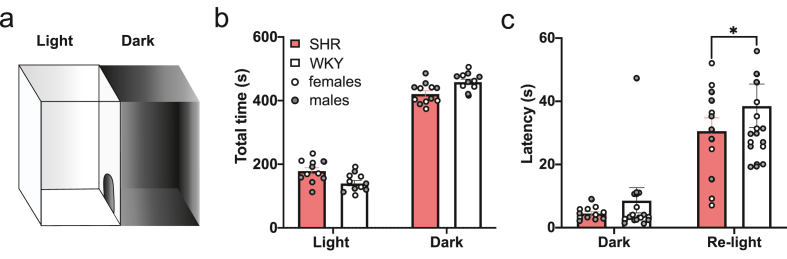
Fig. 3.
Different latencies to re-enter into the light compartment but similar total time in light and dark compartments between SHR and WKY in the LDB test indicative of similar anxiety-like behavior. Unpaired t-test: *p < 0.05. (a) Schematic of the LDB apparatus with light and dark compartments. Rats were initially placed in the light compartment. (b) Average total time that rats of each genotype spent in the light and dark compartments. Male and female SHR (n = 12) and WKY (n = 11) rats spent a similar amount of time in the light and dark compartments. (c) Average latencies to first enter the dark compartment and re-enter the light compartment. WKY rats had a significantly higher latency to re-enter the light compartment, but the latencies to enter the dark compartments were similar between SHR and WKY rats.
2.4. Histological procedures
At the completion of testing, hippocampal- and sham-lesioned rats were administered an overdose of sodium pentobarbital and perfused transcardially with 0.01 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde solution (in 0.01 M PBS). Brains were then removed from the skull and kept in a solution of 4% paraformaldehyde for 24 h before being transferred to a 30% sucrose solution for an average of 48 h. Coronal sections (40-μm thick) were cut with a cryostat beginning at the level of the anterior commissure and continuing posterior through the length of the hippocampus. Every fifth section was mounted and stained with cresyl violet to assess the size of the lesions. Each section was assessed under magnification using the Cavalieri method on ImageJ. The tissue was considered damaged if it was absent or necrotic (i.e., hippocampal tissue was present, but there was no evidence of Nissl staining, or the tissue was gliotic). The volume of the spared tissue in the dentate gyrus (DG), CA1, CA2, and CA3 cell layers was quantified. The percentage of damage was calculated by normalizing the volume of spared tissue in each hippocampal-lesioned animal to the average volume of the hippocampus in animals from the sham group according to the following formula:
2.5. Immunological assays
At least 24 h after completing behavioral testing, SHR and WKY rats were anesthetized with 3% isoflurane and sacrificed. Whole brains were dissected and both ventral and dorsal hippocampus samples were collected, and flash frozen in liquid nitrogen or isopentane before being stored at −80 °C.
2.5.1. Tissue homogenization and sample dilution
Hippocampal samples were thawed and homogenized using a 3.85 μL/mg tissue buffer of Bio-Plex® Cell Lysis containing Factor 1, Factor 2, and 2 mM PMSF (BioRad; catalog #:171304011). Homogenates were then mixed with diluted buffer solution for 20 min at 4 °C in an orbital shaker and centrifuged at 4 °C at 4500 g for 4 min. The resulting supernatant was used for subsequent analyses. Supernatants of each sample were diluted 1:50 in cell lysis buffer and protein concentrations determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific; Cat #23225). The results of the BCA protein assay were used to normalize protein concentration in all samples such that each had a 2000 μg/mL overall protein concentration.
2.5.2. Multiplex bead-based immunoassay
Using Luminex® xMAP® technology, analysis of brain cytokines was performed on diluted homogenate via a 7-plex Millipore Sigma® MILLIPLEX® Rat Cytokine Magnetic Bead panel (#RECYTMAG65K). In specific, concentrations of IL-1ɑ, IL-1β, IL-4, IL-6, IL-10, IL-18, and TNF-ɑ in the dorsal and ventral hippocampus of rats (n = 30) were quantified using a MAGPIX® multiplex reader. We assessed cytokines levels separately in the dorsal and ventral hippocampus, as these regions are known to have separate functions (Fanselow and Dong, 2010; Moser and Moser, 1998). After each brain sample was diluted to a concentration of 2000 μg/mL of protein concentration per sample, 25 μL of premixed magnetic bead solution containing beads for each of the seven cytokines measured (1:60 dilution with assay buffer) was precisely added to the wells along with 25 μL of diluted samples, standards, and quality-controls, as well as 25 μL of assay buffer. After a 2-h incubation at room temperature (RT), the plate was washed two times, 25 μL of detection antibody was added to each well, and the plate was left to incubate at RT. Then, 25 μL of Streptavidin-Phycoerythrin was added to each well before a 30-min incubation at RT in the dark. Finally, the plate was then washed two times and 150 μL of drive fluid was added to all wells. Intervening wash steps were done using Bio-Plex handheld magnet (Bio-Rad Laboratories, Hercules, CA, USA). After the final wash, the bead color and fluorescence were systematically read by a MAGPIX® machine using xPONENT 4.2 software. The machine had been calibrated and verified earlier in the day using MAGPIX® Calibration (#MPX-CAL-K25) and MAGPIX® Performance Verification (#MPX-PVER-K25) kits. Bead color informed analyte identity and amount of fluorescence informed analyte quantity. Fluorescence levels for each bead type in the samples were compared to a 5-parameter logistic regression curve created from known standard concentrations (manufacturer provided) in order to estimate unknown cytokine concentrations. All cytokines with a bead count <50 were excluded. The minimum amounts of detectable cytokine concentrations are as follows: IL-1ɑ: 4.2 pg/mL, IL-1β: 2.8 pg/mL, IL-4: 3.1 pg/mL, IL-6: 30.7 pg/mL, IL-10: 2.7 pg/mL, IL-18: 6.2 pg/mL, and TNF-ɑ:1.9 pg/mL. In total, two 96-well plates were run with sex and genotype of each sample counter-balanced between plates.
2.6. Statistical analysis
For all of the behavioral statistics reported in this article, sex and genotype were always between-subject factors whereas delay and session were always within-subject factors. An alpha value equal to or lower than 0.05 was used for statistical inferences. We analyzed the LBD data using unpaired t-tests and the DA and TDD data using linear regression analyses and two-way ANOVAs, with post-hoc Tukey's and Fisher's pairwise comparisons to identify the source of significant interactions. All behavioral statistics were computed using Prism Version 9.5.1. Immunological data were analyzed using RStudio version 2022.12.0 + 353. To better account for variability resulting from the use of two separate well plates, linear mixed effects modeling was used with each well-plate set as a random effect and genotype and sex set as fixed effects. Model variations were assessed using the likelihood ratio test and the best model was selected based on the lowest Akaike Information Criterion (AIC). Models that included a significant interaction component in the fixed effect were confirmed using pairwise post-hoc analysis.
3. Results
3.1. SHR exhibit delayed-dependent working memory deficits similar to hippocampal-lesioned rats
To examine working memory performance in SHR and WKY rats, as well as hippocampal-lesioned and sham-lesioned Long-Evans rats, we used a DA task with 10- and 60-s delays (Fig. 1a). This task has previously been demonstrated to be dependent on the hippocampus in male rats when 2- or 10-s delays were interposed (Ainge et al., 2007). In our female hippocampal-lesioned animals, 93.7% of the total hippocampus volume was ablated (93.4% of CA1, 95.4% of CA2, 96.3% of CA3 and 89.8% of DG; Supplementary Fig. 1a and b). Fig. 1b shows the accuracy of all SHR, WKY, hippocampal-, and sham-lesioned rats in trials of each delay length. Since the hippocampal- and sham-lesioned rats were all female, we initially only analyzed delayed alternation data from the hippocampal- and sham-lesioned rats in comparison to female SHR and WKY rats (Supplementary Fig 1c). Because there were no sex differences in DA performance among male and female SHR and WKY rats, we decided to combine the sexes in subsequent analyses. In a two-way ANOVA (delay x group) with sphericity assumed, we found a highly significant interaction between delay and group (F(6, 96) = 9.406, p < 0.001), and main effects of both delay (F(2, 96) = 112.1, p < 0.001) and group (F(3, 96) = 18.10, p < 0.001). Although post-hoc analysis revealed that all groups performed similarly in the 0-s delay condition, in the 10-s delay condition, Tukey's multiple comparisons test showed that hippocampal-lesioned rats performed significantly worse than sham-lesioned (CI: −31.18 to −10.00, p < 0.001), SHR (CI: −27.58 to −10.00, p < 0.001), and WKY (CI: −27.02 to −9.444, p < 0.001) rats. In the 60-s delay condition, hippocampal-lesioned rats once again performed worse than sham-lesioned (CI: −34.40 to −14.10, p < 0.001), SHR (CI: −19.36 to −1.778, p = 0.012), and WKY rats (CI: −32.80 to −15.22, p < 0.001). In this case, however, SHR also showed impairment, performing worse than sham-lesioned (CI: −22.47 to −4.888, p < 0.001) and WKY (CI: −20.62 to −6.267, p < 0.001) rats. This deficit in SHR performance in the 60-s delay condition persisted despite SHR and WKY rats performing similarly in the training phases of this task (Supplementary Fig 2).
It has previously been proposed that hyperactivity may act as a confound in assessments of the spatial working memory function of SHR (Sontag et al., 2013). To determine if this may be the case in our delayed alternation task, we plotted average trial accuracy versus average velocity for SHR and WKY rats in each delay condition (Supplementary Fig. 1d and e). Using a linear regression analysis, we found a wider range of velocities in SHR that, in general, tended to be faster than WKY. Moreover, we observed a trend for the average percentage of correct trials to be negatively correlated with average velocity (cm/s) for SHR in the no delay, 10-s and 60-s delay trials. However, the slope of the best fit line was only significantly non-zero in the no delay condition (CI: −1.985 to −0.5320, F(1,10) = 14.9, p = 0.0032, R2 = 0.5983). For the WKY rats, no significant correlation was found between trial accuracy and average velocity across any of the delay conditions. Since there were no significant genotype differences in average trial accuracy in the no delay condition, the effect of hyperactivity on working memory performance was ruled out.
Interestingly, in addition to showing similar overall trial accuracies, SHR and hippocampal-lesioned rats demonstrated comparable patterns of error types, specifically a tendency to make multiple errors consecutively (Fig. 1c). To analyze this behavioral pattern, we quantified the number of error strings of at least four errors in each group and delay condition. A two-way ANOVA (delay x group) revealed a significant interaction (F(6, 64) = 3.844, p = 0.002), as well as main effects of delay (F(2, 64) = 3.844, p < 0.001) and group (F(3, 32) = 6.039, p = 0.002). No groups had error strings of four or more errors in the 0-s delay condition (Fig. 1c). Although only hippocampal-lesioned rats tended to make more than four errors in a row in the 10-s delay trials, both hippocampal-lesioned rats and SHR made prolonged error strings in the 60-s delay trials (Fig. 1c). Post-hoc analysis using Tukey's multiple comparisons test revealed that SHR and hippocampal-lesioned rats made a similar proportion of error strings of at least four errors during the 60-s delay condition. SHR made a significantly higher proportion of these error strings compared to WKY (CI: 0.01642–0.05795, p < 0.001) and sham-lesioned rats (CI: 0.01176–0.06262, p = 0.001) rats. Similarly, hippocampal-lesioned rats also made significantly more of these error strings when compared to WKY (CI: 0.01624–0.06710, p < 0.001) and sham-lesioned rats (CI: 0.01230–0.07103, p = 0.002). In sum, SHR show a similar tendency for behavioral perseverance as hippocampal-lesioned rats, but only in the 60-s delay condition.
3.2. Elapsed time discrimination is initially impaired in female SHR at longer delays
We measured the ability of rats to learn and discriminate elapsed time durations of 10-s versus 20-s using a TDD task (Fig. 2a). Two female WKY rats were excluded from the analyses due to a complete side bias (turning left on 98% of trials during the first seven days of testing). To compare TDD performance in SHR and WKY rats of both sexes during the first 10 days of testing, we ran a three-way repeated measures mixed model analysis in which we found a significant three-way interaction (sex x delay x genotype; F(1,36) = 9.35, p = 0.004; Fig. 2b). There was also a significant two-way interaction (delay x genotype; F(1,36) = 4.45, p = 0.04). Tukey's multiple comparisons tests confirmed that SHR females performed significantly worse on 20-s delay trials compared to 10-s delay trials (CI: 0.92–36.74, p = 0.03) and to WKY females on 20-s delay trials (CI: 3.03–43.08, p = 0.01; Fig. 2b). No significant differences were found amongst males. Using previously unpublished data from Long-Evans males used in Sabariego et al. (2021), we also ran a two-way ANOVA to compare the performance of SHR and WKY males to that of the Long-Evans males during the first 10 days of testing, which can be seen in Supplementary Fig 3a. The delay × genotype interaction was found not significant (F(2,15) = 0.071, p = 0.93), validating the use of WKY rats as controls, and supporting the finding that only female SHR were impaired in this task.
Given that the impairments in performance were seen in SHR females on 20-s delay trials during the initial learning 10-day period, we wanted to determine whether SHR showed sex-related differences in performance across all 30 days of training. Using a linear regression analysis, we found that the percentage of correct trials increased across testing days for both female (F(1,28) = 94.83, p < 0.0001, R2 = 0.77, CI: 1.17–1.79) and male (F(1,28) = 14.02, p = 0.0008, R2 = 0.33, CI: 0.24–0.83) SHR, but that the regression slopes for female versus male SHR rats on 20-s delay trials were significantly different (F(1,56) = 20.40, p < 0.0001), since females performed worse than males during the early trials but eventually had a similar percentage of correct choices towards the later trials (Fig. 2c). Comparing performance for all groups of rats on the 20-s delay trials across all 30 days of training, the male and female WKY rats and male SHR rats performed above chance level throughout all days of testing, whereas the female SHR rats performed at or below chance initially before reaching a comparable level of performance to the other groups of rats (Supplementary Fig 3b).
3.3. Both SHR and WKY rats prefer the dark compartment of the LDB, but SHR show shorter latencies to return to the light compartment
Given that strong differences in anxiety-like behavior have the capacity to disrupt the performance of rodents in memory tasks (Rafaela et al., 2012), we assessed whether there were any differences in anxiety-like behavior between SHR and WKY rats using the LDB test. For all analyses, there were no significant effects of sex and so data were combined to include male and female animals of each genotype. SHR and WKY rats performed similarly in the LDB task, suggesting no significant differences in anxiety-like behaviors (Fig. 3b and c). In two independent samples t-tests, SHR and WKY rats were found to spend a similar amount of time in the light and dark compartments (Fig. 3b). Although significant results were found in an unpaired t-test analysis of the latency to re-enter the light compartment after first entering the dark compartment between SHR and WKY rats (t(33) = 2.234, p = 0.032), the latencies to enter the dark compartment were similar between SHR and WKY rats (Fig. 3c).
3.4. Some sex and genotype differences in TNF-ɑ, IL-4, IL-10, and IL-18 levels in the hippocampus
Cytokines are immune proteins required for many critical neural processes, the dysregulation of which can result in learning and memory impairments (Aubert et al., 1995; Pugh et al., 1998; Cunningham and Sanderson, 2008). To determine if dysregulated inflammatory signaling could be impairing hippocampal function in the SHR, we examined a range of cytokines previously linked to hippocampal-dependent memory processes: IL-1ɑ, IL-1β, IL-4, IL-6, IL-10, IL-18, and TNF-ɑ (delRey et al., 2013 Donzis and Tronson, 2014; Kozłowska et al., 2019). Dorsal and ventral hippocampal samples were analyzed separately using Luminex immunoassay technology, which compares the fluorescence levels of unknown samples to a known concentration curve in order to estimate protein concentrations.
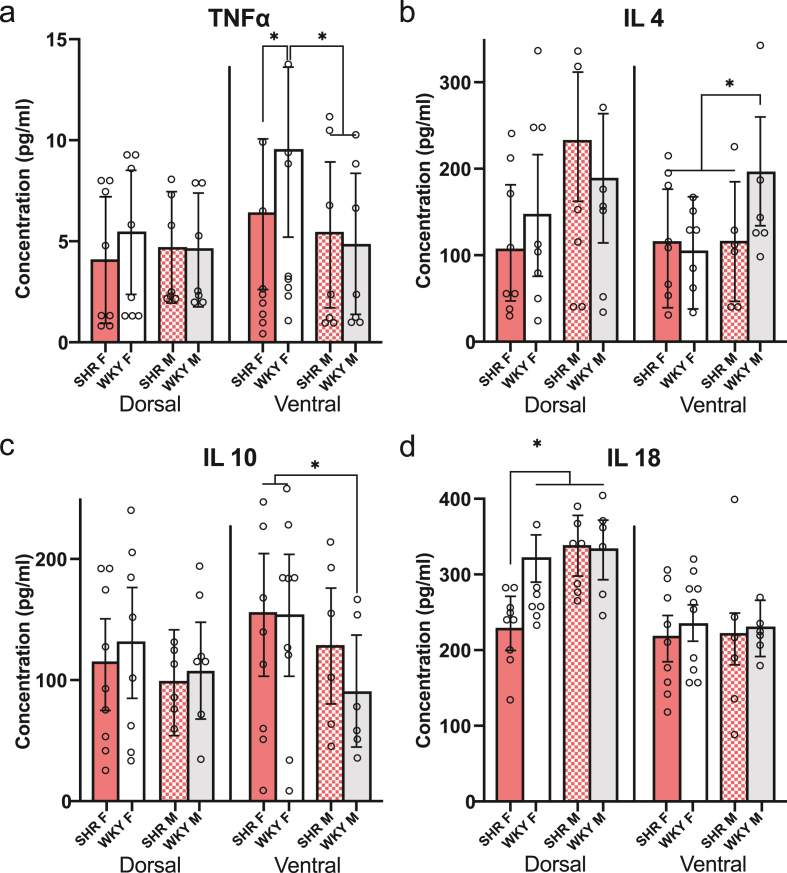
After running a Luminex immunoassay on the dorsal and ventral hippocampal samples of SHR and WKY rats, the concentrations of IL-1ɑ, IL-1β, IL-4, IL-6, IL-10, IL-18, and TNF-ɑ were extrapolated from a known concentration curve and then analyzed using linear mixed-effects modeling. A model that included genotype, sex, and interaction was found to be significant for TNF-ɑ, IL-4, IL-10, and IL-18 (Fig. 4), but not for IL-1ɑ, IL-1β, nor IL-6 (Supplementary Fig 4). Additionally, animal age was found to be a significant covariate in the best fit models for TNF-ɑ and IL-10. In the best fit model for TNF-ɑ (p = 0.015, χ2 = 5.93), WKY females had significantly higher TNF-ɑ concentrations in the ventral hippocampus than WKY males (p = 0.0006), SHR females (p = 0.051), and SHR males (p = 0.002; Fig. 4a). This resulted in a statistically significant effect of genotype in TNF-ɑ concentrations among females (b = −2.42, CI: −4.66 to −0.19, p = 0.051) as well as a significant sex effect among WKY rats (b = −5.17, CI: −7.67 to −2.68, p = 0.001), effects that were still present despite the model including age as a significant source of variability. Pairwise comparisons between male WKY rats and SHR females and males indicated no differences in TNF-ɑ, suggesting that these genotype effects are only present in females (p > 0.05 for all comparisons). Regarding IL-4, the model selected (p = 0.025, χ2 = 7.40) also indicated a sex difference, once again only among WKY rats (b = 91.55, CI: 26.97–156.12, p = 0.013; Fig. 4b). Here, age was not a significant covariate and therefore was excluded from the final model. Post-hoc analysis confirmed that male WKY had higher ventral hippocampal IL-4 concentrations than WKY females (p = 0.022), SHR males (p = 0.041), and SHR females (p = 0.022). Unlike IL-4, WKY males had lower concentrations of IL-10 in the ventral hippocampus than the other groups. Specifically, the best fit model, which included age as a covariate (p = 0.062, χ2 = 7.48), showed a significant effect of sex among WKY rats (b = −74.87, CI: −122.89 to −26.85, p = 0.007; Fig. 4c). Post-hoc analysis confirmed that male WKY had lower ventral hippocampal IL-10 concentrations than both WKY females (p = 0.007) and also SHR females (p = 0.003). No significant differences in ventral hippocampal IL-10 concentrations were found between WKY and SHR males (p > 0.05). Finally, the model found to be significant for IL-18, in which age was not a significant covariate (p = 0.0017, χ2 = 9.82), revealed a significant effect of genotype on IL-18 concentrations among females (b = −102.15, CI: −184.43 to −19.87, p = 0.026) but not males (p = 0.869). More specifically, SHR females had lower IL-18 concentrations in the dorsal hippocampus than WKY females (p = 0.0257), WKY males (p = 0.0458), and SHR males (p = 0.0002; Fig. 4d). Models with fixed effects were not significant for the remaining cytokines, IL-1ɑ, IL-1β, and IL-6 (Supplementary Fig 4).
Fig. 4.
Limited sex and genotype differences in concentrations of TNF-ɑ, IL-4, IL-10, and IL-18. Of the seven cytokines analyzed in the dorsal and ventral hippocampus of SHR (n = 15) and WKY rats (n = 15), a model that included genotype, sex, and interaction was found to be significant for TNF-ɑ, IL-4, IL-10, and IL-18. Pairwise comparisons: *p < 0.05. (a) Average concentrations (pg/mL) of TNF-ɑ in the dorsal and ventral hippocampus of SHR and WKY rats. Although rats of all genotypes and sexes had similar levels of TNF-ɑ in the dorsal hippocampus, WKY females had significantly higher TNF-ɑ concentrations in the ventral hippocampus than WKY males, SHR females, and SHR males. (b) Average concentrations (pg/mL) of IL-4 in the dorsal and ventral hippocampus of SHR and WKY rats. There were no genotype or sex differences in dorsal hippocampal IL-4 concentrations. In the ventral hippocampus, however, male WKY had higher IL-4 concentrations than WKY females, SHR males, and SHR females. (c) Average concentrations (pg/mL) of IL-10 in the dorsal and ventral hippocampus of SHR and WKY rats. In contrast to IL-4 concentrations, WKY males had lower ventral hippocampal IL-10 concentrations than both WKY females and SHR females. No significant differences in ventral hippocampal IL-10 concentrations were found between WKY and SHR males nor were genotype or sex differences present in the dorsal hippocampus. (d) Average concentrations (pg/mL) of IL-18 in the dorsal and ventral hippocampus of SHR and WKY rats. SHR females had lower IL-18 concentrations in the dorsal hippocampus than WKY females, WKY males, and SHR males. IL-18 concentrations were similar across all groups in the ventral hippocampus.
4. Discussion
Although deficits in working memory and memory for elapsed time are key features of ADHD, research investigating the neurobiological correlates of these deficits is notably limited, and often fails to consider sex as a biological variable. The SHR strain serves as the primary animal model for ADHD, yet there is ongoing debate regarding its fidelity modeling both ADHD symptomatology and neurobiology, particularly with respect to cognitive impairments. Cytokines, known to influence both cognitive function (Donzis and Tronson, 2014) and ADHD symptomatology (Oades et al., 2010), could provide insight into the memory impairments associated with ADHD through the lens of neuroinflammation. However, only a few studies have assessed cytokine concentrations in SHR. These studies have generally not analyzed the hippocampus independently, despite its critical role in memory function, nor have they included female animals. To directly examine the memory function and related neurobiology of SHR as compared to their control, the WKY rat, we sought to characterize working and elapsed time memory, as well as investigate potential differences in hippocampal cytokine concentrations, across genotypes and sexes.
We found that SHR exhibit delay-dependent working memory deficits, with particular impairment in the highest working memory load condition (60-s delay) of a DA task. These deficits were similar to those observed in rats with hippocampal lesions, although comparatively less severe. In the TDD task, which assessed memory for elapsed time, female SHR exhibited difficulties in differentiating the delay lengths during the first ten days of testing, particularly on the longer 20-s delay trials. These impairments were not observed in male SHR. Differences in anxiety-like behavior were ruled out as a source of impaired memory performance in SHR using a LDB test. In our exploratory analysis of hippocampal neuroinflammation, we found some differences in hippocampal cytokine concentrations between rat sexes and genotypes, but concentrations were overall similar across groups.
Our results from the DA task validate the SHR as a reliable model for understanding working memory deficits in ADHD, encompassing both sexes for the first time. We observed delay-dependent spatial working memory deficits in SHR, which align with past studies in humans with ADHD (Kasper et al., 2012; Alderson et al., 2013) and those assessing working memory function in male SHR using a radial arm maze task (Nakamura-Palacios et al., 1996; Hernandez et al., 2003; Potter, 2022). Notably, we found no significant sex differences, indicating that working memory deficits are similar in male and female SHR, as is consistent with the limited human data available (Skogli et al., 2013).
In addition to investigating potential sex differences, our study provides a novel comparison between SHR and hippocampal-lesioned rats, as well as WKY and sham-lesioned rats. Despite concerns regarding the validity of WKY rats as controls in previous research (Grauer and Kapon, 1993; Redei et al., 2022; Robertson et al., 2008), our findings reveal highly similar performance between WKY and sham-lesioned Long-Evans rats across both the DA and TDD tasks (Fig. 1; Supplementary Fig. 3a). Moreover, when comparing SHR and hippocampal-lesioned rats, we found that SHR performed similarly, albeit with a less severe deficit primarily observed in the longest delay condition. The shared propensity for both SHR and hippocampal-lesioned rats to make consecutive errors indicates a potential link to the medial temporal lobe, consistent with prior research (Sabariego et al., 2019; Nakamura-Palacios et al., 1996). These similarities in overall trial accuracy and perseverative behaviors further suggest underlying hippocampal malfunction in SHR.
Deficits in memory for elapsed time are another important characteristic of ADHD yet inconclusively measured in SHR (Smith et al., 2002; Toplak and Tannock, 2005; Lee and Yang, 2019). While previous research by Ferguson et al. (2007) found comparable temporal processing performances between SHR and WKY rats, our study employed the TDD task (Sabariego et al., 2021; Tenney et al., 2021) to assess time discrimination of longer delay periods in male and female SHR and WKY rats. Our findings showed that female SHR struggled to differentiate between delay lengths, particularly in longer, 20-s delay trials, aligning with time processing deficits observed in individuals with ADHD (Lee and Yang, 2019; Smith et al., 2002; Toplak and Tannock, 2005). Interestingly, this deficit was transient, disappearing by day 30 of testing, and was not observed in male SHR. These findings, reminiscent of memory deficits in animals with hippocampal and mEC lesions (Sabariego et al., 2021; Vo et al., 2021), further suggest medial temporal lobe involvement in SHR memory deficits and support the SHR as an ADHD model. Nevertheless, further research is needed to explore time processing abilities in SHR beyond the scope of working memory and across various time intervals, spanning from hours to days.
To address potential confounding factors, we examined anxiety-like behavior levels in SHR and WKY rats using a LDB test, as anxiety is frequently comorbid with ADHD in humans (D'Agati et al., 2019) and can impact rodent memory performance (Rafaela et al., 2012). Remarkably, we observed a shorter average latency for SHR to re-enter the light compartment after initially entering the dark compartment, potentially suggesting reduced anxiety-like behavior in SHR compared to WKY rats. However, it is important to consider that this result may be influenced, at least in part, by differences in locomotion and hyperactivity, aligning with the similarity in total time spent in each compartment. These findings parallel concerns raised regarding locomotor activity as a potential confounder in SHR spatial memory assessments (Sontag et al., 2013). To address this, we evaluated working memory performance in the delayed alternation task at different velocities and found no genotype differences at the 60-s delay condition, the only delay where SHR and WKY rats exhibited significant behavioral differences, suggesting that hyperactivity does not account for the observed differences in working memory between the two groups.
Having established that the impairments in working memory are not directly linked to differences in hyperactivity or differences in anxiety-like behaviors, we turned our focus to investigating the neuroimmune environment in the hippocampus by measuring the concentrations of seven cytokines in both the dorsal and ventral regions. We collected separate dorsal and ventral samples, as these regions are known to be associated with distinct functions (Moser and Moser, 1998; Fanselow and Dong, 2010).
Ultimately, we observed higher concentrations of TNF-ɑ in WKY female rats and reduced concentrations of IL-18 in SHR females compared to all other groups. Importantly, both TNF-ɑ and IL-18 signaling can impact neuronal function and long-term potentiation through both direct and indirect mechanisms (Prieto et al., 2019). However, it is important to note that the magnitude of these changes was relatively modest in comparison to reports of cytokine upregulation in response to learning (see Arisi, 2014 for review). The fact that WKY females had elevated TNF-ɑ levels and yet did not perform at a deficit in hippocampal-dependent tasks raises questions about the biological relevance of these statistically significant results.
Similarly, in males, WKY rats showed elevated levels of IL-4 and significantly lower levels of IL-10 in the ventral hippocampus. Both IL-10 and IL-4 are anti-inflammatory cytokines known to regulate neuroinflammation and mitigate memory and plasticity impairments caused by excessive inflammation (Lynch et al., 2004; Nolan et al., 2005; Richwine et al., 2009; Donzis and Tronson, 2014; Kamaltdinova et al., 2021). The elevated IL-4 levels in WKY males suggest potential protection from stress-induced neuroinflammation. However, it is worth noting that IL-10 concentrations showed an opposite trend among male WKY rats, and neither IL-4 nor IL-10 exhibited elevated levels in female WKY rats, despite their equivalent performance in spatial memory tasks. Taken together, our preliminary exploration of hippocampal cytokines in the SHR model provides limited evidence of sustained inflammatory differences between genotypes.
While our data cannot definitively rule out the possibility of neuroinflammatory processes playing a developmental role in later-life memory deficits, it aligns with findings by Kozłowska et al. (2019), who reported elevated levels of several cytokines in 5-week-old SHR that largely normalized by 10 weeks. Since our rats were aged 12 weeks or older at the time of sacrifice, it is possible that neuroinflammation in the hippocampus during earlier developmental stages in SHR could have contributed to the working memory and memory for elapsed time deficits observed in our study. Additionally, our analysis could not fully account for the effects of the different behavioral exposures experienced by each cohort (see Table 1). To delve further into the impact of neuroinflammation on the developing brain of SHR, future experiments should assess cytokine levels in the hippocampi of 5-week-old, experimentally naïve rats.
In summary, our results emphasize the significance of the hippocampus in the working memory and memory for elapsed time-related symptoms of ADHD. We demonstrated that SHR perform worse than controls in a hippocampal-dependent working memory task, approaching performance deficits seen in hippocampal-lesioned rats. Furthermore, female SHR exhibit deficits in discriminating time intervals akin to the impairments observed following hippocampal- (Sabariego et al., 2021) and mEC- (Vo et al., 2021) lesions. Given the substantial evidence linking working and time memory deficits to ADHD, our findings underscore the utility of the SHR as an ADHD model, emphasizing the need to address these deficits in therapeutic interventions (Fried et al., 2019; Huang-Pollock et al., 2017). Investigating the neurobiological sources of these impairments, including the role of the hippocampus, constitutes a crucial step toward this goal. Further research is required to determine whether cytokine neuroinflammation drives hippocampal dysfunction in SHR. While our data demonstrate for the first time the existence of some cytokine concentration differences between SHR and WKY rats in the hippocampus, their biological relevance remains unclear. Future work should assess cytokine concentrations at earlier developmental stages and further investigate how hippocampal dysfunction may impact working and elapsed time memory function in SHR. Understanding the neurobiological basis of memory deficits in ADHD may ultimately inspire the development of novel treatment strategies, potentially improving the lives of people with ADHD.
Author contributions
MS conceived and designed the experiments. LA, JS, JH, and MS led the experimentation. LA, EV, ST, SL, TD, and JG performed the experiments. LA, JS, JH, and MS analyzed the data. LA drafted the manuscript. LA, JS, JH, and MS revised the manuscript. All authors read and approved the final manuscript.
Funding sources
This work was supported by Mount Holyoke College (Program in Neuroscience and Behavior) and the University of San Diego (McNair Scholars Program; College of Arts and Sciences; Office of Undergraduate Research). In particular, we would like to thank the Vorwerk family, Harap family, and friends and colleagues of Curtis Smith for financially supporting the experimentation conducted at Mount Holyoke College.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Olive Aries, Kathleen Byrne, Saee Chitale, Gabriella Gagnon, Laura Johnson, Lauren Kang, Jenny Li, Ryan Nguyen, Divna Scepanovic, Grace Wallsinger, Alexa Watson, Tracy Wen, Madison Wyatt, Zoe Wynter, and Petra Yang for assistance and technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100700.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ainge J.A., Tamosiunaite M., Woergoetter F., Dudchenko P.A. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2007;27(36):9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson R.M., Kasper L.J., Hudec K.L., Patros C.H.G. Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: a meta-analytic review. Neuropsychology. 2013;27(3):287–302. doi: 10.1037/a0032371. [DOI] [PubMed] [Google Scholar]
- Altszuler A.R., Page T.F., Gnagy E.M., Coxe S., Arrieta A., Molina B.S.G., Pelham W.E. Financial dependence of young adults with childhood ADHD. J. Abnorm. Child Psychol. 2016;44(6):1217–1229. doi: 10.1007/s10802-015-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Arisi G.M. Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014;38:43–47. doi: 10.1016/j.yebeh.2014.01.017. Epub 2014 Feb 15. PMID: 24534466. [DOI] [PubMed] [Google Scholar]
- Baddeley A. OUP; Oxford: 2007. Working Memory, Thought, and Action. [Google Scholar]
- Bunford N., Brandt N.E., Golden C., Dykstra J.B., Suhr J.A., Owens J.S. Attention-deficit/hyperactivity disorder symptoms mediate the association between deficits in executive functioning and social impairment in children. J. Abnorm. Child Psychol. 2015;43(1):133–147. doi: 10.1007/s10802-014-9902-9. [DOI] [PubMed] [Google Scholar]
- Campez M., Raiker J.S., Sarver D.E., Friedman L.M., Orban S.A., Rapport M.D. Working memory capacity and ADHD symptoms in boys: examining the heterogeneity of working memory functioning using latent profile analysis. J. Psychopathol. Behav. Assess. 2020;42(3):450–463. doi: 10.1007/s10862-019-09762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control . Centers for Disease Control and Prevention; 2022. Data and Statistics about ADHD | CDC.https://www.cdc.gov/ncbddd/adhd/data.html [Google Scholar]
- D'Agati E., Curatolo P., Mazzone L. Comorbidity between ADHD and anxiety disorders across the lifespan. Int. J. Psychiatr. Clin. Pract. 2019;23(4):238–244. doi: 10.1080/13651501.2019.1628277. [DOI] [PubMed] [Google Scholar]
- Daley D., Birchwood J. ADHD and academic performance: why does ADHD impact on academic performance and what can be done to support ADHD children in the classroom? Child Care Health Dev. 2010;36(4):455–464. doi: 10.1111/j.1365-2214.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- delRey A., Balschun D., Wetzel W., Randolf A., Besedovsky H.O. A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav. Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Donfrancesco R., Nativio P., Borrelli E., Giua E., Andriola E., Villa M.P., DI Trani M. Serum cytokines in pediatric neuropsychiatric syndromes: focus on attention deficit hyperactivity disorder. Minerva Pediatr. 2021;73(5):398–404. doi: 10.23736/s2724-5276.16.04642-9. [DOI] [PubMed] [Google Scholar]
- Donzis E.J., Tronson N.C. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drtilkova I., Sery O., Theiner P., Uhrova A., Zackova M., Balastikova B., Znojil V. Clinical and molecular-genetic markers of ADHD in children. Neuroendocrinol. Lett. 2008;29(3):320–327. [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.-W. Are the Dorsal and Ventral Hippocampus functionally distinct structures? Neuron. 2010;65(1):7. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.A., Paule M.G., Cada A., Fogle C.M., Gray E.P., Berry K.J. Baseline behavior, but not sensitivity to stimulant drugs, differs among spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rat strains. Neurotoxicol. Teratol. 2007;29(5):547–561. doi: 10.1016/j.ntt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Fried R., Abrams J., Hall A., Feinberg L., Pope A., Biederman J. Does working memory impact functional outcomes in individuals with ADHD: a qualitative and comprehensive literature review. J. Atten. Disord. 2019;23(13):1592–1599. doi: 10.1177/1087054717730612. [DOI] [PubMed] [Google Scholar]
- Grauer E., Kapon Y. Wistar-Kyoto rats in the Morris water maze: impaired working memory and hyper-reactivity to stress. Behav. Brain Res. 1993;59(1):147–151. doi: 10.1016/0166-4328(93)90161-I. [DOI] [PubMed] [Google Scholar]
- Hernandez C.M., Høifødt H., Terry A.V. Spontaneously hypertensive rats: further evaluation of age-related memory performance and cholinergic marker expression. J. Psychiatry Neurosci.: JPN. 2003;28(3):197–209. [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock C., Shapiro Z., Galloway-Long H., Weigard A. Is poor working memory a transdiagnostic risk factor for psychopathology? J. Abnorm. Child Psychol. 2017;45(8):1477–1490. doi: 10.1007/s10802-016-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaltdinova E., Pershina E., Mikheeva I., Bugaev-Makarovskiy N., Arkhipov V. Different activation of IL-10 in the Hippocampus and prefrontal cortex during neurodegeneration caused by trimethyltin chloride. J. Mol. Neurosci. 2021;71(3):613–617. doi: 10.1007/s12031-020-01682-w. [DOI] [PubMed] [Google Scholar]
- Kasper L.J., Alderson R.M., Hudec K.L. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin. Psychol. Rev. 2012;32(7):605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kozłowska A., Wojtacha P., Równiak M., Kolenkiewicz M., Huang A.C.W. ADHD pathogenesis in the immune, endocrine and nervous systems of juvenile and maturating SHR and WKY rats. Psychopharmacology. 2019;236(10):2937–2958. doi: 10.1007/s00213-019-5180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-Y., Yang E.-L. Exploring the effects of working memory on time perception in attention deficit hyperactivity disorder. Psychol. Rep. 2019;122(1):23–35. doi: 10.1177/0033294118755674. [DOI] [PubMed] [Google Scholar]
- Lynch A.M., Walsh C., Delaney A., Nolan Y., Campbell V.A., Lynch M.A. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10 – a role for IL-1β? J. Neurochem. 2004;88(3):635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Marin I., Kipnis J. Learning and memory … and the immune system. Learn. Mem. 2013;20(10):601–606. doi: 10.1101/lm.028357.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R., Hayden J., Hogg-johnson S., Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Miller S.M., Piasecki C.C., Lonstein J.S. Use of the light–dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol. Biochem. Behav. 2011;100(1):130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook D.M., Jeffrey J., Neuringer A. Spontaneously hypertensive rats (SHR) readily learn to vary but not repeat instrumental responses. Behav. Neural. Biol. 1993;59(2):126–135. doi: 10.1016/0163-1047(93)90847-B. [DOI] [PubMed] [Google Scholar]
- Mori S., Kato M., Fujishima M. Impaired maze learning and cerebral glucose utilization in aged hypertensive rats. Hypertension. 1995;25(4):545–553. doi: 10.1161/01.HYP.25.4.545. [DOI] [PubMed] [Google Scholar]
- Moser M.-B., Moser E.I. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063. (1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Hartanto T., Iosif A.-M., Dixon J.F., Hinshaw S.P., Pakyurek M., van den Bos W., Guyer A.E., McClure S.M., Schweitzer J.B., Fassbender C. Neural basis of working memory in ADHD: load versus complexity. 2021. [DOI] [PMC free article] [PubMed]
- Nakamura-Palacios E.M., Caldas C.K., Fiorini A., Chagas K.D., Chagas K.N., Vasquez E.C. Deficits of spatial learning and working memory in spontaneously hypertensive rats. Behav. Brain Res. 1996;74(1–2):217–227. doi: 10.1016/0166-4328(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Nolan Y., Maher F.O., Martin D.S., Clarke R.M., Brady M.T., Bolton A.E., Mills K.H.G., Lynch M.A. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J. Biol. Chem. 2005;280(10):9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Oades R.D., Myint A.-M., Dauvermann M.R., Schimmelmann B.G., Schwarz M.J. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: an exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav. Brain Funct.: BBF. 2010;6:32. doi: 10.1186/1744-9081-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963;27(3):282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Orban S.A., Rapport M.D., Friedman L.M., Eckrich S.J., Kofler M.J. Inattentive behavior in boys with ADHD during classroom instruction: the mediating role of working memory processes. J. Abnorm. Child Psychol. 2018;46(4):713–727. doi: 10.1007/s10802-017-0338-x. [DOI] [PubMed] [Google Scholar]
- Pelham W.E., Foster E.M., Robb J.A. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J. Pediatr. Psychol. 2007;32(6):711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- Potter J.L. M.S., The University of Memphis; 2022. Evaluating Executive Functions in a Proposed Animal Model of ADHD: Spontaneously Hypertensive Rats.https://www.proquest.com/docview/2747225332/abstract/6420020F599F4411PQ/1 [Google Scholar]
- Preston A.R., Eichenbaum H. Interplay of Hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto G.A., Tong L., Smith E.D., Cotman C.W. TNFα and IL-1β but not IL-18 suppresses hippocampal long-term potentiation directly at the synapse. Neurochem. Res. 2019;44(1):49–60. doi: 10.1007/s11064-018-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker J.S., Rapport M.D., Kofler M.J., Sarver D.E. Objectively-measured impulsivity and attention-deficit/hyperactivity disorder (ADHD): testing competing predictions from the working memory and behavioral inhibition models of ADHD. J. Abnorm. Child Psychol. 2012;40(5):699–713. doi: 10.1007/s10802-011-9607-2. [DOI] [PubMed] [Google Scholar]
- Redei E.E., Udell M.E., Solberg-Woods L.C., Chen H. The Wistar Kyoto rat: a model of depression traits. Curr. Neuropharmacol. 2022 doi: 10.2174/1570159X21666221129120902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine A.F., Sparkman N.L., Dilger R.N., Buchanan J.B., Johnson R.W. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav. Immun. 2009;23(6):794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B.-A., Clements K.M., Wainwright P.E. The working memory capabilities of the spontaneously hypertensive rat. Physiol. Behav. 2008;94:481–486. doi: 10.1016/j.physbeh.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Sabariego M., Schönwald A., Boublil B.L., Zimmerman D.T., Ahmadi S., Gonzalez N., Leibold C., Clark R.E., Leutgeb J.K., Leutgeb S. Time cells in the Hippocampus are neither dependent on medial entorhinal cortex inputs nor necessary for spatial working memory. Neuron. 2019;102(6):1235–1248. doi: 10.1016/j.neuron.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabariego M., Tabrizi N.S., Marshall G.J., McLagan A.N., Jawad S., Hales J.B. In the temporal organization of episodic memory, the hippocampus supports the experience of elapsed time. Hippocampus. 2021;31(1):46–55. doi: 10.1002/hipo.23261. [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Johansen E.B., Wøien G., Walaas S.I., Storm-Mathisen J., Bergersen L.H., Hvalby Ø., Jensen V., Aase H., Russell V.A., Killeen P.R., DasBanerjee T., Middleton F.A., Faraone S.V. The Spontaneously Hypertensive Rat model of ADHD – the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57(7–8):619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M., Hodgkins P., Caci H., Young S., Kahle J., Woods A.G., Arnold L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogli E.W., Teicher M.H., Andersen P.N., Hovik K.T., Øie M. ADHD in girls and boys – gender differences in co-existing symptoms and executive function measures. BMC Psychiatr. 2013;13(1):298. doi: 10.1186/1471-244X-13-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Taylor E., Rogers J.W., Newman S., Rubia K. Evidence for a pure time perception deficit in children with ADHD. J. Child Psychol. Psychiatry Allied Discip. 2002;43(4):529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- Sontag T.-A., Fuermaier A.B.M., Hauser J., Kaunzinger I., Tucha O., Lange K.W. Spatial memory in spontaneously hypertensive rats (SHR) PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0074660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney S., Vogiatzoglou E., Chohan D., Vo A., Hunt T., Cayanan K., Hales J.B., Sabariego M. A time duration discrimination task for the study of elapsed time processing in rats. Bio-Protocol. 2021;11(6):e3965. doi: 10.21769/BioProtoc.3965. e3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak M.E., Tannock R. Time perception: modality and duration effects in attention-deficit/hyperactivity disorder (ADHD) J. Abnorm. Child Psychol. 2005;33(5):639–654. doi: 10.1007/s10802-005-6743-6. [DOI] [PubMed] [Google Scholar]
- Tsao A., Sugar J., Lu L., Wang C., Knierim J.J., Moser M.-B., Moser E.I. Integrating time from experience in the lateral entorhinal cortex. Nature. 2018;561(7721):57–62. doi: 10.1038/s41586-018-0459-6. [DOI] [PubMed] [Google Scholar]
- Vo A., Tabrizi N.S., Hunt T., Cayanan K., Chitale S., Anderson L.G., Tenney S., White A.O., Sabariego M., Hales J.B. Medial entorhinal cortex lesions produce delay-dependent disruptions in memory for elapsed time. Neurobiol. Learn. Mem. 2021;185 doi: 10.1016/j.nlm.2021.107507. [DOI] [PubMed] [Google Scholar]
- Weissenberger S., Schonova K., Büttiker P., Fazio R., Vnukova M., Stefano G.B., Ptacek R. Time perception is a focal symptom of attention-deficit/hyperactivity disorder in adults. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : International Medical Journal of Experimental and Clinical Research. 2021;27 doi: 10.12659/MSM.933766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss J.M., Fisk G., van Groen T. Impaired learning and memory in mature spontaneously hypertensive rats. Brain Res. 1992;592(1–2):135–140. doi: 10.1016/0006-8993(92)91668-5. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Wang X., Wang S., Li J., Xu X., Wang M., Li G., Shen W. Celastrol attenuates learning and memory deficits in an alzheimer's disease rat model. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/5574207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Young S., Adamo N., Ásgeirsdóttir B.B., Branney P., Beckett M., Colley W., Cubbin S., Deeley Q., Farrag E., Gudjonsson G., Hill P., Hollingdale J., Kilic O., Lloyd T., Mason P., Paliokosta E., Perecherla S., Sedgwick J., Skirrow C., et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatr. 2020;20(1):404. doi: 10.1186/s12888-020-02707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.