Abstract
Background
Schaaf‐Yang syndrome (SYS) is a neurodevelopmental disorder caused by truncating variants in the paternally expressed MAGEL2 gene in the Prader‐Willi syndrome‐region on chromosome 15q. In addition to hypotonia and intellectual disability, individuals with SYS are frequently affected by neonatal contractures and autism spectrum disorder. In this study, we focus on the burden of disease on patients and their families for the first time.
Methods
Based on the online SYS Patient Voices Survey the perspective of 81 primary caregivers on SYS was assessed.
Results
The perceived severity of muscular and developmental manifestations dominated the evaluation of the phenotype in early childhood, while behavioral issues were considered more impactful later in life. Importantly, an apprehension toward symptoms with a later onset was observed in caregivers of younger children. Available therapeutic options, while mostly effective, did not sufficiently alleviate the total burden of disease. Overall, parents stated that caring for an individual with SYS was very challenging, affecting their daily lives and long‐term planning.
Conclusion
Our study demonstrates the necessity for treatments that, adapted to age and in accordance with the caregivers' prioritization, improve the patients' medical condition and thus facilitate their and their families' social participation.
Keywords: disease burden, neurodevelopmental disorders, Schaaf‐Yang syndrome
Individuals with Schaaf‐Yang syndrome manifest symptoms affecting various systems. Disease trajectories, levels of concern, and treatment priorities as perceived by caregivers are illustrated here.
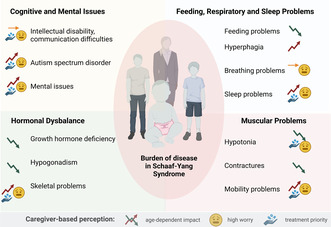
1. INTRODUCTION
Schaaf‐Yang syndrome (SYS) (OMIM: 615547) is a genetic disorder caused by heterozygous nonsense and frameshift variants of the maternally imprinted, paternally expressed MAGEL2 (OMIM: 605283) gene within the Prader‐Willi syndrome (PWS) domain of chromosome 15 (15q11.2‐q13). Described by Schaaf et al. (2013), the first identified individuals with truncating variants in the MAGEL2 gene presented with a phenotype partially overlapping with PWS, including the PWS major criteria neonatal hypotonia, feeding problems, developmental delay/intellectual disability (DD/ID) and hypogonadism (Schaaf et al., 2013). Remarkably, autism spectrum disorder (ASD) and contractures were highly prevalent (Schaaf et al., 2013).
Since then, newly identified cases of SYS have been analyzed, allowing for a more distinct characterization of the phenotype encompassing affections effects of several organ systems. Early in life, severe neonatal contractures are characteristic of the phenotype (Castilla‐Vallmanya et al., 2022; McCarthy, Lupo, et al., 2018), cases of lethal arthrogryposis multiplex congenita have been reported (Mejlachowicz et al., 2015). Temperature instability is frequently present, and seizures and neurological deterioration following febrile episodes have been reported in a cohort of six Japanese children with SYS (McCarthy, Lupo, et al., 2018; Negishi et al., 2019). During early childhood development, feeding difficulties, gastroesophageal reflux, and chronic constipation are eminent and require specialized medical attention (Fountain et al., 2017). Short stature associated with growth hormone deficiency (GHD) and low bone mineral density as indicators of osteoporosis are observed in most patients; the prevalence of scoliosis is elevated compared to the general population (McCarthy, Lupo, et al., 2018; McCarthy, McCann‐Crosby, et al., 2018). Additionally, it has been shown that obstructive sleep apnea occurs in most individuals with SYS (Powell et al., 2020).
Later in life, all individuals with SYS showed intellectual impairment within a broad range of profound to mild ID. Interestingly, the delay in reaching developmental milestones appears to be more severe than typically seen among individuals with PWS (Fountain et al., 2017; McCarthy, Lupo, et al., 2018). Systematic evaluation for neurobehavioral disorders in nine patients confirmed the high prevalence of ASD and associated symptoms as well as low adaptivity and general behavioral issues (Thomason et al., 2020). A minority of patients also presents seizures (Fountain et al., 2017). In adolescents and adults, the phenotype includes hyperphagia and obesity, social withdrawal, and behavioral issues become more pronounced (Marbach et al., 2020). In addition, there is evidence for genotype–phenotype correlations, given that patients with the most common variant NM_019066.5:c.1996dup present a more severe phenotype than individuals with other truncating variant or deletions of the entire gene (Kanber et al., 2009; McCarthy, Lupo, et al., 2018). Importantly, some variants, for example, NM_019066.5:c.1996del, are reported to be pre‐, or perinatally lethal (Fountain et al., 2017).
However, as the medical understanding of this genetic disorder becomes increasingly more detailed, the perception of medical needs and treatment priorities from a caregivers' point of view has not been appraised yet. So far, previous publications mainly focused on the review of medical records and medical staff's perspectives.
To improve the impact of SYS on patients' lives, several treatments that are well‐established for PWS have been assessed in SYS in recent years. For instance, the administration of recombinant human growth hormone (rhGH) increased the height of patients with SYS and suggested a positive effect on the body composition (Hebach et al., 2021). As for advising of caregivers, Schaaf and Marbach (2021) suggest addressing gastrointestinal, respiratory, musculoskeletal, neuro‐psychiatric, ophthalmological, genitourinary, and endocrine concerns as well as genetic counseling and family support. Further treatment options that are in accordance with the families' expectations and needs have yet to be evaluated.
Therefore, a standardized questionnaire was developed to assess the caregivers' perception of their daily challenges, observations, and concerns related to SYS patients. Here, we report the results of an online questionnaire based on the responses of 81 patients and families. In particular, we intend to compare professionals' and caregivers' perspectives.
2. METHODS
2.1. Ethical compliance
The presented study was approved by the ethics board of the North Star Review Board. Caregivers gave their consent for publication of the data.
2.2. Participants
As of 2022, 263 individuals with truncating variants in MAGEL2 (NM_019066.5) have been registered in an internal database (Dr Schaaf, personal communication) and diagnosed with SYS. Primary caregivers of patients in this cohort were directly notified if contact information was available and, additionally, an announcement was posted in a closed SYS community Facebook group. In March and April 2021, the survey was conducted using www.surveymonkey.com or paper forms. The written responses were later added to the SurveyMonkey data by Foundation for Prader‐Willi (FPWR) staff. Included were caregivers of a person with a pathogenic variant in the MAGEL2 gene causing SYS. There were no exclusion criteria. Caregivers were asked to complete the survey for one individual with SYS, however, there was a possibility to report on additional immediate family members with SYS if applicable. In total, 81 caregivers from 19 countries completed the survey, among them 78 parents and three other primary caregivers. Patients' ages ranged from under 1 year to over 18 years. For older patients, the exact age was not registered as not to reveal families' identities. Furthermore, 26 caregivers reported the exact truncating variant causing SYS in the respective individual, among those were 10 patients with a NM_019066.5:c.1996dup variant. For de‐identification purposes, parents' answers were not matched to the respective genotypes.
2.3. Methods
Questions found to be of importance in the US Food and Drug Administration patient‐focused drug development meetings served as guidance for the development of this survey. The collection of data was based on a questionnaire that was later de‐identified. To evaluate the impact of a symptom or the effectiveness of a treatment, Likert‐type scale questions were used. Our survey focused on the assessment of the current importance of a symptom on day‐to‐day living as well as its potential future impact (Supplementary Materials). The possibility of free text answers was provided to identify unregistered medical issues.
For assessment of age‐ and development‐related medical needs and treatment priorities, the cohort was stratified into four age groups relying on clinical experience for determining the cut‐offs (under 4 years, 4–10 years, 11–18 years, over 18 years). The data were analyzed using Microsoft Excel for statistical calculation.
3. RESULTS
Responses for 81 SYS patients were collected. In the different age groups, 26 individuals were below 4 years of age, 32 were between 4 and 10 years, 17 were between 11 and 18 years, and six were over 18 years. The multiple question survey was completed in 98.8%.
Depending on the developmental characteristics of the symptom, the manifestation of phenotypical characteristics remained either stable when assessed in different age groups or it was, apart from breathing and feeding difficulties, increasing. Most prominently, DD/ID was diagnosed in approximately 90% of individuals under the age of 10 years and affected 100% of older patients. Mobility problems including hypotonia had been present in approximately 80% of patients at one point and seemed to decrease in importance with advancing age, whereas symptoms associated with behavior and social interaction were increasingly prevalent in older patients. In total, the mean number [standard deviation] of symptoms reported in individuals under the age of 4 years was 10.2 [2.94], between 4 and 10 years 10.2 [3.34], between 11 and 18 years 12.7 [2.64], and in adults 11.17 [3.31].
Interestingly, for several clinical manifestations, caregivers reported symptom‐related impact more frequently than documented in the official diagnosis.
3.1. Cognitive and mental issues (Table 1a)
TABLE 1a.
Impact of individual symptoms and perceived effectiveness of available treatment options: Cognitive and mental issues.
| Impact | Worry | Treatment option | Ideal treatment | ||||
|---|---|---|---|---|---|---|---|
| Age [years] | Symptom | Very high/most important | Very high/most important | Used | Effective | Very/most important | |
| Developmental delay/intellectual disability | 0–3 | 92.3% | 80.8% | 88.5% | N/A | N/A | 92.3% |
| 4–10 | 87.5% | 81.3% | 78.1% | N/A | N/A | 84.4% | |
| 11–18 | 100.0% | 76.5% | 88.2% | N/A | N/A | 94.1% | |
| >18 | 100.0% | 100.0% | 100.0% | N/A | N/A | 100.0% | |
| Autism spectrum disordera | 0–3 | 23.1% | 30.8% | 34.6% | 11.5% | 11.5% | 46.2% |
| 4–10 | 50.0% | 46.9% | 50.0% | 25.0% | 25.0% | 65.6% | |
| 11–18 | 64.7% | 58.8% | 64.7% | 41.2% | 35.3% | 64.7% | |
| >18 | 83.3% | 66.7% | 33.3% | 50.0% | 33.3% | 83.3% | |
| Communication/speech delays or difficultiesb; 1 | 0–3 | 80.8% | 69.2% | 76.9% | 88.5% | 80.8% | 80.8% |
| 4–10 | 96.9% | 84.4% | 78.1% | 87.5% | 84.4% | 87.5% | |
| 11–18 | 94.1% | 70.6% | 82.4% | 94.1% | 88.2% | 94.1% | |
| >18 | 66.7% | 83.3% | 50.0% | 66.7% | 50.0% | 100.0% | |
| Difficult behaviorc | 0–3 | 11.5% | 30.8% | 30.8% | 88.5% | 88.5% | 46.2% |
| 4–10 | 56.3% | 50.0% | 43.8% | 87.5% | 87.5% | 65.6% | |
| 11–18 | 58.8% | 52.9% | 64.7% | 100.0% | 100.0% | 64.7% | |
| >18 | 33.3% | 66.7% | 50.0% | 66.7% | 50.0% | 83.3% | |
| Anxietyd | 0–3 | 0.0% | 26.9% | 19.2% | 7.7% | 7.7% | N/A |
| 4–10 | 28.1% | 34.4% | 25.0% | 18.8% | 18.8% | N/A | |
| 11–18 | 58.8% | 35.3% | 29.4% | 47.1% | 41.2% | N/A | |
| >18 | 66.7% | 83.3% | 50.0% | 50.0% | 33.3% | N/A | |
| Seizuree | 0–3 | 23.1% | 38.5% | 23.1% | 23.1% | 23.1% | N/A |
| 4–10 | 3.1% | 6.3% | 9.4% | 6.3% | 6.3% | N/A | |
| 11–18 | 29.4% | 11.8% | 23.5% | 17.6% | 17.6% | N/A | |
| >18 | 0.0% | 33.3% | 0.0% | 0.0% | 0.0% | N/A | |
Note: Cognitive problems are present early on and cause high impact. The diagnosis and impact of mental and behavioral issues increase with patients' age. Treatment: aapplied behavioral analysis therapy, bspeech therapy, coccupational therapy, danxiety medication, eseizure mediation; ideal treatment: 1positive social interaction.
Abbreviation: N/A, not asked.
Of the 81 completed questionnaires, the most frequently reported symptoms included ID/DD and communication/speech difficulties, affecting 92.6% and 88.9% of individuals, respectively.
A total of 92.3% of patients under 4 years were reported to show signs of ID/DD, over the age of 11, all individuals had been diagnosed with ID/DD. The impact of this symptom was perceived to be higher in older patients and all caregivers for the oldest subgroup were highly concerned as to what extend ID/DD would impact the person with SYS in the future. With over 90% of all respondents desiring specific therapeutic options, this ranked highest in treatment necessities.
Communication/speech difficulties were stated to strongly affect patients between 4 and 18 years. Treatment options for the issue included speech therapy, which had been used by 87.6% and was found to be helpful by most parents.
Mental health issues, on the other hand, seem to manifest later in life. ASD and associated symptoms like difficult behavior and anxiety largely increased in prevalence and impact in older patients. While only six of 26 patients under 3 years were diagnosed and showed signs of ASD according to their caregivers, five of six individuals over 18 were affected. Interestingly, almost two‐thirds of respondents caring for a child under the age of 4 years reported the impact of ASD was at least somewhat important, and nine of 26 stated it was highly impacting. The highest apprehension toward this symptom was shown by caregivers of adolescent patients. Likewise, the prevalence of anxiety increased in older individuals, with 66.7% of adults having experienced the symptom, while none of the youngest children were diagnosed at the time of the survey. However, 29.6% of caregivers of patients under 4 years and 83.3% of adults described an important impact. Difficult behavior already affected 11.2% of the youngest individuals and was reportedly demonstrated by approximately 60% of adolescents. Despite lacking formal diagnosis, two‐thirds of adult patients were importantly impacted. The highest worry existed among primary caregivers of individuals between 11 and 18 years. Overall, most caregivers worried about how the symptom would affect the individual in the future.
Some therapeutic options can be proposed for mental and behavioral issues. Applied behavior analysis (ABA) therapy to address the symptoms of autism was used by approximately one‐fifth of all patients, ranking highest in the oldest subgroup. Especially younger children under the age of 11 years seemed to have benefitted from the intervention. Nevertheless, all parents whose children received beneficial ABA therapy found ASD to be highly impacting and worrisome. Anxiety medication had been prescribed to almost half of all patients over the age of 4 years and already two of the youngest children. While all patients under the age of 11 years having taken anxiety medication benefitted at least somewhat, adverse side effects were reported for some patients.
Of all therapeutic options addressing behavioral issues, occupational therapy was the most frequently used with 87.7% of patients having undergone this treatment at one time. According to caregivers, with one exception, occupational therapy proved to be at least somewhat helpful. Nevertheless, caregivers considered the development of new treatment options to improve behavior and positive social interaction as particularly important, the desire for amelioration of those symptoms increasing with the age of patients.
3.2. Musculoskeletal and eye problems (Table 1b)
TABLE 1b.
Impact of individual symptoms and perceived effectiveness of available treatment options: Musculoskeletal and eye problems.
| Impact | Worry | Treatment option | Ideal treatment | ||||
|---|---|---|---|---|---|---|---|
| Age [years] | Symptom | Very high/most important | Very high/most important | Used | Effective | Very/most important | |
| Hypotonia/weak muscles1 | 0–3 | 92.3% | 76.9% | 42.3% | N/A | N/A | 73.1% |
| 4–10 | 84.4% | 53.1% | 34.4% | N/A | N/A | 75.0% | |
| 11–18 | 94.1% | 41.2% | 35.3% | N/A | N/A | 64.7% | |
| >18 | 83.3% | 33.3% | 66.7% | N/A | N/A | 83.3% | |
| Contracturesa | 0–3 | 84.6% | 30.8% | 19.2% | 57.7% | 57.7% | N/A |
| 4–10 | 71.9% | 31.3% | 21.9% | 59.4% | 56.3% | N/A | |
| 11–18 | 82.4% | 5.9% | 5.9% | 52.9% | 52.9% | N/A | |
| >18 | 83.3% | 16.7% | 33.3% | 33.3% | 33.3% | N/A | |
| Mobility problems/difficulty walkingb | 0–3 | 84.6% | 73.1% | 73.1% | 100.0% | 100.0% | 88.5% |
| 4–10 | 75.0% | 56.3% | 56.3% | 90.6% | 90.6% | 75.0% | |
| 11–18 | 94.1% | 47.1% | 52.9% | 100.0% | 100.0% | 82.4% | |
| >18 | 83.3% | 83.3% | 83.3% | 83.3% | 83.3% | 100.0% | |
| Scoliosisc | 0–3 | 23.1% | 42.3% | 30.8% | 11.5% | 11.5% | N/A |
| 4–10 | 34.4% | 28.1% | 25.0% | 18.8% | 18.8% | N/A | |
| 11–18 | 64.7% | 47.1% | 47.1% | 41.2% | 29.4% | N/A | |
| >18 | 83.3% | 83.3% | 83.3% | 16.7% | 16.7% | N/A | |
| Eye problems | 0–3 | 76.9% | 42.3% | 30.8% | N/A | N/A | N/A |
| 4–10 | 71.9% | 21.9% | 28.1% | N/A | N/A | N/A | |
| 11–18 | 82.4% | 23.5% | 17.6% | N/A | N/A | N/A | |
| >18 | 50.0% | 33.3% | 0.0% | N/A | N/A | N/A | |
Note: Muscular problems become less important whereas scoliosis causes high impact in older cohorts. n (0–3 years) = 26, n (4–10 years) = 32, n (11–18 years) = 17, n (>18 years) = 6. Therapeutic options: asplints and braces, bphysical therapy, cscoliosis brace; ideal treatment: 1improves stamina and activity.
Abbreviation: N/A, not asked.
Characteristic hypotonia has been present in 88.9% of individuals with no significant differences between age groups. General mobility problems affected 82.7% of individuals at one point and had a great impact, especially in the youngest and oldest subgroup. Likewise, 79% of patients had experienced contractures at one point with no significant differences between the four age subgroups. However, while caregivers of adolescents and adults largely rated the impact of this symptom as low, younger children were reportedly more challenged by contractures. Also, the perceived impact of eye problems decreased as patients grew older. By contrast, the prevalence of scoliosis and osteoporosis increased in the older age groups as did the reported impact. Cumulatively, 40.7% of patients had been diagnosed with scoliosis, among those five of six adults, all of which were highly impacted. Interestingly, without being clinically diagnosed, the impact for younger children was already perceived as very important. Likewise, this apprehension was expressed for osteoporosis: in all subgroups, the number of caregivers who stated to be worried about the development of osteoporosis was higher than the number of currently diagnosed individuals.
All mobility‐associated symptoms can be partially addressed by physical therapy which was used by 95.1% of the individuals, providing at least some alleviation of the symptoms. As for contractures and scoliosis, bracings can be fitted, however, six of 81 caregivers reported significant side effects. Overall, the demand for therapeutic options improving mobility is a high treatment priority for more than 80% of primary caregivers.
3.3. Feeding and respiratory abnormalities, sleep issues (Table 1c)
TABLE 1c.
Impact of individual symptoms and perceived effectiveness of available treatment options: Feeding, respiratory, and sleep issues.
| Impact | Worry | Treatment option | Ideal treatment | ||||
|---|---|---|---|---|---|---|---|
| Age [years] | Symptom | Very high/most important | Very high/most important | Used | Effective | Very/most important | |
| Feeding problems/inability to eat independentlya | 0–3 | 88.5% | 57.7% | 65.4% | 61.5% | 61.5% | N/A |
| 4–10 | 68.8% | 56.3% | 40.6% | 43.8% | 43.8% | N/A | |
| 11–18 | 82.4% | 52.9% | 41.2% | 64.7% | 64.7% | N/A | |
| >18 | 50.0% | 50.0% | 50.0% | 16.7% | 16.7% | N/A | |
| Gastrointestinal problems/chronic constipationb | 0–3 | 80.8% | 50.0% | 46.2% | 69.2% | 69.2% | 50.0% |
| 4–10 | 68.8% | 50.0% | 34.4% | 56.3% | 53.1% | 46.9% | |
| 11–18 | 76.5% | 64.7% | 52.9% | 70.6% | 64.7% | 64.7% | |
| >18 | 66.7% | 66.7% | 50.0% | 100.0% | 100.0% | 83.3% | |
| Sleep problemsc | 0–3 | 65.4% | 53.8% | 46.2% | 26.9% | 26.9% | 65.4% |
| 4–10 | 84.4% | 78.1% | 56.3% | 53.1% | 50.0% | 81.3% | |
| 11–18 | 82.4% | 82.4% | 82.4% | 23.5% | 23.5% | 82.4% | |
| >18 | 66.7% | 66.7% | 50.0% | 16.7% | 16.7% | 83.3% | |
| Breathing problemsd | 0–3 | 42.3% | 50.0% | 46.2% | 34.6% | 34.6% | N/A |
| 4–10 | 37.5% | 46.9% | 40.6% | 34.4% | 28.1% | N/A | |
| 11–18 | 35.3% | 47.1% | 58.8% | 17.6% | 17.6% | N/A | |
| >18 | 33.3% | 33.3% | 66.7% | 16.7% | 16.7% | N/A | |
| Hyperphagia | 0–3 | 11.5% | 19.2% | 23.1% | N/A | N/A | N/A |
| 4–10 | 12.5% | 9.4% | 9.4% | N/A | N/A | N/A | |
| 11–18 | 35.3% | 17.6% | 29.4% | N/A | N/A | N/A | |
| >18 | 16.7% | 50.0% | 33.3% | N/A | N/A | N/A | |
Note: The perceived severity of feeding, respiratory and sleep issues, and effectiveness of applied treatments for use differ between age groups. n (0–3 years) = 26, n (4–10 years) = 32, n (11–18 years) = 17, n (>18 years) = 6. Therapeutic options: aG‐tube/NG‐tube, bGI‐medication, ctonsillectomy, dCPAP.
Abbreviation: N/A, not asked.
Issues concerning eating behavior and digestion show a shift from feeding difficulties in younger children to increasing worries about hyperphagia in adolescence and adulthood. While 88.5% of children until the age of 4 years experienced feeding problems or inability to eat independently, only 50% of SYS‐affected adults showed this symptom. Accordingly, caregivers of younger individuals expressed higher concerns about feeding problems. By contrast, the importance of hyperphagia was perceived invertedly. Overall, 14 of 81 respondents took care of an individual who demonstrated excessive, insatiable hunger, the highest prevalence was reported in adolescents, the lowest in children under the age of 4 years. However, the perceived impact and worry were stated to be higher than the actual diagnosis of hyperphagia in very young and adult patients. Digestion problems were present in approximately two‐thirds, and most parents considered the impact to be substantial.
To address feeding difficulties, more than half of all children had been equipped with a G‐ or NG‐Tube, all of them benefiting at least somewhat. Only one individual over the age of 18 years was reported to be using a feeding tube at the time of the survey. GI medications to treat reflux and constipation, however, were used by and reported to be beneficial for all patients over 18 years old. In total, 54 of 81 individuals received this treatment. When asked to rate the importance of developing further therapeutic options to improve GI health, the need for an ideal treatment increased with the individuals' age.
Breathing problems had an overall prevalence of 38.3% and were most prominently affecting the younger patients. Sleep problems and sleep apnea were present in all age groups and affected 62 of 81 individuals. The perceived impact was highest in adolescents.
To evaluate the treatment of sleep apnea, caregivers could rate the effectiveness of tonsillectomy or adenoidectomy (T/A) and CPAP therapy. Overall, 29 of 81 patients underwent T/A. Only one parent reported it to be not effective and two stated that side effects were significant. Twenty‐four of 81 individuals were treated with CPAP, with a higher usage among patients under the age of 11 years. Three caregivers reported significant side effects and two did not consider the treatment helpful at all. Additionally, in the free‐text section, several parents reported they had been using sleep medication, mostly melatonin, if specified. Improvement of sleep was strongly wished for by 62 of 81 caregivers, the need for an intervention increased with the age of patients.
3.4. Hormonal dysregulation (Table 1d)
TABLE 1d.
Impact of individual symptoms and perceived effectiveness of available treatment options: Hormonal dysbalance.
| Impact | Worry | Treatment option | Ideal treatment | ||||
|---|---|---|---|---|---|---|---|
| Age [years] | Symptom | Very high/most important | Very high/most important | Used | Effective | Very/most important | |
| Growth hormone deficiencya | 0–3 | 76.9% | 42.3% | 34.6% | 65.4% | 65.4% | N/A |
| 4–10 | 62.5% | 34.4% | 18.8% | 65.6% | 65.6% | N/A | |
| 11–18 | 76.5% | 35.3% | 35.3% | 47.1% | 41.2% | N/A | |
| >18 | 50.0% | 33.3% | 33.3% | 66.7% | 33.3% | N/A | |
| Hypogonadism/incomplete sexual development | 0–3 | 34.6% | 19.2% | 19.2% | N/A | N/A | N/A |
| 4–10 | 12.5% | 3.1% | 9.4% | N/A | N/A | N/A | |
| 11–18 | 17.7% | 11.8% | 5.9% | N/A | N/A | N/A | |
| >18 | 16.7% | 0.0% | 0.0% | N/A | N/A | N/A | |
| Osteoporosis/weak bones | 0–3 | 7.7% | 23.1% | 19.2% | N/A | N/A | 61.5% |
| 4–10 | 9.4% | 15.6% | 18.8% | N/A | N/A | 40.6% | |
| 11–18 | 29.4% | 23.5% | 35.3% | N/A | N/A | 52.9% | |
| >18 | 50.0% | 66.7% | 66.7% | N/A | N/A | 100.0% | |
Note: Growth hormone deficiency is comparably well controlled in patient, whereas osteoporosis poses a high concern for caregivers. n (0–3 years) = 26, n (4–10 years) = 32, n (11–18 years) = 17, n (>18 years) = 6. Therapeutic options: arecombinant human growth hormone.
Abbreviation: N/A = not asked.
Largely prevalent in the SYS Patient Voices Cohort, GHD has been diagnosed in 56 of 81 patients. Supplementation of rhGH to address GHD was applied in 50 of 81 patients. In the two younger subgroups, all individuals receiving the treatment experienced at least some benefit, while it was not perceived as effective for three older patients.
The prevalence of hypogonadism and incomplete sexual development was highest in the youngest children, and the perceived impact decreased with age. In contrast, osteoporosis was only diagnosed in two of 26 patients under 4 years; however, it was present in half of the adults. Remarkably, caregivers noticed a severe impact of the symptom in patients of all age groups who had not been formally diagnosed at the time of the survey.
3.5. Additional symptoms and treatments
The possibility to list additional symptoms was also provided by the survey, and 12 caretakers specified these. Manifestations presented by more than one patient included hypersalivation, kyphosis, hearing loss, and harmful repetitive behavior. Furthermore, individuals were diagnosed with endocrine disorders, for example, hypoglycemia, central diabetes insipidus, and hypothyroidism.
Additionally, applied treatments were also mentioned by 31 caregivers, the most frequently used were medications to ameliorate sleep problems and attention deficit hyperactivity disorder. Several patients also underwent spinal surgery, but the exact underlying condition was not specified.
3.6. Impact on (daily) life (Table 2)
TABLE 2.
Extended impact of SYS on individuals, families, and caregivers.
| Moderate to severe impact | ||||||
|---|---|---|---|---|---|---|
| 0–3 years (n = 26) | 4–10 years (n = 32) | 11–18 years (n = 17) | >18 years (n = 6) | All (n = 81) | ||
| Individuals with SYS | ||||||
| Day‐to‐day living | 88.5% | 93.8% | 94.1% | 100.0% | 92.6% | |
| Ability to reach long‐term goals | 92.3% | 90.6% | 100.0% | 100.0% | 93.8% | |
| Families | ||||||
| Overall impact | 84.6% | 84.4% | 94.1% | 100.0% | 87.7% | |
| Caregivers | ||||||
| Financial impact | 80.8% | 75.0% | 70.6% | 100.0% | 77.8% | |
| Social impact | 73.1% | 81.3% | 94.1% | 100.0% | 82.7% | |
| Day‐to‐day living | 84.6% | 87.5% | 88.2% | 100.0% | 87.7% | |
| Ability to work outside of the home | 76.9% | 75.0% | 76.5% | 100.0% | 77.8% | |
| Ability to reach long‐term goals | 76.9% | 78.1% | 82.4% | 100.0% | 80.2% | |
| Overall impact | 92.3% | 96.9% | 100.0% | 100.0% | 96.3% | |
Note: Cumulatively, SYS causes high disruption in the lives of patients, families, and caregivers. The burden of disease also manifests in other aspects of caregivers' lives, namely financially, socially, and in terms of life planning.
Abbreviation: SYS, Schaaf‐Yang syndrome.
Caregivers were also asked to rate the impact of SYS on the affected individual's life as well as theirs' and their families. Most parents also commented in free text, expressing “agony and sadness” over the medical and social conditions. However, some individuals and caregivers were seemingly less affected, one respondent clarified that it was “a positive enrichment to have a child with SYS.” Overall, caregivers reported that the syndrome caused severe disruptions in the lives of 96.3% of primary caregivers and 87.7% of families. Although some symptoms became less prevalent and reportedly less challenging with increasing age, the overall reported burden of disease intensified.
All primary caregivers of individuals 18 years and older reported to be highly affected in terms of financial and social impact, day‐to‐day living, the ability to work outside of home and in achieving long‐term goals. In comparison, respondents taking care of children until the age of 18 years old were slightly less affected concerning their financial and career situation. Nevertheless, the global impact on caregivers for children and adolescents always ranked over 90%.
4. DISCUSSION
In this study, we present the results of an online questionnaire completed by 81 primary caregivers of individuals with SYS, aiming to elucidate the most challenging medical and social needs across the lifespan. To the best of our knowledge, the caregivers' perspective on SYS is addressed for the first time in a scientific publication.
Given the high number of participants and a prevalence of individual symptoms comparable to the literature, the SYS Patient Voices cohort is representative (Castilla‐Vallmanya et al., 2022; Marbach et al., 2020; McCarthy, Lupo, et al., 2018). In general, this survey shows that characteristic symptoms like hypotonia, neonatal contractures, DD, and feeding problems are present in infancy and early childhood, while cognitive and mental issues become more apparent over the course of the years. The severity of the phenotype does not only impair affected individuals, but it also causes high caregiver burden. Available symptomatic treatment is generally effective but does not ease the overall impact of SYS.
4.1. Worries and impact concerning the individual's quality of life
The importance of specific symptoms was perceived variably depending on their severity and patients' ages. In young children, hypotonia, contractures, feeding problems, and hormonal dysregulation were rated as highly impacting and proved to be subject of substantial worry. However, those symptoms seemed to subside or become less important as individuals grow older. In addition to the “natural course” of disease, the amelioration could be due to specific treatments provided to affected patients. In particular, GHD can be treated by administration of rhGH, which does not only lead to an increase in body height, but also positively affects body composition, mobility, and cognitive abilities (Hebach et al., 2021). In congruence with their manifestation, mental and sleep issues, skeletal problems, and hyperphagia have an increasing impact on patients and are also reportedly worrisome for caregivers. It is noteworthy that the perceived importance and apprehension toward those symptoms precede their onset and/or diagnosis, especially in the youngest subgroup. This discrepancy might indicate an underdiagnosis of certain symptoms in SYS, since it has been demonstrated in longitudinal studies on children with non‐curable diseases that physicians tend to under‐report the prevalence of symptoms in comparison to parents (Steele et al., 2014). However, given the strong emphasis on the high prevalence of ASD in patients with SYS as well as the syndrome's close association with PWS, a neurodevelopmental disorder causing morbid hyperphagia and a prominent behavioral phenotype, parents might also anticipate the onset of behavioral issues and evaluate their importance accordingly (McCarthy, Lupo, et al., 2018; Schaaf et al., 2013; Schwartz et al., 2021). Increased parental anxiety has been found to be associated with a stronger perception of symptoms in the affected child (Smith et al., 2020). Therefore, it is within the responsibility of physicians involved in the care of the patient to guide parents and primary caretakers in acquiring information about their child's disorder (Serra et al., 2021). Nevertheless, in their interpretation of the longitudinal assessment of parents' and patients' needs, Steele et al. highlighted the importance of determining why parents are concerned about the impact instead of questioning their diagnostic abilities (Steele et al., 2014).
As demonstrated by the SYS Patient Voices Survey, it is thus crucial to integrate parents' perceptions of the development of symptoms to diagnose and treat possible clinical manifestations early on. Meanwhile, parents should be assured that some symptoms like hypotonia become less impacting over time, in order to partially alleviate caregivers' concerns.
4.2. Treatment and unmet treatment needs
While symptomatic therapeutic options are available and, if used, to some degree effective, caregivers are not yet satisfied with the overall treatment for SYS. Most caregivers considered the development of an ideal treatment in all assessed categories “very important” or “most important.” The respondents were particularly interested in an improvement in intellectual abilities, mobility, and positive social interaction. In addition to the investigation of treatment priorities and unmet medical needs, as performed in the SYS Patient Voices survey, patient‐related outcome measures should be determined using multiple approaches, for example, interviews, focus groups within the family associations, and “shadowing” of day‐to‐day‐living (Morel & Cano, 2017).
Given the fact that SYS is an ultra‐rare disease, development of specific treatments for the disorder is limited by lack of knowledge about the clinical presentation and underlying molecular mechanisms, financial restrictions, and low numbers of participants in clinical trials. One participant in the SYS Patient Survey specifically asked for “more coordination in the investigation.” As research for other orphan diseases has demonstrated, it is crucial to invest in an active communication between researchers, physicians, caregivers/families, and for the industry to further address specific therapeutic needs (Straub et al., 2016). Therefore, specific SYS research has actively been supported by the FPWR in recent years.
4.3. Impact on caregivers and families
The overall impact of SYS on individuals and their families regarding aspects of day‐to‐day living as well as long‐term goals increases as patients get older. This is consistent with studies evaluating the well‐being of caregivers of individuals with PWS (Kayadjanian et al., 2018, 2021). Certain symptoms like sleep difficulties, which affect 76.4% of patients, might not only impair the primary caregiver's sleep quality, but can also be straining for other family members. It has been shown that primary caregivers of children with chronic diseases frequently report severe sleep disturbances affecting their day‐time‐performance, mood as well as mental condition, while neurotypical siblings of individuals with neurodevelopmental disorders have a higher prevalence of sleep disorders themselves (Meltzer & Moore, 2007; Veatch et al., 2021).
Unsurprisingly, caring for a child with a severe disorder like SYS also impacts the social lives of parents. Having to prioritize the patient's needs over social interaction has been shown to be closely associated with a feeling of loneliness and social isolation of caretakers (Currie & Szabo, 2020; Hajek et al., 2021). Therefore, therapeutic strategies should focus on ensuring participation of patients and their caretakers.
In addition to the physical and mental challenges, caring for a child with a rare neurodevelopmental disease has a substantial financial impact on families in terms of direct and indirect health cost, for example, reduced working hours (Stabile & Allin, 2012). Respondents in our survey experienced high financial burdens and limitations in their ability to work outside of home in 77.8% of cases. A European study on patients with PWS, however, demonstrated that economic costs are not the strongest variable on the quality of life of the individual and their caregiver (López‐Bastida et al., 2016).
While access to financial support and necessary treatment options should undoubtedly be ensured for every individual with SYS, it is particularly important to improve the quality of life for parents and families and to alleviate caregiver burden. For short‐term improvement of parent's psychological well‐being, group‐based parent training programs have been beneficial, long‐term interventions should encompass family support in several modalities, for example, organizational support, (in‐home) respite, tele‐counseling, and structural adaptions like employment and healthcare benefits (Barlow et al., 2012; Edelstein et al., 2017). Furthermore, peer support through family interest groups has helped to stabilize caregivers, connecting with other affected families through social media has also become more and more relevant (Titgemeyer & Schaaf, 2022). Therefore, accessibility to online and face‐to‐face SYS groups should actively be promoted (Chakraborti et al., 2021).
4.4. Limitations and outlook
Our study has several limitations. Due to the de‐identification of participants, free‐text answers could not be attributed to individuals and genotype–phenotype‐correlation was not possible. Additionally, the gender of patients with SYS was not assessed, which could have allowed for an analysis of a potential sexual dimorphism of symptoms.
For further analysis of the treatment efficacy in individuals with SYS, the proxy data should be complemented with the patient's perception of symptoms and received medical interventions if acquisition is possible (Ibragimova et al., 2007; U.S. Food and Drug Administration, 2006).
5. CONCLUSION
For the first time, we present the caregivers' perception of disease burden for individuals affected with SYS and their families. It becomes apparent that symptoms associated with the syndrome have an age‐dependent impact and that they limit the patients' well‐being and ability to participate socially. This equally affects primary caregivers, who are also largely restricted in their life planning.
While therapeutic options are available, they do not sufficiently alleviate the impact of the disorder. In addition to an amelioration of the symptoms, future research on treatment interventions should therefore also consider aspects of daily functioning for patients and caregivers.
AUTHOR CONTRIBUTIONS
Christian P. Schaaf, Lisa Matesevac, and Theresa V. Strong contributed to the development of the survey and review of the manuscript, Laura Dötsch and Christian P. Schaaf contributed to the data analysis and preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
FUNDING INFORMATION
This study was supported by the Foundation of Prader‐Willi Research.
ETHICS STATEMENT
Approval was received from the North Star Review Board, IRB, Protocol 2021 04 NB200049. Participants were informed about de‐identification and publication of the data.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We acknowledge the advice and help with the distribution of the SYS Patient Voices survey of Susan Hedstrom and thank the two members of the SYS Community for previewing the survey questions. Open Access funding enabled and organized by Projekt DEAL.
Dötsch, L. , Matesevac, L. , Strong, T. V. , & Schaaf, C. P. (2023). Caregiver‐based perception of disease burden in Schaaf‐Yang syndrome. Molecular Genetics & Genomic Medicine, 11, e2262. 10.1002/mgg3.2262
DATA AVAILABILITY STATEMENT
The de‐identified data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Barlow, J. , Smailagic, N. , Huband, N. , Roloff, V. , & Bennett, C. (2012). Group‐based parent training programmes for improving parental psychosocial health. The Cochrane Database of Systematic Reviews, (6), CD002020. 10.1002/14651858.CD002020.pub3 [DOI] [PubMed] [Google Scholar]
- Castilla‐Vallmanya, L. , Centeno‐Pla, M. , Serrano, M. , Franco‐Valls, H. , Martínez‐Cabrera, R. , Prat‐Planas, A. , Rojano, E. , Ranea, J. A. G. , Seoane, P. , Oliva, C. , Paredes‐Fuentes, A. J. , Marfany, G. , Artuch, R. , Grinberg, D. , Rabionet, R. , Balcells, S. , & Urreizti, R. (2022). Advancing in Schaaf‐Yang syndrome pathophysiology: From bedside to subcellular analyses of truncated MAGEL2. Journal of Medical Genetics, 60, 406–415. 10.1136/jmg-2022-108690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti, M. , Gitimoghaddam, M. , McKellin, W. H. , Miller, A. R. , & Collet, J. P. (2021). Understanding the implications of peer support for families of children with neurodevelopmental and intellectual disabilities: A scoping review. Frontiers in Public Health, 9, 719640. 10.3389/fpubh.2021.719640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie, G. , & Szabo, J. (2020). Social isolation and exclusion: The parents' experience of caring for children with rare neurodevelopmental disorders. International journal of Qualitative Studies on Health and Well‐Being, 15(1), 1725362. 10.1080/17482631.2020.1725362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein, H. , Schippke, J. , Sheffe, S. , & Kingsnorth, S. (2017). Children with medical complexity: A scoping review of interventions to support caregiver stress. Child: Care, Health and Development, 43(3), 323–333. 10.1111/cch.12430 [DOI] [PubMed] [Google Scholar]
- Fountain, M. D. , Aten, E. , Cho, M. T. , Juusola, J. , Walkiewicz, M. A. , Ray, J. W. , Xia, F. , Yang, Y. , Graham, B. H. , Bacino, C. A. , Potocki, L. , van Haeringen, A. , Ruivenkamp, C. A. L. , Mancias, P. , Northrup, H. , Kukolich, M. K. , Weiss, M. M. , van Ravenswaaij‐Arts, C. M. A. , Mathijssen, I. B. , … Schaaf, C. P. (2017). The phenotypic spectrum of Schaaf‐Yang syndrome: 18 new affected individuals from 14 families. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 19(1), 45–52. 10.1038/gim.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, A. , Kretzler, B. , & König, H. H. (2021). Informal caregiving, loneliness and social isolation: A systematic review. International Journal of Environmental Research and Public Health, 18(22). 10.3390/ijerph182212101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebach, N. R. , Caro, P. , Martin‐Giacalone, B. A. , Lupo, P. J. , Marbach, F. , Choukair, D. , & Schaaf, C. P. (2021). A retrospective analysis of growth hormone therapy in children with Schaaf‐Yang syndrome. Clinical Genetics, 100(3), 298–307. 10.1111/cge.14000 [DOI] [PubMed] [Google Scholar]
- Ibragimova, N. , Lillvist, A. , Pless, M. , & Granlund, M. (2007). The utility of ICF for describing interaction in non‐speaking children with disabilities—Caregiver ratings and perceptions. Disability and Rehabilitation, 29(22), 1689–1700. 10.1080/09638280601056186 [DOI] [PubMed] [Google Scholar]
- Kanber, D. , Giltay, J. , Wieczorek, D. , Zogel, C. , Hochstenbach, R. , Caliebe, A. , Kuechler, A. , Horsthemke, B. , & Buiting, K. (2009). A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader–Willi syndrome. European Journal of Human Genetics, 17(5), 582–590. 10.1038/ejhg.2008.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayadjanian, N. , Schwartz, L. , Farrar, E. , Comtois, K. A. , & Strong, T. V. (2018). High levels of caregiver burden in Prader‐Willi syndrome. PLoS One, 13(3), e0194655. 10.1371/journal.pone.0194655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayadjanian, N. , Vrana‐Diaz, C. , Bohonowych, J. , Strong, T. V. , Morin, J. , Potvin, D. , & Schwartz, L. (2021). Characteristics and relationship between hyperphagia, anxiety, behavioral challenges and caregiver burden in Prader‐Willi syndrome. PLoS One, 16(3), e0248739. 10.1371/journal.pone.0248739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Bastida, J. , Linertová, R. , Oliva‐Moreno, J. , Posada‐de‐la‐Paz, M. , Serrano‐Aguilar, P. , Kanavos, P. , Taruscio, D. , Schieppati, A. , Iskrov, G. , Baji, P. , Delgado, C. , von der Schulenburg, J. M. G. , Persson, U. , Chevreul, K. , Fattore, G. , & BURQOL‐RD Research Network . (2016). Social/economic costs and health‐related quality of life in patients with Prader‐Willi syndrome in Europe. The European Journal of Health Economics, 17(1), 99–108. 10.1007/s10198-016-0788-z [DOI] [PubMed] [Google Scholar]
- Marbach, F. , Elgizouli, M. , Rech, M. , Beygo, J. , Erger, F. , Velmans, C. , Stumpel, C. , Stegmann, A. P. A. , Beck‐Wödl, S. , Gillessen‐Kaesbach, G. , Horsthemke, B. , Schaaf, C. P. , & Kuechler, A. (2020). The adult phenotype of Schaaf‐Yang syndrome. Orphanet Journal of Rare Diseases, 15(1), 294. 10.1186/s13023-020-01557-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, J. M. , Lupo, P. J. , Kovar, E. , Rech, M. , Bostwick, B. , Scott, D. , Kraft, K. , Roscioli, T. , Charrow, J. , Schrier Vergano, S. A. , Lose, E. , Smiegel, R. , Lacassie, Y. , & Schaaf, C. P. (2018). Schaaf‐Yang syndrome overview: Report of 78 individuals. American Journal of Medical Genetics. Part A, 176(12), 2564–2574. 10.1002/ajmg.a.40650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, J. M. , McCann‐Crosby, B. M. , Rech, M. E. , Yin, J. , Chen, C. A. , Ali, M. A. , Nguyen, H. N. , Miller, J. L. , & Schaaf, C. P. (2018). Hormonal, metabolic and skeletal phenotype of Schaaf‐Yang syndrome: A comparison to Prader‐Willi syndrome. Journal of Medical Genetics, 55(5), 307–315. 10.1136/jmedgenet-2017-105024 [DOI] [PubMed] [Google Scholar]
- Mejlachowicz, D. , Nolent, F. , Maluenda, J. , Ranjatoelina‐Randrianaivo, H. , Giuliano, F. , Gut, I. , Sternberg, D. , Laquerrière, A. , & Melki, J. (2015). Truncating mutations of MAGEL2, a gene within the Prader‐Willi locus, are responsible for severe arthrogryposis. American Journal of Human Genetics, 97(4), 616–620. 10.1016/j.ajhg.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer, L. J. , & Moore, M. (2007). Sleep disruptions in parents of children and adolescents with chronic illnesses: Prevalence, causes, and consequences. Journal of Pediatric Psychology, 33(3), 279–291. 10.1093/jpepsy/jsm118 [DOI] [PubMed] [Google Scholar]
- Morel, T. , & Cano, S. J. (2017). Measuring what matters to rare disease patients—Reflections on the work by the IRDiRC taskforce on patient‐centered outcome measures. Orphanet Journal of Rare Diseases, 12(1), 171. 10.1186/s13023-017-0718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi, Y. , Ieda, D. , Hori, I. , Nozaki, Y. , Yamagata, T. , Komaki, H. , Tohyama, J. , Nagasaki, K. , Tada, H. , & Saitoh, S. (2019). Schaaf‐Yang syndrome shows a Prader‐Willi syndrome‐like phenotype during infancy. Orphanet Journal of Rare Diseases, 14(1), 277. 10.1186/s13023-019-1249-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, W. T. , Schaaf, C. P. , Rech, M. E. , & Wrede, J. (2020). Polysomnographic characteristics and sleep‐disordered breathing in Schaaf‐Yang syndrome. Pediatric Pulmonology, 55(11), 3162–3167. 10.1002/ppul.25056 [DOI] [PubMed] [Google Scholar]
- Schaaf, C. P. , Gonzalez‐Garay, M. L. , Xia, F. , Potocki, L. , Gripp, K. W. , Zhang, B. , Peters, B. A. , McElwain, M. A. , Drmanac, R. , Beaudet, A. L. , Caskey, C. T. , & Yang, Y. (2013). Truncating mutations of MAGEL2 cause Prader‐Willi phenotypes and autism. Nature Genetics, 45(11), 1405–1408. 10.1038/ng.2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf, C. P. , & Marbach, F. (2021). Schaaf‐Yang syndrome. In Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Gripp K. W., Mirzaa G. M., & Amemiya A. (Eds.), GeneReviews(®). University of Washington, Seattle copyright © 1993–2022, University of Washington. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [Google Scholar]
- Schwartz, L. , Caixàs, A. , Dimitropoulos, A. , Dykens, E. , Duis, J. , Einfeld, S. , Gallagher, L. , Holland, A. , Rice, L. , Roof, E. , Salehi, P. , Strong, T. , Taylor, B. , & Woodcock, K. (2021). Behavioral features in Prader‐Willi syndrome (PWS): Consensus paper from the International PWS Clinical Trial Consortium. Journal of Neurodevelopmental Disorders, 13(1), 25. 10.1186/s11689-021-09373-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, G. , Memo, L. , Coscia, A. , Giuffré, M. , Iuculano, A. , Lanna, M. , Valentini, D. , Contardi, A. , Filippeschi, S. , Frusca, T. , Mosca, F. , Ramenghi, L. A. , Romano, C. , Scopinaro, A. , Villani, A. , Zampino, G. , & Corsello, G. (2021). Recommendations for neonatologists and pediatricians working in first level birthing centers on the first communication of genetic disease and malformation syndrome diagnosis: Consensus issued by 6 Italian scientific societies and 4 parents' associations. Italian Journal of Pediatrics, 47(1), 94. 10.1186/s13052-021-01044-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L. E. , Weinman, J. , Yiend, J. , & Rubin, J. (2020). Psychosocial factors affecting parental report of symptoms in children: A systematic review. Psychosomatic Medicine, 82(2), 187–196. 10.1097/psy.0000000000000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile, M. , & Allin, S. (2012). The economic costs of childhood disability. The Future of Children, 22(1), 65–96. 10.1353/foc.2012.0008 [DOI] [PubMed] [Google Scholar]
- Steele, R. , Siden, H. , Cadell, S. , Davies, B. , Andrews, G. , Feichtinger, L. , & Singh, M. (2014). Charting the territory: Symptoms and functional assessment in children with progressive, non‐curable conditions. Archives of Disease in Childhood, 99(8), 754–762. 10.1136/archdischild-2013-305246 [DOI] [PubMed] [Google Scholar]
- Straub, V. , Balabanov, P. , Bushby, K. , Ensini, M. , Goemans, N. , De Luca, A. , Pereda, A. , Hemmings, R. , Campion, G. , Kaye, E. , Arechavala‐Gomeza, V. , Goyenvalle, A. , Niks, E. , Veldhuizen, O. , Furlong, P. , Stoyanova‐Beninska, V. , Wood, M. J. , Johnson, A. , Mercuri, E. , … Aartsma‐Rus, A. (2016). Stakeholder cooperation to overcome challenges in orphan medicine development: The example of Duchenne muscular dystrophy. Lancet Neurology, 15(8), 882–890. 10.1016/s1474-4422(16)30035-7 [DOI] [PubMed] [Google Scholar]
- Thomason, M. M. , McCarthy, J. , Goin‐Kochel, R. P. , Dowell, L. R. , Schaaf, C. P. , & Berry, L. N. (2020). Neurocognitive and neurobehavioral phenotype of youth with Schaaf‐Yang syndrome. Journal of Autism and Developmental Disorders, 50(7), 2491–2500. 10.1007/s10803-018-3775-7 [DOI] [PubMed] [Google Scholar]
- Titgemeyer, S. C. , & Schaaf, C. P. (2022). Facebook support groups for pediatric rare diseases: Cross‐sectional study to investigate opportunities, limitations, and privacy concerns. JMIR Pediatrics and Parenting, 5(1), e31411. 10.2196/31411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . (2006). Guidance for industry: Patient‐reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health and Quality of Life Outcomes, 4, 79. 10.1186/1477-7525-4-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch, O. J. , Malow, B. A. , Lee, H.‐S. , Knight, A. , Barrish, J. O. , Neul, J. L. , Lane, J. B. , Skinner, S. A. , Kaufmann, W. E. , Miller, J. L. , Driscoll, D. J. , Bird, L. M. , Butler, M. G. , Dykens, E. M. , Gold, J.‐A. , Kimonis, V. , Bacino, C. A. , Tan, W.‐H. , Kothare, S. V. , … Glaze, D. G. (2021). Evaluating sleep disturbances in children with rare genetic neurodevelopmental syndromes. Pediatric Neurology, 123, 30–37. 10.1016/j.pediatrneurol.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The de‐identified data that support the findings of this study are available from the corresponding author upon reasonable request.


