Abstract
BACKGROUND
More than 90% of patients developing heart failure (HF) have an epidemiological background of hypertension. The most frequent concomitant conditions are type 2 diabetes mellitus, obesity, atrial fibrillation, and coronary disease, all disorders/diseases closely related to hypertension.
METHODS
HF outcome research focuses on decreasing mortality and preventing hospitalization for worsening HF syndrome. All drugs that decrease these HF endpoints lower blood pressure. Current drug treatments for HF are (i) angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or angiotensin receptor neprilysin inhibitors, (ii) selected beta-blockers, (iii) steroidal and nonsteroidal mineralocorticoid receptor antagonists, and (iv) sodium-glucose cotransporter 2 inhibitors.
RESULTS
For various reasons, these drug treatments were first studied in HF patients with a reduced ejection fraction (HFrEF). However, subsequently, they have been investigated and, as we see it, documented as beneficial in HF patients with a preserved left ventricular ejection fraction (LVEF, HFpEF) and mostly hypertensive etiology, with effect estimates assessed partly on top of background treatment with the drugs already proven effective in HFrEF. Additionally, diuretics are given on symptomatic indications.
CONCLUSIONS
Considering the totality of evidence and the overall need for antihypertensive treatment and/or treatment of hypertensive complications in almost all HF patients, the principal drug treatment of HF appears to be the same regardless of LVEF. Rather than LVEF-guided treatment of HF, treatment of HF should be directed by symptoms (related to the level of fluid retention), signs (tachycardia), severity (NYHA functional class), and concomitant diseases and conditions. All HF patients should be given all the drug classes mentioned above if well tolerated.
Keywords: angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, angiotensin receptor neprilysin inhibitor, beta-blocker, blood pressure, heart failure, hypertension, mineralocorticoid receptor antagonist, sodium-glucose cotransporter 2 inhibitor
Heart failure (HF) affects 2.4% of the American population and is expected to rise to 3% by 2030.1 Despite extensive developments in many other areas of cardiology, the prognosis of HF remains poor, with 5-year mortality above 50%.2,3
In 1987, the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), a prospective randomized clinical trial (RCT) that enrolled patients with severe HF of New York Heart Association (NYHA) functional class IV treated with the angiotensin-converting enzyme inhibitor (ACEI) enalapril or placebo, demonstrated a 40% reduction in mortality in favor of enalapril.4 CONSENSUS was the first RCT in a new wave of research with modern pharmacotherapy that showed improved HF survival. CONSENSUS included patients without measured left ventricular ejection fraction (LVEF), as LVEF was not yet established as a defining characteristic of HF.
Four years later, Studies of Left Ventricular Dysfunction (SOLVD)5 showed similar benefits with enalapril in HF patients with NYHA class II–III. SOLVD included patients with LVEF <0.35, based on the ability of ACEI therapy to prevent LV dilatation and also because low LVEF was associated with a high event rate, allowing for a limited study sample size in patients with less severe HF. Furthermore, LVEF <0.35 had been a major criterion for defining HF according to Framingham Study criteria6 and therefore was initially acceptable to clinicians as an objective marker of systolic LV dysfunction. Selecting patients with HF and a reduced LVEF (HFrEF) became a standard procedure in subsequent RCTs, which documented the benefit of other drug classes, e.g., selected beta-blockers, mineralocorticoid receptor antagonists (MRAs), and angiotensin receptor blockers (ARBs) over the coming decades.7
The fact that ACEIs or ARBs, selected beta-blockers, MRAs, and good choice and dose of diuretic clinically compensated patients with symptoms and signs of HF, whatever LVEF they might have, and improved the prognosis in HFrEF patients, did not exclude the possibility that these drugs had similar beneficial prognostic effects in patients with HF and a preserved EF (HFpEF). Additionally, for most physicians taking care of decompensated HF patients, dividing into HFrEF and HFpEF subsets when recruiting patients to RCTs and excluding HFpEF patients from these drug benefits did not make sense. When working clinically with patients most HF patients were given these drugs anyway because of hypertension and/or the HF itself with apparent clinical benefits. Later, when RCTs were organized in HFpEF patients, it was too late to do studies in untreated patients without these drugs. They were already well established as background therapies in most HF patients. Surprisingly, it became an uphill effort to document the same beneficial effects in patients with HFpEF as in HFrEF.
Additionally, the RCTs of patients with HFpEF preceded the scientific documentation that systolic LV dysfunction was present in most patients beyond the well-described diastolic LV dysfunction.8 More refined echocardiographic measures of systolic LV function within the preserved LVEF range showed reduced LV mid-wall shortening, stroke volume (SV), and global longitudinal strain. Longitudinal axis shortening could be partly or completely missing as a sign of extensive systolic dysfunction, an echocardiographic observation in patients with HFpEF since the 1990s or even earlier. Thus, most HF patients have systolic dysfunction, whether EF is reduced or preserved.
Furthermore, the event rate in the RCTs in HFpEF was lower than in the corresponding RCTs of patients with HFrEF (Table 1), suggesting difficulties in excluding from RCTs those patients with normal to supra-normal LVEF who did not have HF and thereby could not expect to benefit substantially of the HF medications.9–17
Table 1.
Number of events of comparable RCTs on HFpEF and HFrEF
| Drug classes, study acronyms, and references | HFpEF | HFrEF | ||
|---|---|---|---|---|
| No. events placebo group (n)/size placebo group (n) | Duration of follow-up (mo) | No. events placebo group (n)/size placebo group (n) | Duration of follow-up (mo) | |
|
ARB
CHARM-Preserved9 vs. CHARM-Alternative10 |
366/1,509 | 36.6 | 406/1,015 | 33.7 |
|
ACEi
a
PEP-CHF11 vs. SOLVD5 |
65/426 | 26.2 | 736/1,284 | 41.4 |
|
MRA
TOPCAT12 vs. RALES13 |
351/1,723 | 21.6 | 324 cardiac deaths and 336 hospitalized patients (753 events)/841 | 24 |
|
ARNI
b
PARAGON14 vs. PARADIGM15 |
1,009/2,389 | 35 | 1,117/4,212 | 27 |
|
SGLT2I
EMPEROR Preserved16 vs. EMPEROR Reduced17 |
511/2,991 | 26.6 | 462/1,867 | 16 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; EMPEROR, Empagliflozin in Heart Failure; MRA, mineralocorticoid receptor antagonist; PEP-CHF, Perindopril vs. Placebo in Congestive Heart Disease; RALES, Randomized Aldactone Evaluation Study, and drug classes; SGLT2I, sodium-glucose cotransporter 2 inhibitor; SOLVD, Studies of Left Ventricular Dysfunction; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
aNot the same ACEI (perindopril and enalapril).
Also, the recognition of HFpEF as a disease entity came late in the patent life of medications, which had documented benefits and subsequently were used in patients with HFrEF. Thus, with few exceptions, the pharmaceutical industry did not fund RCTs with drugs whose patent life had expired or soon would expire. Most drugs available for potential investigation in HFpEF became a substantial part of the background therapy in all HF and other cardiac patients because of evidence-based benefits in hypertensive heart disease18,19 and post-myocardial infarction. Therefore, for various reasons, several initial RCTs in HFpEF were too small, had inadequate statistical power, and/or showed results that were difficult to interpret (Figure 1).
Figure 1.
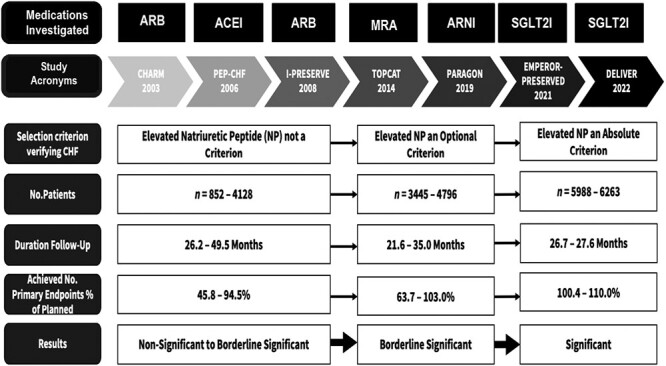
Development of RCTs of heart failure with preserved ejection fraction (HFpEF). The introduction of natriuretic peptides may have contributed to strengthening the diagnosis of HFpEF and, thus, the study quality. The temporal sequence is confounded by the drugs being investigated, which are indicated in the upper part of the figure. Abbreviations: CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; CHF, congestive heart failure; DELIVER, Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction; EMPEROR-PRESERVED, EMPagliflozin outcome tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction; HF, heart failure; I-PRESERVE, Irbesartan in Heart Failure with Preserved Ejection Fraction; NP, natriuretic peptide; Paragon, Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin receptor blocker Global Outcomes in HFpEF; PE, primary endpoint; PEP-CHF, Perindopril vs. Placebo; RCT, randomized clinical trial; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist. For drug classes, see abbreviations in Table 1.
THE AIM OF THIS REVIEW
The aim of the present narrative review is thus to critically assess all RCTs done with HF drugs (Figure 1, Table 2) in patients with HFpEF in order to investigate mortality and hospitalization for worsening of HF, express our opinions on strengths and weaknesses of the development of these RCTs and interpret what this development of initial and newer RCTs means for the treatment of patients with HFpEF. As hypertension is a key driver in the epidemiology, pathophysiology, and treatment of HF,18,19 a central element in the current article is Figure 2, showing our available drug treatments for hypertensive patients if HF is a complication or a concomitant disease.
Table 2.
Characteristics of randomized clinical trials of drug treatment in patients with HFpEF
| Characteristics | CHARM (2003) |
PEP-CHF (2006) |
I-PRESERVE (2008) |
TOPCAT (2014) |
PARAGON (2019) |
EMPEROR PRESERVED (2021) |
DELIVER (2022) |
|
|---|---|---|---|---|---|---|---|---|
| Age (y) | 67 ± 11 | 75 | 72 ± 7 | 68.7 | 72 ± 8 | 71 ± 9 | 71 ± 9 | |
| Women (%) | 40 | 55.5 | 60 | 51.5 | 52 | 45 | 43 | |
| Body mass index (kg/m2) | 29.3 ± 5.9 | 27.5 (25.1–30.0) | 29.7 ± 5.3 | 31 (27–36) | 30.2 ± 4.9 | 29.8 ± 5.8 | 29.8 ± 6.1 | |
| LVEF (%) | 41–49 (35%) 50–59 (35%) |
64.5 | 59.5 | 56 | 57.5 | 54.3 | 54 ± 8 | |
| NYHA (%) | I | – | 75.5 | – | 3 | 3 | 0.1 | – |
| II | 61 | 21.5 | 63 | 77 | 81.5 | 75.2 | ||
| III | 38 | 24.5 | 76.5 | 32.5 | 19.5 | 18 | 24.4 | |
| IV | 1.5 | 3 | 0.4 | 0.4 | 0.3 | 0.3 | ||
| NTproBNP (pg/ml) | – | 335–453 | 320–360 | 887–1,017 | 910 | 970 | 1,387–1,408/704–729 | |
| Systolic BP (mm Hg) | 136 | 139 | 136 | 130 | 131 | 131 ± 15.5 | 125 ± 15 | |
| Comorbidity (%) | AF | 29 | 21 | 29 | 32–51 | 33 | 51 | 56–57 |
| DM | 28 | 20.5 | 27.5 | 13–34 | 43 | 49 | 44.8 | |
| HT | 64 | 79 | 88.5 | 95 | 95 | 90 | 88.6 | |
| MI | 44 | 26 | 23 | 22–40 | 22.5 | – | – | |
Abbreviations: AF, atrial fibrillation; BP, blood pressure; DM, diabetes mellitus; HT, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NTproBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association. Study acronyms are defined in the footnotes in Table 1 and Figure 1.
Figure 2.
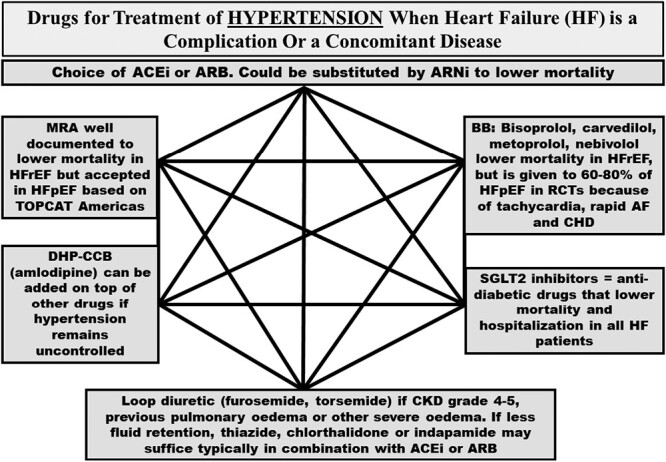
The hexagon is summarizing details of drug treatment of hypertension, when heart failure, HFrEF or HFpEF, is a complication or a concomitant disease. All medications mentioned in the figure are either established antihypertensive medications lowering blood pressure and/or are documented to improve the prognosis in HFrEF and/or HFpEF. For acronyms, see abbreviations in the footnotes in Table 1 and Figure 1. Abbreviations: AF, atrial fibrillation; BB, beta-blocker; BP, blood pressure; CHD, coronary heart disease; CKD, chronic kidney disease.
Physicians who agree that drug treatment is essentially the same in HFrEF and HFpEF may feel that we manufacture a controversy where there is none. American HF guidelines have emphasized the need to treat hypertension as a first and important feature of drug treatment of HF, whether patients have HFrEF or HFpEF.20 Thus, treatment focused on the ACEIs or ARBs and liberal use of MRAs in HF, whatever LVEF, have mostly provided appropriate drug treatment for HF in America (Figure 2). However, European HF guidelines21 have more or less neglected concomitant disorders such as hypertension, and controversy has emerged. It is uncertain whether HFpEF patients are now receiving foundational drug therapies and will receive such drug treatment in the future. Thus, our current paper also aims to promote the implementation of foundational HF therapies in most HFpEF patients.
We consider it an increasing problem that newer drugs like angiotensin receptor neprilysin inhibitor (ARNI) and especially sodium-glucose cotransporter 2 inhibitors (SGLT2Is) are heavily promoted by enthusiastic colleagues and industry in contrast with well-documented drugs currently off patent. Attitudes vary from more fair reviews of the strengths and weaknesses of RCTs22–25 to the eccentric view that SGLT2Is are the only class of medication with documentation good enough for being used in HFpEF.26,27
While we discuss in detail the outcomes of RCTs with the various available HF drugs, administrative and medical controversies, and possible future phenotypes, we do not aim to assess other important nonpharmacological aspects of HF treatment, briefly listed in Table 3.
Table 3.
Various main treatment strategies in patients with heart failure beyond drug treatment not discussed in the present viewpoint article
| • Drug treatments discussed |
| (i) ACEIs, ARBs, or ARNI (sacubitril–valsartan) |
| (ii) Beta-blockers (bisoprolol, carvedilol, metoprolol, nebivolol) |
| (iii) MRAs (spironolactone, eplerenone) and nonsteroidal aldosterone antagonist (finerenone) |
| (iv) SGLT2Is (canagliflozin, dapagliflozin, empagliflozin, sotagliflozin) |
| (v) Diuretics (loop diuretics, and nonloop thiazides, indapamide, chlorthalidone) |
| (vi) Aldosterone synthase inhibitor (baxdrostat) is under clinical development |
| • Cost–benefit assessments, knowing that when patency has expired, the drugs become inexpensive and treatment cost-effective |
| • Nonpharmaceutical treatments, like weight control, physical exercise, and moderate sodium restriction, which, could improve the clinical setting but are less clear regarding “evidence” |
| • Symptomatic effects of the HF drugs, except that hospitalization for HF, in reality, is an expression of symptomatic worsening |
| • Review the treatment of concomitant diseases, though for many diseases like hypertension, diabetes, obesity, coronary heart disease, and atrial fibrillation, the drug treatment, to a large extent, is similar and overlapping with drug treatment for HF |
| • Usefulness of device treatments, including a variety of options: pacemaker (PM), implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy (CRT), left ventricular assist device (LVAD), valve repair (TAVR), coronary surgery (PCI, CABG), heart transplantation (TX), renal denervation (RDN) |
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; SGLT2Is, sodium-glucose cotransporter 2 inhibitors.
OVERVIEW OF THE CURRENT DRUG TREATMENT OF HF IN PATIENTS WITH A PRESERVED EJECTION FRACTION
The objectives of HF treatment are to increase survival, reduce hospitalizations for worsening HF, and improve quality of life. To achieve these objectives, current American20 and European21 HF guidelines have developed specific treatment recommendations for patients with HFrEF. In contrast, most patients with a mildly reduced and a midrange LVEF, HFmrEF, and HFpEF, respectively, have been left with symptomatic supportive care due to an alleged lack of evidence. Due to data that have emerged from recent RCTs, the Food and Drug Administration (FDA) has approved new HF drugs, and American guidelines have been revised accordingly. Also, the European Medical Agency (EMA) and European HF Guidelines may follow, at least partly with the SGLT2Is but not with the traditional drug classes mentioned above.
The treatment recommendations for HFrEF include renin–angiotensin system (RAS) inhibitors (ACEI or ARB, alternatively ARNI), selected beta-blockers, MRAs, and SGLT2Is. The results of major outcome trials and the scientific basis for using these drug classes, “cornerstones,” in HFpEF, are summarized in Figure 3 and are further discussed based on information included in Tables 2 and 5.
Figure 3.
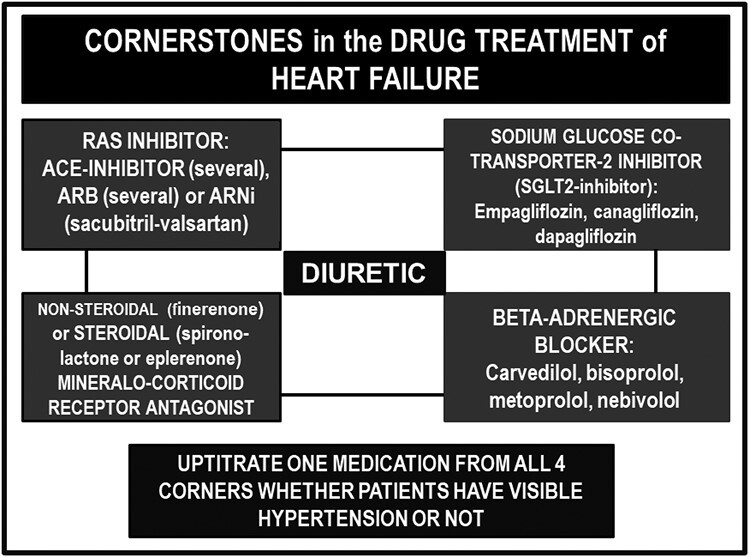
The cornerstones of drug treatment of heart failure. Abbreviations are defined in the footnotes in Table 1 and Figure 1.
Table 5.
Concomitant drug treatment in I-PRESERVEa
| Treatment (%) | Placebo (N = 2,061) | Irbesartan (N = 2,067) | ||
|---|---|---|---|---|
| Baseline | Exposed | Baseline | Exposed | |
| ACE inhibitor | 25 | 40b | 26 | 39b |
| Antiplatelet | 58 | 59 | ||
| Beta-blocker | 58 | 73b | 59 | 73b |
| Calcium channel blocker | 39 | 40 | ||
| Digoxin | 13 | 13 | ||
| Diuretic | 84 | 82 | ||
| Lipid lowering | 30 | 32 | ||
| Spironolactone | 15 | 29b | 15 | 28b |
Shown as fractions at baseline and for the HF (HFrEF) survival medication also as fractions of patients totally exposed during the study. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; RCT, randomized clinical trial.
aI-PRESERVED was an RCT investigating mortality and hospitalization of the ARB irbesartan in patients with HFpEF.
bTotally exposed during the study.29
Bisoprolol, carvedilol, metoprolol, and nebivolol are included because these beta-blockers have been proven to reduce mortality in HFrEF.28 Hypertensive patients with severe HF may have low blood pressure because of very high total peripheral resistance and may need careful in-hospital up-titration of medications from all 4 corners, as indicated in the lower part of Figure 3.
Evidence in favor of ACE-inhibitor treatment in HFpEF
Clinical evidence of ACEI benefit in HFpEF is limited for various reasons outlined above. CONSENSUS4 required increased heart size on chest X-ray, making it likely that many patients had HFrEF. However, chest X-ray is an imperfect means to discriminate between HFrEF and HFpEF. Thus, patients with HF as a consequence of myocardial infarction, hypertensive eccentric hypertrophy (reduced LVEF), and hypertensive concentric hypertrophy (preserved LVEF) were included in the CONSENSUS population, and retrospect, the classification could not be determined. The clinical consequence of CONSENSUS and subsequent RCTs, including SOLVD,5 was straightforward, namely, that enalapril and other ACEIs quickly became the standard treatment for all HF patients with no immediate thoughts of LVEF or at least no thoughts of excluding HFpEF patients from this powerful treatment. It took 20 years from the publication of CONSENSUS4 and 15 years after SOLVD5 before a serious attempt to investigate the prognostic effect of ACEI in HFpEF patients was performed, namely Perindopril vs. Placebo in Congestive Heart Disease (PEP-CHF).11
PEP-CHF was a prospective RCT with older patients but with fewer participants than planned (n = 852) and was hampered by several other challenges regarding the study design and statistical power.11 The investigators overestimated the event rate and underestimated the discontinuation rate, therefore encountering, in a 1-year interim analysis, lower than expected event rate, widespread drop-out and drop-in rates, and a very high rate of open-label ACEI use (Table 4), resulting in the steering committee stopping further inclusion of patients. Although PEP-CHF did not enroll the planned number of participants, the 1-year interim analysis showed a borderline significant effect on the primary endpoint, a composite of mortality and hospitalization for HF worsening. This finding was driven by the prespecified secondary endpoint of HF hospitalization, which was significantly lower in perindopril users. Although the analysis was not planned for year 1, and despite the confounding issues, PEP-CHF showed a preventive effect on hospitalization for the worsening of HF. PEP-CHF likely showed such strong results because patients included after echocardiography had confirmed cardiac disease that could explain HFpEF. The PEP-CHF investigators gave open ACEI treatment to one-third of the participating patients outside the protocol, including patients in the control group.11 Such liberal use of ACEI treatment contributed strongly to minimizing the visible effect of the study drug.
Table 4.
An overview of conditions for calculation of statistical power in the HFpEF trials
| Variables | CHARM (2003) | PEP-CHF (2006) | I-PRESERVE (2008) | TOPCAT (2014) | PARAGON (2019) | EMPEROR PRESERVED (2021) | DELIVER (2022) | |
|---|---|---|---|---|---|---|---|---|
| Predefined conditions for statistical power | ||||||||
| α-Level | Two-sided <0.05 | Two-sided <0.024 | ||||||
| Expected ER (%) or TNE (n) | ER 11 | TNE 451 | ER 18 | ER 17.4 | TNE 1,847 | TNE 841, 10% ER | TNE 1,117 | |
| Planned duration of follow-up (mo) | 24 | 12 | 24 | 18 | 26 | 20 | 13.3 | |
| Planned number of participants (n) | 2,900 | 1,000 | 4,100b | 1,102 orc 1,260 | 4,600 | 4,126 (optionally up to 6,000) | 6,100 | |
| RRR or HR (statistical power %) | RR 0.18 (80) RR 0.16 (70) |
RR 0.40 (90) | RR 0.145 (90) | RR 0.20 (80 or 85) | RR 0.22 (95) RR 0.19 (80) |
HR 0.80 (90) | HR 0.80 (90) | |
| Achieved conditions | ||||||||
| Background medication (%) | Diuretics | 75 | 50 | 83 | 62.5–90 | 95.5 | – | 76.8 |
| ACEI | 19 | – | 25.5 | 47.6–70.6 | 86 | 80% | 36.6 | |
| ARBs | – | – | – | 13–33 | 36.3 | |||
| MRAs | 11.5 | 10 | 15 | – | 25.5 | 37 | 42.4–42.8 | |
| Beta-blocker | 56 | 54.5 | 58.5 | 79–87 | 80 | – | 82.5-82.8 | |
| ARNI | – | – | – | – | – | – | 4.3–5.3 | |
| Primary events (n) ora actual event rate (%) | 699 PE AER 10.4 |
207 PE | 1,505 PE AER 8.8 |
671 PE AER 11.1 |
1,903 PE | 926 PE | 1,122 PE | |
| Number of included participants (n) | 3,023 | 852 | 4,128 | 3,445 | 4,796 | 5,988 | 6,263 | |
| Actual duration of follow-up (mo) | 36.6 | 26.2 | 49.5 | 21.6 | 35 | 26.6 | 27.6 | |
| Detected RR or HR (P value) | HR 0.86 (0.051) | HR 0.92 (0.545) | HR 0.95 (0.35) | HR 0.87 (0.14) | RR 0.87 (0.06) | HR 0.79 (<0.001) | HR 0.82 (<0.01) | |
| Weighted RR or HRd | 0.09 | 0.03 | 0.14 | 0.11 | 0.15 | 0.17 | 0.18 | |
| Overall HR in all trials | 0.85 | |||||||
| Other challenges | – | DR at end of trial 35%–37% | DR at end of trial 33%–34% | – | – | – | DR at end of trial 14.1% | |
Abbreviations: DR, drop-out rate; ENCR, expected noncompliance rate; ER, event rate; HR, hazard ratio; PE, primary events; RRR, relative risk reduction; TNE, target number of events.
aER corresponding to AER, TNE corresponding to PE.
bAdjusted from 3,600 to 4,100 due to miscalculated ER.
cCorresponding to different statistical power.
dWeighted according to the proportion of the trials in the total number of participants in all trials (HR × N/Ntotal).
Evidence in favor of ARB treatment in HFpEF
The effects of ARBs on cardiovascular mortality and morbidity in HFpEF, including candesartan in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) study9 and irbesartan in Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE),29 have been more comprehensively investigated than those of ACEIs. Both trials had surprisingly weak statistical power at the outset because of high rates of background use of other HF medications (with documented prognostic benefit in HFrEF) at baseline (Table 4), which increased further during the trials, as shown for I-PRESERVE29 in Table 5.
Nevertheless, borderline significance (P = 0.051) was achieved in favor of candesartan in the CHARM study after prespecified adjustments for covariates. There was a trend in I-PRESERVE, with Kaplan–Meier curves diverging, apparently favoring irbesartan after 2 years. Some of the investigators published retrospective analyses later after gaining access to the data, with additional statistical methods, up-to-date “repeat-event analysis” for hospital admissions in CHARM,30 and simple adjustment for imbalance in baseline characteristics in the primary endpoint in I-PRESERVE.31 These analyses displayed significant beneficial results on the main endpoints of both RCTs. Our interpretation is that when properly analyzed, treatment with ARB has prognostic beneficial effects in HFpEF despite heavy background medication with HF drugs proven to have beneficial effects in HFrEF.
Evidence in favor of angiotensin receptor neprilysin inhibitor treatment in HFpEF
Dual-acting RAS and neprilysin inhibitors exploit RAS blockade by augmenting natriuretic peptides’ salutary actions.32,33 LCZ696 is the first-in-class ARNI. It consists of the prodrug AHU377 (sacubitril), which is activated to the Neutral Endopeptidase (NEP) inhibitor LBQ657, and the ARB valsartan combined in 1 molecule in equal moieties. Neprilysin, a metallopeptidase, degrades endogenous atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and C-type natriuretic peptide, which all exert powerful diuretic, natriuretic, sympatholytic, antihypertrophic, antifibrotic, and hence antihypertensive effects. In HF and hypertension, excessive NEP activity induces a relative deficiency of these beneficial peptides, thus providing the rationale for dual-acting compounds that both inhibit NEP and block the maladaptive effects of the RAS. In a landmark study in hypertensive patients, LCZ696 exhibited greater blood pressure reduction than stand-alone valsartan with similar tolerability.34 Furthermore, backed by extensive preclinical evidence, recent clinical studies demonstrated additional favorable cardiovascular anti-remodeling effects beyond RAS blockade.35
The effect of sacubitril–valsartan in patients with HFpEF was investigated in the Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin receptor blocker Global Outcomes in the HFpEF (PARAGON-HF) trial,35 which was designed for ethical reasons using valsartan rather than placebo as the comparator. However, 95% of patients received a diuretic and 75% a beta-blocker at baseline. Thus, the comparison was valsartan + hydrochlorothiazide + beta-blockade vs. valsartan–sacubitril + hydrochlorothiazide + beta-blockade. Ideally, the comparison should have been valsartan + hydrochlorothiazide + beta-blockade vs. valsartan–sacubitril + beta-blockade. The double dose of diuretics with different sites of action is probably important. Despite the intrinsically effective comparator, valsartan, considering the results of candesartan in CHARM and irbesartan in I-PRESERVE and the inclusion of a predominance of patients with NYHA class II (not-so-severe HF), PARAGON-HF showed borderline significant results favoring ARNI (hazard ratio [HR] = 0.87, 95% confidence intervals 0.753–1.005, P = 0.058). After subsequent review of the totality of the evidence, and taking into consideration the benefits in HFrEF in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM),15 suggesting benefit across a wide range of LVEF, FDA approved sacubitril–valsartan as a treatment for HF with “up to normal” LVEF. Additionally, the reno-protective properties of ARNI were superior to those of RAS inhibitors—a further benefit for the HFpEF population, in which renal dysfunction is common.36
Evidence in favor of diuretic treatment in HFpEF
The primary intention of diuretics is to prevent hospitalization due to congestion, in addition to the potential blood pressure-lowering effect, a mechanism which is frequently underestimated in HFpEF patients with hypertension. Diuretics are usually required as standard treatment for HF throughout the entire LVEF spectrum. Loop diuretics, such as furosemide, are particularly beneficial in those with renal impairment (eGFR <30 ml/min/1.73 m2) and patients with severe HF with a history of pulmonary edema and/or other forms of severe fluid retention. The Comparative Effectiveness of Torsemide Versus Furosemide in Heart Failure (the TRANSFORM-HF Trial) tested the hypothesis that torsemide was superior to furosemide in treating HF.37,38 This pragmatic, randomized, unblinded trial enrolled 2,859 patients with HF regardless of LVEF during HF hospitalization. Patients were randomized to the loop diuretic torsemide or furosemide with investigator-selected dosing. During a median of 17.4 months, all-cause deaths occurred in 26.1% of patients in the torsemide group and 26.2% in the furosemide group, indicating no difference between treatments.
Hydrochlorothiazide or thiazide-like diuretics such as indapamide or chlorthalidone are preferably given in a single pill combination with a RAS blocker in patients who need hypertension control or in HF patients in whom fluid retention is a minimal problem. Differences in outcomes have never been shown between these diuretics in either HF or hypertension. A recent comparison of hydrochlorothiazide with chlorthalidone in hypertension was neutral.39
Evidence in favor of beta-blocker treatment in HFpEF
Most patients (55%–87%) enrolled in HFpEF RCTs used open-label beta-blocker therapy despite sparse trial evidence28 and mostly as background therapy for concomitant cardiovascular conditions with high heart rate, such as hypertension, ischemic heart disease, or atrial fibrillation. Therefore, designing prospective RCTs in HFpEF with no beta-blocker use in the placebo arm is virtually impossible.
The SENIORS trial with nebivolol40 included HF patients not based on LVEF and demonstrated a beneficial beta-blocker effect independent of LVEF in a prespecified subgroup analysis.41 The effect on the composite primary outcome of all-cause mortality or cardiovascular hospital admission showed an overall HR of 0.86, and in the subgroup possessing LVEF ≥35%, HR was 0.82. Apart from this single RCT, the published literature in HFmrEF or HFpEF patients is based on observational cohorts, on which meta-analyses have also been made. An example is a meta-analysis on beta-blockers in HFmrEF and HFpEF (LVEF >40%), which revealed that beta-blocker use was associated with reduced risk of mortality.42 Beta-blockers also have beneficial effects in HFpEF patients who are in sinus rhythm.43 A specific reason for the beneficial role of beta-blockers in HFpEF beyond their beneficial effects in patients with the concomitant diseases mentioned above is the increased sympathetic activity in this form of HF, whether patients have obesity or not.44 Another interesting aspect of assessing beta-blockers in HF is their effect on increasing natriuretic peptide (NP) concentrations, likely because of their heart rate-reducing properties.45 Thus, although beta-blockers continue to be a major therapy in patients with HFrEF, documented for bisoprolol, carvedilol, metoprolol, and nebivolol, it is unclear how the increase in NPs contributes to their benefits and whether NPs can be used for monitoring HF progression during beta-blocker treatment.
Evidence in favor of steroidal and nonsteroidal MRAs in HFpEF
To evaluate the effect of the steroidal MRA spironolactone in HFpEF, the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial12 was performed with study sites in North and South America, in addition to Russia and Georgia.12 The lack of significant effect of the study drug on the primary endpoint was clouded by the retrospective finding that there were substantial regional differences in participating patients and study conduct, seriously affecting the results of the trial.12 These differences were related to patient inclusion based on either hospitalization due to HF in the previous 12 months or elevated NPs at any time. In the aftermath, it became apparent that centers in Russia and Georgia disproportionately included study participants based on the history of HF hospitalization. In contrast, in most study participants in North America, HF diagnosis was ascertained by elevated NP values. Reanalysis of the data displayed a significant effect of the study drug on the primary endpoint in the subgroup in the Americas, where the event rate was as expected.
In contrast, the event rate of the subset in Russia and Georgia was low and comparable to the general population. This leaves an open question as to whether participants in Russia and Georgia truly had HF. Furthermore, the blood samples of Russian and Georgian patients revealed that many did not receive or take their assigned treatment with spironolactone, as the metabolite canrenone was not detected, and serum potassium and serum creatinine rose much less than expected. These related data appeared in the Online Supplement of the main publication.12 However, FDA reversed course on this matter based on the results from the North American subset of the study (TOPCAT Americas). An FDA advisory committee lit a long-standing fuse, recommending that the totality of evidence from TOPCAT can be used to support a new indication for spironolactone. Several advisors concurred, but ultimately, the Cardiovascular and Renal Drugs Advisory Committee voted 8 to 4, with one abstaining, that TOPCAT provides “sufficient evidence to support any indication.”46
Spironolactone In The Treatment of Heart Failure (SPIRIT-HF) and Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction (SPIRRIT) are 2 distinct ongoing trials comparing spironolactone to placebo in reducing the rate of the composite endpoint of recurrent HF hospitalizations and cardiovascular death in symptomatic HF patients (NYHA II–IV) with mid-range (LVEF 40%–49%) or preserved LVEF (≥50%) (NCT04727073 and NCT02901184).47
Finerenone is a nonsteroidal selective aldosterone receptor antagonist that has been investigated in patients with type 2 diabetes and chronic kidney disease.48–50 In Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD)49 (n = 7,347), the primary endpoint was a composite of cardiovascular events (time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF) and the secondary endpoint was a composite of kidney events (time to kidney failure, sustained ≥40% eGFR decline, or renal death). In Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD)50 (n = 5,734), primary and secondary endpoints were the reverse (kidney and cardiovascular). Among the enrolled patients, >95% had treated hypertension, 46% had a history of cardiovascular disease, and only 7% had a history of HF. Yet, in terms of cardiovascular outcomes, a reduction in the primary cardiovascular endpoint was obtained, but the main and only secondary benefit of finerenone was a significant reduction in hospitalizations for HF (HR = 0.78, 0.66–0.92, P = 0.003). Thus, further evidence obtained from HF patients is needed to implement this medication in HF treatment. The Finerenone in Heart Failure Patients (FINEARTS-HF)51 is an ongoing multicenter, randomized, double-blind, parallel-group, placebo-controlled study to evaluate the efficacy and safety of finerenone on morbidity and mortality in patients with HF (NYHA II–IV) and LVEF ≥40%.
Aldosterone synthase inhibition is a third concept focused on reducing aldosterone activity, and at least 1 compound, baxdrostat, has advanced well into clinical development (Table 3). This compound is well tolerated and lowers blood pressure significantly.52
Evidence in favor of SGLT2I treatment in HFpEF
SGLT2Is were introduced to treat type 2 diabetes mellitus, a disease associated with substantial macro- and microvascular changes in the myocardium and subsequent high risk of HF. Furthermore, RCTs have proven the SGLT2Is to effectively lower cardiovascular and renal outcomes in patients with HF, both with and without diabetes.
Evidence for impact on cardiovascular mortality and hospitalization in HFpEF has been established through 2 randomized controlled trials; Empagliflozin in Heart Failure with a Preserved Ejection Fraction (EMPEROR PRESERVED)16 and Dapagliflozin in Heart failure with Mildly Reduced or Preserved Ejection Fraction (DELIVER).53 EMPEROR-PRESERVED investigated the effect of empagliflozin in HFpEF and found a clear significant reduction of the composite endpoint of cardiovascular mortality or hospitalization—a significant slowing of the decline in kidney function and an improvement in quality of life.16 Thus, FDA and EMA expanded the approval of empagliflozin for HF last year, making it independent of LVEF. Subsequently, and consistently, the DELIVER trial demonstrated a positive effect of dapagliflozin on the composite primary outcome of worsening HF or cardiovascular death in patients with HFmrEF and HFpEF, as well as a significant improvement in quality of life.16
A significant reduction in the composite endpoint of cardiovascular death, total hospitalizations for HF, or urgent visits for HF was demonstrated with the dual SGLT1–2 inhibitor sotagliflozin in HF patients with an LVEF ≥50% and type 2 diabetes in a pooled analysis of 739 patients from the Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) and Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trials.54,55 It is worth mentioning that these are subgroup analysis results in 8% of the total number of enrolled patients from trials that were not specifically HFpEF trials and were both stopped prematurely.
Rather than a poorly characterized diuretic effect, the likely weak contribution of weight reduction and reduction in serum uric acid, many other mechanisms have been suggested to explain the cardiovascular benefits of SGLT2Is beyond blood pressure lowering. The mechanisms are dominated by a reduction in oxidative and endoplasmic reticulum stress, restoration of mitochondrial health and enhanced mitochondrial biogenesis, decreases in proinflammatory and profibrotic pathways, and preservation of cellular and organ integrity and viability related to a distinctive effect on autophagy.56 Some of these critical mechanisms were confirmed in a proteomic analysis in patients enrolled in the empagliflozin HFrEF and HFpEF trials.57
A meta-analysis of all SGLT2I Trials, including HF trials, investigated the effects of these drugs on renal function.58 The authors identified 13 trials and analyzed 90,409 participants, 82.7% with type 2 diabetes and 17.3% without diabetes. Allocation to an SGLT2I reduced the risk of kidney disease progression by 37%, with similar relative risk reductions in patients with and without diabetes. In the 4 chronic kidney disease trials, relative risk reductions were similar irrespective of primary kidney diagnosis. SGLT2I reduced the risk of acute kidney injury and cardiovascular death or hospitalization for HF by 23%. SGLT2I also significantly reduced the risk of cardiovascular death by 14%. Relative risk reductions were similar in patients with and without diabetes for all outcomes, and results were similar irrespective of mean baseline renal function.58
Global evidence of drug treatment of patients with HF: should we still make a difference according to the level of LVEF?
As expected, based on the studies we discussed in the present narrative review, neither a Cochrane review59 nor other standard meta-analyses could show many benefits of HF treatment of patients with HFpEF.60,61 Considering the totality of evidence from available trials and their revised interpretation, including in some instances by regulatory agencies, we suggest that HF drug therapy should not be based on LVEF but on many other aspects of HF, such as presenting symptoms (related to the level of fluid retention), signs (tachycardia), severity (NYHA functional class) and in almost all patients, concomitant diseases such as hypertension, type 2 diabetes mellitus, obesity, atrial fibrillation, coronary heart disease, and chronic kidney disease. Additionally, as mentioned previously, wider echocardiographic findings of more refined measures of LV systolic function within the preserved LVEF range should be taken into account. Consequently, many patients may qualify for all HF drugs documented for HFrEF and HFpEF. Most and probably all HF drugs appear beneficial regardless of the level of LVEF. Similar viewpoints have been expressed by colleagues who have argued that treatment approaches that address aspects of HF syndrome apart from LV remodeling may be expected to be effective across the entire LVEF spectrum.62,63 However, several review papers22–27 of HFpEF are only partly open for the views we express in the present paper based on several decades of clinical experience and extensive HF research.
OTHER IMPORTANT ASPECTS WHEN CONSIDERING OUTCOMES OF RCTs IN HFpEF
Left ventricular ejection fraction
The use of LVEF as a surrogate for systolic LV function and a prognostic marker in the diagnosis and monitoring of HF has increased since the 1990s. Furthermore, clinical trials used the LVEF threshold to enrich the event rates while limiting the sample sizes.5 While LVEF helps to categorize HF into pathophysiological phenotypes, its influence on treatment recommendations is limited for several reasons.
First, the prognostic value of LVEF mainly applies to severely impaired ventricles,60 in which the LVEF measurements are highly operator dependent and reproducible only for experienced echocardiographers.64 LVEF is a characterization of the SV expressed as a fraction of the LV end-diastolic volume (LVEDV). The level of LVEDV is essential to translate SV expressed as a percentage, LVEF, into absolute SV. Before echocardiograms were used, the instantaneous change in SV/LVEDV (i.e., the change in LVEF) was measured invasively as a measure of change in contractility under conditions of constant load. LVEF is influenced by both preload (diastolic) and afterload (systolic) and cannot be interpreted as an index of contractility without knowledge of LV loads; the structural changes leading to increases or decreases in LVEDV will strongly influence the LVEF at a given level of contractility and SV.
Thus, low LVEF may be present even when SV is (sub)normal, depending on the LV dilatation. Furthermore, mitral regurgitation and tethering of the papillary muscles are associated with LV dilatation, resulting in secondary mitral regurgitation, reducing SV and increasing LVEF.65 Other important confounding factors associated with the prognostic value of LVEF are the degree of LV hypertrophy and LV afterload (systolic blood pressure).66 Moreover, it appears that LVEF is a dynamic parameter even under stable conditions. Clarke et al.67 estimated the EF-based HF-phenotype transition probabilities at several follow-up points in patients with a primary discharge diagnosis of HF and ≥2 LVEF measurements separated by at least 30 days. They found that the probabilities for HFpEF to HFrEF transition were 45% and 50% at 1 and 2 years, respectively. Likewise, the probabilities for HFrEF to HFpEF transition were 18% and 20%. Therefore, the guidelines may be ill-guided using strict EF cutoff values.
The statistical power of RCTs in patients with HFpEF
As summarized in Table 4, a recurring issue has been the unsuccessful enrichment of the event rates in HFpEF trials, leading to a lack of statistical power. More recently, HFpEF RCTs relied on more complex “risk-enrichment” criteria, such as the recent history of HF hospitalization, further echocardiographic variables of left ventricular hypertrophy and/or left atrial enlargement, or elevated levels of NPs. Trial power has further been limited by the great extent of background medication and study drug discontinuation in several trials. Hence, several trials did not reach the desired statistical power and had a low probability of detecting a moderate, although clinically meaningful effect.
NPs and selection of patients with HF
An incomplete understanding of HFpEF has been a main driver of weak designs of HFpEF RCTs, the principal issue being the diagnosis of HF in these patients. The guidelines for HFpEF diagnosis highlight the uncertainty and challenges of this diagnosis. Ascertainment of HF risk in HFpEF trials relies on various combinations of structural and/or functional changes in the heart, systolic blood pressure, history of HF hospitalization, and elevated NPs. Consensus is lacking about which structural and functional changes of the heart best describe HFpEF. Elevated NPs have, over the last decade, been incorporated as a standard selection criterion of HFpEF RCTs to address the deficiencies of HF ascertainment in HFpEF trials (Figure 1). Savarese et al.68 described how NPs enriched HF trials due to the positive relation of higher NP levels with higher cardiovascular-to-noncardiovascular event ratios. NTproBNP has been used as an inclusion criterion in TOPCAT,12 PARAGON,14 EMPEROR-PRESERVED,16 and DELIVER53 studies, increasing statistical study power, and consequently showing benefits of the HF drugs being investigated.
The importance of NTproBNP in selecting participants has been underlined in a recent reevaluation of the PARAGON data, in which the investigators conducted a subgroup analysis comparing results from patients enrolled due to elevated NPs with patients enrolled due to hospitalization.69 The inclusion of elevated NPs led to a significant treatment effect on the composite primary endpoint, in contrast to inclusion based on previous hospitalization. An analogous reanalysis with a similar result was carried out on data from TOPCAT.70
In some concomitant diseases, such as mild chronic kidney disease leading to hypervolemia, the sensitivity of NTproBNP as a diagnostic marker of HF may be limited.71,72
Furthermore, too strict requirements regarding the detection of elevated NTproBNP, which may be needed for the inclusion of patients in RCTs, may not necessarily be ideal in clinical practice. Patients who have suffered decompensated HF but have not been diagnosed with elevated NTproBNP may potentially be misclassified and not be given adequate drug treatment.
Potential treatment effects in various phenotypes of HFpEF
Though hypertension is the most common risk factor for HFpEF, more than 90% of the participating patients in some RCTs (Table 2), there are several closely related conditions, including type 2 diabetes mellitus, obesity, atrial fibrillation, and coronary heart disease. Coronary heart disease is a macrovascular disease of epicardial arteries caused by atherosclerosis with a major contribution from hypertension due to systemic high wall tension. In HFpEF, coronary disease causes HF by chronic ischemia, a different mechanism than transmural myocardial infarctions leading to HFrEF. Atrial fibrillation develops as a consequence of poor electrical contact in dilated and fibrotic atria of patients with hypertension and left ventricular hypertrophy. However, the metabolic conditions of diabetes and obesity may be of special interest in the search for phenotypes that may respond to specific therapeutic drug interventions which may improve outcomes in HFpEF.
HFpEF related to hypertension mainly is related to left ventricular hypertrophy of the concentric subtype with wall thickening, narrow cavity, and increased LV mass, with extensive microvascular disorder and accumulation of fibrotic tissue in the myocardium and in the small intramyocardial vessel walls.73 The microvascular disease is advanced in concentric hypertrophy to the degree that a certain perfusion pressure is needed for survival. With most intensive drug treatment of hypertension, systolic blood pressure <130 mm Hg caused by antihypertensive drug treatment is related to increased cardiac and all-cause mortality,74,75 in parallel with the development of HF caused by ischemia and poor contractility or by sudden onset atrial fibrillation. Along the same lines, diabetic myocardial disease is dominated by extensive damage to the arterioles and capillaries. While in type 2 diabetes there may be hypertrophic remodeling of the arterioles, and in hypertension, eutrophic remodeling, the 2 conditions very frequently appear together, and the capillaries show thickening of the basement membrane, reduced lumen diameter, and smaller capillary area.76
Myocardial microvascular density is also decreased in obesity-related HFpEF, which causes cardiac hypoperfusion owing to reduced myocardial oxygen delivery.77,78 Obesity is present in approximately 80% of patients who develop HFpEF, but the myocardial microcirculation is much less described than in hypertension and diabetes. Though the question of a separate phenotype with possible cause-related drug treatment has been raised in obesity, the evidence is missing, possibly because few patients have obesity without concomitant hypertension and/or diabetes. Average body mass index in hypertension RCTs is 28–30 kg/m2,18,19,74,75 identical to the body mass index in the RCT of HFpEF (Table 2). Thus, mostly obesity in HFpEF is a bystander to hypertension, diabetes, or both as a component of the metabolic syndrome. We do not refute obesity as a separate phenotype, though much more research on myocardial microvascular structure and function is needed in obese humans with optimal blood pressure and no diabetes.
Our view that patients with HF should be treated similarly without regard to EF may change in the future if subsets of HFpEF are found to respond to specific therapies. In the present paper, we emphasize the role of hypertension in HFpEF. Hypertension, diabetes, and obesity are the key components of the metabolic syndrome,79,80 very well known in hypertension research for several decades, but may represent a novelty in the setting of HFpEF. Although there is currently no phenotype-based HFpEF treatment, experts78,79 are pursuing the hypothesis that the obesity of HFpEF might need to be treated differently. Until this hypothesis is proven, given that the hypertension phenotype is the dominant HFpEF phenotype, most HFpEF patients should receive the same medications as HFrEF patients.
The cornerstones of treatment of HF to improve outcomes (Figure 3) are RAS inhibitor and ARNI, MRA, and SGLT2 inhibition, and potentially beta-blockers,55 and diuretics are given to alleviate symptoms caused by fluid retention. These foundational therapies in HF treatment are well established in patients with a reduced LVEF. We suggest that the totality of evidence also supports the use of these therapies in HF patients with a preserved LVEF. LVEF is useful in deciding phenotypes in the assessment of HF patients and in detecting patients at the extreme lower end of the scale who need special care. However, regarding decisions for drug treatment, the role of LVEF is, in our opinion, one of many variables and thus limited. Our current discussion of the randomized studies that have been performed in HFpEF aiming to lower mortality and hospitalization for worsening of HF allows extracting a common denominator that supports the interpretation that all analyzed drugs must be part of the therapeutic armamentarium for the treatment of patients with both HFrEF and HFpEF. This conclusion is based on the totality of evidence and is thus different from views or findings in some other recent reviews on this important topic.22–27,59–61
Contributor Information
Kaja Sevre, University of Oslo, Medical School and Institute of Clinical Medicine, Oslo, Norway.
Aurora Rist, University of Oslo, Medical School and Institute of Clinical Medicine, Oslo, Norway.
Kristian Wachtell, Weill-Cornell Medicine, Division of Cardiology, New York City, New York, USA.
Richard B Devereux, Weill-Cornell Medicine, Division of Cardiology, New York City, New York, USA.
Gerard P Aurigemma, Division of Cardiovascular Medicine, Department of Medicine, UMassChan School of Medicine, Worcester, Massachusetts, USA.
Otto A Smiseth, University of Oslo, Institute for Surgical Research and Department of Cardiology, Rikshospitalet, Oslo, Norway.
Sverre E Kjeldsen, University of Oslo, Medical School and Institute of Clinical Medicine, Oslo, Norway; Departments of Cardiology and Nephrology, Ullevaal Hospital, Oslo, Norway; University of Michigan, Division of Cardiovascular Medicine, Ann Arbor, Michigan, USA.
Stevo Julius, University of Michigan, Division of Cardiovascular Medicine, Ann Arbor, Michigan, USA.
Bertram Pitt, University of Michigan, Division of Cardiovascular Medicine, Ann Arbor, Michigan, USA.
Michel Burnier, Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland.
Reinhold Kreutz, Charité – Universitätsmedizin Berlin, Institute of Clinical Pharmacology and Toxicology, Berlin, Germany.
Suzanne Oparil, University of Alabama at Birmingham, Vascular Biology and Hypertension Program, Department of Medicine, Birmingham, Alabama, USA.
Giuseppe Mancia, University of Milan-Bicocca, Milan, Italy.
Faiez Zannad, Universite de Lorraine, Inserm, Centre d’Investigations Cliniques-1433 and F-CRIN INI CRCT, Nancy, France.
CONFLICT OF INTEREST
S.E.K. has received ad hoc lecture honoraria from Getz, J.B. Pharma, Merck, Vector-Intas, and Zydus. B.P. is receiving consulting fees from and owning stock options in Relypsa/Vifor, KBP Pharmaceuticals, Sarfez, SCPharmaceuticals, SQinnovations, G3 Pharmaceuticals, and Tricida, receiving consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, and PhaseBio, and holding patent US 9931412 on site-specific delivery of eplerenone to the myocardium and pending patent US 63/045,784 on histone-acetylation modulating agents for the treatment and prevention of organ damage. M.B. reports honoraria from Bayer, Menarini, Sanofi, and Servier. R.K. reports support for research by Bayer, and honoraria for lectures from Bayer, Berlin-Chemie, Daiichi Sankyo, Ferrer, Merck, Menarini, Sanofi, and Servier. G.M. reports honoraria from Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Menarini, Merck, Novartis, Recordati, Sandoz, Sanofi, and Servier. F.Z. reports personal fees from Boehringer Ingelheim, Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera, other than from CVCT, and Cardiorenal. K.S., A.R., K.W., R.B.D., G.P.A., O.A.S., S.J., and S.O. declare no competing interests.
DATA AVAILABILITY
Not relevant for this review article.
REFERENCES
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang N-Y, Yaffe K, Martin SS.. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022; 145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS.. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018; 6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, Hobbs FDR.. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ 2019; 364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN, Cohn JN; for the SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302. [DOI] [PubMed] [Google Scholar]
- 6. McKee PA, Castelli WP, McNamara M, Kannel WB.. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285:144–146. [DOI] [PubMed] [Google Scholar]
- 7. Lund LH, Pitt B, Metra M.. Left ventricular ejection fraction as the primary heart failure phenotyping parameter. Eur J Heart Fail 2022; 24:1158–1161. [DOI] [PubMed] [Google Scholar]
- 8. Smiseth OA. Evaluation of left ventricular diastolic function: state of the art after 35 years with Doppler assessment. J Echocardiogr 2018; 16:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362:777–781. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362:772–776. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 12. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717. [DOI] [PubMed] [Google Scholar]
- 14. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; for the PARADIGM-HF Investigators and Committees. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004. [DOI] [PubMed] [Google Scholar]
- 16. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 17. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 18. Kjeldsen SE, von Lueder TG, Smiseth OA, Wachtell K, Mistry N, Westheim AS, Hopper I, Julius S, Pitt B, Reid CM, Devereux RB, Zannad F.. Medical therapies for heart failure with preserved ejection fraction. Hypertension 2020; 75:23–32. [DOI] [PubMed] [Google Scholar]
- 19. Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, Ferreira JP, Brguljan J, Januszewicz A, Kahan T, Manolis A, Tsioufis K, Weber T, von Lueder TG, Smiseth OA, Wachtell K, Kjeldsen SE, Zannad F, Mancia G, Kreutz R.. Hypertension and heart failure with preserved ejection fraction: position paper by the European Society of Hypertension. J Hypertens 2021; 39:1522–1545. [DOI] [PubMed] [Google Scholar]
- 20. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW.. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 21. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 22. Peters AE, DeVore AD.. Pharmacologic therapy for heart failure with preserved ejection fraction. Cardiol Clin 2022; 40:473–489. [DOI] [PubMed] [Google Scholar]
- 23. Zhan Q, Peng W, Wang S, Gao J.. Heart failure with preserved ejection fraction: pathogenesis, diagnosis, exercise, and medical therapies. J Cardiovasc Trans Res 2022; 16:310–326. [DOI] [PubMed] [Google Scholar]
- 24. Joury A, Gupta T, Krim SR.. New concepts in heart failure with preserved ejection fraction and hypertension. Curr Opin Cardiol 2022; 37:424–430. [DOI] [PubMed] [Google Scholar]
- 25. Karamichalakis N, Xanthopoulos A, Triposkiadis F, Paraskevaidis I, Tsougos E.. Reshaping treatment of heart failure with preserved ejection fraction. J Clin Med 2022; 11:3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omote K, Verbrugge FH, Borlaug BA.. Heart failure with preserved ejection fraction: mechanisms and treatment strategies. Annu Rev Med 2022; 73:321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redfield MM, Borlaug BA.. Heart failure with preserved ejection fraction: a review. JAMA 2023; 329:827–838. [DOI] [PubMed] [Google Scholar]
- 28. Mancia G, Kjeldsen SE, Kreutz R, Pathak A, Grassi G, Esler M.. Individualized beta-blocker treatment for high blood pressure dictated by medical comorbidities: indications beyond the 2018 European Society of Cardiology/European Society of Hypertension Guidelines. Hypertension 2022; 79:1153–1166. [DOI] [PubMed] [Google Scholar]
- 29. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 30. Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Östergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S.. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail 2014; 16:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferreira JP, Dewan P, Jhund PS, Lorenzo-Almorós A, Duarte K, Petrie MC, Carson PE, McKelvie R, Komajda M, Zile M, Zannad F, McMurray JJV.. Covariate adjusted reanalysis of the I-Preserve trial. Clin Res Cardiol 2020; 109:1358–1365. [DOI] [PubMed] [Google Scholar]
- 32. Kjeldsen SE, Hedner T, Narkiewicz K, Oparil S.. Angiotensin receptor—neprilysin inhibition (ARNI)—a novel therapeutic concept for management of hypertension and heart failure. Blood Press 2012; 21:329–330. [DOI] [PubMed] [Google Scholar]
- 33. von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC Jr, Krum H.. Renin angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP.. Blood pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomized, double-blind, placebo-controlled, active comparator study. Lancet 2010; 375:1255–1266. [DOI] [PubMed] [Google Scholar]
- 35. Schmieder RE, Wagner F, Mayr M, Delles C, Ott C, Keicher C, Hrabak-Paar M, Heye T, Aichner S, Khder Y, Yates D, Albrecht D, Langenickel T, Freyhardt P, Janka R, Bremerich J.. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J 2017; 38:3308–3317. [DOI] [PubMed] [Google Scholar]
- 36. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K.. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018; 391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mentz RJ, Anstrom KJ, Eisenstein EL, Sapp S, Greene SJ, Morgan S, Testani JM, Harrington AH, Sachdev V, Ketema F, Kim D-Y, Desvigne-Nickens P, Pitt B, Velazquez EJ; TRANSFORM-HF Investigators. Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF Randomized Clinical Trial. JAMA 2023; 329:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verbrugge FH, Menon V.. Torsemide comparison with furosemide for management of heart failure (TRANSFORM-HF) trial. Eur Heart J Acute Cardiovasc Care 2022; 11:931–932. [DOI] [PubMed] [Google Scholar]
- 39. Ishani A, Cushman WC, Leatherman SM, Lew A, Woods P, Glassman PA, Taylor AA, Hau C, Klint A, Huang GD, Brophy MT, Fiore LD, Ferguson RE; for the Diuretic Comparison Project Writing Group. Chlorthalidone vs. hydrochlorothiazide for hypertension–cardiovascular events. N Engl J Med 2022; 387:2401–2410. [DOI] [PubMed] [Google Scholar]
- 40. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26:215–225. [DOI] [PubMed] [Google Scholar]
- 41. Ambrosio G, Flather MD, Böhm M, Cohen-Solal A, Murrone A, Mascagni F, Spinucci G, Conti MG, van Veldhuisen DJ, Tavazzi L, Coats AJ.. β-Blockade with nebivolol for prevention of acute ischaemic events in elderly patients with heart failure. Heart 2011; 97:209–214. [DOI] [PubMed] [Google Scholar]
- 42. Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, Ayis S.. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart 2018; 104:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Kotecha D; Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018; 39:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grassi G, Mancia G, Esler M.. Central and peripheral sympathetic activation in heart failure. Cardiovasc Res 2022; 118:1857–1871. [DOI] [PubMed] [Google Scholar]
- 45. Olsen MH, Wachtell K, Nielsen OW, Hall C, Wergeland R, Ibsen H, Kjeldsen SE, Devereux RB, Dahlöf B, Hildebrandt PR.. N-terminal brain natriuretic peptide predicted cardiovascular events stronger than high-sensitivity C-reactive protein in hypertension: a LIFE substudy. J Hypertens 2006; 24:1531–1539. [DOI] [PubMed] [Google Scholar]
- 46. FDA Panel Puts Spironolactone in Play for HF with Preserved EF [Internet]. Medscape; [cited 30 April 2023]. https://www.medscape.com/viewarticle/942799 [Google Scholar]
- 47. Pieske B. Spironolactone in the Treatment of Heart Failure—A Double-Blind, Randomized, Placebo-controlled, Parallel Group Interventional Phase III Study to Determine Efficacy and Safety of Spironolactone on the Composite Endpoint of Recurrent Heart Failure Hospitalizations and Cardiovascular Death in Patients with Heart Failure with Mid-range or Preserved Ejection Fraction [Internet]. Clinicaltrials.gov, 2021. [cited 23 May 2023]. Report no. NCT04727073. https://clinicaltrials.gov/ct2/show/NCT04727073 [Google Scholar]
- 48. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD and for the FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385:2252–2263. [DOI] [PubMed] [Google Scholar]
- 50. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 51. FINEARTS-HF (Finerenone in Heart Failure Patients) [Internet]. Health Research Authority; [cited 30 April 2023]. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/finearts-hf-finerenone-in-heart-failure-patients/ [Google Scholar]
- 52. Freeman MW, Halvorsen YD, Marshall W, Pater M, Isaacsohn J, Pearce C, Murphy B, Alp N, Srivastava A, Bhatt DL, Brown MJ; BrigHTN Investigators. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med 2023; 388:395–405. [DOI] [PubMed] [Google Scholar]
- 53. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang C-E, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387:1089–1098. [DOI] [PubMed] [Google Scholar]
- 54. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021; 384:129–139. [DOI] [PubMed] [Google Scholar]
- 55. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384:117–128. [DOI] [PubMed] [Google Scholar]
- 56. Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 Inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 2022; 146:1383–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zannad F, Ferreira JP, Butler J, Filippatos G, Januzzi JL, Sumin M, Zwick M, Saadati M, Pocock SJ, Sattar N, Anker SD, Packer M.. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur Heart J 2022; 43:4991–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022; 400:1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin N, Manoharan K, Davies C, Lumbers RT.. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev 2021; 5:CD012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sreenivasan J, Malik A, Khan MS, Lloji A, Hooda U, Aronow WS, Lanier GM, Pan S, Greene SJ, Murad MH, Michos ED, Cooper HA, Gass A, Gupta R, Desai NR, Mentz RJ, Frishman WH, Panza JA.. Pharmacotherapies in heart failure with preserved ejection fraction: a systematic review and network meta-analysis. Cardiol Rev 2022; e-pub ahead of print. doi: 10.1097/CRD.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 61. Xiang B, Zhang R, Wu X, Zhou X.. Optimal pharmacologic treatment of heart failure with preserved and mildly reduced ejection fraction: a meta-analysis. JAMA Netw Open 2022; 5:e2231963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kass DA. What’s EF got to do, got to do with it? Circulation 2022; 146:1327–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lam CSP, Solomon SD.. DELIVERing therapeutic efficacy across the ejection fraction spectrum of heart failure. Circulation 2022; 146:1193–1195. [DOI] [PubMed] [Google Scholar]
- 64. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot J-S, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu J-N, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW.. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019; 40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Asgar AW, Mack MJ, Stone GW.. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015; 65:1231–1248. [DOI] [PubMed] [Google Scholar]
- 66. Wachtell K, Gerdts E, Palmieri V, Olsen MH, Nieminen MS, Papademetriou V, Boman K, Dahlöf B, Aurigemma GP, Rokkedal JE, Devereux RB.. In-treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: the Losartan Intervention For Endpoint reduction study. J Hypertens 2010; 28:1541–1546. [DOI] [PubMed] [Google Scholar]
- 67. Clarke CL, Grunwald GK, Allen LA, Barón AE, Peterson PN, Brand DW, Magid DJ, Masoudi FA.. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Savarese G, Orsini N, Hage C, Vedin O, Cosentino F, Rosano GMC, Dahlström U, Lund LH.. Utilizing NT-proBNP for eligibility and enrichment in trials in HFpEF, HFmrEF, and HFrEF. JACC Heart Fail 2018; 6:246–256. [DOI] [PubMed] [Google Scholar]
- 69. Pabón MA, Cunningham JW, Claggett BL, Packer M, Zile M, Pfeffer MA, Lefkowitz M, Shi V, Rizkala A, McMurray JJV, Solomon SD, Vaduganathan M.. Natriuretic peptide-based inclusion criteria in heart failure with preserved ejection fraction clinical trials: insights from PARAGON-HF. Eur J Heart Fail 2022; 24:672–677. [DOI] [PubMed] [Google Scholar]
- 70. Girerd N, Ferreira JP, Rossignol P, Zannad F.. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail 2016; 18:1411–1414. [DOI] [PubMed] [Google Scholar]
- 71. Chenevier-Gobeaux C, Claessens YE, Voyer S, Desmoulins D, Ekindjian OGJC.. Influence of renal function on N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients admitted for dyspnoea in the emergency department: comparison with brain natriuretic peptide (BNP). Clin Chim Acta 2005; 361:167–175. [DOI] [PubMed] [Google Scholar]
- 72. Bansal N, Zelnick L, Go A, Anderson A, Christenson R, Deo R, Defilippi C, Lash J, He J, Ky B, Seliger S, Soliman E, Shlipak M; CRIC Study Investigators †. Cardiac biomarkers and risk of incident heart failure in chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc 2019; 8:e012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strauer BE. Ventricular function and coronary hemodynamics in hypertensive heart disease. Am J Cardiol 1979; 44:999–1006. [DOI] [PubMed] [Google Scholar]
- 74. Okin PM, Hille DA, Kjeldsen SE, Dahlöf B, Devereux RB.. Impact of lower achieved blood pressure on outcomes in hypertensive patients. J Hypertens 2012; 30:802–810; discussion 810. [DOI] [PubMed] [Google Scholar]
- 75. Heimark S, Mehlum MH, Mancia G, Søraas CL, Liestöl K, Wachtell K, Larstorp AC, Rostrup M, Mariampillai JE, Kjeldsen SE, Julius S, Weber MA.. Middle-aged and older patients with left ventricular hypertrophy: higher mortality with drug treated blood pressure below 130/80 mmHg. Hypertension 2023; 80:1739–1748. [DOI] [PubMed] [Google Scholar]
- 76. Strain WD, Paldánius PM.. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 2018; 17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM.. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van Empel VP, Mariani J, Borlaug BA, Kaye DM.. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 2014; 3:e001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Borlaug BA, Sharma K, Shah SJ, Ho JE.. Heart failure with preserved ejection fraction: JACC Scientific Statement. J Am Coll Cardiol 2023; 81:1810–1834. [DOI] [PubMed] [Google Scholar]
- 80. Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, Januzzi JL Jr, Yancy CW.. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023; 81:1835–1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not relevant for this review article.


