Abstract
Background
As an important kidney-sparing treatment for upper urothelial carcinoma (UTUC), whether endoscopic excision can be performed without sacrificing oncologic outcomes remains indefinite. This study aimed to investigate the prevalence and efficacy of endoscopic excision, in patients with non-muscle invasive UTUC (NMIUTUC) and compare them to those of radical nephroureterectomy (RNU).
Methods
Using the Surveillance, Epidemiology, and End Results database, we reviewed 4347 cases with NMIUTUC (cTis/Ta/T1-N0-M0,≤ 5.0 cm) between 2004 and 2020. Surgical treatment modalities included endoscopic excision and RNU. Propensity score matching analysis was used to minimize the selection bias between endoscopic excision and RNU, selecting 1:1 matched patients in the two group.
Results
A total of 794 patients with NMIUTUC were included after matching (397:397). Patients who underwent endoscopic excision had worse survival outcomes compared with those of patients who underwent RNU (5-year OS: 65.3 % vs. 80.3 %, p < 0.0001; 5-year DSS: 83.2 % vs. 94.0 %, p = 0.00021). After stratification by anatomical sites, the effect of endoscopic excision for NMI renal pelvis cancer was worse than RNU (5-year OS, 62.9 % vs. 82.8 %; 5-year DSS, 78.8 % vs. 91.6 %), while in NMI ureteral cancer, there is no statistically significant difference in OS and DSS between endoscopic excision and RNU. Further stratification according to tumor grade revealed equivalent tumor control effects of endoscopic excision and RNU in low-grade NMI ureteral cancer (5-year OS: 67.7 % vs. 72.5 %, p = 0.23; 5-year DSS: 87.2 % vs. 93.1 %, p = 0.17); while for renal pelvis tumor and high-grade ureteral tumor, endoscopic excision was related with significantly inferior prognosis.
Conclusions
Only for low-grade NMI ureteral cancer, endoscopic excision and RNU are oncologically equivalent, indicating that endoscopic excision might be an effective option for low-grade NMI ureteral cancer. This result needs to be further verified in randomized controlled trials.
Keywords: Endoscopic excision, Non-muscle invasive upper tract urothelial carcinoma, Outcomes, Prevalence, Radical nephroureterectomy, SEER
1. Introduction
Urothelial carcinomas (UC) are the fourth most common tumor in the world, >90 % of which are bladder tumors, and upper tract urothelial carcinomas (UTUC) (renal pelvis and ureter cancers) account for only 5–10 % [1,2]. More than 75 % of bladder tumors are non-muscle invasive (NMI), while only one-third of UTUCs are NMI [3]. Disease management in patients with NMIUTUC is receiving increasing attention owing to a lack of high-quality evidence.
In the past two decades, with the development of endoscopy (transurethral ureteroscopy and percutaneous nephoscopy) and ablation technology (new generation lasers represented by holmium laser and thulium laser) [4,5], researchers and clinicians worldwide have attempted to extend the applications of endoscopic kidney-sparing surgery to UTUC in order to reduce surgical trauma and postoperative complications [6]. However, unlike Transurethral resection of bladder tumor, which has become the standard treatment for NMI bladder cancer [7,8], accurately determining preoperative staging and diagnosis of UTUC is difficult for urologists due to the anatomical limitations of the renal pelvis and ureter. Therefore, to yield better tumor clearance [9], radical nephroureterectomy (RNU) with bladder cuff removal remains the gold standard for the treatment of UTUC, as recommended by the European Association of Urology guidelines [2]. The purpose of endoscopic excision is to simultaneously achieve successful complete tumor resection and organ preservation. Studies have shown that, compared with RNU, endoscopic excision prevents the loss of kidney units [10], thus reducing the occurrence of long-term postoperative complications related to RNU, such as chronic kidney disease and cardiovascular disease [11,12]. The current microsurgical management for UTUC aims to determine whether endoscopic excision can achieve organ preservation while attaining favorable oncologic outcomes.
To date, there are no prospective randomized controlled studies on endoscopic excision of UTUC, with only a few retrospective cohort studies investigating this topic. Additionally, most of these studies were single-center and had small sample sizes, which increased their risk of bias [[13], [14], [15], [16], [17], [18]]. The Surveillance, Epidemiology, and End Results (SEER) database covers 18 United States (US) registries and is one of the world's largest cancer cohorts. The SEER database can provide sufficient data on patients treated with endoscopic resection for NMIUTUC. This study aimed to investigate the prevalence and efficiency of endoscopic excision for NMIUTUC using the SEER database, and explore whether its oncological efficacy is equivalent to that of RNU.
2. Patients and methods
2.1. Data sources and study population
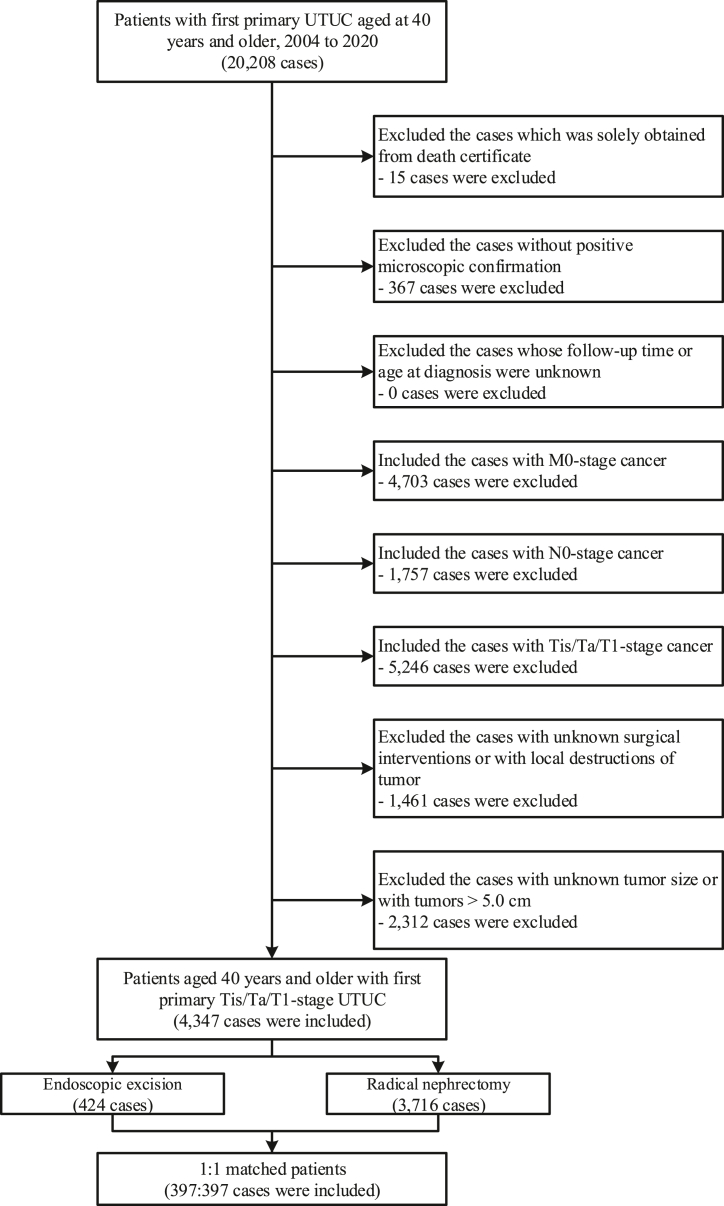
The SEER database broadly represents the US population, covering 29 % of the nation's population and 21 states (http://seer.cancer.gov) [19]. The data of patients diagnosed with first primary UTUC from January 1, 2004, to December 31, 2020, was retrospectively extracted from the SEER database. UTUC was identified using the International Classification of Diseases for Oncology, third edition codes. We included patients with UTUC of the renal pelvis (C65.9), ureter (C66.9), and histology codes of 8020, 8120, 8122, 8130, 8131, and 8082. To accurately determine the specific mortality for UTUC, we excluded data of patients with multiple primary tumors. Detailed inclusion and exclusion criteria are shown in Fig. 1. We only included patients aged 40 years and older with NMI tumors without any metastases or lymph node involvement (i.e., cTa/Tis/T1-N0-M0). The tumors larger than 5.0 cm were also excluded, as theses tumors might be impossible to be removed via endoscopic excision. To improve reporting quality, our study was conducted in accordance with the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) 2021 guidelines [20]. This cohort was registered in the Research Registry (https://www.researchregistry.com) (ID: researchregistry8054).
Fig. 1.
Inclusion and exclusion criteria for patients included in this study. Abbreviations: UTUC, upper tract urothelial carcinomas.
As the SEER database did not directly involve human subjects and all information was anonymized, the Institutional Review Boards of the Union Hospital of the Tongji Medical College waived ethical review and informed consent for this study.
2.2. Definition of variables
Survival time was defined as the date of diagnosis to the date of death, end of the study (December 31, 2017), or early withdrawal. Demographic and socioeconomic variables, including the year of diagnosis, age at diagnosis, sex, race, Hispanic origin, rural–urban residence, and median household income, were evaluated. Clinical features included the American Joint Committee on Cancer Staging (AJCC) TNM stage, anatomical sites, tumor size, and tumor grade. For exposure factors, Surgical treatment modalities were divided into two main types for comparison: endoscopic excision (site-specific surgery codes: 20, 21, 22, 23, 24, 25, 26, and 27) and RNU (site-specific surgery codes: 40 and 50). Patients who underwent local tumor destruction or had missing surgical information were excluded.
2.3. Statistical analysis
We assessed the baseline characteristics of the enrolled patients with UTUC and delineated the differences in patients with different surgical interventions. We used the propensity score matching (PSM) analysis to reduce selection bias in this retrospective cohort studies. The nearest-neighbor matching was used to match patients who underwent endoscopic excision and RNU in a 1:1 ratio. The propensity score takes into account variables that may be relevant to patient treatment selection, including year of diagnosis, age at diagnosis, sex, race, rural-urbane residence, median household income, AJCC T stage, and anatomical sites, tumor size, and tumor grade. Quantify the matching effect of PSM by calculating the Standardized Mean Difference (SMD). SMD = (mean of RNU group - mean of endoscopic excision group)/total standard deviation. Overall survival (OS) and disease-specific survival (DSS) were the main outcomes and were determined using the Kaplan–Meier method. OS was defined as the survival time for all cause-of-death outcomes [21]. DSS was defined as the survival time for specific death associated with UTUC. Further, Survival differences between the two types of surgical procedures were analyzed using a multivariate Cox proportional hazard regression model. A two-tailed P value of less than 0.05 was considered statistically significant. SEER*Stat software, version 8.3.8 and R software, version 3.6.3 (R core team, Vienna, Austria) were used for data collection and statistical analyses.
3. Results
3.1. Baseline characteristics
A total of 6659 patients with NMIUTUC were enrolled in this study; 69.3 % and 30.7 % had renal pelvis and ureter localizations, respectively. Most were 60–79 years old (63.7 %), male (58.8 %), white (87.6 %), non-Hispanic (91.8 %), middle-income (56.8 %), living in urban areas (86.8 %), Ta Stage (55.3 %), and low grade (37.3 %). A total of 1668 deaths was recorded, with a median follow-up of 4.7 years (range: 0–16.9 years) (Table S1).
3.2. Prevalence of surgical interventions in patients with NMIUTUC
Among the 4347 patients, 95.2 % underwent surgery as the initial treatment, of which 9.8 % underwent endoscopic excision and 85.5 % underwent RNU (Table S1). Endoscopic excision treatment was chosen more frequently than RNU in patients who were ≥80 years old (29.7 % vs. 19.2 %), male (64.6 % vs. 58.6 %), diagnosed in 2015–2020 (50.9 % vs. 37.7 %), and had high household incomes (48.1 % vs. 41.8 %), Ta- (71.2 % vs. 54.2 %), ureteral UTUC (53.8 % vs. 27.5 %), tumor size <2.0 cm (64.2 % vs. 28.1 %) and low grade (48.1 % vs. 36.4 %).
For patients aged 40–70 years, there was no significant change between the frequencies of endoscopic excision and RNU. Among patients aged ≥70 years, the total frequencies of surgery for all procedures combined decreased with age (70–74 years: 96.4 %; 75–79 years: 96.0 %; 80–84 years: 93.0 %; and ≥85 years: 83.4 %). The frequency of RNU decreased with age (70–74 years: 88.4 %; 75–79 years: 84.8 %; 80–84 years: 81.8 %; ≥85 years: 67.3 %), while the frequency of endoscopic excision increased with age (70–74 years: 8.0 %; 75–79 years: 11.1 %; 80–84 years: 11.2 %; ≥85 years: 16.1 %) (Figs. S1A–B).
3.3. Survival analysis according to types of surgical intervention after PSM
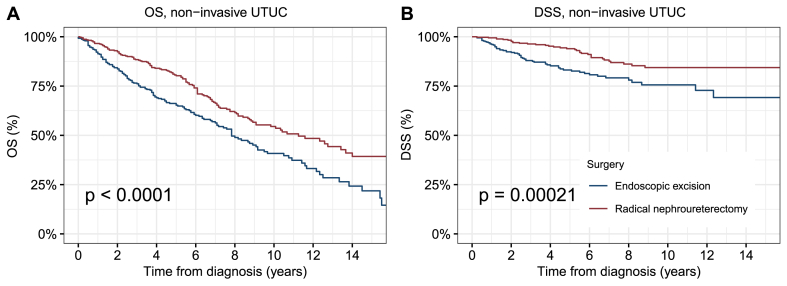
After PSM, 397 patients were enrolled in each of the endoscopic resection group and RNU group, and most characteristics were balanced between the two groups (p values > 0.05, absolute values of SMD <0.1) (Table 1, Fig. S2). K-M curves showed that showed that the OS and DSS after endoscopic excision were significantly worse than those among patients who underwent RNU than those of patients who underwent RNU (5-year OS: 65.3 % vs. 80.3 %, p < 0.0001; 5-year DSS: 83.2 % vs. 94.0 %, p = 0.00021) (Fig. 2A and B).
Table 1.
Baseline characteristics of patients received different surgical operations after propensity score matching.
| Characteristics | Endoscopic excision | Radical nephroureterectomy | P Value |
|---|---|---|---|
| Total | 397 (100 %) | 397 (100 %) | |
| Age at diagnosis | 0.01 | ||
| 40-59 | 51 (12.8 %) | 79 (19.9 %) | |
| 60-79 | 233 (58.7 %) | 198 (49.9 %) | |
| 80+ | 113 (28.5 %) | 120 (30.2 %) | |
| Sex | 0.2 | ||
| Female | 141 (35.5 %) | 157 (39.5 %) | |
| Male | 256 (64.5 %) | 240 (60.5 %) | |
| Race | 0.8 | ||
| White | 354 (89.2 %) | 346 (87.2 %) | |
| Black | 16 (4 %) | 15 (3.8 %) | |
| AI/AN | 2 (0.5 %) | 4 (1 %) | |
| API | 23 (5.8 %) | 30 (7.6 %) | |
| Unknown | 2 (0.5 %) | 2 (0.5 %) | |
| Hispanic origin | 0.1 | ||
| Non-Hispanic | 373 (94 %) | 361 (90.9 %) | |
| Hispanic | 24 (6 %) | 36 (9.1 %) | |
| Year of diagnosis | 0.06 | ||
| 2004–2009 | 78 (19.6 %) | 98 (24.7 %) | |
| 2010–2014 | 122 (30.7 %) | 134 (33.8 %) | |
| 2015–2020 | 197 (49.6 %) | 165 (41.6 %) | |
| Rural/urban status | 0.8 | ||
| Rural | 49 (12.3 %) | 55 (13.9 %) | |
| Urban | 347 (87.4 %) | 341 (85.9 %) | |
| Unknown | 1 (0.3 %) | 1 (0.3 %) | |
| Median house-hold income | 0.5 | ||
| Low | 2 (0.5 %) | 5 (1.3 %) | |
| Median | 206 (51.9 %) | 212 (53.4 %) | |
| High | 189 (47.6 %) | 180 (45.3 %) | |
| AJCC T stage | 0.3 | ||
| Ta | 278 (70 %) | 258 (65 %) | |
| Tis | 14 (3.5 %) | 20 (5 %) | |
| T1 | 105 (26.4 %) | 119 (30 %) | |
| Anatomic sites | 0.8 | ||
| Renal pelvis | 193 (48.6 %) | 188 (47.4 %) | |
| Ureter | 204 (51.4 %) | 209 (52.6 %) | |
| Tumor size | 0.3 | ||
| 0–1.0 | 93 (23.4 %) | 103 (25.9 %) | |
| 1.1–2.0 | 152 (38.3 %) | 128 (32.2 %) | |
| 2.1–3.0 | 97 (24.4 %) | 97 (24.4 %) | |
| 3.1–4.0 | 39 (9.8 %) | 43 (10.8 %) | |
| 4.1–5.0 | 16 (4 %) | 26 (6.5 %) | |
| Tumor grade | 0.1 | ||
| Low grade | 186 (46.9 %) | 188 (47.4 %) | |
| High grade | 73 (18.4 %) | 92 (23.2 %) | |
| Unknown | 138 (34.8 %) | 117 (29.5 %) |
Abbreviation: AI/AN, American Indian/Alaska Native; API, Asian or Pacific Islander.
Fig. 2.
Kaplan–Meier curves depicting (A) OS and (B) DSS of patients with NMIUTUC according to the type of surgical treatment modality. Abbreviations: OS, overall survival; DSS, disease-specific survival; NMIUTUC, non-muscle invasive upper tract urothelial carcinomas.
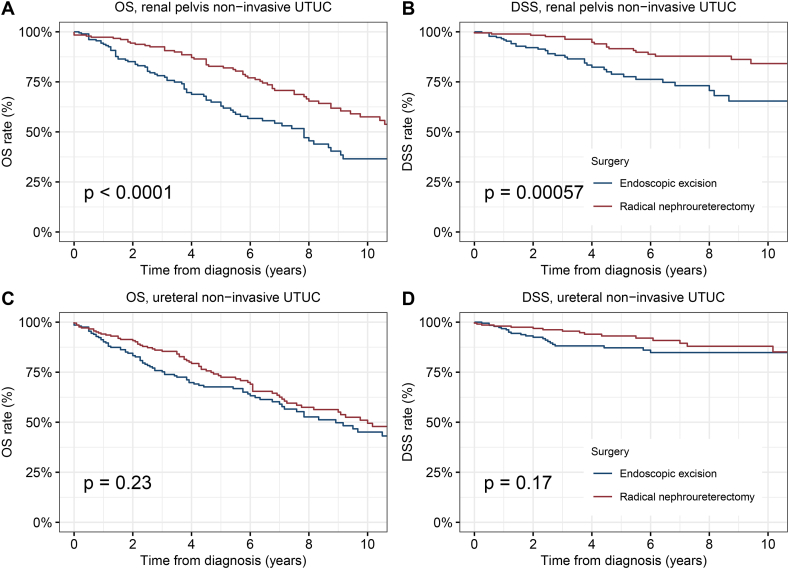
Further, we conducted a stratified analysis according to the anatomical site of NMIUTUC. For NMI renal pelvis cancer, patients who underwent endoscopic excision had significantly worse OS and DSS than those who underwent RNU (5-year OS, 62.9 % vs. 82.8 %, p < 0.0001; 5-year DSS, 78.8 % vs. 91.6 %, p = 0.00057) (Fig. 3A and B). Surprisingly, for NMI ureteral cancer, the difference in survival between endoscopic excision and RNU was small and not statistically significant (5-year OS: 67.7 % vs. 72.5 %, p = 0.23; 5-year DSS: 87.2 % vs. 93.1 %, p = 0.17) (Fig. 3C and D).
Fig. 3.
Kaplan–Meier curves depicting OS and DSS of patients with renal pelvis or ureteral NMIUTUC according to the type of surgical treatment modality. (A) OS of patients with renal pelvis NMIUTUC; (B) DSS of patients with renal pelvis NMIUTUC; (C) OS of patients with ureteral NMIUTUC; (D) DSS of patients with ureteral NMIUTUC.
Abbreviations: OS, overall survival; DSS, disease-specific survival; NMIUTUC, non-muscle invasive upper tract urothelial carcinomas.
Survival analysis by surgical intervention for NMI renal pelvis and ureter cancer of different tumor stages.
After stratifying NMIUTUC patients according to stage, the 5-year OS and 5-year DSS rates were 68.6 % and 86.5 % for patients receiving endoscopic excision for Ta/Tis stage, 57.5 % and 74.7 % for patients receiving endoscopic excision for T1 stage, 77.4 % and 92.5 % for patients receiving RNU for Ta/Tis stage, and 78.1 % and 91.5 % for patients receiving RNU for T1 stage, respectively (Figs. S3A–D).
Further stratified analysis was performed based on tumor stages and anatomic sites. For NMI renal pelvis cancer, except for the DSS of Ta/Tis (p = 0.079), the OS and DSS of the endoscopic excision group in both the Ta/Tis and T1 stages were significantly worse than those in the RNU group (Ta/Tis, 5-year OS: 69.5 % vs. 82.6 %, p = 0.0056; 5-year DSS: 85.1 % vs. 91.0 %, p = 0.08. T1, 5-year OS: 49.3 % vs. 83.0 % p = 0.00067; 5-year DSS: 62.6 % vs. 93.2 % p = 0.00073) (Figs. S4A–D). In contrast, for NMI ureteral cancer, the differences in OS and DSS between endoscopic excision and RNU in Ta/Tis and T1 stages were all small and not statistically significant (p > 0.05) (Figs. S5A–D).
Survival analysis by surgical intervention for NMI renal pelvis and ureter cancer of different tumor grades.
After stratifying NMIUTUC according to tumor grade, the OS and DSS of endoscopic excision group in low-grade NMIUTUC and high-grade NMIUTUC were both significantly worse than those in the RNU group (Low grade, 5-year OS: 71.1 % vs. 77.6 %, p = 0.034; 5-year DSS: 87.2 % vs. 93.6 %, p = 0.023. High grade, 5-year OS: 45.9 % vs. 77.6 %, p < 0.0001; 5-year DSS: 68.3 % vs. 88.7 %, p = 0.0019) (Figs. S6A–D). We observed that compared with the huge gap between the two surgical methods in high-grade patients, the survival rate of endoscopic excision in low-grade patients is close to that of RNU.
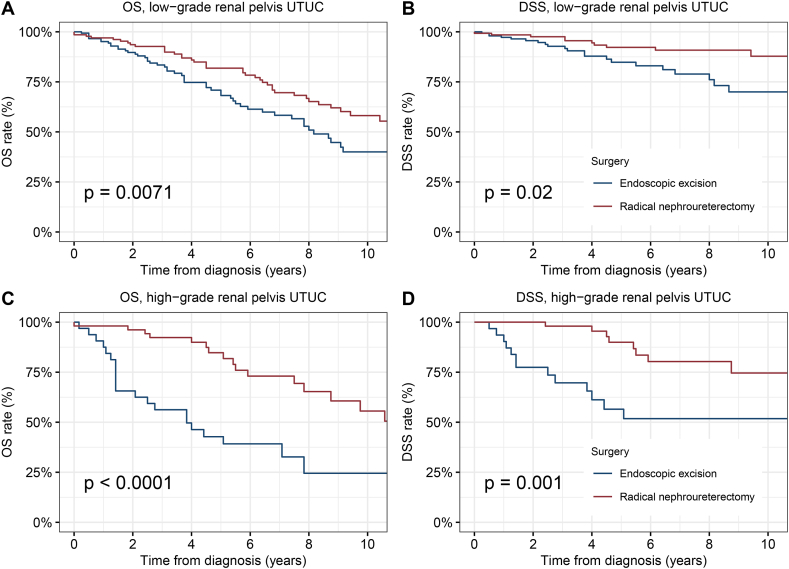
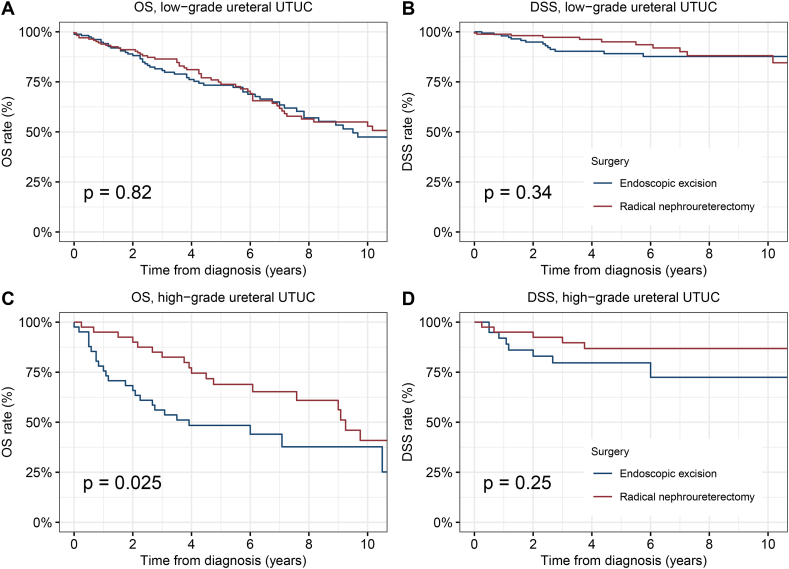
Therefore, we further performed stratified analyzes of tumor grade in NMI renal pelvis and ureter cancer respectively. For NMI renal pelvis cancer, endoscopic excision had significantly worse OS and DSS than RNU in low or high grade (Low-grade, 5-year OS: 68.2 % vs. 81.8 %, p = 0.0071; 5-year DSS: 84.8 % vs. 92.2 % p = 0.02. High-grade, 5-year OS: 42.8 % vs. 84.7 % p < 0.0001; 5-year DSS: 65.6 % vs. 89.9 % p = 0.001) (Fig. 4A–D). For low-grade NMI ureteral cancer, there was no difference in survival between endoscopic excision and RNU (5-year OS: 73.3 % vs. 73.8 % p = 0.82; 5-year DSS: 89.1 % vs. 95.0 % p = 0.34) (Fig. 5A and B). However, for high-grade NMI ureteral cancer, the tumor control effect of endoscopic excision was inferior to that of the RNU (5-year OS: 48.4 % vs. 68.9 % p = 0.025; 5-year DSS: 79.7 % vs. 86.8 % p = 0.25) (Fig. 5C and D).
Fig. 4.
Kaplan-Meier curves depicting OS and DSS of patients with low or high-grade renal pelvis NMIUTUC by type of surgical treatment modality. (A) OS and (B) DSS of patients with Tis/Ta-stage renal pelvis NMIUTUC. (C) OS and (D) DSS of patients with T1-stage renal pelvis NMIUTUC. Abbreviations: OS, overall survival; DSS, disease-specific survival; NMIUTUC, non-muscular invasive upper tract urothelial carcinomas.
Fig. 5.
Kaplan-Meier curves depicting OS and DSS of patients with low or high-grade ureteral NMIUTUC by type of surgical treatment modality. (A) OS and (B) DSS of patients with Tis/Ta-stage ureteral NMIUTUC. (C) OS and (D) DSS of patients with T1-stage ureteral NMIUTUC.
Abbreviations: OS, overall survival; DSS, disease-specific survival; NMIUTUC, non-muscular invasive upper tract urothelial carcinomas.
Further stratified analysis of surgical interventions showed that among patients who underwent endoscopic excision, the 5-year OS and 5-year DSS rates of the low-grade group were 71.1 % and 87.2 %, while the 5-year OS and 5-year DSS rates of the high-grade group were 45.9 % and 68.3 %, yielding huge prognosis gap by tumor grade. In contrast, for patients who underwent RNU, different tumor grades had no significant impact on postoperative survival (Figs. S7A–D).
3.4. Multivariable Cox analyses of OS and DSS of patients with NMIUTUC
After adjusting for confounding variables, the type of surgical procedure was an independent predictor of OS and DSS (Table 2). The risks of overall and disease-specific mortality of the endoscopic excision group were 1.63 times (hazard ratio [HR], 1.6; 95 % confidence interval [CI], 1.37–1.94; p < 0.001) and 2.23 times (HR, 2.11; 95 % CI, 1.67–2.97; p < 0.001) higher compared to the RNU group, respectively. The risks were even higher in the group without surgery, with a 3.79-fold (HR, 3.79; 95 % CI, 3.14–4.58; p < 0.001) and 6.03-fold (HR, 6.03; 95 % CI, 4.59–7.91; p < 0.001) increased risks of death for OS and DSS, respectively, when compared to the RNU group.
Table 2.
Multivariable Cox analyses of overall survival and disease-specific survival of patients diagnosed with upper tract urothelial carcinoma between 2004 and 2020.
| Characteristics | OS |
DSS |
||
|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | |
| Age at diagnosis | ||||
| 40-59 | Reference | Reference | ||
| 60-79 | 3.11 (2.53–3.82) | < 0.001 | 2.04 (1.49–2.8) | < 0.001 |
| 80+ | 8.2 (6.6–10.19) | < 0.001 | 4.48 (3.2–6.28) | < 0.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.18 (1.07–1.3) | 0.001 | 1.15 (0.97–1.36) | 0.11 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.14 (0.9–1.45) | 0.26 | 1.23 (0.86–1.77) | 0.26 |
| AI/AN | 2.09 (1.18–3.72) | 0.01 | 1.48 (0.47–4.63) | 0.5 |
| API | 0.95 (0.77–1.17) | 0.61 | 0.93 (0.65–1.33) | 0.68 |
| Unknown | 0.47 (0.07–3.31) | 0.44 | 0 (0-NA) | 0.99 |
| Year of diagnosis | ||||
| 2004–2009 | Reference | Reference | ||
| 2010–2014 | 0.95 (0.85–1.07) | 0.41 | 1.06 (0.87–1.3) | 0.54 |
| 2015–2020 | 0.92 (0.78–1.08) | 0.29 | 1.04 (0.81–1.32) | 0.77 |
| Rural/urban status | ||||
| Rural | Reference | Reference | ||
| Urban | 1 (0.86–1.16) | 0.99 | 1.19 (0.91–1.55) | 0.2 |
| Unknown | 1.69 (0.22–13.15) | 0.62 | 0 (0-Inf) | 0.99 |
| Median house-hold income | ||||
| High | Reference | Reference | ||
| Low | 1.5 (0.85–2.65) | 0.17 | 2.98 (1.34–6.64) | 0.01 |
| Median | 1.19 (1.07–1.32) | < 0.001 | 1.2 (1–1.44) | 0.047 |
| Anatomic sites | ||||
| Renal pelvis | Reference | Reference | ||
| Ureter | 0.98 (0.88–1.09) | 0.71 | 0.93 (0.77–1.12) | 0.43 |
| AJCC T stage | ||||
| Ta | Reference | Reference | ||
| Tis | 1.17 (0.96–1.43) | 0.12 | 1.36 (0.95–1.96) | 0.1 |
| T1 | 1.18 (1.07–1.31) | 0.001 | 1.58 (1.32–1.9) | < 0.001 |
| Tumor size | ||||
| 0–1.0 | Reference | Reference | ||
| 1.1–2.0 | 1.28 (1.05–1.56) | 0.02 | 1.46 (1.01–2.11) | 0.05 |
| 2.1–3.0 | 1.31 (1.08–1.6) | 0.007 | 1.64 (1.13–2.36) | 0.01 |
| 3.1–4.0 | 1.39 (1.13–1.7) | 0.001 | 1.83 (1.26–2.66) | 0.001 |
| 4.1–5.0 | 1.46 (1.17–1.81) | < 0.001 | 2.22 (1.5–3.29) | < 0.001 |
| Tumor grade | ||||
| High grade | Reference | Reference | ||
| Low grade | 0.91 (0.82–1.02) | 0.09 | 0.7 (0.58–0.85) | < 0.001 |
| Unknown | 0.93 (0.79–1.09) | 0.38 | 0.9 (0.7–1.15) | 0.4 |
| Surgery | ||||
| Radical nephroureterectomy | Reference | Reference | ||
| Endoscopic excision | 1.63 (1.37–1.94) | < 0.001 | 2.23 (1.67–2.97) | < 0.001 |
| None | 3.79 (3.14–4.58) | < 0.001 | 6.03 (4.59–7.91) | < 0.001 |
OS=Overall survival; DSS = Disease-specific survival; CI= Confidence interval; AI/AN = American Indian/Alaska Native; API = Asian or Pacific Islander.
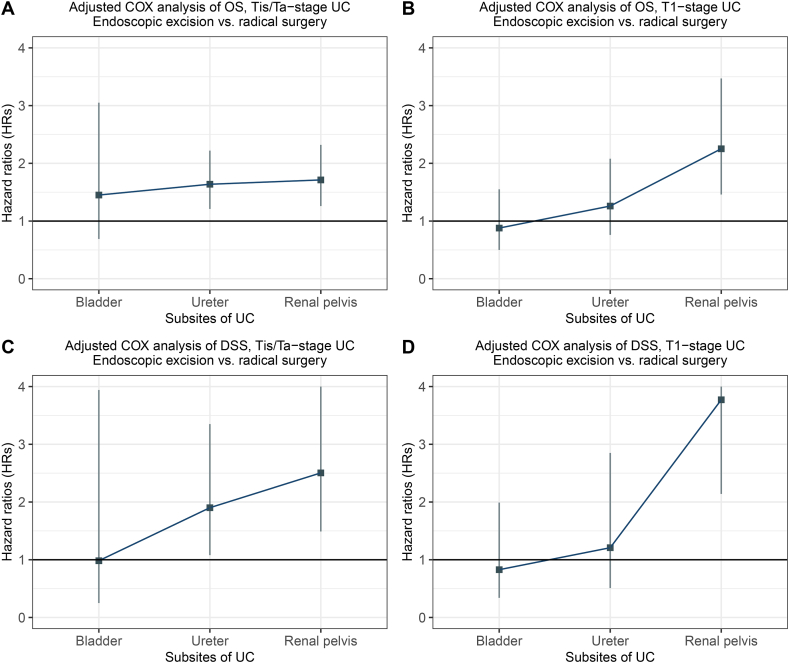
Further, we analyzed the survival of patients with UC at different sites according to the type of surgical intervention using a multivariate Cox regression model (Fig. 6A–D). Compared with RNU, the HRs of endoscopic excision increased with the distance of the tumor site from the external orifice of the urethra. Endoscopic excision was superior or equivalent to radical resection for NMI tumors localized in the bladder, with a HR of less than or close to 1. For ureteral tumors, the HR was slightly greater than 1, but the increase in T1 stage was not statistically significant. Conversely, endoscopic excision was significantly less effective compared to radical resection when the tumor was localized in renal pelvis.
Fig. 6.
Multivariable Cox analyses assessing HRs of endoscopic excision and radical nephroureterectomy for non-muscle invasive UC according to different tumor sites. HR of OS of patients with (A) Tis/Ta-stage UC and (B) T1-stage UC. HR of DSS of patients with (C) Tis/Ta-stage UC and (D) T1-stage UC. Notes: the multivariable Cox regression model was adjusted by sex, race, age at diagnosis, year of diagnosis, ‘rural–urban residence at diagnosis, and median household income. Abbreviations: HR, hazard ratios; UC, urothelial carcinomas; OS, overall survival; DSS, disease-specific survival.
4. Discussion
This study characterized the prevalence and efficacy of endoscopic excision for NMIUTUC using data from over 5600 cases. To date, this study is the longest followed and largest cohort examining endoscopic excision for NMIUTUC [22,23]. Using the PSM model, we compared the efficacy between RNU and endoscopic excision, which can provide population-level evidence for urologists to choose surgical procedures when treating NMIUTUC.
Endoscopic excision has been introduced in the management of localized nonmetastatic UTUC, and its efficacy is under the current spotlight for urologists. Endoscopic excision was initially an expedient procedure for patients with renal atrophy, renal impairment, solitary kidney, and bilateral tumors who cannot undergo RNU [2]. Advances in flexible devices, optics, and laser technology (thulium laser is the most representative) have enabled access to distal tumors localized in the upper ureter and renal pelvis for endoscopic tumor excision, which greatly shortened hospitalization and preserved the nephrons [4,23,24]. Additionally, preservation of normal anatomical cavities can provide opportunities for subsequent local drug treatment, such as Bacillus Calmette Guerin [25,26]. Evidence on the therapeutic efficiency of endoscopic excision of UTUC has been conflicting. Three retrospective cohort studies from Israel and the US concluded that OS and/or DSS of endoscopic excision in low-grade UTUC were comparable to those of RNU [14,15,27], which are consistent with the results of our stratified analysis for low-grade NMIUTUC, especially for low-grade NMI ureteral cancer. In a cohort of 198 low-grade UTUC patients from Austria, OS of endoscopic excision was worse, whereas DSS was equivalent to that of RNU [17]. Unfortunately, most of these studies were retrospective, single-center, and included only few cases of endoscopic excision, increasing the risk of bias.
After detailed stratified analysis, our cohort study showed that the therapeutic efficacy of endoscopic excision is not comparable to that of RNU except in low-grade NMI ureteral tumors. Although there are advantages, current endoscopic excision technology has inherent limitations for UTUC treatment. Compared to lower urinary tract tumors, UTUC occurs in distal anatomic sites, which demand the flexibility of endoscopic instruments and the accuracy of robotic arms [25] Thus, the therapeutic efficiency of endoscopic excision worsens with increased distance of the tumor site from the external urethral orifice. Due to the thin ureteral wall and narrow diameter of the ureter, perforation may occur during the operation; thus, only experienced chief surgeons can perform these operations [28]. A systematic review concluded that the pathological diagnosis obtained through ureteroscopic biopsy may be at risk of undergrading and understaging [29]. It becomes a dilemma for surgeons when the local and/or bladder recurrence rate increases after endoscopic excision, even in the contralateral pelvic ureter [30,31]. In a single-center retrospective cohort of 139 UTUC patients from China, patients who underwent thulium laser ablation had a shorter hospitalization and less loss of renal function, but the tumor recurrence rate was nearly 1.7 times higher than that of the RNU group. (13.1 % vs 21.9 %) [22,23]. UTUC often has a multifocal onset and is prone to spreading along the urothelium. Unlike RNU, endoscopic excision cannot completely remove the urothelium from the renal pelvis to the bladder entrance; therefore, it is more likely to lead to recurrence. Given the many possible risks above, patients should be strictly selected for endoscopic excision, otherwise the protection of normal organs will only occur at the expense of tumor control.
As our results showed, most patients with NMIUTUC selected RNU as the initial treatment for their tumors, while only 9.8 % of the tumors were resected via therapeutic endoscopy. This relatively low preference for endoscopic excision is partly due to the guideline restrictions on indications [32], but could also be attributed to the nature of UTUC surgery itself. RNU coupled with bladder cuff resection provides good tumor control and is considered the gold standard for the management of UTUC patients. Unlike RNU, endoscopic excision has the following shortcomings: 1. it does not provide information on peripheral lymphatic involvement; 2. guaranteeing complete tumor resection with limited excision extension is difficult; 3. obtaining an accurate postoperative pathological diagnosis is difficult, raising concerns regarding its safety. Additionally, the organ-preserving desire of patients with UTUC is low due to the limited impairments of RNU on the patients’ personal image and short-term postoperative quality of life [33]. Clinicians and researchers should perform this procedure in patients with UTUC under strict indications until the efficacy of endoscopic excision has been fully validated.
This study had several limitations. First, the SEER database lacks information on prognostic factors, such as hydronephrosis, obesity, architecture, multifocality and performance status, and comorbidities, including chronic kidney disease and cardiovascular disease. Thus, it is difficult to determine whether these factors affected patient's survival. Additionally, the follow-up outcomes included only OS and DSS, and the results of tumor recurrence and progression could not be further analyzed. Despite these limitations, this study used a large-scale SEER data to provide valuable evidence for the decision-making process between RNU and endoscopic microsurgery in patients with NMIUTUC.
5. Conclusions
In this population-based matched cohort study, endoscopic excision was associated with significantly worse tumor control than RNU in NMIUTUC, except in low-grade NMI ureteral tumors where endoscopic excision was equally effective as RNU. Endoscopic excision shows promising potential as a therapeutic option; however, more studies are needed to improve the efficacy of endoscopic excision. Further research is also needed to validate the efficacy of endoscopic excision for UTUC.
Funding
This work was supported by the National Key Scientific Instrument Development Project (81927807), the Wuhan Science and Technology Plan Application Foundation Frontier Project (2020020601012247) and the National Natural Science Foundation of China (82002706). These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data availability statement
Data associated with this study has been deposited into a publicly available repository. All data used in this study can be freely accessed from the SEER program (https://seer.cancer.gov/).
CRediT authorship contribution statement
Yuzhong Ye: Writing – original draft, Visualization, Software, Project administration, Formal analysis, Data curation. Yongqiang Zheng: Writing – original draft, Visualization, Software, Formal analysis, Data curation. Junteng Li: Visualization, Formal analysis, Data curation. Qi Miao: Formal analysis. Mei Lin: Writing – original draft. Jiawei Chen: Writing – original draft. Hailong Ruan: Writing – review & editing, Writing – original draft. Xiaoping Zhang: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the efforts of the National Cancer Institute and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the public SEER database. We would like to thank Editage (https://www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22408.
Contributor Information
Hailong Ruan, Email: hlruan2010@126.com.
Xiaoping Zhang, Email: xzhang@hust.edu.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Siegel R.L., et al. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 2.Rouprêt M., et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 2021;79(1):62–79. doi: 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Margulis V., et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115(6):1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 4.Korn S.M., et al. Role of lasers in urology. Photochem. Photobiol. Sci. 2019;18(2):295–303. doi: 10.1039/c8pp00409a. [DOI] [PubMed] [Google Scholar]
- 5.Wen J., Ji Z.G., Li H.Z. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: a retrospective single center study. BMC Cancer. 2018;18(1):196. doi: 10.1186/s12885-018-4118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohut R., Zhu H. Treatment of low stage urothelial carcinoma of the ureter: trends in utilization of endoscopic management. J. Urol. 2012;187(4) E256-E256. [Google Scholar]
- 7.Moran G.W., et al. Systematic review and meta-analysis on the efficacy of chemotherapy with transurethral resection of bladder tumors as definitive therapy for muscle invasive bladder cancer. Bladder Cancer. 2017;3(4):245–258. doi: 10.3233/blc-170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witjes J.A., et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur. Urol. 2021;79(1):82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Foerster B., et al. Pretreatment risk stratification for endoscopic kidney-sparing surgery in upper tract urothelial carcinoma: an international collaborative study. Eur. Urol. 2021;80(4):507–515. doi: 10.1016/j.eururo.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Xylinas E., et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013;112(4):453–461. doi: 10.1111/j.1464-410X.2012.11649.x. [DOI] [PubMed] [Google Scholar]
- 11.Weight C.J., et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J. Urol. 2010;183(4):1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Huang W.C., et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–740. doi: 10.1016/s1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bin X., et al. Impact of tumour location and surgical approach on recurrence-free and cancer-specific survival analysis in patients with ureteric tumours. BJU Int. 2012;110(11 Pt B):E514–E519. doi: 10.1111/j.1464-410X.2012.11199.x. [DOI] [PubMed] [Google Scholar]
- 14.Grasso M., et al. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012;110(11):1618–1626. doi: 10.1111/j.1464-410X.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman A., et al. Oncologic results of nephron sparing endoscopic approach for upper tract low grade transitional cell carcinoma in comparison to nephroureterectomy - a case control study. BMC Urol. 2014;14:97. doi: 10.1186/1471-2490-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutress M.L., et al. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J. Urol. 2013;189(6):2054–2060. doi: 10.1016/j.juro.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Fajkovic H., et al. Results and outcomes after endoscopic treatment of upper urinary tract carcinoma: the Austrian experience. World J. Urol. 2013;31(1):37–44. doi: 10.1007/s00345-012-0948-4. [DOI] [PubMed] [Google Scholar]
- 18.Gadzinski A.J., et al. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J. Urol. 2010;183(6):2148–2153. doi: 10.1016/j.juro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and end results (SEER) program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 8 Registries, Nov 2021 Sub (1975-2020) - Linked to County Attributes - Time Dependent (1990-2020) Income/Rurality, 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
- 20.Mathew G., Agha R. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 21.Gschwend J.E., Dahm P., Fair W.R. Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur. Urol. 2002;41(4):440–448. doi: 10.1016/s0302-2838(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 22.Seisen T., et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the eau non-muscle invasive bladder cancer guidelines panel. Eur. Urol. 2016;70(6):1052–1068. doi: 10.1016/j.eururo.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Wen J., Ji Z.G., Li H.Z. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: a retrospective single center study. BMC Cancer. 2018:196. doi: 10.1186/s12885-018-4118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netsch C., et al. Current use of thulium lasers in endourology and future perspectives. Arch. Esp. Urol. 2020;73(8):682–688. [PubMed] [Google Scholar]
- 25.Cutress M.L., et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. 2012;110(5):614–628. doi: 10.1111/j.1464-410X.2012.11068.x. [DOI] [PubMed] [Google Scholar]
- 26.Kleinmann N., et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21(6):776–785. doi: 10.1016/s1470-2045(20)30147-9. [DOI] [PubMed] [Google Scholar]
- 27.Shvero A., et al. Endoscopic treatment for large multifocal upper tract urothelial carcinoma. J. Urol. 2021;205(4):1039–1046. doi: 10.1097/ju.0000000000001505. [DOI] [PubMed] [Google Scholar]
- 28.Zou L., et al. Ureteroscopic cryoablation for patients with upper tract urothelial carcinoma of a solitary kidney: a porcine model and our pilot clinical experience. Ann. Surg Oncol. 2021;28(13):9201–9208. doi: 10.1245/s10434-021-10233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subiela J.D., et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020;46(11):1989–1997. doi: 10.1016/j.ejso.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Raymundo E.M., et al. Third prize: the role of endoscopic nephron-sparing surgery in the management of upper tract urothelial carcinoma. J. Endourol. 2011;25(3):377–384. doi: 10.1089/end.2010.0276. [DOI] [PubMed] [Google Scholar]
- 31.Palou J., et al. Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: recurrence and long-term followup. J. Urol. 2004;172(1):66–69. doi: 10.1097/01.ju.0000132128.79974.db. [DOI] [PubMed] [Google Scholar]
- 32.Rouprêt M., et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur. Urol. 2018;73(1):111–122. doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 33.Babjuk M., et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ) Eur. Urol. 2021;81(1):75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited into a publicly available repository. All data used in this study can be freely accessed from the SEER program (https://seer.cancer.gov/).