Abstract
Insulin resistance, oxidative stress, hyperlipidemia, and inflammation play main roles in the development of nonalcoholic fatty liver disease (NAFLD). Some studies have reported that hesperidin can reduce hyperglycemia and hyperlipidemia by inhibiting inflammatory pathways. In the current study, our purpose was to evaluate whether it can influence the primary parameters in NAFLD and improve the treatment effectiveness for future trials. Various studies have found that hesperidin involves multiple signaling pathways such as cell proliferation, lipid and glucose metabolism, insulin resistance, oxidative stress, and inflammation, which can potentially affect NAFLD development and prognosis. Recent findings indicate that hesperidin also regulates key enzymes and may affect the severity of liver fibrosis. Hesperidin inhibits reactive oxygen species production that potentially interferes with the activation of transcription factors like nuclear factor‐κB. Appropriate adherence to hesperidin may be a promising approach to modulate inflammatory pathways, metabolic indices, hepatic steatosis, and liver injury.
Keywords: fatty liver, hesperidin, inflammation, nonalcoholic, obesity
Various studies have found that hesperidin involves multiple signaling pathways such as cell proliferation, lipid and glucose metabolism, insulin resistance, oxidative stress, and inflammation, which can potentially affect NAFLD development and prognosis. Recent findings indicate that hesperidin also regulates key enzymes and may affect the severity of liver fibrosis. Hesperidin inhibits reactive oxygen species production that potentially interferes with the activation of transcription factors like nuclear factor‐κB.
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has reached epidemic proportions globally. It is estimated that more than 14%–24% of the general adult population worldwide has NAFLD, and of these, more than 80% have clinical morbid obesity with NAFLD (Browning et al., 2004; Youssef & McCullough, 2002). NAFLD is the consequence of a prolonged disorder caused by the agglomeration of triglycerides within the hepatocytes, which is characterized by hepatic steatosis (fat accumulation in the liver) without significant evidence of inflammation. Nonalcoholic steatohepatitis (NASH) occurs when it develops to the next stage, hepatic steatosis, with the initiation of an inflammatory process, eventually leading to liver damage, fibrosis, and cirrhosis (Clark & Diehl, 2003; Sporea et al., 2018). NAFLD is regarded as a multifactorial condition that can be affected by lifestyle, cultural, environmental, genetic, sedentary lifestyle, hypercaloric diets, and physiological and metabolic factors. Interestingly, the consumption of Western diets, especially high consumption of simple sugars and saturated fats, increases the prevalence of obesity and its associated problems, such as hyperlipemia, insulin resistance, and NAFLD among others (Arab et al., 2018; Behrouz & Yari, 2022; Jalilvand et al., 2020; Karimzadeh et al., 2022). Scientific attention is focused on the implementation of revolutionary and helpful solutions for the management of this disease and its comorbidities (Bateni et al., 2022; Jafari & Behrouz, 2023).
Hesperidin is a glycosidic flavanone present generally in citrus fruits like sweet oranges and lemons. It, as a citrus flavonoid, has biological and pharmacological attributes including anticarcinogenic, anti‐inflammatory, antioxidative, vascular protective, and lipid‐decreasing activities (Parhiz et al., 2015). It seems that hesperidin can enhance hypercholesterolemia and fatty liver by persuading fatty liver (steatosis) degeneration, inhibiting cholesterol synthesis and absorption, and moderating mRNA expression of lipid metabolism‐related enzymes (Wang et al., 2011). On the other hand, studies showed that hesperidin was able to ameliorate insulin resistance by modulating oxidative stress signaling pathways (Tian et al., 2021). Actually, hesperidin can perform beneficial and therapeutic impacts on the fatty liver due to several mechanisms including decreasing serum glucose concentration, hepatic lipid levels, fatty acid oxidation, and hepatic steatosis induced by inflammation (Jung et al., 2006). Moreover, evidence has suggested its role in inhibiting tumor cell metastasis, and improving metabolic components, and diabetes complications.
Notwithstanding the considerable interest in phytochemicals, to the best of our knowledge, the effects of hesperidin and probable mechanisms of action in NAFLD have not been discussed in the review literature. For this review, a literature search of Scopus, PubMed, Web of Science, and EMBASE was completed up to January 22, 2023, by the following terms: “NAFLD,” “NASH,” “nonalcoholic fatty liver disease,” “nonalcoholic steatohepatitis,” “lipid profile,” “hypercholesterolemic,” “hypertension,” “glucose metabolism,” “insulin resistance,” “diabetes,” “hyperlipidemia,” “body composition,” “inflammation,” “inflammatory factors,” combined with “hesperidin.” The present review highlights the ongoing development to identify the targets of hesperidin in liver diseases and the possible pathways influenced by hesperidin in NAFLD by reviewing all in vitro studies, human studies, and animal models relevant to the purpose of the present review.
2. HESPERIDIN
Hesperidin, as a derivative of dihydro flavonoids, is widely present in legumes, birch, oleander, Rutaceae, and citrus plants (especially juice) such as lemon, orange, lime, and grapefruit. There may also be Lamiaceae (mint family), honeybush (Cyclopia maculata), and aromatized tea (Hajialyani et al., 2019). Hesperidin (3,5,7 trihydroxy flavanone 7‐rhamnoglucoside, C28H34O15), also known as hesperetin 7‐rutinoside or 7‐O‐glycoside hesperitin, a flavonoid glycoside that was first extracted from citrus peel by the French chemist Lebreton (lemon peel, etc.; Roohbakhsh et al., 2015). The hesperidin‐chitooligosaccharide complex, which is obtained from the reaction of hesperidin with chitooligosaccharide, increases the solubility in water, which improves its antioxidant activity (Xiong et al., 2019).
Hesperidin has anti‐inflammatory features, but it also has low water solubility and, like many other flavonoids, is poorly absorbed in the small intestine. In general, the bioavailability of hesperidin is limited because of its low solubility in water and its being affected by the intestinal microbiota. However, the amount of hesperidin is higher in the layer of the peel and albedo (a white soft middle layer part) and seeds of citrus fruits (Aruoma et al., 2012; Chen, Wang, et al., 2020).
The biochemical properties of hesperidin remain for 2 years if stored at −20°C. Oral or topical administration or injection of hesperidin derivatives in the mice model did not show any side effects (Pyrzynska, 2022). The potential benefits of hesperidin in improving type 2 diabetes, cardiovascular disease, hypertension, glucose metabolism, lipid profile, cancer, and neurodegenerative disorders have been extensively investigated throughout recent decades (Man et al., 2019).
3. HESPERIDIN AND LIPID PROFILE
Investigating the effects of hesperidin on NAFLD is not possible without considering the effects of this compound on lipid profile, glucose homeostasis, body composition, and inflammation (Table 1). The possible hypolipidemic effects of hesperidin against hypercholesterolemia were investigated in some studies (Li et al., 2022; Monforte et al., 1995; Wang et al., 2011). One study examined the effects of consuming hesperidin on indices of lipid parameters in subjects with type 2 diabetes. The results have shown that hesperidin supplementation decreased the plasma level of total cholesterol (TC), although no alterations were observed in triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C; Eghtesadi et al., 2016). Considering that the liver stashes lipids in the form of triglycerides, liver fat aggregation is correlated with increased lipotoxicity due to high levels of free fatty acids, cholesterol, and other lipid metabolites in NAFLD (Schweitzer & Finck, 2014). In a rodent model study, hesperidin decreased hepatic steatosis, adipose tissue, serum TC, and retinol‐binding protein (RBP)‐4 density in rats fed a high‐fat diet, although the reduction in LDL‐C and TG concentration was not significant after hesperidin administration. In addition, hesperidin may ameliorate hypercholesterolemia and fatty liver by downregulating mRNA expression of RBP, heart fatty acid–binding protein (H‐FABP), and cutaneous fatty acid–binding protein (C‐FABP; Wang et al., 2011). Similarly, another study has shown that lipid‐related factors such as H‐FABP and C‐FABP which play key roles in fatty acid metabolism were improved by hesperidin supplementation (Qian et al., 2016). The results of another study conducted on rats with a high‐cholesterol diet showed that diosmin‐hesperidin significantly improved HDL‐C levels, but did not alter other lipid parameters (Yasım et al., 2011).
TABLE 1.
Randomized controlled trials that evaluated the effects of hesperidin on metabolic parameters, liver function, and inflammation.
| References | Condition (n) | Treatment | Duration | Main results |
|---|---|---|---|---|
| Eghtesadi et al. (2016) | T2DM (45) |
|
8 weeks |
|
|
Cheraghpour et al. (2019) |
NAFLD (50) |
|
12 weeks |
|
| Haidari et al. (2015) | Myocardial infarction (75) |
|
4 weeks |
|
| Demonty et al. (2010) | Patients with hypercholesterolemia (204) |
|
4 weeks |
|
| Ribeiro et al. (2017) | Patients with obesity (78) |
|
12 weeks |
|
| Lima et al. (2019) | Healthy individuals (10) |
|
8 weeks |
|
| Homayouni et al. (2017) | T2DM (64) |
|
6 weeks |
|
| Morand et al. (2011) | Healthy people with overweight (24) |
|
4 weeks |
|
| Constans et al. (2015) | Subjects with cardiovascular risk factors (25) |
|
4 weeks |
|
| Yari et al. (2021) | NAFLD (100) |
|
12 weeks |
|
| Hanawa et al. (2008) | Healthy subjects with moderately high BMI (75) |
|
12 weeks |
|
| Aptekmann and Cesar (2010) | Middle‐aged women with overweight (26) |
|
12 weeks |
|
| Yari et al. (2020) | Metabolic syndrome (49) |
|
12 weeks |
|
| Homayouni et al. (2018) | T2DM (64) |
|
6 weeks |
|
Abbreviations: 8‐OHDG, 8‐hydroxydeoxyguanosine; ALT, alanine aminotransferase; ApoA‐I, Apolipoprotein A‐I; apoB, Apolipoprotein B; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; FFM, fat‐free mass; FM, fat mass; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment for insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; ICAM‐1, intercellular adhesion molecule 1; IL‐6: interleukin‐6; LDL, low‐density lipoprotein; LMP, lifestyle modification program; MDA, malondialdehyde; NAFLD, nonalcoholic fatty liver disease; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TAC, total antioxidant capacity; TC, total cholesterol; TG, triglyceride; VCAM‐1, vascular cell adhesion molecule 1.
Moreover, hesperidin can inhibit the expression of genes involved in all phases of adipogenesis including peroxisome proliferator‐activated receptor (PPAR)‐γ, perilipin, sterol regulatory element‐binding protein 1 (SREBP1), fatty acid synthase, and stearoyl‐CoA desaturase (SCD; Chambers et al., 2019; Gómez‐Zorita et al., 2017). Hesperidin also significantly downregulated the expression of other genes that participated in fat metabolism such as fatty‐acid desaturase (FAT‐6 and FAT‐7), acetyl‐CoA carboxylase‐2, and SCD (Peng et al., 2016). Results of an animal study showed that hesperidin enhanced blood lipid profile, reduced hepatic lipid repletion, and ameliorated NASH in mice fed a Western diet (Mosqueda‐Solís, Sánchez, Reynés, et al., 2018).
Additionally, a clinical trial study was conducted to examine the impacts of hesperidin on NAFLD parameters, in which 1 g of hesperidin was administered to subjects with NAFLD for 3 months. The findings demonstrated a considerable reduction in serum concentration of TG, TC, and LDL‐C in the group consuming hesperidin, although only the TG and TC reduction in serum was considerably higher in the hesperidin group compared to the placebo (Cheraghpour et al., 2019). On the other hand, Haidari et al. (2015) reported that consuming 600 mg of hesperidin per day for 4 weeks significantly enhanced HDL‐C in patients with myocardial infarction, although this study did not observe a meaningful improvement in other lipid profiles. These results are incompatible with Demonty et al. (2010) study, in which consuming 800 mg hesperidin in the shape of supplementation for 4 weeks did not affect serum HDL‐C and TG concentrations in individuals with moderate hypercholesterolemia. Moreover, A meta‐analysis investigating the effects of hesperidin supplementation on lipid profile reported that hesperidin might not affect enhancing lipid profile parameters (Mohammadi et al., 2019).
4. HESPERIDIN AND GLUCOSE METABOLISM
Disturbances of glucose metabolism beyond a certain limit can cause NAFLD, so there is a close relationship between glucose homeostasis, fat metabolism in the liver, and NAFLD. Hesperidin can improve glucose and insulin metabolism. A randomized clinical trial study has demonstrated that consumption of hesperidin capsules (500 mg/day) for 12 weeks reduced fasting glucose levels, compared to both baseline values and the control group in patients with metabolic syndrome. Moreover, hesperidin significantly increased insulin sensitivity by assessing the homeostatic model assessment for insulin resistance (HOMA‐IR; Mohammadi et al., 2019). In a study conducted by Ribeiro et al. (2017), 500 mL orange juice consumption for 12 weeks could significantly decrease insulin levels by 18% and HOMA‐IR index by 33% in individuals with obesity. Similarly, in another study, the consumption of 300 mL of orange juice for 2 months in healthy women had a significant reduction in fasting plasma glucose and insulin levels, as well as HOMA‐IR index (Lima et al., 2019). However, the evidence provided by many clinical trial studies in various populations has shown that hesperidin did not significantly change glucose or insulin levels (Constans et al., 2015; Homayouni et al., 2017; Morand et al., 2011). This study was consistent with another study by Cheraghpour et al. (2019), who reported that 3 months of supplementation with 1‐g hesperidin significantly improved blood glucose levels compared to baseline values, although there was no significant difference between intervention and placebo groups. Another study observed that 600 mg hesperidin for 6 weeks could not affect blood glucose and insulin resistance in subjects with type 2 diabetes mellitus (Haidari et al., 2015).
Blood glucose‐lowering effects of hesperidin may be achieved by modulating the main regulatory enzymes of glucose metabolism. Hesperidin downregulated the gene expression of glucose‐6‐phosphatase, glucokinase, alpha‐ketoglutarate, and oxaloacetate and inhibited pyruvate production and hepatic gluconeogenesis, thereby affecting hyperglycemia (Akiyama et al., 2009; Jung et al., 2006). Also, in the liver of rats, hesperidin stimulates glycogenolysis and glycolysis (do Nascimento et al., 2018). Moreover, the hypoglycemic effects of hesperidin have been shown in rat models of diabetes (Franke et al., 2018). Several studies have demonstrated that hesperidin might potentially be used to regulate postprandial blood glucose by reducing the intestinal glucose transporter (Kerimi et al., 2019; Shen et al., 2012).
PPAR‐c, a nuclear receptor, regulates metabolic pathways by regulating energy homeostasis, modulating glucose and fatty acid metabolism, stimulating insulin secretion, and promoting insulin sensitivity. It is worth noting that hesperidin improves glucose metabolism by modulating PPAR‐c activation and reducing the accumulation of body and hepatic fat (Shin et al., 2013). In addition, hesperidin indirectly affects insulin resistance by modulating the composition and functionality of intestinal microbiota to induce the generation of short‐chain fatty acids, thus modulating the metabolism of lipids and inflammation, and ameliorating glucose intolerance and insulin sensitivity (Lima et al., 2019). Growing evidence suggests that hesperidin ameliorates oxidative damage and mitochondrial dysfunction, ultimately reducing insulin resistance in cells stimulated with high glucose. These changes seem to be associated with upregulated phosphorylation of AKT and glycogen synthase kinase‐3β and decreased insulin receptor substrate‐1 (IRS1; Tian et al., 2021).
Treatment of HepG2 cells with naringin and neohesperidin increased glucose uptake, which was accompanied by induced phosphorylation levels of adenosine monophosphate‐activated protein kinase (AMPK; Zhang et al., 2012). In addition, hesperidin modulates insulin resistance by regulating IRS1 and glucose transporter‐2 pathways through the downregulation of toll‐like receptor‐4 and nuclear factor‐κB (NF‐κB) expression in HepG2 cells (Xuguang et al., 2019). A study illustrated that the hypoglycemic activity and antioxidant capacity of hesperidin are due to the inhibition of oxidative stress and the formation of advanced glycation end‐products which play a critical role in the development of diabetes (Dhanya & Jayamurthy, 2020).
Overall, it seems that hesperidin can be effective in reducing insulin resistance and improving glucose metabolism, thereby improving metabolic complications in patients with NAFLD.
5. HESPERIDIN AND LIVER ENZYMES
Oxidative stress markers and mediators in the liver like lipid peroxidation products and liver enzymes have been proposed as indicators of NAFLD. Limited studies have investigated the impacts of hesperidin on subjects affected with NAFLD. It appears that hesperidin ameliorates the progress of NAFLD due to its impacts on the improvement of lipid peroxidation, metabolism of glucose, oxidative stress, and inflammatory pathways.
Hesperidin significantly increased the levels of glutathione peroxidase and superoxide dismutase and reduced the oxidative state in rodents fed a high‐fat diet, which may ameliorate NAFLD (Yasım et al., 2011). Despite the positive results at the preclinical level, the effects of hesperidin consumption on liver enzyme concentration in humans are not certain. The findings of a clinical trial study indicated that 12 weeks of supplementation with 1‐g hesperidin reduced significantly alanine aminotransferase and γ‐glutamyl transferase. Moreover, the results of the FibroScan showed that hepatic steatosis was meaningfully improved in the hesperidin group in comparison with the placebo group (Cheraghpour et al., 2019). Other studies have illustrated that hesperidin supplementation significantly decreased the fatty liver score, serum lipid profiles, liver injury, and hepatic steatosis (Chen et al., 2022; Cheraghpour et al., 2019). Similar to this study, Yari et al. (2021) showed that the combination of hesperidin with flaxseed caused a significant reduction in plasma levels of alanine aminotransferase, and indices of glucose metabolism and improved the grade of hepatic fibrosis.
In experimental animal research carried out by Wang et al. (2020), neohesperidin could significantly reduce hepatic steatosis and systematic insulin resistance in rodents fed a high‐fat diet. Moreover, they showed a significant enhancement in hepatocellular mitochondrial function and fatty acid oxidation with induced expression of peroxisome proliferator‐activated receptor γ coactivator‐1 (PGC‐1α). Although most of the studies focus on modification of the metabolic indicators related to fatty liver, it seems that in the limited studies conducted on the effects of hesperidin in fatty liver, it was able to reduce the process of fibrosis and hepatic steatosis.
6. HESPERIDIN AND INFLAMMATION
It has long been known that inflammation is regarded as one of the critical determinants contributing to the progression and pathogenesis of liver disorders. NAFLD has been identified as a low‐grade and chronic inflammation in the hepatocytes that drives systemic impacts, recognized by systemic changes in humoral factors and circulating immune cell subsets (Behrouz et al., 2021; Gehrke & Schattenberg, 2020). During NAFLD, mitochondria and β‐oxidation dysfunction decrease the hepatic PPARα activity, increase liver triglycerides and free fatty acids, suggest lipotoxicity and oxidative stress, and ultimately result in hepatic inflammation, hepatocellular apoptosis, and fibrosis (Friedman et al., 2018; Ipsen et al., 2018; Spahis et al., 2017; Takaki et al., 2013).
Excessive production of reactive species, which include reactive sulfur species, reactive nitrogen species, and reactive oxygen species (ROS), subsequently results in the secretion of various serum markers of inflammation including C‐reactive protein (CRP), interleukins (ILs), tumor necrosis factor (TNF), and other general immunity factors (Gonzalez et al., 2020; Luo & Lin, 2021). The transcription factor activation of NF‐κB signaling cascade has a key function in the immune and inflammatory responses of the body through the expression of several mediators such as inducible nitric oxide synthase, TNF‐α, cyclooxygenase‐2 (COX‐2), and IL‐6 in subjects affected with NAFLD (Chen, Xing, et al., 2020). In fact, upregulation of the NF‐κB cascade is intimately associated with the stimulation of mitogen‐activated protein kinases (MAPKs), involved in intracellular signaling cascades during pro‐inflammatory immune conditions (Parhiz et al., 2015).
A growing body of evidence has been carried out to investigate the impacts of hesperidin, its synthetic by‐products, or its metabolites on inflammatory reactions in some inflammatory‐based disorders. Experimental studies have demonstrated significant advantageous effects and mechanistic insight into hesperidin (Kumar et al., 2023). However, human studies investigating the impacts of hesperidin in subjects affected with NAFLD are still limited. A 12‐week administration of 1 g of hesperidin combined with lifestyle correction could improve NAFLD‐related risk indicators by preventing NF‐κB activation and reducing hs‐CRP and TNF‐α, compared to lifestyle correction alone in patients with NAFLD. It should be noted that the evaluation of NF‐κB p65 activity was done in peripheral blood mononuclear cells, which is one of the strengths of this study (Cheraghpour et al., 2019). Taking other metabolic disorders into account, it has been observed that taking 500 mg of hesperidin decreased circulating inflammatory molecules in individuals affected with metabolic syndrome via suppressing ORS generation (Ghanim et al., 2010). Homayouni et al. (2017) revealed that 500 mg/day hesperidin improved several inflammatory molecules such as circulating IL‐6, TNF‐α, and hs‐CRP after 6 weeks of supplementation compared to the control group in individuals affected with type 2 diabetes.
In contrast, another research found that circulating IL‐6 and hs‐CRP are not improved in subjects affected with diabetes after consuming 500 mg/day of hesperidin for 2 months. This could be due to the short duration of the intervention of 2 months (Eghtesadi et al., 2016). These findings are noteworthy because insulin resistance has a main function in the pathogenesis of NAFLD. Also, it is worth mentioning that 7‐day supplementation with 500 mL red orange juice, one of the main sources of hesperidin, had considerable anti‐inflammatory effects that led to decreasing hs‐CRP, TNF‐α, and IL‐6 concentrations in subjects with increased cardiovascular risk (Buscemi et al., 2012). However, a before‐after clinical study showed no significant alterations in circulating concentrations of CRP and IL‐6 after 4‐week supplementation with orange juice or hesperidin in healthy volunteers, which could be explained by the almost normal inflammatory levels in the healthy population (Morand et al., 2011).
In NAFLD rodent models, 12‐week supplementation with hesperidin was related to a meaningful reduction in some inflammatory molecules such as TNF‐α, IL‐6, and IL‐1β. It is believed that the suppression of endoplasmic reticulum stress‐related biomarkers in the liver immune cells prevents the activation of inflammatory pathways including NF‐κB, and consequently NAFLD development (Xie et al., 2022). Hesperidin could attenuate inflammatory and oxidative damage induced by hyperglycemia by reducing malondialdehyde, nitric oxide, and IL‐6 concentrations and improving adiponectin expression and glutathione levels in rodent models of diabetes (Mahmoud, 2013; Mahmoud et al., 2012). It can also alter oxidative damage in hepatocytes by affecting factors associated with hepatic fatty acid oxidation (Constantin et al., 2013). Moreover, hesperidin has been reported to decrease hs‐CRP, macrophage chemoattractant protein 1 (MCP‐1), and IL‐6 and increase the serum total antioxidant capacity, thereby preventing oxidative stress and inflammation caused by hyperlipidemia and hyperglycemia in rodents fed a high‐fat diet (Ferreira et al., 2016).
Considering that the majority of studies report positive effects of hesperidin on inflammation, there is still insufficient data to demonstrate the exact mechanisms of action. Nonetheless, it seems that hesperidin, as an antioxidant, inhibits ROS production that potentially interferes with the activation of transcription factors like NF‐κB and nuclear translocation by blocking the phosphorylation of NF‐κβ inhibitor (IκB). Additionally, hesperidin inhibits the phosphorylation of MAPKs, extracellular signal‐regulated kinase (ERK), and a lesser degree C‐jun N‐terminal kinase (JNK), which are important signaling pathways in the inflammatory processes. Other potential mechanism includes the upregulation of PPARγ, a nuclear transcription factor involved in inhibiting NF‐κB activation and the production of different inflammatory agents, chemokines, and adhesion molecules (Cheraghpour et al., 2019; Tejada et al., 2018). Cyclooxygenase‐1 and cyclooxygenase‐2 are the main enzymes for the production of pro‐inflammatory prostaglandins from arachidonic acid, which in turn contribute to inflammatory responses. Studies highlighted that hesperidin can suppress cyclooxygenase‐2 gene expression and block the generation of prostaglandins leading to the inhibition of inflammatory markers (Hirata et al., 2005; Lawrence, 2009; Vabeiryureilai et al., 2015).
7. HESPERIDIN AND BODY COMPOSITION
The major objective of NAFLD control is to achieve at least a 5% reduction in body weight (Chalasani et al., 2012). Multiple lines of evidence report that hesperidin has anti‐obesity activity. It can induce the secretion of cholecystokinin, an anorexigenic gut hormone, in enteroendocrine (Kim et al., 2013). It has been suggested that dietary bioflavonoid hesperidin may induce its anti‐obesity effects partially via the prevention of hepatic lipogenesis (Ohara et al., 2015). Citrus flavonoids like hesperidin have been found to induce the browning of white adipocytes, promote energy balance and thermogenesis, and reduce plasma lipids concentrations and obesity through an AMPK‐mediated pathway (Mosqueda‐Solís, Sánchez, Portillo, et al., 2018; Xiong et al., 2019; Zhang et al., 2019).
In addition, the beneficial effect of hesperidin treatment on obesity was related to increased expression of uncoupling protein 3, which in turn improves energy expenditure from lipids (Kim et al., 2019). Accumulating data demonstrated that hesperidin has positive effects on lipid accumulation and adiposity (Kim et al., 2019; Mosqueda‐Solís et al., 2017; Serino & Salazar, 2018). Several animal models of obesity or metabolic syndrome have reported a body‐weight‐reducing effect in response to hesperidin supplementation (Mayneris‐Perxachs et al., 2019; Pu, 2016; Sun et al., 2017; Wu et al., 2017), as well as a reduction in adiposity (Mayneris‐Perxachs et al., 2019; Pu, 2016; Wang et al., 2011; Wu et al., 2017). For example, in a rodent model, hesperidin supplementation decreased body weight, fat mass, and plasma lipids in rodents fed a high‐fat diet. This effect is mediated by improving intestinal barrier integrity and modulating the composition of intestinal microbiota (Liu et al., 2020). In contrast, daily hesperidin supplementation (100 mg/kg body weight) for 2 months had no meaningful alterations in the body weight of rodents fed a Western diet, although hesperidin was able to reduce adipocyte size (Mosqueda‐Solís, Sánchez, Reynés, et al., 2018).
In a clinical trial, a daily intake of 500 mg α‐glucosyl hesperidin, the soluble hesperidin derivative, reduced abdominal fat significantly in individuals with a moderately high body mass index compared to baseline after 12 weeks of supplementation. However, this reduction was not significantly different from those of subjects taking a placebo (Hanawa et al., 2008). These findings are similar to Aptekmann et al. study which reported a significant body weight reduction after daily use of orange juice for 13 weeks in individuals with hypercholesterolemia, without significant change between the intervention and control groups (Aptekmann & Cesar, 2010). Also, other human clinical trials revealed that 500 mg α‐glucosyl hesperidin did not exert anti‐obesity effects in individuals with moderate obesity (Ohara et al., 2015). This result is consistent with the Yari et al. (2021) study, in which hesperidin supplementation failed to induce weight‐loss effects. Overall, hesperidin or orange juice supplementation in subjects with overweight or obesity does not reveal the beneficial effects observed in animal models.
8. LIMITATIONS AND STRENGTHS
To the best of our knowledge, this is the first review study demonstrating the potential effects of hesperidin on NAFLD parameters. Our review also discussed the mechanisms of actions of hesperidin on several aspects of NAFLD that may be less well‐regarded. The lack of sufficient clinical data on the therapeutic effects of hesperidin is an important limitation that can be mentioned in most of the previous studies. Therefore, clinical studies, especially those focused on the appropriate dosage, bioavailability, efficacy, and safety of hesperidin and its metabolites, are warranted before extending flavonoid therapy to humans.
9. CONCLUSION
The NAFLD represents a significant burden of disease worldwide, which is alarmingly worsening each year. The important pathophysiological mechanisms underlying NAFLD are several including oxidative stress, inflammation, liver fibrosis, and apoptosis. Currently, therapeutic approaches are not ideal for managing NAFLD, thus new approaches and treatments are still needed. Hesperidin has a remarkable effect on hepatocytes and brings new hope to people with NAFLD around the world. Primary evidence has suggested a reverse association between hesperidin and NAFLD risk factors including oxidative stress, inflammation, dyslipidemia, hyperglycemia, and obesity (Figure 1). Animal studies have demonstrated that taking hesperidin could decrease NAFLD severity. Accordingly, it was expected that the use of hesperidin might improve NAFLD. Overall, human studies investigating the impacts of hesperidin on NAFLD are rare. It has been indicated that hesperidin might contribute to the improvement of NAFLD through glucose‐lowering effect, alterations in liver fat content and lipid profile, and anti‐inflammatory and antioxidant properties. Due to the limited number and heterogeneity of existing human studies, further research is required to confirm these results in human beings.
FIGURE 1.
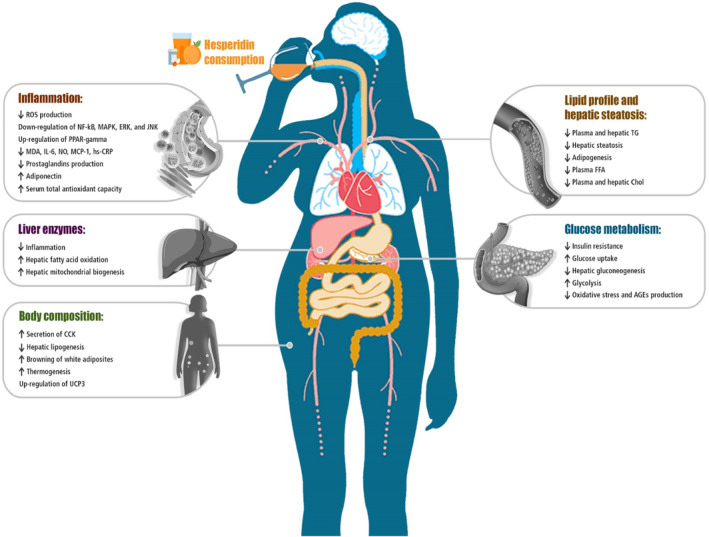
Summary of the most important effects of hesperidin consumption on proposed mechanisms of action in NAFLD. CCK, cholecystokinin; CRP, C‐reactive protein; IL‐6, interleukin‐6; MCP‐1, monocyte chemoattractant protein‐1; MDA, malondialdehyde; NO, nitric oxide; PPAR, peroxisome proliferator‐activated receptors; ROS, reactive oxygen species; TG, triglyceride; UCP3, uncoupling protein 3.
AUTHOR CONTRIBUTIONS
Nava Morshedzadeh: Conceptualization (equal); investigation (equal); methodology (equal); writing – original draft (equal). Amirhossein Ramezani: Data curation (equal); investigation (equal); writing – original draft (equal). Vahideh Behrouz: Investigation (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Elias Mir: Software (lead).
FUNDING INFORMATION
No external funding was received to support this work.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This article is the result of the research project approved by the student research committee of Kerman University of Medical Sciences under the number 402000015 which was carried out with the financial support of the research and technology vice‐chancellor of this university.
Morshedzadeh, N. , Ramezani Ahmadi, A. , Behrouz, V. , & Mir, E. (2023). A narrative review on the role of hesperidin on metabolic parameters, liver enzymes, and inflammatory markers in nonalcoholic fatty liver disease. Food Science & Nutrition, 11, 7523–7533. 10.1002/fsn3.3729
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Akiyama, S. , Katsumata, S.‐I. , Suzuki, K. , Nakaya, Y. , Ishimi, Y. , & Uehara, M. (2009). Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin‐clathrated hesperetin in Goto‐Kakizaki rats with type 2 diabetes. Bioscience, Biotechnology, and Biochemistry, 73(12), 2779–2782. [DOI] [PubMed] [Google Scholar]
- Aptekmann, N. P. , & Cesar, T. B. (2010). Orange juice improved lipid profile and blood lactate of overweight middle‐aged women subjected to aerobic training. Maturitas, 67(4), 343–347. [DOI] [PubMed] [Google Scholar]
- Arab, J. P. , Arrese, M. , & Trauner, M. (2018). Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annual Review of Pathology: Mechanisms of Disease, 13, 321–350. [DOI] [PubMed] [Google Scholar]
- Aruoma, O. I. , Landes, B. , Ramful‐Baboolall, D. , Bourdon, E. , Neergheen‐Bhujun, V. , Wagner, K.‐H. , & Bahorun, T. (2012). Functional benefits of citrus fruits in the management of diabetes. Preventive Medicine, 54, S12–S16. [DOI] [PubMed] [Google Scholar]
- Bateni, Z. , Behrouz, V. , Rahimi, H. R. , Hedayati, M. , Afsharian, S. , & Sohrab, G. (2022). Effects of nano‐curcumin supplementation on oxidative stress, systemic inflammation, adiponectin, and NF‐κB in patients with metabolic syndrome: A randomized, double‐blind clinical trial. Journal of Herbal Medicine, 31, 100531. [Google Scholar]
- Behrouz, V. , Sohrab, G. , Hedayati, M. , & Sedaghat, M. (2021). Inflammatory markers response to crocin supplementation in patients with type 2 diabetes mellitus: A randomized controlled trial. Phytotherapy Research, 35(7), 4022–4031. [DOI] [PubMed] [Google Scholar]
- Behrouz, V. , & Yari, Z. (2022). A review on differential effects of dietary fatty acids on weight, appetite and energy expenditure. Critical Reviews in Food Science and Nutrition, 62(8), 2235–2249. [DOI] [PubMed] [Google Scholar]
- Browning, J. D. , Szczepaniak, L. S. , Dobbins, R. , Nuremberg, P. , Horton, J. D. , Cohen, J. C. , Grundy, S. M. , & Hobbs, H. H. (2004). Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology, 40(6), 1387–1395. [DOI] [PubMed] [Google Scholar]
- Buscemi, S. , Rosafio, G. , Arcoleo, G. , Mattina, A. , Canino, B. , Montana, M. , Verga, S. , & Rini, G. (2012). Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. The American Journal of Clinical Nutrition, 95(5), 1089–1095. [DOI] [PubMed] [Google Scholar]
- Chalasani, N. , Younossi, Z. , Lavine, J. E. , Diehl, A. M. , Brunt, E. M. , Cusi, K. , Charlton, M. , & Sanyal, A. J. (2012). The diagnosis and management of non‐alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology, 55(6), 2005–2023. [DOI] [PubMed] [Google Scholar]
- Chambers, C. S. , Biedermann, D. , Valentová, K. , Petrásková, L. , Viktorová, J. , Kuzma, M. , & Křen, V. (2019). Preparation of retinoyl‐flavonolignan hybrids and their antioxidant properties. Antioxidants, 8(7), 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Nie, T. , Zhang, P. , Ma, J. , & Shan, A. (2022). Hesperidin attenuates hepatic lipid accumulation in mice fed high‐fat diet and oleic acid induced HepG2 via AMPK activation. Life Sciences, 296, 120428. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Xing, J. , Pan, D. , Peng, X. , & Gao, P. (2020). Chinese herbal medicine mixture 919 syrup alleviates nonalcoholic fatty liver disease in rats by inhibiting the NF‐κB pathway. Biomedicine & Pharmacotherapy, 128, 110286. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Wang, D. , Tan, C. , Hu, Y. , Sundararajan, B. , & Zhou, Z. (2020). Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants, 9(2), 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraghpour, M. , Imani, H. , Ommi, S. , Alavian, S. M. , Karimi‐Shahrbabak, E. , Hedayati, M. , Yari, Z. , & Hekmatdoost, A. (2019). Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo‐controlled, double‐blind clinical trial. Phytotherapy Research, 33(8), 2118–2125. [DOI] [PubMed] [Google Scholar]
- Clark, J. M. , & Diehl, A. M. (2003). Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA, 289(22), 3000–3004. [DOI] [PubMed] [Google Scholar]
- Constans, J. , Bennetau‐Pelissero, C. , Martin, J.‐F. , Rock, E. , Mazur, A. , Bedel, A. , Morand, C. , & Bérard, A. M. (2015). Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross‐over trial on mild hypercholesterolemic men. Clinical Nutrition, 34(6), 1093–1100. [DOI] [PubMed] [Google Scholar]
- Constantin, R. P. , Nascimento, G. S. D. , Constantin, R. P. , Salgueiro, C. L. , Bracht, A. , Ishii‐Iwamoto, E. L. , Yamamoto, N. S. , & Constantin, J. (2013). Citrus flavanones affect hepatic fatty acid oxidation in rats by acting as prooxidant agents. BioMed Research International, 2013, 342973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonty, I. , Lin, Y. , Zebregs, Y. E. , Vermeer, M. A. , van der Knaap, H. C. , Jäkel, M. , & Trautwein, E. A. (2010). The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. The Journal of Nutrition, 140(9), 1615–1620. [DOI] [PubMed] [Google Scholar]
- Dhanya, R. , & Jayamurthy, P. (2020). In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochemistry and Function, 38(4), 419–427. [DOI] [PubMed] [Google Scholar]
- do Nascimento, G. S. , Constantin, R. P. , Gilglioni, E. H. , de Castro Ghizoni, C. V. , Bracht, A. , Utsunomiya, K. S. , Yamamoto, N. S. , Ishii‐Iwamot, E. L. , Constantin, J. , & Constantin, R. P. (2018). The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver. Toxicology Letters, 291, 158–172. [DOI] [PubMed] [Google Scholar]
- Eghtesadi, S. , Mohammadi, M. , Vafa, M. , Heidari, I. , Salehi, M. , Khadem, H. H. , Amiri, F. , Alipour, R. , & Eghtesadi, M. (2016). Effects of hesperidin supplementation on glycemic control, lipid profile and inflammatory factors in patients with type 2 diabetes: a randomized, double‐blind and placebo‐controlled clinical trial . Paper presented at the Endocrine Abstracts.
- Ferreira, P. S. , Spolidorio, L. C. , Manthey, J. A. , & Cesar, T. B. (2016). Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high‐fat diet. Food & Function, 7(6), 2675–2681. [DOI] [PubMed] [Google Scholar]
- Franke, S. I. , Molz, P. , Mai, C. , Ellwanger, J. H. , Zenkner, F. F. , Horta, J. A. , & Prá, D. (2018). Influence of hesperidin and vitamin C on glycemic parameters, lipid profile, and DNA damage in rats treated with sucrose overload. Anais da Academia Brasileira de Ciências, 90, 2203–2210. [DOI] [PubMed] [Google Scholar]
- Friedman, S. L. , Neuschwander‐Tetri, B. A. , Rinella, M. , & Sanyal, A. J. (2018). Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine, 24(7), 908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, N. , & Schattenberg, J. M. (2020). Metabolic inflammation—A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology, 158(7), 1929–1947. e1926. [DOI] [PubMed] [Google Scholar]
- Ghanim, H. , Sia, C. L. , Upadhyay, M. , Korzeniewski, K. , Viswanathan, P. , Abuaysheh, S. , Mohanty, P. , & Dandona, P. (2010). Orange juice neutralizes the proinflammatory effect of a high‐fat, high‐carbohydrate meal and prevents endotoxin increase and Toll‐like receptor expression. The American Journal of Clinical Nutrition, 91(4), 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Zorita, S. , Lasa, A. , Abendaño, N. , Fernández‐Quintela, A. , Mosqueda‐Solís, A. , Garcia‐Sobreviela, M. P. , Arbonés‐Mainar, J. M. , & Portillo, M. P. (2017). Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. Journal of Translational Medicine, 15(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, A. , Huerta‐Salgado, C. , Orozco‐Aguilar, J. , Aguirre, F. , Tacchi, F. , Simon, F. , & Cabello‐Verrugio, C. (2020). Role of oxidative stress in hepatic and extrahepatic dysfunctions during nonalcoholic fatty liver disease (NAFLD). Oxidative Medicine and Cellular Longevity, 2020, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidari, F. , Heybar, H. , Jalali, M. , Ahmadi Engali, K. , Helli, B. , & Shirbeigi, E. (2015). Hesperidin supplementation modulates inflammatory responses following myocardial infarction. Journal of the American College of Nutrition, 34(3), 205–211. [DOI] [PubMed] [Google Scholar]
- Hajialyani, M. , Hosein Farzaei, M. , Echeverría, J. , Nabavi, S. M. , Uriarte, E. , & Sobarzo‐Sánchez, E. (2019). Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules, 24(3), 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa, M. , Morimoto, Y. , Yokomizo, A. , Akaogi, A. , Mafune, E. , Tsunoda, K. , Azuma, M. , Nishitani, M. , Kajimoto, Y. , & Kadowaki, T. (2008). Effect of long‐term intake of the tablet containing glucosyl hesperidin on body weight and body fat. Journal of Nutritional Food, 11, 1–17. [Google Scholar]
- Hirata, A. , Murakami, Y. , Shoji, M. , Kadoma, Y. , & Fujisawa, S. (2005). Kinetics of radical‐scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX‐2 expression. Anticancer Research, 25(5), 3367–3374. [PubMed] [Google Scholar]
- Homayouni, F. , Haidari, F. , Hedayati, M. , Zakerkish, M. , & Ahmadi, K. (2017). Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: A randomized double‐blind placebo‐controlled clinical trial. Phytotherapy Research, 31(10), 1539–1545. [DOI] [PubMed] [Google Scholar]
- Homayouni, F. , Haidari, F. , Hedayati, M. , Zakerkish, M. , & Ahmadi, K. (2018). Blood pressure lowering and anti‐inflammatory effects of hesperidin in type 2 diabetes; a randomized double‐blind controlled clinical trial. Phytotherapy Research, 32(6), 1073–1079. [DOI] [PubMed] [Google Scholar]
- Ipsen, D. H. , Lykkesfeldt, J. , & Tveden‐Nyborg, P. (2018). Molecular mechanisms of hepatic lipid accumulation in non‐alcoholic fatty liver disease. Cellular and Molecular Life Sciences, 75(18), 3313–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari, R. S. , & Behrouz, V. (2023). Nordic diet and its benefits in neurological function: A systematic review of observational and intervention studies. Frontiers in Nutrition, 10, 1215358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilvand, A. , Behrouz, V. , Nikpayam, O. , Sohrab, G. , & Hekmatdoost, A. (2020). Effects of low fructose diet on glycemic control, lipid profile and systemic inflammation in patients with type 2 diabetes: A single‐blind randomized controlled trial. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 14(5), 849–855. [DOI] [PubMed] [Google Scholar]
- Jung, U. J. , Lee, M.‐K. , Park, Y. B. , Kang, M. A. , & Choi, M.‐S. (2006). Effect of citrus flavonoids on lipid metabolism and glucose‐regulating enzyme mRNA levels in type‐2 diabetic mice. The International Journal of Biochemistry & Cell Biology, 38(7), 1134–1145. [DOI] [PubMed] [Google Scholar]
- Karimzadeh, L. , Behrouz, V. , Sohrab, G. , Hedayati, M. , & Emami, G. (2022). A randomized clinical trial of beetroot juice consumption on inflammatory markers and oxidative stress in patients with type 2 diabetes. Journal of Food Science, 87(12), 5430–5441. [DOI] [PubMed] [Google Scholar]
- Kerimi, A. , Gauer, J. S. , Crabbe, S. , Cheah, J. W. , Lau, J. , Walsh, R. , Cancalon, P. F. , & Williamson, G. (2019). Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. British Journal of Nutrition, 121(7), 782–792. [DOI] [PubMed] [Google Scholar]
- Kim, H. Y. , Park, M. , Kim, K. , Lee, Y. M. , & Rhyu, M. R. (2013). Hesperetin stimulates cholecystokinin secretion in enteroendocrine STC‐1 cells. Biomolecules & Therapeutics, 21(2), 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. K. , Jeong, H. W. , Kim, A. Y. , Hong, Y. D. , Lee, J. H. , Choi, J. K. , & Hwang, J. S. (2019). Green satsuma mandarin orange (Citrus unshiu) extract reduces adiposity and induces uncoupling protein expression in skeletal muscle of obese mice. Food Science and Biotechnology, 28(3), 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Khan, M. I. , Ashfaq, F. , Alsayegh, A. A. , Khatoon, F. , Altamimi, T. N. , & Rizvi, S. I. (2023). Hesperidin supplementation improves altered PON‐1, LDL oxidation, inflammatory response and hepatic function in an experimental rat model of hyperlipidemia. Indian Journal of Clinical Biochemistry, 1–7. 10.1007/s12291-023-01140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, T. (2009). The nuclear factor NF‐κB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1(6), a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Yao, Y. , Wang, Y. , Hua, L. , Wu, M. , Chen, F. , Deng, Z. Y. , & Luo, T. (2022). Effect of hesperidin supplementation on liver metabolomics and gut microbiota in a high‐fat diet‐induced NAFLD mice model. Journal of Agricultural and Food Chemistry, 70(36), 11224–11235. [DOI] [PubMed] [Google Scholar]
- Lima, A. C. D. , Cecatti, C. , Fidélix, M. P. , Adorno, M. A. T. , Sakamoto, I. K. , Cesar, T. B. , & Sivieri, K. (2019). Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: Controlled clinical trials. Journal of Medicinal Food, 22(2), 202–210. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Liu, T. , Lei, C. , Song, W. , Fang, R. , Chen, H. , Huang, Q. , Li, C. , Li, X. , Liang, X. , Huang, Q. , Ti, H. , Sun, N. , … Ma, Y. (2020). Novel role of hesperidin improve obesity in HFD mice by modulating the composition of the gut microbiota. 10.21203/rs.2.21089/v1 [DOI]
- Luo, Y. , & Lin, H. (2021). Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immunity, Inflammation and Disease, 9(1), 59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, A. M. (2013). Hematological alterations in diabetic rats‐role of adipocytokines and effect of citrus flavonoids. EXCLI Journal, 12, 647–657. [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, A. M. , Ashour, M. B. , Abdel‐Moneim, A. , & Ahmed, O. M. (2012). Hesperidin and naringin attenuate hyperglycemia‐mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin‐induced type 2 diabetic rats. Journal of Diabetes and its Complications, 26(6), 483–490. [DOI] [PubMed] [Google Scholar]
- Man, M.‐Q. , Yang, B. , & Elias, P. M. (2019). Benefits of hesperidin for cutaneous functions. Evidence‐Based Complementary and Alternative Medicine, 2019, 2676307. 10.1155/2019/2676307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayneris‐Perxachs, J. , Alcaide‐Hidalgo, J. M. , de la Hera, E. , del Bas, J. M. , Arola, L. , & Caimari, A. (2019). Supplementation with biscuits enriched with hesperidin and naringenin is associated with an improvement of the metabolic syndrome induced by a cafeteria diet in rats. Journal of Functional Foods, 61, 103504. [Google Scholar]
- Mohammadi, M. , Ramezani‐Jolfaie, N. , Lorzadeh, E. , Khoshbakht, Y. , & Salehi‐Abargouei, A. (2019). Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta‐analysis of randomized controlled clinical trials. Phytotherapy Research, 33(3), 534–545. [DOI] [PubMed] [Google Scholar]
- Monforte, M. , Trovato, A. , Kirjavainen, S. , Forestieri, A. , Galati, E. , & Lo Curto, R. B. (1995). Biological effects of hesperidin, a citrus flavonoid.(note II): Hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco (Società Chimica Italiana: 1989), 50(9), 595–599. [PubMed] [Google Scholar]
- Morand, C. , Dubray, C. , Milenkovic, D. , Lioger, D. , Martin, J. F. , Scalbert, A. , & Mazur, A. (2011). Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. The American Journal of Clinical Nutrition, 93(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Mosqueda‐Solís, A. , Lasa, A. , Gómez‐Zorita, S. , Eseberri, I. , Picó, C. , & Portillo, M. P. (2017). Screening of potential anti‐adipogenic effects of phenolic compounds showing different chemical structure in 3T3‐L1 preadipocytes. Food & Function, 8(10), 3576–3586. [DOI] [PubMed] [Google Scholar]
- Mosqueda‐Solís, A. , Sánchez, J. , Portillo, M. P. , Palou, A. , & Picó, C. (2018). Combination of capsaicin and hesperidin reduces the effectiveness of each compound to decrease the adipocyte size and to induce browning features in adipose tissue of western diet fed rats. Journal of Agricultural and Food Chemistry, 66(37), 9679–9689. [DOI] [PubMed] [Google Scholar]
- Mosqueda‐Solís, A. , Sánchez, J. , Reynés, B. , Palou, M. , Portillo, M. P. , Palou, A. , & Picó, C. (2018). Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome‐related alterations in western diet‐fed rats. Scientific Reports, 8(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara, T. , Muroyama, K. , Yamamoto, Y. , & Murosaki, S. (2015). A combination of glucosyl hesperidin and caffeine exhibits an anti‐obesity effect by inhibition of hepatic lipogenesis in mice. Phytotherapy Research, 29(2), 310–316. [DOI] [PubMed] [Google Scholar]
- Parhiz, H. , Roohbakhsh, A. , Soltani, F. , Rezaee, R. , & Iranshahi, M. (2015). Antioxidant and anti‐inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytotherapy Research, 29(3), 323–331. [DOI] [PubMed] [Google Scholar]
- Peng, H. , Wei, Z. , Luo, H. , Yang, Y. , Wu, Z. , Gan, L. , & Yang, X. (2016). Inhibition of fat accumulation by hesperidin in Caenorhabditis elegans . Journal of Agricultural and Food Chemistry, 64(25), 5207–5214. [DOI] [PubMed] [Google Scholar]
- Pu, P. (2016). Protection mechanisms of hesperidin on mouse with insulin resistance. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica, 41(17), 3290–3295. [DOI] [PubMed] [Google Scholar]
- Pyrzynska, K. (2022). Hesperidin: A review on extraction methods, stability and biological activities. Nutrients, 14(12), 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, W. , Hasegawa, J. , Cai, X. , Yang, J. , Ishihara, Y. , Ping, B. , Tsuno, S. , Endo, Y. , Matsuda, A. , & Miura, N. (2016). Components of boiogito suppress the progression of hypercholesterolemia and fatty liver induced by high‐cholesterol diet in rats. Yonago Acta Medica, 59(1), 67–80. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, C. , Dourado, G. , & Cesar, T. (2017). Orange juice allied to a reduced‐calorie diet results in weight loss and ameliorates obesity‐related biomarkers: A randomized controlled trial. Nutrition, 38, 13–19. [DOI] [PubMed] [Google Scholar]
- Roohbakhsh, A. , Parhiz, H. , Soltani, F. , Rezaee, R. , & Iranshahi, M. (2015). Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sciences, 124, 64–74. [DOI] [PubMed] [Google Scholar]
- Schweitzer, G. G. , & Finck, B. N. (2014). Targeting hepatic glycerolipid synthesis and turnover to treat fatty liver disease. Advances in Hepatology, 2014, 1–14. [Google Scholar]
- Serino, A. , & Salazar, G. (2018). Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients, 11(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. , Xu, Y. , & Lu, Y.‐H. (2012). Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. Journal of Agricultural and Food Chemistry, 60(38), 9609–9619. [DOI] [PubMed] [Google Scholar]
- Shin, E. J. , Hur, H. J. , Sung, M. J. , Park, J. H. , Yang, H. J. , Kim, M. S. , Kwon, D. Y. , & Hwang, J.‐T. (2013). Ethanol extract of the Prunus mume fruits stimulates glucose uptake by regulating PPAR‐γ in C2C12 myotubes and ameliorates glucose intolerance and fat accumulation in mice fed a high‐fat diet. Food Chemistry, 141(4), 4115–4121. [DOI] [PubMed] [Google Scholar]
- Spahis, S. , Delvin, E. , Borys, J.‐M. , & Levy, E. (2017). Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxidants & Redox Signaling, 26(10), 519–541. [DOI] [PubMed] [Google Scholar]
- Sporea, I. , Popescu, A. , Dumitraşcu, D. , Brisc, C. , Nedelcu, L. , Trifan, A. , Gheorghe, L. , & Braticevici, C. F. (2018). Nonalcoholic fatty liver disease: Status quo. Journal of Gastrointestinal & Liver Diseases, 27(4), 439–448. [DOI] [PubMed] [Google Scholar]
- Sun, Y.‐Z. , Chen, J.‐F. , Shen, L.‐M. , Zhou, J. , & Wang, C.‐F. (2017). Anti‐atherosclerotic effect of hesperidin in LDLr−/− mice and its possible mechanism. European Journal of Pharmacology, 815, 109–117. [DOI] [PubMed] [Google Scholar]
- Takaki, A. , Kawai, D. , & Yamamoto, K. (2013). Multiple hits, including oxidative stress, as pathogenesis and treatment target in non‐alcoholic steatohepatitis (NASH). International Journal of Molecular Sciences, 14(10), 20704–20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada, S. , Pinya, S. , Martorell, M. , Capó, X. , Tur, J. A. , Pons, A. , & Sureda, A. (2018). Potential anti‐inflammatory effects of hesperidin from the genus citrus. Current Medicinal Chemistry, 25(37), 4929–4945. [DOI] [PubMed] [Google Scholar]
- Tian, M. , Han, Y.‐B. , Zhao, C.‐C. , Liu, L. , & Zhang, F.‐L. (2021). Hesperidin alleviates insulin resistance by improving HG‐induced oxidative stress and mitochondrial dysfunction by restoring miR‐149. Diabetology & Metabolic Syndrome, 13(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabeiryureilai, M. , Lalrinzuali, K. , & Jagetia, G. (2015). Determination of anti‐inflammatory and analgesic activities of a citrus bioflavanoid, hesperidin in mice. Immunochem Immunopathol: Open Access, 1(107), 2. [Google Scholar]
- Wang, S.‐W. , Sheng, H. , Bai, Y.‐F. , Weng, Y.‐Y. , Fan, X.‐Y. , Lou, L.‐J. , & Zhang, F. (2020). Neohesperidin enhances PGC‐1α‐mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutrition & Diabetes, 10(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Hasegawa, J. , Kitamura, Y. , Wang, Z. , Matsuda, A. , Shinoda, W. , Miura, N. , & Kimura, K. (2011). Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high‐cholesterol diet in rats. Journal of Pharmacological Sciences, 117(3), 129–138. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Liu, Y. , Chen, X. , Zhu, D. , Ma, J. , Yan, Y. , Si, M. , Li, X. , Sun, C. , Yang, B. , He, Q. , & Chen, K. (2017). Neohesperidin exerts lipid‐regulating effects in vitro and in vivo via fibroblast growth factor 21 and AMP‐activated protein kinase/sirtuin type 1/peroxisome proliferator‐activated receptor gamma coactivator 1α signaling axis. Pharmacology, 100(3–4), 115–126. [DOI] [PubMed] [Google Scholar]
- Xie, Q. , Gao, S. , Lei, M. , & Li, Z. (2022). Hesperidin suppresses ERS‐induced inflammation in the pathogenesis of non‐alcoholic fatty liver disease. Aging (Albany NY), 14(3), 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, H. , Wang, J. , Ran, Q. , Lou, G. , Peng, C. , Gan, Q. , Hu, J. , Sun, J. , Yao, R. , & Huang, Q. (2019). Hesperidin: A therapeutic agent for obesity. Drug Design, Development and Therapy, 13, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuguang, H. , Aofei, T. , Tao, L. , Longyan, Z. , Weijian, B. , & Jiao, G. (2019). Hesperidin ameliorates insulin resistance by regulating the IRS1‐GLUT2 pathway via TLR4 in HepG2 cells. Phytotherapy Research, 33(6), 1697–1705. [DOI] [PubMed] [Google Scholar]
- Yari, Z. , Cheraghpour, M. , Alavian, S. M. , Hedayati, M. , Eini‐Zinab, H. , & Hekmatdoost, A. (2021). The efficacy of flaxseed and hesperidin on non‐alcoholic fatty liver disease: An open‐labeled randomized controlled trial. European Journal of Clinical Nutrition, 75(1), 99–111. [DOI] [PubMed] [Google Scholar]
- Yari, Z. , Movahedian, M. , Imani, H. , Alavian, S. M. , Hedayati, M. , & Hekmatdoost, A. (2020). The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double‐blind, placebo‐controlled clinical trial. European Journal of Nutrition, 59, 2569–2577. [DOI] [PubMed] [Google Scholar]
- Yasım, A. , Özbağ, D. , Kılınç, M. , Çıralık, H. , & Toru, İ. (2011). The effect of diosmin‐hesperidin combination treatment on the lipid profile and oxidativeantioxidative system in high‐cholesterol diet‐fed rats. Türk Göğüs Kalp Damar Cerrahisi Dergisi, 1, 55–61. [Google Scholar]
- Youssef, W. I. , & McCullough, A. J. (2002). Steatohepatitis in obese individuals. Best Practice & Research Clinical Gastroenterology, 16(5), 733–747. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Sun, C. , Yan, Y. , Chen, Q. , Luo, F. , Zhu, X. , Li, X. , & Chen, K. (2012). Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chemistry, 135(3), 1471–1478. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Li, X. , Fang, H. , Guo, F. , Li, F. , Chen, A. , & Huang, S. (2019). Flavonoids as inducers of white adipose tissue browning and thermogenesis: Signalling pathways and molecular triggers. Nutrition & Metabolism, 16(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


