Abstract
Objectives
The aim of the present study was to assess the differences between BAV and TAV patients with chronic moderate to severe or severe AS regarding presentation, incidence of TAVR, survival, ascending aorta diameter and dilatation rate before and after TAVR.
Methods
The study included 667 consecutive patients with chronic moderate to severe or severe AS from January 2012 and December 2022. Outcomes included all-cause mortality, incidence of TAVR, and ascending aorta diameter and dilatation rate.
Results
There were 185 BAV-AS and 482 TAV-AS patients, and BAV-AS patients were younger (67 vs 78 years, P = 0.027). Total follow-up was 4.5 years (IQR: 2.7-8.9 years), 290 patients underwent TAVR, and 165 patients died. The 8-year TAVR incidence was higher in BAV-AS (55% ± 4%) vs TAV-AS (41% ± 5%; P = 0.02). The 8-year survival was higher in BAV-AS (85% ± 6%) vs TAV-AS (71% ± 6%; P < 0.0001) and became insignificant after age adjustment (P = 0.33). The dilatation rate of ascending aorta was significantly faster in BAV-AS patients compared with TAV-AS patients before TAVR. However, the ascending aorta dilatation rate for BAV-AS and TAV-AS patients was not significantly different after TAVR.
Conclusions
Compared with TAV-AS, BAV-AS patients were younger and underwent TAVR more frequently, resulting in a considerable survival advantage. After TAVR, ascending aorta dilatation rates were similar in BAV-AS and TAV-AS patients, suggesting an important role of hemodynamics on ascending aorta dilatation in BAV-AS.
Keywords: Transcatheter aortic valve replacement, Bicuspid aortic valve, Tricuspid aortic valve, Aortic stenosis, Ascending aorta dilatation
1. Introduction
Transcatheter aortic valve replacement (TAVR) has evolved from a novel technology to an established therapy for high-risk patients with symptomatic severe aortic stenosis (AS) [1]. Bicuspid aortic valve (BAV) is the most common congenital valvular disease that occurs in approximately 1–2 % of the population [2]. BAV patients with AS present unique challenges for TAVR because of the morphologic variants in valve anatomical structure, annular and left ventricular outflow tract shape, and dynamic flow patterns [3]. Nevertheless, satisfactory results are accumulating regarding BAV-AS patients undergoing TAVR [4]. Comparable outcomes have been achieved in BAV-AS patients versus tricuspid aortic valve (TAV) patients with AS, especially with new-generation devices [5], [6].
BAV anatomy is commonly associated with an increased dilatation rate of the ascending aorta compared with TAV, which can be explained by the intrinsic differences in the aortic wall structure due to an underlying genetic substrate and abnormal aortic wall stress distribution in BAV patients [7]. TAVR could change valvular hemodynamics and their impact on aortic wall stress distribution [8], and therefore we hypothesized that after TAVR, differences in the ascending aorta dilatation rate between patients with TAV-AS and those with BAV-AS would be secondary to the underlying genetic substrate. We also hypothesized that survival of BAV-AS patients would be superior to TAV-AS, and that the progression of aortic dilatation will be decelerated with abnormal hemodynamics being corrected by TAVR in BAV-AS patients.
2. Methods
2.1. Study population
Between January 2012 and December 2022, all consecutive patients aged ≥65 years with chronic moderate to severe or severe AS by transthoracic echocardiogram (TTE) were retrospectively identified in our institution. AS was graded by either transthoracic or transoesophageal echocardiography following the European Guidelines of Echocardiography [9]. Severe AS was defined as the aortic valve area ≤1.0 cm2, peak aortic velocity >4 m/s or mean pressure gradient >40 mmHg. Moderate AS was defined as aortic valve area between 1.0 and 1.5 cm2, peak aortic velocity between 3.0 and 4.0 m/s or mean pressure gradient between 20 and 40 mmHg.
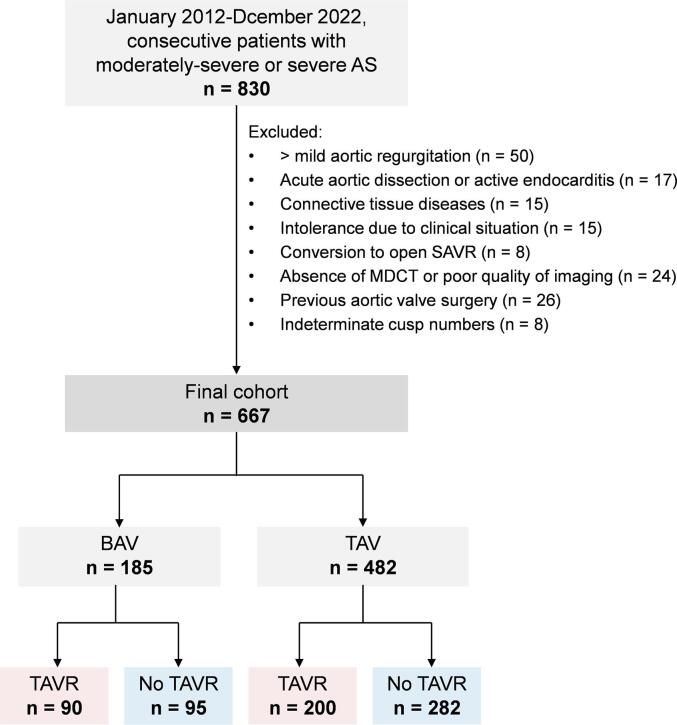
All cases were manually reviewed to determine eligibility. Exclusion criteria included: (1) aortic regurgitation more than mild; (2) acute aortic dissection or active endocarditis; (3) connective tissue diseases; (4) intolerance due to clinical situation; (5) conversion to open surgical aortic valve replacement (SAVR); (6) absence of MDCT or poor quality of imaging; (7) previous aortic valve surgery; (8) indeterminate cusp numbers (Fig. 1). After exclusions, 667 patients constituted the study cohort.
Fig. 1.
Flow diagram of patient inclusion and exclusion. AS = aortic stenosis; BAV = bicuspid aortic valve; MDCT = multidetector computed tomography; SAVR = surgical aortic valve replacement; TAV = tricuspid aortic valve; TAVR = transcatheter aortic valve replacement.
This study was approved by the Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) waived the need for informed consent.
2.2. Imaging measurement
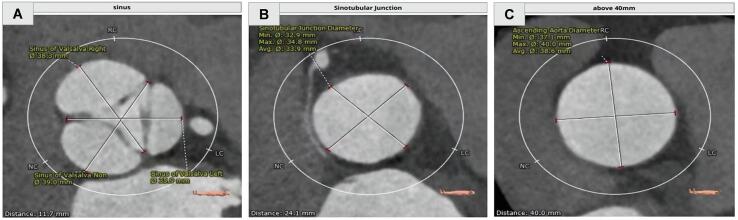
Patients underwent a standard screening algorithm including echocardiography and contrast-enhanced multidetector computed tomography (MDCT). The type of aortic valve was distinguished based on transthoracic echocardiography by two professional cardiologists (Wei Cheng and Jun Li) following the description by Sievers et al. [10]. Ascending aorta diameter was measured by end-systolic images of MDCT in 3mensio software (3mensio Medical Imaging BV, Bilthoven, the Netherlands). To make sure that the measurement of the aortic diameter was comparable at the same slice for each patient, the left coronary ostia was identified as a mark. The circumferences of the sinus of Valsalva (SOV), sinotubular junction (STJ), and ascending aorta 40 mm distal from the aortic valve were measured (Fig. 2), and the average diameter of the plane was defined by dividing the perimeter by 3.14. Ascending aorta dilatation rates were calculated by dividing the change of ascending aorta diameter by the time interval in years for each patient.
Fig. 2.
Ascending aorta measurements. (A) Sinus of Valsalva (SOV) plane cross section. (B) Sinotubular junction (STJ) plane cross section. (C) The diameter of ascending aorta was measured at the broadest level of ascending aorta plane cross section.
2.3. Transcatheter aortic valve replacement procedure
The decision to perform TAVR was made by a multidisciplinary heart team, including an interventional cardiologist, a cardiovascular surgeon, an echocardiographer, and an anesthesiologist. Detailed procedures of TAVR were previously reported [11]. A large proportion of patients were implanted with self-expanding valves, such as: VitaFlow (Microport, Shanghai, China), Taurus One-Valve (Peijia Medical, Suzhou, China), and CoreValve (Medtronic Inc., Minnesota, USA). The rest of the patients were implanted with the Lotus valve (Boston Scientific, Marlborough, MA) or Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, California). The valve size was determined on the basis of MDCT measurements. A transfemoral approach was the preferred access route, but if that was not viable, transiliac, transapical, trans-subclavian, or transaortic approaches were considered. Almost all patients were treated with dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) with no indication of anticoagulation after TAVR; when anticoagulation treatment was indicated, patients received warfarin or new oral anticoagulants.
2.4. Data collection
Preoperative and perioperative data and postoperative outcomes were collected retrospectively from databases at our institution. Postoperative follow-up data were collected by review of medical records or patient telephone interviews. Mortality status, dates of death, and cause of death were retrieved from medical records and China’s National Health Insurance Research Database.
The primary endpoint was all-cause mortality during total follow-up (observation stopped at death or last follow-up) and after TAVR. Secondary endpoints were: 1) cumulative incidence of TAVR; 2) ascending aorta diameter and dilatation rate (at baseline, directly preoperatively, immediately postoperatively and at the last follow-up).
2.5. Statistical analysis
Continuous variables are shown as mean ± standard deviation (SD) or as median (interquartile range [IQR]: 25th to 75th percentile) in cases of skewed distribution. Categorical variables are presented as raw counts and percentages. Assessment of normality was performed using the Shapiro-Wilk test. For normally distributed continuous data, two-tailed unpaired Student’s t tests were used for comparisons between groups. For non-normally distributed data, Mann-Whitney U-test was used. Categorical variables were compared using the Chi-square test. Survival was estimated with the Kaplan-Meier method. The endpoints of mortality and TAVR incidence between BAV-AS and TAV-AS were analyzed by using the Cox proportional hazards model, while adjusting for age, sex, and STS score in multivariable analysis. Repeated-measurement analysis of variance was performed to assess the impact of BAV on the ascending aorta diameters at each point in time. BAV was incorporated into the model as a factor. Estimated marginal means ± standard error of the mean for the ascending aorta diameters were reported. The ascending aorta dilatation was assumed to be linear, and therefore linear regression analysis without including an intercept was performed separately in BAV and TAV to assess ascending aorta dilatation in mm/year in both groups before and after TAVR. We included BAV multiplied by follow-up duration in years to assess the difference in dilatation of the ascending aorta between BAV and TAV [12]. All statistical tests were 2-sided. A P value < 0.05 was considered significant. Analyses were performed using the statistical packages SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. Results
3.1. Patient characteristics
Baseline characteristics of the total cohort (n = 667), including 185 (27.7%) BAV-AS and 482 (72.3%) TAV-AS patients are displayed in Table 1. Compared to TAV-AS patients, BAV-AS patients were over one decade younger (67 vs 78 years, P = 0.027), and predominantly male (69.2 vs 58.1, P = 0.033), and had a lower proportion of patients with comorbidities. According to echocardiography, BAV-AS patients had a smaller aortic valve area (0.54 vs 0.65, P = 0.024) and a larger mean gradient (59.5 vs 52.6, P = 0.037).
Table 1.
Baseline characteristics.
| Total (n = 667) | BAV (n = 185) | TAV (n = 482) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 74 (67-82) | 67 (65-76) | 78 (74-85) | 0.027 |
| Male | 418 (62.7) | 128 (69.2) | 280 (58.1) | 0.033 |
| Body surface area (m2) | 2.06 ± 0.25 | 1.99 ± 0.27 | 2.15 ± 0.24 | 0.659 |
| Hypertension | 135 (20.2) | 33 (17.8) | 102 (21.2) | 0.028 |
| Diabetes mellitus | 76 (11.4) | 17 (9.2) | 59 (12.2) | 0.043 |
| Hyperlipidemia | 112 (16.8) | 28 (15.1) | 84 (17.4) | 0.081 |
| COPD | 105 (15.7) | 25 (13.5) | 80 (16.6) | 0.046 |
| Chronic kidney disease | 35 (5.2) | 9 (4.9) | 26 (5.4) | 0.317 |
| Coronary artery disease | 99 (14.8) | 20 (10.8) | 75 (15.6) | 0.019 |
| Previous myocardial infarction | 30 (4.5) | 8 (4.3) | 22 (4.6) | 0.801 |
| Previous atrial fibrillation | 118 (17.7) | 19 (10.3) | 89 (18.5) | 0.015 |
| Previous cerebrovascular accident | 22 (3.3) | 5 (2.7) | 15 (3.1) | 0.429 |
| NYHA functional class III or IV | 100 (15.0) | 23 (12.4) | 77 (16.0) | 0.035 |
| STS score (%) | 5.6 (3.7-9.4) | 5.0 (3.5-7.3) | 6.2 (4.6-10.5) | 0.028 |
| Echocardiographic variables | ||||
| LVEF (%) | 53.2 (41.7-60.5) | 51.0 (40.9-62.3) | 54.0 (42.5-62.6) | 0.651 |
| LVEF <55% | 211 (31.6) | 56 (30.3) | 155 (32.2) | 0.719 |
| Max velocity (m/s) | 4.8 ± 0.73 | 4.9 ± 0.88 | 4.7 ± 0.66 | 0.185 |
| Mean gradient (mmHg) | 55.7 ± 16.6 | 59.5 ± 15.6 | 52.6 ± 15.3 | 0.037 |
| Aortic valve area (cm2) | 0.59 ± 0.17 | 0.54 ± 0.14 | 0.65 ± 0.19 | 0.024 |
Data are presented as n (%) or mean ± SD or median (interquartile range, IQR).
BAV = bicuspid aortic valve; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; STS = Society of Thoracic Surgeons; TAV = tricuspid aortic valve.
3.2. TAVR incidence and periprocedural outcomes
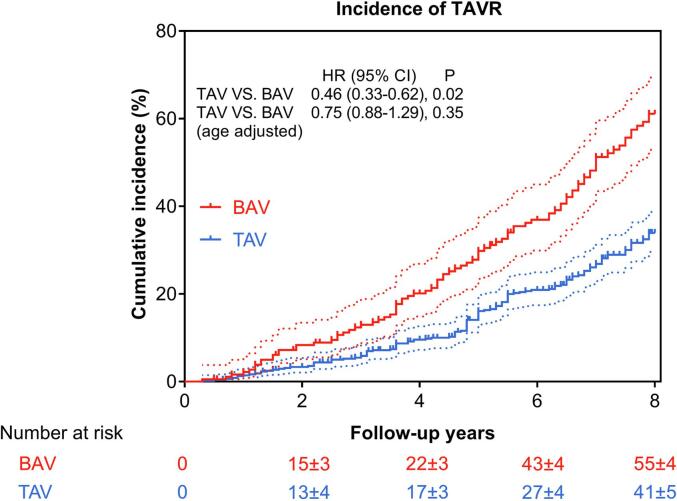
Overall, 290/667 (43.5%) patients underwent TAVR, including 90 BAV-AS patients and 200 TAV-AS patients; no patients had received open SAVR. The 8-year incidence of TAVR (55% ± 4% in BAV and 41% ± 5% in TAV, P = 0.02) was 1.3-fold higher for BAV-AS and became insignificant after adjustment for age (Fig. 3) and after additional adjustment for sex, and STS score (HR: 0.77; 95% CI: 0.68–1.90; P = 0.62). The procedural details and in-hospital outcomes are summarized in Table 2, and no significant differences were observed between BAV-AS and TAV-AS patients.
Fig. 3.
Kaplan–Meier curve showing cumulative incidence of TAVR. BAV patients had higher incidence of TAVR. Dotted lines depict 95% confidence limits accordingly. BAV = bicuspid aortic valve; TAV = tricuspid aortic valve; TAVR = transcatheter aortic valve replacement.
Table 2.
Procedural details and in-hospital outcomes.
| BAV (n = 90) | TAV (n = 200) | P Value | |
|---|---|---|---|
| Access routes | |||
| Transfemoral | 77 (85.6) | 165 (82.5) | 0.765 |
| Non-transfemoral (Carotid, Subclavian) | 13 (14.4) | 35 (17.5) | 0.584 |
| Post-procedural variables | |||
| Valve-in-valve implantation | 7 (7.8) | 15 (7.5) | 0.642 |
| Coronary occlusion | 3 (3.4) | 7 (3.5) | 0.598 |
| New myocardial infarction | 2 (2.2) | 5 (2.5) | 0.723 |
| New permanent pacemaker implantation | 8 (8.9) | 20 (10.0) | 0.567 |
| New cerebrovascular accident | 3 (3.3) | 8 (4.0) | 0.689 |
| Conversion to open SAVR | 3 (3.3) | 5 (2.5) | 0.592 |
| Procedure-related death | 0 | 0 | - |
| In-hospital mortality | 0 | 0 | - |
| Post-operation in-hospital stay, days | 5.5 ± 2.0 | 6.7 ± 3.0 | 0.503 |
| Echocardiography before discharge | |||
| LVEF (%) | 57.7 (52.5-65.0) | 58.4 (52.7-65.2) | 0.783 |
| Max velocity (m/s) | 2.49 ± 0.71 | 2.43±0.52 | 0.653 |
| Mean gradient (mmHg) | 12.37 ± 5.42 | 12.44 ± 5.16 | 0.719 |
| Aortic valve area (cm2) | 1.59 ± 0.23 | 1.60 ± 0.31 | 0.628 |
| Moderate/severe paravalvular leakage | 6 (6.7) | 13 (6.5) | 0.612 |
Data are presented as n (%) or mean ± SD or median.
BAV = bicuspid aortic valve; LVEF = left ventricular ejection fraction; TAV = tricuspid aortic valve; SAVR = surgical aortic valve replacement.
3.3. Follow-up survival
During a median follow-up of 4.5 years (IQR: 2.7–8.9 years), 165 patients died (25 died after TAVR), including 145 (30.1%) TAV-AS and 20 (10.8%) BAV-AS patients. The mortality follow-up was 100% by December 2022.
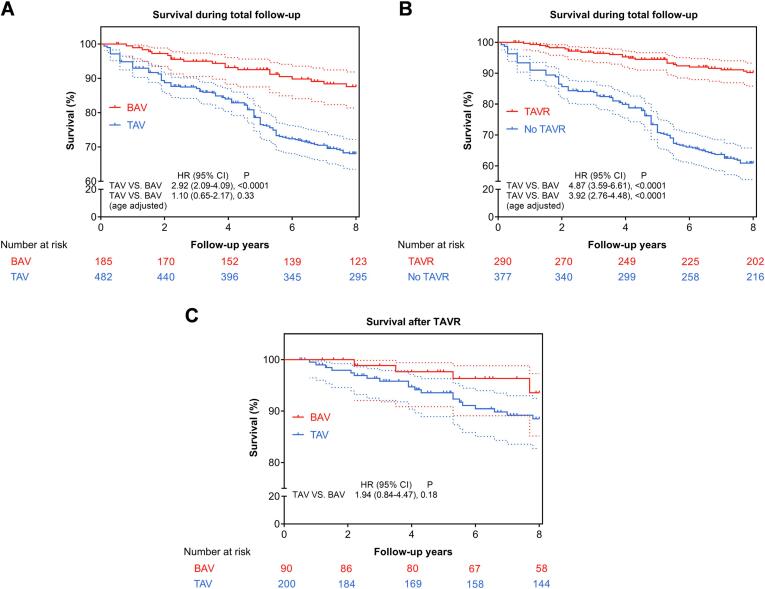
Unadjusted (real-life) Kaplan-Meier models showed that BAV-AS patients had significantly better survival during total follow-up (8-year survival 85% ± 6% in BAV vs 71% ± 6% in TAV, P < 0.0001) (Fig. 4A). However, after adjusting exclusively for age, BAV-AS and TAV-AS had similar survival (P = 0.33). Therefore, age was a critical determinant of mortality and not the presence of BAV or TAV. Also, Kaplan-Meier curves showed that patients with TAVR had better overall survival, whereas those without TAVR had the worst survival (Fig. 4B).
Fig. 4.
Kaplan–Meier curve showing survival differences. (A) BAV patients had better survival during total follow-up. (B) Patients with TAVR had better survival whereas those without TAVR during total follow-up had the worst survival. (C) When patients were further classified into BAV or TAV, there were no inter-group survival differences after TAVR. Dotted lines depict 95% confidence limits accordingly. BAV = bicuspid aortic valve; TAV = tricuspid aortic valve; TAVR = transcatheter aortic valve replacement.
Furthermore, 25 patients died after TAVR during a median follow-up of 3.4 years (IQR: 1.0–5.6 years). The 8-year post-TAVR survival was 93% ± 6% in BAV-AS and 88% ± 5% in TAV-AS (Fig. 4C), and there were no inter-group survival differences (P = 0.18). However, there were only 5 deaths in BAV-AS and 20 deaths in TAV-AS. Therefore, the impact of BAV and TAV on post-TAVR survival was uncertain due to limited statistical power.
3.4. Ascending aorta diameter and dilatation rate
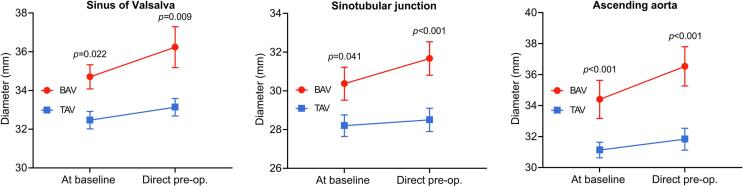
The mean duration from baseline to preoperative MDCT measurements was similar between BAV-AS and TAV-AS (1.2 [IQR: 0.5–4.8] years vs 0.8 [IQR: 0.5–4.0] years; P = 0.87). The diameters of ascending aorta were significantly larger in BAV-AS patients compared with TAV-AS patients at baseline and directly preoperatively (Fig. 5 and Table 3). The preoperative ascending aorta dilatation rate for BAV-AS and TAV-AS patients is displayed in Table 4. The dilatation rate of the STJ and ascending aorta was significantly faster in BAV-AS patients compared with TAV-AS patients.
Fig. 5.
Preoperative change in aortic diameters over time. BAV = bicuspid aortic valve; TAV = tricuspid aortic valve.
Table 3.
Aorta diameters by MDCT at baseline, direct preoperatively, immediate postoperatively, and at the last follow-up.
| BAV | TAV | P Value | |
|---|---|---|---|
| Sinus of Valsalva (mm) | |||
| At baseline | 34.6 ± 0.9 | 32.3 ± 0.5 | 0.022 |
| Direct preoperatively | 36.3 ± 0.7 | 33.5 ± 0.5 | 0.009 |
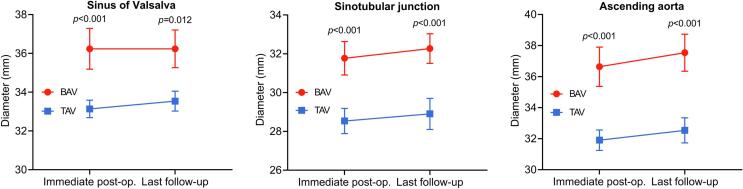
| Immediate postoperatively | 36.5 ± 0.8 | 33.7 ± 0.4 | <0.001 |
| Last follow-up | 36.8 ± 0.7 | 34.2 ± 0.6 | 0.012 |
| Sinotubular junction (mm) | |||
| At baseline | 30.7 ± 0.7 | 28.5 ± 0.6 | 0.041 |
| Direct preoperatively | 31.8 ± 0.9 | 28.4 ± 0.6 | <0.001 |
| Immediate postoperatively | 31.9 ± 0.8 | 28.6 ± 0.5 | <0.001 |
| Last follow-up | 32.8 ± 0.7 | 29.0 ± 0.5 | <0.001 |
| Ascending aorta (mm) | |||
| At baseline | 34.8 ± 0.8 | 31.5 ± 0.6 | <0.001 |
| Direct preoperatively | 36.8 ± 0.9 | 32.2 ± 0.6 | <0.001 |
| Immediate postoperatively | 37.1 ± 0.6 | 32.7 ± 0.5 | <0.001 |
| Last follow-up | 38.9 ± 0.7 | 34.7 ± 0.7 | <0.001 |
Data are presented as n (%) or mean ± SD or median.
BAV = bicuspid aortic valve; TAV = tricuspid aortic valve.
Table 4.
Average preoperative and postoperative annual dilatation rates in BAV and TAV patients.
| BAV |
TAV |
BAV Versus TAV P Value |
|||
|---|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | ||
| Preoperative | |||||
| Sinus of Valsalva | 0.23 (0.07–0.39) | 0.045 | 0.15 (0.02–0.15) | 0.574 | 0.302 |
| Sinotubular junction | 0.29 (0.09–0.40) | 0.021 | 0.02 (0.01–0.12) | 0.763 | 0.037 |
| Ascending aorta | 0.36 (0.22–0.65) | <0.001 | 0.13 (0.09–0.22) | 0.679 | 0.029 |
| Postoperative | |||||
| Sinus of Valsalva | 0.02 (−0.25–0.27) | 0.688 | 0.16 (0.02–0.23) | 0.832 | 0.291 |
| Sinotubular junction | 0.05 (−0.05–0.29) | 0.549 | 0.06 (−0.03–0.19) | 0.595 | 0.698 |
| Ascending aorta | 0.18 (0.10–0.38) | <0.001 | 0.16 (0.1–0.26) | 0.721 | 0.647 |
Data are presented as regression coefficient and 95% confidence interval (95% CI), indicating annual dilatation rates in millimeters per year.
BAV = bicuspid aortic valve; TAV = tricuspid aortic valve.
The mean duration from immediately postoperative to the late follow-up MDCT measurement was similar between BAV-AS and TAV-AS (3.0 [IQR: 1.6–4.5] years vs 3.5 [IQR: 1.5–4.9] years; P = 0.68). The ascending aorta diameter after TAVR is presented in Fig. 6 and Table 3. The diameter of the ascending aorta remained significantly larger in BAV-AS patients compared with TAV-AS patients directly postoperatively and during follow-up. Table 4 shows the postoperative ascending aorta dilatation rate for patients with BAV-AS and TAV-AS, which was not significantly different at all levels.
Fig. 6.
Postoperative change in aortic diameters over time. BAV = bicuspid aortic valve; TAV = tricuspid aortic valve.
4. Discussion
In this large cohort of consecutive patients with chronic moderate to severe or severe AS, we compared differences between TAV-AS and BAV-AS patients before and after TAVR. Our major findings are: (1) compared with TAV-AS patients, BAV-AS patients were one decade younger, and had a higher incidence of TAVR; (2) BAV-AS patients showed superior survival during total follow-up (mainly determined by age); (3) BAV-AS patients had larger ascending aorta and significantly faster dilatation rate before TAVR; However, after TAVR, the ascending aorta diameters remained relatively stable, and dilatation rate were similar to that of TAV-AS patients.
Unadjusted (real-life) survival analyses demonstrated that BAV-AS patients exhibited a significant survival advantage (total follow-up and after TAVR) compared with TAV-AS patients; however, when adjusted exclusively for age, the survival difference was significantly reduced during total follow-up. These observations strongly suggest that age and not valve anatomy is the major determinant of survival. In addition to age, a higher cumulative incidence of TAVR in the BAV-AS group could also contribute to their better outcome.
The diameter of the ascending aorta is changing throughout the lifetime. The average growth rate of ascending aorta in the population with TAV is found to be 0.15–0.20 mm/year [13]. In the setting of BAV patients, the growth rate of ascending aorta is altered by different phases of BAV natural history [14]. In “normally functioning” BAV, the growth rate of ascending aorta is reported to be 0.39–0.77 mm/year, which is 2–4 times faster than that in the healthy TAV population [15], [16]. The faster growth rate may be related to the genetically impaired aortic wall in BAV patients and altered blood flow generated by normal functioning BAV [17], [18]. When BAV becomes dysfunctional, ascending aorta seems to dilate faster with a growth rate of 0.6–0.9 mm/year [19]. As a result, aortic dilatation and aneurysm are more commonly seen in BAV-AS than TAV-AS patients [20], which is consistent with this study.
Currently there are 2 main hypotheses explaining the relation between BAV and ascending aorta dilatation. The first factor that might explain the difference in aortic dilatation between BAV and TAV is an underlying genetic substrate [21]. Histopathologic studies showed increased smooth muscle cell apoptosis, increased matrix metalloproteinase-9 levels, and lower expression of α-smooth muscle actin, smooth muscle 22α, and calponin in the aortic wall of patients with BAV [22], [23]. Although collagen orientation is almost identical in BAV and TAV, there are some differences in biomechanical properties of the aortic wall that may explain the differences in dilatation rate, such as decreased wall thickness, lowered aortic distensibility, and increased aortic stiffness in BAV patients [24]. The second hypothesis on the association between BAV and ascending aorta dilatation is the hemodynamics theory. In TAV, the flow is directed along the curvature of the aorta. In BAV, the flow angle is disturbed, resulting in different increased wall shear stress, depending on the orientation of the cusps [25]. In BAV with fusion of the right and left coronary cusps, the flow is directed toward the right anterior, with increased wall shear stress in this region, resulting in ascending aorta dilatation. In BAV, with fusion of the right and noncoronary cusps, the flow is directed higher into the ascending aorta toward the posterior aortic wall, resulting in ascending aorta dilatation [25].
If the genetics hypothesis is the only factor determining aortic dilatation in BAV, the ascending aorta dilatation rate after TAVR would be as high as it was preoperatively. However, if the hemodynamics theory were the only factor explaining the different aortic dilatation rate between BAV and TAV, once the dysfunctional aortic valve has been replaced, the aortic dilatation rate and the risk of adverse aortic events during follow-up would be similar between BAV and TAV. In addition, these two theories may overlap because increased wall shear stress influences gene expression in the aortic wall [26]. In our study, after TAVR, the mean ascending aortic dilatation rate of BAV group was 0.18 mm/year, which was comparable to that of the TAV group after TAVR, as well as the previously reported average growth rate in normal TAV population (0.15–0.20 mm/year) [26], [27]. These results indicate that after hemodynamic abnormality is corrected, growth rate returns to be normal range, which supports the hemodynamic theory of BAV related aortic dilatation.
5. Clinical implications
BAV is a congenital cardiac malformation which can cause valve dysfunction and increase the risk of aortic dilation or aneurysm. Hemodynamic and genetic components have been suggested to be related to the pathogenesis of ascending aortic dilatation in BAV patients. With abnormal hemodynamics being corrected after TAVR, the progression of ascending aorta was significantly decelerated. This suggested that TAVR could prevent the further progression of ascending aortic diameter for patients with BAV and aortic dilation. However, our results need further confirmation with future investigations in a larger population with longer-term follow-up.
6. Study limitations
There are several limitations in this study. (1) This study was conducted at a single medical center, which may limit the generalizability of the findings to a broader population. (2) The study design is retrospective, which means that it is susceptible to issues related to data accuracy, missing data, and potential bias in data collection. (3) The sample size of the study, while significant, may still be limited in capturing rare outcomes or subgroups within the BAV and TAV patient populations. (4) The study may be subject to selection bias, as patients who underwent TAVR may have been selected based on certain clinical criteria, which could influence the outcomes. (5) While the study adjusted for age as a confounding variable, other potential confounders such as comorbidities, genetic factors, or socio-economic status were not fully accounted for. (6) The study may have a relatively limited follow-up period, which might not capture long-term changes in aortic dimensions and outcomes. (7) The study did not comprehensively differentiate of BAV anatomical variations, which might have different effects on aortic dilatation. (8) The study primarily focused on aortic dimensions and survival but did not include data on aortic valve function.
7. Conclusions
Compared with TAV-AS, BAV-AS patients were one decade younger and underwent TAVR more frequently, resulting in a considerable real-life survival advantage that was determined primarily by age and not valve anatomy. The ascending aorta dilatation rates were significantly higher in BAV-AS patients before TAVR. However, after TAVR, ascending aorta dilatation rates were similar in BAV-AS and TAV-AS patients, suggesting an important role of hemodynamics on ascending aorta dilatation in BAV-AS.
Ethical approval and consent to participate
This retrospective study was approved by the Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) waived the need for informed consent.
Consent for publication: Not applicable.
Author contributions
JL and WC were responsible for the study concept and design. HJZ, YBC, DQL, and CJY were responsible for the acquisition and analysis of data. All authors contributed to the interpretation of the data. HJZ and WC drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria. All authors read and approved the final manuscript.
Funding
This work was supported by the Chongqing Science and Health Joint Medical Research Project (No. 2023MSXM110) and the Science and Technology Innovation Capacity Improvement Project of University (No. 2019XYY13).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jun Li, Email: lijun10461046@163.com.
Wei Cheng, Email: yjchw@126.com.
References
- 1.Zaid S., Atkins M.D., Kleiman N.S., Reardon M.J., Tang G. What's new with TAVR? An update on device technology. Methodist Debakey Cardiovasc. J. 2023;19(3):4–14. doi: 10.14797/mdcvj.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeb G.M., Reardon M.J., Ramlawi B., Yakubov S.J., Chetcuti S.J., Kleiman N.S., et al. Propensity-matched 1-year outcomes following transcatheter aortic valve replacement in low-risk bicuspid and tricuspid patients. J. Am. Coll. Cardiol. Intv. 2022;15(5):511–522. doi: 10.1016/j.jcin.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Yeats B.B., Yadav P.K., Dasi L.P., Thourani V.H. Treatment of bicuspid aortic valve stenosis with TAVR: filling knowledge gaps towards reducing complications. Curr. Cardiol. Rep. 2022;24(1):33–41. doi: 10.1007/s11886-021-01617-w. [DOI] [PubMed] [Google Scholar]
- 4.Vincent F., Ternacle J., Denimal T., Shen M., Redfors B., Delhaye C., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021;143(10):1043–1061. doi: 10.1161/CIRCULATIONAHA.120.048048. [DOI] [PubMed] [Google Scholar]
- 5.Nuyens P., De Backer O., Sathananthan J., Hojsgaard J.T., Treede H., Leipsic J.A., et al. TAVR in bicuspid aortic stenosis: current evidence and proposal for a randomized controlled trial design. J. Am. Coll. Cardiol. Intv. 2023;16(13):1682–1687. doi: 10.1016/j.jcin.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Kang J.J., Fialka N.M., El-Andari R., Watkins A., Hong Y., Mathew A., et al. Surgical vs transcatheter aortic valve replacement in bicuspid aortic valve stenosis: a systematic review and meta-analysis. Trends Cardiovasc. Med. 2023 doi: 10.1016/j.tcm.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Verma R., Cohen G., Colbert J., Fedak P. Bicuspid aortic valve associated aortopathy: 2022 guideline update. Curr. Opin. Cardiol. 2023;38(2):61–67. doi: 10.1097/HCO.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 8.Engel G.P., Kumbhani D.J. Treatment of bicuspid aortic valve stenosis using transcatheter heart valves. Interv. Cardiol. Clin. 2021;10(4):541–552. doi: 10.1016/j.iccl.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Rev. Esp Cardiol. (Engl. Ed.) 2022;75(6):524. doi: 10.1016/j.rec.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Sievers H.H., Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007;133(5):1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Gherasie F.A., Udroiu C.A. Bicuspid aortic valves and transcatheter valve replacement: feasibility and safety. Maedica (Bucur) 2023;18(1):117–120. doi: 10.26574/maedica.2023.18.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo J.A., Coady M.A., Elefteriades J.A. Procedures for estimating growth rates in thoracic aortic aneurysms. J. Clin. Epidemiol. 1998;51(9):747–754. doi: 10.1016/s0895-4356(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 13.Kauhanen P., Korpela T., Hedman M. Dilatation of the ascending aorta - growth rate, risk factors and clinical outcomes in the long-term follow-up. Eur. J. Radiol. 2022;150 doi: 10.1016/j.ejrad.2022.110234. [DOI] [PubMed] [Google Scholar]
- 14.Bellaire C.P., Tharakan S.M., Roy J., Puskas J.D., Di Luozzo G. Natural history of bicuspid aortic valves and ascending aortic aneurysms: aortic center experience. J. Card. Surg. 2022;37(8):2326–2335. doi: 10.1111/jocs.16597. [DOI] [PubMed] [Google Scholar]
- 15.Guala A., Dux-Santoy L., Teixido-Tura G., Ruiz-Munoz A., Galian-Gay L., Servato M.L., et al. Wall shear stress predicts aortic dilation in patients with bicuspid aortic valve. J. Am. Coll. Cardiol. Img. 2022;15(1):46–56. doi: 10.1016/j.jcmg.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Rooprai J., Boodhwani M., Beauchesne L., Chan K.L., Dennie C., Nagpal S., et al. Thoracic aortic aneurysm growth in bicuspid aortic valve patients: role of aortic stiffness and pulsatile hemodynamics. J. Am. Heart Assoc. 2019;8(8):e010885. doi: 10.1161/JAHA.118.010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka T., Furukawa T., Mochizuki S., Okubo S., Go S., Yamada K., et al. Non-aneurysmal ascending aorta diameter changes after aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve. Gen. Thorac. Cardiovasc. Surg. 2022;70(1):33–43. doi: 10.1007/s11748-021-01669-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.S., Kim J.H., Lee S.H., Lee S., Youn Y.N., Yoo K.J., et al. Long-term fate of dilated ascending aorta after aortic valve replacement for bicuspid versus tricuspid aortic valve disease. Am. J. Cardiol. 2020;129:53–59. doi: 10.1016/j.amjcard.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Nucifora G., Miller J., Gillebert C., Shah R., Perry R., Raven C., et al. Ascending aorta and myocardial mechanics in patients with “Clinically Normal” bicuspid aortic valve. Int. Heart J. 2018;59(4):741–749. doi: 10.1536/ihj.17-230. [DOI] [PubMed] [Google Scholar]
- 20.Kerneis C., Pasi N., Arangalage D., Nguyen V., Mathieu T., Verdonk C., et al. Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur. Heart J. Cardiovasc. Imaging. 2018;19(7):792–799. doi: 10.1093/ehjci/jex176. [DOI] [PubMed] [Google Scholar]
- 21.Lin A., Rajagopalan A., Nguyen H.H., White A.J., Vincent A.J., Mottram P.M. Dilatation of the ascending aorta in turner syndrome: influence of bicuspid aortic valve morphology and body composition. Heart Lung Circ. 2021;30(1):e29–e36. doi: 10.1016/j.hlc.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Nappi F., Giacinto O., Lusini M., Garo M., Caponio C., Nenna A., et al. Patients with bicuspid aortopathy and aortic dilatation. J. Clin. Med. 2022;11(20) doi: 10.3390/jcm11206002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia H., Kang L., Ma Z., Lu S., Huang B., Wang C., et al. MicroRNAs involve in bicuspid aortic aneurysm: pathogenesis and biomarkers. J. Cardiothorac. Surg. 2021;16(1):230. doi: 10.1186/s13019-021-01613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junco-Vicente A., Del R.A., Martin M., Rodriguez I. Update in biomolecular and genetic bases of bicuspid aortopathy. Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geeraert P., Jamalidinan F., Fatehi H.A., Sojoudi A., Bristow M., Lydell C., et al. Bicuspid aortic valve disease is associated with abnormal wall shear stress, viscous energy loss, and pressure drop within the ascending thoracic aorta: a cross-sectional study. Medicine (Baltimore) 2021;100(26):e26518. doi: 10.1097/MD.0000000000026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira D., Rosa S.A., Tiago J., Ferreira R.C., Agapito A.F., Sequeira A. Bicuspid aortic valve aortopathies: an hemodynamics characterization in dilated aortas. Comput. Methods Biomech. Biomed. Eng. 2019;22(8):815–826. doi: 10.1080/10255842.2019.1597860. [DOI] [PubMed] [Google Scholar]
- 27.He Y.X., Fan J.Q., Zhu Q.F., Zhou Q.J., Jiang J.B., Wang L.H., et al. Ascending aortic dilatation rate after transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis: a multidetector computed tomography follow-up study. World J. Emerg. Med. 2019;10(4):197–204. doi: 10.5847/wjem.j.1920-8642.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]