Abstract
The mammalian host microbiome affects many targets throughout the body, at least in part through an integrated gut-brain-immune axis and neuropeptide hormone oxytocin. It was discovered in animal models that microbial symbionts, such as Lactobacillus reuteri, leverage perinatal niches to promote multigenerational good health and reproductive fitness. While roles for oxytocin were once limited to women, such as giving birth and nurturing offspring, oxytocin is now also proposed to have important roles linking microbial symbionts with overall host fitness and survival throughout the evolutionary journey.
Keywords: Symbiotic, Symbiont, Probiotic, L. reuteri, Gut-brain-immune axis, Vagus nerve
Highlights
-
•
Oxytocin and the Microbiome Highlights.
-
•
Humans and microbes have evolved alongside each other in a symbiotic relationship that transcends generations.
-
•
Gut microbes communicate with the brain via the vagus nerve modulating host oxytocin production and release, with downstream targets including the immune system.
-
•
Lactobacillus reuteri upregulates endogenous oxytocin which yields beneficial health effects.
-
•
The gut-brain-immune axis mechanism could be leveraged to improve sociability, mental health and physical wellness.
Trillions of cells in the human body work together in complex regulatory and immune mechanisms to maintain homeostasis and achieve good health. Over the course of human evolution, thousands of microbial species have colonized the human host and now comprise more than half of the cells in our bodies. These organisms are primarily found on the major barriers between the human body and its environment: the skin, the mouth, airways, and most extensively the gastrointestinal tract [1]. While hominids have evolved to have a gut microbiome, every individual's gut microbiome is unique. The architecture of a person's gut microbiome develops alongside its host and is influenced by elements of its environment like diet, exposure to pathogens, and host genetics [2]. However, everyone's gut microbiome is initially influenced by their mother's; pioneer microbes inhabit the gut microbiome perinatally and derive from the mother's vaginal and fecal microbiota (Fig. 1) [3,4]. The gut microbiome has a significant impact by influencing metabolic pathways and the maturation of the immune system [[5], [6], [7]]. This is accomplished via a paradigm known as the gut-brain-axis wherein gut microbiota can communicate with the rest of the human body through the vagus nerve, thus controlling many elements of human growth and development (Fig. 1) [8,9]. The gut microbiome similarly communicates directly and indirectly with the host's immune system and can signal the release of various immune cells and factors as needed to mitigate risk for disease and infection [10]. A part of this response is controlled via the hypothalamic-adrenal-pituitary axis, which releases hormones particularly important in a host's stress response [11].
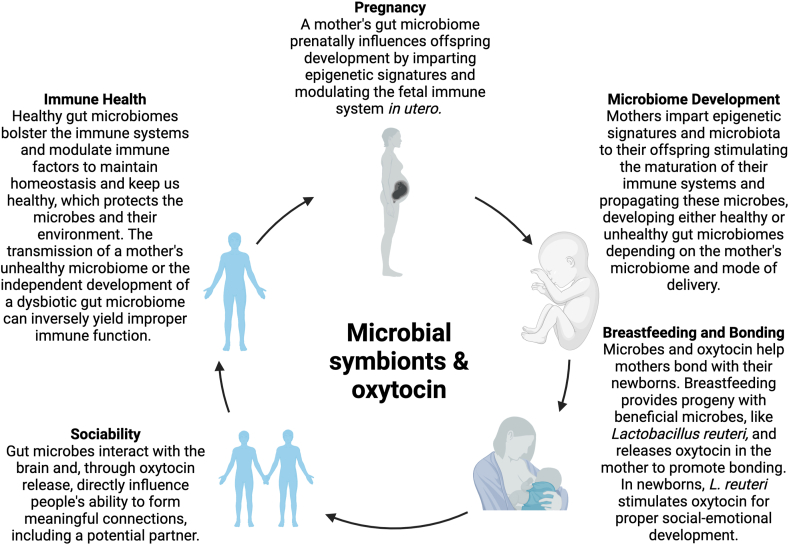
Fig. 1.
Humans and the microbes that colonize their guts have developed a symbiotic relationship over the course of evolution. Microbes live on the surfaces where humans interface with their environments. This causes factors like diet, pathogens, the environment, and genetic predispositions to influence our relationship with our gut microbiomes and its outputs. Oxytocin and the gut microbiome are at the center of many of the human body's functions. Both oxytocin and the gut microbes are integral in various capacities to the stages of human life like pregnancy and breastfeeding, immune health and microbiome development, and bonding and sociability. Taken together with parallel evidence from animal models this paradigm supports multigenerational health for both the microbes and their hosts.
Including these immune factors, the gut microbiome influences many targets throughout the body by means of the gut-immune-brain axis. One contributor that has drawn significant research interest over the last few decades is oxytocin, a neuropeptide hormone primarily synthesized in the hypothalamus. Oxytocin's importance to human health and survival begins with social bonds prior to conception and continues as oxytocin is essential for stimulating contractions inducing labor in pregnant mothers [12]. Once their offspring are born, mothers rely on oxytocin to bond with their babies and to stimulate lactation, making it easier for the offspring to breastfeed [13]. For a while, the scope of knowledge about oxytocin was limited to women and giving birth. However, a growing body of research suggests that oxytocin is exceedingly important to human health [14] and development, and may even be a driving force of human evolution [15,16]. Humans are an exceptionally social species and rely on communities for all facets of our lives, including sharing knowledge, child rearing, and partnership. Oxytocin is fundamental to the mechanisms that allow humans to form relationships, both social and intimate, which inevitably leads to building strong communities and the prospect of reproduction across generations [12,[17], [18], [19]].
Like the gut microbiome, oxytocin plays a role in maintaining homeostasis by modulating the immune system [10,11,[20], [21], [22], [23]]. In 2013, fundamental discoveries involving interactions between oxytocin and the gut microbiome and the immune system paved the way for further exploring these mechanisms in whole body health [10]. To study these pathways, several studies have examined a subject's ability to heal skin wounds efficiently. Wound healing is accomplished through a balance of pro- and anti-inflammatory factors working in tandem with each other. The importance of the immune system in this process suggests that a host's ability to recover after injury could be a reasonable indicator to the health of the organism [10,11,24]. Like wound healing, the process of sustained good health also involves a complex balance and interplay of pro-inflammatory and anti-inflammatory responses and immune interplay throughout the body. In the 2013 study, Poutahidis et al. used an isolate of Lactobacillus reuteri, a beneficial probiotic microbe that was originally isolated from human breast milk, and administered the microbial treatment to mice as a supplement in regular drinking water in order to measure its effect on tissue injury repairs [10]. Amazingly, the mice that were treated with L. reuteri exhibited not only accelerated wound healing and displays of reproductive fitness, but also demonstrated increased circulating levels of oxytocin [10,24,25]. These studies also revealed that treatment with L. reuteri and the accompanying boost in endogenous oxytocin levels accelerated the wound infiltrating pro-inflammatory neutrophils, for example, and at the same time enhanced the potency of anti-inflammatory TREG cells in immune cell titration assays [10]. These results exhibited that microbiota were interacting with host mechanisms to control oxytocin production and improve wound repair in some capacity.
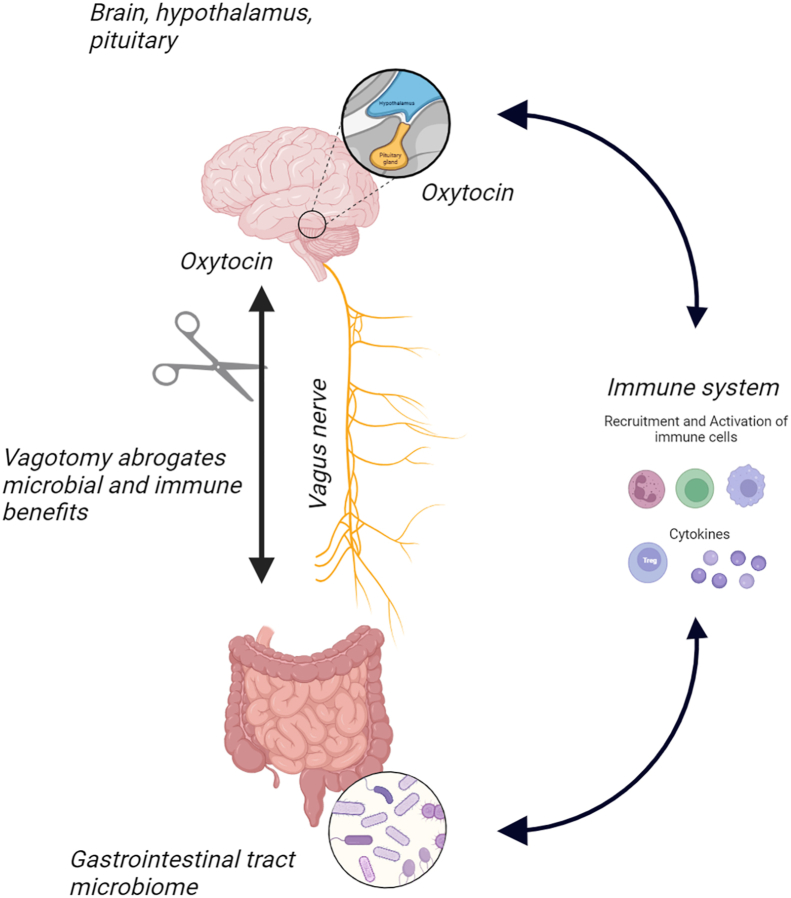
To further investigate the pathways of communication between the gut microbiome and brain mechanisms that regulate oxytocin levels and release, vagotomies were performed to interrupt the vagus nerve transmissions between brain and gut in a cohort of mice as previously done in Bravo et al. 2011 [26]. Even when treated with L. reuteri, mice with vagotomy surgery did not show any of the previous signs of accelerated wound healing nor upregulated oxytocin. This revealed that the microbial mechanism involved the gut-brain-axis via the vagus nerve to communicate and influence oxytocin circulation to favor mammalian host and microbial survival (Fig. 2) [10]. Interestingly, the study found that wounds healed faster in vagus-intact mice. L. reuteri supplementation accelerated the timing of immune cells reaching the skin wound, but did not alter the types of immune cells involved. Thus, both beneficial pro-inflammatory and anti-inflammatory immune cells were normally represented in the robust wound repair process albeit more rapidly in probiotic-treated animals. Elegant adoptive immune cell transfer experiments demonstrated that oxytocin was required for that effect. Taken together, findings in the 2013 study showed that a human breast milk-derived microbe boosted host injury repair via a vagus nerve-dependent gut-brain-immune axis mechanism, leading to unifying hypotheses of microbial symbionts boosting oxytocin in ways pivotal in mammalian health and survival [10]. These results further support that this symbiotic relationship between microbe and human yields beneficial immunological effects like physically healing wounds and restoring an individual's healthy phenotype (Fig. 1).
Fig. 2.
The gut microbiome modulates host immune responses via the gut-brain-immune axis through a variety of mechanisms including microbial community dynamics and circulating microbial products and immune factors. Among these mechanisms, the vagus nerve allows gut microbes to communicate with the brain and modulate the immune system and maintain homeostasis in the host's body. As demonstrated in Poutahidis et al., 2013, vagotomies, which sever this line of communication, block gut-mediated immune modulation affecting the circulation, distribution, activation and potency of various immune cells [10].
The physical manifestation of this beneficial immune paradigm extends beyond cutaneous barriers. Various studies investigated mouse weanlings that inherited a dysbiotic gut microbiome from their mothers who either had an infection or were fed a high-fat and low-fiber diet during pregnancy and eventually developed ASD-like phenotypes [[27], [28], [29]]. The prevalence of autism spectrum disorder (ASD) has been steadily climbing over the last few decades [30]. Social deficits and metabolic comorbidities, like constipation and diarrhea [31], caused by an unbalanced gut microbiome are two common symptoms that people with ASD experience [32]. While the occurrence of ASD is influenced by both environmental and genetic factors, researchers have discovered that oxytocin upregulation could be leveraged as a treatment for social deficits in individuals with ASD. Indeed, there is abundant evidence for a gut microbe-brain connection in this paradigm. Researchers discovered that germ-free mice, or mice that were treated with an abundance of antibiotics to wipe out the gut's microbiota, exhibited abnormal behaviors and some phenotypic characteristics of ASD [33,34], similar to those fed the high-fat and low-fiber diet [27,28]. Expanding on the research conducted by Poutahidis et al. (2013), these mice that were fed a poor diet were then treated with the same strain of L. reuteri (ATCC-PTA-6475) which upregulated endogenous oxytocin [10,27,28,39]. These mice exhibited fewer sociability deficits compared to their untreated counterparts.
The pregnant females that were fed a fast-food-style diet had lower counts of L. reuteri. As a result, their weanlings' gut microbiomes also had an altered ratio of good bacteria, specifically from Bacteroidetes and Firmicutes families, in their gastrointestinal tract [27,28,35]. A battery of sociability behavioral tests on these animals demonstrated that the mice with dysbiotic guts also had stunted social capacities. It's not conclusive how the relative abundance of microbes from the Lactobacillus family affects social capabilities in humans. Further testing needs to be done to determine whether microbiota stimulating endogenous oxytocin for therapeutics would be effective and safe for human subjects. However, specific microbes and pathways that are responsible for altering social behavior in individuals with ASD have not yet been extensively tested in this regard. Several other treatments are being tested for their efficacy in ameliorating typical ASD symptoms. Administration of exogenous oxytocin treated sociability deficits in patients with ASD or other psychiatric disorders are underway in various clinical trials [32,36]. Studies using fecal microbiome transplants to treat the social symptoms in ASD have also shown promise when tested in human subjects [37,38].
Difficulties with sociability is one of the major symptoms of ASD, but it is also a component of several other neurological and psychological disorders [1,35]. Separately, building upon a previous study implicating fast food-style diets and microbial dysbiosis with multigenerational effects of obesity, infertility, cancer and progeria [27,28,39], it was discovered that offspring had higher risk of hyperactivity, hyper-aggression, and antisocial tendencies, similar to symptoms of Fragile X Syndrome, a neurodevelopmental disorder mechanistically related to ASD. When mothers of these male offspring were administered L. reuteri, the severity of their symptoms were ameliorated [40,41]. Studies investigating anxiety, depression, and other psychological disorders demonstrated that boosting levels of oxytocin, whether administered exogenously or upregulated endogenously using microbe interventions, improved symptoms of deficient or low sociability [36,[42], [43], [44]].
There is no universal approach for leveraging the gut microbiome and oxytocin for prevention and treatment of these and other human diseases. In some instances, pre-emptive administration of probiotics like L. reuteri or synthetic oxytocin could reduce the possibility of developing these disorders from an early age, even in utero [39,41,45]. In others, oxytocin administration is coupled with probiotics to alleviate symptoms of neurological disorders, like ASD [46]. It was discovered that sterile lysates of L. reuteri alone could be used in place of whole organisms in order to stimulate endogenous oxytocin production in the hypothalamus and avoid concerns over live microbes entering the bloodstream in rodents [11]. Oxytocin-boosting therapy using microbiota like L. reuteri could be harnessed to prevent and treat obesity [14,47,48]. L. reuteri is one of several recognized microbiota that interact with the immune system and the brain during wound repair. Other Lactobacillus strains including L. plantarum are successful microbial species for improving wound healing [46,49,50]. The microbe Bacteroides fragilis is particularly effective at inhibiting infections from other microbes, bolstering the immune system against foreign bodies [51].
Humans and their microbes are inextricably linked. The gut-brain-immune axis is integral to our health, both physical and mental. The entire extent to which our gut microbiome interplay with oxytocin influences our lives, however, is still unknown. Humans are complex beings that strive for holistic health, meaning they aspire to maintain mental, physical, emotional, and spiritual health, and gut microbes and oxytocin undoubtedly impact all spheres of human lives (Fig. 1). If our microbes impact our ability to form connections and be social with others, then perhaps they also influence the groups that we join, how we spend our time, and the people with whom we acquaint ourselves. While this is a burgeoning field of research that encompasses many biological and philosophical aspects of human life, researchers have started asking these questions [52]. Further research into the pathways of communication between the gut microbiome, the brain, and the immune system could yield better therapies and interventions for individuals diagnosed with dysbiotic guts and the neurological disorders associated with them. A reasonable place to start may be mapping the microbial species most often associated with a healthy gut to understand how to heal an unhealthy one. Some researchers have already started this work [53,54]. Slowly unraveling the mysteries of these microbial symbionts continues to bring us closer to answers and continues to shed light on the complex mechanisms that keep us healthy, build societies, and make us what we are.
Funding
This research was funded by NIH [#P30-ES002109]; NIH U01 [#CA164337]; and Anonymous Fund for Climate Action.
CRediT authorship contribution statement
Bernard J. Varian: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Katherine T. Weber: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Susan E. Erdman: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Figures were created using Biorender.com.
References
- 1.Sarkar A., Harty S., Johnson K.V.A., Moeller A.H., Carmody R.N., Lehto S.M., Erdman S.E., Dunbar R.I.M., Burnet P.W.J. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. 2020;95(5):1131–1166. doi: 10.1111/brv.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M., Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017;66(4):515–522. doi: 10.1016/j.alit.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Gomez de Aguero M., Ganal-Vonarburg S.C., Fuhrer T., Rupp S., Uchimura Y., Li H., Steinert A., Heikenwalder M., Hapfelmeier S., Sauer U., McCoy K.D., Macpherson A.J. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 4.Mueller N.T., Bakacs E., Combellick J., Grigoryan Z., Dominguez-Bello M.G. The infant microbiome development: mom matters. Trends Mol. Med. 2015;21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung H., Sünje Jonathan, Neeraj Sanna, Erin Nicola, Eduardo S., Wang Jorge, Umesaki Y., Mathis D., Benoist C., David, Dennis Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan J.F., O'Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neuro Gastroenterol. Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 8.Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long-Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O'Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., Van De Wouw M., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The microbiota-gut-brain Axis. Physiol. Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 9.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- 10.Poutahidis T., Kearney S.M., Levkovich T., Qi P., Varian B.J., Lakritz J.R., Ibrahim Y.M., Chatzigiagkos A., Alm E.J., Erdman S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078898. PONE-D-13-13551 ([pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varian B.J., Poutahidis T., DiBenedictis B.T., Levkovich T., Ibrahim Y., Didyk E., Shikhman L., Cheung H.K., Hardas A., Ricciardi C.E., Kolandaivelu K., Veenema A.H., Alm E.J., Erdman S.E. Microbial lysate upregulates host oxytocin. Brain Behav. Immun. 2017;61:36–49. doi: 10.1016/j.bbi.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendrick K.M. Oxytocin, motherhood and bonding. Exp. Physiol. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. Spec No: p. [DOI] [PubMed] [Google Scholar]
- 13.Nagasawa M., Okabe S., Mogi K., Kikusui T. Oxytocin and mutual communication in mother-infant bonding. Front. Hum. Neurosci. 2012;6:31. doi: 10.3389/fnhum.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson E.A., Olszewski P.K., Weller A., Blevins J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020;32(4) doi: 10.1111/jne.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter C.S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 16.Carter C.S., Kingsbury M.A. vol. 377. Philosophical Transactions of the Royal Society B: Biological Sciences; 2022. p. 1858. (Oxytocin and Oxygen: the Evolution of a Solution to the ‘stress of Life’). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman R. Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Dev. Psychopathol. 2015;27(2):369–395. doi: 10.1017/S0954579415000048. [DOI] [PubMed] [Google Scholar]
- 18.Bernaerts S., Prinsen J., Berra E., Bosmans G., Steyaert J., Alaerts K. Long-term oxytocin administration enhances the experience of attachment. Psychoneuroendocrinology. 2017;78:1–9. doi: 10.1016/j.psyneuen.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Horn A.J., Carter C.S. Love and longevity: a social dependency hypothesis. Compr. Psychoneuroendocrinol. 2021;8 doi: 10.1016/j.cpnec.2021.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T., Wang P., Wang S.C., Wang Y.F. Approaches mediating oxytocin regulation of the immune system. Front. Immunol. 2016;7:693. doi: 10.3389/fimmu.2016.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsbury M.A., Bilbo S.D. The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocrinol. 2019;55 doi: 10.1016/j.yfrne.2019.100794. [DOI] [PubMed] [Google Scholar]
- 22.Yuan L., Liu S., Bai X., Gao Y., Liu G., Wang X., Liu D., Li T., Hao A., Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammation. 2016;13(1) doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szeto A., Nation D.A., Mendez A.J., Dominguez-Bendala J., Brooks L.G., Schneiderman N., McCabe P.M. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am. J. Physiol. Endocrinol. Metab. 2008;295(6):E1495–E1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levkovich T., Poutahidis T., Smillie C., Varian B.J., Ibrahim Y.M., Lakritz J.R., Alm E.J., Erdman S.E. Probiotic bacteria induce a 'glow of health'. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim Y.M., Kearney S.M., Levkovich T., Springer A., Mirabal S., Poutahidis T., Varian B.J., Lakritz J.R., Alm E.J., Erdman S.E. Maternal gut microbes control offspring sex and survival. J. Probiotics Health. 2014;2:6. [Google Scholar]
- 26.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sgritta M., Dooling S.W., Buffington S.A., Momin E.N., Francis M.B., Britton R.A., Costa-Mattioli M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246–259 e246. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baio J. Prevalence of autism spectrum disorders — autism and developmental disabilities monitoring network, 14 sites, United States, 2008. CDC Morbidity and Mortality Weekly Rep. 2012;61(SS03):1–19. [PubMed] [Google Scholar]
- 31.Krajmalnik-Brown R., Lozupone C., Kang D.W., Adams J.B. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb. Ecol. Health Dis. 2015;26 doi: 10.3402/mehd.v26.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frye R.E. Social skills deficits in autism spectrum disorder: potential biological origins and progress in developing therapeutic agents. CNS Drugs. 2018;32(8):713–734. doi: 10.1007/s40263-018-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desbonnet L., Clarke G., O'Sullivan O., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;50:335–336. doi: 10.1016/j.bbi.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Desbonnet L., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatr. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erdman S.E., Poutahidis T. Microbes and oxytocin: benefits for host physiology and behavior. Int. Rev. Neurobiol. 2016;131:91–126. doi: 10.1016/bs.irn.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Kirsch P. Oxytocin in the socioemotional brain: implications for psychiatric disorders. Dialogues Clin. Neurosci. 2015;17(4):463–476. doi: 10.31887/dcns.2015.17.4/pkirsch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang D.-W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A., Khoruts A., Geis E., Maldonado J., McDonough-Means S., Pollard E.L., Roux S., Sadowsky M.J., Lipson K.S., Sullivan M.B., Caporaso J.G., Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1) doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan Q., Orsso C.E., Deehan E.C., Kung J.Y., Tun H.M., Wine E., Madsen K.L., Zwaigenbaum L., Haqq A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: a systematic review. Autism Res. 2021;14(9):1820–1836. doi: 10.1002/aur.2560. [DOI] [PubMed] [Google Scholar]
- 39.Poutahidis T., Varian B.J., Levkovich T., Lakritz J.R., Mirabal S., Kwok C., Ibrahim Y.M., Kearney S.M., Chatzigiagkos A., Alm E.J., Erdman S.E. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75(7):1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Olaby R.R., Zafarullah M., Barboza M., Solakyildirim K., Peng G., Alvarez M.R., Erdman S.E., Lebrilla C., Tassone F. Differenital methylation profile in Fragile X syndrome-prone offspring mice after in utero exposure to Lactobacillus reuteri. Genes. 2022;13 doi: 10.3390/genes13081300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varian B.J., Weber K.T., Kim L.J., Chavarria T.E., Carrasco S.E., Muthupalani S., Poutahidis T., Zafarullah M., Al Olaby R.R., Barboza M., Solakyildirim K., Lebrilla C., Tassone F., Wu F., Alm E.J., Erdman S.E. Maternal microbiota modulate a fragile X-like syndrome in offspring mice. Genes. 2022;13(8):1409. doi: 10.3390/genes13081409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lach G., Schellekens H., Dinan T.G., Cryan J.F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Foster J.A., McVey Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013 May;36(5) doi: 10.1016/j.tins.2013.01.005. 305-12. [DOI] [PubMed] [Google Scholar]
- 45.Tyzio R., Nardou R., Ferrari D.C., Tsintsadze T., Shahrokhi A., Eftekhari S., Khalilov I., Tsintsadze V., Brouchoud C., Chazal G., Lemonnier E., Lozovaya N., Burnashev N., Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343(6171):675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 46.Kong X.-J., Liu J., Liu K., Koh M., Sherman H., Liu S., Tian R., Sukijthamapan P., Wang J., Fong M., Xu L., Clairmont C., Jeong M.-S., Li A., Lopes M., Hagan V., Dutton T., Chan S.-T., Lee H., Kendall A., Kwong K., Song Y. Probiotic and oxytocin combination therapy in patients with autism spectrum disorder: a randomized, double-blinded, placebo-controlled pilot trial. Nutrients. 2021;13(5):1552. doi: 10.3390/nu13051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poutahidis T., Kleinewietfeld M., Smillie C., Levkovich T., Perrotta A., Bhela S., Varian B.J., Ibrahim Y.M., Lakritz J.R., Kearney S.M., Chatzigiagkos A., Hafler D.A., Alm E.J., Erdman S.E. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068596. PONE-D-13-14662 ([pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson E.A., Marengi D.A., DeSanti R.L., Holmes T.M., Schoenfeld D.A., Tolley C.J. Oxytocin reduces caloric intake in men. Obesity. 2015;23(5):950–956. doi: 10.1002/oby.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gudadappanavar A.M., Hombal P.R., Timashetti S.S., Javali S.B. Influence of Lactobacillus acidophilus and Lactobacillus plantarum on wound healing in male Wistar rats - an experimental study. Int. J. Appl. Basic Med. Res. 2017;7(4):233–238. doi: 10.4103/ijabmr.IJABMR_329_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong J.S., Taylor T.D., Yong C.C., Khoo B.Y., Sasidharan S., Choi S.B., Ohno H., Liong M.T. Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrob. Proteins. 2020;12(1):125–137. doi: 10.1007/s12602-018-9505-9. [DOI] [PubMed] [Google Scholar]
- 51.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 52.Erdman S.E. Microbial muses: threads of our inner wisdom. Challenges. 2021;12(1):10. doi: 10.3390/challe12010010. [DOI] [Google Scholar]
- 53.Greenblum S., Carr R., Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species. Cell. 2015;160(4):583–594. doi: 10.1016/j.cell.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonnell L., Gilkes A., Ashworth M., Rowland V., Harries T.H., Armstrong D., White P. Association between antibiotics and gut microbiome dysbiosis in children: systematic review and meta-analysis. Gut Microb. 2021;13(1):1–18. doi: 10.1080/19490976.2020.1870402. [DOI] [PMC free article] [PubMed] [Google Scholar]