Abstract
Objective:
This study aimed to compare histological and radiological union in the bone transport of 3 segments of different sizes to reconstruct the rabbit femur's bone defects. Methods: Thirty rabbits were divided into 3 groups; a 1-cm defect was created in the femur in all rabbits. The length of the segment to be transferred was 10% of the femur length in group 1, 15% in group 2, and 20% in group 3. All defects were reconstructed by applying bone transport. At the end of the consolidation period, the distraction zone was compared radiologically and histologically.
Results:
While there was no radiological difference between the groups, the highest histological scores were obtained from group 3. Osteocalcin staining revealed similar involvement in groups 2 and 3, butless involvement in group 1.
Conclusion:
Evidence from this study has shown that as the size of the segment used for bone transport increases, more stable fixation and better histological union tissue can be obtained in the rabbit femoral defect model.
Keywords: Bone transport, Distraction osteogenesis, Bone regeneration
Highlights
Bone transport is a biological approach that yields positive outcomes for long bone defects. This study sought to compare histological and radiological healing in bone transport, using three different segment sizes in rabbit femur.
The results indicated no radiological differences based on segment size. However, the group with the largest segment transfer achieved the highest histological scores.
The length of the transported bone should be sufficient to provide adequate stabilization and not impair vascularization, additionally as the size of the segment used for bone transport increases, more stable fixation and better histological union tissue can be obtained.
Introduction
The treatment of long bone defects is still a challenge for orthopedic surgeons.1 Several surgical options have been described for long bone defects, including autogenous bone grafts,2 pedicled or free vascularized fibular transfer,3 and the Ilizarov transport technique,4 which involves an osteotomy above or below the defect followed by the transport of the vascularized bone segment into the bone defect after the latency period. New bone formation occurs in the gap created at the osteotomy site5-6 based on the principles of distraction osteogenesis,1,5 which refers to the regeneration of bone between vascularized bone surfaces that are separated by gradual distraction.1,5-7 Although the bone transport technique is a reliable method in the treatment of large bone defects, it also has difficulties and results in complications, such as infection, non-union or delayed union, insufficient bone regeneration, refracture, joint stiffness, and muscle contracture.8-9
Insufficient bone regeneration in the distraction zone is a known complication of the bone transport method.8 In the literature, factors affecting bone regeneration has been widely investigated.8-10 However, studies on this subject have generally focused on distraction speed and rhythm.9-11 There is only limited research concerning the size of the transported fragment.12
The aim of this study was to compare histological and radiological bone regeneration in bone transport performed with segments of 3 different sizes for the reconstruction of bone defects in the rabbit femur.
Material and methods
Animals
This study was approved by the Animal Experiments Local Ethics Committee of Mehmet Akif Ersoy Istanbul Experimental Research Development and Training Center (Date: February 15, 2018, Decision Number: 2018/02) and carried out under the supervision of the animal laboratory authorities and veterinarians of the institution.
Thirty white New Zealand rabbits (male, 20 weeks, body weight: 2.7-3.2 kg) were included in the study. The length of the femur was determined during the surgical procedure by measuring the distance from the trochanter major to the lateral epicondyle. The right femur was used in all rabbits, and the mean femur length was 10.8 cm (10.1-11.6 cm). The rabbits were randomly divided into 3 groups. A 1-cm bone defect was created at the diaphyseal level of the right femur in all rabbits. The length of the bone transported from the distal defect was planned as 10% of the entire femur in group 1, 15% in group 2, and 20% in group 3.
Surgical method
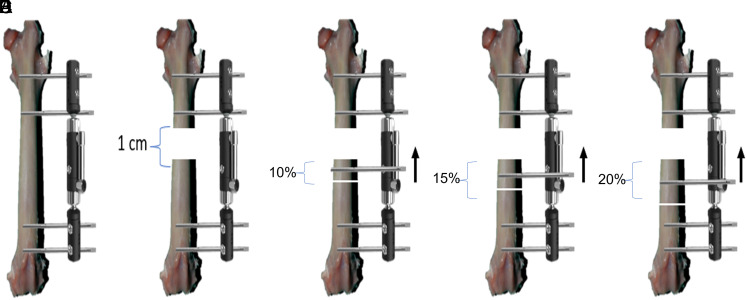
Surgical procedures were performed by a single surgeon on the right femur of each rabbit under sterile conditions. As a pre-anesthetic, 7 mg/kg xylazine hydrochloride (Rompun, Bayer, 23.32 mg/mL) was administered intramuscularly into the thigh. Approximately 10 minutes after the animal was sedated, 35 mg/kg ketamine hydrochloride (Ketalar, Pfizer, 50 mg/mL) was administered intramuscularly into the thigh as an anesthetic. Before surgery, 1 dose of 25 mg/kg of cefazolin sodium (Sefazol, Mustafa Nevzat, Turkey, intramuscular) was applied for prophylaxis. Then, the hair on the lateral right thigh was shaved to expose the surgical site. A sterile environment was provided by cleaning the surgical site with a povidone-iodine (10%) solution. Two 2-mm Schanze screws were placed proximal to the femur. Then, a further 2 2-mm Schanze screws were placed on the distal femur. Mini-slide monolateral finger external fixator was installed (Tasarım medical, Istanbul, Turkey). An approximately 3 cm incision was made from the lateral thigh. In all rabbits, a 1 cm segment was removed with the drill osteotomy technique from the middle of the femur to create a defect. The osteotomy line was selected from the distal part of the defect as 10% of the femur length in group 1, 15% in group 2, and 20% in group 3. For each rabbit, 1 Schanze screw was placed in the middle of the segment to be used in bone transport. Then, osteotomy was performed in the targeted area using a drill and osteotome (Figure 1). The procedure was terminated by suturing the skin (Figure 1). After the procedure, the rabbits were allowed to move freely in the cage. All the rabbits received daily doses of intramuscular penicillin (40 000 IU/day) and streptomycin (12 mg/kg) for 3 days postoperatively. Bone transport was started as 1 mm per day on the seventh day of surgery and continued for 10 days. After waiting for 20 days for consolidation, each animal was given a lethal overdose of an anesthetic agent (propofol) (AstraZeneca Products, Cambridge, England).
Figure 1.
Schematic view of the surgical technique. (A) Establishment of the fixator system by placing 2 Schanze screws on the proximal and distal femur. (B) A 1-cm defect was created in the middle of the diaphysis in all femurs. (C) The rabbits in group 1 underwent osteotomy distal to the defect using a segment covering 10% of femur length, and the segment was fixed with a Schanze screw and attached to the system. (D) The rabbits in group 2 underwent osteotomy distal to the defect using a segment covering 15% of femur length, and the segment was fixed with a Schanze screw and attached to the system. (E) The rabbits in group 3 underwent osteotomy distal to the defect using a segment covering 20% of femur length, and the segment was fixed with a Schanze screw and attached to the system.
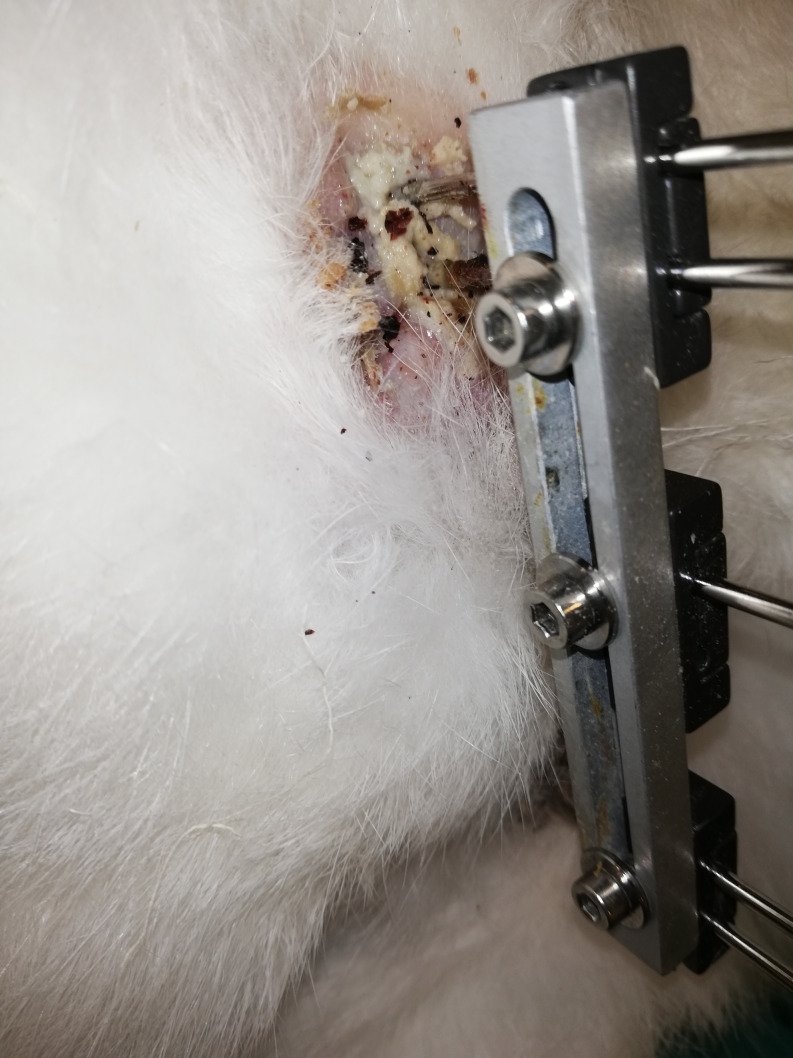
During the study, 4 rabbits (3 in group 1 and 1 in group 2) were excluded from the evaluation because sufficient stability could not be achieved with the surgical procedure, and 3 rabbits (2 in group 3 and 1 in group 2) died during the consolidation period due to infection (Figure 2). The remaining 23 rabbits (7 in group 1, 8 in group 2, and 8 in group 3) were included in the study.
Figure 2.
Pin tract infection.
Radiological evaluation
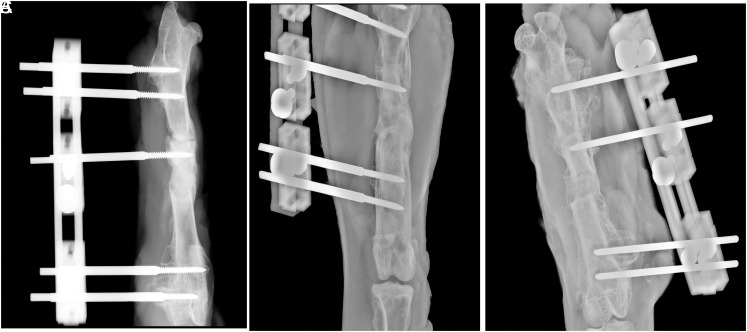
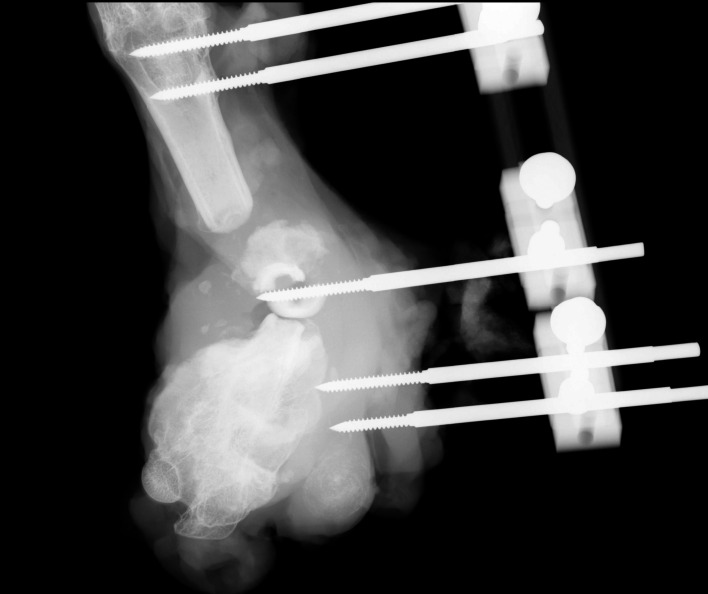
The lateral and anterior–posterior radiographies of the femur were taken for each rabbit. The radiographies were evaluated by a blinded radiologist according to the modified Lane and Sandhu13 radiologic scoring system (Table 1) (Figure 3).
Table 1.
Modified Lane and Sandhu radiological scoring system
| Bone formation | |
| No evidence of bone formation | 0 |
| Bone formation occupying 25% of defect | 1 |
| Bone formation occupying 50% of defect | 2 |
| Bone formation occupying 75% of defect | 3 |
| Full gap bone formation | 4 |
| Union | |
| Non-union | 0 |
| Possible union | 1 |
| Radiographic union | 2 |
| Remodeling | |
| No evidence of remodeling | 0 |
| Remodeling of medullary canal | 1 |
| Full remodeling | 2 |
Figure 3.
Radiological images of the rabbit femurs in (A) group 1, (B) group 2, and (C) group 3.
Histopathological and immunohistochemical evaluation
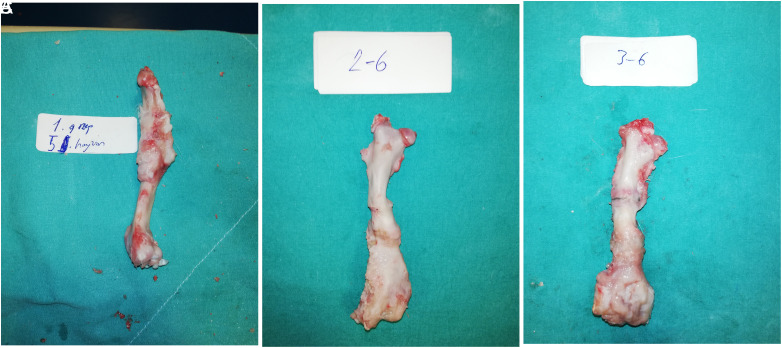
After the radiologic evaluation, the operated femur was dissected (Figure 4). For the histopathological examination, the femurs kept in 10% formalin solution for 1 day were treated with 0.6 N hydrochloric acid. Longitudinal sections were taken from the bones, washed with distilled water, and embedded in paraffin blocks. From the prepared paraffin blocks, 6-µm thick sections were cut with a microtome and stained with hematoxylin–eosin. These preparations were examined under a light microscope at 100 magnification (Nikon Eclipse E600, Nikon Corporation, Japan). The histopathological sections were analyzed by a pathologist in a single-blinded manner. The histopathological evaluation was performed according to the method described by Lane and Sandhu13 and modified by Heiple and et al14 (Table 2).
Figure 4.
Macroscopic view of the femur after dissection. (A) The femur of the seventh rabbit in group 1. (B) The femur of the sixth rabbit in group 2. (C) The femur of the fifth rabbit in group 3.
Table 2.
Lane–Sandhu histopathological scoring system
| Union | |
| No evidence of union | 0 |
| Fibrous union | 1 |
| Osteochondral union | 2 |
| Bone union | 3 |
| Complete organization of shaft | 4 |
| Spongiosa | |
| No osseous cellular activity | 0 |
| Early apposition of new bone | 1 |
| Active apposition of new bone | 2 |
| Reorganizing cancellous bone | 3 |
| Complete reorganization of cancellous bone | 4 |
| Cortical bone | |
| None | 0 |
| Early appearance | 1 |
| Formation under way | 2 |
| Mostly reorganized | 2 |
| Completely formed | 4 |
Osteocalcin staining was carried out in the sections obtained from the bone tissue in the distraction region of the rabbits. The streptavidin–peroxidase method was used to determine the localization and expression of osteocalcin. Positive cells were scored for their staining intensities15 (Table 3).
Table 3.
Osteocalcin staining degree
| Grade 0 | No staining |
| Grade 1 | Minimal staining |
| Grade 2 | Moderate staining |
| Grade 3 | Severe Staining |
Statistical evaluation
Statistical analyses were performed with Number Cruncher Statistical System (NCSS) software, NCSS, LLC, Kaysville, Utah 84037 USA. Data were presented as mean ± SD, minimum, and maximum values. The normality of the distribution of variables was analyzed with the Shapiro–Wilk test. The groups were compared with the parametric 1-way analysis of variance and post hoc Tukey Honest Significant Difference (HSD) tests and the nonparametric Kruskal–Wallis and post hoc Dunn’s test. A P value of < .05 was considered statistically significant.
Results
In the radiological evaluation, union was obtained in the distraction area in all rabbits except 2 in group 1, where both had loss of reduction (Figure 5). These 2 rabbits were excluded from scoring. The mean radiological Lane–Sandhu scores of groups 1, 2, and 3 were found to be 6.20, 6.87, and 7.0, respectively (Table 4). The Lane–Sandhu radiological scores did not statistically significantly differ between the 3 groups (P = .016) (Table 5).
Figure 5.
Radiological image of the rabbit in which the procedure resulted in failure in group 1.
Table 4.
Radiological and histologic scores of the groups
| n | Mean | SD | Minimum | Maximum | P | ||
|---|---|---|---|---|---|---|---|
| Lane–Sandhu radiological score | Group 1 | 5 | 6.20 | .447 | 6.00 | 7.00 | |
| Group 2 | 8 | 6.87 | .353 | 6.00 | 7.00 | .016 | |
| Group 3 | 8 | 7.00 | .534 | 6.00 | 8.00 | ||
| Total | 21 | 6.76 | .538 | 6.00 | 8.00 | ||
| Lane–Sandhu histological score | Group 1 | 5 | 5.20 | .447 | 5.00 | 6.00 | |
| Group 2 | 8 | 6.37 | .744 | 5.00 | 7.00 | .002 | |
| Group 3 | 8 | 6.62 | .517 | 6.00 | 7.00 | ||
| Total | 21 | 6.19 | .813 | 5.00 | 7.00 | ||
| Osteocalcin | Group 1 | 5 | 2.20 | .447 | 2.00 | 3.00 | |
| Group 2 | 8 | 3.00 | .000 | 3.00 | 3.00 | .00 | |
| Group 3 | 8 | 3.00 | .000 | 3.00 | 3.00 | ||
| Total | 21 | 2.80 | .402 | 2.00 | 3.00 |
Group 1, fragment size of 10%; group 2, fragment size of 15%; group 3, fragment size of 20%. Total and p values are written in bold.
Table 5.
Pairwise comparisons of the groups
| Samples | Lane–Sandhu radiological score (P) | Lane–Sandhu histological score (P) | Osteocalcin (P) |
|---|---|---|---|
| Group 1 vs. group 2 | .052 | .009 | .018 |
| Group 1 vs. group 3 | .18 | .002 | .032 |
| Group 2 vs. group 3 | .810 | .633 | 1.000 |
Statistically significant ones are written in bold.
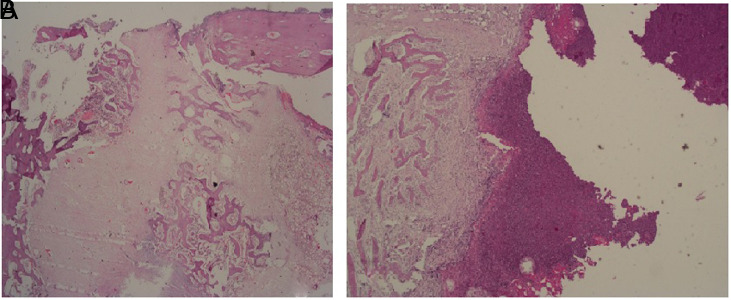
When the groups were compared according to the modified Lane–Sandhu histological scoring system, the highest score was obtained from group 3 (Table 4). A statistically significant difference was found between the groups (P = .002). The paired comparisons revealed no significant difference between groups 2 and 3 (P = .633), but there was a significant difference between groups 3 and 1 and between groups 2 and 1 (P = .002 and P = .009, respectively) (Table 5) (Figure 6).
Figure 6.
(A) New bone development area in fibrous tissue in group 3 (H&E, ×100). (B) New bone development in fibrous tissue in group 1, and an unorganized area filled with fibrin and exudate in its vicinity (H&E, ×20).
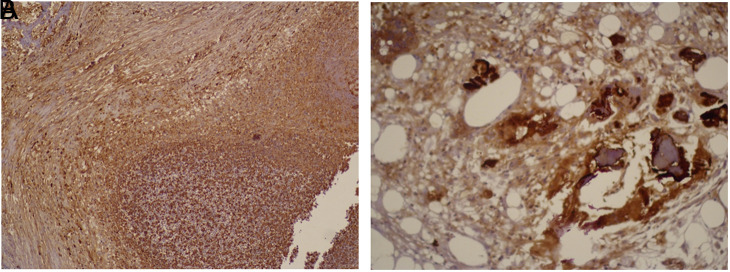
The expression of osteocalcin was similar in groups 2 and 3 and higher in these 2 groups compared to group 1 (Tables 4 and 5, Figure 7).
Figure 7.
(A) Staining with osteocalcin in group 1 (osteocalcin ×100). (B) Staining with osteocalcin in group 2 (osteocalcin ×200).
Discussion
This study was designed to evaluate radiologic and histologic recovery after bone transport using segments of 3 different lengths. Although the radiological score was found to be similar in all segments, better results were obtained from the groups with longer segment lengths in terms of the histological score and osteocalcin staining. This differential healing potential can be explained by the better vascularization of large segments.
Bone transport is an excellent biological method to achieve good results in long bone defects. With this method, the defect can be filled with the original bone tissue similar to the natural bone, mechanically and biologically, using the transported bone.1,6,16 In the literature, we found only few literature reports evaluating the size of the transported bone.12-17 Windhager et al12 reported that as a result of bone transport from the rabbit tibia by creating 2 different segment lengths, the osteogenic potential for both segments was similar. However, endosteal osteogenesis predominates in small segments and periosteal osteogenesis in large segments. This distinctive new pattern of bone formation appeared to be associated with a higher revascularization rate of the endosteum in small segments but with the better preservation of the periosteum in large segments. In the same study, it was emphasized that while determining the length of the piece to be transported, it should have sufficient length to provide stable fixation. In our experimental study, 4 rabbits were excluded from the study because stability could not be achieved during the surgical procedure. In the follow-up, complications due to the loss of reduction developed in 2 more rabbits at the end of the consultation period. Five of these 6 rabbits were in the group where the shortest segment was used. Therefore, we also consider that the length to provide sufficient stability is an important criterion when determining the segment length.
One of the basic principles of the bone transport technique is that periosteal integrity should be maintained during osteotomy to preserve vascularization and facilitate bone formation during bone transport.18-19 The periosteum, a highly vascularized thin tissue, plays an important role in bone formation and regeneration.20 Damage to the periosteum during osteotomy may complicate regenerate formation due to the loss of vascularization.16,18-20 The higher incidence of complications in the group in which the smallest segment was used in this study may be due to the failure in the preservation of the periosteum and soft tissue. The lower histological scores in this group can also be explained by the loss of osteogenic feature due to the lower vascularization of the periosteum.
Osteocalcin is a protein commonly produced by osteoblasts and effective in bone mineralization. It is deposited in the extracellular matrix and indicates bone formation.21-23 The increase in osteocalcin staining uptake in the newly formed bone matrix has been reported to indicate characteristic mature bone formation.24,25 In the current animal study, higher osteocalcin staining involvement in groups 2 and 3 compared to group 1 suggests that the increase in segment length is effective in bone mineralization capacity and new bone formation.
The main limitation of this study is that it was an experimental study. Since the study could not be performed with real patients, animals with human-like histological features were selected to obtain safe and meaningful results. In addition, the rabbits of the same sex, same species, and homogeneous age and weight distribution were used to minimize individual variations. Another limitation is the small sample size.
In the bone transport method, which is frequently used to treat bone defects, the length of the transported bone should be sufficient to provide adequate stabilization and not impair vascularization. Although union can be achieved with smaller segments, more stable fixation and histologically better union tissue can be obtained as the size of the segment increases.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of Mehmet Akif Ersoy Istanbul Experimental Research Development and Training Center (Approval No: 2018/02, Date: February 15, 2018).
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – F.F., S.S., M.K., O.L., F.K., A.P.; Design – F.F., S.S., M.K., O.L., F.K., A.P.; Supervision – F.F., S.S., M.K., O.L., F.K., A.P.; Resources – F.F., S.S., M.K., O.L., F.K., A.P.; Materials – F.F., S.S., M.K., O.L., F.K., A.P.; Data Collection and/or Processing – F.F., S.S., M.K., O.L., F.K., A.P.; Analysis and/or Interpretation – F.F., S.S., M.K., O.L., F.K., A.P.; Literature Search – F.F., S.S., M.K., O.L., F.K., A.P.; Writing – F.F., S.S., M.K., O.L., F.K., A.P.; Critical Review – F.F., S.S., M.K., O.L., F.K., A.P.
Declaration of Interests: The authors have no conflict of interest to declare.
References
- 1. Aktuglu K, Erol K, Vahabi A. Ilizarov bone transport and treatment of critical-sized tibial bone defects: a narrative review. J Orthop Traumatol. 2019;20(1):22. ( 10.1186/s10195-019-0527-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeCoster TA, Gehlert RJ, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg. 2004;12(1):28 38. ( 10.5435/00124635-200401000-00005) [DOI] [PubMed] [Google Scholar]
- 3. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421 428. ( 10.1055/s-2007-1006751) [DOI] [PubMed] [Google Scholar]
- 4. Green SA, Jackson JM, Wall DM, Marinow H, Ishkanian J. Management of segmental defects by the Ilizarov intercalary bone transport method. Clin Orthop Relat Res. 1992;(280):136 142. ( 10.1097/00003086-199207000-00016) [DOI] [PubMed] [Google Scholar]
- 5. Barinaga G, Beason AM, Gardner MP. Novel surgical approach to segmental bone transport using a magnetic intramedullary limb lengthening system. J Am Acad Orthop Surg. 2018;26(22):e477 e482. ( 10.5435/JAAOS-D-17-00487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl MT, Morrison S. Segmental bone defects and the history of bone transport. J Orthop Trauma. 2021;35(suppl 4):S1 S7. ( 10.1097/BOT.0000000000002124) [DOI] [PubMed] [Google Scholar]
- 7. Lu Y, Ma T, Ren C, et al. Treatment of segmental tibial defects by bone transport with circular external fixation and a locking plate J Int Med Res. 2020;48(4):300060520920407. ( 10.1177/0300060520920407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Yushan M, Liu Z, Liu J, Ma C, Yusufu A. Complications of bone transport technique using the Ilizarov method in the lower extremity: a retrospective analysis of 282 consecutive cases over 10 years. BMC Musculoskelet Disord. 2020;21(1):354. ( 10.1186/s12891-020-03335-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kenawey M, Krettek C, Liodakis E, Meller R, Hankemeier S. Insufficient bone regenerate after intramedullary femoral lengthening: risk factors and classification system. Clin Orthop Relat Res. 2011;469(1):264 273. ( 10.1007/s11999-010-1332-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;(239):263 285. ( 10.1097/00003086-198902000-00029) [DOI] [PubMed] [Google Scholar]
- 11. Yasui N, Kojimoto H, Sasaki K, Kitada A, Shimizu H, Shimomura Y. Factors affecting callus distraction in limb lengthening. Clin Orthop Relat Res. 1993;(293):55 60. ( 10.1097/00003086-199308000-00008) [DOI] [PubMed] [Google Scholar]
- 12. Windhager R, Tsuboyama T, Siegl H, et al. Effect of bone cylinder length on distraction osteogenesis in the rabbit tibia. J Orthop Res. 1995;13(4):620 628. ( 10.1002/jor.1100130419) [DOI] [PubMed] [Google Scholar]
- 13. Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213 225. ( 10.1016/S0030-5898(20)30385-0) [DOI] [PubMed] [Google Scholar]
- 14. Heiple KG, Goldberg VM, Powell AE, Bos GD, Zika JM. Biology of cancellous bone grafts. Orthop Clin North Am. 1987;18(2):179 185. ( 10.1016/S0030-5898(20)30381-3) [DOI] [PubMed] [Google Scholar]
- 15. Einhorn TA, Gundberg CM, Devlin VJ, Warman J. Fracture healing and osteocalcin metabolism in vitamin K deficiency. Clin Orthop Relat Res. 1988;(237):219 225. ( 10.1097/00003086-198812000-00033) [DOI] [PubMed] [Google Scholar]
- 16. Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23:143 153. [DOI] [PubMed] [Google Scholar]
- 17. Ma Y, Yin Q, Wu Y, et al. Retraction of transporting bone segment during Ilizarov bone transport. BMC Musculoskelet Disord 2020;21:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nayagam S. Femoral lengthening with a rail external fixator: tips and tricks. Strateg Trauma Limb Reconstr. 2010;5(3):137 144. ( 10.1007/s11751-010-0098-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Z, Tao H, Ye Z, Jin L, Lin N, Yang D. Bone transport for reconstruction of large bone defects after tibial tumor resection: a report of five cases. J Int Med Res. 2018;46(8):3219 3225. ( 10.1177/0300060518774992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang W, Wang N, Yang M, et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Translat. 2022;33:41 54. ( 10.1016/j.jot.2022.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chenu C, Colucci S, Grano M, et al. Osteocalcin induces chemotaxis, secretion of matrix proteins and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994;127(4):1149 1158. ( 10.1083/jcb.127.4.1149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knabe C, Kraska B, Koch C, Gross U, Zreiqat H, Stiller M. A method for immunohistochemical detection of osteogenic markers in undecalcified bone sections. Biotech Histochem. 2006;81(1):31 39. ( 10.1080/10520290600725474) [DOI] [PubMed] [Google Scholar]
- 23. Verbicaro T, Giovanini AF, Zielak JC, Baratto Filho F, de Araujo MR, Deliberador TM. Osteocalcin immunohistochemical expression during repair of critical-sized bone defects treated with subcutaneous adipose tissue in rat and rabbit animal model. Braz Dent J. 2013;24(6):559 564. ( 10.1590/0103-6440201302362) [DOI] [PubMed] [Google Scholar]
- 24. Tera T, Nascimento RD, Prado RF, et al. Immunolocalization of markers for bone formation during guided bone regeneration in osteopenic rats. J Appl Oral Sci. 2014;22(6):541 553. ( 10.1590/1678-775720140190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ivanovski S, Li H, Daley T, Bartold PM. An immunohistochemical study of matrix molecules associated with barrier membrane-mediated periodontal wound healing. J Periodont Res. 2000;35(3):115 126. ( 10.1034/j.1600-0765.2000.035003115.x) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a