Abstract
The gene encoding the DNA gyrase A subunit of Streptococcus pneumoniae was cloned and sequenced. The gyrA gene codes for a protein of 822 amino acids homologous to the gyrase A subunit of eubacteria. Translation of the gene in an Escherichia coli expression system revealed a 92-kDa polypeptide. A sequence-directed DNA curvature was identified in the promoter region of gyrA. The bend center was mapped and located between the −35 and −10 regions of the promoter. Primer extension analysis showed that gyrA transcription initiates 6 bp downstream of an extended −10 promoter. The possible implications of the bent DNA region as a regulatory element in the transcription of gyrA are discussed.
DNA topoisomerases control bacterial DNA topology, which is implicated in the processes of DNA replication, recombination, and transcription. These physiological effects made the bacterial type II DNA topoisomerases, DNA gyrase (gyrase) and DNA topoisomerase IV (topo IV), essential for cell viability. Gyrase is composed of two A (GyrA) and two B (GyrB) subunits, which are encoded by the gyrA and gyrB genes, respectively. The enzyme introduces negative supercoils into DNA by wrapping DNA around the A2B2 protein complex, cleaving both DNA strands (which involves the formation of DNA-protein covalent bonds) and using ATP hydrolysis to pass another portion of DNA through this break. Resealing of the break results in the introduction of two negative supercoils (10, 44). The A subunit is required for the double-stranded breakage and reunion of DNA (41), and the B subunit is required for energy transduction via ATP hydrolysis (25, 40). Topo IV is composed of two C (ParC) and two E (ParE) subunits encoded by the parC and parE genes, respectively. This enzyme is essential for chromosome partitioning (1, 16). The amino acid sequences of ParC and ParE are homologous to those of GyrA and GyrB, respectively.
Gyrase and topo IV can be inhibited by different types of drugs (22). Among them are the fluoroquinolones, a relatively new class of potent, broad-spectrum antimicrobial agents (45). However, their limited activity against Streptococcus pneumoniae (the pneumococcus) and the increasing resistance observed in this species worldwide (2) have led to the continued search for more active compounds. Several studies have shown that the primary target for quinolones in gram-negative bacteria is gyrase, while in the gram-positive bacteria topo IV is the primary target for most quinolones, although it has been reported that sparfloxacin targets gyrase in S. pneumoniae (30). Previous studies on Escherichia coli have identified quinolone resistance mutations in the GyrA quinolone resistance-determining region (QRDR), located between amino acid residues 67 and 106 (48). This region has the highest sequence conservation between GyrA and ParC. Recent studies have identified similar mutations in the analogous region of ParC (18). However, E. coli parC resistance mutations are expressed only in the presence of gyrA mutations (18), and purified E. coli topo IV is less sensitive to quinolones than E. coli gyrase (18). Likewise, Neisseria gonorrhoeae (4) and Haemophilus influenzae (11) strains with low-level resistance contain gyrA mutations, while those with higher levels of resistance have mutations in both gyrA and parC. The opposite is true for S. pneumoniae: mutations altering amino acid residues within the QRDR of ParC confer low-level resistance; mutations altering both QRDRs of ParC and GyrA confer high-level resistance (15, 29, 31, 43). For enterococci, the absence of gyrA mutations in first-step fluoroquinolone-resistant mutants and their presence in second-step mutants (19) suggest the possibility that first-step mutants contain parC mutations, as in the pneumococcus. Moreover, genetic as well as biochemical evidence shows that in Staphylococcus aureus, topo IV is also the primary target for these antimicrobial agents (5, 9).
The genes encoding the two subunits of S. pneumoniae topo IV have been cloned and sequenced (29, 32). We have also previously reported the genetic characterization of the pneumococcal gyrB gene (28) and of a region of the gyrA gene encoding 127 amino acids which includes the QRDR (29). We report here on the characterization of the complete gyrA gene and shown that it is transcribed from a promoter containing a −10 extended promoter that is located in a region showing intrinsic DNA bending. This work complements the genetic characterization of the type II DNA topoisomerases of the pneumococcus and open new ways for the study of the regulation of gyrA gene expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and DNA manipulations.
The E. coli strains used for plasmid transformation were DH5α (13) and XL1-Blue (Stratagene). Plasmids used for cloning were pUC18 (47) and pEMBL18+ (7). Chromosomal DNA from S. pneumoniae wild-type strain R6 was obtained as described previously (8). Plasmids were prepared from E. coli by the alkaline lysis method or by equilibrium centrifugation in CsCl-ethidium bromide gradients (37). Manipulations of DNA, including electrophoresis, and Southern blotting were carried out by standard methods (37). For in situ colony hybridization, DNA was radiolabeled with 50 μCi of [α-32P]dCTP (300 Ci/mmol), using the Multiprime DNA labeling system (Amersham).
PCR amplification and cloning procedures.
PCR amplifications were performed as described previously (8). Amplification was achieved with an initial cycle of 5 min of denaturation at 95°C, 15 min of annealing at 55°C (7 min before and 8 min after adding the enzyme), and 6 min of polymerase extension at 72°C, followed by 20 cycles of 1 min at 95°C, 2 min at 55°C, and 2.5 min at 72°C, with a final 20 min 72°C extension step and slow cooling at 4°C. For PCR reactions with oligonucleotides gyrA46 and gyrA435 (see below), conditions were an initial cycle of 5 min at 95°C and then 60 cycles of 1 min at 95°C, 1 min at 40°C, and 2.5 min at 72°C. The synthetic oligonucleotide primers used were gyrAUP1 (5′-gcgctctagaTGGTTTAGAGGCTGAAATAGAC-3′), gyrADOWN (5′-gcgctctagAGTAATATCAGAAATCCTGCTAGG-3′), 512 (8), gyrA46 (29), gyrA172 (29), gyrA435 (5′-gcgcgtcgACNGATCA(A/G)GATGA(A/G)GTT-3′), and gyrA607 (5′-gcgcgtcgacGA(T/C)GCNTA(T/C)CTNTT(C/T)TT(T/C)ACNAC-3′). The 5′ ends of some of the primers contained sequences including either a PstI (gyrA435), SalI (gyrA607), or XbaI (gyrAUP1 and gyrADOWN) restriction site (lowercase letters).
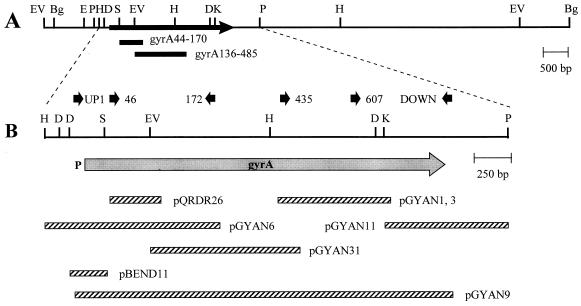
Cloning of the gyrA gene was done by PCR amplifications of overlapping DNA fragments. The upstream gyrA region was amplified by using the degenerate primer gyrA172, (Fig. 1 and 2) and the A-T-rich primer 512. PCR products were hybridized with the gyrA44-170 probe, isolated from agarose gel slices, cut with HindIII (a target included both in gyrA172 and in the chromosomal region upstream of gyrA), and cloned into pEMBL18+ cut with the same enzyme. Plasmid pGYAN6 was selected by in situ colony hybridization with the gyrA44-170 probe. Its chromosomal insert showed the sequence corresponding to the N terminus of GyrA, and the gyrA172 oligonucleotide at one of its ends (Fig. 1). To clone the downstream gyrA region, we performed PCR experiments using three single degenerate primers (Fig. 1 and 2): gyrA46, gyrA435, and gyrA607. Amplification of R6 DNA by using the gyrA46 primer, cutting of the PCR products with EcoRV (a target located within gyrA) (Fig. 1) plus XbaI (a target included in the primer), and ligation into XbaI-SmaI-cut pUC18 allowed the isolation of plasmid pGYAN31 (Fig. 1). Its gyrA origin was detected by hybridization with the gyrA44-170 DNA probe. For the isolation of plasmids pGYAN1 and pGYAN3, PCR amplification was done with primer gyrA435, and a gyrA332-488 probe obtained from plasmid pGYAN31 was used for hybridization. PCR fragments were cut with PstI (a target included into the gyrA435 oligonucleotide) and cloned into pEMBL18+ cut with the same enzyme. Isolation of plasmid pGYAN11 was achieved after a PCR amplification of R6 DNA with oligonucleotide gyrA607. After cutting of the PCR products with KpnI-PstI (targets included in the chromosomal gyrA region [Fig. 1]), bands of the appropriate size that hybridized with the insert of plasmid pGYAN1 were isolated and cloned into pEMBL18+, cut with the same enzymes.
FIG. 1.
Restriction map of the gyrA region of S. pneumoniae (A) and genetic structure as deduced from the nucleotide sequence (B). P at the left of the gray arrow indicates the promoter. The physical maps of the inserts of pertinent plasmids are also indicated (hatched bars). Bg, BglII; D, DraI; E, EcoRI; EV, EcoRV; H, HindIII; K, KpnI; P, PstI; S, SacI. The DNA probes used in Southern blot experiments are indicated as bars labeled gyrA44-70 (insert of plasmid pQRDR26) and gyrA136-485 (insert of pGYAN31). The oligonucleotides used in PCR experiments are indicated by black arrows (not drawn to scale).
FIG. 2.
Nucleotide sequence of a 2,685-bp fragment of S. pneumoniae R6 which contains the gyrA gene. The strand corresponding to the mRNA is shown. Nucleotides and amino acids (italics) are numbered by taking the first gyrA nucleotide as nt 1 and the first GyrA residue as residue 1. The −35 and the extended −10 promoter regions and the putative ribosome-binding site (RBS) are underlined and in boldface. The first nucleotide of the mRNA is indicated as +1. The amino acid residues encoded by the degenerate oligonucleotides used in PCR experiments are also underlined and in boldface. Other pertinent oligonucleotides and restriction endonuclease sites are labeled and underlined.
Circular permutation analysis.
A 533-bp DraI-EcoRV fragment from plasmid pGYAN6 was cloned into the SmaI site of plasmid pCY7 (35), a pBR322 derivative in which the 375-bp EcoRI-BamHI fragment is present as a tandem repeat separated by a SacI-SmaI-XbaI-BglII polylinker. To shorten the size of the insert, the resultant plasmid was digested with SacI, and a 222-bp SacI fragment was then cloned into the SacI site of the plasmid pCY7 polylinker. The orientation of the 222-bp pneumococcal insert into plasmid pBEND-11 was tested by sequence analysis. Plasmid pBEND-11 was digested separately with restriction enzymes EcoRI, HindIII, EcoRV, NheI, and BamHI to generate a set of 609-bp fragments that differ in the position of the pneumococcal insert relative to their ends. DNA samples were fractionated by 5% polyacrylamide gel electrophoresis at 4°C, and the bands were visualized by staining with ethidium bromide.
DNA sequence determination and analysis.
DNA sequencing was carried out with protocols and materials from the Sequenase system (U.S. Biochemical). All sequences shown in this report were determined for both strands of the DNA. DNA and protein sequence comparisons were done with software from Intelligenetics (PCGENE 6.0). Bending analysis was performed by the use of the DNASTAR computer program (DNASTAR, Inc., London, United Kingdom).
RNA purification and analysis.
Cells from a mid-log-phase culture (optical density at 600 nm of 0.50) of E. coli XL1-Blue(pGYAN6) were collected and resuspended in 1/20 volume of 20 mM sodium acetate (pH 5.5)–1 mM EDTA–1 mg of lysozyme per ml. The suspension was lysed by freezing (−70°C) and fast thawing (37°C) several times. The solution was then made 0.5% sodium dodecyl sulfate (SDS), and RNAs were extracted three times with phenol (buffered with 20 mM sodium acetate [pH 5.5]) at 70°C and precipitated twice. For primer extension analysis, cellular RNA (3 to 15 μg) was annealed with 1 pmol of oligonucleotide gyrA20 (5′-CACTCATGGCGTAGTCG-3′) during slow cooling from 65 to 20°C in 14 μl of 50 mM Tris (pH 8.3)–75 mM KCl–3 mM MgCl2. The sample was brought to a final volume of 20.5 μl and incubated for 30 min at 37°C with 8 U of Moloney murine leukemia virus reverse transcriptase (Gibco-BRL) in the presence of 100 μM deoxynucleoside triphosphates (except dATP), 10 mM dithiothreitol, 9.75 μM cold dATP, and 15 μCi of [α-32P]dATP (300 Ci/mmol). The products were phenol extracted, precipitated, and dissolved in 4 μl of formamide dye solution for storage at −20°C.
Analysis of plasmid-encoded proteins.
The pGEM3Z vector/BL21(DE3) host cloning system permits the specific synthesis and labeling of protein products encoded by genes placed under the control of the phage T7 φ10 promoter (39). The cell harbors a defective λ prophage that contains the T7 RNA polymerase gene under the control of lacUV5, which can be induced by isopropyl-β-d-thiogalactopyranoside (IPTG). One-milliliter cultures of E. coli BL21(DE3) carrying the desired plasmids were grown at 37°C in M9 or LB medium containing 200 μg of ampicillin. When the culture reached an A600 of 0.5, cells grown in M9 were induced with IPTG (1 μmol), incubation proceeded for 30 min, and 200 μg of rifampin was added. After 90 min, 10 pmol of [35S]methionine (1,000 Ci/mmol) was added, and incorporation was terminated 10 min later by chilling the cultures. Cells grown in LB were induced with IPTG for 25 min. The cells were centrifuged, suspended, and lysed in sample loading solution for gel electrophoresis. Proteins were analyzed by electrophoresis in 10% (wt/vol) polyacrylamide gels, and they were revealed by staining the gels with Coomassie blue, photography, and autoradiography.
Nucleotide sequence accession number.
The DNA sequence corresponding to the gyrA gene has been assigned GenBank accession no. AF053121.
RESULTS AND DISCUSSION
Cloning and sequencing the gyrA gene of S. pneumoniae.
We had previously cloned into plasmid pQRDR26 (29) a region of S. pneumoniae R6 encoding GyrA amino acids 44 to 170 (Fig. 1), which includes the QRDR region. Attempts to clone the complete gyrA gene from a λgt11 library of pneumococcal DNA were unsuccessful (29). In this work we designed a cloning strategy based on a PCR-walking approach. We constructed degenerate oligonucleotide primers based on the known amino acid sequence, designed to account for the codon usage of S. pneumoniae. These primers were used in PCR DNA amplifications, individually or in combination with an A-T-rich oligonucleotide that was assumed to prime in any region of the (A-T-rich) pneumococcal chromosome. PCR products from the gyrA region were identified through hybridization with specific DNA probes derived from the previously cloned regions and by the presence of restriction targets on the chromosomal DNA, as deduced from Southern blot experiments (Fig. 1). Positive fragments with appropriate sizes were isolated, digested with restriction endonucleases (target sequences included in the oligonucleotide primers and/or in the chromosomal gyrA region), and cloned into E. coli plasmid vectors. Recombinant clones were detected once more by in situ colony hybridization with the same DNA probe used for the identification of the PCR products. This PCR-cloning approach allowed the genetic characterization of the pneumococcal gyrA gene (Fig. 2).
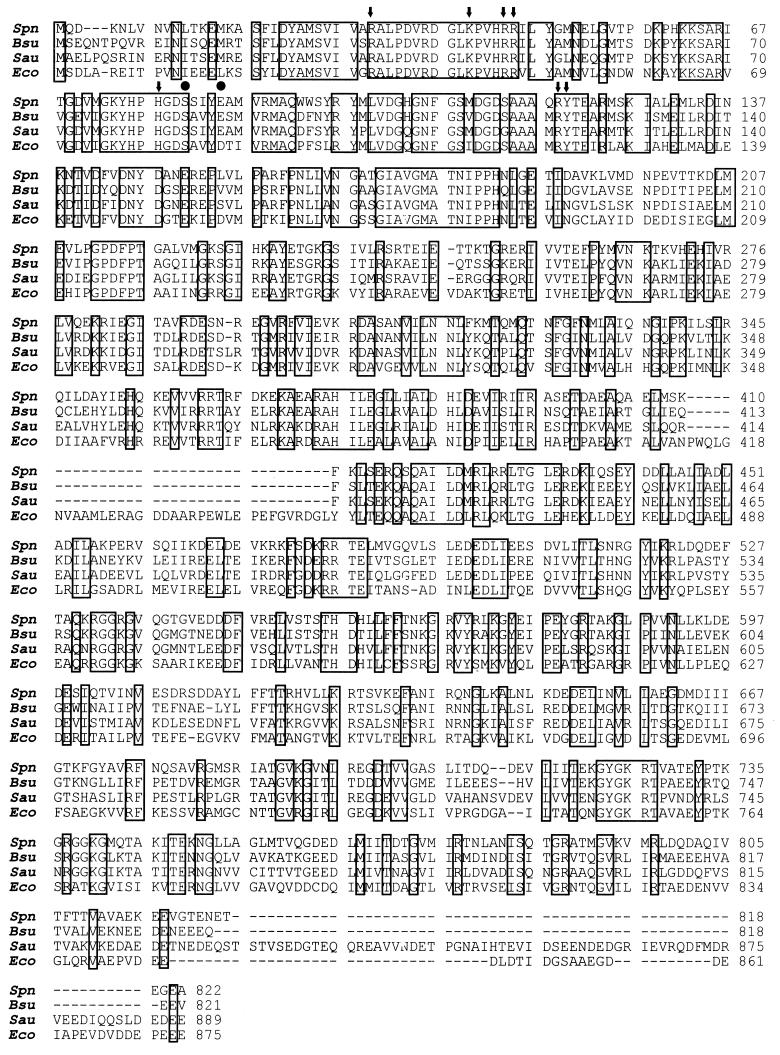
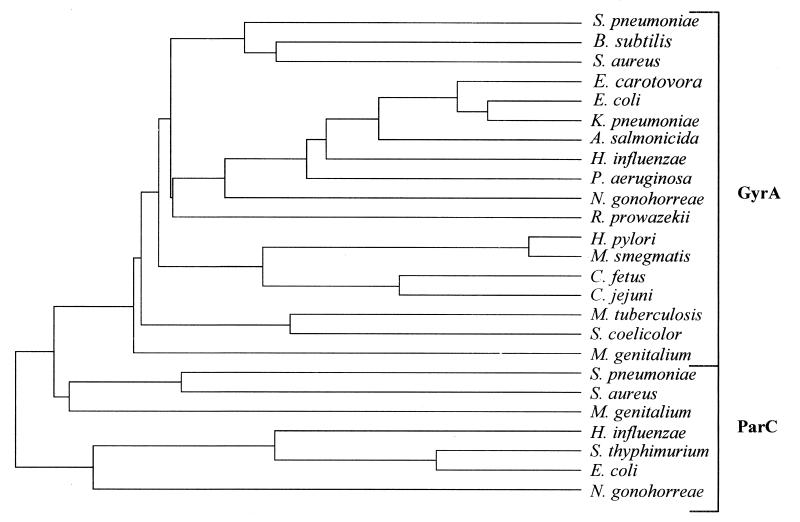
Analysis of the nucleotide sequence revealed the presence of a putative promoter for gyrA transcription, and the gene was preceded by a putative ribosome-binding site (38). The deduced product of gyrA is a protein of 822 amino acid residues that shows about 60% identity with the GyrA subunit of Bacillus subtilis and S. aureus and near 50% identity with GyrA of E. coli (Fig. 3). The residues that form the active site of the breakage-reunion reaction, as revealed by the crystal structure of this domain of E. coli GyrA (residues 2 to 523) (26), are conserved in the pneumococcal GyrA protein (Fig. 3). A FASTA search on the Swiss-Prot sequence database was performed to select sequences to be used in the construction of the protein tree shown on Fig. 4. Only the 25 more similar full-length protein sequences that corresponded to the GyrA and ParC subunits of gyrase and topo IV were used. These GyrA and ParC sequences formed separate groups within the tree. The identities among S. pneumoniae GyrA and the other GyrA proteins varied between 43 and 60%, while identities for ParC proteins were between 31 and 39%. S. pneumoniae GyrA and ParC sequences were 39% identical. The GyrA and ParC sequences of the gram-positive bacteria with low G+C content (S. pneumoniae, S. aureus, and B. subtilis) formed differentiated clusters. These data are in agreement with the phylogenetic comparisons of type II topoisomerases reported by Huang (14).
FIG. 3.
Comparison of the amino acid sequences of the DNA gyrase A subunits from S. pneumoniae (Spn), B. subtilis (Bsu) (27), S. aureus (Sau) (21), and E. coli (Eco) (42). Identical amino acids are boxed. Residues in E. coli GyrA that form the active site of the breakage-reunion reaction, including the active Tyr-122 residue that links to DNA, are indicated by arrows. Residues involved in quinolone resistance in S. pneumoniae are indicated by circles (15, 29, 31, 43).
FIG. 4.
Protein tree of full-length GyrA and ParC subunits from bacteria. The tree was compiled by using the CLUSTAL multiple sequence alignment program from PCGENE.
Identification of the gyrA gene product.
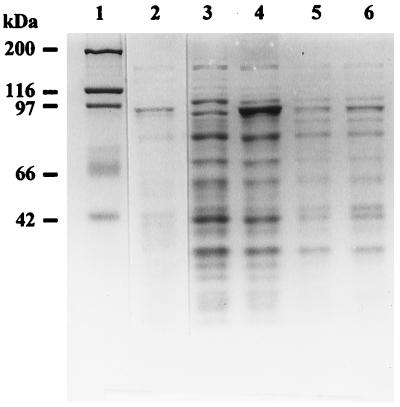
To identify the protein encoded by gyrA, the complete gene was cloned into plasmid pGYAN9 (Fig. 1). A PCR amplification of R6 chromosomal DNA with oligonucleotides gyrAUP1 (nucleotides [nt] −77 to −56 of the sequence in Fig. 2) and gyrA DOWN (nt 2548 to 2525) was performed. PCR products were cut with XbaI, cloned into the XbaI site of pGEM3Z, which contains the T7 polymerase promoter (39), and established in E. coli BL21(DE3). When a culture of this strain was induced with IPTG, one polypeptide, which is not expressed by the pGEM3Z vector, of 92 kDa was produced and specifically labeled with [35S]methionine (Fig. 5). This identified the pneumococcal DNA gyrase A subunit as a 92-kDa polypeptide. This value is in agreement with the molecular mass (92.096 kDa) predicted from sequence analysis.
FIG. 5.
Expression of the GyrA protein. Cultures of E. coli BL21(DE3) containing pGEM3Z (lanes 3 and 5) or pGYAN9 (lanes 4 and 6) were grown in M9 (lanes 5 and 6) or in LB (lanes 3 and 4) medium and induced with IPTG as described in Materials and Methods. Samples containing 15 μg of protein were electrophoresed in an SDS-polyacrylamide gel. Lanes 3 to 6, polypeptides revealed by Coomassie blue staining. The dried gel was exposed for 8 h to Kodak X-Omat for autoradiography. Lane 2 shows the autoradiogram of lane 6. Lane 1, molecular mass protein standards.
Characterization of a sequence-directed DNA bend in the promoter region and the transcription start site.
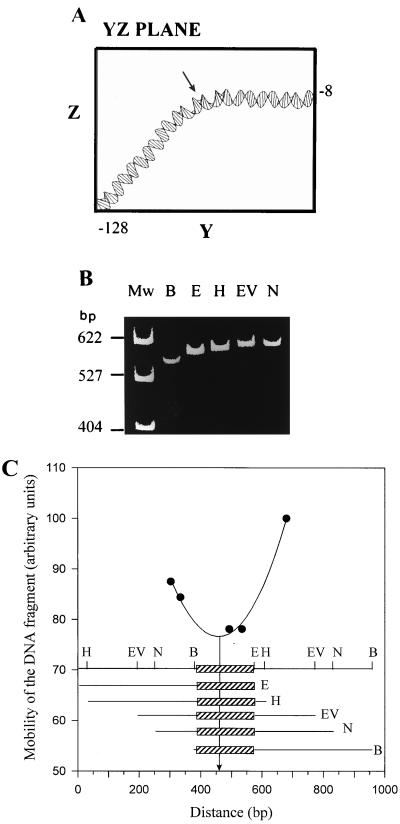
Analysis of the nucleotide sequence in the region preceding the putative ATG initiation codon for gyrA revealed the presence of a putative promoter (Fig. 2). We had previously observed an anomalous mobility of some restriction fragments carrying the upstream gyrA region. Because this behavior is normally associated with intrinsic DNA curvatures, we performed a computer modeling of the promoter region including nt −128 to −8 of the sequence in Fig. 2. The modeling (Fig. 6A) predicted a bent region located at position −67, i.e., centered at about the −35 promoter region. Two-dimensional gel electrophoresis in polyacrylamide gels, the first dimension run at 60°C and the second run at 4°C, showed that the 403-bp HindIII-SacI fragment contained the promoter region (Fig. 1) separate from the diagonal of noncurved DNA fragments (not shown), indicating the presence of bent DNA. To map precisely the center of curvature, we used the circular permutation assay of Wu and Crothers (46). This method is based on the observation that the electrophoretic mobility of a DNA fragment in polyacrylamide gels decreases as the center of the bending approaches the center of the fragment. Thus, plasmid pBEND-11, which carries a 222-bp pneumococcal insert (including nt −128 to 94 of the sequence in Fig. 2), was cut with a series of restriction enzymes with cutting sites present only once in the EcoRI-BamHI duplicated fragment to produce a family of fragments of identical size (609 bp) (Fig. 6B). These molecules, which differ only in the position of the bending center, were analyzed in a polyacrylamide gel run at 4°C. Their relative mobilities were plotted against the distance between the 5′ end (the EcoRI site) of the duplicated fragment in the vector and the half point of the fragment generated by each of the restriction enzymes used (Fig. 6C). In such plots, the lowest point of the best-fitting curve corresponds to the bending center (46). In our case, the position of the bend center mapped at 74 bp from the 5′ end of the fragment (nt −128), i.e., at around nt −55 of the sequence in Fig. 2, between the −35 and −10 extended regions of the putative promoter for gyrA transcription.
FIG. 6.
Mapping of the center of curvature by circular permutation analysis. (A) Computer-generated structure of the DNA region from coordinates −128 to −8 of the sequence in Fig. 2. Plot is shown in the YZ plane to show the predicted bend region. (B) Plasmid pBEND-11 was digested separately with the indicated enzymes (E, EcoRI; H, HindIII; EV, EcoRV; N, NheI; B, BamHI) to generate 609-bp fragments containing the insert at different positions relative to their ends. These fragments were isolated from agarose gel slices, and mobility was analyzed by electrophoresis in a 5% polyacrylamide gel run at 4°C. Plasmid pBR322 digested with HpaII was used as the size marker (lane Mw). (C) Physical structure of plasmid pBEND-11. The restriction sites that occur only once within each duplicated region flanking the insert (box) are indicated. The mobility of each permuted fragment was determined and plotted against the distance from the 5′ end (EcoRI site) of the duplicated fragment in the vector to the midpoint of the fragment generated by each of the restriction enzymes used. The line represents the best fit to the experimental data, and the arrow points to their minima, which correspond to the center of curvature (see text for details).
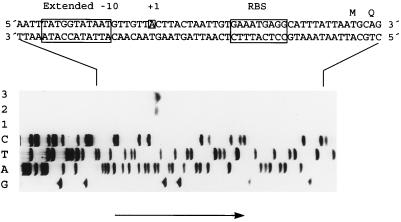
To map the transcription initiation site of the gyrA gene, a 17-base oligonucleotide (gyrA20) was used in a primer extension assay with RNA extracted from E. coli XL1-Blue carrying plasmid pGYAN6 and from the same strain carrying pEMBL18+ as a negative control. This yielded an 89-nt runoff product (Fig. 7) mapping to the A (position −31 of the sequence in Fig. 2) that is 6 bp downstream from the 3′ terminus of a −10 extended promoter sequence (Fig. 7). Thus, the pneumococcal gyrA gene is transcribed from a promoter that shows the sequence TGAAA(N)12TATGGTATAAT, with a −10 extended region (17). Such −10 extended sites have been demonstrated to function in E. coli (17, 34), but extended −10 sites occur rarely in this species (20). Extended −10 sites occur more frequently in gram-positive bacteria (12), and they commonly occur in the pneumococcus (36). It has been suggested that the presence of this kind of promoter could result in excessive expression in E. coli, probably accounting for difficulties in cloning pneumococcal DNA fragments in E. coli, either by producing high levels of toxic proteins or by interfering with vector functions (36). This might explain why we did not find any lambda recombinant clone carrying the gyrA gene when we looked for it in a λgt11 library of pneumococcal DNA (29). Another factor that can influence the strength of the pneumococcal gyrA promoter is the presence of a static curvature centered at the spacer region located between the −35 and −10 regions (Fig. 6) (33). Interestingly, an extended −10 region, which matches the consensus TaTGgTATAAT (36), is also present in the E. coli gyrA gene (42). It is well known that expression of the E. coli gyrA gene is regulated by DNA supercoiling (24). The activity of the promoter is stimulated by gyrase inhibition (i.e., by DNA relaxation), and the sequence required for this response is a 20-bp segment spanning positions −19 to +1 of the promoter region, which excludes the −35 region (23). Whether that −10 extended sequence is involved in regulation of expression of the gyrA genes by targeting of a specific factor is unknown, but it has been suggested that an activator would be required for the transcription of E. coli gyrA (6). However, it has been reported that the E. coli RNA polymerase sigma 70 subunit recognizes the extended −10 motif at promoters (3).
FIG. 7.
DNA sequence of the 5′ region of gyrA and localization of the transcription initiation site. Sequenase reactions using plasmid pGYAN6 as the template and gyrA20 as the primer provided a reference sequence ladder. G, A, T, and C indicate the dideoxynucleotides used during the sequencing assay. For primer extension experiments, RNAs obtained from E. coli XL1-Blue containing either pEMBL18+ (lane 1, 15 μg of RNA) or pGYAN6 (lane 2, 3 μg of RNA; lane 3, 15 μg of RNA) were used. The arrow indicates the direction of electrophoresis. The extended −10 region, the first nucleotide of the mRNA (+1), and the putative ribosome-binding site (RBS) are framed. The double-strand DNA sequence of the 5′ gyrA region and the deduced amino acid sequence are shown.
DNA supercoiling is known to influence the curvature of DNA (33). If a bend site is located in the promoter region, as is the case for the S. pneumoniae gyrA gene, then DNA supercoiling may influence the transcription of that gene by modifying the bend. The presence of an intrinsic DNA curvature in the pneumococcal gyrA promoter would make this promoter very sensitive to changes in supercoiling, allowing the expression of gyrA to act as a regulator of DNA supercoiling in the cell.
ACKNOWLEDGMENTS
We thank R. Muñoz for construction of plasmid pGYAN31, M. Espinosa for computer bending analysis and critical reading of the manuscript, and P. A. Lazo for allowing us to use the PCGENE program on his computer.
D.B. has a Beca de Perfeccionamiento from the Fondo de Investigación Sanitaria, and E. F.-M. has a Beca de Formación de Personal Investigador from Comunidad Autónoma de Madrid. This work was supported by grant FIS 97/2026 from Fondo de Investigación Sanitaria.
REFERENCES
- 1.Adams D E, Shekhtman E M, Zechiedrich E L, Schmid M B, Cozzarelli N R. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell. 1992;71:277–288. doi: 10.1016/0092-8674(92)90356-h. [DOI] [PubMed] [Google Scholar]
- 2.Baquero F. Epidemiology and management of penicillin-resistant pneumococci. Curr Opin Infect Dis. 1996;9:372–379. [Google Scholar]
- 3.Barne K A, Bown J A, Busby S J W, Minchin S D. Region 2.5 of the Escherichia coli RNA polymerase ς70 subunit is responsible for the recognition of the ‘extended-10’ motif at promoters. EMBO J. 1997;13:4034–4040. doi: 10.1093/emboj/16.13.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belland R, Morrison S, Ison C, Huang W. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanche F, Cameron B, Bernard F X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carty M, Menzel R. Inhibition of DNA gyrase activity in an in vitro transcription-translation system stimulates gyrA expression in a DNA concentration manner. J Mol Biol. 1990;214:397–406. doi: 10.1016/0022-2836(90)90189-S. [DOI] [PubMed] [Google Scholar]
- 7.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenoll A, Muñoz R, García E, de la Campa A G. Molecular basis of the optochin-sensitive phenotype of pneumococcus: characterization of the genes encoding the F0 complex of the Streptococcus pneumoniae and Streptococcus oralis H+-ATPases. Mol Microbiol. 1994;12:587–598. doi: 10.1111/j.1365-2958.1994.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero L, Cameron B, Manse B, Langeaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 10.Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- 11.Georgiou M, Muñoz R, Román F, Cantón R, Gómez-Lus R, Campos J, de la Campa A G. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob Agents Chemother. 1996;40:1741–1744. doi: 10.1128/aac.40.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves M C, Rabonowitz J C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for extended promoter elements in gram-positive organisms. J Biol Chem. 1986;25:11409–11415. [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1985;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Huang W M. Bacterial diversity based on type II DNA topoisomerase genes. Annu Rev Genet. 1996;30:79–107. doi: 10.1146/annurev.genet.30.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Janoir C, Zeller V, Kitzis M-D, Moreau N J, Gutmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato J, Nishimura Y, Imamura R, Niki H, Higara S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 17.Keilty S, Rosenberg M. Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J Biol Chem. 1987;262:6389–6395. [PubMed] [Google Scholar]
- 18.Khodursky A B, Zechiedrich E L, Cozarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korten V, Huang W M, Murray B E. Analysis by PCR and direct sequencing of gyrA mutations associated with fluoroquinolone resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1994;38:2091–2094. doi: 10.1128/aac.38.9.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 21.Magerrison E E C, Hopewell R, Fisher L M. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992;174:1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell A. DNA gyrase as a drug target. Trends Microbiol. 1997;5:102–109. doi: 10.1016/S0966-842X(96)10085-8. [DOI] [PubMed] [Google Scholar]
- 23.Menzel R, Gellert M. Modulation of transcription by DNA supercoiling: a deletion analysis of the Escherichia coli gyrA and gyrB promoters. Proc Natl Acad Sci USA. 1987;84:4185–4189. doi: 10.1073/pnas.84.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzel R, Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983;34:105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- 25.Mizuuchi K, O’Dea H, Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci USA. 1978;75:5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morals Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R C. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 27.Moriya S, Ogasawara N, Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985;13:2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz R, Bustamante M, de la Campa A G. Ser-127-to-Leu substitution in the DNA gyrase B subunit of Streptococcus pneumoniae is implicated in novobiocin resistance. J Bacteriol. 1995;177:4166–4170. doi: 10.1128/jb.177.14.4166-4170.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz R, de la Campa A G. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan X S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan X-S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-Martín J, Rojo F, de Lorenzo V. Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol Rev. 1994;58:268–290. doi: 10.1128/mr.58.2.268-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponnambalan S, Webster C, Bingham A, Busby S. Transcription initiation at the E. coli galactose operon promoters in the absence of the normal −35 region sequences. J Biol Chem. 1986;261:16043–16048. [PubMed] [Google Scholar]
- 35.Prentki P, Pham M-H, Galas D J. Plasmid permutation vectors to monitor DNA bending. Nucleic Acids Res. 1987;15:10060. doi: 10.1093/nar/15.23.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabelnikov A G, Greenberg B, Lacks S A. An extended −10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J Mol Biol. 1995;250:144–155. doi: 10.1006/jmbi.1995.0366. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Shine J, Dalgarno L. Determinants of cistron specificity in bacterial ribosomes. Nature. 1975;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 39.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 40.Sugino A, Higgins N P, Brown P O, Peebles C L, Cozzarelli N R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci USA. 1978;75:4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugino A, Peebles C L, Kreuzer K N, Cozzarelli N R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase, a novel nicking-closing enzyme. Proc Natl Acad Sci USA. 1977;74:4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanberg S L, Wang J C. Cloning and sequencing of the Escherichia coli DNA gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987;197:729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- 43.Tankovic J, Perichon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2505–250. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- 45.Wolfson J S, Hooper D C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989;2:378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H-M, Crothers D M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984;308:509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- 47.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC9 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]