Abstract
Background
The plant kingdom has long been considered a valuable source for therapeutic agents, however, some plant species still untapped and need to be phytochemically and biologically explored. Although several Atriplex species have been investigated in depth, A. leucoclada, a halophytic plant native to Saudi Arabian desert, remains to be explored for its phytochemical content and biological potentials. Herein, the current study investigated the metabolic content and the anti-inflammatory potential of A. leucoclada.
Methods
Powdered aerial parts of the plant were defatted with n-hexane then the defatted powder was extracted with 80% methanol. n-Hexane extract (ATH) was analyzed using GC–MS, while the defatted extract (ATD) was subjected to different chromatographic methods to isolate the major phytoconstituents. The structures of the purified compounds were elucidated using different spectroscopic methods including advanced NMR techniques. Anti-inflammatory activity of both extracts against COX-1 and COX-2 enzymes were examined in vitro. Molecular docking of the identified compounds into the active sites of COX-1 and COX-2 enzymes was conducted using pdb entries 6Y3C and 5IKV, respectively.
Results
Phytochemical investigation of ATD extract led to purification and identification of nine compounds. Interestingly, all the compounds, except for 20-hydroxy ecdysone (1), are reported for the first time from A. leucoclada, also luteolin (6) and pallidol (8) are isolated for the first time from genus Atriplex. Inhibitory activity of ATD and ATH extracts against COX-1 and COX-2 enzymes revealed concentration dependent activity of both fractions with IC50 41.22, 14.40 μg/ml for ATD and 16.74 and 5.96 μg/ml for ATH against COX-1 and COX-2, respectively. Both extracts displayed selectivity indices of 2.86 and 2.80, respectively as compared to 2.56 for Ibuprofen indicating a promising selectivity towards COX-2. Molecular docking study supported in vitro testing results, where purified metabolites showed binding affinity scores ranged from -9 to -6.4 and -8.5 to -6.6 kcal/mol for COX-1 and 2, respectively, in addition the binding energies of GC–MS detected compounds ranged from -8.9 to -5.5 and -8.3 to -5.1 kcal/mol for COX-1 and 2, respectively as compared to Ibuprofen (-6.9 and -7.5 kcal/mol, respectively), indicating high binding affinities of most of the compounds. Analysis of the binding orientations revealed variable binding patterns depending on the nature of the compounds. Our study suggested A. leucoclada as a generous source for anti-inflammatory agents.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04281-5.
Keywords: A. leucoclada, NMR, GC–MS, COX, Anti-inflammatory, Molecular docking simulation
Introduction
Inflammation is a self-defense mechanism that is triggered by pathogens, tissue injury, trauma or dysregulation of the normal physiological processes. Inflammatory response rises from the production of prostaglandins that is synthesized from the unsaturated arachidonic acid via cyclooxygenase (COX) enzymes [1, 2]. Cyclooxygenase enzymes are responsible for the formation of important biological mediators called prostanoids, including prostaglandins, prostacyclin and thromboxane. There are two known isoenzymes: COX-1 and COX-2. COX-1 is constitutively expressed in many tissues and is the predominant form in gastric mucosa and in kidney. COX-2 is not normally expressed in most cells, but elevated levels are found at sites of inflammation. Pharmacological inhibition of COX can provide relief signs of inflammation and pain [3].
Anti-inflammatory drugs include steroids and non-steroid drugs. Steroid drugs have some serious side effects such as osteoporosis and fractures, immunosuppression, myopathy, cardiovascular disease, glaucoma and cataracts, diabetes and hyperglycemia, psychiatric disturbances, gastrointestinal and dermatologic adverse effects. These adverse effects limit their utility and make them less popular to be used in inflammatory diseases compared with nonsteroidal drugs [4]. However, nonsteroidal drugs may have some side effects too such as bronchospasm, renal failure, thrombosis, and gastrointestinal bleeding [5]. To overcome these problems, herbal medicines and phytochemicals have been submitted to studies to identify and develop natural products that can be used as anti-inflammatory agents [6–8] or as a combinatorial therapy with these synthetic drugs [9].
The Atriplex genus (Amaranthaceae) constitutes herbaceous halophytes that include about 260 species distributed throughout the world, especially in the arid and semi-arid regions of Europe, Asia, Africa, Australia, and North America [10, 11]. Recent studies have shown that some species have high nutritional value and protein content and can be used as cereal grains as A. hortensis seeds [12]. Phytochemical investigations of some Atriplex species revealed various chemical constituents belonging to different chemical classes as: phenolics [13, 14], triterpenes, sterols [15], phytoecdysteroids [16, 17] and triterpene saponins [13, 15, 18, 19]. From biological point of view some species have been reported to have anti-inflammatory [20], antioxidants, anticholinestrase [13], antidiabetic [21], antimicrobial [22], hepatoprotective [23], immunomodulatory [14], analgesic, antipyretic, and, cytotoxic activities [24, 25].
Atriplex leucoclada Boiss. (English name: cut-leaf saltbush, orach, Arabic name: Ragal, رغل), is a low perennial shrub commonly growing in Saudi Arabian desert. This species has an agricultural importance in arid regions. It can adapt high salt habitats via different strategies [13, 26]. Reviewing the relevant literature little research was found discussing the metabolic content and/or the biological activity of A. leucoclada, where, one previous study reported the isolation of five triterpenoidal saponins and highlighted their molluscidal potential [27]. Accordingly, this study was designed to add more research about the phytochemical constituents and anti-inflammatory activity of A. leucoclada.
Materials and methods
General experimental procedures
NMR spectra were obtained on Bruker Avance III 400 MHz with BBFO Smart Probe and Bruker 400 MHz AEON Nitrogen-Free Magnet (Bruker AG, Switzerland) operating at 400 MHz for proton and 100 MHz for carbon. Data were analyzed using Topspin 3.1 Software (Bruker AG, Fallanden, Switzerland). 1D and 2D-NMR spectra (1H, 13C, HSQC and HMBC) were obtained using standard Bruker pulse programs. All deuterated solvents (CDCl3, CD3OD and pyridine-d5) for NMR measurement were obtained from (Cambridge Isotopes, USA). Column chromatography was performed using silica gel 60 (Fluka, St. Louis, MO, USA, particle size 0.063–0.2 mm, 70–230 mesh), polyamide-6 (50–160 μm), and Sephadex LH-20 (Sigma-Aldrich, Germany). Solvents used in chromatographic isolation of secondary metabolites were of analytical grade; n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), methanol (MeOH), and n-butanol (n-BuOH). Pre-coated silica gel 60 TLC plates used for the analysis of fractions and isolated compounds were purchased from Merck (Darmstadt, Germany). Visualization of the TLC plates was achieved with portable UV lamp (254 and 365 nm), AlCl3 and p-anisaldehyde′s spray reagent [28]
Plant material
Aerial parts of A. leucoclada were collected in October 2020 from the Qassim area, Kingdom of Saudi Arabia. The plant identity was verified by Ibrahim Aldakhil, area botanical expert, Qassim, KSA. Voucher sample number QPP-103 was deposited at the College of Pharmacy, Qassim University, KSA.
Preparation of extract
The dried aerial parts of A. leucoclada (700 g) were pulverized by a grinder and defatted with n-hexane (4 × 750 mL, at room temperature) to provide 1.7 g ATH extract. Afterwards, air-dried defatted powdered aerial parts were extracted with 80% methanol (4 × 1000 mL, at room temperature) to yield 45 g crude ATD extract.
Chromatographic isolation of phytochemicals
The defatted fraction (30 g) was fractionated on polyamide-6 using H2O-MeOH gradient to obtain two main sub-fractions after TLC monitoring; (A-I and A-II); A-I (eluted by 10–30% MeOH in H2O) was purified on Sephadex LH-20 column using MeOH to obtain compound 1 (5.0 mg). A-II (eluted with 70–100% MeOH in H2O) was chromatographed on a silica gel column using gradient elution with CH2Cl2-MeOH as eluent in 5% increments to obtain five sub-fractions; A-IIa (100 mg, eluted with 5% MeOH in CH2Cl2), A-IIb (40 mg, eluted with 15% MeOH in CH2Cl2), A-IIc (70 mg, eluted with 15% MeOH in CH2Cl2), A-IId (20 mg, eluted with 20% MeOH in CH2Cl2), A-IIe (25 mg, eluted with 25–30% MeOH in CH2Cl2). A-IIa was purified on Sephadex LH-20 using MeOH to afford two sub-fractions; the first one was purified on Sephadex LH-20 column using MeOH as eluent to obtain compound 2 (6.0 mg), the other was chromatographed on a silica gel column using mixtures of n-hexane–EtOAc as eluent in 5% increments to obtain mixture of compound 3 &4 (10.0 mg) and compound 5 (15.0 mg), respectively. A-IIb, A-IId and A-IIe were separately filtered through Sephadex LH-20 column using MeOH as eluent to obtain compounds 6 (8.0 mg), 8 (20.0 mg) & 9 (7.0 mg); respectively. A-IIc was recrystallized to obtain compound 7 (25 mg).
Gas chromatography–mass spectrometry analysis
GC–MS system: thermo scientific trace 1310 gas chromatograph attached with ISQ LT single quadrupole mass spectrometer. Column used for separation was db5-ms, 30m; 0.25 mm id (J&W scientific) with temperature program; 40°c (3 min)—280°c (5 min) at 5°c/min. -290°c (1 min) at 7.5°c/min. Ionization mode: EI. Ionization voltage: 70eV. Detector temperature: 300°c. Injector temperature was adjusted at 200°c. Helium was used as a carrier gas at 1 ml/min flow rate. identification of components was based on Willey and NIST mass spectral data base [29].
In vitro determination of COX-1 and COX-2 enzymatic activity
COX-1 and COX-2 inhibition assays are based on the detection of the florescence produced by prostaglandin G2 (i.e., the intermediate product produced by the COX-1 and 2 enzymes). The assay was performed by using COX-1 inhibitor screening kit (#K548-100, BioVision Inc.) and COX-2 inhibitor screening kit (#K547-100, BioVision Inc.) to measure in vitro COX-1 and COX-2 enzymatic activities respectively. Ibuprofen was used as a positive control. Samples and control were used at different concentrations: 0.01–100 ug/ml. According to the manufacturer’s instructions [2, 30], 10 μL of samples or Ibuprofen was added to each well, and 80 μL of reaction master mix was prepared (76 μL of COX buffer assay, 1 μL COX probe, 2 μL diluted COX cofactor, 1 μL COX-1 or COX-2) and added to each well, and the fluorescence was measured kinetically at (Ex/Em = 535/587 nm) at 25°C for 5–10 min. The experiments were performed in triplicate. The relative percentage of inhibition of COX-1 and COX-2 was calculated according to the following Equation:
Statistical analysis
All measurements were performed in triplicate and results were expressed as mean ± standard deviation (SD).
In silico studies
Isolated metabolites from defatted methanol (ATD) extract as well as compounds detected during GC–MS analysis of n-hexane (ATH) extract were docked into the active sites of COX-1 and COX-2 enzymes using pdb entries; 6Y3C [31] and 5IKV [32], respectively that were retrieved from Protein Data Bank (https://www4.rcsb.org/). Structures of all compound were downloaded from PubChem [33] [July, 2023] and their energies were minimized using Chem Bio 3D (Chem Bio Office Ultra 12.0 suite). Docking studies were performed using Autodock Vina in Pyrx [34]. XYZ coordinates were set as; 6Y3C: -30.39, -44.13, 7.77; 5IKV: 166.48, 183.18, 186.96 BIOVIA Discovery Studio visualizer v21.1.0.20298 (Dassault systems Biovia Corp., San Diego, CA, USA) and Pymol software [35] were used to visualize and analyze the docked ligand poses.
Results
Structural determination of isolated compounds
Chromatographic fractionation of defatted fraction of aerial parts (ATD) led to the isolation and characterization of nine known compounds. The structures of the isolated compounds were identified upon spectral data analysis (Spectroscopic data of compounds were shown in supplementary materials) and confirmed by comparison with those published in the literature (Fig. 1) as: 20-hdroxy ecdysone (1) [36], phytol (2) [37], β-sitosterol (3) [38], stigmasterol (4) [38], palmitic acid (5) [39], luteolin (6) [38, 40], β-sitosterol-3-O-β-d-glucopyranoside (7) [40, 41], pallidol (8) [42, 43] and isorhamnetin 3-O-β-galactopyranoside (9) [44, 45].
Fig. 1.

Chemical structures of the isolated compounds from the defatted methanolic extract (ATD) of A. leucoclada
Gas Chromatography–Mass Spectrometry (GC–MS) analysis
The chemical composition of the ATH extract of A. leucoclada was investigated using gas chromatography-mass spectrometry (GC–MS) analysis. A total of 27 metabolites were identified (Table 1); accounting for 85.97% of the total compounds. The identified compounds (Fig. 2) belong to three main classes including terpenoids, fatty acids and their derivatives and steroids (31.27, 21.18, and 20.25%; respectively). Other identified compounds included straight-chain hydrocarbons and derivatives (5.59%), 1,2-benzene dicarboxylic acid, bis(2-ethylhexyl) ester, 1,4-benzenediol, 2-(1,1-dımethylethyl)-5-(2-propenyl)-, and chamazulene (5.43, 1.11, and 1.14%). Phytol and cholest-5-en-3-ol (Fig. 2) were the main components (21.24, 12.50%; respectively).
Table 1.
Chemical profile of n-hexane extract (ATH) of A. leucoclada using GC–MS analysis
| NO | Compound | Chemical class | M.F | M.Wt | R.T (m) | Area (%) |
|---|---|---|---|---|---|---|
| 1. | 4-Thujanol, Cis-(± .) | Bicyclic monoterpene alcohol | C10H18O | 154 | 9.08 | 0.96 |
| 2. | 1,4-Benzenediol, 2-(1,1-dimethylethyl)-5-(2-propenyl)- | Hydroquinone | C13H18O2 | 206 | 17.31 | 1.11 |
| 3. | Spathulenol | Sesquiterpene | C15H24O | 220 | 18.82 | 0.77 |
| 4. | Neophytadiene | Diterpene | C20H38 | 278 | 24.50 | 3.48 |
| 5. | 2-Pentadecanone, 6,10,14-trimethyl- | Ketone | C18H36O | 268 | 24.60 | 3.51 |
| 6. | Chamazulene | Azulene derivative | C14H16 | 184 | 25.00 | 1.14 |
| 7. | 13-Heptadecyn-1-ol | Long-chain fatty alcohol | C17H32O | 252 | 25.37 | 1.17 |
| 8. | Hexadecanoic acid, methyl ester | Fatty acid ester | C17H34O2 | 270 | 26.30 | 6.45 |
| 9. | 9-Octadecenoic acid (Z) | Fatty acid | C18H34O2 | 282 | 27.63 | 0.65 |
| 10. | 7,10-Octadecadienoic acid, methyl ester | Fatty acid ester | C19H34O2 | 294 | 29.43 | 2.24 |
| 11. | 9-Octadecenoic Acid (Z)-, methyl ester | Fatty acid ester | C19H36O2 | 296 | 29.57 | 4.01 |
| 12. | Phytol | Acyclic diterpene alcohol | C20H40O | 296 | 29.77 | 21.24 |
| 13. | Heptadecanoic acid, 16-methyl-, methyl ester | Fatty acid ester | C19H38O2 | 298 | 30.09 | 1.86 |
| 14. | [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | Fatty acid ester | C21H38O2 | 322 | 30.64 | 0.84 |
| 15. | 2-Hydroxy-3-[(9e)-9-octadecenoyloxy]propyl (9e)-9-octadecenoate | Fatty acid ester | C39H72O5 | 620 | 33.84 | 1.09 |
| 16. | Villosin | Diterpene | C20H28O2 | 300 | 35.21 | 1.13 |
| 17. | 1-Heptatriacotanol | Alcohol | C37H76O | 536 | 36.24 | 0.91 |
| 18. | 9,19-Cyclolanostan-3-ol, 24,24-epoxymethano-, acetate | Steroid | C33H54O3 | 498 | 36.35 | 0.81 |
| 19. | Ethyl iso-allocholate | Steroid | C26H44O5 | 436 | 36.63 | 1.15 |
| 20. | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | Benzenedicarboxylic acid | C24H38O4 | 390 | 36.75 | 5.43 |
| 21. | 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E, E, E)- | Fatty acid ester | C57H104O6 | 884 | 39.39 | 1.12 |
| 22. | Glycidyl oleate | Fatty acid ester | C21H38O3 | 338 | 39.55 | 0.94 |
| 23. | Trilinolein | Triacylglycerol | C57H98O6 | 878 | 40.82 | 1.98 |
| 24. | Rhodopin | Carotenoid (tetraterpenoid) | C40H58O | 554 | 41.02 | 3.69 |
| 25. | β- Sitosterol | Steroid | C29H50O | 414 | 43.12 | 2.07 |
| 26. | Cholest-5-en-3-ol | Steroid | C27H46O | 414 | 43.79 | 12.50 |
| 27. | Ursodeoxycholic acid | Steroid | C24H40O4 | 392 | 45.34 | 3.72 |
| Terpenoids | 31,27 | |||||
| Steroids | 20.25 | |||||
| Fatty acids and fatty acids derivatives | 21.18 | |||||
| Straight-chain hydrocarbons and derivatives | 5.59 | |||||
| Others | 7.68 | |||||
| Total identified compounds % | 85.97 | |||||
Fig. 2.
The most characteristic compounds identified in n-hexane (ATH) extract of A. leucoclada using GC–MS analysis
In vitro determination of COX-1 and COX-2 inhibitory activity
In the present study, inhibitory effects of the defatted methanolic extract (ATD) and n-hexane (ATH) extract against COX-1 and COX-2 enzymes were examined in vitro. The results (Fig. 3A and B) showed that the tested extracts displayed inhibition of COX-1 and COX-2 enzymes in a concentration dependent manner being more selective towards COX-2 enzyme. Where IC50 of ATD were 41.22, 14.4 μg/mL and of ATH were 16.74 and 5.96 μg/mL, against COX-1 and COX-2, respectively while ibuprofen IC50 was 6.88 and 2.68, respectively (Table 2).
Fig. 3.
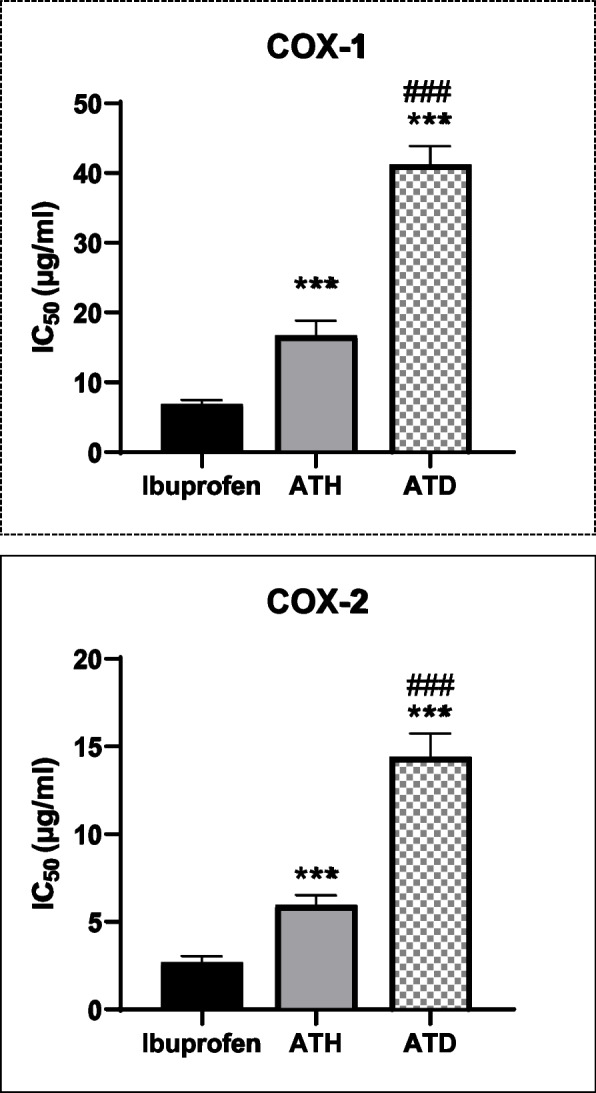
The in vitro inhibitory effect of n-hexane (ATH) and defatted methanol (ATD) extracts of A. leucoclada against COX-1 (A) and COX-2 (B) enzymes using Ibuprofen as a positive control. Data in the figures expressed mean ± SEM (n = 3). ***P < 0.001 consider statistically significant compared to ibuprofen. ###P < 0.001 consider statistically significant compared to ATH group using one way ANOVA followed by Tukey’s post hoc test
Table 2.
IC50 (μg/ml) values against COX-1 and COX-2 for n-hexane and defatted methanol extracts from A. Leucoclada and the COX-1/COX-2 selectivityy index
| COX-1 | COX-2 | SI | |
|---|---|---|---|
| Ibuprofen | 6.88 ± 0.27 | 2.68 ± 0.1 | 2.56 |
| ATH | 16.74 ± 0.94*** | 5.96 ± 0.22*** | 2.81 |
| ATD | 41.22 ± 1.18***, ### | 14.4 ± 0.54***, ### | 2.86 |
Defatted methanol extract (ATD); n-hexane extract (ATH); Cyclooxygenase (COX); The half of the inhibitory concentration (IC50). Selectivity index (SI) = IC50 COX-1/IC50 COX-2. Data in the Table expressed mean ± SEM (n = 3). Where ***P < 0.001 consider statistically significant compared to ibuprofen and ###P < 0.001 consider statistically significant compared to ATH group using one-way ANOVA followed by Tukey’s post hoc test
In silico studies
Isolated compounds from ATD extract along with compounds identified by GC–MS profiling of ATH were subjected to molecular docking with COX-1 and COX-2 proteins. The majority of the compounds manifested high binding affinities and good binding interactions. The docked purified metabolites from ATD showed scores for COX-1 and COX-2 ranged from -9 to -6.4 and -8.5 to -6.6 kcal/mol, respectively, while docking scores of metabolites that identified by GC–MS in ATH ranged from -8.9 to -5.5 and -8.3 to -5.1 kcal/mol, respectively (Table 3), compared with binding scores of the drug reference; Ibuprofen for COX-1 and 2 (-6.9 and -7.5 kcal/mol, respectively). The flavone; luteolin exhibited the highest binding affinity to COX-2 (-8.5 kcal/mol) followed by the steroids; β-sitosterol-3-O-β-D-glucoside (-8.4 kcal/mol), stigmasterol (-8.4 kcal/mol), 9,19-Cyclolanostan-3-ol, 24,24-epoxymethano-, acetate (-8.3 kcal/mol), and 20-hydroxy ecdysone (-8.1 kcal/mol). Among terpenoids, the diterpene villosin and the tetraterpene rhodopsin revealed utmost binding affinities (-7.7 and -7.6 kcal/mol, respectively), while 2-Hydroxy-3-[(9e)-9-octadecenoyloxy]propyl (9e)-9-octadecenoate unveiled the best score (-7.1 kcal/mol) among other fatty acid esters.
Table 3.
Docking scores (kcal/mol) of isolated compounds from defatted methanolic (ATD) extract and compounds detected by GC–MS in n-hexane (ATH) extract of A. leucoclada against cyclooxygenase enzymes
| No | Compound | COX-1 | COX-2 |
|---|---|---|---|
| Compounds isolated from ATD extract of A. leucoclada | |||
| 1 | 20-hydroxy ecdysone | -8.0 | -8.1 |
| 4 | Stigmasterol | -9.0 | -8.4 |
| 5 | Palmitic acid | -6.4 | -6.6 |
| 6 | Luteolin | -8.2 | -8.5 |
| 7 | β-sitosterol-3-O- β-D glucoside | -8.1 | -8.4 |
| 8 | Pallidol | -8.5 | -8.1 |
| 9 | Isorhamnetin3-O-β-D-galactopyranoside | -7.7 | -7.8 |
| Compounds detected in ATH extract of A. leucoclada | |||
| 1 | 4-Thujanol, Cis | -5.8 | -5.4 |
| 2 | 1,4-Benzenediol, 2-(1,1-dimethylethyl)-5-(2-propenyl)- | -6.4 | -6.3 |
| 3 | Spathulenol | -5.9 | -6.5 |
| 4 | Neophytadiene | -6.5 | -5.5 |
| 5 | 2-Pentadecanone, 6,10,14-trimethyl- | -6.2 | -7.0 |
| 6 | Chamazulene | -7.7 | -7.6 |
| 7 | 13-Heptadecyn-1-ol | -6.4 | -6.5 |
| 8 | Hexadecanoic acid, methyl ester | -6.1 | -6.1 |
| 9 | 9-Octadecenoic acid (Z) | -5.5 | -6.7 |
| 10 | 7,10-Octadecadienoic acid, methyl ester | -7.3 | -6.0 |
| 11 | 9-Octadecenoic Acid (Z)-, methyl ester | -7.1 | -6.1 |
| 12 | Phytol | -6.2 | -5.6 |
| 13 | Heptadecanoic acid, 16-methyl-, methyl ester | -6.5 | -6.4 |
| 14 | [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | -6.4 | -5.6 |
| 15 | 2-Hydroxy-3-[(9e)-9-octadecenoyloxy]propyl (9e)-9-octadecenoate | -6.5 | -7.1 |
| 16 | Villosin | -7.3 | -7.7 |
| 17 | 1-Heptatriacotanol | -6.5 | -5.2 |
| 18 | 9,19-Cyclolanostan-3-ol, 24,24-epoxymethano-, acetate | -8.4 | -8.3 |
| 19 | Ethyl iso-allocholate | -7.4 | -7.4 |
| 20 | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | -5.7 | -6.3 |
| 21 | 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E, E, E)- | -6.1 | -6.7 |
| 22 | Glycidyl oleate | -7.3 | -5.1 |
| 23 | Trilinolein | -6.6 | -6.7 |
| 24 | Rhodopin | -8.0 | -7.6 |
| 25 | β-sitosterol | -8.9 | -7.5 |
| 26 | Cholest-5-en-3-ol | -7.6 | -7.4 |
| 27 | Ursodeoxycholic acid | -7.4 | -7.4 |
Discussion
Reviewing the relevant literature several studies were found reporting diverse chemical structures of the metabolic contents of some Atriplex species. While, in regards of A. leucoclada, previous investigations were not sufficient to describe the chemical profile of the plant. To achieve this purpose, the chemical composition of both defatted methanol (ATD) and hexane (ATH) extracts were investigated. The current findings showed that all purified compounds, except for compound (1), were isolated for the first time from A. leucoclada [46]. Furthermore, compounds (6) and (8) were isolated for the first time from this genus. Compound (1) was previously obtained from other Atriplex species as A. inflata and A. nummularia [16, 17], compounds (3) and (4) were previously reported in A. stocksii [15], compound (7) in A. canescens [47] and compound (9) in A. inflate [46], while compound (2) and (5) were identified by GC–MS only in the methanol extract of A. halimus [48, 49].
Interestingly, this study is the first to report GC–MS investigation of A. leucoclada. The current GC–MS analysis results enabled the tentative qualitative identification of numerous phytochemicals in ATH. The compounds identified have been interpreted as given in Table 1. Oxygenated and non-oxygenated hydrocarbons, alcohols, phenolics, steroidal and terpenoidal compounds were identified. Among the isolated compounds: phytol (21.24%) and cholest-5-en-3-ol (12.50%) are the major detected compounds. These results were similar to those reported for GC–MS analysis for A. lindleyi Moq [50].
Based upon the above recorded results, the anti-inflammatory activity of A. leucoclada may be attributed to its content of palmitic (5) and β-sitosterol (3) that were previously reported to reduce expression of COX- 1 and COX-2 [51–53]. Moreover, 20-hdroxy ecdysone (1) [54], stigmasterol (4) [55], luteolin (6) [56], β-sitosterol-3-O-β-D-glucopyranoside (7) [57], and isorhamnetin 3-O-β-galactopyranoside (9) [58] were also reported to suppress COX-2 expression. Also, computational study on phytol (2) indicated its efficient interaction with COX-1 and 2 enzymes [59]. Concerning pallidol (8), a resveratrol dimer, it was reported to have weak activity against COX enzymes [60, 61]
In regard of GC–MS results, the high anti-inflammatory activity of the ATH fraction may be attributed to the synergistic effect of certain compounds e.g. 2-hexadecen-1-ol [62] and hexadecanoic acid, methyl ester [63]. Also, ursodeoxycholic acid was reported to show COX-2 inhibition [64]. From another point of view, neophytadiene significantly inhibited NO production and inflammatory cytokines TNF-α, IL-6 and IL-10 both in vitro and in vivo [65, 66], azulene derivative reverses osteoarthritic inflammation through regulation of matrix metalloproteinases and NF-kβ pathway in in vitro and in vivo models [67] and villosin exerted inhibitory effects against NO production with IC50 = 15.5 μM [68].
Most of the compounds identified in the ATD or ATH extracts were previously reported to be more selective for COX-2. This is compatible with the current results that indicated high selectivity towards COX-2 enzyme. As selective COX-2, anti-inflammatory agents have minimum GIT side effects, and so they are more appreciated as per safety concern. Accordingly, the current findings acknowledge the metabolic content of A. leucoclada as a rich mixture of chemical entities that have promising potential of anti-inflammatory activity with limited side effects.
It is already stated that, computational studies played an effective role in drug development as it can provides a fast, cheap and easy method for expectation of the possible promising bioactive drugs. Herein, we examined the binding affinity of isolated (from ATD extract) as well as GC–MS identified compounds (in ATH extract) in this plant with key anti-inflammatory targets. Analysis of the binding with the two proteins COX-1 and COX-2 revealed variable binding patterns depending on the nature of the compounds. Steroids that constitute 44.44% of isolated compounds from ATD extract and 20.25% of detected compounds in ATH extract exhibited docking score with COX-2 ranged from -8.4 to -7.4 kcal/mol. During this study, we explored some data that can be gleaned from analysis of steroids conformation and interactions at the cyclooxygenase active site. Previous studies that examined the binding of the substrate “arachidonic acid” into COX active site suggested that the active site could be viewed as including three parts; proximal, central, and distal binding pockets and that the distal and proximal binding pockets are important for stabilization of the substrate, while the central part that contains the catalytic Tyr385 is the place where substrate is transformed into PGG2 [69]. Analysis of our docking results unveiled that steroids occupied the proximal binding pocket and may extends towards the central binding pocket. It was noted that presence of more hydrophilic groups at C-3 may contribute to the increased binding affinity as exemplified in β-sitosterol3-O-β-D-glucopyranoside (-8.4 kcal/mol) with glucosylation at C-3 where it exhibited flat inverted conformation compared to that of β-sitosterol, furthermore, one of the sugar hydroxyl groups formed two hydrogen bonds with His90 and Gln192 in the side pocket, a pocket that was generated in COX-2 due to Ile523 mutation in COX-1 to the smaller Val523 in COX-2 [69] (Fig. 4B), while β-sitosterol (-7.4 kcal/mol) exhibited only covalent bonds with Tyr355 at the constriction located near the entrance of the active site and with His90, Ser353, and His356 (Fig. 4A). Similarly, presence of carbonyl group at C-6 and hydroxyl groups at C-14, C-20, C-22, and C-25 as in the case of 20-hydroxyecdysone (-8.4 kcal/mol) may play a role in improving its binding affinity via formation of two hydrogen bonds between His356 and OH groups at C-20 and C-25, respectively and oxygen of the carbonyl group at C-6 exhibited two hydrogen bonds with Phe 580, and Ser581, in addition to hydrogen bonding between hydroxyl group at C-14 and Gln350 (Fig. 4C). Additionally, the current findings highlighted the flavonoid luteolin that was one of the lead metabolites exhibiting the highest binding affinity to COX-2 (-8.5 kcal/mol); analysis of the binding orientation of luteolin revealed that it formed one hydrogen bond with Trp387 in the catalytic region of the active site and another hydrogen bond with Asn382 in addition to π-π stacking with His388 and three π-alkyl interactions with Ala202, His207, and His386 (Fig. 4D).
Fig. 4.
3D docking view of β-sitosterol (A), β-sitosterol-3-O-β-D-glucopyranoside (B), 20-hydroxy-ocdysone (C), and luteolin (D) in the active site of COX-2. Labeled residues that constitute the proximal (yellow), central (magenta), and distal (cyan) binding pockets and other interacting residues in the active site (gray) are depicted in line models. Ligands are represented by green stick model. Hydrogen bond (blue), π-π stacking (pink), alkyl (yellow), and carbon-hydrogen bond (green) interactions are depicted in dashed lines
Conclusion
The current study presented a detailed chromatographic exploration of A. leucoclada with successful isolation of nine compounds, eight of which were isolated for the first time from this species, moreover, GC–MS analysis identified the non-polar components of the hexane extract of the plant. These findings will add to chemical profile of this species, as well as to Atriplex genus. Additionally, COX-1 and COX-2 inhibitory activity testing noted the n-hexane and defatted methanol extracts for selective inhibitory activity against COX-2 enzyme. The molecular docking studies revealed high binding affinities and good binding interactions of most of the compounds with binding scores ranged from -8.5 to -6.6 kcal/mol for isolated compounds from ATD and -8.3 to -5.1 kcal/mol for compounds identified by GC–MS in ATH. Accordingly, A. leucoclada is suggested as a valuable source of safe anti-inflammatory agents. Future studies are recommended for evaluating the anti-inflammatory potential of the highlighted metabolites whether, alone or in combination with commercially used anti-inflammatory drugs.
Supplementary Information
Additional file 1: Figure S1. 2D Docking poses of β-sitosterol (A), β-sitosterol-3-O-β-D-glucopyranoside (B), 20-hydroxy-ocdysone (C), and luteolin (D) in the active site of COX-2.
Acknowledgements
There is none to be clarified.
Authors’ contributions
H.S.A, N.A: Isolation and identification of compounds, Investigation, Writing original draft preparation. E.I.M: Theoretical calculation, writing original draft preparation. E.A: Resources, Review, Editing and Supervision. A.S.M, M.A, S.A.A: Reviewing and Editing. All authors have read and approved the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
All obtained data have been included in the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hayam S. Ahmed and Enas I. A. Mohamed contributed equally to this work.
References
- 1.Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaaeldin R, Hassan HA, Abdel-Rahman IM, Mohyeldin RH, Youssef N, Allam AE, Abdelwahab SF, Zhao Q-L, Fathy M. A new EGFR inhibitor from ficus benghalensis exerted potential anti-inflammatory activity via Akt/PI3K pathway inhibition. Curr Issues Mol Biol. 2022;44(7):2967–2981. doi: 10.3390/cimb44070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgens A, Sharman T: Corticosteroids. In: StatPearls [Internet]. edn.: StatPearls Publishing; 2021. [PubMed]
- 5.Bozimowski G. A review of nonsteroidal anti-inflammatory drugs. AANA J. 2015;83(6):425–433. [PubMed] [Google Scholar]
- 6.Macedo S, Ferreira L, Perazzo F, Carvalho JT. Anti-inflammatory activity of Arnica Montana 6cH: preclinical study in animals. Homeopathy. 2004;93(02):84–87. doi: 10.1016/j.homp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Alghaithy A, El-Beshbishy H, AbdelNaim A, Nagy A, Abdel-Sattar E. Anti-inflammatory effects of the chloroform extract of Pulicaria guestii ameliorated the neutrophil infiltration and nitric oxide generation in rats. Toxicol Ind Health. 2011;27(10):899–910. doi: 10.1177/0748233711399320. [DOI] [PubMed] [Google Scholar]
- 8.Fathy M, Fawzy MA, Hintzsche H, Nikaido T, Dandekar T, Othman EM. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Molecules. 2019;24(21):3979. doi: 10.3390/molecules24213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaterra G, Kelber O, Weiser D, Kinscherf R. Mechanisms of the anti-proliferative and anti-inflammatory effects of the herbal fixed combination STW 5 (Iberogast®) on colon adenocarcinoma (HT29) cells in vitro. Phytomedicine. 2013;20(8–9):691–698. doi: 10.1016/j.phymed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Grabowska K, Pietrzak W, Paśko P, Sołtys A, Galanty A, Żmudzki P, Nowak R, Podolak I. Antihyaluronidase and antioxidant potential of Atriplex sagittata Borkh. in relation to phenolic compounds and triterpene saponins. Molecules. 2023;28(3):982. doi: 10.3390/molecules28030982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafari M, Tahmoures M, Ehteram M, Ghorbani M, Panahi F. Soil Erosion Control in Drylands. Cham: Springer; 2022.
- 12.Wright K, Pike O, Fairbanks D, Huber C. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. J Food Sci. 2002;67(4):1383–1385. doi: 10.1111/j.1365-2621.2002.tb10294.x. [DOI] [Google Scholar]
- 13.Kamal Z, Ullah F, Ayaz M, Sadiq A, Ahmad S, Zeb A, Hussain A, Imran M. Anticholinesterse and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of atriplex laciniata L: potential effectiveness in Alzheimer’s and other neurological disorders. Biolog Res. 2015;48:1–11. doi: 10.1186/s40659-015-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Aasr M, Kabbash A, El-Seoud KAA, Al-Madboly LA, Ikeda T. Antimicrobial and Immunomodulatory activities of flavonol glycosides isolated from Atriplex halimus L. herb. J Pharmaceut Sci Res. 2016;8(10):1159. [Google Scholar]
- 15.Siddiqui BS, Ahmed S. Khan MA-U: triterpenoids of Atriplex stocksii. Phytochemistry. 1994;37(4):1123–1125. doi: 10.1016/S0031-9422(00)89541-2. [DOI] [Google Scholar]
- 16.Ben Nejma A, Znati M, Nguir A, Daich A, Othman M, Lawson AM, Ben Jannet H. Phytochemical and biological studies of Atriplex inflata f. Muell.: Isolation of secondary bioactive metabolites. J Pharmacy Pharmacol. 2017;69(8):1064–1074. doi: 10.1111/jphp.12735. [DOI] [PubMed] [Google Scholar]
- 17.Keckeis K, Sarker S, Dinan L. Phytoecdysteroids from Atriplex nummularia. Fitoterapia. 2000;71(4):456–458. doi: 10.1016/S0367-326X(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 18.Ali B, Tabassum R, Riaz N, Yaqoob A, Khatoon T, Tareen RB, Jabbar A, Nasim F-u-H, Saleem M. Bioactive triterpenoids from Atriplex lasiantha. J Asian Natur Products Res. 2015;17(8):843–850. doi: 10.1080/10286020.2015.1008463. [DOI] [PubMed] [Google Scholar]
- 19.Kamal Z, Ullah F, Ahmad S, Ayaz M, Sadiq A, Imran M, Ahmad S, Rahman FU, Zeb A. Saponins and solvent extracts from Atriplex laciniata L. exhibited high anthelmintic and insecticidal activities. J Tradit Chinese Med. 2017;37(5):599–606. doi: 10.1016/S0254-6272(17)30312-6. [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Kim H, Ju E, Lee S-G, Kong C-S, Seo Y. Antiinflammatory activity of solvent-partitioned fractions from Atriplex gmelinii CA Mey in LPS-stimulated RAW264 7 macrophages. Journal of Life Science. 2017;27(2):187–193. doi: 10.5352/JLS.2017.27.2.187. [DOI] [Google Scholar]
- 21.Chikhi I, Allali H, Dib MEA, Medjdoub H, Tabti B. Antidiabetic activity of aqueous leaf extract of Atriplex halimus L. (Chenopodiaceae) in streptozotocin–induced diabetic rats. Asian Pac J Trop Dis. 2014;4(3):181–184. doi: 10.1016/S2222-1808(14)60501-6. [DOI] [Google Scholar]
- 22.Zohra T, Ovais M, Khalil AT, Qasim M, Ayaz M, Shinwari ZK, Ahmad S, Zahoor M. Bio-guided profiling and HPLC-DAD finger printing of Atriplex lasiantha Boiss. BMC Complement Altern Med. 2019;19(1):1–14. doi: 10.1186/s12906-018-2416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slama K, Boumendjel M, Taibi F, Boumendjel A, Messarah M. Atriplex halimus aqueous extract abrogates carbon tetrachloride-induced hepatotoxicity by modulating biochemical and histological changes in rats. Arch Physiol Biochem. 2020;126(1):49–60. doi: 10.1080/13813455.2018.1489852. [DOI] [PubMed] [Google Scholar]
- 24.Zahran MA. Climate-Vegetation: Afro-Asian Mediterranean and Red sea coastal lands. London: Springer Dordrecht; 2010. [Google Scholar]
- 25.Mikaili P, Shayegh J, Asghari MH. Review on the indigenous use and ethnopharmacology of hot and cold natures of phytomedicines in the Iranian traditional medicine. Asian Pac J Trop Biomed. 2012;2(2):S1189–S1193. doi: 10.1016/S2221-1691(12)60382-7. [DOI] [Google Scholar]
- 26.Alam H, Zamin M, Adnan M, Ahmad N, Nawaz T, Saud S, Basir A, Liu K, Harrison MT, Hassan S. Evaluating the resistance mechanism of Atriplex leucoclada (Orache) to salt and water stress; A potential crop for biosaline agriculture. Front Plant Sci. 2022;13:948736. [DOI] [PMC free article] [PubMed]
- 27.El-Sayed M. Molluscicidal saponins from Atriplex leucoclada. Zagazig J Pharmaceut Sci. 1995;4(2):143–146. doi: 10.21608/zjps.1995.186266. [DOI] [Google Scholar]
- 28.Waksmundzka-Hajnos M, Sherma J, Kowalska T. Thin layer chromatography in phytochemistry. Boca Raton: CRC Press; 2008. [Google Scholar]
- 29.Shafaie F, Aramideh S, Valizadegan O, Safaralizadeh MH, Pesyan NN. GC/MS analysis of the essential oils of Cupressus arizonica Greene, Juniperus communis L. and Mentha longifolia L. Bull Chem Soc Ethiopia. 2019;33(3):389–400. doi: 10.4314/bcse.v33i3.1. [DOI] [Google Scholar]
- 30.Al-Khalaf AA, Hassan HM, Alrajhi AM, Mohamed RAEH, Hozzein WN. Anti-cancer and anti-inflammatory potential of the green synthesized silver nanoparticles of the red sea sponge phyllospongia lamellosa supported by metabolomics analysis and docking study. Antibiotics. 2021;10(10):1155. doi: 10.3390/antibiotics10101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miciaccia M, Belviso BD, Iaselli M, Cingolani G, Ferorelli S, Cappellari M, Loguercio Polosa P, Perrone MG, Caliandro R, Scilimati A. Three-dimensional structure of human cyclooxygenase (h COX)-1. Sci Rep. 2021;11(1):4312. doi: 10.1038/s41598-021-83438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando BJ, Malkowski MG. Substrate-selective inhibition of cyclooxygeanse-2 by fenamic acid derivatives is dependent on peroxide tone. J Biol Chem. 2016;291(29):15069–15081. doi: 10.1074/jbc.M116.725713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B. PubChem 2023 update. Nucleic Acids Res. 2023;51(D1):D1373–D1380. doi: 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lill MA, Danielson ML. Computer-aided drug design platform using PyMOL. J Comput Aided Mol Des. 2011;25:13–19. doi: 10.1007/s10822-010-9395-8. [DOI] [PubMed] [Google Scholar]
- 36.Girault J, Lafont RD. The complete 1H-NMR assignment of ecdysone and 20-hydroxyecdysone. J Insect Physiol. 1988;34(7):701–706. doi: 10.1016/0022-1910(88)90080-7. [DOI] [Google Scholar]
- 37.Thang PT, Dung NA, Giap TH, Oanh VTK, Hang NTM, Huong TT, Thanh LN, Huong DTM, Van Cuong P. Preliminary study on the chemical constituents of the leaves of Macaranga balansae Gagnep. Vietnam J Chem. 2018;56(5):632–636. doi: 10.1002/vjch.201800061. [DOI] [Google Scholar]
- 38.Elwekeel AH, Amin E, Khairallah A, Moawad AS. Terminalia arjuna flowers: secondary metabolites and antifungal activity. Pharmaceutical Sciences Asia. 2022;49(3):249–256. doi: 10.29090/psa.2022.03.21.149. [DOI] [Google Scholar]
- 39.Ruksilp T. Fatty acids and an Ester from the leaves of Millettia utilis Dunn. Naresuan Univ J: Sci Technol (NUJST) 2020;28(3):63–68. [Google Scholar]
- 40.Afifi NI, Moawad AS, Hetta MH, Mohammed RM. Phytochemical composition and antioxidant activity of two species related to family Arecaceae. Pharm Sci Asia. 2022;49(1):43–50. doi: 10.29090/psa.2022.01.21.045. [DOI] [Google Scholar]
- 41.Sadikun A, Aminah I, Ismail N, Ibrahim P. Sterols and sterol glycosides from the leaves of Gynura procumbens. Nat Prod Sci. 1996;2(1):19–23. [Google Scholar]
- 42.Li L, Henry GE, Seeram NP. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem. 2009;57(16):7282–7287. doi: 10.1021/jf901716j. [DOI] [PubMed] [Google Scholar]
- 43.Liu W-B, Hu L, Hu Q, Chen N-N, Yang Q-S, Wang F-F. New resveratrol oligomer derivatives from the roots of Rheum lhasaense. Molecules. 2013;18(6):7093–7102. doi: 10.3390/molecules18067093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Güvenalp Z, Demirezer L. Flavonol glycosides from Asperula arvensis L. Turk J Chem. 2005;29(2):163–169. [Google Scholar]
- 45.Wang Y, Guo T, Li JY, Zhou SZ, Zhao P, Fan MT: Four flavonoid glycosides from the pulps of Elaeagnus angustifolia and their antioxidant activities. In: Advanced Materials Research: 2013: Trans Tech Publ; 2013: 16–20.
- 46.El-Kersh DM, El-Sakhawy FS, Abou-Hussein DR, Sleem AA. Anabolic and androgenic effects of certain Atriplex species grown in Egypt. Egyptian J Biomed Sci. 2013;40:97–113. [Google Scholar]
- 47.Ali B, Musaddiq S, Iqbal S, Rehman T, Shafiq N, Hussain A. The therapeutic properties, ethno pharmacology and phytochemistry of Atriplex species: a review. Pakistan J Biochem Biotechnol. 2021;2(1):49–64. doi: 10.52700/pjbb.v2i1.38. [DOI] [Google Scholar]
- 48.Bouzidi A, Zayen N, Saidana D, Gam W, Achour L, Mighri Z. Protein contents, total lipids and fatty acids profiles of Tunisian halophyte species: Limonium echioides, Tamarix bovena and Atriplex halimus. Tunisian J Med Plants Natural Prod. 2012;8(1):82–89. [Google Scholar]
- 49.Morad MY, El-Sayed H, El-Khadragy MF, Abdelsalam A, Ahmed EZ, Ibrahim AM. Metabolomic profiling, antibacterial, and molluscicidal properties of the medicinal plants Calotropis procera and Atriplex halimus: in silico molecular docking study. Plants. 2023;12(3):477. doi: 10.3390/plants12030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matloub A, El Souda S, Hamed M. Phytoconstituent of petroleum ether extract of Atriplex lindleyi Moq aerial part and its hepato-renal protection. Planta Medica. 2011;77(12):PF36. doi: 10.1055/s-0031-1282424. [DOI] [Google Scholar]
- 51.Zhang Y, Mills G, Nair M. Cyclooxygenase inhibitory and antioxidant compounds from the fruiting body of an edible mushroom. Agrocybe aegerita Phytomed. 2003;10(5):386–390. doi: 10.1078/0944-7113-00272. [DOI] [PubMed] [Google Scholar]
- 52.Yuan L, Zhang F, Shen M, Jia S, Xie J. Phytosterols suppress phagocytosis and inhibit inflammatory mediators via ERK pathway on LPS-triggered inflammatory responses in RAW264. 7 macrophages and the correlation with their structure. Foods. 2019;8(11):582. doi: 10.3390/foods8110582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C, Shen J-L, Liang C-S, Sun Z-C, Jiang H-F. First discovery of beta-sitosterol as a novel antiviral agent against white spot syndrome virus. Int J Mol Sci. 2022;23(18):10448. doi: 10.3390/ijms231810448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y, Zhao DL, Liu ZX, Sun XH, Li Y. Beneficial effect of 20-hydroxyecdysone exerted by modulating antioxidants and inflammatory cytokine levels in collagen-induced arthritis: a model for rheumatoid arthritis. Mol Med Rep. 2017;16(5):6162–6169. doi: 10.3892/mmr.2017.7389. [DOI] [PubMed] [Google Scholar]
- 55.Bakrim S, Benkhaira N, Bourais I, Benali T, Lee L-H, El Omari N, Sheikh RA, Goh KW, Ming LC, Bouyahya A. Health benefits and pharmacological properties of stigmasterol. Antioxidants. 2022;11(10):1912. doi: 10.3390/antiox11101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziyan L, Yongmei Z, Nan Z, Ning T, Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007;73(03):221–226. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Jayaprakasam B, Seeram NP, Olson LK, DeWitt D, Nair MG. Insulin secretion and cyclooxygenase enzyme inhibition by cabernet sauvignon grape skin compounds. J Agric Food Chem. 2004;52(2):228–233. doi: 10.1021/jf034616u. [DOI] [PubMed] [Google Scholar]
- 58.Kim D-W, Cho H-I, Kim K-M, Kim S-J, Choi JS, Kim YS, Lee S-M. Isorhamnetin-3-O-galactoside protects against CCl4-induced hepatic injury in mice. Biomol Therapeut. 2012;20(4):406. doi: 10.4062/biomolther.2012.20.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam MT, Ayatollahi SA, Zihad SNK, Sifat N, Khan MR, Paul A, Salehi B, Islam T, Mubarak MS, Martins N. Phytol anti-inflammatory activity: pre-clinical assessment and possible mechanism of action elucidation. Cell Mol Biol (Noisy-le-grand) 2020;66(4):264–269. doi: 10.14715/cmb/2020.66.4.31. [DOI] [PubMed] [Google Scholar]
- 60.Waffo-Teguo P, Lee D, Cuendet M, Mérillon J-M, Pezzuto JM, Kinghorn AD. Two new stilbene dimer glucosides from grape (vitis v inifera) cell cultures. J Nat Prod. 2001;64(1):136–138. doi: 10.1021/np000426r. [DOI] [PubMed] [Google Scholar]
- 61.Cichewicz RH, Kouzi SA, Hamann MT. Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea. J Nat Prod. 2000;63(1):29–33. doi: 10.1021/np990266n. [DOI] [PubMed] [Google Scholar]
- 62.Chansiw N, Chotinantakul K, Srichairatanakool S. Anti-inflammatory and antioxidant activities of the extracts from leaves and stems of Polygonum odoratum lour. AntiInflammatory Antiallergy Agents Med Chem. 2019;18(1):45–54. doi: 10.2174/1871523017666181109144548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Othman AR, Abdullah N, Ahmad S, Ismail IS, Zakaria MP. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L plant root. BMC Complement Altern Med. 2015;15:1–10. doi: 10.1186/s12906-015-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khare S, Cerda S, Wali RK, Von Lintig FC, Tretiakova M, Joseph L, Stoiber D, Cohen G, Nimmagadda K, Hart J. Ursodeoxycholic acid inhibits Ras mutations, wild-type Ras activation, and cyclooxygenase-2 expression in colon cancer. Can Res. 2003;63(13):3517–3523. [PubMed] [Google Scholar]
- 65.Bhardwaj M, Sali VK, Mani S, Vasanthi HR. Neophytadiene from Turbinaria ornata suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and Sprague Dawley rats. Inflammation. 2020;43:937–950. doi: 10.1007/s10753-020-01179-z. [DOI] [PubMed] [Google Scholar]
- 66.Amin E, Elwekeel A, Alshariedh NF, Abdel-Bakky MS, Hassan MH. GC-MS analysis and bioactivities of the essential oil of Suaeda aegyptiaca. Separations. 2022;9(12):439. doi: 10.3390/separations9120439. [DOI] [Google Scholar]
- 67.Ma D, He J, He D. Chamazulene reverses osteoarthritic inflammation through regulation of matrix metalloproteinases (MMPs) and NF-kβ pathway in in-vitro and in-vivo models. Biosci Biotechnol Biochem. 2020;84(2):402–410. doi: 10.1080/09168451.2019.1682511. [DOI] [PubMed] [Google Scholar]
- 68.Hu H-J, Zhou Y, Han Z-Z, Shi Y-H, Zhang S-S, Wang Z-T, Yang L. Abietane diterpenoids from the roots of Clerodendrum trichotomum and their nitric oxide inhibitory activities. J Nat Prod. 2018;81(7):1508–1516. doi: 10.1021/acs.jnatprod.7b00814. [DOI] [PubMed] [Google Scholar]
- 69.Rouzer CA, Marnett LJ. Structural and chemical biology of the interaction of cyclooxygenase with substrates and non-steroidal anti-inflammatory drugs. Chem Rev. 2020;120(15):7592–7641. doi: 10.1021/acs.chemrev.0c00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. 2D Docking poses of β-sitosterol (A), β-sitosterol-3-O-β-D-glucopyranoside (B), 20-hydroxy-ocdysone (C), and luteolin (D) in the active site of COX-2.
Data Availability Statement
All obtained data have been included in the manuscript.




