Abstract
Artificial intelligence (AI) has shown excellent diagnostic performance in detecting various complex problems related to many areas of healthcare including ophthalmology. AI diagnostic systems developed from fundus images have become state-of-the-art tools in diagnosing retinal conditions and glaucoma as well as other ocular diseases. However, designing and implementing AI models using large imaging data is challenging. In this study, we review different machine learning (ML) and deep learning (DL) techniques applied to multiple modalities of retinal data, such as fundus images and visual fields for glaucoma detection, progression assessment, staging and so on. We summarize findings and provide several taxonomies to help the reader understand the evolution of conventional and emerging AI models in glaucoma. We discuss opportunities and challenges facing AI application in glaucoma and highlight some key themes from the existing literature that may help to explore future studies. Our goal in this systematic review is to help readers and researchers to understand critical aspects of AI related to glaucoma as well as determine the necessary steps and requirements for the successful development of AI models in glaucoma.
Keywords: Artificial intelligence, Glaucoma, Machine learning, Deep learning
Introduction
Glaucoma is an optic neuropathy accompanied by characteristic structural and functional changes [1]. It affects over 90 million individuals worldwide and constitutes the second leading cause of blindness and subsequent disability overall [2, 3]. The number of people aged 40–80 years with glaucoma worldwide was estimated to be 64.3 million in 2013, with projections that this number will increase to 76.0 million in 2020, and 111.8 million in 2040 [4]. Because older people make up the fastest-growing part of the US population, glaucoma will become even more prevalent in the US in the coming decades. As such, population-based screening for glaucoma becomes critical.
Glaucoma detection is challenging particularly at the early stages of the disease; however, early detection may lead to slowing its progression and future vision loss [5]. A major challenge in detecting glaucoma is that signs and symptoms may manifest only when significant vision has been already lost. Therefore, it is critical to diagnose glaucoma early to prevent future vision loss [6]. Glaucoma diagnosis typically includes assessment of the optic nerve head (ONH) through retinal examination, intraocular pressure (IOP) measurement, evaluation of visual fields (VFs), and examining other related factors.
Assessing the ONH in glaucoma is an important diagnostic step, as the primary implication for glaucoma is glaucomatous optic neuropathy (GON), which is widely identified through fundus photographs or optical coherence tomography (OCT) images. Currently, fundus photography is better suited for glaucoma screening because fundus cameras are affordable and more importantly, portable. However, development of low-cost and portable OCT systems is advancing as well, and these portable OCT technologies are poised to gain popularity in the coming years. Fundus photography has been the most established modality for documenting the status of the optic nerve and detecting GON since 1857. As a result, large, annotated datasets of fundus photographs are currently available and importantly, they are appropriate for machine learning (ML) and deep learning (DL) models. In contrast, clinical evaluations of the ONH are subjective and prone to error. According to prior research, it has been reported that both ophthalmology trainees and comprehensive ophthalmologists underestimated the likelihood of glaucoma in 20% of disc photographs. Additionally, ophthalmology trainees and comprehensive ophthalmologists were twice as likely to underestimate or overestimate the likelihood of glaucoma due to various factors, such as the variability in cup-to-disc ratio, peripapillary RNFL atrophy, and the presence of disc hemorrhage [7]. Optic nerve assessment is primarily performed in a subjective manner while most of the useful structural and functional features of healthy and diseased patients are overlapping therefore leading to inter- and intra-observer variability. The problem is worse for monitoring as glaucoma progression is often slow and happens over decades, making prediction of progression highly challenging. As such, automated ML models may provide more objective, consistent, and more accurate outcomes.
Artificial intelligence (AI) and DL models are emerging areas that automate the interpretation of retinal images. Advancements in computer systems and availability of large datasets and algorithms allow these systems to mimic human thought processes such as learning, reasoning, and self-correction. DL, a subfield of ML and AI, has made significant progress over the past few years and development of objective systems to automate glaucoma detection has been highly promising [8]. Although many studies demonstrated promising results in detecting glaucoma using AI, limitations still exist in many perspectives. For example, lack of standardized and consensus glaucoma definitions makes it difficult to evaluate the results consistently in different datasets; the shortage of large, well-annotated datasets of good quality limited the generalizability of AI model; the limited interpretability and liability of the DL model hurdled the implementation of it in clinic.
This review aims to identify ML-based models applied to glaucoma over the past few years and create a reference of those models and their performance. We also generate several taxonomies including categories of ML models, input data types, and level of performance and compare different ML types to identify better performing approaches. Lastly, we provide insight into current challenges and future directions as well.
The paper is organized as follows:
This paragraph ends the introductory section. An overview of AI in glaucoma is presented in Section “Literature review”. Section "Overview of the AI models in glaucoma" outlines applications of specific AI models in glaucoma based on various categories. Section "Discussion” presents open issues and future directions of AI in glaucoma. Finally, Section “Conclusions” concludes this review. Section "Methods” presents literature search and filtering strategies for this review.
Literature review
Glaucoma
Glaucoma is a group of heterogenous diseases that may lead to irreversible vision loss [1]. In some forms of glaucoma, increased IOP impacts the retina and ONH, which in turn, may lead to irreversible vision loss [9]. Lowering IOP is a proven treatment for open-angle glaucoma (OAG).
Although glaucoma detection is challenging, particularly at the early stages of the disease, early detection is critical in order to provide timely treatments that may be effective in slowing its progression [5]. Glaucoma is typically diagnosed by evaluating the ONH and the adjacent retinal nerve fiber layer (RNFL) through retinal examination and imaging tools, assessing VFs, and evaluating IOP levels.
AI for glaucoma
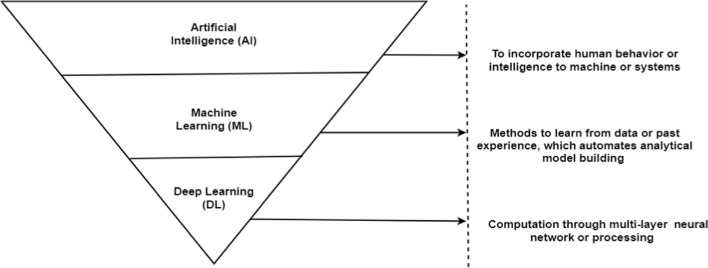
Not only is glaucoma diagnosis potentially time-consuming and costly, but also its reliance on an individual clinician’s knowledge and ability makes it subjective and prone to over/under estimation [10]. Alternatively, automated AI models could minimize subjectivity by interpreting and quantifying retinal and optic nerve images. AI has several other applications for glaucoma. For instance, AI can be used to optimize workflows and processes in glaucoma clinics that may lead to more time for clinicians to interact with patients thus enhancing overall care. AI could be used to quantify optic cup, disc, and rim characteristics in fundus images, retinal layers in OCT images, and patterns of VF loss. Such applications hold promise for providing improved glaucoma assessment, as well as forecasting, screening, diagnosing, and prognosing glaucoma. Figure 1 describes the overall AI domain and its subcategories including ML and DL. While DL models are more appropriate for analysis of image-based glaucoma data, other categories of ML models may be more appropriate for VF and other non-image data. Different categories of AI models including expert systems may be utilized to optimize glaucoma clinic workflows and processes.
Fig. 1.
Illustration and definition of artificial intelligence (AI), machine learning (ML), and deep learning (DL)
Overview of the AI models in glaucoma
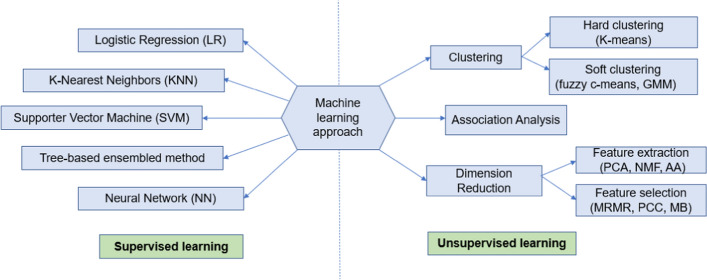
This section reviews different AI models from the literature that have been used for glaucoma assessment. Figure 2 shows different ML-based models that have been applied to detect glaucoma. These models have been grouped into two major categories including supervised and unsupervised learning models. In supervised learning, the model is trained on labeled data with each data instance having a known outcome. Unsupervised learning describes algorithms for finding patterns in data, without prior knowledge of outcomes for data instances [11]. Based on this taxonomy, we will highlight some of the representative ML studies applied to glaucoma and provide their strengths and limitations.
Fig. 2.
Various types of ML models applied to glaucoma. GMM: Gaussian Mixture Modeling; PCA: Principal Component Analysis; NMF: Non-negative Matrix Factorization; AA: Archetypal Analysis; PCC: Pearson Correlation Coefficient, MB: Markov Blanket; mRMR: Minimum Redundancy Maximum Relevance
Machine learning
Supervised machine learning
Supervised ML [12–80] has been widely used in glaucoma detection, severity classification, progression prediction, segmentation, etc., based on different modalities, such as VF, fundus, OCT, clinical data, transcriptomic data, etc.
Several metrics were used for model evaluation, such as: accuracy (the proportion of correctly classified samples relative to the total number of samples); sensitivity/recall (the rate of positive samples correctly classified, reflecting the ratio of correctly classified positive samples to all samples assigned to the positive class); specificity (measures the rate of negative samples correctly classified, determined by the ratio of correctly classified negative samples to all samples belonging to the negative class); error rates (the ratio of the incorrectly classified samples to the total number of samples); precision (the proportion of true positive predictions out of all positive predictions from the model); true positive rate (TPR, the proportion of actual positive samples that the model correctly identified as positive); false positive rate (FPR, the proportion of actual negative samples that the model incorrectly identified as positive); area under the receiver operating characteristic (ROC) curve (AUC, the model’s performance across various thresholds, presenting TPR against FPR at various threshold settings), area under precision—recall curve (AUPRC, the classification model performance appropriate for imbalanced classes, demonstrating the precision against the recall at different threshold settings).
Logistic regression (LR) is a supervised model designed to estimate the probability between categorical classes. LR has also been used in glaucoma diagnosis in various studies. Lu et al. [28] used four ML classifiers to detect glaucoma based on biomechanical data from 52 patients including 20 glaucoma (40 eyes) and 32 healthy (64 eyes). The LR model obtained the best accuracy of 0.983, AUC of 0.990, sensitivity of 98.9% (at 80% specificity), and sensitivity of 97.7% (at 95% specificity) based on 3-fold cross-validation (CV). Baxter et al. [35] developed machine models to predict the requirement for surgical intervention in individuals diagnosed with primary open-angle glaucoma (POAG) utilizing systemic parameters obtained from the electronic health records (EHR) system from 385 POAG patients. They used leave-one-out CV, and the best model was multivariable LR with an AUC of 0.67. This model also provided the odds ratio of the factors which were associated with the risk of glaucoma surgery. Higher mean systolic blood pressure (OR: 1.09, P < 0.001) and use of anticoagulant medication (OR: 2.75, p = 0.042) were significantly associated with increased risk of glaucoma surgery. The major advantage of the LR models is their simplicity and explainability, which would be an essential advantage in glaucoma research and clinics.
K-Nearest Neighbor (KNN) is a supervised classification technique to estimate the likelihood that a data point belonging to a specific group by analyzing the groups to which its nearest neighboring data points belong. The KNN model has been used in glaucoma detection and some of the studies obtained better results using KNN than the other models. Singh, et al. [40] developed several ML models for glaucoma diagnosis from 70 glaucomatous and 70 healthy eyes based on OCT data. They extracted 45 features from OCT images and the highest AUC of 0.97 was achieved by a KNN model tested using 5-fold CV. Singh et al. [48] also developed an interconnected architecture with Customized Particle Swarm Optimization (CPSO) and four machine-learning classifiers based on 110 fundus images and found CPSO-KNN demonstrated superior performance compared to other models, achieving an accuracy of 0.99, specificity of 0.96, sensitivity of 0.97 and precision of 0.97, F1-score of 0.97 and kappa of 0.94 by utilizing 5-fold CV.
Support Vector Machine (SVM): SVM is a supervised ML technique that has the capability to tackle both classification and regression problems. The algorithm aims to identify the optimal line or decision boundary that separates different groups, enabling accurate classification of additional data points into their respective categories. SVM classifier has been widely reported in the literature for detecting glaucoma. For instance, Goldbaum et al. [12] compared various ML models for glaucoma diagnosis based on Standard Automated Perimetry (SAP) data collected from 189 normal eyes and 156 glaucomatous eyes, and SVM with Gaussian kernel with the input of VF sensitivities at each of 52 locations plus age. Results from this study obtained the second highest performance with AUC of 0.903, sensitivity of 0.53 (at specificity of 1.0) and sensitivity of 0.71 (at specificity of 0.9) based on 10-fold CV. Zangwill et al. [13] employed SVM models to detect glaucoma based on Heidelberg Retina Tomograph (HRT) data collected from 95 glaucomatous eyes and 135 normal eyes, and obtained the best performance with AUC of 0.964, sensitivity of 97% (at 75% specificity), and sensitivity of 85% (at 90% specificity) with the input of all parameters combined, including RNFL regional and global parameters, sectoral mean height contour along the disc margin, sectoral parapapillary mean height contour, sectoral RNFL thickness along the disc margin, and sectoral parapapillary RNFL thickness. Evaluations were performed based on 10-fold CV. Burgansky-Eliash et al. [14] developed different ML models for glaucoma detection from 47 glaucomatous eyes and 42 healthy eyes based on OCT parameters and found SVM with eight OCT parameters achieved the best performance with AUC of 0.981, accuracy of 0.966, sensitivity of 97.9% (at the specificity of 80%), and sensitivity of 92.5% (at specificity of 92.5%) using 6-fold CV. Townsend et al. [15] developed several ML models for glaucoma detection based on HRT3 data collected from 60 healthy subjects and 140 glaucomatous subjects. The SVM-radial applied on all HRT3 parameters showed significant improvement over the other models and obtained an AUC of 0.904, accuracy of 85.0%, sensitivity of 85.7% (at 80% specificity) and 64.8% sensitivity (at 95% specificity) based on leave-one-out CV. Garcia-Morate et al. [16] developed an SVM model to detection glaucoma using 136 glaucomatous eyes and 117 non-glaucomatous eyes based on HRT2 parameters. The SVM model exploiting 22 parameters obtained the highest performance with AUC of 0.905, sensitivity of 85.3% (at 75% specificity), and 79.4% sensitivity (at 90% specificity) using 10-fold CV. Bizios et al. [17] developed numerous AI models to detect glaucoma based on OCT-A scans collected from 90 healthy and 62 glaucomatous subjects and reported that SVM achieved the best performance with an AUC of 0.989 (95% confidence interval: 0.979–1.0) using 10-fold CV.
In summary, SVM worked well in dealing with VF, HRT, and OCT parameters to detect glaucoma mostly in earlier studies from 2002 to 2010. SVM is straightforward to implement and has relatively high explainability.
Tree-based ensembled model: The tree-based ensembled method has been reported to have better performance than a single tree-based model. Random Forest (RF), XGboost, and gradient boosting models from this family have been widely used in glaucoma diagnosis based on VFs and OCT parameters and have shown reasonable performance. Barella et al. [19] developed multiple ML models to detect early to moderate POAG from 57 early to moderate POAG and 46 healthy patients based on RNFL and optic nerve parameters collected from SD-OCT instrument. RF obtained the best AUC of 0.877 based on 13 input parameters with sensitivity of 64.9% (at 80% specificity) and sensitivity of 49.1% (at 90% specificity) using 10-fold CV. Hirasawa et al. [18] used various ML models to predict vision-related quality of life (VRQoL) based on VF and visual acuity from 164 glaucomatous patients. Based on this regression problem, RF and boosting models obtained the lowest error rate with root mean square error (RMSE) of 1.99. Silva et al. [20] developed models for glaucoma diagnosis based on SD-OCT and SAP (24–2) data collected from 62 glaucomatous patients and 48 healthy subjects. Based on four features, RF achieved the best AUC of 0.946 with the sensitivity of 95.16% (at 80% specificity), and the sensitivity of 82.25% (at 90% specificity) using 10-fold CV. Kim et al. [25] developed several ML models for diagnosis of glaucoma based on RNFL thickness and VFs collected from 399 cases and RF showed the best performance with an accuracy of 98%, sensitivity of 98.3%, specificity of 97.5%, and AUC of 0.979 when using seven features on internal testing set with 100 cases. Oh et al. [44]applied various ML models to detect glaucoma based on clinical data (IOP, OCT measurements, VF examinations) collected from 1244 eyes and observed XGboost the best performing model with an accuracy of 94.7%, sensitivity of 94.1%, specificity of 95.0%, and AUC of 0.945 with 10-fold CV.
Neural network (NN), also known as artificial neural networks (ANNs), are structured with layers of nodes and usually consist of an input layer, one or multiple hidden layers, and an output layer. Within this network, each node, also known as an artificial neuron, establishes connections with other nodes and possesses an assigned weight and threshold. When the output of a node surpasses a predetermined threshold, it becomes activated and transmits its output to the subsequent layer of the network; otherwise, no output is passed along to the next layer of the network. NNs have long been used for glaucoma-related tasks based on VFs and other imaging parameters. Omodaka et al. [24] used an NN with a structure of nine input layer units, eight hidden layer units, and four output layer units to identify the status of 163 eyes based on 15 features selected by minimum redundancy maximum relevance (mRMR) from 91 OCT parameters. The model achieved an accuracy of 87.8% (Cohen’s Kappa of 0.83) based on 10-fold CV. An et al. [27] used the same dataset and compared the performance of NN, SVM, and Naïve Bayesian models based on nine parameters selected by combining mRMR and genetic-algorithm-based feature selection. They identified the NN model as the best performing algorithm, with accuracy of 87.8% using 10-fold CV.
Overall, these conventional supervised ML models have been used widely in glaucoma diagnosis based on VFs, RNFL parameters, or other clinical factors. Among these models, we observed that SVM was used more frequently and obtained better performance, compared to other conventional ML models.
Unsupervised machine learning
Unsupervised learning is used for learning representative features and extracting patterns from data. Many studies applied unsupervised learning for extracting VF or RNFL loss patterns, glaucoma staging, segmentation and other features by using clustering, association analysis, dimension reduction, etc. [37, 41, 43, 49, 56, 60, 62, 81–92].
Clustering
We can broadly group the clustering models into either of two categories: hard or soft clustering algorithms.
Hard clustering: In hard clustering, each data point is clustered or grouped to just one cluster and not others. K-Means is a hard clustering algorithm. In K-Means, the algorithm determines the optimal initial centroid points by minimizing the sum of the squared distances between each point and its assigned centroid across all clusters. Huang et al. [87] applied K-Means clustering to identify different stages of glaucoma without any supervision by experts. They identified four severity levels based on 13,231 VFs and determined objective thresholds of − 2.2, − 8.0 and − 17.3 dB for VF mean deviation for distinguishing normal, early, moderate, and advanced stages of glaucoma. Ammal et al. [90] used K-Means to segregate optic disc (OD) and optic cup (OC) for further glaucoma diagnosis model development based on fundus images from the Retinal Fundus Images for Glaucoma Analysis (RIGA) dataset, and validated the model using another dataset with 90 images. The result of the K-Means was compared with severity levels determined by ophthalmologists on the same data set, and authors showed that the outcome was similar.
Soft clustering: In soft or fuzzy clustering, instead of putting each data points into only one cluster, the data point can be assigned to different clusters with different likelihoods. Fuzzy c-Means and Gaussian Mixture Model (GMM) are examples of soft clustering. Fuzzy-c Means: Praveena et al. [86] applied K-Means, Fuzzy c-Means (FCM) and Spatially Weighted fuzzy C-Means Clustering (SWFCM) to automatically determine the cup-to-disc ratio (CDR) from the fundus photographs of 50 normal and 50 glaucoma eyes. The K-value is determined by hill climbing algorithm. K-Means was used for segmenting OD, while FCM was used for segmenting optic cup. SWFCM was used for segmenting both OD and OC. The error rate was calculated with reference to the manually determined CDR value (considered the gold standard) provided by ophthalmologists for comparison. The mean error of the K-Means clustering method for elliptical and morphological fitting was 4.5% and 4.1%, respectively. The mean error was reduced by the FCM clustering to 3.83% and 3.52%, and the mean error was minimized to 3.06% and 1.67% using SWFCM. GMM: GMM attempts to find a mixture of multidimensional Gaussian probability distributions. Yousefi et al. [82] applied GMM to identify different patterns of VF loss. Their GMM successfully detected three distinct clusters, which included normal eyes, eyes in the early stage of glaucoma, and eyes in the advanced stage of glaucoma based on SAP VFs collected from 2085 eyes. Based on another subset with 270 eyes, they showed that GMM detected progressing eyes at an earlier stage, compared to other methods.
Association analysis
Association analysis: Association rule algorithms aims to find interesting relationships among variables within extensive datasets, provided they satisfy the predetermined minimum support (a threshold for determining the minimum association) and confidence level (or accuracy, minimum threshold of the correct rule or prediction) set by the user, in order to discover a pattern with strong association. Apriori algorithms, one of the association rule categories, focus on identifying frequent associations in order to unveil intriguing relationships between attributes. Al-Shamiri et al. [89] applied Apriori algorithm to discover risk factors of glaucoma based on a survey dataset from 4000 patients. Their association analysis revealed several glaucoma risk factors including family history of glaucoma, high IOP, optic nerve damage, high myopia, diabetes, hypertension, history of eye surgery, use of some medicines, psychological stress, pressure work or study-related pressure, gastroenterology disease, and chronic constipation.
Dimensionality reduction
A comprehensive eye examination to diagnose glaucoma typically includes collecting numerous imaging, VF, and ocular measurements, and thus provides high-dimensional datasets. A challenge is that ML models typically tend to overfit when dealing with glaucoma data and thus is not generalizable when tested on new data. However, reducing the dimensionality may address the issue by selecting a smaller subset of the features or deriving new features from a pool of features, while preserving the information of the original features as much as feasible.
Transformations: Transformation methods typically perform linear or nonlinear transformations to map a high-dimensional space of initial input data into a lower dimensional space of the features to reduce dimensionality. The widely used methods in glaucoma research are principal component analysis (PCA), archetypal analysis, and non-negative matrix factorization (NMF).
PCA: PCA [93] refers to a mathematical technique that employs an orthogonal transformation to convert a collection of observations, which may consist of correlated variables, into a new set of linearly uncorrelated variables known as principal components (PCs). Christopher et al. [29] applied PCA to structural RNFL features from RNFL thickness maps and retained 10 PCs for glaucoma diagnosis and glaucoma progression prediction based on 235 eyes and compared OCT and SAP features. The LR model based on PCA from RNFL data obtained a better performance than other available parameters such as mean cpRNFL, SAP 24–2 MD, and FDT MD in both glaucoma diagnosis (AUC of 0.95; CI 0.92–0.98) and progression prediction (AUC of 0.74; CI 0.62–0.85) using leave-one-out CV. Yousefi et al. [88] applied PCA to 52 TD values of 13,231 VFs and selected 10 PCs (explained 90% of variance in VFs) which were subsequently input to a manifold learning model, two features (dimensions) were ultimately retained. They applied unsupervised ML and identified 30 clusters based on those two features and used those clusters for glaucoma progression detection.
Archetypal Analysis: Wang et al. [83] applied Archetypal Analysis to identify the central VF patterns of loss from 13,951 VF tests (Humphery, 10–2). They discovered 17 distinct central VF patterns and noted that incorporating coefficients from central VF archetypical patterns strongly enhances the prediction of central VF loss compared to using global indices only. In another study, Wang et al. [84] employed Archetypal Analysis to identify the central VF patterns in end-stage glaucoma based on 2912 reliable VFs (10–2) and found central VF loss in end-stage glaucoma exhibits characteristic patterns that could potentially be associated with various subtypes. Nasal loss is likely the initial central VF loss, and a particular subtype of nasal loss is highly prone to progress into complete or total loss. Such explainable models may also shed light on some aspects of glaucoma pathophysiology. Follow-up studies based on deep archetypal analysis (DAA) identified more patterns of VF loss, their role in forecasting glaucoma, and their association with rapid glaucoma progression [94–96].
NMF: Wang et al. [81] utilized NMF to identify distinct patterns of the RNFL thickness (RNFLT) based on RNFLT collected from 691 eyes. NMF identified 16 distinct RNFLT patterns (RPs). Using these RPs resulted in a substantial enhancement in the prediction of VF sensitivities. The AI-based RNFLT patterns hold promise in assisting clinicians to more effectively evaluate and interpret RNFLT maps.
Feature selection: Feature selection models to identify the most optimal subset of original input variables. Various feature selection methods have been used in glaucoma research and some improved the performance of ML models. Examples include Pearson Correlation Coefficient (PCC)-based variable selection, Markov Blanket (MB) variable selection, and the minimum Redundancy Maximum Relevance (mRMR) approaches. Lee et al. [33] developed a set of ML models for glaucoma diagnosis based on VF data collected from 632 eyes and compared the result of models with different input features which were selected by PCC-based variable selection, MB variable selection, mRMR, and features extracted by PCA. By employing a combination of total deviation (TD) values, the GHT sector map, and variable selection using SVM and MB methods, the researchers achieved the highest performance, as evaluated through 5-fold CV, with an AUC of 0.912. Omodaka et al. [24] used mRMR to select 15 features from 91 quantified ocular parameters and used an NN model for OD classification based on 165 eyes of 105 POAG patients, and obtained an accuracy of 87.8%, Cohen’s kappa of 0.83 using 10 -fold CV. An et al. [27] applied a combination of mRMR and genetic algorithm-based feature selection to identify nine most valid and relevant features from a pool of 91 ocular parameters and patients’ background information of 163 glaucomatous eyes to develop ML model for classifying glaucomatous optic discs, and using the selected nine parameters yielded the highest accuracy of 87.8% with an NN model evaluated based on 10 -fold CV.
Deep learning
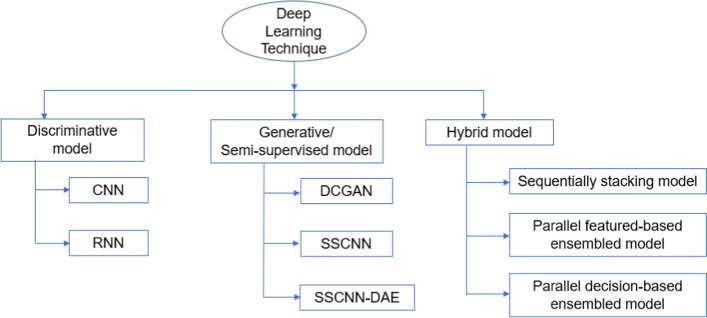
Emerging DL models have been widely used in ophthalmology and many DL models have shown promising performance in glaucoma screening, diagnosis, or quantification and segmentation of fundus photographs, VFs, and OCT images [31, 34, 51, 53, 85, 97–290]. We will discuss some of the applications in glaucoma in three broad categories of discriminative, generative, and hybrid models, as shown in Fig. 3.
Fig. 3.
Classification of DL models. CNN: Convolutional Neural Network; RNN: Recurrent Neural Network; LSTM: long short-term memory; DCGAN: Deep Convolutional Generative Adversarial Network; SSCNN: Convolutional Neural Network model with self-learning; SSCNN-DAE: Semi-supervised Convolutional Neural Network model with autoencoder
Discriminative models
Discriminative models separate data points into different classes and learn the boundaries using probability estimates and maximum likelihood. Discriminative models are most common in glaucoma and have been extensively used in detection, optic disc/cup, and region of interest (ROI) segmentation.
Convolutional Neural Network (CNN): CNN has been widely used in glaucoma diagnosis based on retinal images such as fundus photograph and OCT images. Chen et al. [97] developed one of the earliest CNN models for glaucoma diagnosis based on fundus images from ORIGA and SCES datasets. The CNN model with five layers obtained the best performance with AUCs of 0.838 and 0.898 based on fundus images from the ORIGA (internal testing) and SCES (independent validation) datasets, respectively. Ahn et al. [100] developed a CNN model to discriminate glaucomatous from normal eyes based on 1542 fundus images. The model outperformed LR and InceptionV3 model with an accuracy of 87.9% and AUC of 0.94 on internal testing set. Norouzifard et al. [102] applied transfer learning based on VGG19 and Inception-ResNet-V2 architectures in identifying glaucoma from 447 fundus photograph and re-tested the model on an independent dataset (HRF with 30 fundus images). They reported that that VGG19 obtained 80.0% accuracy on independent dataset. Masumoto et al. [103] developed a CNN model to classify glaucoma patients into four severity levels based on fundus images from 1399 patients/images. The model obtained AUCs of 0.872, 0.830, 0.864, 0.934 for normal vs all glaucoma, early glaucoma, moderate glaucoma, and severe glaucoma, respectively, on the internal testing set. Fuentes-Hurtado et al. [104] applied DenseNet-201 in classifying 1912 rat OCT images into healthy and pathological using leave-P-out CV (P = 15) and obtained an AUC of 0.99. Shibata et al. [105] developed a CNN model using the ResNet architecture based on 1364 glaucomatous and 1768 non-glaucomatous fundus images and tested on independent dataset with 60 glaucomatous eyes and 50 normal eyes. The model obtained an AUC of 0.965 (95% CI 0.935–0.996) that was higher than the performance of ophthalmology residents with an AUC between 0.762 and 0.912 and other models, such as VGG16, RF and SVM. Asaoka et al. [31] developed a six-layer DL model to diagnose early-glaucoma based on 4316 OCT images and obtained an AUC of 0.937 (95% CI 0.906–0.968) based on an independent dataset with 114 patients with glaucoma and 82 normal subjects using a DL transfer model, which was significantly higher than the AUC of 0.631 to 0.862 obtained based on other models (RF and SVM). Using the Youden method, the model attained optimal discrimination with a sensitivity of 82.5% and specificity of 93.9%. Phene et al. [109] developed a CNN model based on Inception-v3 architecture to predict referable GON from ONH features using 86,618 color fundus images and validated the model using three independent datasets, then compared the outcome with glaucoma specialists. For referable GON, they achieved AUCs of 0.945 (0.929–0.960), 0.855 (0.841–0.870), and 0.881 (0.838–0.918) based on the fundus images in the first dataset (with 1205 images), second dataset (with 9642 images), and third dataset (with 346 images), respectively. The model’s AUCs ranged from 0.661 to 0.973 based on glaucomatous ONH features. The CNN model detected referable GON with higher sensitivity than compared with eye care providers.
Al-Aswad et al. [289] evaluated the performance of Pegasus (an AI system based on deep learning) in glaucoma screening based on color fundus photographs by comparing with six ophthalmologists. They found there was no statistically significant distinction between Pegasus (AUC of 0.926, sensitivity and specificity of 83.7% and 88.2%, respectively) and the “optimal” consensus among ophthalmologists (AUC of 0.891, sensitivity and specificity of 61.3–81.6% and 80.0–94.1%, respectively). The correspondence between Pegasus and the gold standard yielded a score of 0.715, whereas the highest level of agreement between ophthalmologists and the gold standard stood at 0.613. Jammal et al. [112] developed a CNN model using ResNet34 architecture (M2M DL) to predict RNFL thickness and grading glaucomatous eyes based on 32,820 pairs of fundus photographs and SD-OCT scans. A total of 490 images were used for testing the model, then the outcome was compared with two glaucoma specialists, the predicted RNFL thickness obtained through M2M DL exhibited a notably stronger absolute correlation with SAP mean deviation (r = 0.54) compared to the probability of GON determined by human graders (r = 0.48; P < 0.001). Furthermore, the M2M DL algorithm demonstrated a significantly higher partial AUC compared to the probability of GON assessed by human graders (partial AUC = 0.529 vs 0.411, respectively; P = 0.016). Yu et al. [274] trained a 3D CNN to estimate global VF indices based on macula and optic disc OCT scans from 10,370 eyes, and showed that integrating information from macula and optic disc scan achieved better result compared with inputting separate scan. The combined scan obtained 0.76 Spearman’s correlation coefficient and 0.87 Pearson’s correlation based on VFI and MD while the median absolute error was 2.7 for VFI and 1.57 dB for MD from one of the 8-fold CVs. Wang et al. [34] compared four different CNN models for detecting glaucoma based on RNFL thickness maps from 93 glaucomatous eyes and 128 healthy eyes and found ResNet-18 and a customized CNN architecture called GlaucomaNet had higher performance compared to SVM and KNN. The ResNet-18 architecture obtained the highest accuracy of 90.5%, with sensitivity of 86.0%, specificity of 93.8%, and AUC of 0.906 using 5-fold CV. Kim et al. [113] developed DL models for glaucoma diagnosis based on 1903 fundus images using VGG16 and ResNet-152-M architectures and employed Grad-CAM to visualize regions that were more important for the model to make diagnosis. They used both the whole fundus image as well as cropped versions with OD region only and observed that the ResNet-152-M model achieved an accuracy of 96%, sensitivity of 96%, and specificity of 100% based on 220 fundus images from an independent dataset.
CNN models have been also applied to VFs to detect glaucoma. Kucur et al. [101] proposed an eight-layer CNN model for discrimination of early-glaucoma versus control samples, trained on Glaucoma Center of Semmelweis University in Budapest (BD) dataset with 2267 OCTOPUS G1 VFs (30°) and Rotterdam Eye Hospital (RT) dataset with 2573 HFA VFs. The CNN model had the highest average precision (AP) with 0.874 and 0.986 based on BD and RT datasets, respectively, using 10-fold CV. Performance was similar to other methods using MD thresholds and NN model.
CNN models were also applied to ROI localization in glaucoma studies. Mitra et al. [108] developed a CNN mode to detect the bounding box coordinates of OD that acts as a ROI based on fundus images from MESSIDOR and Kaggle datasets and tested the model using fundus images in the DRIVE and STARE datasets. The average IOU of their model was 96.83%, 95.45%, 96.19%, 95.93% on internal testing set of MESSIDOR and Kaggle, independent datasets of DRIVE and STARE, respectively.
CNN models have been widely employed in optic disc/cup segmentation, which plays an important role in glaucoma detection as CDR is a glaucoma risk factor. Kim et al. [114] developed a fully convolutional networks (FCN) with U-Net architectures for optic disc/cup segmentation based on fundus ROI region from 750 fundus images of RIGA dataset. The best segmentation results for OD showed Jaccard index of 0.95, F-measure of 0.98, and accuracy of 99%. The best segmentation results for OC showed Jaccard index of 0.80, F-measure of 0.88, and accuracy of 99% evaluated by 5-fold CV. Xie et al. [210] developed a new method to segment inner retina thickness using 41 OCT macular scans. The approach addressed spike-like segmentation errors and lack of contextual data by reconstructing more B-scans, concatenating smoothed and contrast-enhanced images into a six-channel input image stack, and merging predicted surfaces from both horizontal and vertical B-scans. The suggested method surpassed the performance of cutting-edge techniques when it came to mean absolute surface distances (normal: 2.18, glaucoma: 3.02), Dice coefficients (GCIPL normal: 0.952, GCIPL glaucoma: 0.899), and Hausdorff distance (RNFL-GCL normal: 12.1; RNFL-GCL glaucoma: 28.9) in an independent test dataset. Li et al. [182] developed a joint OD and OC segmentation model using a region-based DCNN (R-DCNN) based on 2440 fundus images and validated the model based on both in-house testing dataset and public datasets (DRISHIT-GS and RIM-ONE v3) and compared with that of ophthalmologists. The model achieved high Dice similarity coefficient (DC) and Jaccard coefficient (JC) for both OD (DC: 98.51%, JC: 97.07%) and OC (DC: 97.63%, JC: 95.39%) segmentation on the in-house dataset, comparable to that of ophthalmologists. On DRISHTI-GS and RIM-ONE v3 datasets, the model obtained higher DC (DRISHTI-GS: OD: 97. 23%, OC: 94.56%; RIM-ONE v3: OD: 96.89%, OC: 88.94%) and JC (DRISHTI-GS: OD: 94.17%, OC: 89.92%; RIM-ONE v3: OD: 91.32%, OC: 78.21%) values than previous studies.
An automatic two-stage glaucoma screening system was developed by Sreng et al. [291] and was evaluated on 2787 retinal images from 5 public datasets (REFUGE, ACRIMA, ORIGA, RIM-ONE and DRISHTI-GS1). The system utilized DeepLabv3 + combined with pretrained networks for OD segmentation in the first stage and pretrained networks ensembled with SVM for glaucoma classification in the second stage. The best model for OD segmentation achieved high accuracy (99.70%), Dice coefficient (91.73%), and IoU (84.89%) on REFUGE dataset based on the combination of DeepLabv3 + and MobileNet. The ensembled classification model outperformed conventional methods with high accuracy (97.37%, 90.00%, 86.84%, 99.53% and 95.59%) and AUC (100%, 92.06%, 91.67%, 99.98% and 95.10%) values on RIM-ONE, ORIGA, DRISHTI-GS1, ACRIMA and REFUGE datasets.
Recurrent Neural Network (RNN): RNN is specifically designed to handle sequential data by preserving an internal state, enabling the network to remember information from prior inputs. The network takes a sequence of inputs, one at a time, and updates its internal state based on the current input and its previous state. The output of the network at each step depends on the current input and the current state. Long Short-Term Memory (LSTM) networks were introduced as a specialized type of RNN. LSTM networks incorporate memory cells and forget gates, allowing them to manage information as it enters and exits the memory, thus mitigating the drawbacks of traditional RNNs. Veena et al. [145] used an RNN-LSTM model with three dense and three dropout layers and one batch normalization layer for glaucoma diagnosis based on the segmentation result of the fundus images from DRISHTI-GS (101 images) database (no report of classification accuracy). LSTM has been successfully applied to longitudinal data in glaucoma studies as well. Dixit et al. [147] used LSTM to assess glaucoma progression based on longitudinal VF data from 11,242 eyes. Using four consecutive VFs for each subject, the convolutional LSTM network achieved an accuracy of 91–93% when evaluated against various conventional glaucoma progression algorithms. The model trained on both VFs and clinical data displayed superior diagnostic capabilities (AUC:0.89–0.93) compared to a model exclusively trained on VF (AUC:0.79–0.82, P < 0.001) using 3-fold CV. In summary, the majority of the studies using discriminative DL models in glaucoma have applied transfer learning and compared performance of different CNN model architectures in a specific task, such as classification based on fundus, VFs, or OCT images. Among the pretrained CNN architectures in glaucoma studies, ResNet has been the most popular architecture used in CNN models and has achieved a higher accuracy compared to the other CNN architectures.
Generative Adversarial Network (GAN)/semi-supervised model
Generative Adversarial Networks (GANs) represent a recent breakthrough in DL. First proposed by Goodfellow et al. [292], it constitutes two networks one for image generation and the other for discrimination (between the generated image and authentic). This model has demonstrated high levels of performance in a variety of applications including glaucoma to generate synthesized retinal images in a semi-supervised learning fashion. Diaz-Pinto et al. [240] developed a new retinal image synthesizer and a semi-supervised learning approach for glaucoma assessment utilizing a Deep Convolutional Generative Adversarial Network (DCGAN) based on a dataset consisting of 86,926 fundus images. The model was able to generate (close to) realistic retinal images and discriminate glaucomatous eyes from normal ones with an AUC of 0.9017, specificity of 79.86%, sensitivity of 82.90%, and F1-score of 0.8429 based on the internal testing set. Tang et al. [205] developed a semi-supervised model using a multi-level amplification iterative training method to detect glaucoma based on three different datasets; Sanyuan dataset (11,443 images), Tongren dataset (7806 images), and Xiehe dataset (4363 images). They tested the model based on REFUGE dataset and obtained an accuracy of 95.75%, sensitivity of 87.5%, specificity of 96.7%, and F1-score of 0.919, which were higher than the accuracy of the models previously published. Alghamdi et al. [241] developed a semi-supervised CNN model with self-learning (SSCNN) and Semi-supervised CNN model with autoencoder (SSCNN-DAE) based on both labeled and unlabeled data from RIM-ONE and RIGA datasets. Compared with transfer CNN (TCNN), SSCNN-DAE obtained a higher accuracy of 93.8%, sensitivity of 98.9% and AUC of 0.95 based on the internal testing set.
Overall, GAN and semi-supervised learning can improve the model performance significantly compared to supervised DL models when dealing with small datasets or datasets with limited number of labeled images. Because this is a typical problem in glaucoma studies, such models may be applicable to address related glaucoma challenges. However, GAN has been not widely used in glaucoma studies probably because there is a huge concern related to synthesizing retinal images in ophthalmology [293].
Hybrid models
Hybrid models, or fusion networks, are formed based on combining multiple DL models using a single modality or multiple modalities. It has demonstrated a better performance in some glaucoma diagnosis scenarios than a single CNN model [294–298]. In addition, Muhammad et al. [107] developed a hybrid deep CNN model based on AlexNet architecture to extract features from OCT scans, coupled with RF classifier to distinguish healthy suspects and mild glaucoma using 102 eyes. The model with the input of RNFL probability map had the best accuracy of 93.1% using leave-one-out validation. An et al. [110] developed a hybrid CNN mode based on fundus and OCT scans from 208 glaucomatous eyes and 149 healthy eyes. They first trained a VGG19 architecture separately based on fundus images cantered at optic disc, disc RNFL thickness maps, macular GCC thickness maps, disc RNFL deviation maps, and macular GCC deviation maps, then combined the feature vector representation of each CNN model and used a RF classifier to combine models for glaucoma diagnosis. The hybrid model achieved an AUC of 0.963 based on 10-fold CV. Patil et al. [111] developed GlaucoNet which stacked an autoencoder with a CNN model for glaucoma diagnosis based on fundus images from DRISHTI-GS and DRION-DB datasets. The accuracy, precision, F1-score, recall, specificity, and AUC of the model based on the DRISHTI-GS (internal testing set) were 98.2%, 94.6%, 0.979, 99.6%, 94.6%, and 0.94, respectively. Based on the DRION-DB (internal testing set), the corresponding performance metrics were 96.3%, 93.9%, 0.941, 94.2%, 92.6%, and 0.90, respectively. The model outperformed the previous state-of-the-art studies. Cho et al. [247] developed a hybrid model composed of an ensemble of 56 CNNs with different architectures by averaging the outcome of those models based on 3460 fundus photographs to identify unaffected controls, early-stage, and late-stage glaucoma. The proposed hybrid model demonstrated a significantly better performance compared with the best single CNN model, with an accuracy of 88.1% and an average AUC of 0.975 based on 10-fold CV. Akbar et al. [248] developed a hybrid model by combining the DenseNet and DarkNet CNN architectures for glaucoma diagnosis based on 1270 fundus images from HRF, RIM 1, and ACRIMA databases. The hybrid model outperformed the two single CNN networks and achieved an accuracy of 99.7%, sensitivity of 98.9%, and specificity of 100% based on the HRF as the internal validation set. Based on the RIM1 as the internal validation set, accuracy, sensitivity, and specificity were 89.3%, 93.3%, 88.46%, respectively, while based on ACRIMA as the internal validation set, accuracy, sensitivity, and specificity were 99.0%, 100%, and 99%, respectively. Joshi et al. [178] developed a hybrid model based on the ensemble of VGGNet-16, ResNet-50, and GoogLeNet CNN architectures using fundus images collected from a private dataset (PSGIMSR with 1150 images) and three publicly available datasets (DRISHTI-GS with 101 images; DRIONS-DB with 110 images; and HRF with 30 images). The hybrid model outcome was formed based on the majority voting of the three models. The hybrid model yielded an accuracy of 91.13%, sensitivity of 86.58%, and specificity of 95.21% on PSGIMSR dataset, and achieved accuracies of 95.63%, 98.67%, 95.64%, and 88.96%, respectively, on the DRIONS-DB, HRF, DRISHTI-GS, and combined datasets based on 10-fold CV.
It is becoming evident that hybrid CNN models with fusion of different single CNN architectures using a single data modality or multi-modality are being increasingly applied and receiving more attention from investigators. Because glaucoma is a complex and multifactorial disease, hybrid models that utilize different data modalities to detect glaucoma may provide different pieces of information regarding glaucoma to better portray the disease. This is true based on information theory. In addition, multiple sources of information may increase the overall information about the disease. Thus, we predict attention to, and utilization of, hybrid CNN models will continue to increase in glaucoma studies.
Overall, diagnosis, screening of glaucoma and glaucoma progression detection are the major goals in studies discussing various applications of AI in glaucoma. Currently, most of the studies are focused on glaucoma diagnosis or screening and have applied both conventional ML and DL approaches. However, detecting glaucoma progression remains a significant hurdle in clinical practice since detecting true changes due to the disease is challenging. Some of the imaging modalities generate substantial test–retest variability, which complicates distinction between genuine change and fluctuations. Furthermore, there is a lack of consensus regarding specific criteria for glaucoma progression based on VF or structural parameters. Despite these obstacles, several research groups have proposed models for detecting glaucoma progression using traditional ML based on VF test [46, 82, 299] or both VF test and OCT parameters [29, 38]. Wang et al. [299] applied archetypal analysis to detect VF progression from 11,817 eyes and validated it on 397 eyes and achieved an agreement (kappa) and accuracy of 0.51 and 77%, respectively. They showed that archetypal analysis was more accurate than Advanced Glaucoma Intervention Study (AGIS) scoring, Collaborative Initial Glaucoma Treatment Study (CIGTS) scoring, MD slope, and permutation of pointwise linear regression (PoPLR). Shuldiner et al. [46] assessed several ML models to predict rapid progression based on 22,925 initial VFs from 14,217 patients and found SVM as the best performing model with an AUC of 0.72 (95% CI 0.70–0.75). Lee et al. [38] included 33 initial clinical parameters in ML models to predict normal-tension glaucoma (NTG) progression in young myopic patients based on 155 patients and obtained an AUC of 0.881 (95% CI 0.814–0.945) based on extremely randomized tree which was better than RF. There are also several studies that applied DL models to detect or predict progression based on VF and clinical data [147], OCT images [237, 243] or fundus images [164]. Bowd et al. [243] developed a DL-autoencoder (AE) to detect glaucoma progression based on OCT RNFLROI from 44 progressing, 189 non-progressing, and 109 healthy eyes. The DL-AE ROIs achieved sensitivity and specificity of 0.90 and 0.92, respectively, which was higher than models based on global cpRNFL annulus thicknesses. Li et al. [164] developed AI models to predict glaucoma progression based on color fundus photographs and demonstrated AUCs of 0.87 (0.81–0.92) and 0.88 (0.83–0.94) based on two external datasets. Successful detection of progression may facilitate earlier interventions thus diminishing the likelihood of patients experiencing vision impairment due to glaucoma over their lifetimes. Nevertheless, existing studies have not showed solid results in forecasting the rate of progression or the timing of its occurrence.
Discussion
AI is an active research area that encompasses a wide range of approaches of ML, employing different applications such as ML and DL algorithms, which have been successfully applied in various domains such as image processing, pattern recognition, speech recognition, and natural language processing. When applied in medical fields such as ophthalmology, these models show promising potential for improving access to health care and enhancing patient outcomes. Early ML models in the form of neural networks were applied to VFs for diagnosing glaucoma in the 1990’s [300, 301]. With later advancements in AI models, various groups demonstrated the efficacy of these models in detecting glaucoma. The following is a summary of our findings regarding AI models in glaucoma. DL-based models are typically more accurate than conventional ML approaches, as evidenced by their performance in glaucoma applications such as image processing, pattern recognition, diagnosis, and prognosis. ML/DL techniques, such as CNNs, RNNs, LSTM, GAN, DBN, etc., can be easily adapted to various glaucoma problems including screening, diagnosis, and prognosis. DL-based techniques can handle more complicated problems such as high-dimensional data and interactions between different data modalities. Ensemble DL models can improve the performance of glaucoma detection. Combination of multiple modalities of the data can improve the model performance. However, there are also several limitations with respect to AI liability, reimbursement, and ethical principles, including non-maleficence of AI models in glaucoma, patient autonomy, and equity and absence of bias in benefits and rights, that are out of the scope of this article and may be discussed in more focused future studies.
Datasets
Datasets serve as the key element for developing AI models and they play a critical role in reproducibility and generalizability. Conventional statistical learning models can be applied to clinical datasets to identify associations even based on a relatively small sample size. However, in addition to quality, quantity is also important in developing emerging DL models. For instance, labeling fundus images by non-glaucoma experts may degrade the quality of the dataset, leading to non-solid constructs. Increased quantity, along with improved quality, usually leads to better model performance and higher likelihood of generalizability. Well-annotated, multi-modal datasets can also positively impact AI models for more accurate detection of the disease. However, obtaining a sufficiently large dataset with several modalities can be challenging due to numerous hurdles such as low disease prevalence, data confidentiality, data protection regulations, and labor-intensiveness of the process.
Moreover, lack of standardized definitions for glaucoma poses another challenge in achieving consistent evaluation of the AI models. Christopher et al. [136] investigated the impact of study population, labeling, and training on glaucoma detection using AI models, and found that the diagnosis performance varied based on the reference standard (RS) and labeling strategy. To develop solid AI models for detecting glaucoma, it is vital to train the models based on dependable datasets and evaluate the models based on consistent ground truths. Thakoor et al. [281] attempted to improve the generalizability of AI models in glaucoma detection and found that the model trained and tested with the same RS demonstrated the highest accuracy. However, substantial disagreement, even among experienced glaucoma specialists, makes it challenging to establish a uniform reference for ground-truth labeling [281].
Many of the currently available datasets have been gathered from populations with limited representation of different ethnicities, used specific hardware and imaging settings to collect data, and used high-quality images that are far from real-world settings. One potential drawback of lack of diversity within the models, is that these datasets may not readily generalize to real-world patient populations. Asaoka et al. [118] applied ResNet model based on 3132 fundus images 0.877 to 0.948 and from 0.945 to 0.997 based on two independent validations datasets. Nonetheless, their model was not evaluated based on diverse ethnic groups, which is important as fundus images from patients with different ethnic backgrounds may exhibit distinct characteristics such as variation in retina color as well as optic disc structure.
AI models
Although there are several different CNN-based AI models reported in the literature with high performance in detecting glaucoma, the generalizability of those models is questionable since independent validation is lacking in many studies. Independent validation is a crucial step in developing AI models because it can determine whether a model has learned meaningful disease-related features and patterns to generalize well to unseen data. It also aids uncovering potential biases or overfitting issues when the model performs exceptionally well based on the training data but performs poorly based on the new data. Moreover, assessing the robustness of AI models against variations and changes in the data distribution is especially important when deploying models in real-world applications where input data may change over time. Additionally, validating AI models based on independent (accessible) datasets allows for fair comparison of AI models using the same evaluation dataset, ensuring transparency. For the literature we reviewed, most of the articles only used internal testing or CV, and less than about one-third of the articles used independent validation, which are summarized in Table 1. Also, many models were developed based on very clean datasets, for example including only high-quality images and clear pathological features, whereas this does not reflect the case in real-world data. In addition, many models demonstrated good performance in differentiating healthy versus moderate/severe glaucoma; however, this does not add significant clinical value, as differentiation between these two types of conditions is also easily achieved by clinicians. Thus, more helpful models should be developed based on a wide spectrum of glaucoma severity subjects, including early-stage glaucoma.
Table 1.
Summary of studies that have used independent validation or compared the model against human experts
| References | Task | Output label | Data modality | No. of sample for model development | No. of sample for independent validation | No. of human expert | Model used | Key results | Strengths/limitations |
|---|---|---|---|---|---|---|---|---|---|
| Nawaz et al. [166] | Glaucoma detection | Healthy, glaucoma | Fundus |
ORIGA (650 images) |
HRF (30 images), RIM ONE (485 images) |
Bi-directional Feature Pyramid Network (BiFPN) EfficientDet-D0 (EfficientNet-B0 as backbone) |
Train on ORIGA, test accuracy: HRF-98.21%; RIM ONE–97.96%; train on ROM-ONE, test accuracy: ORIGA–97.83%, HRF–98.19%. |
Pros: high performance. Cons: validation dataset is small. |
|
| Gong et al. [249] | Glaucoma diagnosis | Normal, glaucoma | Fundus | 1000 images | 4 doctors | Hierarchical structure (HDLS) AI system + SVM |
Doctors’ performance was improved with the assistance of AI. Overall doctors' performance without AI assistance (round1): sensitivity: 65%, specificity: 78%; accuracy: 71.5%; Overall doctors' performance with AI assistance (round 2): sensitivity: 91%, specificity: 88%; accuracy: 89.5%. |
Pros: AI and human comparison. Cons: small number of tested samples (200 each round) and number of experts; self-learning effect exists. | |
| Yugha et al. [239] | Glaucoma detection | Healthy, glaucoma | Fundus |
ORIGA (650 images) |
RIM-1-DL (485 images); HRF (45 images) |
Bi-Directional Feature Pyramid system modules of EfficientDet-DO (EfficientNet-B0 backbone) | Accuracy: HRF: 97.89%; RIM 1: 97.64%. |
Pros: high performance. Cons: validation dataset is small. |
|
| Ko et al. [287] | Glaucoma detection | Non-glaucoma, glaucoma | Fundus |
TVGH (944 images) |
CHGH (158 images); DRISHTI-GS1 (101 images); RIM-ONE r2 (455 images) |
EfficientNet B3 |
CHGH: AUC: 0.910 (0.798–1.000); accuracy: 80%; sensitivity: 65%; specificity: 95%; RIM-ONE r2: AUC: 0.624 (0.501–0.748); accuracy: 52.5%; sensitivity: 15%; specificity: 90%; DRISHTI-GS1: AUC: 0.770 (0.558–0.982); accuracy: 55%; sensitivity: 10%; specificity: 100%. |
Pros: validated on multiple datasets. Cons: performance was not generalizable in RIM-ONE r2 and DRISHTI-GS1. | |
| Xue et al. [228] | Glaucoma detection; severity classification | Normal, mild, moderate, severe | IOP, fundus, VF | 6131 samples | 240 samples | 8 juniors,3 seniors,3 experts | Multi- feature deep learning (MFDL) (DetectionNet, ClassificationNet) (ResNet backbone) |
MFDL achieved a higher accuracy of 0.842 (95% CI, 0.795–0.888) than the direct four classification deep learning (DFC-DL, accuracy of 0.513 [0.449–0.576]), CFP-based single-feature deep learning (CFP-DL, accuracy of 0.483 [0.420–0.547]) and VF-based single-feature deep learning (VF-DL, accuracy of 0.725 [0.668–0.782]) Its performance outperformed 8 juniors,3 seniors and 1 expert and was comparable with 2 glaucoma experts |
Pros: compared with human expert. Cons: validation dataset is small and not from multi-center. |
| Wu et al. [47] |
Glaucoma screening; subtyping; early diagnosis |
Screening (glaucoma, healthy); subtyping (POAG, PAAG); early POAG | Tear metabolic fingerprinting (TMF) | 266 samples | 54 samples | Ridge regression (RR) |
Identified metabolic biomarker (Lac, Thr, Mer, Sul, Bar, or DPAE) for glaucoma characterization Glaucoma Screening AUC: 0.856 (95% CI: 0.757–0.954). |
Pros: biomarkers were identified; simple model with good performance. Cons: mass spectrometer is essential for the data; larger dataset is needed for further validation. | |
| Singh et al. [173] | Glaucoma diagnosis | Normal, glaucoma | Fundus |
ACRIMA(705 mages), ORIGA (650 images), HRF (30 images) |
DRISHTI-GS (101 images); PRIVATE (33 images) |
InceptionResNet-V2 | AUC: 0.9042; accuracy: 90%; sensitivity: 86.748%; specificity: 94.11%; F1-score: 91.13%. |
Pros: compared multiple models. Cons: small validation and training dataset. |
|
| Noury et al. [175] |
Glaucoma diagnosis; severity classification |
Normal, glaucoma; mild, moderate severe | SD-OCT ONH scans | 2461 OCT scans volumes |
Hong Kong (HK): 1625 scans; India: 672 scans; Nepal: 380 scans |
1 | DiagFind: 3D CNN |
AUC: HK: 0.80 (95% CI, 0.78–0.82), India:0.94 (95% CI, 0.93–0.96), Nepal: 0.87 (95% CI, 0.85–0.90); sensitivity: HK: 0.73 (0.67–0.79); India: 0.93 (0.88–0.99); Nepa: 0.79 (0.68–0.90); specificity: HK: 0.73 (0.61–0.85); India: 0.71 (0.51–0.91); Nepa: 0.79 (0.66–0.92); F1-score: HK: 0.76 (0.75–0.77); India: 0.91 (0.90–0.92); Nepal: 0.80 (0.78–0.83) testing set from Stanford (100 cases): AUC: 0.92 (95% CI, 0.90–0.93), human grader: 0.91 |
Pros: validated result on rea-world datasets from multiple sites. Cons: exclude cases without consensus labeling and difficulty to be diagnosed by skilled clinicians. |
| Fan et al. [176] | Glaucoma diagnosis | Healthy, glaucoma | Fundus | OHTS: 66,715 images |
ACRIMA (705 images); LAG (4854 images); DIGS (9473 images) |
ResNet-50 |
AUC: DIGS: 0.74 (0.69–0.79); ACRIMA: 0.74 (0.70–0.77); LAG: 0.79 (0.78–0.81); Sensitivity (at 85% specificity): DIGS: 0.52; ACRIMA: 0.46; LAG: 0.59; Sensitivity (at 95% specificity): DIGS: 0.30; ACRIMA: 0.29; LAG: 0.42 |
Pros: validated results on multiple external datasets. Cons: the model was not generalizable in external datasets. |
|
| Fan et al. [222] | Glaucoma diagnosis | Healthy, glaucoma | Fundus | OHTS: 66,715 images |
DIGS (10,473 images), ACRIMA (705 images), LAG (4854 images), RIM-ONE (455 images), ORIGA (650 images) |
Data-efficient image Transformer (DeiT) | AUC: OHTS: 0.91 (0.87, 0.93), DIGS:0.77 (0.71, 0.82); ACRIMA: 0.740.74 (0.70, 0.77); LAG: 0.88 (0.87, 0.89); RIM-ONE:0.91 (0.88, 0.94); ORIGA: 0.73 (0.68, 0.77); sensitivity (at 85% specificity): OHTS:0.79; DIGS: 0.57; ACRIMA: 0.46; LAG: 0.77; RIM-ONE: 0.83; ORIGA: 0.40; sensitivity (at 95% specificity): OHTS:0.56; DIGS: 0.34; ACRIMA: 0.31; LAG: 0.59; RIM-ONE: 0.73; ORIGA: 0.21. |
Pros: validated in multiple external datasets; Vision Transformers have the potential to improve generalizability. Cons: cropped images may lose information. |
|
| Huang et al. [231] | Glaucoma diagnosis | Normal, glaucoma | Fundus, VF | 1655 samples | 196 samples | Probabilistic deep learning model (EffientientNetB4 backbone) | AUC: 0.98 (0.98–0.99); accuracy: 93% (92–95%); sensitivity: 91% (87–95%); specificity: 95% (94–96%). |
Pros: quantifying the uncertainty of the model Cons: dataset was from COMPASS instrument; more external validation is needed. |
|
| Huang et al. [146] | Glaucoma VF grading | Clear, mild, moderate, severe, diffuse | VF (HFA and Octopus) |
3805 VFs (Octopus); 13,231 VFs (HFA) |
150 VFs (HFA) | 2 ophthalmic clinicians, 6 medical students | Fine-grained grading deep learning system (FGGDL: FGG-O, FGG-H); Interactive Interface |
AI outperformed human experts and their performance was improved with the assistance of AI AUC: FGGDL: 0.893 (0.862–0.923); clinician 1: 0.838 (0.801–0.874); clinician 2: 0.833 (0.796–0.869); all the other medical students' performance was lower than 0.80. |
Pros: external validation is applied and compared with human experts. Cons: high test–retest variability was not considered. |
| Li et al. [182] | OD/OC segmentation, glaucoma screening | Glaucoma, non-glaucoma | Fundus | In-house (2440 images) | DRISHIT-GS (101 images); RIM-ONE v3 (159 images) | 4 ophthalmologists | R-DCNN (DAC-ResNet34) |
The segmentation results of in-house testing set were comparable to that of human experts', OD: DC-98.51%, JC-97.07%; OC: DC-97.63%, JC-95.39% Segmentation results of DRISHTI-GS and RIM-ONE v3 are better than existing studies: DRISHTI-GS: OD: DC-97.23%, JC-94.17%; OD: DC-94.56%, JC-89.92%. RIM-ONE v3: OD: DC-96.89%, JC-91.32%; OC: DC-88.94%, JC-78.21%. glaucoma screening: AUC: DRISHTI-GS:0.968; RIM-ONE v3:0.941. |
Pros: compared with human expert and existing studies. Cons: small sample size of training and validation dataset. |
| Li et al. [164] | Glaucoma diagnosis, glaucoma incidence/progression prediction |
Diagnosis (glaucoma, non-glaucoma); glaucoma incidence prediction: with/without glaucoma development; glaucoma progression prediction: with/without progression |
Fundus |
Diagnosis: 24,054 eyes; predict glaucoma incidence: 11,548 eyes; predict glaucoma progression: 3425 eyes |
Glaucoma diagnosis: external test 1: 6162 images (eyes), external test 2: 824 images (eyes); glaucoma incidence: external test 1: 955 images, external test 2: 719 images; glaucoma progression: external test 1: 337 images, external test 2: 513 images |
DiagnoseNet, PredictNet |
Glaucoma diagnosis: AUC: test 1: 0.94 (0.93–0.94); test 2–0.91 (0.89–0.93), sensitivity: glaucoma diagnosis: test 1:0.89(0.87–0.90); test 2: 0.92 (0.88–0.96), specificity: test 1: 0.83 (0.81–0.84); test 2: 0.71 (0.67–0.74); Predict glaucoma incidence: AUC: test 1: 0.89 (0.83–0.95); test 2: 0.88 (0.79–0.97); sensitivity: test 1: 0.84 (0.81–0.86); test 2: 0.84 (0.81–0.86); specificity: test 1: 0.68 (0.43–0.87); test 2: 0.80 (0.44–0.97); Predict glaucoma progression: AUC: test 1: 0.87 (0.81–0.92) and test 2: 0.88 (0.83–0.94); sensitivity: test 1: 0.82 (0.78–0.87) and test 2: 0.81 (0.77–0.84); specificity: test 1: 0.59 (0.39–0.76) and test 2: 0.74 (0.55–0.88). |
Pros: performed multiple tasks (diagnosis, incidence and progression prediction) and include multiple external validation. Cons: exclude low quality images, validation dataset were only Chinese datasets. |
|
| Mehta et al. [149] | Glaucoma detection | Healthy, glaucoma, PTG (progress to glaucoma) | Demographic, systemic and ocular data, color fundus, OCT | UK Biobank (2574 eyes, glaucoma-1193 eyes, healthy-1283 eyes, PTG-98 eyes) | 200 eyes | 5 glaucoma-fellowship trained ophthalmologists | InceptionResnetV4 |
Best model with input of OCT, color fundus, systemic and ocular data obtained AUC = 0.967 (95% CI 0.93–1.0); Human expert (only made diagnosis on color fundus): AUC: 0.79–0.84. |
Pros: used multiple modalities, several methods for interpret DL model (SHAP, saliency map); Cons: poor quality of the fundus images may contribute low AUC. |
| Hemelings et al. [151] | Glaucoma detection, VCDR regression | Glaucoma, non-glaucoma | Fundus | UZL (13,551 images) | REFUGE (1200 images) | ResNet50 | AUC: original fundus: 0.87(95% CI 0.83–0.91); 60% ONH cropping: 0.80 (95% CI 0.76–0.84). |
Pros: report explainability analysis by cropping ONH area; Cons: applying masks of fixed size might lead to a small variation in visible features across fundus images due to variation in ONH size across the study population. |
|
| Thakoor et al. [279] | Glaucoma detection | Glaucoma, non-glaucoma | OCT images (RNFL probability maps) | 737 eyes | 135 eyes | 2 expert OCT readers | InceptionV3 + FC (with concept activation vectors (TCAVs)) |
The TCAVs scores were consistent with features used by human experts based on eye fixations AUC:0.911. |
Pros: applied test with concept activation vectors (TCAVs) for model interpretability; Cons: multi-center datasets were not in external validation. |
| Thakoor et al. [281] | Glaucoma detection | Glaucoma, non-glaucoma | OCT B-Scans, RNFL probability maps | RNFL maps: 737 eyes; B-scans: 771 eyes | RNFL maps:135 eyes; B-scans: 125 eyes |
CNN A (ResNet18 + RF) with RNFL-map as input |
CNN generalizability can be improved with data augmentation, multiple input image modalities, and training on images with confident ratings. choosing a thorough and consistent RS for training and testing improves generalization to new datasets Best result was with RNFL map input and data augmentation on CNN A: AUC = 0.918 (95% CI 0.866–0.970), accuracy = 85.9%; |
Pros: improved generalizability by several techniques (multi-modalities, consistent labels, data augmentation); Cons: independent validation can be improved by a larger dataset. | |
| Natarajan et al. [143] | Glaucoma detection, OD segmentation | Glaucoma, normal | Fundus | RIGA (750 images), RIM-ONEv2 (455 images) | ACRIMA (705 images), Drishti-GS1 (101 images), RIMONEv1 (169 images) | UNet-Snet (SqueezeNet) | glaucoma detection: AUC: ACRIMA: 100%; Drishti-GS1: 99.90%, RIMONEv1: 100%; accuracy: ACRIMA: 99.86%; Drishti-GS1: 97.05%, RIMONEv1: 100%; sensitivity: ACRIMA: 100%; Drishti-GS1: 100%, RIMONEv1: 100%; specificity: ACRIMA: 99.75%; Drishti-GS1: 90.32%, RIMONEv1: 100%. | Pros: achieved high performance; Cons: small dataset for training and independent validation. | |
| Kenichi et al. [129] | Glaucoma diagnosis | Glaucoma, normal | Fundus | 3132 images (from ordinary camera) | 162 images (from ordinary camera and smartphone) | ResNet34 |
AUC: camera-98.9%; smartphone-84.2% advanced glaucoma: AUC: camera-99.3%; smartphone-90.0%. |
Pros: validated performance between fundus from ordinary camera and smartphone; Cons: training images were all from ordinary camera, poor image quality of fundus from smartphone. |
|
| Xu et al. [242] | Glaucoma diagnosis | Referable glaucomatous optic neuropathy (GON), unlikely GON | Fundus | 1791 images |
dataset1: 6301 images dataset2: 1964 images dataset3: 400 images |
12 (4 senior ophthalmologists, 4 junior ophthalmologists, and 4 technicians) | Hierarchical deep learning system (HDLS)(segmentation- classification, Inception-v3 backbone) |
Reliable region has higher sensitivity and specificity than suspicious region datset1: AUC = 0.981 (95% CI, 0.978–0.985), reliable region: sensitivity = 97.7% (95% CI, 97.0–98.3%), specificity = 97.8% (95% CI, 97.2–98.4%); dataset2: AUC = 0.983 (95% CI, 0.977–0.989); reliable region: sensitivity: 98.4% (95% CI, 97.3–99.5%), specificity = 98.2% (95% CI, 97.4–99.1%) Dataset 3: performance of human experts were improved with the referring of HDLS: senior group: sensitivity: 0.93 (diagnose independently), 0.96 (referring to HDLS); specificity: 0.88 (diagnose independently), 0.95 (referring to HDLS). |
Pros: this system is transparent and interpretable. the results were validated on three large validation datasets and results were comparable to that of human experts. Cons: validation dataset did not include data from other ethnics than Chinese. |
| Bhuiyan et al. [246] | Glaucoma diagnosis | Glaucoma-suspect, not-suspect | Fundus | 1546 disc-centered fundus images (AREDS, SiMES, RIM-ONE) |
ORIGA (638 gradable images) |
Ensemble of Xception, Inception-Resnet-V2, NasNet, Inception-V3 | AUC: 0.85; accuracy: 83.54%; sensitivity: 80.11%; specificity: 84.96%. |
Pros: screening glaucoma suspect is important; Cons: CDR is not the only biomarker for glaucoma suspect, other biomarkers, such as CDR asymmetry can be added in future studies. |
|
| Tang et al. [205] | Glaucoma diagnosis | Glaucoma, non-glaucoma | Fundus | Sanyuan (11,443 images), Tongren (7806 images), Xiehe (4363 images) | REFUGE (1200 images) | AMNet (semi-supervised learning) | accuracy: 95.75%; sensitivity: 87.5%; specificity: 96.7%; F1-score: 91.9%. |
Pros: the model boosted the robustness of model with limited labeled data. Cons: diverse validation dataset might be needed to future validate the model. |
|
| Alghamdi et al. [241] | Glaucoma diagnosis | Glaucoma, normal | Fundus |
RIM-ONE (455 images) RIGA (750 images) |
2 ophthalmologists | TCNN-Transfer Convolutional Neural Network (VGG16); SSCNN- Semi-supervised Convolutional Neural Network model with self-learning; SSCNN-DAE-Semi-supervised Convolutional Neural Network model with autoencoder | three deep learning CNN models outperform the performances of both ophthalmologists with clear margins. RIM-ONE: accuracy: two experts attain 59.2% and 55.4.0% SSCNN-DAE: AUC: 0.95, accuracy: 93.8%; sensitivity: 98.90%; specificity: 90.50%. |
Pros: compared with human experts and outperformed them. Cons: small datasets were used for training; external validation was not included. |
|
| Li et al. [278] | Glaucoma diagnosis in myopia | Glaucoma, non-glaucoma | RNFL profile | 2223 eyes | 508 eyes | FCN + RBFN (radial basis function network) + RNFL compensation | By applying the RNFL compensation algorithm, the AUC for detecting glaucoma increased from 0.70 to 0.84, from 0.75 to 0.89, from 0.77 to 0.89, and from 0.78 to 0.87 for eyes in the highest 10%, 20%, 30% and any axial length (AL), respectively. | Pros: An RBFN has good generalization, strong tolerance to input noise, and online learning ability made it possible to interpret the patterns to a reliable compensation. Cons: compensation of the RNFL profile was based on data from participants with an age ≥ 50 years, this was not validated in younger participants; the validation dataset included a 1:1 ratio of glaucomatous to non-glaucomatous eyes, this is not the case in read world data. | |
| Chiang et al. [277] |
Primary angle closure glaucoma (PACG) detection |
POAG, PACG | Goniophotography | 32,635 images | 1000 images | 9 graders | CNN (ResNet-50 backbone) | CNN achieved excellent performance based on single-grader (AUC = 0.969) and consensus (AUC = 0.952) labels. The agreement between the CNN classifier and consensus labels (κ = 0.746) surpassed that of all non-reference human graders (κ = 0.578–0.702). | Pros: the model obtained comparable performance with human graders. Cons: the model has limitation of generalizable to other ethnic groups and goniophotography taken from other devices. |
| Zhao et al. [196] | Cup-to-disc ratio (CDR) estimation, glaucoma screening | Glaucoma, normal | Fundus | Direct-CSU (934 images) | ORIGA (650 images) | Unsupervised feature representation of fundus image with a CNN (MFPPNet-3 blocks DenseNet) + RF |
glaucoma screening: AUC: 0.88 CDR estimation: MAE: 0.0606; coefficient of correlation r = 0.68. |
Pros: estimated the CDR value more effectively than results obtained from traditional segmentation-based methods; potential to handle unlabeled data. Cons: further validation on diverse data sources would be needed. |
|
| Jammal et al. [112] | Fundus predict RNFL; glaucoma detection | Glaucoma, normal | Fundus | 32,820 pairs of fundus photos and SD-OCT scans | 2 graders | M2M DL (ResNet34 backbone) |
DL algorithm outperformed human graders in detecting signs of glaucomatous damage on fundus photographs. glaucoma detection: AUC: DL: 0.801 [95% CI: 0.757, 0.845]; human: 0.775 [95% CI: 0.728, 0.823]; AUPRC: DL: 0.810 [95% CI: 0.765, 0.851]; human: 0.761 [95% CI: 0.703, 0.819] RNFL prediction (DL): MAE: 7.39 um; Pearson’s r = 0.832. |
Pros: the M2M model is able to provide a quantitative output and outperformed human graders. Cons: used the presence of visual field defects as the gold standard may be biased and influence accuracy. |
|
| Wang et al. [142] | Glaucoma detection | Glaucoma, normal | OCT images | HK dataset: 975,400 B-scans (4877 volumns) |
Stanford dataset: 246,200 B-scans (1231 volumns) |
2 glaucoma experts | 2D-ResNet18-SEMT (SEmi-supervised Multi-Task) |
Stanford (volumn based): AUC: 0.933, accuracy:86%; F1-score: 0.889; human vs model (HK, volumn based): AUC: 0.977 (DL), 0.918 (human); accuracy: 0.927 (DL), 0.912 (human); F1 score: 0.941 (DL); 0.917 (human). |
Pros: semi-supervised learning addressed the miss VF measurement label problem in the training set, the multi-task learning network to explore the relationship between the functional and structural, which was beneficial to accuracy improvement. Cons: the framework at the training stage is not end-to-end, where the hard assignment for VF measurement and the multi-tasking training work in a cascaded manner. |
| Russakoff et al. [119] | Glaucoma diagnosis | Referable, nonreferable glaucoma | 3D-OCT | 2805 scans | Hongkong:505 eyes, India:336 eyes | gNet3D (3DCNN) | AUC: HK:0.78; India: 0.95 |
Pros: first studies to use machine learning in risk stratification; multinational external datasets across geographical and ethnicity distribution from Hong Kong and India. Cons: lack of inclusion of suspects in external datasets; different definitions of glaucoma between development and external dataset. |
|
| Zaleska-Żmijewska et al. [121] | Glaucoma diagnosis | Glaucoma, healthy | Fundus, IOP | 1687 images |
Campaign1:752 images (C1) campaign2: 352 images (C2) |
AlexNet |
images classifier: accuracy: C1:80%; C2:78%; sensitivity: C1:0.73, C2:0.84; specificity: C1:0.83; C2:0.67; fundus + IOP: accuracy: C1:71%; C2:79%; sensitivity: C1:0.79, C2:0.92; specificity: C1:0.67; C2:0.42. |
Pros: include IOP risk factor in model; IOP inclusion improved the sensitivity. Cons: small number of datasets, performance need to be improved. | |
| Zheng et al. [130] | Glaucoma diagnosis | Glaucoma, normal | SD-OCT images (hand-craft features (HCFs), peripapillary RNFL OCT images) | 1501 images | 104 images | Inception-V3 | AUC: 0.990 (0.974, 1.000), accuracy: 0. 990(0.974, 1.000); sensitivity: 0.981 (at 80%, 90% specificity). |
Pros: achieved higher sensitivity and specificity compared to traditional HCFs. Cons: external validation test is needed from different centers or OCT devices; most of the glaucoma cases were quite severe and this made classification easier in the current study; used images of Chinese eyes only, the results may not be applicable to other populations. |
|
| Li et al. [131] | Glaucoma detection | Glaucoma, non-glaucoma |
VF (deviation probability plots (PDPs), numerical pattern deviation plots (NDPs), and numeric displays (NDs)) |
9022 VFs |
phase1: test 1: 200 VFs, test 2:406 VFs, test 3: 507VFs phase 2: 649 VFs |
6 ophthalmologists | iGlaucoma (2D-Fusion-CNN (with input of ND + NDP + PDP)) |
In Phase I, the DLS outperformed all six ophthalmologists in the three test sets (AUC of 0.834–0.877, with a sensitivity of 0.831–0.922 and a specificity of 0.676–0.709); In Phase II, iGlaucoma had 0.99 accuracy in recognizing different patterns in pattern deviation probability plots region, with corresponding AUC, sensitivity and specificity of 0.966 (0.953–0.979), 0.954 (0.930–0.977), and 0.873 (0.838–0.908). |
Pros: developed ‘iGlaucoma’, a smartphone application-based deep learning system (DLS); the performance outperformed human expert. Cons: this study is limited to the Chinese population; this DLS only utilizes VF, no other modality included. |
| Kim et al. [132] | Glaucoma diagnosis | Glaucoma, normal | OCT (RNFL thickness, RNFL deviation maps, GCIPL thickness, GCIPL deviation maps, ocular axial length) | 8988 images | 1420 images | 2 glaucoma specialists | VGG-19 |
The glaucoma-diagnostic ability was highest when the DL system used the RNFL thickness map alone, among combination sets, use of the RNFL and GCIPL deviation map showed the highest diagnostic ability. It showed detection patterns similar to those of glaucoma specialists External (RNFL + GCIPL deviation map): AUC: 0.985 (95% CI 0.966–0.995), sensitivity (at 90% specificity): 97.2%, sensitivity (at 80% specificity): 98.2%. |
Pros: include interpretability and compared with human, with agreement pattern; multiple modalities and wide severity level included. Cons: external validation only include good quality OCT images and only from Asian ethnicity. |
| Christopher et al. [136] | Glaucoma diagnosis | Glaucoma, normal | Fundus | DIGS/ADAGES: 14,822 images; MRCH: 3132 images |
Iinan Dataset: 215 images Hiroshima Dataset: 171 images ACRIMA: 705 images |
UCSD (ResNet50) UTokyo (ResNet34) |
Utokyo (sequential) obtained highest performance in external datasets: AUC: linan:0.97 (0.94–0.99), Hiroshima: 0.99 (0.99–0.99), ACRIMA: 0.86 (0.83–0.89). |
Pros: DIGS/ADAGES and MRCH consisted diverse population, the study computed model performance stratified by disease severity, myopia status, and race. Cons: the datasets have inconsistent glaucoma definition and labeling strategy. |
|
| Maadi et al. [194] | OD/OC segmentation, glaucoma detection | GLAUCOMA, non-glaucoma | Fundus |
Drishti-GS1: 101 images RIMONE v3: 159 images |
REFUGE: 1200 images | Modified U-Net (SE-ResNet50) | OD/OC segmentation: F1-score: OD:0.91; OC:0.79. Diagnosis: AUC: 0.939. |
Pros: external validation was used and performed well. Cons: larger training and validation datasets might be needed for further improvement. |
|
| Phene et al. [109] | Detect referable GON | Non-referable GON, referable GON | Fundus | 88,126 images |
A: 1205 images B: 9642 images C: 346 images |
10 graders (grade 411 images in validation A dataset) | Inception-v3 |
The performance of DL was better than human on a subset (411 images) of validation dataset A AUC: A:0.945 (0.929–0.960); B: 0.855 (0.841–0.870); C: 0.881 (0.838–0.918). |
Pros: this DL algorithm has higher sensitivity and comparable specificity to eye care providers in detecting referable GON in color fundus images; provides insight into which ONH features drive GON assessment by glaucoma specialists. Cons: diagnosis of glaucoma is not based on ONH appearance alone but also relies on the compilation of risk factors. |
| Gómez-Valverde et al. [261] | Glaucoma detection | Glaucoma, normal | Fundus | 2313 images (RIM-ONE, DRISHTI-GS, ESPERANZA) | 4 glaucoma experts | VGG19 |
human expert (ESPERANZA): specificity = 0.8914, sensitivity = 0.7662; model (train on all 3 dataset): AUC: 0.94; sensitivity: 87.01%; specificity: 89.01%. |
Pros: model performance was comparable to human expert. Cons: no independent validation dataset provided. |
|
| Asaoka et al. [118] | Glaucoma diagnosis | Glaucoma, normal | Fundus | 3132 images (camera: Kowa) |
Test 1: 205 images (camera: Kowa) test2: 171 images (camera: Topcon) |
ResNet |
Data augmentation improved model performance With augmentation: AUC: test 1: 0.948 (0.903–0.968); test 2: 0.997 (0.994–1); Without augmentation: AUC: test 1:0.877 (0.828–0.926); test 2: 0.945(0.913–0.976). |
Pros: model was validated using images obtained from different fundus cameras and it had a high diagnostic ability irrespective of the type of fundus camera. Cons: the model has not been validated in different ethnicities. |
|
| Kim et al. [113] | Glaucoma diagnosis and localization | Glaucoma, normal | Fundus | 2123 images |
RIM-ONE r3 (159 images) |
ResNet152 | accuracy: 93.5%; sensitivity: 92.9%, specificity: 92.9%. | Pros: a web application was developed with output pd Grad-CAM. Cons: larger datasets from different instrument may be incorporated to improve the performance. | |
| Asaoka et al. [31] | Diagnose early glaucoma | Glaucoma, normal | Macula SD-OCT (RNFL and GCC thicknesses) |
Pretrain: 4316 OCT images train: 178 eyes (94 POAG, 84 normal) |
196 eyes (114 POAG, 82 normal) |
DL transform (CNN using 8*8 grid RNFL (in the first channel) and GCC data (in the second channel), use both pretraining and training) |
With pretraining: AUC: 93.7% (90.6–96.8). Optimum discrimination at sensitivity of 82.5%, specificity of 93.9%. Specificity (at 80% sensitivity): 83.3%; Specificity (at 90% sensitivity): 86.6% Without pretraining: AUC: 76.6–78.8% |
Pros: the study tested importance of pretraining process in model performance improvement Cons: the results were obtained using a homogeneous patient population (only Japanese patients), further study might prepare OCT data from multiple ethnicities, to generalize the results |
|
| Al-Aswad et al. [289] | Glaucoma diagnosis | Glaucoma, non-glaucoma | Fundus | 110 images | 6 ophthalmologists | Pegasus |
AUC: Pegasus: 92.6%, ophthalmologist: 69.6% -84.9%, the "best case" consensus scenario AUC of 89.1%. Sensitivity: Pegasus: 83.7%; ophthalmologists: 61.3%-81.6%. Specificity: Pegasus: 88.2%, ophthalmologists:80.0% -94.1% Agreement with gold standard: Pegasus: 0.715; highest ophthalmologist: 0.613. |
Pros: Pegasus outperformed 5 of the 6 ophthalmologists in terms of diagnostic performance, and there was no statistically significant difference between the deep learning system and the “best case” consensus between the ophthalmologists. Cons: small sample size and fundus from same camera. |
|
| Norouzifard et al. [102] | Glaucoma diagnosis | Glaucoma, normal | Fundus | 447 images | HRF (30 images) | Inception-ResNet-V2 | accuracy: 80% |
Pros: validated on independent dataset. Cons: both training and validation datasets were small. |
|
| Shibata et al. [105] | Glaucoma screening | Glaucoma, normal | Fundus | 3132 images (1364 glaucoma, 1768 normal) | 110 images (60 glaucoma, 50 normal) | 3 residents | ResNet | AUC: model:96.5% (93.5–99.6%); residents: 72.6–91.2%. |
Pros: the performance was compared with human expert and the model outperformed them. Cons: the current study excluded photographs with features that could interfere with an expert diagnosis of glaucoma, which was not “real world” setting. |
| Li et al. [256] | VF classification | Glaucoma, non-glaucoma | VF (VF PD images) | 4012 VFs | 300 VFs | 9 ophthalmologists | CNN |
Model: accuracy: 0.876 (95% CI 0.838–0.914); sensitivity: 0.932; specificity: 0.826 Ophthalmologists: the average accuracies are 0.607, 0.585 and 0.626 for resident ophthalmologists, attending ophthalmologists and glaucoma experts, respectively. |
Pros: CNN has achieved higher accuracy compared to human ophthalmologists and traditional rules; Cons: used only pattern deviation images as the input of CNN, preperimetric glaucoma may not be effectively detected by machine. |
| Andersson et al. [75] | Glaucoma Diagnosis | Glaucoma, healthy |
VF (30–2 VF) |
165 subjects (99 glaucoma,66 healthy) |
30 physicians | ANN |
ANN: sensitivity: 93%, specificity: 91% Physicians: sensitivity: 61%- 96% (mean 83%); specificity:59%-100% (mean 90%). |
Pros: ANN performs at least as well as physicians in assessments of visual fields for the diagnosis of glaucoma; Cons: external and larger datasets are needed for further validation. | |
| Goldbaum et al. [12] | Glaucoma diagnosis | Glaucoma, normal | VF (24–2 VF) + age | 345 eyes (189 normal, 156 glaucoma) | 2 human experts | Mixture of Gaussian (MoG) |
MoG had significantly greater ROC area than PSD and CPSD. Human experts were not better at classifying visual fields than the machine classifiers or the global indices. MoG(PCA): AUC: 0.922; sensitivity: 0.67(at specificity = 1), 0.79 (at specificity = 0.9); Expert 1: sensitivity = 0.75, specificity = 0.96; Expert 2: sensitivity = 0.88, specificity = 0.59. |
Pros: compared multiple methods based on different parameters. Cons: larger and external validation are needed for further evaluation. |
|
| Goldbaum et al. [63] | Glaucoma diagnosis | Glaucoma, normal | VF | 120 eyes (60 normal, 60 glaucoma) | 2 glaucoma specialists | ANN (2 layered) |
The experts and the network were in agreement about 74% of the time ANN: accuracy: 67%; sensitivity: 65%; specificity: 71%; glaucoma specialists: accuracy: 67%; sensitivity: 59%; specificity: 74%. |
Pros: compared with human expert. Cons: dataset was small, performance needs to be improved and external validation was needed. |
It is also challenging to compare the performance of different AI models as they typically used differing definitions of glaucoma, different methodologies to develop models and various strategies for labeling, as well as images from glaucoma patients at varying levels of severity. In addition, they typically employed images with diverse quality under different instrument settings and included different populations from primary, secondary, or tertiary centers. As a result, for example, it is unfair to compare a model trained on normal subjects and glaucoma patients at the moderate to advanced stages of the disease with another model trained on a dataset with patients at a wider spectrum of glaucoma severity. Model reliability is also a barrier for real-life clinical applications, as most DL models are deterministic and provide an output regardless of whether the input image is even relevant. Finally, most DL models provide a “black box” architecture without appropriate visualization and interpretability, thus lowering user trust and creating another challenge for a successful integration.
Future directions
Two autonomous AI models have been approved by the FDA to detect more than mild diabetic retinopathy and diabetic macular edema [302]; however, there is no FDA-approved autonomous AI instrument for detecting glaucoma. Therefore, the development of innovative AI models to autonomously assess and detect glaucoma remains an important goal for improving treatment outcomes for this major blinding disorder. To achieve this goal, we suggest implementing the following directions in the future studies:
Lack of consistent and objective definitions of glaucoma and its progression leads to improper AI model evaluations. As such, addressing this challenge is a primary step to improve integration of AI glaucoma research and clinical applications. One potential solution may be using quantified parameters from VF and OCT data to create objective criteria for glaucoma definition. More recently, some groups have worked on identifying more objective criteria for defining GON and glaucoma staging based on OCT and VFs [87, 303, 304]. However, this active area of research requires further studies. Firstly, these criteria need to be validated further based on larger and more diverse datasets. Secondly, data collected from different OCT or VF instruments may impact the identified objective criteria. Lastly, comorbidity and ethnic-specific criteria may influence findings thus considering the effect of these parameters is vital. The other area that needs attention is the architecture of the AI models. In particular, DL models sometimes can be fragile, unexplainable, and non-interpretable. Many teams are currently working on these limiting aspects of the DL models and hopefully some limitations will be addressed in the near future. New studies may incorporate interpretability into the AI model through various techniques, such as feature importance, SHAP value, Grad-CAM, saliency map, and Poly-CAM. Some current studies have already included interpretability elements like feature importance and Grad-CAM. Thakoor et al. [279] developed end-to-end CNN architectures to detect glaucoma based on OCT images. They applied Grad-CAM and tested with concept activation vectors (TCAVs) to infer what image concepts CNN models rely on to make predictions and compared with that of human experts by tracking eye fixations. They identified consistent regions of OCT are evaluated based on CNN and OCT experts in detecting glaucoma. Such studies can shed lights on improving interpretability of AI models by applying multiple consistent methods and comparing with clinicians’ eye fixations. Future work may validate these studies using more diverse datasets. Also, comparative studies can be conducted based on multiple data modalities by including or masking human focused region to indirectly identify areas that are more important for CNN models.
Another practical approach to partially address some of these limitations is to perform accurate quantification of CDR in fundus and RNFL thickness profiles in OCT images to enhance performance and improve interpretability. Moreover, longitudinal assessment of these clinically relevant quantified parameters may allow a more consistent and accurate monitoring and progression detection.
Another future direction could be improving datasets for training AI models. Some of the reference datasets are annotated by non-glaucoma experts thus may not represent GON accurately. Therefore, generating more accurate reference datasets with panels of glaucoma specialists are warranted. We also suggest selecting diverse populations of patients (for instance, datasets for population-based screening and diagnosis for intended use) from different ethnicities across all glaucoma severity levels to minimize selective bias. Due to the complex nature of glaucoma (e.g., different disease processes can lead to the same optic nerve degeneration, glaucoma can look and progress differently due to different disease mechanisms, glaucoma has several phenotypes such as open angle, closed angle, secondary glaucoma, low tension, etc.), making it difficult to standardize outcomes of glaucoma studies across different investigations, we strongly recommend generating multi-modal glaucoma datasets given the fact that glaucoma is multi-factorial and complex, thus several modalities may enhance detection and monitoring tasks. However, obtaining a sufficiently large dataset with several modalities can be challenging. A primary priority for future AI studies could be implementing guidelines regarding designing and reporting AI studies [305, 305–307] to minimize evaluation and comparison challenges.
Another promising solution is developing foundational model using self-supervised learning [308] based on known diverse datasets to improve the performance and generalizability. This is particularly important given the fact that the performance of the AI models can vary depending on the severity level of the patients with glaucoma in the dataset, as AI models may perform better on datasets with a greater number of patients at the advanced level of glaucoma compared to datasets with a greater number of patients at the early stages of glaucoma.
Improving dependability of AI models can build trust. One direction could be developing probabilistic DL models [231] that can generate the likelihood as well as the level of confidence of the model on generated outcome. This way, clinicians have two levels of outcome to make a final decision and thus may trust the class of AI models better. However, probabilistic models are more challenging to develop due primarily to more complexity that typically leads to suboptimal performance, thus only a few studies have utilized probabilistic models. Recent CNN architectures such as ConvNeXt [309] and availability of larger datasets may however address these challenges.
The paradigm of applications of AI in glaucoma changed in 2016 with applications of deep CNN models in glaucoma. [310]. However, we believe a second major impact will result from the application of ChatGPT [311], first initiated in late 2022. ChatGPT can be a great tool for many different aspects of glaucoma, and a recent study showed that ChatGPT can assist glaucoma diagnosis based on clinical case reports and obtained comparable performance with senior ophthalmology residents [312]. We believe the development of large language models (LLMs) with glaucoma domain-specific knowledge that leverage multi-modal data in combination with active learning holds more promise for future integration into clinical practice.
Conclusions
The advancement of AI in glaucoma detection and monitoring is progressing rapidly. In recent years, numerous innovative DL models have been developed specifically for diagnosing glaucoma, showcasing remarkable performance. However, despite their promising results, none of these models have received FDA approval for being used in glaucoma clinical practice. This is partly due to obstacles such as inconsistencies in defining glaucoma, the generalizability and reliability of the models, and their interpretability. To enhance the integration of these technologies into healthcare settings, future research is essential to address these potential challenges, including generation of dependable gold standards, improving model generalizability, reliability, interpretability as well as legal, ethical, and patient privacy issues, among several others. Successful integration of AI in glaucoma clinical practice, and ophthalmology, requires addressing challenges facing all elements of the healthcare pipeline.
Methods
We searched PubMed, Scopus, and Embase databases for the AI-related studies in glaucoma conducted through 2022. Figure 4 shows the methodology utilized in this review. We first used “glaucoma”, “machine learning”, “deep learning”, “artificial intelligence”, and “neural network” keywords and searched through the title of papers in these three databases to identify related papers. Our initial search identified 986 articles. We then screened the collected articles for duplicates and irrelevant topics and included 430 relevant and unique papers. Finally, we went through the remaining articles and excluded 139 articles that were review papers, or the contents were irrelevant, or essential information (number of samples) was missed, the full texts were not in English or the full text cannot be obtained. The final study included 291 full-text articles discussing various applications of AI in glaucoma.
Fig. 4.
Proposed review methodology for sample collection and analysis
Author contributions
Conceptualization: XH, RI and SY. Methodology: XH, RI, SF, and SY; writing: XH, RI, SF, SY. Review and editing: XH, RI, SF, HAS, AAA, EK and SY. Supervision: SY. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH Grants EY033005, EY031725, and a challenge Grant from Research to Prevent Blindness (RPB), New York. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Declarations
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoqin Huang and Md Rafiqul Islam are shares co-first authorship.
References
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686. doi: 10.7759/cureus.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg LF. Glaucoma: early detection and therapy for prevention of vision loss. Am Fam Physician. 1995;52(8):2289–2298. [PubMed] [Google Scholar]
- 6.Lucy KA, Wollstein G. Structural and functional evaluations for the early detection of glaucoma. Expert Rev Ophthalmol. 2016;11(5):367–376. doi: 10.1080/17469899.2016.1229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi M, Dubey S. Evaluation of the optic nerve head in glaucoma. J Curr Glaucoma Pract. 2013;7(3):106–114. doi: 10.5005/jp-journals-10008-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi S. Clinical applications of artificial intelligence in glaucoma. J Ophthalmic Vis Res. 2023;18(1):97–112. doi: 10.18502/jovr.v18i1.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahabadi N, Foris LA, Tripathy K. Open angle glaucoma. Treasure Island: StatPearls; 2022. [PubMed] [Google Scholar]
- 10.Susanna R, Jr, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1–1. doi: 10.1167/tvst.4.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldbaum MH, Sample PA, Chan K, et al. Comparing machine learning classifiers for diagnosing glaucoma from standard automated perimetry. Invest Ophthalmol Vis Sci. 2002;43(1):162–169. [PubMed] [Google Scholar]
- 13.Zangwill LM, Chan K, Bowd C, et al. Heidelberg retina tomograph measurements of the optic disc and parapapillary retina for detecting glaucoma analyzed by machine learning classifiers. Invest Ophthalmol Vis Sci. 2004;45(9):3144–3151. doi: 10.1167/iovs.04-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgansky-Eliash Z, Wollstein G, Chu T, et al. Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest Ophthalmol Vis Sci. 2005;46(11):4147–4152. doi: 10.1167/iovs.05-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend KA, Wollstein G, Danks D, et al. Heidelberg retina tomograph 3 machine learning classifiers for glaucoma detection. Br J Ophthalmol. 2008;92(6):814–818. doi: 10.1136/bjo.2007.133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Morate D, Simón-Hurtado A, Vivaracho-Pascual C, Antón-López A. A new methodology for feature selection based on machine learning methods applied to glaucoma. In: Cabestany J, Sandoval F, Prieto A, Corchado JM, editors. Bio-inspired systems: computational and ambient intelligence. Berlin: Springer; 2009. [Google Scholar]
- 17.Bizios D, Heijl A, Hougaard JL, Bengtsson B. Machine learning classifiers for glaucoma diagnosis based on classification of retinal nerve fibre layer thickness parameters measured by Stratus OCT. Acta Ophthalmol. 2010;88(1):44–52. doi: 10.1111/j.1755-3768.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa H, Murata H, Mayama C, Araie M, Asaoka R. Evaluation of various machine learning methods to predict vision-related quality of life from visual field data and visual acuity in patients with glaucoma. Acta Ophthalmol. 2010 doi: 10.1111/j.1755-3768.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 19.Barella KA, Costa VP, Goncalves Vidotti V, Silva FR, Dias M, Gomi ES. Glaucoma diagnostic accuracy of machine learning classifiers using retinal nerve fiber layer and optic nerve data from SD-OCT. J Ophthalmol. 2013;2013:789129. doi: 10.1155/2013/789129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva FR, Vidotti VG, Cremasco F, Dias M, Gomi ES, Costa VP. Sensitivity and specificity of machine learning classifiers for glaucoma diagnosis using spectral domain OCT and standard automated perimetry. Arq Bras Oftalmol. 2013;76(3):170–174. doi: 10.1590/S0004-27492013000300008. [DOI] [PubMed] [Google Scholar]
- 21.Vidotti VG, Costa VP, Silva FR, et al. Sensitivity and specificity of machine learning classifiers and spectral domain OCT for the diagnosis of glaucoma. Eur J Ophthalmol. 2012 doi: 10.5301/ejo.5000183. [DOI] [PubMed] [Google Scholar]
- 22.Kavitha S, Duraiswamy K, Karthikeyan S. Assessment of glaucoma using extreme learning machine and fractal feature analysis. Int J Ophthalmol. 2015;8(6):1255–1257. doi: 10.3980/j.issn.2222-3959.2015.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuppusamy PG. An artificial intelligence formulation and the investigation of glaucoma in color fundus images by using BAT algorithm. J Comput Theor Nanosci. 2017;14:1–5. doi: 10.1166/jctn.2017.6542. [DOI] [Google Scholar]
- 24.Omodaka K, An G, Tsuda S, et al. Classification of optic disc shape in glaucoma using machine learning based on quantified ocular parameters. PLoS ONE. 2017;12(12):e0190012. doi: 10.1371/journal.pone.0190012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Cho KJ, Oh S. Development of machine learning models for diagnosis of glaucoma. PLoS ONE. 2017;12(5):e0177726. doi: 10.1371/journal.pone.0177726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abidi SSR, Roy PC, Shah MS, Yu J, Yan S. A data mining framework for glaucoma decision support based on optic nerve image analysis using machine learning methods. J Healthc Inform Res. 2018;2(4):370–401. doi: 10.1007/s41666-018-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An G, Omodaka K, Tsuda S, et al. Comparison of machine-learning classification models for glaucoma management. J Healthc Eng. 2018;2018:6874765. doi: 10.1155/2018/6874765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu SH, Lee KY, Chong JIT, Lam AKC, Lai JSM, Lam DCC. Comparison of Ocular Biomechanical Machine Learning Classifiers for Glaucoma Diagnosis. In: 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); 3–6 Dec. 2018, 2018.
- 29.Christopher M, Belghith A, Weinreb RN, et al. Retinal nerve fiber layer features identified by unsupervised machine learning on optical coherence tomography scans predict glaucoma progression. Invest Ophthalmol Vis Sci. 2018;59(7):2748–2756. doi: 10.1167/iovs.17-23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin KR, Mansouri K, Weinreb RN, et al. Use of machine learning on contact lens sensor-derived parameters for the diagnosis of primary open-angle glaucoma. Am J Ophthalmol. 2018;194:46–53. doi: 10.1016/j.ajo.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Asaoka R, Murata H, Hirasawa K, et al. Using deep learning and transfer learning to accurately diagnose early-onset glaucoma from macular optical coherence tomography images. Am J Ophthalmol. 2019;198:136–145. doi: 10.1016/j.ajo.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Thomas PBM, Chan T, Nixon T, Muthusamy B, White A. Feasibility of simple machine learning approaches to support detection of non-glaucomatous visual fields in future automated glaucoma clinics. Eye (Lond) 2019;33(7):1133–1139. doi: 10.1038/s41433-019-0386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SD, Lee JH, Choi YG, You HC, Kang JH, Jun CH. Machine learning models based on the dimensionality reduction of standard automated perimetry data for glaucoma diagnosis. Artif Intell Med. 2019;94:110–116. doi: 10.1016/j.artmed.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Shen J, Chang R, et al. Machine learning models for diagnosing glaucoma from retinal nerve fiber layer thickness maps. Ophthalmol Glaucoma. 2019;2(6):422–428. doi: 10.1016/j.ogla.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter SL, Marks C, Kuo TT, Ohno-Machado L, Weinreb RN. Machine learning-based predictive modeling of surgical intervention in glaucoma using systemic data from electronic health records. Am J Ophthalmol. 2019;208:30–40. doi: 10.1016/j.ajo.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee R, Kundu S, Dutta K, Sen A, Majumdar S. Predictive diagnosis of glaucoma based on analysis of focal notching along the neuro-retinal rim using machine learning. Pattern Recognit Image Anal. 2019;29(3):523–532. doi: 10.1134/S1054661819030155. [DOI] [Google Scholar]
- 37.Thakur N, Juneja M. Classification of glaucoma using hybrid features with machine learning approaches. Biomed Signal Process Control. 2020;62:102137. doi: 10.1016/j.bspc.2020.102137. [DOI] [Google Scholar]
- 38.Lee J, Kim YK, Jeoung JW, Ha A, Kim YW, Park KH. Machine learning classifiers-based prediction of normal-tension glaucoma progression in young myopic patients. Jpn J Ophthalmol. 2020;64(1):68–76. doi: 10.1007/s10384-019-00706-2. [DOI] [PubMed] [Google Scholar]
- 39.Brandao-de-Resende C, Cronemberger S, Veloso AW, et al. Use of machine learning to predict the risk of early morning intraocular pressure peaks in glaucoma patients and suspects. Arq Bras Oftalmol. 2021;84(6):569–575. doi: 10.5935/0004-2749.20210101. [DOI] [PubMed] [Google Scholar]
- 40.Singh LK, Garg H, Khanna M. An artificial intelligence-based smart system for early glaucoma recognition using OCT images. Int J E-Health Med Commun (IJEHMC). 2021;12(4):32–59. doi: 10.4018/IJEHMC.20210701.oa3. [DOI] [Google Scholar]
- 41.Yoon BW, Lim SH, Shin JH, Lee JW, Lee Y, Seo JH. Analysis of oral microbiome in glaucoma patients using machine learning prediction models. J Oral Microbiol. 2021;13(1):1962125. doi: 10.1080/20002297.2021.1962125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CW, Shen HL, Lu CJ, Chen SH, Chen HY. Comparison of different machine learning classifiers for glaucoma diagnosis based on spectralis OCT. Diagnostics (Basel). 2021;11(9):1718. doi: 10.3390/diagnostics11091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elizabeth Jesi V, Mohamed Aslam S, Ramkumar G, Sabarivani A, Gnanasekar AK, Thomas P. Energetic glaucoma segmentation and classification strategies using depth optimized machine learning strategies. Contrast Media Mol Imaging. 2021;2021:5709257. doi: 10.1155/2021/5709257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh S, Park Y, Cho KJ, Kim SJ. Explainable machine learning model for glaucoma diagnosis and its interpretation. Diagnostics (Basel). 2021;11(3):510. doi: 10.3390/diagnostics11030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez Escamez CS, Martin Giral E, Perucho Martinez S, Toledano FN. High interpretable machine learning classifier for early glaucoma diagnosis. Int J Ophthalmol. 2021;14(3):393–398. doi: 10.18240/ijo.2021.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuldiner SR, Boland MV, Ramulu PY, et al. Predicting eyes at risk for rapid glaucoma progression based on an initial visual field test using machine learning. PLoS ONE. 2021;16(4):e0249856. doi: 10.1371/journal.pone.0249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Xu M, Liu W, et al. Glaucoma characterization by machine learning of tear metabolic fingerprinting. Small Methods. 2022;6(5):2200264. doi: 10.1002/smtd.202200264. [DOI] [PubMed] [Google Scholar]
- 48.Singh LK, Khanna M, Thawkar S. A novel hybrid robust architecture for automatic screening of glaucoma using fundus photos, built on feature selection and machine learning-nature driven computing. Expert Syst. 2022;39(10):e13069. doi: 10.1111/exsy.13069. [DOI] [Google Scholar]
- 49.Khan SI, Choubey SB, Choubey A, Bhatt A, Naishadhkumar PV, Basha MM. Automated glaucoma detection from fundus images using wavelet-based denoising and machine learning. Concurr Eng. 2022;30(1):103–115. doi: 10.1177/1063293X211026620. [DOI] [Google Scholar]
- 50.Dai M, Hu Z, Kang Z, Zheng Z. Based on multiple machine learning to identify the ENO2 as diagnosis biomarkers of glaucoma. BMC Ophthalmol. 2022;22(1):155. doi: 10.1186/s12886-022-02350-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong D, Chua J, Bujor I, et al. Comparison of machine learning approaches for structure-function modeling in glaucoma. Ann N Y Acad Sci. 2022;1515(1):237–248. doi: 10.1111/nyas.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banna HU, Zanabli A, McMillan B, et al. Evaluation of machine learning algorithms for trabeculectomy outcome prediction in patients with glaucoma. Sci Rep. 2022;12(1):2473. doi: 10.1038/s41598-022-06438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kooner KS, Angirekula A, Treacher AH, et al. Glaucoma diagnosis through the integration of optical coherence tomography/angiography and machine learning diagnostic models. Clin Ophthalmol. 2022;16:2685–2697. doi: 10.2147/OPTH.S367722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen RB, Zhong YL, Liu H, Huang X. Machine learning analysis reveals abnormal functional network hubs in the primary angle-closure glaucoma patients. Front Hum Neurosci. 2022;16:935213. doi: 10.3389/fnhum.2022.935213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leite D, Campelos M, Fernandes A, et al. Machine Learning automatic assessment for glaucoma and myopia based on Corvis ST data. Proc Comput Sci. 2022;196:454–460. doi: 10.1016/j.procs.2021.12.036. [DOI] [Google Scholar]
- 56.Gajendran MK, Rohowetz LJ, Koulen P, Mehdizadeh A. Novel machine-learning based framework using electroretinography data for the detection of early-stage glaucoma. Front Neurosci. 2022;16:869137. doi: 10.3389/fnins.2022.869137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Y, Hu M, Xie X, Li H-X. Knowledge-based machine learning for glaucoma diagnosis from fundus image data. J Med Imaging Health Inform. 2014;4:776. doi: 10.1166/jmihi.2014.1319. [DOI] [Google Scholar]
- 58.Wyawahare M, Patil PM. Machine learning classifiers based on structural ONH measurements for glaucoma diagnosis. Int J Biomed Eng Technol. 2016;21:343. doi: 10.1504/IJBET.2016.078338. [DOI] [Google Scholar]
- 59.Tekouabou SCK, Alaoui EAA, Chabbar I, Cherif W, Silkan H. Machine learning approach for early detection of glaucoma from visual fields. Niss2020. 2020.
- 60.Singh LK, Garg HP, Khanna M. Performance analysis of machine learning techniques for glaucoma detection based on textural and intensity features. Int J Innov Comput Appl. 2020;11(4):216–230. doi: 10.1504/IJICA.2020.111230. [DOI] [Google Scholar]
- 61.Eswari MS, Balamurali S. An intelligent machine learning support system for glaucoma prediction among diabetic patients. In: 2021 International conference on advance computing and innovative technologies in engineering (ICACITE); 4–5 March 2021, 2021.
- 62.Shyla NSJ, Emmanuel WRS. Automated classification of glaucoma using DWT and HOG features with extreme learning machine. In: 2021 Third international conference on intelligent communication technologies and virtual mobile networks (ICICV); 4–6 Feb. 2021, 2021.
- 63.Goldbaum MH, Sample PA, White H, et al. Interpretation of automated perimetry for glaucoma by neural network. Invest Ophthalmol Vis Sci. 1994;35(9):3362–3373. [PubMed] [Google Scholar]
- 64.Henson DB, Spenceley SE, Bull DR. Artificial neural network analysis of noisy visual field data in glaucoma. Artif Intell Med. 1997;10(2):99–113. doi: 10.1016/S0933-3657(97)00388-6. [DOI] [PubMed] [Google Scholar]
- 65.Brigatti L, Hoffman D, Caprioli J. Neural networks to identify glaucoma with structural and functional measurements. Am J Ophthalmol. 1996;121(5):511–521. doi: 10.1016/S0002-9394(14)75425-X. [DOI] [PubMed] [Google Scholar]
- 66.Poinoosawmy D, Tan JC, Bunce C, Hitchings RA. The ability of the GDx nerve fibre analyser neural network to diagnose glaucoma. Graefes Arch Clin Exp Ophthalmol. 2001;239(2):122–127. doi: 10.1007/s004170100256. [DOI] [PubMed] [Google Scholar]
- 67.Bowd C, Chan K, Zangwill LM, et al. Comparing neural networks and linear discriminant functions for glaucoma detection using confocal scanning laser ophthalmoscopy of the optic disc. Invest Ophthalmol Vis Sci. 2002;43(11):3444–3454. [PubMed] [Google Scholar]
- 68.Hitzl W, Reitsamer HA, Hornykewycz K, Mistlberger A, Grabner G. Application of discriminant, classification tree and neural network analysis to differentiate between potential glaucoma suspects with and without visual field defects. J Theor Med. 2003;5(3–4):161–170. doi: 10.1080/10273360410001728011. [DOI] [Google Scholar]
- 69.Bengtsson B, Bizios D, Heijl A. Effects of input data on the performance of a neural network in distinguishing normal and glaucomatous visual fields. Invest Ophthalmol Vis Sci. 2005;46(10):3730–3736. doi: 10.1167/iovs.05-0175. [DOI] [PubMed] [Google Scholar]
- 70.Bizios D, Heijl A, Bengtsson B. Trained artificial neural network for glaucoma diagnosis using visual field data: a comparison with conventional algorithms. J Glaucoma. 2007;16(1):20–28. doi: 10.1097/IJG.0b013e31802b34e4. [DOI] [PubMed] [Google Scholar]
- 71.Grewal DS, Jain R, Grewal SP, Rihani V. Artificial neural network-based glaucoma diagnosis using retinal nerve fiber layer analysis. Eur J Ophthalmol. 2008;18(6):915–921. doi: 10.1177/112067210801800610. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira D, Vellasco M, Oliveira M, Yamane R. Application of neural networks in aid for diagnosis for patients with glaucoma. 2009.
- 73.Huang ML, Chen HY, Huang WC, Tsai YY. Linear discriminant analysis and artificial neural network for glaucoma diagnosis using scanning laser polarimetry-variable cornea compensation measurements in Taiwan Chinese population. Graefes Arch Clin Exp Ophthalmol. 2010;248(3):435–441. doi: 10.1007/s00417-009-1259-3. [DOI] [PubMed] [Google Scholar]
- 74.Boquete L, Miguel-Jiménez JM, Ortega S, Rodríguez-Ascariz JM, Pérez-Rico C, Blanco R. Multifocal electroretinogram diagnosis of glaucoma applying neural networks and structural pattern analysis. Expert Syst Appl. 2012;39(1):234–238. doi: 10.1016/j.eswa.2011.07.013. [DOI] [Google Scholar]
- 75.Andersson S, Heijl A, Bizios D, Bengtsson B. Comparison of clinicians and an artificial neural network regarding accuracy and certainty in performance of visual field assessment for the diagnosis of glaucoma. Acta Ophthalmol. 2013;91(5):413–417. doi: 10.1111/j.1755-3768.2012.02435.x. [DOI] [PubMed] [Google Scholar]
- 76.Sheeba O, George J, Rajin PK, Thomas N, George S. Glaucoma detection using artificial neural network. Int J Eng Technol. 2014;6(2):158–161. doi: 10.7763/IJET.2014.V6.687. [DOI] [Google Scholar]
- 77.Oh E, Yoo TK, Hong S. Artificial neural network approach for differentiating open-angle glaucoma from glaucoma suspect without a visual field test. Invest Ophthalmol Vis Sci. 2015;56(6):3957–3966. doi: 10.1167/iovs.15-16805. [DOI] [PubMed] [Google Scholar]
- 78.Larrosa JM, Polo V, Ferreras A, Garcia-Martin E, Calvo P, Pablo LE. Neural network analysis of different segmentation strategies of nerve fiber layer assessment for glaucoma diagnosis. J Glaucoma. 2015;24(9):672–678. doi: 10.1097/IJG.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 79.Jerith GG, Kumar PN. Recognition of glaucoma by means of Gray wolf optimized neural network. Multimedia Tools and Applications. 2020;79(15):10341–10361. doi: 10.1007/s11042-019-7224-1. [DOI] [Google Scholar]
- 80.Anton N, Lisa C, Doroftei B, et al. Use of artificial neural networks to predict the progression of glaucoma in patients with sleep apnea. Appl Sci. 2022;12:6061. doi: 10.3390/app12126061. [DOI] [Google Scholar]
- 81.Wang M, Shen LQ, Pasquale LR, et al. An artificial intelligence approach to assess spatial patterns of retinal nerve fiber layer thickness maps in glaucoma. Transl Vis Sci Technol. 2020;9(9):41. doi: 10.1167/tvst.9.9.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yousefi S, Kiwaki T, Zheng Y, et al. Detection of longitudinal visual field progression in glaucoma using machine learning. Am J Ophthalmol. 2018;193:71–79. doi: 10.1016/j.ajo.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Wang M, Shen LQ, Pasquale LR, et al. Artificial intelligence classification of central visual field patterns in glaucoma. Ophthalmology. 2020;127(6):731–738. doi: 10.1016/j.ophtha.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M, Tichelaar J, Pasquale LR, et al. Characterization of central visual field loss in end-stage glaucoma by unsupervised artificial intelligence. JAMA Ophthalmol. 2020;138(2):190–198. doi: 10.1001/jamaophthalmol.2019.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Natarajan D, Sankaralingam E, Balraj K, Thangaraj V. Automated segmentation algorithm with deep learning framework for early detection of glaucoma. Concurr Comput. 2021;33(10):e6181. doi: 10.1002/cpe.6181. [DOI] [Google Scholar]
- 86.Praveena R, GaneshBabu TR. Determination of cup to disc ratio using unsupervised machine learning techniques for glaucoma detection. Mol Cell Biomech. 2021;18(2):69. doi: 10.32604/mcb.2021.014622. [DOI] [Google Scholar]
- 87.Huang X, Saki F, Wang M, et al. An objective and easy-to-use glaucoma functional severity staging system based on artificial intelligence. J Glaucoma. 2022;31(8):626–633. doi: 10.1097/IJG.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yousefi S, Elze T, Pasquale LR, Boland M. Glaucoma monitoring using manifold learning and unsupervised clustering. In: 2018 International conference on image and vision computing New Zealand (IVCNZ); 19–21 Nov. 2018, 2018.
- 89.Al-Shamiri AYR. Employing machine learning algorithms to discover risk factors of glaucoma. In: 2021 4th International conference on pattern recognition and artificial intelligence (PRAI); 20–22 Aug. 2021, 2021.
- 90.Antony Ammal M, Gladis D, Shaik A. Metric measures of optic nerve head in screening glaucoma with machine learning. In: Peng SL, Hao RX, Pal S, editors. Proceedings of first international conference on mathematical modeling and computational science. Singapore: Springer; 2021. [Google Scholar]
- 91.Saini C, Shen LQ, Pasquale LR, et al. Assessing surface shapes of the optic nerve head and peripapillary retinal nerve fiber layer in glaucoma with artificial intelligence. Ophthalmol Sci. 2022;2(3):100161. doi: 10.1016/j.xops.2022.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Surendiran J, Meena M. Analysis and detection of glaucoma from fundus eye image by cup to disc ratio by unsupervised machine learning. In: 2022 IEEE International conference on data science and information system (ICDSIS); 29–30 July 2022, 2022.
- 93.Jolliffe I. Principal component analysis. In: Kenett RS, Longford NT, Piegorsch WW, Ruggeri F, editors. Wiley statsref: statistics reference online. Hoboken: Wiley; 2014. [Google Scholar]
- 94.Thakur A, Goldbaum M, Yousefi S. Convex representations using deep archetypal analysis for predicting glaucoma. IEEE J Transl Eng Health Med. 2020;8:3800107. doi: 10.1109/JTEHM.2020.2982150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yousefi S, Pasquale LR, Boland MV, Johnson CA. Machine-identified patterns of visual field loss and an association with rapid progression in the ocular hypertension treatment study. Ophthalmology. 2022;129(12):1402–1411. doi: 10.1016/j.ophtha.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta K, Thakur A, Goldbaum M, Yousefi S. Glaucoma precognition: recognizing preclinical visual functional signs of glaucoma.In: 2020 IEEE/CVF conference on computer vision and pattern recognition workshops (CVPRW); 14–19 June 2020, 2020.
- 97.Chen X, Xu Y, Yan S, Wong DWK, Wong TY, Liu J. Automatic feature learning for glaucoma detection based on deep learning. In: Navab N, Hornegger J, Wells WM, Frangi AF, editors. Medical image computing and computer-assisted intervention–MICCAI 2015: 18th International Conference, Munich, Germany, October 5–9, 2015, proceedings, part III. Cham: Springer; 2015. [Google Scholar]
- 98.Asaoka R, Murata H, Iwase A, Araie M. Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology. 2016;123(9):1974–1980. doi: 10.1016/j.ophtha.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 99.Cerentini A, Welfer D, Cordeiro d'Ornellas M, Pereira Haygert CJ, Dotto GN. Automatic identification of glaucoma using deep learning methods. Stud Health Technol Inform. 2017;245:318–321. [PubMed] [Google Scholar]
- 100.Ahn JM, Kim S, Ahn K-S, Cho S-H, Lee KB, Kim US. A deep learning model for the detection of both advanced and early glaucoma using fundus photography. PLoS ONE. 2018;13(11):e0207982. doi: 10.1371/journal.pone.0207982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kucur SS, Hollo G, Sznitman R. A deep learning approach to automatic detection of early glaucoma from visual fields. PLoS ONE. 2018;13(11):e0206081. doi: 10.1371/journal.pone.0206081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Norouzifard M, Nemati A, GholamHosseini H, Klette R, Nouri-Mahdavi K, Yousefi S. Automated glaucoma diagnosis using deep and transfer learning: proposal of a system for clinical testing. In: 2018 International conference on image and vision computing New Zealand (IVCNZ); 19–21 Nov. 2018, 2018.
- 103.Masumoto H, Tabuchi H, Nakakura S, Ishitobi N, Miki M, Enno H. Deep-learning classifier with an ultrawide-field scanning laser ophthalmoscope detects glaucoma visual field severity. J Glaucoma. 2018;27(7):647–652. doi: 10.1097/IJG.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 104.Fuentes-Hurtado F, Morales S, Mossi JM, et al. Deep-learning-based classification of rat OCT images after intravitreal injection of ET-1 for glaucoma understanding. In: Yin H, Camacho D, Novais P, Tallón-Ballesteros AJ, et al., editors. Intelligent data engineering and automated learning–IDEAL 2018: 19th international conference, Madrid, Spain, November 21–23, 2018, proceedings, part I. Cham: Springer; 2018. [Google Scholar]
- 105.Shibata N, Tanito M, Mitsuhashi K, et al. Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Sci Rep. 2018;8(1):14665. doi: 10.1038/s41598-018-33013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chai Y, Liu H, Xu J. Glaucoma diagnosis based on both hidden features and domain knowledge through deep learning models. Knowl Based Syst. 2018;161:147–156. doi: 10.1016/j.knosys.2018.07.043. [DOI] [Google Scholar]
- 107.Muhammad H, Fuchs TJ, De Cuir N, et al. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J Glaucoma. 2017;26(12):1086–1094. doi: 10.1097/IJG.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mitra A, Banerjee PS, Roy S, Roy S, Setua SK. The region of interest localization for glaucoma analysis from retinal fundus image using deep learning. Comput Methods Prog Biomed. 2018;165:25–35. doi: 10.1016/j.cmpb.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Phene S, Dunn RC, Hammel N, et al. Deep learning and glaucoma specialists: the relative importance of optic disc features to predict glaucoma referral in fundus photographs. Ophthalmology. 2019;126(12):1627–1639. doi: 10.1016/j.ophtha.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 110.An G, Omodaka K, Hashimoto K, et al. Glaucoma diagnosis with machine learning based on optical coherence tomography and color fundus images. J Healthc Eng. 2019;2019:4061313. doi: 10.1155/2019/4061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rao PVPN. GlaucoNet: a highly robust stacked auto-encoder assisted deep learning model for glaucoma detection system. Int J Eng Adv Technol (IJEAT) 2019;9(1):5293–5303. doi: 10.35940/ijeat.A2960.109119. [DOI] [Google Scholar]
- 112.Jammal AA, Thompson AC, Mariottoni EB, et al. Human versus machine: comparing a deep learning algorithm to human gradings for detecting glaucoma on fundus photographs. Am J Ophthalmol. 2020;211:123–131. doi: 10.1016/j.ajo.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim M, Han JC, Hyun SH, et al. Medinoid: computer-aided diagnosis and localization of glaucoma using deep learning †. Appl Sci. 2019;9(15):3064. doi: 10.3390/app9153064. [DOI] [Google Scholar]
- 114.Kim J, Tran L, Chew EY, Antani S. Optic disc and cup segmentation for glaucoma characterization using deep learning. In: 2019 IEEE 32nd international symposium on computer-based medical systems (CBMS); 5–7 June 2019, 2019.
- 115.Yu S, Xiao D, Frost S, Kanagasingam Y. Robust optic disc and cup segmentation with deep learning for glaucoma detection. Comput Med Imaging Graph. 2019;74:61–71. doi: 10.1016/j.compmedimag.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Lee J, Kim Y, Kim JH, Park KH. Screening glaucoma with red-free fundus photography using deep learning classifier and polar transformation. J Glaucoma. 2019;28(3):258–264. doi: 10.1097/IJG.0000000000001187. [DOI] [PubMed] [Google Scholar]
- 117.Bajwa MN, Malik MI, Siddiqui SA, et al. Two-stage framework for optic disc localization and glaucoma classification in retinal fundus images using deep learning. BMC Med Inform Decis Mak. 2019;19(1):136. doi: 10.1186/s12911-019-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Asaoka R, Tanito M, Shibata N, et al. Validation of a deep learning model to screen for glaucoma using images from different fundus cameras and data augmentation. Ophthalmol Glaucoma. 2019;2(4):224–231. doi: 10.1016/j.ogla.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Russakoff DB, Mannil SS, Oakley JD, et al. A 3D deep learning system for detecting referable glaucoma using full OCT macular cube scans. Transl Vis Sci Technol. 2020;9(2):12. doi: 10.1167/tvst.9.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peroni A, Cutolo CA, Pinto LA, et al. A deep learning approach for semantic segmentation of gonioscopic images to support glaucoma categorization. In: Papież BW, Namburete AIL, Yaqub M, Noble JAN, et al., editors. Medical image understanding and analysis. Cham: Springer; 2020. [Google Scholar]
- 121.Zaleska-Zmijewska A, Szaflik J, Borowiecki P, et al. A new platform designed for glaucoma screening: identifying the risk of glaucomatous optic neuropathy using fundus photography with deep learning architecture together with intraocular pressure measurements. Klin Oczna. 2020;2020:1–6. doi: 10.5114/ko.2020.94205. [DOI] [Google Scholar]
- 122.Mariottoni EB, Datta S, Dov D, et al. Artificial intelligence mapping of structure to function in glaucoma. Transl Vis Sci Technol. 2020;9(2):19. doi: 10.1167/tvst.9.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zapata MA, Royo-Fibla D, Font O, et al. Artificial intelligence to identify retinal fundus images, quality validation, laterality evaluation, macular degeneration, and suspected glaucoma. Clin Ophthalmol. 2020;14:419–429. doi: 10.2147/OPTH.S235751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thompson AC, Jammal AA, Berchuck SI, Mariottoni EB, Medeiros FA. Assessment of a segmentation-free deep learning algorithm for diagnosing glaucoma from optical coherence tomography scans. JAMA Ophthalmol. 2020;138(4):333–339. doi: 10.1001/jamaophthalmol.2019.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Juneja M, Singh S, Agarwal N, et al. Automated detection of glaucoma using deep learning convolution network (G-net) Multimed Tools Appl. 2020;79(21):15531–15553. doi: 10.1007/s11042-019-7460-4. [DOI] [Google Scholar]
- 126.Božić-Štulić D, Braović M, Stipanicev D. Deep learning based approach for optic disc and optic cup semantic segmentation for glaucoma analysis in retinal fundus images. Int J Elec Comput Eng Syst. 2020;11:111–120. [Google Scholar]
- 127.Sb S, Cho H-k. Deep learning classification of early normal-tension glaucoma and glaucoma suspects using Bruch’s membrane opening-minimum rim width and RNFL. Sci Rep. 2020;10(1):19042. doi: 10.1038/s41598-020-76154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yohei H, Ryo A, Taichi K, et al. Deep learning model to predict visual field in central 10° from optical coherence tomography measurement in glaucoma. Br J Ophthalmol. 2021;105(4):507. doi: 10.1136/bjophthalmol-2019-315600. [DOI] [PubMed] [Google Scholar]
- 129.Kenichi N, Ryo A, Masaki T, et al. Deep learning-assisted (automatic) diagnosis of glaucoma using a smartphone. Br J Ophthalmol. 2022;106(4):587. doi: 10.1136/bjophthalmol-2020-318107. [DOI] [PubMed] [Google Scholar]
- 130.Zheng C, Xie X, Huang L, et al. Detecting glaucoma based on spectral domain optical coherence tomography imaging of peripapillary retinal nerve fiber layer: a comparison study between hand-crafted features and deep learning model. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):577–585. doi: 10.1007/s00417-019-04543-4. [DOI] [PubMed] [Google Scholar]
- 131.Li F, Song D, Chen H, et al. Development and clinical deployment of a smartphone-based visual field deep learning system for glaucoma detection. NPJ Digit Med. 2020;3(1):123. doi: 10.1038/s41746-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim KE, Kim JM, Song JE, Kee C, Han JC, Hyun SH. Development and validation of a deep learning system for diagnosing glaucoma using optical coherence tomography. J Clin Med. 2020;9(7):2167. doi: 10.3390/jcm9072167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee J, Kim YK, Park KH, Jeoung JW. Diagnosing glaucoma with spectral-domain optical coherence tomography using deep learning classifier. J Glaucoma. 2020;29(4):287–294. doi: 10.1097/IJG.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 134.Civit-Masot J, Domínguez-Morales MJ, Vicente-Díaz S, Civit A. Dual machine-learning system to aid glaucoma diagnosis using disc and cup feature extraction. IEEE Access. 2020;8:127519–127529. doi: 10.1109/ACCESS.2020.3008539. [DOI] [Google Scholar]
- 135.Hirota M, Mizota A, Mimura T, et al. Effect of color information on the diagnostic performance of glaucoma in deep learning using few fundus images. Int Ophthalmol. 2020;40(11):3013–3022. doi: 10.1007/s10792-020-01485-3. [DOI] [PubMed] [Google Scholar]
- 136.Christopher M, Nakahara K, Bowd C, et al. Effects of study population, labeling and training on glaucoma detection using deep learning algorithms. Transl Vis Sci Technol. 2020;9(2):27. doi: 10.1167/tvst.9.2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang J, Lee J, Ha A, et al. Explaining the rationale of deep learning glaucoma decisions with adversarial examples. Ophthalmology. 2021;128(1):78–88. doi: 10.1016/j.ophtha.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 138.Rajasekaran A, Indirani G. IoT based automatic detection of glaucoma disease in OCT and fundus images using deep learning techniques. J Green Eng. 2020;10:13621–13643. [Google Scholar]
- 139.Singh LK, Garg H, Khanna M. Performance evaluation of various deep learning based models for effective glaucoma evaluation using optical coherence tomography images. Multimed Tools Appl. 2022;81(19):27737–27781. doi: 10.1007/s11042-022-12826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thakur A, Goldbaum M, Yousefi S. Predicting glaucoma before onset using deep learning. Ophthalmol Glaucoma. 2020;3(4):262–268. doi: 10.1016/j.ogla.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fumero F, Diaz-Aleman T, Sigut J, Alayón S, Arnay R, Angel-Pereira D. RIM-ONE DL: a unified retinal image database for assessing glaucoma using deep learning. Image Anal Stereol. 2020;39:161. doi: 10.5566/ias.2346. [DOI] [Google Scholar]
- 142.Wang X, Chen H, Ran A-R, et al. Towards multi-center glaucoma OCT image screening with semi-supervised joint structure and function multi-task learning. Med Image Anal. 2020;63:101695. doi: 10.1016/j.media.2020.101695. [DOI] [PubMed] [Google Scholar]
- 143.Natarajan D, Sankaralingam E, Balraj K, Karuppusamy S. A deep learning framework for glaucoma detection based on robust optic disc segmentation and transfer learning. Int J Imaging Syst Technol. 2022;32(1):230–250. doi: 10.1002/ima.22609. [DOI] [Google Scholar]
- 144.Rakesh G, Rajamanickam V. A novel deep learning algorithm for optical disc segmentation for glaucoma diagnosis. Traitement Signal. 2022;39(1):305–311. doi: 10.18280/ts.390132. [DOI] [Google Scholar]
- 145.Veena HN, Muruganandham A, Kumaran TS. Enhanced CNN-RNN deep learning-based framework for the detection of glaucoma. Int J Biomed Eng Technol. 2021;36(2):133–147. doi: 10.1504/IJBET.2021.116116. [DOI] [Google Scholar]
- 146.Huang X, Jin K, Zhu J, et al. A structure-related fine-grained deep learning system with diversity data for universal glaucoma visual field grading. Front Med (Lausanne) 2022;9:832920. doi: 10.3389/fmed.2022.832920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dixit A, Yohannan J, Boland MV. Assessing glaucoma progression using machine learning trained on longitudinal visual field and clinical data. Ophthalmology. 2021;128(7):1016–1026. doi: 10.1016/j.ophtha.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shanmugam P, Raja J, Pitchai R. An automatic recognition of glaucoma in fundus images using deep learning and random forest classifier. Appl Soft Comput. 2021;109:107512. doi: 10.1016/j.asoc.2021.107512. [DOI] [Google Scholar]
- 149.Mehta P, Petersen CA, Wen JC, et al. Automated detection of glaucoma with interpretable machine learning using clinical data and multimodal retinal images. Am J Ophthalmol. 2021;231:154–169. doi: 10.1016/j.ajo.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bowd C, Belghith A, Zangwill LM, et al. Deep learning image analysis of optical coherence tomography angiography measured vessel density improves classification of healthy and glaucoma eyes. Am J Ophthalmol. 2022;236:298–308. doi: 10.1016/j.ajo.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hemelings R, Elen B, Barbosa-Breda J, Blaschko MB, De Boever P, Stalmans I. Deep learning on fundus images detects glaucoma beyond the optic disc. Sci Rep. 2021;11(1):20313. doi: 10.1038/s41598-021-99605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shin Y, Cho H, Jeong HC, Seong M, Choi J-W, Lee WJ. Deep learning-based diagnosis of glaucoma using wide-field optical coherence tomography images. J Glaucoma. 2021;30(9):803. doi: 10.1097/IJG.0000000000001885. [DOI] [PubMed] [Google Scholar]
- 153.Latif J, Tu S, Xiao C, et al. Digital forensics use case for glaucoma detection using transfer learning based on deep convolutional neural networks. Sec Commun Netw. 2021;2021:1. doi: 10.1155/2021/4494447. [DOI] [Google Scholar]
- 154.Thakur N, Juneja M. Early-stage prediction of glaucoma disease to reduce surgical requirements using deep-learning. Mater Today Proc. 2021;45:5660. doi: 10.1016/j.matpr.2021.02.458. [DOI] [Google Scholar]
- 155.Gonzalez-Hernandez M, Gonzalez-Hernandez D, Perez-Barbudo D, Rodriguez-Esteve P, Betancor-Caro N, Gonzalez de la Rosa M. Fully automated colorimetric analysis of the optic nerve aided by deep learning and its association with perimetry and OCT for the study of glaucoma. J Clin Med. 2021;10(15):3231. doi: 10.3390/jcm10153231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schottenhamml J, Wurfl T, Mardin S, et al. Glaucoma classification in 3 x 3 mm en face macular scans using deep learning in a different plexus. Biomed Opt Express. 2021;12(12):7434–7444. doi: 10.1364/BOE.439991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chai Y, Bian Y, Liu H, Li J, Xu J. Glaucoma diagnosis in the Chinese context: an uncertainty information-centric Bayesian deep learning model. Inf Process Manage. 2021;58(2):102454. doi: 10.1016/j.ipm.2020.102454. [DOI] [Google Scholar]
- 158.Ajitha S, Akkara JD, Judy MV. Identification of glaucoma from fundus images using deep learning techniques. Indian J Ophthalmol. 2021;69(10):2702–2709. doi: 10.4103/ijo.IJO_92_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sandoval-Cuellar HJ, Alfonso-Francia G, Vázquez-Membrillo MA, Ramos-Arreguín JM, Tovar-Arriaga S. Image-based glaucoma classification using fundus images and deep learning. Rev Mex Ing Bioméd. 2021;42(3):29–41. [Google Scholar]
- 160.Hashimoto Y, Kiwaki T, Sugiura H, et al. Predicting 10–2 visual field from optical coherence tomography in glaucoma using deep learning corrected with 24–2/30-2 visual field. Transl Vis Sci Technol. 2021;10(13):28–28. doi: 10.1167/tvst.10.13.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee T, Jammal AA, Mariottoni EB, Medeiros FA. Predicting glaucoma development with longitudinal deep learning predictions from fundus photographs. Am J Ophthalmol. 2021;225:86–94. doi: 10.1016/j.ajo.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Asano S, Asaoka R, Murata H, et al. Predicting the central 10 degrees visual field in glaucoma by applying a deep learning algorithm to optical coherence tomography images. Sci Rep. 2021;11(1):2214. doi: 10.1038/s41598-020-79494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abdel-Hamid L. TWEEC: Computer-aided glaucoma diagnosis from retinal images using deep learning techniques. Int J Imaging Syst Technol. 2022;32(1):387–401. doi: 10.1002/ima.22621. [DOI] [Google Scholar]
- 164.Li F, Su Y, Lin F, et al. A deep-learning system predicts glaucoma incidence and progression using retinal photographs. J Clin Invest. 2022;132(11):e157968. doi: 10.1172/JCI157968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yi S, Zhang G, Qian C, Lu Y, Zhong H, He J. A multimodal classification architecture for the severity diagnosis of glaucoma based on deep learning. Front Neurosci. 2022;16:939472. doi: 10.3389/fnins.2022.939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nawaz M, Nazir T, Javed A, et al. An efficient deep learning approach to automatic glaucoma detection using optic disc and optic cup localization. Sensors. 2022;22(2):434. doi: 10.3390/s22020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haider A, Arsalan M, Lee MB, et al. Artificial intelligence-based computer-aided diagnosis of glaucoma using retinal fundus images. Expert Syst Appl. 2022;207:117968. doi: 10.1016/j.eswa.2022.117968. [DOI] [Google Scholar]
- 168.Lin M, Hou B, Liu L, et al. Automated diagnosing primary open-angle glaucoma from fundus image by simulating human’s grading with deep learning. Sci Rep. 2022;12(1):14080. doi: 10.1038/s41598-022-17753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Geetha A, Prakash NB. Classification of glaucoma in retinal images using EfficientnetB4 deep learning model. Comput Syst Sci Eng. 2022;43(3):1041. doi: 10.32604/csse.2022.023680. [DOI] [Google Scholar]
- 170.Shin Y, Cho H, Shin YU, Seong M, Choi JW, Lee WJ. Comparison between deep-learning-based ultra-wide-field fundus imaging and true-colour confocal scanning for diagnosing glaucoma. J Clin Med. 2022;11(11):3168. doi: 10.3390/jcm11113168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Saravanan V, Samuel RDJ, Krishnamoorthy S, Manickam A. Deep learning assisted convolutional auto-encoders framework for glaucoma detection and anterior visual pathway recognition from retinal fundus images. J Ambient Intell Humaniz Comput. 2022 doi: 10.1007/s12652-021-02928-0. [DOI] [Google Scholar]
- 172.Juneja M, Thakur S, Uniyal A, Wani A, Thakur N, Jindal P. Deep learning-based classification network for glaucoma in retinal images. Comput Electr Eng. 2022;101:108009. doi: 10.1016/j.compeleceng.2022.108009. [DOI] [Google Scholar]
- 173.Singh LK, Garg H, Khanna M. Deep learning system applicability for rapid glaucoma prediction from fundus images across various data sets. Evol Syst. 2022;13(6):807–836. doi: 10.1007/s12530-022-09426-4. [DOI] [Google Scholar]
- 174.Islam MT, Mashfu ST, Faisal A, Siam SC, Naheen IT, Khan R. Deep learning-based glaucoma detection with cropped optic cup and disc and blood vessel segmentation. IEEE Access. 2022;10:2828–2841. doi: 10.1109/ACCESS.2021.3139160. [DOI] [Google Scholar]
- 175.Noury E, Mannil SS, Chang RT, et al. Deep learning for glaucoma detection and identification of novel diagnostic areas in diverse real-world datasets. Transl Vis Sci Technol. 2022;11(5):11–11. doi: 10.1167/tvst.11.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan R, Bowd C, Christopher M, et al. Detecting glaucoma in the ocular hypertension study using deep learning. JAMA Ophthalmol. 2022;140(4):383–391. doi: 10.1001/jamaophthalmol.2022.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Neto A, Camara J, Cunha A. Evaluations of deep learning approaches for glaucoma screening using retinal images from mobile device. Sensors (Basel). 2022;22(4):1449. doi: 10.3390/s22041449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joshi S, Partibane B, Hatamleh WA, Tarazi H, Yadav CS, Krah D. Glaucoma detection using image processing and supervised learning for classification. J Healthc Eng. 2022;2022:2988262. doi: 10.1155/2022/2988262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Akter N, Fletcher J, Perry S, Simunovic MP, Briggs N, Roy M. Glaucoma diagnosis using multi-feature analysis and a deep learning technique. Sci Rep. 2022;12(1):8064. doi: 10.1038/s41598-022-12147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ibrahim MH, Hacibeyoglu M, Agaoglu A, Ucar F. Glaucoma disease diagnosis with an artificial algae-based deep learning algorithm. Med Biol Eng Comput. 2022;60(3):785–796. doi: 10.1007/s11517-022-02510-6. [DOI] [PubMed] [Google Scholar]
- 181.Kim J, Tran L, Peto T, Chew EY. Identifying those at risk of glaucoma: a deep learning approach for optic disc and cup segmentation and their boundary analysis. Diagnostics. 2022;12(5):1063. doi: 10.3390/diagnostics12051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li F, Xiang W, Zhang L, et al. Joint optic disk and cup segmentation for glaucoma screening using a region-based deep learning network. Eye. 2023;37(6):1080–1087. doi: 10.1038/s41433-022-02055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pascal L, Perdomo OJ, Bost X, Huet B, Otálora S, Zuluaga MA. Multi-task deep learning for glaucoma detection from color fundus images. Sci Rep. 2022;12(1):12361. doi: 10.1038/s41598-022-16262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Latif J, Tu S, Xiao C, Ur Rehman S, Imran A, Latif Y. ODGNet: a deep learning model for automated optic disc localization and glaucoma classification using fundus images. SN Applied Sciences. 2022;4(4):98. doi: 10.1007/s42452-022-04984-3. [DOI] [Google Scholar]
- 185.Hemelings R, Elen B, Barbosa-Breda J, et al. Pointwise visual field estimation from optical coherence tomography in glaucoma using deep learning. Transl Vis Sci Technol. 2022;11(8):22. doi: 10.1167/tvst.11.8.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sudhan MB, Sinthuja M, Pravinth Raja S, et al. Segmentation and classification of glaucoma using U-net with deep learning model. J Healthc Eng. 2022;2022:1601354. doi: 10.1155/2022/1601354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Al-Bander B, Al-Nuaimy W, Al-Taee MA, Zheng Y. Automated glaucoma diagnosis using deep learning approach. In: 2017 14th International multi-conference on systems, signals and devices (SSD); 28–31 March 2017, 2017.
- 188.Chai Y, He L, Mei Q, Liu H, Xu L. Deep learning through two-branch convolutional neuron network for glaucoma diagnosis. In: Chen H, Zeng DD, Karahanna E, Bardhan I, editors. Smart health. Cham: Springer; 2017. [Google Scholar]
- 189.Serte S, Serener A. A generalized deep learning model for glaucoma detection. In: 2019 3rd International symposium on multidisciplinary studies and innovative technologies (ISMSIT); 11–13 Oct. 2019, 2019.
- 190.Phasuk S, Poopresert P, Yaemsuk A, et al. Automated glaucoma screening from retinal fundus image using deep learning. In: 2019 41st Annual international conference of the ieee engineering in medicine and biology society (EMBC); 23–27 July 2019, 2019. [DOI] [PubMed]
- 191.Kim M, Park Hm, Zuallaert J, Janssens O, Hoecke SV, Neve WD. Computer-aided diagnosis and localization of glaucoma using deep learning. In: 2018 IEEE International conference on bioinformatics and biomedicine (BIBM); 3–6 Dec. 2018, 2018.
- 192.Raja PMS, Ramanan K. Damped least-squares recurrent deep neural learning classification for glaucoma detection. In: 2019 International conference on data science and engineering (ICDSE); 26–28 Sept. 2019, 2019.
- 193.Mojab N, Noroozi V, Yu P, Hallak J. Deep multi-task learning for interpretable glaucoma detection. In: 2019 IEEE 20th International conference on information reuse and integration for data science (IRI). 2019; Los Angeles.
- 194.Maadi F, Faraji N, Bibalan MH. A robust glaucoma screening method for fundus images using deep learning technique. In: 2020 27th National and 5th International Iranian conference on biomedical engineering (ICBME); 26–27 Nov. 2020, 2020.
- 195.Pandey A, Patre P, Minj J. Detection of glaucoma disease using image processing, soft computing and deep learning approaches. In: 2020 Fourth International conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC); 7–9 Oct. 2020, 2020.
- 196.Zhao R, Chen X, Liu X, Chen Z, Guo F, Li S. Direct cup-to-disc ratio estimation for glaucoma screening via semi-supervised learning. IEEE J Biomed Health Inform. 2020;24(4):1104–1113. doi: 10.1109/JBHI.2019.2934477. [DOI] [PubMed] [Google Scholar]
- 197.Borwankar S, Sen R, Kakani B. Improved glaucoma diagnosis using deep learning. In: 2020 IEEE International conference on electronics, computing and communication technologies (CONECCT); 2–4 July 2020, 2020.
- 198.Shoukat A, Akbar S, Safdar K. A deep learning-based automatic method for early detection of the glaucoma using fundus images. In: 2021 International conference on innovative computing (ICIC); 9–10 Nov. 2021, 2021.
- 199.Shoukat A, Akbar S, Hassan SAE, Rehman A, Ayesha N. An automated deep learning approach to diagnose glaucoma using retinal fundus images. In: 2021 International conference on frontiers of information technology (FIT); 13–14 Dec. 2021, 2021.
- 200.Ovreiu S, Paraschiv EA, Ovreiu E. Deep learning and digital fundus images: glaucoma detection using DenseNet. In: 2021 13th International conference on electronics, computers and artificial intelligence (ECAI); 1–3 July 2021, 2021.
- 201.Venkatachalam K, Bacanin N, Kabir E, Prabu P. Effective tensor based PCA machine learning techniques for glaucoma detection and ASPP–EffUnet classification. Health Information Science: 10th International Conference, HIS 2021, Melbourne, VIC, Australia, October 25–28, 2021, Proceedings; 2021; Melbourne.
- 202.Phankokkruad M. Evaluation of Deep transfer learning models in glaucoma detection for clinical application. In: 2021 4th International conference on information and communications technology (ICOIACT); 30–31 Aug. 2021, 2021.
- 203.Díaz-Alemán VT, Fumero Batista FJ, Alayón Miranda S, Pereira DÁ, Arteaga-Hernández VJ, Sigut Saavedra JF. Ganglion cell layer analysis with deep learning in glaucoma diagnosis. Arch Soc Esp Oftalmol (English Edition) 2021;96(4):181–188. doi: 10.1016/j.oftal.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 204.Lavric A, Petrariu AI, Havriliuc S, Coca E. Glaucoma detection by artificial intelligence: GlauNet A deep learning framework. In: 2021 International Conference on e-Health and Bioengineering (EHB); 18–19 Nov. 2021, 2021.
- 205.Tang Y, Yang G, Ding D, Cheng G. Multi-level Amplified iterative training of semi-supervision deep learning for glaucoma diagnosis. In: 2021 IEEE International conference on bioinformatics and biomedicine (BIBM); 9–12 Dec. 2021, 2021.
- 206.Christopher M, Hoseini P, Walker E, et al. A deep learning approach to improve retinal structural predictions and aid glaucoma neuroprotective clinical trial design. Ophthalmol Glaucoma. 2023;6(2):147–159. doi: 10.1016/j.ogla.2022.08.014. [DOI] [PubMed] [Google Scholar]
- 207.Nahar L, Hossain MS, Das P, Alam T, Andersson K. A deep learning-based ophthalmologic approach for retinal fundus image analysis to detect glaucoma. In: Kaiser MS, Ray K, Bandyopadhyay A, Jacob K, Long KS, editors. Third international conference on trends in computational and cognitive engineering. Singapore: Springer; 2022. [Google Scholar]
- 208.Sattar M, Ghani F, Khan H, Narmeen M, Mehmood A. A methodology for glaucoma disease detection using deep learning techniques. Int J Compu Dig Syst. 2022;11:401–411. doi: 10.12785/ijcds/110133. [DOI] [Google Scholar]
- 209.Kalin YA, Bhardwaj Y, Dwivedi SA, Agrawal AP, Risariya S, Jha V. Analogizing the potency of deep learning models for glaucoma detection. In: 2022 12th International conference on cloud computing, data science and engineering (Confluence); 27–28 Jan. 2022, 2022.
- 210.Xie H, Wang J-K, Kardon RH, Garvin MK, Wu X. Automated macular OCT retinal surface segmentation in cases of severe glaucoma using deep learning. 2022.
- 211.Deperlioglu O. 9 - Diagnosing glaucoma with optic disk segmenting and deep learning from color retinal fundus images. In: Gupta D, Kose U, Khanna A, Balas VE, editors. Deep learning for medical applications with unique data. Cambridge: Academic Press; 2022. pp. 181–195. [Google Scholar]
- 212.Muramatsu C. Diagnosis of glaucoma on retinal fundus images using deep learning: detection of nerve fiber layer defect and optic disc analysis. In: Lee G, Fujita H, editors. Deep learning in medical image analysis : challenges and applications. Cham: Springer International Publishing; 2020. pp. 121–132. [DOI] [PubMed] [Google Scholar]
- 213.Ganapathy J, Vijaykumar K, Sundar S, Sadasivam T, Chandrasekaran R, Lakshmanan V. Evaluation of deep learning framework for detection and diagnosis of glaucoma. In: 2022 6th International conference on trends in electronics and informatics (ICOEI); 28–30 April 2022, 2022.
- 214.Shadin NS, Sanjana S, Chakraborty S, Sharmin N. Performance analysis of glaucoma detection using deep learning models. In: 2022 International conference on innovations in science, engineering and technology (ICISET); 26–27 Feb. 2022, 2022.
- 215.Hung KH, Kao YC, Tang YH, et al. Application of a deep learning system in glaucoma screening and further classification with colour fundus photographs: a case control study. BMC Ophthalmol. 2022;22(1):483. doi: 10.1186/s12886-022-02730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Akter N, Gordon J, Li S, et al. Glaucoma detection and staging from visual field images using machine learning techniques. medRxiv. 2022;4:279. [Google Scholar]
- 217.Yang H, Ahn Y, Askaruly S, You JS, Kim SW, Jung W. Deep learning-based glaucoma screening using regional RNFL thickness in fundus photography. Diagnostics (Basel). 2022;12(11):2894. doi: 10.3390/diagnostics12112894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Almansour A, Alawad M, Aljouie A, et al. Peripapillary atrophy classification using CNN deep learning for glaucoma screening. PLoS ONE. 2022;17(10):e0275446. doi: 10.1371/journal.pone.0275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou Q, Guo J, Chen Z, et al. Deep learning-based classification of the anterior chamber angle in glaucoma gonioscopy. Biomed Opt Express. 2022;13(9):4668–4683. doi: 10.1364/BOE.465286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zang P, Hormel TT, Hwang TS, Bailey ST, Huang D, Jia Y. Deep-learning-aided diagnosis of diabetic retinopathy, age-related macular degeneration, and glaucoma based on structural and angiographic OCT. Ophthalmol Sci. 2023;3(1):100245. doi: 10.1016/j.xops.2022.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Moon S, Lee JH, Choi H, Lee SY, Lee J. Deep learning approaches to predict 10–2 visual field from wide-field swept-source optical coherence tomography en face images in glaucoma. Sci Rep. 2022;12(1):21041. doi: 10.1038/s41598-022-25660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fan R, Alipour K, Bowd C, et al. Detecting glaucoma from fundus photographs using deep learning without convolutions: transformer for improved generalization. Ophthalmol Sci. 2023;3(1):100233. doi: 10.1016/j.xops.2022.100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shroff S, Rao DP, Savoy FM, et al. Agreement of a novel artificial intelligence software with optical coherence tomography and manual grading of the optic disc in glaucoma. J Glaucoma. 2023;32(4):280. doi: 10.1097/IJG.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 224.Omodaka K, Horie J, Tokairin H, et al. Deep learning-based noise reduction improves optical coherence tomography angiography imaging of radial peripapillary capillaries in advanced glaucoma. Curr Eye Res. 2022;47(12):1600–1608. doi: 10.1080/02713683.2022.2124275. [DOI] [PubMed] [Google Scholar]
- 225.Yang L, Xie Y, Islam MR, Xu G. Big Data and Artificial Intelligence (AI) to Detect Glaucoma. In: 2022 9th International Conference on Behavioural and Social Computing (BESC); 29–31 Oct. 2022, 2022.
- 226.Braganca CP, Torres JM, Soares CPA, Macedo LO. Detection of glaucoma on fundus images using deep learning on a new image set obtained with a smartphone and handheld ophthalmoscope. Healthcare (Basel). 2022;10(12):2345. doi: 10.3390/healthcare10122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kashyap R, Nair R, Gangadharan SM, Botto-Tobar M, Farooq S, Rizwan A. Glaucoma detection and classification using improved U-Net deep learning model. Healthcare (Switzerland). 2022;10:2497. doi: 10.3390/healthcare10122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xue Y, Zhu J, Huang X, et al. A multi-feature deep learning system to enhance glaucoma severity diagnosis with high accuracy and fast speed. J Biomed Inform. 2022;136:104233. doi: 10.1016/j.jbi.2022.104233. [DOI] [PubMed] [Google Scholar]
- 229.Elmoufidi A, Skouta A, Jai-andaloussi S, Ouchetto O. Deep multiple instance learning for automatic glaucoma prevention and auto-annotation using color fundus photography. Prog Artif Intell. 2022;11(4):397–409. doi: 10.1007/s13748-022-00292-4. [DOI] [Google Scholar]
- 230.Seo SB, Cho HK. Deep learning classification of early normal-tension glaucoma and glaucoma suspect eyes using Bruch’s membrane opening-based disc photography. Front Med (Lausanne) 2022;9:1037647. doi: 10.3389/fmed.2022.1037647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Huang X, Sun J, Gupta K, et al. Detecting glaucoma from multi-modal data using probabilistic deep learning. Front Med (Lausanne) 2022;9:923096. doi: 10.3389/fmed.2022.923096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Panahi A, Askari Moghadam R, Tarvirdizadeh B, Madani K. Simplified U-Net as a deep learning intelligent medical assistive tool in glaucoma detection. Evol Intell. 2022 doi: 10.1007/s12065-022-00775-2. [DOI] [Google Scholar]
- 233.Cordero-Mendieta MI, Pinos-Vélez E, Buri-Abad E, Coronel-Berrezueta R. Support tool for presumptive diagnosis of Glaucoma using fundus image processing and artificial intelligence implementation. In: 2022 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC); 9–11 Nov. 2022, 2022.
- 234.Danao DPR, Mababangloob DMN, Cruz JCD. Machine learning-based glaucoma detection through frontal eye features analysis. In: 2022 IEEE 13th control and system graduate research colloquium (ICSGRC); 23–23 July 2022, 2022.
- 235.Jibhakate P, Gole S, Yeskar P, Rangwani N, Vyas A, Dhote K. Early glaucoma detection using machine learning algorithms of VGG-16 and Resnet-50. In: 2022 IEEE region 10 symposium (TENSYMP); 1–3 July 2022, 2022.
- 236.Doan D, Ho PTT, Nguyen TT, Ngo TN, Pham TTT, Nguyen MS. Implementation of complete glaucoma diagnostic system using machine learning and retinal fundus image processing. In: 2022 International conference on advanced computing and analytics (ACOMPA); 21–23 Nov. 2022, 2022.
- 237.Mariottoni EB, Datta S, Shigueoka LS, et al. Deep learning-assisted detection of glaucoma progression in spectral-domain OCT. Ophthalmol Glaucoma. 2022 doi: 10.1016/j.ogla.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lu DW, Hsu WW, Huang YC, et al. Visual interpretability of deep learning models in glaucoma detection using color fundus images. In: 2022 IET International conference on engineering technologies and applications (IET-ICETA); 14–16 Oct. 2022, 2022.
- 239.Yugha R, Vinodhini V, Arunkumar JR, Varalakshmi K, Karthikeyan G, Ramkumar G. An automated glaucoma detection from fundus images based on deep learning network. In: 2022 Sixth international conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC); 10–12 Nov. 2022, 2022.
- 240.Diaz-Pinto A, Colomer A, Naranjo V, Morales S, Xu Y, Frangi AF. Retinal image synthesis and semi-supervised learning for glaucoma assessment. IEEE Trans Med Imaging. 2019;38(9):2211–2218. doi: 10.1109/TMI.2019.2903434. [DOI] [PubMed] [Google Scholar]
- 241.Alghamdi M, Abdel-Mottaleb M. A comparative study of deep learning models for diagnosing glaucoma from fundus images. IEEE Access. 2021;9:23894–23906. doi: 10.1109/ACCESS.2021.3056641. [DOI] [Google Scholar]
- 242.Xu Y, Hu M, Liu H, et al. A hierarchical deep learning approach with transparency and interpretability based on small samples for glaucoma diagnosis. NPJ Digit Med. 2021;4(1):48. doi: 10.1038/s41746-021-00417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bowd C, Belghith A, Christopher M, et al. Individualized glaucoma change detection using deep learning auto encoder-based regions of interest. Transl Vis Sci Technol. 2021;10(8):19. doi: 10.1167/tvst.10.8.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ghamdi MA, Li M, Abdel-Mottaleb M, Shousha MA. Semi-supervised transfer learning for convolutional neural networks for glaucoma detection. In: ICASSP 2019 - 2019 IEEE International conference on acoustics, speech and signal processing (ICASSP); 12–17 May 2019, 2019.
- 245.Sreng S, Maneerat N, Hamamoto K, Win K. Deep learning for optic disc segmentation and glaucoma diagnosis on retinal images. Appl Sci. 2020;10:4916. doi: 10.3390/app10144916. [DOI] [Google Scholar]
- 246.Bhuiyan A, Govindaiah A, Smith RT. An artificial-intelligence- and telemedicine-based screening tool to identify glaucoma suspects from color fundus imaging. J Ophthalmol. 2021;2021:6694784. doi: 10.1155/2021/6694784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cho H, Hwang YH, Chung JK, et al. Deep learning ensemble method for classifying glaucoma stages using fundus photographs and convolutional neural networks. Curr Eye Res. 2021;46(10):1516–1524. doi: 10.1080/02713683.2021.1900268. [DOI] [PubMed] [Google Scholar]
- 248.Akbar S, Hassan SA, Shoukat A, Alyami J, Bahaj SA. Detection of microscopic glaucoma through fundus images using deep transfer learning approach. Microsc Res Tech. 2022;85(6):2259–2276. doi: 10.1002/jemt.24083. [DOI] [PubMed] [Google Scholar]
- 249.Gong D, Hu M, Yin Y, et al. Practical application of artificial intelligence technology in glaucoma diagnosis. J Ophthalmol. 2022;2022:5212128. doi: 10.1155/2022/5212128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fu H, Cheng J, Xu Y, Liu J. Glaucoma detection based on deep learning network in fundus image. In: Lu L, Wang X, Carneiro G, Yang L, editors. Deep learning and convolutional neural networks for medical imaging and clinical informatics. Cham: Springer International Publishing; 2019. pp. 119–137. [Google Scholar]
- 251.Deepa N, Esakkirajan S, Keerthiveena B, Dhanalakshmi SB. Automatic diagnosis of glaucoma using ensemble based deep learning model. In: 2021 7th International conference on advanced computing and communication systems (ICACCS); 19–20 March 2021, 2021.
- 252.Serener A, Serte S. Glaucoma classification via deep learning ensembles. In: 2021 International conference on INnovations in Intelligent SysTems and Applications (INISTA); 25–27 Aug. 2021, 2021.
- 253.Liao W, Zou B, Zhao R, Chen Y, He Z, Zhou M. Clinical interpretable deep learning model for glaucoma diagnosis. IEEE J Biomed Health Inform. 2020;24(5):1405–1412. doi: 10.1109/JBHI.2019.2949075. [DOI] [PubMed] [Google Scholar]
- 254.Orlando JI, Prokofyeva E, del Fresno M, Blaschko MB. Convolutional neural network transfer for automated glaucoma identification. 2017.
- 255.Sevastopolsky A. Optic disc and cup segmentation methods for glaucoma detection with modification of U-Net convolutional neural network. Pattern Recognit Image Anal. 2017;27(3):618–624. doi: 10.1134/S1054661817030269. [DOI] [Google Scholar]
- 256.Li F, Wang Z, Qu G, et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med Imaging. 2018;18(1):35. doi: 10.1186/s12880-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.dos Santos V, Ferreira M, de Carvalho O, Filho A, Dalília de Sousa A, Corrêa Silva A, Gattass M. Convolutional neural network and texture descriptor-based automatic detection and diagnosis of glaucoma. Expert Syst Appl. 2018;110:250–263. doi: 10.1016/j.eswa.2018.06.010. [DOI] [Google Scholar]
- 258.Raghavendra U, Fujita H, Bhandary SV, Gudigar A, Tan JH, Acharya UR. Deep convolution neural network for accurate diagnosis of glaucoma using digital fundus images. Inf Sci. 2018;441:41–49. doi: 10.1016/j.ins.2018.01.051. [DOI] [Google Scholar]
- 259.Panda R, Puhan NB, Rao A, Mandal B, Padhy D, Panda G. Deep convolutional neural network-based patch classification for retinal nerve fiber layer defect detection in early glaucoma. J Med Imaging (Bellingham) 2018;5(4):044003. doi: 10.1117/1.JMI.5.4.044003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mari K, Venugopal N. Automated optic disc segmentation and classification model using optimal convolutional neural network for glaucoma diagnosis system. Int J Eng Adv Technol. 2019;9:7555–7561. doi: 10.35940/ijeat.A1928.109119. [DOI] [Google Scholar]
- 261.Gomez-Valverde JJ, Anton A, Fatti G, et al. Automatic glaucoma classification using color fundus images based on convolutional neural networks and transfer learning. Biomed Opt Express. 2019;10(2):892–913. doi: 10.1364/BOE.10.000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Phan S, Satoh S, Yoda Y, Kashiwagi K, Oshika T, Japan Ocular Imaging Registry Research G Evaluation of deep convolutional neural networks for glaucoma detection. Jpn J Ophthalmol. 2019;63(3):276–283. doi: 10.1007/s10384-019-00659-6. [DOI] [PubMed] [Google Scholar]
- 263.Hemelings R, Elen B, Barbosa-Breda J, et al. Accurate prediction of glaucoma from colour fundus images with a convolutional neural network that relies on active and transfer learning. Acta Ophthalmol. 2020;98(1):e94–e100. doi: 10.1111/aos.14193. [DOI] [PubMed] [Google Scholar]
- 264.Aamir M, Irfan M, Ali T, et al. An adoptive threshold-based multi-level deep convolutional neural network for glaucoma eye disease detection and classification. Diagnostics. 2020;10(8):602. doi: 10.3390/diagnostics10080602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Musial G, Queener HM, Adhikari S, et al. Automatic segmentation of retinal capillaries in adaptive optics scanning laser ophthalmoscope perfusion images using a convolutional neural network. Transl Vis Sci Technol. 2020;9(2):43–43. doi: 10.1167/tvst.9.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Raja H, Akram MU, Shaukat A, et al. Extraction of retinal layers through convolution neural network (CNN) in an OCT image for glaucoma diagnosis. J Digit Imaging. 2020;33(6):1428–1442. doi: 10.1007/s10278-020-00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mvoulana A, Kachouri R, Akil M. Fine-tuning convolutional neural networks: a comprehensive guide and benchmark analysis for Glaucoma Screening. In: 2020 25th International conference on pattern recognition (ICPR); 10–15 Jan. 2021, 2021.
- 268.García G, Amor Rd, Colomer A, Naranjo V. Glaucoma Detection from raw circumpapillary OCT images using fully convolutional neural networks. In: 2020 IEEE international conference on image processing (ICIP); 25–28 Oct. 2020, 2020.
- 269.Ajesh F, Ravi R. Hybrid features and optimization-driven recurrent neural network for glaucoma detection. Int J Imaging Syst Technol. 2020;30(4):1143–1161. doi: 10.1002/ima.22435. [DOI] [Google Scholar]
- 270.Yuan X, Zhou L, Yu S, Li M, Wang X, Zheng X. A multi-scale convolutional neural network with context for joint segmentation of optic disc and cup. Artif Intell Med. 2021;113:102035. doi: 10.1016/j.artmed.2021.102035. [DOI] [PubMed] [Google Scholar]
- 271.de Sales Carvalho NR, Rodrigues MD, de Carvalho Filho AO, Mathew MJ. Automatic method for glaucoma diagnosis using a three-dimensional convoluted neural network. Neurocomputing. 2021;438:72–83. doi: 10.1016/j.neucom.2020.07.146. [DOI] [Google Scholar]
- 272.Patil N, Patil PN, Rao PV. Convolution neural network and deep-belief network (DBN) based automatic detection and diagnosis of Glaucoma. Multimed Tools Appl. 2021;80(19):29481–29495. doi: 10.1007/s11042-021-11087-5. [DOI] [Google Scholar]
- 273.Sukkyu S, Ahnul H, Young Kook K, Byeong Wook Y, Hee Chan K, Ki HP. Dual-input convolutional neural network for glaucoma diagnosis using spectral-domain optical coherence tomography. Br J Ophthalmol. 2021;105(11):1555. doi: 10.1136/bjophthalmol-2020-316274. [DOI] [PubMed] [Google Scholar]
- 274.Yu H-H, Maetschke SR, Antony BJ, et al. Estimating global visual field indices in glaucoma by combining macula and optic disc OCT scans using 3-dimensional convolutional neural networks. Ophthalmol Glaucoma. 2021;4(1):102–112. doi: 10.1016/j.ogla.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Elangovan P, Nath MK. Glaucoma assessment from color fundus images using convolutional neural network. Int J Imaging Syst Technol. 2021;31(2):955–971. doi: 10.1002/ima.22494. [DOI] [Google Scholar]
- 276.Afroze T, Akther S, Chowdhury MA, Hossain E, Hossain MS, Andersson K. Glaucoma detection using inception convolutional neural network V3. In: Mahmud M, Kaiser MS, Kasabov N, Iftekharuddin K, Zhong N, editors. Applied intelligence and informatics. Cham: Springer; 2021. [Google Scholar]
- 277.Chiang M, Guth D, Pardeshi AA, et al. Glaucoma expert-level detection of angle closure in goniophotographs with convolutional neural networks: the Chinese American eye study. Am J Ophthalmol. 2021;226:100–107. doi: 10.1016/j.ajo.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Li L, Zhu H, Zhang Z, et al. Neural network-based retinal nerve fiber layer profile compensation for glaucoma diagnosis in myopia: model development and validation. JMIR Med Inform. 2021;9(5):e22664. doi: 10.2196/22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Thakoor KA, Koorathota SC, Hood DC, Sajda P. Robust and interpretable convolutional neural networks to detect glaucoma in optical coherence tomography images. IEEE Trans Biomed Eng. 2021;68(8):2456–2466. doi: 10.1109/TBME.2020.3043215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang H, Hu J, Zhang J. SCRD-Net: a deep convolutional neural network model for glaucoma detection in retina tomography. Complexity. 2021;2021:9858343. [Google Scholar]
- 281.Thakoor KA, Li X, Tsamis E, et al. Strategies to improve convolutional neural network generalizability and reference standards for glaucoma detection from OCT scans. Transl Vis Sci Technol. 2021;10(4):16–16. doi: 10.1167/tvst.10.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Madhumalini M, Devi TM. Detection of glaucoma from fundus images using novel evolutionary-based deep neural network. J Digit Imaging. 2022;35(4):1008–1022. doi: 10.1007/s10278-021-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.David DS, Selvi SAM, Sivaprakash S, et al. Enhanced detection of glaucoma on ensemble convolutional neural network for clinical informatics. Comput Mater Contin. 2022 doi: 10.32604/cmc.2022.020059. [DOI] [Google Scholar]
- 284.Deperlioglu O, Kose U, Gupta D, Khanna A, Giampaolo F, Fortino G. Explainable framework for Glaucoma diagnosis by image processing and convolutional neural network synergy: analysis with doctor evaluation. Fut Gener Comput Syst. 2022;129:152–169. doi: 10.1016/j.future.2021.11.018. [DOI] [Google Scholar]
- 285.Rakhmetulayeva S, Syrymbet Z. Implementation of convolutional neural network for predicting glaucoma from fundus images. East Eur J Enterp Technol. 2022;6:70–77. [Google Scholar]
- 286.Atalay E, Devecioglu O, Özalp O, Erdoğan H, Yildirim N. Investigation of the role of convolutional neural network architectures in the diagnosis of glaucoma using color fundus photography. Turk J Ophthalmol. 2022;52:193–200. doi: 10.4274/tjo.galenos.2021.29726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ko Y-C, Chen W-S, Chen H-H, et al. Widen the applicability of a convolutional neural-network-assisted glaucoma detection algorithm of limited training images across different datasets. Biomedicines. 2022;10(6):1314. doi: 10.3390/biomedicines10061314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang P, Yuan M, He Y, Sun J. 3D augmented fundus images for identifying glaucoma via transferred convolutional neural networks. Int Ophthalmol. 2021;41(6):2065–2072. doi: 10.1007/s10792-021-01762-9. [DOI] [PubMed] [Google Scholar]
- 289.Al-Aswad LA, Kapoor R, Chu CK, et al. Evaluation of a deep learning system for identifying glaucomatous optic neuropathy based on color fundus photographs. J Glaucoma. 2019;28(12):1029–1034. doi: 10.1097/IJG.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 290.Raja J, Shanmugam P, Pitchai R. An automated early detection of glaucoma using support vector machine based visual geometry group 19 (VGG-19) convolutional neural network. Wireless Pers Commun. 2021;118(1):523–534. doi: 10.1007/s11277-020-08029-z. [DOI] [Google Scholar]
- 291.Sreng S, Maneerat N, Hamamoto K, Win KY. Deep learning for optic disc segmentation and glaucoma diagnosis on retinal images. Appl Sci. 2020;10(14):4916. doi: 10.3390/app10144916. [DOI] [Google Scholar]
- 292.Goodfellow IJ, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y. Generative adversarial networks. arXiv. 2014 doi: 10.48550/arXiv.1406.2661. [DOI] [Google Scholar]
- 293.Li X, Jiang Y, Rodriguez-Andina JJ, Luo H, Yin S, Kaynak O. When medical images meet generative adversarial network: recent development and research opportunities. Discover Artif Intell. 2021;1(1):5. doi: 10.1007/s44163-021-00006-0. [DOI] [Google Scholar]
- 294.Gheisari S, Shariflou S, Phu J, et al. A combined convolutional and recurrent neural network for enhanced glaucoma detection. Sci Rep. 2021;11(1):1945. doi: 10.1038/s41598-021-81554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.García G, del Amor R, Colomer A, Verdú-Monedero R, Morales-Sánchez J, Naranjo V. Circumpapillary OCT-focused hybrid learning for glaucoma grading using tailored prototypical neural networks. Artif Intell Med. 2021;118:102132. doi: 10.1016/j.artmed.2021.102132. [DOI] [PubMed] [Google Scholar]
- 296.Gupta K, Goldbaum M, Yousefi S. Glaucoma precognition based on confocal scanning laser ophthalmoscopy images of the optic disc using convolutional neural network. In: 2021 IEEE/CVF conference on computer vision and pattern recognition workshops (CVPRW); 19–25 June 2021, 2021.
- 297.Serte S, Serener A. Graph-based saliency and ensembles of convolutional neural networks for glaucoma detection. IET Image Proc. 2021;15(3):797–804. doi: 10.1049/ipr2.12063. [DOI] [Google Scholar]
- 298.Li Q, Wang N, Liu Z, et al. Approach to glaucoma diagnosis and prediction based on multiparameter neural network. Int Ophthalmol. 2023;43(3):837–845. doi: 10.1007/s10792-022-02485-1. [DOI] [PubMed] [Google Scholar]
- 299.Wang M, Shen LQ, Pasquale LR, et al. An artificial intelligence approach to detect visual field progression in glaucoma based on spatial pattern analysis. Invest Ophthalmol Vis Sci. 2019;60(1):365–375. doi: 10.1167/iovs.18-25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kelman SE PH, D’Autrechy L, Scott RJ. A neural network can differentiate glaucoma and optic neuropathy visual fields through pattern recognition. Perimetry Update 1990/1991, Proceedings of the IXth International Perimetric Society Meeting. 1991: 291–295.
- 301.Nagata S KK, Sugiyama A. A computer assisted visual field diagnosis system using neural networks perimetry update 1990/1991, Proceedings of the IXth international perimetric society meeting. 1991:291–295.
- 302.Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digit Med. 2018;1(1):39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jayant Venkatramani I, Michael VB, Joan J, Harry Q. Defining glaucomatous optic neuropathy using objective criteria from structural and functional testing. Br J Ophthalmol. 2021;105(6):789. doi: 10.1136/bjophthalmol-2020-316237. [DOI] [PubMed] [Google Scholar]
- 304.Yousefi S, Huang X, Poursoroush A, et al. An artificial intelligence enabled system for retinal nerve fiber layer thickness damage severity staging. Ophthalmology Science. 2023;4:100389. doi: 10.1016/j.xops.2023.100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sounderajah V, Ashrafian H, Golub RM, et al. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: the STARD-AI protocol. BMJ Open. 2021;11(6):e047709. doi: 10.1136/bmjopen-2020-047709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Cruz Rivera S, Liu X, Chan A-W, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351–1363. doi: 10.1038/s41591-020-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Liu X, Rivera SC, Moher D, Calvert MJ, Denniston AK. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension. BMJ. 2020;370:m3164. doi: 10.1136/bmj.m3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhou Y, Chia MA, Wagner SK, et al. A foundation model for generalizable disease detection from retinal images. Nature. 2023;622:156. doi: 10.1038/s41586-023-06555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liu Z, Mao H, Wu CY, Feichtenhofer C, Darrell T, Xie S. A ConvNet for the 2020s. arXiv. 2022 doi: 10.48550/arXiv.2201.03545. [DOI] [Google Scholar]
- 310.Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Leiter C, Zhang R, Chen Y, Belouadi J, Larionov D, Fresen V, Eger S. ChatGPT: a meta-analysis after 2.5 months. arXiv. 2023 doi: 10.48550/arXiv.2302.13795. [DOI] [Google Scholar]
- 312.Delsoz M, Raja H, Madadi Y, et al. The use of ChatGPT to assist in diagnosing glaucoma based on clinical case reports. Ophthalmol Ther. 2023 doi: 10.1007/s40123-023-00805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.