Abstract
Sex is a significant source of heterogeneity in schizophrenia, with more negative symptoms in males and more affective symptoms and internalizing comorbidity in females. In this narrative review, we argue that there are likely sex differences in the pathophysiological mechanisms of schizophrenia-spectrum disorders (SZ) that originate during puberty and relate to the sex-specific impacts of pubertal maturation on brain development. Pubertal maturation might also trigger underlying (genetic or other) vulnerabilities in at-risk individuals, influencing brain development trajectories that contribute to the emergence of SZ. This review is the first to integrate links between pubertal development and neural development with cognitive neuroscience research in SZ to form and evaluate these hypotheses, with a focus on the frontal-striatal and frontal-limbic networks and their hypothesized contribution to negative and mood symptoms respectively. To test these hypotheses, longitudinal research with human adolescents is needed that examines the role of sex and pubertal development using large cohorts or high risk samples. We provide recommendations for such studies, which will integrate the fields of psychiatry, developmental cognitive neuroscience, and developmental endocrinology towards a more nuanced understanding of the role of pubertal factors in the hypothesized sex-specific pathophysiological mechanisms of schizophrenia.
Keywords: hormones, sex-specific, pathophysiology, psychosis, MRI
1. Introduction
Despite years of research efforts, long-term outcomes for individuals with schizophrenia-spectrum disorders (SZ) remain poor (Lally et al., 2017) and associated healthcare and societal costs are disproportionately high (Cloutier et al., 2016; Desai et al., 2013). Although remission of psychotic symptoms is achievable (Masand et al., 2009), 80% of individuals relapse within five years (Eisner et al., 2013). Moreover, existing pharmacological treatments do not alleviate negative symptoms, cognitive symptoms, or social impairments (Dodell-Feder et al., 2015). One barrier to effective treatments is the striking heterogeneity in clinical presentations of SZ, with varying symptom types (hallucination vs. delusion), content (paranoid vs. grandiose delusion), and comorbidities. The mechanisms underlying this heterogeneity are unknown, leaving a significant gap in knowledge that is important to address for the development of more efficacious treatments (Cooper et al., 2019; Leucht et al., 2009; Samara et al., 2018).
A significant source of heterogeneity in schizophrenia is sex (note: this review broadly uses the term sex without further differentiating between biological sex, sex assigned at birth, and gender identity1). Age of SZ onset peaks in early adulthood, but is 3–4 years earlier in males than females, and there is a secondary peak around menopause in females (Häfner, 2003; Kirkbride et al., 2012; Mancuso et al., 2015). Additionally, sex differences exist in symptoms, clinical presentation, and treatment outcomes (Hanlon et al., 2017; Heila et al., 1997; Mancuso et al., 2015; Schultz et al., 1997) and are already visible in clinical-high-risk stage (Walder et al., 2013). Specifically, males have worse negative symptoms (e.g., anhedonia, avolition) (Schultz et al., 1997) and occupational and social outcomes (Hanlon et al., 2017; Mancuso et al., 2015); females have higher rates of affective symptoms and comorbid depression (Mancuso et al., 2015), anxiety (Cosoff and Häfner, 1998), and suicidal behaviors (Heila et al., 1997). Further, although females respond better to antipsychotic medication (Ceskova et al., 2015), they experience more serious side effects (Seeman, 2009). Given these sex differences, it is plausible that the pathophysiological mechanisms of SZ vary by sex, and may begin to manifest during puberty.
Sex differences in SZ pathophysiology might manifest during puberty through the sex-specific impacts of pubertal maturation and associated psychosocial experiences on brain development. SZ is conceptualized as a neurodevelopmental disorder (Murray et al., 2017) typically emerging in late adolescence. Adolescence starts at the onset of puberty and entails substantial physical and neural maturation and adaptation to new expectations for social and cultural roles and responsibilities (Herting and Sowell, 2017). Puberty involves hormonal and physical changes, which unfold in a sex-dependent manner. Animal and human research demonstrates that the effects of pubertal hormones on brain structure and function vary by sex.
Of particular interest are two brain networks whose development is affected by pubertal processes, the frontal-striatal and frontal-limbic networks. The frontal-striatal network encompasses the subcortical regions of the striatum and medial prefrontal cortex (PFC), and has an important role in reward and motivational processes (Liu et al., 2011). The frontal-limbic network encompasses limbic regions (e.g., amygdala) and medial and lateral PFC, and is known for its importance in emotion regulation (Etkin et al., 2015). Later sections detail how these networks have been associated with symptoms that show a sex discrepancy in SZ, i.e., frontal-striatal with negative symptoms (Chung and Barch, 2016; Katthagen et al., 2020; Murray et al., 2008; Waltz et al., 2009; Zhang et al., 2016) and frontal-limbic with affective and psychotic symptoms (Modinos et al., 2015; Park et al., 2018; Vai et al., 2015).
Puberty might also trigger underlying genetic, epigenetic, and fetal/early environmental vulnerabilities for SZ, putting at-risk individuals on a different brain development trajectory that could lead to development of SZ. This idea combines evidence of genetic and early environmental risk factors of SZ (Birnbaum and Weinberger, 2017; Rapoport et al., 2012) with theories that SZ arises from neurobiological abnormalities originating, in part, from disruptions in adolescent brain development (Rapoport et al., 2012). However, research examining neurodevelopmental pathways to SZ has largely ignored pubertal effects. Consequently, very little is known about their effects on the development of brain networks associated with SZ, and whether these effects are altered in at-risk individuals.
Understanding the mechanisms that contribute to the onset and maintenance of SZ requires systematic investigation of the impact of pubertal development, including steroid hormones, on brain development. First, pubertal development might cause sex differences in symptom profile and course of illness in SZ, through its effect on brain development. Second, puberty might express underlying (genetic or other) vulnerabilities, resulting in an altered neurodevelopmental path that can lead to SZ. Although this current directions paper is not the first to mention the relevance of puberty and hormonal factors (Owens et al., 2018; Patel et al., 2021), it is the first to integrate links between pubertal development and neural development with cognitive neuroscience research in SZ to shape and evaluate the two above-mentioned theories on the pathophysiological mechanisms of SZ (summarized in Figure 1), with a focus on the frontal-striatal and frontal-limbic networks and their hypothesized contribution to negative and mood symptoms respectively. We aim to form testable hypotheses and methodological recommendations for the research required to better evaluate these theories.
Figure 1.
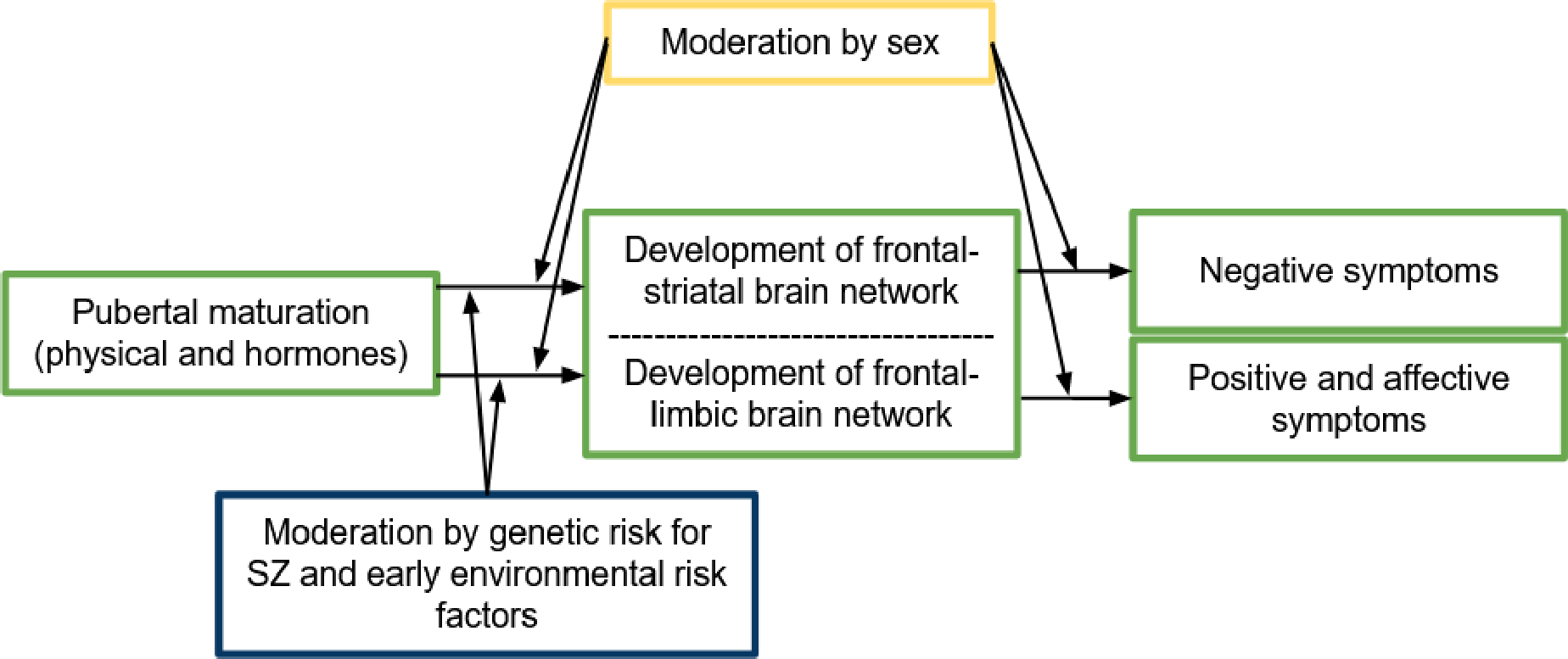
Theoretical model of how sex and genetic and early-environmental risk factors for schizophrenia-spectrum disorders (SZ) are hypothesized to moderate the influence of pubertal maturation on the development of frontal-limbic & frontal-striatal brain networks, changing the risk for the emergence of psychotic, affective and negative symptoms. The hypothesized pathways are based on the literature discussed in sections 2 to 4 and can be tested using recommendations described in section 5.
2. Puberty, steroid hormones, and brain network development in relation to schizophrenia symptoms
2.1. Direct links between steroid hormones and SZ
Given the sex-related differences in SZ symptom presentation, steroid hormones may play a role in this differentiation. Indeed, a large literature has examined the role of estrogen in female adults with SZ. Findings indicate an antipsychotic effect of estrogen, demonstrated by reduced psychotic symptom severity during high estrogen periods in the menstrual cycle (Bergemann et al., 2007) and in response to exogenous estrogen administration (Begemann et al., 2012), and a secondary peak in incidence after menopause. A smaller and more varied literature has addressed testosterone. Some studies report lower testosterone levels in males with SZ (Huber et al., 2005) and male adolescents at clinical high risk(Van Rijn et al., 2011); others have failed to replicate these findings (Ceskova et al., 2015), possibly due to individual differences in negative symptoms masking group effects (Markham, 2012). Low testosterone is consistently associated with more severe negative symptoms, and administration of exogenous testosterone improves negative symptoms (Ko et al., 2008). Notably, no studies have examined links between changes in steroid hormones in adolescence and SZ symptoms.
2.2. Pubertal timing and SZ
Motivated by the theory that estrogen is protective, studies tested whether earlier onset of puberty is associated with later onset of psychosis and lower symptom severity in females. Non-conclusive results are reported, likely due to small samples using retrospective cross-sectional designs: some indicate associations in the predicted direction (Cohen et al., 1999; Galdos et al., 1993) whilst others indicate no association (Hochman and Lewine, 2004; Rubio-Abadal et al., 2016; Ruiz et al., 2000). To better understand pubertal influences prior to the onset of SZ, recent work has focused on high-risk samples. The North American Prodrome Longitudinal Study (NAPLS), the largest study of high-risk individuals to date, reported no difference in self-reported physical pubertal development between high- and low-risk adolescents of the same age (Moskow et al., 2016). However, another study indicated later timing of self-reported physical pubertal development in males (but not females) at genetic high risk for SZ relates to more severe prodromal negative symptoms (Ramanathan et al., 2015). In sum, no consistent association has been found between the timing of puberty and risk for SZ.
2.3. Frontal-limbic and frontal-striatal brain networks in SZ
The frontal-limbic and frontal-striatal networks are two key circuits implicated in SZ. The frontal-limbic network is involved in processing and regulating response to emotionally salient stimuli; its functioning is relevant for psychotic, depression, and anxiety symptoms. Reduced frontal-limbic connectivity during emotion processing in adults with SZ relates to more severe psychotic symptoms (Modinos et al., 2015; Park et al., 2018; Vai et al., 2015), worse symptoms overall (Anticevic et al., 2013), and worse social functioning(Bjorkquist et al., 2016). Additionally, reduced cognitive control of emotion is associated with worse paranoia in adults with SZ (Hooker et al., 2011), suggesting a mechanism through which frontal-limbic function might influence positive symptoms. Studies in adolescent samples (non-SZ) demonstrate involvement of frontal-limbic network activation (Bertocci et al., 2017; Swartz et al., 2014) and connectivity (Gard et al., 2018; Swartz et al., 2014) in anxiety and depression, putatively given involvement of this network in emotion regulation. Considering these links with both psychotic and internalizing disorders, abnormalities in frontal-limbic function might explain comorbid risk for internalizing disorders in those at clinical high risk for psychosis (Lin et al., 2015). This transdiagnostic risk is also illustrated by the effectiveness of cognitive behavioral therapy, which targets emotion regulation and strengthens lateral prefrontal cortex activation (Yang et al., 2018), for reducing the risk of transition to psychosis in high-risk individuals (van der Gaag et al., 2013). A role for estrogen receptors in modulating the frontal-limbic network is suggested by a placebo-controlled study in adults with SZ, which reported increased lateral PFC activation during implicit emotion regulation after treatment with a selective estrogen receptor modulator, although symptoms did not significantly improve (Kindler et al., 2016). However, the full pathway from estrogen receptor functioning to psychotic symptoms through frontal-limbic function is not yet established. The frontal-limbic system has also been considered in the context of the stress sensitivity model: stressful life events are a risk factor for SZ and internalizing disorders, and heightened cortisol levels have been found in participants with first-episode psychosis or clinical-high-risk (Hubbard and Miller, 2019), but not in studies focused on schizophrenia only (Hubbard and Miller, 2019; Zorn et al., 2017). Lower hippocampal volume has been considered as a mechanism linking cortisol to psychosis because of its high number of glucocorticoid receptors, but meta-analyses concluded hippocampal volume is normal in high-risk (Walter et al., 2016) and first-episode patients (Radua et al., 2012).
The frontal-striatal network supports motivational, reward, and several cognitive processes. Negative symptoms in SZ (e.g., avolition, anhedonia) are thought primarily to reflect impairments in initiating rewarding activities driven by disruptions in the frontal-striatal network. This is supported by a robust literature demonstrating dysfunction of striatal regions during anticipation and receipt of rewards (Chung and Barch, 2016; Katthagen et al., 2020; Murray et al., 2008; Waltz et al., 2009; Zhang et al., 2016) and aberrant medial PFC activation during reward-related tasks (Lee et al., 2019; Segarra et al., 2016; Waltz et al., 2013; Zhang et al., 2016) in SZ. Reduced ventral striatum activation during reward anticipation has also been consistently associated with negative symptoms (Juckel et al., 2006; Simon et al., 2010; Waltz et al., 2009) in adults, although recent work reports hypoactivation in the dorsal striatum during reward anticipation in relation to anhedonia in early adolescents (Pornpattananangkul et al., 2019). A study in male adults demonstrated a positive association between testosterone and ventral striatal response to unexpected rewards that was absent in males with SZ, which the authors speculate is due to dysfunctional sex steroid receptor signaling in the midbrain of people with SZ (Morris et al., 2015). Similar research in younger SZ-relevant populations, such as prodromal or high-risk adolescents, in relation to puberty and steroid hormones, is needed.
2.4. Summary and next steps
Findings so far suggest a link between higher estrogen and fewer positive symptoms and between higher testosterone and fewer negative symptoms. Neuroimaging research reports involvement of the frontal-limbic network in positive and affective symptoms, and associations of reduced activation of the frontal-striatal network during rewards with negative symptoms. To overcome limitations of the current research, we need well-powered, longitudinal studies that include both sexes to discern the neurobiological mechanisms behind steroid hormone associations with (sex differences in) SZ symptoms. There is also a need for more research on brain function and pubertal maturation in adolescents with psychosis or at high risk for psychosis.
3. Understanding puberty and its relevance to frontal-limbic and frontal-striatal brain development
3.1. Processes of Puberty: Adrenarche and Gonadarche
Puberty encompasses hormonal and physical changes, broadly categorized as adrenarche and gonadarche. Puberty begins with adrenarche when adrenal glands mature following activation of the hypothalamic-pituitary-adrenal (HPA) axis (starting age 6–9) culminates in increased production of adrenal androgens like androstenedione, dehydroepiandrosterone (DHEA) dehydroepiandrosterone sulfate (DHEAS), and testosterone. These hormones are responsible for physical changes like pubic hair and acne, and continue to increase into the mid-20s (Herting and Sowell, 2017; Søeborg et al., 2014; Vijayakumar et al., 2018).
Gonadarche involves activation of the hypothalamic-pituitary-gonadal (HPG) axis (starting age 9–10): gonadotropin-releasing hormone pulses from the hypothalamus occur more frequently, stimulating the anterior pituitary to secrete luteinizing hormone and follicle stimulating hormone (FSH) which, in turn, act on the ovaries and testes to produce sex steroids like estrogen, progesterone and testosterone (Mendle et al., 2019a; Vijayakumar et al., 2018). Sex steroids drive reproductive maturity and development of secondary sex characteristics. These hormones can increase into the early-to-mid 20s (Frederiksen et al., 2020; Søeborg et al., 2014). Gonadarche begins approximately 1–1.5 years later in males than females (Mendle et al., 2019a).
Of note, the HPA axis also produces cortisol, and cortisol levels and reactivity increase with pubertal development (Gunnar et al., 2009; Shirtcliff et al., 2012). The HPG and HPA axes are generally positively coupled but can interact in various, complex ways (which are beyond the scope of this review but see (Shirtcliff et al., 2015)).
Thus, pubertal development encapsulates several underlying processes expressed in sex-specific ways. Next we review how these processes impact, directly or indirectly (e.g., through physique/motivation changes that influence psychosocial interactions), development of the frontal-striatal and frontal-limbic brain networks.
3.2. Puberty and Frontal-Limbic Development
Pubertal processes contribute to structural development of the frontal-limbic network in adolescence. Increasing testosterone and physical pubertal maturation have been consistently associated with increased amygdala and hippocampus volumes in males but not females (Barendse and Pfeifer, 2021). Pubertal development, including hormones, contributes to the normative process of cortical thinning across many regions of the cortex (Barendse and Pfeifer, 2021; Vijayakumar et al., 2018).
A prominent neurodevelopmental theory posits an increase in subcortical reactivity (e.g., amygdala response) to emotional stimuli starting at puberty, along with a slower, linear increase in lateral PFC function that supports emotion regulation (Casey et al., 2011). However, pubertal maturation has been associated with increased, decreased, and unaltered amygdala response to emotional faces (Barendse and Pfeifer, 2021; Vijayakumar et al., 2018). Discrepant findings are likely influenced by small sample sizes, varying age ranges, sex-specific effects, and fMRI task design variations. Evidence for stronger lateral PFC activation during emotion regulation in adults compared to adolescents is also inconsistent (Pozzi et al., 2021). More advanced pubertal development has been associated with less ventrolateral PFC activation and higher DHEA with less dorsolateral PFC activation to emotional faces, although null findings have been reported (Vijayakumar et al., 2018). Recent models of frontal-limbic development and emotion regulation more prominently include social brain regions and suggest that adolescent development of the PFC can have both ‘good’ and ‘bad’ consequences (the ‘bad’ including contributions to rumination and anticipating problems) (Pfeifer and Allen, 2020).
3.3. Puberty and Frontal-Striatal Development
Reward sensitivity and sensation seeking behaviors peak in late adolescence, accompanied by a peak in ventral striatum response to rewards (Braams et al., 2015; Shulman et al., 2015; Silverman et al., 2015). Pubertal processes might play a role in these developmental changes in reward processing: More advanced physical development and higher testosterone have been associated with smaller nucleus accumbens volume (Goddings et al., 2014; Wierenga et al., 2018) (although null findings are reported (Koolschijn et al., 2014; Neufang et al., 2009)) and pubertal stage is positively associated with orbitofrontal-accumbal white matter development in males (Chahal et al., 2021). Several studies have reported positive associations between adolescents’ testosterone and striatal activation during reward feedback in both sexes (Alarcón et al., 2017; Braams et al., 2015; Op de Macks et al., 2011), although there are null findings as well (Forbes et al., 2010; Ladouceur et al., 2019; Poon et al., 2019). Striatal response to reward anticipation has been studied less often, but results show a similar positive association with testosterone in males (Forbes et al., 2010). Higher testosterone has further been related to stronger connectivity between ventral striatum and anterior cingulate during reward anticipation in female but not male adolescents (Ladouceur et al., 2019).
3.4. Summary and next steps
Prior literature indicates that steroid hormones and pubertal development are associated with variations in frontal-limbic and frontal-striatal networks and that some of these associations are moderated by sex. Adolescence is characterized by increased striatal activation to reward and these effects may be driven in part by testosterone, independent of age (Alarcón et al., 2017; Braams et al., 2015; Op de Macks et al., 2011). This is consistent with the neurodevelopmental model positing a mismatch between the development of subcortical systems supporting reactivity to rewarding and emotional stimuli and prefrontal cortical systems supporting regulation of those stimuli (Casey et al., 2011). This model proposes a similar mismatch in the frontal-limbic network, but evidence for a puberty-driven peak in limbic reactivity to emotional stimuli is inconsistent (Barendse and Pfeifer, 2021). Research is needed to establish pubertal processes in relation to frontal-striatal and frontal-limbic connectivity (as opposed to regional activation), and to determine whether developmental changes are linear. One study (Vijayakumar et al., 2019) reported nonlinear relations between changes in testosterone levels and limbic activation in response to emotional faces, highlighting the importance of longitudinal study designs and the modeling of non-linear associations.
4. Puberty, Steroid Hormones, and Brain Chemistry in SZ
4.1. Hormonal mechanisms
Steroid hormones released from the gonads and adrenal cortex across sexes influence functioning of a wide range of organs, including the brain. Estrogens and androgens bind to estrogen and androgen receptors, respectively, after which the receptor relocates to the nucleus to impact gene transcription. Testosterone can be converted to estradiol through aromatization. Steroid hormones can also bind to membrane receptors for rapid influence on neuronal function, acting as receptor agonists or antagonists. In these ways, the hormones modulate functioning of neurotransmitters and their receptors, including in the dopamine, glutamate, γ-Aminobutyric acid (GABA) and serotonin systems (Maninger et al., 2009; McCarthy, 2008; Schulz et al., 2009). Effects of steroid hormones on neurotransmission vary by brain region and sex. Importantly, functioning of these neurotransmitter systems or their receptors have been found to be disrupted in SZ, possibly contributing to hallucinations and delusions, and occurring in regions underlying cognitive and social functioning (Stahl, 2018). Below we highlight ways in which steroid hormones modulate neurotransmitter functioning (for expansive reviews, see (Maninger et al., 2009; McCarthy, 2008; Schulz et al., 2009)), with particular focus on contributions to SZ symptoms and pathophysiology.
4.2. Dopamine
A core mechanism underlying positive symptoms in SZ is thought to be dopamine hyperactivity at D2 dopamine receptors in the ventral striatum (Stahl, 2018). Simultaneously, dopamine is a key neurotransmitter in the frontal-striatal network, and dysfunction of this network has been consistently linked to negative symptoms (Juckel et al., 2006; Simon et al., 2010; Waltz et al., 2009). Prenatal stress, a well-known risk factor for SZ, causes increased dopamine activity and receptor expression in the ventral striatum, with implications for prepulse inhibition and animal phenotypes of SZ (Markham and Koenig, 2011). These effects are often not visible until after pubertal onset, suggesting the rise in steroid hormones and their impact on the frontal-striatal network at puberty might expose the early vulnerabilities. Animal studies support the idea that gonadal hormones modulate the dopamine system. For example, testosterone enhances midbrain dopamine synthesis and D2 receptor expression and normalizes medial PFC dopamine after gonadectomy (Sinclair et al., 2014). In female rats, estrogen appears protective against sensorimotor gating disruptions induced by DR1 and DR2 agonists (Gogos et al., 2010). Most research on gonadal hormones and dopamine was done in adult animals though, despite differences between adolescents and adults in hormone levels and dopamine function; more research during adolescence is needed.
4.3. Glutamate
Glutamate is another key neurotransmitter involved in SZ pathophysiology, given consistent findings of N-methyl-D-aspartate receptor hypofunction and elevated glutamatergic levels in SZ. Proton Magnetic Resonance Spectroscopy (MRS) investigation of glutamate and glutamine levels in the medial PFC of adolescents at high genetic risk for SZ (Tibbo et al., 2004) support findings in unmedicated patients (Poels et al., 2014) that there are increased levels of glutamate in this region, which may lead to excitotoxicity that later contributes to the decreased levels in chronic patients (Tibbo et al., 2004). Animal research indicates estradiol can modulate PFC glutamate function in adolescence: in adolescent rodents, estrogen protects against the negative impact of repeated stress on glutamatergic transmission and PFC-dependent cognition (Wei et al., 2014); conversely repeated stress exposure in males leads to impaired cognition and reductions in glutamate transmission and receptor expression in the PFC, consistent with chronic SZ.
4.4. GABA
GABA has been of interest in SZ research because of its inhibitory function, opposing excitation by glutamate. Proton MRS studies have not consistently found altered GABA levels in any region in SZ compared to controls, despite indications from postmortem studies of impaired GABA function in SZ (Egerton et al., 2017). Impairments in GABA function might be limited to a subgroup of individuals or specific to the synapse (which cannot be measured with MRS). Animal studies on sex steroid hormones suggest estradiol and progesterone influence GABA function. Estradiol can induce temporary changes in the number of dendritic spines in the hippocampus and arcuate nucleus of the hypothalamus in adult females, such that numbers fluctuate with the menstrual cycle, and, in the arcuate nucleus, is likely due to estradiol’s influence on GABA synthesis (McCarthy, 2008). Female puberty in mice increases GABA release and inhibitory neurotransmission in the anterior cingulate cortex (Piekarski et al., 2017), a region implicated in SZ and adolescent depression (Fornito et al., 2009; Lichenstein et al., 2016). Further, progesterone reductions have been linked to premenstrual and postpartum depressive symptoms in high-risk females (Bloch et al., 2000; Hantsoo and Epperson, 2015), which might be partially mediated by the impact of progesterone and its metabolites on GABAa receptors in e.g. the hippocampus (Andréen et al., 2009). Together, these findings suggest that estradiol and progesterone influence GABA function in subcortical regions and the anterior cingulate. These patterns have relevance for mood problems, warranting further investigation of these hormones and GABA function in subgroups of psychoses with affective symptoms.
4.5. Serotonin
Serotonin also plays a role in psychosis. Hyperactivity of serotonin receptor 5-HT2A on glutamate neurons in the visual cortex is a potential pathway to visual hallucinations (Stahl, 2018). Animal and human research has found steroid hormones, especially estrogens, to modulate serotonin functioning, including modulation of serotonin biosynthesis and receptor and transporter expression (Barth et al., 2015; Bethea et al., 2002). However, whether estrogen has a facilitatory or inhibitory impact varies by serotonin receptor subtype, and receptor subtypes show a complex pattern of neurochemical actions. Few studies have examined these modulatory impacts in the context of SZ. In a small sample of healthy women, treatment with estradiol prevented the disruption of prepulse inhibition induced by a partial serotonin 5-HT1A receptor agonist (Gogos et al., 2006). Disruption of prepulse inhibition is a common cognitive deficit in SZ. In summary, estrogens can alter serotonin functioning through a variety of molecular and cellular mechanisms, although there is limited research on the clinical implications for psychotic symptoms.
4.6. Summary and next steps
Steroid hormones have sex-specific and region-specific impact on the functioning of many neurotransmitter systems. Examples include estradiol augmenting striatal dopamine function in female rats; testosterone regulating cortical dopamine function in male animals; and estradiol and progesterone increasing GABA release and inhibitory neurotransmission in medial frontal regions of female pubertal mice. Notably, other neurotransmitter systems are likely influenced by steroid hormones and contribute to brain mechanisms underlying psychosis, such as cannabinoids (Viveros et al., 2012), but are beyond the scope of this review.
An important consideration is that findings on steroid hormone effects on neurotransmitter function from other phases of life or from rodent models are not always translatable to human adolescence. Proton MRS is an effective way to study neurotransmitter function, mainly of glutamate and GABA, in living humans, and could be used as a next step to elucidate associations between changes in hormone levels in human adolescents and changes in neurotransmitter function implicated in SZ (e.g., glutamate in the medial PFC).
5. Methodological Considerations
We reviewed evidence supporting the idea that changes in steroid hormones in adolescence and their sex-specific impacts on brain development might contribute to sex differences in symptom profile and course of illness in SZ; or might express underlying vulnerabilities, placing some individuals on a neurodevelopmental trajectory toward higher SZ risk. These hypotheses remain to be tested in large, longitudinal studies in human adolescents. Below we describe recommendations for such studies.
5.1. Study Design
A key starting point is to conduct prospective longitudinal studies. Longitudinal designs permit examination of both inter- and intra-individual variation; they allow modeling of developmental trajectories and evaluating differences between (groups of) people or effects of biological or social factors on these trajectories over time. Mixed effects modeling and latent change score modeling are powerful and flexible methods for modeling change over time and estimating the associations between (changes in) two variables in longitudinal research. These models can give insight into processes that unfold over years (e.g., many pathophysiological processes of SZ).
Adolescence is a particularly important developmental period of focus for longitudinal research on SZ. Considering the timeframe of pubertal processes (starting in late childhood and continuing through adolescence), relevant neurodevelopmental processes (all through adolescence), and emergence of SZ and other psychotic illnesses (likely late adolescence and early adulthood), longitudinal studies will ideally follow participants for all of adolescence. Additionally, data collection would begin before symptoms typically onset, and would include a very large sample to have enough adolescents who go on to develop psychosis. Examples of such studies already underway are the Human Connectome Project-Development (HCP-D) and Adolescent Brain and Cognitive Development (ABCD) studies. Another promising approach is to collect a moderately large sample of high-risk adolescents, such as in NAPLS. This latter design is especially useful to determine whether the contribution of physical and hormonal pubertal processes to brain development in adolescence differs between those with versus without early risk factors for SZ.
5.2. Measuring relevant frontal-limbic & frontal-striatal processes
In addition to longitudinal study designs (which may be out of scope for some researchers), thorough investigation of the brain networks of interest (frontal-limbic and frontal-striatal) is achievable via well designed tasks for use both inside and outside of the MRI scanner. Recommendations for tasks that activate the frontal-limbic network include those that engage cognitive control of emotion, which encompasses both the effortful control of the experience and expression of emotional states (i.e., explicit emotion regulation, or ER) as well as the automatic control of the influence of emotional information on decision-making and behavior (i.e., implicit emotional conflict adaptation, or ECA). Neuroimaging studies demonstrate both ER and ECA tasks engage the frontal-limbic network (Tully and Niendam, 2014) and relate to key real-world experiences such as psychotic symptoms and social functioning (Hooker et al., 2014, 2011; Tully et al., 2012). Reappraisal tasks that require individuals to explicitly change the intensity of their emotional experience are used to investigate ER processes(Gross and Thompson, 2007), whereas tasks that require the inhibition of irrelevant emotional information in order to give task-relevant responses are used to investigate ECA (e.g., emotional n-back tasks (Casey et al., 2018), the faces–houses task (Bishop et al., 2004), the facial-emotional Stroop task (Etkin et al., 2006), and emotional flanker tasks (Hooker et al., 2014)). Recommendations for tasks that activate the frontal-striatal network include those that target reward processes, comprising four stages of reward prediction/anticipation (typically measured using the monetary incentive delay task [MID]), decision (i.e., choosing between options, typically measured using a gambling task), action (typically measured by modeling participant task responses), and reward receipt (typically measured during the feedback phase of a task like the MID) (Keren et al., 2018). Given that frontal-striatal activation during both reward anticipation and reward receipt has been linked to negative symptoms in SZ (Chung and Barch, 2016; Katthagen et al., 2020; Murray et al., 2008; Waltz et al., 2009; Zhang et al., 2016), we recommend using a task that measures both stages of reward processing. In an ideal scenario, researchers would include both frontal-limbic and frontal-striatal tasks in the context of cross-sectional experimental design with a broad age range, enabling the examination of inter-individual pubertal and age effects in both frontal-limbic and frontal-striatal networks.
5.3. Measuring Sex
Reminiscent of the state of the field (Hartung and Lefler, 2019), studies reviewed do not sufficiently define their sex variable or differentiate sex from gender. Sex-assigned-at-birth based on visual inspection of external genitalia often reflects biological sex (e.g., chromosomes, hormones, gonads) but not always (Calleja-Agius et al., 2012). Sex should be measured in a way it can be distinguished from gender identity, for questions for older adolescents and adults see Schmalenberger et al. (2021) and for measures for younger adolescents and parents see Potter et al. (2022). Researchers should describe how intersex and gender-diverse participants were considered. One promising side effect of increased integration of hormone measurement in studies is that it permits the investigation of biological aspects of sex (e.g., hormonal profile) in a non-binary manner.
5.4. Measuring Puberty
Measuring puberty can be divided into assessing physical pubertal development (i.e., development of secondary sex characteristics) and pubertal hormones (i.e., sex steroid hormones). Pubertal development encapsulates three constructs: pubertal status or stage, where an individual is in the process of puberty, pubertal timing, a person’s pubertal status relative to others their age and sex, and pubertal tempo, the pace of pubertal maturation, i.e. how fast an individual goes through puberty (Berenbaum et al., 2015; Mendle et al., 2019b). Pubertal status, timing, and tempo are measured by a widening range of methods.
5.4.1. Physical Measures
Table 1 provides a brief description of common methods for assessing physical pubertal development, summarizing advantages and limitations. Self-report or parent-report of secondary sex characteristics is an easy and inexpensive way to capture overall pubertal development. These are often scored as Tanner Stages (Tanner and Whitehouse, 1976), a classification method with five stages: pre-pubertal, early pubertal, mid-pubertal, late pubertal, and adult development. We recommend parent-report when the sample is young and/or early in puberty (e.g. 9–10), but self-report at older ages (e.g. 12+), see Table 1. Clinician assessment is also a valid method, but has high cost and time requirements, and it does not systematically outperform questionnaire methods for research purposes (Chavarro et al., 2017; Shirtcliff et al., 2009). Gonadal and adrenal aspects in physical development can be distinguished with a coding system for the PDS (Shirtcliff et al., 2009).
Table 1.
Advantages and Limitations of Pubertal Physical Assessment Measures for Research Purposes
| Measure | Construct | Advantages | Limitations |
|---|---|---|---|
| Tanner Stages by clinician administered physical examination (Tanner and Whitehouse, 1976) Physician/researcher categorizes adolescent into one of five Tanner Stages of puberty from 1) no development to 5) adult development, based on breast/genital development and pubic hair. |
Visible secondary sex characteristics | - Stages are associated with hormonal changes in puberty (Berenbaum et al., 2015; Shirtcliff et al., 2009) | - High time- and financial investment - Exam may be embarrassing - Developed with reference to a single ethnic group |
| Tanner Stage line drawings (Morris and Udry, 1980) Self-report or parent-report. Two sets of five line drawings (breast and hips/pubic hair for females; testicular and pubic hair for males). The adolescent picks the drawing that looks most like them for each set. Scored as Tanner Stages as described above. |
Secondary sex characteristics | - Maps directly onto Tanner Staging - Easy, quick, cheap - Captures basal hormones in parallel to the clinician-administered physical exam (Shirtcliff et al., 2009) |
- Not completely objective - Parent might not know their child’s body well enough, especially at older ages |
| Pubertal Development Scale (Petersen et al., 1988) Self-report or parent-report. Questions regarding height spurt, body hair growth, skin changes, breast (female) or facial hair (male) development, and menarche (female) or voice changes (male). Scored on a 4-point scale from ‘not started yet’ to ‘seems complete’ |
Secondary sex characteristics | - Valid and reliable in comparison to clinician Tanner Staging (Carskadon, 1993) - Easy, quick, cheap - Captures basal hormones in parallel with the physical exam in males, in females, the gonadal score performed slightly better than the exam (Shirtcliff et al., 2009) |
- Not completely objective, males tend to overestimate their development (Herting et al., 2021a) - Young participants might not know enough about the pubertal process to understand and accurately answer the questions - Parent might not know their child’s body well enough, especially at older ages |
5.4.2. Hormonal Measures
Measuring hormone levels is of particular interest if researchers aim to examine a specific neurobiological pathway or neurotransmitter system (see Section 4). Hormone measurement is also valuable at the earliest (e.g., adrenarche) and the latest (e.g., early 20s) phases of pubertal development, when Tanner staging may show little variation but hormonal maturation occurs. If the research only requires a broad index of pubertal status or timing, then questionnaire methods will be most cost-effective and hormone assessment can be seen as complementary. Most pubertal hormones can be detected in blood, saliva, urine and hair. See Table 2 for advantages and limitations of each collection method. Saliva is the most commonly used biospecimen in pubertal development research, because it is less invasive and easier to collect than blood (Gröschl, 2008; Herting et al., 2021b; Liening et al., 2010). Hair sampling has recently gained interest because it is less sensitive to short-term fluctuations (e.g., diurnal and menstrual cycling), and this method is actively being validated in puberty research (with mixed results (Byrne et al., 2019; Smith et al., 2019; Wang et al., 2019)). We recommend repeated sampling of biospecimens to improve reliability and allow for correction of short-term variations in hormone levels. Sex should always be taken into account when modeling pubertal hormones.
Table 2.
Advantages and Limitations to Venipuncture-Alternative Hormone Collection Methods for Research Purposes
| Collection Method | Common Hormones | Advantages | Limitations |
|---|---|---|---|
| Passive-drool saliva (Gröschl, 2008; Herting et al., 2021b; Liening et al., 2010) | Testosterone DHEA Estradiol Progesterone |
- Non-invasive - Self-sampling and repeated sampling feasible - Cost-effective - Well-validated - Measures free hormone |
- Cold storage and transport required - Cannot measure FSH and LH - Might need to correct for oral hygiene and need to eliminate contamination by blood and phlegm - Need to consider diurnal and menstrual cycling - Less well-validated for estradiol in males compared to females |
| Dried blood spot (Fischer et al., 2019; McDade, 2014) | DHEAS Testosterone Estradiol Progesterone FSH LH Prolactin |
- Self-sampling feasible, easier to collect than venipuncture - Transportation and storage may be more cost-effective than serum or saliva storage and transport - Measures free+bound hormone (need to measure SHBG to distinguish them) |
- Low sample volume - Self-collection procedures vary in how complicated and well-tolerated they are - Need to consider diurnal and menstrual cycling - Additional validation in comparison to serum samples may be needed(Fischer et al., 2019) |
| Hair (Byrne et al., 2019; Smith et al., 2019; Wang et al., 2019) | DHEA Testosterone Estradiol Progesterone |
- Captures average hormone levels across larger time windows, i.e. months - Easy to collect, store and transport |
- Relatively new approach, more validation needed - Participants with shorter hair may not have enough for sample |
| Urine (Kesner et al., 1998; Singh et al., 2015) | DHEA Testosterone Estradiol Progesterone LH FSH |
- Captures average levels over several hours - Self-sampling feasible - Generally accepted by participants |
- Cold storage and transport required - Not well-correlated with hormone levels from other biospecimens - Need to correct for concentration of urine - How well-validated it is, depends on the hormone |
5.4.3. Pubertal Timing and Tempo
Pubertal timing and tempo capture individual differences in pubertal development. Early pubertal timing has consistently been associated with higher risk for internalizing and externalizing psychopathology in both sexes (Ullsperger and Nikolas, 2017), as well as with threatening forms of early life adversity (Colich et al., 2020), but pubertal timing has not consistently been linked with SZ risk (see Pubertal timing and SZ). There is very little knowledge regarding the relevance of pubertal tempo for any form of psychopathology, and limited knowledge on how pubertal timing and tempo relate to brain function and structure (Barendse et al., 2019; Chahal et al., 2021). Pubertal tempo is theoretically interesting since puberty is often considered a sensitive window for brain development and pubertal tempo would influence the length of that window. Research is needed to examine whether pubertal timing and tempo are relevant to brain developmental processes contributing to SZ risk/symptomatology. The measurement method of pubertal timing matters for associations with internalizing and externalizing psychopathology, with the strongest effects for physical markers relative to age (Ullsperger and Nikolas, 2017). Extracting the residuals from physical maturation regressed on age can be a cost-effective and valid way to examine pubertal timing in SZ studies. We do not recommend adult reports of age at menarche, since these are less reliable than prospective measurement and menarche is only one component of pubertal development. Pubertal tempo requires longitudinal tracking of pubertal status and focusing on within-person change. A robust alternative for modeling timing and tempo is latent growth modeling (Mendle et al., 2010).
6. Conclusion
More effective treatments for SZ are urgently needed. One barrier to this goal is heterogeneity in clinical presentations and treatment response. A significant source of heterogeneity in schizophrenia is sex, with more negative symptoms in males and more internalizing comorbidity and stronger response to antipsychotics in females. In this current directions paper, we presented a theoretical model based on integration of multiple disparate fields to encourage and inform future research on sex differences in SZ. Specifically, we argue that there are likely sex differences in the pathophysiological mechanisms of SZ that originate in puberty and in the sex-specific impacts of pubertal maturation on brain development.
One area of focus in the literature is the association between testosterone, the frontal-striatal network, and negative symptoms. Increased testosterone in adolescence is associated with stronger striatal response to reward, and higher testosterone relates to less negative symptoms in males with SZ. Weakened frontal-striatal function also relates to negative symptoms in the general population. This suggests, although based on limited direct evidence, that disruptions in the way testosterone shapes frontal-striatal function during adolescence might be a mechanism of the more prevalent presentation of negative symptoms in males with SZ. Conversely, affective and positive symptoms relate to frontal-limbic network function and its role in cognitive control of emotion. Some evidence suggests this network is also influenced by pubertal development and estradiol, but variations in methodology and inconsistent results limit specific conclusions.
Additionally, pubertal development might trigger underlying genetic or prepubertal environmental vulnerabilities in at-risk individuals, altering neurodevelopmental trajectories and contributing to the development of SZ. Prenatal stress, for example, alters dopamine levels and function, but much of its impact on the dopamine system is not yet visible prior to puberty. Although direct evidence for this idea is limited, it is a promising avenue for future research, considering (a) genetic and perinatal environmental factors are important risk factors for SZ, (b) these factors, potentially in interaction with steroid hormones, shape fetal brain development, and (c) steroid hormones during puberty act on receptors and modulate systems laid out during fetal development.
Altogether, there is a need for longitudinal research on SZ with human adolescents; to date, investigations of the neurodevelopment of SZ have largely ignored sex and pubertal influences on relevant brain networks. We argue that SZ researchers ought to include measures of sex and puberty in longitudinal studies of adolescents (indeed, longitudinal examination of sex and pubertal influences on neurodevelopment could also aid in a better understanding of other expressions of psychopathology and neurodiversity, but this is beyond the scope of this paper; see e.g. (Walsh et al., 2021) for further discussion). Finally, we make a variety of methodological recommendations. First, we recommend proton MRS studies that examine how steroid hormone changes relate to neurotransmitter function, especially of glutamate given its central role in SZ pathophysiology. Second, we recommend using large, community samples to test sex-specific pubertal influences on brain development and if these influences predict the development of SZ symptoms in each sex. Third, research should be conducted using high-risk populations to reveal how pubertal processes interact with genetic or prepubertal environmental risk factors to alter the course of neurodevelopmental trajectories relevant to SZ. These approaches will unite the fields of psychiatry, cognitive neuroscience, and endocrinology towards a comprehensive understanding of the role of pubertal factors in the hypothesized sex-specific pathophysiological mechanisms of schizophrenia.
Supplementary Material
Funding
This research was supported by the National Institute of Mental Health (grant number R01MH125873) to LMT; and a Building Interdisciplinary Research Careers in Women’s Health award (grant number K12 HD051958) to LMT, funded by the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health, Office of Dietary Supplements, and the National Institute of Aging. The funding sources had no role in the study design, data collection and analysis, or submission process.
Footnotes
Conflicts of interest
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
We don’t differentiate between biological sex, sex assigned at birth, and gender identity given that, unfortunately, the extant literature reviewed does not consistently report how sex is defined or how/whether gender-diverse youth are included. Given the focus on neural functioning, we emphasize sex as a biological variable although we acknowledge sex and gender as intertwined but non-redundant constructs, see Methodological Considerations for more information.
7. References
- Alarcón G, Cservenka A, Nagel BJ, 2017. Adolescent neural response to reward is related to participant sex and task motivation. Brain and Cognition 111, 51–62. 10.1016/j.bandc.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T, 2009. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology 34, 1121–1132. 10.1016/j.psyneuen.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Anticevic A, Tang Y, Cho YT, Repovs G, Cole MW, Savic A, Wang F, Krystal JH, Xu K, 2013. Amygdala Connectivity Differs Among Chronic, Early Course, and Individuals at Risk for Developing Schizophrenia. Schizophrenia Bulletin 40, 1105–1116. 10.1093/schbul/sbt165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse MEA, Pfeifer JH, 2021. Puberty and social brain development, in: The Oxford Handbook of Developmental Cognitive Neuroscience. Oxford University Press. 10.1093/oxfordhb/9780198827474.013.25 [DOI] [Google Scholar]
- Barendse MEA, Simmons JG, Patton G, Mundy L, Byrne ML, Seal ML, Allen NB, Whittle S, 2019. Adrenarcheal Timing Longitudinally Predicts Anxiety Symptoms via Amygdala Connectivity During Emotion Processing. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2019.04.018 [DOI] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J, 2015. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE, 2012. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophrenia research 141, 179–184. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, Corley R, 2015. The Importance of Puberty for Adolescent Development, in: Advances in Child Development and Behavior. Elsevier, pp. 53–92. 10.1016/bs.acdb.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C, 2007. Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychological medicine 37, 1427–1436. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Dwojak A, Iyengar S, Ladouceur CD, Fournier JC, Versace A, Perlman SB, Almeida JRC, Travis MJ, Gill MK, Bonar L, Schirda C, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Frazier T, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML, 2017. Longitudinal Relationships Among Activity in Attention Redirection Neural Circuitry and Symptom Severity in Youth. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2, 336–345. 10.1016/j.bpsc.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Su A, Michelson D, 2002. Effects of oral estrogen, raloxifene and arzoxifene on gene expression in serotonin neurons of macaques. Psychoneuroendocrinology 27, 431–445. 10.1016/S0306-4530(01)00054-3 [DOI] [PubMed] [Google Scholar]
- Birnbaum R, Weinberger DR, 2017. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci 18, 727–740. 10.1038/nrn.2017.125 [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD, 2004. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7, 184–188. 10.1038/nn1173 [DOI] [PubMed] [Google Scholar]
- Bjorkquist OA, Olsen EK, Nelson BD, Herbener ES, 2016. Altered amygdala-prefrontal connectivity during emotion perception in schizophrenia. Schizophrenia Research 175, 35–41. 10.1016/j.schres.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR, 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157, 924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA, 2015. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 35, 7226–7238. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, Chavez S, Vijayakumar N, Cheng TW, Flournoy JC, Barendse M, Shirtcliff E, Allen NB, Pfeifer JH, 2019. Multi-method confirmatory factor analyses of puberty in early adolescent girls (preprint). PsyArXiv. 10.31234/osf.io/pue6f [DOI] [Google Scholar]
- Calleja-Agius J, Mallia P, Sapiano K, Schembri-Wismayer P, 2012. A Review of the Management of Intersex. Neonatal Network 31, 97–104. 10.1891/0730-0832.31.2.97 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, 1993. A Self-Administered sting Scale for Development 14, 6. [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ, Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM, 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Somerville LH, 2011. Braking and Accelerating of the Adolescent Brain. Journal of Research on Adolescence 21, 21–33. 10.1111/j.1532-7795.2010.00712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceskova E, Prikryl R, Libiger J, Svancara J, Jarkovsky J, 2015. Gender differences in the treatment of first-episode schizophrenia: Results from the European First Episode Schizophrenia Trial. Schizophrenia Research 169, 303–307. 10.1016/j.schres.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Chahal R, Delevich K, Kirshenbaum JS, Borchers LR, Ho TC, Gotlib IH, 2021. Sex differences in pubertal associations with fronto-accumbal white matter morphometry: Implications for understanding sensitivity to reward and punishment. NeuroImage 226, 117598. 10.1016/j.neuroimage.2020.117598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Watkins DJ, Afeiche MC, Zhang Z, Sánchez BN, Cantonwine D, Mercado-García A, Blank-Goldenberg C, Meeker JD, Téllez-Rojo MM, Peterson KE, 2017. Validity of Self-Assessed Sexual Maturation Against Physician Assessments and Hormone Levels. The Journal of Pediatrics 186, 172–178.e3. 10.1016/j.jpeds.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Barch DM, 2016. Frontal-striatum dysfunction during reward processing: Relationships to amotivation in schizophrenia. Journal of abnormal psychology 125, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, 2016. The economic burden of schizophrenia in the United States in 2013. The Journal of clinical psychiatry 77, 764–771. [DOI] [PubMed] [Google Scholar]
- Cohen RZ, Seeman MV, Gotowiec A, Kopala L, 1999. Earlier puberty as a predictor of later onset of schizophrenia in women. American Journal of Psychiatry 156, 1059–1065. [DOI] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, McLaughlin KA, 2020. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin 146, 721–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RE, Laxhman N, Crellin N, Moncrieff J, Priebe S, 2019. Psychosocial interventions for people with schizophrenia or psychosis on minimal or no antipsychotic medication: A systematic review. Schizophrenia Research. 10.1016/j.schres.2019.05.020 [DOI] [PubMed] [Google Scholar]
- Cosoff SJ, Häfner J, 1998. The prevalence of comorbid anxiety in schizophrenia, schizoaffective disorder and bipolar disorder. Australian and New Zealand Journal of Psychiatry 32, 67–72. [DOI] [PubMed] [Google Scholar]
- Desai PR, Lawson KA, Barner JC, Rascati KL, 2013. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. Journal of Pharmaceutical Health Services Research 4, 187–194. [Google Scholar]
- Dodell-Feder D, Tully LM, Hooker CI, 2015. Social impairment in schizophrenia: New approaches for treating a persistent problem. Curr Opin Psychiatry 28, 236–242. 10.1097/YCO.0000000000000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Modinos G, Ferrera D, McGuire P, 2017. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry 7, e1147–e1147. 10.1038/tp.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner E, Drake R, Barrowclough C, 2013. Assessing early signs of relapse in psychosis: Review and future directions. Clinical Psychology Review 33, 637–653. 10.1016/j.cpr.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ, 2015. The neural bases of emotion regulation. Nature Reviews Neuroscience 16, 693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 51, 871–882. 10.1016/j.neuron.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Fischer S, Obrist R, Ehlert U, 2019. How and when to use dried blood spots in psychoneuroendocrinological research. Psychoneuroendocrinology 108, 190–196. 10.1016/j.psyneuen.2019.06.011 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE, 2010. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry 49, 162–172. e5. 10.1016/j.jaac.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C, 2009. Anatomical Abnormalities of the Anterior Cingulate Cortex in Schizophrenia: Bridging the Gap Between Neuroimaging and Neuropathology. Schizophrenia Bulletin 35, 973–993. 10.1093/schbul/sbn025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, Andersen AN, Vestergaard ET, Schorring ME, Linneberg A, Main KM, Andersson A-M, Juul A, 2020. Sex-specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. The Journal of Clinical Endocrinology & Metabolism 105, 754–768. 10.1210/clinem/dgz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdos PM, Van Os JJ, Murray RM, 1993. Puberty and the onset of psychosis. Schizophrenia Research 10, 7–14. [DOI] [PubMed] [Google Scholar]
- Gard AM, Waller R, Swartz JR, Shaw DS, Forbes EE, Hyde LW, 2018. Amygdala functional connectivity during socioemotional processing prospectively predicts increases in internalizing symptoms in a sample of low-income, urban, young men. NeuroImage 178, 562–573. 10.1016/j.neuroimage.2018.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S-J, 2014. The influence of puberty on subcortical brain development. NeuroImage 88, 242–51. 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, Kwek P, Chavez C, van den Buuse M, 2010. Estrogen treatment blocks 8-hydroxy-2-dipropylaminotetralin-and apomorphine-induced disruptions of prepulse inhibition: involvement of dopamine D1 or D2 or serotonin 5-HT1A, 5-HT2A, or 5-HT7 receptors. Journal of Pharmacology and Experimental Therapeutics 333, 218–227. [DOI] [PubMed] [Google Scholar]
- Gogos A, Nathan PJ, Guille V, Croft RJ, van den Buuse M, 2006. Estrogen prevents 5-HT1A receptor-induced disruptions of prepulse inhibition in healthy women. Neuropsychopharmacology 31, 885–889. 10.1038/sj.npp.1300933 [DOI] [PubMed] [Google Scholar]
- Gröschl M, 2008. Current Status of Salivary Hormone Analysis. Clinical Chemistry 54, 1759–1769. 10.1373/clinchem.2008.108910 [DOI] [PubMed] [Google Scholar]
- Gross J, Thompson R, 2007. Emotion regulation: Conceptual foundations, in: Handbook of Emotion Regulation. New York: The Guilford Press, pp. 3–26. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C, 2009. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and psychopathology 21, 69–85. 10.1017/S0954579409000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, 2003. Gender differences in schizophrenia. Psychoneuroendocrinology 28, 17–54. 10.1016/S0306-4530(02)00125-7 [DOI] [PubMed] [Google Scholar]
- Hanlon M-C, Campbell LE, Single N, Coleman C, Morgan VA, Cotton SM, Stain HJ, Castle DJ, 2017. Men and women with psychosis and the impact of illness-duration on sex-differences: The second Australian national survey of psychosis. Psychiatry Research 256, 130–143. 10.1016/j.psychres.2017.06.024 [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Epperson CN, 2015. Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Curr Psychiatry Rep 17, 87. 10.1007/s11920-015-0628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung CM, Lefler EK, 2019. Sex and gender in psychopathology: DSM–5 and beyond. Psychological Bulletin 145, 390–409. 10.1037/bul0000183 [DOI] [PubMed] [Google Scholar]
- Heila H, Isometsa ET, Henriksson MM, Heikkinen ME, 1997. Suicide and schizophrenia: a nationwide psychological autopsy study on age-and sex-specific clinical characteristics of 92 suicide victims with schizophrenia. The American journal of psychiatry 154, 1235. [DOI] [PubMed] [Google Scholar]
- Herting MM, Sowell ER, 2017. Puberty and structural brain development in humans. Frontiers in Neuroendocrinology 44. 10.1016/j.yfrne.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Uban KA, Gonzalez MR, Baker FC, Kan EC, Thompson WK, Granger DA, Albaugh MD, Anokhin AP, Bagot KS, Banich MT, Barch DM, Baskin-Sommers A, Breslin FJ, Casey BJ, Chaarani B, Chang L, Clark DB, Cloak CC, Constable RT, Cottler LB, Dagher RK, Dapretto M, Dick AS, Dosenbach N, Dowling GJ, Dumas JA, Edwards S, Ernst T, Fair DA, Feldstein-Ewing SW, Freedman EG, Fuemmeler BF, Garavan H, Gee DG, Giedd JN, Glaser PEA, Goldstone A, Gray KM, Hawes SW, Heath AC, Heitzeg MM, Hewitt JK, Heyser CJ, Hoffman EA, Huber RS, Huestis MA, Hyde LW, Infante MA, Ivanova MY, Jacobus J, Jernigan TL, Karcher NR, Laird AR, LeBlanc KH, Lisdahl K, Luciana M, Luna B, Maes HH, Marshall AT, Mason MJ, McGlade EC, Morris AS, Nagel BJ, Neigh GN, Palmer CE, Paulus MP, Potter AS, Puttler LI, Rajapakse N, Rapuano K, Reeves G, Renshaw PF, Schirda C, Sher KJ, Sheth C, Shilling PD, Squeglia LM, Sutherland MT, Tapert SF, Tomko RL, Yurgelun-Todd D, Wade NE, Weiss SRB, Zucker RA, Sowell ER, 2021a. Correspondence Between Perceived Pubertal Development and Hormone Levels in 9–10 Year-Olds From the Adolescent Brain Cognitive Development Study. Front. Endocrinol. 11, 549928. 10.3389/fendo.2020.549928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Uban KA, Gonzalez MR, Baker FC, Kan EC, Thompson WK, Granger DA, Albaugh MD, Anokhin AP, Bagot KS, Banich MT, Barch DM, Baskin-Sommers A, Breslin FJ, Casey BJ, Chaarani B, Chang L, Clark DB, Cloak CC, Constable RT, Cottler LB, Dagher RK, Dapretto M, Dick AS, Dosenbach N, Dowling GJ, Dumas JA, Edwards S, Ernst T, Fair DA, Feldstein-Ewing SW, Freedman EG, Fuemmeler BF, Garavan H, Gee DG, Giedd JN, Glaser PEA, Goldstone A, Gray KM, Hawes SW, Heath AC, Heitzeg MM, Hewitt JK, Heyser CJ, Hoffman EA, Huber RS, Huestis MA, Hyde LW, Infante MA, Ivanova MY, Jacobus J, Jernigan TL, Karcher NR, Laird AR, LeBlanc KH, Lisdahl K, Luciana M, Luna B, Maes HH, Marshall AT, Mason MJ, McGlade EC, Morris AS, Nagel BJ, Neigh GN, Palmer CE, Paulus MP, Potter AS, Puttler LI, Rajapakse N, Rapuano K, Reeves G, Renshaw PF, Schirda C, Sher KJ, Sheth C, Shilling PD, Squeglia LM, Sutherland MT, Tapert SF, Tomko RL, Yurgelun-Todd D, Wade NE, Weiss SRB, Zucker RA, Sowell ER, 2021b. Correspondence Between Perceived Pubertal Development and Hormone Levels in 9–10 Year-Olds From the Adolescent Brain Cognitive Development Study. Front. Endocrinol. 11. 10.3389/fendo.2020.549928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman KM, Lewine RR, 2004. Age of menarche and schizophrenia onset in women. Schizophrenia research 69, 183–188. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Benson TL, Gyurak A, Yin H, Tully LM, Lincoln SH, 2014. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J Abnorm Psychol 123, 190–204. 10.1037/a0035223 [DOI] [PubMed] [Google Scholar]
- Hooker CI, Tully LM, Verosky SC, Fisher M, Holland C, Vinogradov S, 2011. Can I trust you? Negative affective priming influences social judgments in schizophrenia. Journal of Abnormal Psychology 120, 98–107. 10.1037/a0020630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard DB, Miller BJ, 2019. Meta-analysis of blood cortisol levels in individuals with first-episode psychosis. Psychoneuroendocrinology 104, 269–275. 10.1016/j.psyneuen.2019.03.014 [DOI] [PubMed] [Google Scholar]
- Huber TJ, Tettenborn C, Leifke E, Emrich HM, 2005. Sex hormones in psychotic men. Psychoneuroendocrinology 30, 111–114. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, Wrase J, Heinz A, 2006. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29, 409–416. [DOI] [PubMed] [Google Scholar]
- Katthagen T, Kaminski J, Heinz A, Buchert R, Schlagenhauf F, 2020. Striatal Dopamine and Reward Prediction Error Signaling in Unmedicated Schizophrenia Patients. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A, 2018. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. AJP 175, 1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg EF, Wilcox AJ, O’Connor JF, 1998. Detecting pre-ovulatory luteinizing hormone surges in urine. Human Reproduction 13, 15–21. 10.1093/humrep/13.1.15 [DOI] [PubMed] [Google Scholar]
- Kindler J, Weickert CS, Schofield PR, Lenroot R, Weickert TW, 2016. Raloxifene increases prefrontal activity during emotional inhibition in schizophrenia based on estrogen receptor genotype. European Neuropsychopharmacology 26, 1930–1940. 10.1016/j.euroneuro.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, Boydell J, Murray RM, Jones PB, 2012. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PloS one 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y-H, Lew Y-M, Jung S-W, Joe S-H, Lee C-H, Jung H-G, Lee M-S, 2008. Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. Journal of clinical psychopharmacology 28, 375–383. [DOI] [PubMed] [Google Scholar]
- Koolschijn PCMP, Peper JS, Crone EA, 2014. The influence of sex steroids on structural brain maturation in adolescence. PloS one 9, e83929. 10.1371/journal.pone.0083929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Kerestes R, Schlund MW, Shirtcliff EA, Lee Y, Dahl RE, 2019. Neural systems underlying reward cue processing in early adolescence: The role of puberty and pubertal hormones. Psychoneuroendocrinology 102, 281–291. 10.1016/j.psyneuen.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally J, Ajnakina O, Stubbs B, Cullinane M, Murphy KC, Gaughran F, Murray RM, 2017. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. The British Journal of Psychiatry 211, 350–358. [DOI] [PubMed] [Google Scholar]
- Lee J, Jimenez AM, Reavis EA, Horan WP, Wynn JK, Green MF, 2019. Reduced neural sensitivity to social vs nonsocial reward in schizophrenia. Schizophrenia Bulletin 45, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis J, 2009. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Molecular psychiatry 14, 429–447. [DOI] [PubMed] [Google Scholar]
- Lichenstein SD, Verstynen T, Forbes EE, 2016. Adolescent brain development and depression: A case for the importance of connectivity of the anterior cingulate cortex. Neuroscience & Biobehavioral Reviews 70, 271–287. 10.1016/J.NEUBIOREV.2016.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC, 2010. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & Behavior 99, 8–16. 10.1016/j.physbeh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR, 2015. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry 172, 249–258. 10.1176/appi.ajp.2014.13030418 [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J, 2011. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews 35, 1219–1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso SG, Morgan VA, Mitchell PB, Berk M, Young A, Castle DJ, 2015. A comparison of schizophrenia, schizoaffective disorder, and bipolar disorder: Results from the Second Australian national psychosis survey. Journal of affective disorders 172, 30–37. 10.1016/j.jad.2014.09.035 [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in neuroendocrinology 30, 65–91. 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, 2012. Sex steroids and schizophrenia. Reviews in Endocrine and Metabolic Disorders 13, 187–207. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI, 2011. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology 214, 89–106. 10.1007/s00213-010-2035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand PS, Roca M, Turner MS, Kane JM, 2009. Partial Adherence to Antipsychotic Medication Impacts the Course of Illness in Patients With Schizophrenia: A Review. Prim Care Companion J Clin Psychiatry 11, 147–54. 10.4088/PCC.08r00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, 2008. Estradiol and the developing brain. Physiological reviews 88, 91–124. 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, 2014. Development and validation of assay protocols for use with dried blood spot samples: T.W. McDade. Am. J. Hum. Biol. 26, 1–9. 10.1002/ajhb.22463 [DOI] [PubMed] [Google Scholar]
- Mendle J, Beltz AM, Carter R, Dorn LD, 2019a. Understanding Puberty and Its Measurement: Ideas for Research in a New Generation. Journal of Research on Adolescence 29, 82–95. 10.1111/jora.12371 [DOI] [PubMed] [Google Scholar]
- Mendle J, Beltz AM, Carter R, Dorn LD, 2019b. Understanding Puberty and Its Measurement: Ideas for Research in a New Generation. J Res Adolesc 29, 82–95. 10.1111/jora.12371 [DOI] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, Graber JA, 2010. Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol 46, 1341–1353. 10.1037/a0020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Tseng H-H, Falkenberg I, Samson C, McGuire P, Allen P, 2015. Neural correlates of aberrant emotional salience predict psychotic symptoms and global functioning in high-risk and first-episode psychosis. Social Cognitive and Affective Neuroscience 10, 1429–1436. 10.1093/scan/nsv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence 9, 271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- Morris RW, Purves-Tyson TD, Weickert CS, Rothmond D, Lenroot R, Weickert TW, 2015. Testosterone and reward prediction-errors in healthy men and men with schizophrenia. Schizophrenia Research, Reproductive hormones and schizophrenia 168, 649–660. 10.1016/j.schres.2015.06.030 [DOI] [PubMed] [Google Scholar]
- Moskow DM, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, Woods SW, Walker EF, 2016. The relations of age and pubertal development with cortisol and daily stress in youth at clinical risk for psychosis. Schizophrenia Research 172, 29–34. 10.1016/j.schres.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Corlett P, Clark L, Pessiglione M, Blackwell A, Honey G, Jones P, Bullmore E, Robbins T, Fletcher P, 2008. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular psychiatry 13, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Bhavsar V, Tripoli G, Howes O, 2017. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed into the Developmental Risk Factor Model of Psychosis. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, Konrad K, 2009. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral cortex 19, 464–73. 10.1093/cercor/bhn100 [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Gunther Moor B, Overgaauw S, Güroğlu B, Dahl RE, Crone EA, 2011. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental cognitive neuroscience 1, 506–16. 10.1016/j.dcn.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SJ, Murphy CE, Purves-Tyson TD, Weickert TW, Weickert CS, 2018. Considering the role of adolescent sex steroids in schizophrenia. Journal of Neuroendocrinology 30, e12538. 10.1111/jne.12538 [DOI] [PubMed] [Google Scholar]
- Park J, Chun J, Park H, Kim E, Kim J, 2018. Involvement of amygdala–prefrontal dysfunction in the influence of negative emotion on the resolution of cognitive conflict in patients with schizophrenia. Brain and behavior 8, e01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PK, Leathem LD, Currin DL, Karlsgodt KH, 2021. Adolescent Neurodevelopment and Vulnerability to Psychosis. Biological Psychiatry, Adolescent Brain Development and Psychopathology 89, 184–193. 10.1016/j.biopsych.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolescence 17, 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB, 2020. Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biological Psychiatry. 10.1016/j.biopsych.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski DJ, Boivin JR, Wilbrecht L, 2017. Ovarian Hormones Organize the Maturation of Inhibitory Neurotransmission in the Frontal Cortex at Puberty Onset in Female Mice. Current Biology 27, 1735–1745.e3. 10.1016/j.cub.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EMP, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR, 2014. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 19, 20–29. 10.1038/mp.2013.136 [DOI] [PubMed] [Google Scholar]
- Poon JA, Niehaus CE, Thompson JC, Chaplin TM, 2019. Adolescents’ pubertal development: Links between testosterone, estradiol, and neural reward processing. Horm Behav 114, 104504. 10.1016/j.yhbeh.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornpattananangkul N, Leibenluft E, Pine DS, Stringaris A, 2019. Association between childhood anhedonia and alterations in large-scale resting-state networks and task-evoked activation. JAMA psychiatry 76, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Dube SL, Barrios LC, Bookheimer S, Espinoza A, Feldstein Ewing SW, Freedman EG, Hoffman EA, Ivanova M, Jefferys H, McGlade EC, Tapert SF, Johns MM, 2022. Measurement of gender and sexuality in the Adolescent Brain Cognitive Development (ABCD) study. Developmental Cognitive Neuroscience 53, 101057. 10.1016/j.dcn.2022.101057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi E, Vijayakumar N, Rakesh D, Whittle S, 2021. Neural Correlates of Emotion Regulation in Adolescents and Emerging Adults: A Meta-analytic Study. Biological Psychiatry 89, 194–204. 10.1016/j.biopsych.2020.08.006 [DOI] [PubMed] [Google Scholar]
- Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P, 2012. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience & Biobehavioral Reviews 36, 2325–2333. 10.1016/j.neubiorev.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Miewald J, Montrose D, Keshavan MS, 2015. Can age at sexual maturity act as a predictive biomarker for prodromal negative symptoms? Schizophrenia Research 164, 35–39. 10.1016/j.schres.2015.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport J, Giedd J, Gogtay N, 2012. Neurodevelopmental model of schizophrenia: update 2012. Molecular psychiatry 17, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Abadal E, Usall J, Barajas A, Carlson J, Iniesta R, Huerta-Ramos E, Baños I, Dolz M, Sánchez B, Group G, 2016. Relationship between menarche and psychosis onset in women with first episode of psychosis. Early intervention in psychiatry 10, 419–425. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Blanco R, Santander J, Miranda E, 2000. Relationship between sex differences in onset of schizophrenia and puberty. Journal of psychiatric research 34, 349–353. [DOI] [PubMed] [Google Scholar]
- Samara MT, Nikolakopoulou A, Salanti G, Leucht S, 2018. How Many Patients With Schizophrenia Do Not Respond to Antipsychotic Drugs in the Short Term? An Analysis Based on Individual Patient Data From Randomized Controlled Trials. Schizophrenia Bulletin 45, 639–646. 10.1093/schbul/sby095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, Girdler SS, Kiesner J, Ditzen B, Eisenlohr-Moul TA, 2021. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 123, 104895. 10.1016/j.psyneuen.2020.104895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, Miller DD, Oliver SE, Arndt S, Flaum M, Andreasen NC, 1997. The life course of schizophrenia: age and symptom dimensions. Schizophrenia Research 23, 15–23. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL, 2009. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and behavior 55, 597–604. 10.1016/j.yhbeh.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, 2009. Secondary effects of antipsychotics: women at greater risk than men. Schizophrenia bulletin 35, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, Arrondo G, Robbins TW, Clark L, Fletcher PC, Murray GK, 2016. Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology 41, 2001–2010. 10.1038/npp.2015.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ, 2012. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology 54, 493–502. 10.1002/dev.20607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal Development: Correspondence Between Hormonal and Physical Development: Hormonal Correlates of Pubertal Stage. Child Development 80, 327–337. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dismukes AR, Marceau K, Ruttle PL, Simmons JG, Han G, 2015. A dual-axis approach to understanding neuroendocrine development. Developmental psychobiology 57, 643–53. 10.1002/dev.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Harden KP, Chein JM, Steinberg L, 2015. Sex Differences in the Developmental Trajectories of Impulse Control and Sensation-Seeking from Early Adolescence to Early Adulthood. J Youth Adolescence 44, 1–17. 10.1007/s10964-014-0116-9 [DOI] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M, 2015. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage 122, 427–439. 10.1016/j.neuroimage.2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S, 2010. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophrenia research 118, 154–161. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS, 2014. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 231, 1581–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GKS, Balzer BWR, Kelly PJ, Paxton K, Hawke CI, Handelsman DJ, Steinbeck KS, 2015. Urinary Sex Steroids and Anthropometric Markers of Puberty - A Novel Approach to Characterising Within-Person Changes of Puberty Hormones. PLOS ONE 10, e0143555. 10.1371/journal.pone.0143555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Johnson KA, Whittle S, Allen NB, Simmons JG, 2019. Measurement of cortisol, dehydroepiandrosterone, and testosterone in the hair of children: Preliminary results and promising indications. Dev Psychobiol 61, 962–970. 10.1002/dev.21807 [DOI] [PubMed] [Google Scholar]
- Søeborg T, Frederiksen H, Mouritsen A, Johannsen TH, Main KM, Jørgensen N, Petersen JH, Andersson A-M, Juul A, 2014. Sex, age, pubertal development and use of oral contraceptives in relation to serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, Δ4-androstenedione, testosterone and their ratios in children, adolescents and young adults. Clinica chimica acta; international journal of clinical chemistry 437, 6–13. 10.1016/j.cca.2014.06.018 [DOI] [PubMed] [Google Scholar]
- Stahl SM, 2018. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectrums 23, 187–191. 10.1017/S1092852918001013 [DOI] [PubMed] [Google Scholar]
- Swartz JR, Phan KL, Angstadt M, Fitzgerald KD, Monk CS, 2014. Dynamic changes in amygdala activation and functional connectivity in children and adolescents with anxiety disorders. Development and Psychopathology 26, 1305–1319. 10.1017/S0954579414001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH, 1976. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of Disease in Childhood 51, 170–179. 10.1136/adc.51.3.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbo P, Hanstock C, Valiakalayil A, Allen P, 2004. 3-T Proton MRS Investigation of Glutamate and Glutamine in Adolescents at High Genetic Risk for Schizophrenia. AJP 161, 1116–1118. 10.1176/appi.ajp.161.6.1116 [DOI] [PubMed] [Google Scholar]
- Tully LM, Lincoln SH, Hooker CI, 2012. Impaired executive control of emotional information in social anhedonia. Psychiatry Research 197, 29–35. 10.1016/j.psychres.2011.12.023 [DOI] [PubMed] [Google Scholar]
- Tully LM, Niendam TA, 2014. Beyond “Cold” Cognition: Exploring Cognitive Control of Emotion as a Risk Factor for Psychosis. Curr Behav Neurosci Rep 1, 170–181. 10.1007/s40473-014-0016-z [DOI] [Google Scholar]
- Ullsperger JM, Nikolas MA, 2017. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk? Psychological Bulletin 143, 903–938. 10.1037/bul0000106 [DOI] [PubMed] [Google Scholar]
- Vai B, Sferrazza Papa G, Poletti S, Radaelli D, Donnici E, Bollettini I, Falini A, Cavallaro R, Smeraldi E, Benedetti F, 2015. Abnormal cortico-limbic connectivity during emotional processing correlates with symptom severity in schizophrenia. European Psychiatry 30, 590–597. 10.1016/j.eurpsy.2015.01.002 [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, McGorry P, Cuijpers P, 2013. Preventing a first episode of psychosis: Meta-analysis of randomized controlled prevention trials of 12month and longer-term follow-ups. Schizophrenia Research 149, 56–62. 10.1016/j.schres.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Van Rijn S, Aleman A, De Sonneville L, Sprong M, Ziermans T, Schothorst P, van Engeland H, Swaab H, 2011. Neuroendocrine markers of high risk for psychosis: salivary testosterone in adolescent boys with prodromal symptoms. Psychological medicine 41, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH, 2018. Puberty and the human brain: Insights into adolescent development. Neuroscience & Biobehavioral Reviews 92, 417–436. 10.1016/j.neubiorev.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Pfeifer JH, Flournoy JC, Hernandez LM, Dapretto M, 2019. Affective reactivity during adolescence: Associations with age, puberty and testosterone. Cortex 117, 336–350. 10.1016/j.cortex.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros M, Llorente R, Suarez J, Llorente-Berzal A, López-Gallardo M, Rodriguez de Fonseca F, 2012. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol 26, 164–176. 10.1177/0269881111408956 [DOI] [PubMed] [Google Scholar]
- Walder DJ, Holtzman CW, Addington J, Cadenhead K, Tsuang M, Cornblatt B, Cannon TD, McGlashan TH, Woods SW, Perkins DO, Seidman LJ, Heinssen R, Walker EF, 2013. Sexual dimorphisms and prediction of conversion in the NAPLS psychosis prodrome. Schizophrenia Research 144, 43–50. 10.1016/j.schres.2012.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJM, Wallace GL, Gallegos SM, Braden BB, 2021. Brain-based sex differences in autism spectrum disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. NeuroImage: Clinical 31, 102719. 10.1016/j.nicl.2021.102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Suenderhauf C, Harrisberger F, Lenz C, Smieskova R, Chung Y, Cannon TD, Bearden CE, Rapp C, Bendfeldt K, Borgwardt S, Vogel T, 2016. Hippocampal volume in subjects at clinical high-risk for psychosis: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 71, 680–690. 10.1016/j.neubiorev.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Waltz JA, Kasanova Z, Ross TJ, Salmeron BJ, McMahon RP, Gold JM, Stein EA, 2013. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PloS one 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, Rose EJ, McClure SM, Stein EA, 2009. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 34, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Moody SN, Kiesner J, Tonon Appiani A, Robertson OC, Shirtcliff EA, 2019. Assay validation of hair androgens across the menstrual cycle. Psychoneuroendocrinology 101, 175–181. 10.1016/j.psyneuen.2018.10.029 [DOI] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z, 2014. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry 19, 588–598. 10.1038/mp.2013.83 [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, Schreuders E, vd Kamp F, Peper JS, Tamnes CK, Crone EA, 2018. Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology 91, 105–114. 10.1016/j.psyneuen.2018.02.034 [DOI] [PubMed] [Google Scholar]
- Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, Satchell EK, Shou H, Sheline YI, 2018. Cognitive Behavioral Therapy Is Associated With Enhanced Cognitive Control Network Activity in Major Depression and Posttraumatic Stress Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3, 311–319. 10.1016/j.bpsc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Lin P, Shi H, Öngür D, Auerbach RP, Wang Xiaosheng, Yao S, Wang Xiang, 2016. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging and Behavior 10, 920–939. 10.1007/s11682-015-9457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH, 2017. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36. 10.1016/j.psyneuen.2016.11.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


