Summary
Mammalian embryos differentiate into the inner cell mass (ICM) and trophectoderm at the 8–16 cell stage. The ICM forms a single cluster that develops into a single fetus. However, the factors that determine differentiation and single cluster formation are unknown. Here we investigated whether embryos could develop normally without gravity. As the embryos cannot be handled by an untrained astronaut, a new device was developed for this purpose. Using this device, two-cell frozen mouse embryos launched to the International Space Station were thawed and cultured by the astronauts under microgravity for 4 days. The embryos cultured under microgravity conditions developed into blastocysts with normal cell numbers, ICM, trophectoderm, and gene expression profiles similar to those cultured under artificial-1 g control on the International Space Station and ground-1 g control, which clearly demonstrated that gravity had no significant effect on the blastocyst formation and initial differentiation of mammalian embryos.
Subject areas: Biological sciences, Developmental biology, Embryology, Space sciences
Graphical abstract

Highlights
-
•
Mouse 2-cell embryos can develop into blastocysts under microgravity
-
•
Gravity did not affect initial differentiation of mammalian embryos
-
•
Mammals can thrive in space
Biological sciences; Developmental biology; Embryology; Space sciences
Introduction
Elucidating the effect of space conditions, such as microgravity and radiation, on mammalian reproduction is essential to ensure the long-term survival of humans in space.1 Although a few studies have been conducted on the effects of space radiation on mammalian spermatozoa,2,3,4,5 the impact of microgravity on embryo development has only been reported in sea urchins, fish, and amphibians.6,7,8,9,10,11,12 Mammalian reproduction is complicated and highly specialized and is unlike that of other taxa. Fertilization and implantation occur in the uterus, and the mother supports fetal growth through the placenta till full-term fetal development. Thus, research outcomes from non-mammalian species cannot be extrapolated to understand mammalian reproduction in space.1,13
In preimplantation mammalian embryos, after first cell fate specification occurs, the blastocyst develops into two types of cells: the outer epithelial trophectoderm (TE) layer and inner cell mass (ICM). The TE cell layer expresses Cdx2 and contributes to the placenta formation,14 and the ICM cell expresses transcription factors such as Oct4 and Nanog, which contribute to the fetus formation.15,16 The first cell fate was controlled by the inside-outside position of the blastomeres, and it is unknown how this position is controlled.17,18,19,20 When embryos develop into blastocysts, the ICM cells always cluster at one place in the blastocyst cavity, suggesting that the ICM cells are heavier than other cells and sink to the bottom of the blastocyst cavity.
If gravity is the cause of the inside-outside positioning of the cells and gathering of ICM cells in one place, then it may not be possible for the cells to differentiate correctly into ICM and TE cells or for ICM cells to gather in one place in the blastocyst cavity in space. If the ICM cells accidentally split into two in the cavity of the blastocyst, identical monozygotic twins sharing one placenta will be born, which may lead to an increased burden on the fetuses as well as mother with an increased risk of miscarriage, especially in single-gestation species.21,22 Thus, if gravity affects cell differentiation and localization in mammalian embryos, humans would be unable to reproduce in space.
Herein, we aimed to determine whether mammals are able to reproduce normally in space, where there is approximately zero gravity. Although mice and rats should be used for reproductive experiments in space, it is currently impossible to keep them in the International Space Station (ISS) for an extended time period. Moreover, reproduction experiments on mammals have special criteria. For instance, changing the breeding room alters the estrous cycle, which may lead to failure to mate.23,24 Instead of conducting an actual space experiment, our group previously conducted an experiment on mammalian embryonic development in vitro under simulated μ g conditions using a three-dimensional clinostat.25 Embryos cultured for 4 days under μ g exhibited significant anomalies, such as delayed embryonic development, trophectoderm deterioration, impaired differentiation rates, and significantly reduced birth rates after transfer to a female recipient. Similar results were also reported by other laboratories. When early-stage embryos were cultured under simulated microgravity conditions, many of the embryos died by 72 h,26 or a decrease in the number of embryos reaching the blastocyst stages was observed after 96 h.27 Thus, although the impact on embryos varied somewhat depending on the type of microgravity simulator, the adverse effects on embryo development occurred only in those cultured under the simulated microgravity condition.
To thoroughly assess the actual impact of space, frozen embryos should be transported, thawed, and cultured by highly skilled experimentalists rather than relying on astronauts at the ISS (because the embryos are very small; 80–100 μm); however, this is not practical. We have devised several methods and tools to perform these experiments, such as a simple device for frozen embryo thawing and culturing on the ISS,28 and identified the appropriate stage of two-cell mouse embryos and the suitable culture medium for this project.29,30 Our findings will allow untrained personnel on Earth and the astronauts on the ISS to perform embryo experiments. Using this method, frozen mouse 2-cell stage embryos were launched to the ISS, thawed by astronauts, cultured on the ISS for 4 days, and used to investigate if mammalian embryos can develop into normal blastocysts under the microgravity conditions of the ISS.
Results
Preliminary results
The experiments conducted on the ISS were carefully planned to overcome the limitations associated with μ g. To conduct this experiment, we developed a new embryo thawing and culturing unit (ETC), which will help to thaw and culture frozen mouse 2-cell embryos on the ISS without directly contacting the embryos.28 A lot of preliminary experiments were performed to modify and improve the ETC; thus, before performing space experiment, we thought that it could be used in every conceivable situation in space. The results of our preliminary experiment are shown in Figures S1–S11 and Tables S1–S13. For example, in microgravity, convection of liquid is absent; therefore, exchange from the cryoprotectant to the culture medium in the ETC might be unsuccessful. Therefore, we shook the ETC during solution exchange to confirm that this process does not affect embryo development (Table S8). On the ISS, the use of toxins, including 4% paraformaldehyde (PFA) (a common chemical fixation agent), is restricted. Therefore, we investigated whether 0.99% PFA, which can be used on the ISS, could be used for the fixation of blastocysts. After 0.99% PFA fixation, the blastocysts would be refrigerated for about one month before returning to Earth. We found that the morphology of these blastocysts was unaffected by the drop test from the second floor, which simulates landing on the ground, and immunostaining/gene expression analysis is possible for these blastocysts (Figures S7 and S9–S11; and Table S10). In addition, four untrained lab personnel performed the entire experiment simulating conditions at the ISS and obtained many blastocysts (Figure S11; Table S13). Thus, space experiments can be conducted on the ISS using the ETCs and our protocol.
Launch of frozen embryos and experimentation at the ISS
Our project is named “Space Embryo.” We prepared frozen embryos on the ground for conducting the ISS experiment (Figures 1, 2A–C, and S12). We proposed to launch eight ETCs, containing 90 frozen two-cell stage mouse embryos/ETC, to the ISS. The astronauts would thaw the embryos of eight ETSs continuously. Four ETCs would be incubated in μ g and the other four under artificial-1 g conditions (created by rotating the samples inside an incubator) for 4 days. Simultaneously, four ETCs, preserved at the Tsukuba Space Center, Japan, Earth, would be thawed at ground-1 gas control.
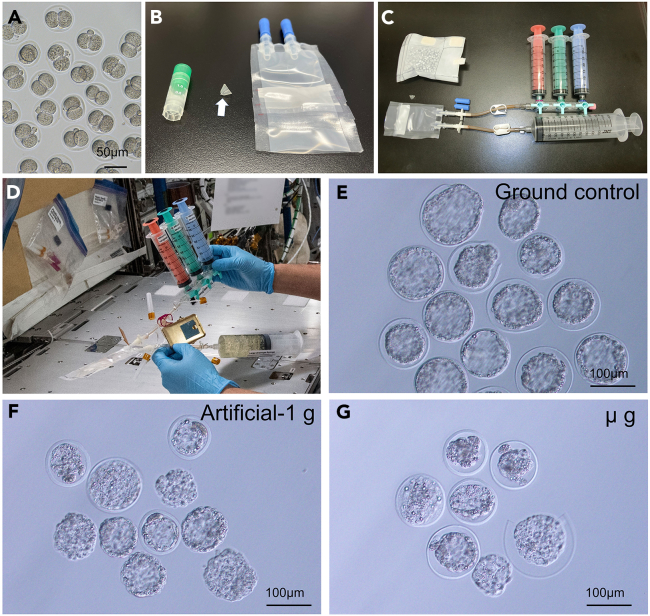
Figure 1.
Study outline
Diagram showing the timeline of this project. The dates are shown when the frozen embryo were prepared, lunched to the ISS, thawed and cultured on the ISS, fixed with PFA, returned to Earth, and the blastocysts were analyzed.
Figure 2.
The development of embryos to blastocysts under microgravity on the ISS
(A) Embryos at the two-cell stage before frozen.
(B) Embryos were frozen in a cryotube (V-tube: arrow) bottom and packed into the ETC in liquid nitrogen.
(C) The ETC will be connected to three syringes for solution replacement and a syringe for waste solutions.
(D) Thawing by astronaut under microgravity.
(E–G) Blastocysts collected from the ETC cultured on ground control (E), artificial-1G on the ISS (F), and microgravity on the ISS (G).
If the launch was postponed after the ETCs were loaded into a rocket, they would be replaced with a backup set stored in liquid nitrogen to shorten the storage period to −95°C. Five sets (each set containing 8 ETCs) were prepared for replacement in case the launch was postponed several times. The samples were transported from Japan to the USA using the dry shipping method (Figure S13).
The frozen embryos in the ETCs were launched to the ISS on August 28, 2021, by Space X 23 and transported to the ISS on August 29, 2021 (Figure 1). Because the launch was postponed by only 1 day, the ETCs did not require replacement. On September 4, 2021 (2 days before the start of the experiment on the ISS), the culture medium was placed in a carbon dioxide incubator for gas equilibration. On September 6, 2021, eight ETCs (with 90 embryos each) were thawed by an astronaut (Figure 2D and Video S1), and four ETCs were cultured in μ g while the other four ETCs were cultured in artificial-1 g31 for 4 days at 37°C (Figure S14). On September 10, 2021, 0.99% PFA was injected into the ETCs to fix the embryos, and PFA was replaced with PBS. The ETCs were refrigerated until their return to Earth. During solution exchange from medium to fixative on the ISS, some ETCs showed a slightly cloudy culture medium, when we were observing through live image from ISS, which suggested that the infection of bacteria occurred into the ETCs. However, bacterial infection could not be confirmed in the ETCs that returned to Earth, possibly because multiple solution changes (with PFA and PBS on the ISS) occurred in the ETC and washed out those bacteria from ETCs. All ETCs were recovered safely from the return vessel that splashed downed in Earth’s ocean on September 30, 2021 (Figure S15).
Three syringes are attached to the ETC in the cooling bag using a three-way stopcock. The blue syringe contains thawing solution, green syringe contains washing solution, and pink syringe contains the culture medium. After attaching the syringe for the waste fluid, remove the ETC from the cooling bag. Next, remove the air inside the ETC and slowly push the blue syringe to inject the thawing solution into the bag. Shake the ETC from side to side to mix the solution inside. After exchanging all solutions in this manner, the embryos in the ETC are cultured on the ISS for 4 days.
Dose of space radiation
The radiation dose received by the embryos during the experiment was measured with a biological dosimeter “PADLES” placed in a box containing the sample (Figures S14 and S16). The average space radiation exposure during the total stay on the ISS was 0.33 mGy (0.55 mSv) per day. After the ETCs were launched with frozen embryos to the ISS, they were preserved for 9 days in the freezer before the start of experiments. The embryos were thawed and cultured for 4 days. Therefore, total exposure time of embryos to space radiation was 13 days, and the total dose that the embryos were exposed to was 4.29 mGy (7.15 mSv) at the end of the experiment (Figure S16 and Table S14). The effects of space radiation during cryopreservation (9 days) and culturing of the live embryos (4 days) may be different on embryo development, but we were unable to examine it separately.
Embryo development under microgravity on the ISS
As shown in Tables 1 and 134 embryos (37.2%) were recovered from the ETCs at ground-1 g control. For the 1 g control (artificial-1 g) and μ g experiment, 61 (16.9%) and 72 (20.0%) embryos were recovered, respectively, which are significantly less than those for ground-1 g. Some embryos died before PFA fixation or disappeared, and only zona pellucida was collected (Figure S17). Embryos that stopped development at the two-cell stage were 5–6 times more common under artificial-1 g and μ g conditions than at the ground-1 g (47.5%, 44.4%, and 8.2%, respectively). The number of embryos that formed blastocysts was 82 (ground-1 g: 61.2%), 19 (artificial-1 g: 29.5%), and 17 (μ g: 23.6%), respectively (Table 1; Figures 2E–2G). Although the rate of formation of blastocysts was significantly lower in both artificial-1 g and μ g experiments than at ground-1 g, two-cell mouse embryos could develop blastocysts in microgravity on the ISS. Because ground-1 g control cannot mimic the space experiment not only microgravity but also some factors (such as convection of liquid, differences in the experimenter, and vibration/hypergravity during launch), it is possible that those factors may cause higher embryo development of the ground-1 g.
Table 1.
In vitro development of vitrified 2-cell embryos cultured on the ISS
| Place | Gravity | No. of embryos vitrified | No. (%) of embryos retrieved | No. (%) of embryos developed to |
||||
|---|---|---|---|---|---|---|---|---|
| Dead/Zona only embryosa | 2-cell | 3- 4-cell | 8-cell | Blastocyst | ||||
| Earth | 1 g | 360 | 134 (37.2) | 26 (19.4) | 11 (8.2) | 9 (6.7) | 6 (4.5) | 82 (61.2) |
| Space | Artificial-1 g | 360 | 61 (16.9) | 5 (8.2) | 29 (47.5) | 6 (9.8) | 2 (3.3) | 19 (31.1) |
| μ g | 360 | 72 (20.0) | 8 (11.1) | 32 (44.4) | 10 (13.9) | 5 (6.9) | 17 (23.6) | |
No significant difference between methods; one-way ANOVA test.
Developmental rate was calculated against embryos retrieved.
This includes cases where a portion of the embryos is found.
First cell differentiation
The blastocysts were divided into three groups: 1) to analyze the cell numbers in the ICM cells and outer epithelial TE cell layer; 2) to detect DNA damage in the cells; and 3) for Next Generation Sequencing (NGS) analysis (Table S15). The ICM and TE cells could be visualized in all blastocysts, including the μ g-cultured embryos, using Nanog and CDX2 antibodies (Figures 3A–3D). When the number of ICM cells was examined (Figure 3I; and Table S16), no difference was observed between the ground-1 g control and μ g experiment (both contained 5.9 cells/blastocyst). Although the number of TE cells was fewer in μ g (58.0 cells) than in ground-1 g (69.9 cells), the total number of cells in the blastocysts was also lower in the μ g experiment than in ground-1 g; thus, the proportion of TE cells of the μ g-derived blastocysts was similar to that of ground-1 g. Therefore, microgravity did not affect the differentiation of embryos into ICM and TE cells. In the artificial-1 g on the ISS, the numbers of ICM and TE cells were 3.1 and 49.5, respectively, which are lower than those in ground-1 g and μ g. This may be due to adverse effects of the artificial-1 g created by the rotation on the ISS.
Figure 3.
The quality of blastocysts developed in microgravity on the ISS
(A–D) ICM and TE cells were immunostained using Nanog and Cdx2 antibodies, respectively. The blastocysts were obtained from ETCs cultured on the ground (A), artificial-1 g (B), and μ g (C, D) on the ISS. One blastocyst from μ g showing ectopic expression of NANOG (D). Nanog-positive cells (ICM) are in red, and CDX2-positive cells (TE) are in green. All images are shown by z stack, except (D). The numbers of ICM cells (red bar), TE cells (green bar), and the total cell (blue) are shown in (I). Data are represented as mean ± SEM.
(E–H) γ-H2A.x-positive cells and ICM cells were immunostained. Blastocysts were obtained same as above. (H) A blastocyst with ectopic expression of NANOG. Embryonic nuclei were detected using DAPI (blue). Nanog-positive cells are in red, and γ-H2A.x-positive cells are in green.
(J) The rate of blastocysts with ectopic expression of NANOG was compared between 1 g control cultured in a dish (n = 135), ETC on Earth (n = 146), artificial-1 g (n = 12), and μ g (n = 9) on the ISS.
(K) Principal-component analysis of expression profiles of 5 blastocysts for each of the three experimental groups, μ g, a-1 g, and ground, was analyzed by RNA-seq. No separation was observed in principa components 1 and 2. Individual blastocysts are in brown: ground; pink: artificial-1 g; and blue: μ g.
Interestingly, one of the seven blastocysts examined in the μ g experiment showed blastomeres with ectopic expression of NANOG in the blastocyst cavity (Figure 3D), whereas no such activity in the blastocysts was observed under artificial-1 g conditions.
DNA damage in the blastocyst
Immunostaining for γH2A.x and Nanog were performed to examine DNA damage in the cells and the localization of the ICM cells in the blastocyst cavity. Cells with a minimum of one γ-H2A.x focus were labeled γ-H2A.x-positive cells. No difference in γ-H2A.x-positive cell number was found between the ground-1 g (44%) and μ g experiment (46%) (Figures 3E–3H; Table S17). Although fewer blastocysts were examined, we concluded that microgravity and space radiation do not cause DNA damage in the blastocysts. By contrast, the number of γ-H2A.x-positive cells was reduced in the artificial-1 g experiment compared with the others; however, only four blastocysts were examined. Notably, two of the five μ g-cultured blastocysts showed blastomeres with an ectopic expression of NANOG (Figure 3H).
Blastocysts with ectopic expression of NANOG
In total, we found that three of the 12 (25%) blastocysts had blastomeres with ectopic expression of NANOG in the microgravity experiment on the ISS (Figures 3D, 3H, S18, and S19). We performed two additional ground-1 g experiments to investigate whether this was because the embryos were frozen using ETCs. We examine 157 blastocysts obtained by thawing, culturing, fixing, and washing embryos from four ETCs and found 11 (7.0%) blastocysts with ectopic expression of NANOG cells. This is comparable to the dish-cultured controls (9/144 blastocysts, 6.2%). Thus, the use of ETCs for culturing did not affect the cluster formation of the ICM cell (Figure 3J; Table S18).
Probability of ICM cells being present at the bottom of the blastocyst
To determine the possibility that ICM cells gathered at the bottom of the blastocyst cavity due to gravity, we examined whether the ICM cells are located at the upper, middle, or lower positions in the cavity of intact blastocysts. We selected 10 expanded and unhatched blastocysts and dropped them from the top of the medium onto the dish. After all the blastocysts had sunk and attached to the bottom of the dish, the location of the ICM cells was examined using an inverted microscope; this process was repeated 10 times. Although the localization of ICM cells before dropping the blastocyst could not be observed, it is assumed that the localization of ICM cells at the time of drop from the pipette was random because the blastocyst entered the pipette while rotating. In 89% of the blastocysts, the ICM cells were located at the bottom, whereas in the remaining 11 cells (11%), the ICM cells were located in the middle. None of the ICM cells were located on the upper side of the blastocyst (Table S19). Thus, the ICM cells localize to the bottom of the blastocyst possibly due to gravity.
Next-generation sequencing analysis
Finally, to detect differences between the blastocysts derived from ground-1 g, artificial-1 g, and μ g, we analyzed the global gene expression profile of the blastocysts (Figure S20) using RNA sequencing (RNA-seq) analysis. Relatively well-developed blastocysts were selected, RNA was extracted individually from single blastocyst, and RNA-seq was performed. All blastocysts showed reads that were probably derived from nucleic acids of bacterial origin, suggesting bacterial contamination. There were variations in the mapping rate of the mouse genome to the reference sequence among the analyzed samples, confirming the contamination. Because the development of blastocysts was favorable, RNA-seq data for the three groups were compared to search for differences in gene expression. There were 13 genes that were differentially expressed in the μ g experiment compared with ground-1 g and/or artificial-1 g (Table S20). However, these genes were not significant difference and were either overexpressed or not expressed compared with the ground and artificial-1g controls, which indicate no common trend (Figure S21). Comparison using principal-component analysis (Figure 3 K) showed that the artificial-1 g (red dots) and ground-1 g (brown dots) embryos seemed to show more variation than the μ g (blue dots) embryos, and ground-1 g and μ g embryos appeared to be slightly separated. However, the provability ellipse of the three groups overlapped overall, and no apparent differences were observed.
Embryo development under simulated microgravity conditions on the ground
A simulated microgravity (artificial-μ g) experiment was also conducted using a 3D clinostat on the ground to compare its findings with those of the space experiment (Figure S22). When 2-cell stage embryos were frozen, thawed, and cultured for 4 days, similar to the ISS experiment, the developmental rate to the blastocyst stage was found to be 67.1% in the artificial-μ g experiment, which was significantly lower than that of the embryos cultured under control 1 g conditions without using clinostat (82.4%) (Table S21). Similarly, when experiments were conducted using fresh, non-frozen 2-cell stage embryos, the embryos reached the blastocyst stage within 3 days, showing a high development rate even in artificial-μ g conditions (90.9%), which was comparable to the growth of embryos cultured under 1 g control conditions (100%) (Table S21). There was no difference in the number of ICM cells observed between 1 g and artificial-μ g conditions (7.2 cell and 6.8 cells, respectively), but the number of TE cells was slightly less in the artificial-μ g (40.1 cells) than in the 1 g conditions (44.4 cells) (Table S22). However, when experiments were conducted using fresh, non-frozen 2-cell stage embryos, there were few differences between the 1 g and artificial-μ g conditions with respect to the number of both ICM and TE cells (Table S22). The ectopic expression of NANOG in the blastomeres was observed in both the 1 g (6.8%) and artificial-μ g (7.2%) cultured blastocysts.
Discussion
Here we studied, for the first time, the impact of microgravity on embryo development, exploring both μ g and artificial-1 g conditions experienced on the ISS, and compared our findings with similar experiments performed on Earth. This study clearly demonstrated that the mammalian embryos can develop to blastocyst and differentiate into ICM and TE cells even in the absence of gravity.
When launching living embryos, in addition to the waiting time between loading and rocket launch, the embryos were exposed to strong vibrations and hypergravity during the rocket launch. To overcome this limitation, we developed the new device “ETC” for thawing and culturing embryos on the ISS by astronaut. The ETC is the first device that allows the entire process to be performed on the ISS by untrained astronauts. However, despite taking into account all the conditions expected in space experiments, the ETC performed well on the ground, but many embryos in the ETC died or stopped developing at the two-cell stage. We believe that the cryoprotectant agent, which is toxic to the embryos, was not washed out from the ETCs smoothly, thereby causing cytotoxicity. However, because some embryos developed into blastocysts, the lack of liquid convection likely created different culture areas in the same ETC—some where the cryoprotectant remained and others, where it was cleaned out. In addition, although the embryos could develop into blastocysts, bacterial contamination occurred in all ETCs. This must have been a significant factor in the decreased rate of development of the blastocyst. We determined these results as potential limitations and took steps to address them, such as shaking the ETCs during solution exchange (Table S8). However, experiments on the ISS are much more complex than we had anticipated. Moreover, sterile operations are impossible on the ISS because alcohol disinfection on the bench is not allowed. Despite the challenging conditions, the astronauts were able to produce blastocysts through this project and we were able to analyze them.
Recently, Lei et al. pointed out the possibility that space radiation may have affected the development of embryos in space experiments. They cultured embryos at an orbital altitude of ∼252 km32 and found that the exposure to space radiation (∼0.15 mGy/d, 64 h of incubation; <0.4 mGy in total) reduced the rate of blastocyst development and resulted in severe DNA damage and epigenetic abnormalities. However, despite the test embryos being exposed to a 10-fold higher dose of space radiation (4.29 mGy), the quality of the blastocysts that were developed under the space and ground-1 g conditions was found to be comparable in this study. Although space radiation could not entirely be replicated in the ground-based irradiation experiment, the ground studies demonstrated that the early-stage embryos are radiosensitive. Moreover, the lethal dose 50 (LD50) of the two-cell mouse embryos is 300 mGy or higher.33,34 We are doubtful whether 0.4 mGy (used by Lei et al.) or 4.29 mGy (used in our study) of space radiation was the reason for the poor survival and development rate of space experiment groups.
Previously, several ground-based simulated microgravity experiments using a clinostat have reported developmental arrest of embryos and a decrease in the cell number of TE cell, suggesting that microgravity may have a detrimental effect on early embryo development.25,26,27 However, in this study, we clearly show that differentiation into ICM and TE cells is possible under real microgravity on the ISS. Additionally, similar result was observed by Lei et al., who performed an orbital experiment.32 The negative effect on embryonic development derived from the clinostat experiment was probably caused by the rotational culture induced by the clinostat rather than being solely attributed to the effect of microgravity. When embryos were cultured on the ISS, it was observed that the artificial-1 g generated by rotation resulted in a lower number of TE cells compared with μ g.
Maitre et al. reported that coupling of cell positioning and fate specification via contractility enables blastomeres to anticipate their final position and initiate their differentiation accordingly. If the Earth’s gravity exerted a positional and even slight tension to the embryos, it may impact the cell position and initial differentiation under microgravity. Interestingly, when embryos were cultured in μ g on the ISS, blastocysts with ectopic expression of NANOG cell were found in 3 of 12 (25%) embryos, which is a higher incidence rate than that on artificial-1 g (0%) or ground-1 g embryos (7%). Additionally, our ground base experiment suggests that the ICM cell is heavier than other cells and sinks to the bottom of the blastocyst cavity (Table S19). In ISS, where there is approximately zero gravity, the ICM cell would not sink or cluster at one location in the blastocyst cavity. In this study, it is unclear whether the ectopically expressed Nanog-positive cells were ICM cells or not; however, if they were ICM cells, they may tend to cluster less in one place in microgravity. When the ICM cell is separated into two pieces, identical twins are formed that share the placenta.21 During armadillo reproduction, the ICM cell is split into four, resulting in the birth of monozygotic quadruplets. However, it is important to note that the separation of the ICM cell is a rare occurrence in mammalian reproduction.21,22,35 In such cases, it increases the burden on both fetuses and mothers, with a higher risk of miscarriage, especially for single-gestation species. Because mammals evolved in a 1 g environment on Earth, it is likely that they use Earth’s gravity for safe pregnancy and delivery.
It is known that microgravity during space flight causes subtle abnormalities in the fertilization and embryonic development of sea urchins and amphibians.7,8,12 However, mating, fertilization, and hatching of Medaka fish during an orbit experiment resulted in offspring with apparently normal ovaries and fertility.36 Additionally, space flight in mice during mid-to-late gestation of mice caused modest effects on the birth rate, litter size, birth weights, and neonatal mortality.37,38 Based on these reports and our results, perhaps mammalian space reproduction is possible, although it may be somewhat affected. Unfortunately, the number of blastocysts obtained from the ISS experiment was not abundant; and we have not been able to confirm the impact on offspring because we have not produced offspring from embryos developed in space. We believe that the ETC will allow blastocysts to be frozen on the ISS if a cryoprotectant is used in place of PFA. Then, the frozen blastocysts could be brought back to Earth for transfer to a female recipient, and the viability of the blastocysts could be evaluated. Moreover, we could design a device to launch frozen oocytes and spermatozoa to the ISS, where in vitro fertilization experiments could be performed in microgravity. The use of this approach would be cheaper. Furthermore, the study of mammalian reproduction in space is essential to start the space age, making it necessary to study and clarify the effect of space environment before the ISS is no longer operational.
Limitations of the study
The limitations of this study are that only one experiment could be conducted because it was a space experiment on the ISS, and the number of blastocysts obtained from the ISS was small. Additional experiments should be conducted based on these results.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 568 | Thermos Fisher | A-11011; RRID: AB_143157 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | Thermos Fisher | A-11001; RRID: AB_2534069 |
| Anti-phospho-H2A.X (Ser139) Antibody | Millipore-Merck | 07-164; RRID: AB_11213838 |
| anti-CDX2 mouse monoclonal antibody | Proteintech Group, Inc. | 60243-1-Ig |
| anti-Nanog rabbit polyclonal antibody | Abcam, Cambridge, UK, | ab80892; RRID: AB_2150114 |
| Chemicals, peptides, and recombinant proteins | ||
| 4′,6-Diamidine-2′-phenylindole dihydrochloride | Roche | 10236276001 |
| Critical commercial assays | ||
| PicoPure RNA Isolation Kit | ThermoFisher Scientific, MA, USA | KIT0204 |
| SMART-seq Stranded kit | Takara Bio, Shiga, Japan | 634442 |
| Experimental models: Organisms/strains | ||
| B6D2F1 female mice, 10-12 weeks of age | SLC Inc. | B6C3F1/Slc |
| B6D2F1 male mice, 10-12 weeks of age | SLC Inc. | B6C3F1/Slc |
| ICR female mice, 8-10 weeks of age | SLC Inc. | Slc:ICR |
| ICR male mice, 10-12 weeks of age | SLC Inc. | Slc:ICR |
| Software and algorithms | ||
| DESeq2 package | Wang et al., 201045 | N/A |
| bowtie2 | Langmead et al., 200944 | N/A |
| Bioconductor package “DEGseq” | Love et al., 201449 | N/A |
| Cluster 3.0 | de Hoon et al., 200446 | N/A |
| Deposited data | ||
| DNA Data Bank of Japan Sequence Read Archive | This paper | accession number: PRJDB14277 |
| Other | ||
| Bio PADLES (TLD/CR39) monitoring devices | Fukuvi Chemical Industry, Co. Ltd., Fukui, Japan | N/A |
| ETC | Wakayama et al., 202228 | N/A |
| CultiLife™ Culture bag) | NIPRO&JAXA | N/A |
| V-tube (Cryotube | SUMITOMO BAKELITE CO.LTD Wakayama et al., 202228 | MS-4601 |
| 3D clinostat (Portable Microgravity Simulator) | Advanced Engineering Services Co., Ltd. (AES) | PMS-CSTI |
Resource availability
Lead contact
Further information and requests for reagents should be directed to and will be fulfilled by the lead contact, Teruhiko Wakayama (twakayama@yamanashi.ac.jp)
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Animals
The SLC Inc. (Hamamatsu, Japan) provided the female and male BDF1 mice. ICR mice were bred at our mouse facility. For embryo collection, female mice were aged 8–10 weeks. Surrogate pseudopregnant ICR females, which would be embryo-recipients, were mated with vasectomized ICR males, which were sterile. On the day of the experiment or upon completion of all experiments, the mice were euthanized using CO2 inhalation or cervical dislocation. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee of Laboratory Animal Experimentation of the University of Yamanashi (reference number: A29-24), which follow the ARRIVE guidelines.
Method details
Media
The HEPES-CZB (H-CZB) medium39 was used for embryo collection from the oviduct and CZB medium40 was used for embryo incubation in 5% CO2 at 37°C. Solutions EFS20 and EFS42.5 for vitrification; 0.25- and 0.75-M sucrose-PBI (S-PBI) for thawing41 were the vitrification solutions used for the HOV method. Equilibrated CZB (eCZB) medium29 was prepared 1 day before the start of the experiment by the astronaut, as previously described.29
Preparation of in vivo fertilized embryos
Female mice were induced to superovulate by injection with 5 IU of equine chorionic gonadotropin (eCG). After 48 h, the mice were injected with 5 IU of human chorionic gonadotropin (hCG). Immediately after, each female mouse was mated with a male mouse. The mated female mice were examined the following day for vaginal plug presence and separated from the males. The next afternoon, two-cell embryos were recovered by flushing the oviducts with H-CZB medium, as previously described.30 The collected embryos were placed in CZB medium and incubated at 37°C with 5% CO2 until use.
Vitrified two-cell mouse embryos preparation of for launch
The vitrification method for two-cell mouse embryos has been described.42 Briefly, 30 embryos were suspended in the EFS20 equilibrium solution for 2 min and then transferred into the V-tube (part of the cryotube that we processed) containing 50 μL of EFS42.5. After 1 min, the V-tube was soaked in LN2, where it was stored until the ETCs were assembled.
Assembly and ETCs preservation
ETC assembly was as described.28 Three V-tubes and small cryotube pieces were first inserted into the empty ETC on the LN2 gas layer. The ETC open side was pulled out of LN2 minimally and its sides were heat-sealed. The ETC was placed back into LN2. Each ETC contained 90 embryos (30 embryos in one V-tube × 3). The four ETCs were used for the μG experiment and four ETCs for the artificial-1G experiment at the ISS (Figure 1). A total of five sets (one set: 8 ETCs; total 40 ETCs) were produced as a backup if launch was postponed and preserved in LN2 until use. The four ETCs were used at the Tsukuba Space Center, Japan, as ground-1 g.
At the time of embryo freezing in V-tubes and at the time of ETC assembly, a lot-check was performed to examine the completion degree on the day, because this was an expensive space experiment where failure was not an option. Therefore, >100 ETCs were prepared for preliminary studies, rehearsals, and lot-checks.
Launching of ETCs by space X23 and embryo culture experiments at the ISS
The five sets of 40 ETCs were sent to NASA, Florida, using dry shippers at liquid nitrogen temperature. At 3 days before launch, one set of ETCs was transferred to a −95°C freezer and the freezer was loaded onto Space X23. ETCs were stored at −95°C when loaded onto the rocket, so frozen embryos cannot be stored for long time at this temperature. In the event of postponement of launch, the ETCs were to be replaced by another back up ETCs that was stored in liquid nitrogen on the ground. Space X23 was launched on August 29, 2021, just one day behind schedule. Therefore, the ETC was not replaced with a backup ETC stored in liquid nitrogen. After arriving at the ISS, the astronauts moved the freezer from the rocket to the ISS. The culture medium, thawing solution, and syringes to be used were also transported to the ISS in the same rocket at room temperature or under refrigeration. On September 4, 2021, the astronauts placed the medium in a CO2 incubator to initiate temperature and CO2 gas pressure equilibration. On September 6, 2021, the astronauts removed eight ETCs from the freezer one at a time and switched solutions using syringes in the following order: thawing solution, washing solution, and culture medium (Video S1), as previously described.28 The ETCs containing the culture medium were alternately divided into μ g group and artificial-1 g group, so total four ETCs were directly placed in an incubator as μ g experiment, and other were rotated in an incubator to generate artificial-1 g, then cultured at 37°C for 4 days. Simultaneously, four ETCs were thawed and cultured as ground-1 g controls at Tsukuba JAXA in Japan. On September 10, 2021, all ETCs were removed from the incubator. The medium was replaced with 0.99% PFA and 1–2 h later, 0.99% PFA was replaced with PBS. Finally, the ETCs were refrigerated. On September 30, 2021, the ETCs were retrieved on a return rocket to Earth and transported to the University of Yamanashi to initiate analysis.
Collection of embryos from ETCs
The ETCs’ end was cut and the contents were transferred to a 10-cm dish (Video S2). The ETC was washed two times with PBS and its contents were transferred to the same dish. The embryos were collected from the dish using a stereomicroscope and refrigerated until use.
Cut off the end of the ETC and transfer all the solution (PBS) inside the dish. Collect the embryo from the dish under a stereomicroscope.
ICM and TE cells number examination in blastocysts using immunostaining
Cell numbers were examined using immunofluorescence staining, as described5 to evaluate the quality of the blastocysts derived from the space experiments. The anti-CDX2 mouse monoclonal antibody (1:500; BioGenex, San Ramon, CA, USA, MU392A-UC) to detect TE cells and anti-Nanog rabbit polyclonal antibody (1:500; Abcam, Cambridge, UK, ab80892) to detect the ICM cells were the primary antibodies used. The Alexa Fluor 488-labeled goat anti-mouse IgG (1:500; Molecular Probes Inc., Oregon, USA, A11004) and Alexa Fluor 568-labeled goat anti-rabbit IgG (1:500 dilution; Molecular Probes) were the secondary antibodies used. DNA was stained with DAPI (2 μg/mL; Molecular Probes). In some case (Figure S11B), the secondary antibodies, Alexa 488 and 568, were used to identify the ICM and TE cells, respectively.
DNA damage observation in blastocysts using γH2A.x immunostaining
PBS–polyvinyl alcohol (0.1 mg/mL PVA, Sigma-Aldrich, St Louis, MO) for 10 min was used to wash the blastocysts three times and then stored overnight at 4°C in PBS supplemented with 1% (w/v) bovine serum albumin (BSA/PBS; Sigma-Aldrich) and 0.1% (v/v) Triton X-100 (Nacalai Tesque, Inc., Kyoto, Japan). The following procedures were previously described in.43 Primary anti-phospho-H2AX (Ser139) mouse monoclonal antibody (1:500; Millipore-Merck, Darmstadt, Germany) and anti-Nanog rabbit polyclonal antibody (1:500; Abcam, Cambridge, UK, ab80892) were used to detect ICM cells. The secondary antibodies used were Alexa Fluor 488-labeled goat anti-mouse IgG (1:500, Molecular Probes, Eugene, OR, USA) and Alexa Fluor 568-labeled goat anti-rabbit IgG (1:500 dilution; Molecular Probes). DNA was stained with 4′6-diamidino-2-phenylindole (DAPI; 2 μg/mL; Molecular Probes). In some cases (Figure S11C), the second antibodies, Alexa 568 was used to identify the γH2AX foci.
Embryo development under simulated microgravity on the ground
Frozen 2-cell stage embryos were thawed and placed in 5-mL plastic tubes (Assist tube, 60.9921.530S, Sarstedt K.K., Tokyo, Japan) filled with CO2 gas pressure equilibration medium as described above. The lid was then tightly closed and sealed with Parafilm. Some plastic tubes were taped to the 3D-Clinostat (PMS-VII, Advanced Engineering Services Co., Ltd, Japan) tray (artificial-μ g) and the other tubes were placed next to the clinostat as controls (clino-1 g) and cultured for 4 days. The same experiment was also conducted with fresh, unfrozen embryos, but culture period comprised 3 days because of the rapid development of the embryos to blastocysts. At the end of embryo culture, blastocysts were collected from the plastic tubes and, as in other experiments, their developmental rate into blastocysts was examined, and the cell number of ICM and TE cells, rate of ectopic expression of NANOG were determined by immunostaining.
ICM cells position observation in intact blastocysts
The blastocysts percentage was evaluated with ICM cell positioned at the bottom. The 10 blastocysts were selected that were as round as possible because an irregularly shaped blastocyst would affect the ICM cell position. The 10 blastocysts were then dropped separately from the top of the medium on the dish covered by mineral oil, shaking the dish slightly, and waiting for the blastocysts to reach the bottom. Once all blastocysts had fallen to the bottom, the upper, middle, and lower portions of the blastocysts were observed under an inverted microscope with varying focus. This measurement was repeated 10 times using the same blastocysts.
RNA-seq analysis
RNA-seq analysis was performed as previously described.5 Briefly, five blastocysts each from ground-1 g, artificial-1 g experiment, and μ g experiment were examined using RNA-Seq. The PicoPure RNA Isolation Kit (Arcturus, CA, USA) was used to purify the total RNA from single blastocyst, following the manufacturer’s instructions with slight modifications. For reverse-crosslinking of RNA, each blastocysts sample was placed in the 0.2 mL microtubes with 1 μl PBS. A 1 μl of Proteinase K solution consisting of 0.1 % polysorbate 20, 0.1 % mercaptoethanol, and 80 U/ml Proteinase K, Molecular Biology Grade (New England Biolabs, MA, USA) in the PBS was added and incubated at 50°C for 15 min. Then, 10 μl of PicoPure kit extraction buffer and incubated at 70°C for 30 min to reverse-crosslink the formaldehyde. The library for RNA-seq studies was prepared using the SMART-seq Stranded kit (Takara Bio, Shiga, Japan). The RNA-seq library was sequenced for 75-base single-end RNAs using the Illumina miniSeq system (Illumina, San Diego, CA, USA). The sequence data were mapped against the mouse reference genome sequence (GRCm38/mm10) using bowtie2,44 followed by a calculation of the “reads per kilobase of exon per million mapped reads (RPKM)” value for each gene using the Bioconductor package “DEGseq”.45 Clustering analysis and principal component analysis (PCA) were performed using “Cluster 3.0”.46
Embryo transfer
To ensure that the embryos thawed and cultured in the ETC were normal, embryo transfers were performed to determine if they could develop into offspring. Embryos at the two-cell stage collected from the ETC just after thawing, morulae or blastocysts collected after 3 days of culture or blastocysts collected after 4 days of culture, were transferred into the oviduct on day 0.5 or uteri on day 2.5 of the pseudopregnant ICR mice that had been mated with vasectomized males.47 Hence, five to eight embryos were transferred into each oviduct. On day 18.5, the offspring were delivered by cesarean section and allowed to mature.
Space radiation dosimetry
The Bio PADLES (TLD/CR39) monitoring devices (Fukuvi Chemical Industry, Co. Ltd., Fukui, Japan) were used to measure the radiation dosages. These included CR-39 PNTDs. They were placed inside the space or ground control sample cases. We took pictures of the radiation-etched pits that reflected the tracks of the atomic nuclei that had accumulated during the space flight (Figures S14 and S15; Table S14) and measured the TLD brightness, which increased after receiving radiation energy and used these values to calculate the total radiation dose.48
Quantification and statistical analysis
All embryos were recovered from BDF1 female mice that were approximately the same age, and the technical influences during embryo freezing and ETC assembly were eliminated by checking the lot. However, since the thawing of the embryos was performed by astronauts who had no experience in this area, there may be a difference between the first half of the thawing process, which is performed for the first time, and the second half, which is more familiar. Therefore, of the four ETCs thawed in the first half, two were cultured in μ g and the other two in artificial-1 g. The second half was performed in the same manner to reduce the technical differences that may affect the embryo thawing results.
The survival rates and development rates were evaluated using chi-squared tests, unpaired t-test, or One-way ANOVA. The number of cells was evaluated using One-way ANOVA. The Tukey-Kramer test was used for multiple comparisons. Statistically significant differences between the variables were determined at p-values <0.05. RNA-seq analyses were performed using the Wilcoxon–Mann–Whitney nonparametric test adjusted for a false discovery rate. Differentially-expressed genes between the space and control pups were statistically analyzed using the DESeq2 package49 with the raw sequence read count.
Acknowledgments
We thank Drs M. Shirakawa, S. Ogawa, Y. Fujimoto, Mr. M. Nakamura, Mrs. Y. Kanda, and C. Yamaguchi for providing critical comments on the study. We would also like to ardently thank our Astronaut, Mr. Akihiko Hoshide, for his enormous efforts. This work was partially funded by the grants from Challenging Researchers for the Next Generation (VUCA) to Y.Kikuchi; JST, the establishment of university fellowships toward the creation of science technology innovation to L.L.Y. (JPMJFS2117); the Naito Foundation (S.W.), Takahashi Industrial and Economic Research Foundation (189), and the Japan Society for the Promotion of Science (23K08843) to S.W.; the Japan Society for the Promotion of Science (23K18124), Asada Science Foundation and the Canon Foundation (M20-0008) to T.W.
Author contributions
S.W. and T.W. conceived and designed the study. S.W., Y.Kikuchi, M.S., E.H., N.U., C.Y., T.Suzuki., T.Shimaz., T.Y., I.O., H.S., M.U., A.Hasegawa, K.M., L.Y., R.E., K.K., K.I., Y.Kurokawa., Y.S., A.Higashibata, H.M., H.N., A.O., T.K., and T.W. performed experiments, analyzed the data, and interpreted the results. S.W. and T.W. wrote the manuscript. All authors read and edited the manuscript.
Declaration of interests
Patent Publication: May 5, 2023 “Frozen egg cultivation apparatus, and method for cultivating frozen eggs” Teruhiko Wakayama, Sayaka Wakayama, Tomomi Suzuki, Chiaki Yamazaki. International application number: PCT/JP2021/027820. International publication number: WO2022/025092.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: October 27, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108177.
Contributor Information
Sayaka Wakayama, Email: sayakaw@yamanashi.ac.jp.
Teruhiko Wakayama, Email: twakayama@yamanashi.ac.jp.
Supplemental information
Date and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request. The accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Mishra B., Luderer U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019;15:713–730. doi: 10.1038/s41574-019-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakayama S., Ito D., Kamada Y., Shimazu T., Suzuki T., Nagamatsu A., Araki R., Ishikawa T., Kamimura S., Hirose N., et al. Evaluating the long-term effect of space radiation on the reproductive normality of mammalian sperm preserved on the International Space Station. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg5554. eabg5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumura T., Noda T., Muratani M., Okada R., Yamane M., Isotani A., Kudo T., Takahashi S., Ikawa M. Male mice, caged in the International Space Station for 35 days, sire healthy offspring. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50128-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida K., Fujita S.I., Isotani A., Kudo T., Takahashi S., Ikawa M., Shiba D., Shirakawa M., Muratani M., Ishii S. Intergenerational effect of short-term spaceflight in mice. iScience. 2021;24 doi: 10.1016/j.isci.2021.102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakayama S., Kamada Y., Yamanaka K., Kohda T., Suzuki H., Shimazu T., Tada M.N., Osada I., Nagamatsu A., Kamimura S., et al. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc. Natl. Acad. Sci. USA. 2017;114:5988–5993. doi: 10.1073/pnas.1701425114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijiri K. Ten years after medaka fish mated and laid eggs in space and further preparation for the life-cycle experiment on ISS. Biol. Sci. Space. 2004;18:138–139. [PubMed] [Google Scholar]
- 7.Aimar C., Bautz A., Durand D., Membre H., Chardard D., Gualandris-Parisot L., Husson D., Dournon C. Microgravity and hypergravity effects on fertilization of the salamander Pleurodeles waltl (urodele amphibian) Biol. Reprod. 2000;63:551–558. doi: 10.1095/biolreprod63.2.551. [DOI] [PubMed] [Google Scholar]
- 8.Schatten H., Chakrabarti A., Taylor M., Sommer L., Levine H., Anderson K., Runco M., Kemp R. Effects of spaceflight conditions on fertilization and embryogenesis in the sea urchin Lytechinus pictus. Cell Biol. Int. 1999;23:407–415. doi: 10.1006/cbir.1999.0371. [DOI] [PubMed] [Google Scholar]
- 9.Serova L.V. [Effect of weightlessness on the reproductive system of mammals] Kosm. Biol. Aviakosm. Med. 1989;23:11–16. [PubMed] [Google Scholar]
- 10.Tash J.S., Kim S., Schuber M., Seibt D., Kinsey W.H. Fertilization of sea urchin eggs and sperm motility are negatively impacted under low hypergravitational forces significant to space flight. Biol. Reprod. 2001;65:1224–1231. doi: 10.1095/biolreprod65.4.1224. [DOI] [PubMed] [Google Scholar]
- 11.Ubbels G.A., Berendsen W., Narraway J. Fertilization of frog eggs on a Sounding Rocket in space. Adv. Space Res. 1989;9:187–197. doi: 10.1016/0273-1177(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 12.Souza K.A., Black S.D., Wassersug R.J. Amphibian development in the virtual absence of gravity. Proc. Natl. Acad. Sci. USA. 1995;92:1975–1978. doi: 10.1073/pnas.92.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proshchina A., Gulimova V., Kharlamova A., Krivova Y., Besova N., Berdiev R., Saveliev S. Reproduction and the early development of vertebrates in space: problems, results, opportunities. Life. 2021;11 doi: 10.3390/life11020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strumpf D., Mao C.A., Yamanaka Y., Ralston A., Chawengsaksophak K., Beck F., Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 15.Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 16.Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schöler H., Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 17.Hirate Y., Hirahara S., Inoue K.I., Suzuki A., Alarcon V.B., Akimoto K., Hirai T., Hara T., Adachi M., Chida K., et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson M.H., Ziomek C.A. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka Y., Ralston A., Stephenson R.O., Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev. Dyn. 2006;235:2301–2314. doi: 10.1002/dvdy.20844. [DOI] [PubMed] [Google Scholar]
- 20.Maître J.L., Turlier H., Illukkumbura R., Eismann B., Niwayama R., Nédélec F., Hiiragi T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall J.G. Twinning. Lancet. 2003;362:735–743. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 22.Steinman G. The mechanism initiating and controlling monozygotic twinning in humans remains to be elucidated. Twin Res. 2000;3:337. [PubMed] [Google Scholar]
- 23.Keefe J.R., editor. Final report of the NASA mammalian developmental biology working group. NASA; 1985. [Google Scholar]
- 24.Nagy A., Gertsenstein M., Vintersten K., Behringer R. 3 Edition. Cold Spring Harbor Laboratory Press; 2003. Manipulating the mouse embryo; A Laboratory Manual. [Google Scholar]
- 25.Wakayama S., Kawahara Y., Li C., Yamagata K., Yuge L., Wakayama T. Detrimental effects of microgravity on mouse preimplantation development in vitro. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Xie Y., Wygle D., Shen H.H., Puscheck E.E., Rappolee D.A. A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK. Reprod. Sci. 2009;16:947–959. doi: 10.1177/1933719109337544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima Y., Sasaki S., Kubota Y., Ikeuchi T., Hayashi Y., Kohri K. Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil. Steril. 2000;74:1142–1147. doi: 10.1016/s0015-0282(00)01583-1. [DOI] [PubMed] [Google Scholar]
- 28.Wakayama S., Soejima M., Kikuchi Y., Hayashi E., Ushigome N., Hasegawa A., Mochida K., Suzuki T., Yamazaki C., Shimazu T., et al. Development of a new device for manipulating frozen mouse 2-cell embryos on the International Space Station. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi Y., Wakayama S., Ito D., Ooga M., Wakayama T. Optimised CO2-containing medium for in vitro culture and transportation of mouse preimplantation embryos without CO2 incubator. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi E., Wakayama S., Ito D., Hasegawa A., Mochida K., Ooga M., Ogura A., Wakayama T. Mouse in vivo-derived late 2-cell embryos have higher developmental competence after high osmolality vitrification and -80 degrees C preservation than IVF or ICSI embryos. J. Reprod. Dev. 2022;68:118–124. doi: 10.1262/jrd.2021-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida T., Sakashita Y., Kitahata K., Yamashita Y., Tomida C., Kimori Y., Komatsu A., Hirasaka K., Ohno A., Nakao R., et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2018;314:C721–C731. doi: 10.1152/ajpcell.00184.2017. [DOI] [PubMed] [Google Scholar]
- 32.Lei X., Cao Y., Ma B., Zhang Y., Ning L., Qian J., Zhang L., Qu Y., Zhang T., Li D., et al. Development of mouse preimplantation embryos in space. Natl. Sci. Rev. 2020;7:1437–1446. doi: 10.1093/nsr/nwaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honjo Y., Ichinohe T. Stage-specific effects of ionizing radiation during early development. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21113975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugh R., Duhamel L., Somogyi C., Chandler A., Cooper W.R., Smith R., Stanford G. Sequelae of the LD/50 x-ray exposure of the pre-implantation mouse embryo: days 0.0 to 5.0. Biol. Bull. 1966;131:145–154. doi: 10.2307/1539654. [DOI] [PubMed] [Google Scholar]
- 35.Blickstein I., Keith L.G. On the possible cause of monozygotic twinning: lessons from the 9-banded armadillo and from assisted reproduction. Twin Res. Hum. Genet. 2007;10:394–399. doi: 10.1375/twin.10.2.394. [DOI] [PubMed] [Google Scholar]
- 36.Ijiri K. Development of space-fertilized eggs and formation of primordial germ cells in the embryos of Medaka fish. Adv. Space Res. 1998;21:1155–1158. doi: 10.1016/s0273-1177(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 37.Ronca A.E., Alberts J.R. Physiology of a microgravity environment selected contribution: effects of spaceflight during pregnancy on labor and birth at 1 G. J. Appl. Physiol. 2000;89:849–854. doi: 10.1152/jappl.2000.89.2.849. 1985. [DOI] [PubMed] [Google Scholar]
- 38.Burden H.W., Poole M.C., Zary J., Jeansonne B., Alberts J.R. The effects of space flight during gestation on rat uterine smooth muscle. J. Gravitational Physiol. 1998;5:23–29. [PubMed] [Google Scholar]
- 39.Kimura Y., Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 40.Chatot C.L., Lewis J.L., Torres I., Ziomek C.A. Development of 1-cell embryos from different strains of mice in CZB medium. Biol. Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 41.Mochida K., Hasegawa A., Li M.W., Fray M.D., Kito S., Vallelunga J.M., Lloyd K.C.K., Yoshiki A., Obata Y., Ogura A. High osmolality vitrification: a new method for the simple and temperature-permissive cryopreservation of mouse embryos. PLoS One. 2013;8 doi: 10.1371/journal.pone.0049316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mochida K. Development of assisted reproductive technologies in small animal species for their efficient preservation and production. J. Reprod. Dev. 2020;66:299–306. doi: 10.1262/jrd.2020-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamada Y., Wakayama S., Shibasaki I., Ito D., Kamimura S., Ooga M., Wakayama T. Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-28896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Feng Z., Wang X., Wang X., Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 46.de Hoon M.J.L., Imoto S., Nolan J., Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 47.Ushigome N., Wakayama S., Yamaji K., Ito D., Ooga M., Wakayama T. Production of offspring from vacuum-dried mouse spermatozoa and assessing the effect of drying conditions on sperm DNA and embryo development. J. Reprod. Dev. 2022;68:262–270. doi: 10.1262/jrd.2022-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagamatsu A., Murakami K., Kitajo K., Shimada K., Kumagai H., Tawara H. Area radiation monitoring on ISS increments 17 to 22 using PADLES in the Japanese Experiment Module Kibo. Radiat. Meas. 2013;59:84–93. [Google Scholar]
- 49.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three syringes are attached to the ETC in the cooling bag using a three-way stopcock. The blue syringe contains thawing solution, green syringe contains washing solution, and pink syringe contains the culture medium. After attaching the syringe for the waste fluid, remove the ETC from the cooling bag. Next, remove the air inside the ETC and slowly push the blue syringe to inject the thawing solution into the bag. Shake the ETC from side to side to mix the solution inside. After exchanging all solutions in this manner, the embryos in the ETC are cultured on the ISS for 4 days.
Cut off the end of the ETC and transfer all the solution (PBS) inside the dish. Collect the embryo from the dish under a stereomicroscope.
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request. The accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.