Abstract
Loeys-Dietz syndrome (LDS) is a rare connective tissue disorder. Vessel tortuosity and aneurysms throughout the vasculature are unique to LDS. Aortic root enlargement is ubiquitous, with most patients undergoing root replacement at some point in their lifetime. Multiple vascular procedures are required to prolong life expectancy. We describe a staged hybrid approach to a 17-year-old patient with LDS presenting with ascending aorta, arch, and bilateral subclavian artery aneurysms and prominent tortuosity. Transposition of the left vertebral and subclavian arteries onto the common carotid artery was performed. Total aortic arch replacement with frozen elephant trunk extension into the descending thoracic aorta was performed as a second stage. Bilateral subclavian artery aneurysms were excluded with the use of a four-branched graft.
Keywords: Loeys-Dietz syndrome, Subclavian artery aneurysm, Frozen elephant trunk
Loeys-Dietz syndrome (LDS) was first described in 2005 and is characterized by a triad of hypertelorism, cleft palate or bifid/broad uvula, and arterial aneurysm/tortuosity.1 Patients with LDS are at high risk for aortic dissection and rupture at earlier ages and smaller aortic diameters compared with other connective tissue disorders.2 Aortic root dilatation is nearly universal and surgery is recommended at aortic diameters of >4 cm.3 Moreover, aneurysms in the arch branches, descending thoracic aorta, and abdominal aorta are key distinguishing features of LDS.
We describe a 17-year-old patient with LDS with prior valve-sparing root replacement who presented with enlarging residual ascending aorta, arch, and bilateral subclavian artery (SCA) aneurysms and prominent tortuosity. The patient underwent staged repair with transposition of the left vertebral artery and SCA onto the common carotid artery before total arch reconstruction with frozen elephant trunk (FET). Informed consent was obtained from the patient and her parents for the publication of this report and accompanying images.
Case report
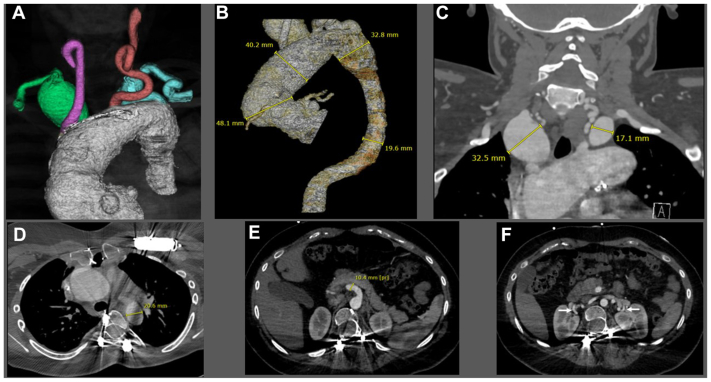
The patient is a 17-year-old female who was diagnosed with type 2 LDS (TGBR2 c.1379 G>A, p.Arg460His; heterozygote, missense, de novo variant) at birth. She underwent transcatheter Amplatzer occlusion of patent ductus arteriosus at 1 year of age and valve-sparing root replacement with a 24-mm Valsalva graft for a 3.4cm root aneurysm (20-mm aortic annulus) at age 3. She had three eye surgeries for strabismus (ages 3-5), spinal fusion for scoliosis (age 10), bilateral through-knee amputations after failed surgeries for clubfoot (age 11), placement of automatic implantable cardioverter defibrillator (age 12), and mechanical mitral valve replacement for prolapse (age 14). She has been maintained on an antihypertensive regimen of atenolol and losartan. She has been undergoing annual computed tomographic angiography (CTA) from the head to the pelvis (magnetic resonance angiography was contraindicated owing to defibrillator and spinal hardware) per clinical practice guidelines.3 She had normal cognitive function and no complaints. Relevant physical examination findings included dysmorphic facial features, marked hypertelorism, cleft palate, and bifid uvula. CTA indicated ascending aorta and arch (4.8 cm), right SCA (3.2 cm), and left SCA (1.7 cm) aneurysms (Fig 1, A-C). Bilateral SCA aneurysms were intrathoracic. There was severe tortuosity of the vertebral and carotid arteries; the vertebral tortuosity index was 183 (an index >50 is associated with an increased risk of adverse cardiovascular outcomes).4 The left vertebral artery was dominant. There were no intracranial aneurysms. The descending thoracic and abdominal aorta were of normal caliber (19-22 mm) (Fig 1, D). There was ectasia of the superior mesenteric artery and bilateral renal arteries were tortuous (Fig 1, E, F). Echocardiogram revealed normal valvular function.
Fig 1.
(A) Three-dimensional computed tomographic angiography (CTA) reconstruction of the aortic root and dilated ascending aortic aneurysm and arch. The right subclavian artery (SCA) aneurysm is denoted in green, the right common carotid artery in pink, left common carotid artery in red, and left SCA in blue. (B) Configuration and dimensions of the aortic arch and descending thoracic aorta. (C) Coronal CTA depicting bilateral SCA aneurysms. (D) Planned distal landing zone in the descending thoracic aorta for the frozen elephant trunk (FET) prosthesis. (E) Ectasia of the superior mesenteric artery. (F) Bilateral renal arteries demonstrate hairpin tortuosity (arrows).
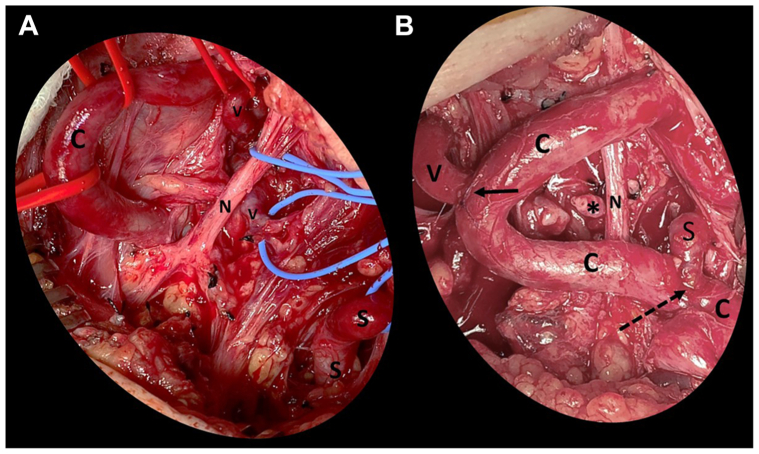
After multidisciplinary discussion, we decided on a staged approach with the primary indication for repair being the enlarging pseudoaneurysm at the prior aortic root repair and the enlarging native ascending aorta, arch, and right SCA aneurysms. She first underwent transposition of the left vertebral artery and SCA onto the left common carotid artery (Fig 2). Left transverse supraclavicular skin incision was made 2 cm superior to the clavicle. The clavicular head of the sternocleidomastoid muscle was divided. We extended the incision longitudinally along the anterior neck to enhance exposure, creating a hockey-stick incision. The common carotid artery was noted to be redundant with two hairpin turns in the area of exposure. The vertebral artery was dissected; it was fragile and tortuous. The scalene fat pad was reflected superiorly. The anterior scalene muscle was divided. The SCA was exposed and was also redundant. The proximal vertebral artery was ligated. The distal end of the vertebral artery was anastomosed to the side of the common carotid artery using 7-0 pledgeted Prolene suture. The SCA was ligated distal to the aneurysm. The thyrocervical trunk was ligated off the aneurysm. The distal SCA was anastomosed to the side of the common carotid artery 5 cm proximal to the vertebral anastomosis using 6-0 Prolene sutures. Both the vertebral and SCA anastomoses were situated within one hairpin turn of the common carotid artery.
Fig 2.
Intraoperative photographs of the first stage of repair: left vertebral and left subclavian artery (SCA) transposition onto the left common carotid artery. (A) Exposure of the left common carotid (C), vertebral artery (V), and SCA (S). The vagus nerve is identified and preserved (N). The head is oriented toward the top of the photo; the torso towards the bottom. The exposed arteries demonstrate severe tortuosity. (B) Completion of the left vertebral artery transposition to the right lateral aspect of the common carotid artery (solid arrow) and the left subclavian artery transposition to the left lateral aspect of the common carotid artery (dotted arrow). The ligated proximal end of the vertebral artery is marked with an asterisk. The SCA anastomosis is 5 cm proximal to the vertebral anastomosis. Note the hairpin turns of the common carotid artery.
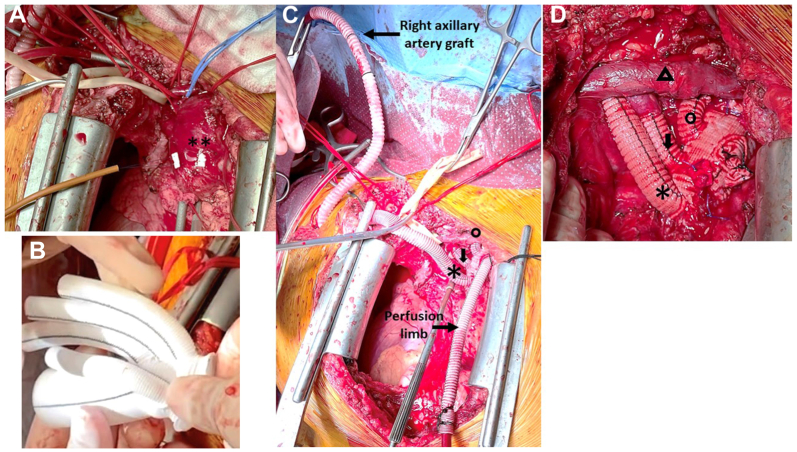
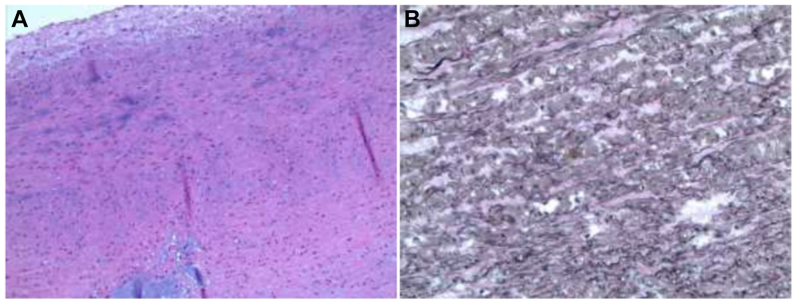
Three days later, she underwent total arch replacement with FET extension (Fig 3). Venous cannulation was performed using a 21F cannula passed percutaneously from the right common femoral vein up to the right atrium under transesophageal echocardiographic guidance. A right transverse infraclavicular incision in the deltopectoral groove was made to expose the right axillary artery. An 8-mm Dacron graft was anastomosed end-to-side to the axillary artery. Arterial cannula was placed into the axillary Dacron graft and connected to the arterial limb of cardiopulmonary bypass circuit. Cardiopulmonary bypass was initiated and cooling to 18° centigrade was begun. During cooling, redo median sternotomy with incision extending transversely along the right supraclavicular area was performed. The distal suture line of the previous Valsalva root graft was noted to be dehisced and contained within a pseudoaneurysm capsule. The innominate, left common carotid, and left subclavian arteries were exposed and circumferentially controlled. The left SCA was ligated at the origin. Through the supraclavicular incision, the right common carotid artery and right SCA were exposed. The patient was placed in Trendelenburg position. The ascending aorta was cross-clamped proximal to the pseudoaneurysm. Cardioplegia was infused into the aortic root and the heart arrested. Selective antegrade cerebral perfusion was started through the right axillary artery after clamps were placed on the innominate and left common carotid arteries. The distal ascending aorta was opened longitudinally and the aneurysm excised. The distal arch was transected between the left carotid artery and left SCA (zone 2). A 21 mm × 10 cm Gore C-TAG thoracic stent graft (W. L. Gore & Associates, Flagstaff, AZ) was deployed antegrade into the descending thoracic aorta (20-mm diameter) through the opened aortic arch. A Teflon felt strip was positioned circumferentially on the outer portion of the distal aorta and sewn in place with mattress 4-0 Prolene sutures, incorporating the stent graft on the inside of the aorta. A quadrifurcated graft (24-mm Gelweave Siena Plexus graft with three arch branches [10 × 8 × 8 mm] and a side perfusion limb; Terumo Aortic, Sunrise, FL) was modified by excising the distal end and trimming the sewing collar (Fig 3, B). It was then sewn end-to-end to the distal transverse arch, incorporating the stent graft, native aortic wall, and felt strip using 4-0 Prolene sutures under low-flow hypothermic bypass. Once the distal anastomosis was completed, the arterial cannula was placed into the side limb of the arch graft and reperfusion to the lower body commenced. End-to-end anastomosis of the 8-mm arch branch to the left common artery was performed with 6-0 Prolene. The proximal anastomosis with the previous Valsalva graft was performed end-to-end with 4-0 Prolene. Circulatory arrest time was 43 minutes. Selective antegrade cerebral perfusion time was 86 minutes. As the patient was being rewarmed, end-to-end anastomosis of the middle 8-mm arch branch to the right carotid artery was performed with 6-0 Prolene. The 10-mm arch branch was tunneled beneath the strap muscles to reach the right SCA. The right internal mammary artery and the thyrocervical trunk emerging from the aneurysm were ligated. End-to-end anastomosis to the right SCA was performed with 6-0 Prolene. The innominate, proximal left common carotid, and left internal mammary arteries were ligated. After de-airing and rewarming, the patient was successfully weaned from the bypass circuit. Total cardiopulmonary bypass time was 317 minutes. Total operative time was 607 minutes. Her postoperative course was uneventful. Warfarin anticoagulation was resumed for her mechanical mitral valve. She was discharged on postoperative day 10. Histopathological examination indicated cystic medial degeneration and fragmentation of the elastic lamina consistent with LDS (Fig 4). CTA at 1 year demonstrated patent reconstruction and exclusion of bilateral SCA aneurysms (Fig 5).
Fig 3.
Intraoperative photographs of the second stage of repair: total arch reconstruction with frozen elephant extension. (A) Median sternotomy with right supraclavicular extension. Exposure of the ascending aorta (∗∗) is obtained. (B) Modified quadrifurcated graft 24 mm Gelweave Siena Plexus graft with three arch branches (10 × 8 × 8 mm) and a side perfusion limb. The sewing collar has been trimmed. (C) Total arch reconstruction is nearly complete. Selective antegrade cerebral perfusion is performed via a Dacron graft anastomosed to the right axillary artery and the perfusion limb is seen. The right arch limb (∗) will be tunneled beneath the strap muscles to complete the right SCA anastomosis. The middle arch limb (short downward arrow) has been anastomosed to the right common carotid artery. The left arch limb (open circle) has been anastomosed to the left common carotid artery. (D) Completed arch reconstruction. Note the innominate vein (open triangle) and ligated perfusion limb offset from the arch branches.
Fig 4.
(A) Cystic medial degeneration on histopathological examination of the aneurysm wall. (B) Fragmentation of the elastic fibers is noted (elastin stain).
Fig 5.
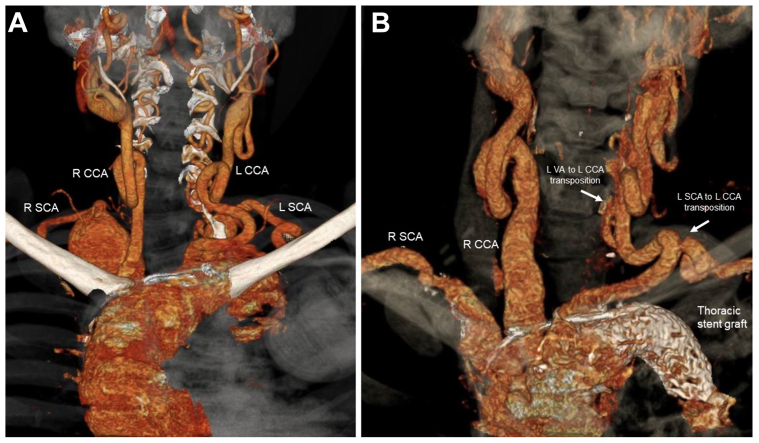
(A) Three-dimensional computed tomographic angiography (CTA) reconstruction of the arch vessels with prominent tortuosity of bilateral carotid and vertebral arteries before ascending aortic, arch, and bilateral SCA repairs. (B) Three-dimensional CTA reconstruction of the hybrid repair with frozen elephant trunk (FET). Transposition of the left subclavian and vertebral arteries onto the left common carotid artery is patent at 1 year. CCA, common carotid artery; L, left; R, right; SCA, subclavian artery; VA, vertebral artery.
Discussion
LDS is an autosomal-dominant connective tissue disorder characterized by perturbations in the transforming growth factor (TGF)-β signaling cascade, which lead to elastic fiber fragmentation and collagen deposition in the media.5 It is stratified into six subtypes based on heterozygous pathogenic variants in six genes: TGF-β receptor-1 (type 1), TGF-β receptor-2 (type 2), suppressor of mothers against decapentaplegic-3 (type 3), TGF-β ligand 2 (type 4), TGF-β ligand 3 (type 5), and suppressor of mothers against decapentaplegic-2 (type 6). Types 1 and 2 LDS have a more sinister course. Aortic root enlargement is ubiquitous. The decision to proceed with prophylactic aortic root replacement depends on the absolute diameter, rate of growth, aortic valve function, severity of craniofacial features, genetic variant, and family history. Current guidelines recommend elective replacement of the aortic root in adults when the diameter is >4 cm or if there is rapid expansion of >0.5 cm per year.2,3,6 Valve-sparing root replacement is considered the operation of choice for the LDS patient with root dilatation and suitable leaflet anatomy and avoids lifetime anticoagulation.2,7, 8, 9, 10 Our patient had type 2 LDS, which is associated with a more aggressive vascular pathology.11,12 She underwent valve-sparing root replacement at 3 years of age, mitral valve replacement at age 14, and total arch replacement at age 17.
LDS has a wide spectrum of phenotypic expression, including cardiovascular findings (thoracic, abdominal, cerebral arterial aneurysms and/or dissections), skeletal manifestations (pectus deformities, scoliosis, joint laxity, arachnodactyly, cervical spine instability, clubfeet), craniofacial features (hypertelorism, bifid/broad uvula, cleft palate, blue sclera, strabismus, craniosynostosis), and dermatologic findings (velvety translucent skin, easy bruising, dystrophic scarring). Mean survival is 37 years; causes of death are related to aortic dissection/rupture or intracranial bleeding.5 Life expectancy can be prolonged by scrupulous surveillance and timely surgery. In contrast with vascular Ehlers-Danlos syndrome, where the friable aortic wall makes reconstruction efforts treacherous, surgical interventions are generally successful in LDS, even in redo cases.2,5 In our case, three sternotomies had been performed with uncomplicated postoperative courses. Multiple vascular procedures have been reported in up to one-third of patients with LDS, are well-tolerated, and prolong life expectancy.2,13,14
There is a much higher need for subsequent arch interventions after root and ascending aortic replacement in LDS compared with Marfan syndrome.8,15, 16, 17 In our case, graft dehiscence at the prior root repair and aneurysmal degeneration of the residual ascending aorta and arch required total arch replacement 14 years later. The amount of aorta that should be replaced at the time of root replacement is contentious (partial/entire ascending aorta, hemiarch, total arch). Some studies have proposed performing concomitant total arch replacement regardless of arch dimension at the time of initial proximal ascending aortic intervention.14, 15, 16, 17 However, it remains unknown whether performing prophylactic total arch replacement surgery is beneficial to long-term outcomes.17, 18, 19, 20
SCA aneurysms are rare, accounting for 0.13% to 0.50% of aneurysms overall.21,22 SCA aneurysms in patients with LDS have been reported.7,23, 24, 25, 26 Our patient had asymptomatic bilateral SCA aneurysms (right 3.2 cm, left 1.7 cm) in addition to ascending aorta and arch aneurysms (>4 cm). Size threshold for SCA aneurysm repair has not been clearly defined.27,28 Indications for SCA aneurysm repair have included size of >2 cm, symptoms, or size of >1.5 cm and concurrent operation for other aortic pathology.26 The surgical approach to SCA aneurysm repair is often complicated by the presence of hereditary connective tissue diseases, concomitant aortic pathology, and prior aortic interventions.29 SCA aneurysms are classified as intrathoracic or extrathoracic. In our case, both SCA aneurysms were intrathoracic. Exposure of an intrathoracic deep left SCA from a sternotomy-based approach can be difficult, particularly when the arch is aneurysmal. In our patient, the left vertebral artery was dominant. We elected to perform transposition of the left vertebral artery and SCA onto the left common carotid artery 3 days before total arch replacement. The hairpin turns in the carotid and vertebral arteries made surgery challenging. We used meticulous tissue handling, soft atraumatic clamps, and interrupted pledgeted sutures on the back wall. The distal arch was transected in zone 2 where the anastomosis is less technically demanding compared with zone 3. Most commonly, the three branches of a prefabricated graft are anastomosed to the innominate artery, the left common carotid artery, and the left SCA in total arch reconstruction. In our patient, the three arch branches were anastomosed to the right SCA and bilateral common carotid arteries after the left SCA had been previously transposed to achieve exclusion of bilateral SCA aneurysms. The stent graft was fixed to the aorta using a sandwich technique, incorporating a felt strip and the soft branched graft.30
The use of FET and quadrifurcated graft to reconstruct the aortic arch and bilateral SCA aneurysms in a patient with LDS has not been described previously. The placement of FET may be controversial in a patient with heritable thoracic aortic disease, but is being increasingly advocated as a prophylactic strategy in younger patients with connective tissue disorders.31,32 We felt that prophylactic FET was prudent in our patient because of the aggressive vascular nature of type 2 LDS.11,12 Her severe craniofacial features and tortuosity also portend a more ominous phenotype.4,6,11 FET can simplify subsequent operation of the thoracic/thoracoabdominal aorta. The FET can be clamped and a standard vascular graft can be sewn to the stent graft portion, obviating the need for hypothermic circulatory arrest and clamping of the distal aortic arch. Complications of a conventional elephant trunk may include graft occlusion or kinking and clot formation around the free-floating end of the elephant trunk with thromboembolism, which may be circumvented by a more rigid FET. However, the outward radial force of FET may injure fragile aortic tissue and promote dilatation.13 FET has been associated with distal stent graft-induced new entry tears, particularly when oversized.33 There is no consensus on the optimal size selection method for FET. The FET should be selected so that distal end is snugly positioned in the aorta to prevent retrograde flow and type IB endoleak. We sized a C-TAG graft to the CTA measurement of the planned distal FET implantation site (20 mm) and used the smallest available 21-mm stent graft. The two most common commercially available hybrid FET prostheses are Thoraflex Hybrid Plexus and E-vita Open Neo. The Thoraflex graft is the only device approved for use in the United States and was not available at the time of our procedure in 2021. FET has also been associated with an increased risk of spinal cord ischemia compared with conventional elephant trunk, although shorter stent lengths have reduced this risk.34,35 We used a short, 10-cm graft to decrease the risk of spinal cord ischemia with the distal landing zone at T4 to T5. Cerebrospinal fluid drainage was not used. Our spinal cord protection maneuvers included revascularization of the left SCA before total arch replacement, deep hypothermic circulatory arrest, selective antegrade cerebral perfusion, and the use of lower body perfusion. Perioperative maintenance of a mean arterial pressure of 90 mm Hg and hemoglobin level of >9 g/L are also components of our spinal protection protocol.
Conclusions
Repair of the ascending aortic, arch, and bilateral SCA aneurysms in a patient with LDS is feasible using staged left subclavian/vertebral carotid transposition and total arch reconstruction with FET.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Loeys B.L., Chen J., Neptune E.R., et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 2.Williams J.A., Loeys B.L., Nwakanma L.U., et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg. 2007;83:S757–S763. doi: 10.1016/j.athoracsur.2006.10.091. [DOI] [PubMed] [Google Scholar]
- 3.Isselbacher E.M., Preventza O., Hamilton Black Iii J., et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;80:e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris S.A., Orbach D.B., Geva T., Singh M.N., Gauvreau K., Lacro R.V. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–396. doi: 10.1161/CIRCULATIONAHA.110.990549. [DOI] [PubMed] [Google Scholar]
- 5.Loeys B.L., Schwarze U., Holm T., et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 6.MacCarrick G., Black J.H., 3rd, Bowdin S., et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams J.A., Hanna J.M., Shah A.A., et al. Adult surgical experience with Loeys-Dietz syndrome. Ann Thorac Surg. 2015;99:1275–1281. doi: 10.1016/j.athoracsur.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Iba Y., Minatoya K., Matsuda H., et al. Surgical experience with aggressive aortic pathologic process in Loeys-Dietz syndrome. Ann Thorac Surg. 2012;94:1413–1417. doi: 10.1016/j.athoracsur.2012.05.111. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H., Ogino H., Matsuda H., Minatoya K., Sasaki H., Iba Y. Midterm outcome of valve-sparing aortic root replacement in inherited connective tissue disorders. Ann Thorac Surg. 2011;92:1646–1649. doi: 10.1016/j.athoracsur.2011.06.090. [DOI] [PubMed] [Google Scholar]
- 10.Patel N.D., Arnaoutakis G.J., George T.J., et al. Valve-sparing aortic root replacement in Loeys-Dietz syndrome. Ann Thorac Surg. 2011;92:556–560. doi: 10.1016/j.athoracsur.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Jondeau G., Ropers J., Regalado E., et al. Montalcino Aortic Consortium International registry of patients carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium) Circ Cardiovasc Genet. 2016;9:548–558. doi: 10.1161/CIRCGENETICS.116.001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seike Y., Matsuda H., Ishibashi-Ueda H., et al. Surgical outcome and histological differences between individuals with TGFBR1 and TGFBR2 mutations in Loeys-Dietz Syndrome. Ann Thorac Cardiovasc Surg. 2021;27:56–63. doi: 10.5761/atcs.oa.20-00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalhub S., Eagle K.A., Asch F.M., LeMaire S.A., Milewicz D.M., GenTAC investigators for the genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC) consortium Endovascular thoracic aortic repair in confirmed or suspected genetically triggered thoracic aortic dissection. J Vasc Surg. 2018;68:364–371. doi: 10.1016/j.jvs.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 14.Patel N.D., Crawford T., Magruder J.T., et al. Cardiovascular operations for Loeys-Dietz syndrome: intermediate-term results. J Thorac Cardiovasc Surg. 2017;153:406–412. doi: 10.1016/j.jtcvs.2016.10.088. [DOI] [PubMed] [Google Scholar]
- 15.Schoenhoff F.S., Alejo D.E., Black J.H., et al. Management of the aortic arch in patients with Loeys-Dietz syndrome. J Thorac Cardiovasc Surg. 2020;160:1166–1175. doi: 10.1016/j.jtcvs.2019.07.130. [DOI] [PubMed] [Google Scholar]
- 16.Seike Y., Matsuda H., Inoue Y., et al. The differences in surgical long-term outcomes between Marfan syndrome and Loeys-Dietz syndrome. J Thorac Cardiovasc Surg. 2022;164:16–25. doi: 10.1016/j.jtcvs.2020.07.089. [DOI] [PubMed] [Google Scholar]
- 17.Aftab M., Cikach F.S., Zhu Y., et al. Loeys-Dietz syndrome: intermediate-term outcomes of medically and surgically managed patients. J Thorac Cardiovasc Surg. 2019;157:439–450. doi: 10.1016/j.jtcvs.2018.03.172. [DOI] [PubMed] [Google Scholar]
- 18.Weininger G., Zafar M., Ziganshin B.A., et al. Long-term risk of arch complications in Loeys Dietz syndrome patients undergoing proximal ascending aortic replacement. J Card Surg. 2022;37:3688–3692. doi: 10.1111/jocs.16855. [DOI] [PubMed] [Google Scholar]
- 19.Augoustides J.G., Plappert T., Bavaria J.E. Aortic decision-making in the Loeys-Dietz syndrome: aortic root aneurysm and a normal-caliber ascending aorta and aortic arch. J Thorac Cardiovasc Surg. 2009;138:502–503. doi: 10.1016/j.jtcvs.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Yang B. Commentary: how aggressive should we be in a prophylactic surgery for patients with Loeys-Dietz or Marfan syndrome? J Thorac Cardiovasc Surg. 2022;164:26–27. doi: 10.1016/j.jtcvs.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Dent T.L., Lindenauer S.M., Ernst C.B., Fry W.J. Multiple arteriosclerotic arterial aneurysms. Arch Surg. 1972;105:338–344. doi: 10.1001/archsurg.1972.04180080184031. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence P.F., Gazak C., Bhirangi L., et al. The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg. 1999;30:632–640. doi: 10.1016/s0741-5214(99)70102-3. [DOI] [PubMed] [Google Scholar]
- 23.Dueppers P., Prêtre R., Hofmann M., Bettex D., Huber F.A., Zimmermann A. Complex multi-stage total aortic and subclavian artery replacement in a 9-year old boy with Loeys-Dietz-Syndrome. Ann Vasc Surg. 2022;80:396.e1–396.e6. doi: 10.1016/j.avsg.2021.10.051. [DOI] [PubMed] [Google Scholar]
- 24.Sobh M., Voges I., Attmann T., Scheewe J. Prosthetic graft replacement of a large subclavian aneurysm in a child with Loeys-Dietz syndrome: a case report. Eur Heart J Case Rep. 2020;4:1–4. doi: 10.1093/ehjcr/ytaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaulieu R.J., Lue J., Ehlert B.A., Grimm J.C., Hicks C.W., Black J.H., 3rd Surgical management of peripheral vascular manifestations of Loeys-Dietz Syndrome. Ann Vasc Surg. 2017;38:10–16. doi: 10.1016/j.avsg.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Andersen N.D., Barfield M.E., Hanna J.M., et al. Intrathoracic subclavian artery aneurysm repair in the thoracic endovascular aortic repair era. J Vasc Surg. 2013;57:915–925. doi: 10.1016/j.jvs.2012.09.074. [DOI] [PubMed] [Google Scholar]
- 27.Vierhout B.P., Zeebregts C.J., van den Dungen J.J., Reijnen M.M. Changing profiles of diagnostic and treatment options in subclavian artery aneurysms. Eur J Vasc Endovasc Surg. 2010;40:27–34. doi: 10.1016/j.ejvs.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Coselli J.S., Crawford E.S. Surgical treatment of aneurysms of the intrathoracic segment of the subclavian artery. Chest. 1987;91:704–708. doi: 10.1378/chest.91.5.704. [DOI] [PubMed] [Google Scholar]
- 29.French B.L., Menghini A.M., Burke C.R., Byers P.H., Shalhub S. Operative repair of right intrathoracic subclavian artery aneurysms in patients with genetic arteriopathy. J Vasc Surg Cases Innov Tech. 2023;9:101081. doi: 10.1016/j.jvscit.2022.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleck T.M., Tschernich H., Grabenwoger M., et al. A double patch sandwich technique for surgical repair of acute aortic dissection type A. Ann Thorac Surg. 2003;76:499–502. doi: 10.1016/s0003-4975(03)00459-4. [DOI] [PubMed] [Google Scholar]
- 31.Acharya M., Sherzad H., Bashir M., Mariscalco G. The frozen elephant trunk procedure: indications, outcomes and future directions. Cardiovasc Diagn Ther. 2022;12:708–721. doi: 10.21037/cdt-22-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldeiry M., Miyamoto S., Chatfield K., Reece T.B., Mitchell M.B. Frozen elephant trunk aortic reconstruction in a patient with Loeys-Dietz syndrome. JTCVS Tech. 2022;16:8–10. doi: 10.1016/j.xjtc.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreibich M., Bünte D., Berger T., et al. Distal stent graft-induced new entries after the frozen elephant trunk procedure. Ann Thorac Surg. 2020;110:1271–1279. doi: 10.1016/j.athoracsur.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Leontyev S., Borger M.A., Etz C.D., et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardio Thorac Surg. 2013;44:1076–1082. doi: 10.1093/ejcts/ezt252. [DOI] [PubMed] [Google Scholar]
- 35.Preventza O., Liao J.L., Olive J.K., et al. Neurologic complications after the frozen elephant trunk procedure: a meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. 2020;160:20–33. doi: 10.1016/j.jtcvs.2019.10.031. [DOI] [PubMed] [Google Scholar]