Abstract
Tolerizing mice polygenically predisposed to lupus-like disease (NZB/NZW F1 females) with a peptide mimicking anti-DNA IgG sequences containing MHC class I and class II T cell determinants (pConsensus, pCons) results in protection from full-blown disease attributable in part to the induction of CD4+ CD25+ Foxp3+ and CD8+ Foxp3+ regulatory T cells. We compared 45 000 murine genes in total white blood cells (WBC), CD4+ T cells, and CD8+ T cells from splenocytes of (NZBxNZW) F1 lupus-prone mice tolerized with pCons vs untreated naïve mice and found two-fold or greater differential expression for 448 WBC, 174 CD4, and 60 CD8 genes. We identified differentially expressed genes that played roles in the immune response and apoptosis. Using real-time PCR, we validated differential expression of selected genes (IFI202B, Bcl2, Foxp3, Trp-53, CCR7 and IFNar1) in the CD8+ T cell microarray and determined expression of selected highly upregulated genes in different immune cell subsets. We also determined Smads expression in different immune cell subsets, including CD4+ T cells and CD8+ T cells, to detect the effects of TGF-β, known to be the major cytokine that accounts for the suppressive capacity of CD8+ Treg in this system. Silencing of anti-apoptotic gene Bcl2 or interferon genes (IFI202b and IFNar1 in combination) in CD8+ T cells from tolerized mice did not affect the expression of the other selected genes. However, silencing of Foxp3 reduced expression of Foxp3, Ifi202b and PD1—all of which are involved in the suppressive capacity of CD8+ Treg in this model.
Keywords: autoimmunity, systemic lupus erythematosus, T cells, gene expression, tolerance/suppression
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease associated with the hyperactivation of helper T cells and B cells and subsequent increases in the production of autoantibodies (including anti-DNA IgG), which can form immune complexes that cause organ damage.1-7 Studies on human autoimmune diseases including SLE have reported amelioration of inflammatory responses and increases in CD4+ CD25+ Treg cells following treatment with self-antigen-derived peptides.8-16 Similarly, self-antigen-derived peptides have also been used to induce immune tolerance and ameliorate disease in murine lupus models.15,17-25
Our group has developed a novel peptide pConsensus (pCons) that is based on MHC Class I and Class II T-cell determinants in the heavy chain region of murine anti-DNA IgG.26 Intravenous administration of the peptide in BWF1 mice has been shown to reduce anti-DNA antibodies, reduce CD4+ Th secreted Interferon-γ, and prolong survival through a mechanism at least partially dependent on the generation of CD4+ CD25+ FoxP3+ Tregs (CD4+ Tregs) and CD8+ T inhibitory cells (CD8+ Ti).6,7,26,27 These CD4+ Tregs and CD8+ Ti have been shown to inhibit autoimmunity through CD4+ Treg-mediated cell–cell contact (dependent on Foxp3, cell surface GITR, and membrane bound TGFβ) and CD8+ Treg secretion of TGFβ.28-31
In an attempt to understand the mechanisms by which pCons mediates immune tolerance, we employed microarray technology to examine differences in gene expression profiles between naive BWF1 mice and BWF1 mice treated with pCons. Recently, microarray analyses has been used by other investigators to study differential gene expression patterns in SLE patients as compared with healthy controls.32-37 In this study, we investigated differential gene expression in the splenic cells of pCons-tolerized BWF1 mice to cells from unmanipulated BWF1 mice to better understand the molecular mechanisms of suppression/tolerance. We investigated genes that are differentially expressed after pCons treatment in BWF1 mice in splenic white blood cells (WBC), CD4+ T cells and CD8+ T cells. We identified a subset of differentially expressed genes in WBC, CD4+ T cells and CD8+ T cells from tolerized mice 1 week after a single dose of pCons—at which time tolerance (that is, suppression of anti-DNA synthesis by CD8+ Treg or CD4+ CD25+ Treg) is well established.6,7,24,25
We examine here the differential expression of several immune response, defense response, signal transduction and apoptosis genes. We assessed the reproducibility of CD8+ T cells arrays and validated the gene expression patterns of some selected genes in WBC and CD8+ T cells using real-time PCR. As the CD8+ Treg induced in this tolerance system depend on the secretion of TGF-β for their suppressive effects, we determined Smads expression in tolerized CD4+ and CD8+ T cells. With siRNA gene silencing, we for the first time showed that silencing of one gene, B-cell leukemia/lymphoma 2 (Bcl2), in CD8+ T cells from tolerized mice did not affect the expression of the other selected genes. Furthermore, we showed that silencing of interferon genes (interferon-activated gene 202b (IFI202b) and interferon (α and β) receptor 1 (IFNar1) combination) has no effect on programmed death 1 (PD1), forkhead box P3 (Foxp3) and bcl2 gene expression. Finally, we showed that silencing of critical molecule Foxp3 also suppressed expression of Ifi202b and PD1—both of which are molecules that contribute to the suppressive capacity of CD8+ Treg in this model.
Results
Differential gene expression in splenic WBC from naive vs pCons-treated mice
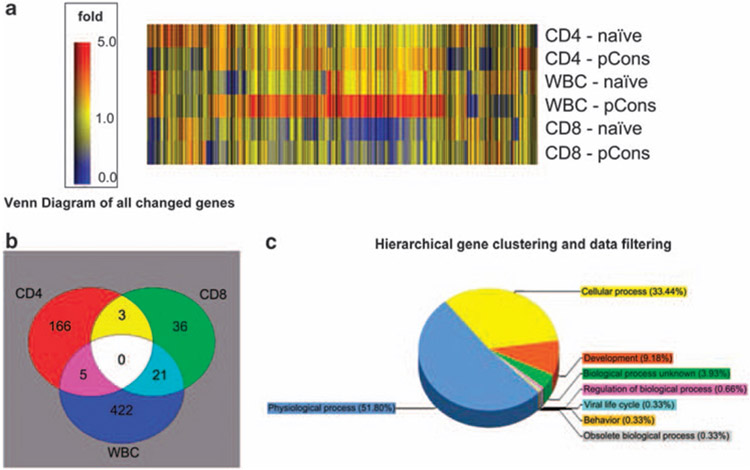
To examine the profile of differentially expressed genes induced by pCons treatment, total RNA was isolated from WBC, CD4+ T cells and CD8+ T cells of pCons-treated and naive untreated mice splenocytes. Subsequent analysis and comparison identified 448 genes in WBC, 174 genes in CD4+ T cells and 60 genes in CD8+ T cells that were differentially expressed after pCons treatment (Figure 1a). As expected, larger numbers of genes were differentially upregulated in WBC than in isolated CD4 or CD8 subsets (Figure 1b), suggesting that pCons tolerance has a significant effect on several peripheral blood cell subsets.
Figure 1.
(a) Gene and condition cluster with all differentially expressed genes together. Heat map diagram comparing changes in gene expression in splenic WBC, CD4+ T cells and CD8+ T cells from naive BWF1 mice (1st, 3rd and 5th horizontal row) to splenic WBC, CD4+ CD8+ T cells from BWF1 mice treated 1 week earlier with pCons (2nd, 4th and 6th horizontal row). Cells were pooled from spleens of 4–5 mice in tolerized vs naive groups. Note the increased expression of multiple genes in the cells from tolerized mice. (b) Venn diagram of all differentially expressed genes together in various cell types. Overlap of differentially regulated gene expression in different cell types is relatively small, although for a small number of genes, expression is changed in more than one cell type. (c) Gene ontology (amiGO) and pathways graphs were generated using Gene Sifter Net Software (Seattle, WA, USA), Gene and condition clustering and Venn Diagram graphs were generated by Gene Spring 6.0 software.
Gene and condition cluster with all changed genes in WBC
Gene and condition clustering indicate that pCons treatment induces significant upregulation of many genes (Figures 1a and b). The Venn diagram of all changed gene expressions shows that some gene expression is changed in more than one cell type (Figure 1b). However, except for the sharing of 21 (5%) of the differentially expressed genes between total WBC and CD8+ T cells, there is remarkably little sharing of similarities between the three cell subsets. White blood cells (WBC) from tolerized mice showed upregulation of more than 48 genes (Table 1a) having roles in transcriptional regulation (Tal1, 5-fold, E2f8, 6.5-fold), signal transduction (Tetraspanin-8 (Tm4sf3), 19.7-fold; G protein-signaling modulator 2; 14.9-fold; Discoidin domain receptor family, member 1, 8-fold), cell division (S17 protein (Spag 5), 26-fold; 6720463M24Rik (9.2-fold)), DNA-protein binding (Ribonucleotide reductase M2, 5-fold; Tal1, 5-fold; Synuclein α (Snca), 5-fold; BC004690, 8-fold; Klf1, 5.7-fold) and coding for integral membrane proteins (Apol2, 4.6-fold; Tetraspanin 33 (1300010 A20Rik), 4.6-fold). There were 11 downregulated genes showing more than two-fold changes in expression (Table 1b). Notable decreases in expression were seen in genes coding for rRNA processing (WD repeat domain 3, 42-fold), transaminase activity/lipid metabolism (glutamic pyruvate transaminase, 9-fold), signal transduction (platelet-activating factor receptor, 9-fold) and proteolysis (Elastase 3B, 5-fold).
Table 1.
Differentially expressed genes in pCons-tolerized splenocytes vs naive splenocytes from BWF1 mice
| Gene ID | Gene name | Fold change |
Function/gene ontology |
|---|---|---|---|
| (a) Microarray analyses of downregulated splenocyte (WBC) genes from pCons-tolerized vs naive mice | |||
| Wdr3 | WD repeat domain 3 | −42.2 | rRNA processing |
| Ptafr | Predicted: platelet-activating factor receptor | −9.2 | G protein-coupled receptor activity/signal transduction/chemotaxis/immune response |
| Gpt2 | Glutamic pyruvate transaminase | −9.2 | Transaminase activity/lipid metabolism |
| 4921507P07Rik | TISP74 | −8 | Transporter activity |
| — | RIKEN cDNA 4930534I15 gene | −8 | Unknown |
| 1200015N20Rik | RIKEN cDNA 1200015N20 gene | −6.1 | Unknown |
| 4732465J09Rik | Myosin IXa | −5.7 | Cytoskeletal organization and biogenesis/intracellular signaling cascade |
| Kif13a | KIF13A | −5.3 | Kinesin complex/microtubule cytoskeleton/protein transport |
| Ela3b | Elastase 3B, pancreatic | −4.9 | Serine-type peptidase activity/proteolysis |
| Ovca2 | DPH1 homolog | −4.6 | Diphthamide biosynthesis/translation/candidate tumor suppressor gene |
| (b) Microarray analyses of upregulated splenocyte ( WBC) genes from pCons-tolerized vs naive mice | |||
| Spag5 | S17 protein | 26 | Kineticore component/mitosis |
| Tm4sf3 | Tetraspanin 8 | 19.7 | Cytoskeletal component/mell motility/mignal transduction |
| A330102K04Rik | Predicted: similar to hypothetical protein 9030421J09 | 18.4 | Unknown |
| — | Transcribed locus | 17.1 | Unknown |
| A330102K04Rik | Predicted: similar to hypothetical protein 9030421J09 | 16 | Unknown |
| Gpsm2 | G-protein-signalling modulator 2 | 14.9 | GTPase activator activity/signal transduction |
| Gm944 | MKIAA4238 protein | 13.9 | Unknown |
| — | Gag | 13 | Intracisternal A-type retroviral particle (IAP) |
| 2410004I17Rik | Establishment of cohesion 1 homolog 2 | 9.8 | Acyltransferase activity/organ development |
| Xtrp3s1 | X transporter protein 3 similar 1 | 9.2 | Transporter activity |
| 6720463M24Rik | RIKEN cDNA 6720463M24 gene | 9.2 | Cell division |
| Slc38a5 | Solute carrier family 38, member 5 | 8.6 | Membrane component |
| BC004690 | CDNA sequence BC004690 | 8 | Protein binding |
| Ddr1 | Discoidin domain receptor family, member 1 | 8 | Protein kinase activity/signal transduction |
| Gypa | Glycophorin Aa | 7.5 | Cytoskeletal anchoring/Response to abiotic stimulus, osmotic stress |
| Add2 | Beta-2 adducin (ADD2), alternatively spliced | 7.5 | Calmodulin binding/Cytoskeletal component/hemopoietic or lymphoid organ development |
| Hmbs | Hydroxymethylbilane synthase | 7.5 | Porphyrin, heme biosynthesis |
| Ptdss2 | Phosphatidylserine synthase 2 | 7.5 | Phosphatidylserine biosynthesis/Lipid metabolism |
| 8-Sep | Septin 8 | 7 | GTP binding/platelet granular secretion |
| Arhgap19 | MFLJ00368 protein | 7 | GTPase activator activity |
| Anln | Anillin, actin-binding protein | 6.5 | Contractile ring/cytoskeletal component |
| 4432406C08Rik | E2F transcription factor 8 (E2f8) | 6.5 | DNA binding/transcription repression/cell cycle regulation |
| C920005C14Rik | RIKEN cDNA C920005C14 gene | 6.5 | Unknown |
| bcl2 | bcl2 | 6.4 | Anti-apoptosis/transcription factor activity |
| Hemgn | Hemogen | 6.1 | Anti-apoptosis/hemopoietic or lymphoid development |
| Rhag | Rhesus blood group-associated A glycoprotein | 6.1 | Cysteine-type endopeptidase activity/proteolysis |
| — | Adult male urinary bladder cDNA, RIKEN library | 6.1 | Unknown |
| Klf1 | Kruppel-like factor 1 (erythroid) | 5.7 | DNA binding/regulation of transcription/chromatin remodeling |
| 1110063G11Rik | MKIAA0481 protein | 5.7 | Protein binding/vesicle-mediated transport |
| Rrm2 | Ribonucleotide reductase M2 | 5.3 | Nucleotide metabolism/DNA metabolism |
| Snca | Synuclein, alpha (Snca) | 4.9 | Lipid binding/protein binding/regulation of biogen amine biosynthesis/lipid metabolism |
| Tal1 | T-cell acute lymphocytic leukemia 1 | 4.9 | DNA binding/regulation of transcription/hemopoietic or lymphoid development |
| Aqp1 | Aquaporin 1 | 4.6 | Water channel activity |
| Slc4a1 | Solute carrier family 4, member 1 | 4.6 | Inorganic anion transporter activity |
| Trim10 | Tripartite motif protein TRIM10 | 4.6 | Ubiquitin-protein ligase activity/protein metabolism |
| Cpox | Coproporphyrinogen oxidase | 4.6 | Oxidoreductase activity/porphyrin, heme biosynthesis |
| Abcg2 | Breast cancer resistance protein 1 | 4.6 | ATPase activity/xenobiotic transporter |
| Cldn13 | Claudin 13 | 4.6 | structural molecule/apical junction complex |
| Icam4 | Intercellular adhesion molecule 4 | 4.6 | Cell adhesion |
| Asns | Asparagine synthetase | 4.6 | Carbon-nitrogen ligase activity/amino acid metabolism |
| Apol2 | Predicted: hypothetical protein XP_139463 | 4.6 | Integral membrane component |
| 1300010A20Rik | Tetraspanin 33 | 4.6 | Integral membrane component |
| 4432416J03Rik | RIKEN cDNA 4432416J03 gene | 4.6 | Unknown |
| Ifi202b | Interferon inducible 202b | 2 | Anti-apoptosis/cell survival |
| Ifnar1 | Ifnar1 | 1.5 | T-cell activation/Type 1 interferon receptor activity |
| CCR7 | Chemokine receptor seven-7 | 1.5 | Chemotaxis/G-protein coupled receptor protein/inflammatory response |
| Foxp3 | Forkhead box p3 | 1.3 | Regulation of transcription/DNA binding/protein binding |
| Trp53 | Transformation-related protein53 | 1.3 | Regulation of transcription/apoptosis/cell cycle regulation |
Differentially expressed genes in tolerized vs naive BWF1 splenocytes were obtained after microarray analyses. Fluorescence intensity and signal to log ratios were obtained as described in the Materials and methods, and fold changes and P-values were determined. Table shows microarray analysis of WBC RNA from pairs of pCons tolerized vs naive mice. (Probe sets called ‘Present’ in at least one sample and showing a 1.6 fold or greater change in expression are shown) Experiment = WBC-pCons vs WBC-naive. Fluorescence intensities of arrays were analyzed using MAS 5.0 (Tukey Bi-weight) algorithms resident in Gene-Chip Operating Software (GCOS) 1.1 (Affymetrix) and normalized by global scaling to a target value of 250. Signal to log ratios were obtained and fold changes were determined.
Replicate probe sets.
Functional analysis of differentially expressed genes in total WBC revealed three major categories: (1) apoptotic genes, (2) signal transduction genes and (3) immune response genes. The 27 genes listed in Table 2 are considered to be involved in apoptosis and their fold changes and gene function/ontology have been depicted. Signal transduction genes and immune response genes have been described in Tables 1, 3 and 4.
Table 2.
Apoptosis-related genes in pCons-tolerized BWF1 mice splenocytes vs naive mice splenocytes
| Gene ID | Gene name | Fold change |
Gene function/ontology |
|---|---|---|---|
| Pim2 | Proviral integration site 2 | −6.06 | Apoptosis |
| Siah2 | Seven in absentia 2 | −6.06 | Proteolysis and peptidolysis |
| Birc2 | Baculoviral IAP repeat-containing 2 | −5.27 | Physiological process |
| Sgk | Serum/glucocorticoid-regulated kinase | −5.27 | Physiological process |
| Inpp5d | Inositol polyphosphate-5-phosphatase D | −4.28 | Response to biotic stimulus |
| Axud1 | AXIN1 upregulated 1 | −4 | Physiological process |
| Gadd45b | Growth arrest and DNA-damage-inducible 45 beta | −3.73 | Cell communication |
| Notch1 | Notch gene homolog 1 (Drosophila) | −3.73 | Determination of bilateral symmetry |
| Bclaf1 | BCL2-associated transcription factor 1 | −3.48 | Apoptosis |
| Stk4 | Serine/threonine kinase 4 | −3.24 | Cell communication |
| Tcf7 | Transcription factor 7, T-cell specific | −3.24 | Cell communication |
| Pdcd5 | Programmed cell death 5 | −3.031 | Physiological process |
| Cd28 | CD28 antigen | −2.63 | Response to biotic stimulus |
| Mcl1 | Myeloid cell leukemia sequence 1 | −2.63 | Physiological process |
| Scotin | Scotin gene | −2.46 | Apoptosis |
| Sirt1 | Sirtuin 1 ((silent mating type information regulation 2, homolog) 1 (S. cerevisiae) | −2.46 | Negative regulation of gene expression, epigenetic |
| Tnfaip3 | Tumor necrosis factor, α-induced protein 3 | −2.46 | Physiological process |
| Trp53inp1 | Transformation-related protein 53 inducible nuclear protein 1 | −2.46 | Apoptosis |
| Btg2 | B-cell translocation gene 2, anti-proliferative | −2.29 | Transcription |
| Dsip1 | Delta sleep-inducing peptide, immunoreactor | −2.29 | Apoptosis |
| Rhob | Ras homolog gene family, member B | −2.29 | Cell communication |
| Cebpb | CCAAT/enhancer-binding protein (C/EBP), β | −2.14 | Response to biotic stimulus |
| Bcl10 | B-cell leukemia/lymphoma 10 | −2 | Response to biotic stimulus |
| Eif5a | Eukaryotic translation initiation factor 5A | −2 | Physiological process |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | −2 | Apoptosis |
| Stk17b | Serine/threonine kinase 17b (apoptosis-inducing) | −2 | Positive regulation of programmed cell death |
| Prdx2 | Peroxiredoxin 2 | 2.82 | Apoptosis |
Apoptosis-related genes were identified from microarray analyses of pCons-tolerized BWF1 mice splenocytes vs untreated naive mice splenocytes. Fluorescence intensities of arrays were analyzed using MAS 5.0 (Tukey Bi-weight) algorithms resident in Gene-Chip Operating Software (GCOS) 1.1 (Affymetrix) and normalized by global scaling to a target value of 250. Signal to log ratios were obtained and fold changes were determined.
Table 3.
Differentially expressed genes in CD4+T cell subsets
| Gene ID | Gene name | Fold change |
Function/gene ontology |
|---|---|---|---|
| (a) Microarray analyses of downregulated CD4+T cell genes from pCons-tolerized vs. naïve mice | |||
| Zfp146 | Zinc finger protein 146 | −24.3 | DNA binding/regulation of transcription |
| 9230110C19Rik | RIKEN cDNA 9230110C19 gene | −17.1 | Unknown |
| Wbscr1 | Williams–Beuren syndrome chromosome region 1 homolog | −17.1 | RNA binding/translation initiation factor activity |
| Dixdc1 | DIX domain containing 1 | −14.9 | Actin binding/signal transduction |
| - | 9 days embryo whole body cDNA, RIKEN library | −13.0 | Unknown |
| - | cyclin D binding myb-like transcription factor 1 | −12.1 | DNA binding/regulation of transcription/cell cycle regulation |
| - | nuclear receptor subfamily 2, group C, member 2 | −9.8 | DNA binding/regulation of transcription/signal transduction |
| 2210010C04Rik | RIKEN cDNA 2210010C04 gene | −8.6 | Serine-type peptidase activity/proteolysis |
| Mfhas1 | Predicted: similar to malignant fibrous histiocytoma amplified sequence 1 | −8.6 | Protein binding |
| - | RIKEN cDNA 1300007C21 gene | −5.7 | Unknown |
| Hrb | HIV-1 Rev-binding protein | −5.7 | Regulation of GTPase activity/macromolecular localization/spermatogenesis |
| - | ESTs | −5.7 | Unknown |
| Amy1 | Amylase 1, salivary | −4.9 | Hydrolase activity/carbohydrate metabolism |
| 9030625G08Rik | RIKEN cDNA 9030625G08 gene | −4.9 | DNA binding/Zinc ion binding/metal ion binding |
| 2210401K01Rik | Adult male stomach cDNA, RIKEN library, receptor (calcitonin) activity | −4.6 | Unknown |
| Pnlip | Pancreatic lipase | −4.6 | Lipid/sterol metabolism |
| Zfhx1b | Zinc finger homeobox 1b | −4.3 | DNA binding/regulation of transcription/cell migration |
| Igh-VJ558 | Clone X1AC2145 immunoglobulin heavy chain variable region | −4.3 | antigen binding/immune response |
| 1700012F10Rik | WD repeat domain 68 | −4.3 | Signal transduction |
| Rbm5 | RNA binding motif protein 5a | −4.3 | RNA binding |
| Peli1 | Pellino 1 | −4.3 | Ubiquitin-protein ligase activity/scaffold protein |
| Ela3b | Elastase 3B, pancreatic | −3.7 | Serine-type endopeptidase activity/proteolysis |
| Trip12 | Thyroid hormone receptor interactor 12 | −3.7 | Ubiquitin-protein ligase activity/protein metabolism/signal transduction |
| - | ESTs | −3.7 | Unknown |
| 2210401K01Rik | Adult male stomach cDNA, RIKEN library | −3.5 | Unknown |
| Luc7l2 | LUC7-like 2 | −3.5 | Development |
| - | RIKEN cDNA 9030402K04 gene | −3.5 | Unknown |
| D12Ertd771e | DNA segment, Chr 12, ERATO Doi 771, expressed | −3.5 | Unknown |
| 9030612M13Rik | MKIAA3006 protein | −3.2 | Nucleic acid binding/regulation of transcription |
| Ddx6 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 6 | −3.0 | ATPase activity/RNA binding/RNA helicase activity |
| Akt1 | Thymoma viral proto-oncogene 1 | −3.0 | Protein kinase activity/signal transduction/Anti-apoptosis/cell cycle regulation |
| 1110014N23Rik | Predicted: hypothetical protein LOC68505 | −3.0 | Unknown |
| - | ESTs | −3.0 | Unknown |
| Sec61a1 | Sec61 α-1 subunit | −2.8 | Protein translocase activity/protein secretion |
| Snrpd3 | Small nuclear ribonucleoprotein D3 | −2.8 | RNA splicing |
| Tcf12 | Transcription factor 12 | −2.8 | DNA binding/regulation of transcription |
| D12Ertd551e | DNA segment, Chr 12, ERATO Doi 551, expressed (D12Ertd551e) | −2.8 | Unknown |
| Ddx50 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 50 | −2.8 | ATPase activity/RNA binding |
| (b) Microarray analyses of upregulated CD4+ T cell genes from pCons-tolerized vs. naive mice | |||
| Acly | ATP citrate lyase | 18.4 | ATP citrate synthase activity/Acetyl-CoA biosynthesis/lipid metabolism |
| Vrk2 | Vaccinia-related kinase 2 | 18.4 | Protein kinase activity |
| Ocil | C-type lectin domain family 2, member d (Clec2d) | 8.0 | Transmembrane receptor activity/signal transduction/regulation of myeloid differentiation, immune cell-mediated cytotoxicity, osteoclast differentiation |
| - | ESTs | 5.7 | Unknown |
| Bbx | HMG-box transcription factor BBX, alternatively spliced | 4.6 | DNA binding/regulation of transcription |
| Thsd2 | Predicted: Thrombospondin, type I, domain 2 | 3.5 | Iron ion binding/electron transporter activity |
| Tacr2 | Tachykinin receptor 2 | 3.2 | Neuropeptide receptor activity/signal transduction |
| Hsp105 | Heat-shock protein 105a | 3.2 | Chaperone cofactor dependent protein folding/response to stress |
| Riok3 | RIO kinase 3 | 3.2 | Protein kinase activity |
Differentially expressed genes in tolerized vs naive BWF1 CD4+ T cells were obtained after microarray analyses. Fluorescence intensity and signal to log ratios were obtained as described in the Materials and methods section, and fold changes and P-values were determined.
Replicate probe sets.
Table 4.
Differentially expressed genes in CD8+ T cell subsets
| Gene ID | Gene name | Fold change |
Function/gene ontology |
|---|---|---|---|
| (a) Microarray analyses of downregulated CD8+ T cell genes from pCons-tolerized vs naive mice | |||
| Igl-V1 | Immunoglobulin lambda chain (IgL)a | −8.0 | Endogenous antigen binding and processing/MHC class I receptor activity/immune response |
| - | IgG light chain gene, V region | −6.1 | Antigen binding/immune response |
| - | RIKEN full-length enriched library, clone:A430018E01 | −5.7 | Unknown |
| Pde4b | Phosphodiesterase 4B | −5.3 | cAMP-specific phosphodiesterase activity/regulation of PKA signal transduction |
| Pde7a | Phosphodiesterase 7A | −5.3 | cAMP-specific phosphodiesterase activity/regulation of PKA signal transduction |
| Lasp1 | LIM and SH3 protein 1 | −4.6 | Actin binding/cytoskeletal organization/focal adhesion-mediated signal transduction |
| Pabpc1 | Poly A-binding protein, cytoplasmic 1 | −4.0 | RNA binding/RNA stability/translation |
| Smc4l1 | SMC4 structural maintenance of chromosomes 4-like 1 | −3.7 | ATPase activity/mitosis/sister chromatid exchange |
| Igh-VJ558 | Immunoglobulin heavy chain (J558 family) | −3.5 | Antigen binding/immune response |
| Capza1 | Capping protein (actin filament) muscle Z-line, α-1 | −3.0 | Barbed-end actin filament capping/negative regulation of actin filament depolymerization |
| Dnaja2 | DnaJ (Hsp40) homolog, subfamily A, member 2 | −2.8 | HSP70 co-chaperone/protein folding/protein transport |
| - | ESTs | −2.8 | Unknown |
| Mcl1 | Myeloid cell leukemia sequence 1 | −2.8 | Regulation of programmed cell death |
| Sesn3 | Sestrin 3 | −2.5 | Negative regulation of cell cycle progression |
| Arid4b | AT-rich interactive domain 4B (Rbp1 like) | −2.5 | Possible involvement in repression of transcription or RNA processing/splicing |
| Igtp | Interferon-inducible GTPase 2 | −2.5 | GTPase activity/Response to pest, pathogen or parasite |
| - | Mus musculus expressed sequence AI893585 | −2.3 | Unknown |
| Utrn | Utrophin | −2.3 | Cytoskeletal binding/calcium ion binding/muscle contraction/chemotaxis |
| Igl-V1 | IgL variable regiona | −2.3 | Endogenous antigen binding and processing/MHC class I receptor activity/immune response |
| Igh-VJ558 | Immunoglobulin heavy chain (J558 family) | −1.5 | Antigen binding/immune response |
| (b) Microarray analyses of upregulated CD8+ T cell genes from pCons-tolerized vs naive mice | |||
| S100a8 | S100 calcium-binding protein A8 (calgranulin A) | 3.2 | Calcium ion binding/chemotaxis/cellular locomotion/inflammation |
| H2-K1 | MHC class I heavy chain precursor (H-2K(d)) | 3.2 | Antigen binding and processing/MHC class I protein complex/immune response |
| Gypa | Glycophorin A | 3.2 | Cell adhesion molecule/cytoskeletal anchoring/response to osmotic stress |
| Prdx2 | Peroxiredoxin 2 | 2.8 | Thioredoxin peroxidase activity/response to oxidative stress/anti-apoptosis |
| S100a9 | S100 calcium-binding protein A9 (calgranulin B) | 2.8 | Calcium ion binding/chemotaxis/cellular locomotion/inflammation |
| Rsad2 | Viperin (Vig1)a | 2.6 | Interferon-inducible anti-viral activity |
| Alas2 | Aminolevulinic acid synthase 2 | 2.6 | N-succinyltransferase activity/porphyrin, heme biosynthesis |
| Cd24a | CD24a antigena | 2.5 | Cell surface antigen/regulation of T-cell expansion |
| 5730469M10Rik | RIKEN cDNA 5730469M10 gene | 2.5 | Unknown |
| Eraf | Erythroid-associated factor | 2.5 | Hemoglobin binding/hemopoiesis |
| Car2 | Carbonic anhydrase 2 | 2.3 | carbon-oxygen lyase activity/interconverts carbon dioxide and carbonic acid/respiration |
| 1110063G11Rik | MKIAA0481 proteina | 2.3 | Integral membrane protein |
| Hba-a1 | Hemoglobin-α, adult chain 1a | 2.1 | heme, iron, oxygen binding/oxygen transport |
| Slc4a1 | Solute carrier family 4 (anion exchanger), member 1 | 2.1 | Inorganic anion transport/intrinsic to plasma membrane |
| Mscp | Solute carrier family 25, member 37 (Slc25a37), variant 1 | 1.9 | Ion and metabolite exchange/mitochondrial transport |
| Alad | Aminolevulinate, delta-, dehydratase | 1.7 | Carbon-oxygen lyase activity/porphyrin, heme biosynthesis |
| Klf1 | Kruppel-like factor 1 | 1.7 | DNA binding/regulation of transcription/chromatin remodeling |
| Hbb-b1 | Hemoglobin, β-adult major chain | 1.6 | Heme, iron, oxygen binding/oxygen transport/respiration |
| Bpgm | 2,3-bisphosphoglycerate mutase | 1.4 | Bisphosphoglycerate phosphatase activity/glucose metabolism |
Differentially expressed genes in tolerized vs naive BWF1 CD8+T cells were obtained after microarray analyses. Fluorescence intensity and signal to log ratios were obtained as described in the Materials and methods section, and fold changes and P-values were determined.
Replicate probe sets.
Affymetrix Gene chip analysis of naive WBC vs pCons-treated WBC showed 448 genes that were differentially expressed (Figure 1 and Table 1). The differentially expressed genes were at least two-fold increased or decreased in their normalized expression value. Gene ontology (amiGO), shown in Figure 1c, showed distribution of more than 50% of differentially regulated WBC genes in physiological processes, with more than one-third of the genes involved in cellular processes. The other categories of genes influenced significantly, as shown by hierarchical gene clustering were development (9.18%) and unknown biological processes (3.93%). In summary, the differences in gene expression associated with pCons-induced tolerance showed effects mainly on physiological and cellular processes.
Expression of selected highly upregulated genes in different cell subsets within WBC
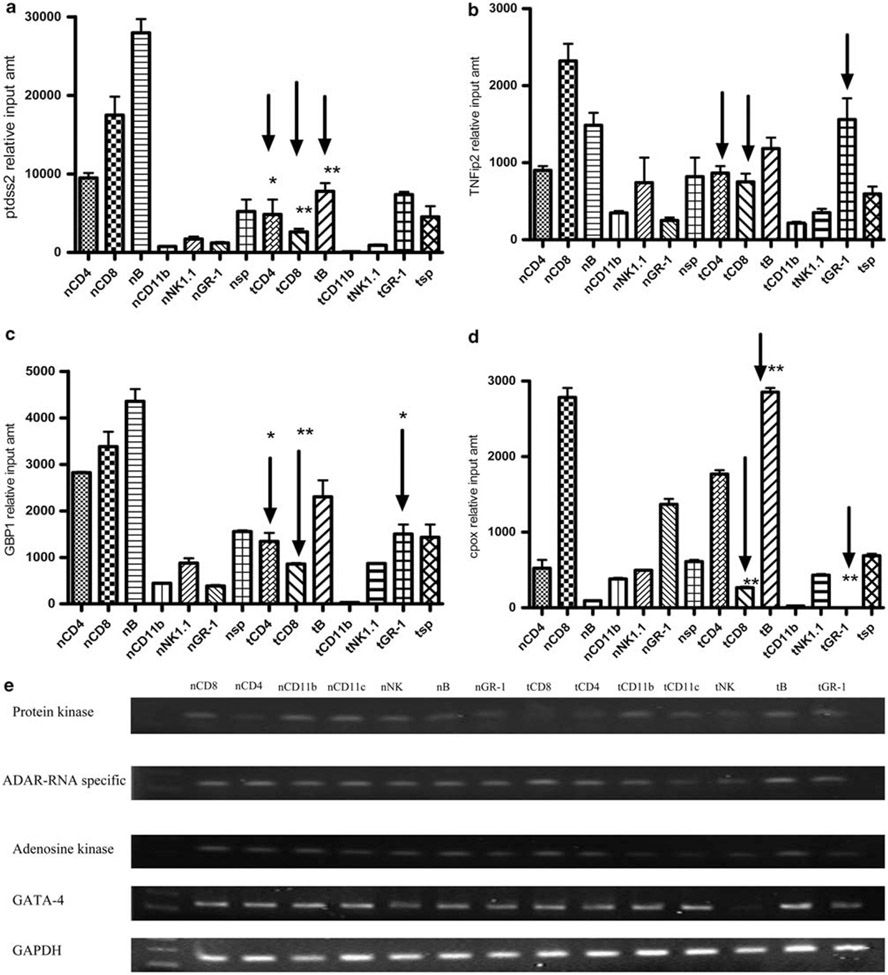
As upregulation of gene expression was most dramatic in WBC, we determined whether a few of the highly upregulated genes (three-fold or more) were similar in different immune cell subsets, using real-time PCR on RNA from purified and isolated immune cell populations. The results are shown in Figure 2. In tolerized (t) mice compared with naive (n) mice, phosphatidylserine synthetase-2 (Figure 2a) was significantly decreased in CD4, CD8 and B cells and increased in granulocytes. Tnfaip2 tumor necrosis factor, α-induced protein 2 (Figure 2b) was decreased in tolerized CD8+ T cells. mRNA expression of GATA-binding protein-1 (Figure 2c) was decreased in CD4+ T cells, CD8+ T cells and B cells from tolerized mice compared with naive mice. Tolerized granulocytes have increased Guanylate-binding protein 1 expression. The mRNA gene expression of adenosine kinase and protein kinase are reduced in pCons-treated groups in tested CD8+ T cell, CD11b and CD11c cell subsets (Figure 2e). Similarly the adenosine deaminase, RNA-specific and GATA 4 expression was reduced in tolerized CD8, CD11c and Gr-1 subsets (Figure 2e). Finally, copropyrinogen oxidase (Figure 2d) was increased in tolerized B cells from pCons-treated mice. Cpox gene expression was significantly reduced in tolerized CD8+ T cells and in granulocytes. These data indicate that the upregulation of some genes in WBC shown in Figure 1 and Table 1 is primarily attributable to changes in single cell subsets, and that such changes may be unique to selected immune cell subpopulations and may even be downregulated in other subsets in the same tolerized mice.
Figure 2.
(a–d) Gene expression pattern in splenocyte subsets before and after pCons treatment in BWF1 mice. Non-T cells show large differential expression of some genes after pCons treatment. Different cell subsets (CD4+ T cells, CD8+ T cells, B cells, NK cells, macrophages, granulocytes and dendritic cells) were isolated from saline-treated naive and pCons-treated BWF1 mice spleen by AutoMACS using specific microbeads. RNA from cell subsets, pooled from 4–5 mice in each group was isolated, measured and used for RT-PCR. 100 ng of RNA was used for real-time PCR. A separate standard curve for each gene was constructed and the relative input amount was calculated. Real-time PCR was performed with gene-specific primers and TaqMan probes. (e) RT-PCR was performed from the isolated RNA from different cell subsets (CD8, CD4, CD11b+ macrophages, CD11c+ DC, NK1.1+ NK cells, B 220+B cells and Gr-1+ granulocytes from naive and pCons-treated mice). cDNA was prepared from isolated RNA with reverse transcriptase enzyme and individual gene-specific primers were designed and used for subsequent PCR analysis. The amplified products were size-fractionated by 2% agarose gel electrophoresis. GAPDH was used as a housekeeping gene. *P<0.05, **P<0.005.
CD4 genes differentially expressed in naive vs pCons-treated mice
The differentially expressed genes in naive CD4+ T cells vs pCons-treated CD4+ T cells showed 174 genes that are differentially expressed (Figure 1a). pCons treatment upregulated some genes and downregulated others (Figure 1a— top panel —right 2nd row). There was little overlap in the regulation of genes between CD4+ and CD8+ T cells, with only 3 of the 174 differentially regulated in both types of T cells (Figure 1b). Comparisons between CD4+ T cells from tolerized and naive mice displayed a distinct gene expression pattern (Table 3a and 3b). Notable genes upregulated in CD4+ T cells from tolerized mice (Table 3a) were those coding for protein kinases (Vaccinia-related kinase 2, 18-fold), Rio kinase 3 (Rio kinase 3, 3-fold), DNA binding (Bbx, 4.6-fold), signal transduction (C-type lectin domain family 2, member d (Ocil), 8-fold; Tachykinin receptor 2, 3.5-fold)) and heat-shock proteins (Hsp105, 3-fold). Tolerization of CD4+ T cells seems to have had the effect of downregulating several genes (Table 3b), notably genes that have a role in the regulation of transcription (Zinc finger protein 146 (Zfp146), 24.3-fold; transcription factor 12, 2.8-fold; and Zinc finger homeobox 1b, 4-fold among others), proteolysis (Elastase 3B, pancreatic, 3.7-fold; 2210010C04Rik, 3.5 fold), ubiquitin protein ligase activity (Pellino 1 (Peli1), 4.3-fold; thyroid hormone receptor interactor 12, 3.7-fold), ATPase activity (DEAD box polypeptide 6 (Ddx6), 3.0-fold; DEAD box polypeptide 6 (Ddx50), 2.8-fold), and RNA binding/modification (RNA binding motif protein 5, 4.3-fold; DEAD box polypeptide 6 (Ddx6), 3-fold; and Williams–Beuren syndrome chromosome region 1 homolog (Wbscr1), 17-fold among others).
CD8 genes differentially expressed in naive vs pCons-treated mice
We have long been interested in the role of CD8+ T cells in murine lupus and in their specific role in the system of pCons-induced tolerance.5,7,24-26 The analysis of CD8+ T cells from naive vs pCons-treated mice indicated that 60 genes were differentially expressed in this cell subset (Figure 1a). Gene and condition clustering of CD8+ T cells showed that large numbers of genes were downregulated in naive cells with higher expression in tolerized cells (Figure 1a bottom two lines).
CD8+ T cells from tolerized mice had a variety of genes that were either upregulated or downregulated relative to naive cells. Upregulated genes (Table 4a) included those that have a role in calcium ion binding and chemotaxis (S100 calcium-binding protein A8 (S100a8), 3.2-fold; S100 calcium-binding protein A9 (S100a9), 2.8-fold), the regulation of T-cell expansion (CD24a antigen, 2.5-fold), anti-apoptotic activity (Prdx2, 2.8-fold), and interferon-inducible viral activity (Viperin (Rsad2), 2.6-fold). Downregulated genes (Table 4b) include those having a role in ATPase and GTPase activity (Smc4l1, 3.7-fold; Igtp, 2.5-fold), cytoskeletal organization (LIM and SH3 protein 1 (Lasp1), 4.6 fold; Capping protein (actin filament) muscle Z-line, α-1 (Capza1), 3.0-fold; and Utrophin, 2.3-fold), and Phosphodiesterase activity (Phosphodiesterase 4B, 5.3-fold; Phosphodiesterase 7A (Pde7a), 5.3-fold).
Apoptosis-related genes
We have found earlier that both CD4+ and CD8+ T cells from tolerized mice are protected from apoptosis.7 A microarray focusing on genes related to apoptosis revealed the differential regulation of 27 genes by at least two-fold in tolerized vs naive splenocytes (Table 2). These genes encoded proteins that had either indirect or direct roles in apoptosis and had been reported to have either pro-apoptotic or anti-apoptotic functions. Twenty-six genes in the apoptosis microarray were downregulated and one, Peroxiredoxin 2, was upregulated. Of the downregulated genes, 12 (44%) were downregulated more than 3-fold.
Signal transduction genes
Genes involved in signal transduction were differentially regulated among the CD4+, CD8+ and splenocyte subsets. In the splenocyte microarray (Tables 1a and b), these genes included Tetraspanin 8 (Tm4sf3), G protein-signaling modulator 2, Myosin Ixa, platelet-activating factor receptor, and Discoidin domain receptor family, member 1. In CD4+ T cells (Tables 3a and b), the differentially expressed genes in signal transduction were Tachykinin receptor 2, Thymoma viral proto-oncogene 1 (Akt1), Ocil and thyroid hormone receptor interactor 12. Genes that were differentially regulated in the CD8+ subset (Tables 4a and b) were LIM and SH3 protein 1 (Lasp1), phosphodiesterase 4B (Pde4b), and phosphodiesterase 7A (Pde7a).
Reproducibility
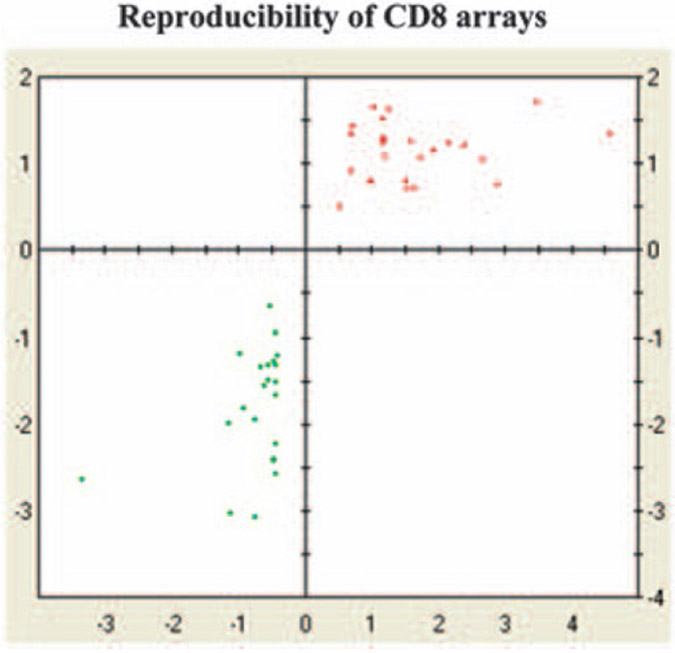
To determine the reproducibility of CD8+ T cells genes from naive and tolerized animals, microarray analyses were performed twice. As shown in Figure 3, excellent reproducibility of the CD8 arrays for differentially expressed genes was found.
Figure 3.
Reproducibility of CD8 arrays. Reproducibility of CD8 arrays were performed by analyzing microarray analysis twice from isolated CD8+ T cells (pooled from five mice in each group). RNA was isolated and Affymetrix 430 2.0 chips were hybridized with RNA. The scatter plot of signal log ratios of up- and downregulated genes from the first CD8 experiments against the signal log ratios of the up- and downregulated genes from the second CD8 experiment are shown. Red, increased expression. Green, decreased expression. Both the upregulated and downregulated genes were found in both arrays indicating excellent reproducibility of the data.
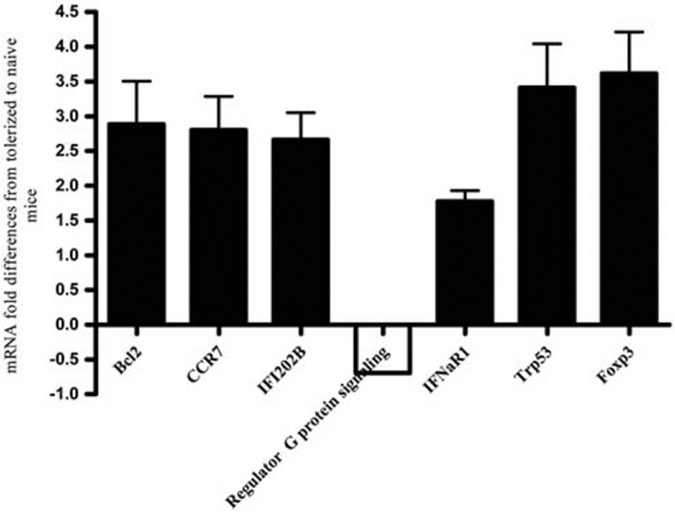
Real-time PCR validation shows consistent upregulation of selected genes in CD8+ T cells
From the WBC arrays, we selected 10 upregulated genes that were also upregulated at least 2-fold in the CD8+ arrays of pCons-treated vs untreated naive mice, and that could be related to specific immune functions in autoimmunity and immune tolerance. Using real-time PCR, we confirmed the upregulation of 6 of those 10 genes tested. The 10 genes studied were: interferon inducible Ifi202b, Foxp3, bcl2, bcl-2 modifying factor, muscle intestine and stomach expression 1, tumor necrosis factor receptor super family member 11b (osteoprotegerin), orosomucoid-2, transformation-related protein 53 (Trp-53), interferon α receptor IFNar1 and CCR7. Regulator of G protein signaling was chosen as a negative control, as the microarray analyses suggested that it is downregulated in CD8+ T cells from tolerized mice. Statistically significant increased expression patterns with real-time PCR of six genes in CD8+ T cells from tolerized mice was repeatedly found, as shown in Figure 4. Bcl2, CCR7, Ifi202b, IFNar1, Trp53 and Foxp3 were upregulated two- to threefold as compared with cells from naive control mice. In contrast, regulator of G protein signaling was downregulated in pCons-treated cells compared with naive CD8+ T cells (Figure 4). The expression pattern of muscle intestine and stomach expression 1, OPG or orosomucoid-2 could not be confirmed as different from that in naive mice. Of significance was the upregulated expression of Foxp3 in tolerized CD8+ T cells. We have shown previously that interference with the expression of this gene via siRNA abrogated 60–70% of the suppressive capacity of the CD8+ T cells from tolerized mice.7,24,25
Figure 4.
Validation of microarray analysis by real-time PCR for selected genes differently regulated in CD8+ T cells from pCons-treated mice compared with saline-treated controls. mRNA-fold differences by real time PCR are shown on the y axis for tolerized CD8+ Ti cells compared with saline-treated naive CD8+ T cells by real-time PCR. RNA was isolated from splenic CD8+ T cells from naive and pCons-tolerized mice. Splenocytes were pooled from 4–5 mice in each group. 100 ng of RNA was used with TaqMan Primer and probe from Applied Biosystems. Expression values are normalized to house keeping gene GAPDH. Data are means ± s.e.m. of two to three independent experiments for each gene.
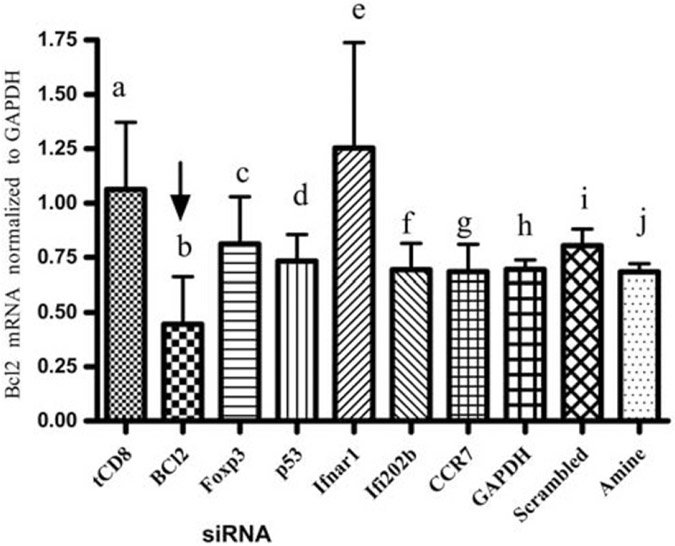
Silencing of one gene (Bcl2) in CD8+ T cells from tolerized mice does not effect expression of other selected genes known to be involved (or not) in the suppressive capacity of CD8+ Treg in our system
To test whether silencing of bcl2 in tolerized CD8+ T cells affects expression of other genes, we silenced tolerized CD8+ T cells with different siRNA as described in the Materials and methods section and in the figure legends. Results shown in Figure 5 illustrate that silencing of bcl2 did not significantly affect the expression of other genes studied.
Figure 5.
Silencing of one gene (Bcl2) in CD8+ T cells from tolerized mice does not effect expression of other selected genes known to be involved (or not) in suppressive capacity of CD8+ Treg in our system. CD8+ T cells were isolated from pCons-treated mice 1 week after pCons tolerization using antibody-specific microbeads from AUTOMACS. Cells were pooled from 3–4 mice in each group. The cells were transfected with siRNA of Foxp3, CCR7, IFI202b, IFNar1, bcl2 (50–100 nm) or with siRNA controls (GAPDH or scrambled siRNA, 50–100 nm). The negative control scrambled sequence has random sequences with no homology to mouse, rat or human genomes. Incubation with siPORT amine served as a control for the transfection reagent. Then the transfected CD8+ T cells (1 × 105) were cultured for 3 days and RNA was isolated. Real-time PCR for Bcl2 was performed with isolated RNA (100 ng). tolerized CD8=tCD8. Columns: a; tCD8; b; tCD8+ Bcl2 siRNA, c; tCD8+ Foxp3 siRNA, d; tCD8+ p53 siRNA, e; tCD8+ IFNar1 siRNA, f; tCD8+ ifi202b siRNA, g; tCD8+ CCR7 siRNA, h; tCD8+ GAPDH siRNA, I; tCD8+ scrambled siRNA, j; tCD8+ si PORT amine.
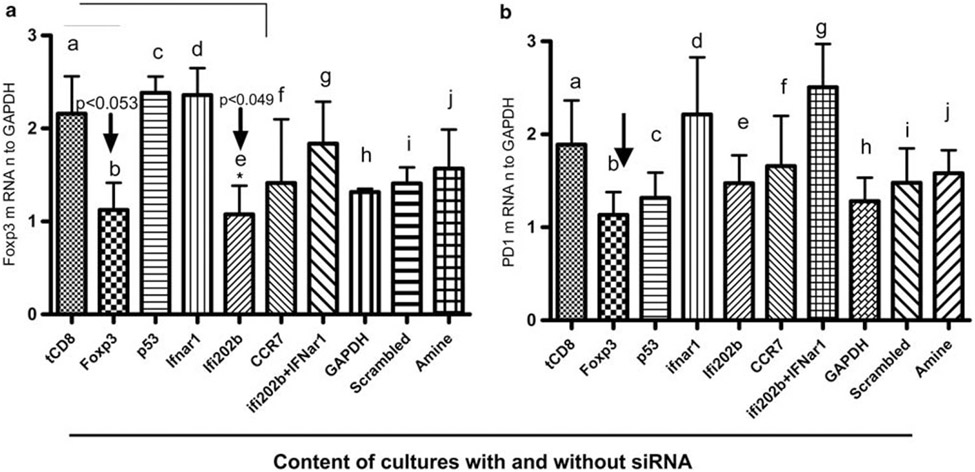
Silencing of Foxp3 affects expression of other genes in addition to silenced gene/genes in tolerized CD8+ T cells
To address whether silencing of selected genes and combinations of these genes affects other genes in tolerized CD8+ T cells, we did siRNA-silencing experiments for single genes, known to be involved in immune tolerance in our system 7,24,25 and for the combination of Ifi202b and IFNar1. As shown in Figure 6, we found that silencing of the Foxp3 gene or of the Ifi202b gene both downregulated Foxp3 expression (Figure 6 panel a, compare columns b and e with column a). Silencing of Foxp3 also downregulated expression of PD-1 mRNA (Figure 6 panel b, column b). In contrast, silencing of the p53 and CCR7 genes had no significant effect on either Foxp3 or PD-1 expression (Figure 6 panels a and b) These data suggest that there is a correlation between Foxp3 and PD1 gene expression in our model of tolerance.
Figure 6.
Silencing of Foxp3 affects expression of other genes in addition to the silenced gene in tolerized CD8+ T cells; silencing of interferon genes (IFI202b and IFNar1 combination) has no effect on PD1, Foxp3 and bcl2 gene expression. CD8+ T cells were isolated from pCons-treated mice after 1 week of pCons tolerization using antibody-specific microbeads from AutoMACS. Cells were pooled from 3 to 4 mice in each group. Isolated CD8+ T cells from pCons-tolerized mice were transfected with siRNA of Foxp3, PD1, CCR7, IFI202b, IFNar1 and bcl2 (50–100 nm) alone and in combinations, and with controls (GAPDH-50–100 nm), as indicated on the x axis. Real-time PCR was performed with isolated RNA (100 ng). House keeping gene GAPDH-positive siRNA and scrambled-negative siRNA were used as controls. Another control, the transfectant siPORT amine by itself, was also used. The negative control scrambled sequence has random sequences with no homology to mouse, rat or human genomes. (a): Foxp3 mRNA gene expression (b): PD1 mRNA gene expression. Gene expression values were normalized to GAPDH. *P<0.05.
Silencing of interferon genes (IFI202b and IFNar1 combination) has no effect on PD1, Foxp3 and bcl2 gene expression
As interferon gene signatures have been found in SLE patients,37-40 we were interested to see whether silencing/blocking of interferon genes has any affect on PD1, bcl2 and Foxp3, all of which participate in immune tolerance in our system. Silencing with an IFNar1 and IFI202b combination had no effect on PD1, Foxp3 and bcl2 expression (Figures 6A and B, column i). These data suggest that PD1, Foxp3 and bcl2 expression and their regulation is independent of the interferon genes tested (IFI202b and IFNar1 combination). A future detailed study will be required to understand these interactions and their biological significance.
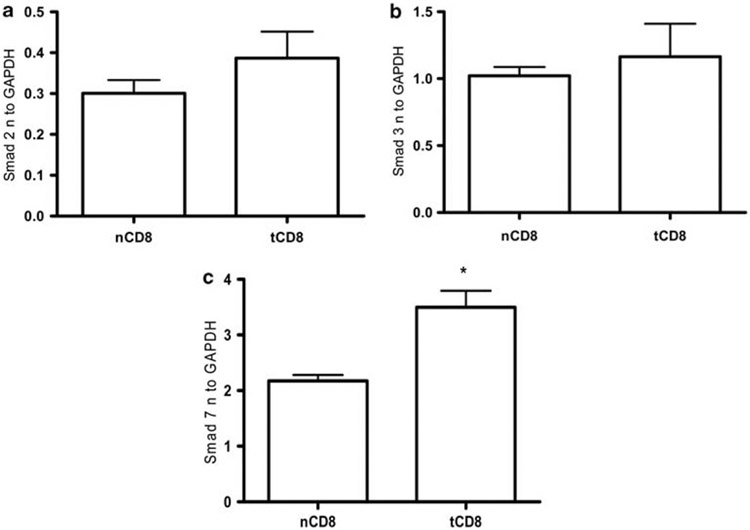
Smads expression is increased in tolerized CD8+ T cells
Earlier we found that TGFβ was significantly increased in tolerized CD8+ T cells and was at least in part responsible for the suppression of anti-DNA Ab in our tolerance model.7 Therefore, we examine here the possibility of changes in the expression of TGFβ signaling molecules, such as Smad2, Smad3 and Smad7. We found increased expression of Smad 2 and 3 in tolerized CD8+ T cells but these changes were not significant (Figures 7 a and b). However, we found significantly increased expression of Smad7 in tolerized CD8+ T cells (Figure 7c, P<0.0147). These data suggest TGFβ receptor signaling through Smad 7 has an important role in CD8+ inhibitory T cells in our tolerance model.
Figure 7.
Expression of Smad2 (a), Smad3 (b), and Smad7 (c) increases in tolerized CD8+ T cells. CD8+ T cells were isolated from BWF1 mice splenocytes treated with negative control peptide (p58) or pCons. After 1 week, cells were isolated from splenocytes by AUTOMACS and RNA isolated. Real-time PCR was performed for Smad 2, Smad3 and Smad7 with specific primers and probes from Applied Biosystem. 100 ng of RNA was used. Data were normalized with GAPDH. *P<0.05 was considered significant.
Discussion
The new information provided in this study of our pCons-induced model of immune tolerance includes the differential expression of genes in peripheral white blood cell subsets, the expansion of our work in CD8+ Treg showing the importance of Foxp3 in controlling expression of genes that have a role in tolerance, and finally, initial studies of Smad expression that display the effect of TGFβ in CD8+ Tregs. We show herein that splenic WBC harvested 1 week after a tolerizing injection of pCons have altered gene expression in many cell subsets. The upregulation of genes was dramatic in whole WBC. Gene ontology and Venn diagrams indicated that although pCons treatment affected gene expression widely, only a few genes differentially expressed were common to CD4, CD8 and WBC. We also showed the reproducibility of CD8+ T cell arrays and validated some of the selected target genes. We identified differentially expressed genes having roles in the immune response and apoptosis. With the gene silencing study, we identified the role of certain candidate genes influencing immune tolerance.
We analyzed the information from WBC scans by testing a few highly upregulated genes by real-time PCR, and found that upregulation occurred primarily in one or two cell subsets for each gene. For example, phosphatidylserine synthetase-2 and TNF inducible protein-2 were highly upregulated in CD8+ T cells and B cells from tolerized mice, compared with CD8+ T cells and B cells from naive mice. For some genes, downregulation occurred at the same time in other cell subsets. Thus, understanding the role each gene has in tolerance would require isolation of different cell types for functional studies, with or without gene silencing, and knock-out or knock-in strategies.
A variety of genes in the different immune subsets are differentially expressed and a few are of interest because of their possible significance in the pCons-mediated induction of tolerance. Among these genes of interest were those that have roles in apoptosis and the immune response. We performed a microarray focusing on genes related to apoptosis because previous studies have shown that peptide tolerization decreases apoptosis of CD4+ and CD8+ T cells in our model of autoimmunity.7,24,25 The apoptosis microarray revealed the differential regulation of 27 genes by at least two-fold in tolerized vs naive splenocytes (Table 2). Of these genes, several have been reported to have roles in immunity and autoimmunity. Of significance was apoptosis-related gene peroxiredoxin-2 whose expression was increased approximately threefold in tolerized splenocytes compared with naive splenocytes. Peroxiredoxin 2 (Prdx2) encodes for a protein that is a member of the Peroxiredoxin family of antioxidant proteins. Expression of Prdx2 has been reported to be important for the maintenance of anti-viral activity in CD8+ T cells.41 The knockout of Prdx2 in mice has been shown to cause an increase in T cells and dendritic cells42 and increases in cellular senescence and aging.43 In addition, Prdx2 has been reported to have an important role in the inhibition of apoptosis in a novel pathway distinct from Bcl-2.44,45 The increased expression of Prdx2 following treatment with pCons might shed light on the mechanism by which peptide tolerization decreases apoptosis.
The CD28 antigen is one of the molecules responsible for providing co-stimulatory signals for TCR activation and is downregulated 2.63-fold in our microarray analyses of CD8+ T cells. There have been reports of CD28 having both pro-apoptotic46,47 and anti-apoptotic effects.48-50 Studies on the aging immune system have found that the number of CD8+ CD28− T cells increases with age and that these cells are resistant to apoptosis, having increased bcl-2 expression and decreased caspase 3 expression.51-53 In addition, CD8+ CD28− T cells have been reported to have regulatory functions; CD8+ CD28− T cells have been implicated in tolerance in studies of cardiac transplant recipients,54 in decreased antibody production following immunization in elderly patients55 and tumor susceptibility and growth in cancer patients.56,57 The downregulation of CD28 following tolerization might also explain the anti apoptotic effect of pCons treatment.
Both of the aforementioned genes were reported to have a role in the induction of apoptosis and were interestingly differentially expressed in the apoptosis microarray. Further study of the differential regulation of these genes might provide a mechanistic basis for reduced apoptosis in CD8+ T cells following tolerization.
In addition, a few differentially expressed genes in the microarray were shown to be involved in the immune response. The platelet-activating factor receptor is a G-protein-coupled receptor that binds the platelet-activating factor, a proinflammatory phospholipid that has an important role in inflammation and platelet aggregation.58 Blocking of the Ptfar reduces proteinuria and increases survival in BWF1 lupus mice.59 Furthermore, the use of antagonists against Ptfar reduces chronic arthritis in CIA mice60 and has an anti-inflammatory effect in the treatment of patients with rheumatoid arthritis.61 Platelet-activating factor receptor was downregulated 9.2-fold (Table 1b) in splenocytes, indicating that a possible method of disease attenuation after treatment is the downregulation of genes involved in the immune response. C-type lectin domain family 2, member D (Ocil, also Clec2d, LLT1) encodes for a protein that is a lectin superfamily member and a ligand for the CD161 receptor.62 Ocil expressed on target cells is able to inhibit NK-mediated cytotoxicity and IFN-ã production by binding to CD161, but Ocil/CD161 interaction along with TCR stimulation increased IFN-ã production in T cells.62,63 Ocil was upregulated 8.0-fold (Table 3a) in the CD4+ T cell subset, indicating that cytotoxic NK cell activity in pCons-tolerized mice is being suppressed.
One group recently reported microarray studies in BWF1 mice treated with the tolerogenic peptide hCDR1 (GYYWSWIRQPPGKGEEWIG) that derives from the Ig of a human anti-DNA antibody.64 pConsensus, an artificial peptide based on sequences in the Ig of murine anti-DNA (FIEWNKLRFRQGLEW), is a 15-mer with similarity in numbers of positive or negatively charged amino acids and in some amino acid sequences. A study by Mozes’ group64 and the study reported here have quite different designs, which is likely to explain why the genes with differential expression are not the same in the two studies. Mozes and co-workers isolated RNA from splenic whole WBC of young BWF1, diseased older BWF1 and diseased older BWF1 mice treated for 10 weeks subcutaneously with hCDR1.64 Their main objective in screening 22 000 genes was to compare the ability of peptide therapy to regulate the genes overexpressed or underexpressed in diseased older mice back toward normal (that is, compared with young mice). In contrast, our design consisted of one high dose i.v. injection of pCons in young healthy BWF1 mice followed by the harvesting of spleen cells 1 week later (a time at which the generation of CD4+ and CD8+ regulatory T cells is robust).7,24,25
Recent evidence indicated that defective CD8+ T-cell-suppressive function is a feature of both murine and human SLE.1,4,5 As BWF1 mice age, their CD8+ T cells become defective, in that they are deleted rather than activated in response to activating signals.5 However, in BWF1 mice treated with pCons, some CD8+ T cells become Foxp3+ inhibitory cells secreting TGFβ; they downregulate autoantibody production and delay the onset of nephritis.7,24,25 In addition, they suppress the onset of disease when adoptively transferred to syngeneic BWF1 females.7 Therefore, we focused most of our effort on applying microarray data to further understand the molecular basis of the suppressive capacity of CD8+ T cells from tolerized animals. Our data suggest that some of the differential gene expression identified in CD8+ T cells from tolerized mice participate in this change. We also found changes in the TGFβ-signaling molecule Smad 7, which increased in tolerized CD8+ T cells suggesting a role for TGFβ and the TGFβ-signaling pathway in the tolerance mechanisms induced in our model. Our data are in agreement with others who have shown the role of TGFβ and Smad signaling in induced CD4+ regulatory T cells.65,66
Two independent analyses of the same RNA showed excellent reproducibility (Figure 3). An increased expression pattern (more than two-fold) was found for six genes in CD8+ T cells from tolerized mice using real time PCR (Ifi202b, bcl2, Trp-53, IFNaR1, CCR7 and Foxp3).
Previously,7,24,25 we showed that silencing of Foxp3 in CD8+ T cells from tolerized mice abrogated on average 70–80% of the suppressive capacity of those cells. In contrast, silencing of Trp53 or CCR7 did not alter the ability of those cells from tolerized mice to suppress in vitro IgG anti-DNA production by B cells cultured with helper CD4+ T cells from unmanipulated mice. In this study, we show that both Ifi202b and Foxp3 silencing affect other genes also. Our data are in agreement with others who have suggested that silencing a single gene with targeted siRNA or shRNA can influence multiple additional genes in a culture system.67,68
In conclusion, we have identified differentially expressed genes in WBC, CD4, CD8 and other immune cell subsets following anti-DNA Ig peptide tolerance in BWF1 mice. We showed the reproducibility of the CD8+ T cells arrays. We verified the expression pattern of selected candidate genes including interferon-inducible genes that have important roles in autoimmunity. With siRNA gene-silencing studies, we showed that silencing with siRNA for Foxp3 or Ifi202b affects expression of other genes involved in the suppressive capacity of CD8+ Treg induced in our system of immune tolerance. These studies show the potential for future therapeutic interventions and modulation of immune responses in an autoimmune model involving certain candidate genes.
Materials and methods
Mice
NZB (H-2d/d), NZW (H-2z/z) and (NZB × NZW) F1 (H-2d/z) mice were purchased from The Jackson Laboratories (Bar Harbor, ME, USA) or bred and taken care of at the University of California Los Angeles (UCLA). All mice were treated in accordance with the guidelines of the University of California Los Angeles Animal Research Committee, an Institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice were housed in pathogen-free conditions. Female mice were used for all experiments.
Peptides
The peptides used in this study, their sources, and the MHC molecules they bind are described in detail elsewhere.7 pCons (FIEWNKLRFRQGLEW), the artificial tolerizing peptide, contains T-cell determinants based on the J558 VH regions of several murine mAb anti-dsDNA from BWF1 mice.27,69,70 Peptides were synthesized at Chiron Biochemicals (San Diego, CA, USA), purified to a single peak on high-performance liquid chromatography, and analyzed by mass spectroscopy for expected amino acid content.
Treatment of mice
Ten- to twelve-week-old BWF1 mice received a single i.v. dose of 1 mg of one of the peptides, dissolved in saline, as reported previously26 for tolerance induction.
Cell isolation and staining
Spleen cells were isolated 1 week after administration of the pCons peptide from tolerized, saline-treated or naive BWF1 mice. ACK lysing buffer (Sigma, St Louis, MO, USA) was used to lyse red blood cells. Cell subsets were enriched for following incubation with anti-CD4, anti-B, anti-CD8, anti NK1.1, anti-Mac-3, anti-CD11C or anti-Gr-1 microbeads from Miltenyi Biotech (Auburn, CA, USA).
Microarray sample preparation and Gene-chip hybridization
Total RNA was isolated from different cell subsets (obtained from pooled splenocytes of 4–5 mice in each treatment group) with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and the quality of isolated RNA assessed by Agilent Bioanalyzer 2100 (Agilent Technologies, Foster City, CA, USA). Briefly, in vitro two-cycle amplification was performed to prepare biotinylated anti-sense cRNA using target labeling and a control reagent kit according to Affymetrix protocol. Biotinylated cRNA (10–15 μg) was fragmented and hybridized to Affymetrix Gene Chip array 430 2.0 (Affymetrix, Santa Clara, CA, USA) containing −45 000 murine genes. The hybridized arrays were scanned with a GCS 3000 high-resolution confocal laser scanner (Affymetrix). The reproducibility of the array was confirmed in two replicate experiments.
Microarray data analysis
Fluorescence intensities of arrays were analyzed using MAS 5.0 (Tukey Bi-weight) algorithms resident in Gene-Chip Operating Software (GCOS) 1.1 (Affymetrix) and normalized by global scaling to a target value of 250. Classification of differentially expressed genes required a minimum 2-fold change in normalized expression values between the naive and pCons-tolerized groups, a MAS 5.0 detection call of ‘present’ (P) in at least one sample, and a MAS 5.0 change call of ‘Increase’ (I) or ‘Decrease’ (D). Gene Spring 6.0 (Silicon Genetics, Santa Clara, CA, USA) and GeneSifter.net (visXlabs, Seattle, WA, USA) were used for gene ontology and pathway analysis, conditional clustering and boolean operations (Venn Diagrams). Microarray data has been deposited to the NCBI Gene Expression Ominbus (GEO) with accession number GSE13364 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13364).
Real-time PCR
Real time PCR was analyzed as described earlier.7,24 Briefly, total cellular RNA was isolated from purified cell subsets or total splenocytes from saline-treated or pCons-tolerized BWF1 mice with TRIzol (Invitrogen, Carlsbad, CA, USA) as per manufacturer’s protocol. Each experimental group consists of the pooled spleen cells of 3–4 mice from each group. 100 ng of total RNA was used with one-step RT-PCR reagents (Applied Biosystems, Foster City, CA, USA). The oligonucleotide sequences used for the primers and TaqMan probes are described in Table 5. GAPDH was used as an endogenous control in each experimental set.
Table 5.
Primers and gene ID
| Gene | Unigene ID | Forward | Reverse |
|---|---|---|---|
| Copropyrinogen oxidase | Mm291519 | CACCCCCAGGGACAAGCT | CGATCATACAACAGATTAAACTCCACAT |
| Protein kinase 2-β | Mm25594 | CCCTGGTCACTAACAAACCAAGA | TCCATGGCTAAGCATTTGACAGT |
| ADAR–RNA specific | Mm316628 | GAGATGGAGCCCTCTTTGACAA | TGGCGGGACTCTGTGCTT |
| TNFip2 | Mm255332 | GCCACATACGCCACTTGGTA | TGCTTGGTAGATTGCCCTTGA |
| Adenosine deaminase | Mm316628 | GAGATGGAGCCCTCTTTGACAA | GGCGGGACTCTGTGCTTTC |
| Ribonucleotide reductase | Mm99 | CCGCCGAGCTGGAAAGT | TTTCTCTCAGTAACGGCTCATCCT |
| GATA-binding protein-1 | Mm335973 | GCCCAAGAAGCGAATGATTG | GTGGTCGTTTGACAGTTAGTGCAT |
| Phosphatidylserine synthase 2 | Mm293591 | TGGGCTACGTGACTCTCCTAGAA | CAAAATACTGGCCACAATACCTCTCT |
| GAPDH | Mm317779 | TGAAGGTCGGTGTGAACGGATTTGGC | CTCTTTGATGTCACGCACGATTTC |
| Smad2 | Mm.391091 | TGTGCAGAGCCCCAACTGT | GCCTGGTGGGATCTTACACACT |
| Smad3 | Mm.7320 | TGGGCCTACTGTCCAATGTCA | TCCCAATGTGTCGCCTTGTA |
| Smad7 | Mm.34407 | CTGACGCGGGAAGTGGAT | TGGCGGACTTGATGAAGATG |
Table 5 indicates unigene ID, primers and probe sequences used in the article.
siRNA transfection
CD8+ T cells isolated as described above were plated and cultured in complete medium containing 10% FCS for 24 h in 12–24 well plates. Silencer siRNA Transfection Kit from Ambion (Austin, TX, USA) was used for transfection. OptiMEM reduced serum medium (Gibco/Invitrogen, Carlsbad, CA, USA) was used to dilute the siPORT amine. Validated siRNA of IFI202b, Bcl2, CCR7, PD1, IFNar1 and GAPDH as well as positive and negative siRNA controls were obtained from Ambion. The transfection agent (siPORT amine) alone served as another control. siRNA of IFI202b, IFNar1, CCR7, PD1, Bcl2 (50–100 nm), GAPDH (50–100 nm) or controls was mixed with the agent in serum-free medium and detailed siRNA transfection was performed as described earlier.7,24,25
Statistical analyses
Statistical analyses were executed using Prism 4 software (GraphPad, San Diego, CA, USA). Paired t-test, two-sample t–tests or the Mann–Whitney U-test were used for comparisons between groups. Comparisons between more than two groups were carried out by analysis of variance followed by Tukey’s test for comparison of individual groups. P-values less than 0.05 were considered significant. All tests were two-sided.
Acknowledgements
We thank the UCLA flow core facility for FACS analysis and cell sorting, Fanny Ebling for pCons tolerization, Vineet Dixit for computational assistance in figure drawing, and Dr Desmond Smith’s lab for the real-time PCR. This study was supported by NIH Grants AR54034, AI 083894, AI65645 to RPS, AR53239 to ALC, AI46776 to BHH and UCLA Senate Core Grant and Southern California Arthritis Foundation (SCAF) grant to BHH, and RPS, and gifts from the Maltz and Horchow families and from Jeanne Rappaport.
Footnotes
Conflict of interest
Dr Divyen Patel, PhD and Dr Robert J Rooney, PhD, are employed at Genome Explorations Inc., Memphis, TN, USA. All other authors are employed at UCLA, Los Angeles, CA, USA and have no conflict of interest. Drs. Hahn, La Cava and Singh have a patent through the University of California, Los Angeles for the use of pCons as an immune modulator in systemic lupus erythematosus.
References
- 1.Linker-Israeli M, Quismorio FP Jr, Horwitz DA. CD 8 lymphocytes from patients with systemic lupus erythematosus sustain, rather than suppress, spontaneous polyclonal IgG production and synergize with CD4+ cells to support autoantibody synthesis. Arthritis Rheum 1990; 33: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 2.Kotzin BL. Systemic lupus erythematosus. Cell 1996; 85: 303–306. [DOI] [PubMed] [Google Scholar]
- 3.Hahn BH. Lessons in lupus: the mighty mouse. Lupus 2001; 10: 589–593. [DOI] [PubMed] [Google Scholar]
- 4.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol 2001; 166: 6452–6457. [DOI] [PubMed] [Google Scholar]
- 5.Karpouzas GA, La Cava A, Ebling FM, Singh RR, Hahn BH. Differences between CD8+ T cells in lupus-prone (NZB × NZW) F1 mice and healthy (BALB/c × NZW) F1 mice may influence autoimmunity in the lupus model. Eur J Immunol 2004; 34: 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol 2004; 173: 3542–3548. [DOI] [PubMed] [Google Scholar]
- 7.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFβ-secreting CD8+ T cell suppressors. J Immunol 2005; 175: 7728–7737. [DOI] [PubMed] [Google Scholar]
- 8.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 1993; 259: 1321–1324. [DOI] [PubMed] [Google Scholar]
- 9.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000; 6: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 2000; 6: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 11.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR et al. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 2001; 358: 1749–1753. [DOI] [PubMed] [Google Scholar]
- 12.Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci USA 2004; 101: 4228–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22: 531–562. [DOI] [PubMed] [Google Scholar]
- 14.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD 4+CD25 regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA 2005; 102: 6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharabi A, Haviv A, Zinger H, Dayan M, Mozes E. Amelioration of murine lupus by a peptide, based on the complementarity determining region-1 of an autoantibody as compared to dexamethasone: different effects on cytokines and apoptosis. Clin Immunol 2006; 119: 146–155. [DOI] [PubMed] [Google Scholar]
- 16.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum 2008; 58: 2488–2497. [DOI] [PubMed] [Google Scholar]
- 17.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: tolerance spreading impairs pathogenic function of autoimmune T and B cells. J Immunol 1999; 162: 5775–5783. [PubMed] [Google Scholar]
- 18.Eilat E, Dayan M, Zinger H, Mozes E. The mechanism by which a peptide based on complementarity-determining region-1 of a pathogenic anti-DNA auto-Ab ameliorates experimental systemic lupus erythematosus. Proc Natl Acad Sci USA 2001; 98: 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riemekasten G, Riemekasten G, Langnickel D, Enghard P, Undeutsch R, Humrich J et al. Intravenous injection of a D1 protein of the Smith proteins postpones murine lupus and induces type 1 regulatory T cells. J Immunol 2004; 173: 5835–5842. [DOI] [PubMed] [Google Scholar]
- 20.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol 2005; 174: 3247–3255. [DOI] [PubMed] [Google Scholar]
- 21.Hahn BH, Hahn BH, Ebling F, Singh RR, Singh RP, Karpouzas G et al. Cellular and molecular mechanisms of regulation of autoantibody production in lupus. Ann N Y Acad Sci 2005; 1051: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharabi A, Sharabi A, Luger D, Ben-David H, Dayan M, Zinger H et al. The role of apoptosis in the ameliorating effects of a CDR1-based peptide on lupus manifestations in a mouse model. J Immunol 2007; 179: 4979–4987. [DOI] [PubMed] [Google Scholar]
- 23.Kang SM, Tang Q, Bluestone JA. CD4(+)CD25(+) regulatory T Cells in transplantation: progress, challenges and prospects. Am J Transplant 2007; 7: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 24.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol 2007; 178: 7649–7657. [DOI] [PubMed] [Google Scholar]
- 25.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB x NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol 2008; 180: 2069–2080. [DOI] [PubMed] [Google Scholar]
- 26.Hahn BH, Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K et al. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum 2001; 44: 432–441. [DOI] [PubMed] [Google Scholar]
- 27.Singh RR, Singh RR, Ebling FM, Albuquerque DA, Saxena V, Kumar V et al. Induction of autoantibody production is limited in nonautoimmune mice. J Immunol 2002; 169: 587–594. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Kitani A. Strober WCell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001; 194: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura K, Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol 2004; 172: 834–842. [DOI] [PubMed] [Google Scholar]
- 30.McHugh RS, McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 2002; 16: 311–323. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389–400. [DOI] [PubMed] [Google Scholar]
- 32.Rus V, Rus V, Atamas SP, Shustova V, Luzina IG, Selaru F et al. Expression of cytokine- and chemokine-related genes in peripheral blood mononuclear cells from lupus patients by cDNA array. Clin Immunol 2002; 102: 283–290. [DOI] [PubMed] [Google Scholar]
- 33.Maas K, Maas K, Chan S, Parker J, Slater A, Moore J et al. Cutting edge: molecular portrait of human autoimmune disease. J Immunol 2002; 169: 5–9. [DOI] [PubMed] [Google Scholar]
- 34.Deng YJ, Deng YJ, Huang ZX, Zhou CJ, Wang JW, You Y et al. Gene profiling involved in immature CD4+ T lymphocyte responsible for systemic lupus erythematosus. Mol Immunol 2006; 43: 1497–1507. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Zhang L, Yi Y, Kang HK, Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med 2004; 10: 411–415. [DOI] [PubMed] [Google Scholar]
- 36.Qing X, Putterman C. Gene expression profiling in the study of the pathogenesis of systemic lupus erythematosus. Auto-immun Rev 2004; 3: 505–509. [DOI] [PubMed] [Google Scholar]
- 37.Baechler EC, Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 2003; 100: 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett L, Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003; 197: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 2006; 25: 383–392. [DOI] [PubMed] [Google Scholar]
- 40.Feng X, Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 2006; 54: 2951–2962. [DOI] [PubMed] [Google Scholar]
- 41.Geiben-Lynn R, Geiben-Lynn R, Kursar M, Brown NV, Addo MM, Shau H et al. HIV-1 antiviral activity of recombinant natural killer cell enhancing factors, NKEF-A and NKEF-B, members of the peroxiredoxin family. J Biol Chem 2003; 278: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 42.Moon EY, Moon EY, Noh YW, Han YH, Kim SU, Kim JM et al. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (Prx II) gene. Immunol Lett 2006; 102: 184–190. [DOI] [PubMed] [Google Scholar]
- 43.Han YH, Han YH, Kim HS, Kim JM, Kim SK, Yu DY et al. Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett 2005; 579: 4897–4902. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Zhang P, Liu B, Kang SW, Seo MS, Rhee SG et al. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem 1997; 272: 30615–30618. [DOI] [PubMed] [Google Scholar]
- 45.Chikamori K, Chikamori K, Hill JE, Grabowski DR, Zarkhin E, Grozav AG et al. Downregulation of topoisomerase IIbeta in myeloid leukemia cell lines leads to activation of apoptosis following all-trans retinoic acid-induced differentiation/growth arrest. Leukemia 2006; 20: 1809–1818. [DOI] [PubMed] [Google Scholar]
- 46.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med 1994; 179: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu XZ, Martin PJ, Anasetti C. CD28 signal enhances apoptosis of CD8 T cells after strong TCR ligation. J Immunol 2003; 170: 3002–3006. [DOI] [PubMed] [Google Scholar]
- 48.Radvanyi LG, Radvanyi LG, Shi Y, Vaziri H, Sharma A, Dhala R et al. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol 1996; 156: 1788–1798. [PubMed] [Google Scholar]
- 49.Daniel PT, Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankenstein T et al. Costimulatory signals through B7 1/CD28 prevent T cell apoptosis during target cell lysis. J Immunol 1997; 159: 3808–3815. [PubMed] [Google Scholar]
- 50.Kerstan A, Armbruster N, Leverkus M, Hunig T. Cyclosporin A abolishes CD28-mediated resistance to CD95-induced apoptosis via superinduction of caspase-3. J Immunol 2006; 177: 7689–7697. [DOI] [PubMed] [Google Scholar]
- 51.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol 1999; 34: 633–644. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol 2002; 105: 117–125. [DOI] [PubMed] [Google Scholar]
- 53.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev 2005; 205: 147–157. [DOI] [PubMed] [Google Scholar]
- 54.Colovai AI, Colovai AI, Mirza M, Vlad G, Wang S, Ho E et al. Regulatory CD8+CD28− T cells in heart transplant recipients. Hum Immunol 2003; 64: 31–37. [DOI] [PubMed] [Google Scholar]
- 55.Saurwein-Teissl M, Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 2002; 168: 5893–5899. [DOI] [PubMed] [Google Scholar]
- 56.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother 2003; 52: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filaci G, Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol 2007; 179: 4323–4334. [DOI] [PubMed] [Google Scholar]
- 58.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci 2003; 40: 643–672. [DOI] [PubMed] [Google Scholar]
- 59.Morigi M, Morigi M, Macconi D, Riccardi E, Boccardo P, Zilio P et al. Platelet-activating factor receptor blocking reduces proteinuria and improves survival in lupus autoimmune mice. J Pharmacol Exp Ther 1991; 258: 601–606. [PubMed] [Google Scholar]
- 60.Palacios I, Palacios I, Miguélez R, Sánchez-Pernaute O, Gutierrez S, Egido J et al. A platelet-activating factor receptor antagonist prevents the development of chronic arthritis in mice. J Rheumatol 1999; 26: 1080–1086. [PubMed] [Google Scholar]
- 61.Hilliquin P, Guinot P, Chermat-Izard V, Puechal X, Menkes CJ. Treatment of rheumatoid arthritis with platelet activating factor antagonist BN 50730. J Rheumatol 1995; 22: 1651–1654. [PubMed] [Google Scholar]
- 62.Aldemir H, Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol 2005; 175: 7791–7795. [DOI] [PubMed] [Google Scholar]
- 63.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL et al. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol 2005; 175: 7796–7799. [DOI] [PubMed] [Google Scholar]
- 64.Elmann A, Elmann A, Sharabi A, Dayan M, Zinger H, Ophir R et al. Altered gene expression in mice with lupus treated with edratide, a peptide that ameliorates the disease manifestations. Arthritis Rheum 2007; 56: 2371–2381. [DOI] [PubMed] [Google Scholar]
- 65.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007; 178: 2018–2027. [DOI] [PubMed] [Google Scholar]
- 66.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 2008; 180: 7112–7116. [DOI] [PubMed] [Google Scholar]
- 67.Butz NV, Gronostajski RM, Campbell CE. T-box proteins differentially activate the expression of the endogenous interferon gamma gene versus transfected reporter genes in non-immune cells. Gene 2006; 377: 130–139. [DOI] [PubMed] [Google Scholar]
- 68.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 2005; 307: 430–433. [DOI] [PubMed] [Google Scholar]
- 69.Eilat E, Zinger H, Nyska A, Mozes E. Prevention of systemic lupus erythematosus-like disease in (NZBxNZW)F1 mice by treating with CDR1− and CDR3-based peptides of a pathogenic autoantibody. J Clin Immunol 2000; 20: 268–278. [DOI] [PubMed] [Google Scholar]
- 70.Tsao BP, Tsao BP, Ebling FM, Roman C, Panosian-Sahakian N, Calame K et al. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J Clin Invest 1990; 85: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]