Abstract
Background:
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hematological disease. The development of complement inhibitors such as eculizumab, ravulizumab, and pegcetacoplan has revolutionized the management of PNH, leading to improvements in overall survival and quality of life for patients.
Objectives:
This systematic review aims to provide comprehensive evidence of the efficacy of complement inhibitors in relation to treatment duration.
Design:
This is a systematic review and meta-analysis.
Data sources and methods:
A thorough literature search was conducted in MEDLINE, EMBASE, and the Cochrane Library up to 3 May 2022. We included all prospective interventional studies including single-arm trials. The primary outcomes of interest were lactate dehydrogenase (LDH) levels, hemoglobin (Hb) concentrations, transfusion avoidance, and Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) scores.
Results:
Our study included a total of 27 studies, comprising 5 randomized controlled trials and 11 single-arm trials, with a total of 912 patients with PNH. We stratified the studies according to treatment duration, based on the most frequently reported period of 26 weeks. Our analysis showed that treatment-naïve patients who received complement inhibitors had a pooled estimate of a decrease in LDH levels from baseline by −1462.0 U/L (95% CI: −1735.6 to −1188.5) for treatment ⩽26 weeks and −1696.5 U/L (95% CI: −2122.7 to −1270.2) for treatment >26 weeks. The mean Hb levels were increased by 1.4 g/dL (95% CI: 0.5–2.3) and 1.9 g/dL (95% CI: 0.7−3.1) in each group. Treatment with any complement inhibitor prevented the need for transfusion in at least 50% of patients with PNH in all treatment periods. Clinically meaningful improvements in FACIT-F were observed both before and after 26 weeks, with a pooled estimate of 6.8 (95% CI: 6.0−7.6) and 9.5 (95% CI: 7.0−12.0), respectively.
Conclusion:
Our findings suggest that complement inhibitors can result in positive treatment outcomes and sustained benefits for patients with PNH.
Keywords: clinical outcomes, complement inhibitors, eculizumab, paroxysmal nocturnal hemoglobinuria, pegcetacoplan, rare disease, ravulizumab
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hematological disorder with an estimated incidence of 1.5–2 new cases per 1 million individuals per year.1,2 PNH is characterized by complement-mediated hemolysis, bone marrow dysfunction, and thrombosis caused by a mutation in the phosphatidylinositol glycan anchor biosynthesis class A (PIGA) gene.3,4 Before the introduction of eculizumab, the first-in-class complement component 5 inhibitor (C5 inhibitor) approved in 2007, treatment options for patients with PNH were primarily supportive, including blood transfusions, erythropoiesis-stimulating agents and steroids, or allogeneic bone marrow transplantation, which involves considerable risks of transplant-related mortality. 5
The development of eculizumab has changed the paradigm of PNH management, resulting in improvements in overall survival and quality of life for patients.6,7 However, several unmet needs persisted, including residual intravascular hemolysis due to incomplete C5 inhibition, 8 component 3 (C3)-mediated extravascular hemolysis, 9 and the treatment burden associated with frequent infusions. 10 To address these issues, novel complement inhibitors have been developed, resulting in the approval of two additional drugs: ravulizumab, a long-acting C5 inhibitor, in 2018, and pegcetacoplan, a complement C3 inhibitor, in 2021. Nevertheless, despite recent advancements, there are limited guidelines or consensus on how to incorporate these new treatments into clinical practice for PNH. This may be attributed to the scarcity of robust clinical evidence derived from large-scale trials owing to the rarity of PNH. While pivotal randomized clinical trials (RCTs) have been conducted to obtain regulatory approval for complement inhibitors, their sample sizes are relatively small, and the comparative efficacy data are restricted to a 26-week randomization period.11–15 Although several systematic reviews on specific complement inhibitors exist,16–18 a comprehensive analysis of short- and long-term efficacy data for all available treatments using unified criteria is still lacking. Considering that clinical trials of rare diseases have limited sample sizes, a comprehensive approach is required to establish optimal treatment strategies. 19
Therefore, this study aims to conduct a systematic review and meta-analysis of the latest data on all complement inhibitors approved for PNH. The primary objective is to provide comprehensive evidence on the efficacy of these inhibitors according to treatment duration to support clinical decision-making. With the rapidly evolving landscape of complement inhibitors for PNH treatment, this study can help fill the knowledge gap and provide insights for clinicians on the optimal treatment strategies.
Materials and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.20,21 The study protocol was registered with the National Institute Preferred Reporting Items for Systematic for Health Research PROSPERO, International Prospective Register of Systematic Reviews (CRD42023394298; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023394298).
Database search
A comprehensive literature search was conducted without any restrictions on language, date, or document type, using the MEDLINE, EMBASE, and Cochrane Registry of Clinical Trials (CENTRAL) databases. The search covered the inception of each database until 3 May 2022, and used the following search terms: ‘paroxysmal nocturnal hemoglobinuria’, ‘eculizumab’, ‘ravulizumab’, and ‘pegcetacoplan’. The search strategies used for each database are listed in Supplemental Materials. On 8 March 2023, we conducted a manual search of MEDLINE using trial titles and identification numbers as search terms, to identify any publications from May 2022 to March 2023 that reported additional data for the trials originally included in our review.
Inclusion criteria
The inclusion criteria were as follows: prospective interventional studies [including single-arm studies and randomized controlled trials (RCTs)]; patients diagnosed with PNH; patients treated with eculizumab, ravulizumab, or pegcetacoplan; and studies reporting efficacy endpoints, including changes in lactate dehydrogenase (LDH) levels, hemoglobin (Hb) levels, transfusion avoidance, and Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) scores.
The exclusion criteria were as follows: retrospective studies, observational studies, in vitro or in vivo experiments, and studies with incomplete data on the targeted outcomes. Review articles reporting relevant outcome data from clinical trials, conference abstracts, and posters were not excluded from consideration.
Two reviewers (JL and HL) independently screened the titles and abstracts of all retrieved studies for eligibility after removing duplicates using EndNote 20 software (Clarivate). Eligible studies underwent full-text analysis to confirm their inclusion in the meta-analysis. The reviewers compared their findings at each selection stage and resolved any disagreements through discussion. In cases where further inconsistencies arose, a third reviewer (HSS) was consulted to resolve them.
Outcome measures
LDH and Hb levels, as well as transfusion dependence, are directly associated with the clinical symptoms and complications of PNH. 22 LDH is released into the bloodstream after cellular damage or destruction and is useful for assessing the response to treatment because LDH levels decrease with a reduction in the rate of hemolysis. 23 An increase in Hb levels indicates an improvement in anemia, which is a common complication of PNH. Transfusion avoidance, defined as the proportion of patients not requiring transfusion during treatment, indicates sufficient improvement in the patient’s condition to avoid the need for regular blood transfusions. The FACIT-F is a validated measure that evaluates the impact of disease and treatment on fatigue in patients with chronic illnesses such as PNH.24,25 It is a 13-item questionnaire with scores ranging from 0 to 52, with higher scores indicating less fatigue. An increase in FACIT-F scores signifies a reduction in the impact of fatigue on the patient’s quality of life. 25
Data extraction
Two reviewers (JL and HL) independently extracted data from the studies using a standardized form, including information on the author, publication year, study design and title, duration, number and sex ratio of participants, prior exposure to complement inhibitors, treatment type, and dose, LDH and Hb levels, transfusion avoidance, and FACIT-F scores. Any differences in opinion between the reviewers were resolved through discussion with a third reviewer (HSS). Continuous data for the meta-analyses were presented as mean and standard deviation (SD), and different data formats from the studies were converted as necessary. For example, data reported as the standard error of the mean were converted to SD using the formula SD = SEM × √n (where n is the sample size). Median and interquartile range or minimum to maximum values were converted to mean and SD using a method proposed by Wan et al. 26 In cases where changes from baseline values for continuous outcomes were not reported, they were calculated using baseline and endpoint values with an imputed correlation factor derived from the average correlation factor of the other included studies that reported all necessary data.
Data analysis
The study employed a narrative summary approach with summary tables to describe the efficacy data of the RCTs. Single-arm meta-analyses were conducted using the statistical software R (version 4.2.2; R Core Team 2022) and the ‘meta’ package to determine the pooled effect size. The analysis was stratified by the treatment period and divided into two groups: those reported within 26 weeks and those reported after more than 26 weeks. For continuous variables, such as LDH, Hb, and FACIT-F, we calculated the pooled effect size of the change from baseline for each substance and the integrated total change from baseline for all three complement inhibitors combined. The estimated effect size was reported as the mean difference with a 95% confidence interval. For transfusion avoidance, which is defined as the proportion of patients not requiring transfusion during treatment, a meta-analysis of event rates was performed using logit transformation. Heterogeneity among studies was assessed using χ2 and I2 statistics. A random-effects model was applied when significant heterogeneity was detected among the included studies (defined as I2 > 50%), a fixed-effects model was adopted otherwise. Statistical significance was set at p < 0.05.
Quality assessment
For the RCTs, we assessed the quality and risk of bias using the Cochrane Risk of Bias 2 tool, 27 which assigned each trial a risk of bias grade of ‘low’, ‘high’, or ‘some concerns’ based on our assessment. The methodological quality of non-randomized studies was evaluated using the Methodological Index for Non-Randomized Studies (MINORS) tool. 28 As all non-randomized studies in this meta-analysis were single-arm trials, we evaluated eight items from the MINORS tool for non-comparative studies. Each item was scored on a scale of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate), with a maximum possible score of 16. Two reviewers (JL and HL) independently assessed the quality of all studies included in this systematic review, and any discrepancies were resolved through discussion and consensus.
Results
Selection of studies
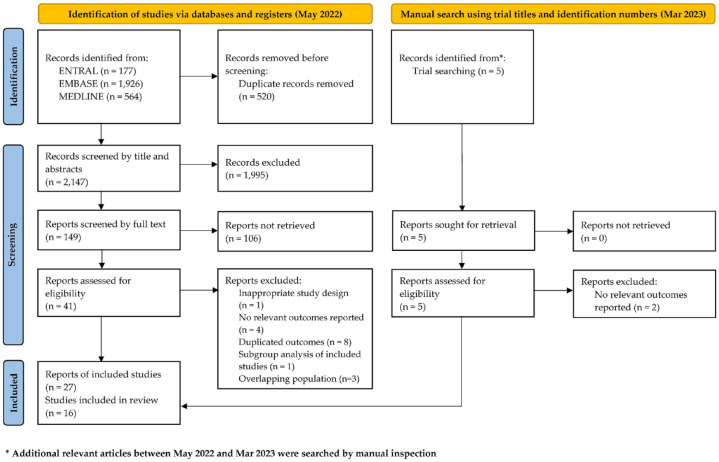
An initial electronic database search identified a total of 2667 records, which were subsequently screened for eligibility. After removing duplicates and manually screening titles and abstracts, 2515 records were excluded. We evaluated the full text of the remaining 149 records and excluded 125 that did not meet the eligibility criteria for the meta-analysis. A total of 24 records that reported the outcomes of 16 clinical trials were included. In a manual search conducted on 8 March, we found five publications and added three with relevant outcomes. Therefore, our final analysis included 27 records from 16 clinical trials (Figure 1). Although 11 of the included records reported on overlapping cohorts, we decided to include them because each article provided complementary data (Figure 1).
Figure 1.
Study flow chart based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline.
The studies included in our analysis involved 912 patients with PNH, of whom 50.5% were female. The patients treated with eculizumab, ravulizumab, or pegcetacoplan were in 5 RCTs, and 11 were in single-arm trials. In all, 13 studies enrolled treatment-naïve patients,12,14,15,29–47 while two enrolled patients previously treated with eculizumab,11,13,48,49 and one enrolled both treatment-naïve and eculizumab-treated patients. 50 Two studies focused on pediatric populations,35,50 while the remaining 14 enrolled adult patients. The duration of the studies varied from 5 weeks to over 66 weeks. Table 1 provides a summary of the characteristics of the included studies.
Table 1.
Characteristics of included studies.
| Study title | Design | PNH patients | Intervention/comparator | Patients no. (% female) | References | Study duration | Outcomes reported (used in analysis) |
|---|---|---|---|---|---|---|---|
| TRIUMPH | RCT | Naïve | Eculizumab/placebo | 87 (59.8%) | Hillmen et al. 200612 | 26 weeks | LDH, Hb, TA, FACIT |
| STUDY-301 | RCT | Naïve | Ravulizumab/eculizumab | 246 (45.5%) | Lee et al. 201914 | 26 weeks | LDH, Hb, TA, FACIT |
| Schrezenmeier et al. 202029 | 52 weeks | TA, FACIT | |||||
| STUDY-302 | RCT | Treated | Ravulizumab/eculizumab | 195 (49.7%) | Kulasekararaj et al. 201913 | 26 weeks | LDH, Hb, TA, FACIT |
| Kulasekararaj et al. 202148 | 52 weeks | LDH, TA, FACIT | |||||
| PRINCE | RCT | Naïve | Pegcetacoplan/SOC | 53 (45.3%) | Wong et al. 202130 | 26 weeks | LDH, Hb, TA |
| Wong 202215 | 26 weeks | LDH, Hb, TA, FACIT | |||||
| PEGASUS | RCT | Treated | Pegcetacoplan/eculizumab | 80 (61.3%) | Hillmen et al. 202111 | 16 weeks | LDH, Hb, TA, FACIT |
| de Latour et al. 202249 | 48 weeks | LDH, Hb, TA | |||||
| SHEPHERD | NRSI | Naïve | Eculizumab | 97 (50.5%) | Young et al. 200632 | 26 weeks | TF |
| Muus et al. 200733 | 52 weeks | LDH | |||||
| Schubert et al. 200834 | 52 weeks | Hb | |||||
| Brodsky et al. 200831 | 52 weeks | LDH, Hb, TA, FACIT | |||||
| M07-005 | NRSI | Naïve | Eculizumab | 7 (57.1%) | Reiss et al. 201435 | 12 weeks | LDH, Hb, FACIT |
| AEGIS | NRSI | Naïve | Eculizumab | 29 (51.7%) | Kanakura et al. 201137 | 12 weeks | LDH, Hb, TA, FACIT |
| Kanakura et al. 201336 | 66 weeks | LDH, Hb, FACIT | |||||
| X03-001 | NRSI | Naïve | Eculizumab | 11 (45.5%) | Hillmen et al. 200439 | 12 weeks | LDH |
| Hill et al. 200538 | 64 weeks | LDH, Hb | |||||
| KOREA | NRSI | Naïve | Eculizumab | 6 (50.0%) | Kim et al. 201040 | 24 weeks | LDH, Hb, TA, FACIT |
| BRAZIL | NRSI | Naïve | Eculizumab | 16 (62.5%) | Ghidettti et al. 201241 | 5 weeks | LDH |
| STUDY-103 | NRSI | Naïve | Ravulizumab | 13 (53.8%) | Lee et al. 201642 | 24 weeks | LDH, TA |
| STUDY-201 | NRSI | Naïve | Ravulizumab | 26 (23.1%) | Roth et al. 201843 | 40 weeks | LDH, Hb, FACIT |
| STUDY-304 | NRSI | Mixed | ravulizumab | 13 (69.2%) | Kulagin et al. 202150 | 26 weeks | LDH, TA |
| PADDOCK | NRSI | Naïve | Pegcetacoplan | 23 (43.5%) | Wong et al. 201845 | 12 weeks | Hb, TA |
| Wong et al. 201944 | 12 weeks | FACIT | |||||
| PALOMINO | NRSI | Naïve | Pegcetacoplan | 4 (75.0%) | Wong et al. 202146 | 52 weeks | LDH, Hb, FACIT |
| Wong et al. 202247 | 52 weeks | LDH, TA, FACIT |
FACIT, Functional Assessment of Chronic Illness Therapy; Hb, hemoglobin; LDH, lactate dehydrogenase; NRSI, non-randomized studies on interventions; PNH, paroxysmal nocturnal hemoglobinuria; RCT, randomized controlled trial; SOC, standard of care; TA, transfusion avoidance.
Summary of RCTs
Five RCTs were conducted on three complement inhibitors: one study compared eculizumab and placebo, 12 two studies compared ravulizumab and eculizumab,13,14,29,48 and two studies compared pegcetacoplan with standard of care (SOC)15,30 or eculizumab11,49 as controls. All studies were multicenter phase III clinical trials and reported four common outcomes of interest, with a primary endpoint of fewer than 26 weeks. The subjects in the RCTs were divided into two groups: treatment-naïve patients and patients who had previously been treated with eculizumab; treated patients showed better baseline values than treatment-naïve patients. Although there were no direct comparison trials between ravulizumab and SOC, ravulizumab is expected to be more effective because previous studies have demonstrated that eculizumab is superior to placebo 12 and that ravulizumab is non-inferior to eculizumab.13,14,29,48 Due to the absence of head-to-head RCTs on ravulizumab and pegcetacoplan, it was challenging to directly compare the effectiveness of the two treatments.Table 2 summarizes the efficacy outcomes reported in the RCTs.
Table 2.
Summary of efficacy outcomes from RCTs.
| Study title | Treatment | Endpoint | LDH concentration* | Hb concentration | Transfusion avoidance | FACIT-F score |
|---|---|---|---|---|---|---|
| TRIUMPH (Naïve) | Eculizumab | 26 weeks | Baseline: 2199.7 ± 157.7 U/L$
Endpoint: 327.3 ± 67.6 U/L$ |
Baseline: 10.0 ± 0.2 g/dL$
Endpoint: 10.1 ± 0.2 g/dL$ |
22/43 (52.1%) | Baseline: 36.7 ± 10.5 CFB: 6.4 ± 1.2$ |
| Placebo | Baseline: 2258.0 ± 154.8 U/L$
Endpoint: 2418.9 ± 140.3 U/L$ |
Baseline: 9.7 ± 0.2 g/dL$
Endpoint: 8.9 ± 0.2 g/dL$ |
0/44 (0.0%) | Baseline: 34.3 ± 12.0 CFB: −4.0 ± 1.7$ |
||
| Difference |
p < 0.001 Superior |
p < 0.001 Superior |
p < 0.0001 Superior |
p < 0.0001 Superior |
||
| STUDY-301 (Naïve) | Ravulizumab | 26 weeks | Baseline: 1633.5 ± 778.8 U/L %CFB: −76.84% (−79.96, −73.73) |
Hemoglobin stabilization rate: 68.0% (59.82, 76.18) | 92/125 (73.6%) | CFB: 7.07‡ |
| Eculizumab | Baseline: 1578.3 ± 727.1 U/L %CFB: −76.02% (−79.20, −72.83) |
Hemoglobin stabilization rate: 64.5% (55.93, 72.99) | 80/121 (66.1%) | CFB: 6.40‡ | ||
| Difference | −0.83% (−5.21, 3.56) Non-inferior |
2.9% (−8.80, 14.64) Non-inferior |
6.8% (−4.66, 18.14) Non-inferior |
0.67 points (−1.21, 2.55) Non-inferior |
||
| STUDY-302 (Treated) | Ravulizumab | 26 weeks | Baseline: 228 ± 48.7 U/L %CFB: −0.82% (−7.80, 6.10) |
Hemoglobin stabilization rate: 76.3% (67.0, 84.8) | 85/97 (87.6%) | Baseline: 43 CFB: 2.01 ± 0.697$,‡ |
| Eculizumab | Baseline: 235.2 ± 49.7 U/L %CFB: 8.40% (1.50, 15.30) |
Hemoglobin stabilization rate: 75.5% (67.0, 84.0) | 81/98 (82.7%) | Baseline: 41 CFB: 0.54 ± 0.704$,‡ |
||
| Difference | −9.2% (−18.84, 0.42) Non-inferior |
1.4% (−10.4, 13.3) Non-inferior |
5.5% (−4.27, 15.68) Non-inferior |
1.47 points (−0.21, 3.15) Non-inferior |
||
| PRINCE (Naïve) | Pegcetacoplan | 26 weeks | CFB: −1870.5 ± 101.0 U/L$,‡
Endpoint: 204.6 ± 90.0 U/L |
CFB: 2.9 ± 0.4 g/dL$,‡
Endpoint:12.8 ± 2.1 g/dL |
32/35 (91.4%) | CFB: 7.78 ± 1.210$,‡
Endpoint: 45.3 ± 7.3 |
| SOC | CFB: −400.1 ± 313.0 U/L$,‡
Endpoint: 1535.0 ± 751.6 U/L |
CFB: 0.3 ± 0.8 g/dL$,‡
Endpoint: 9.8 ± 2.4 g/dL |
1/18 (5.6%) | CFB: 3.26 ± 2.113$,‡
Endpoint: 39.6 ± 10.3 |
||
| Difference |
p < 0.0001 Superior |
p = 0.0019 Superior |
p < 0.0001 Superior |
p = 0.061 Not significant |
||
| PEGASUS (Treated) | Pegcetacoplan | 16 weeks | Baseline: 257.5 ± 97.6 U/L CFB: −15 ± 42.7 U/L |
Baseline: 8.69 ± 1.08 g/dL CFB: 2.4 ± 0.4 g/dL$ |
35/41 (85.4%) | Baseline: 32.2 ± 11.4 CFB: 9.2 ± 1.6$,‡ |
| Eculizumab | Baseline: 308.6 ± 284.8 U/L CFB: −10 ± 71.0 U/L |
Baseline: 8.68 ± 0.89 g/dL CFB: −1.5 ± 0.7 g/dL$ |
6/39 (15.4%) | Baseline: 31.6 ± 12.5 CFB: −2.7 ± 2.8$,‡ |
||
| Difference | –5.0 U/L (–181.3, 172.0) Non-inferiority not proven |
p < 0.0001 Superior |
p < 0.001 Superior |
11.9 points (5.49, 18.25) Non-inferiority not tested |
||
CFB, change from baseline; FACIT, Functional Assessment of Chronic Illness Therapy; Hb, hemoglobin; LDH, lactate dehydrogenase; RCT, randomized controlled trial; SOC, standard of care; TA, transfusion avoidance.
Of note, the upper limit of the normal range for LDH is generally under 250 U/L in adults, although the reference values exhibit slight variability among different laboratory settings. In the TRIUMPH, STUDY-301, STUDY-302, and PEGASUS trials, the upper limits of the normal LDH range were referenced as 223, 246, 246, and 226 U/L, respectively.
Continuous outcomes are presented as mean and standard deviation unless otherwise specified; $standard error, ‡least-square mean.
Effect of complement inhibitors on LDH level
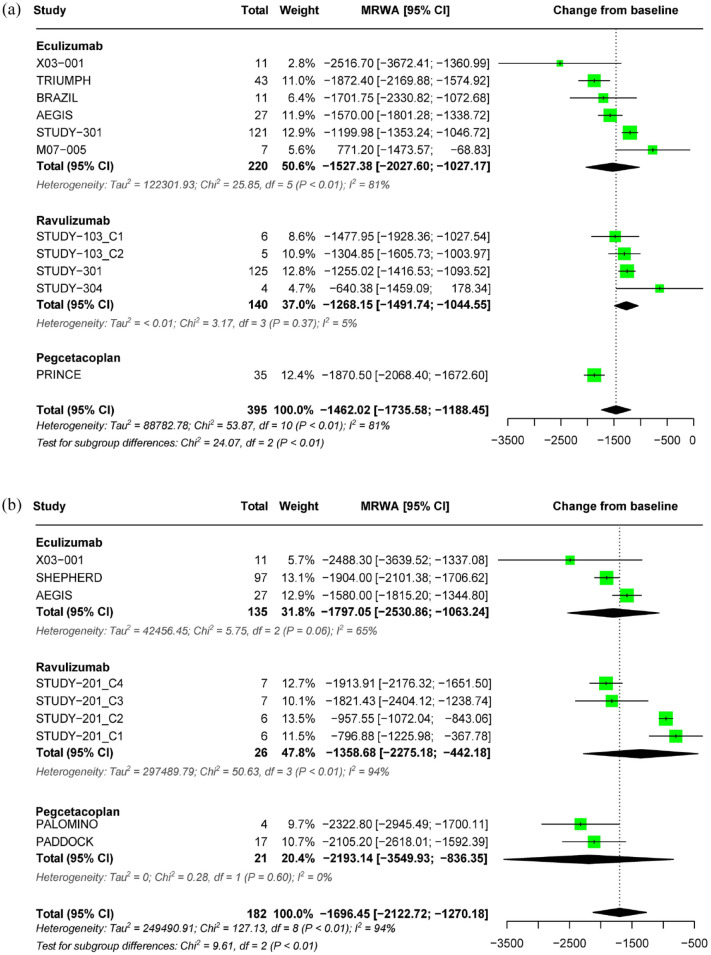
In the RCTs, both eculizumab and pegcetacoplan demonstrated better efficacy in LDH outcomes than placebo in treatment-naïve patients with PNH. Ravulizumab is non-inferior to eculizumab. In patients with PNH treated with eculizumab, switching to ravulizumab or pegcetacoplan did not result in a significant change in LDH levels (Table 2). The meta-analysis showed that, in treatment-naïve patients with PNH who received complement inhibitors for less than 26 weeks, there was a pooled estimate of a decrease in LDH levels from baseline by −1462.02 U/L (95% CI: −1735.58 to −1188.45), with considerable heterogeneity observed (I2 = 81%, p < 0.01). Subgroup analysis revealed that the decrease in LDH levels was –1527.38 U/L (95% CI: −2027.60 to −1027.17) for eculizumab, −1268.15 U/L (95% CI: −1491.74 to −1044.55) for ravulizumab, and −1870.50 U/L (95% CI: −2068.4 to −1672.6) for pegcetacoplan (Figure 2(a)). In patients treated for more than 26 weeks, the pooled estimate showed a decrease in LDH levels from baseline by −1696.45 U/L (95% CI: −2122.72 to −1270.18, I2 = 94%). There was a trend toward a decrease in LDH with longer treatment (>26 weeks) in all treatment subgroups (Figure 2(b)).
Figure 2.
Forest plot of LDH outcomes. (a) Pooled effect of complement inhibitors on LDH concentration in treatment-naïve PNH patients (⩽26 weeks) and (b) pooled effect of complement inhibitors on LDH concentration in treatment-naïve PNH patients (>26 weeks).
LDH, lactate dehydrogenase; PNH, paroxysmal nocturnal hemoglobinuria.
Effect of complement inhibitors on Hb level
In an RCT, eculizumab showed a significant increase in Hb concentration compared to placebo in treatment-naïve patients. Ravulizumab demonstrated non-inferiority to eculizumab in terms of the Hb stabilization rate. In treatment-naïve patients, pegcetacoplan resulted in a significant increase in Hb concentration compared to SOC, and in patients who switched from eculizumab, it produced a greater increase compared to eculizumab (Table 2).
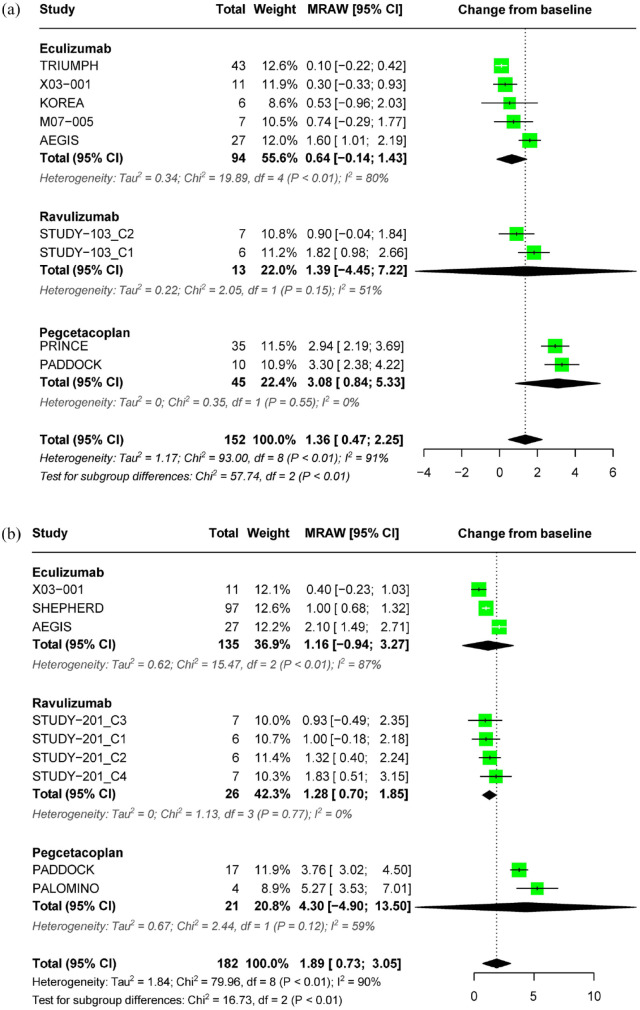
In treatment-naïve patients with PNH who received complement inhibitor for less than 26 weeks, a pooled estimate showed a significant increase in Hb levels from baseline (1.36 g/dL; 95% CI: 0.47–2.25) with considerable heterogeneity across included studies (I2 = 91%, p < 0.01). Subgroup analysis revealed a pooled estimate of 0.64 g/dL (95% CI: –0.14–1.43) for eculizumab, 1.39 g/dL (95% CI: −4.45–7.22) for ravulizumab, and 3.08 g/dL (95% CI: 0.84–5.33) for pegcetacoplan. Heterogeneity was particularly high in the eculizumab subgroup (I2 = 80%, p < 0.01) (Figure 3(a)). In patients treated for over 26 weeks, the pooled estimate showed a significant increase in Hb levels from baseline by 1.89 g/dL (95% CI: 0.73–3.05, I2 = 90%) (Figure 3(b)). The effect size of pegcetacoplan on Hb change was numerically higher than that of C5 inhibitors in both analyses of treatment duration ⩽26 weeks and >26 weeks.
Figure 3.
Forest plot of hemoglobin outcomes. (a) Pooled effect of complement inhibitors on Hb concentration in treatment-naïve PNH patients (⩽26 weeks) and (b) pooled effect of complement inhibitors on Hb concentration in treatment-naïve PNH patients (>26 weeks).
Hb, hemoglobin; PNH, paroxysmal nocturnal hemoglobinuria.
Effect of complement inhibitors on transfusion avoidance
The results of RCTs indicate that eculizumab and pegcetacoplan were effective in reducing transfusion dependence compared to placebo in treatment-naïve patients with PNH. Ravulizumab was found to be non-inferior to eculizumab in both treatment-naïve and eculizumab-treated patients. Furthermore, switching to pegcetacoplan resulted in a significant improvement in transfusion dependence for patients previously treated with eculizumab, as compared to those who continued with eculizumab treatment (Table 2).
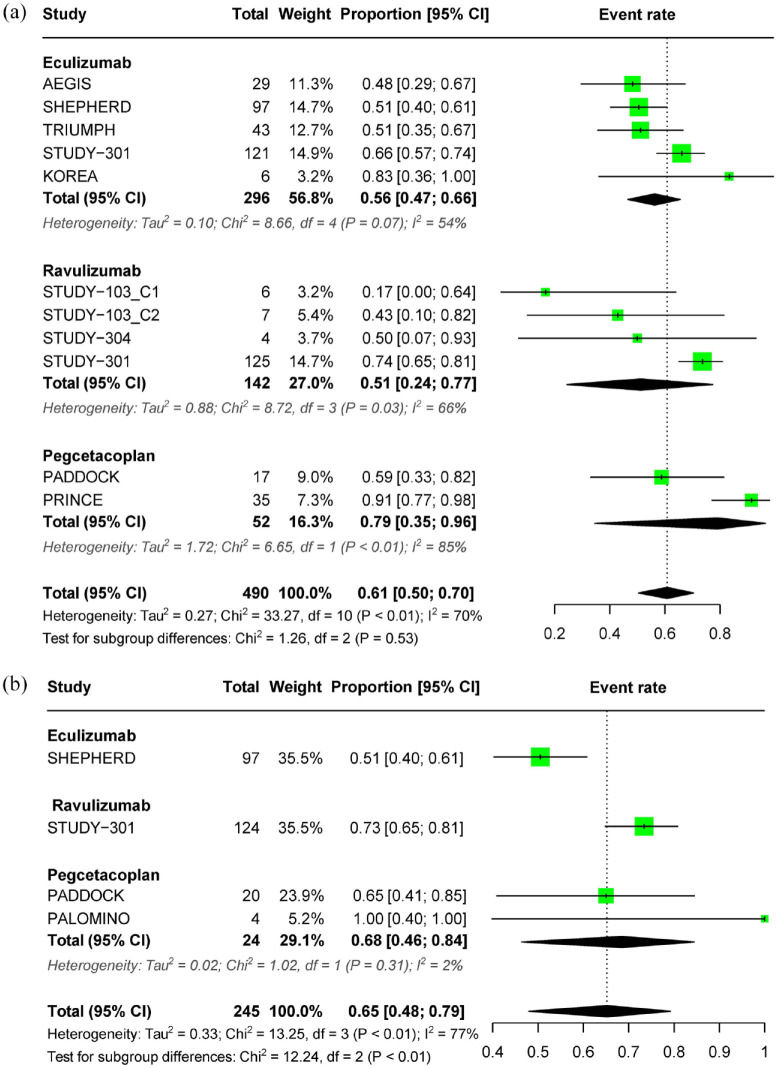
In treatment-naïve patients with PNH who received complement inhibitors for less than 26 weeks, the pooled estimate for transfusion avoidance was 0.61 (95% CI: 0.50–0.70), with significant heterogeneity observed (I2 = 70%, p < 0.01). The respective subgroup estimates for eculizumab, ravulizumab, and pegcetacoplan were 0.56 (95% CI: 0.47–0.66), 0.51 (95% CI: 0.24–0.77), and 0.79 (95% CI: 0.35–0.96) (Figure 4(a)). For patients treated for more than 26 weeks, the pooled estimate was 0.65 (95% CI: 0.48–0.79, I2 = 77%) (Figure 4(b)). Treatment with any complement inhibitor prevented the need for transfusion in at least 50% of patients with PNH, not only for treatment lasting ⩽26 weeks but also for a period longer than 26 weeks.
Figure 4.
Forest plot of transfusion outcomes. (a) Pooled effect of complement inhibitors on transfusion avoidance in treatment-naïve PNH patients (⩽26 weeks) and (b) pooled effect of complement inhibitors on transfusion avoidance in treatment-naïve PNH patients (>26 weeks).
PNH, paroxysmal nocturnal hemoglobinuria.
Effect of complement inhibitors on FACIT-F score
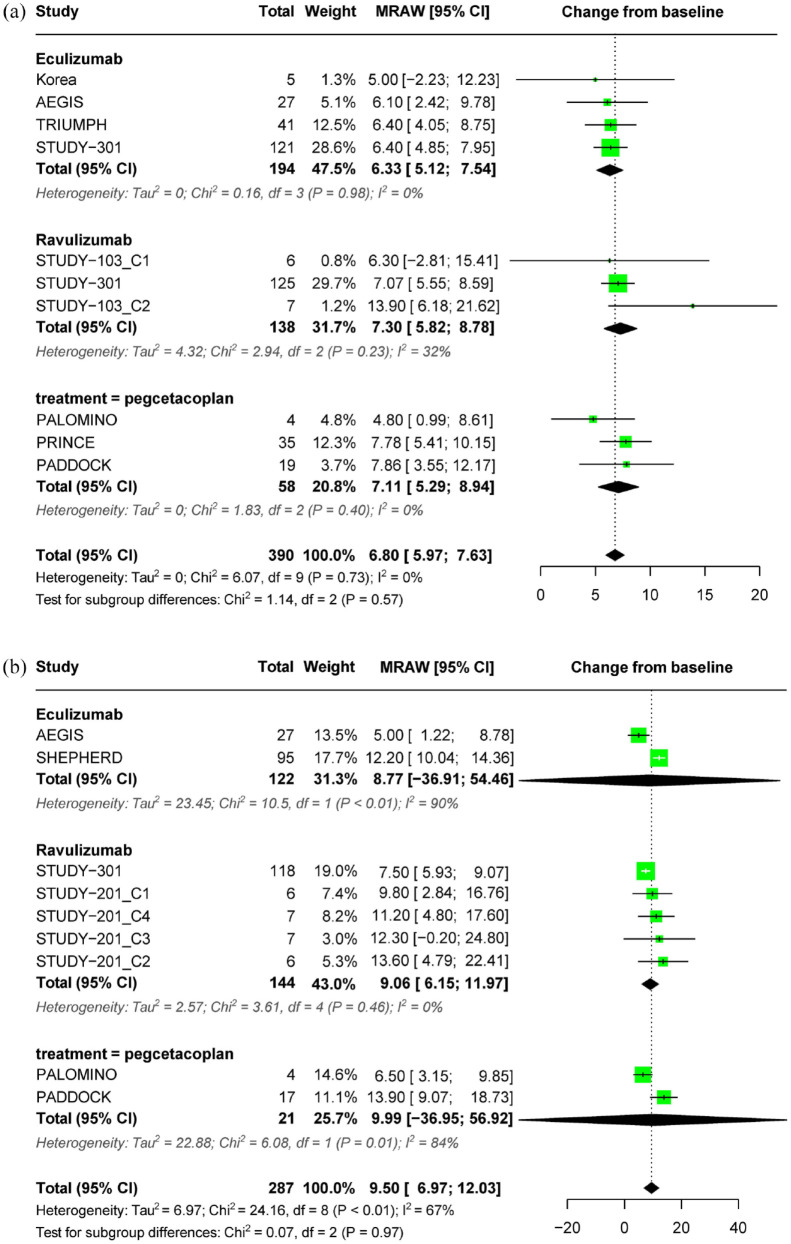
In a pivotal RCT, eculizumab demonstrated a significant improvement in fatigue levels compared to placebo in treatment-naïve patients with PNH (Table 2). Ravulizumab was non-inferior to eculizumab in terms of changes in FACIT-F scores, regardless of prior eculizumab treatment. On the other hand, in patients who had previously received eculizumab treatment, pegcetacoplan resulted in a significant improvement in fatigue levels compared to eculizumab. However, in treatment-naïve patients, pegcetacoplan did not show a significant improvement compared to the standard care.
For treatment-naïve patients with PNH who received complement inhibitors for less than 26 weeks, the mean change in fatigue levels from baseline, as measured by the FACIT-F score, was 6.80 (95% CI: 5.97–7.63), with no significant heterogeneity observed (I2 = 0%, p = 0.72). The subgroup estimates for eculizumab, ravulizumab, and pegcetacoplan were 6.33 (95% CI: 5.12–7.54), 7.30 (95% CI: 5.82–8.78), and 7.11 (95% CI: 5.29–8.94), respectively (Figure 5(a)). For patients treated for more than 26 weeks, the mean change from baseline was 9.50 (95% CI: 6.97–12.03, I2 = 67%) (Figure 5(b)).
Figure 5.
Forest plot of FACIT-F outcomes. (a) Pooled effect of complement inhibitors on FACIT-F score in treatment-naïve PNH patients (⩽26 weeks) and (b) pooled effect of complement inhibitors on FACIT-F score in treatment-naïve PNH patients (>26 weeks).
FACIT-F, Functional Assessment of Chronic Illness Therapy Fatigue; PNH, paroxysmal nocturnal hemoglobinuria.
In the treatment duration of less than 26 weeks, the average FACIT-F score for treatment-naïve PNH patients was 44.61 (95% CI: 42.91–46.32) points, with subgroup estimates for eculizumab, ravulizumab, and pegcetacoplan at 44.40 (95% CI: 40.72–48.08), 40.68 (95% CI: 35.47–45.88), and 45.30 (95% CI: 43.24–47.36) points, respectively. Beyond the 26-week mark, these scores shifted to 43.30 (95% CI: 39.40–47.20), 42.27 (95% CI: 40.08–44.46), and 47.28 (95% CI: 39.86–54.70) points, which remained consistent with those observed within the initial 26 weeks.
Quality assessment of included studies
Nine RCTs and 18 non-randomized trials were evaluated using the ROB 2.0 and MINORS tools, respectively. The risk of bias in the RCTs was assessed and one study (11.1%) was rated as low risk, five (55.6%) as having some concern, and three (33.3%) as high risk. Potential sources of bias included randomization (88.9%), deviation from the intended intervention (44.4%), and selective reporting (22.2%). The overall quality of the non-randomized studies was limited due to their single-arm design and abstract publications. The scores of the included publications ranged from 6 to 12, with an average score of 8.8 as evaluated using the MINORS tool. The results of the quality assessment are presented in the Supplemental Materials.
Discussion
This study conducted a systematic review to gather all available evidence from prospective interventional studies on complement inhibitors currently approved for patients with PNH. Subsequently, a comprehensive descriptive analysis of the comparative efficacy reported in the RCTs was conducted, and the pooled effect size of complement inhibitors was estimated by performing a meta-analysis that integrated data from both RCTs and single-arm studies. The RCTs included in this study were pivotal clinical trials conducted to evaluate the safety and efficacy of eculizumab, ravulizumab, and pegcetacoplan. Randomization was maintained until the primary endpoint, which was 26 weeks, except for one trial of pegcetacoplan for 16 weeks. The results from the RCTs indicated that eculizumab and pegcetacoplan were more efficacious than the SOC in treatment-naïve patients with PNH. In eculizumab-treated patients, switching to pegcetacoplan generally resulted in better outcomes than maintaining eculizumab, except for LDH levels, which were not significantly different. Ravulizumab was not inferior to eculizumab in treatment-naïve or eculizumab-treated patients. While there has been no head-to-head trial comparing ravulizumab and pegcetacoplan, recent matching-adjusted indirect comparison (MAIC) studies have provided additional evidence in favor of pegcetacoplan.51,52 However, the evidence was constrained by examining outcomes only up to the primary endpoint of each trial, limiting the demonstration of treatment effectiveness beyond 26 weeks. In addition, the limited availability of head-to-head trials comparing the outcomes of complement inhibitors prevents the possibility of conducting meta-analyses to establish their comparative efficacy.
To address the limitations of previous research, we conducted a study aimed at estimating the efficacy of complement inhibitors both within and beyond the 26-week primary endpoint using consistent outcome measures for comparability. Our meta-analysis of pooled data from all available prospective studies revealed the consistent efficacy of complement inhibitors in improving various clinical outcomes with continued treatment for over 26 weeks. As the studies included in the analysis reported results from different endpoints, we extracted outcomes reported from 2 to 26 weeks and 32 to 66 weeks to estimate the pooled effect size within and beyond 26 weeks.
The LDH changes within 26 weeks were similar to those observed in the RCTs, and when used beyond 26 weeks, LDH levels tended to decrease slightly more in all treatments. Notably, pegcetacoplan showed the greatest reduction across all periods, which is consistent with the findings of an MAIC study indicating that pegcetacoplan was associated with statistically significant improvements in both absolute and percent reductions in LDH levels (p < 0.0001). 49 Although previous research only evaluated the improvement in Hb levels up to 26 weeks in RCTs, meta-analyses that included single-arm studies indicated that the change in Hb levels achieved within the first 26 weeks remained stable over time. Pegcetacoplan is superior to C5 inhibitors in terms of the change in Hb levels from baseline in RCT and MAIC studies, with the largest numerical change observed in meta-analysis as well.
In patients with PNH, LDH is primarily considered as a marker for intravascular hemolysis, and Hb levels are associated with the overall clinical response, including both intravascular hemolysis and extravascular hemolysis.53,54 Eculizumab and ravulizumab aim to reduce hemolysis by inhibiting terminal complement C5, whereas pegcetacoplan inhibits proximal complement C3. Proximal inhibitors act at an early stage of the complement activation system, effectively controlling both intravascular and C3-mediated extravascular hemolysis. 55 By contrast, terminal inhibitors do not adequately address extravascular hemolysis. 9 This difference may account for the superior hematologic response compared to terminal inhibitors. However, it is notable that incomplete inhibition of proximal C3 may potentially lead to a more substantial breakthrough intravascular hemolysis through the enzymatic cascade, as compared to incomplete terminal inhibition. 55 Breakthrough hemolysis (BTH), which refers to a sudden reappearance of intravascular hemolysis despite treatment with complement inhibitors, is a critical concern in current PNH therapy. 8 It is important to note that clinical endpoints more relevant to the prognosis of PNH patients, including BTH and thrombo-embolism, are gaining significance in the era of complement inhibitors. 53 While the phase III RCTs of the long-acting C5 inhibitor ravulizumab did not demonstrate a significant difference in the average change in LDH levels compared to eculizumab, there was a noticeable decrease in the occurrence of BTH attributed to improved C5 inhibition. 8 Pegcetacoplan also demonstrated a lower incidence of BTH in its phase III trial compared to eculizumab. However, all patients who experienced BTH in the pegcetacoplan treatment group reported a rapid increase in LDH levels exceeding three times the ULN, and three out of four patients with BTH returned to treatment with eculizumab. 11
In our meta-analysis, more than 50% of the patients with PNH treated with complement inhibitors avoided transfusion during all periods, and the initial efficacy was sustained over 26 weeks. However, it is notable that many patients continued to receive blood transfusions. Despite the clear therapeutic benefits of complement inhibitors, unmet needs persist in patients with PNH. While newer drugs have shown higher potential for efficacy, a significant proportion of patients with PNH still experience suboptimal hematological recovery and remain reliant on transfusions,54,56 highlighting the need for further research and the development of novel therapies.
The use of complement inhibitors may have a positive impact on the quality of life of patients with PNH. Notably, all treatments showed a score change exceeding 5 points within 26 weeks of treatment, which represents a clinically important difference in the FACIT-F scale for PNH patients. 57 The meta-analysis showed similar outcomes beyond 26 weeks, suggesting that the three treatments have comparable long-term effectiveness. Our findings were consistent with the RCTs and MAIC results in that there were no significant differences in the FACIT-F scores among the three complement inhibitors.
In 2022, Kulasekararaj et al. 58 reported a 2-year extension of pivotal trials for ravulizumab. Although this report was not included in our meta-analysis due to the format of the outcome reporting, it is highly regarded as a significant clinical outcome, as it represents the longest follow-up for more than 400 patients with PNH. Similar to the context of our results, where effects within 26 weeks of treatment initiation persisted beyond 26 weeks for all complement inhibitors (utilizing data up to 66 weeks), ravulizumab demonstrated that the effects observed during the randomization period (0–26 weeks) were sustained into the extension period (27 weeks to 2 years) across most evaluated outcomes.
Until very recently, the options for complement inhibitors in the clinical field of PNH treatment were limited to just three original products. However, the landscape has expanded with the development of biosimilar medicines that have demonstrated equivalence to eculizumab, even in terms of non-inferiority.59–61 In the first half of 2023, two biosimilar medicines consecutively received approval from the European Medicines Agency. It is worth noting that approximately 70% of PNH patients treated with eculizumab do not adhere to the regimen, and over 60% discontinue treatment within 5 years, often due to considerable healthcare costs. 56 This suggests the ongoing need for the development of biosimilar medicines or innovative therapies to address issues of limited access to care and adherence. Novel proximal complement inhibitors directed toward factor B and factor D have also shown promising results in recent developments.62–65 As treatment options for PNH continue to increase, it is crucial to establish a consensus on optimal treatment strategies using existing options while ensuring the seamless integration of new ones. Although our study has limitations, it provides valuable insights into enhancing clinical treatment strategies for PNH by evaluating the efficacy of all current treatment options on key outcome measures. Given the challenges of conducting large-scale, long-term clinical trials for rare diseases such as PNH, it may be necessary to consider alternative approaches that use real-world data, such as registry and claims data, to assess the comparative effectiveness of complement inhibitors. Such an approach can provide valuable evidence on long-term outcomes, safety, and cost-effectiveness and may help identify subgroups of patients who are most likely to benefit from each treatment.
Limitations
While several systematic reviews have been conducted previously on complement inhibitors for PNH treatment,16–18 they have focused on individual substances or provided a descriptive review of results from clinical trials. No systematic reviews, including those of ravulizumab, have been conducted. This study is the first comprehensive evaluation of the effectiveness of all approved complement inhibitors in treating PNH by quantifying their collective impact on critical efficacy outcomes, including patient-reported quality-of-life measures.
However, our findings should be interpreted with caution due to several limitations. To conduct a comprehensive assessment of all available evidence on complement inhibitors for PNH treatment, we performed single-arm meta-analyses to provide integrated effect sizes by treatment duration, supplementing the results of the RCTs. However, the use of a single-arm meta-analysis may introduce bias, potentially affecting the validity of the results and limiting their generalizability. Furthermore, substantial heterogeneity was observed for most outcome measures. Sensitivity analyses were performed to understand the source of heterogeneity, taking into account factors such as age group (pediatric), sample size (<10), treatment duration (<12 weeks), the dose of treatment (out of recommended dose in the label), and outcome measure (least-square mean). However, these factors did not fully explain the heterogeneity. The inclusion of studies with a high risk of bias and low-quality records to permit a comprehensive review may have contributed to the observed heterogeneity.
It is important to note that the majority of the trials included in our study involved treatment-naïve patient cohorts and the meta-analysis was exclusively based on these treatment-naïve cohorts due to the limited number of trials conducted with treatment-experienced PNH patients. In addition, there was an imbalance in sample sizes among treatments in the meta-analysis, with a relatively small patient population for pegcetacoplan compared to eculizumab or ravulizumab. Furthermore, with regard to Hb outcomes, we were unable to include the pivotal phase III clinical trials for ravulizumab, specifically STUDY-301 14 along with its extension study. 29 This was due to the reporting of the outcome as the proportion of patients with stabilized Hb in the study, making it unfeasible to derive numerical values for estimating changes from baseline in Hb. These limitations could potentially influence the generalizability and robustness of the results.
In our analysis of continuous outcomes, we used several imputed means and SDs. This was due to inconsistent reporting of data in various formats across different studies and the absence of reported variability in some instances, particularly in abstracts. While it is preferable to report the original trial data instead of trying to recover missing mean or standard deviation values, excluding data can introduce potential bias and decrease precision. 66 In the case of PNH, a rare disease with limited data from large-scale clinical trials, we chose to incorporate all available data for a more robust analysis rather than exclude it.
Conclusions
This study demonstrated that the use of complement inhibitors can lead to positive treatment outcomes in PNH, including improvements in LDH and Hb levels, transfusion dependence, and FACIT-F scores within and beyond 26 weeks of treatment. However, the lack of head-to-head trials comparing the efficacy of different complement inhibitors makes it difficult to confirm their effectiveness. Alternative approaches using real-world data may be necessary to confirm the comparative effectiveness of drugs for the treatment of rare diseases.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207231216080 for Efficacy of complement inhibitors for patients with paroxysmal nocturnal hemoglobinuria: a systematic review and meta-analysis by Jiyeon Lee, Haeseon Lee, Siin Kim and Hae Sun Suh in Therapeutic Advances in Hematology
Acknowledgments
None.
Footnotes
ORCID iDs: Jiyeon Lee  https://orcid.org/0009-0003-8687-2722
https://orcid.org/0009-0003-8687-2722
Haeseon Lee  https://orcid.org/0000-0003-3956-9758
https://orcid.org/0000-0003-3956-9758
Siin Kim  https://orcid.org/0000-0002-4368-7389
https://orcid.org/0000-0002-4368-7389
Hae Sun Suh  https://orcid.org/0000-0003-3445-6607
https://orcid.org/0000-0003-3445-6607
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jiyeon Lee, Department of Regulatory Science, Graduate School, Kyung Hee University, Seoul, Republic of Korea; Institute of Regulatory Innovation Through Science, Kyung Hee University, Seoul, Republic of Korea.
Haeseon Lee, Department of Regulatory Science, Graduate School, Kyung Hee University, Seoul, Republic of Korea; Institute of Regulatory Innovation Through Science, Kyung Hee University, Seoul, Republic of Korea.
Siin Kim, College of Pharmacy, Woosuk University, Wanju-gun, Republic of Korea.
Hae Sun Suh, Department of Regulatory Science, Graduate School, Kyung Hee University, Seoul, Republic of Korea; Institute of Regulatory Innovation Through Science, Kyung Hee University, Seoul, Republic of Korea; College of Pharmacy, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 02447, Republic of Korea.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Jiyeon Lee: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing – original draft; Writing – review & editing.
Haeseon Lee: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Writing – original draft; Writing – review & editing.
Siin Kim: Methodology; Supervision; Validation; Writing – review & editing.
Hae Sun Suh: Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant (21153MFDS601) from the Ministry of Food and Drug Safety in 2023.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers 2017; 3: 17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahin F, Ozkan MC, Mete NG, et al. Multidisciplinary clinical management of paroxysmal nocturnal hemoglobinuria. Am J Blood Res 2015; 5: 1–9. [PMC free article] [PubMed] [Google Scholar]
- 3. Brodsky RA, Hu R. PIG-A mutations in paroxysmal nocturnal hemoglobinuria and in normal hematopoiesis. Leuk Lymphoma 2006; 47: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 4. Martí-Carvajal AJ, Anand V, Cardona AF, et al. Eculizumab for treating patients with paroxysmal nocturnal hemoglobinuria. Cochrane Database Syst Rev 2014; 10: CD010340. [DOI] [PubMed] [Google Scholar]
- 5. Santarone S, Bacigalupo A, Risitano AM, et al. Hematopoietic stem cell transplantation for paroxysmal nocturnal hemoglobinuria: long-term results of a retrospective study on behalf of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Haematologica 2010; 95: 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Fontbrune FS, de Latour RP. Ten years of clinical experience with eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Semin Hematol 2018; 55: 124–129. [DOI] [PubMed] [Google Scholar]
- 7. Peipert JD, Kulasekararaj AG, Gaya A, et al. Patient preferences and quality of life implications of ravulizumab (every 8 weeks) and eculizumab (every 2 weeks) for the treatment of paroxysmal nocturnal hemoglobinuria. PLoS One 2020; 15: e0237497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodsky RA, Peffault de, Latour R, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica 2021; 106: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood 2009; 113: 4094–4100. [DOI] [PubMed] [Google Scholar]
- 10. Groth M, Singer S, Niedeggen C, et al. Development of a disease-specific quality of life questionnaire for patients with aplastic anemia and/or paroxysmal nocturnal hemoglobinuria (QLQ-AA/PNH)-report on phases I and II. Ann Hematol 2017; 96: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2021; 384: 1028–1037. [DOI] [PubMed] [Google Scholar]
- 12. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2006; 355: 1233–1243. [DOI] [PubMed] [Google Scholar]
- 13. Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood 2019; 133: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood 2019; 133: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong RSM. Safety and efficacy of pegcetacoplan in paroxysmal nocturnal hemoglobinuria. Ther Adv Hematol 2022; 13: 20406207221114673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva Pires YM, de Fátima Bonetti A, Ciecilinsky JT, et al. Efficacy and safety of current treatments for paroxysmal nocturnal hemoglobinuria: a systematic review. Clin Immunol Commun 2023; 3: 37–41. [Google Scholar]
- 17. Shah S, Chamlagain R, Musalman ZH, et al. Pegcetacoplan in paroxysmal nocturnal hemoglobinuria: a systematic review on efficacy and safety. Res Pract Thromb Haemost 2022; 6: e12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou S, Dong X, Chen C, et al. Efficacy and safety of eculizumab for paroxysmal nocturnal hemoglobinuria: a systematic review and meta-analysis. J Pediatr Hematol/Oncol 2021; 43: 203–210. [DOI] [PubMed] [Google Scholar]
- 19. Forman J, Taruscio D, Llera VA, et al. The need for worldwide policy and action plans for rare diseases. Acta Paediatr 2012; 101: 805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88: 105906. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waheed A, Kaufhold S, Liu C-R, et al. A systematic literature review of clinical and economic outcomes for patients with paroxysmal nocturnal hemoglobinuria in clinical trials and real-world settings. Blood 2022; 140: 11451–11452. [Google Scholar]
- 23. Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers 2015; 2015: 635670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13: 63–74. [DOI] [PubMed] [Google Scholar]
- 25. Weitz I, Meyers G, Lamy T, et al. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern Med J 2013; 43: 298–307. [DOI] [PubMed] [Google Scholar]
- 26. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 28. Slim K, Nini E, Forestier D, et al. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 29. Schrezenmeier H, Kulasekararaj A, Mitchell L, et al. One-year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naive to complement inhibitor therapy: open-label extension of a randomized study. Ther Adv Hematol 2020; 11: 2040620720966137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong RS, Navarro JR, Comia NS, et al. Efficacy and safety of pegcetacoplan treatment in complement-inhibitor naïve patients with paroxysmal nocturnal hemoglobinuria: results from the Phase 3 PRINCE study. Blood 2021; 138: 606. [Google Scholar]
- 31. Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 2008; 111: 1840–1847. [DOI] [PubMed] [Google Scholar]
- 32. Young NS, Antonioli E, Rotoli B, et al. Safety and efficacy of the terminal complement inhibitor eculizumab in patients with paroxysmal nocturnal hemoglobinuria: interim Shepherd Phase III clinical study. Blood 2006; 108: 971. [Google Scholar]
- 33. Muus P, Luzzatto L, Rotoli B, et al. OP04 Safety and efficacy of the terminal complement inhibitor eculizumab in patients with paroxysmal nocturnal hemoglobinuria: Shepherd Phase III clinical study results. Leuk Res 2007; 31: S32–S33. [Google Scholar]
- 34. Schubert J, Hillmen P, Roth A, et al. Eculizumab, a terminal complement inhibitor, improves anaemia in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2008; 142: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reiss UM, Schwartz J, Sakamoto KM, et al. Efficacy and safety of eculizumab in children and adolescents with paroxysmal nocturnal hemoglobinuria. Pediatr Blood Cancer 2014; 61: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 36. Kanakura Y, Ohyashiki K, Shichishima T, et al. Long-term efficacy and safety of eculizumab in Japanese patients with PNH: AEGIS trial. Int J Hematol 2013; 98: 406–416. [DOI] [PubMed] [Google Scholar]
- 37. Kanakura Y, Ohyashiki K, Shichishima T, et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: the AEGIS clinical trial. Int J Hematol 2011; 93: 36–46. [DOI] [PubMed] [Google Scholar]
- 38. Hill A, Hillmen P, Richards SJ, et al. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood 2005; 106: 2559–2565. [DOI] [PubMed] [Google Scholar]
- 39. Hillmen P, Hall C, Marsh JCW, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 2004; 350: 552–559. [DOI] [PubMed] [Google Scholar]
- 40. Kim JS, Lee JW, Kim BK, et al. The use of the complement inhibitor eculizumab (Soliris(R)) for treating Korean patients with paroxysmal nocturnal hemoglobinuria. Korean J Hematol 2010; 45: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghidettti M, Catarino D, Carvalho A, et al. Clinical features and outcome of 16 patients with PNH treated with eculizumab in Sao Paulo-Brazil. Haematologica 2012; 97: 681. Conference Abstract. [Google Scholar]
- 42. Lee JW, Bachman ES, Aguzzi R, et al. Immediate, complete, and sustained inhibition of C5 with ALXN1210 reduces complement-mediated hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH): interim analysis of a dose-escalation study. Blood 2016; 128: 2428. Conference Abstract. [Google Scholar]
- 43. Roth A, Rottinghaus ST, Hill A, et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv 2018; 2: 2176–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong R, Pullon H, Deschatelets P, et al. Inhibition of C3 with APL-2 results in normalisation of markers of intravascular and extravascular haemolysis in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2019; 185: 7. Conference Abstract.30426468 [Google Scholar]
- 45. Wong RSM, Pullon HWH, Deschatelets P, et al. Inhibition of C3 with APL-2 results in normalisation of markers of intravascular and extravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH). Blood 2018; 132: 2314. Conference Abstract.30467190 [Google Scholar]
- 46. Wong R, Ignatova K, Pullon H, et al. C3 Inhibition with pegcetacoplan in patients with paroxysmal nocturnal hemoglobinuria: results from the Paddock and Palomino trials. Br J Haematol 2021; 193: 187–188. Conference Abstract. [Google Scholar]
- 47. Wong RSM, Pullon HWH, Amine I, et al. Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria. Ann Hematol 2022; 101: 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kulasekararaj AG, Hill A, Langemeijer S, et al. One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab. Eur J Haematol 2021; 106: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Latour RP, Szer J, Weitz IC, et al. Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial. Lancet Haematol 2022; 9: e648–e659. [DOI] [PubMed] [Google Scholar]
- 50. Kulagin A, Chonat S, Maschan A, et al. Pharmacokinetics, pharmacodynamics, efficacy and safety of ravulizumab in children and adolescents with paroxysmal nocturnal hemoglobinuria: interim analysis of a phase 3, openlabel study. HemaSphere 2021; 5: 260–261. Conference Abstract. [Google Scholar]
- 51. Bhak RH, Mody-Patel N, Baver SB, et al. Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison. Curr Med Res Opin 2021; 37: 1913–1923. [DOI] [PubMed] [Google Scholar]
- 52. Wong R, Fishman J, Wilson K, et al. Comparative effectiveness of pegcetacoplan versus ravulizumab and eculizumab in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria: a matching-adjusted indirect comparison. Adv Ther 2023; 40: 1571–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol 2019; 10: 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kulasekararaj AG, Kuter DJ, Griffin M, et al. Biomarkers and laboratory assessments for monitoring the treatment of patients with paroxysmal nocturnal hemoglobinuria: differences between terminal and proximal complement inhibition. Blood Rev 2023; 59: 101041. [DOI] [PubMed] [Google Scholar]
- 55. Notaro R, Luzzatto L, et al. Breakthrough hemolysis in PNH with proximal or terminal complement inhibition. N Engl J Med 2022; 387: 160–166. [DOI] [PubMed] [Google Scholar]
- 56. Cheng WY, Sarda SP, Mody-Patel N, et al. Real-world healthcare resource utilization (HRU) and costs of patients with paroxysmal nocturnal hemoglobinuria (PNH) receiving eculizumab in a US population. Adv Ther 2021; 38: 4461–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cella D, Johansson P, Ueda Y, et al. Clinically important difference for the FACIT-Fatigue scale in paroxysmal nocturnal hemoglobinuria: a derivation from international PNH registry patient data. Blood 2021; 138: 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kulasekararaj AG, Griffin M, Langemeijer S, et al. Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies. Eur J Haematol 2022; 109: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chow V, Pan J, Chien D, et al. A randomized, double-blind, single-dose, three-arm, parallel group study to determine pharmacokinetic similarity of ABP 959 and eculizumab (Soliris((R))) in healthy male subjects. Eur J Haematol 2020; 105: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kulagin AD, Ptushkin VV, Lukina EA, et al. Randomized multicenter noninferiority phase III clinical trial of the first biosimilar of eculizumab. Ann Hematol 2021; 100: 2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jang JH, Gomez RD, Bumbea H, et al. A phase III, randomised, double-blind, multi-national clinical trial comparing SB12 (proposed eculizumab biosimilar) and reference eculizumab in patients with paroxysmal nocturnal haemoglobinuria. EJHaem 2023; 4: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jang JH, Wong L, Ko BS, et al. Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study. Blood Adv 2022; 6: 4450–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kulasekararaj AG, Risitano AM, Maciejewski JP, et al. Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab. Blood 2021; 138: 1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Risitano AM, Kulasekararaj AG, Lee JW, et al. Danicopan: an oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica 2021; 106: 3188–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Risitano AM, Röth A, Soret J, et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol 2021; 8: e344–e354. [DOI] [PubMed] [Google Scholar]
- 66. Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol 2018; 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207231216080 for Efficacy of complement inhibitors for patients with paroxysmal nocturnal hemoglobinuria: a systematic review and meta-analysis by Jiyeon Lee, Haeseon Lee, Siin Kim and Hae Sun Suh in Therapeutic Advances in Hematology