Abstract
Utilizing the comprehensive Nationwide Inpatient Sample (NIS) database, we examined the impact of thrombotic thrombocytopenic purpura (TTP) on the outcomes of patients with coronavirus disease-19 (COVID-19), emphasizing the potential role of the ADAMTS13 enzyme in disease pathogenesis and evolution. We analyzed extensive data from the NIS database using STATA v.14.2 and accounted for potential confounders using multivariate regression analysis to uphold the validity and reliability of the study. Among 1 050 045 adult patients hospitalized with COVID-19, only 300 (0.03%) developed TTP. These patients were younger (mean age 57.47 vs 64.74, P < .01) and exhibited a higher prevalence of preexisting conditions, such as congestive heart failure (13.33% vs 16.82%, P value not provided) and end-stage renal disease (3.33% vs 3.69%, P value not provided). On multivariate regression analysis, COVID-19 patients with concomitant TTP demonstrated a significant increase in mortality (adjusted odds ratio [AOR] 3.99, P < .01), venous thromboembolism (AOR 3.33, P < .01), acute kidney injury (AOR 7.36, P < .01), gastrointestinal bleeding (AOR 10.75, P < .01), intensive care unit admission (AOR 14.42, P < .01), length of hospital stay (17.42 days, P < .01), and total hospitalization charges ($298 476, P < .01). Thrombotic thrombocytopenic purpura in COVID-19 patients elevates the risk of mortality and complications, likely driven by the thrombotic nature of TTP. Our data underline the potential significance of ADAMTS13 in COVID-19 and TTP pathophysiology, suggesting its possible role as a therapeutic target.
Keywords: COVID-19, thrombotic thrombocytopenic purpura, Nationwide Inpatient Sample, thrombosis, venous thromboembolism, ADAMTS13
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare, life-threatening thrombotic microangiopathy (TMA) characterized by hemolytic anemia, thrombocytopenia, fever, neurological symptoms, and renal failure. 1 Thrombotic thrombocytopenic purpura can be initiated by a virus-induced immune response, leading to a decrease in the level of ADAMTS-13, an enzyme responsible for the cleavage of von Willebrand factor (vWF). 2 Although no established link exists between coronavirus disease-19 (COVID-19) and TTP, reports suggest the development of TTP as a complication in some COVID-19 patients. 3 Uniquely, patients with COVID-19-associated TTP have presented atypical manifestations compared to traditional TTP cases, with the absence of fever in all reported instances and neurological symptoms in 27.3% of cases. 3
At present, existing data are not robust enough to deduce the influence of TTP onset on the prognosis of COVID-19 patients. Remarkably, no large-scale observational studies have evaluated the impact of COVID-19-associated TTP on morbidity and mortality. To fill this knowledge gap, we conducted a retrospective study on a national scale utilizing the National Inpatient Sample (NIS) database. Our investigation centered on adults aged 18 years and over who were diagnosed with COVID-19, with the specific goal of discerning the impact of TTP on COVID-19 outcomes, as well as the associated healthcare costs.
Methodology
Database Information
This retrospective cohort study leveraged data from the NIS database for 2020. Recognized as the largest publicly accessible all-payer inpatient healthcare database, the NIS covers 48 states and captures more than 98% of the US population. It contains de-identified data from approximately 7 million hospitalizations annually, which, when weighted, represents approximately 35 million hospital stays across the US Key variables in NIS include the International Classification of Diseases, Clinical Modification diagnoses and procedure codes, gender, age, race, median household income for the ZIP Code, length of hospital stay (LOS), and total hospitalization costs. Hospitals are sampled based on 5 characteristics: geographic region, control, bed size, teaching status, and location. A 20% stratified sample is subsequently extracted from all US community hospitals, barring rehabilitation, and long-term acute care facilities. Each discharge within this data set is then weighed to ensure national representation. 4
Study Population and Variables
Our study included adult patients (aged ≥ 18 years) admitted with a principal diagnosis of COVID-19, which was established using the International Classification of Diseases, Tenth Revision, Clinical Modifications (ICD-10-CM) codes. These patients were further categorized based on the presence of a concomitant TTP. To identify patients who had a concurrent TTP, we combined patients who had a secondary diagnosis TTP and received therapeutic plasma exchange and/or Caplacizumab. The ICD-10-CM diagnoses and procedure codes employed in our study are presented in Supplemental Table 1.
This study aimed to evaluate the outcomes of COVID-19 in patients with and without TTP. The outcomes assessed included all-cause inpatient mortality, acute myocardial infarction (AMI), acute cerebrovascular accident (CVA), lower extremity or pulmonary vessel venous thromboembolism (LE DVT/PE), acute kidney injury (AKI), AKI requiring dialysis, gastrointestinal bleeding (GIB), blood transfusion, and intensive care unit (ICU) admission. Further, resource utilization, measured by total hospitalization charges and LOS, was compared between the 2 groups. Although the NIS contains only de-identified data, approval from the institutional review board was sought, and our study was exempted from a comprehensive review process.
Statistical Analysis
The statistical analysis was performed using STATA, version 14.2 (StataCorp). Multivariate linear and logistic regression analyses were employed for confounders with a P value ≤.2 in the univariate regression analysis. Additionally, variables deemed clinically significant to the outcome as per previous literature were included in the analysis irrespective of their statistical significance in the univariate analysis. The final set of confounders comprised the patient's age, gender, race, median income in the patient's ZIP code, insurance status, hospital bed size, hospital teaching status, hospital location and region, end-stage renal disease (ESRD), obesity, and the Charlson Comorbidity Index that accounts for 17 comorbidities. 5 The analysis was further adjusted for acute respiratory failure (ARF), sepsis, and organ failure to account for the severity of COVID-19 infection. Linear regression analysis was employed to calculate the mean for continuous outcomes, whereas logistic regression analysis was used for comparison of binary outcomes. Student t test was utilized to compare continuous variables, while Fisher exact test was employed for categorical variables. All P values were 2-sided, with the threshold for statistical significance set at P < .05.
Results
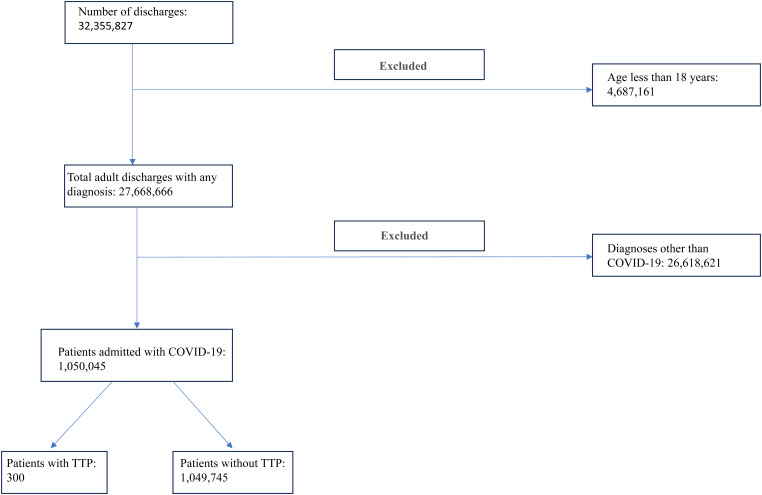
The study encompassed 1 050 045 adult patients hospitalized for COVID-19. Of this pool, a mere 300 (0.03%) patients developed TTP (Figure 1). Patients whose COVID-19 infection was complicated by TTP were comparatively younger (mean age 57.47 vs 64.74, P < .03) (Table 1). Relative to patients without TTP, those with TTP were more likely to have preexisting conditions, such as congestive heart failure (CHF) (13.33% vs 16.82%, P = .49) and ESRD (3.33% vs 3.69%, P = .88). Moreover, patients with TTP were more frequently admitted to teaching hospitals (90% vs 68.95%, P < .01), predominantly in the Midwest (18.33% vs 23.29%) and West (11.67% vs 17.19%) regions (P = .51).
Figure 1.
Flowchart depicting the patient selection process.
Table 1.
Baseline Characteristics.
| Baseline characteristics | COVID-19 + age >18 y = 1,050,045 | P value | |
|---|---|---|---|
| With TTP (n = 300) | Without TTP (n = 1,049,745) | ||
| Age [mean (95% confidence interval)] (years) | 57.47 (53.04-61.90) | 64.74 (64.58-66.90) | <.01 |
| Female gender, % | 53.33 | 47.19 | .24 |
| Race, % | .36 | ||
| White | 45.45 | 55.51 | |
| Black | 25.45 | 19.46 | |
| Hispanic | 27.27 | 21.63 | |
| Asians | 1.82 | 3.40 | |
| Charlson's Comorbidity Index, % | .37 | ||
| 1 | 28.57 | 38.59 | |
| 2 | 28.57 | 22.59 | |
| ≥3 | 42.86 | 38.81 | |
| Median household income in the patient's zip code (quartile), % | .44 | ||
| First (0-25th) | 32.76 | 34.24 | |
| Second (26th-50th) | 24.14 | 27.67 | |
| Third (51st-75th) | 31.03 | 21.91 | |
| Fourth (76th-100th) | 12.07 | 16.18 | |
| Insurance status, % | .34 | ||
| Medicare | 43.86 | 55.05 | |
| Medicaid | 15.79 | 12.21 | |
| Private | 33.33 | 29.15 | |
| Uninsured | 7.02 | 3.59 | |
| Hospital region, % | .51 | ||
| Northeast | 25.00 | 17.67 | |
| Midwest | 18.33 | 23.29 | |
| South | 45.00 | 41.84 | |
| West | 11.67 | 17.19 | |
| Hospital bed size, % | .22 | ||
| Small | 8.05 | 25.71 | |
| Medium | 35.63 | 28.91 | |
| Large | 56.32 | 45.38 | |
| Hospital teaching status, % | <.01 | ||
| Nonteaching | 10.00 | 31.05 | |
| Teaching | 90.00 | 68.95 | |
| Hospital location, % | .21 | ||
| Rural | 5.00 | 11.75 | |
| Urban | 95.00 | 88.25 | |
| Hypertension, % | 43.33 | 41.92 | .82 |
| Chronic obstructive pulmonary disease, % | 15.00 | 23.49 | .08 |
| Congestive heart failure, % | 13.33 | 16.82 | .49 |
| End-stage renal disease, % | 3.33 | 3.69 | .88 |
| Obesity, % | 40.00 | 28.41 | .07 |
Abbreviations: TTP, thrombotic thrombocytopenia purpura; COVID-19, coronavirus disease.
Upon conducting multivariate regression analysis to adjust for confounders, including severity of the COVID-19 infection (ARF, sepsis, and organ failure), we discovered that COVID-19 patients with TTP had a significantly elevated mortality rate compared to those without TTP (adjusted odds ratio [AOR] 3.99, 95% CI 1.93-8.28, P < .01) (Table 2). Furthermore, COVID-19 patients with TTP had a higher likelihood of LE DVT/PE (AOR 3.33, 95% CI 1.40-7.91, P < .01), AKI (AOR 7.36, 95% CI 3.74-14.50, P < .01), AKI necessitating dialysis (AOR 23.78, 95% CI 12.31-45.94, P < .01), GIB (AOR 10.75, 95% CI 4.77-24.23, P < .01), requiring blood transfusion (AOR 20.32, 95% CI 12.19-42.33, P < .01), and ICU admission (AOR 14.42, 95% CI 8.52-24.43, P < .01). As for resource utilization, patients with TTP had a significantly longer adjusted LOS (adjusted mean difference [AMD] 17.42 days, 95% CI 12.73-22.10, P < .01) and incurred higher total hospitalization charges (AMD $298 476, 95% CI 195 883-401 069, P < .01).
Table 2.
TTP Outcomes in COVID-19 Patients.
| Outcomes | COVID-19 + age >18 y = 1,050,045 | |||
|---|---|---|---|---|
| With TTP (n = 300) | Without TTP (n = 1,049,745) | *Adjusted OR (95% CI) |
P value | |
| Mortality, % | 25.00 | 11.17 | 3.99 (1.93-8.28) | <.01 |
| Acute myocardial infarction, % | 5.00 | 1.38 | 4.34 (1.02-18.49) | .05 |
| Acute stroke or transient ischemic attack (TIA), % | 1.67 | 1.09 | 2.22 (0.37-13.45) | .39 |
| LE DVT &/or PE, % | 13.33 | 3.83 | 3.33 (1.40-7.91) | <.01 |
| Acute kidney injury (AKI), % | 63.33 | 25.26 | 7.36 (3.74-14.50) | <.01 |
| AKI requiring dialysis, % | 23.33 | 1.73 | 23.78 (12.31-45.94) | <.01 |
| Gastrointestinal bleeding, % | 25.00 | 2.80 | 10.75 (4.77-24.23) | <.01 |
| Blood transfusion, % | 35.00 | 2.36 | 20.32 (12.19-42.33) | <.01 |
| Requiring ICU admission, % | 61.67 | 10.03 | 14.42 (8.52-24.43) | <.01 |
| Adjusted mean difference | ||||
| Mean length of hospital stay, days (95% CI) | 27.00 (22.09-31.87) | 7.48 (7.41-7.54) | 17.42 (12.73-22.10) | <.01 |
| Total hospitalization charges, mean, USD | 474 596 (335 886-613 306) | 78 478 (76 273-80 684) | 298 476 (195 883-401 069) | <.01 |
Abbreviations: TTP, thrombotic thrombocytopenia purpura; COVID, coronavirus disease; LE DVT, lower extremity deep venous thrombosis; PE, pulmonary embolism; ESRD, end-stage renal disease; CI, confidence interval.
Discussion
Our large-scale study included 1 050 045 adult patients hospitalized with COVID-19, among whom 300 (0.03%) developed TTP during their hospital stay. Notably, patients with TTP were relatively younger (57.47 vs 64.74, P < .01) and had a higher prevalence of CHF (13.33% vs 16.82%, P value not provided) and ESRD (3.33% vs 3.69%, P value not provided). After adjusting for potential confounders, including the severity of COVID-19, we found a significantly increased mortality rate in COVID-19 patients with TTP (AOR 3.99, 95% CI 1.93-8.28, P < .01). In addition, these patients exhibited higher odds of complications, such as venous thromboembolism (AOR 3.33, 95% CI 1.40-7.91, P < .01), AKI (AOR 7.36, 95% CI 3.74-14.50, P < .01), GIB (AOR 10.75, 95% CI 4.77-24.23, P < .01), need for blood transfusion (AOR 20.32, 95% CI 12.19-42.33, P < .01), and ICU admission (AOR 14.42, 95% CI 8.52-24.43, P < .01). Additionally, the TTP group had longer hospital stays (AMD 17.42 days, 95% CI 12.73-22.10, P < .01) and higher total hospitalization costs (AMD $298 476, 95% CI 195 883-401 069, P < .01). This underlines the significant impact of TTP on clinical outcomes and resource allocation among hospitalized COVID-19 patients.
Coronavirus disease-19 typically presents with a diverse range of symptoms, which can vary significantly from patient to patient. An analysis of more than 370 000 confirmed COVID-19 cases from January to May 2020 revealed that the most common symptoms were cough (50%), fever (43%), myalgias (36%), and headache (34%). 6 However, when comparing the 2 periods of predominant Delta and Omicron variants, different patterns emerged, with nasal congestion (77%-82%), headache (75%-78%), sneezing (63%-71%), and sore throat (61%-71%) being the most common presenting symptoms. 7 This clinical picture can be contrasted with TTP, a subtype of TMA that typically presents with neurological symptoms such as headaches and seizures in approximately 44% of idiopathic TTP patients. 8 It is worth noting that the classical pentad of TTP symptoms, including thrombocytopenia, microangiopathic hemolytic anemia, neurological symptoms, renal dysfunction, and fever, is observed in less than 5% of cases. 8
Thrombotic thrombocytopenic purpura patients also often exhibit organ-specific symptoms, such as abdominal pain, which is noted in 23.5% of cases. Renal and pulmonary involvement are less common in TTP than in the frequently observed respiratory complications in severe COVID-19 cases. 8 For instance, in a study involving 138 COVID-19 patients, acute respiratory distress syndrome developed in 20% of patients, a median of 8 days after symptom onset. 9 Similarly, thromboembolic complications pose a significant risk for both severely ill COVID-19 and TTP patients. For COVID-19, the reported rates of venous thromboembolism range from 10% to 40% among patients in the ICU. Gastrointestinal symptoms are another common feature of COVID-19, with an 18% prevalence, but they also occur in TTP, albeit less commonly.10–14 In our study, patients who developed TTP exhibited a significantly higher mortality rate of 26.44%, whereas a study by Vrečko et al in 2022 reported a mortality rate of 16.7%, with only 3 deaths among 18 TTP patients. 15 We found no significant difference in the rates of AMI and CVA between TTP and non-TTP groups. However, we found that patients with TTP were at an increased risk of developing venous thromboembolism.
Tan and colleagues highlighted a substantial prevalence of leg deep vein thrombosis (DVT; 11.2%) and pulmonary embolism (PE; 7.8%) in COVID-19 patients, 16 underscoring the dual susceptibility to hypercoagulability and venous thromboembolic events in patients experiencing both TTP and COVID-19. 16 This dual whammy situation accentuates the heightened risk of thrombotic complications due to the combined influence of TTP and COVID-19's hypercoagulable state. Consistent with our findings, and as reported by Yarranton et al, TTP is known to contribute to an elevated risk of certain complications, such as GI bleeding, with a lower prevalence of venous thromboembolic phenomena. 17 However, our study emphasizes the additive effect of these phenomena when TTP is compounded with external factors, as seen in the increased thromboembolic risk associated with COVID-19.
Building on our findings, it is important to mention that unique to TTP, among TMA conditions, is the presence of anti-ADAMTS13 antibodies. These antibodies neutralize or accelerate the clearance of ADAMTS13, thereby inhibiting the breakdown of vWF.18,19 This results in the accumulation of oversized vWF multimers in arterioles and capillaries. In light of the recent pandemic, a novel link has been discovered between COVID-19 and TMA conditions, including TTP. This link stems from the COVID-19-mediated increase in procoagulant factors, such as factor VIII, vWF, and fibrinogen, coupled with cytokine-induced endothelial damage. 20 An intriguing observation is that COVID-19 patients frequently exhibit diminished ADAMTS13 levels likely due to endothelial dysfunction. 21 This reduction has significant implications, as a clear inverse correlation exists between ADAMTS13 levels and COVID-19 patient mortality, whether TTP is present or not. 22
In a study by Sweeney et al, it was strikingly observed that COVID-19 patients with ADAMTS13 levels below 43% had a notably higher mortality rate than those with higher levels (70% vs 40%, P < .001), although none of the patients had anti-ADAMTS13 antibodies. 23 Corroborating this association further, Alhomoud et al reported 6 instances of COVID-19-induced TTP in their study. 24 Five of these patients had ADAMTS13 levels less than 10%. Remarkably, all patients recovered after treatment with plasma exchange, rituximab, and corticosteroids. 24 These observations highlight the pivotal role of ADAMTS13 in the context of TTP and the broader spectrum of COVID-19.25–28 Comprehending this relationship can shed new light on the pathophysiology of COVID-19 and its associated coagulopathies.
Strengths
The strength of our study stems from the inclusion of a diverse patient population derived from the US NIS database, which covers hospitals across 48 states of the United States. This vast coverage enhances the external validity and generalizability of our findings, helping to offset the potential biases seen in single or multicenter studies. We also considered various socioeconomic and hospital-related factors, including estimates of household income and hospitalization costs. These considerations would not be feasible in studies confined to single institutions, thus adding depth and nuances to our results.
Limitations
Our study had certain limitations. First, the retrospective design of the study has inherent limitations, including the inability to fully randomize cases. Although we attempted to control for known and potential confounding factors, including the severity of COVID infection through multivariate regression analysis, the possibility of residual confounding remains. Furthermore, our utilization of the NIS, a claims-based database, is susceptible to coding inaccuracies. 29 Diagnosis misclassification is another potential limitation, as we relied on diagnosis codes rather than clinical parameters. On the positive side, our use of the ICD-10 coding system for data extraction offers greater specificity than its predecessor ICD-9. 30 We acknowledge the lack of quantitative data on the oxygen requirements of our patients; however, we adjusted for ARF, sepsis, and organ failure to reflect the severity of COVID-19 infection. It is important to highlight that few of the outcomes exhibit relatively wide confidence intervals, which can be attributed to the smaller size of the TTP patient cohort. However, the rigorous adjustment of confounding factors during multivariate regression model building ensures the reliability of our results. Finally, the timing of TTP diagnosis during COVID-19 hospitalization and the levels of ADAMTS13 could not be assessed due to the limitations of the NIS database.
Conclusion
Our study demonstrates a significant association between TTP and heightened mortality, as well as an increased incidence of complications such as DVT, PE, AKI, and GIB in COVID-19 patients. This surge in complications can be attributed to the hypercoagulable state induced by the TTP. Our findings highlight the need for future research to delve deeper into this relationship, potentially shaping better diagnostic and therapeutic strategies for the management of TTP in the context of COVID-19.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296231219252 for Impact of Concomitant Thrombotic Thrombocytopenic Purpura on COVID-19 Mortality and Morbidity: A Nationwide Inpatient Sample Analysis by Ali Jaan, Zouina Sarfraz, Farhan Khalid and Junaid Anwar in Clinical and Applied Thrombosis/Hemostasis
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zouina Sarfraz https://orcid.org/0000-0002-5132-7455
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017;1(10):590-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furlan M, Robles R, Galbusera M, et al. Von Willebrand factor–cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic–uremic syndrome. N Engl J Med. 1998;339(22):1578-1584. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary H, Nasir U, Syed K, et al. COVID-19-associated thrombotic thrombocytopenic purpura: a case report and systematic review. Hematol Rep. 2022;14(3):253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD [Internet]. www.hcup-us.ahrq.gov/nisoverview.jsp
- 5.Kuswardhani RAT, Henrina J, Pranata R, Lim MA, Lawrensia S, Suastika K. Charlson Comorbidity Index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):2103-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance–United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399(10335):1618-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley M, Killeen RB, Michalski JM. Thrombotic thrombocytopenic purpura. In: StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK430721/ [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll M, Zon RL, Sylvester KW, et al. VTE In ICU patients with COVID-19. Chest. 2020;158(5):2130-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. Br Med J. 2022:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malgaj Vrečko M, Aleš Rigler A, Večerić-Haler Ž. Coronavirus disease 2019-associated thrombotic microangiopathy: literature review. Int J Mol Sci. 2022;23(19):11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021;76(10):970-979. [DOI] [PubMed] [Google Scholar]
- 17.Yarranton H, Cohen H, Pavord SR, Benjamin S, Hagger D, Machin SJ. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. Br J Haematol. 2003;121(5):778-785. [DOI] [PubMed] [Google Scholar]
- 18.Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav Immun. 2021;95:514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafez W, Ziade MA, Arya A, et al. Reduced ADAMTS13 activity in correlation with pathophysiology, severity, and outcome of COVID-19: a retrospective observational study. Int J Infect Dis. 2022;117:334-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program. 2018;2018(1):530-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwaegermann M-K, Hobohm L, Rausch J, et al. COVID-19 as a potential trigger for immune thrombotic thrombocytopenic purpura and reason for an unusual treatment: a case report. Hamostaseologie. 2021;43(3):215-218. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DM, Patriquin CJ, Nazy I. Thrombotic microangiopathies: a general approach to diagnosis and management. CMAJ. 2017;189(4):E153-E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney JM, Barouqa M, Krause GJ, Gonzalez-Lugo JD, Rahman S, Gil MR. Low ADAMTS13 activity correlates with increased mortality in COVID-19 patients. TH Open. 2021;5(01):e89-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhomoud M, Alhobayb T, Armitage K. COVID-19 infection triggering thrombotic thrombocytopenic purpura. IDCases. 2021;26:e01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13-von willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021;19(2):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favaloro EJ, Henry BM, Lippi G. Von Willebrand factor and ADAMTS13 in COVID-19 and beyond: a question of balance. EMJ Hematol. 2021;9(1):55-68. [Google Scholar]
- 27.Doevelaar AAN, Bachmann M, Hölzer B, et al. Generation of potentially inhibitory autoantibodies to ADAMTS13 in coronavirus disease 2019. Sci Rep. 2023;13(1):10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favaloro EJ, Mohammed S, Chapman K, et al. A multicenter laboratory assessment of a new automated chemiluminescent assay for ADAMTS13 activity. J Thromb Haemost. 2021;19(2):417-428. [DOI] [PubMed] [Google Scholar]
- 29.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV26-IV35. [DOI] [PubMed] [Google Scholar]
- 30.Cartwright DJ. ICD-9-CM to ICD-10-CM codes: What? Why? How? Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296231219252 for Impact of Concomitant Thrombotic Thrombocytopenic Purpura on COVID-19 Mortality and Morbidity: A Nationwide Inpatient Sample Analysis by Ali Jaan, Zouina Sarfraz, Farhan Khalid and Junaid Anwar in Clinical and Applied Thrombosis/Hemostasis