Abstract
Background:
The utilization of herbal medicine has been noteworthy for treating cancer; however, there is not enough information regarding the characteristics of clinical trials of herbal medicine interventions. This study aimed to evaluate the characteristic of registered trials using herbal medicine interventions for cancer.
Methods:
A cross-sectional study was performed via the website ClinicalTrials.gov, ISRCTN registry, Chinese clinical trial registry, and international clinical trials registry platform to gather associated registered clinical trials using an advanced search with the developed keyword strategy as of March 26, 2023. All obtainable information from the trials was collected without any restrictions to conduct a comprehensive review.
Results:
A total of 169 registered trials were included for evaluation. Of all trials, 102 trials were eligible for this study. Countries from Asia registered the most trials (62.75%), and hospitals sponsored most of the trials (42.16%). Randomized, Phase 2, interventional trials were dominant, and approximately 64.71% of the trials anticipated recruiting less than 100 participants. More than half of the trials were from 2016 to 2023 (53.92%). While 45 trials were completed, only 16 trials had results for further analysis. According to the completed results, the types of herbal medicines from the trials mainly focused on lung, breast, and colorectal cancer.
Conclusion:
This study is the first to explore the characteristics of clinical trials of herbal medicine for cancer registered in large clinical databases. The acquired trials had relatively informative data; however, better-designed trials may be needed for health professionals to consider herbal medicine as an option when treating cancer patients.
Keywords: herbal medicine, integrative medicine, cancer patients, herbal drug, clinical trials
Introduction
Cancer has been known as the primary cause of death worldwide. 1 With the rapid increment of the aging population, cancer has become a major problem for health systems. 2 The leading cause of cancer cases was breast cancer, accounting for 2.26 million cases; the most common cause of cancer death was lung cancer, accounting for 1.8 million deaths in 2020. 3 As the number of cancer patients has been rising each year, various types of cancer care services have been introduced apart from chemo- and radiotherapies. There has been a growing number of patients seeking several modalities of complementary and alternative medicine (CAM) when coping with cancer. Among the therapies, herbal medicine therapy has been popular for improving physical symptoms, stimulating the immune system, improving mental health, etc. 4
According to the World Health Organization (WHO) report, herbal medicine is defined as herbs, herbal preparations, and herbal finished products containing plant or inorganic ingredients. 5 It is vital to note that the highest users of herbal medicines were known as cancer patients. 6 Cancer patients with experience of herbal medicines have said that it relieves symptoms, improves adverse effects from conventional treatments for cancer, and prevents further recurrence or metastasis. 4 According to a systematic review on the use of herbal supplements in the United Kingdom, 22% of cancer patients were taking herbal supplements for the purpose of treating symptoms and improving quality of life. 7
Furthermore, the utilization of herbal medicines has received increasing attention as an auxiliary cancer treatment strategy from various countries for alleviating the side effects of chemotherapy or radiation while improving the overall quality of life of cancer patients. 8 As a well-known example, herbal medicines showed positive results in treating gastrointestinal side effects of cancer patients, such as vomiting, diarrhea, constipation, and nausea. 9 More importantly, combination therapies, using both herbal medicines and conventional drugs, to treat cancer and its related adverse effects have gained much attraction among researchers to maximize its effects. 10
Even though numerous cancer patients are in favor of using herbal medicines to treat the adverse effects of cancer, there still is a paucity of evidence proving the positive effects of herbal medicine interventions for treating cancer. 11 Exploring and analyzing registered clinical trials may be vital to assist future clinical practice, as clinical trials provide a reliable source of efficacy and safety. Additionally, it is the most widely used among researchers to find comprehensive information regarding completed or ongoing trials, identifying the characteristics of the registered trials in the fields or topics of interest. 12 Hence, the aim of this cross-sectional study is to investigate the current status of the characteristic of registered trials using herbal medicine interventions for cancer via clinical databases.
Materials and Methods
Reporting Guideline
As a cross-sectional study, this work was reported according to the STROBE guideline. 13
Searching of Registered Trials
ClinicalTrials.gov was first chosen since it is the largest clinical trial registry, with high transparency and free accessibility for detailed information on past and present clinical trials, which provides valid evidence of efficacy and safety for clinical practice. 14 In order to ensure adequate coverage of the existing clinical trials, the ISRCTN registry, the Chinese clinical trial registry (ChiCTR), and the international clinical trials registry platform (ICTRP) were additionally chosen.
The advanced search function was utilized along with the preliminary search terms, including (herbal medicine* OR phytotherap* OR herbal therap* OR botanical therap* OR botanical medicine* OR Chinese herbal OR plant extract* OR ethnobotany, ethnopharmacology, herbalism) AND (cancer OR neoplasm*OR tumor* OR oncology OR malignanc* OR tumor* OR carcinoma OR malignanc* OR neoplasia*) to find the most significant number of registered trials on March 26, 2023. Although the keyword “herbal medicine” and its similar words were utilized to locate trials using herbal medicines, this keyword strategy was intended not to survey all the literature of clinical trials on herbs for cancer.
Inclusion and Exclusion Criteria
The searched results from each database were exported and tailored in CSV file format. All trials were included inception to the search date with the keyword strategy, presuming that there were limited trials using herbal medicines. The trials not related to herbal compounds or herbal medicines, trials not related to cancer patients, terminated or suspended trials, and non-clinical trials, such as questionnaire survey studies, were excluded. Further, the final chosen trials were checked via PubMed, Google Scholar, SCOPUS, and Chinese National Knowledge Infrastructure (CNKI) database to locate any publications that may have results. Three authors (BY, SY, and SK) individually reviewed all trials regarding herbal medicine interventions. If there were any disagreements during the inclusion and exclusion process, other authors (JK, JI, and JL) were invited to reach a consensus.
Data Extraction and Statistical Analysis
The following accessible data of all clinical trials were extracted from the searched results: database number, title, status, study results, conditions, outcome measures, lead sponsors, gender, age, phases, enrollment, primary funding, study type, all available study designs, start date, completion date, results posted, location, and URL. The final dataset was imported to Microsoft Excel 2019 software to perform descriptive analysis, and the categorial data were reported as frequency and percentage.
Results
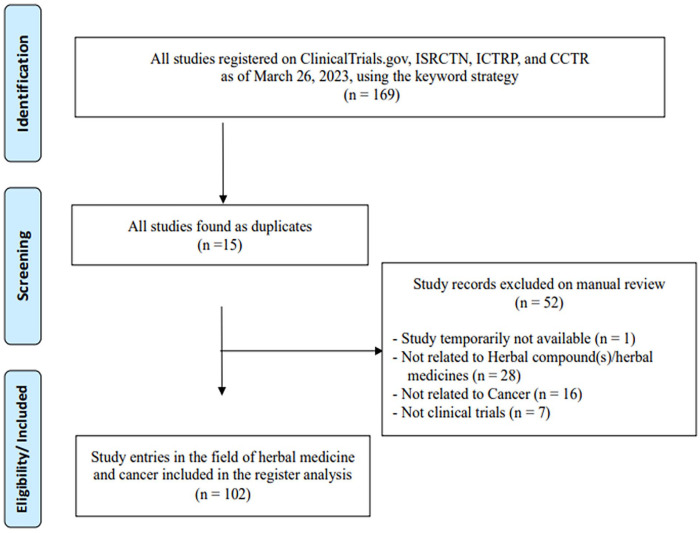
The initial search yielded 169 registered trials on the aforementioned databases. With the keyword search strategy, a total of 154 trials were available for review. After the exclusion criteria process, 102 trials remained for analysis (Figure 1).
Figure 1.
Flowchart of selection trials.
General Characteristics of Included Trials
The characteristics of the included trials are shown in Table 1. The largest number of trials were initiated between 2016 and 2020, with 36 trials (35.29%) in this period. Although 45 trials (44.12%) were completed, only 8 trials (7.84%) were indicated to have results. Asia was the most frequently identified study location (N = 64, 62.75%), followed by North America (N = 21, 20.59%). Most of the primary funding came from other funding sources (N = 82, 80.39%). Hospitals have sponsored the most trials (N = 43, 42.16%). Most trials had participants ages 18 and older; however, 4 trials included child participants (3.92%).
Table 1.
General Characteristics of the Included Trials.
| Variable | Number | Percent |
|---|---|---|
| Study start date | ||
| Prior to 2005 | 11 | 10.78 |
| 2005-2010 | 11 | 10.78 |
| 2011-2015 | 25 | 24.51 |
| 2016-2020 | 36 | 35.29 |
| 2021-2023 | 19 | 18.63 |
| Recruitment status | ||
| Not yet recruiting | 13 | 12.75 |
| Recruiting | 24 | 23.53 |
| Enrolling by invitation | 1 | 0.98 |
| Terminated | 4 | 3.92 |
| Completed | 45 | 44.12 |
| Withdrawn | 2 | 1.96 |
| Unknown Status | 13 | 12.75 |
| Study results | ||
| Has results | 8 | 7.84 |
| No results available | 94 | 92.16 |
| Locations | ||
| Asia | 64 | 62.75 |
| Europe | 9 | 8.82 |
| North America | 21 | 20.59 |
| South America | 1 | 0.98 |
| Middle East | 6 | 5.88 |
| Unknown | 1 | 0.98 |
| Lead Sponsors | ||
| University | 37 | 36.27 |
| Hospital | 43 | 42.16 |
| Industry | 12 | 11.76 |
| Other | 10 | 9.80 |
| Primary Funding | ||
| Industry | 10 | 9.80 |
| NIH | 8 | 7.84 |
| Other | 82 | 80.39 |
| None | 2 | 1.96 |
| Ages | ||
| Child, Adult, Old Adult | 4 | 3.92 |
| 18 y and older | 97 | 95.10 |
| Not provided | 1 | 0.98 |
| Sex/gender | ||
| All | 77 | 75.49 |
| Female | 21 | 20.59 |
| Male | 4 | 3.92 |
Study Designs of Included Trials
Most trials were found to be interventional (N = 94, 92.16%) and in phase II (N = 29, 28.43%). The primary purpose of the trials was treatment (N = 71, 69.61%), followed by supportive care (N = 18, 17.65%). The majority of the trials were randomized (N = 69, 67.65%), and most trials’ anticipated enrollment was less than 100 (N = 66, 64.71%). Most trials had a double-arm design (N = 63, 61.76%), and parallel assignment (N = 72, 70.59%) was used the most as an interventional model. Less than half of the trials were masked; single, double, and quadruple masking was used the most among the trials. The details of all study designs are shown in Table 2.
Table 2.
Study Design of the Included Trials.
| Variable | Number | Percent |
|---|---|---|
| Study type | ||
| Interventional | 94 | 92.16 |
| Observational | 8 | 7.84 |
| Formulation type | ||
| Single herbs | 7 | 6.86 |
| Multi herbs | 95 | 93.14 |
| Primary purpose | ||
| Prevention | 5 | 4.90 |
| Treatment | 71 | 69.61 |
| Supportive Care | 18 | 17.65 |
| Other | 2 | 1.96 |
| Unknown | 6 | 5.88 |
| Phase | ||
| Phase 1 | 8 | 7.84 |
| Phase 1 & Phase 2 | 7 | 6.86 |
| Phase 2 | 29 | 28.43 |
| Phase 2 & Phase 3 | 3 | 2.94 |
| Phase 3 | 13 | 12.75 |
| Phase 4 | 5 | 4.90 |
| Unknown | 37 | 36.27 |
| Allocation | ||
| Randomized | 69 | 67.65 |
| Non-randomized | 16 | 15.69 |
| Unknown | 8 | 7.84 |
| Not applicable | 9 | 8.82 |
| Anticipated Enrollment | ||
| <100 | 66 | 64.71 |
| 100-600 | 35 | 34.31 |
| Not provided | 1 | 0.98 |
| Interventional model | ||
| Single group assignment | 14 | 13.73 |
| Parallel assignment | 72 | 70.59 |
| Crossover assignment | 4 | 3.92 |
| Case-control | 5 | 4.90 |
| Cohort | 3 | 2.94 |
| Sequential Assignment | 1 | 0.98 |
| Unknown | 3 | 2.94 |
| Masking | ||
| None (Open label) | 49 | 48.04 |
| Single | 10 | 9.80 |
| Double | 22 | 21.57 |
| Triple | 4 | 3.92 |
| Quadruple | 10 | 9.80 |
| Unknown | 7 | 6.86 |
| Number of arms | ||
| 1 | 14 | 13.73 |
| 2 | 63 | 61.76 |
| 3 | 9 | 8.82 |
| 4 | 3 | 2.94 |
| Not provided | 13 | 12.75 |
Description of Herbal Medicines in Various Cancer Types of Included Trials
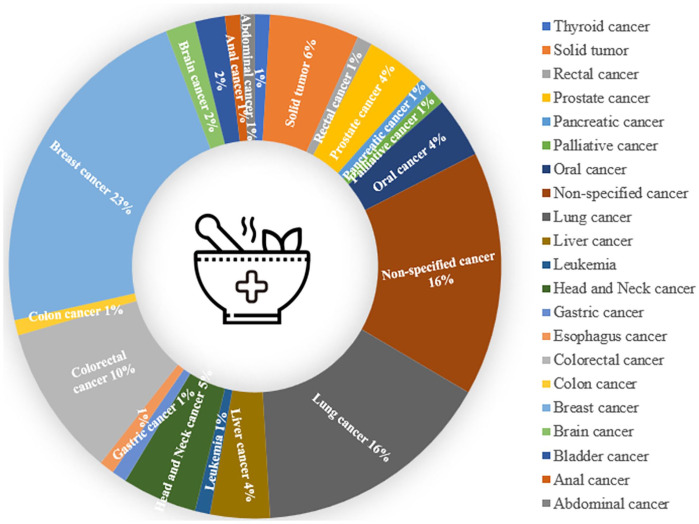
The cancer types of the included trials are shown in Figure 2. Cancer types adopted for clinical trials were mostly concentrated on breast cancer, followed by lung cancer and non-specified cancer. According to Table 3, all of the utilized herbal medicines were categorized into 16 different conditions in clinical trials. In addition, herbal medicines adopted for clinical trials were mostly concentrated on the decoction of herbal mixtures; lung cancer trials were found to have well-known prescriptions the most frequently. It is noteworthy that various herbal combinations of herbal mouthwash, oil, and tea were used for trials. YangYinFang, YiQi Fang, and YiQi Yang Yin Fang were most adopted in lung cancer trials. Traditional Chinese medicines were most applied to breast cancer; however, the majority of the trials did not reveal the actual descriptions of the prescriptions. PHY906 (KD018) has appeared most in colorectal cancer trials. PHY906 commonly appeared in hepatocellular carcinoma and colorectal cancer.
Figure 2.
The cancer types of the included trials.
Table 3.
Description of Herbal Medicines in Various Cancer Types.
| Conditions | Types of herbal medicines |
|---|---|
| Anal cancer | Arnebia Indigo Jade Pearl |
| Acute Leukemia | Sheng-Yu-Tang |
| Brain tumor | Boswellia serrata extract; Elemene |
| Breast cancer | A combined herbal medication of selenium, milk thistle, goldenrod and bromelain; Chinese herbal compound combined with capecitabine; Chinese herbal medicine (Shu Gan Liang Xue Decoction); Endocrine therapy combined with Chinese herbal medicines; Scutellaria Barbata; Herbal medicine ES001; Herbal ointment (pomegranate peel, alum, honey, and oak apple); Nigella sativa; Sipjeondaebotang; TPE-1; Wenshen Bugu prescription. |
| Colorectal cancer | Roman chamomile and ginger essential oils of sweet almond oil; Samryungbaekchulsan; Standard western medicine treatment and taking herbal granules (Jiedu Sangen granules); Teng Long Bu Zhong Tang; Traditional Chinese medicine (taro mushroom formula granules); PHY906 (KD018). |
| Esophagus cancer | Qizhu Yuling Prescription |
| Gastric cancer | Yiqi Wenyang Jiedu |
| Head and neck cancer | 2 formulations of herbal mouthwash containing 5 plant ingredients-Emblica officinalis (dried fruit), Terminalia chebula (dried fruit), Terminalia bellerica (dried fruit), Azadiracta indica (leaf & Bark), Glycyrrhiza glabra (root); Herbal gel containing 2.5% of the active standardized arrabidaea chica extract; Zi Yin Liang Ge San. |
| Liver cancer | PHY906 (KD018); Sho-saiko-to. |
| Lung cancer | Astragalus; Cimicifuga foetida; Codonopsis; Coix seed; Cordate houttuynia; Dried orange peel; Hedyotis diffusa; Herba Patriniae; Iscar; Jin Fu Kang; Kanglaite Injection; Maekmoondong-tang; Radix Ophiopogonis; Rhizoma Alismatis; Solanum nigrum; Standard chemotherapy and Chinese herbal (basil); Thunberg fritillary bulb; Traditional Chinese medicine (Prescriptions from Professor Liu Jiaxiang); Traditional Chinese medicine (Yiqi Jianpi Jiedu Fang Radix Astragali Mongolici, Radix Codonopsis, Rhizoma Atractylodis Macr); Wolfiporia extensa; YangYinFang; YiQi Fang; YiQi Yang Yin Fang; Yiqi Yangyin Jiedu; YQ1; Yupingfeng; Pinellia; Prunella vulgaris; Chinese herbal medicine Yi-Qi-Tong-Luo-Jie-Du formula. |
| Non-specified cancer | Aarewat Rasayan (Sonamukhi ghana and Argavadha ghana); DaHuang GanTsao Tang; Herbal powder (Gymnema, Sylvestre, Azadiracta, Indica, Citrullus colocynthis, Berberis aristata, Tribulus terrestris, Withania somnifera, Simarouba glauca, and Ocimum tenuiflorum); Medicinal cannabis (Bedrocan); Renshen Yangrong Tang (RSYRT decoction); Routine mouthwash plus mouthwash containing pomegranate peel and extract of turmeric; Tongkuaixiao herbal medicine ointment; Traditional Chinese Herbal medicine combination of Astragalus membranaceus and Ligustrum lucidum; Wormwood (Registered in Germany); Xiang Bei Yang Rong Tang; Shen-Mai San. |
| Oral cancer | Homeodent®; SAMITAL. |
| Palliative cancer | HuangQi; HuangQi. |
| Prostate cancer | An herbal medicine combination of green tea (Camellia sinensis) standardized to contain green tea catechins, turmeric (Curcuma longa) standardized to contain curcumin, resveratrol (from Polygonum cuspidatum); and broccoli sprout extract (Brassica oleracea) in capsule form; Herbal formula BYSH; Oral herbal medicines of hochuekkito and keishibukuryougan. |
| Solid tumor | Herbal combination including Trigonella, Foeniculum, Chichorium, Hornleum, and Glycine provided in Dineh company named General Tonic; Herbal combination of Cichorium inyubus, Trigonella foenum-graecum and Foeniculum vulgare; SH003. |
| Thyroid cancer | Ginseng |
Characteristics of Completed Trials With Final Results
Of the 102 trials, only 8 trials reported results on the website. Six trials started between 2004 and 2009; 2 trials began in 2013 and 2014, respectively. One trial published a meeting abstract (NCT00411762), 15 and four trials had publications (NCT00730158, NCT02638051, ISRCTN7208885, ChiCTR-TRC-10001017).16 -19 Six trials investigated herbal medicines as adjuvant therapy in combination with conventional therapy, and 2 trials tested single therapy. Four trials recruited more than 50 participants, and the other 4 recruited less than 50 participants. The highest number of recruited patients was 260. One of the trials, NCT00243022, was terminated due to low patient accrual even though it is indicated as “has results” via ClinicalTrials.gov.
NCT00411762 concluded that a combination of capecitabine and PHY906 is safe for patients with solid tumors; the combination of cationic anti-microbial peptides and PHY906 has also shown insufficient evidence in patients with solid tumors for its effectiveness. 20 The results of NCT00622440 indicated that Arnebia Indigo Jade Pearl (AIJP) cream showed a better clinical response for treatment of precancerous anal lesions in order to prevent their progression to anal cancer. 21 AIJP is a multiherbal topical cream based on Chinese herbal medicine (CHM) that has been specifically designed to treat people with the cancer precursor lesions caused by human papillomavirus. 22 NCT00730158 showed that KD018 (traditional Chinese medicine formulation) relieves the gastrointestinal toxicity of irinotecan in patients with metastatic colorectal cancer. 23 NCT02638051 reported that Shi Pi (TCM herbal decoction) combined with modulated electro-hyperthermia is safe and tolerable in patients with malignant ascites; it was found that the intervention group had a higher level of quality of life than the control group, yet adverse effects such as bone marrow depression might become an issue. 24 According to the trial, Shi Pi decoction invigorates the spleen, promotes qi circulation to induce diuresis and treat foot-taiyin meridian in Gu Zhang. NCT01898091 used the herbal mouth rinse for adults with head and neck cancer receiving radiation therapy, testing whether the study mouth rinse may lessen oral mucositis. 25 Unfortunately, the trial study indicated that there is not an agreement between principal investigators and the sponsor to discuss or publish trial results after the trial is completed. ISRCTN7208885 concluded that administering medicinal cannabis with either irinotecan or docetaxel did not have much impact on pharmacokinetics of the drugs. 26 ChiCTR-TRC-10001017 noted that after 2 cycles of maintenance TCM treatment, the serum concentration of sCTLA-4 in patients with advanced NSCLC was significantly lower. The study indicated that controlling the serum concentration of sCTLA-4 may be a potential mechanism for TCM maintenance treatment of NSCLC drugs. 27
Further exploration was carried out to locate more publications of the final trials despite the indication as “has no results” on trial databases since researchers may have been delayed in updating the results. As mentioned, the extra search was discovered via PubMed, Google Scholar, SCOPUS, and CNKI and found 8 more publications. Among the 8 publications, only one was published as a meeting abstract (NCT00669656), 28 and others were journal articles (NCT02795390, NCT02468141, NCT00076609, NCT03911921, NCT02900742, IRCT20180722040556N2, IRCT20180722040556N1).29 -35 The highest number of enrolled participants was 83; the lowest was 32. Only 2 studies (NCT00669656, NCT00076609) used the single-arm design, having the intervention group, while all others used the double-arm. Four studies (NCT00076609, NCT02900742, IRCT20180722040556N2, IRCT20180722040556N1) investigated herbal medicines as adjuvant therapy combined with conventional treatment, and the rest utilized herbal medicine only. The complete list of the results of the trials is summarized in Table 4.
Table 4.
Characteristics of Completed Trials with Results.
| Trial Number | Title | Start date | Publication date | Groups | No. of patients | Conclusion |
|---|---|---|---|---|---|---|
| NCT00243022 | Dietary, Herbal and Alternative Medicine in Glioblastoma Multiforme | 2004/09/01 | None | [Intervention] Boswellia serrata extract + Cyanocobalamin [Control] Cyanocobalamin | 12 | Certain outcome analysis not done due to low patient accrual (terminated). |
| NCT00411762 | A Phase I/II, Multi-Center, Open-Label, Dose-Escalation, Safety and Efficacy Study of PHY906 Plus Capecitabine in Patients with Advanced Pancreatic Carcinoma | 2006/12/01 | 2008/05/20 (Gastrointestinal Cancer Symposium) 15 | [Intervention] PHY906 + Capecitabine | 25 | A combination of capecitabine and PHY906 has shown limited evidence in patients with solid tumors for its effectiveness. Efficacy and quality of life in gemcitabine-refractory advanced pancreatic cancer patients will be assessed by Phase II study. |
| NCT00622440 | Treatment of Anal High-grade Squamous Intraepithelial Lesions (HSIL) Through Use of a Chinese Herbal Topical Cream | 2008/05/14 | None | [Intervention] Arnebia Indigo Jade Pearl [Control] Placebo | 70 | Considering the final response of anal high-grade squamous intraepithelial lesions, AIJP showed a better clinical response. There were no all-cause of mortality or adverse events in patients treated with AIJP. |
| NCT00730158 | A Phase II Multicenter, Randomized, Placebo Controlled, Double Blinded Clinical Study of KD018 as a Modulator of Irinotecan Chemotherapy in Patients with Metastatic Colorectal Cancer | 2008/12/08 | 2010/08/1816 | [Intervention] Irinotecan+ KD018 [Control] Irinotecan + Placebo | 33 | PHY906, a four-herb formulation, reduced the gastrointestinal toxicity of CPT-11 through multiple mechanisms of action that included the inhibition of multiple steps of inflammation and the promotion of intestinal progenitor cell repopulation. |
| NCT01898091 | Herbal Mouthrinse for Oral Mucositis Study (OM) | 2013/07/01 | None | [Intervention] Neem Mouthrinse [Control] Placebo Mouthrinse | 50 | Not available as there is no agreement between principal investigators and the sponsor. |
| NCT02638051 | Local mEHT + TCM Versus Intraperitoneal Chemoinfusion in Treatment of Malignant Ascites: Phase II RCT | 2014/01/01 | 2017/04/1017 | [Intervention] Modulated Electro-Hyperthermia + TCM herbal decoction Shi Pi [Control] IPCI (CDDP + 5FU) | 260 | The combination of mEHT with TCM achieves better control of PCMA compared with standard IPCI, having a higher quality of life of Group A than Group B. The combination of mEHT with TCM may be a preferred treatment option; however, it should be cautious of adverse effects, such as bone marrow depression. |
| ISRCTN72088851 | Influence of Medicinal Cannabis (Bedrocan) on the Pharmacokinetics of Irinotecan and Docetaxel in Cancer Patients | 2004/01/01 | 2007/03/0118 | [Intervention] Irinotecan + medicinal cannabis [Control] Docetaxel + medicinal cannabis | 24 | Co-administration of medicinal cannabis, as herbal tea, in cancer patients treated with irinotecan or docetaxel does not significantly influence the plasma pharmacokinetics of the drugs. |
| ChiCTR-TRC-10001017 | Effect of Traditional Chinese Medicine Treatment as Maintenance Therapy on Regulating the Serum Concentration of sCTLA-4 in Patients with Advanced Non-Small-Cell Lung Cancer and its Relationship with Prognosis | 2009/07/01 | 2016/10/2319 | [Intervention] TCM treatment (treated with cinobufacini injection, herbal decoction and Chinese acupoint application) [Control] Single-agent maintenance chemotherapy regimen |
64 | After two cycles of maintenance treatment, TCM treatment lowered the serum concentration of sCTLA-4 compared to chemotherapy (12.77 ± 2.37vs 46.64 ± 11.21 pg/ml, P = .004). Regulating the serum concentration of sCTLA-4 may be a mechanism for TCM maintenance treatment of NSCLC. |
| NCT00669656 | Final Results from a Trial of a Combination Herbal Supplement for Biochemically Recurrent Prostate Cancer | 2006/07/08 | 2013/05/20 | [Intervention] Prostate health cocktail (PHC) 3 capsules daily for four-week cycles | 43 | PHC-induced PSA declines in 37% of patients with biochemically recurrent prostate cancer (bcrPC); there was no association with changes in serum androgens or significant toxicities. PHC can be considered a potential alternative in select patients with bcrPC. |
| NCT02795390 | A Pilot Randomized Placebo-Controlled Study on Modified MaZiRenWan: A Formulated Chinese Medicine to Relieve Constipation for Palliative Cancer Patients | 2016/11/01 | 2022/03/02 | [Intervention] Modified MaZiRenWan (MZRW, traditional Chinese herbal formula) [Control] Placebo |
60 | The study suggested that modified MZRW is well-tolerated and effective for relieving constipation in patients with advanced cancer. It could be considered a potential treatment option for constipation in palliative care. |
| NCT02468141 | Efficacy and Safety of Sipjeondaebo-Tang for Anorexia in Patients with Cancer: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial | 2016/06/01 | 2017/12/26 | [Intervention] Sipjeondaebo-tang 3 times a day for 4 wk [Control] placebo 3 times a day for 4 wk |
32 | Sipjeondaebo-tang can be considered as a potential option for anorexia management in patients with cancer as anorexia and quality of life were improved after 4 wk of Sipjeondaebo-tang treatment, measured by functional assessment of anorexia/cachexia therapy and visual analog scale. However, no significant difference was noted between the Sipjeondaebo-tang group and the placebo group. |
| NCT00076609 | A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma | 2003/10/01 | 2020/11/25 | [Intervention] Capecitabine twice a day for 14 d plus received PHY906 (a pharmaceutical-grade formulation of four traditional Chinese herbs) twice a day on days 1 to 4 and days 8 to 11 every 21-day cycle | 39 | PHY906 increases the index of capecitabine by enhancing its antitumor activity while reducing its toxicity in advanced hepatocellular carcinoma. The median progression-free survival was 1.5 mo, and the median overall survival was 6 mo, with a 51.3% 6-month survival rate. |
| NCT03911921 | A Phase II Randomized Controlled Trial of Renshen Yangrong Tang Herbal Extract Granules for Fatigue Reduction in Cancer Survivors | 2015/06/01 | 2019/10/24 | [Intervention] RSYRT (ChongGuanYaoYe, Beijing, People’s Republic of China) twice a day for 6 wk [Control] Huangqi (an expected most effective agent under the RSYRT by TCM) twice a day for 6 wk |
83 | The efficacy of RSYRT for reducing cancer-related fatigue has been found to be positive. A more significant MD Anderson Symptom Inventory—fatigue score reduction in the intervention group was indicated; more patients in the intervention group had a two-point decrease in fatigue than the control group (90.2%vs 52.4%). By Week 4, between-group differences of fatigue reduction on mean severity reached a large effect size. |
| NCT02900742 | Maintenance Chemotherapy with Chinese Herb Medicine Formulas vs. With Placebo in Patients with Advanced Non-small Cell Lung Cancer After First-Line Chemotherapy: A Multicenter, Randomized, Double-Blind Trial | 2013/03/01 | 2016/10/23 | [Intervention] Maintenance chemotherapy plus Chinese herb medicine formulas (CHMF) group [Control] Maintenance chemotherapy plus placebo group |
71 | The intervention group showed significant improvements in median progression-free survival (PFS) (HR = 0.55, 95% CI 0.28-0.88, P = .019), Karnosfsky performance status scores (P = .047), fatigue (cycle [C] 3: P = .03), interference with daily activities (C3: P = .04) and dyspnea (C2: P = .03) compared with patients in the placebo group. Thus, maintenance chemotherapy combined with CHMF may prolong PFS, relieve symptoms, and improve quality of life. |
| IRCT20180722040556N2 | The Role of Trigonella, Cichorium, and Foeniculum Herbal Combination in the Treatment of Cancer-Induced Anorexia/Cachexia: A Quasi-Experimental Study | 2018/07/01 | 2020/08/26 | [Intervention] Megestrol plus herbal combination for a 2-month follow-up [Control] Megestrol plus placebo tablets for a 2-month follow-up |
47 | Patients in the herbal combination group experienced a mean weight gain of 1.5 kg, while patients in the placebo group had an average weight loss of 0.6 kg. Anthropometric indices, including triceps skinfold thickness, mid-arm muscle circumference index, and grip strength, were significantly improved in the herbal combination group. The quality of life, FAACT, and some factors of Anderson criteria were considerably enhanced in the herbal combination group. Hence, the herbal combination could be an adjunctive treatment option for managing patients suffering from cancer-induced cachexia and anorexia. |
| IRCT20180722040556N1 | Combination of traditional herbal medicine for the treatment of cancer-induced Anorexia/Cachexia: A pilot, randomized, double-blinded and placebo-controlled clinical trial | 2018/07/01 | 2018/07/01 | [Intervention] Megestrol plus one sachet of herbal combination, three times per day for 4 wk [Control] Megestrol plus placebo, three times per day for 4 wk |
55 | Patients who had received an herbal combination gained 0.9 kg weight, compared with a weight loss of 0.53 kg in the placebo group (P < .001). Improvement in quality of life and FAACT scores were not significant in each group (P > .05); the Edmonton symptom assessment scale questionnaire assessment indicated that there was a significant improvement in two indices in the intervention group, including appetite (P < 0.001) and fatigue (P = .008). Thus, megestrol plus traditional herbal combination may effectively prevent weight loss and improve appetite and fatigue for advanced cancer patients. |
NCT00669656 concluded that the prostate health cocktail had decreased the prostate-specific antigen level by 37% of the patients who experienced biochemically recurrent prostate cancer. 36 NCT02795390 suggested that a modified traditional Chinese herbal formula, MaZiRenWan, was effective for relieving constipation in patients in palliative cancer care. 37 NCT02468141 indicated SDT could be a potential option for anorexia management in cancer patients, showing improvement in both anorexia and quality of life. 38 NCT00076609 mentioned that PHY906, a pharmaceutical-grade formulation of 4 traditional Chinese herbs, increased the index of capecitabine by enhancing its antitumor activity while reducing toxicity in advanced hepatocellular carcinoma. 39 NCT03911921 appeared that RSYRT, also known as ChongGuanYaoYe in TCM, for reducing cancer-related fatigue was found to be effective. 40 Regarding the trial of NCT02900742, the intervention group (chemotherapy with Chinese herbal medicine formula) showed significant improvements in progression-free survival rate and relieved symptoms while improving overall quality of life. 41 IRCT20180722040556N2 and IRCT20180722040556N1 have shown effectiveness in preventing weight loss and improving appetite for advanced cancer patients.42,43 Both trials used megestrol with an herbal combination in the intervention group; the type of herbal combination was unknown.
Discussion
Cancer patients have recently been utilizing CAM modalities, including herbal medicine therapy, for treating cancer and improving patients’ quality of life. The present cross-sectional study investigated the current status of herbal medicine interventions for cancer by analyzing registered clinical trials since there has been a lack of the characteristics of the registered trials. Thus, this may be the first study to report registered trials in such field.
As the global herbal medicine market grew rapidly, the consumption of herbal medicine has also up-surged. 44 Herbal medicines have been applied to cancer patients to relieve symptoms and improving adverse effects from conventional therapies, including chemotherapy and immunotherapy.4,7 Several studies reported that herbal therapy for cancer patients is applied to treat cancer and anorexia, cachexia, insomnia, neuropathic pain, nausea, vomiting, and so on.45 -49 Meanwhile, misuse of herbal medicine can be harmful for cancer patients. 50 Hence, it is essential to detect the gradually increasing registered trials regarding the use of herbal medicines in the field of cancer research.
It is also vital to note that most trials were focused on treatment. According to a recent systematic review, including 155 studies and 809 065 participants, a large percentage of cancer patients sought herbal medicine during the course of conventional therapy, and the prevalence of herbal medicine use by cancer patients worldwide was 22%. 6 Moreover, the use of traditional and complementary medicine in Europe by cancer patients has been high, varying from 44.7% to 72%.51 -53 As an example, a multi-institutional cohort study with long-term use of CHM in patients with esophageal cancer stage 4 found that patients with CHM had a higher 5-year survival rate than those without CHM, and the risk of death was also 46% lower than those without CHM. 54
The rapid growth of cancer patients seeking herbal medicine therapies, may have occurred in part due to the synergistic combination of herb-drug interactions. 55 For example, the combination treatment of gemcitabine, a chemical used in the treatment of pancreatic cancer, and C5E, an herbal mixture extract, has been found to be more effective than individual treatments since the mRNA expression levels of sonic hedgehog, which is implicated in the development of types of cancer, were downregulated when the co-treatment with C5E and gemcitabine was compared with the treatment with either C5E or gemcitabine alone. 56 Herbal/dietary supplements (HDS) are the most popular CAM treatment among cancer patients. According to a survey, 127 out of 375 patients used HDS before and after a cancer diagnosis, and most patients stated that other drugs were used at the same time as HDS. 57 Moreover, herbal medicine has positive effects, such as inhibiting tumor growth and cancer cell metastasis by inducing tumor cell apoptosis and reversing the resistance of existing chemotherapy drugs; therefore, adjuvant herbal treatment can make chemotherapy treatment more effective and less toxic than traditional chemotherapy drugs. 58
Various modalities of CAM therapies have been known to be helpful for cancer patients. Among the trials, 2 trials (NCT04584775, ChiCTR2000038393) indicated that herbal medicines were combined with acupuncture, and 2 more trials (ChiCTR2000037192, ChiCTR-INR-16009557) also mentioned that herbal medicines were combined with moxibustion. According to a recent comprehensive narrative review regarding using CAM treatments for cancer pain, the study stated that a multimodal approach to cancer pain management, including herbal medicines, might improve the quality of pain treatment and patients’ quality of life. 59 Moreover, a pilot study describes a multimodal outpatient complementary therapy program including herbs, conducted during adjuvant radiotherapy and/or chemotherapy or outpatient aftercare, showing its potential to improve the overall quality of life and reduce treatment-associated adverse effects. 60
Regarding the final selection of trials from the databases, numerous trials were heavily concentrated on lung, breast, and colorectal cancer. According to cancer statistics 2022 by the America Cancer Society, the greatest number of cancer-related death are associated with lung cancer (21%), prostate cancer (11%), and colorectal cancer (9%) in men and with lung cancer (21%), breast cancer (15%) and colorectal cancer (8%) in women, which indicates that the selected trials seem to have focused on the types of cancer due to clinical needs. 61
Furthermore, the herbal medicines utilized for clinical trials were mainly concentrated on the decoction of herbal mixtures. SH003 is a decoction of medicinal herbs consisting of Astragalus membranaceus, Angelica gigas, Trichosanthes Kirilowii. 62 PHY906 is a decoction of a mixture of 4-herbs, Scutellarin baicalensis Georgi, Glycyrrhiza uralensis Fisch, Paeonia lactiflora Pall, and Ziziphus jujuba Mill. 63 Besides, the aforementioned trials investigated DaHuang GanTsao Tang, Maekmoondong-tang, Xiang Bei Yang Rong Tang, Sheng-Yu-Tang, and others. Decoction is the most common form of administration of herbal medicine; formulas can be varied according to clinical need. When using decoctions, the herb-herb combined effect would act as multi-targeted in the clinical outcome. For instance, the decoction of Ephedra (Mahuang Tang et al ), which contains ephedra, cinnamon twig, bitter apricot seed, and licorice root, is used for its diaphoretic effect but also the relief of coughing and general aching during the common cold as well as reducing headaches.64,65
With regard to the results of the published trials, 8 trials used combinations of chemotherapy and herbal medicine compared to 6 monotherapies only using herbal medicines. Notably, all the outcomes were positive among the trials that utilized the combined therapies. Considering the increasing popularity of herbal medicine among cancer patients, safety and effectiveness approaches to integrative cancer care remain undisclosed. According to a scoping review of pharmacoepidemiological studies based on real-world data on herb-drug interactions in cancer, most clinical interactions had no negative consequences. 66 The study also stated that well-designed, multicenter, double-blind, and placebo-controlled randomized clinical trials are needed to elucidate the risks and benefits of using combined therapies. Another review further suggested it is imperative to seek more reliable evidence from other parts of the world, which provides an in-depth global perspective on herbal-drug combination therapy in cancer patients since most of the herbal medicine clinical trials are from Asia. 67
Additionally, the essential potential for alleviating adverse events of chemotherapies was recognized; the outcomes from the published articles have revealed to prevent weight loss, reduce fatigue, and improve overall quality of life. A recent systematic review regarding using East Asian herbal medicine in cancer patients identified that combined therapies may benefit patients with cancer pain by increasing the response rate, relieving pain intensity, improving pain-related performance status, and improving quality of life. 68
Several researchers and industry professionals have requested that a transparent scientific verification of traditional herbal medicines be needed to ensure safety and efficacy. Based on the gathered data via ClinicalTrials.gov, the ISRCTN registry, the ChiCTR, and the ICTRP, more trials are still required to provide evidence for clinical practice. However, it is vital to note the upward trajectory of the number of clinical trial registrations during the survey period. It is also noteworthy that the majority of the trials are in Phase 2. Although herbal medicine has long been used in practice, there always has been a lack of scientifically proven therapeutic effects and safety. 69 This phenomenon perhaps may be associated with various challenges. First, the treatments in herbal medicines are complex, consisting of a mixture of active components and difficult-to-define inclusion and exclusion criteria. Randomized controlled trials (RCT) are usually double-blinded; however, it may be challenging to maintain double-blind when herbal treatment involves a multidimensional approach to prescribing herbal medicines. In addition, the selection of herbal medicine for the control group may also be challenging as the medicines somewhat closely match with the intervention group in order to provide evidence of a specific effect; however, it is incredibly arduous to match the standardized herbal medicines which have the credibility of the treatment to the patient in the similar clinical setting as the intervention group. 70
This study has several limitations. First, all clinical trial data were only obtained from ClinicalTrials.gov, the ISRCTN registry, the ChiCTR, and the ICTRP; some missed clinical trials may have been registered in other registries. However, ClinicalTrials.gov does contain most global trials. Second, the search term “herbal medicine” does not retrieve every registered herbal medicine trial since some trials may have used the names of herbal extract and herbal decoction. Most of the single and multi-herbal trials registered in registries can only be retrieved by searching on the name of the plant, not with the “herbal medicine” search term.71 -74 Third, most of the trials are interventional trials so that observational trials might have been registered with other databases. Additional information on study protocols and in-depth results details may serve as valuable information for clinicians and researchers to further scrutinize the factors for using herbal medicine for cancer patients. As a general limitation of a clinical registry, the data quality of each trial may vary.
Conclusion
This cross-sectional study comprehensively analyzed the characteristics of the registered clinical trials related to herbal medicine and cancer via ClinicalTrials.gov, the ISRCTN registry, the ChiCTR, and the ICTRP. Most clinical trials were interventional studies in phase 2. Unsurprisingly, the highest number of trials were registered in Asia since traditional, complementary, and alternative medicine has been widely practiced for a long time. Almost half of the trials were found to be still ongoing, and only 16 trials had complete results for further analysis. Based on the results of the analysis, herbal medicines were primarily used for lung, breast, and colorectal cancer; however, this may change as more complete results are to be added in the near future. Overall, it can be assumed that an herbal medicine therapy will be another potential option to consider for healthcare professionals; the combination of herbal medicine and chemotherapy has attracted attention in the medical community due to various synergies. More importantly, there is not enough research solely using herbal medicine for treating cancer, it may be significant to design well-established study protocols to generate more concrete evidence for the cancer community.
Supplemental Material
Supplemental material, sj-docx-2-ict-10.1177_15347354231218255 for Current Characteristics of Herbal Medicine Interventions for Cancer on Clinical Databases: A Cross-Sectional Study by Bo-Young Youn, Ji-Hyun Kim, Yong-Kyu Jo, Sanghoon Yoon, Ji-Yeong Im, Hyo-Jung Kim, Jae-Dong Lee and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-xlsx-1-ict-10.1177_15347354231218255 for Current Characteristics of Herbal Medicine Interventions for Cancer on Clinical Databases: A Cross-Sectional Study by Bo-Young Youn, Ji-Hyun Kim, Yong-Kyu Jo, Sanghoon Yoon, Ji-Yeong Im, Hyo-Jung Kim, Jae-Dong Lee and Seong-Gyu Ko in Integrative Cancer Therapies
Footnotes
Authorship: Bo-Young Youn: Conceptualization, data analysis, creating statistical tables and figures, writing the initial manuscript, and formatting and preparation of the manuscript submission. Ji-Hyun Kim: Conceptualization, design, and research implementation. Yong-Kyu Jo and Sanghoon Yoon: Data collection, and cleaning. Ji-Yeong Im and Hyo-Jung Kim: Data cleaning and analysis. Jae-Dong Lee and Seong-Gyu Ko: Conceptualization, design, research implementation, and overall supervision. All authors contributed to interpretations of the findings, writing, review, and editing of the manuscript, and approval of the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (no. 2020R1A5A2019413).
ORCID iD: Seong-Gyu Ko  https://orcid.org/0000-0002-2345-430X
https://orcid.org/0000-0002-2345-430X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2019;10:1614-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Youn BY, Lee SY, Cho W, et al. Global trends of nutrition in cancer research: A bibliometric and visualized analysis study over the past 10 years. Int J Environ Res Public Health. 2022;19:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Cancer key factors. Accessed December 2, 2022. https://www.who.int/news-room/fact-sheets/detail/cancer
- 4. Poonthananiwatkul B, Howard RL, Williamson EM, Lim RH. Cancer patients taking herbal medicines: A review of clinical purposes, associated factors, and perceptions of benefit or harm. J Ethnopharmacol. 2015;175:58-66. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Key technical issues of herbal medicine with references to interactions with other medicines. Accessed December 3, 2022. https://www.who.int/publications/i/item/9789240019140
- 6. Asiimwe JB, Nagendrappa PB, Atukunda EC, et al. Prevalence of the use of herbal medicines among patients with cancer: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2021;2021:9963038-9963118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theuser AK, Hack CC, Fasching PA, et al. Patterns and trends of herbal medicine use among patients with gynecologic cancer. Geburtshilfe Frauenheilkd. 2021;81:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ying J, Zhang M, Qiu X, Lu Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed Pharmacother. 2018;103:381-390. [DOI] [PubMed] [Google Scholar]
- 9. Aiello P, Sharghi M, Mansourkhani SM, et al. Medicinal plants in the prevention and treatment of colon cancer. Ovix Med Cell Longev. 2019;2019:1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee YK, Bae K, Yoo HS, Cho SH. Benefit of adjuvant traditional herbal medicine with chemotherapy for resectable gastric cancer. Integr Cancer Ther. 2018;17:619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong TY, Park BK, Cho JH, et al. A prospective study on the safety of herbal medicines, used alone or with conventional medicines. J Ethnopharmacol. 2012;143:884-888. [DOI] [PubMed] [Google Scholar]
- 12. Glanville JM, Duffy S, McCool R, Varley D. Searching clinicaltrials.gov and the international clinical trials registry platform to inform systematic reviews: what are the optimal search approaches? J Med Libr Assoc. 2014;102:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J Evid Based Med. 2015;8:2-10. [DOI] [PubMed] [Google Scholar]
- 14. Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in clinicaltrials.gov, 2007-2010. JAMA. 2012;307:1838-1847. [DOI] [PubMed] [Google Scholar]
- 15. Hoimes CJ, Lamb L, Ruta S, et al. A phase I/II study of PHY906 plus capecitabine (CAP) in patients (pts) with advanced pancreatic cancer (APC). J Clin Oncol. 2008;26(15_suppl):15538. [Google Scholar]
- 16. Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2(45):45ra59. [DOI] [PubMed] [Google Scholar]
- 17. Pang CLK, Zhang X, Wang Z, et al. Local modulated electro-hyperthermia in combination with traditional Chinese medicine vs. Intraperitoneal chemoinfusion for the treatment of peritoneal carcinomatosis with malignant ascites: A phase II randomized trial. Mol Clin Oncol. 2017;6(5):723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engels FK, de Jong FA, Sparreboom A, et al. Medicinal cannabis does not influence the clinical pharmacokinetics of irinotecan and docetaxel. Oncologist. 2007;12(3):291-300. [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y, Wu L, Shen L, et al. Effect of traditional Chinese medicine treatment as maintenance therapy on regulating the serum concentration of sCTLA-4 in patients with advanced non-small-cell lung cancer and its relationship with prognosis. Zhonghua Zhong Liu Za Zhi. 2016;38(10):757-762. [DOI] [PubMed] [Google Scholar]
- 20. ClinicalTrials.gov. A Phase I/II, Multi-Center, Open-Label, Dose-Escalation, Safety and Efficacy Study of PHY906 Plus Capecitabine in Patients With Advanced Pancreatic Carcinoma. Accessed July 29, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT00411762
- 21. ClinicalTrials.gov. Treatment of Anal High-grade Squamous Intraepithelial Lesions (HSIL) Through Use of a Chinese Herbal Topical Cream (AIJP). Accessed July 29, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT00622440
- 22. Macaya A, Muñoz-Santos C, Balaguer A, Barberà MJ. Interventions for anal canal intraepithelial neoplasia. Cochrane Database Syst Rev. 2012;12:CD009244-NaN23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov. A Phase II Multicenter, Randomized, Placebo Controlled, Double Blinded Clinical Study of KD018 as a Modulator of Irinotecan Chemotherapy in Patients With Metastatic Colorectal Cancer. Accessed July 29, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT00730158
- 24. ClinicalTrials.gov. Local mEHT + TCM Versus Intraperitoneal Chemoinfusion in Treatment of Malignant Ascites: Phase II RCT (OTMA-RII). Accessed July 29, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT02638051
- 25. ClinicalTrials.gov. Herbal Mouthrinse for Oral Mucositis Study (OM). Accessed July 29, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01898091
- 26. ISRCTN Registry. Influence of Medicinal Cannabis (Bedrocan) on the Ppharmacokinetics of Irinotecan and Docetaxel in Cancer Patients. Accessed July 29, 2023. https://www.isrctn.com/ISRCTN72088851
- 27. Chinese Clinical Trial Registry. Randomized Controlled Clinical Study to Evaluate the Efficacy and Safety of Integrated Traditional Chinese Medicine Treatment as Maintenance Therapy for Patients with Advanced Non-small-cell Lung Cancer. Accessed July 29, 2023. https://www.chictr.org.cn/showprojEN.html?proj=8521
- 28. Dorff TB, Tsao-Wei DD, Hawes D, et al. Final results from a trial of a combination herbal supplement for biochemically recurrent prostate cancer. J Clin Oncol. 2013;31(15_suppl):316020. [Google Scholar]
- 29. Cheng CW, Mok HF, Yau CWS, et al. A pilot randomized placebo-controlled study on modified MaZiRenWan: a formulated Chinese medicine to relieve constipation for palliative cancer patients. Chin Med. 2022;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheon C, Yoo JE, Yoo HS, et al. Efficacy and safety of Sipjeondaebo-Tang for anorexia in patients with cancer: A pilot, randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2017;2017:8780325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Changou CA, Shiah HS, Chen LT, et al. A phase II clinical trial on the combination therapy of PHY906 plus capecitabine in hepatocellular carcinoma. Oncologist. 2021;26(3):e367-e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y, Wang XS, Chen Y, et al. A phase II randomized controlled trial of Renshen Yangrong Tang herbal extract granules for fatigue reduction in cancer survivors. J Pain Symptom Manag. 2020;59(5):966-973. [DOI] [PubMed] [Google Scholar]
- 33. Wang Q, Jiao L, Wang S, et al. Maintenance chemotherapy with Chinese herb medicine formulas vs. With placebo in patients with advanced non-small cell lung cancer after first-line chemotherapy: a multicenter, randomized, double-blind trial. Front Pharmacol. 2018;9:1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Famil-Dardashti A, Hajigholami A, Badri S, Yekdaneh A, Moghaddas A. The role of trigonella, cichorium, and foeniculum herbal combination in the treatment of cancer-induced Anorexia/Cachexia: A quasi-experimental study. Int J Cancer Manag. 2020;13(8):e102515. [Google Scholar]
- 35. Yaghobi N, Mehrzad V, Badri S, Yegdaneh A, Moghaddas A. Combination of traditional herbal medicine for the treatment of cancer-induced Anorexia/Cachexia: A pilot, randomized, double-blinded and placebo-controlled clinical trial. J Herb Med. 2021;29:100499. [Google Scholar]
- 36. ClinicalTrials.gov. Herbal Therapy for Treatment of Recurrent Prostate Cancer. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT00669656
- 37. ClinicalTrials.gov. Clinical Trial on Palliative Cancer Patients With Constipation. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT02795390
- 38. ClinicalTrials.gov. Effect of Sipjeondaebo-tang for Cancer Related Anorexia in Cancer Patients. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT02468141
- 39. ClinicalTrials.gov. Study of Botanical PHY906 Plus Capecitabine for Advanced Unresectable Hepatocellular Carcinoma. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT00076609
- 40. ClinicalTrials.gov. RSYR for Fatigue Reduction in Cancer Survivors. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03911921
- 41. ClinicalTrials.gov. Clinical Study of Chinese Medicine Plus Chemotherapy Maintenance in Advanced Non Small Cell Lung Cancer. Accessed August 23, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT02900742
- 42. International Clinical Trials Registry Platform. Trigonella, Foeniculum, Chichorium in prevention of cancer-induced cachexia/anorexia. Accessed August 23, 2023. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180722040556N2
- 43. International Clinical Trials Registry Platform. Trigonella, Foeniculum, Chichorium, Hornleum and Glycine in prevention of cancer-induced cachexia/anorexia. Accessed August 23, 2023. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180722040556N1
- 44. Polaris Market Research. The global herbal medicine market size. Accessed December 9, 2022. https://www.polarismarketresearch.com/industry-analysis/herbal-medicine-market, 2020
- 45. Colige A, Sokolov BP, Nugent P, Baserga R, Prockop DJ. Use of an antisense oligonucleotide to inhibit expression of a mutated human procollagen gene (COL1A1) in transfected mouse 3T3 cells. Biochemistry. 1993;32:7-11. [DOI] [PubMed] [Google Scholar]
- 46. Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013:302426-302515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nayeri ND, Bakhshi F, Khosravi A, Najafi Z. The effect of complementary and alternative medicines on quality of life in patients with breast cancer: a systematic review. Indian J Palliat Care. 2020;26:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoon JH, Kim EH, Park SB, Lee JY, Yoon SW. Traditional herbal medicine for insomnia in patients with cancer: a systematic review and meta-analysis. Front Pharmacol. 2021;12:753140-753211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henke O, Bruchhausen W, Massawe A. Use of herbal medicine is associated with late-stage presentation in Tanzanian patients with cancer: A survey to assess the utilization of and reasons for the use of herbal medicine. J Glob Oncol. 2022;8(8):e2200069-NaN8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Werneke U, Earl J, Seydel C, et al. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer. 2004;90:408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lüthi E, Diezi M, Danon N, et al. Complementary and alternative medicine use by pediatric oncology patients before, during, and after treatment. BMC Complement Med Ther. 2021;21:96-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ciarlo G, Ahmadi E, Welter S, Hübner J. Factors influencing the usage of complementary and alternative medicine by patients with cancer. Complement Ther Clin Pract. 2021;44:101389-101398. [DOI] [PubMed] [Google Scholar]
- 53. Bonucci M, Geraci A, Pero D, et al. Complementary and integrative approaches to cancer: a pilot survey of attitudes and habits among cancer patients in Italy. Evid Based Complement Alternat Med. 2022;2022:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen SL, Lin WC, Chen YC, et al. The association between mortality and use of Chinese herbal medicine among incident stage IV esophageal cancer patients: A retrospective cohort study with core herbs exploration. Front Pharmacol. 2022;13:1018281-1018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu XQ, Sun Y, Lau E, Zhao M, Su SB. Advances in synergistic combinations of Chinese herbal medicine for the treatment of cancer. Curr Cancer Drug Targets. 2016;16:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pak PJ, Lee DG, Sung JH, et al. Synergistic effect of the herbal mixture C5E on gemcitabine treatment in PANC-1 cells. Mol Med Rep. 2021;23:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alsanad SM, Howard RL, Williamson EM. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complement Altern Med. 2016;16:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang J, Li JX, Ma LR, et al. Traditional herbal medicine: A potential therapeutic approach for adjuvant treatment of non-small cell lung cancer in the future. Integr Cancer Ther. 2022;21:15347354221144312-15347354221144313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kocot-Kępska M, Zajączkowska R, Zhao J, et al. The role of complementary and alternative methods in the treatment of pain in patients with cancer - current evidence and clinical practice: a narrative review. Contemp Oncol. 2021;25(2):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Domnick M, Domnick M, Wiebelitz KR, Beer AM. Evaluation of the effectiveness of a multimodal complementary medicine program for improving the quality of life of cancer patients during adjuvant radiotherapy and/or chemotherapy or outpatient aftercare. Oncology. 2017;93(2):83-91. [DOI] [PubMed] [Google Scholar]
- 61. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [DOI] [PubMed] [Google Scholar]
- 62. Lee K, Youn BY, Choi YJ, et al. State of the art and future implications of SH003: acting as a therapeutic anticancer agent. Cancers. 2022;14(4):1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu SH, Cheng YC. Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol. 2012;140:614-623. [DOI] [PubMed] [Google Scholar]
- 64. Che CT, Wang ZJ, Chow MS, Lam CW. Herb-herb combination for therapeutic enhancement and advancement: theory, practice and future perspectives. Molecules. 2013;18:5125-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang S, Ren J, Kong L, et al. Ephedrae herba: A review of its phytochemistry, pharmacology, clinical application, and alkaloid toxicity. Molecules. 2023;28(2):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lam CS, Koon HK, Ma CT, et al. Real-world data on herb-drug interactions in oncology: a scoping review of pharmacoepidemiological studies. Phytomedicine. 2022;103:154247. [DOI] [PubMed] [Google Scholar]
- 67. Okem A, Henstra C, Lambert M, Hayeshi R. A review of the pharmacodynamic effect of chemo-herbal drug combinations therapy for cancer treatment. Med Drug Discov. 2023;17:100147. [Google Scholar]
- 68. Jo HG, Seo J, Choi S, Lee D. East Asian herbal medicine to reduce primary pain and adverse events in Cancer Patients : A systematic review and meta-analysis with association rule mining to identify core herb combination. Front Pharmacol. 2021;12:800571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang J, Onakpoya IJ, Posadzki P, Eddouks M. The safety of herbal medicine: from prejudice to evidence. Evid Based Complement Alternat Med. 2015;2015:316706-316713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parveen A, Parveen B, Parveen R, Ahmad S. Challenges and guidelines for clinical trial of herbal drugs. J Pharm Bioallied Sci. 2015;7:329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li X, Qin Y, Liu W, et al. Efficacy of Ginger in ameliorating acute and delayed chemotherapy-induced nausea and vomiting among patients with lung cancer receiving cisplatin-based regimens: a randomized controlled trial. Integr Cancer Ther. 2018;17(3):747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barton DL, Liu H, Dakhil SR, et al. Wisconsin ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Howells LM, Iwuji COO, Irving GRB, et al. Curcumin combined with FOLFOX Chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa Trial. J Nutr. 2019;149(7):1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tröger W, Galun D, Reif M, et al. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: a randomized controlled trial. Dtsch Arztebl Int. 2014;111(29-30):493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-ict-10.1177_15347354231218255 for Current Characteristics of Herbal Medicine Interventions for Cancer on Clinical Databases: A Cross-Sectional Study by Bo-Young Youn, Ji-Hyun Kim, Yong-Kyu Jo, Sanghoon Yoon, Ji-Yeong Im, Hyo-Jung Kim, Jae-Dong Lee and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-xlsx-1-ict-10.1177_15347354231218255 for Current Characteristics of Herbal Medicine Interventions for Cancer on Clinical Databases: A Cross-Sectional Study by Bo-Young Youn, Ji-Hyun Kim, Yong-Kyu Jo, Sanghoon Yoon, Ji-Yeong Im, Hyo-Jung Kim, Jae-Dong Lee and Seong-Gyu Ko in Integrative Cancer Therapies