Abstract
The entomopathogenic fungus Beauveria majiangensis strain MJ1015, recently isolated from white grubs on a blueberry farm in Guizhou, China, could be used as a biocontrol agent. As a first step toward determining the effect of different solid culture media, temperature, and pH on colony growth rate and sporulation, we evaluated the optimum solid medium for mycelial growth and conidia production on a commercial scale. Subsequently, we also used single-factor analysis and response surface optimization to optimize the composition of the solid culture medium. On potato dextrose agar (PDA) medium, MJ1015 grew fastest and produced the highest spore yield at 29°C and pH 5. The best solid medium for the growth and sporulation of strain MJ1015 comprised 64.70 g/l of rice, 13.00 g/l of wheat, 0.30 g/l of NaNO3, 0.36 g/l of K2HPO4 · 3H2O, and 1.00 g/l of CaCO3. Rice, NaNO3, and K2HPO4 · 3H2O were the main influencing factors. The predicted value of cultured spores using the optimal medium was 4.56 x 1010 conidia/l. The validation test results showed that the average growth rate of strain MJ1015 on the optimal medium was 85% and 96% faster than that on Sabouraud dextrose agar with yeast extracts medium (SDAY) and PDA, respectively. Sporulation was 43.90 times and 9.65 times of that produced on SDAY and PDA, respectively. Our findings provide a theoretical basis for the commercial production of B. majiangensis to control white grubs.
Keywords: Beauveria, biocontrol agent, biological characteristics, solid media, growth rate, spore sporulation
Introduction
Entomopathogenic fungi are widely used as biocontrol agents against many insect pests like lepidopteran larvae (Wraight et al. 2010; Fite et al. 2020; Gielen et al. 2022), thrips (Ugine et al. 2005; Bara and Laing 2020) and aphids (Castillo Lopez et al. 2014; Mantzoukas et al. 2022). In 1879, Metchnikoff used Metarhizium to control Anisoplia austriaca, a pest of cereal crops (Pu and Li 1996), demonstrating that entomopathogenic fungi could be used against white grubs. Subsequently, many studies have focused on the biological characteristics, production, and application of entomopathogenic fungi (mainly Metarhizium and Beauveria) against white grubs (Erler and Ates 2015; Kim et al. 2020). The use of Beauveria to control grubs on peanut (Nong et al. 2011), sugarcane (Visalakshi et al. 2015; Nozipho 2016), maize (Tamayo-Sánchez et al. 2022) and potato (Soni et al. 2018) has achieved remarkable results. Therefore, as an environmentally friendly fungal insecticide, Beauveria has a broad application potential and significant commercial value as a biological pest control agent for white grubs.
Beauveria majiangensis (Ascomycota: Cordycipitaceae) was first isolated from the larva of Holotrichia scrobiculata on a blueberry farm in Guizhou, China (Chen et al. 2018). The fungus shows a high level of infectivity and virulence toward the larvae of three species of beetle: H. scrobiculata, Holotrichia parallela, and Oxycetonia bealiae (Liu et al. 2020). However, not all members are suitable for mycoinsecticide formulations for controlling scarab beetles (Wang et al. 2022). Although B. majiangensis has shown potential as a biological control agent and host specificity, further investigations are needed to determine the optimum culture conditions and to identify a suitable growth medium for the commercialscale production of stable B. majiangensis spore powder for the large-scale control of underground pests.
Liquid fermentation, solid fermentation, and liquid-solid two-phase fermentation systems are used for the large-scale production of biopathogenic fungi. Earlier studies (Ibrahim and Low 1993; Ovruski et al. 2003; Feng et al. 2004) have suggested that fungi could be economically and efficiently mass-produced on different solid substrates, such as sorghum, rice, rice straw, wheat, wheat bran or Italian millet (Sikura and Primak 1970; Ibrahim and Low 1993; Machado et al. 2010; Kankale et al. 2017; Hassan et al. 2020; Yu et al. 2020), to produce stable and easily preserved conidia (Pandey 1994; Soccol et al. 2017). Therefore, the solid fermentation method became the medium of choice in the mass production of Beauveria. A strain’s growth rate and sporulation yield differ on different culture substrates (de Farias et al. 2010; Bhadauria et al. 2012; Ibrahim et al. 2015; Song et al. 2019). Similarly, the sporulation yield of different strains on the same solid medium also differs (Goffré et al. 2018; Song et al. 2019). Optimization studies are therefore needed to establish the optimal production conditions by different strains.
In this study, the biological characteristics of B. majiangensis strain MJ1015 were studied, and colony growth and conidial production on different solid media were assessed to provide a theoretical basis for expanding this research into other fields and for the large-scale industrial production of this strain.
Experimental Materials and Methods
Test strain
B. majiangensis strain MJ1015 was isolated from the larva of H. scrobiculata on a blueberry farm in Guizhou, China (Chen et al. 2018), and is stored in the Species Preservation Room at Guizhou Institute of Biology (preservation number CGMCC No. 15090).
Preparation of spore suspension
Aerial conidia of MJ1015 were obtained from a stock culture growing on a potato dextrose agar (PDA) plate by suspending them in sterile water containing 0.05% Tween-80. The number of conidia in the suspension was counted using a hemocytometer (Yue Cheng Trading Co., Ltd., China) and then diluted to 1.0 × 1010 conidia/l with 0.05% Tween 80 for use in subsequent experiments.
Inoculation and cultivation of strain MJ1015
Media plates were inoculated in the center with 1 μl of spore suspension using a pipette and then incubated in the dark in a climate chamber with 90 ± 5% relative humidity for 14 days. The colony growth rate and spore production were measured.
Effect of different media on colony growth rate and sporulation
To assess the effects of different media on colony growth rate and sporulation, four different media were inoculated with MJ1015 spore suspension: Sabouraud dextrose agar with yeast extract (SDAY), which comprised 10 g/l of tryptone, 40 g/l of dextrose, 20 g/l of agar, 10 g/l of yeast extract, and, with a pH 6.0 ± 0.1; PDA medium; ¼ Sabouraud dextrose agar with yeast extract (¼SDAY, 2.5 g/l of tryptone, 10 g/l of dextrose, 20 g/l of agar, 5 g/l of yeast extract); and base medium (CZM), which comprised 30 g/l of dextrose, 2 g/l NaNO3, 0.5 g/l MgSO4·7H2O, 1 g/l K2HPO4, 0.5 g/l KCl, 0.001 g/l FeSO4 · 7H2O, 20 g/l of agar. The inoculated plates were cultivated in a climate chamber at 26.5 ± 1°C, with at least three replications of each treatment.
Effect of different temperatures on colony growth rate and sporulation
To assess the effects of different temperatures on colony growth rate and sporulation, plates of PDA medium were inoculated with spore suspension and then incubated in the climate chamber at 20 ± 1°C, 23 ± 1°C, 26 ± 1°C, 29 ± 1°C, or 30 ± 1°C, with at least three replications of each temperature treatment.
Effect of pH on colony growth rate and sporulation
To assess the effects of different pH values on colony growth rate and sporulation, plates of PDA medium were prepared with pH values adjusted to pH 4, 5, 6, 7, 9, 11, or 13 with 0.1 mol/l HCl or 0.1 mol/l NaOH under sterile conditions. The PDA plates were inoculated with spore suspension and cultured at 29°C, with at least three replications of each pH value.
Solid medium single-factor screening
Carbon source
To assess the effects of media with different carbon sources on colony growth rate and sporulation, the 40 g/l of glucose in SDAY medium was replaced with 40 g/l of sucrose, fructose, brown sugar, corn meal, corn cob meal, wheat bran, wheat meal, rice stalk meal, bran, rice meal, potato meal, or sweet potato meal, or with a mixture of rice and wheat (1 : 1 mass ratio). The plates were inoculated with spore suspension and cultured at 29°C. SDAY was used as a control. Each medium treatment comprised at least three replications, and three solid medium plates were used for each biological replicate experiment.
Proportion of rice and wheat
To assess the effects of media with different proportions of rice and wheat as the carbon source on colony growth rate and sporulation, the 40 g/l of glucose in SDAY medium were replaced with rice and wheat mass ratios of 1 : 1, 2 : 1, 3 : 1, 5 : 1, 7 : 1, 9 : 1, 1 : 2, 1 : 3, 1 : 5, 1 : 7, or 1 : 9. Plates of yeast extract plus tryptone, rice or wheat as the carbon source and SDAY were used as control groups. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Nitrogen source
To assess the effects of different nitrogen sources on colony growth rate and sporulation, plates of medium were prepared with a 2 : 1 ratio of rice and wheat as the carbon source and 20 g/l of yeast extract, yeast paste, peanut powder, cottonseed powder, silkworm pupal powder, soybean powder, or fish meal, or 1 g/l of ammonium sulfate, sodium nitrate, or urea. Plates of SDAY and rice: wheat ratio of 2 : 1 as the carbon source without the nitrogen source was used as control groups. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Inorganic salt
To assess the effects of different inorganic salts on colony growth rate and sporulation, agar plates were prepared with rice: wheat ratio of 2 : 1 and NaNO3 as the carbon and nitrogen source, respectively. To this medium, 1 g/l of KH2PO4, K2HPO4 · 3H2O, NaH2PO4, KNO3, MgSO4 · 7H2O, CaSO4 · 2H2O, ZnSO4, FeSO4, MgCl2 · 6H2O, KCl, CaCl2, NaCl, or CaCO3 were added. Medium without the addition of an inorganic salt acted as the control group. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Plackett-Burman design
Based on the results of the single-factor screening experiments, we used a Plackett-Burman design (N = 12) to assess five factors: two carbon sources, a nitrogen source, and two inorganic salts. Three dummy variables were used to estimate the error. For each factor, two levels were assessed: high (+1) and low (-1) (Table I). The high level was 1.25 times that of the low level. Colony growth rate and sporulation measurements were taken as response value Y. The design code and the coding values are shown in Table I. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Table I.
Range of different factors investigated with Plackett-Burman design.
| Symbol | Variables | Experimental value | |
|---|---|---|---|
| Low (−1) | High (+1) | ||
| X1 | Rice (g/l) | 27 | 33.75 |
| X2 | Wheat (g/l) | 13 | 16.25 |
| X3 | Virtual 1 | −1 | 1 |
| X4 | NaNO3 (g/l) | 1 | 1.25 |
| X5 | CaCO3 (g/l) | 1 | 1.25 |
| X6 | Virtual 2 | −1 | 1 |
| X7 | K2HPO4 · 3H.O (g/l) | 1 | 1 .25 |
| X8 | Virtual 3 | −1 | 1 |
Steepest ascent path
Based on the main factors promoting sporulation identified by the Plackett-Burman method, the steepest ascent method was designed using rice, NaNO3, and K2HPO4 · 3H2O. The step size and the direction of each significant factor were determined by the coefficient of sporulation regression equation of the Plackett-Burman design model. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Response surface optimization experiment
Based on our analysis of the significance factors and levels obtained using the Plackett-Burman test and steepest ascent method, we performed a response surface analysis experiment comprising three factors and five levels on a solid medium, which was designed using a central combination experimental design. The design is shown in Table II. The culture conditions and the number of replicates were the same as those used in the carbon source screening experiment.
Table II.
Factors and levels of response surface central composite design.
| Symbol | Variables | Code level | ||||
| −1.6828 | −1 | 0 | 1 | 1.6818 | ||
| A | Rice (g/l) | 58.4489 | 60.75 | 64.125 | 67.50 | 69.8011 |
| B | NaNO3 (g/l) | 0.1755 | 0.23 | 0.31 | 0.39 | 0.44459 |
| C | K2HPO4 · 3H2O (g/l) | 0.2389 | 0.29 | 0.365 | 0.44 | 0.49119 |
Statistical analysis
Colony growth rate (mm/d) and sporulation (conidia/l) data are presented as means ± the standard error (SE) and were statistically analyzed by performing a one-way analysis of variance (ANOVA) or Welch’s ANOVA using SPSS 21.0. SigmaPlot 14 software was used to plot the experimental results. Calculation of growth rate – colony growth diameter was measured with vernier caliper crossovers after 14 days of culture and recorded:
Calculation of spore number – the sterilized hole punch of 1 cm2 was used to take bacteria cakes from the center of the colony ½ distance from the edge, and three bacteria cakes were taken from each treatment. The cakes were placed in 20 ml sterile water containing 0.05% Tween-80 and fully oscillated and mixed on the vortex oscillator to make spore suspension.
The spore concentration was measured with the blood cell counting plate and the number of spores (spores/l) was calculated:
where N is the total number of spores in the 5 squares in the middle.
Results
Effect of different media on colony growth rate and sporulation
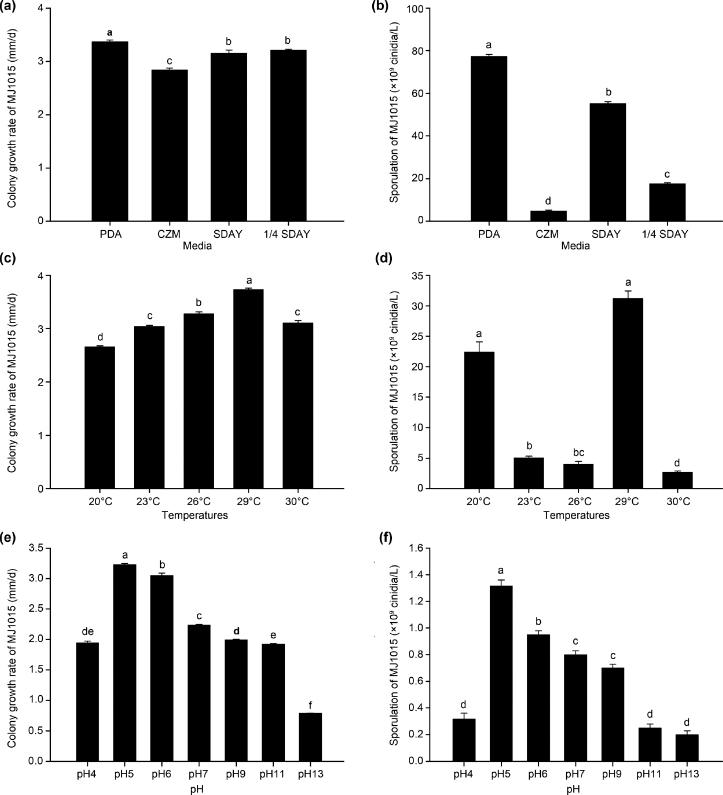
The colony growth rate of strain MJ1015 on PDA was significantly faster (3.37 mm/d; Fig. 1a), and sporulation was significantly higher (7.73 × 1010 conidia/l; Fig. 1b) than on the other three media. Although there was no significant difference in the colony growth rate on SDAY and ¼ SDAY, sporulation was significantly higher on SDAY than on ¼ SDAY. The colony growth rate on CZM was significantly slower (2.84 mm/d), and sporulation was significantly lower (4.80 × 109 conidia/l) than on the other media.
Fig. 1.
Effect of different culture conditions on colony growth rate and sporulation. Effect of different media (a–b), temperatures (c–d), and pH values (e–f) on colony growth rate and sporulation of Beauveria majiangensis strain MJ1015. Values shown are means ± the standard error. Different lowercase letters indicate significant differences between values (p < 0.05).
PDA – potato dextrose agar medium, CZM – base medium, SDAY – Sabouraud dextrose agar with yeast extract medium, ¼ SDAY – ¼ Sabouraud dextrose agar with yeast extract medium.
Effect of different temperatures on colony growth rate and sporulation
Colony growth and sporulation were observed under all the different temperature treatments (i.e., from 20°C to 30°C; Fig. 1c). The growth rate increased with increasing temperature up to 29°C. The optimal temperature for mycelial growth was 29°C and spore production was significantly higher than at 23°C, 26°C, or 30°C, but was not significantly different from that at 20°C (Fig. 1d). Moreover, the lowest level of sporulation occurred at 30°C, and the growth rate was significantly lower than at 26°C or 29°C.
Effects of different pH on colony growth rate and sporulation
Colony growth and sporulation were observed under all the different pH treatments (i.e., from pH 4 to 13; Fig. 1e and 1f). However, the optimal pH for mycelial growth (3.23 mm/d) and sporulation (1.32 × 109 conidia/l) was pH 5.
Effect of different carbon sources on colony growth rate and sporulation
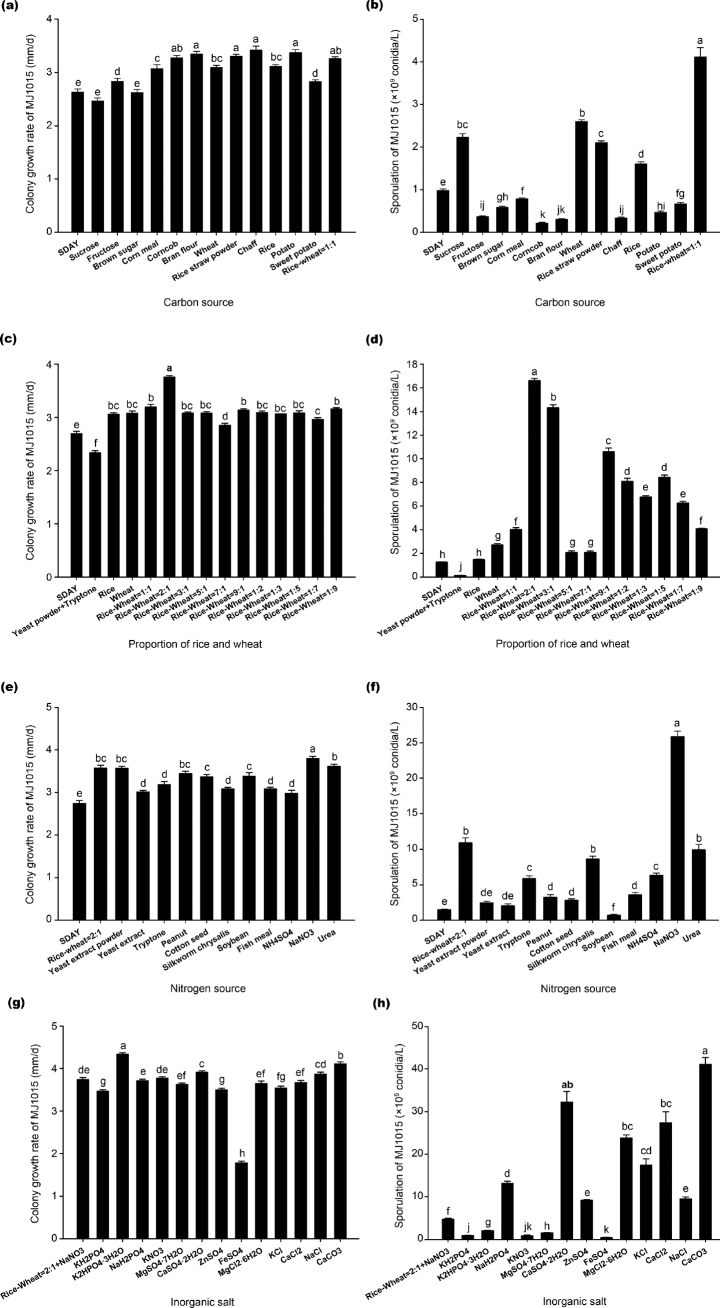
When we replaced the 40 g/l of dextrose in the SDAY medium with one of 13 different carbon sources, the colony growth rate of strain MJ1015 was either not significantly different or was better than that on SDAY (Fig. 2a and 2b). It appears that the growth rate on a 1:1 ratio of rice and wheat (3.26 ± 0.03 mm/d) was not significantly different from that of corncob, bran flour, wheat, rice straw powder, chaff, rice, and potato. Moreover, a 1 : 1 ratio of rice and wheat was the optimal carbon source for promoting sporulation ((4.11 ± 0.22) × 109 conidia/l), significantly higher than that produced on media with other carbon sources.
Fig. 2.
a–f. Solid medium single-factor screening. Effect of different carbon sources (a–b), proportions of rice and wheat (c–d), nitrogen sources (e–f), and inorganic salts (g–h) on colony growth rate and sporulation of Beauveria majiangensis strain MJ1015. Values shown are means ± the standard error. Different lowercase letters indicate a significant difference between values (p < 0.05).
Effect of different proportions of rice and wheat on colony growth rate and sporulation
To determine the optimal proportion of rice and wheat in the medium for colony growth and sporulation, we assessed 11 different rice: wheat ratios (Fig. 2c and 2d). A significantly higher growth rate (3.02 ± 0.03 mm/d) and greater sporulation ((1.66 ± 0.02) × 1010 conidia/l) were achieved on medium with rice: wheat ratio of 2 : 1 compared with the other treatments.
Effect of different nitrogen sources on colony growth rate and sporulation
When we replaced the yeast extract powder and tryptone in the SDAY medium with one of 11 different nitrogen sources, the colony growth rate of strain MJ1015 was significantly better than that on SDAY (Fig. 2e and 2f). When strain MJ1015 was grown on media containing NaNO3, the mycelial growth rate (3.81 ± 0.04 mm/d) was significantly faster, and sporulation was significantly higher ((25.83 ± 0.80) × 109 conidia/l) than on other media, suggesting that NaNO3 was the optimal nitrogen source for the growth and sporulation of strain MJ1015.
Effect of inorganic salts on colony growth rate and sporulation
When strain MJ1015 was grown on media containing rice: wheat ratio of 2 : 1, NaNO3, and either 0.10% K2HPO4 · 3H2O or CaCO3, the average mycelial growth rate (4.34 ± 0.03 mm/d or 4.12 ± 0.04 mm/d, respectively) was significantly higher than that on other media (Fig. 2g and 2h). In addition, significantly higher sporulation (41.01 ± 1.56) × 109 conidia/l) was achieved on media containing CaCO3 than on other media.
Plackett-Burman design
Based on the test results shown in Table III, multiple regression equation fitting and ANOVA of sporulation by MJ1015 colonies were performed using Design-Expert 12 software (Table IV). The regression equation for colony sporulation was:
Table III.
Plackett-Burman design and response values.
| Order | Variable code | Y Sporulation (conidia/l) 1 × 109 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | ||
| 1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 22.370 ± 1.833 |
| 2 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 2.860 ± 0.282 |
| 3 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 4.267 ± 0.466 |
| 4 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 0.923 ± 0.117 |
| 5 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 9.043 ± 1.283 |
| 6 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1.487 ± 0.289 |
| 7 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 33.453 ± 3.836 |
| 8 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 30.840 ± 2.155 |
| 9 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 11.113 ± 0.934 |
| 10 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 5.773 ± 0.636 |
| 11 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 6.950 ± 0.865 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 5.650 ± 1.211 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10.628 ± 1.353 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13.027 ± 1.477 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19.129 ± 1.886 |
Table IV.
Results of regression analysis of Plackett-Burman design.
| Source | Factor | df | Sporulation (Y) | |||
|---|---|---|---|---|---|---|
| Sum of squares | Mean square | F-value | p-value | |||
| Model | 5 | 11.69 | 2.34 | 7.84 | 0.0060** | |
| X1 | 0.7365 | 1 | 6.51 | 6.51 | 21.81 | 0.0016** |
| X2 | 0.0313 | 1 | 0.0117 | 0.0117 | 0.0393 | 0.8477 |
| X4 | -0.4789 | 1 | 2.75 | 2.75 | 9.22 | 0.0162* |
| X5 | 0.0448 | 1 | 0.0241 | 0.0241 | 0.0807 | 0.7836 |
| X7 | -0.4468 | 1 | 2.40 | 2.40 | 8.03 | 0.0220* |
| Residual | 8 | 2.39 | 0.2985 | |||
| Cor total | 14 | 15.30 | ||||
indicates significant (p < 0.05)
indicates very significant (p < 0.001)
Regression analysis showed that the Plackett-Burman model (Table IV) was highly significant (p < 0.01), indicating that the model could be used to analyze significant factors of B. majiangensis MJ1015 sporulation. The results of the regression analysis (Table IV) showed that rice was positively correlated with sporulation (p < 0.01), increasing its content promoted the sporulation of strain MJ1015, whereas NaNO3 and K2HPO4 · 3H2O were negatively correlated with sporulation (p < 0.05). Increasing the NaNO3 and K2HPO4 · 3H2O content of solid media could inhibit sporulation. However, the other factors were not significant (Table IV). Because the added reagents did not affect the colony growth rate in our laboratory, the colony growth rate was not analyzed here or in sub-sequent experiments.
Steepest ascent path
Steepest ascent tests of rice, NaNO3, and K2HPO4 · 3H2O were carried out with sporulation as the main factor from Plackett-Burman result. Based on the coefficient of the sporulation regression equation of the Plackett-Burman design model, the step size and direction of each significant factor were determined, as shown in Table V. Colony spore production peaked at the “origin + 10Δ” factor level and then decreased. The response interval of the maximum value was around factor level 10, which was selected as the center point.
Table V.
Experimental design and results of steepest ascent path.
| Level of factor | Factor coded value | Factor actual value | Sporulation (conidia/l) 1 × 109 | ||||
|---|---|---|---|---|---|---|---|
| Rice | NaNO3 | K2HPO4 · 3H2O | Rice | NaNO3 | K2HPO4 · 3H2O | ||
| Step lengthΔ | 1 | 0.125 | 0.125 | 3.375 | 0.125 | 0.125 | n.d. |
| Origin | 0 | 0.000 | 0.000 | 30.375 | 1.125 | 1.125 | 10.627 ± 0.342 |
| Origin + 1Δ | 1 | 0.125 | 0.125 | 33.750 | 1.044 | 1.049 | 9.043 ± 0.532 |
| Origin + 2Δ | 2 | 0.250 | 0.250 | 37.125 | 0.962 | 0.973 | 5.273 ± 0.136 |
| Origin + 3Δ | 3 | 0.375 | 0.375 | 40.500 | 0.881 | 0.898 | 4.837 ± 0.229 |
| Origin + 4Δ | 4 | 0.500 | 0.500 | 43.875 | 0.800 | 0.822 | 1.880 ± 0.218 |
| Origin + 5Δ | 5 | 0.625 | 0.625 | 47.250 | 0.719 | 0.746 | 0.730 ± 0.063 |
| Origin + 6Δ | 6 | 0.750 | 0.750 | 50.625 | 0.637 | 0.670 | 0.620 ± 0.008 |
| Origin + 7Δ | 7 | 0.875 | 0.875 | 54.000 | 0.556 | 0.594 | 29.487 ± 0.546 |
| Origin + 8Δ | 8 | 1.000 | 1.000 | 57.375 | 0.475 | 0.518 | 28.773 ± 0.540 |
| Origin + 9Δ | 9 | 1.125 | 1.125 | 60.750 | 0.393 | 0.443 | 35.627 ± 1.806 |
| Origin + 10Δ | 10 | 1.250 | 1.250 | 64.125 | 0.312 | 0.367 | 47.587 ± 4.056 |
| Origin + 11Δ | 11 | 1.375 | 1.375 | 67.500 | 0.231 | 0.291 | 9.917 ± 0.451 |
| Origin + 12Δ | 12 | 1.500 | 1.500 | 70.875 | 0.150 | 0.215 | 2.420 ± 0.079 |
| Origin + 13Δ | 13 | 1.625 | 1.625 | 74.250 | 0.068 | 0.139 | 3.967 ± 0.138 |
Response surface optimization experiment
According to the Plackett-Burman test results and steepest ascent path, a three-factor and five-level response surface analysis was conducted on rice, NaNO3 and K2HPO4 · 3H2O using a central combination design. The central combination design and its levels are shown in Table II, and the experimental results are shown in Table VI.
Table VI.
Response surface central composite design and corresponding results.
| Run | Coded variable level | Y Sporulation (conidia/l) 1 × 109 | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | −1 | −1 | −1 | 1.95 + 0.108 |
| 2 | 1 | −1 | −1 | 2.75 + 0.085 |
| 3 | −1 | 1 | −1 | 1.60 + 0.070 |
| 4 | 1 | 1 | −1 | 2.34 + 0.098 |
| 5 | −1 | −1 | 1 | 1.75 + 0.060 |
| 6 | 1 | −1 | 1 | 2.16 + 0.117 |
| 7 | −1 | 1 | 1 | 1.45 + 0.062 |
| 8 | 1 | 1 | 1 | 1.55 + 0.076 |
| 9 | −1.6818 | 0 | 0 | 2.13 + 0.061 |
| 10 | 1.6818 | 0 | 0 | 2.91 + 0.077 |
| 11 | 0 | −1.6818 | 0 | 2.70 + 0.102 |
| 12 | 0 | 1.6818 | 0 | 1.23 + 0.038 |
| 13 | 0 | 0 | −1.6818 | 2.58 + 0.076 |
| 14 | 0 | 0 | 1.6818 | 2.85 + 0.059 |
| 15 | 0 | 0 | 0 | 4.90 + 0.077 |
| 16 | 0 | 0 | 0 | 4.88 + 0.088 |
| 17 | 0 | 0 | 0 | 4.67 + 0.119 |
| 18 | 0 | 0 | 0 | 4.25 + 0.110 |
| 19 | 0 | 0 | 0 | 4.57 + 0.085 |
| 20 | 0 | 0 | 0 | 3.70 + 0.082 |
Multiple regression equation fitting and ANOVA with Table VI test data using Design-Expert 12 software yielded the regression equation:
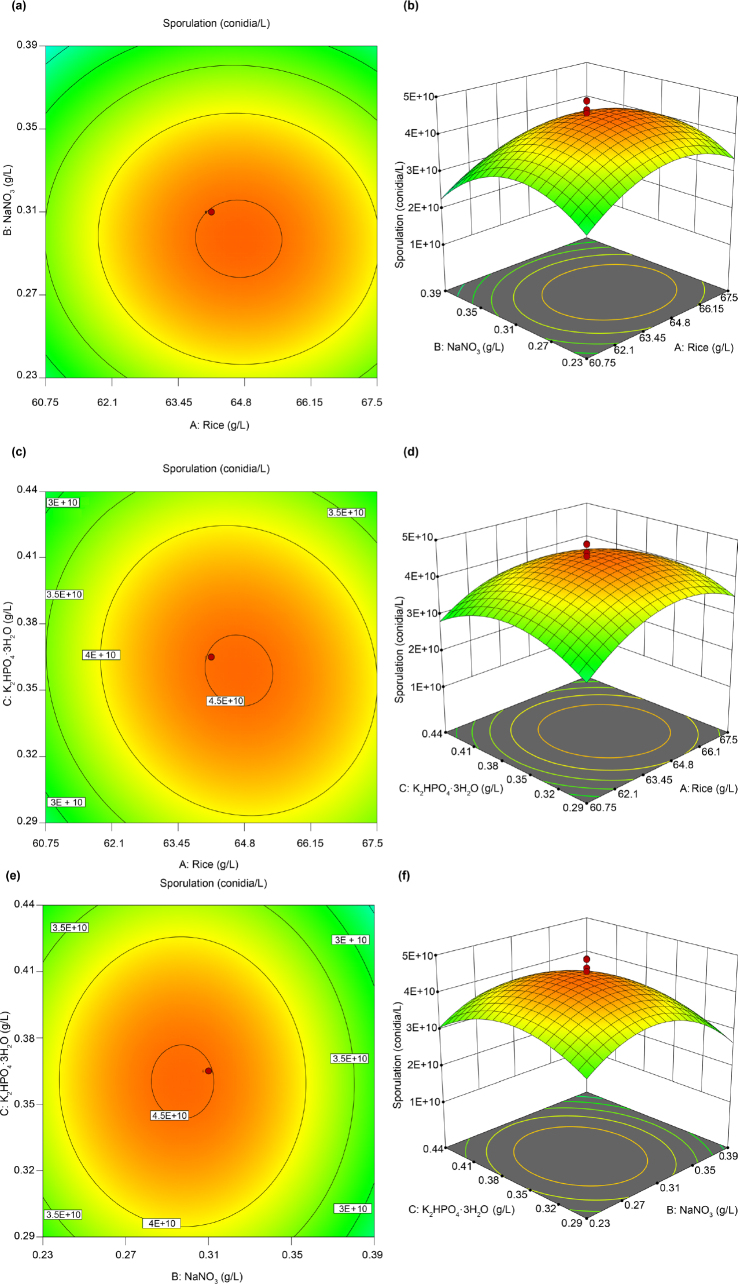
The regression equation results (Table VII) revealed that the regression model has relevance (p < 0.0001) and that the model has a high goodness of fit value (R2 = 0.9348), indicating that the model is a good reflection of the relationship between the investigated factors and response values. The colony spore yield lack of fit was not significant (p = 0.6030), indicating that the model did not lose fit; that is, the modified model could be used for the theoretical prediction of solid spore yield optimization of strain MJ1015. According to the response surface diagrams showing the effects of rice (A), NaNO3 (B), and K2HPO4 · 3H2O (C), on sporulation (Fig. 3), A, B, and C have maximum values.
Table VII.
ANOVA for response surface quadratic polynomial model.
| Source | df | Sporulation (Y) | ||||
|---|---|---|---|---|---|---|
| Sum of squares | Mean square | F-value | p-value | |||
| Model | 9 | 2.668 × 1021 | 2.965 × 1020 | 15.93 | < 0.0001 | significant |
| A-Rice | 1 | 8.275 × 1019 | 8.275 × 1019 | 4.450 | 0.0612 | |
| B-NaNO3 | 1 | 1.256 × 1020 | 1.256 × 1020 | 6.750 | 0.0266 | |
| C-K2HPO4 · 3H2O | 1 | 1.180 × 1019 | 1.180 × 1019 | 0.6336 | 0.4445 | |
| AB | 1 | 1.650 × 1018 | 1.650 × 1018 | 0.0886 | 0.7720 | |
| AC | 1 | 1.343 × 1019 | 1.343 × 1019 | 0.7126 | 0.4155 | |
| BC | 1 | 3.335 × 1017 | 3.335 × 1017 | 0.0179 | 0.8962 | |
| A2 | 1 | 8.529 × 1020 | 8.529 × 1020 | 45.810 | < 0.0001 | |
| B2 | 1 | 1.339 × 1021 | 1.339 × 1021 | 71.900 | < 0.0001 | |
| C2 | 1 | 7.069 × 1020 | 7.069 × 1018 | 37.970 | 0.0001 | |
| Residual | 10 | 1.862 × 1020 | 1.862 × 1019 | |||
| Lack of fit | 5 | 8.170 × 1019 | 1.634 × 1019 | 0.7821 | 0.6030 | not significant |
| Pure error | 5 | 1.045 × 1020 | 2.089 × 1019 | |||
| Cor Total | 19 | 2.855 × 1021 | ||||
| R2 | 0.9348 | |||||
| Adjusted R2 | 0.8761 | |||||
Fig. 3.
(a–d). Response surface diagrams showing the effects of rice and NaNO3 (contour (a) and 3D plots (b)), rice and K2HPO4 · 3H2O (contour (c) and 3D plots (d)), NaNO3 and K2HPO4 · 3H2O (contour (e) and 3D plots (f)) on the sporulation of Beauveria majiangensis strain MJ1015.
In summary, based on changes in sporulation with nutrient composition, combined with response surface optimization experiments, Design-Expert 12 software was used to analyze spore production as the primary response value and indicated that the optimal medium conditions for MJ1015 sporulation were 64.70 g/l of rice, 0.30 g/l of NaNO3, and 0.36 g/l of K2HPO4 · 3H2O. The spore yield of strain MJ1015 when grown on the optimal medium, was predicted to be 4.56 × 1010 conidia/l.
Validation test
The average growth rate of strain MJ1015 on the optimized solid phase medium was (4.46 ± 0.04) mm/d, 85% and 96% faster than that on SDAY and PDA, respectively. Furthermore, the sporulation of strain MJ1015 ((4.54 ± 0.16) × 1010 conidia/l) was 43.90 times and 9.65 times that on SDAY and PDA, respectively.
Discussion
Entomopathogenic fungi have been reported to tolerate a wide range of temperatures (i.e., 0 ~ 40°C), with most entomopathogenic fungi showing optimal growth and sporulation at approximately 20 ~ 30°C (Ibrahim and Low 1993; Vega and Kaya 2012; Tumuhaise et al. 2018). This study showed that a temperature of 29°C is most conducive for B. majiangensis MJ1015 colony
growth and sporulation. Our study supports previous reports that growth rates depend on the fungal isolate (genotype) and temperature. The colony growth rate and the number of spores produced by a fungus are not necessarily related (Safavi et al. 2007; Rangel et al. 2008; Campos-Esquivel et al. 2022), which was also confirmed by our study. Colonies that grew the fastest did not necessarily produce the most spores. The number of conidia produced when grown at 20°C did not differ significantly from that produced at 29°C even though the colony growth rate at 20°C was significantly slower than at 29°C. This may indicate that MJ1015 performed asexual reproduction to produce offspring when grown under the harsher environment of the 20°C climate chamber. This finding should help to guide the production of MJ1015 and its use in the field. Previous reports have shown that different Beauveria species have different pH tolerance ranges, such as 5–6 (Sanzhimitupova 1980), 6–8 (Galani 1988), as well as 10 and above (Shimazu and Sato 1996). An acidic pH (i.e., pH 5) was more suitable for the growth and sporulation of strain MJ1015, unlike Beauveria bassiana, which shows optimal growth and spore in media with a pH of 6 to 8 (Karthikeyan et al. 2008). However, the optimum pH for maximal growth of three other B. bassiana stains (i.e., BbR1, BbR2, Bbr3) was found to be 6 to 7 (Dhar et al. 2016). A preference for an acidic environment might reflect that B. majiagensis strain MJ1015 was isolated from blue-berry grubs and that the biological characteristics of Beauveria isolated from different geographical sources and hosts are different.
Although entomopathogenic fungi can grow and sporulate on different media, the utilization rate of different media depends on the fungal species (Lin et al. 1988; Aregger 1992). Bhadauria et al. (2012) used the mass ratio method to evaluate 15 different grains as a solid medium for the mass production of B. bassiana, with fungal dry weights of 0.38 and 0.56 g/100 g obtained on wheat and rice. Ibrahim et al. (2015) used the same method to show that the dry weight of B. bassiana per 100 g of substrate varied greatly, with 20.7, 5.0, and 1.3 g/100 g recorded when grown on burghul, rice, or wheat, respectively. After 14 days of culture, Pham et al. (2010) observed that B. bassiana KK5 conidial production was greatest when steamed rice was used as a substrate (2.69 g conidia/100 g substrate), followed by brown rice (1.43 g conidia/100 g substrate).
Table VIII.
Response surface optimization results validation.
| Medium | Colony growth (mm/d) | Sporulation (conidia/l) 1 × 1010 |
|---|---|---|
| New medium | 4.46 ± 0.04 | 4.54 ± 0.16 |
| SDAY | 2.41 ± 0.03 | 0.10 ± 0.02 |
| PDA | 2.28 ± 0.03 | 0.47 ± 0.04 |
SDAY – Sabouraud dextrose agar with yeast extract medium,
PDA – potato dextrose agar medium
However, rice husks were not found to be suitable for producing B. bassiana KK5 conidia. Some researchers use a method of spore counting to compare the effect of different media on the growth or sporulation of strains. According to Feng et al. (2000), conidial production of Verticillium lecanii on cooked rice and rice bran solid medium was 1.5 × 109 and 1.4 × 109 conidia/g, respectively, which was significantly higher than that on rice husks or a mixture of rice and rice bran under the same culture conditions. In addition, wheat bran and rice bran in the proportion of 3 : 1 supported maximum conidiospores yields for strain BbR2 (1.9 × 107 conidia/ ml) and stain BbR1(1.66 × 107 conidia/ml) and significantly superior over other strains (Dhar et al. 2016). However, there were some differences in our study, the best growth rate and sporulation were achieved when strain MJ1015 was grown on a medium comprising a mixture of rice and wheat, NaNO3, K2HPO4 · 3H2O, and CaCO3. Our results confirm the findings of earlier studies (Ibrahim and Low 1993; Feng et al. 2004; Bhadauria et al. 2012), showing that the fungal species may cause differences in carbon and nitrogen source utilization among strains.
In our study, rice and wheat impart essential factors favoring fungal growth and conidial yields. A possible explanation for higher levels of conidial production on rice and wheat than on other substrates could be the carbon to nitrogen ratio of these substrates. A high carbon-to-nitrogen ratio could promote conidiation under nitrogen starvation (Gao et al. 2007; Safavi et al. 2007; Uzma and Gurvinder 2009; Pham et al. 2010; Goffré et al. 2018; Song et al. 2019; Hassan et al. 2020). However, further investigations are needed to analyze the carbon: nitrogen ratios of the substrates used in this study to verify this idea.
Culture conditions also affect the conidial production of fungi, and, therefore, culture parameters, such as water, temperature, and light, also need to be optimized (Rodríguez-Gómez et al. 2009; Pham et al. 2010; Rizal et al. 2022). In addition, the influence of the medium on the infectivity of the strain should also be considered when selecting a medium (Rodríguez-Gómez et al. 2009; Garza-López et al. 2012; Doolotkeldieva et al. 2019) to ensure that applications of B. majiangensis as a pest control agent in the field are practical.
In conclusion, we have taken a first step toward identifying the culture conditions needed for a future commercial-scale and the optimal solid-state fermentation medium operation to produce B. majiangensis conidia to control white grubs. It to pave the way towards commercialization of strain MJ1015.
Acknowledgments
The authors want to thank Chao Kang from the Guizhou Institute of Biology for his suggestions on experimental design.
Funding Statement
This work was supported by the Natural Science Foundation of Guizhou Province ([2020]1Y110) and (ZK[2022]283).
Footnotes
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author.
Author contributions
Conception and design of the study: XW and ML. Material preparation, data collection, and analysis: XW, ZH, CL, GY, LL, YS, YR and JW. The first draft of the manuscript was written by WX and all authors commented on previous versions of the manuscript and approved the submitted version.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Aregger E Conidia production of the fungus Beauveria brongniartii on barley and quality evaluation during storage at 2°C J Invertebr Pathol 1992;59(1):2. doi: 10.1016/0022-2011(92)90104-c. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Bara GT, Laing MD . In: Horticultural Reviews. Warrington I, editor. Vol. 47 Hoboken (USA): John Wiley and Sons, Inc; 2020. Entomopathogens: potential to control thrips in avocado, with special reference to Beauveria bassiana; pp. 325–368. . , editor. , vol. . : ; . p. –. . https://doi.org/ [DOI] [Google Scholar]
- Bhadauria BP, Puri S, Singh PK Mass production of entomopatho-genic fungi using agricultural products Bioscan 2012;7(2):229. . . . ; ( ): –. . [Google Scholar]
- Campos-Esquivel L, Hanson PE, Escudero-Leyva E, Chaverri P Virulence of native isolates of entomopathogenic fungi (Hypocreales) against the “sweetpotato whitefly” Bemisia tabaci (Hemiptera: Aleyrodidae), including the effects of temperature and fungicides J Invertebr Pathol 2022 NaN192:107787. doi: 10.1016/j.jip.2022.107787. . . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Castillo Lopez D, Zhu-Salzman K, Ek-Ramos MJ, Sword GA The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions PLoS One 2014 NaN9(8):e103891. doi: 10.1371/journal.pone.0103891. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Liu M, Huang ZX, Yang GM, Han YF, Liang JD, Liang ZQ Beauveria majiangensis, a new entomopathogenic fungus from Guizhou, China Phytotaxa 2018;333(2):243. doi: 10.11646/phytotaxa.333.2.8. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- de Farias LV, Ximenes Monteiro K, Rodrigues S, André Narciso Fernandes F, Adolfo Saavedra Pinto G Comparison of Aspergillus niger spore production on Potato Dextrose Agar (PDA) and crushed corncob medium J Gen Appl Microbiol 2010 NaN56(5):399. doi: 10.2323/jgam.56.399. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dhar S, Jindal V, Gupta VK Optimization of growth conditions and medium composition for improved conidiation of newly isolated Beauveria bassiana strains Indian J Exp Biol 2016 NaN54(10):634. . . . ; ( ): –. . [PubMed] [Google Scholar]
- Doolotkeldieva T, Bobusheva S, Kulmanbetova A, Zholdoshbekova S, Amanbek Kyzy A Characterization of Beauveria bassiana isolates from Kyrgyzstan J Invertebr Pathol 2019 NaN167:107243. doi: 10.1016/j.jip.2019.107243. . . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Erler F, Ates AO Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle J Insect Sci 2015 NaN15(1):44. doi: 10.1093/jisesa/iev029. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng KC, Liu BL, Tzeng YM Verticillium lecanii spore production in solid-state and liquid-state fermentations Bioprocess Biosyst Eng 2000 NaN23(1):25. doi: 10.1007/s004499900115. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Feng MG, Pu XY, Ying SH, Wang YG Field trials of an oil-based emulsifiable formulation of Beauveria bassiana conidia and low application rates of imidacloprid for control of false-eye lcafhopper Empoasca vitis on tea in southern China Crop Prot 2004 NaN23(6):489. doi: 10.1016/j.cropro.2003.10.004. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Tamayo-Sánchez F, Tamayo-Mejía F, Marín-Jarrillo A, Santillán--Galicia MT, Pérez-Valdez A, Guzmán-Franco AW Dynamics of natural infection of white grub larvae by Beauveria and Metarhizium in maize crops from Mexico Biocontrol Sci Technol 2022;32(10):1177. doi: 10.1080/09583157.2022.2098925. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Fite T, Tefera T, Negeri M, Damte T, Sori W Evaluation of Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis for the management of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) under laboratory and field conditions Biocontrol Sci Technol 2020;30(3):278. doi: 10.1080/09583157.2019.1707481. . . . : ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Galani G . Cultivation of some entomopathogenic fungi in liquid media with various initial pH values. Vol. 21 Analel Institutului de Cercetari pentru Protecta Plantelor; 1988. p. 54. . . . ; : . [Google Scholar]
- Gao L, Sun MH, Liu XZ, Che YS Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi Mycol Res 2007 NaN111(1):87. doi: 10.1016/j.mycres.2006.07.019. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Garza-López PM, Konigsberg M, Gómez-Quiroz LE, Loera O Physiological and antioxidant response by Beauveria bassiana Bals (Vuill.) to different oxygen concentrations World J Microbiol Biotechnol 2012 NaN28(1):353. doi: 10.1007/s11274-011-0827-y. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Goffrè D, Cavalloa EC, Cavalitto SF, Folgarait PJ Selection and yield optimisation of a Beauveria bassiana isolate for the biological control of leaf cutter ants Biocontrol Sci Technol 2018;28(7):672. doi: 10.1080/09583157.2018.1479730. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Hassan F, Abdullah S, Assaf L Molecular identification and biomass production of an endophytic Beauveria bassiana isolated from cucumber leaves in Iraq J Duhok Univ 2020;22:38. doi: 10.26682/cajuod.2020.22.2.5. . . . ; (s2): - . https://doi.org/ [DOI] [Google Scholar]
- Ibrahim L, Laham L, Touma A, Ibrahim S Mass production, yield, quality, formulation and efficacy of entomopathogenic Metarhizium anisopliae conidia Br J Appl Sci Technol 2015;9(5):427. doi: 10.9734/BJAST/2015/17882. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Ibrahim YB, Low W Potential of mass production and field efficacy of isolates of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus against Plutella xylostella Int J Pest Manage 1993;39(3):288. doi: 10.1080/09670879309371807. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Kankale MD, Kelwatkar NM, Das SB, Sontakke BK Selection of suitable and economic substrate for mass production of fungus, Beauveria bassiana J Appl Zool Res 2017;28(2):157. . . . ; ( ): –. . [Google Scholar]
- Karthikeyan A, Shanthi V, Nagasathya A Effect of different media and pH on the growth of Beauveria bassiana and its parasitism on leaf eating caterpillars Res J Agric Biol Sci 2008;4(2):117. . . . ; ( ): –. . [Google Scholar]
- Kim S, Kim JC, Lee SJ, Lee MR, Park SE, Li D, Baek S, Shin TY, Gasmi L, Kim JS Soil application of Metarhizium anisopliaeJEF-314 granules to control, flower chafer beetle, Protaetia brevitarsis seulensis Mycobiology 2020 NaN 11;48(2):139. doi: 10.1080/12298093.2020.1735765. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LX, Yu YC, Hu JW . In: Study and application of entomogenous fungi in China. Li YW, Li ZZ, Liang ZQ, Wu JW, Wu ZK, Xu QF, editors. Vol. 1 Beijing (China): Academic Periodical Press; 1988. Study on white grub control by Beauveria brongiartii in soybean field, in study and application of Entomogenous Fungi in China; pp. 135–139. . , editors. , vol. . : ; . –. . [Google Scholar]
- Liu M, Wang XH, Hauang ZX, Yang GM, Luo LL, Wang H, Zhang SH [Primary study on the virulence of Beauveria majiangensis strain MJ1015 against three species of white grubs] (in Chinese) China Fruits 2020;2020(3):80. doi: 10.16626/j.cnki.issn1000-8047.2020.03.009. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Machado ACR, Monteiro AC, de Almeida AMB, Martins MIEG Production technology for entomopathogenic fungus using a biphasic culture system Pesq Agropec Bras 2010;45(10):1157. doi: 10.1590/s0100-204x2010001000015. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Mantzoukas S, Tamez-Guerra P, Zavala-Garcia F, Lagogiannis I, Ek-Ramos MJ Entomopathogenic fungi tested in planta on pepper and in field on sorghum, to control commercially important species of aphids World J Microbiol Biotechnol 2022 NaN38(5):84. doi: 10.1007/s11274-022-03268-7. . . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Nong XQ, Liu CQ, Lu X, Wang QL, Wang GJ, Zhang ZH Laboratory evaluation of entomopathogenic fungi against the white grubs, Holotrichia oblita and Anomala corpulenta (Coleoptera: Scarabaeidae) from the field of peanut, Arachis hypogaea Biocontrol Sci Technol 2011;21(5):593. doi: 10.1080/09583157.2011.566324. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Nozipho K . Development of Beauveria brongniartii as a bio-insecticide to control white grub (Coleoptera: Scarabaeidae) species attacking sugarcane in South Africa [Master Thesis] Pietermaritzburg (South Africa): University of KwaZulu-Natal; 2016. . . : ; . [Google Scholar]
- Ovruski S, Schliserman P, Aluja M Native and introduced host plants of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in northwestern Argentina J Econ Entomol 2003 NaN96(4):1108. doi: 10.1093/jee/96.4.1108. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Pandey A . In: Solid state fermentation. Pandey A, editor. New Delhi (Indie): Wiley Eastern; 1994. Solid-state fermentation: An overview; pp. 3–10. . , editor. . : ; . p. –. . [Google Scholar]
- Pham TA, Kim JJ, Kim K Optimization of solid-state fermentation for improved conidia production of Beauveria bassiana as a mycoin-secticide Mycobiology 2010 NaN38(2):137. doi: 10.4489/myco.2010.38.2.137. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu ZL. and Li ZZ . Insect mycology. Hefei (China): Anhui Science and Technology Press; 1996. pp. 387–446. . . : ; . p. –. . [Google Scholar]
- Rangel DE, Alston DG, Roberts DW Effects of physical and nutritional stress conditions during mycelial growth on conidial germination speed, adhesion to host cuticle, and virulence of Metarhizium anisopliae, an entomopathogenic fungus Mycol Res 2008 NaN112(11):1355. doi: 10.1016/j.mycres.2008.04.011. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Rizal LM, Furlong MJ, Walter GH Responses of diamondback moth to diverse entomopathogenic fungi collected from nonagricultural habitats – effects of dose, temperature and starvation Fungal Biol 2022 NaN126(10):648. doi: 10.1016/j.funbio.2022.08.005. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Gielen R, Robledo G, Zapata AI, Tammaru T, Pöldmaa K. Entomopathogenic fungi infecting lepidopteran larvae: A case from central Argentina Life 2022 NaN12(7):974. doi: 10.3390/life12070974. . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Gómez D, Loera O, Saucedo-Castañeda G, Viniegra-González G. Substrate influence on physiology and virulence of Beauveria bassiana acting on larvae and adults of Tenebrio molitor World J Microbiol Biotechnol 2009;25(3):513. doi: 10.1007/s11274-008-9917-x. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Safavi SA, Shah FA, Pakdel AK, Reza Rasoulian G, Bandani AR, Butt TM Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana FEMS Microbiol Lett 2007 NaN270(1):116. doi: 10.1111/j.1574-6968.2007.00666.x. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Sanzhimitupova RD Effect of the pH of the medium on the growth and development of the causal agent of mycosis of the seabuckthorn moth (Gelecjia hippophaella Schrk) Izvestiya Sibirskogo Otdeleniya Akademii Nauk SSSR Biology 1980;15:39. . . . ; : –. . [Google Scholar]
- Shimazu M, Sato H Media for selective isolation of an entomogenous fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) Appl Entomol Zool 1996;31(2):291. doi: 10.1303/aez.31.291. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Sikura AI, Primak TA . Native formulation of Boverin. Moscow (USSR): Microbiological Industry of Soviet Union, Department of Information; 1970. pp. 1–44. . . : ; . p. –. . [Google Scholar]
- Soccol CR, da Costa ESF, Letti LAJ, Karp SC, Woiciechowski AL, de Souza Vandenberghe LP Recent developments and innovations in solid state fermentation Biotechnol Res Innovation 2017;1(1):52. doi: 10.1016/j.biori.2017.01.002. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Song MH, Yu JS, Kim S, Lee SJ, Kim JC, Nai YS, Shin TY, Kim JS Downstream processing of Beauveria bassiana and Metarhizium anisopliae-based fungal biopesticides against Riptortus pedestris: solid culture and delivery of conidia Biocontrol Sci Technol 2019;29(6):514. doi: 10.1080/09583157.2019.1566951. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Soni S, Mehta PK, Chandel RS Susceptibility of white grub, Brahmina coriacea (Hope) infesting potato to local strains of Beauveria brongniartii (Saccardo) in Himachal Pradesh J Biol Control 2018;32(1):41. doi: 10.18311/jbc/2018/16288. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Tumuhaise V, Ekesi S, Maniania NK, Tonnang HEZ, Tanga CM, Ndegwa PN, Irungu LW, Srinivasan R, Mohamed SA Temperature-dependent growth and virulence, and mass production potential of two candidate isolates of Metarhizium anisopliae (Metschnikoff) Sorokin for managing Maruca vitrata Fabricius (Lepidoptera: Crambidae) on cowpea Afr Entom 2018;26(1):73. doi: 10.4001/003.026.0073. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Ugine TA, Wraight SP, Brownbridge M, Sanderson JP Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana, against western flower thrips, Frankliniella occidentalis J Invertebr Pathol 2005 NaN89(3):210. doi: 10.1016/j.jip.2005.05.010. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Uzma M, Gurvinder K Effects of carbon and nitrogen sources and ratio on the germination, growth and sporulation characteristics of Metarhizium anisopliae and Beauveria bassiana isolates Afr J Agric Res 2009;3(10):922. doi: 10.5897/AJAR.9000244. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Vega FE, Kaya HK . Insect pathology. Second edition Waltham (USA): Academic Press; 2012. . . . : ; . [Google Scholar]
- Visalakshi M, Bhavani B, Rao SG Field evaluation of entomopathogenic fungi against white grub, Holotrichia consanguinea blanch in sugarcane J Biol Control 2015;29(2):103. doi: 10.18311/jbc/2015/3228. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Wang Y, Fan Q, Wang D, Zou WQ, Tang DX, Hongthong P, Yu H Species diversity and virulence potential of the Beauveria bassiana complex and Beauveria scarabaeidicola complex Front Microbiol 2022 NaN13:841604. doi: 10.3389/fmicb.2022.841604. . . . ; : . http://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight SP, Ramos ME, Avery PB, Jaronski ST, Vandenberg JD Comparative virulence of Beauveria bassiana isolates against lepidopteran pests of vegetable crops J Invertebr Pathol 2010 NaN103(3):186. doi: 10.1016/j.jip.2010.01.001. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Yu JS, Lee SJ, Shin TY, Kim WJ, Kim JS Enhanced thermotolerance of entomopathogenic Beauveria bassiana and Metarhizium anisopliae JEF-isolates by substrate modification Int J Ind Entomol 2020;41(2):28. doi: 10.7852/ijie.2020.41.2.28. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]