Abstract
The transmission of human immunodeficiency virus (HIV) through blood poses a slightly increased risk. As a result, patients requiring blood transfusions should be screened for HIV antibodies. This study examined the diagnostic effectiveness of the photostimulated chemiluminescence assay in detecting anti-HIV antibodies and determined the cut-off value for this method. The performance of the fully automated photostimulated chemiluminescence assay system was validated according to CNAS-GL038:2019 (2020) and CNAS-GL037:2019 (2019) guidelines. A retrospective study was conducted at the Department of Medical Laboratory, Nanjing Tongren Hospital, affiliated with Southeast University, from January 2020 to December 2022. A total of 77,386 cases were tested for anti-HIV antibodies using the photostimulated chemiluminescence assay, with 79 cases initially testing positive. The method’s performance in detecting anti-HIV antibodies was evaluated using the Receiver Operating Characteristic (ROC) curve and the average Coefficient of Variation (CV) value of 3-year in-house quality control. The precision, detection limit, coincidence rate, and critical value of the performance verification results met the requirements. Using Western blotting (WB) as the reference method, positive cases were initially screened using the light-induced chemiluminescence method to determine the cut-off index (COI) value and draw the ROC curve. The maximum area under the ROC curve using the chemiluminescence method was 0.997, with a cutoff value of < 28.56, sensitivity of 98%, specificity of 100%, Jordan index of 0.98, and an average CV value of 3.55%. In conclusion, the photostimulated chemiluminescence assay has good diagnostic efficacy in detecting anti-HIV antibodies and is suitable for rapid screening before blood transfusion and surgery.
Keywords: anti-HIV, blood transfusion, diagnostic efficiency, photostimulated chemiluminescence assay
Introduction
Acquired Immune Deficiency Syndrome (AIDS) is a severe infectious disease caused by the Human Immunodeficiency Virus (HIV). HIV infection primarily results in severe damage to the immune system, destruction of CD4+ T lymphocytes, weakened resistance, susceptibility to severe infections, and some rare cancers such as Kaposi’s sarcoma. Patients with AIDS are prone to various rare diseases and eventually die due to long-term consumption and systemic failure (Cheng et al. 2016; Li et al. 2019b).
In clinical diagnosis and treatment, blood transfusion is a standard treatment method. However, due to the blood transmission characteristics of HIV, there is a slightly increased risk of HIV transmission through blood transfusion (Stramer et al. 2004; Busch et al. 2005). To ensure medical safety, the “Clinical Blood Transfusion Technical Specification” requires that every patient needing a blood transfusion undergo HIV antibody screening. Patients must also be tested for HIV antibodies before surgery. Effective detection and precise diagnosis are important for controlling transmission, guiding treatment, and determining prognosis (Zhang et al. 2019).
Currently, the primary methods used by domestic laboratories for screening anti-HIV antibodies are the enzyme-linked immunosorbent assay (ELISA) method and the chemiluminescence method. The Centers for Disease Control and Prevention (CDC) mainly use the Western blot method for confirming HIV diagnosis (Alhajj et al. 2023). ELISA is a widely used screening method for detecting anti-HIV antibodies in blood samples. It is based on antigen-antibody reactions and can be performed in a high-throughput manner, making it cost-effective for large-scale screening. Mazziotta et al. (2021a; 2021b; 2021c) utilized an indirect ELISA to identify serum IgG antibodies against Merkel cell polyomavirus (MCPyV) in two groups: females with spontaneous abortion (SA, n =94) and those who underwent voluntary pregnancy interruption (VI, n = 96). This indirect ELISA, employing two linear synthetic peptides/mimotopes to detect circulating IgGs against MCPyV capsid protein mimotopes, demonstrated reliability in identifying these antibodies in SA and VI females. However, false positives and negatives may occur due to cross-reactivity or incomplete seroconversion. Chemiluminescence immunoassays use chemical reactions to produce light, allowing for sensitive and rapid detection of anti-HIV antibodies. These assays have been shown to be more sensitive and specific than ELISA, with fewer false positives and negatives. However, they may be more expensive and require specialized equipment (Bronstein and Olesen 1995). Western blot is a confirmatory test used to verify the presence of anti-HIV antibodies in samples that have tested positive by ELISA or chemiluminescence. It involves separating HIV antigens by electrophoresis and detecting specific antibodies against these antigens. Western blot is highly specific and accurate but is more time-consuming and labor-intensive than other methods (Kirchner 2007). Photostimulated chemiluminescence is a type of chemiluminescence that occurs when a sample is exposed to light, which causes light emission from the sample. This phenomenon is used in various analytical techniques, such as immunoassays and DNA hybridization assays, to detect and quantify analytes (Li et al. 2019a). Chemiluminescence is the emission of light due to a chemical reaction, and photostimulated chemiluminescence is a specific type of chemiluminescence that requires light to be present (Zhang et al. 2022).
The objective of this research was to assess the diagnostic accuracy of the photostimulated chemiluminescence assay for detecting anti-HIV antibodies and establish the cutoff value.
Experimental
Materials and Methods
Sample collection
This retrospective study included 77,386 patients who underwent anti-HIV antibody screening at our hospital from January 2020 to December 2022, including inpatients and outpatients. Of these, 79 cases initially tested positive and were confirmed by the Nanjing Center for Disease Control and Prevention AIDS Laboratory. The ethics committee of Nanjing Tongren Hospital (TRLLKY2021005) approved this study, and written informed consent was obtained from each participant.
Instrumentation
The photostimulated chemiluminescence assay was performed using a LiCA®500 chemiluminescence analyzer (Kemei Boyang Diagnostic Technology Co., Ltd., China) with corresponding reagents. With Western blotting (WB) serving as the benchmark technique, the light-induced chemiluminescence method was employed for the initial screening of positive cases, with a cut-off index (COI) established as the reference point. A COI value exceeding 1.0 denoted a positive outcome, whereas a COI value below 1.0 signified a negative outcome (Su et al. 2019).
Internal quality control
In addition to routine sample testing, internal quality control was performed in the laboratory using quality control material provided by the manufacturer.
Validation of the photostimulated chemiluminescence assay system
The photostimulated chemiluminescence assay system underwent a comprehensive performance validation to ascertain its ability to execute chemiluminescence assays with precision and reliability. This fully automated system was evaluated following China National Accreditation Service for Conformity Assessment CNAS-GL038:2019 “Guidance of verification of qualitative measurement; procedures used in the clinical immunology” and CNAS-GL037:2019 “Guidance for validation of quantitative; procedures in clinical chemistry”. The purpose of this rigorous validation process was to confirm the effectiveness of the system.
Within-batch precision
Low-value, high-value, and negative control quality control materials were tested using the L1908 batch of HIV reagents (Chemclin Diagnostics Co., Ltd., China), with 20 repetitions for each. The Coefficient of Variation (CV) was calculated based on these results.
Between-batch precision
The L1908 batch of HIV reagents was used to test high and low concentration levels of quality control materials and the negative control, with four replicates for each concentration tested per day. The experiment was repeated for five consecutive days, with 20 data points, from which the CV was calculated.
Limit of detection
The HIV standard substance – a stable and controllable form of the HIV (Beijing Conch-Tech Biotech Co., Ltd., China) was diluted at a specific ratio and tested in undiluted and diluted states. The highest dilution ratio at which a positive result was still detectable was determined as the detection limit. The sample was tested in different batches over three days, with 1–2 batches tested per day and each batch tested four times. A total of 20 tests were conducted, with a minimum of 19 positive results required for validation to pass.
Agreement of inter-test measure
To ensure that test results can be replicated across different machines, we conducted a validation study in which 20 controlled samples were analyzed on our hospital’s LiCA®500 analyzer and tested on the same analyzer at Taizhou People’s Hospital. The sample set consisted of 10 HIVnegative and 10 HIV-positive serum quality control samples. To pass validation, a concordance rate of at least 80% between the two sets of test results was required for both positive and negative samples.
Verification of critical values
We selected 40 fresh serum samples from healthy individuals for testing. The verification criterion was that if all 40 samples tested below the critical value provided in the instructions or if no more than two samples exceeded it, the verification was considered to have passed. All initial positive samples were sent to the HIV confirmation laboratory at the Centers for Disease Control and Prevention in Nanjing, China, where detection was performed using the Western blotting method (GS HIV-1/HIV-2 PLUS O EIA kit, Bio-Rad Laboratories (Shanghai) Co., Ltd., China). The diagnostic results obtained were regarded as the gold standard. In this sample set, 50 samples tested positive for HIV-1 antibodies, while 29 tested negative by WB.
In-house quality control
In addition to routine sample testing, we performed in-house quality control using the manufacturer’s matching quality control product with National Medical Products Administration (NMPA) 20193400471 registration number.
Statistical methods
We recorded initial screening positive results and diagnostic test results in SPSS® 23.0 software (IBM® SPSS® Statistics for Windows, Version 23.0, IBM Corp., USA). To calculate the corresponding specificity, sensitivity, and area under the Receiver Operating Characteristic (ROC) curve of the method (Nahm 2022). We also obtained the method’s three-year in-house quality control CV value to determine its diagnostic efficacy and reproducibility. The intra- and interassay coefficients of variation (CVs) remained within an acceptable range, ensuring reliable and consistent measurements (Meesters and Voswinkel 2018).
Results
Basic information
Between January 2020 and December 2022, 77,386 samples were screened using photostimulated chemiluminescence assay, with 79 cases initially testing positive, resulting in a positive rate of 0.10%. Among the initial positive cases, there were 57 males and 22 females, averaging 42.8 years.
Performance verification results
In the batch precision test, the average value, standard deviation, and CV for low, high, and negative value samples were calculated and reported in Table I. The mean of the low-value samples was 3.734, with a standard deviation of 0.063 and a CV of 1.68%. The mean of the high-value samples was 15.495, with a standard deviation of 0.268 and a CV of 1.73%. The mean of the negative value samples was 0.461, with a standard deviation of 0.014 and a CV of 3.03%.
Table I.
Coefficient of variation of the intra test for photostimulated chemiluminescence assay.
| Indicator | Low S/CO | High S/CO | Negative S/CO |
|---|---|---|---|
| Mean | 3.734 | 15.495 | 0.461 |
| SD | 0.063 | 0.268 | 0.014 |
| CV | 1.68% | 1.73% | 3.03% |
S/CO – signal value/cut-off value, CV – coefficient of variation,
SD – standard deviation
Table II shows the between-batch precision results. The mean of the low-value samples was 4.018, with a standard deviation of 0.065 and a CV of 1.63%. The mean of the high-value samples was 16.284, with a standard deviation of 0.365 and a CV of 2.24%. The mean of the negative value samples was 0.474, with a standard deviation of 0.016 and a CV of 3.38%.
Table II.
Coefficient of variation of the inter test for photostimulated chemiluminescence assay.
| Indicator | Low S/CO | High S/CO | Negative S/CO |
|---|---|---|---|
| Mean | 4.018 | 16.284 | 0.474 |
| SD | 0.065 | 0.365 | 0.016 |
| CV | 1.63% | 2.24% | 3.38% |
S/CO – signal value/cut-off value, CV – coefficient of variation, SD – standard deviation
The experiment’s detection limit for anti-HIV was determined to be 0.5 NCU/ml, with a dilution factor of 4. All 20 positive control samples showed positive results, indicating that the experiment passed the test for detecting anti-HIV.
The conformity rate of test results obtained using the LiCA®500 kit is reported in Table III. A total of 20 samples were tested, with all positive samples conforming to reference LiCA®500 positive results and all negative samples conforming to reference LiCA®500 negative results. To establish the critical value for the specificity test of healthy serum samples, all 40 samples from healthy individuals were examined and produced negative results.
Table III.
Agreement of inter-test measure photostimulated chemiluminescence assay.
| LICA500 in our hospital | LICA500 in Taizhou People’s Hospital | Agreement | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 10 | 0 | 100% |
| Negative | 0 | 10 | 100% |
Distribution of COI values in positive cases screened by photostimulated chemiluminescence assay
All 79 initially positive samples had COI values greater than 1.03. The lowest COI value diagnosed as HIVpositive by the WB test was 9.23, while the highest COI value diagnosed as HIV-negative was 26.46.
Distribution ranges of COI values and WB test results in photostimulated chemiluminescence assay
Western blots were performed for 79 HIV-positive patients. Table IV shows that in the range of 1.0–10.0 COI value, 26 samples were positive in the chemiluminescence assay, while only one was positive in the WB. All samples in the 10.0–20.0 and 20.0–30.0 COI value ranges were negative in both assays. In the range of 30.0–40.0 COI value, two samples were positive in both assays. In the COI value range greater than 40.0, 47 samples were positive in both assays. In total, 79 samples were positive in the chemiluminescence assay, of which 50 were positive in WB.
Table IV.
Distribution ranges of COI values and WB test results in photostimulated chemiluminescence assay.
| COI | Photostimulated chemiluminescence assay positive | WB positive | WB negative |
|---|---|---|---|
| 1.0–10.0 | 26 | 1 | 25 |
| 10.0–20.0 | 2 | 0 | 2 |
| 20.0–30.0 | 2 | 0 | 2 |
| 30.0–40.0 | 2 | 2 | 0 |
| > 40.0 | 47 | 47 | 0 |
| Total | 79 | 50 | 29 |
COI – cut-off index, WB – Western blotting
Evaluating the ability of two methods to detect HIV antibodies using ROC curves
The ability of two methods to detect HIV antibodies was evaluated using a ROC curve, with the WB result as the gold standard. The provided data signifies a highly accurate chemiluminescence assay with a cut-off < 28.56. It exhibits exceptional sensitivity (98%) and specificity (100%), ensuring accurate identification of positive and negative cases. The AUC value of 0.997 indicates excellent overall performance. The study’s robustness is supported by a narrow 95% confidence interval (0.99–1.00) and a statistically significant p-value (< 0.0001, Fig. 1).
Fig. 1.
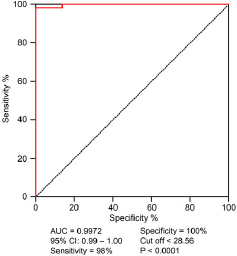
ROC curve of photostimulated chemiluminescence assay.
CV values for three-year in-house quality control
Table V presents the CV% values for in-house quality control for three consecutive years. The average CV% was 3.61% for 2020, 3.36% for 2021, and 3.67% for 2022, with a mean CV% of 3.55% for the three years. These values indicate good precision of laboratory test results over the three years.
Table V.
CV values for three years of in-house quality control.
| Year | CV% |
|---|---|
| 2020 | 3.61% |
| 2021 | 3.36% |
| 2022 | 3.67% |
| Mean | 3.55% |
CV – coefficient of variation
Discussion
We conducted a retrospective study to verify the performance of a photostimulated chemiluminescence assay. The precision, detection limit, compliance rate, and critical value all met the requirements. Using immunoblotting as the gold standard, we plotted a ROC curve for positive samples detected by the assay. The maximum area under the curve was 0.997, with a cut-off value of < 28.56, sensitivity of 98%, specificity of 100%, and a Youden index of 0.98. The average CV value for 3 years of in-house quality control was 3.55%, far lower than the requirement of CV ≤ 15% in the Beijing Comei Reagent Instructions and batch precision CV ≤ 15% in the HIV quality control product instructions. The inter-test uniformity CV was also less than 10% (Meesters and Voswinkel 2018). Of the 26 samples with COI values between 1–10, only one was truly positive, indicating a high false positive rate. There were 49 confirmed positive cases with COI values greater than 30. Therefore, we determined the cut-off value for the assay to be < 28.56. Results lower than this value have a relatively high probability of being false positives and require WB for diagnosis for medical safety. Results higher than this value have a relatively high probability of being true positives. In follow-up work, it is necessary to actively communicate with clinical doctors and patients to avoid adverse consequences. The diagnostic efficacy of photostimulated chemiluminescence assay in this experimental system is suitable for rapid screening of patients before blood transfusion and surgery.
ELISA is an initial HIV antibody detection method that adsorbs the antigen or antibody to a solid-phase carrier and uses an enzyme-labeled antigen-antibody reaction. It has the advantage of easy carrier standardization. However, ELISA requires the separation of free and bound antigens and antibodies, repeated sample addition, and plate washing. The operational steps could be more convenient, the detection time is long, and it requires a higher level of operator skill. Different personnel may produce significantly different results, and differences between wells on the enzyme plate can increase the CV, making clinical diagnosis more difficult (Cai 2018).
The photostimulated chemiluminescence assay is a qualitative detection method for human immunodeficiency virus (HIV) antibodies in human serum. Its basic principle is a homogeneous immune reaction based on antigen or antibody coated on microparticles. Under laser excitation, ion oxygen transfer between microparticles produces high-energy red light. The number of photons is converted into target molecule concentration through a single-photon counter and mathematical fitting. No high-energy red light is produced during detection when there is no target molecule in the sample. This method avoids the disadvantages of other luminescence methods, such as cross-contamination from repeated use of circulating flow cells, high cost of periodic replacement of flow cells, poor stability of small molecule detection, low signal intensity, and susceptibility to interference (Su et al. 2019).
Following CLSI (2011) and ISO 15189:2012 (2012) standards, Su et al. (2019) validated the precision, linearity, and reporting range of Thyroglobulin (Tg) measurements using photostimulated chemiluminescence. Compared with Roche’s electrochemiluminescence, their method showed excellent precision (CV 3.96–4.97%), linear range (0.49–456 ng/ml), and reporting range (0.2–4,502 ng/ml). Results from both methods were highly correlated and clinically consistent, confirming the suitability of photostimulated chemiluminescence for precise Tg detection in clinical settings.
Compared with ELISA, the photostimulated chemiluminescence assay has a longer effective period, higher sensitivity, fewer operational steps, faster reaction rate, better specificity, wider dynamic range, smaller CV and sample requirement, and is easy to implement rapid, automated, and high-throughput detection (Sommese et al. 2016; Chen et al. 2019; Song et al. 2019; Li 2020; Guan et al. 2021; Tang et al. 2022). This can significantly improve the accuracy of HIV antibody detection. Additionally, this method allows for adding specimens at any time, making it more suitable for emergency surgery patients than ELISA, which requires centralized specimen detection. The shortcomings include fluorescence quenching, possible interference from other fluorescent substances during the experiment, difficulty controlling cross-reactions and reaction target quality, and difficulty maintaining batch consistency (Tang et al. 2022).
ELISA and photostimulated chemiluminescence assay are both methods for detecting antibody content in specimens. However, the two methods have separate control and signal conversion formulas, such as ELISA’s signal value/cut-off value (S/CO) and photostimulated chemiluminescence assay’s COI value. The magnitude of these values is directly proportional to the amount of HIV antibody in the specimen, meaning that a more significant HIV antibody detection signal indicates a higher probability of HIV diagnosis (Guan et al. 2021).
Limitations of the study include its conduct at a single hospital, which may limit the generalizability of the findings to other populations or settings, and its focus on evaluating the diagnostic efficacy of the photostimulated chemiluminescence assay for detecting anti-HIV antibodies without assessing other potential uses or applications. These limitations highlight the need for further research to confirm and expand upon the study’s findings and evaluate the potential uses and limitations of the assay in other clinical contexts.
Conclusions
The photostimulated chemiluminescence assay is a susceptible and specific method for detecting HIV antibodies, with the advantages of high throughput, automation, and easy implementation. It provides an alternative to ELISA, which requires a higher level of operator skill and has a higher CV and longer detection time. Establishing a cutoff value of < 28.56 for COI value in this study can help improve the diagnostic accuracy of HIV antibody detection using this assay.
Footnotes
Ethical statement
This study was approved by the ethics committee of Nanjing Tongren Hospital (TRLLKY2021005). Written informed consent was obtained from each participant. All methods were carried out in accordance with relevant guidelines and regulations.
Author contributions
Jianxiang Han: guarantor of integrity of the entire study; study concepts; study design; definition of intellectual content; literature research; clinical studies; experimental studies; statistical analysis; manuscript preparation; manuscript editing; manuscript review
Yong Wang: study design; definition of intellectual content; literature research; manuscript preparation; manuscript editing; manuscript review
Bei Wang: data acquisition; statistical analysis; literature research Huacheng Tong: study design; experimental studies; data analysis; manuscript editing; manuscript review
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Alhajj M, Zubair M, Farhana A . Enzyme Linked Immunosorbent Assay. Treasure Island (USA): StatPearls Publishing; 2023. [cited 2023 Jun 21]. . . [Updated 2023 Apr 23] . : ; [ ]. Available from https://www.ncbi.nlm.nih.gov/books/NBK555922/ [PubMed] [Google Scholar]
- Bronstein I, Olesen CEM . In: Molecular methods for virus detection. Wiedbrauk DL, Farkas DH, editors. San Diego (USA): Academic Press; 1995. 7– Detection methods using chemiluminescence; pp. 147–174. . , editors. . : ; . p. –. . https://doi.org/ [DOI] [Google Scholar]
- Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, Pappalardo B, Kleinman SH NHLBI-REDS NAT Study Group A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors Transfusion 2005 NaN45(2):254. doi: 10.1111/j.1537-2995.2004.04215.x. ; . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Cai YX [Discussion on two different methods for HIV antibody detection] (in Chinese) Dermatol Venereol 2018;04:504. . . . ; : –. . [Google Scholar]
- Chen J, Feng X, Tian S, Chen S, Guo J, Liu Y, Sun L, Li Y, Wei H Study on the COI value of the 4th generation electrochemical luminescence assay as HIV diagnosis threshold Chin J AIDS STD 2019;10:1006. doi: 10.13419/j.cnki.aids.2019.10.06. . . . ; : –. . https://doi.org/ [DOI] [Google Scholar]
- Cheng M, Wang Y, Gao G [A contrastive study on chemiluminescence immunoassay and ELISA for detecting HIV antibody] (in Chinese) Modern Hospital 2016;01:30. . . . ; ; –. . [Google Scholar]
- CLSI Criteria for laboratory testing and diagnosis of Human Immunodeficiency Virus infection; Approved guideline. CLSI document M53-A. Wayne (USA): Clinical and Laboratory Standards Institute; 2011. . . : ; . [Google Scholar]
- CNAS-GL037:2019 Guidance for validation of quantitative; procedures in clinical chemistry [in Chinese] Beijing (China): China National Accreditation Service for Conformity Assessment; 2019. [cited 2023 Jul 03]. . . : ; [ ]. Available from https://www.cnas.org.cn/rkgf/sysrk/rkzn/2019/04/896307.shtml. [Google Scholar]
- CNAS-GL038:2019 Guidance of verification of qualitative measurement; procedures used in the clinical immunology [in Chinese] Beijing (China): China National Accreditation Service for Conformity Assessment; 2020. [cited 2023 Jul 03]. . . : ; [ ]. Available from https://www.cnas.org.cn/rkgf/sysrk/rkzn/2019/04/896309. . [Google Scholar]
- shtml Guan S, Zhang H, Fang J, Wang Y [Performance analysis of light initiated chemiluminescence assay in detecting anti-HCV, anti-HIV and syphilis] (in Chinese) Chin J Blood Transfus 2021:649. doi: 10.13303/j.cjbt.issn.1004-549x.2021.06.022. . . . ;06: –. . https://doi.org/ [DOI] [Google Scholar]
- ISO 15189:2012 Medical laboratories – Particular requirements for quality and competence. Geneva (Switzerland): International Organization for Standardization; 2012. . . : ; . [Google Scholar]
- Kirchner JT It’s time to normalize testing for HIV Am Fam Physician 2007 NaN76(10):1459. . . . ; ( ): –. . [PubMed] [Google Scholar]
- Li Q, Zeng J, Miao Q, Gao M Self-illuminating agents for deeptissue optical imaging Front Bioeng Biotechnol 2019a NaN7:326. doi: 10.3389/fbioe.2019.00326. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL [Analysis of the effectiveness of HIV antibody detection using chemiluminescence method and enzyme-linked immunosorbent method] (in Chinese) Electron J Clin Med Lit 2020;46:124. doi: 10.16281/j.cnki.jocml.2020.46.086. . . . ; : –. . https://doi.org/ [DOI] [Google Scholar]
- Li Y, Li N, Yi F [Electrochemiluminescence immunoassay versus enzyme-linked immunosorbent assay in detection of antibodies of acquired immune deficiency syndrome] (in Chinese) J Clin Med Pract 2019b;23:14. . . . ; : –. . [Google Scholar]
- Mazziotta C, Lanzillotti C, Govoni M, Pellielo G, Mazzoni E, Tognon M, Martini F, Rotondo JC Decreased IgG antibody response to viral protein mimotopes of oncogenic Merkel cell polyomavirus in sera from healthy elderly subjects Front Immunol 2021a NaN12:738486 doi: 10.3389/fimmu.2021.738486. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta C, Lanzillotti C, Torreggiani E, Oton-Gonzalez L, Iaquinta MR, Mazzoni E, Gaboriaud P, Touzé A, Silvagni E, Govoni M, et al Serum antibodies against the oncogenic Merkel cell polyomavirus detected by an innovative immunological assay with mimotopes in healthy subjects Front Immunol 2021b 12 NaN:676-627 doi: 10.3389/fimmu.2021.676627. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta C, Pellielo G, Tognon M, Martini F, Rotondo JC Significantly low levels of igg antibodies against oncogenic Merkel cell polyomavirus in sera from females affected by spontaneous abortion Front Microbiol 2021c;12:789991 doi: 10.3389/fmicb.2021.789991. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters RJ, Voswinkel S Bioanalytical method development and validation: From the USFDA 2001 to the USFDA 2018 guidance for industry J Appl Bioanal 2018;4(3):67. doi: 10.17145/jab.18.010. . . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Nahm FS Receiver operating characteristic curve: Overview and practical use for clinicians Korean J Anesthesiol 2022 NaN75(1):25. doi: 10.4097/kja.21209. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommese L, Sabia C, Esposito A, Iannone C, Montesano ML, Napoli C Comparison of performance of two Treponema pallidumautomated chemiluminescent immunoassays in blood donors Infect Dis 2016;48(6):483. doi: 10.3109/23744235.2016.1142674. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Song SJ, Ma XL, Yang XL, Liu YN, Chen LJ, Li J, Wang YJ [The Evaluation of reagents for joint detection of HIV antigen and antibody by light initiated chemiluminescence assay] (in Chinese) Labeled Immunoassays Clin Med 2019;10:1758. . . . ; : –. . [Google Scholar]
- Stramer SL, Glynn SA, Kleinman SH, Strong DM, Caglioti S, Wright DJ, Dodd RY, Busch MP National Heart, Lung, and Blood Institute Nucleic Acid Test Study Group. Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing N Engl J Med 2004 NaN351(8):760. doi: 10.1056/NEJMoa040085. ; . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Su P, Fu J, Zhang Z [The performance evaluation of photostimulated chemiluminescence for the detection of Thyroglobulin] (in Chinese) Labeled Immunoassays Clin Med 2019;12:2151. . . . ; : –. [Google Scholar]
- Tang X, Yang Z, Zhao G, Liu F, Chen Y [A comparative analysis of HIV antibody screening test and confirmatory test] (in Chinese) Labeled Immunoassays Clin Med 2022;04:604. . . . ; : –. , 691. . [Google Scholar]
- Zhang B, Liu W, Liu Z, Fu X, Du D Establishment of a chemiluminescence immunoassay combined with immunomagnetic beads for rapid analysis of ochratoxin A J AOAC Int 2022 NaN105(2):346. doi: 10.1093/jaoacint/qsab104. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang M, Li JJ, Liu JF, Yang CX [Analysis of the uncertain results of HIV-1 antibody Western blotting test in 33 cases] (in Chinese) J Trop Med 2019;10:1217. . . . ; : –. , 1253. . [Google Scholar]


