Abstract
Background:
Juvenile urinary bladder rhabdomyosarcoma (ubRMS) is a known entity; however, literature regarding its clinical behavior and endoscopic features is scarce. The aim of this study was to describe clinical and endoscopic features, and outcomes of ubRMS in dogs.
Case Description:
Dogs undergoing transurethral endoscopy and with a histological diagnosis of ubRMS were retrospectively collected. Seven dogs with a median age of 18 months (range 6–32 months) were included in this retrospective, multicenter, and descriptive study. Median tumor size was 58 mm (range 30–65 mm), and tumor location was bladder neck in three cases, trigone in two cases, and bladder body in two cases. Two dogs had monolateral ureteral obstruction. Two dogs presented with regional lymphadenopathy and one dog had lung lesions suggestive of metastatic disease. A grape-like mass was reported in four cases and solid in two, with variable consistency (two friables, two firms, and two not reported). Tumor treatments included surgery in three cases, surgery, and adjuvant doxorubicin in one case, and palliative therapy in three cases. The overall median survival time (ST) was 45 days. STs were shorter (range 20–45 days) for dogs treated with palliative care than for dogs treated with curative-intent treatment (range 70–120 days).
Conclusion:
ubRMS should be considered as a differential diagnosis in young dogs presenting with bladder masses. In this study, ubRMS confirmed its aggressive clinical behavior. Surgery and chemotherapy seem to increase STs but the prognosis remains poor.
Keywords: Canine, Chemotherapy, Endoscopy, Prostatectomy, Sarcoma
Introduction
Rhabdomyosarcomas (RMSs) are the most common malignant tumor of skeletal muscle in domestic animals (Cooper and Valentine, 2017). RMS can also occur in organs that normally lack striated muscle tissue (Cooper and Valentine, 2017). In a retrospective review of 20-year collected records at Cornell University, only 14 of the 58 presumed skeletal muscle tumors of approximately 83,000 cases of neoplasia were confirmed by immunohistochemistry as RMS (Cooper and Valentine, 2017).
Urinary bladder tumors are relatively uncommon in dogs, accounting for 2% of all canine neoplasms. Among these tumors, transitional cell carcinoma is the most represented (Knapp et al., 2014; Cooper and Valentine, 2017), while RMS is extremely rare (Cooper and Valentine, 2017). Only 2 of the 12 confirmed RMS at Cornell University originated from the urinary bladder (Cooper and Valentine, 2017).
Urinary bladder rhabdomyosarcoma (ubRMS) is rarely reported in dogs. A comparative review of canine and human RMSs reported 27 published cases of canine ubRMS from 1968 to 2009 (Caserto, 2013). To the best of the authors’ knowledge, no other canine RMS case reports have been published since 2009 (Gerber and Rees, 2009).
RMSs are histologically classified into three major classes in both humans and animals: embryonal, alveolar, and pleomorphic (Caserto, 2013; Cooper and Valentine, 2017). Embryonal is further subclassified as botryoid when specific characteristic gross appearance and anatomic location are present. The term botryoid refers to the grape-like masses protruding into a mucosa-lined organ, such as the urinary bladder, which is the most common location of botryoid RMS (Caserto, 2013; Cooper and Valentine, 2017).
UbRMS commonly occurs in dogs 2 years of age or younger. Large-breed dogs are mainly affected, with Saint Bernards more represented (Kelly, 1973). UbRMS is generally located in the trigone and may also involve the urethra and/or ureters leading to urinary obstruction, hydroureter, and hydronephrosis (Kelly, 1973; Cooper and Valentine, 2017). Clinical presentation of dogs with ubRMS overlaps with other lower urinary tract diseases with hematuria and dysuria most reported (Caserto, 2013).
Macroscopic appearance consists of a polypoid mass with a grape-like and lobulated shape protruding from the mucosa of the urinary bladder, with a firm or friable consistency (Cooper and Valentine, 2017). For this reason, ubRMS are generally classified as botryoid RMS.
Metastatic rate, prognostic factors, treatment success rate, and overall survival for dogs with ubRMS are still little known as dogs are often euthanized shortly after diagnosis (Kelly, 1973; Halliwell and Ackerman, 1974; Van Vechten et al., 1990; Caserto, 2013).
Of 27 canine ubRMS reviewed by Caserto (2013), 6 dogs presented with metastases. The most frequent sites of metastasis were regional lymph nodes, liver, spleen, lungs, kidney, and mesentery. Other sites of metastases included the ovary, skin, heart, adrenal glands, and small intestine (Stamps and Harris, 1968; Kelly, 1973; Van Vechten et al., 1990; Takiguchi et al., 2002; Kobayashi et al., 2004; Gerber and Rees, 2009).
To date, no treatment seems to improve the prognosis of ubRMS in dogs. Surgery failed to control tumor progression because of the high risk of local recurrence and distant metastases (Stamps and Harris, 1968; Kelly, 1973; Van Vechten et al., 1990; Takiguchi et al., 2002; Kobayashi et al., 2004; Gerber and Rees, 2009). There is not much data available on chemotherapy’s efficacy and rarely do dogs experience long survival times (STs) when treated with a combination of surgery and adjuvant chemotherapy (Van Vechten et al., 1990; Senior et al., 1993; Saulnier-Troff et al., 2008).
The aim of this study is to describe the clinical presentation, endoscopic appearances, and outcome of ubRMS in a case series of dogs.
Case Details
In this multinstitutional retrospective study, cases of canine ubRMS were searched for from medical records between January 2013 and December 2020. Dogs undergoing transurethral endoscopy and with a histological diagnosis of ubRMS were included.
Information collected included signalment, body weight, clinical signs, laboratory test results (blood and urine analysis), imaging and endoscopic findings, tumor size and location, histological diagnosis, type of treatment, and outcome. Information on signs of urinary tract occlusion, and loco-regional or distant metastases was also collected. The presence of metastases was assessed by ultrasound or CT although no further cytological or histological investigations were performed.
Urethral and urinary bladder endoscopy was performed with rigid cystoscopy in female dogs (12 F and 30°, working channel 6 F, Karl Storz®, Tuttlingen, Germany) and flexible cystoscopy in male dogs (3 F and working channel 4 F Karl Storz®, Tuttlingen, Germany). During endoscopy, multiple biopsies of the target lesion were collected under direct vision. Surgical biopsies were performed at the clinicians’ discretion. Both endoscopic and surgical biopsies were fixed in 10% neutral-buffered formalin, processed routinely, and stained with hematoxylin and eosin. Cytological smears were collected by percutaneous ultrasound-guided fine-needle aspiration from the tumors or by squash smears using fresh endoscopy biopsies as previously described (Riondato et al., 2014). Then, the slides were air-dried and stained with rapid Romanowsky stain. Neither cytological nor histological slides were reviewed for the purpose of this study.
Treatments included palliative care, surgery (radical with curative intent or marginal with tumor debulking intent), chemotherapy, or a combination of these. Since all dogs died from tumor-related causes, the date of diagnosis, first progression, and death were collected to calculate disease-free interval (DFI), time from surgery to recurrence or relapse, time to progression (TTP), time from diagnosis to local disease progression or development of metastasis and ST, time from diagnosis to death.
Data were shown as descriptive statistics. Continuous parameters were shown as median and range. Categorical parameters were shown as absolute and relative frequency.
Clinical information, imaging, endoscopic and pathological findings, treatment modalities, and outcomes of the seven dogs included in the study are shown in Tables 1 and 2.
Table 1. Clinical information, imaging findings, and endoscopic appearances of juvenile ubRMS at the time of diagnosis.
| Signalment and clinical signs | Imaging findings | Endoscopy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Breed | Sex | Body weight (kg) | Age (months) | Complaints | Location | Size (mm) | Other | Shape and consistency | Ureteral papillae involvement |
| 1 | French Bouledouge | Female | 12 | 11 | Dysuria, hematuria, vesical tenesmus | Trigone | 60 | Monolateral pyelectasis | Grape-like | Yes (bilateral) |
| 2 | English Setter | Male | 21 | 12 | Dysuria, hematuria | Dorsal body | 60 | - | Solid and firm | No |
| 3 | German Shepherd | Male | 33 | 32 | Dysuria, urgency, hyporexia | Bladder neck | 65 × 58 × 28 | Inguinal and medial iliac lymphadenopathy, lung metastasis | Grape-like and friable | No |
| 4 | Cane Corso | Female | 25 | 6 | Dysuria, hematuria | Dorsal body | 58 × 57 × 28 | - | Solid and firm | No |
| 5 | Jack Russel Terrier | Female | 6.5 | 18 | Hematuria, urgency | Bladder neck | 30 | Regional lymphadenopathy | Grape-like | No |
| 6 | Mixed-breed | Male | 17.5 | 26 | Dysuria, vesical and rectal tenesmus | Bladder neck and urethra | 30 × 23 × 20 | - | Not applicable | Not applicable |
| 7 | English Setter | Female | 16 | 24 | Dysuria, hematuria | Trigone | 30 | Monolateral hydroureter | Grape-like and friable | Yes (bilateral) |
Table 2. Pathological findings, treatment modalities, and outcomes of the seven dogs with juvenile ubRMS were included in the study.
| Histology | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Cytology | Morphological diagnosis | MC | IHC | Margins | Surgery | Chemotherapy | Recurrence/progression (local/distant)) | DFI/TTP (days) | ST (days) |
| 1 | NA | Spindle cell tumor | <1/HPF* | Yes (desmin) | NA | No | No | NA | NA | 20 |
| 2 | Suspected spindle cell tumor | RMS | NR | No | NR | Yes (radical) | No | Distant (lung) | 45 | 70 |
| 3 | Neutrophilic inflammation | Embryonal RMS, mixoid variant | 3/HPF* | No | Incomplete | Yes (debulking) | No | Local and distant (lung) | 20 | 20 |
| 4 | Neutrophilic inflammation | Embryonal RMS (spindle cells) | High | No | Complete | Yes (radical) | No | Distant (peritoneal) | 60 | 70 |
| 5 | Neutrophilic inflammation and urothelial cells | Botryoid RMS | Low | No | NA | No | No | NA | NA | 45 |
| 6 | Spindle cell tumor | Embryonal RMS | up to 10/HPF* | No | Incomplete | Yes (radical) | Yes (doxorubicin) | Local | 113 | 120 |
| 7 | Neutrophilic inflammation and urothelial cells | Undifferentiated round cell tumor | High | Yes (desmin) | NA | No | No | NA | NA | 30 |
400× magnification.
DFI: disease-free interval; HPF (high-power field); IHC: immunohistochemistry; MC: mitotic count; NA: not applicable; NR: measure not reported; RMS: rhabdomyosarcoma; ST: survival time; TTP: time to progression.
Four dogs were female and three were male. All dogs were sexually intact. Six dogs were purebred including: two English setters, one Cane Corso, one French Bouledogue, one German Shepherd, and one Jack Russel Terrier. The last was a mixed-breed dog. The median age was 18 months (range 6–32 months) and the median weight was 17.5 kg (range 6.5–33 kg).
All dogs presented urinary disorders including dysuria (6/7), hematuria (5/7), urgency (2/7), and vesical tenesmus (2/7). One dog had hyporexia and another had rectal tenesmus.
Before endoscopy, dogs received nonsteroidal anti-inflammatory drugs (3/7) and/or antibiotics (6/7) with no improvement of clinical signs.
Median tumor size was 58 mm (range 30–65 mm), and tumor location was bladder neck in three dogs, trigone in two dogs, and bladder body in two dogs (Table 2). Both dogs with trigone location had indirect signs of urinary obstruction: one dog showed pyelectasia while the other showed hydroureter. Computed tomography revealed regional lymphadenopathy in two dogs and multiple pulmonary metastases in one of them.
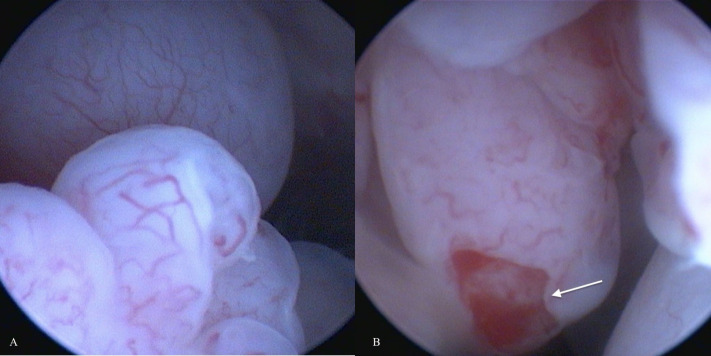
An obstructive urethral mass did not allow endoscopic examination of the urinary bladder in one case (number 6). Both dogs with indirect signs of ureteral occlusion showed bilateral ureteral papillae involvement at endoscopic examination. Four of six endoscopically evaluable tumors appeared as grape-like shaped masses (Fig. 1) and two as solid, and sessile masses. Both latter were located dorsally on the bladder body. The grape-like shape was associated with a friable consistency, while solid and sessile masses resulted in firmness. The cytological examination was performed in six cases but failed to diagnose RMS in four cases. In two cases an atypical spindle cell population was observed, suggesting mesenchymal origin. Histological diagnosis of RMS was reported in five of seven cases. Two cases were classified as round cell tumors and not further classified as spindle cell tumors at morphological examination and as RMS after immunohistochemistry (desmin +). Mitotic count was reported in six of seven cases and resulted low in two cases and a high in four (Table 2).
Fig. 1. (A and B): Endoscopic appearance of canine ubRMSs: grape-like shaped vascularized masses with pale pink color and friable consistency. The arrow indicates tumor ulceration.
One dog received radical surgery and adjuvant doxorubicin, two dogs received radical surgery, one received debulking surgery and three dogs received palliative medications only (Table 2). Overall median ST was 45 days (range 20–120).
The present study confirms that ubRMS occurs rarely in dogs, frequently in young dogs. Moreover, the dogs included in the present study died soon after diagnosis from causes related to tumor progression, confirming that ubRMS is an aggressive tumor typically associated with a poor prognosis.
In the authors’ opinion, the present study raised some interesting points of discussion including tumor appearance and treatment.
Discussion
UbRMSs are usually described as masses located in the trigone and with friable consistency and grape-like appearance, from which the term botryoid (Cooper and Valentine, 2017). Differently, in the present study ubRMSs were in the dorsal wall of the urinary bladder in two cases (Case no. 2 and 4) and they had firm consistency. The location of the tumor allowed for a partial cystectomy with curative intent; however, both dogs died shortly after due to metastases.
The main limit of this study is that no immunohistochemistry confirmed the skeletal muscle origin in most cases but there are some reasons to suppose that histological diagnosis of RMS was correct for dogs included in this study. Non-RMS urinary bladder sarcomas in dogs are described rarely including several histological subtypes: fibrosarcoma, osteosarcoma, hemangiosarcoma, leiomyosarcoma, histiocytic sarcoma, lymphangiosarcoma (Seely et al., 1978; Liptak et al., 2004; Olausson et al., 2005; Amores-Fuster et al., 2011; Woldemeskel, 2017; Delaune et al., 2018; Michishita et al., 2020; Townsend et al., 2020; Shigihara et al., 2021). In comparison to ubRMS, other histological forms of bladder sarcomas tend to occur in adult or older dogs. However, an aggressive and metastatic fibrosarcoma in a 14-month-old dog has been reported (Olausson et al., 2005). The tumor was described as a multilobulated firm mass involving the bladder neck, microscopically classified as fibrosarcoma because of the fibroblastic-like cell appearance with high mitotic activity and vimentin expression. However, no further antibodies were used to define the tumor histotype. In the current study, all cases of ubRMS occurred in dogs younger than 3 years. All five morphological diagnoses of ubRMS included in the present study showed atypical spindle cells frequently with high mitotic activity. In the authors’ opinion, a misclassification of ubRMS is unlikely even with the lack of immunohistochemistry investigation due to the lack of consistent literature on bladder sarcomas other than RMS in young dogs.
Despite palliative or curative surgery, STs after tumor resection have been reported to be short (4–8 weeks) due to local recurrence or distant metastases (Kelly, 1973; Takiguchi et al., 2002).
The impact of adjuvant chemotherapy in ubRMS treatment is still unknown. To the best of the author’s knowledge, in literature, there are only two cases of canine ubRMS treated with both radical surgery and adjuvant chemotherapy (Van Vechten et al., 1990; Senior et al., 1993). One dog survived for 21 months starting chemotherapy the day after surgery (four cycles of doxorubicin on day 1 followed by cyclophosphamide on day 3–6) (Senior et al., 1993). The other dog started chemotherapy (doxorubicin and cyclophosphamide IV on day 1 followed by vincristine on day 4) 46 days after surgery because of local recurrence (Van Vechten et al., 1990). This dog had progressive disease and was euthanatized shortly after (ST 96 days).
The present study observed that the more aggressive the treatment the longer the ST, as previously reported. However, all dogs died relatively soon after diagnosis confirming that ubRMS prognosis is poor.
There were several limitations in this study, including its retrospective nature, the small number of dogs, the absence of tissue biopsy review, and the lack of immunohistochemistry in five of seven cases to confirm the diagnosis of ubRMS. However, for this latter point, the authors think that ubRMS was the most likely differential diagnosis due to the dog’s history, signalment, tumor location and appearance, histopathological findings, and outcome, making misclassification unlikely.
In conclusion, UbRMS should be considered as a differential diagnosis in young dogs presenting with bladder masses. The aggressive clinical behavior associated with poor prognosis of ubRMS observed in this study, may add useful information to clinicians in the treatment decision-making processes. Moreover, this study raises the need for wider multicenter studies to deeply explore this rare and fatal disease.
Acknowledgments
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any financial support.
Authors’ contributions
All authors discussed the results and drafted, revised, and approved the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Amores-Fuster I, Elliott J.W, Freeman A.I, Blackwood L. Histiocytic sarcoma of the urinary bladder in a dog. J. Small. Anim. Pract. 2011;52:665. doi: 10.1111/j.1748-5827.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- Caserto B.G. A comparative review of canine and human rhabdomyosarcoma with emphasis on classification and pathogenesis. Vet. Pathol. 2013;50:806–826. doi: 10.1177/0300985813476069. [DOI] [PubMed] [Google Scholar]
- Cooper B, Valentine B. Tumors of muscle. In: Meuten D.J, editor. In Tumors in domestic animals. Hoboken, NJ: Wiley-Blackwell; 2017. pp. 425–466. [Google Scholar]
- Delaune T, Bernard F, Matres-Lorenzo L, Bernardé A. Radical cystectomy and subsequent ureterohysterostomy in a bitch. Vet. Surg. 2018;47:1106–1111. doi: 10.1111/vsu.12950. [DOI] [PubMed] [Google Scholar]
- Gerber K, Rees P. Urinary bladder botryoid rhabdomyosarcoma with widespread metastases in an 8-month-old Labrador cross dog. J. S. Afr. Vet. Assoc. 2009;80:199–203. doi: 10.4102/jsava.v80i3.202. [DOI] [PubMed] [Google Scholar]
- Halliwell W.H, Ackerman N. Botryoid rhabdomyosarcoma of the urinary bladder and hypertrophic osteoarthropathy in a young dog. J. Am. Vet. Med. Assoc. 1974;165:911–913. [PubMed] [Google Scholar]
- Kelly D.F. Rhabdomyosarcoma of the urinary bladder in dogs. Vet. Pathol. 1973;10:375–384. [Google Scholar]
- Knapp D.W, Ramos-Vara J.A, Moore G.E, Dhawan D, Bonney P.L, Young K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR. J. 2014;55:100–118. doi: 10.1093/ilar/ilu018. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Sakai H, Hirata A, Yonemaru K, Yanai T, Watanabe K, Yamazoe K, Kudo T, Masegi T. Expression of myogenic regulating factors, myogenin and MyoD, in two canine botryoid rhabdomyosarcomas. Vet. Pathol. 2004;41:275–277. doi: 10.1354/vp.41-3-275. [DOI] [PubMed] [Google Scholar]
- Liptak J, Dernell W, Withrow S. Haemangiosarcoma of the urinary bladder in a dog. Aust. Vet. J. 2004;82:215–217. doi: 10.1111/j.1751-0813.2004.tb12679.x. [DOI] [PubMed] [Google Scholar]
- Michishita M, Ishizaki Y, Konnai M, Machida Y, Nakahira R, Hatakeyama H, Yoshimura H, Yamamoto M, Soeta S, Ochiai K, Misawa K, Yugeta N, Azakami D. Primary lymphangiosarcoma of the urinary bladder in a dog. J. Comp. Pathol. 2020;179:31–35. doi: 10.1016/j.jcpa.2020.06.014. [DOI] [PubMed] [Google Scholar]
- Olausson A, Stieger S.M, Loefgren S, Gillingstam M. A urinary bladder fibrosarcoma in a young dog. Vet. Radiol. Ultrasound. 2005;46:135–138. doi: 10.1111/j.1740-8261.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- Riondato F, Miniscalco B, Berio E, Lepri E, Rossi S, Bottero E. Diagnosis of canine gastric adenocarcinoma using squash preparation cytology. Vet. J. 2014;201(3):390–394. doi: 10.1016/j.tvjl.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Saulnier-Troff F.G, Busoni V, Hamaide A. A technique for resection of invasive tumors involving the trigone area of the bladder in dogs: preliminary results in two dogs. Vet. Surg. 2008;37:427–437. doi: 10.1111/j.1532-950X.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- Seely J.C, Cosenza S.F, Montgomery C.A. Leiomyosarcoma of the canine urinary bladder, with metastases. J. Am. Vet. Med. Assoc. 1978;172:1427–1429. [PubMed] [Google Scholar]
- Senior D, Lawrence D.T, Gunson C, Fox L, Thompson J.P, Buergelt C. Successful treatment of botryoid rhabdomyosarcoma in the bladder of a dog. J. Am. Anim. Hosp. Assoc. 1993;29:386–390. [Google Scholar]
- Shigihara K, Shimonohara N, Stanley B.J. Outcome of a dog with urinary bladder osteosarcoma treated with a total cystectomy and ureterocutaneostomy. Can. Vet. J. 2021;62:1089–1094. [PMC free article] [PubMed] [Google Scholar]
- Stamps P, Harris D.L. Botryoid rhabdomyosarcoma of the urinary bladder of a dog. J. Am. Vet. Med. Assoc. 1968;153:1064–8. [PubMed] [Google Scholar]
- Takiguchi M, Watanabe T, Okada H, Kudo T, Yamada K, Yasuda J, Hashimoto A. Rhabdomyosarcoma (botryoid sarcoma) of the urinary bladder in a maltese. J. Small. Anim. Pract. 2002;43:269–271. doi: 10.1111/j.1748-5827.2002.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Townsend S, Regier P.J, More S.N. Successful treatment of urinary bladder hemangiosarcoma by partial cystectomy in a dog. J. Am. Anim. Hosp. Assoc. 2020;56:231–235. doi: 10.5326/JAAHA-MS-6910. [DOI] [PubMed] [Google Scholar]
- Van Vechten M, Goldschmidt M.H, Wortmn J.A. Embryonal rhabdomyosarcoma of the urinary bladder in dogs. Compend. Contin. Educ. Pract. Vet. 1990;12:783–793. [Google Scholar]
- Woldemeskel M. Primary urinary bladder osteosarcoma in a dog. J. Comp. Pathol. 2017;157:141–144. doi: 10.1016/j.jcpa.2017.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.