Abstract
Background
This work aimed to prepare niosomal formulations of an anticancer agent [mefenamic acid (MEF)] to enhance its cancer targeting. 131I was utilized as a radiolabeling isotope to study the radio-kinetics of MEF niosomes.
Methods
niosomal formulations were prepared by the ether injection method and assessed for entrapment efficiency (EE%), zeta potential (ZP), polydispersity index (PDI) and particle size (PS). MEF was labeled with 131I by direct electrophilic substitution reaction through optimization of radiolabeling-related parameters. In the radio-kinetic study, the optimal 131I-MEF niosomal formula was administered intravenously (I.V.) to solid tumor-bearing mice and compared to I.V. 131I-MEF solution as a control.
Results
the average PS and ZP values of the optimal formulation were 247.23 ± 2.32 nm and − 28.3 ± 1.21, respectively. The highest 131I-MEF labeling yield was 98.7 ± 0.8%. The biodistribution study revealed that the highest tumor uptake of 131I-MEF niosomal formula and 131I-MEF solution at 60 min post-injection were 2.73 and 1.94% ID/g, respectively.
Conclusion
MEF-loaded niosomes could be a hopeful candidate in cancer treatment due to their potent tumor uptake. Such high targeting was attributed to passive targeting of the nanosized niosomes and confirmed by radiokinetic evaluation.
Keywords: Mefenamic acid, Niosomes, Anticancer, 131I, Radiokinetics
Introduction
Cancer is a condition in which cells proliferate uncontrollably and produce aggressive malignancies, which results in millions of deaths annually (Anand et al. 2022). Chemotherapy is a widely used type of cancer treatment that depends on the use of toxic substances and medications to kill cancerous cells (Anand et al. 2022; Zugazagoitia et al. 2016). High dose of chemotherapy results in a variety of side effects and damage to other healthy cells. Additionally, it has been well documented that cancer cells develop drug resistance after prolonged drug exposure (Abbas and Rehman 2018; Kumar et al. 2022). As a result, avoiding healthy cells and specifically targeting malignant ones has been the main aim of cancer research. For this purpose, the new drugs are being developed into novel nanosystems (Yao et al. 2020; Edis et al. 2021).
Chronic inflammation raises the risk of various cancer types, indicating that inflammation and cancer are inextricably linked. Consequently, reducing inflammation may be an effective strategy for both cancer treatment and prevention. Numerous non-steroidal anti-inflammatory drugs (NSAIDs) are used as anticancer medications (Zappavigna et al. 2020; Kay et al. 2019). Additionally, they have been discovered to improve and accelerate tumor radio-response (Bernardi et al. 2008; Wong 2019).
MEF is an NSAID that is commonly used to treat headache, tooth pain, and osteoarthritis. It is classified as a class II biopharmaceutical that has low water solubility and high permeability (Wichianphong and Charoenchaitrakool 2018). The ability of MEF to inhibit the cyclooxygenase-2 (COX-2) enzyme, as an important component of the inflammatory process, is part of its mechanism of action (Hosseinimehr et al. 2019). MEF inhibits the multiplication of cancer cells such as those seen in in-vitro anti-proliferative assays for liver and colon cell lines (Altay et al. 2019). MEF, along with other fenamates, has demonstrated an anticancer effect also in cervical-uterine (Soriano-Hernandez et al. 2015) and prostate cancer cells (Soriano-Hernández et al. 2012). But it has also been demonstrated in tumors from animal models of colon cancer (Seyyedi et al. 2022), and its anti-cancer effect has even been tested in human clinical trials in prostate cancer(Guzman-Esquivel et al. 2020).
The activation of the caspases-3 pathway, inhibition of hyaluronic acid synthesis and cyclooxygenase inhibition are among the hypothesized mechanisms underlying MEF’s anticancer action (Woo et al. 2004). Suppression of COX-2, which is usually overexpressed in cancer tissues, by NSAIDs has been suggested as a cancer treatment. Pro-inflammatory cytokines are essential in cancer cell proliferation. So, the inflammatory environment is associated with DNA damage and death in normal cells (Hosseinimehr et al. 2015; Zha et al. 2001). It is indicated that NSAIDs decrease cancer growth by blocking such inflammation process (Wong 2019). In addition, MEF has been shown to selectively inhibit the NLRP3 inflammasome and the release of IL-1β, independent of its COX-mediated anti-inflammatory activity (Guzman-Esquivel et al. 2022), which is also a mechanism that could have an antineoplastic effect (Guzman-Esquivel et al. 2022; Blevins et al. 2022). MEF not only has anticancer activity but also protects normal cells from ionizing radiation-induced DNA damage in vitro (Hosseinimehr et al. 2015).
Nanotechnology was applied for the formulation of chemotherapeutic drugs to increase their therapeutic efficacy (Faheela and Malathi 2022; He et al. 2019). Nanocarriers can accumulate specifically in cancerous tissue due to the enhanced permeability and retention effect (EPR). This passive process of drug accumulation at tumor sites necessitates extended drug circulation for effective drug delivery (Edis et al. 2021; Xiao et al. 2021; Navya et al. 2019). Numerous carriers, including immunoglobulin, serum proteins, synthetic polymers, liposomes, microspheres, erythrocytes and niosomes have been used for enhancing drug targeting (Hua et al. 2015; Parveen et al. 2012; Lohumi 2012).
Niosomes are non-ionic surfactant based, bi-layered multilamellar or unilamellar structures ranging in size from 10 to 10,000 nm (Ahmed 2013; Akbarzadeh et al. 2021). It forms only when surfactant and cholesterol are combined in the right ratio and the temperature is above the gel-liquid transition point (Bhardwaj et al. 2020). Niosomes can encapsulate both hydrophilic and hydrophobic drugs because of their special geometry. Niosomal formulation is essential for drug delivery and tumor targeting to create a stable, nontoxic, biodegradable, nonimmunogenic drug-loaded formula with good bioavailability (Masjedi and Montahaei 2021; Targhi et al. 2021). Niosomes also extend the time that the drug spends in the bloodstream (Faheela and Malathi 2022). They, therefore, exhibit promise as carriers for the sustained and/or controlled delivery of drugs to target sites (Amoabediny et al. 2018).
Radio-kinetic technique is used as a surrogate for liquid chromatography − mass spectrometry (LC–MS) to evaluate the pharmacokinetics of new drug dosage forms (Atzrodt et al. 2020; Jafarieh et al. 2015; Boseila et al. 2019; Rashed et al. 2017a; Ezz Eldin 2023). Major drawbacks of LC–MS include isobaric interferences, ion suppression effect, high cost and large samples number (Manohar and Marzinke 2016; Seger 2012). Radiokinetic studies allow following the radiolabeled drug biodistribution throughout the body and excretion over time. The radioactivity uptake, corresponding to drug uptake, in different body organs and fluids can be accurately tracked (Vogel et al. 2006, 1990; Ramakrishnan et al.; Rashed et al. 2017b). Iodine-131 is a radioisotope with a half-life of nearly 8 days and an energy of 364 keV of gamma radiation (Jimenez et al. 2019; Saha and Saha 2004). Such properties of 131I made it a suitable radionuclide to be applied in radiokinetic studies (Rashed et al. 2017b; Avcıbaşı et al. 2013; Bayrak et al. 2010).
In this research, niosomes were used as nanocarriers for MEF to increase its water solubility, stability and cancer targeting (Akbarzadeh et al. 2021). The Box-Behnken experimental design was used to investigate numerous niosomal formulation components to find the ideal formula. Fourier Transform Infrared Spectroscopy (FTIR), Transmission Electron Microscopy (TEM), Differential Scanning Calorimetry (DSC), ZP and EE % measures were performed for the formulated niosomes. In addition, 131I was used in the radiolabeling of MEF for the in vivo study to make a radiokinetic evaluation for MEF-loaded niosomes.
Materials and methods
Materials
Mefenamic acid and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO, USA). A dialysis bag (12 kDa MWCO) bought from SERVAPOR® Dialysis Membranes, Heidelberg, Germany. Tween 20, Span 20, disodium hydrogen phosphate, sodium dihydrogen phosphate, methanol and diethyl ether were purchased from ADWIC, El-Nasr Pharmaceutical Co., Egypt. All other reagents were of analytical grade. The Egyptian Atomic Energy Authority’s radioisotope production facility (RPF) provided iodine-131 (131I) as a no-carrier-added solution (Cairo, Egypt). Sigma Aldrich Chemical Co. (St. Louis, MO, USA) was the source of Chloramine-T (CAT).
Methods
Preparation of MEF-loaded niosomes
Using the ether injection method, MEF-loaded niosomes were formulated. Methanol and diethyl ether (10 ml) with a ratio of 2.5:7.5 v/v were used as organic solvents to dissolve lipid (cholesterol), drug (MEF) and non-ionic surfactants (Span 20 and Tween 20 with a 1:1 (w/w) ratio). Then, organic solvent was injected slowly at a rate of 1 ml/min with a syringe into phosphate buffer solution (PBS) pH 7.4, as an aqueous solution that was preheated at 60 °C. According to the design, the buffer volume added was 10, 15, or 20 ml. This formulation was stirred for 30 min using a magnetic stirrer. Complete organic solvent evaporation was achieved at approximately 60 °C. The gradual addition of the lipid solution into the aqueous phase caused rapid ether vaporization due to the temperature differences between the two phases, which led to spontaneous vesiculation and the formation of niosomes. For additional investigation, the prepared niosomal formulations were kept refrigerated (Kulkarni et al. 2021; Yasamineh et al. 1878).
Box-Behnken experimental design
In this research, different formulations were created using a Box-Behnken design (BBD) for three factors, resulting in 15 experimental runs. Table 1 lists variables (dependent and independent), their respective levels, and the desired results. The cholesterol amount (A), surfactant amount (B), and hydration volume (C) were selected as independent variables (factors). To investigate their impact on dependent variables (responses) including: (Y1) vesicle size, (Y2) zeta potential, and (Y3) entrapment efficiency%. Design Expert® (Version 12, Stat-Ease, Inc. USA) was utilized to examine three levels for each factor (low, medium, and high) and complete the statistical optimization process. Response surface methodology including ANOVA, multiple regression analysis, and statistical optimization were carried out using the software.
Table 1.
Independent variables with their levels and dependent variables with desired values of the Box-Behnken design
| Independent variables | ||||
|---|---|---|---|---|
| Factor coding | Name | Low level | Medium level | High level |
| A | Cholesterol amount (mg) | 50.00 | 125.00 | 200.00 |
| B | Surfactant amount (mg) | 100.00 | 150.00 | 200.00 |
| C | Hydration volume (ml) | 10.00 | 15.00 | 20.00 |
| Dependent variables | |||
|---|---|---|---|
| Responses | Unit | Constraints | |
| Y1 | Particle size | nm | Minimum |
| Y2 | Zeta potential | mv | Minimum |
| Y3 | Entrapment efficiency | % | Maximum |
Particle size
Dynamic light scattering (DLS) was used to evaluate the vesicle size of MEF-loaded niosomes at 25 °C using the Malvern Zetasizer (Nano ZS, Malvern, UK). The niosomal suspension was sonicated for 4 min using a water bath sonicatior then diluted (100 ×) with distilled water and vortexed for 3 min prior to every measuring. Each measurement repeated three times (Allam et al. 2021).
Zeta potential measurement
Zeta potential for niosomal preparations was measured in a zeta sizer (Malvern equipment, UK) using the Laser Doppler Micro-electrophoresis technique. Samples were put in single-use zeta cells after being diluted with distilled water. For each sample, measurements were performed three times, and average values ± SD were assessed (Shewaiter et al. 2021).
Entrapment efficiency
The quantity of the entrapped MEF in the MEF-loaded niosomes was evaluated with cooling centrifuge equipment (Remi C -24, Mumbai, India). Firstly, centrifugation of one ml of MEF-loaded niosomes for 1 h at 14,000 rpm was carried out. The supernatant containing unentrapped drug (0.1 ml) was then diluted with PBS pH 7.4 (5 ml) and tested in a UV–visible spectrophotometer against a blank (phosphate buffer). At a UV wavelength of 285 nm in a spectrophotometer (Shimadzu- UV, 1601 PC), sample absorbance was investigated for the determination of the quantity of encapsulated drug (Asaithambi et al. 2020). Entrapment efficiency (EE%) was assessed using the equation shown below (Haroun et al. 2022):
| 1 |
Optimization of MEF niosomes
To create a MEF niosomal formulation with the greatest desirable responses, formulation parameters were numerically and graphically optimized using Design Expert® 12 software. The goal was to produce niosomes with the smallest vesicle size, the best and greatest zeta potential value (ZP), and the highest entrapment efficiency (EE%). The optimal formula, which had the greatest desirability, was chosen for further investigations.
Morphology of niosomes
To study the morphology of niosomes, TEM test was used. Before imaging, a droplet of the optimal formula was dropped on carbon grids that had been coated with copper, dyed by 2% (w/v) phosphotungstic acid, and then allowed for air drying. Using an 80 kV acceleration voltage, the formula was investigated through the TEM (JEM-100-CX11, Tokyo, Japan) (Mousa et al. 2022; Thabet et al. 2022).
Fourier transform infrared spectroscopy (FTIR)
To discover if there was any interaction between the excipients or polymers used to formulate niosomes and the drug, drug-excipient compatibility experiments were conducted. FTIR spectroscopy was used to determine if MEF and excipients being used were compatible (Manohar and Marzinke 2016). The dispersion method of potassium bromide (KBr) was used to determine the infrared spectra of MEF using a Fourier Transform Infrared Spectrophotometer (FTIR-4100 JASCO, Japan). Using dried KBr, the baseline correction was completed. After being oven-dried for 30 min, the sample to be tested and the KBr were completely blended at a ratio of 1:300 in a glass mortar. In a sample holder, these samples were scanned at a resolution of 2 cm−1 from 4000 to 400 cm−1 (Hassan et al. 2021; Chaudhari and Desai 2019).
Differential scanning calorimetry (DSC)
The DSC experiment was carried out using a differential scanning calorimeter (DSC-60, SHIMADZU). The MEF pure powder, physical drug mixture with excipients, plain niosomes and the optimal MEF niosomal formula were investigated. All samples were carefully weighed (about 2–3 mg) and then loaded in closed aluminum pans, where they were heated under 30 ml/min nitrogen gas flow from 40 to 200 °C at a rate of 10 °C/min. As a reference, an unfilled aluminum pan was utilized (Nishu et al. 2018).
Niosomes stability
The optimal MEF-loaded niosomes were split into two groups and their stability was investigated over a period of three months at 4 °C and 25 °C. The optimum formulation was placed in 20 ml glass vials and stored at the previously mentioned conditions. Zeta potential, entrapment efficiency and particle size were evaluated as stability indicating parameters at a specific time (0 and 90 days). Additionally, a visual examination of the two groups of the optimum formula was performed to evaluate the probability of color changes and sedimentation (Kulkarni et al. 2021; Khan et al. 2016).
In vitro drug release study
In comparison to the release of MEF from suspension, the MEF release from the optimal niosomal formulation was assessed. The dialysis method is the technique of choice for assessing the criteria of drug release from the niosomal formula. MEF release from niosomal formulation and suspension was monitored using a dialysis bag (MWCO12000 Da) at 37 °C for 24 h versus PBS (pH 7.4) to simulate the biological conditions. The optimal niosomes formula and suspension (1 ml of each) were loaded separately inside the dialysis tube, and the released quantity of MEF was evaluated in 100 ml of PBS with continuous stirring. At predefined intervals, (1, 2, 3, 4, 5, 6, 8, and 24 h), 2 ml of freshly formed PBS was added to replace two ml that were withdrawn from the release medium to assess the drug release rate. Lastly, the released concentrations of MEF were determined by examining the samples at 285 nm wavelength using a UV–visible spectrophotometer. This study was performed in triplicate (Targhi et al. 2021).
The mathematical methodology utilized to determine the kinetics of the total released percentages (cumulative) of MEF from the niosomes formulation was linear regression analysis. These models include Higuchi, Korsmeyer Peppas, First Order, and Zero Order. In the end, the correlation coefficient values were compared to select the model that best fit the data. The model with a correlation coefficient (R2) near to 1 would be chosen for drug release kinetics.
Radio-iodination of MEF using Iodine-131 (131I)
An oxidizing agent, Chloramine-T (CAT), was utilized in this study to radiolabel MEF with 131I through a direct electrophilic substitution reaction. An amber-colored vial containing the required quantity of MEF was filled with the proper quantity of CAT (dissolved in 70% ethanol). Under vigorous stirring, about 18–37 MBq of 131I were added to this reaction vial. The reaction of MEF labeling was run under various time intervals and different pH (5–9). By adding a droplet of sodium metabisulfite solution (10 mg/ml H2O), the reaction was stopped because of the extra amount of iodine (I2) was changed to iodide (I−) form. Ascending paper chromatography was used to quantify the radiochemical yield % of 131I-MEF (Ahmed et al. 2020; Aboumanei and Mahmoud 2022).
Paper Chromatographic Analysis of 131I-MEF
The 131I-MEF’s labeling efficiency was estimated by Paper Chromatography using 1 cm in width and 13 cm in length sheet of Whatman paper. Briefly, 1 to 2 µl of the reaction mixture was dropped at a distance of 1 cm from the lower edge of the sheet. Chloroform and methanol mixture with a ratio of 3:1 v/v was used as the mobile phase. When the mobile phase had totally developed, the sheet was dried and cut into small slices (1 cm length). Each piece was counted with a Scalar Ratemeter SR7 gamma counter (Nuclear Enterprises Ltd., USA). The 131I-MEF was assessed at Rf = 1, whereas at Rf = 0–0.1 free radioiodine settles (Ahmed et al. 2020; Aboumanei and Mahmoud 2022). To calculate the radiochemical yield percentage (RCY %), we applied the following equation:
| 2 |
We investigated and assessed how various reaction conditions affected the efficiency of radioiodination to increase the radiochemical yield. These factors included the quantity of MEF, the reaction time, the reaction pH, and the oxidizing agent (CAT) amount.
In vitro stability of 131I-MEF
During the 24-h period, at various time intervals, samples (about 1–2 µl) were withdrawn from the prepared 131I-MEF solution and assessed for RCY % (Rashed et al. 2014).
Preparation of 131I-MEF loaded niosomes
The 131I-MEF niosomal formula was made using the prepared 131I-MEF complex in the same way as the optimum MEF niosomal formula (Ahmed et al. 2020; Aboumanei and Mahmoud 2022).
Biodistribution study
For in vivo biodistribution studies in mice, the radiokinetic technique was employed to monitor the effectiveness of the prepared MEF-loaded niosomes in targeting tumor sites in comparison to MEF solution. The animal ethics committee of the Egyptian Atomic Energy Authority approved this experiment. Swiss albino mice, with 20–25 g weight, were utilized in all experiments. Mice were divided into groups and provided with water and food throughout the experiment period.
Tumor induction in mice
In order to generate solid tumor in mice, Ehrlich ascites carcinoma cell line diluted with sterilized saline solution was used. Swiss albino male mice were injected carefully into their right thigh muscles with 0.2 ml of the previous solution. After 7–10 days, a detectable solid tumor developed in the right thigh muscle of the previously injected mice (Eldin et al. 2022; Nasr et al. 2018).
Biodistribution of 131I-MEF niosomes in tumor-bearing mice
Solid tumor-bearing mice were separated into two groups, A and B. Aliquots of 150 μl of 131I-MEF niosomes and 131I-MEF solution containing 5.2 MBq were injected intravenously into the two groups A and B, respectively. Mice injection was followed by dissection of anesthetized mice (n = 3) at different time interval post injection (0.5, 1, 2, 3, 4, 6 and 24 h). Bone, muscle and fresh blood samples were taken, weighted and counted for their radioactivity content. The percentages of blood, bone, and muscle mass in comparison with the total body weight were measured to be 7, 10 and 40%, respectively (Rashed et al. 2018; Sakr et al. 2017). The tumor muscle was totally separated from the mouse leg, rinsed with physiological saline, and then allowed to dry before being weighted. The contralateral muscle served as a normal control muscle to estimate the radiolabeled formula uptake into the tumor muscle (Haroun et al. 2022; Aboud et al. 2022). The other tissues and organs’ radioactivity were measured with a gamma counter. A group of three mice was used to determine the radioactivity uptake for each point of time, and the percent of injected dosage per gram (% ID/g ± S.D.) was reported for each organ (El-Sharawy et al. 2022; Khedr et al. 2019).
Statistical analysis
The maximal plasma concentration of 131I-MEF (Cmax) and the time to achieve it (Tmax) were simply calculated by graphing the average 131I-MEF uptake (% ID/g) in samples of blood and various tissues against time (hr). The area under the curve from 0 to 24 h (AUC0–24 h, % ID/g), the tumor AUC value (AUC0–24 h), the tumor drug targeting efficiency (AUCtarget /AUCnon-target) and the time to achieve half plasma concentration (T1/2, hr) were also reported.
All data of statistics were presented as means value ± SD. The statistical analysis was performed using the Design Expert® program. Tukey’s post hoc assessment following a one-way ANOVA was applied, where the p value less than 0.05 was regarded as statistically significant.
Results
MEF-loaded niosomes optimization
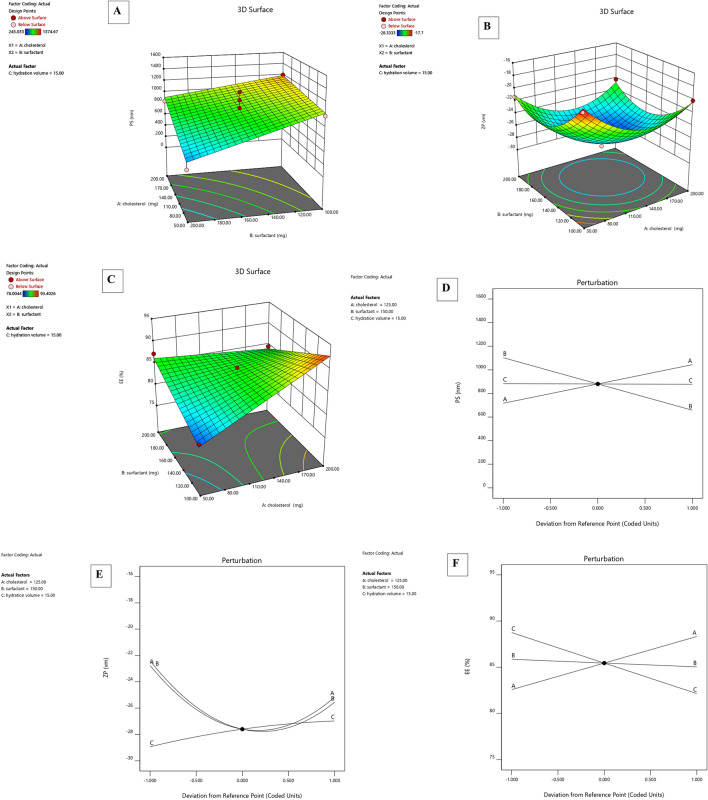
The effect of independent factors (A- Cholesterol amount (mg), B- Surfactant amount (mg) and C- Hydration volume (ml)) on dependent responses (Y1-PS, Y2-ZP and Y3- EE %) was studied for optimization of MEF-loaded niosomes (Fig. 1). Box–Behnken design (Design-Expert® Software version 12.0.1) was used for this optimization study.
Fig. 1.
Response surface plots for A particle size, B zeta potential and C entrapment efficiency. Three-factor interaction plots D particle size, E zeta potential and F entrapment efficiency
Fifteen batches of MEF-loaded niosomes with three center points were prepared and tested according to the experimental design [Table 2]. The optimized MEF niosomal formula, which Design-Expert® Software selected based on desirability value, was used for additional studies and characterizations.
Table 2.
Composition of niosomal formulations and observed responses (means ± SD)
| Run | A:cholesterol amount | B:surfactant amount | C:hydration time | PS | ZP | EE |
|---|---|---|---|---|---|---|
| mg | mg | min | nm | mv | % | |
| 1 | 200 | 100 | 15 | 1195.4 ± 3.1 | − 20.13 ± 1.1 | 71.39 ± 2.4 |
| 2 | 50 | 150 | 20 | 1142.3 ± 4.7 | − 18.8 ± 0.9 | 78.00 ± 2.7 |
| 3 | 50 | 100 | 15 | 976.6 ± 1.4 | − 17.7 ± 1.3 | 79.58 ± 3.1 |
| 4 | 125 | 100 | 10 | 1255.33 ± 5.2 | − 23.4 ± 1.5 | 93.40 ± 1.6 |
| 5 | 125 | 100 | 20 | 849.7 ± 3.6 | − 21.8 ± 0.7 | 90.41 ± 2.1 |
| 6 | 200 | 150 | 10 | 1374.66 ± 4.4 | − 24.63 ± 1.3 | 90.83 ± 1.8 |
| 7 | 50 | 200 | 15 | 295.55 ± 1.2 | − 21.33 ± 1.7 | 87.20 ± 3.7 |
| 8 | 125 | 200 | 20 | 180.8 ± 1.6 | − 25.63 ± 1.4 | 85.38 ± 2.6 |
| 9 | 50 | 150 | 10 | 245.03 ± 1.8 | − 26.7 ± 2.1 | 86.72 ± 3.9 |
| 10 | 125 | 200 | 10 | 531 ± 2.3 | − 26.5 ± 1.8 | 83.66 ± 2.3 |
| 11 | 200 | 200 | 15 | 838.56 ± 3.7 | − 22.2 ± 1.2 | 84.52 ± 1.9 |
| 12 | 125 | 150 | 15 | 1169.33 ± 6.4 | − 28.33 ± 2.7 | 83.53 ± 3.3 |
| 13 | 125 | 150 | 15 | 904.16 ± 4.1 | − 26.86 ± 2.9 | 84.25 ± 4.2 |
| 14 | 200 | 150 | 20 | 558.83 ± 2.9 | − 27.16 ± 3.2 | 86.47 ± 1.5 |
| 15 | 125 | 150 | 15 | 1037 ± 5.2 | − 27.6 ± 2.4 | 87.15 ± 0.6 |
Particle size
Table 2 displays particle size measurement results of the prepared niosomes. The particle size of the samples ranged between 245.03 and 1374.66 nm. The PS of niosomes was affected by many factors, such as the cholesterol amount in the formula. The results indicated that the niosomal size increased with increasing the cholesterol amount (p = 0.0164). This could be due to the increased hydrophobicity of the bilayer caused by higher cholesterol levels leading to MEF sequestering in the hydrophobic bilayer (Witika and Walker 2021). This result is in agreement with previous findings by Moghddam et al. (Moghddam et al. 2016). The vesicles’ formation and properties are well known to be affected by the HLB of the used surfactant. Two classes of surfactants were used, Span 20 and Tween 20, to decrease the nonionic surfactants’ hydrophilic head group effect on the vesicles’ properties. Generally, preparing niosomes using polysorbate 20 (Tween 20) produces much larger particles than those prepared using sorbitan esters (Spans) due to the high HLB value of this polysorbate surfactant. In this study, the increase in surfactant amount resulted in a decreasing PS that may be due to using a combined mixture of surfactants (Tween 20 with Span 20) (p = 0.007) (Witika and Walker 2021).
The best-fit model for the niosomal particle size analysis was the 2FI model with a significant p value of 0.0026. The P-value of lack of fit was 1.33 (not significant), and the predicted and adjusted R-squared difference was < 0.2, indicating a good fit.
Final equation in terms of coded factors:
Polydispersity index (PDI) of niosomes ranged between 0.2 and 0.8, indicating that the produced niosomes were uniform in size and homogeneous. A PDI ≤ 0.5 is regarded as suitable for drug delivery applications because it demonstrates a relatively homogeneous and uniform distribution of nanocarriers. To provide proper particle distribution, the optimal formulation must have a PDI value ≤ 0.5.
Zeta potential
Niosomes were physically stable since the ZP values of all formulations ranged from − 28.3 to − 17.7 mV (Allam et al. 2021; Ghanem et al. 2021). Cholesterol had a significant effect on ZP with a p value of 0.0094. Zeta potential increased, as an absolute value, by increasing the cholesterol amount leading to enhancing niosomes stability. As a result, cholesterol is an important excipient in the preparation of niosomes because it enhances the stability of niosomes’ bilayers and reduces drug leakage due to the retarded solute permeability of these vesicles’ aqueous core (Auda et al. 2016).
In addition, ZP increased by raising the surfactant amount until reaching a particular level, after this level, the ZP decreased. This could be because the hydrophilic–lipophilic balance (HLB) influenced ZP values. Because Span 20 (HLB 8.6) has a higher hydrophilic content than the other surfactants, it may have resulted in fewer counter ions adsorbed on the vesicles’ surface, leading to a rise in the ZP value at high surfactant amount (Abou-Taleb et al. 2018). Moreover, ZP decreased with increasing hydration volume, with a p value < 0.0206.
Final equation in terms of coded factors:
Entrapment efficiency
There was a decrease in EE% observed with an increase in the amount of surfactant. This may be due to the incorporation of a surfactant with a high HLB value, such as Tween 20 (HLB 16.7), into niosomes which decreased the hydrophobicity of the system and consequently decreased the EE% of MEF (Sharma et al. 2021). However, high entrapment efficiency ranged from 78 to 93.4% was due to the inclusion of Span 20 in formulations (Witika and Walker 2021). Cholesterol addition led to an increase in the amount of MEF being sequestered in the hydrophobic bilayer and a subsequent increase in EE% (Witika and Walker 2021; Sharma et al. 2021).
The ideal volume of hydration media was ten ml which effectively hydrated the produced film. In this study, larger volumes were found to have a negative effect on EE%. That was explained by the fact that increasing the drug dispersion by increasing the hydration medium volume results in small vesicles with a low EE% (Aziz et al. 2018).
Final equation in terms of coded factors:
Selection of optimal formulation
All models were statistically significant, as indicated in Table 3. The Y1 and Y3 factors had a 2FI model fit, whereas the Y2 factor had a Quadratic model fit, as presented in Table 3.
Table 3.
Responses of regression analysis for particle size (PS), Zeta Potential (ZP) and %Entrapment Efficency (%EE)
| Response | R2 | Adjusted R2 | Predicted R2 | Adequate precision | Minimum | Maximum | p value | Lack of fit | Trans | Model |
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 = PS (nm) | 0.9075 | 0.8283 | 0.7314 | 11.357 | 180.80 | 1374.666 | 0.0026 | 0.4818 | None | 2FI |
| Y2 = ZP (mv) | 0.9790 | 0.9411 | 0.7540 | 15.395 | − 28.333 | − 17.7 | 0.0011 | 0.4299 | None | Quadratic |
| Y3 = EE (%) | 0.9468 | 0.8937 | 0.8180 | 15.524 | 78.0044 | 93.402 | 0.0014 | 0.9040 | None | 2FI |
According to the design optimization, the optimal formula with the highest desirability value (0.819) was prepared with the following composition (170.207 mg surfactant, 67.716 mg cholesterol, and a 10 ml hydration volume). This selected optimum formula had a vesicle size of 247.23 nm, a zeta potential of -28.3 mV, a PDI of 0.44, and an entrapment efficiency of 85.81% (Table 4).
Table 4.
Composition, actual and predicted responses for the optimum MEF niosomes
| Factor optimized level | |
|---|---|
| X1 = cholesterol amount | 67.716 mg |
| X2 = surfactant amount | 170.207 mg |
| X3 = hydration volume | 10 ml |
| Response | Predicted | Observed | Residual |
|---|---|---|---|
| Y1 = PS (nm) | 245.033 | 247.23 ± 1.3 | − 2.197 |
| Y2 = ZP (mV) | − 27.987 | − 28.3 ± 2.1 | 0.313 |
| Y3 = EE (%) | 86.752 | 85.81 ± 1.7 | 0.942 |
Transmission electron microscopy (TEM)
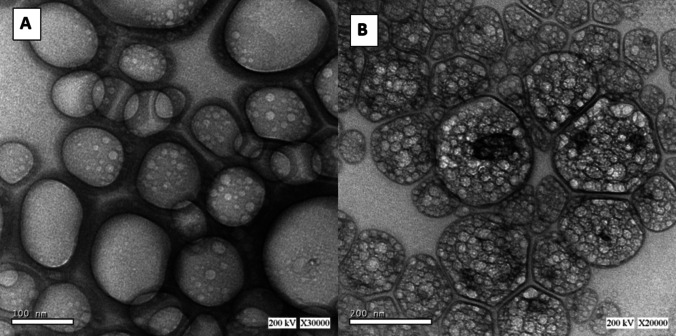
TEM is frequently used to examine the morphology of the niosomal formulation. The MEF niosomal formulation panoramic image showed niosomes vesicles with a spherical shape without aggregation at 30,000× (Fig. 2A) and 20,000× (Fig. 2B) magnification. The image obtained by TEM of MEF niosomes presented spherical vesicles with a size of around 100–200 nm that was similar to the size obtained via the DLS experiment (de Siqueira et al. , 2022). Additionally, TEM scans demonstrated that MEF-loaded niosomes appeared as distributed spherical particles and had a hollow vesicular shape with well-defined edges (Shilakari Asthana et al. 2016).
Fig. 2.
Transmission electron microscopy of optimum niosomal formulation: A 30,000 × and B 20,000 × magnification
FTIR study
MEF had a secondary amine characteristic peak at 3310 cm−1 in its FTIR spectrum (Fig. 3). Due to the presence of the –OH group, there was a broad band in the range of 3010–2858 cm−1. The –OH group also represented intra- and intermolecular hydrogen bonding, which overlapped with the (–CH3) group. The presence of the peak at 1649–1733 cm−1 was due to the appearance of the C=O group. The phenyl group presence is indicated by a peak presence at 1076 cm−1. In the formula, the secondary amine group was presented at 3455 cm−1 and also overlapped with the (–OH) group. The C=O of the carboxyl salt was presented at 1641 cm−1 and the phenyl group was presented at 1084 cm−1 (Bhardwaj et al. 2015; Puspaningtyas 2017).
Fig. 3.
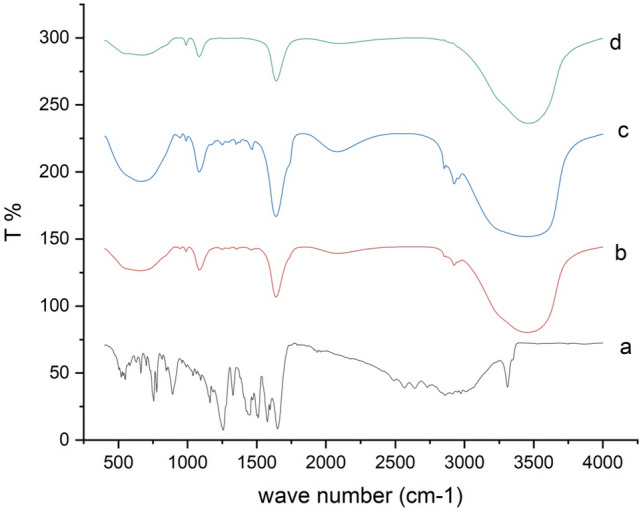
Fourier Transform Infrared Spectra of a MEF, b plain niosomes, c physical mixture and d MEF-loaded niosomes
As a result, all the characteristic peaks of the drug were presented and there were no noticeable additional peaks or peak shifts of MEF detected in the formula. Such results indicated that MEF had not been exposed to chemical degradation or lost compatibility in the presence of the additives. Therefore, FTIR confirmed the stability of the niosomal formula.
Differential scanning calorimetry (DSC)
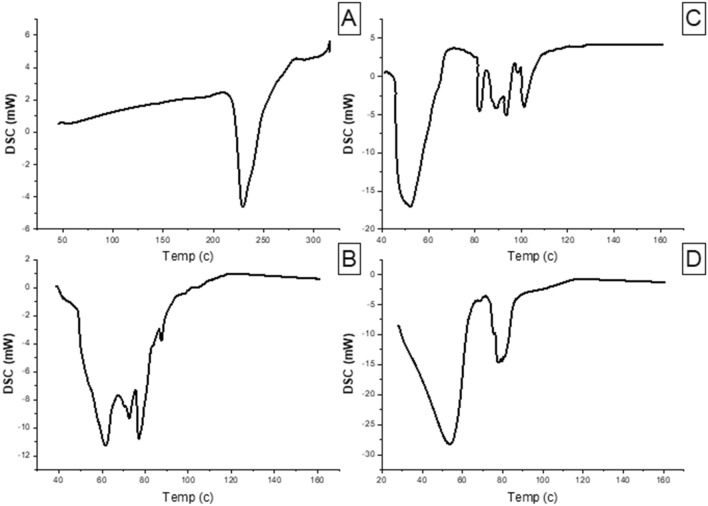
DSC was applied at a temperature range of 40–300 °C to investigate if the niosomal formulation has any excipients that are incompatible with MEF. The obtained thermogram presented an endothermic peak for MEF at 229.3 °C. The crystalline peak of MEF disappeared in the niosomal formula curve. The absence of a distinctive MEF peak indicates that the drug was fully dissolved in the niosomal system and converted to an amorphous form. In addition to that the thermogram curves showed that the additives and the drug were compatible with each other (Utami et al. 2017) (Fig. 4).
Fig. 4.
Differential scanning calorimetry of A MEF, B plain niosomes, C physical mixture and D MEF-loaded niosomes
Stability study
After 3 months of storage, the niosomal formulations showed no change in color. They kept their homogeneous, opaque white appearance and there wasn’t any precipitation. To obtain more detailed knowledge about the niosomes’ stability, any changes in zeta potential, vesicle size and EE% were used as stability-indicating factors (Temprom et al. 2022; Ghafelehbashi et al. 2019). These variables were assessed at predetermined intervals. According to Table 5, it could be seen that the MEF niosomal formula showed a small change in vesicle size during the 3 months of storage. Under refrigeration at 4 °C, the distribution of vesicle size changed by an insignificant value due to the existence of the appropriate electrostatic repulsion force, which prevents agglomeration. However, at room temperature (25 ± 2 °C), the niosomal formula was found to aggregate, indicating that the fluidity of the bilayer membrane increased at higher temperatures (Kulkarni et al. 2021). Because the lipophilic part rigidity in the niosomes rises at lower temperatures, the sample held at 4 °C was more stable than that kept at 25 °C (Sayyad et al. 2021). Another essential stability parameter is the niosomes’ capability to retain the drug in its encapsulated form during the storage period. The entrapment efficiency percentage decreased from 85.81% to 81.62%, after three months of storage at 25 °C. This was due to the surfactant reaching its superior alternation temperature, which resulted in the formation of a more stable membrane. Such stability data suggested that these formulations were more stable at low temperatures (Ghafelehbashi et al. 2019).
Table 5.
Stability of the optimized MEF-loaded niosomes
| PS (nm) | ZP (mV) | EE% | |
|---|---|---|---|
| At zero day | 247.23 ± 1.3 | – 28.3 ± 2.1 | 85.81 ± 1.7 |
| After 90 days at 4 °C | 250.9 ± 1.7 | – 27.5 ± 1.9 | 84.98 ± 2.6 |
| After 90 days at 25 °C | 274.2 ± 2.4 | – 24.2 ± 1.5 | 81.62 ± 2.2 |
In vitro release study
A quick initial drug release was followed by a prolonged release phase (Fig. 5) indicating a biphasic cumulative release pattern for MEF. The quick burst release could be due to the drug desorption from the niosomes surface. Drug diffusion across vesicular bilayers may be responsible for the slower phase, indicating that the entrapped drug was relatively stable. Niosomes were good drug carrier with good release since MEF was released cumulatively from suspension and niosomes formulation at rates of 55.36% and 77.73%, respectively, after 24 h (Targhi et al. 2021).
Fig. 5.
In vitro release of MEF suspension and optimal MEF niosomes (cumulative release % ± SD)
The in vitro release data gained from the optimal niosomal formulation was fitted to different kinetic models to predict the mechanism of MEF release from the niosomes. Table 6 displays the regression coefficient (R2) values for each model. The results showed that Higuchi’s model best explained niosomal formulation release, indicating that the drug diffused more slowly as the distance for diffusion increased. So, the diffusion mechanism controlled the release process of MEF from the niosomal formula suggesting a matrix diffusion-based release, which was unpredicted with such a liquid formula (Temprom et al. 2022). The responsibility for this behavior lies in the fact that the lipophilic drug was trapped within the lipid bilayer of the produced niosomes, despite the test system’s fluidity. Firstly, the drug was released quickly from the multi-lamellar structure’s surface layer of niosomes, followed by a diffusion-controlled release from the interior layers (Fayed et al. 2021).
Table 6.
Different release kinetics R2 values for MEF suspension and optimal MEF niosomes
| Formula | Zero (R2) | First (R2) | Diffusion (R2) | Krosmear (R2) | Hixon (R2) | Order of permeation |
|---|---|---|---|---|---|---|
| MEF niosomes | 0.7552 | 0.4917 | 0.9016 | 0.8712 | 0.5856 | Diffusion |
| MEF suspension | 0.8398 | 0.7199 | 0.9442 | 0.9211 | 0.7622 | Diffusion |
Radiolabeling of MEF using 131I
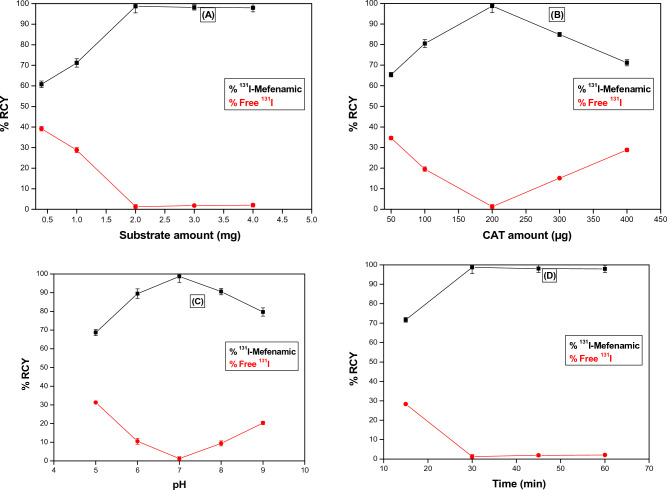
Figure 6 presents how different factors of radiolabeling influenced the 131I-MEF niosomes RCY%. The highest RCY% of the 131I-MEF complex was 98.7 ± 0.8% was achieved using 200 µg of CAT, 18–37 MBq of 131I and 2 mg of MEF at 30 min reaction time, pH of 7 and room temperature (25 °C). In vitro, the 131I-MEF complex maintained its stability for at least 24 h. The physical and chemical characteristics of the niosomes, such as EE%, PS, PDI, ZP or in vitro release, were unaffected by the use of radiolabeled MEF in the niosomes’ creation (Ahmed et al. 2020).
Fig. 6.
Influence of various radiolabeling parameters; A MEF quantity, B CAT quantity, C pH and D reaction time, on 131I-MEF radiochemical yield
Biodistribution of 131I-MEF niosomes
The two groups of tumor-bearing mice (each consisting of 21 mice) were used to study the in vivo biodistribution of 131I-MEF niosomes. Groups A and B were intravenously (I.V) injected with 131I-MEF niosomes and 131I-MEF solution, respectively. Using three mice for each time interval, the radioactivity (measured as %ID/g) was calculated in different organs and body fluids during the 24-h period (Haroun et al. 2022).
The accumulation of drug in tumor tissues started to rise within 60 min after I.V. injection, while the uptake of drug in the blood started to gradually decline. The enhanced permeability and retention (EPR) effect was the reason for enhancing the 131I-MEF niosomes’ accumulation and permeability into malignant cells. In addition, the nanoparticles (niosomes) were targeted to cancer tissues passively due to the small niosomal particle size. Particles with a small diameter avoided internalization into the Mononuclear Phagocyte System (MPS), resulting in a longer circulation time due to reduced clearance by macrophages. In comparison to group A, group B’s biodistribution profile showed less 131I-MEF accumulation at the tumor site. There were noticeable differences between groups A and B in tumor uptake of 131I-MEF and accumulation at the tumor site, as reported in Table 7. The highest accumulation of 131I-MEF at cancer cells in groups A and B post-I.V injection was 2.73% ID/g at 60 min and 1.94% ID/g at 30 min, respectively. Examination of the target (solid tumor) to non-target (normal muscle) ratio (T/NT) of 131I-MEF was an essential parameter (Fig. 7). Regarding group A, the highest T/NT ratio was attained after 2 h of the I.V injection, which was 4.692. However, it was only 2.978 in group B after 24 h post I.V. injection. The Tumor/Blood (T/B) ratio, which should be higher than 1, determines the potentiation of any novel nano-radiopharmaceutical (Witika and Walker 2021). The 131I-MEF niosomes had a T/B ratio greater than 1 at 1 and 2 h after I.V. injection (Table 7) (El-Safoury et al. 2021). The pharmacokinetic differences between 131I-MEF solution and 131I-MEF niosomes were mathematically calculated using Cmax, Tmax, T1/2, AUC0–24, tumor AUC0–24 and the tumor drug targeting efficiency (AUCtarget/AUCnon-target) as shown in Table 8. Consequently, 131I-MEF niosomes improved MEF uptake in tumor tissues following intravenous injection and it could be considered as a hopeful nano-radiopharmaceutical that potentiates tumor treatment.
Table 7.
Radioactivity uptake (%ID/g ± S.D) in tumor and normal muscle at different time intervals post-injection (hr), target/non-target ratio (T/NT) and target/blood ratio (T/B)
| Organs and body fluids | Detected dose/gram % at different time intervals post-injection (hr) | |||||
|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 2 h | 4 h | 6 h | 24 h | |
| 131I-MEF niosomes | ||||||
| Tumor (T) | 2.176 ± 0.25 | 2.738 ± 0.16 | 1.607 ± 0.09 | 1.21 ± 0.14 | 1.053 ± 0.06 | 0.791 ± 0.07 |
| Muscle (NT) | 1.066 ± 0.19 | 1.039 ± 0.42 | 0.343 ± 0.26 | 0.446 ± 0.11 | 0.358 ± 0.32 | 0.581 ± 0.08 |
| T/NT | 2.041 | 2.635 | 4.692 | 2.713 | 2.941 | 1.363 |
| T/B | 0.486 | 1.107 | 1.152 | 0.840 | 0.941 | 0.505 |
| 131I-MEF solution | ||||||
| Tumor (T) | 1.94 ± 0.14 | 1.62 ± 0.27 | 1.47 ± 0.34 | 1.43 ± 0.19 | 1.39 ± 0.15 | 1.34 ± 0.18 |
| Muscle (NT) | 1.47 ± 0.12 | 1.17 ± 0.28 | 0.77 ± 0.16 | 0.71 ± 0.08 | 0.52 ± 0.01 | 0.45 ± 0.02 |
| T/NT | 1.32 | 1.385 | 1.909 | 2.014 | 2.673 | 2.978 |
| T/B | 0.144 | 0.360 | 0.369 | 0.475 | 0.556 | 0.720 |
Fig. 7.
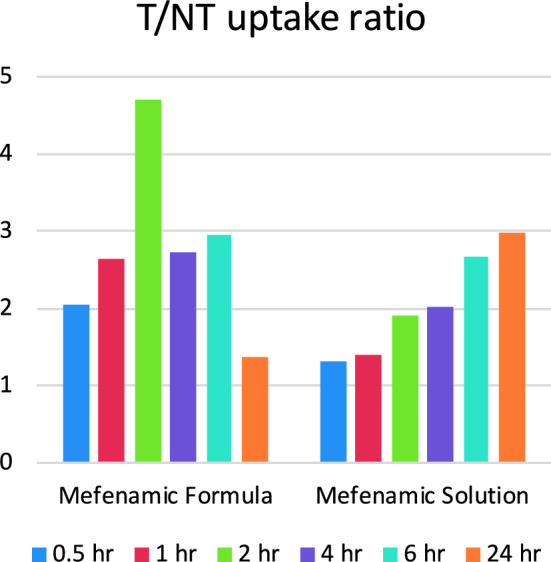
Target/non-target ratio (T/NT) for 131I-MEF niosomes and 131I-MEF solution at different time intervals post-injection
Table 8.
Cmax, Tmax, T1/2, AUC0–24, Tumor AUC0–24 and Tumor drug targeting efficiency for 131I-MEF niosomes and 131I-MEF solution
| Formula | Cmax (ID/g) | Tmax (hr) | T1/2 (hr) | AUC0–24 | Tumor AUC0–24 | Tumor drug targeting efficiency (AUCtarget /AUCnon-target) |
|---|---|---|---|---|---|---|
| 131I-MEF niosomes | 4.471 | 0.5 | 0.213 | 59.079 | 42.3348 | 2.4691 |
| 131I-MEF solution | 13.40 | 0.5 | 0.261 | 155.144 | 34.2033 | 1.4782 |
Conclusion
By using the ether injection method, a niosomal formulation of MEF made of cholesterol and non-ionic surfactants (Tween 20 and Span 20) was created. DSC demonstrated that MEF was trapped in an amorphous form and that the nanovesicle size was less than 300 nm. After formulation, the drug’s molecular chemistry did not change, according to FTIR. The optimum niosomal formula of MEF displayed a high entrapment efficiency of 85.81%. The niosomal formula continuously released the drug in a sustained manner for 24 h. MEF was radiolabeled successfully by 131I with a radiochemical yield of 98.7%. The radiokinetic analysis revealed that passive targeting of the nanosized niosomes increased the delivery of MEF to the tumor cells. More importantly, optimum 131I-MEF niosomes showed promising properties and potentiated cancer activity due to enhanced targeting, encapsulation efficiency and release rate and showed a high accumulation in tumor tissues. In conclusion, the optimal niosomal formula of MEF was effective in improving MEF delivery as an anticancer agent. Further histopathology, pharmacodynamics and clinical studies are required to improve the findings of this research.
Author contributions
MAS and AAS: performed the experiments. HMR and YMM: prepared the figures and analyzed data. SG: wrote and revieed the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hassan M. Rashed, Email: Hassan_vodafone@hotmail.com, Email: Hassan.Medhat@su.edu.eg
Shadeed Gad, Email: Shaded_abdelrahman@pharm.Suez.edu.eg.
References
- Abbas Z, Rehman SJN (2018) An overview of cancer treatment modalities. Neoplasm 1:139–157 [Google Scholar]
- Aboud HM et al (2022) Tailoring of selenium-plated novasomes for fine-tuning pharmacokinetic and tumor uptake of quercetin: in vitro optimization and in vivo radiobiodistribution assessment in ehrlich tumor-bearing mice. Pharmaceutics 14(4):875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboumanei MH, Mahmoud AF (2022) Development of tamoxifen in situ gel nanoemulsion for ocular delivery in photoreceptor degeneration disorder: in vitro characterization, 131I-Radiolabeling, and in vivo biodistribution studies. J Pharm Innov 18:369–380 [Google Scholar]
- Abou-Taleb HA, Khallaf RA, Abdel-Aleem JA (2018) Intranasal niosomes of nefopam with improved bioavailability: preparation, optimization, and in-vivo evaluation. Drug Des Dev Ther 12:3501–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AM et al (2013) The application of Plackett-Burman design and response surface methodology for optimization of formulation variables to produce Piroxicam niosomes. Int J Drug Dev Res 5(2):121–130 [Google Scholar]
- Ahmed IS et al (2020) Nanoparticle-mediated dual targeting: an approach for enhanced baicalin delivery to the liver. Pharmaceutics 12(2):107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh I et al (2021) Niosomes: a novel targeted drug delivery system. Preprints, p 2021120315
- Allam A et al (2021) Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int J Pharm 598:120380 [DOI] [PubMed] [Google Scholar]
- Altay A et al (2019) Novel silver(I) complexes bearing mefenamic acid and pyridine derivatives: synthesis, chemical characterization and in vitro anticancer evaluation. Inorg Chim Acta 493:61–71 [Google Scholar]
- Amoabediny G et al (2018) Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int J Polym Mater Polym Biomater 67(6):383–400 [Google Scholar]
- Anand U et al (2022) Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis 10(4):1367–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaithambi K et al (2020) Synthesis and characterization of turAmoabedinymeric oil loaded non-ionic surfactant vesicles (niosomes) and its enhanced larvicidal activity against mosquito vectors. Biocatal Agric Biotechnol 29:101737 [Google Scholar]
- Atzrodt J, Derdau V, Loewe C (2020) Synthesis of radiolabelled compounds for clinical studies. In: Drug discovery and Evaluation: methods in clinical Ppharmacology, pp 807–825
- Auda SH et al (2016) Niosomes as transdermal drug delivery system for celecoxib: in vitro and in vivo studies. Polym Bull 73(5):1229–1245 [Google Scholar]
- Avcıbaşı U et al (2013) Synthesis and biodistribution of novel magnetic-poly (HEMA–APH) nanopolymer radiolabeled with iodine-131 and investigation its fate in vivo for cancer therapy. J Nanopart Res 15:1–24 [Google Scholar]
- Aziz DE, Abdelbary AA, Elassasy AI (2018) Implementing central composite design for developing transdermal diacerein-loaded niosomes: ex vivo permeation and in vivo deposition. Curr Drug Deliv 15(9):1330–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak E et al (2010) In vitro evaluation, biodistribution in rats of radiolabeled raloxifene. Appl Radiat Isot 68(1):33–36 [DOI] [PubMed] [Google Scholar]
- Bernardi A et al (2008) Selective cytotoxicity of indomethacin and indomethacin ethyl ester-loaded nanocapsules against glioma cell lines: an in vitro study. Eur J Pharmacol 586(1–3):24–34 [DOI] [PubMed] [Google Scholar]
- Bhardwaj P et al (2020) Niosomes: a review on niosomal research in the last decade. J Drug Deliv Sci Technol 56:101581 [Google Scholar]
- Bhardwaj G, Singh L, Sharma V (2015) Development of taste masked oral suspension of mefenamic acid and paracetamol for increase acceptability. Res J Pharm Biol Chem Sci 6(6)
- Blevins HM et al (2022) The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci 14:879021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseila AA et al (2019) Superiority of DEAE-Dx-stabilized cationic bile-based vesicles over conventional vesicles for enhanced hepatic delivery of daclatasvir. Mol Pharm 16(10):4190–4199 [DOI] [PubMed] [Google Scholar]
- Chaudhari PD, Desai US (2019) Formulation and evaluation of niosomal in situ gel of prednisolone sodium phosphate for ocular drug delivery. Int J Appl Pharm 11:97–116 [Google Scholar]
- de Siqueira LBDO et al (2022) Encapsulation of photosensitizer in niosomes for promotion of antitumor and antimicrobial photodynamic therapy. J Drug Deliv Sci Technol. 68:103031 [Google Scholar]
- Edis Z et al (2021) Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int J Nanomed 16:1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin SSE et al (2023) Multifunctional 99mTc-5-azacitidine gold nanoparticles: formulation, in vitro cytotoxicity, radiosynthesis, and in vivo pharmacokinetic study. Curr Drug Deliv 20:387–399 [DOI] [PubMed] [Google Scholar]
- El-Safoury D et al (2021) Amelioration of tumor targeting and in vivo biodistribution of 99mTc-methotrexate-gold nanoparticles (99mTc-Mex-AuNPs). J Pharm Sci 110(8):2955–2965 [DOI] [PubMed] [Google Scholar]
- El-Sharawy DM et al (2022) Radiolabeling, biological distribution, docking and ADME studies of 99mTc-Ros as a promising natural tumor tracer. Appl Radiat Isot 184:110196 [DOI] [PubMed] [Google Scholar]
- Faheela M, Malathi S (2022) In-vitro characterization of pluronic P 123 based niosome for targeted delivery of doxorubicin. Mater Today Proc 58:795–801 [Google Scholar]
- Fayed ND et al (2021) Chitosan-encapsulated niosomes for enhanced oral delivery of atorvastatin. J Drug Deliv Sci Technol 66:102866 [Google Scholar]
- Ghafelehbashi R et al (2019) Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int J Pharm 569:118580 [DOI] [PubMed] [Google Scholar]
- Ghanem HA et al (2021) Comprehensive study of atorvastatin nanostructured lipid carriers through multivariate conceptualization and optimization. Pharmaceutics 13(2):178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Esquivel J et al (2020) Decreased biochemical progression in patients with castration-resistant prostate cancer using a novel mefenamic acid anti-inflammatory therapy: a randomized controlled trial. Oncol Lett 19(6):4151–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Esquivel J et al (2022) Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID-19: a randomized double-blind placebo-controlled trial. Int J Mol Med 49(3):1–9 [DOI] [PubMed] [Google Scholar]
- Haroun M et al (2022) Significant of injectable brucine PEGylated niosomes in treatment of MDA cancer cells. J Drug Deliv Sci Technol 71:103322 [Google Scholar]
- Hassan TH et al (2021) Refinement of Simvastatin and Nifedipine combined delivery through multivariate conceptualization and optimization of the nanostructured lipid carriers. J Drug Deliv Sci Technol 64:102570 [Google Scholar]
- He J et al (2019) Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Adv Mater 31(40):1902409 [DOI] [PubMed] [Google Scholar]
- Hosseinimehr SJ et al (2015) Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat Oncol J 33(3):256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinimehr SJ et al (2019) The synergistic effect of mefenamic acid with ionizing radiation in colon cancer. J Bioenerg Biomembr 51(3):249–257 [DOI] [PubMed] [Google Scholar]
- Hua S et al (2015) Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomed Nanotechnol Biol Med 11(5):1117–1132 [DOI] [PubMed] [Google Scholar]
- Jafarieh O et al (2015) Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev Ind Pharm 41(10):1674–1681 [DOI] [PubMed] [Google Scholar]
- Jimenez C, Erwin W, Chasen B (2019) Targeted radionuclide therapy for patients with metastatic pheochromocytoma and paraganglioma: from low-specific-activity to high-specific-activity iodine-131 metaiodobenzylguanidine. Cancers 11(7):1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J et al (2019) Inflammation-induced DNA damage, mutations and cancer. DNA Repair 83:102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MI, Madni A, Peltonen L (2016) Development and in-vitro characterization of sorbitan monolaurate and poloxamer 184 based niosomes for oral delivery of diacerein. Eur J Pharm Sci 95:88–95 [DOI] [PubMed] [Google Scholar]
- Khedr MA et al (2019) Rational design of some substituted phenyl azanediyl (bis) methylene phosphonic acid derivatives as potential anticancer agents and imaging probes: Computational inputs, chemical synthesis, radiolabeling, biodistribution and gamma scintigraphy. Bioorg Chem 92:103282 [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Rawtani D, Barot T (2021) Design, development and in-vitro/in-vivo evaluation of intranasally delivered Rivastigmine and N-Acetyl cysteine loaded bifunctional niosomes for applications in combinative treatment of Alzheimer’s disease. Eur J Pharm Biopharm 163:1–15 [DOI] [PubMed] [Google Scholar]
- Kumar S et al (2022) Therapeutics effect of mesenchymal stromal cells in reactive oxygen species-induced damages. Hum Cell 35(1):37–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohumi A (2012) A novel drug delivery system: niosomes review. J Drug Deliv Ther 2(5):129–135
- Manohar M, Marzinke MA (2016) Application of chromatography combined with mass spectrometry in therapeutic drug monitoring. Clinical challenges in therapeutic drug monitoring. Elsevier, pp 45–70 [Google Scholar]
- Masjedi M, Montahaei T (2021) An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: fabrication, characterization, pharmaceutical, and cosmetic applications. J Drug Deliv Sci Technol 61:102234 [Google Scholar]
- Moghddam SRM et al (2016) Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater Sci Eng C 69:789–797 [DOI] [PubMed] [Google Scholar]
- Mousa IA et al (2022) Formulation and characterization of metformin-loaded ethosomes for topical application to experimentally induced skin cancer in mice. Pharmaceuticals 15(6):657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr T et al (2018) Novel hydrazide-hydrazone and amide substituted coumarin derivatives: synthesis, cytotoxicity screening, microarray, radiolabeling and in vivo pharmacokinetic studies. Eur J Med Chem 151:723–739 [DOI] [PubMed] [Google Scholar]
- Navya P et al (2019) Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg 6:1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishu SBN et al (2018) In vitro and ex vivo studies of linagliptin loaded non-ionic surfactant vesicles using statistical optimization. J Adv Med Pharm Sci 18(2):1–16 [Google Scholar]
- Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed Nanotechnol Biol Med 8(2):147–166 [DOI] [PubMed] [Google Scholar]
- Puspaningtyas AR (2017) Drug development of mefenamic acid derivatives as analgesic by molecular approach. Int J Pharm Clin Res 9(2):123–130 [Google Scholar]
- Ramakrishnan R et al (1990) Nonuniform radiolabeling of VLDL apolipoprotein B: implications for the analysis of studies of the kinetics of the metabolism of lipoproteins containing apolipoprotein B. J Lipid Res 31(6):1031–1042 [PMC free article] [PubMed] [Google Scholar]
- Rashed H et al (2014) Preparation of radioiodinated ritodrine as a potential agent for lung imaging. J Radioanal Nucl Chem 300(3):1227–1233 [Google Scholar]
- Rashed HM, Shamma RN, Basalious EB (2017a) Contribution of both olfactory and systemic pathways for brain targeting of nimodipine-loaded lipo-pluronics micelles: in vitro characterization and in vivo biodistribution study after intranasal and intravenous delivery. Drug Delivery 24(1):181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed H, Ibrahim I, Motaleb M (2017b) 99m Tc-hexoprenaline and 131 I-dapoxetine: preparation, in silico modeling and biological evaluation as promising lung scintigraphy radiopharmaceuticals. J Radioanal Nucl Chem 314:1297–1307 [Google Scholar]
- Rashed HM, Shamma RN, El-Sabagh HA (2018) Preparation of 99mTc-levetiracetam intranasal microemulsion as the first radiotracer for SPECT imaging of the synaptic vesicle protein SV2A. Eur J Pharm Sci 121:29–33 [DOI] [PubMed] [Google Scholar]
- Saha GB, Saha GB (2004) Fundamentals of nuclear pharmacy, vol 6. Springer [Google Scholar]
- Sakr TM et al (2017) Preparation and biological profile of 99m Tc-lidocaine as a cardioselective imaging agent using 99m Tc eluted from 99 Mo/99m Tc generator based on Al–Mo gel. J Radioanal Nucl Chem 314:2091–2098 [Google Scholar]
- Sayyad N et al (2021) Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension. Int J Pharm 609:121191 [DOI] [PubMed] [Google Scholar]
- Seger C (2012) Usage and limitations of liquid chromatography-tandem mass spectrometry (LC–MS/MS) in clinical routine laboratories. Wien Med Wochenschr 162(21):499–504 [DOI] [PubMed] [Google Scholar]
- Seyyedi R et al (2022) Mefenamic acid as a promising therapeutic medicine against colon cancer in tumor-bearing mice. Med Oncol 39(2):18 [DOI] [PubMed] [Google Scholar]
- Sharma S, Bhatt S, Saini V (2021) Formulation development and evaluation of novel vesicular carrier for enhancement of bioavailability of poorly soluble drug. Pharm Nanotechnol 9(1):70–82 [DOI] [PubMed] [Google Scholar]
- Shewaiter MA et al (2021) Formulation and characterization of leflunomide/diclofenac sodium microemulsion base-gel for the transdermal treatment of inflammatory joint diseases. J Drug Deliv Sci Technol 61:102110 [Google Scholar]
- Shilakari Asthana G, Sharma PK, Asthana A (2016) In vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Scientifica 2016:6492953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Hernández AD et al (2012) Antitumor effect of meclofenamic acid on human androgen-independent prostate cancer: a preclinical evaluation. Int Urol Nephrol 44:471–477 [DOI] [PubMed] [Google Scholar]
- Soriano-Hernandez AD et al (2015) Anti-inflammatory drugs and uterine cervical cancer cells: Antineoplastic effect of meclofenamic acid. Oncol Lett 10(4):2574–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targhi AA et al (2021) Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: enhanced antibacterial and anti-biofilm activities. Bioorg Chem 115:105116 [DOI] [PubMed] [Google Scholar]
- Temprom L et al (2022) A novel preparation and characterization of melatonin loaded niosomes based on using a ball milling method. Mater Today Commun 31:103340 [Google Scholar]
- Thabet Y, Elsabahy M, Eissa NG (2022) Methods for preparation of niosomes: a focus on thin-film hydration method. Methods 199:9–15 [DOI] [PubMed] [Google Scholar]
- Utami D, Nugrahani I, Ibrahim S (2017) Mefenamic acid-nicotinamide co-crystal synthesized by using melt crystallization method and its solubility study. Asian J Pharm Clin Res 10:135–139 [Google Scholar]
- Vogel HG, Maas J, Mayer D (2006) Drug discovery and evaluation: safety and pharmacokinetic assays; with 125 tables. Springer Science & Business Media [Google Scholar]
- Wichianphong N, Charoenchaitrakool M (2018) Statistical optimization for production of mefenamic acid–nicotinamide cocrystals using gas anti-solvent (GAS) process. J Ind Eng Chem 62:375–382 [Google Scholar]
- Witika BA, Walker RB (2021) Preformulation characterization and identification of excipients for nevirapine loaded niosomes. Die Pharmazie-an Int J Pharm Sci 76(2–3):77–83 [DOI] [PubMed] [Google Scholar]
- Wong RS (2019) Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv Pharm Sci 2019:3418975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DH, Han I-S, Jung G (2004) Mefenamic acid-induced apoptosis in human liver cancer cell-lines through caspase-3 pathway. Life Sci 75(20):2439–2449 [DOI] [PubMed] [Google Scholar]
- Xiao C et al (2021) Biomimetic nanoparticles loading with gamabutolin-indomethacin for chemo/photothermal therapy of cervical cancer and anti-inflammation. J Control Release 339:259–273 [DOI] [PubMed] [Google Scholar]
- Yao Y et al (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7:193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasamineh S et al (1878) A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm 2022:12 [DOI] [PubMed] [Google Scholar]
- Zappavigna S et al (2020) Anti-inflammatory drugs as anticancer agents. Int J Mol Sci 21(7):2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha S et al (2001) Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Can Res 61(24):8617–8623 [PubMed] [Google Scholar]
- Zugazagoitia J et al (2016) Current challenges in cancer treatment. Clin Ther 38(7):1551–1566 [DOI] [PubMed] [Google Scholar]