Abstract
Background
The physiological effects of renin-angiotensin system modulation in acute respiratory distress syndrome (ARDS) remain controversial and have not been investigated in randomized trials. We sought to determine whether angiotensin-II treatment is associated with improved oxygenation in shock-associated ARDS.
Methods
Post-hoc subgroup analysis of the Angiotensin Therapy for High Output Shock (ATHOS-3) trial. We studied patients who met modified Berlin ARDS criteria at enrollment. The primary outcome was PaO2/FiO2-ratio (P:F) at 48-h adjusted for baseline P:F. Secondary outcomes included oxygenation index, ventilatory ratio, PEEP, minute-ventilation, hemodynamic measures, patients alive and ventilator-free by day-7, and mortality.
Results
Of 81 ARDS patients, 34 (42%) and 47 (58%) were randomized to angiotensin-II or placebo, respectively. In angiotensin-II patients, mean P:F increased from 155 mmHg (SD: 69) at baseline to 265 mmHg (SD: 160) at hour-48 compared with no change with placebo (148 mmHg (SD: 63) at baseline versus 164 mmHg (SD: 74) at hour-48)(baseline-adjusted difference: + 98.4 mmHg [95%CI 35.2–161.5], p = 0.0028). Similarly, oxygenation index decreased by − 6.0 cmH2O/mmHg at hour-48 with angiotensin-II versus − 0.4 cmH2O/mmHg with placebo (baseline-adjusted difference: -4.8 cmH2O/mmHg, [95%CI − 8.6 to − 1.1], p = 0.0273). There was no difference in PEEP, minute ventilation, or ventilatory ratio. Twenty-two (64.7%) angiotensin-II patients had sustained hemodynamic response to treatment at hour-3 versus 17 (36.2%) placebo patients (absolute risk-difference: 28.5% [95%CI 6.5–47.0%], p = 0.0120). At day-7, 7/34 (20.6%) angiotensin-II patients were alive and ventilator-free versus 5/47(10.6%) placebo patients. Day-28 mortality was 55.9% in the angiotensin-II group versus 68.1% in the placebo group.
Conclusions
In post-hoc analysis of the ATHOS-3 trial, angiotensin-II was associated with improved oxygenation versus placebo among patients with ARDS and catecholamine-refractory vasodilatory shock. These findings provide a physiologic rationale for trials of angiotensin-II as treatment for ARDS with vasodilatory shock.
Trial Registration: ClinicalTrials.Gov Identifier: NCT02338843 (Registered January 14th 2015).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-023-01227-5.
Keywords: Angiotensin II, Renin–angiotensin system, ARDS, Norepinephrine, Shock, Septic
Introduction
Critically ill patients with acute respiratory distress syndrome (ARDS) frequently have associated vasodilatory shock requiring infusions of vasopressors. Angiotensin-II is a non-catecholamine endogenous hormone of the renin-angiotensin system (RAS) that elicits vasoconstriction. Synthetic angiotensin-II was approved to increase blood pressure in vasodilatory shock after the Angiotensin-II for the Treatment of High-Output Shock-3 (ATHOS-3) trial demonstrated the peptide’s efficacy as a vasopressor in catecholamine-refractory vasodilatory shock [1]. However, the biological and clinical effects of RAS modulation in patients with ARDS are controversial [2].
Inflammatory excess likely contributes to the progression of a meaningful subset of acute lung injuries that produce ARDS [3, 4]. While the angiotensin-II/type-1 receptor (AT1R) signaling axis has well established pro-inflammatory effects in chronic cardiorenovascular disease that could theoretically worsen ARDS, a randomized trial in critically-ill COVID-19 patients ended enrollment early due to high probability that RAS-inhibition caused harm [5]. On the other hand, experimental studies report divergent effects of catecholamines and angiotensin-II on the pulmonary vasculature [6, 7], and in COVID-19 ARDS, several observational studies independently reported increased systemic arterial oxygenation after the initiation of angiotensin-II treatment [8–11]. However, there are no randomized clinical trials of angiotensin-II in ARDS. Thus, the impact of angiotensin-II therapy in ARDS remains uncertain. Yet, determining if and how angiotensin-II impacts pulmonary function in ARDS would have immediate implications for both clinical practice and our understanding of disease biology.
Accordingly, to investigate whether angiotensin-II treatment is associated with clinically relevant effects on pulmonary function, we conducted a post-hoc analysis of ATHOS-3, a phase-III multicenter placebo-controlled, randomized clinical trial of angiotensin-II treatment in catecholamine-refractory vasodilatory shock [1]. We hypothesized that, compared to placebo, among patients with ARDS and refractory shock, angiotensin-II therapy would be associated with improved systemic oxygenation.
Methods
ATHOS-3 trial
The ATHOS-3 trial has been previously described (clinicaltrials.gov identifier NCT 02338843) [1]. Briefly, adults with persistent vasodilatory shock after ≥ 25 mL/kg of volume resuscitation requiring high-dose vasopressors (i.e., norepinephrine-equivalent dose (NED) > 0.2 μg/kg/min) were randomly assigned 1:1 to receive synthetic human angiotensin-II (La Jolla Pharmaceutical Co.) or saline placebo plus standard vasopressors. Randomization was stratified by mean arterial pressure (MAP) at screening and Acute Physiology and Chronic Health Evaluation II (APACHE-II) score. The trial was completed before the COVID-19 pandemic began.
Objectives
The present study reflects a post-hoc analysis of ATHOS-3 in the subset of patients who had ARDS at enrollment that aimed to answer the following questions:
Was angiotensin-II associated with improved oxygenation independent of other differences in ventilatory support in ARDS?
What were the clinical outcomes of ARDS patients treated with angiotensin-II versus placebo?
Patients
The present study included all ATHOS-3 participants who met modified Berlin criteria for ARDS at enrollment [12]. All patients had bilateral opacities on chest imaging, a PaO2/FiO2 ratio (P:F) ≤ 300 mmHg despite mechanical ventilation with ≥ 5 cmH2O of positive end-expiratory pressure (PEEP). To ensure the PEEP criterion was met, we included only patients receiving positive-pressure ventilation at enrollment. We refer to these criteria as modified because a volume overload component of hypoxemia could not be definitively excluded for all cases. However, low output states, defined as cardiac index < 2.3 L/min m2 or central venous oxygenation saturation (ScvO2) < 70% with central venous pressure (CVP) < 8 mmHg were exclusion criteria in ATHOS-3.
Interventions
Study drug infusion was started at 20 ng/kg/min and titrated during the first 3 h to achieve MAP ≥ 75 mmHg while keeping other vasopressor doses constant. Thereafter, study drug and other vasopressors were titrated at treating clinicians’ discretion to maintain MAP between 65 and 75 mmHg. At 48 h, study drug infusion was discontinued according to a protocol-specified tapering process but could be continued for up to 7 days per clinician discretion.
Respiratory physiologic outcomes
The primary aim of this study was to determine whether angiotensin-II treatment was associated with improved oxygenation versus placebo. The primary outcome for this analysis was P:F at 48 h post-treatment. As an additional measure of oxygenation, we determined the oxygenation index (OI), defined:
where mAwP = mean airway pressure. The OI is a validated measure of oxygenation in ARDS that, unlike P:F, also incorporates the level of ventilatory support [13, 14]. Lower OI corresponds to greater oxygenation.
As another measure of pulmonary function, we calculated the ventilatory ratio (VR), which reflects the degree of dead space ventilation [15], defined:
Because improvements in oxygenation could be driven by changes in ventilator management independent of an effect of angiotensin-II, we also assessed PEEP, respiratory rate, tidal volumes, minute ventilation, PaCO2, and mAwP.
All pulmonary and ventilator measures were assessed at baseline, hour-3, and hour-48.
Cardiovascular outcomes
The primary efficacy outcome of the ATHOS-3 trial was treatment response at hour-3, defined as a sustained increase in MAP, either > 75 mmHg or > 10 mmHg above baseline MAP, without an increase in background vasopressors [1]. We additionally assessed the total NED and the MAP over the 48-h study period.
Exploratory vasopressor dose analysis
Experimental studies suggest angiotensin-II augments hypoxic pulmonary vasoconstriction (HPV) [16, 17]. Literature also suggests that catecholamines inhibit HPV [18, 19]. Therefore, an association of angiotensin-II treatment with improved oxygenation could either suggest a direct effect of angiotensin-II (e.g., by augmenting HPV) or an indirect effect of catecholamine dose reduction (e.g., by reducing inhibition of HPV). We explored these hypotheses, summarized in Additional file 1: Fig. S1, as follows.
Under the hypothesis that an association of angiotensin-II treatment with increased oxygenation predominantly reflects a catecholamine-sparing (i.e., indirect) effect, we hypothesized that:
Adjusting for NED will attenuate the association of angiotensin-II with P:F.
NED will be strongly associated with P:F over time.
Conversely, under the hypothesis that an association of angiotensin-II with increased oxygenation predominantly reflects a direct effect of treatment, we instead hypothesized that:
Angiotensin-II treatment should remain associated with P:F after adjustment for NED
NED should not be associated with P:F.
As sensitivity analysis, we performed this analysis first using the total vasopressor dose in norepinephrine-equivalents (NEDTotal), and again using only the catecholamine vasopressor dose (NEDCatechol).
Exploratory clinical outcomes
We anticipated the study to be underpowered to detect differences in clinical outcomes. However, as an exploratory analysis, we tabulated clinical outcomes between groups. Outcomes of interest included by day-7, whether patients were alive and liberated from the ventilator or alive and off vasopressors, respectively, and by day-28, whether patients had died or were alive and discharged from the hospital.
Statistical analysis
Continuous variables are reported as mean (SD) or median (interquartile range) as appropriate, categorical variables as frequency (percent). Missing data management is detailed in Additional file 1: Supplemental Methods. Analyses were performed in SAS (SAS Institute, Cary, NC, USA.)
In the primary analysis, respiratory indices at hour-3 and hour-48 were modeled in a linear regression that included terms for treatment and baseline value of the dependent variable in accordance with best statistical practice for evaluating continuous outcomes in clinical trials [20, 21]. We also report mean differences between hour-48 and baseline measurements by treatment group, with 95%CI and p-values calculated using paired T-tests.
For multivariable analyses, we additionally included prespecified covariates that could potentially confound the outcome. The prespecified covariates were age, sex, BMI, and baseline APACHE-II score, MAP, NED, PEEP, and minute ventilation.
For the exploratory analysis of treatment and NED effects over time, we used longitudinal mixed-effects repeated-measures models. This approach was chosen to assess time-varying associations of NED with oxygenation and to account for within-subject correlation and the sequence of measurements. Individual patients were entered as random-effects and time was treated as a 3-level categorical repeated-measure variable with a compound symmetry covariance structure. The model included fixed-effects for treatment, NED as a continuous variable, and interaction terms for these effects with time. We iterated the model twice, using NEDTotal, and NEDCatechol, respectively. To facilitate intuitive interpretation, least-squares means and errors were constructed and graphed at different levels of NED across timepoints and treatment groups.
For the exploratory clinical outcomes, we report the absolute risk-difference, relative risk, and 95% confidence intervals.
Results
Of the 321 patients enrolled in ATHOS-3, 81 (25.2%) met Berlin criteria for ARDS at enrollment and were included in this study. Among these, 34 (42%) were randomized to angiotensin-II and 47 (58%) to placebo. The baseline characteristics are shown in Table 1. All patients were invasively ventilated at baseline. Sepsis was the likely or definite cause of shock in more than 90% of the cohort. Overall, the angiotensin-II group had more women (64.7%) than the placebo group (34.0%). The groups were otherwise well balanced for age, body-mass index, cause of vasodilatory shock, as well as baseline vasopressor support level, cardiac index, CVP, ScvO2, APACHE-II score, and albumin level.
Table 1.
Baseline and treatment characteristics
| Outcome | Placebo | Angiotensin-II | All Patients |
|---|---|---|---|
| N | 47 | 34 | 81 |
| Demographics and clinical factors | |||
| Age (years) | 60 (17) | 57 (18) | 59 (17) |
| Female—n (%) | 16 (34%) | 22 (65%) | 38 (47%) |
| Body Mass Index (kg/m2) | 30.2 (8.4) | 28.9 (7.5) | 29.6 (8.0) |
| Ideal body weight (kg) | 63.6 (10.6) | 59.1 (10.2) | 61.7 (10.6) |
| Exposure to ACE inhibitors or ARBs—n (%) | 5 (11%) | 6 (18%) | 11 (14%) |
| Cause of vasodilatory shock—n (%) | |||
| Sepsis | 41 (87%) | 28 (82%) | 69 (85) |
| Other—potentially sepsis | 4 (9%) | 4 (12%) | 8 (10%) |
| Other—not sepsis | 2 (4%) | 2 (6%) | 4 (5%) |
| Baseline APACHE II Score | 30.9 (7.9) | 29.1 (8.2) | 30.1 (8.0) |
| Baseline albumin (g/dl) | 2.4 (0.6) | 2.3 (0.8) | 2.3 (0.7) |
| Baseline cardiovascular status | |||
| Mean arterial pressure (mmHg) | 65 (7) | 66 (5) | 65 (6) |
| Average NED in past 6 h (µg/kg/min) | 0.55 (0.32) | 0.54 (0.35) | 0.54 (0.33) |
| Vasopressin use in past 6 h—n (%) | 40 (85%) | 24 (71%) | 64 (79%) |
| Central venous pressure (mmHg) | 13.6 (4.5) | 15.1 (5.3) | 14.3 (4.9) |
| Cardiac Index (L/min/m2) | 3.4 (0.9) | 3.4 (0.7) | 3.4 (0.8) |
| ScvO2 (%) | 78 (7) | 77 (8) | 78 (8) |
| Baseline respiratory status | |||
| PaO2:FiO2 ratio (mmHg) | 148 (63) | 155 (69) | 151 (65) |
| < 100—n (%) | 15 (31.9%) | 9 (27%) | 20 (25%) |
| 100–199—n (%) | 21 (44.7%) | 16 (47%) | 37 (46%) |
| 200–299—n (%) | 11 (23.4%) | 9 (27%) | 24 (30%) |
| PaO2 (mmHg) | 84 (25) | 84 (27) | 84 (26) |
| FiO2 | 0.64 (0.21) | 0.62 (0.25) | 0.63 (0.23) |
| Oxygenation Index (cmH2O/mmHg) | 13.8 (12.8) | 13.3 (11.2) | 13.6 (12.1) |
| mAwP (cmH2O) | 16.5 (5.4) | 17.4 (6.9) | 16.9 (6.0) |
| PEEP (cmH2O) | 10.4 (4.1) | 10.1 (3.3) | 10.3 (3.7) |
| Tidal volume ≤ 8 mL/kg—n (%) | 32 (68%) | 21 (62%) | 53 (65%) |
| PaCO2 (mmHg) | 42 (16) | 44 (13) | 43 (15) |
| pH | 7.286 (0.118) | 7.263 (0.114) | 7.276 (0.116) |
| Minute ventilation (L/min) | 10.8 (4.6) | 10.4 (3.3) | 10.6 (4.1) |
| Ventilatory ratio (L mmHg/min kg) | 1.9 (1.0) | 2.0 (0.8) | 1.9 (0.9) |
| Ventilatory ratio ≥ 2.0—n (%) | 15 (32%) | 11 (32%) | 26 (32%) |
| Additional therapies | |||
| Glucocorticoids—n (%) | 33 (70%) | 25 (74%) | 58 (72%) |
| Neuromuscular blockade—n (%) | 25 (53%) | 19 (56%) | 44 (54%) |
| Pulmonary vasodilators—n (%) | 6 (13%) | 4 (12%) | 10 (12%) |
| Nitric oxide scavengers—n (%) | 1 (2%) | 1 (3%) | 2 (3%) |
| Venovenous-ECMO | 2 (4%) | 2 (6%) | 4 (5%) |
| Treatment characteristics | |||
| Study drug exposure duration (hrs)—median [IQR] | 48 [30, 49] | 47 [38, 49] | 48 [36, 49] |
| Mean study drug dose (ng/kg/min) | 39 (13) | 31 (25) | 35 (19) |
Baseline characteristics of the study population. Results displayed as mean (SD) unless otherwise indicated
ACE angiotensin-converting enzyme, ARB angiotensin receptor blockers, NED norepinephrine equivalent dose, ScvO2 central venous oxygenation saturation, PaO2 arterial partial pressure of oxygen, FiO2 fraction inspired oxygen, mAwP mean airway pressure, PEEP positive end-expiratory pressure, PaCO2 arterial partial pressure of carbon dioxide
Groups had similar baseline P:F, OI, and ventilatory ratio. There were no baseline differences in PEEP, mAwP, minute-ventilation, tidal volume, PaCO2, pH, or the proportion of patients receiving lung-protective ventilation.
Missing data prevalence is summarized in Additional file 1: Table S1; all missing hour-48 P:F data were due to death before hour-48. The fluid volume administered during the study-drug titration period is shown in Additional file 1: Table S2. The duration of study-drug exposure is shown in Additional file 1: Fig. S2.
Respiratory measures
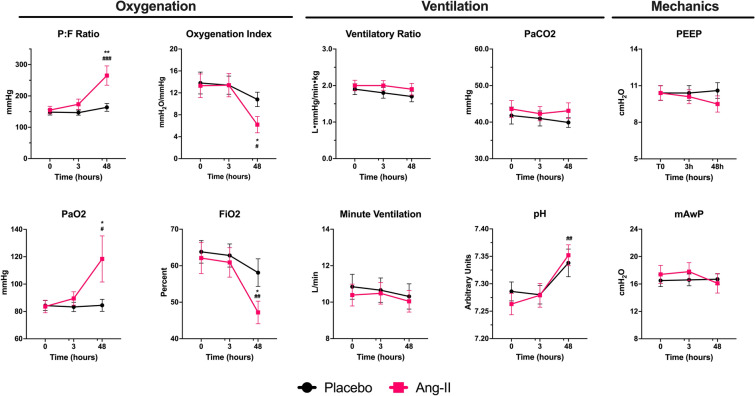
Among ARDS patients treated with angiotensin-II, mean P:F increased by 106 mmHg from 155 mmHg (SD: 69) at baseline to 265 mmHg (SD: 160) at hour-48. In contrast, mean P:F did not change (difference: 8 mmHg) in the placebo group (148 mmHg at baseline versus 164 mmHg at hour-48). In the primary analysis model, baseline-adjusted P:F at hour-48 was 98.4 mmHg higher in angiotensin-II patients versus placebo (95%CI: 35.2–161.5 mmHg, p = 0.0028) (Table 2 and Fig. 1). The increased P:F at hour-48 in the angiotensin-II group was driven by improvement in both PaO2 and FiO2, which were 34.4 mmHg higher (95%CI 1.8–66.9 mmHg, p = 0.0392) and 10% lower (95%CI − 0.18 to − 0.02, p = 0.0200), respectively, than for placebo (Table 2). In the full multivariable model, angiotensin-II treatment was associated with 120.6 mmHg higher P:F at hour-48 than placebo (95%CI 51.0–190.3, p = 0.0010) (Additional file 1: Table S3).
Table 2.
Respiratory measures at hour 48 for angiotensin-II versus placebo
| Outcome | Placebo | Angiotensin-II | |||
|---|---|---|---|---|---|
| Hour-48 | ∆ vs. hour-0 | Hour-48 | ∆ vs. hour-0 | Baseline-adjusted ∆ | |
| Mean (SD) | Mean (SD) [95%CI] p-value |
Mean (SD) | Mean (SD) [95%CI] p-value |
Mean [95%CI] p-value |
|
| Oxygenation | |||||
| P:F (mmHg) | 163.6 (73.7) |
7.9 (84.2) [− 21.5 to 37.3] p = 0.32 |
265.1 (159.8) |
105.9 (172.8) [37.6 to 174.3] p = 0.0006 |
98.4 [35.2 to 161.5] p = 0.0028 |
| PaO2 (mmHg) | 84.5 (25.8) |
− 2.4 (30.4) [− 13.0 to 8.3] p = 0.98 |
118.4 (87.6) |
34.9 (89.1) [− 0.3 to 70.2] p = 0.0322 |
34.4 [1.8 to 66.9] p = 0.0392 |
| FiO2 | 0.58 (0.22) |
− 0.02 (0.24) [− 0.11 to 0.06] p = 0.24 |
0.47 (0.16) |
− 0.12 (0.19) [− 0.20 to − 0.05] p = 0.0092 |
− 0.10 [− 0.18 to − 0.02] p = 0.0200 |
| Oxygenation Index (cmH2O/mmHg) | 10.8 (7.1) |
− 0.4 (8.1) [− 3.6 to 2.7] p = 0.26 |
6.2 (7.0) |
− 6.0 (9.2) [− 10.1 to − 1.9] p = 0.0118 |
− 4.8 [− 8.6 to − 1.1] p = 0.0121 |
| Ventilation | |||||
| Ventilatory ratio | 1.8 (0.6) |
− 0.2 (0.3) [− 0.35 to 0.02] p = 0.42 |
2.0 (0.7) |
− 0.1 (0.4) [− 0.35 to 0.14] p = 0.84 |
0.20 [ − 0.41 to 0.82] p = 0.50 |
| PaCO2 (mmHg) | 39.9 (7.8) |
0.2 (8.8) [− 2.9 to 3.3] p = 0.51 |
43.1 (11.3) |
− 2.0 (8.8) [− 5.5 to 1.4] p = 0.89 |
0.9 [ − 3.5 to 5.3] p = 0.68 |
| pH | 7.338 (0.147) |
0.023 (0.136) [− 0.020 to 0.070] p = 0.0858 |
7.352 (0.099) |
0.059 (0.086) [0.020 to 0.090] p = 0.0021 |
0.010 [− 0.040 to 0.070] p = 0.63 |
| Respiratory rate (breaths/minute) | 23.9 (7.7) |
− 0.3 (6.1) [− 2.29 to 1.71] p = 0.91 |
23.3 (7.9) |
− 0.2 (4.1) [− 1.81 to 1.43] p = 0.81 |
− 1.1 [− 3.8 to 1.5] p = 0.39 |
| Tidal volume (mL) | 425.5 (142.4) |
− 2.9 (75.0) [− 28.7 to 22.8] p = 0.58 |
436.5 (151.2) |
1.7 (79.7) [− 30.4 to 33.9] p = 0.98 |
16.5 [ − 26.9 to 59.9] p = 0.45 |
| Minute ventilation (L/min) | 10.31 (4.2) |
− 0.46 (2.6) [− 1.36 to 0.43] p = 0.59 |
10.04 (2.95) |
− 0.11 (2.49) [− 1.14 to 0.92] p = 0.69 |
− 0.20 [− 1.36 to 0.95] p = 0.73 |
| Mechanics | |||||
| PEEP (cmH2O) | 10.6 (3.9) |
− 0.1 (2.8) [− 0.99 to 0.89] p = 0.86 |
9.5 (3.5) |
− 0.7 (1.9) [− 1.46 to − 0.03] p = 0.36 |
− 0.59 [− 1.61 to 0.43] p = 0.26 |
| mAwP (cmH2O) | 16.7 (4.2) |
− 0.4 (3.8) [− 1.90 to 1.09] p = 0.85 |
16.1 (6.2) |
− 1.5 (2.4) [− 2.67 to − 0.39] p = 0.51 |
− 1.35 [− 3.07 to 0.38] p = 0.12 |
Displays respiratory physiologic measures at hour-48 in Angiotensin-II vs. placebo. The Hour-48 columns show the average measures within the treatment group. The ∆ vs. hour-0 columns show the average difference at Hour-48 versus baseline within the treatment group, displayed as: Hour-48–Hour-0. The 95% CI and p-values in these columns reflect the results of a paired T-test. The baseline-adjusted ∆ column reflects the estimate for difference in means by for Angiotensin-II vs. Placebo groups from the linear model adjusted for the hour-0 value (primary analysis)
∆ difference, SD standard deviation, P:F PaO2/FiO2 ratio, PaO2 arterial partial pressure of oxygen, FiO2 fraction of inspired oxygen, mAwP mean airway pressure, PEEP positive end-expiratory pressure, PaCO2 arterial partial pressure of carbon dioxide
Fig. 1.
Respiratory Measures Over Time for Angiotensin-II versus Placebo. Displays respiratory variables over time by treatment group. Black indicates the placebo group, pink the Ang-II group. Error bars display the SEM. Asterisks display the p-value for the between-treatment group difference at the indicated timepoint, adjusted for the baseline value, from the regression model as follows: *p < 0.05; **p < 0.01; ***p < 0.001. Hashmarks show the within-treatment difference versus the baseline as follows: #p < 0.05; ##p < 0.01; ###p < 0.001. Ang-II angiotensin-II, PEEP positive end-expiratory pressure, mAwP mean airway pressure, SEM standard error of the mean
Similarly, the OI decreased by hour-48 for the angiotensin-II (-6.0 cmH2O/mmHg, SD: 9.2) but not the placebo group (− 0.4 cmH2O/mmHg, SD: 8.1) (baseline-adjusted hour-48 difference: − 4.8 cmH2O/mmHg, [95%CI − 8.6 to − 1.1], p = 0.0273). In the multivariable model (Additional file 1: Table S4), angiotensin-II treatment was numerically but not significantly associated with improved OI at hour-48 (effect-estimate: − 4.2 cmH2O/mmHg [95%CI − 8.7 to 0.4], p = 0.0694).
In both the baseline-adjusted and multivariable models, VR was similar between groups, suggesting no difference in dead space ventilation (Table 2, Additional file 1: Table S5). There were also no significant differences between treatment groups in PEEP, PaCO2, pH, minute-ventilation, lung-protective tidal volume, or mAwP (Fig. 1).
Cardiovascular measures
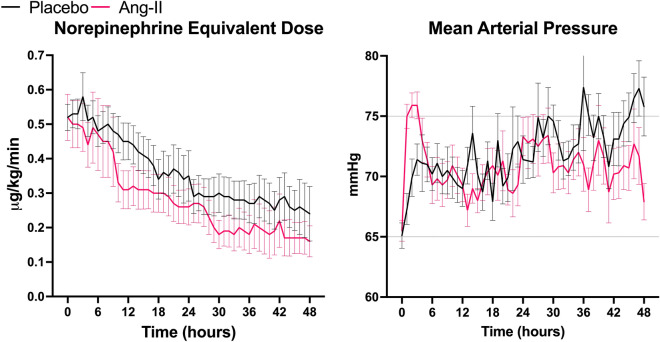
The original ATHOS-3 trial’s primary endpoint, a sustained MAP > 75 mmHg or > 10 mmHg from baseline without an increase in background vasopressor dose at hour-3, was met in 22/34 (64.7%) angiotensin-II and 17/47 (36.2%) placebo patients (absolute risk-difference: 28.5% [95%CI 6.5–47.0], p = 0.0112) (Table 3). The average NED over the 48-h study period was significantly lower in the angiotensin-II group (0.28 µg/kg/min) than the placebo group (0.36 µg/kg/min) (difference: 0.08 µg/kg/min [95%CI 0.05–0.10 µg/kg/min], p < 0.0001). Differences over time in NED and MAP are shown in Fig. 2.
Table 3.
Efficacy and exploratory clinical outcomes for angiotensin-II versus placebo
| Outcome | Placebo | Angiotensin-II | Absolute difference (CI95%) | Relative risk (CI95%) | p-value |
|---|---|---|---|---|---|
| n = 47 | n = 34 | ||||
| Hour-3 | |||||
| Treatment (MAP) response | 17 (36.2%) | 22 (64.7%) | 28.5% (6.5% to 47.0%) | 1.79 (1.14 to 2.82) | p = 0.0112 |
| Hour-48 | |||||
| Alive and ventilator-free | 1 (2.1%) | 0 (0.0%) | − 2.1% (− 12.7% to 10.7%) | 0.98 (0.88 to 1.09) | p = 0.39 |
| Alive and vasopressor-free | 3 (6.4%) | 7 (20.6%) | 14.2% (− 4.3% to 30.8%) | 1.18 (1.00 to 1.49) | p = 0.0551 |
| Mortality | 14 (29.8%) | 7 (20.6%) | − 9.2% (− 30.7% to 9.8%) | 0.69 (0.31 to 1.47) | p = 0.35 |
| Day-7 | |||||
| Alive and ventilator-free | 5 (10.6%) | 7 (20.6%) | 10.0% (− 5.8% to 27.2%) | 1.94 (0.62 to 6.12) | p = 0.21 |
| Alive and vasopressor-free | 18 (38.3%) | 17 (50.0%) | 11.7% (− 9.9% to 32.0%) | 1.44 (0.74 to 2.79) | p = 0.29 |
| Mortality | 25 (53.2%) | 14 (41.2%) | − 12.0% (− 32.0% to 9.7%) | 0.70 (0.36 to 1.34) | p = 0.29 |
| Day-28 | |||||
| Mortality | 32 (68.1%) | 19 (55.9%) | − 12.2% (− 32.3% to 8.7%) | 0.71 (0.40 to 1.26) | p = 0.26 |
| Discharged alive from the hospital | 8 (17.0%) | 7 (20.6%) | 3.6% (− 13.1% to 21.7%) | 1.22 (0.44 to 3.37) | p = 0.68 |
Absolute differences and relative risks are reported for the angiotensin-II group versus placebo. Treatment response refers to the primary outcome of the ATHOS-3 trial, which was a MAP either ≥ 75 mmHg or a ≥ 10 mmHg increase from baseline at hour-3
CI95% 95% Confidence interval, MAP mean arterial pressure
Fig. 2.
Cardiovascular support measures over time for angiotensin-II versus placebo. Displays the hourly total vasopressor dose in norepinephrine equivalents and mean arterial pressure over time by treatment group. Black curves show the placebo group. Pink curves show the Ang-II group. Error bars display the SEM. Ang-II angiotensin-II, SEM standard error of the mean
Exploratory vasopressor dose analysis
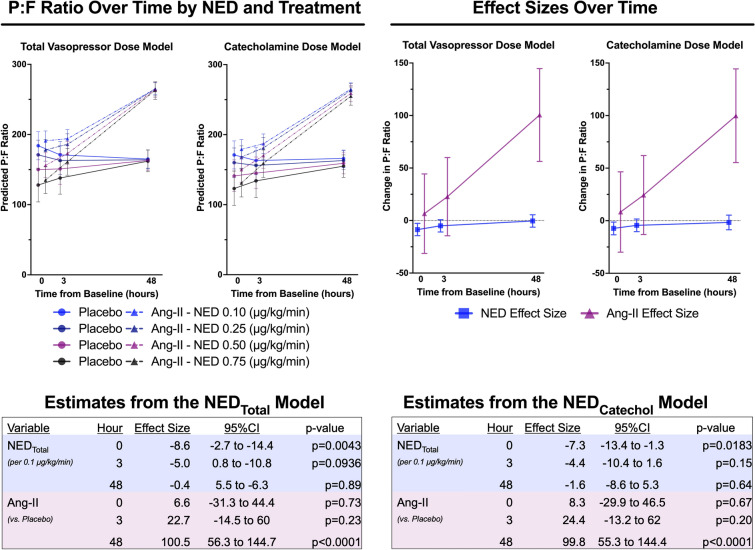
Figure 3 displays the results of the longitudinal models assessing the total and catecholamine-specific vasopressor dose association with oxygenation between treatment groups. All models estimated no difference in P:F at baseline between angiotensin-II and placebo. P:F between groups began to diverge at hour-3 and was significantly higher in the angiotensin-II group than placebo at hour-48 (Fig. 3). This relationship was preserved across adjustments for both NEDTotal and NEDCatechol (Hour-48 effect size: 100 mmHg and p < 0.0001 for both).
Fig. 3.
P:F over time by treatment group and vasopressor support level. Left graphs display the predicted P:F over time by treatment group at varying vasopressor dose levels from the longitudinal mixed-effects repeated measures models. Circle markers with solid lines display model estimates for the placebo group. Triangle markers with dashed lines, the angiotensin-II group. Colors are used to display the vasopressor dose: light blue = 0.10 mcg/kg/min; dark blue = 0.25 mcg/kg/min; purple = 0.50 mcg/kg/min; black = 0.75 mcg/kg/min. Right graphs show the model effect size for angiotensin-II treatment (purple triangles) and NED (blue squares) at each time point. Error bars indicate the 95%CIs. The model estimates for both variables are tabulated with 95%CIs and p-values at the bottom of the figure. Ang-II angiotensin-II, NED norepinephrine equivalent dose, P:F PaO2/FiO2 ratio, 95%CI 95% confidence interval, NEDTotal total vasopressor dose in NED, NEDCatechol total catecholamine dose in NED
In contrast, at baseline, higher NEDTotal was associated with lower P:F, independent of treatment group (NEDTotal: − 8.6 mmHg per 0.1 µg/kg/min [95%CI: − 14.4 to − 2.7], p = 0.0043). The association between NEDTotal and P:F dissipated over time and was not significant at hour-3 or hour-48 (Hour-48 NEDTotal: − 0.4 mmHg per 0.1 µg/kg/min [95%CI − 6.3–5.5], p = 0.89). The same relationship was seen for NEDCatechol. In summary, adjustment for total or catecholamine-specific vasopressor dose did not attenuate the association of angiotensin-II treatment with improved P:F.
Exploratory clinical outcomes
Table 3 displays exploratory clinical outcomes. At day-7, the angiotensin-II group had 7/34 (20.6%) patients alive and liberated from the ventilator versus 5/47 (10.6%) placebo patients (difference: 10.0% [95%CI − 5.8 to 27.2%], p = 0.21). Similarly, 17/34 (50.0%) patients were alive and off vasopressors in the angiotensin-II group versus 18/47 (38.3%) for placebo (difference: 11.7% [95%CI − 9.9 to 32.0%], p = 0.29). By day-28, 19/34 (55.9%) and 32/47 (68.1%) angiotensin-II and placebo patients, respectively, had died while 7/34 (20.6%) and 8/47 (17.0%) were alive and discharged from the hospital.
Sensitivity analyses
Sensitivity analyses for main study findings are shown in Additional file 1: Tables S6–S10, throughout which, angiotensin-II treatment remained associated with improved oxygenation at hour-48.
Discussion
In this post-hoc analysis of the ATHOS-3 trial that compared angiotensin-II treatment to placebo in ARDS patients, angiotensin-II was associated with significantly improved oxygenation within 48-h of treatment initiation among patients with ARDS and vasodilatory shock. Moreover, this increase in oxygenation after angiotensin-II treatment was not attributable to measured differences in ventilator management between groups and was independent of catecholamine dose. Finally, twice as many angiotensin-II patients as placebo patients were alive and ventilator-free by day-7, although the study was underpowered to define the significance of this finding.
Relationship to previous literature
The role of the renin-angiotensin system (RAS) in ARDS remains controversial. Compelling preclinical experiments suggested excess pulmonary AT1R signaling mediates the early development of inflammation-induced and ventilator-induced acute lung injury in rodents [22, 23]. These data led to small trials of RAS antagonism in ARDS and, more recently, larger trials in COVID-19 pneumonia [5, 24–27]. All but one of the COVID-19 studies examined high-dose RAS blockade exclusively within moderate severity disease with very low prevalence of ARDS [25–27]. Therefore, their results cannot be extrapolated to ARDS patients, particularly with concomitant vasodilatory shock. In contrast, the only randomized trial to date of RAS-inhibition in critically-ill COVID-19 patients stopped prematurely for safety [5]. That trial, which compared angiotensin-receptor blockers (ARB) and angiotensin-converting enzyme inhibitors (ACE-i) versus placebo, found > 95% probability of harm from both ARB and ACE-i on organ support-free days, in-hospital mortality, and 90-day survival [5]. Notably, differences in organ support-free days were not driven by vasopressors alone: both RAS-inhibition groups had fewer ventilator-free and respiratory support-free days than placebo (> 90% probability of harm for both). [5]
It is possible that angiotensin-II could benefit patients with ARDS and shock. Several studies in COVID-19 ARDS found improved P:F following angiotensin-II treatment [8–10], although these reports were observational. Another observational study found a similar increase in P:F after angiotensin-II in patients without COVID-19, though this analysis included patients without ARDS [28]. The current analysis demonstrates improved oxygenation after angiotensin-II treatment among non-COVID-19 ARDS patients versus placebo-treated controls.
The mechanism underlying these observations remains uncertain. One possibility is the presence of a relative angiotensin-II deficiency in ARDS. Experimental inflammatory lung injury causes pulmonary endothelial angiotensin-converting enzyme-1 (ACE-1) shedding, which, after transiently increasing systemic angiotensin-II, reduces systemic angiotensin-II levels over time [29, 30]. Several longitudinal biomarker studies report precisely this pattern of RAS dynamics among patients with COVID-19 induced ARDS [31–33]. Small observational studies in non-COVID-19 ARDS also report evidence of endogenous ACE-inhibition [34]. However, why correcting such a deficiency would improve oxygenation is not readily apparent. An anti-inflammatory effect of angiotensin-II elicited through AT2R agonism could be considered, though we note that angiotensin-II has 15-fold higher affinity for AT1R than AT2R [35], making this explanation less likely. Another possibility is that infused angiotensin-II is catalyzed to angiotensin (1–7) by ACE-2, producing anti-inflammatory effects through Mas receptor signaling. Further studies are needed, as the effect of angiotensin-II infusion on non-classical RAS peptide concentrations in ARDS or shock are not known.
Alternatively, or in addition, the relationship between pulmonary vasopressor effects and hypoxic pulmonary vasoconstriction (HPV) may offer insight. Previous literature demonstrates divergent effects of catecholamines and angiotensin-II on the lung vasculature [6, 7]. Norepinephrine, via α1-adrenergic signaling, is a potent pulmonary vasoconstrictor that increases total pulmonary vascular resistance (PVR) disproportionately to systemic vascular resistance (SVR) [36]. Unlike angiotensin-II, norepinephrine constricts both the pulmonary arterial and venous systems. [6]
Under normoxic conditions, lung vasculature is more sensitive to angiotensin-II than the systemic circulation [37], where angiotensin-II acts as an arterial-selective pulmonary vasoconstrictor [6]. Hypoxia, however, alters the intrapulmonary effects of angiotensin-II. Cargil et al. first reported that alveolar hypoxemia lessens the in vivo vasoconstrictive effects of angiotensin-II in human lung [38]. Similarly, Kiley and colleagues showed, in human volunteers, that during normoxia, AT1R-blockade with losartan did not alter mean pulmonary artery pressure (mPAP) or PVR, but during hypoxemia, losartan reduced both mPAP and PVR [16]. In analogous human experiments, AT1R-inhibition with saralasin inhibited hypoxic vasoconstriction [17]. The improved oxygenation in ARDS after angiotensin-II treatment in the ATHOS-3 study could therefore indicate that angiotensin-II augments HPV, increasing oxygenation by improving V/Q matching.
In contrast to angiotensin-II, catecholamines inhibit HPV, increasing V/Q mismatch. Several studies demonstrate that HPV requires α1-adrenergic signaling [18, 39]. These investigations also show that non-specific β-adrenergic blockade heightens the vasoconstrictive response and PVR increase to hypoxemia [39]. Experiments in critically-ill patients and large mammals reported that β-agonists both attenuate HPV and increase shunt fraction. [19, 40, 41]
The interaction of hypoxia and catecholamines raises the possibility that high-dose norepinephrine increases overall pulmonary vasoconstriction while reducing HPV, inhibiting a compensatory mechanism for hypoxemia in ARDS. Sarkar et al. showed in porcine hemorrhagic shock that norepinephrine infusion both doubled the PVR/SVR ratio and reduced P:F by 20%. [42]
This literature led us to query whether the difference in oxygenation trajectories in the ARDS subset of ATHOS-3 between angiotensin-II and placebo was attributable to catecholamine dose-reduction. Supporting this hypothesis, placebo group oxygenation was substantially lower at hour-48, when these patients were receiving 50% higher NED (0.24 µg/kg/min) than the angiotensin-II group (0.16 µg/kg/min). At hour-3, when oxygenation was more similar between groups, placebo group NED (0.58 µg/kg/min) was only 18% higher than the angiotensin-II group (0.49 µg/kg/min) and both groups remained on high catecholamine doses. However, when we analyzed the associations of angiotensin-II treatment and longitudinal vasopressor dose with oxygenation, we found angiotensin-II was robustly associated with improved P:F independent of NED, whereas NED was not significantly associated with P:F. We found identical results in sensitivity analysis of the catecholamine-specific NED. These results are consistent with a direct effect of angiotensin-II to increase oxygenation, and not indirect effects mediated through catecholamine dose reduction.
However, while this analysis suggests a direct effect of angiotensin-II, it does not necessarily implicate HPV. Extrapolating from the nitric oxide literature, improving V/Q matching does not appear inherently disease modifying in ARDS [43, 44]. Improved oxygenation in the treatment arm could alternatively relate to the complex effects of the RAAS on immune regulation [45, 46], or simply represent an epiphenomenon of better treatment of shock.
Implications of study findings
Our study findings imply angiotensin-II improves oxygenation to a clinically relevant degree in patients with ARDS and vasodilatory shock. Prospective clinical trials investigating angiotensin-II as a treatment in ARDS are now needed to validate or refute these findings.
Moreover, these data suggest enhanced oxygenation with angiotensin-II treatment more likely reflects a direct effect of angiotensin-II rather than an effect of catecholamine dose reduction. Thus, our study highlights a need for renewed physiologic investigation into the effect of vasoactive therapies on respiratory gas exchange in ARDS.
Finally, we note that a previous criticism of the ATHOS-3 trial had been the higher prevalence of ARDS in the placebo group [47]. We now show that in that subset of ATHOS-3 patients with ARDS at enrollment, angiotensin-II met the trial’s primary efficacy endpoint and was associated with improved oxygenation. Therefore, higher ARDS prevalence in the control arm of the overall ATHOS-3 cohort cannot explain the favorable outcomes found in the trial’s primary and pre-specified analyses [1, 48]. At a minimum, angiotensin-II appears to be a safe treatment in patients with catecholamine-refractory vasodilatory shock with concomitant ARDS.
Strengths and limitations
The multicenter international structure, randomized treatment allocation, placebo-comparator, and double-blinded design of the ATHOS-3 trial all increase our confidence in these results. However, we also stress that post-hoc subgroup analyses cannot confirm causal effects of treatment [49]. Additionally, increased oxygenation following angiotensin-II infusion was not attributable to differences in ventilator management or baseline severity-of-illness. Initial vasopressor requirements, hemodynamic and respiratory parameters, and APACHE-II scores were well balanced between treatment groups. There was no difference between treatment groups in PEEP, minute-ventilation, or implementation of lung-protective tidal volumes that would explain improved oxygenation among only the angiotensin-treated patients. The similar levels of PEEP during the study period are particularly important: more robust systemic hemodynamics could contribute to improved oxygenation if they facilitated more aggressive PEEP titration and alveolar recruitment. This potential bias seems unlikely in the absence of a difference in PEEP management between groups.
However, several limitations impact this study. First, fluid balance, filling pressures, and serial echocardiography were not measured. The placebo group could have failed to improve oxygenation if they more often developed volume overload. Second, this was a post-hoc analysis of 81 patients which incurs the risk of small-sample bias and other type-I errors [49]. Third, the primary trial protocol was focused on refractory shock and did not protocolize respiratory management. All included patients met criteria for ARDS at baseline but 35% of patients were not receiving low tidal volume ventilation at that time. The frequency of low tidal volume ventilation was similar between treatment groups suggesting this factor does not confound our results, but findings could differ in a population where a higher proportion of patients received lung-protective management. Relatedly, PEEP titration and lung recruitment were also not protocolized, and data on the strategies and timing used for these factors were not available. Data on the ventilation strategy, (e.g., assisted vs. controlled) were also not available). Fourth, collecting respiratory data at hour-3 and hour-48 leaves a significant gap that could coincide with a dynamic part of our subjects’ respiratory course. It was also not known how early or late in an ARDS course patients were. Fifth, VR is an imperfect surrogate for dead space ventilation [15]. Sixth, we applied modified Berlin criteria to select patients and cannot exclude that some patients may have had a fluid overload component of their hypoxemia at baseline. Seventh, prone positioning is an important intervention for severe refractory hypoxemia in ARDS, but data on its implementation were not available. Eighth, this analysis was underpowered to assess patient outcomes in the ARDS subset and we cannot attribute differences in these endpoints to the effects of treatment. Still, whether due to chance or other causes, the fact that twice as many angiotensin-treated as placebo-treated patients were alive and ventilator-free on day-7 provides reassurance that the physiological effects seen were not dissociated from clinical outcomes.
Conclusion
In post-hoc subgroup analysis of an international, multicenter, double-blind randomized trial among patients with ARDS and catecholamine-refractory distributive shock, angiotensin-II was associated with improved oxygenation within 48-h of therapy versus placebo independent of ventilator management. Targeted, experimental studies are now needed in ARDS to determine the mechanisms driving this observation. These findings additionally provide a physiological rationale for prospective clinical trials testing angiotensin-II as a treatment in ARDS.
Supplementary Information
Additional file 1: Figure S1. DAG for Direct vs. Indirect Effect of Ang-II on Oxygenation. Figure S2. Duration of Study Drug Exposure. Table S1: Prevalence of Missing Data. Table S2. Fluid Administration During Study Drug Titration Period. Table S3. Full Multivariable Model for PaO2/FiO2 Ratio at Hour 48. Table S4. Full Multivariable Model for Oxygenation Index at Hour 48. Table S5. Full Multivariable Model for Ventilatory Ratio at Hour 48. Table S6. Sensitivity Analyses Using Different Missing Data Strategies. Table S7. Sensitivity Analyses Excluding Patients with Prior ACEi/ARB Exposure. Table S8. Model for PaO2/FiO2 Ratio at Hour 48 Adjusting for ACEi/ARB Exposure. Table S9. Sensitivity Analyses Excluding Patients with ECMO Exposure. Table S10. PaO2/FiO2 Ratio at Hour 48 According to Baseline Hypoxemia Severity.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ACE-i
Angiotensin-converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- APACHE-II
Acute physiology and Chronic Health Evaluation II score
- AT1R
Angiotensin-II Type-1 Receptor
- ATHOS-3
Angiotensin-II for the Treatment of High-Output Shock-3 (ATHOS-3) trial
- HPV
Hypoxic pulmonary vasoconstriction
- MAP
Mean arterial pressure
- mAwP
Mean airway pressure
- NED
Norepinephrine equivalent dose (in µg/kg/min)
- OI
Oxygenation index
- P:F
PaO2/FiO2 ratio
- PEEP
Positive end-expiratory pressure
- RAS
Renin-angiotensin system
- VR
Ventilatory ratio
Author contributions
Conceptualization: DEL, LSC, CDA, TNH, RB. Study design: DEL, RB. Data acquisition: LSC, TEA, LWB, DWB, AMD, MNG, KRH, AKK, MO, MTM, BTT, JST, RB. Analysis: DEL, DRH. Manuscript (first draft): DEL. Manuscript editing and revision: All authors. All authors have reviewed and approved the final version of this manuscript and agree to be accountable for all aspects of the work.
Funding
Data and statistical analysis support for this study was provided by Innoviva Specialty Therapeutics (Waltham, MA, USA).
Availability of data and materials
The data that support the findings of this study were used under license from La Jolla Pharmaceutical Company for the current study. Data are available from the authors upon reasonable request and with permission of La Jolla Pharmaceutical Company.
Declarations
Ethics approval and consent to participate
The trial was conducted in accordance with Good Clinical Practice guidelines, applicable local regulations, and Declaration of Helsinki principles. The respective independent institutional review boards reviewed the protocol, informed-consent form, and all other documents before study initiation.
Consent to for publication
Not applicable.
Competing interests
DEL, AMD, KRH, and RB declare no competing interests. DRH, CDA, and TNH are employees of Innoviva Specialty Therapeutics, of which La Jolla Pharmaceutical Company (LJPC) is a subsidiary. LSC declares that he was formerly an employee of LJPC. TEA received consulting fees from LJPC. LWB served on the Speaker’s Bureau for LJPC and received consulting fees from LJPC. DWB served on the Speaker’s Bureau for LJPC and received consulting fees from LJPC. MNG received NIH and CDC grants for research unrelated to this study, fees for serving on scientific advisory panel for Philips Healthcare and Endpoint for advice on AI and personalized approach to sepsis, and previously received funding from LJPC for the conduct of the Athos-3 trial. AKK served on the Speaker’s Bureau for LJPC, received consulting fees from LJPC, and received research grant funding from La Jolla Pharmaceutical Company for the ATHOS-3 study and through the Wake Forest Center for Hypertension and Vascular Research for RAAS in septic shock. MTM served on the Speaker’s Bureau for LJPC. MO declares that her institution received research funding from LJPC. BTT declares that during a portion of this research, the author had a financial interest in Direct Biologics, a developer and manufacturer of regenerative biologic products, including an investigational treatment of COVID-19 associated ARDS. These interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. JTT received research grant support and institutional funding from LJPC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 2.Lawler PR, Derde LPG, McVerry BJ, Russell JA, van de Veerdonk FL. The renin–angiotensin system in acute lung injury. Crit Care Med. 2022;50(9):1411–1415. doi: 10.1097/CCM.0000000000005567. [DOI] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawler PR, Derde LPG, van de Veerdonk FL, et al. Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial. JAMA. 2023;329(14):1183–1196. doi: 10.1001/jama.2023.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum MD, Kot PA. Response of pulmonary vascular segments to angiotensin and norepinephrine. J Thorac Cardiovasc Surg. 1972;63(2):322–328. doi: 10.1016/S0022-5223(19)41947-8. [DOI] [PubMed] [Google Scholar]
- 7.Sai Y, Okamura T, Amakata Y, Toda N. Comparison of responses of canine pulmonary artery and vein to angiotensin II, bradykinin and vasopressin. Eur J Pharmacol. 1995;282(1–3):235–241. doi: 10.1016/0014-2999(95)00343-j. [DOI] [PubMed] [Google Scholar]
- 8.Serpa Neto A, Landoni G, Ostermann M, et al. Angiotensin II infusion in COVID-19: an international, multicenter, registry-based study. J Med Virol. 2022 doi: 10.1002/jmv.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leisman DE, Mastroianni F, Fisler G, et al. Physiologic response to angiotensin II treatment for coronavirus disease 2019-induced vasodilatory shock: a retrospective matched cohort study. Crit Care Explor. 2020;2(10):e0230. doi: 10.1097/CCE.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zangrillo A, Landoni G, Beretta L, et al. Angiotensin II infusion in COVID-19-associated vasodilatory shock: a case series. Crit Care. 2020;24(1):227. doi: 10.1186/s13054-020-02928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieruszewski PM, Coleman PJ, Levine AR, et al. Trajectory of PaO. J Intensive Care Med. 2023;38:939. doi: 10.1177/08850666231174870. [DOI] [PubMed] [Google Scholar]
- 12.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 14.Dechert RE, Park PK, Bartlett RH. Evaluation of the oxygenation index in adult respiratory failure. J Trauma Acute Care Surg. 2014;76(2):469–473. doi: 10.1097/TA.0b013e3182ab0d27. [DOI] [PubMed] [Google Scholar]
- 15.Sinha P, Calfee CS, Beitler JR, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiely DG, Cargill RI, Lipworth BJ. Acute hypoxic pulmonary vasoconstriction in man is attenuated by type I angiotensin II receptor blockade. Cardiovasc Res. 1995;30(6):875–880. doi: 10.1016/S0008-6363(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 17.Kiely DG, Cargill RI, Lipworth BJ. Angiotensin II receptor blockade and effects on pulmonary hemodynamics and hypoxic pulmonary vasoconstriction in humans. Chest. 1996;110(3):698–703. doi: 10.1378/chest.110.3.698. [DOI] [PubMed] [Google Scholar]
- 18.Porcelli RJ, Cutaia MV. Pulmonary vascular reactivity to biogenic amines during acute hypoxia. Am J Physiol. 1988;255(2 Pt 2):H329–H334. doi: 10.1152/ajpheart.1988.255.2.H329. [DOI] [PubMed] [Google Scholar]
- 19.Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC, Stickland MK. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol (1985). 2012;113(4):541–8. doi: 10.1152/japplphysiol.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials. 2011;12:264. doi: 10.1186/1745-6215-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell F. Biostatistics for biomedical research. Vanderbilt Institute for Clinical and Translational Research; 2022. https://hbiostat.org/bbr/change.html.
- 22.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao X, Krenn K, Tripp T, et al. Tidal volume-dependent activation of the renin–angiotensin system in experimental ventilator-induced lung injury. Crit Care Med. 2022;50(9):e696–e706. doi: 10.1097/CCM.0000000000005495. [DOI] [PubMed] [Google Scholar]
- 24.Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puskarich MA, Ingraham NE, Merck LH, et al. Efficacy of losartan in hospitalized patients with COVID-19-induced lung injury: a randomized clinical trial. JAMA Netw Open. 2022;5(3):e222735. doi: 10.1001/jamanetworkopen.2022.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Self WH, Shotwell MS, Gibbs KW, et al. Renin-angiotensin system modulation with synthetic angiotensin (1–7) and angiotensin II type 1 receptor-biased ligand in adults with COVID-19: two randomized clinical trials. JAMA. 2023;329(14):1170–1182. doi: 10.1001/jama.2023.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieruszewski PM, Coleman PJ, Levine AR, et al. Trajectory of PaO2/FiO2 ratio in shock after angiotensin II. J Intensive Care Med. 2023;38:939. doi: 10.1177/08850666231174870. [DOI] [PubMed] [Google Scholar]
- 29.Nukiwa T, Matsuoka R, Takagi H, Ishii Y, Arai T, Kira S. Responses of serum and lung angiotensin-converting enzyme activities in the early phase of pulmonary damage induced by oleic acid in dogs. Am Rev Respir Dis. 1982;126(6):1080–1086. doi: 10.1164/arrd.1982.126.6.1080. [DOI] [PubMed] [Google Scholar]
- 30.Orfanos SE, Armaganidis A, Glynos C, et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102(16):2011–2018. doi: 10.1161/01.cir.102.16.2011. [DOI] [PubMed] [Google Scholar]
- 31.Leisman DE, Mehta A, Thompson BT, et al. Alveolar, endothelial, and organ injury marker dynamics in severe COVID-19. Am J Respir Crit Care Med. 2022;205(5):507–519. doi: 10.1164/rccm.202106-1514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akin S, Schriek P, van Nieuwkoop C, et al. A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity. J Hypertens. 2022;40(3):606–614. doi: 10.1097/HJH.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkan S, Cakmak F, Konukoglu D, et al. Efficacy of serum angiotensin II levels in prognosis of patients with coronavirus disease 2019. Crit Care Med. 2021;49(6):e613–e623. doi: 10.1097/CCM.0000000000004967. [DOI] [PubMed] [Google Scholar]
- 34.Krenn K, Höbart P, Poglitsch M, Croizé A, Ullrich R. Equilibrium angiotensin metabolite profiling in patients with acute respiratory distress syndrome indicates angiotensin-converting enzyme inhibition. Am J Respir Crit Care Med. 2020;202(10):1468–1471. doi: 10.1164/rccm.201912-2504LE. [DOI] [PubMed] [Google Scholar]
- 35.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121(7):297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 36.Schreuder WO, Schneider AJ, Groeneveld AB, Thijs LG. Effect of dopamine vs norepinephrine on hemodynamics in septic shock. Emphasis on right ventricular performance. Chest. 1989;95(6):1282–1288. doi: 10.1378/chest.95.6.1282. [DOI] [PubMed] [Google Scholar]
- 37.Lipworth BJ, Dagg KD. Vasoconstrictor effects of angiotensin II on the pulmonary vascular bed. Chest. 1994;105(5):1360–1364. doi: 10.1378/chest.105.5.1360. [DOI] [PubMed] [Google Scholar]
- 38.Cargill RI, Lipworth BJ. Acute effects of hypoxaemia and angiotensin II in the human pulmonary vascular bed. Pulm Pharmacol. 1994;7(5):305–310. doi: 10.1006/pulp.1994.1036. [DOI] [PubMed] [Google Scholar]
- 39.Porcelli RJ, Viau A, Demeny M, Naftchi NE, Bergofsky EH. Relation between hypoxic pulmonary vasoconstriction, its humoral mediators and alpha-beta adrenergic receptors. Chest. 1977;71(2 suppl):249–251. doi: 10.1378/chest.71.2.249. [DOI] [PubMed] [Google Scholar]
- 40.Furman WR, Summer WR, Kennedy TP, Sylvester JT. Comparison of the effects of dobutamine, dopamine, and isoproterenol on hypoxic pulmonary vasoconstriction in the pig. Crit Care Med. 1982;10(6):371–374. doi: 10.1097/00003246-198206000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Lejeune P, Naeije R, Leeman M, Melot C, Deloof T, Delcroix M. Effects of dopamine and dobutamine on hyperoxic and hypoxic pulmonary vascular tone in dogs. Am Rev Respir Dis. 1987;136(1):29–35. doi: 10.1164/ajrccm/136.1.29. [DOI] [PubMed] [Google Scholar]
- 42.Sarkar J, Golden PJ, Kajiura LN, Murata LA, Uyehara CF. Vasopressin decreases pulmonary-to-systemic vascular resistance ratio in a porcine model of severe hemorrhagic shock. Shock. 2015;43(5):475–482. doi: 10.1097/SHK.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 44.Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014;42(2):404–412. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 45.Leisman DE, Privratsky JR, Lehman JR, et al. Angiotensin II enhances bacterial clearance via myeloid signaling in a murine sepsis model. Proc Natl Acad Sci USA. 2022;119(34):e2211370119. doi: 10.1073/pnas.2211370119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crowley SD, Rudemiller NP. Immunologic effects of the renin–angiotensin system. J Am Soc Nephrol. 2017;28(5):1350–1361. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey JD, Semler MW. Renin, angiotensin II, and the journey to evidence-based individual treatment effects. Am J Respir Crit Care Med. 2020;202(9):1209–1211. doi: 10.1164/rccm.202007-2731ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellomo R, Forni LG, Busse LW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. A clinical trial. Am J Respir Crit Care Med. 2020;202(9):1253–1261. doi: 10.1164/rccm.201911-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallach JD, Sullivan PG, Trepanowski JF, Sainani KL, Steyerberg EW, Ioannidis JP. Evaluation of evidence of statistical support and corroboration of subgroup claims in randomized clinical trials. JAMA Intern Med. 2017;177(4):554–560. doi: 10.1001/jamainternmed.2016.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. DAG for Direct vs. Indirect Effect of Ang-II on Oxygenation. Figure S2. Duration of Study Drug Exposure. Table S1: Prevalence of Missing Data. Table S2. Fluid Administration During Study Drug Titration Period. Table S3. Full Multivariable Model for PaO2/FiO2 Ratio at Hour 48. Table S4. Full Multivariable Model for Oxygenation Index at Hour 48. Table S5. Full Multivariable Model for Ventilatory Ratio at Hour 48. Table S6. Sensitivity Analyses Using Different Missing Data Strategies. Table S7. Sensitivity Analyses Excluding Patients with Prior ACEi/ARB Exposure. Table S8. Model for PaO2/FiO2 Ratio at Hour 48 Adjusting for ACEi/ARB Exposure. Table S9. Sensitivity Analyses Excluding Patients with ECMO Exposure. Table S10. PaO2/FiO2 Ratio at Hour 48 According to Baseline Hypoxemia Severity.
Data Availability Statement
The data that support the findings of this study were used under license from La Jolla Pharmaceutical Company for the current study. Data are available from the authors upon reasonable request and with permission of La Jolla Pharmaceutical Company.