Version Changes
Revised. Amendments from Version 2
This version of our article incorporates valuable suggestions from the reviewer, including minor corrections and changes to the existing content. The abstract has been modified. A brief introduction about natural products and isoflavones has been added. Additionally, a few references have been added, updating the bibliography.
Abstract
This review was aimed at summarizing the cellular and molecular mechanisms behind the various pharmacological actions of biochanin-A. Many studies have been reported claiming its application in cancers, metabolic disorders, airway hyperresponsiveness, cardiac disorders, neurological disorders, etc. With regard to hormone-dependent cancers like breast, prostate, and other malignancies like pancreatic, colon, lung, osteosarcoma, glioma that has limited treatment options, biochanin-A revealed agreeable results in arresting cancer development. Biochanin-A has also shown therapeutic benefits when administered for neurological disorders, diabetes, hyperlipidaemia, and other chronic diseases/disorders. Isoflavones are considered phenomenal due to their high efficiency in modifying the physiological functions of the human body. Biochanin-A is one among the prominent isoflavones found in soy (glycine max), red clover (Trifolium pratense), and alfalfa sprouts, etc., with proven potency in modulating vital cellular mechanisms in various diseases. It has been popular for ages among menopausal women in controlling symptoms. In view of the multi-targeted functions of biochanin-A, it is essential to summarize it's mechanism of action in various disorders. The safety and efficacy of biochanin-A needs to be established in clinical trials involving human subjects. Biochanin-A might be able to modify various systems of the human body like the cardiovascular system, CNS, respiratory system, etc. It has shown a remarkable effect on hormonal cancers and other cancers. Many types of research on biochanin-A, particularly in breast, lung, colon, prostate, and pancreatic cancers, have shown a positive impact. Through modulating oxidative stress, SIRT-1 expression, PPAR gamma receptors, and other multiple mechanisms biochanin-A produces anti-diabetic action. The diverse molecular mechanistic pathways involved in the pharmacological ability of biochanin-A indicate that it is a very promising molecule and can play a major impact in modifying several physiological functions.
Keywords: Biochanin-A, Isoflavones, Mechanism, Cancer, Diabetes, Neuroprotection, Cardiovascular, Anti-oxidant
1. Introduction
Traditionally, going back to nature has always been an answer, and in reality, natural products have been essential in the development of new drugs, particularly for infectious and cancerous disorders ( Atanasov et al., 2021). Natural products have been a rich source of new drugs for centuries. ‘Anticancer drugs such as Taxol ( Taxus brevifolia) and Vinblastine ( Catharanthus roseus) and antimalarial drugs such as quinine ( Cinchona spp.) and Artemisinin (Artemisia annua)’ were all discovered from natural products and have been effective in treating these diseases ( Singh et al., 2021).
Phytoestrogens are naturally occurring nonsteroidal phenolic plant compounds that can be categorized into flavonoids and non-flavonoids ( Boyle et al., 2003). Flavonoids encompass isoflavones, coumestans, and prenylflavonoids, while non-flavonoids comprise lignans. The major isoflavones are genistein (7,4′-dihydroxy-6-methoxyisoflavone), daidzein (7,4′-dihydroxyisoflavone), glycitein (7,4′-dihydroxy-6-methoxyisoflavone), biochanin-A (5,7-dihydroxy-4′-methoxyisoflavone), and formononetin (7-hydroxy-4′-methoxyisoflavone). Isoflavones, renowned for their estrogenic properties, are predominantly found in legumes from the Fabaceae family ( Dixon and Sumner, 2003), with soybean (Glycine max) as a source of daidzein, genistein, glycitein, and red clover (Trifolium pratense) as a source of formononetin and biochanin-A.
Isoflavones predominantly found in soybean and soybean products serve as major dietary sources for humans. Isoflavones are recognized for their chemoprotective properties, offering an alternative therapeutic approach for various hormonal disorders, including breast cancer, prostate cancer, cardiovascular diseases, osteoporosis, and menopausal symptoms ( Křížová et al., 2019). Biochanin-A, a phytochemical obtained from soy, alfalfa sprouts, red clover plants, chickpeas, etc., has recently gained attention in research due to its various pharmacological applications ( Ferrer and Thurman, 2013). It can benefit humans in various systems. Biochanin-A has been tested for its effect in various cancers, inflammation, osteoarthritis, metabolic disorders, cardiovascular diseases, anti-oxidant properties, hormone-dependent diseases, etc ( Sehdev, Lai and Bhushan, 2009; Kole et al., 2011; Szliszka et al., 2013; Bhardwaj et al., 2014; D. Q. Wu et al., 2014). Biochanin-A has been shown to have a potential neuroprotective impact by modulating multiple critical neurological pathways. Further, biochanin-A is a chief phytoconstituent of the red clover plant, which is well known for alleviating menopause symptoms through its oestrogenic and antioxidant properties ( Romm et al., 2010; Raheja et al., 2018). Patients with prostate and breast malignancies have proven to show a defensive effect from an isoflavone-rich diet with evidence to various epidemiological studies and the mechanism behind such an action is by isoflavone and oestrogen receptor binding resulting in osteoprotective actions ( Messina and Hilakivi-Clarke, 2009; Shu et al., 2009; Lecomte et al., 2017). Common dietary mixtures have gained consideration because of their synergistic impacts with several anticancer drugs seen in different kinds of malignancy. The pleiotropic effects of isoflavones on tumour cells work through modulation of various cellular signalling pathways. As indicated by various in vitro investigations, genistein is more powerful than biochanin-A as far as both oestrogenic activity and cancer prevention ability are concerned ( Ullah et al., 2009). However, biochanin-A is effectively changed over to genistein in first-pass digestion, and genistein can be identified in human plasma after treatment with biochanin-A ( Setchell et al., 2001).
Administration of isoflavones is known to produce a significant pharmacokinetic problem identified with their deprived bioavailability ( Passamonti et al., 2009). Isoflavones are non-nutrient plant components and a subclass of flavonoids. They principally exist as β-glucosides ( Panche, Diwan and Chandra, 2016). Higher content of biochanin-A is found in red clover plants ( Lemežienė et al., 2015). The harmful clastogenic effect presented in genistein is less exhibited in biochanin-A, adding to its more clinical acceptance ( Snyder and Gillies, 2002; Michael McClain et al., 2006). Mutagenicity related to geinstein use is not presented in biochanin-A associated studies. The low incidence of cancer exhibited in the Asian population has been a widely discussed topic globally ( Jin et al., 2016; Tran et al., 2018). It has a close connection to the soy-rich diet of the population ( Hawrylewicz, Zapata and Blair, 1995; He and Chen, 2013; Wei et al., 2017; Ziaei and Halaby, 2017). Soy isoflavones have significance over selective oestrogen receptor modulators (SERMs) and hormone replacement in breast cancer treatment as they possess oestrogen-like rings in their structure ( Sarkar et al., 2009). Though there are no conclusive reports, isoflavones are preferred by women due to their best safety profile and improved quality of life in comparison with hormone replacement therapy to treat menopausal symptoms ( Franco et al., 2016; Ahsan and Mallick, 2017; Chen, Ko and Chen, 2019). Thus, this review was focused on summarizing the pharmacological applications of biochanin-A along with the various cellular and molecular pathways involved in it. The deprived water solubility along with minimal oral bioavailability confined the application of biochanin-A as a drug molecule. Nano-sized biochanin-A phospholipid complex “nBCA-PLCs” have the potential to overcome this limitation and boost its oral bioavailability. When compared to other formulations such as normal biochanin-A phospholipid complex and the suspension of biochanin-A, nBCA-PLCs have relatively higher bioavailability ( Singh et al., 2021). There is compelling evidence that biochanin-A is a bioactive compound with a wide range of biological and pharmacological activities. To help understand the beneficial and myriad therapeutic effects of biochanin-A, our review evaluated past and current findings of the literature and proposed molecular mechanisms behind various disorders such as different types of cancers, diabetes, airway and cardiovascular disorders, neurological disorders etc.
2. Biochanin-A and its pharmacological benefits
2.1 Cancer
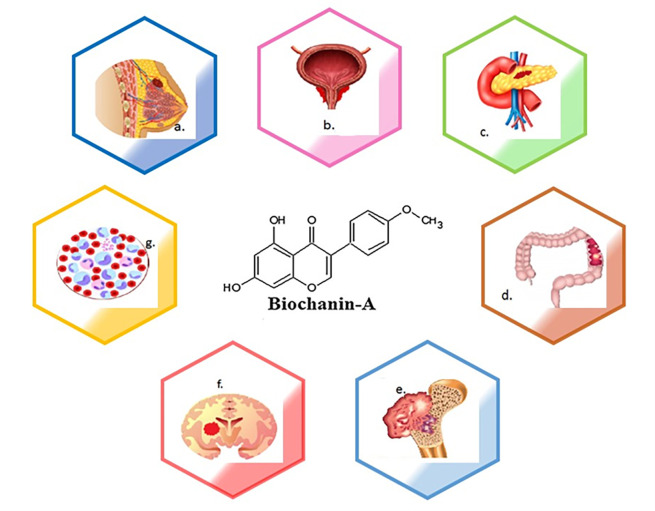
Biochanin-A has prevented/inhibited the development of different types of cancers. Figure 1 depicts the types of cancers where biochanin-A has an impact in controlling the disease. Various kinds of cancer are a) breast cancer, b) prostate cancer, c) lung cancer, d) pancreatic cancer, e) colon cancer, f) osteosarcoma, g) glioma, h) leukaemia. The molecular structure of biochanin-A is depicted in the centre of diagram ( Feng and Lai, 2023).
Figure 1. The schematic diagram represents pharmacological actions of biochanin-A in different types of cancer.
Biochanin-A has prevented the development of different types of cancers. a) breast cancer, b) prostate cancer, c) lung cancer, d) pancreatic cancer, e) colon cancer, f) osteosarcoma, g) glioma, h) leukaemia. The molecular structure of biochanin-A is depicted in the centre of diagram.
2.1.1 Breast cancer
Breast cancer is the most widely reported form of cancer, presented with several subtypes and varying vulnerability to anti-cancer agents. Tumour cells have shown a unique pattern in terms of uncontrolled growth, dedifferentiated morphology, and resistance to apoptosis ( Baba and Câtoi, 2007). In most types of solid cancers like breast cancer, the normal signalling pathways are interrupted, stimulating refractory growth, no cell death, and progressive invasion of neighbouring tissues. Soy-rich diets in controlling hormone-dependent cancers have gained wide attention lately ( Shu et al., 2009; Kang et al., 2010; Varinska et al., 2015). Good levels of isoflavones in serum serve as protection from the risk of breast cancer ( Kang et al., 2010). Various studies have been tested for the effect of biochanin-A being one of the most beneficial constituents of red clover and soy isoflavones on breast cancer. Unlike chemical agents such as chemotherapeutic agents, isoflavones have shown zero toxicity to humans ( Pop et al., 2008).
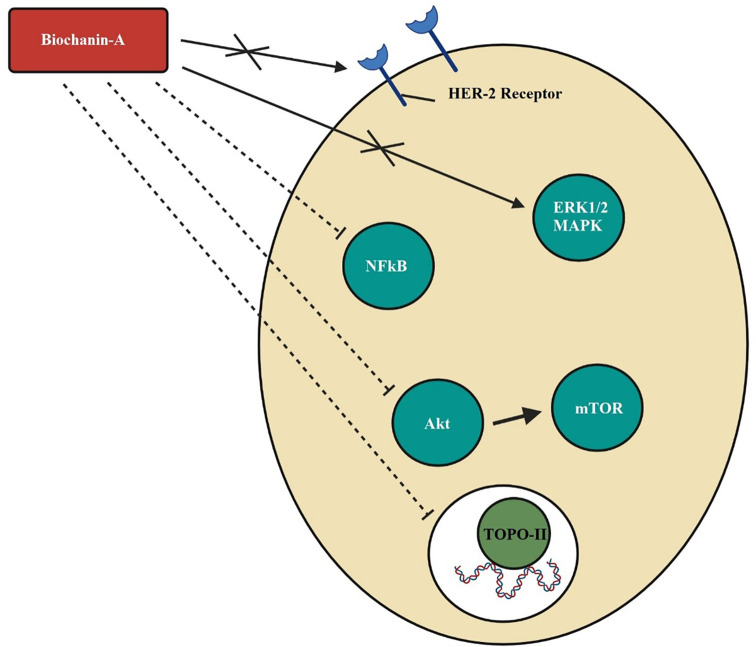
With the influence of biochanin-A, HER2 receptor activation is inhibited, resulting in blockade of downstream signalling pathways of cancer cell development, viability, and metastasis. In HER2-positive breast cancer, the transcriptional unit nuclear factor (NF)-κB is suppressed ( Sehdev, Lai and Bhushan, 2009). MAPK or ERK 1/2 phosphorylation is inhibited causing the poor mitogenic effect ( Sehdev, Lai and Bhushan, 2009; Bhardwaj et al., 2014). The major downstream signalling pathway Akt is dephosphorylated, consequently down-regulates the mTOR signalling pathway which regulates the cell cycle in SK-BR-3 breast cancer cells ( Sehdev, Lai and Bhushan, 2009). MMP-9 enzyme which facilitates metastasis of cancer cells using the extracellular matrix is repressed in SK-BR-3 cells treated with biochanin-A ( Sehdev, Lai and Bhushan, 2009). Flavonoids, having structural similarity to oestrogens, enable oestrogen receptor binding, and possess anti-oestrogenic and oestrogenic properties. Phytoestrogen inhibits oestrogen alpha receptors hence effective in oestrogen receptor-based treatment for breast cancer ( Collins, McLachlan and Arnold, 1997; Le Bail et al., 1998). Reducing endogenous oestrogen levels in the body by inhibition of enzymes such as HSD and Cyp19 would defend against breast cancer. Phytoestrogen intake decreases oestrogen biosynthesis and prolongs menstrual cycle length thereby decreases lifetime exposure to oestrogen ( Mense et al., 2008). Biochanin-A which is an AhR activator act as a cell cycle apoptotic stimulator, inhibiting DMBA (7,12 Dimethylbenz [a]anthracene) which can implicate in hormone-dependent cancer therapy and prevention ( Han, Ji and Hye, 2006; Medjakovic and Jungbauer, 2008). Biochanin-A inhibits CYP19 and negatively affects the synthesis of oestrogen in the body which enhances the anti-oestrogenic property in hormone-influenced cancer such as prostate cancer and breast cancer ( Mense et al., 2008). Biochanin-A when combined with genistein and daidzein, significantly reduced Cyp19 enzyme activity and eliminated transcription of Cyp19 mRNA ( Rice, Mason and Whitehead, 2006). Biochanin-A blocks the cell proliferation in ER+ve MCF7 breast cancer cells ( Collins, McLachlan and Arnold, 1997). Oestradiol inhibition of biochanin-A at half maximal inhibitory concentration (IC 50) dose is found with the highest inhibitory effect to 3-galactosidase action. The anti-oestrogenic activity of biochanin-A follows a mechanism analogous to tamoxifen ( Collins, McLachlan and Arnold, 1997). Topoisomerase II inhibition affects DNA replication. Biochanin-A through Topoisomerase II inhibition prevented the mammary tumor growth in N-nitro -N-methyl urea treated rat, interleukin 2-dependent CTLL-2 cells ( Azuma et al., 1995; Gotoh et al., 1998). Biochanin-A has shown synergism with 5-fluorouracil (5FU) in oestrogen receptor (ER) positive breast cancer cell lines such as MCF7, and triple-negative breast cancer cells such as MDA-MB231. The combination of 5FU and biochanin-A producing a synergistic anti-tumour effect partly attributed to the inhibitory capability of biochanin-A through ER-α/Akt ( Mahmoud et al., 2022). The mechanism of action of biochanin-A in breast cancer by dephosphorylation of HER-2 receptor and MAPK or ERK1/2 causing blockade of cancer cell development, growth, metastasis, and mitogenesis, the inhibition of Akt phosphorylation and downregulation of mTOR signals that disrupt the cell cycle, the inhibition of NF-kB and interrupted transcription and blockade of topoisomerase-II and DNA replication is depicted in Figure 2.
Figure 2. Mechanism of action of biochanin-A in breast cancer.
Biochanin-A dephosphorylates HER-2 receptor and MAPK or ERK1/2 causing blockade of cancer cell development, growth, metastasis, and mitogenesis. Biochanin-A inhibits Akt phosphorylation thereby downregulates mTOR signals and disrupts the cell cycle. It inhibits NFkB and interrupts transcription. It inhibits topoisomerase-ll and DNA replication ( Azuma et al., 1995; Gotoh et al., 1998; Sehdev, Lai and Bhushan, 2009; Bhardwaj et al., 2014).
2.1.2 Prostate cancer
Prostate cancer has been reported as a commonly found cancer in men and the second prominent death reason in western countries ( Siegel, Miller and Jemal, 2020). Diet can influence the prostate carcinogenesis process ( Bostwick et al., 2004). Isoflavone contained dietary intake had presented with an association of reduced prostate cancer risk in different countries ( Jacobsen, Knutsen and Fraser, 1998; Applegate et al., 2018).
Biochanin-A elevates the level of testosterone-UDPGT (Uridine 5′-diphospho-glucuronosyltransferase) enzyme activity and disrupts the androgen metabolism in connection with UDP-glucuronic acid. PLK-1 (Polo-like kinase-1) is responsible for various cell cycles activities such as Cdc2 (Cyclin-dependent kinase) stimulation and mitosis. Biochanin-A induces p21 which is a negative regulator for PLK-1 leading to prostate cancer cell apoptosis ( Seo et al., 2011). The EGF (epidermal growth factor)-stimulated growth of cell lines, such as DU-145 and LNCaP prostatic cancer, were inhibited by biochanin-A without affecting its autophosphorylation. Biochanin-A has shown inhibitory behaviour in prostate cancer by antagonizing tyrosine kinase events within the signal transduction pathway ( Peterson and Barnes, 1993). The level of testosterone and development of prostate carcinoma in Lobund-Wistar rats were influenced through a soy-rich diet, signifying preventive action of soy isoflavones in prostate cancer ( Pollard, Wolter and Sun, 2000). Using the orthotopic prostate tumour animal model, the influence of soy proteins on cancer advancement has been studied. PSA androgen sensitivity, and cancer metastasis was inhibited significantly by different soy-derived compounds ( Zhou et al., 2002). ER-β, when activated in prostate cells, inhibits cell proliferation, and exerts anti-cancer effects. Red clover supplemented diet in mice exhibited increased ER-β and E-cadherin levels leading to the disruption of cell morphology and cancer formation ( Slater, Brown and Husband, 2002). Biochanin-A in a LNCaP cell line induced apoptosis incorporated by 3H-thymidine with increased DNA fragmentation, low p21, and cyclin B expression. Animal study with LNCaP xenografts, biochanin-A subsided the prostate cancer load and size of the tumour ( Rice et al., 2002). The prostatic androgen 5α-dihydrotestosterone synthesized using a 5α-reductase enzyme which is responsible for prostate cell development and function has been influenced by biochanin-A, thus generate a role in the prevention of prostate malignancy ( Evans, Griffiths and Morton, 1995).
Phytoestrogens have been predicted as compounds liable for chemoprotective activity on prolonged exposure. Prostatic cell proliferation in PC-3, LNCaP, and DU145 were inhibited by biochanin-A with variable mechanisms ( Hempstock, Kavanagh and George, 1998). Aromatase enzyme has an impact on the level of oestrogen. Biochanin-A upon competitive inhibitory action on aromatase minimizes oestrogen level and exhibit anti-cancer activity ( Campbell and Kurzer, 1993). TRAIL-induced cell death is an epitome in cancer prevention. Biochanin-A through deactivating NF-kB and death receptor (DR) 4/5 mediated caspases causes TRAIL-associated cell death in prostatic cancer cell lines ( Szliszka et al., 2013). Increased activity of UDP-glucuronosyltransferase (UDGPT) is found in biochanin-A-exposed LNCaP cells. It enhanced intracellular glucuronidation of testosterone, steroid UDGPT transcript, and lowered prostate-specific antigen (PSA), hence showed an effect in prostate cancer prevention ( Sun et al., 1998). The patients with clinically significant prostate cancer were treated with red clover isoflavones such as biochanin-A before surgical intervention. The apoptosis markers in prostate tumor cells from radical prostatectomy specimens were analysed. Markedly, higher apoptosis was found in the treatment group with a dietary supplement of isoflavones, indicating cessation of prostate cancer progression in low to moderate-grade malignancy ( Jarred et al., 2002).
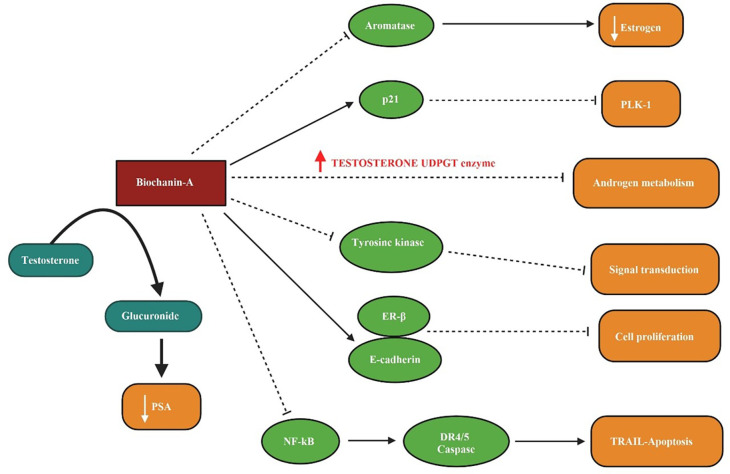
In Figure 3 the inhibition of aromatase enzyme and lowering of oestrogen level by biochanin-A, the activation of p21 and antagonization of PLK-1 action, increased level of testosterone UDGPT enzyme disrupting androgen metabolism with the treatment of biochanin-A, the interrupted level of tyrosine kinase blocking the signal transduction, the prompted ER- β and E-cadherin level leading to the inhibition of cell proliferation, via biochanin-A inhibition of NF-kB inducing the TRAIL associated apoptosis and the increased conversion of testosterone into glucuronide resulting low appearance of prostate-specific antigen (PSA) is illustrated.
Figure 3. Biochanin-A on prostate cancer.
Biochanin-A inhibits aromatase enzyme and decreases estrogen levels. It activates p21 and antagonizes PLK-1 action. Increased level of testosterone UDGPT enzyme disrupts androgen metabolism with the treatment of biochanin-A. The level of tyrosine kinase is interrupted and inhibits signal transduction. It induces ER-β and E-cadherin and inhibits cell proliferation. Biochanin-A inhibits NF-kB and induced TRAIL associated apoptosis. It increased conversion of testosterone into glucuronide resulting low appearance of prostate-specific antigen (PSA) ( Sun et al., 1998; Seo et al., 2011; Szliszka et al., 2013).
2.1.3 Lung cancer
Cancer affecting the most vital body parts elucidate the difficulty of its therapy. Lung cancer has often been reported and is one of the supreme death causes in cancer patients. The fight against cancer with phytochemicals will benefit mankind greatly. Studies have been carried out on soy isoflavones, utilized as an integral system for the treatment and to boost the radiation viability on lung tumors ( Hillman and Singh-Gupta, 2011; Singh-Gupta et al., 2011). Soy-rich foods may reduce the occurrence of cancer development in the general population, according to epidemiology research ( Yang et al., 2011). Like genistein which has proven its effectiveness in cancer, biochanin-A displays anti-cancer properties in lung tissue.
The mechanism with which soy isoflavones boost radiation therapeutic effect is through inhibiting APE1/Ref-1 DNA repair in A549 cells, which leads to cell killing ( Singh-Gupta et al., 2011). Soy isoflavones exhibited a synergistic effect and significantly improved the radiation-induced cell killing. in vitro observation on biochanin-A treatment in 95D and A549 lung cancer cells revealed that the level of P21 (cyclin dependent kinase-1), Caspase-3, and Bcl-2 were stimulated causing cell cycle arrest and death. Dose-proportional apoptosis and prevention of DNA replication in the S phase by biochanin-A were observed. A good level of caspase-3 and reduced Bcl-2/Bax proportion facilitates apoptosis and lung cancer prevention ( Li et al., 2018). Biochanin-A elicited pro-inflammatory properties beneficial to anti-cancer effect in lung cancer. AML 193 and A427 were tested with exposure of biochanin-A, the release of IL-6 cytokines and TNF-α, low level of E cadherin, and Snail blocking to epithelial–mesenchymal transition (EMT) which is essential in tumour growth and metastasis ( Wang, Li and Chen, 2018). In an in vivo study with benzo(a)pyrine-induced lung cancer, the biochanin-A treated group had displayed a marked decrease in the development of tumour at a dose less than dose causing a 10% lethality (LD10) ( Lee et al., 1991). When soy and red clover extracts were tested in NSCLC in combination with anti-cancer agents like gefitinib, erlotinib, afatinib, and osimertinib, the results were satisfactory. Particularly, the red clover extract which essentially contains biochanin-A showed a synergic effect with EGFR inhibitors and significant inhibition of tumor growth ( Ambrosio et al., 2016).
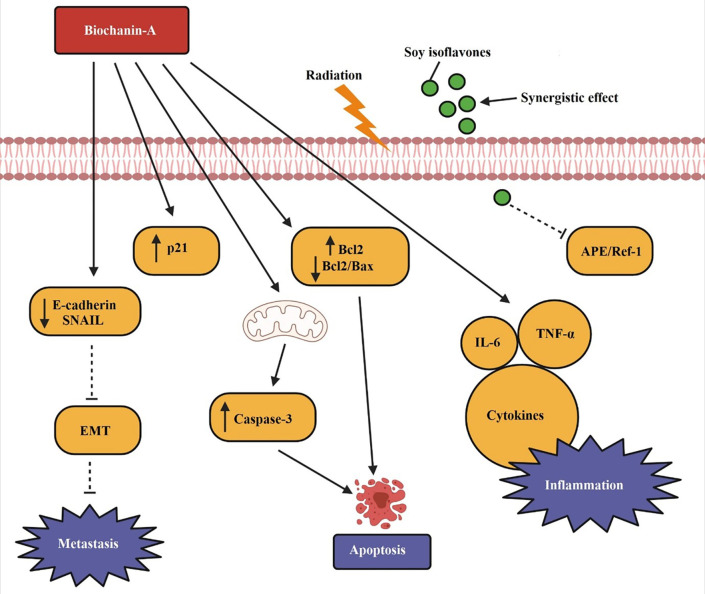
Figure 4 demonstrates the apoptotic pathway by biochanin-A on lung cancer. p21, caspase-3, and Bcl-2 levels are elevated by biochanin-A, which causes lung cancer to undergo apoptosis. Both metastasis and E-cadherin are reduced. TNF-a, IL-6, and cytokines are only a few examples of the pro-inflammatory mediators that are generated to aid in apoptosis. Soy isoflavones along with radiation therapy shows a synergistic effect in causing cell death by inhibiting APE/Ref-1 as depicted in the figure.
Figure 4. Apoptotic pathway by biochanin-A on lung cancer.
Biochanin-A cause apoptosis in lung cancer by increasing p21, caspase-3, and Bcl-2 levels. It lowers E-cadherin and blocks metastasis. Pro-inflammatory mediators such as TNF-α, IL-6, and cytokines are released to facilitate apoptosis. Soy isoflavones along with radiation therapy shows a synergistic effect in causing cell death by inhibiting APE/Ref-1 ( Singh-Gupta et al., 2011; Li et al., 2018; Wang, Li and Chen, 2018).
2.1.4 Pancreatic cancer
The low survival rate of pancreatic cancer with limited anti-cancer agents makes it difficult to manage the disease. It is also known to be the most aggressive one among other cancers ( Baghurst et al., 1991; Moore et al., 2003). Mutations in the tumour-suppressor and tumour-promoting gene are attributed to the aggressiveness of the disease ( Saif, 2007). Association with high-calorie diet and increased incidence of pancreatic cancer are reported ( Lowenfels and Maisonneuve, 2005; Li, 2009; Arslan et al., 2010; Chang et al., 2017). Consequently, it is important to conduct research on the role of isoflavones in pancreatic cancer ( Silverman et al., 1998). Biochanin-A had a negative influence on pancreatic cancer progression with variable mechanisms.
The cluster formation ability of pancreatic cancer cells Panc1 was hindered by biochanin-A with dose-dependent toxicity. It inhibited mitosis, migration, and invasion of pancreatic cancer progression. EGFR, Akt, and MAPK pathways are deactivated resulting in apoptosis in Panc1 and AsPC-1 cell lines suggesting combination therapy with biochanin-A could be considered for treatment ( Bhardwaj et al., 2014). Biochanin-A along with atorvastatin enhanced anti-cancer properties on AsPC1, MIAPaCa-2, and PANC-1 cell lines by lowering cell invasiveness and cell cycle progression. Biochanin-A interferes with cell survival by decreasing MAPK and Akt hence affects mitogenic signalling ( Desai et al., 2018). Concentration-dependent cellular invasiveness and migration are found with biochanin-A by reducing the level of matrix metalloproteases (MMP) indicating pancreatic cancer cell migration is inhibited ( Bhardwaj et al., 2014).
2.1.5 Colon cancer
It is the most prevalent type of cancer in existence today. Statistics by the American Cancer Society, 2019, shows that colon cancer is the second leading source of mortality in cancer. As specified by American Institute for Cancer Research and World Cancer Research Fund reports dietary factors may elucidate the risk of having colorectal cancer. Intake of isoflavone-contained food like soy influences gastric cancer occurrence ( Yan, Spitznagel and Bosland, 2010; Ko et al., 2013).
The synergism of biochanin-A with 5-fluorouracil evidenced in Caco-2 and HCT-116 cell lines indicates the modulatory influence of biochanin-A in colon cancer treatment. The biochanin-A on its own shows cytotoxicity in the cell lines. It blocked the “Akt and GSK3β phosphorylation and boosted the degradation of β-catenin” ( Mahmoud et al., 2017). Biochanin-A when combined with gamma radiation on HT29 cells, which is resistant to radiation, had revealed a reduction in cell proliferation. Raised levels of ROS, lipid peroxidation, MMP, caspase-3 have been observed more in the treatment group with significant apoptosis ( Puthli, Tiwari and Mishra, 2013). Biochanin-A and other isoflavones displayed a growth-retarded effect on HCT-116/SW-480 in a time and dose-reliant manner ( ZHANG et al., 2013). Stomach cancer cell lines SH101-P4, HSC-45M2, HSC-41E6, and colon cancer cell lines have been treated with isoflavones including biochanin-A and observed cytostatic effect. DNA fragmentation, chromatin condensation, and nuclear fragmentation of each cell line are seen with the apoptotic result ( Yanagihara et al., 1993). Oestrogen sensitive cancer cell lines including colon cancer cell 320DM when treated with biochanin-A and other isoflavones revealed antiproliferative effect which is beneficial as a cancer preventative ( Kohen et al., 2007).
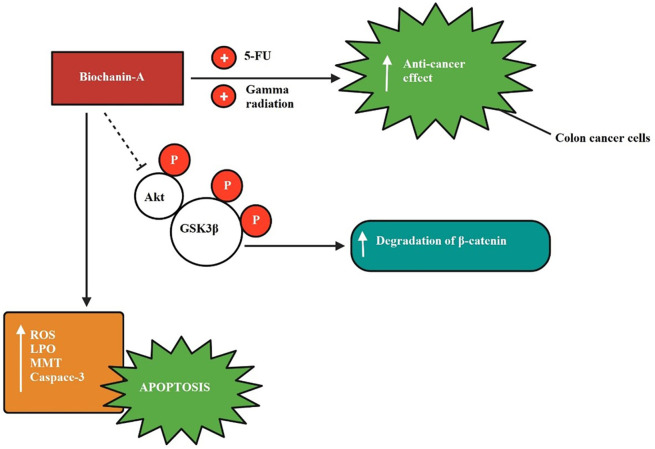
The inhibitory mechanism of biochanin-A on colon cancer is shown in Figure 5 explaining biochanin-A when given in combination enhanced the anti-cancer effect exerted by 5-fluorouracil and gamma radiation in colon cancer cells. Biochanin-A has inhibited Akt and GSK3β phosphorylation.
Figure 5. Colon cancer inhibition by biochanin-A.
Biochanin-A boosted the anti-cancer effect exerted by 5-fluorouracil and gamma radiation when given in combination in colon cancer cells. Biochanin-A has inhibited Akt and GSK3β phosphorylation ( Puthli, Tiwari and Mishra, 2013; Mahmoud et al., 2017).
2.1.6 Glioma
Glioblastoma multiforme (GBM), the most widely reported and destructive brain malignancy, has a high death rate. GBM tends to reappear since it displays both intra- and inter-tumoral heterogeneity ( Shergalis et al., 2018). The therapy becomes especially challenging due to the existence of BBB and rapid penetration to neighbouring tissue ( Desai and Bhushan, 2017). The chemo preventive and anti-angiogenic properties of isoflavones along with refining the adequacy of chemotherapy and radiotherapy for the treatment of GBM have received much attention lately ( Tedeschi-Blok et al., 2006; Sarkar et al., 2009).
In a dose-dependent manner, biochanin-A influenced the tumour invasion capacity by lowering matrix-degrading enzymes (MMP 2 and MMP 9) tested in U87MG cells ( Puli, Lai and Bhushan, 2006). Biochanin-A inhibited endothelial cell functions in rat brain tumour, brain endothelial cells, and chick chorioallantoic membrane model with its anti-angiogenic properties through ERK/AKT ex vivo/mTOR dephosphorylation ( Jain, Lai and Bhushan, 2015). Biochanin-A along with temozolomide disclosed exceptional anti-cancer activities in human glioblastoma cells, U87 MG, and T98-G. Biochanin-A by lowering EGFR, p-ERK (Extracellular signal related kinases), p-AKT (Protein kinase-B), c-myc, and MT-MMP1 (Membrane type matrix metalloproteinase) activation, inhibited cell survival. It influenced the abilities of cancer cells in viability, DNA repair, proliferation, and cell cycle arrest. Biochanin-A synergistically improved temozolomide anti-cancer ability in GBM ( Desai et al., 2019). Biochanin-A augments temozolomide by lowering the number of colonies and p-EGFR, p-ERK, uPAR (Urokinase type plasminogen activator receptor), MMP-2 levels in GBM cells ( Jain, Lai and Bhushan, 2011). Biochanin-A has proven to be a better candidate in GBM management in comparison with other isoflavones. It is found that biochanin-A exhibits a protective effect in a multimodal treatment methodology via testing in glioma cells (in-vitro), IP injection to tumour-implanted Fisher rats (in-vivo), and organotypic brain slices as ex-vivo experiments ( Sehm et al., 2014). Cell signalling pathways MAP kinase, PI3 kinase, mTOR, matrix metalloproteases, hypoxia-inducible factor, and VEGF were inhibited by biochanin-A, making it suitable in treating GBM ( Bhushan, Jain and Lai, 2015). In glioma C6 cells, the activation of ERK/Akt, the pro-angiogenic proteins were blocked by biochanin-A, and also VEGF and HIF-1α (hypoxia-inducible factor 1 alpha) were inhibited ( Jain, Lai and Bhushan, 2015). While testing the effect of rapamycin combined with biochanin-A in U87 glioma cells, there was decrease in cancer invasion and matrix-degrading enzymes. The combination dephosphorylated Akt and eIF4E (Eukaryotic translation initiation factor) augmenting rapamycin drug effect ( Puli, 2006). In individuals with glioblastoma, tolerance to temozolomide (TMZ) chemotherapy is the most common cause of the relapse of GBM. Biochanin-A was found to be a strong TMZ sensitizer in GBM, by increasing the cell sensitivity through AMPK/ULK-1 pathway ( Dong et al., 2022).
2.1.7 Osteosarcoma
Osteosarcoma (OS), a malignant bone tumour, is generally seen in children and adolescents. The low survival rate of OS is associated with drug resistance causing the poor response to chemotherapy. Hence, phytochemicals that can contribute to OS treatment are significant.
Biochanin-A and doxorubicin together suppressed the tumour development by promoting the release of apoptotic factors, damaging mitochondrial membrane potential, eliciting “the intrinsic mitochondrial pathway, caspase-9 and -3 activation” and increasing “Bax: Bcl-2/Bcl-XL ratio” ( Hsu et al., 2018). MG63 and U2OS osteosarcoma cells treated with biochanin-A revealed cytotoxicity at the molecular level. It is an apoptosis inducer (caspase-3), cell proliferation and invasion inhibitor, and dephosphorylates PCNA (proliferating cell nuclear antigen) and cyclin D1 gene expression ( Zhao et al., 2018b). The expression of caspase-3 controlled by biochanin-A while regulating cell death is one possible mechanism to manage osteosarcoma. With the molecular docking technique it was found that certain proteins were identified as effective targets of biochanin-A for osteosarcoma. Among those “BGLAP, BAX and ATF3” were recognized as the potential target of interest in blocking cancer cell proliferation using biochanin-A ( Luo et al., 2019). Biochanin-A tested in MG63 and U2OS cell lines exhibited a time and dose-related inhibitory effect on cancer proliferation, cell death, infiltration, and metastasis ( Zhao et al., 2018b).
2.1.8 Leukaemia & other cancers
Leukaemia or blood cancer affects the most important connective tissue of our body: blood, and the blood-forming tissue. It is considered a deadly and serious form of cancer ( Leukaemia Care, 2020). However, isoflavones have revealed their protective role in several investigations ( Xu et al., 2008). Soy derivative isoflavones obstruct the cell cycle of leukemic cells ( Yamasaki et al., 2007).
Biochanin-A in JCS cells prompted monocytic differentiation to macrophages (markers “Mac-l and F4/80”) showing increased phagocytic activity. The cytokines production (“IL-la, IL-lo, IL-4 and TNF-α”) is regulated in the late stage of monocytic differentiation of JCS cells by biochanin-A ( Fung et al., 1997). Biochanin-A affects intracellular antioxidant response system via Nrf2-Anti oxidant response element signalling pathway in tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in HepG2 cell line. Biochanin-A binds to Keap1’s pocket, causing Nrf2 signalling to be activated. These results suggest that dietary isoflavones may protect liver cancer patients from oxidative damage ( Liang et al., 2019). A study has found that Biochanin-A can be used to treat multiple myeloma, which is a type of cancer that affects plasma B cells in the bone marrow. The application of Biochanin-A has been shown to reduce the level of CD38, which is one of the key therapeutic targets for combating Multiple Myeloma. It has also been found to trigger apoptosis in Multiple Myeloma cells and reduce cytokine expression. The study further explored NOD/SCID mice with U266-induced tumors, Biochanin-A treatment was found to significantly reduce tumour growth. Mechanistic studies have shown that Biochanin-A’s anti-cancer effects are achieved by modulating the NF-κB and MAPK signalling pathways. This study suggests that Biochanin-A may offer a new, more effective, and less toxic type of treatment for multiple myeloma ( Jaina et al., 2022).
Anti-cancer effects of biochanin-A substantiated through in vivo and in vitro experiments is summarized in Table 1.
Table 1. Anti-cancer effects of biochanin-A substantiated through in-vivo and in-vitro experiments is summarized in Table 1.
( ↑increase, ↓decrease or × inhibit, + activation).
| Pharmacological Application | Study models | Dose used | Major findings\mechanisms | Reference |
|---|---|---|---|---|
| Breast cancer | HER-2 Positive breast cancer cell lines | 2–100 μM | NFκB ↓ Unique anticancer agent MAPK or ERK 1/2 phosphorylation × | ( Sehdev, Lai and Bhushan, 2009) |
| MCF-7 human breast carcinoma cells | 10–50 μM | Cell cycle apoptotis + DMBA × | ( Han et al., 2006) | |
| Human granular luteal cells | 10–100 nm | Cyp19 enzyme ↓ Have role in aromatase activity | ( Rice, Mason and Whitehead, 2006) | |
| MCF-7 breast cancer cell lines | 500 nm | Anti-oestrogenic property | ( Collins, McLachlan and Arnold, 1997) | |
| CTLL-2 cells (Murine IL-2 dependent T cell clone | 5–50 μM | Topoisomerase II × | ( Azuma et al., 1995) | |
| Prostate cancer | LNCaP cell lines | 0.5–50 μM | testosterone-UDPGT ↑ PSA ↓ Androgen metabolism × | ( Sun et al., 1998) |
| PC-3 (p53 mutant) and LNCaP (p53 wild type) | 100 μM | p21 ↑ PLK-1 ↓ Cell apoptosis + | ( Seo et al., 2011) | |
| DU-145 and LNCaP | 8.0 μg/mL for LNCaP 9.0 μg/mL for DU-145 cells | EGF-stimulated growth × tyrosine kinase events × | ( Peterson and Barnes, 1993) | |
| Red clover diet Model (Isoflavones constitute 1.26% of the diet) | Biochanin-A 5.74 mg isoflavone/g pellet | ER-β and E-cadherin levels ↑ Cancer formation × | ( Slater, Brown and Husband, 2002) | |
| LNCaP cells Athymic mice with LNCaP flank tumors | 10 μg/mL 400 μg for animal xenographs model | DNA fragmentation ↑ p21 and cyclin B ↓ Tumor size and incidence ↓ | ( Rice et al., 2002) | |
| Fibroblast cell | 100 μg/mL | 5 α-reductase isozymes × hormone-dependent tumours ↓ | ( Evans, Griffiths and Morton, 1995) | |
| LNCaP cells and DU145 cells, | 20–100 μM | TRAIL-associated cell death NF-κB × | ( Szliszka et al., 2013) | |
| PC-3, LnCaP, and DU145 | 100 μmol/L | Cell proliferation × Growth and metabolism × | ( Hempstock, Kavanagh and George, 1998) | |
| Lung cancer | 95D and A549 cancer cells injected to male nude mice | Biochanin-A IP 15,60 mg/kg group of A549 18,75 mg/kg group of 95D | Cell proliferation of lung cancer cells × | ( Li et al., 2018). |
| Human lung adenocarcinoma cell line(A427),Human monocyte leukaemia cell line (AML-193) | 5, 20, 40 μM | Hinderes proinflammatory effects triggered from leukaemia | ( Wang et al., 2018 | |
| New born mouse model | 65 mg/kg | Carcinogenesis × | ( Lee et al., 1991) | |
| Non-small cell lung cancer | Soy red clover isoflavones | Isoflavones combined with EGFR inhibitors improve NSCLC cell growth | ( Ambrosio et al., 2016) | |
| Pancreatic cancer | Panc/AsPC-1 | 5 mg/mL | Cell proliferation × Apoptosis + | ( Bhardwaj et al., 2014) |
| Colon cancer | Caco-2,HCT-116 cell lines | 34 μM | Biochanin-A combination with 5 FU promotes management of colon cancer | ( Mahmoud et al., 2017) |
| HT29cells | 1–100 μM | Biochanin-A increases radiotoxicity | ( Puthli, Tiwari and Mishra, 2013) | |
| HCT-116 Sw-480 cell lines | 2.5–100 μM | Biochanin-A enhances genotoxYangiharaic effects and antitumor mechanism | Yanagihara et al., 1993) | |
| 320 DM cell line | 20 μM | Biochanin-A is a promising target for cancer | ( Kohen et al., 2007) | |
| Glioblastoma multiforme | U87MG | 50 μM | MMP 2 and MMP 9 ↓ | ( Puli, Lai and Bhushan, 2006) |
| Rat brain tumour (C6) Murine brain endothelial (bEnd.3) cells Ex-vivo chick chorioallantoic membrane model | 5, 35, and 70 μmol/L | Anti-angiogenic properties through ERK/AKT/mTOR dephosphorylation | ( Jain, Lai and Bhushan, 2015) | |
| U-87 MG, and T98 G | 70 μM | EGFR, p-ERK, p-AKT, c-myc, and MT-MMP1 activation ↓ cell survival * Synergism to anti-cancer ability of Temozolomide | ( Desai et al., 2019) | |
| U-87 human glioblastoma cell line | 20 μM and 70 μM | p-EGFR, p-ERK, uPAR, MMP-2 ↓ | ( Jain, Lai and Bhushan, 2011) | |
| Osteosarcoma | MG63 and U2OS Cells | 20±0.3 μg/mL | Bax: Bcl-2/Bcl-XL ratio ↑ caspase 9 & 3 + MMP ↓ | ( Hsu et al., 2018) |
| MG63 and U2OS Cells | 40 μM | Apoptosis + caspase-3 ↑ Cell proliferation and invasion × | ( Zhao et al., 2018b) | |
| Leukaemia | JCS | Monocytic differentiation to macrophages + Phagocytic activity ↑ | ( Fung et al., 1997) |
2.2 Metabolic disorders
2.2.1 Diabetes
Diabetes mellitus, an age-old metabolic disorder, has a high rate of occurrence around the world. It is described as “hyperglycaemia” caused by deformities in insulin secretion, insulin activity, or both ( American Diabetes Association, 2009). The long-term presence of diabetes is associated with many other complications. Even though currently, accessible medications might be significant in the control of diabetes, these medications are joined with certain side effects as well. A few varieties of phytochemicals have shown potential for the management of diabetes with minor or no side effects ( S.Mohana Lakshmi, Rani and Reddy, 2012; Surya et al., 2014). Bioflavonoids are remarkable for their hypoglycaemic abilities ( Vinayagam and Xu, 2015). It has been exhibited that flavonoids can go about as “insulin secretagogue or insulin-mimetic agents” ( Patel et al., 2012; Singh and Sahu, 2018).
Biochanin-A action in streptozotocin-induced diabetic rats displayed improved glucose digestion and dropped HbA1C levels. Serum visfatin amount was enhanced ( Azizi, Goodarzi and Salemi, 2014). STZ diabetic rats on oral treatment with biochanin-A exposed anti-diabetic properties by lowering FBS and hyperglycaemia-induced free radicals ( Sadri et al., 2017). Red clover extract was tested in “db/db diabetic mice” to see the anti-diabetic and anti-hyperlipidemic activities. Increased hepatic PPARα/γ stimulation and reduced hepatic fatty acid synthase levels contributed to achieving glucose and lipid homeostasis by red clover compounds ( Qiu, Ye, et al., 2012b). STZ-diabetic C57BL/6 mice were treated with red clover extracts including biochanin-A and formononetin. The lipid profile of the animal was influenced by biochanin-A rather than glucose levels through mechanisms in connection with hepatic PPARa ( Qiu, Ye, et al., 2012a). The raised levels of plasma glucose, HbA1C, and gained weight were normalized with biochanin-A treatment ( Harini, Ezhumalai and Pugalendi, 2012). At certain doses, biochanin-A reduced glucose tolerance and insulin resistance, developed insulin sensitivity and increased SIRT-1 expression which might explain the anti-diabetic properties of the drug ( Oza and Kulkarni, 2018). Biochanin-A acts as a strong PPAR receptor activator (PPARalpha/PPARgamma) even at low doses signifying its anti-diabetic and anti-hyperlipidaemic properties ( Shen et al., 2006). Nesfatin-1, a regulatory peptide level, and insulin were increased upon biochanin-A treatment in type-1 diabetic rats, which can hint to its one probable mechanism behind the hypoglycaemic property ( Eskandari Mehrabadi and Salemi, 2016). The structural relationship and activity of biochanin-A and other isoflavones were checked. Biochanin-A derivative (7-diethyl phosphite-O-biochanin-A) had shown greater anti-hyperglycaemic activity compared to all other compounds ( Wei et al., 2017). The role of serum adiponectin and serum resistin as a glucose metabolism regulator in diabetes is discoursed and it is found that biochanin-A improved adiponectin release and augments insulin activity. The elevated resistin usually observed in the type-1 diabetic condition is decreased after biochanin-A intake. The oxidative stress produced in diabetes was also taken care of by biochanin-A ( Salemi et al., 2018).
The influence of biochanin-A in diabetic neuropathy using an STZ-induced rat model revealed that, mechanical allodynia and hyperalgesia (paw withdrawal threshold) were reversed upon treatment. Hence, biochanin-A could be a drug of choice in diabetic neuropathy ( Chundi et al., 2016). The retina of diabetic rats was tested to rule out diabetic retinopathy after biochanin-A treatment. It was uncovered that it significantly prolonged the event of retinal damage with its anti-inflammatory and anti-angiogenic property ( Mehrabadi et al., 2018). Diabetic nephropathy caused due to increased oxidative stress and TGF-β, was monitored in type 2 diabetes mellitus-induced rats to know if biochanin-A can play a role. It had significantly improved kidney function through modulating TGF-β expression and minimizing oxidative stress ( Ahad, 2013). Hyperlipidaemia is a common comorbidity seen in diabetic patients. Biochanin-A administered to diabetic animals demonstrated low fasting blood sugar (FBS) as well as small dense low density lipoprotein cholesterol (sd-LDLC), favourable in diabetic dyslipidaemia conditions ( Ghadimi et al., 2019). A formulation containing biochanin-A with or without its analogues will be beneficial in treating diabetes and diabetic cardiomyopathy, with evidence through increasing IGF1R (insulin-like growth factor 1 receptor), INSR (insulin receptor), and IRS2 (insulin receptor substrate 2) levels, thereby leading to the up-regulation of Lin28 gene and insulin sensitivity ( Boominathan L, 2017). Biochanin-A was administered for 16 weeks orally once in a day to HFD-fed rats with single dose streptozotocin and showed that it has the ability to increase SIRT1 expression in heart tissue while also controlling hyperglycaemia and oxidative stress. Biochanin-A could be a promising candidate for lowering the advancement of cardiomyopathy in people with type 2 diabetes ( Oza and Kulkarni, 2022). A study designed to examine the diabetic and diabetic nephropathy effects on diabetic rats revealed that administering biochanin-A markedly reduced the expression of transforming growth factor-β1 (TGF-β1), protease-activated receptors 2 (PAR-2) genes, and fasting blood glucose (FBG) ( Amri et al., 2021).
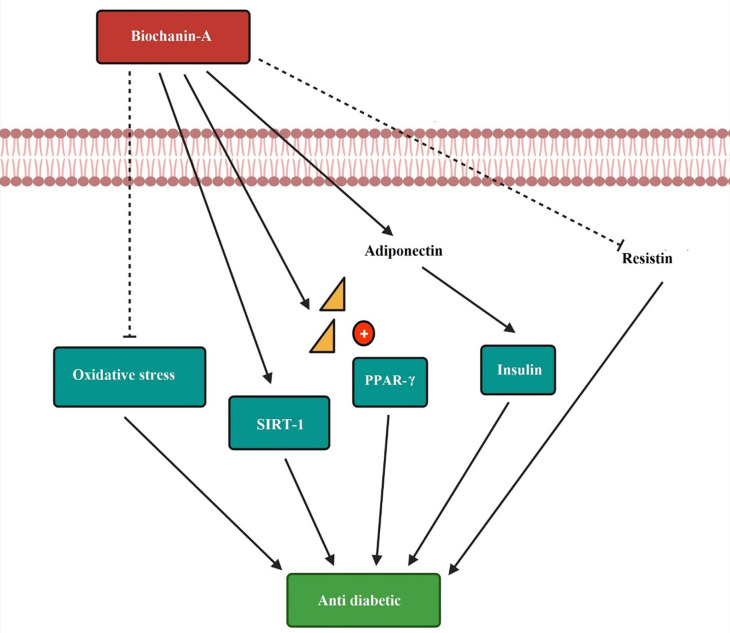
The anti-diabetic mechanism of biochanin-A is by decreasing oxidative stress. SIRT-1 influences the progression of insulin sensitivity. Biochanin-A act as a PPAR gamma receptor activator and produces anti-diabetic effect. The increased release of adiponectin and low resistin level to improve the diabetic condition is depicted in Figure 6.
Figure 6. Anti-diabetic mechanism of biochanin-A.
Biochanin-A decreases the oxidative stress and helps in diabetes condition. It increases the expression of SIRT-1 and progresses insulin sensitivity. Biochanin-A is a PPAR-γ receptor activator producing an anti-diabetic effect. It increases the release of adiponectin and decreases the resistin level to improve the diabetic condition ( Shen et al., 2006; Oza and Kulkarni, 2018; Salemi et al., 2018).
2.2.2 Dyslipidaemia
Dyslipidaemia or hyperlipidaemia is a very common metabolic disorder characterized by a high level of triglycerides and low-density lipoproteins, responsible for cardiovascular diseases ( Thompson, 2004). The association between soy diet and hyperlipidaemia is a discussed topic among researchers. Soy can normalize the increased cholesterol level while taken in combination with conventional hyperlipidaemic drugs ( Costa and Summa, 2000). A randomized control trial on isoflavones in hypercholesterolaemia showed that there was a minor significant positive impact on triglycerides levels supporting this fact ( Qin et al., 2013).
Treatment with a moderate dose of biochanin-A in HFD mice has shown a substantial lowering of LDL and total cholesterol. Lipoprotein lipase and hepatic triglyceride lipase levels are increased. Molecular docking studies on biochanin-A displayed a noteworthy role in reducing cholesterol-ester transport ( Xue et al., 2017). Biochanin-A over formononetin reduced LDL cholesterol in men, though the same was not observed in women ( Nestel et al., 2004). Biochanin-A lowers blood lipid levels, blood fibrinogen, and blood thickness levels of rats with hyperlipidaemia, increases blood circulation, and alters the blood coagulation system in lipid metabolism disorders ( Ling-hui et al., 2012). Plant sterol combined with soy constituent such as biochanin-A was administered to a human to check LDL cholesterol level and the impact on atherosclerosis. It was shown to be efficacious to co-administer plant sterol and isoflavones ( Waggle, Potter and Henley, 2003).
2.2.3 Obesity
Obesity has been the most serious global health concern that is rapidly turning into an outbreak, currently affecting both developing and developed countries to varying degrees. Despite the fact that obesity and overweight are on the rise in modern society, there are no pharmacological treatments available. As a result, both researchers and health-care systems must prioritise the development of safe and effective treatments for obesity.
In HFD-induced obesity, oral treatment of biochanin-A significantly reduced the physiological changes that have occurred during trace element metabolism. This could be due to the inhibition of pathological mechanisms that derange trace elements, possibly by reverting hyperglycemia and insulin resistance and changing hepcidin and HO-1 levels. These findings strongly suggest that biochanin-A has therapeutic potential in the treatment of obesity and the prevention of cardiovascular disease ( Antony Rathinasamy et al., 2020). Biochanin-A enhanced the expression of PPAR-α and its regulatory proteins in the liver by stimulating the transcriptional activation of PPAR-α in vitro. In the livers of obese mice, biochanin-A treatment increased the recovery of metabolites involved in phosphatidylcholine production, lipogenesis, and beta-oxidation. Biochanin-A also inhibited the expression of glucose 6-phosphatase and pyruvate kinase, two enzymes involved in gluconeogenesis. In diet-induced obesity, biochanin-A modulated lipid and glucose metabolism, improving metabolic abnormalities such as hepatic steatosis and insulin resistance ( Park et al., 2016). Furthermore, biochanin-A administration in obese rats had a higher therapeutic effect, returning the altered parameters to near-normal levels. Biochanin-A up-regulated the Nrf-2 pathway while suppressing the NF-κB cascade, increasing the activity and mRNA expressions of enzymatic antioxidants. By activating the Nrf-2 pathway and inhibiting NF-κB activation, biochanin-A may reduce obesity and its related cardiomyopathy by decreasing oxidative stress and inflammation ( Rani A, et al., 2021).
Biochanin-A promotes AMPK signalling in C3H10T1/2 MSCs, leading to upregulation of brown fat adipocyte. According to the findings, biochanin-A treatment improves mitochondrial biogenesis and lipolysis, modulating the thermogenic process. Biochanin-A improves energy expenditure by boosting mitochondrial respiration while preserving the functional mitochondria. These data imply that biochanin-A might be a new antiobesity drug ( Rahman et al., 2021). Leptin is a hormone that regulates energy intake and body weight. Leptin resistance has been identified as a significant component in the development of obesity in recent studies. Endoplasmic reticulum (ER) stress, induced by the development of unfolded protein in the ER, causes leptin resistance. Biochanin-A decreased the ER stress related cell death in neuronal cells, restricted the glucose-regulated protein expression and modified the leptin signalling induced by ER stress. These findings imply that biochanin-A may have pharmacological characteristics that might reduce ER stress and hence alleviate leptin resistance ( Horiuchi et al., 2021). A study deals with the evaluation of the cholesterol esterase inhibitory activity of biochanin-A using in silico docking approach. Biochanin -A contributed cholesterol esterase inhibitory activity, these molecular docking analyses could lead to the further development of potent cholesterol esterase inhibitors for the treatment of obesity ( Sivashanmugam et al., 2013).
2.3 Cardiovascular disorders
Asian countries usually presented lower cardiovascular disease (CVD) mortality rates as it has very different dietary patterns from that of Western countries. Benefits over cardiovascular disease risk is an appreciated capability of soy protein and isoflavones. Isoflavones restore the disrupted endothelial function ( Sacks et al., 2006; A. Gil-Izquierdo et al., 2012; Sathyapalan et al., 2018). By the year 1999, the US Food and Drug Administration (FDA) permitted to give soy protein-enriched foods as a protective agent in coronary heart disease routinely to lower the risk of cardiovascular disease ( U.S. FDA, 1999).
Biochanin-A mitigated myocardial injury by inhibiting the anti-inflammatory pathway, TLR4/NF-kB/NLRP3 signalling. It perfected the injury area and stopped the release of aspartate transaminase (AST), creatine kinase (CK-MB) and lactic dehydrogenase (LDH) enzyme. Biochanin-A further decreased inflammatory cytokines and protected rats from myocardial infarction ( Bai et al., 2019). Reverse cholesterol transport (RCT) stimulated by biochanin-A and lowered pro-inflammatory cytokines make it a remarkable drug of choice in managing atherosclerotic cardiovascular disorder ( Yu et al., 2020). Treatment with biochanin-A in isoproterenol-induced myocardial infarction rats normalized anti-oxidant levels and produced cardio-protective effects by controlling lipid peroxidation and detoxifying enzyme systems ( Govindasami et al., 2020). In a study of screening various flavonoids, it was found that biochanin-A produces vasodilation in rat aorta via a mechanism of inhibiting the L-type calcium channel by interfering cGMP pathway of the coronary artery and can be of potential clinical interest in the management of the CVDs ( Migkos et al., 2020).
2.4 Airway hyperresponsiveness
In the 19th and 20th centuries, people were drinking red clover tea or tincture (ethanolic extract) as an antispasmodic to give relief in whooping cough, measles, bronchitis, laryngitis, and tuberculosis ( Felter and Lloyd, 1999). Biochanin-A being the major constituent of red clover can act as an anti-spasmodic agent in asthma and COPD (chronic obstructive pulmonary disease) ( Ko et al., 2011).
It has been proven that biochanin-A diminishes airway resistance and improves respiratory health in methacholine (MCh) induced mice. Inflammatory mediators released were under control and ovalbumin (OVA)-specific immunoglobulin E (IgE) levels were low, hence, evidencing significant effect in allergic asthma and COPD ( Ko et al., 2011). Biochanin-A reduced allergic asthma in mice with histological evidence through inhibitory effects on inflammatory cytokines, cell infiltration, protein leakage into the airways and expression of haem oxygenase-1 in OVA-induced lungs (ovalbumain). The action is mediated through PPAR-γ activation ( Derangula, Panati and Narala, 2021). Biochanin-A exposed defensive effect in particulate matter with an aerodynamic diameter of 2.5 μm (PM2.5)-associated pulmonary disease rat model, it decreased cell death, the release of pro-inflammatory mediators, malondialdehyde (MDA), lactate dehydrogenase (LDH), and alkaline phosphatase (AKP) while increasing antioxidant enzymes levels ( Xue et al., 2020). The risk of pulmonary and heart injury can be exacerbated by particulate matter with an aerodynamic diameter less than 10 μm (PM10). When examined in an in vitro model of lung injury caused by PM10, biochanin-A showed an anti-inflammatory effect that lessened lung injury and produced low levels of intracellular catalase and LDH. It regulates the phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) signalling pathway and activates PI3K protein ( Li et al., 2021a).
2.5 Osteoarthritis
Osteoarthritis is a condition that affects articular cartilage and synovial joints with structural and functional failure and diminishes the quality of life ( Hunter and Felson, 2006). It is a chronic and irreversible disease causing pain and disability. Soybean isoflavones stopped the cartilage damage in animals with an ovarian hormone deficiency, which is indicative of its effect on osteoarthritis ( Toda, Sugioka and Koike, 2020).
Biochanin-A controlled the cartilage damage by deactivating the expression of MMP, NF-κB, and activation of TIMP-1, hence effective in OA cases ( D. Q. Wu et al., 2014). Biochanin-A blocked the adipocyte differentiation considerably and the level of PPAR-γ, lipoprotein lipase (LPL), and leptin and osteopontin (OPN) mRNA expression were lowered and prompted the osteoprotegerin (OPG) to put forward its ability of osteoblast differentiation stimulation and adipogenesis inhibition ( Su et al., 2013). The significance of biochanin-A on the resolution of the neutrophilic inflammatory response in an antigen-induced arthritis model, using wild-type BALB/c mice showed that biochanin-A reduced the number of migrating neutrophils which was linked to decreased levels of myeloperoxidase activity, IL-1 and CXCL1, as well as the histological score in periarticular tissues. Treatment with biochanin-A improved joint dysfunction as indicated by mechanical hyper-nociception ( Felix et al., 2021).
2.6 Inflammation
Inflammation, biological feedback of the human body to damaging stimuli, is also associated with a wide range of diseases such as “obesity, atherosclerosis, rheumatoid arthritis, and even cancer”. Isoflavones are famous for their anti-inflammatory properties. Underlining the evidence for isoflavones is required in managing chronic diseases in which inflammation plays a vital role. Isoflavones that are an assured agent in various inflammatory diseases, show exciting anti-inflammatory effects proven in animal and human studies through better anti-oxidant properties, reduced pro-inflammatory enzymes, and NF-κB regulation ( Su et al., 2013).
Biochanin-A repressed LPS induced TNF-α and IL-8 production, NF-κB. Through PPAR-γ activation, biochanin-A displayed an anti-inflammatory effect hence, can be considered as an agent in the therapeutic management of inflammatory cardiovascular disease ( Su et al., 2013). Biochanin-A is considered an anti-inflammatory agent with regards to its inhibitory effect in the release of nitric oxide (NO) production by LPS (Lipopolysaccharide), IkB kinase (IKK) activity, and NF-κB activation and lowered IL-6, IL-1β, and TNF-α production in RAW264.7 cells ( Kole et al., 2011). It competes with the inflammation by impeding release of pro-inflammatory cytokines and modulating NF-κB and MAPKs pathways.
2.7 Anti-oxidant activity
Antioxidant rich foods may lower the chance of developing a number of ailments including heart disease and certain cancers. Free radicals are removed from cells by antioxidants, which minimizes oxidation related damage in the body. Biochanin -A being a good natural anti-oxidant can produce various health benefit in human biological system.
Water-soluble urban particulate matter is a major lung toxicant shown to induce oxidative damage in human alveolar basal-epithelial cells. Biochanin-A tested in this model produced a protective effect by increasing anti-oxidant markers such as catalase, superoxide dismutase and glutathione. The malondialdehyde (MDA) and nitric oxide (NO) levels were found to be reduced and mitigated the lung injury by regulating MEK5/extracellular signal-related kinase 5 (ERK5) nuclear factor-erythroid factor 2-related factor 2 (Nrf-2) pathway ( Xue et al., 2021). Several anti-oxidant assays have shown biochanin-A to be a powerful free radical scavenger molecule with its activity comparable to ascorbic acid. Biochanin-A when assessed in anti-oxidant assays such as nitric oxide scavenging activity assay, 1,1-diphenyl-2-picryl hydrazyl (DPPH), 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ferric reducing antioxidant power (FRAP), hydroxy-radical activity assay, superoxide anion scavenging activity, hydrogen peroxide radical assay, metal ion chelating activity and phosphomolybdenum assay, it was observed that biochanin-A is able to produce efficient free radical scavenging ability in a dose reliant manner ( Vennila L, Asaikumar L, Sivasangari S, Jayaraj D, 2019).
2.8 Hepatotoxicity
The hepatoprotective abilities of biochanin-A were explored in carbon-tetrachloride hepatotoxicity animal model. The anti-oxidant and anti-inflammatory capacity of biochanin-A influence the elevated hepatic enzyme level, such as AST, ALP, ALT, bilirubin, etc., and found to be a promising molecule in hepatotoxicity models ( Breikaa et al., 2013b).
2.9 Anti-bacterial activity
Natural isoflavones have exposed anti-microbial activity in various studies. The specific inhibitory action of biochanin-A was explored against possible Clostridium spp. bacterial infections of the human digestive system and was found to be beneficial to the human microbiota. “Clostridium tertium and clostridium clostridioforme” had exhibited high sensitivity to biochanin-A with a minimum inhibitory drug concentration of 0.13 mM whereas, Lactobacillus spp. or bifidobacteria showed resistant activity ( Flesar et al., 2009; Sklenickova et al., 2010). Out of many isoflavones, biochanin-A exhibited a growth inhibitory effect against a number of Gram-positive and Gram-negative bacteria ( Hummelova et al., 2015). Augmenting the immune response of host is a novel strategy to fight against microbial infections. Biochanin-A has proven its effect as intra-cellar and extra-cellular bactericidal on HeLa cells and inhibited the Salmonella spp. infection through mTOR/AMPK/ULK1 pathway ( Zhao et al., 2018a). Biochanin-A was the most effective Chlamydia spp. growth inhibitor among the various isoflavones tested, with an IC 50 of 12 μM on Chlamydia pneumoniae inclusion counts and 6.5 μM on infectious progeny generation ( Hanski et al., 2014). Biochanin-A by moderating the alterations associated with starch fermentation ex vivo, may be an efficient alternative to antibiotics for mitigating sub-acute rumen acidosis (SARA), according to a research to investigate the effect of biochanin-A on amylolytic bacteria and rumen pH during a SARA challenge ( Harlow et al., 2021). The effects of biochanin-A were similar to that of monensin, an antibiotic that is used conventionally for SARA treatment in cattles. The results indicate that biochanin-A may be an effective alternate to antibiotics for alleviating SARA in cattles ( Harlow et al., 2021).
2.10 Neurological disorders
Neurological disorders hampering the brain and nervous system are associated with a wide group of disorders as well as varying pathophysiology and symptoms. Inflammation is thought to be one important pathogenesis to cause peripheral (neuropathic pain, fibromyalgia) and central nervous systems disorders (e.g., Parkinson's disease, ischaemia, and traumatic brain injury, etc.) ( Skaper et al., 2018). Multiple sclerosis (MS) is another chronic inflammatory neurodegenerative disease of the central nervous system. In a study conducted to explore the positive impacts of biochanin-A on cuprizone (CPZ)-induced MS model on mice, found that biochanin-A was able to modify the neurological harm of the condition with five weeks of treatment period. When compared to the CPZ group, biochanin-A boosted up the spatial memory in the Y-maze and recognition memory in the novel arm discrimination task (NADT) and novel object recognition task (NORT) of the animals ( Aldhahri et al., 2022).
2.10.1 Cerebral ischaemia
Two key pathways in the development of cerebral ischaemia/reperfusion damage are oxidative stress and neuroinflammation. Biochanin-A pretreatment on experimental animals induced with stroke, showed that the neurological deficiency is improved and the size of neural infarct and brain oedema was reduced. Biochanin-A reduced oxidative stress in the brain by augmenting SOD (superoxide dismutase) and GSH-Px (glutathione peroxidase) and repressing MDA (malondialdehyde) levels. The neuroprotective effects of biochanin-A might be attributed to the activation of the Nrf2 pathway and suppression of the NF-κB pathway ( Guo et al., 2019). An L-glutamate-induced cytotoxic PC12 cell line when treated with biochanin-A exposed a protective effect by reducing cytotoxicity. It aided the release of glutathione while stopping LDH, caspase-3 effects, and act as an anti-apoptotic agent to produce neuroprotective activity ( Tan et al., 2013). Middle cerebral artery occlusion (MCAO) subjected animals treated with biochanin-A, aiming to produce a protective effect against cerebral ischaemia/injury, presented with suppression of inflammatory response like TNF-α and IL-1β levels, MPO activity, and downregulation of p38 signalling (W. Wang et al., 2015). The elevated level of glutamate may be the principle cause that leads to cerebral ischaemia. By screening various phytomolecules, biochanin-A was found to be the most effective GOT (glutamate oxaloacetate transaminase) gene expression inducer in neural cells to alleviate ischemic injury. The glutamate induced cell death was lowered by biochanin-A administration, which was also proven when tested in GOT knock-down model as it did not have any protective effect. The ischaemic stroke presented animals were injected with biochanin-A and experienced a high level GOT protein in their brain tissues. Biochanin-A diminishes the stroke volume and amended the sensory motor abilities ( Khanna et al., 2017).
2.10.2 Parkinson’s disease
Parkinson’s disease (PD) is related to the degeneration of dopaminergic neurons in the SNpc. Oxidative stress in connection with the neurodegenerative symptoms has been found in this condition. Studies on lipopolysaccharide (LPS)-injected animals revealed that treatment with O-methylated biochanin-A amended the behavioural patterns of animals, stopped the dopamine neuronal loss, and prevented the harmful microglia activation. Biochanin-A additionally blocked the activation of NADPH-oxidase, MDA formation, SOD, and GPx actions in the brain preferring its choice as an anti-oxidant in PD management (J. Wang et al., 2015). Neurodegeneration due to inflammatory response through activating microglia is one of the reason in PD pathophysiology. It is found in rat mesencephalic neuron-glia cultures treated with biochanin-A, decreased dopamine uptake, and blocked LPS-related microglia activation. Low levels of TNF release, NO/SO release is associated with a protective effect towards LPS induced neurodegeneration ( Chen, Jin and Li, 2007). While proving the anti-inflammatory ability of biochanin-A on LPS-treated mice BV 2 microglial cells observed that the levels of TNF-α, IL-1β, nitric oxide, and ROS were lowered. Biochanin-A influences pro-inflammatory responses induced by LPS and produces a protective effect on glial cells ( Wu et al., 2015). Additionally, it up-regulated PPAR-γ levels, limited NF-κB release, and acts as an anti-inflammatory agent ( Zhang and Chen, 2015). Increased neonatal iron supplementation (120 μg/g body weight) to the rats may contribute to the pathogenic mechanism of PD, and iron and rotenone co-treatment may worsen neurochemical and behavioural impairments by generating a redox imbalance. Biochanin-A had considerably lowered the malondialdehyde levels and significantly improved the glutathione levels in the substantia nigra amongst iron and rotenone co-treated male and female rats. It reduced the behavioural impairments by reducing striatal dopamine depletion. Furthermore, biochanin-A may protect dopaminergic neurons by preserving redox equilibrium ( Yu et al., 2017). In another study where iron co-treated with 1-methyl-4-phenylpyridinium (MPP +), it raised the level of superoxide in microglia via p38 mitogen-activated protein kinase (MAPK) stimulation and resulted in deteriorated neurochemical and behavioral features of animals. Biochanin-A has been shown to repress the activation of p38 MAPK ( Li et al., 2021b).
2.10.3 Alzheimer's disease
The neuroprotective ability of various phytoestrogenic isoflavones including biochanin-A was screened against oxidative stress-induced cell death in the HCN 1-A (human cortical cell line) maintained in culture using the Alzheimer's disease-associated hydrogen peroxide (H 2O 2) model. Due to the anti-oxidant capacity of isoflavones, the concentration-reliant reduction in neuron viability by H 2O 2 was prevented ( Occhiuto et al., 2009). The neuroprotective benefits of biochanin-A as a possible alternative to oestrogen replacement treatment, against Aβ25–35 (amyloid beta) related toxicity, as well as its putative modes of action in PC12 cells, were investigated. In the presence of biochanin-A, the effects of Aβ25–35 were considerably reversed as it lowered cell death, LDH release, and cellular caspase activity, reducing the cytotoxic impact of the Aβ25–35 protein. Furthermore, it was found that in the presence of biochanin-A, there was decreased cytochrome-c and Puma (p53 up-regulated modulator of apoptosis) expression, along with reduced Bcl-2/Bax and Bcl-xL/Bax ratio ( Tan and Kim, 2016).
The neuroprotective effects of biochanin-A on LPS-induced dopaminergic neuron injury in in vivo and in vitro models, as well as the molecular processes involved were explored. Biochanin-A therapy for 21 days substantially reduced behavioural impairment in PD rats, lowered dopaminergic neuronal loss, and suppressed microglia activation in LPS-induced PD rats. Biochanin-A protects LPS-induced PD rats, and the mechanisms are thought to be related to the suppression of the inflammatory response and the MAPK signalling pathway. Biochanin-A prevented primary microglial activation and protected dopaminergic neurons, reduced the amount of nitric oxide, IL-1, and TNF-α in supernatants, and suppressed the formation of reactive oxygen species ( Wang et al., 2016). Rats lesioned with the PD-related neurotoxin 6-OHDA (6-hydroxydopamine) showed less motor impairment after receiving isoflavone-rich soy extract. These results imply that plant-derived isoflavones might be used as a dietary supplement to prevent the onset of Parkinson's disease in at-risk patients and to reduce neurodegeneration in the brains ( de Rus Jacquet et al., 2021).
The behavioural and neurochemical effects of biochanin-A among cognitive-impaired animals in scopolamine-induced amnesia and naturally occurring aged animal amnesia models were assessed. In exteroceptive behavioural paradigms such as the elevated plus maze and the passive shock avoidance paradigm, biochanin-A decreased the transfer latency and increased the step through latency considerably in scopolamine-treated and natural aged mice. Acetylcholinesterase activity was found decreased in a dose-reliant manner amongst biochanin-A treated animals. A high level of GSH suppressed the pyknotic neurons formation, noradrenalin and dopamine expression thereby revealed the protective ability of biochanin-A against Alzheimer's disease ( Biradar, Joshi and Chheda, 2014). The enzyme beta-site amyloid precursor protein cleaving enzyme-1 (BACE1) is involved in the aberrant synthesis of the amyloidogenic peptide Aβ, and is one of the primary causes of Alzheimer's disease (AD). BACE1 is found to be a crucial target protein in the identification of novel strategies to minimize and prevent Alzheimer's disease. Biochanin-A non-competitively inhibited BACE1 with an IC 50 value of 28 μM. With a binding energy of −8.4 kcal/mol, biochanin-A might strengthen the chemical's strong interaction with the allosteric site of BACE1, leading to more effective BACE1 inhibition ( Youn et al., 2016).
In postmenopausal women, oestrogen insufficiency is a major risk factor for Alzheimer's disease. In the Morris water-maze test, chronic treatment with biochanin-A replicated the capacity of β-estradiol (E2) to restore learning and memory deficits in ovariectomized (OVX) rats. Biochanin-A also lowered MDA levels and increased SOD and GSH-Px enzyme levels eventually produced neuro-protective effect and can be used to treat memory loss in postmenopausal women who are suffering from an oestrogen imbalance ( Zhou et al., 2021).
2.11 Anti-fibrotic activity
Studies have been conducted to explore the role of biochanin-A in idiopathic pulmonary fibrosis (IPF), a chronic inflammatory disease characterised by fibrotic cascade events such as epithelial-mesenchymal transition, synthesis of extracellular matrix, and collagen formation in the lungs. The study was conducted on LL29 cells (lung fibroblast from IPF patient), NHLF (normal human lung fibroblast), and DHLF (diseased human lung fibroblast). Furthermore, the research focused on evaluating the effect of biochanin-A in bleomycin-induced pulmonary fibrosis. Biochanin-A treatment produced increased levels of Smad7 expression and decreased Smad2mRNA expression in cell lines, suggesting that biochanin-A contributes to pulmonary fibrosis by inhibiting TGF-β/Smad signalling. In vivo results of the research revealed that lung index was increased in the bleomycin-induced pulmonary fibrosis group (BML) and biochanin-A reversed the same. The study proved that via modulating the TGF-β/Smad Pathway, biochanin-A prevented the onset and progression of pulmonary fibrosis ( Andugulapati et al., 2020).
Biochanin-A was investigated for its antifibrotic effects on a rat liver model wherein hepatotoxicity was induced through intraperitoneal chloroform. Hepatic fibrosis is the consequence of the wound-healing action of the liver which results in the accumulation of high fibrous scar tissue. The fibrotic lesions in the biochanin-A treated group were found to be minimum, and it also decreased α-SMA levels which confirms its anti-fibrotic effect. The study observed that biochanin-A improved blood flow and have good antioxidant properties. Biochanin-A decreased the TNF-α and NO levels. Thus, biochanin-A in the liver has anti-fibrotic properties by reducing oxidative stress while preserving hepatic function ( Breikaa et al., 2013a).
The several pharmacological models explained in the review are collectively summarized in Table 2 and Table 3 emphasizing the model either in vitro or in vivo along with the dose of biochanin-A used in the study and the molecular mechanism behind the activity.
Table 2. Pharmacological findings of biochanin-A validated via in vivo experiments.
(↑increase, ↓decrease, × inhibit, + activation).
| Pharmacological application | Study model | Dose and route used | Findings | Reference |
|---|---|---|---|---|
| Diabetes | STZ-rat model | 10 mg/kg bodyweight per os (p/o) | HbAIc level ↓ Glucose tolerance, Insulin resistance ↓ | ( Harini, Ezhumalai and Pugalendi, 2012) |
| 10–15 mg/kg p/o | Glucose digestion ↑ HbA1C levels ↓ Serum visfatin amount ↑ | ( Azizi, Goodarzi and Salemi, 2014) | ||
| 10–15 mg/kg p/o | FBS ↓ hyperglycemia-induced free radicals | ( Sadri et al., 2017) | ||
| 15 mg/kg p/o | Nesfatin-1 ↑ Insulin ↑ FBG level ↓ | ( Eskandari Mehrabadi and Salemi, 2016) | ||
| 10–15 mg/kg p/o | Adiponectin ↑ insulin ↑ serum resistin ↓ serum adiponectin ↑ | ( Salemi et al., 2018) | ||
| 0.1, 1 and 5 mg/kg intraperitonial (i/p) | Mechanical allodynia ↓ Hyperalgesia ↓ | ( Chundi et al., 2016) | ||
| 10–15 mg/kg p/o | The concentrations of VEGF, TNF-α and IL-1β in retina ↓ blood sugar ↓ inflammation ↓ angiogenesis ↓ | ( Mehrabadi et al., 2018) | ||
| 10–20 mg/kg/day p/o | Diabetic nephropathy × Renal TGF-β expression↑ | ( Ahad, 2013) | ||
| db/db diabetic mice model | 10–50 mg/kg/day red clover extract (Red clover extract containing 9.6% biochanin-A) p/o | Hepatic PPARα/γ stimulation ↑ Hepatic fatty acid synthase levels ↓ | ( Qiu et al., 2012b) | |
| STZ-diabetic C57BL/6 mice | 1 mg/kg/day p/o | Hepatic PPARα ↑ Lipid profile ↓ Glucose levels ↓ | ( Qiu et al., 2012a) | |
| High fat diet + streptozotocin | 10, 20 and 40 mg/kg | Glucose tolerance ↓ Insulin resistance ↓ Insulin sensitivity, SIRT-1 expression ↑ | ( Oza and Kulkarni, 2018) | |
| Streptozotocin- Nicotinamide rat model | 5 mg/kg | Fasting blood glucose ↓ Body weight ↑ | ( Ghadimi et al., 2019) | |
| Dyslipidaemia | HFD Mice model | 30mg/kg/day,60mg/kg/day, and 120mg/kg/day | LDL,total cholesterol ↓ Lipoprotein lipase and hepatic triglyceride lipase ↑ | ( Xue et al., 2017) |
| Obesity | HFD-induced obese rats. | p/o | HFD induced trace elements metabolism ↓ Glucose, insulin, ferritin, total cholesterol, phospholipids, free fatty acids ↓ | ( Antony Rathinasamy et al., 2020). |
| C57BL/6 mice HFD | 0.05% biochanin-A p/o | PPAR ↑ glucose 6-phosphatase and pyruvate kinase × Obesity-induced hepatic steatosis and insulin resistance × | ( Park et al., 2016) | |
| Cardiovascular disorders | Transient coronary ligation in Sprague-Dawley rats | 12.5, 25 and 50 mg/kg intragastrically | TLR4/NF-kB/NLRP3 signalling × AST, CK-MB, LDH enzyme releases ↓ | ( Bai et al., 2019) |
| apoE-/- mice fed with Western diet. | 50 mg/kg intragastrically | pro-inflammatory cytokines ↓ | ( Yu et al., 2020) | |
| isoproterenol-induced MI rats | 10 mg/kg body weight subcutaneously | antioxidant levels ↑ lipid peroxidation and detoxifying enzyme systems ↓ | Govindasami et al., 2020 | |
| Airway hyper responsiveness | Ovalbumin-Induced Airway Hyperresponsiveness BABL/c mice model | 100 μmol/kg, p/o | Total inflammatory cells ↓ Eosinophils ↓ Neutrophils ↓ Th1-released IL-2 & TNF-α ↓ Th2-released IL-4 & IL-5 ↓ | ( Ko et al., 2011) |
| Male Hartley guinea pigs | 10–30 μm | baseline tension and cumulative OVA-induced contractions in isolated sensitized guinea pig tracheal × degranulation of mast cells× inflammation ↓ | ( Ko et al., 2011) | |
| PM2.5-induced lung toxicity in SD rats | 5, 50,100 mg/kg intragastric administration | cell death ↓ release of pro-inflammatory mediators, MDA, LDH, AKP ↑ antioxidant enzymes levels ↑ | ( Xue et al., 2020). | |
| Osteoarthritis | Osteoarthritis rabbit model | 5–25 mM intra-articular injection | Cartilage damage ↓ MMP, NF-κB × TIMP-1 + | ( D. Q. Wu et al., 2014) |
| Anti-microbial activity | BALB/c mice+ intragastric administration of Salmonella | 2 μg/mL | Salmonella infection × AMPK/ULK1/mTOR pathway | Zhao et al., 2018a) |
| Neurological disorders: | ||||
| Cerebral ischemia: | C57BL/6 male mice middle cerebral artery occlusion induced Focal cerebral ischemia | 5,10 mg/kg intraperitoneal | GOT protein expression ↑ stroke lesion volume ↓ | ( Khanna, Stewart, Gnyawali, Harris, Balch, Spieldenner, Chandan K. Sen, et al., 2017) |
| Ischemic stroke Rat model | 10, 20, or 40 mg/kg/day | infarct size and brain edema ↓ SOD,GSH-Px ↑ MDA,NF-κB × Nrf2 nuclear translocation, HO-1 + | Guo et al., 2019) | |
| Middle cerebral artery occlusion (MCAO) rat model | 10, 20, or 40 mg/kg/day | inflammatory response TNF-α and IL-1β levels, MPO activity↓ p38 signalling ↓ | ( Wang et al., 2015b) | |
| Parkinson’s disease | SD Rats with Iron and Rotenone Co-treatment | 30 mg/kg | malondialdehyde ↓ glutathione levels ↑ striatal dopamine depletion↓ behavioural impairments ↓ maintains redox equilibrium | ( Yu et al., 2017) |
| C57BL/6 with enhanced neonatal Iron and MPTP co-treatment | 10–60 mg/kg | microglial p38 MAPK activation × behavioral and neurochemical deficits ↑ | ( Li et al., 2021b) | |
| Alzheimer's disease | LPS-induced PD rats | 12.5, 25, 50 mg/kg | inflammatory response ↓ MAPK signalling pathway × nitric oxide, IL-1, and TNF-α | ( Wang et al., 2016). |
| scopolamine-treated mice and natural aged cognitive deficit mice | 40 mg/kg | AchE activity ↓ GSH ↑ thiobarbituric acid, ROS ↓ pyknotic neurons ↓ | ( Biradar et al., 2014) | |
| ovariectomized (OVX) rats | 5,20,60 mg/kg | restore learning and memory deficits, MDA ↓ SOD and GSH-Px ↑ | ( Youn et al., 2016) |
Table 3. Pharmacological findings of biochanin-A established via in vitro experiments.
(↑increase, ↓decrease, × inhibit, + activation).
| Pharmacological application | Study model | Dose and route used | Findings | Reference |
|---|---|---|---|---|
| Diabetes | Cervical carcinoma (HeLa) cells | EC 50 = 1–4 mmol/L | PPAR receptor activator | ( Shen et al., 2006). |
| Obesity | C3H10T1/2 cells | _ | Mitochondrial respiration ↑ AMPK signalling + | ( Rahman et al., 2021) |
| Neuronal cells | _ | ER stress ↓ leptin signalling × | ( Horiuchi et al., 2021). | |
| Airway hyper responsiveness | OVA-Induced Tracheal Contractions In Vitro. | 10–30 μm | anti-spasmodic agent | ( Ko et al., 2011) |
| Osteoarthritis | IL-1β induced rabbit chondrocytes | 5, 25, 50 μM | Cartilage damage ↓ MMP, NF-κB × TIMP-1 + | ( D. Q. Wu et al., 2014) |
| Primary Rat Adipose-Derived Stem Cells (ADSCs). | 0.3 μM | Adipocyte differentiation × PPARγ, LPL, OPN and leptin ↓ OPG ↑ osteoblast differentiation ↑ adipogenesis × | Su et al., 2013) | |
| Inflammation | ADSCs | 0.1–1 μM | cytoplasmic lipid droplet accumulation × PPAR-γ × Runx2, OPG, RhoA protein, OCN ↑ | ( Su et al., 2013) |
| RAW 264.7 and HT-29 cell lines | 100 μM and 50 μM | NO production, LPS, IKK activity × NF-κB + IL-6, IL-1β, and TNF-α production in RAW264.7 cells ↓ cell proliferation in HT-29 cell line × | ( Kole et al., 2011) | |
| Anti-microbial activity | Potential bacterial pathogens of the human digestive system | 0.13mM 0.26–0.51 mM | Clostridium tertium, clostridium clostridioforme × | ( Flesar et al., 2009; Sklenickova et al., 2010 |
| in vitro antibacterial activity | 16 to 128 μg/mL | Growth inhibitory effect to gram-positive and gram-negative bacteria | ( Hummelova et al., 2015) | |
| C. pneumoniae, C. trachomatis | 12 μM & 6.5 μM buccal formulation | Chlamydia growth × Antichlamydial action + | ( Hanski et al., 2014). | |
| HeLa cells/Macrophages | _ | Salmonella infection × AMPK/ULK1/mTOR pathway | Zhao et al., 2018a) | |
| Neurological disorders: | ||||
| Cerebral ischemia: | Mouse hippocampal HT4 neural cells or primary cortical neurons | 25 and 50 μM | GOT mRNA expression ↑ glutamate-induced cell death × | ( Khanna, Stewart, Gnyawali, Harris, Balch, Spieldenner, Chandan K. Sen, et al., 2017) |
| L-glutamate-induced cytotoxic PC12 cell line | 1, 10, 50, and 100 μM | cytotoxicity ↓ glutathione ↑ LDH, caspase-3 | ( Tan et al., 2013) | |
| Parkinson’s disease | Rat mesencephalic neuron-glia cultures | 12.5, 25, 50 mg/kg i.p. | NADPH-oxidase, MDA formation, SOD, and GPx × dopamine neuronal loss ↓ | ( Wang et al., 2015a) |
| BV2 microglial cells | 1.25, 2.5, 5 μM | LPS related microglia activation × TNFα, IL-1β, nitric-oxide, and ROS ↓ | Chen et al., 2007) | |
| LPS treated mice BV 2 microglial cells | 1.25, 2.5, 5 μM | TNFα, IL-1β, nitric oxide, and ROS ↓ MAPK signalling × | ( WU et al., 2015) | |
| BV2 Microglia | 5, 10, 20 μM | PPAR-γ levels ↑ NF-κB release × | ( Zhang and Chen 2015) | |
| Alzheimer's disease | HCN 1-A cell line with H2O2 model | 0.5, 1 and 2 μg/mL | H2O2 induced neurotoxicity ↓ | ( Occhiuto et al., 2009) |
| PC12 cells with β-Amyloid-Induced Neurotoxicity | 100 μM | Amyloid beta induced cell toxicity ↓ cytochrome-c and Puma Bcl-2/Bax ↓ | ( Tan and Kim 2016) | |
| Microglial cells | 1.25, 2.5, 5, 10, 15, 20, 25, 30 μM | microglial activation × nitric oxide, IL-1b, and TNF-α ↓ ROS × MAPK signaling pathway × | ( Wang et al., 2016) | |
| In-vitro BACE-1 activity assay | 28 μM | BACE1 × strong binding between the chemical and the allosteric site of BACE1 |
3. Clinical trial on biochanin-A
A clinical trial is underway to check the effect of red clover extract including biochanin-A among post-menopausal women with osteopenia. The results are yet to be concluded ( University of Aarhus, 2015). The clinical trial data are summarized in Table 4
Table 4. Clinical trial on biochanin-A.
| Sl no. | Study design | Title of the study | Objective | Comparison to: |
|---|---|---|---|---|
| 1 | Double-blind parallel, randomized intervention trial ( University of Aarhus, 2015) | Postmenopausal women with osteopenia (low bone mineral density) | Effects of daily intake of fermented red clover extract on oestrogen dependent bone mineral resorption | Placebo |
4. Limitation of biochanin-A
Biochanin-A is a poorly soluble bioflavonoid, which prevents its oral absorption, despite having a rapid clearance and a broad apparent volume of distribution. The bioavailability of biochanin-A is poor. It was reported that biochanin-A undergoes extensive metabolism. The pharmacological value of biochanin-A was limited by its poor water solubility and low bioavailability. Numerous attempts have been made to increase the solubility and bioavailability of biochanin-A, including the use of dispersants, silver nanoparticles, liposomes, different film formulations for buccal delivery, nanostructured lipid carriers with and without polyethylene glycol, and cyclodextrin inclusion complexes. Esters of biochanin-A and carbamate ester derivatives have also been developed, and they have higher metabolic stability than biochanin-A. Thus, several attempts are being made worldwide to enhance biochanin-A’s solubility and bioavailability without compromising its effectiveness ( Yu et al., 2019).
5. Conclusion
This review discusses various pharmacological applications of biochanin-A focussing on the molecular pathway that might be responsible for its beneficial action. Biochanin-A might be able to modify various systems of the human body like the cardiovascular system, CNS, respiratory system, etc. It has a remarkable effect on hormonal cancers and other types of cancers. The growing amount of research on biochanin-A in breast, lung, colon, prostate, pancreatic cancers is an illustration of its impact in medicine. Through modulating oxidative stress, SIRT-1 expression, PPAR-γ receptors, and other multiple mechanisms, biochanin-A produces anti-diabetic action. The diverse molecular mechanistic pathways involved in the pharmacological ability of biochanin-A indicate that it is a very promising molecule and have the potential to stimulate drug development for multiple disorders.
Acknowledgments
The authors would like to express their gratitude to Manipal Academy of Higher Education and Manipal College of Pharmaceutical Sciences for the support provided in the preparation of this manuscript.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 3; peer review: 2 approved]
Data availability
No data are associated with this article.
References
- Ahad A: Biochanin-A ameliorates experimental diabetic nephropathy by reducing the hyperglycemia induced oxidative stress and renal TGF-β expression. Journal of Diabetes & Metabolism. 2013;s4:6156. 10.4172/2155-6156.S1.024 [DOI] [Google Scholar]
- Ahsan M, Mallick AK: The effect of soy isoflavones on the menopause rating scale scoring in perimenopausal and postmenopausal women: A pilot study. J. Clin. Diagn. Res. 2017;11(9):FC13. 10.7860/JCDR/2017/26034.10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldhahri RS, et al. : Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis. Behav. Sci. 2022;12(3):70. 10.3390/bs12030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio R, et al. : Isoflavone Extracts Enhance the Effect of Epidermal Growth Factor Receptor Inhibitors in NSCLC Cell Lines. Anticancer Res. 2016;36(11):5827–5834. 10.21873/anticanres.11167 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 Suppl 1(Supplement_1):S62–S67. 10.2337/dc09-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri J, et al. : Biochanin-A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF-β1 and PAR-2 genes expression in kidney tissues of STZ-induced diabetic rats. Biotechnol. Appl. Biochem. 2021;69:2112–2121. 10.1002/bab.2272 [DOI] [PubMed] [Google Scholar]
- Andugulapati SB, et al. : Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine. 2020;78:153298. 10.1016/j.phymed.2020.153298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony Rathinasamy JIR, et al. : Antiobesity Effect of Biochanin-A: Effect on Trace Element Metabolism in High Fat Diet-Induced Obesity in Rats. Cardiovasc. Hematol. Agents Med. Chem. 2020;18(1):21–30. 10.2174/1871524920666200207101920 [DOI] [PubMed] [Google Scholar]
- Applegate CC, et al. : Soy consumption and the risk of prostate cancer: An updated systematic review and meta-analysis. Nutrients. 2018;10(1). 10.3390/nu10010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan AA, et al. : Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 2010;170(9):791–802. 10.1001/archinternmed.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov AG, Zotchev SB, Dirsch VM, et al. : Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021;20(3):200–216. 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi R, Goodarzi MT, Salemi Z: Effect of Biochanin A on Serum Visfatin Level of Streptozocin-Induced Diabetic Rats. Iran Red Crescent Med J. 2014;16(9):e15424. 10.5812/ircmj.15424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, et al. : Effects of Protein Tyrosine Kinase Inhibitors with Different Modes of Action on Topoisomerase Activity and Death of IL-2-Dependent CTLL-2 Cells1. The Journal of Biochemistry. 1995;118(2):312–318. 10.1093/oxfordjournals.jbchem.a124908 [DOI] [PubMed] [Google Scholar]
- Baba AI, Câtoi C: TUMOR CELL MORPHOLOGY. The Publishing House of the Romanian Academy;2007. [PubMed] [Google Scholar]
- Baghurst PA, et al. : A case-control study of diet and cancer of the pancreas. Am. J. Epidemiol. 1991;134(2):167–179. 10.1093/oxfordjournals.aje.a116069 [DOI] [PubMed] [Google Scholar]
- Bai Y, et al. : Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir. Bras. 2019;34(11):e201901104. 10.1590/s0102-865020190110000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj V, et al. : Biochanin A reduces pancreatic cancer survival and progression. Anti-Cancer Drugs. 2014;25(3):296–302. 10.1097/CAD.0000000000000044 [DOI] [PubMed] [Google Scholar]
- Bhushan A, Jain A, Lai JCK: Biochanin A: An isoflavone with anti-cancer and anti-invasive properties. J. Nutr. Food Sci. 2015;05. 10.4172/2155-9600.S1.017 [DOI] [Google Scholar]
- Biradar S, Joshi H, Chheda T: Biochanin-A ameliorates behavioural and neurochemical derangements in cognitive-deficit mice for the betterment of Alzheimer’s disease. Hum. Exp. Toxicol. 2014;33(4):369–382. 10.1177/0960327113497772 [DOI] [PubMed] [Google Scholar]
- Boominathan L: Natural product derived-therapy for Regenerating the lost pancreatic β-cells in Diabetic patients: Biochanin A, an O-methylated isoflavone found in r. clover, in soy, in alfalfa sprouts, in peanuts, and in chickpea, increases the expression of IGF1R, INSR, Genome-2-Bio-Medicine Discovery center (GBMD). 2017. (Accessed: 30 July 2020). Reference Source
- Bostwick DG, et al. : Human prostate cancer risk factors. Cancer. 2004;101(10 SUPPL):2371–2490. 10.1002/cncr.20408 [DOI] [PubMed] [Google Scholar]
- Boyle C, et al. : Phytoestrogens and healt. Phytochemical Functional Foods. 2003(May), pp.65–87. 10.1016/B978-1-85573-672-6.50009-5 [DOI] [Google Scholar]
- Breikaa RM, et al. : Multimechanistic Antifibrotic Effect of Biochanin A in Rats: Implications of Proinflammatory and Profibrogenic Mediators. PLoS One. Edited by Mukhopadhyay P.2013a;8(7):e69276. 10.1371/journal.pone.0069276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breikaa RM, et al. : Biochanin A Protects against Acute Carbon Tetrachloride-Induced Hepatotoxicity in Rats. Biosci. Biotechnol. Biochem. 2013b;77(5):909–916. 10.1271/bbb.120675 [DOI] [PubMed] [Google Scholar]
- Campbell DR, Kurzer MS: Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1993;46(3):381–388. 10.1016/0960-0760(93)90228-O [DOI] [PubMed] [Google Scholar]
- Chang H-H, et al. : Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One. 2017;12(9):e0184455. Edited by I. Rooman. 10.1371/journal.pone.0184455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-Q, Jin Z-Y, Li G-H: Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007;417(2):112–117. 10.1016/j.neulet.2006.11.045 [DOI] [PubMed] [Google Scholar]
- Chen LR, Ko NY, Chen KH: Isoflavone supplements for menopausal women: A systematic review. Nutrients. 2019;11(11):1–15. 10.3390/nu11112649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chundi V, et al. : Biochanin-A attenuates neuropathic pain in diabetic rats. Journal of Ayurveda and Integrative Medicine. 2016;7(4):231–237. Elsevier Limited and Science Press. 10.1016/j.jaim.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, McLachlan JA, Arnold SF: The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids. 1997;62(4):365–372. 10.1016/S0039-128X(96)00246-2 [DOI] [PubMed] [Google Scholar]
- Costa RL, Summa MA: Soy Protein in the Management of Hyperlipidemia. Ann. Pharmacother. 2000;34(7–8):931–935. 10.1345/aph.19371 [DOI] [PubMed] [Google Scholar]
- Derangula M, Panati K, Narala VR: Biochanin A Ameliorates Ovalbumin-induced Airway Inflammation through Peroxisome Proliferator-Activated Receptor-Gamma in a Mouse Model. Endocr. Metab. Immune Disord. Drug Targets. 2021;21(1):145–155. Bentham Science Publishers Ltd. 10.2174/1871530320666200503051609 [DOI] [PubMed] [Google Scholar]
- Desai V, Bhushan A: Natural Bioactive Compounds: Alternative Approach to the Treatment of Glioblastoma Multiforme. Biomed. Res. Int. 2017;2017:1–10. Hindawi. 10.1155/2017/9363040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai V, et al. : Abstract 4193: Biochanin A in pancreatic cancer. Tumor Biol. 2018;78:4193–4193. American Association for Cancer Research. 10.1158/1538-7445.AM2018-4193 [DOI] [Google Scholar]
- Desai V, et al. : Combination of Biochanin A and Temozolomide Impairs Tumor Growth by Modulating Cell Metabolism in Glioblastoma Multiforme. Anticancer Res. 2019;39(1):57–66. 10.21873/anticanres.13079 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Sumner LW: Legume Natural Products: Understanding and Manipulating Complex Pathways for Human and Animal Health. Plant Physiol. 2003;131(3):878–885. 10.1104/pp.102.017319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, et al. : Biochanin A Sensitizes Glioblastoma to Temozolomide by Inhibiting Autophagy. Mol. Neurobiol. 2022;59(2):1262–1272. 10.1007/s12035-021-02674-6 [DOI] [PubMed] [Google Scholar]
- Eskandari Mehrabadi M, Salemi Z: Effect of Biochanin A on serum nesfatin-1 Level in STZ induced type 1 diabetic rat. Diabetologie und Stoffwechsel. 2016;11(S 01):P225. Georg Thieme Verlag KG. 10.1055/s-0036-1580972 [DOI] [Google Scholar]
- Evans BAJ, Griffiths K, Morton MS: Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995;147(2):295–302. 10.1677/joe.0.1470295 [DOI] [PubMed] [Google Scholar]
- Felix FB, et al. : Biochanin A Regulates Key Steps of Inflammation Resolution in a Model of Antigen-Induced Arthritis via GPR30/PKA-Dependent Mechanism. Front. Pharmacol. 2021;12. 10.3389/fphar.2021.662308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter HW, Lloyd JU: King`s American Dispensatory. 1999.
- Feng Z-J, Lai W-F: Chemical and Biological Properties of Biochanin A and Its Pharmaceutical Applications. Pharmaceutics. 2023;15(4):1105. 10.3390/pharmaceutics15041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Thurman EM: GC–MS–MS for the Analysis of Phytoestrogens in the Environment. Compr. Anal. Chem. 2013. Elsevier. 10.1016/B978-0-444-62623-3.00010-1 [DOI] [Google Scholar]
- Flesar J, et al. : Selective antimicrobial activity of biochanin A. Planta Med. 2009;75(09). 10.1055/s-0029-1234894 [DOI] [Google Scholar]
- Franco OH, et al. : Use of plant-based therapies and menopausal symptoms: A systematic review and meta-analysis. JAMA - Journal of the American Medical Association. 2016;315(23):2554–2563. 10.1001/jama.2016.8012 [DOI] [PubMed] [Google Scholar]
- Fung MC, et al. : Effects of biochanin A on the growth and differentiation of myeloid leukemia WEHI-3B (JCS) cells. Life Sci. 1997;61(2):105–115. 10.1016/S0024-3205(97)00365-2 [DOI] [PubMed] [Google Scholar]
- Ghadimi D, et al. : The Effect of Biochanin A as PPAR γ agonist on LDL Particles Diameter and Type 2 Diabetic Dyslipidemia. International Journal of Medical Laboratory. 2019;6(2):107–114. 10.18502/ijml.v6i2.1028 [DOI] [Google Scholar]
- Gil-Izquierdo A, et al. : Soy Isoflavones and Cardiovascular Disease Epidemiological, Clinical and -Omics Perspectives. Curr. Pharm. Biotechnol. 2012;13(5):624–631. 10.2174/138920112799857585 [DOI] [PubMed] [Google Scholar]
- Gotoh T, et al. : Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn. J. Cancer Res. 1998;89(2):137–142. 10.1111/j.1349-7006.1998.tb00541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasami S, et al. : Therapeutic Potential of Biochanin-A Against Isoproterenol-Induced Myocardial Infarction in Rats. Cardiovasc. Hematol. Agents Med. Chem. 2020;18(1):31–36. Bentham Science Publishers Ltd. 10.2174/1871525718666200206114304 [DOI] [PubMed] [Google Scholar]
- Guo M, et al. : Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury by Nrf2-Mediated Inhibition of Oxidative Stress and Inflammation Signaling Pathway in Rats. Medical science monitor: international medical journal of experimental and clinical research. 2019;25:8975–8983. 10.12659/MSM.918665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EH, Ji YK, Hye GJ: Effect of biochanin A on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Arch. Pharm. Res. 2006;29(7):570–576. 10.1007/BF02969267 [DOI] [PubMed] [Google Scholar]
- Hanski L, et al. : Inhibitory Activity of the Isoflavone Biochanin A on Intracellular Bacteria of Genus Chlamydia and Initial Development of a Buccal Formulation. PLoS One. 2014;9(12):e115115. Edited by J.-P. Gorvel. 10.1371/journal.pone.0115115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harini R, Ezhumalai M, Pugalendi KV: Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 2012;676(1–3):89–94. Elsevier B.V. 10.1016/j.ejphar.2011.11.051 [DOI] [PubMed] [Google Scholar]
- Harlow BE, Flythe MD, Klotz JL, et al. : Effect of biochanin A on the rumen microbial community of Holstein steers consuming a high fiber diet and subjected to a subacute acidosis challenge. PLoS One. 2021;16(7):e0253754. Edited by J. J. Loor. 10.1371/journal.pone.0253754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylewicz ÂJ, Zapata JJ, Blair AVH: Soy Intake and Cancer Risk: Animal and Epidemiologie Studies Soy And Experimental Cancer: Animal Studies i. 1995; pp.709–712.
- He FJ, Chen JQ: Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Human Wellness. 2013;2(3–4):146–161. Beijing Academy of Food Sciences. 10.1016/j.fshw.2013.08.002 [DOI] [Google Scholar]
- Hempstock, Kavanagh, George: Growth inhibition of prostate cell lines in vitro by phyto-oestrogens. BJU Int. 1998;82(4):560–563. 10.1046/j.1464-410X.1998.00769.x [DOI] [PubMed] [Google Scholar]
- Hillman GG, Singh-Gupta V: Soy isoflavones sensitize cancer cells to radiotherapy. Free Radic. Biol. Med. 2011;51(2):289–298. 10.1016/j.freeradbiomed.2011.04.039 [DOI] [PubMed] [Google Scholar]
- Horiuchi K, et al. : The possible role of biochanin A in ameliorating endoplasmic reticulum stress-induced leptin resistance. NeuroReport. 2021;32(12):983–987. 10.1097/WNR.0000000000001674 [DOI] [PubMed] [Google Scholar]
- Hsu Y-N, et al. : Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem. Toxicol. 2018;112(March 2017):194–204. Elsevier. 10.1016/j.fct.2017.12.062 [DOI] [PubMed] [Google Scholar]
- Hummelova J, et al. : The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett. Appl. Microbiol. 2015;60(3):242–247. 10.1111/lam.12361 [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Felson DT: Osteoarthritis. BMJ. 2006;332(7542):639–642. BMJ Publishing Group. 10.1136/bmj.332.7542.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen BK, Knutsen SF, Fraser GE: Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States). Cancer Causes Control. 1998;9(6):553–557. 10.1023/A:1008819500080 [DOI] [PubMed] [Google Scholar]
- Jain A, Lai JCK, Bhushan A: Abstract 3523: Biochanin A enhances anticancer activity of temozolomide in glioblastoma multiforme. Experimental and Molecular Therapeutics. American Association for Cancer Research;2011; pp.3523–3523. 10.1158/1538-7445.AM2011-3523 [DOI] [Google Scholar]
- Jain A, Lai JCK, Bhushan A: Biochanin A inhibits endothelial cell functions and proangiogenic pathways. Anti-Cancer Drugs. 2015;26(3):323–330. 10.1097/CAD.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Jaina VK, et al. : Anti-cancer activity of Biochanin A against multiple myeloma by targeting the CD38 and cancer stem-like cells. Process Biochem. 2022;123:11–26. 10.1016/j.procbio.2022.10.029 [DOI] [Google Scholar]
- Jarred RA, et al. : Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol. Biomarkers Prev. 2002;11(12):1689–1696. Reference Source [PubMed] [Google Scholar]
- Jin H, et al. : Cancer incidence among Asian American populations in the United States, 2009-2011. Int. J. Cancer. 2016;138(9):2136–2145. 10.1002/ijc.29958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, et al. : Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. 2010;182(17):1857–1862. 10.1503/cmaj.091298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, et al. : Phytoestrogen isoflavone intervention to engage the neuroprotective effect of glutamate oxaloacetate transaminase against stroke. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2017;31(10):4533–4544. 10.1096/fj.201700353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W-C, et al. : Biochanin A, a Phytoestrogenic Isoflavone with Selective Inhibition of Phosphodiesterase 4, Suppresses Ovalbumin-Induced Airway Hyperresponsiveness. Evid. Based Complement. Alternat. Med. 2011;2011:1–13. 10.1155/2011/635058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K-P, et al. : Intake of soy products and other foods and gastric cancer risk: a prospective study. J. Epidemiol. 2013;23(5):337–343. 10.2188/jea.je20120232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen F, et al. : Synthesis and Evaluation of the Antiproliferative Activities of Derivatives of Carboxyalkyl Isoflavones linked to N-t-Boc-hexylenediamine. J. Med. Chem. 2007;50(25):6405–6410. 10.1021/jm070727z [DOI] [PubMed] [Google Scholar]
- Kole L, et al. : Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011;653(1–3):8–15. Elsevier B.V. 10.1016/j.ejphar.2010.11.026 [DOI] [PubMed] [Google Scholar]
- Křížová L, Dadáková K, Kašparovská J, et al. : Isoflavones. Molecules. 2019;24(6):1076. 10.3390/molecules24061076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail JC, et al. : Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett. 1998;133(1):101–106. 10.1016/S0304-3835(98)00211-0 [DOI] [PubMed] [Google Scholar]
- Lecomte S, et al. : Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int. J. Mol. Sci. 2017;18(7):1–19. 10.3390/ijms18071381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, et al. : Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene. J. Korean Med. Sci. 1991;6(4):325–328. 10.3346/jkms.1991.6.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemežienė N, et al. : The concentration of isoflavones in red clover (Trifolium pratense L.) at flowering stage. Zemdirbyste-Agriculture. 2015;102(4):443–448. 10.13080/z-a.2015.102.057 [DOI] [Google Scholar]
- Leukaemia Care: Leukaemia|Leukaemia Care. 2020. (Accessed: 17 November 2020). Reference Source
- Li D: Body Mass Index and Risk, Age of Onset, and Survival in Patients With Pancreatic Cancer. JAMA. 2009;301(24):2553–2562. 10.1001/jama.2009.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. : Biochanin A Induces S Phase Arrest and Apoptosis in Lung Cancer Cells. Biomed. Res. Int. 2018;2018:1–12. Hindawi. 10.1155/2018/3545376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. : Investigation on the mechanisms of biochanin A alleviate PM10-induced acute pulmonary cell injury. Ecotoxicol. Environ. Saf. 2021a;228:112953. 10.1016/j.ecoenv.2021.112953 [DOI] [PubMed] [Google Scholar]
- Li Y, et al. : Aggravated behavioral and neurochemical deficits and redox imbalance in mice with enhanced neonatal iron intake: improvement by biochanin A and role of microglial p38 activation. Nutr. Neurosci. 2021b;24(3):161–172. 10.1080/1028415X.2019.1611021 [DOI] [PubMed] [Google Scholar]
- Liang F, et al. : Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors. 2019;45:biof.1514–biof.1574. 10.1002/biof.1514 [DOI] [PubMed] [Google Scholar]
- Ling-hui Z, et al. : Antiatheroscloresis effects of biochanin A on hyperlipidemia rats. Chinese Journal of Biochemical Pharmaceutics. 2012. [Google Scholar]
- Lowenfels AB, Maisonneuve P: Risk factors for pancreatic cancer. J. Cell. Biochem. 2005;95(4):649–656. 10.1002/jcb.20461 [DOI] [PubMed] [Google Scholar]
- Luo Q, et al. : Network Pharmacology Integrated Molecular Docking Reveals the Antiosteosarcoma Mechanism of Biochanin A. Evid. Based Complement. Alternat. Med. 2019;2019:1–10. 10.1155/2019/1410495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M, et al. : Abstract B47: A novel combination of Biochanin-A/5-Fluorouracil against colon cancer: Impact on Wnt/beta-catenin signaling. Oncogenic Signals Translate the Cancer Genome. 2017;77:B47–B47. American Association for Cancer Research. 10.1158/1538-7445.Transcontrol16-B47 [DOI] [Google Scholar]
- Mahmoud M, et al. : The natural isoflavone Biochanin-A synergizes 5-fluorouracil anticancer activity in vitro and in vivo in Ehrlich solid-phase carcinoma model. Phytotherapy research: PTR. 2022;36(3):1310–1325. 10.1002/ptr.7388 [DOI] [PubMed] [Google Scholar]
- Medjakovic S, Jungbauer A: Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008;108(1–2):171–177. 10.1016/j.jsbmb.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Mehrabadi ME, et al. : Effect of Biochanin A on Retina Levels of Vascular Endothelial Growth Factor, Tumor Necrosis Factor-Alpha and Interleukin-1Beta in Rats With Streptozotocin-Induced Diabetes. Can. J. Diabetes. 2018;42(6):639–644. Elsevier Inc. 10.1016/j.jcjd.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Mense SM, et al. : Review Phytoestrogens and Breast Cancer Prevention: Possible Mechanisms of Action. 2008;426(4): pp.426–433. 10.1289/ehp.10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M, Hilakivi-Clarke L: Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr. Cancer. 2009;61(6):792–798. 10.1080/01635580903285015 [DOI] [PubMed] [Google Scholar]
- Michael McClain R, et al. : Genetic toxicity studies with genistein. Food Chem. Toxicol. 2006;44(1):42–55. 10.1016/j.fct.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Migkos T, et al. : Biochanin A, the Most Potent of 16 Isoflavones, Induces Relaxation of the Coronary Artery Through the Calcium Channel and cGMP-dependent Pathway. Planta Med. 2020;86(10):708–716. 10.1055/a-1158-9422 [DOI] [PubMed] [Google Scholar]
- Mohana Lakshmi S, Rani KSS, Reddy UTK: A review on diabetes milletus and the herbal plants used for its treatment. Asian J. Pharm. Clin. Res. 2012;5(4):15–21. [Google Scholar]
- Moore PS, et al. : Genetic abnormalities in pancreatic cancer. Mol. Cancer. 2003;2(June 2014):7. 10.1186/1476-4598-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P, et al. : A biochanin-enriched isoflavone from red clover lowers LDL cholesterol in men. Eur. J. Clin. Nutr. 2004;58(3):403–408. 10.1038/sj.ejcn.1601796 [DOI] [PubMed] [Google Scholar]
- Occhiuto F, et al. : The isoflavones mixture from Trifolium pratense L. protects HCN 1-A neurons from oxidative stress. Phytother. Res. 2009;23(2):192–196. 10.1002/ptr.2584 [DOI] [PubMed] [Google Scholar]
- Oza MJ, Kulkarni YA: Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018;107(April):1119–1127. Elsevier. 10.1016/j.biopha.2018.08.073 [DOI] [PubMed] [Google Scholar]
- Oza MJ, Kulkarni YA: Biochanin A Attenuates Cardiomyopathy in Type 2 Diabetic Rats by Increasing SIRT1 Expression and Reducing Oxidative Stress. Chem. Biodivers. 2022;19(3):e202100591. 10.1002/cbdv.202100591 [DOI] [PubMed] [Google Scholar]
- Panche AN, Diwan AD, Chandra SR: Flavonoids: An overview. Journal of Nutritional Science. 2016;5:e47. 10.1017/jns.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-S, et al. : Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 2016;60(9):1944–1955. 10.1002/mnfr.201500689 [DOI] [PubMed] [Google Scholar]
- Passamonti S, et al. : Bioavailability of Flavonoids: A Review of Their Membrane Transport and the Function of Bilitranslocase in Animal and Plant Organisms. Curr. Drug Metab. 2009;10(4):369–394. 10.2174/138920009788498950 [DOI] [PubMed] [Google Scholar]
- Patel DK, et al. : An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012;2(4):320–330. 10.1016/S2221-1691(12)60032-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G, Barnes S: Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22(4):335–345. 10.1002/pros.2990220408 [DOI] [PubMed] [Google Scholar]
- Pollard M, Wolter W, Sun L: Prevention of induced prostate-related cancer by soy protein isolate/isoflavone-supplemented diet in Lobund-Wistar rats. In vivo (Athens, Greece). 2000;14(3), pp.389–392. (Accessed: 12 July 2020). [PubMed] [Google Scholar]
- Pop EA, et al. : Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: A phase I clinical trial. Menopause. 2008;15(4):684–692. 10.1097/gme.0b013e318167b8f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puli S: Regulation of invasion, signaling, and gene expression in human glioblastoma cells (U87): Effect of isoflavones, and manganese. Idaho State University;2006. [Google Scholar]
- Puli S, Lai JCK, Bhushan A: Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) Cells by isoflavones. J. Neuro-Oncol. 2006;79(2):135–142. 10.1007/s11060-006-9126-0 [DOI] [PubMed] [Google Scholar]
- Puthli A, Tiwari R, Mishra KP: Biochanin a enhances the radiotoxicity in colon tumor cells in vitro. J. Environ. Pathol. Toxicol. Oncol. 2013;32(3):189–203. 10.1615/JEnvironPatholToxicolOncol.2013007280 [DOI] [PubMed] [Google Scholar]
- Qin Y, et al. : Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst. Rev. 2013;2013(6):CD009518. 10.1002/14651858.CD009518.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Ye H, et al. : Red Clover Extract Ameliorates Dyslipidemia in Streptozotocin-induced Diabetic C57BL/6 Mice by Activating Hepatic PPARα. Phytother. Res. 2012a;26(6):860–864. 10.1002/ptr.3641 [DOI] [PubMed] [Google Scholar]
- Qiu L, Chen T, et al. : Red clover extract exerts antidiabetic and hypolipidemic effects in db/db mice. Exp. Ther. Med. 2012b;4(4):699–704. 10.3892/etm.2012.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheja S, et al. : Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018;79:55–66. Elsevier Ltd. 10.1016/j.tifs.2018.07.001 [DOI] [Google Scholar]
- Rahman MS, et al. : Biochanin A induces a brown-fat phenotype via improvement of mitochondrial biogenesis and activation of AMPK signaling in murine C 3H 10T 1/2 mesenchymal stem cells. Phytother. Res. 2021;35(2):920–931. 10.1002/ptr.6845 [DOI] [PubMed] [Google Scholar]
- Rani A, et al. : Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Arch. Physiol. Biochem. 2021;1–16. 10.1080/13813455.2021.1874017 [DOI] [PubMed] [Google Scholar]
- Rice L, et al. : Mechanisms of the growth inhibitory effects of the isoflavonoid biochanin A on LNCaP cells and xenografts. Prostate. 2002;52(3):201–212. 10.1002/pros.10100 [DOI] [PubMed] [Google Scholar]
- Rice S, Mason HD, Whitehead SA: Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. 2006;101: pp.216–225. 10.1016/j.jsbmb.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Romm A, et al. : 19 - Menopausal Health. First Edit, Botanical Medicine for Women’s Health. First Edit. Elsevier Inc.;2010. 10.1016/B978-0-443-07277-2.00021-0 [DOI] [Google Scholar]
- Rus Jacquet A, et al. : Neuroprotective mechanisms of red clover and soy isoflavones in Parkinson’s disease models. Food Funct. 2021;12:11987–12007. 10.1039/D1FO00007A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks FM, et al. : Soy Protein, Isoflavones, and Cardiovascular Health. Circulation. 2006;113(7):1034–1044. 10.1161/CIRCULATIONAHA.106.171052 [DOI] [PubMed] [Google Scholar]
- Sadri H, et al. : Antioxidant Effects of Biochanin A in Streptozotocin Induced Diabetic Rats. Braz. Arch. Biol. Technol. 2017;60(December):1–10. 10.1590/1678-4324-2017160741 [DOI] [Google Scholar]
- Saif MW: Genetic alterations in pancreatic cancer. World J. Gastroenterol. 2007;13(33):4423–4430. 10.3748/wjg.v13.i33.4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi Z, et al. : Effects of Biochanin A on Resistin, Adiponectin and Some Stress Oxidative Markers in Normal and STZ-Induced Diabetic Rats. Arch. Med. Lab. Sci. 2018; (4):9–16. 10.22037/amls.v4i2.24908 [DOI] [Google Scholar]
- Sarkar FH, et al. : Cellular signaling perturbation by natural products. Cell. Signal. 2009;21(11):1541–1547. Elsevier Inc. 10.1016/j.cellsig.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapalan T, et al. : Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018;28(7):691–697. The societies SID, SISA and SINU and the Department of Clinical Medicine and Surgery at Federico II University in Italy. 10.1016/j.numecd.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Sehdev V, Lai JCK, Bhushan A: Biochanin A Modulates Cell Viability, Invasion, and Growth Promoting Signaling Pathways in HER-2-Positive Breast Cancer Cells. J. Oncol. 2009;2009:1–10. 10.1155/2009/121458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm T, et al. : The impact of dietary isoflavonoids on malignant brain tumors. Cancer Med. 2014;3(4):865–877. 10.1002/cam4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YJ, et al. : Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011;26(11):1489–1494. 10.3346/jkms.2011.26.11.1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell KDR, et al. : Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2001;131(4):1362S–1375S. 10.1093/jn/131.4.1362S [DOI] [PubMed] [Google Scholar]
- Shen P, et al. : Differential Effects of Isoflavones, from Astragalus Membranaceus and Pueraria Thomsonii, on the Activation of PPARα, PPARγ, and Adipocyte Differentiation In Vitro. J. Nutr. 2006;136(4):899–905. 10.1093/jn/136.4.899 [DOI] [PubMed] [Google Scholar]
- Shergalis A, et al. : Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018;70(3):412–445. Edited by E. L. Barker. 10.1124/pr.117.014944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XO, et al. : ‘Soy Food Intake and Breast Cancer Survival [Original Contribution]. JAMA: J. Am. Med. Assoc. 2009;302(22):2437–2443. 10.1001/jama.2009.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Silverman DT, et al. : Dietary and Nutritional Factors and Pancreatic Cancer: a Case-Control Study Based on Direct Interviews. JNCI Journal of the National Cancer Institute. 1998;90(22):1710–1719. 10.1093/jnci/90.22.1710 [DOI] [PubMed] [Google Scholar]
- Singh IP, et al. : Natural Products: Drug Discovery and Development. Drug Discovery and Development. Singapore: Springer Singapore;2021; pp.11–65. 10.1007/978-981-15-5534-3_2 [DOI] [Google Scholar]
- Singh VK, Sahu MK: Antidiabetic Medicinal Plants Having Insulin Mimetic Property: A Review. Kenkyu Journal of Pharmacy Practice & Health Care. 2018;60:56–60. 10.31872/2018/KJPHC-100115 [DOI] [Google Scholar]
- Singh SK, et al. : A novel nanosized phospholipid complex of Biochanin A for improving oral bioavailability: Preparation and in-vitro/in-vivo characterizations. Journal of Drug Delivery Science and Technology. 2021;61:102254. 10.1016/j.jddst.2020.102254 [DOI] [Google Scholar]
- Singh-Gupta V, et al. : Soy Isoflavones Augment Radiation Effect by Inhibiting APE1/Ref-1 DNA Repair Activity in Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011;6(4):688–698. International Association for the Study of Lung Cancer. 10.1097/JTO.0b013e31821034ae [DOI] [PubMed] [Google Scholar]
- Sivashanmugam T, et al. : Discovery of potential cholesterol esterase inhibitors using in silico docking studies. Bangladesh Journal of Pharmacology. 2013;8(3). 10.3329/bjp.v8i3.14521 [DOI] [Google Scholar]
- Skaper SD, et al. : An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018;12. 10.3389/fncel.2018.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklenickova O, et al. : Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria. Molecules. 2010;15(3):1270–1279. 10.3390/molecules15031270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M, Brown D, Husband A: In the prostatic epithelium, dietary isoflavones from red clover significantly increase estrogen receptor β and E-cadherin expression but decrease transforming growth factor β1. Prostate Cancer Prostatic Dis. 2002;5(1):16–21. 10.1038/sj/pcan/4500546 [DOI] [PubMed] [Google Scholar]
- Snyder RD, Gillies PJ: Evaluation of the clastogenic, DNA intercalative, and topoisomerase II-interactive properties of bioflavonoids in Chinese hamster V79 cells. Environ. Mol. Mutagen. 2002;40(4):266–276. 10.1002/em.10121 [DOI] [PubMed] [Google Scholar]
- Su S-J, et al. : Biochanin A Promotes Osteogenic but Inhibits Adipogenic Differentiation: Evidence with Primary Adipose-Derived Stem Cells. Evid. Based Complement. Alternat. Med. 2013;2013:1–12. 10.1155/2013/846039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XY, et al. : Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 1998;58(11):2379–2384. [PubMed] [Google Scholar]
- Surya S, et al. : Diabetes mellitus and medicinal plants-a review. Asian Pacific Journal of Tropical Disease. 2014;4(5):337–347. 10.1016/S2222-1808(14)60585-5 [DOI] [Google Scholar]
- Szliszka E, et al. : The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urologic Oncology: Seminars and Original Investigations. 2013;31(3):331–342. Elsevier Inc. 10.1016/j.urolonc.2011.01.019 [DOI] [PubMed] [Google Scholar]
- Tan J, Kim M: Neuroprotective Effects of Biochanin A against β-Amyloid-Induced Neurotoxicity in PC12 Cells via a Mitochondrial-Dependent Apoptosis Pathway. Molecules. 2016;21(5):548. 10.3390/molecules21050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JW, et al. : Neuroprotective Effects of Biochanin A Against Glutamate-Induced Cytotoxicity in PC12 Cells Via Apoptosis Inhibition. Neurochem. Res. 2013;38(3):512–518. 10.1007/s11064-012-0943-6 [DOI] [PubMed] [Google Scholar]
- Tedeschi-Blok N, et al. : Inverse association of antioxidant and phytoestrogen nutrient intake with adult glioma in the San Francisco Bay Area: a case-control study. BMC Cancer. 2006;6(1):148. 10.1186/1471-2407-6-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR: Management of dyslipidaemia. Heart. 2004;90(8):949–955. 10.1136/hrt.2003.021287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Sugioka Y, Koike T: Soybean isoflavone can protect against osteoarthritis in ovariectomized rats. J. Food Sci. Technol. 2020;57(9):3409–3414. Springer India. 10.1007/s13197-020-04374-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HN, et al. : Low Cancer Risk of South Asians: A Brief Report. Perm. J. 2018;22:15–17. 10.7812/TPP/17-095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. FDA: Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed. Regist. 1999;64(206):57700–57733. [PubMed] [Google Scholar]
- Ullah MF, et al. : Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol. Nutr. Food Res. 2009;53(11):1376–1385. 10.1002/mnfr.200800547 [DOI] [PubMed] [Google Scholar]
- University of Aarhus: Isoflavone Treatment for Postmenopausal Osteopenia. 2015. NCT02174666.
- Varinska L, et al. : Soy and breast cancer: Focus on angiogenesis. Int. J. Mol. Sci. 2015;16(5):11728–11749. 10.3390/ijms160511728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennila L, Asaikumar L, Sivasangari S, et al. : Evaluation of In-Vitro Antioxidant Activity of Biochanin A. Journal of Drug Delivery and Therapeutics. 2019;9(4-A):594–600. 10.22270/jddt.v9i4-A.3404 [DOI] [Google Scholar]
- Vinayagam R, Xu B: Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr. Metab. 2015;12(1):60. 10.1186/s12986-015-0057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggle DH, Potter SM, Henley EC: (12) United States Patent. united states. 2003.
- Wang J, et al. : Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2015a;138:96–103. Elsevier B.V. 10.1016/j.pbb.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Wang W, et al. : Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 2015b;348(1–2):121–125. 10.1016/j.jns.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Wang J, et al. : Biochanin A Protects Against Lipopolysaccharide-Induced Damage of Dopaminergic Neurons Both in vivo and in vitro via Inhibition of Microglial Activation. Neurotox. Res. 2016;30(3):486–498. 10.1007/s12640-016-9648-y [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Chen Y: Biochanin A extirpates the epithelial-mesenchymal transition in a human lung cancer. Exp. Ther. Med. 2018;15(3):2830–2836. 10.3892/etm.2018.5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, et al. : Study of the Hypoglycemic Activity of Derivatives of Isoflavones from Cicer arietinum L. Evid. Based Complement. Alternat. Med. 2017;2017:1–18. 10.1155/2017/8746823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DQ, et al. : Protective effects of biochanin A on articular cartilage: in vitro and in vivo studies. BMC Complement. Altern. Med. 2014;14(1):1–10. 10.1186/1472-6882-14-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-Y, et al. : Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015;35(2):391–398. 10.3892/ijmm.2014.2020 [DOI] [PubMed] [Google Scholar]
- Xu J-W, et al. : Isoflavones regulate secretion of leukemia inhibitory factor and transforming growth factor β and expression of glycodelin in human endometrial epithelial cells. J. Endocrinol. 2008;196(2):425–433. 10.1677/JOE-07-0416 [DOI] [PubMed] [Google Scholar]
- Xue Z, et al. : Potential Lipid-Lowering Mechanisms of Biochanin A. J. Agric. Food Chem. 2017;65(19):3842–3850. 10.1021/acs.jafc.7b00967 [DOI] [PubMed] [Google Scholar]
- Xue Z, et al. : Amelioration of PM2.5-induced lung toxicity in rats by nutritional supplementation with biochanin A. Ecotoxicol. Environ. Saf. 2020;202(May):110878. Elsevier Inc. 10.1016/j.ecoenv.2020.110878 [DOI] [PubMed] [Google Scholar]
- Xue Z, et al. : Biochanin A alleviates oxidative damage caused by the urban particulate matter. Food Funct. 2021;12(5):1958–1972. 10.1039/D0FO02582H [DOI] [PubMed] [Google Scholar]
- Yamasaki M, et al. : Soy-derived isoflavones inhibit the growth of adult T-cell leukemia cells in vitro and in vivo. Cancer Sci. 2007;98(11):1740–1746. 10.1111/j.1349-7006.2007.00595.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Spitznagel EL, Bosland MC: Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2010;19(1):148–158. 10.1158/1055-9965.EPI-09-0856 [DOI] [PubMed] [Google Scholar]
- Yanagihara K, et al. : Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 1993;53(23):5815–5821. [PubMed] [Google Scholar]
- Yang W-S, et al. : Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2011;94(6):1575–1583. 10.3945/ajcn.111.020966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn K, et al. : The Identification of Biochanin A as a Potent and Selective β-Site App-Cleaving Enzyme 1 (Bace1) Inhibitor. Nutrients. 2016;8(10):637. 10.3390/nu8100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, et al. : Perspectives Regarding the Role of Biochanin A in Humans. Front. Pharmacol. 2019;10:793. 10.3389/fphar.2019.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, et al. : Neurochemical and Behavior Deficits in Rats with Iron and Rotenone Co-treatment: Role of Redox Imbalance and Neuroprotection by Biochanin A. Front. Neurosci. 2017;11. 10.3389/fnins.2017.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, et al. : Biochanin A Mitigates Atherosclerosis by Inhibiting Lipid Accumulation and Inflammatory Response. Oxidative Med. Cell. Longev. 2020;2020:1–15. Edited by A. Lloret. 10.1155/2020/8965047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen W: Biochanin A Inhibits Lipopolysaccharide-Induced Inflammatory Cytokines and Mediators Production in BV2 Microglia. Neurochem. Res. 2015;40(1):165–171. 10.1007/s11064-014-1480-2 [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. : Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013;43(1):289–296. 10.3892/ijo.2013.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. : Biochanin a Enhances the Defense Against Salmonella enterica Infection Through AMPK/ULK1/mTOR-Mediated Autophagy and Extracellular Traps and Reversing SPI-1-Dependent Macrophage (MΦ) M2 Polarization. Front. Cell. Infect. Microbiol. 2018a;8. 10.3389/fcimb.2018.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al. : The effect of biochanin A on cell growth, apoptosis, and migration in osteosarcoma cells. Pharmazie. 2018b;73(6):335–341. 10.1691/ph.2018.7351 [DOI] [PubMed] [Google Scholar]
- Zhou J-R, et al. : Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate. 2002;53(2):143–153. 10.1002/pros.10141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. : Biochanin A Attenuates Ovariectomy-Induced Cognition Deficit via Antioxidant Effects in Female Rats. Front. Pharmacol. 2021;12. 10.3389/fphar.2021.603316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei S, Halaby R: Dietary Isoflavones and Breast Cancer Risk. Medicines. 2017;4(4):18. MDPI AG. 10.3390/medicines4020018 [DOI] [PMC free article] [PubMed] [Google Scholar]