Abstract
The incidence of cholecystitis is relatively high in developed countries and may usually be attributed to gallstones, the treatment for which involves complete surgical removal of the gallbladder (cholecystectomy). Bile acids produced following cholecystectomy continue to flow into the duodenum but are poorly absorbed by the colon. Excessive bile acids in the colon stimulate mucosal secretion of water and electrolytes leading, in severe cases, to diarrhoea. Bile acid diarrhoea (BAD) is difficult to diagnose, requiring a comprehensive medical history and physical examination in combination with laboratory evaluation. The current work reviews the diagnosis and treatment of BAD following cholecystectomy.
Keywords: Cholecystitis, Gallstones, Bile acids, Colon, Bile acid diarrhoea
Core Tip: The incidence of cholecystitis is relatively high in developed countries, the treatment for which involves complete surgical removal of the gallbladder. Bile acids produced following cholecystectomy are poorly absorbed by the colon. Excessive bile acids in the colon stimulate mucosal secretion of water and electrolytes leading, in severe cases, to diarrhoea. The current work reviews the diagnosis and treatment of bile acid diarrhoea following cholecystectomy.
INTRODUCTION
The incidence of diarrhoea after cholecystectomy is extremely high, affecting 57.2%[1-3] of patients in a multimedical center study in which only a small proportion of post-cholecystectomy patients were investigated and for which there was a time delay in diagnosis. The true prevalence of bile acid diarrhoea (BAD) after cholecystectomy may be much higher and clinicians need to raise awareness of this treatable disease which seriously impacts patient quality of life[4-6]. Post-cholecystectomy diarrhoea (PCD) may be multifactorial[2]. No storage of bile acids is possible following cholecystectomy, resulting in the slow release of unconcentrated bile into the small intestine. Postprandial gastrointestinal reflex causes large-scale movement of bile from the small intestine to the colon, resulting in biliary acid-mediated diarrhoea[1,6-8]. Indeed, the frequency of bowel movement may change from a pre-operative once a day to a postoperative 4-5 times per day. The risk of postoperative diarrhoea depends on age, weight, and sex[2]. One review published in the US has estimated that, of the 750000 cases of cholecystectomy per annum, 5%-12% are followed by diarrhoea[9]. A prospective study which assessed 93 patients in two years before and after surgery found that eight had recurrent watery diarrhoea during the two years following cholecystectomy[10].
DIAGNOSIS OF BAD
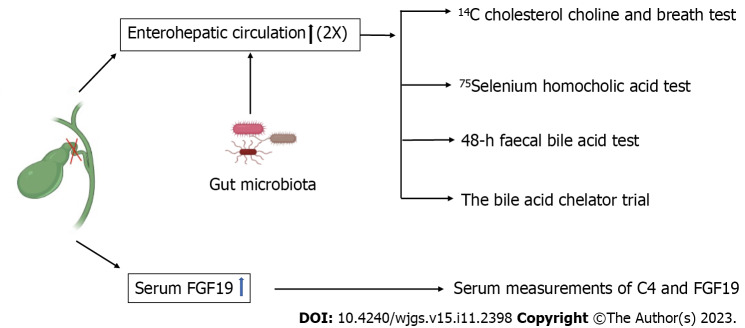
BAD is more common among patients with chronic watery diarrhoea and prior cholecystectomy[11,12] and diagnosis requires a comprehensive medical history and examination (including digital rectal examination) in combination with laboratory assessments, including total blood count, catabolic status, C-reactive protein level, faecal occult blood test, faecal lactoferritin test, white blood cell smear test, and microbiological assessment[13]. Laboratory confirmation of BAD remains a challenge. Five diagnostic tools are available: (1) 14C cholesterol choline and breath test; (2) 75Selenium homocholic acid taurine (SeHCAT) test[12,14-18]; (3) serum measurements of C4 and FGF19[18]; (4) 48-h faecal bile acid test[18-20]; and (5) the bile acid chelator trial[18,21-23] (Figure 1).
Figure 1.
Five diagnostic tools for bile acid diarrhoea.
The British Society of Gastroenterology Guidelines for Chronic Diarrhoea Investigation recommend endoscopy and the SeHCAT test as the first-line diagnostic modality[24]. Currently, clinicians pay insufficient attention to the diagnosis of diarrhoea after cholecystectomy and approximately 10%-30% of BAD patients are misdiagnosed with irritable bowel syndrome (IBS-D)[5,25-27]. The SeHCAT test allows differentiation between PCD and IBS-D[28]. The 48-h faecal bile acid test is the alternative to the SeHCAT test and may reduce healthcare utilization and costs in patients with chronic non-bloody, unexplained diarrhoea[19].
TREATMENT AND MANAGEMENT OF BAD
Cholecystectomy has an effect on bile concentration and excretion and changes the circulation of bile acids between the liver and intestine. The orthodox explanation for altered intestinal function refers to the loss of gallbladder fluid storage and changes in bile acid metabolism. In particular, the concentration of deoxycholic acid in the faeces increases, which enhances rectal sensitivity, causing an urge to defecate[29]. The accompanying physiological changes expose therapeutic targets for the post-cholecystectomy syndrome. BAD is usually regarded as incurable[17,28,30], and the chronic condition may be treated by drugs targeted to bile acid receptors and transporters or which aim to change bile acid pools. The diarrhoea is considered difficult to treat and is only reduced in half of cases, significantly affecting quality of life. Long-term follow-up is thus necessary, accompanied by adjustment of treatment methods and the development of new approaches[12] (Table 1).
Table 1.
Treatment of post-cholecystectomy bile acid diarrhoea
|
Treatment
|
Target
|
Limited
|
| Bile acid sequestrant trial | Bile acids secreted into the intestine are bound to reduce damage to intestinal tissues | Poorly tolerated due to stomach pain, bloating, flatulence, nausea and vomiting |
| Bile acid receptor agonists | Receptor agonists reduce bile acid synthesis to relieve symptoms of diarrhoea | Potent FXR agonists may have adverse side effects |
| Glucagon-like peptide 1 receptor agonist | Slows upper gastrointestinal motility and increases small intestine transit time | Further clinical trials and follow-up required |
| Intestinal microbiota | Increased bile acid binding, excretion in faeces, and hepatic synthesis via an FGF-dependent mechanism after probiotic administration | Not intended to target the entire intestinal microbial community as a therapeutic approach |
| Ursodeoxycholic acid | Reduces mucosal cytokine levels, inhibiting release of antimicrobial peptides and preventing apoptosis. | LCA metabolism may be required to allow full pharmacological effects of ursodeoxycholic acid |
| Anti-diarrhoeal agents | Inhibit intestinal secretion and peristalsis, slowing intestinal transit and allowing increased fluid reabsorption to alleviate diarrheal symptoms | High doses or abuse may cause cardiotoxicity |
| Dietary therapy | Vegetable dietary fiber prevents gastrointestinal diarrhea by reducing gastric emptying | May respond to a reduction of dietary cholesterol and fats |
Bile acid sequestrant trial
The Canadian Association of Gastroenterology clinical practice guidelines recommend bile acid sequestrants (BAS) as the first-line treatment for BAD[31,32]. For patients with suspected or confirmed BAD, a BAS trial (BAST), initially with cholestyramine, is suggested. However, the BAST must be carefully managed to avoid under- or over-treatment[33]. BAS, such as cholestyramine, colestipol, and colesevelam, bind bile acids secreted into the intestine to reduce damage to intestinal tissues. Cholestyramine was the first BAS used to treat BAD in 1972, and Hoffman and Poley found favourable results in patients following resection of the small intestine. A study of eight patients with PCD, defined as more than four loose stools in a 24-h period for 1 to 20 years, included six subjects with elevated stool bile acids and stool weight greater than 200 g/24 h. Treatment with 4 to 16 g/d oral cholestyramine reduced the number of daily bowel movements within 72 h. Diarrhoea recurred in all patients after cessation of cholestyramine treatment[28,32]. A meta-analysis of the medical records of 291 patients with chronic watery diarrhoea tested by the SeHCAT test including 74 patients with a previous cholecystectomy[11] and a multi-centre study across three sites in the United Kingdom[5] found that 60%-70% of patients discontinue cholestyramine and colestipol within 5 years due to adverse reactions, including constipation, excessive diarrhoea, stomach pain, bloating, flatulence, and nausea and vomiting. Colesevelam is often better tolerated and results in firmer stools but may be less effective in improving stool frequency than colestyramine. In addition, colesevelam may be prohibitively expensive in countries such as Spain. The need for superior BAD treatments is clear and the colon release preparation of bile glycol, A3384, has been shown to be well-tolerated and effective in clinical trials.
Bile acid receptor agonist therapy
Sequestrants bind and remove excessive bile acids to reduce colon secretion but the primary causes of bile acid production remain unresolved[34]. The farnesoid X receptor (FXR) is highly expressed in the intestine and liver[35-38] and receptor agonists reduce bile acid synthesis to relieve symptoms of diarrhoea. Both FXR and Takeda G-protein receptor 5 are bile acid receptors on the nucleus and cell surface and have been considered to participate in the mechanisms by which bile acids regulate physiological functions since 2000. FXR has since been assigned roles in the extrahepatic metabolism of cholesterol, lipid, and glucose. FXR agonists are under development for treatment of liver and intestinal diseases and have excellent potential as anti-diarrheal drugs due to inhibition of calcium- and cyclic adenosine monophosphate-dependent chloride secretion by the colonic epithelium. The impact of FXR agonists on fluid and electrolyte transport by colonic epithelial cells gives these drugs a broader efficacy than preexisting treatments while generating fewer side effects[39]. Walters et al[37] have proposed that FXR agonists that influence the fibroblast growth factor 15/19 (FGF15/19) pathway may alleviate cholestatic liver injury and diarrhoea. A reduction of FGF19 synthesis by the ileum would lead to impaired feedback inhibition of hepatic CYP7A1 in the liver and increased bile acid synthesis, reflected by increased C4 Levels. It is the excessive production of bile acids by the liver which are secreted into the small intestine and exceed the reabsorption capacity of the ileum that allows bile acids to enter the colon and cause diarrhoea. Many agonists, including GW4064, PX-102, LJN452, and Ec001, have been developed and INT-747 obberic acid (OCA) was approved by the United States Food and Drug Administration (FDA) for clinical use in 2016. OCA has been shown to be 100 times more potent as an FXR agonist than goose deoxycholic acid[40]. OCA is often combined with ursodeoxycholic acid (UDCA) to treat primary biliary cholangitis and other liver diseases, such as non-alcoholic steatohepatitis and primary sclerosing cholangitis. A recent phase II clinical trial at Imperial College London demonstrated that OCA improved serum FGF19 Levels and decreased C4 and faecal bile acids, reducing diarrheal symptoms in BAD patients. OCA inhibited colonic fluid secretion and reduced bile acid biosynthesis, decreasing the flow of bile acids into the colon[41]. FXR agonists also have the potential for the treatment of inflammatory diseases and reduced FXR expression was found in intestinal epithelial cells of patients with inflammatory bowel disease (IBD). This finding suggests that changes in bile acid synthesis and FXR expression may be involved in the dysregulation of the immune response and development of inflammatory diseases, such as IBD, an observation that merits further study. A 2-wk clinical study of patients with primary and secondary bile acid malabsorption (BAM)-induced diarrhoea had improved stool frequency, stool morphology, and total diarrhoea index with increased FGF19 and decreased C4 and faecal bile acids after treatment with OCA[42]. However, potent FXR agonists may have adverse side effects, such as lowering HDL levels, and receptors must be carefully selected to achieve the desired treatment effects.
Glucagon-like peptide 1 receptor agonist: Liraglutide
Liraglutide is a commonly used drug for type 2 diabetes and obesity. It also has utility as second-line antisecretory therapy for BAD after cholecystectomy. Liraglutide delays gastric emptying and inhibits duodenal and small intestine motility[43-45]. The peptide glucagon-like peptide 1 (GLP-1) is known to slow upper gastrointestinal motility and increase small intestine transit time, which may enhance the passive resorption of bile acids from the gut to the bloodstream and reduce bile acid flow to the colon[46]. GLP-1 receptor agonist therapy has been reported to reduce cholecystokinin (CCK)-induced gallbladder emptying[47,48] and a study of liraglutide confirmed its safety for pancreatitis but indicated an increased risk of cholelithiasis[49]. However, this adverse effect does not apply to post-cholecystectomy patients. A clinical trial at the Center for Clinical Metabolic Research at Copenhagen University Hospital compared the efficacy of liraglutide and colesevelam in reducing defecation frequency in BAD patients and indicated the superiority of liraglutide[50]. Furthermore, case reports exist of two patients who experienced total remission of BAD symptoms after liraglutide treatment[46]. GLP-1 receptor agonists have also been shown to have beneficial effects on outcomes and mortality for patients with cardiovascular and chronic kidney disease[51-57]. Post-cholecystectomy patients experience mild disturbances in glucose homeostasis and slight deterioration in postprandial blood glucose, GLP-1, and insulin and glucagon concentrations[58]. Cholecystectomy has been reported to be associated with an increased diabetes risk[59]. Therefore, we consider liraglutide to be very suitable for BAD patients with additional benefits for those who also suffer from diabetes, cardiovascular disease, chronic kidney disease, or more than one of these conditions. Furthermore, treatment of PCD with liraglutide may prevent the development of type 2 diabetes. However, contrary views have been recorded previously. Smits et al[60] reported the results of a clinical trial in which liraglutide was found to be a cause of BAD. Further clinical trials and follow-up regarding the application of liraglutide for BAD are required.
Treatment targeting the intestinal microbiota
The changes in bowel habits and loss of bile acids during BAD following cholecystectomy may cause changes in the gut microbiota in some patients[61,62]. However, a controlled study in South Korea in which stool samples were collected from 39 gallstone patients and 26 healthy controls found that cholecystectomy did not affect the gut microbiome 3 mo after surgery, although an elevated relationship between microbes in the gallstone patients after surgery was found by network analysis. We suggest that PCD is a delayed postoperative complication that requires long-term follow-up data to determine changes in the gut microbiome[61]. Previous studies have used microbial metabolomics to demonstrate differences between post-cholecystectomy patients and healthy controls, raising the question of whether the gut microbiome could be targeted as a treatment for BAD[3,63-65]. The increasing scrutiny of probiotics in basic and clinical research has illustrated the potential health benefits that may follow sufficient dosages of these sterilized living microorganisms. Unlike drugs, probiotics may be taken by healthy subjects to reduce the risk of developing disease or to optimize physiological functions. Probiotics survive transit through stomach acid and bile, successfully reaching the small intestine and colon. A recent study has demonstrated increased BA binding, excretion in faeces, and hepatic synthesis via an FGF-dependent mechanism after probiotic administration. Thus, a beneficial effect of BA on the gut microbiome and systemic metabolism is indicated. Treatment of post-cholecystectomy patients with probiotics to enhance intestinal microecology improves gut microbiome balance through an impact on Bacteroidetes and Firmicutes levels. A healthy gut microbiome balance has the effect of suppressing the growth of opportunistic pathogens and promoting intestinal microecology. Lactobacillus plantarum (pCBH1) is a genetically engineered strain which overexpresses bile salt hydrolase and degrades glycodeoxycholic acid and taurodeoxycholic acid in vitro. It has the potential to reduce bile acids in BAD patients. Genetic engineering of microbial strains allows the targeting of pathogenic molecules or metabolic pathways of interest, rather than affecting the entire intestinal microbial community and may represent a superior form of BAD treatment. Intestinal dysbiosis may play a key role in PCD, exposing therapeutic targets for this disorder[66].
UDCA
UDCA from bear bile has been used in traditional Chinese medicine for hundreds of years to treat a range of diseases, including liver and intestinal disorders. In Western medicine, UDCA has been used for many years to treat liver diseases, especially primary biliary cholangitis, and as a bile acid replacement therapy to reduce bile acid toxicity in patients with deficient bile acid synthesis, gallstone dissolution, and digestive diseases[67,68]. Its potential for the prevention of primary sclerosing cholangitis and IBD has also been investigated. UDCA has shown therapeutic efficacy in treating a variety of extrahepatic diseases, including IBD, in clinical and preclinical studies[69] and UDCA or taurine conjugated derivatives have demonstrated pharmacological effects in reducing disease severity, mucosal cytokine levels, and the release of antimicrobial peptides and preventing apoptosis in animal models of IBD. UDCA also inhibited activation of and release of pro-inflammatory cytokines by mucosal immune cells. However, the UDCA metabolite LCA is considered the most toxic of the colonic bile acids and it may be necessary for LCA metabolism to take place to allow the full pharmacological effects of UDCA[70]. Clearly, much work is still needed to elucidate the relationship among UDCA, its metabolites, the microbiome, and mucosal inflammatory responses.
Anti-diarrhoeal agents
Loperamide is a synthetic phenylpiperidine derivative approved by the FDA in 1976 for the treatment of diarrhoea. Loperamide inhibits intestinal secretion and peristalsis, slowing intestinal transit and allowing increased fluid reabsorption to alleviate diarrheal symptoms. Diphenoxylate–atropine is a combination treatment for acute and chronic diarrhoea symptoms[71] but exposure to high doses during use and abuse may cause cardiotoxicity[72,73].
Dietary therapy
PCD may respond to a reduction of dietary cholesterol, fats, and animal protein and eggs and an increase in dietary fruit and vegetables[74-77]. Vegetable dietary fiber can prevent gastrointestinal diarrhea by reducing gastric emptying, improving intestinal barrier function, increasing epithelial cell regrowth, and increasing colonic fluid and electrolyte uptake[78,79].
PROSPECTS
The last two decades has seen great progress in the understanding of the role of bile acids in modulating the intestinal epithelium in health and disease. There has been corresponding interest from the pharmaceutical industry in the utilization of bile acids for the treatment of enteric and parenteral diseases. The discovery of novel bile acid receptors has driven an appreciation of the sensing of luminal bile acid characteristics by intestinal epithelial cells with the resulting activation of molecular pathways. Understanding of the endogenous and exogenous factors that influence bile acid pool size and composition has increased but there remain many unknown areas.
An ideal therapeutic approach would involve a gut-specific FXR activator to alleviate bile enterostasis by inducing FGF19 and reducing hepatic bile acid synthesis. Side effects of hepatic FXR activation would thus be avoided. However, whereas the main site of FGF19 secretion into bile was found to be the gallbladder mucosa, FGF19 is also an endocrine hormone which exerts metabolic effects on distant tissues. FGF19 has a pro-mitogenic function and a concern is potential tumorigenic activity. Long-term treatment of diarrhoea with FXR agonists requires consideration of this possible side effect. A clearer understanding of the regulation of cellular signalling pathways involved in bile acid synthesis, transport, and metabolism is required to avoid bile acid toxicity in the gut and liver. In addition to selective enterohepatic circulating FXR modulators, genetic and metabolic pathway-specific FXR modulators may be possible therapeutic strategies to treat cholestasis and metabolic diseases.
Liraglutide has utility as a second-line treatment for PCD associated with diabetes, metabolic syndrome, and obesity. It is an effective anti-diarrhoea treatment but remains too expensive to be used as a first-line anti-diarrhoea treatment alone.
Examination of faecal samples indicates that post-cholecystectomy patients have significant gut microflora differences compared with controls but gut microbiome changes could not be accurately correlated with the time after cholecystectomy during the current review. Studies are underway to elucidate the association between cholecystectomy and changes to the intestinal microbiota at 3 mo, 1 year, and 5 years after surgery. A study of signalling by bile acids as intermediaries between host and gut microbes and the integration and transduction of signals into biological responses is planned and may expose novel therapeutic targets for diarrhoea.
The apical sodium-dependent bile acid transporter (ASBT) has a theoretical role in hepatic and intestinal bile acid circulation with the potential to influence liver disease. Several ASBT inhibitors are under development, although none has so far been FDA-approved. These small-molecule inhibitors lower plasma LDL levels and have shown therapeutic promise for chronic constipation in preclinical and clinical studies.
CONCLUSION
Finally, the influence of diet should be stressed. Patient follow-up after surgery indicates that diarrhoea is often linked to diet, particularly to the consumption of greasy foods. A diet low in fat and animal protein may alleviate PCD.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 23, 2023
First decision: July 8, 2023
Article in press: September 22, 2023
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Sitkin S, Russia S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
Contributor Information
Rang-Lang Huang, Department of Hepatobiliary and Pancreatic Surgery, The Third Xiangya Hospital of The Central South University, Changsha 410013, Hunan Province, China.
Wen-Kai Huang, Department of General Medicine, The Third Xiangya Hospital of The Central South University, Changsha 410013, Hunan Province, China.
Xiang-Yi Xiao, The Xiangya School of Medicine, The Central South University, Changsha 410013, Hunan Province, China.
Lin-Feng Ma, The Xiangya School of Medicine, The Central South University, Changsha 410013, Hunan Province, China.
He-Zi-Rui Gu, The Xiangya School of Medicine, The Central South University, Changsha 410013, Hunan Province, China.
Guo-Ping Yang, Department of Clinical Pharmacy, The Third Hospital of The Central South University, Changsha 410013, Hunan Province, China. ygp9880@126.com.
References
- 1.Lamberts MP, Lugtenberg M, Rovers MM, Roukema AJ, Drenth JP, Westert GP, van Laarhoven CJ. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc. 2013;27:709–718. doi: 10.1007/s00464-012-2516-9. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M, Spilias DC, Tong LK. Diarrhoea after laparoscopic cholecystectomy: incidence and main determinants. ANZ J Surg. 2008;78:482–486. doi: 10.1111/j.1445-2197.2008.04539.x. [DOI] [PubMed] [Google Scholar]
- 3.Sauter GH, Moussavian AC, Meyer G, Steitz HO, Parhofer KG, Jüngst D. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–1735. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 4.Farrugia A, Attard JA, Hanmer S, Bullock S, McKay S, Al-Azzawi M, Ali R, Bond-Smith G, Colleypriest B, Dyer S, Masterman B, Okocha M, Osborne A, Patel R, Sallam M, Selveraj E, Shalaby S, Sun W, Todd F, Ward J, Windle R, Khan S, Williams N, Arasaradnam RP. Rates of Bile Acid Diarrhoea After Cholecystectomy: A Multicentre Audit. World J Surg. 2021;45:2447–2453. doi: 10.1007/s00268-021-06147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Galbraith N, Al-Hassi HO, Jain M, Phipps O, Butterworth J, Steed H, McLaughlin J, Brookes MJ. The impact of treatment with bile acid sequestrants on quality of life in patients with bile acid diarrhoea. BMC Gastroenterol. 2022;22:325. doi: 10.1186/s12876-022-02404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fort JM, Azpiroz F, Casellas F, Andreu J, Malagelada JR. Bowel habit after cholecystectomy: physiological changes and clinical implications. Gastroenterology. 1996;111:617–622. doi: 10.1053/gast.1996.v111.pm8780565. [DOI] [PubMed] [Google Scholar]
- 7.Hutcheon DF, Bayless TM, Gadacz TR. Postcholecystectomy diarrhea. JAMA. 1979;241:823–824. [PubMed] [Google Scholar]
- 8.Laing AW, Pardi DS, Loftus EV Jr, Smyrk TC, Kammer PP, Tremaine WJ, Schleck CD, Harmsen WS, Zinsmeister AR, Melton LJ 3rd, Sandborn WJ. Microscopic colitis is not associated with cholecystectomy or appendectomy. Inflamm Bowel Dis. 2006;12:708–711. doi: 10.1097/00054725-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad DS, Faulx A. Management of Postcholecystectomy Biliary Complications: A Narrative Review. Am J Gastroenterol. 2020;115:1191–1198. doi: 10.14309/ajg.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 10.Ros E, Zambon D. Postcholecystectomy symptoms. A prospective study of gall stone patients before and two years after surgery. Gut. 1987;28:1500–1504. doi: 10.1136/gut.28.11.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Campos L, Gisbert JP, Ysamat M, Arau B, Loras C, Esteve M, Fernández-Bañares F. Systematic review with meta-analysis: the prevalence of bile acid malabsorption and response to colestyramine in patients with chronic watery diarrhoea and previous cholecystectomy. Aliment Pharmacol Ther. 2019;49:242–250. doi: 10.1111/apt.15099. [DOI] [PubMed] [Google Scholar]
- 12.Damsgaard B, Dalby HR, Krogh K, Jørgensen SMD, Arveschough AK, Agnholt J, Dahlerup JF, Jørgensen SP. Long-term effect of medical treatment of diarrhoea in 377 patients with SeHCAT scan diagnosed bile acid malabsorption from 2003 to 2016; a retrospective study. Aliment Pharmacol Ther. 2018;47:951–957. doi: 10.1111/apt.14533. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA. 2021;325:865–877. doi: 10.1001/jama.2020.22532. [DOI] [PubMed] [Google Scholar]
- 14.Lim SJ, Gracie DJ, Kane JS, Mumtaz S, Scarsbrook AF, Chowdhury FU, Ford AC, Black CJ. Prevalence of, and predictors of, bile acid diarrhea in outpatients with chronic diarrhea: A follow-up study. Neurogastroenterol Motil. 2019;31:e13666. doi: 10.1111/nmo.13666. [DOI] [PubMed] [Google Scholar]
- 15.Murray IA, Murray LK, Woolson KL, Sherfi H, Dixon I, Palmer J, Sulkin T. Incidence and predictive factors for positive (75)SeHCAT test: improving the diagnosis of bile acid diarrhoea. Scand J Gastroenterol. 2017;52:698–703. doi: 10.1080/00365521.2017.1298153. [DOI] [PubMed] [Google Scholar]
- 16.Sciarretta G, Fagioli G, Furno A, Vicini G, Cecchetti L, Grigolo B, Verri A, Malaguti P. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28:970–975. doi: 10.1136/gut.28.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford GA, Preece JD, Davies IH, Wilkinson SP. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J. 1992;68:272–276. doi: 10.1136/pgmj.68.798.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Vijayvargiya P. The Role of Bile Acids in Chronic Diarrhea. Am J Gastroenterol. 2020;115:1596–1603. doi: 10.14309/ajg.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Camilleri M. Fecal Bile Acid Testing in Assessing Patients With Chronic Unexplained Diarrhea: Implications for Healthcare Utilization. Am J Gastroenterol. 2020;115:1094–1102. doi: 10.14309/ajg.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Al-Hassi HO, Jain M, Phipps O, Ford C, Gama R, Steed H, Butterworth J, McLaughlin J, Galbraith N, Brookes MJ, Hughes LE. A single faecal bile acid stool test demonstrates potential efficacy in replacing SeHCAT testing for bile acid diarrhoea in selected patients. Sci Rep. 2022;12:8313. doi: 10.1038/s41598-022-12003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11:1232–1239. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes LE, Ford C, Brookes MJ, Gama R. Bile acid diarrhoea: Current and potential methods of diagnosis. Ann Clin Biochem. 2021;58:22–28. doi: 10.1177/0004563220966139. [DOI] [PubMed] [Google Scholar]
- 23.Reid F, Peacock J, Coker B, McMillan V, Lewis C, Keevil S, Sherwood R, Vivian G, Logan R, Summers J. A Multicenter Prospective Study to Investigate the Diagnostic Accuracy of the SeHCAT Test in Measuring Bile Acid Malabsorption: Research Protocol. JMIR Res Protoc. 2016;5:e13. doi: 10.2196/resprot.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, Major G, O'Connor M, Sanders DS, Sinha R, Smith SC, Thomas P, Walters JRF. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67:1380–1399. doi: 10.1136/gutjnl-2017-315909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 26.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Carlson P, BouSaba J, McKinzie S, Vijayvargiya P, Magnus Y, Sannaa W, Wang XJ, Chedid V, Zheng T, Maselli D, Atieh J, Taylor A, Nair AA, Kengunte Nagaraj N, Johnson S, Chen J, Burton D, Busciglio I. Comparison of biochemical, microbial and mucosal mRNA expression in bile acid diarrhoea and irritable bowel syndrome with diarrhoea. Gut. 2023;72:54–65. doi: 10.1136/gutjnl-2022-327471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sciarretta G, Furno A, Mazzoni M, Malaguti P. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol. 1992;87:1852–1854. [PubMed] [Google Scholar]
- 29.Hearing SD, Thomas LA, Heaton KW, Hunt L. Effect of cholecystectomy on bowel function: a prospective, controlled study. Gut. 1999;45:889–894. doi: 10.1136/gut.45.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol. 2013;27:653–659. doi: 10.1155/2013/485631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadowski DC, Camilleri M, Chey WD, Leontiadis GI, Marshall JK, Shaffer EA, Tse F, Walters JRF. Canadian Association of Gastroenterology Clinical Practice Guideline on the Management of Bile Acid Diarrhea. Clin Gastroenterol Hepatol. 2020;18:24–41.e1. doi: 10.1016/j.cgh.2019.08.062. [DOI] [PubMed] [Google Scholar]
- 32.Danley T, St Anna L. Clinical inquiry. Postcholecystectomy diarrhea: what relieves it? J Fam Pract. 2011;60:632c–632d. [PubMed] [Google Scholar]
- 33.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39:923–939. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]
- 34.Walters JRF. Letter: long-term treatment of severe bile acid diarrhoea-obeticholic acid can normalise SeHCAT retention. Aliment Pharmacol Ther. 2018;48:1032–1034. doi: 10.1111/apt.14979. [DOI] [PubMed] [Google Scholar]
- 35.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keely SJ, Walters JR. The Farnesoid X Receptor: Good for BAD. Cell Mol Gastroenterol Hepatol. 2016;2:725–732. doi: 10.1016/j.jcmgh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Han CY. Update on FXR Biology: Promising Therapeutic Target? Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, Donowitz M, Fallon PG, Keely SJ. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63:808–817. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]
- 40.Jose S, Mukherjee A, Horrigan O, Setchell KDR, Zhang W, Moreno-Fernandez ME, Andersen H, Sharma D, Haslam DB, Divanovic S, Madan R. Obeticholic acid ameliorates severity of Clostridioides difficile infection in high fat diet-induced obese mice. Mucosal Immunol. 2021;14:500–510. doi: 10.1038/s41385-020-00338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 42.Walters JR. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2014;11:426–434. doi: 10.1038/nrgastro.2014.32. [DOI] [PubMed] [Google Scholar]
- 43.Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y, Yasu T, Harasawa H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017;43:430–437. doi: 10.1016/j.diabet.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Schirra J, Houck P, Wank U, Arnold R, Göke B, Katschinski M. Effects of glucagon-like peptide-1(7-36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans. Gut. 2000;46:622–631. doi: 10.1136/gut.46.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55:243–251. doi: 10.1136/gut.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kårhus ML, Brønden A, Røder ME, Leotta S, Sonne DP, Knop FK. Remission of Bile Acid Malabsorption Symptoms Following Treatment With the Glucagon-Like Peptide 1 Receptor Agonist Liraglutide. Gastroenterology. 2019;157:569–571. doi: 10.1053/j.gastro.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Shaddinger BC, Young MA, Billiard J, Collins DA, Hussaini A, Nino A. Effect of Albiglutide on Cholecystokinin-Induced Gallbladder Emptying in Healthy Individuals: A Randomized Crossover Study. J Clin Pharmacol. 2017;57:1322–1329. doi: 10.1002/jcph.940. [DOI] [PubMed] [Google Scholar]
- 48.Nexøe-Larsen CC, Sørensen PH, Hausner H, Agersnap M, Baekdal M, Brønden A, Gustafsson LN, Sonne DP, Vedtofte L, Vilsbøll T, Knop FK. Effects of liraglutide on gallbladder emptying: A randomized, placebo-controlled trial in adults with overweight or obesity. Diabetes Obes Metab. 2018;20:2557–2564. doi: 10.1111/dom.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab. 2017;19:1233–1241. doi: 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- 50.Kårhus ML, Brønden A, Forman JL, Haaber A, Knudsen E, Langholz E, Dragsted LO, Hansen SH, Krakauer M, Vilsbøll T, Sonne DP, Knop FK. Safety and efficacy of liraglutide vs colesevelam for the treatment of bile acid diarrhoea: a randomised, double-blind, active-comparator, non-inferiority clinical trial. Lancet Gastroenterol Hepatol. 2022;7:922–931. doi: 10.1016/S2468-1253(22)00198-4. [DOI] [PubMed] [Google Scholar]
- 51.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 52.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imprialos KP, Stavropoulos K, Doumas M. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:2196. doi: 10.1056/NEJMc1713042. [DOI] [PubMed] [Google Scholar]
- 54.Neeland IJ, Marso SP, Ayers CR, Lewis B, Oslica R, Francis W, Rodder S, Pandey A, Joshi PH. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021;9:595–605. doi: 10.1016/S2213-8587(21)00179-0. [DOI] [PubMed] [Google Scholar]
- 55.Svanström H, Ueda P, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Pasternak B. Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. 2019;7:106–114. doi: 10.1016/S2213-8587(18)30320-6. [DOI] [PubMed] [Google Scholar]
- 56.Shaman AM, Bain SC, Bakris GL, Buse JB, Idorn T, Mahaffey KW, Mann JFE, Nauck MA, Rasmussen S, Rossing P, Wolthers B, Zinman B, Perkovic V. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145:575–585. doi: 10.1161/CIRCULATIONAHA.121.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 58.Sonne DP, Hare KJ, Martens P, Rehfeld JF, Holst JJ, Vilsbøll T, Knop FK. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am J Physiol Gastrointest Liver Physiol. 2013;304:G413–G419. doi: 10.1152/ajpgi.00435.2012. [DOI] [PubMed] [Google Scholar]
- 59.Sang M, Xie C, Qiu S, Wang X, Horowitz M, Jones KL, Rayner CK, Sun Z, Wu T. Cholecystectomy is associated with dysglycaemia: Cross-sectional and prospective analyses. Diabetes Obes Metab. 2022;24:1656–1660. doi: 10.1111/dom.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smits MM, Tonneijck L, Muskiet MH, Hoekstra T, Kramer MH, Diamant M, Nieuwdorp M, Groen AK, Cahen DL, van Raalte DH. Biliary effects of liraglutide and sitagliptin, a 12-week randomized placebo-controlled trial in type 2 diabetes patients. Diabetes Obes Metab. 2016;18:1217–1225. doi: 10.1111/dom.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noh CK, Jung W, Yang MJ, Kim WH, Hwang JC. Alteration of the fecal microbiome in patients with cholecystectomy: potential relationship with postcholecystectomy diarrhea - before and after study. Int J Surg. 2023 doi: 10.1097/JS9.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Jing H, Wang J, Zhang S, Chang Q, Li Z, Wu X, Zhang Z. Disordered Gut Microbiota Correlates With Altered Fecal Bile Acid Metabolism and Post-cholecystectomy Diarrhea. Front Microbiol. 2022;13:800604. doi: 10.3389/fmicb.2022.800604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang Z, Lu M, Jiang M, Zhou D, Huang H. Proteobacteria Acts as a Pathogenic Risk-Factor for Chronic Abdominal Pain and Diarrhea in Post-Cholecystectomy Syndrome Patients: A Gut Microbiome Metabolomics Study. Med Sci Monit. 2019;25:7312–7320. doi: 10.12659/MSM.915984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma Y, Qu R, Zhang Y, Jiang C, Zhang Z, Fu W. Progress in the Study of Colorectal Cancer Caused by Altered Gut Microbiota After Cholecystectomy. Front Endocrinol (Lausanne) 2022;13:815999. doi: 10.3389/fendo.2022.815999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li YD, Liu BN, Zhao SH, Zhou YL, Bai L, Liu EQ. Changes in gut microbiota composition and diversity associated with post-cholecystectomy diarrhea. World J Gastroenterol. 2021;27:391–403. doi: 10.3748/wjg.v27.i5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Giorgio A, Vergani D, Mieli-Vergani G. Cutting edge issues in juvenile sclerosing cholangitis. Dig Liver Dis. 2022;54:417–427. doi: 10.1016/j.dld.2021.06.028. [DOI] [PubMed] [Google Scholar]
- 68.Floreani A, De Martin S. Treatment of primary sclerosing cholangitis. Dig Liver Dis. 2021;53:1531–1538. doi: 10.1016/j.dld.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 69.Liberal R, Gaspar R, Lopes S, Macedo G. Primary biliary cholangitis in patients with inflammatory bowel disease. Clin Res Hepatol Gastroenterol. 2020;44:e5–e9. doi: 10.1016/j.clinre.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Mroz MS, Lajczak NK, Goggins BJ, Keely S, Keely SJ. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing. Am J Physiol Gastrointest Liver Physiol. 2018;314:G378–G387. doi: 10.1152/ajpgi.00435.2016. [DOI] [PubMed] [Google Scholar]
- 71.Lacy BE, Weiser K, De Lee R. The treatment of irritable bowel syndrome. Therap Adv Gastroenterol. 2009;2:221–238. doi: 10.1177/1756283X09104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu PE, Juurlink DN. Clinical Review: Loperamide Toxicity. Ann Emerg Med. 2017;70:245–252. doi: 10.1016/j.annemergmed.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Wu PE, Juurlink DN. Loperamide Cardiac Toxicity: Pathophysiology, Presentation, and Management. Can J Cardiol. 2022;38:1378–1383. doi: 10.1016/j.cjca.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Shin Y, Choi D, Lee KG, Choi HS, Park Y. Association between dietary intake and postlaparoscopic cholecystectomic symptoms in patients with gallbladder disease. Korean J Intern Med. 2018;33:829–836. doi: 10.3904/kjim.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altomare DF, Rotelli MT, Palasciano N. Diet After Cholecystectomy. Curr Med Chem. 2019;26:3662–3665. doi: 10.2174/0929867324666170518100053. [DOI] [PubMed] [Google Scholar]
- 76.Yueh TP, Chen FY, Lin TE, Chuang MT. Diarrhea after laparoscopic cholecystectomy: associated factors and predictors. Asian J Surg. 2014;37:171–177. doi: 10.1016/j.asjsur.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 77.McKenzie YA, Sremanakova J, Todd C, Burden S. Effectiveness of diet, psychological, and exercise therapies for the management of bile acid diarrhoea in adults: A systematic review. J Hum Nutr Diet. 2022;35:1087–1104. doi: 10.1111/jhn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi X, Tester RF. Utilisation of dietary fibre (non-starch polysaccharide and resistant starch) molecules for diarrhoea therapy: A mini-review. Int J Biol Macromol. 2019;122:572–577. doi: 10.1016/j.ijbiomac.2018.10.195. [DOI] [PubMed] [Google Scholar]
- 79.Baker SS. Why dietary supplements? Pediatrics. 2014;133:e1740–e1741. doi: 10.1542/peds.2014-0883. [DOI] [PubMed] [Google Scholar]