Abstract
Neural guidance mechanisms ensure the precise targeting and synaptogenesis events essential for normal circuit function. Neuronal growth cones encounter numerous attractive and repulsive cues as they navigate toward their intermediate and final targets; temporal and spatial regulation of these responses is critical for circuit assembly. Recent work highlights the complexity of these events throughout neural development and the multifaceted functions of a wide range of guidance cues. Here, we discuss recent studies that leverage advances in genetics, single cell tracing, transcriptomics and proteomics to further our understanding of the molecular mechanisms underlying neural guidance and overall circuit organization.
Introduction
Complex neural circuit formation requires spatial and temporal coordination of neuronal responses to extrinsic guidance cues. Growing axons and dendrites must filter a myriad of signals to identify relevant cues and navigate to their appropriate targets [1–3]. Not only are these cues essential for determining overall neuronal architecture by regulating directed neurite extension, they also function in synapse formation and refinement [4].
Here, we highlight recent advances that elucidate or refine roles for guidance cues in each step of circuit formation, focusing primarily on activity-independent mechanisms. Through use of novel genetic tools, live imaging, and anatomical tracing approaches we have gained further insight into how guidance cues function in growth cone navigation and circuit assembly. We have also learned how these cues function in the context of laminar or topographic frameworks, in some cases providing a scaffold for building specific circuits that underlie discrete neural connections. Further, several recent advances in systems biology, using transcriptomic and proteomic profiling, facilitate understanding neural circuit establishment at the level of cellular and subcellular responses to guidance cues. Progress has also been made defining intracellular signaling events critical for appropriate guidance responses to extrinsic guidance cues. Though space limitations restrict our ability to discuss all progress in the field of neural guidance over the past several years, we hope that focusing on key advances will point the way towards future areas of investigation into cellular and molecular mechanisms underlying neural circuit assembly and refinement.
Guidance at the CNS midline
New observations on netrin-mediated axon guidance
Netrins are secreted, evolutionarily conserved, proteins that act as both attractive and repulsive guidance cues. The netrin-1 orthologue in C. elegans, Unc6, regulates neuronal migration and axon extension toward and away from the central nervous system (CNS) midline [1]. In vertebrates, netrin-1 was shown to be secreted from the spinal cord floorplate and to be required for dorsally-located spinal cord commissural neurons to extend their axons ventrally toward the floorplate, strongly supporting a model that netrin-1 functions as a long-range attractant and is presented in a gradient diffusing from the CNS midline. With the more recent generation of a netrin-1 conditional allele in the mouse, it was possible to revisit the specific cellular contributions to netrin-1 function through select deletion of netrin-1 from either the floorplate or ventricular zone (VZ), both known locations of netrin-1 expression in the spinal cord and hindbrain [5–9]. Deletion of netrin-1 from VZ progenitor cells results in commissural axon guidance defects both near and far from the floorplate in both the spinal cord and hindbrain. Additionally, netrin-1 released at the pial surface by ventricular zone progenitor cells was also observed to be responsible for the confinement of commissural axons and precerebellar neurons within the hindbrain, establishing a peripheral nervous system (PNS)/CNS boundary [10]. On the other hand, select removal of netrin-1 only from the floorplate did not phenocopy the commissural axon guidance defects observed in the netrin-1 LOF mutants far from the floorplate in either the spinal cord or hindbrain [9,11•]. These findings suggest that VZ netrin-1 acts locally far from the floorplate in both the hindbrain and spinal cord to guide commissural axons ventrally.
Most recently, an even closer look into the role of netrin-1 at the midline confirms both long-range and short-range attraction of commissural axons toward the midline (Figure 1a). Deletion of netrin-1 only from the floorplate results in a reduction of commissural axon bundle thickness in addition to commissural axon guidance defects adjacent to the floorplate and also misprojections into the motor column [11•,12•]. These results show that netrin-1 can act at a distance of several hundred mircometers from where it is secreted at the floor plate to attract and guide commissural axons. Simultaneous deletion of netrin-1 from both the floorplate and the VZ results in severe commissural axon guidance defects and shows that both sources of netrin-1 contribute to commissural axon guidance [11•]. Interestingly, loss of the sonic hedgehog (Shh) receptor Boc enhances phenotypes observed following floorplate deletion of netrin-1, demonstrating that Shh and netrin-1 act together in the ventral spinal cord to guide commissural axons [12•]. Unlike in the spinal cord, deletion of netrin-1 from the hindbrain floorplate does not result in commissural axon guidance defects [5]. This difference is likely due to the larger size of the hindbrain, and so netrin-1 secreted from the hindbrain floorplate is far from navigating commissural axon growth cones, requiring these neurons to rely more heavily on VZ netrin-1 for proper guidance to the hindbrain midline. These recent studies suggest that VZ and floorplate netrin-1 each contribute to commissural axon guidance. Further, netrin-1 removal from both the floorplate and VZ does not completely phenocopy the netrin-1 null allele, suggesting that netrin-1 is secreted from an additional location [11•]. Taken together, these studies highlight how coordinated action of netrin-1 secreted from VZ neural progenitors provides a short-range commissural axon guidance function when localized at the pial surface. They also present compelling evidence that netrin-1 secreted from the spinal cord floor plate influences guidance of commissural axons at a distance. Netrin-1 protein distribution in the VZ and along commissural axons requires the deleted in colorectal cancer (DCC) netrin-1 receptor [9,11•], and so future work will most certainly explore the mechanisms by which DCC, and other netrin receptors, contribute to commissural axons guidance both near and far from the floor plate.
Figure 1. Molecular mechanisms underlying commissural and motor axon guidance in the developing spinal cord.
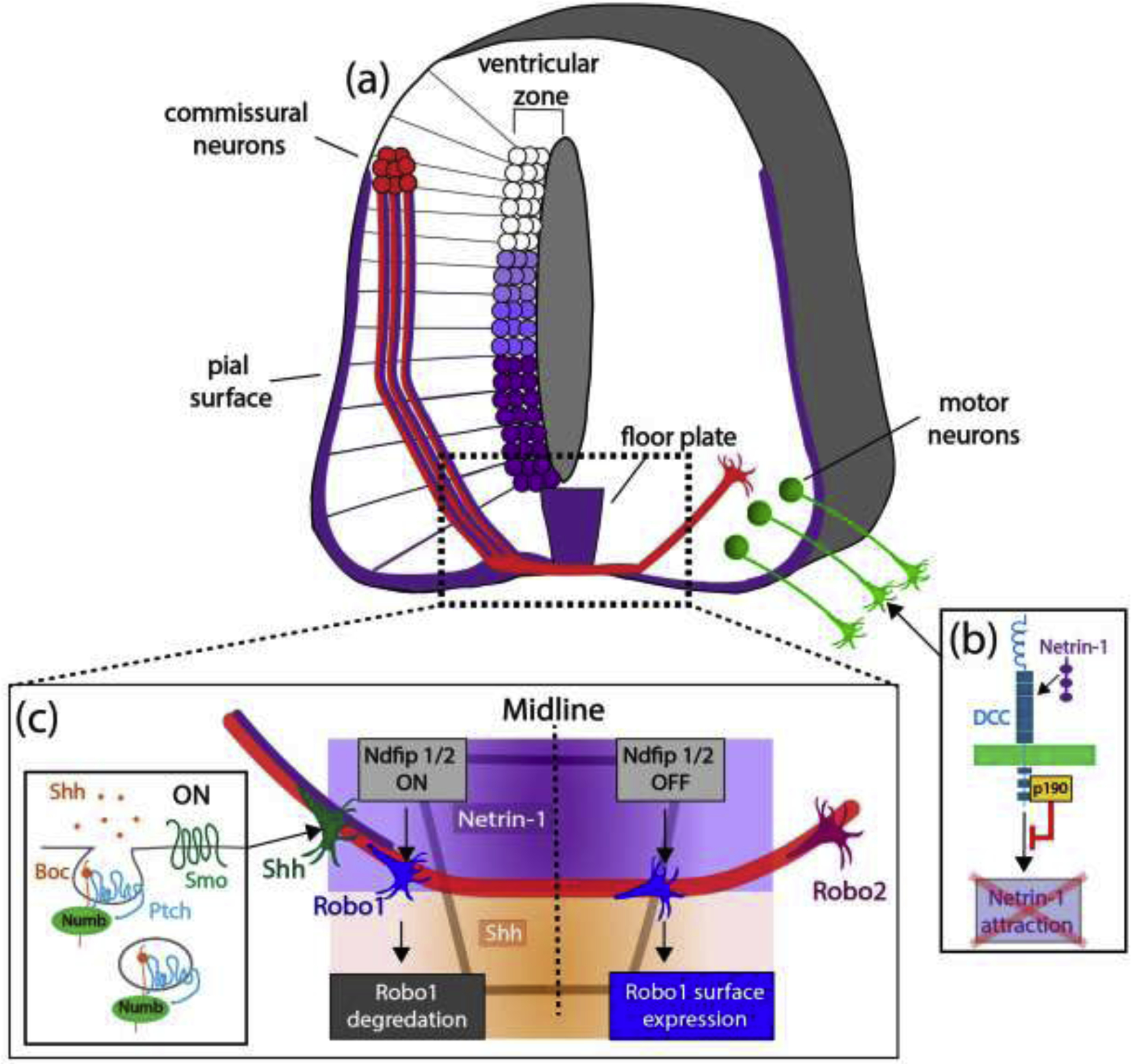
(a) Schematic of commissural axons navigating towards the floor plate (purple gradient: netrin-1 expression in the floorplate (FP), ventricular zone (VZ) and pial surface). Commissural neurons in the mouse dorsal spinal cord (red) extend their axons ventrally towards the floorplate (FP) and are attracted by netrin-1 (adapted from reference #6). (b) p190RhoGAP associates with DCC and prevents inappropriate attraction of spinal motor neuron axons to netrin-1, allowing exit from the spinal cord. (c) Schematic of molecular mechanisms underlying axon guidance at the midline. Shh secreted from the FP also attracts commissural axons (orange gradient). Shh-mediated endocytosis of the co-receptors Boc and Ptch1 (along with the adaptor Numb1) is necessary for Shh attraction. Robo1 (blue growth cones) and Robo2 (magenta growth cone) are differentially sorted to the cell surface and regulated during commissural guidance (see text for details).
Another population of axons, those of spinal motor neurons, also express DCC. Interestingly, though they express the attractive netrin1 receptor DCC these axons are not attracted towards the midline, and instead they exit the spinal cord and project into the periphery. This is due to a balance between netrin-1/DCC-mediated attraction and slit/Robo-mediated repulsion that directs the precise position of motor axon exit points from the CNS [13]. Recent work demonstrates that selective silencing of DCC ensures proper exit and subsequent extension of motor axons away from the CNS midline and toward their muscle targets [14•]. Following a forward genetic screen in the mouse for mutations that affect motor axon guidance, the p190RhoGAP (p190) cytosolic signaling protein was found to interact with the cytoplasmic domain of the DCC receptor. In this context, p190 modulates motor axon responses to netrin-1, ensuring that motor axons follow their proper trajectories away from spinal cord. p190 mutant motor neuron axons are more sensitive to netrin-1 attraction, and as a result they exhibit aberrant trajectories toward sources of netrin-1, suggesting an inappropriate activation of netrin-1/DCC signaling in the absence of p190 (Figure 1b). It is notable that p190 regulation of DCC is independent of its GTPase activation activity, highlighting a non-canonical signaling mechanism by which p190RhoGAP silences DCC activation by netrin-1 to facilitate motor axon exit from the spinal cord [14•]. However, inhibition of Rho GTPase signaling by p190 is required by some motor axons for guidance in the periphery in both mice [14•] and in Drosophila [15], again revealing that complex regulation of guidance cue signaling is required for proper wiring during neural development.
Spatiotemporal control of midline guidance cue responsiveness
A common theme when considering axon guidance at the CNS midline is that growing axons can change their sensitivity to guidance cues, for example to semaphorin and slit repellents, upon midline crossing [16–19]. Recent work provides real-time, super-resolution, examination of guidance cue receptor expression dynamics during commissural growth cone navigation in the developing chicken spinal cord [20•]. Robo1 and Robo2 both signal slit-mediated repulsion away from the CNS midline and are known to have distinct roles in post-crossing commissural axons [21]. Visualization of pHLourin-tagged Robo1 and Robo2 receptors as commissural axons traverse the midline shows that these receptors are differentially sorted to the cell surface at different stages of axon navigation. Whereas Robo1 is present in commissural axons as they extend toward and cross the CNS midline, it is sorted to the axon cell surface only just after axons have crossed the CNS midline and are exiting the floor plate. Robo2 is initially absent from the surface of commissural growth cones navigating the floorplate and is sorted to the cell surface in post-crossing axons only after they have turned longitudinally in the lateral funiculus (Figure 1c). Interestingly, Robos1 and 2 are differentially distributed within the growth cone such that Robo1 accumulates toward the rear of the growth cone, whereas Robo2 cell-surface expression is homogenous throughout. This suggests that Robos1 and 2 are likely responding to different sources of slit that mediate different stages of midline navigation and receptor membrane insertion. Select localization of other guidance receptors, including PlexA1 [17], also demonstrates distinct patterns of cell surface guidance cue receptor distribution, providing insight into the regulation of commissural axon guidance.
At the subcellular level, Robo1 cell surface expression in the mouse has recently been shown to be regulated by members of Nedd4 family of interacting adaptor proteins: Ndfip1 and Ndfip2 [22•]. These proteins participate in CNS midline guidance by promoting Robo1 localization to endosomes in commissural axons prior to midline crossing, facilitating its ubiquitylation and subsequent degradation by recruiting E3 ligases. This leads to low levels of Robo1 specifically on pre-crossing axons, and therefore reduced responsiveness to the midline-derived repellent slit (Figure 1c). Posttranslational regulation of Robo1 cell surface levels constrains slit responsiveness to post crossing commissural axons and is remarkably similar to regulation of Robo receptors at the Drosophila CNS midline [23]. Taken together, these recent studies underscore the importance of regulating guidance cue receptor cell surface levels in order to control growth cone responses to guidance cues.
Additional evidence for the importance of regulating cell surface distribution of guidance cue receptors comes from work on Shh and its co-receptor brother of CDO (Boc) in the context of midline axon guidance. In spinal commissural axons, Shh binding to Boc leads to endocytosis of the Boc/Ptch-1 complex into Rab5+ early endosomes, and this endocytosis is required to regulate Shh-mediated growth cone turning in vitro and axon guidance in vivo (Figure 1c) [24•]. The endocytic adaptor protein numb binds directly to Boc and is necessary for Boc/Ptch-1 endocytosis. This enables the activation of smoothened and subsequent non-canonical Shh signaling that includes activation of Src family kinases, which is required for growth-cone turning in response to Shh [24•]. Interestingly, in the developing visual system Shh can function as a repellent for ipsilaterally-extending retinal ganglion cell (RGC) axons approaching the optic chiasm and participates in preventing them from crossing the midline [25]. In this context Shh is transported and secreted by earlier arriving contralaterally projecting RGCs, and repulsion of ipsilaterally projecting RGCs is dependent upon signaling through Boc and Smo. It will be of interest to determine whether internalization of Shh receptors is also required for Shh repulsion as it is for Shh-mediated attraction.
Regulation of intermediate and terminal axon targeting
Axons extend over long distances, often through intermediate targets, to find their synaptic partners in the CNS. In the cerebral cortex, for example, excitatory neurons migrate to their appropriate layers and then extend axons to establish long-range connections to cortical and subcortical target regions [26]. Though the specific cues that regulate these targeting events are under intense investigation, the large transmembrane glycoprotein dystroglycan is known to regulate multiple stages of axon guidance and targeting. Dystroglycan is required for the organization of laminins and several other extracellular matrix (ECM) proteins in the basement membrane, and this is essential for the development of multiple axon tracts in the brain and spinal cord, including those harboring retinal ganglion cell axons and commissural axons [27–31]. Interestingly, dystroglycan coordinates slit-mediated spinal commissural axon guidance by regulating the spatial distribution of this secreted guidance cue along the murine spinal cord basement membrane [32]. Recent work demonstrates that dystroglycan functions non-cell autonomously during spinal commissural axon guidance, and that it is similarly required for the formation of multiple forebrain axon tracts and also corticothalamic and thalamocortical connectivity [33•]. In addition, a novel interaction between dystroglycan and the adhesion GPCR Celsr3 was found to be necessary for post-crossing guidance of spinal commissural axons, but not for the formation of several other CNS tracts that require dystroglycan. One attractive model is that Celsr3 functions cell-autonomously in commissural axons in cis with the frizzled 3 receptor, but that Celsr3-dystroglycan trans interactions selectively regulate this segment of commissural axon trajectories. Therefore, dystroglycan coordinates multiple interactions with secreted guidance cues, ECM components and receptors to regulate the establishment of distinct axon trajectories.
Recent work on murine hippocampal subregion connectivity informs our understanding of the molecular basis underlying target recognition following long-range axon pathfinding [34•]. Establishing connections among hippocampal subregions, and also with adjacent cortical regions, involves a series of topographic axon targeting events. The transmembrane protein teneurin-3 (Ten3) exhibits graded expression in several hippocampal regions that are topographically interconnected, including the proximal CA1 region, the distal subiculum, and the medial entorhinal cortex [34•]. Members of the teneurin protein family are known to instruct select synaptic partner matching in Drosophila and to play roles in the development of vertebrate visual system [35]. In the hippocampus, teneurin-3 (Ten3) promotes precise topographic targeting between proximal CA1 axons and target dendrites of the distal subiculum, and it can also promote homophilic cell adhesion in vitro [34•]. Thus, Ten3 acts both pre- and post-synaptically to control the assembly of this topographic hippocampal projection. Ten3 mutants show a wider spread of CA1 axons projecting proximally within the subiculum, and overall less refined topographic targeting of the CA1 to subiculum projection compared to controls, suggesting that Ten3 is required for precise targeting of proximal CA1 axons to distal subiculum. Further, Ten3 mutants show a reduction in EPSC amplitudes in distal subicular neurons even though residual axons from CA1 still reach the distal subiculum. This suggests Ten3 is required for the formation or function of synapses in this region. It will be important to determine whether mistargeted axons actually form functional synapses and whether these are retained in the adult, ultimately linking developmental mechanisms of circuit wiring to circuit function.
Teneurins engage in complex interactions with a variety of proteins, and these interactions underlie their diverse roles in the developing brain, including neuronal migration and synapse formation [36•,37]. Very recently, the adhesion GPCR latrophilin-2 (Lphn2) was shown to act as a repulsive ligand for Ten3-expressing pCA1 axons targeting the distal subiculum [38]. This demonstrates a remarkable dual role for Ten3, such that loss of either Lphn2/Ten3 heterophilic repulsion or Ten3 homophilic attraction disrupts pCA1 topographic targeting in the subiculum.
Structural analyses of teneurin proteins reveal the highly atypical structures these proteins can adopt, suggesting possible mechanisms that underlie their diverse biological activities. Teneurin structural data also define interactions in trans between teneurins and Lphns, and also in trans between Lphns and FLRT membrane proteins [36•,39,40]. These Lphn in trans associations with teneurins control radial neuron migration in the developing mouse neocortex [36•]. Similarly, the function of Lphn3 in synapse formation in CA1 pyramidal neurons also requires simultaneous binding in trans to FLRTs and to and teneurins, defining a coincidence signaling mechanism that can control both cell migration and synapse formation [40]. Future work will investigate how these transmembrane receptors, both alone and in combination with various other cell surface proteins, regulate neural circuit formation and function.
Lamination
The segregation of neural projections into separate layers, or laminae, in order to facilitate appropriate connectivity is a common CNS feature. Examples of laminated structures include the vertebrate retina, the insect visual system neuropils, the dorsal horn of the spinal cord, and the cerebral cortex [26,41–43]. Recent work reveals new roles for guidance cues in the regulation of lamina-specific innervation by axons and dendrites during circuit formation.
The larval zebrafish optic tectum (OT) is a prime example of a laminated structure; there are at least ten retinorecipient synaptic laminae targeted by RGCs, and the terminal regions of individual RGC axons remain confined to a single lamina throughout development [44]. Recent work shows that components of the reelin signaling pathway are required for proper targeting of RGC axons within select OT laminae, such that loss of reelin in the OT, or the very low density lipoprotein receptor (VLDLR) or Dab1 in RGCs, leads to aberrant RGC targeting within the OT [45•]. Tectum-derived reelin is distributed in a superficial (high)-to-deep (low) gradient that is stabilized by heparan sulfate proteoglycans (HSPGs), and this gradient functions to attract RGC axons to their appropriate laminae, counterbalancing previously described repulsive slit signaling that constrains incoming RGCs in the OT [46]. Further, RGCs expressing different levels of VLDLR exhibit differential sensitivity to reelin in the OT [45•]. This underscores the importance of receptor expression levels during axon guidance events, suggesting they are also important for targeting axons to their final laminar destinations.
Guidance cue regulation of lamination has also been recently observed in the ellipsoid body (EB) and fan shaped body (FB), both of which are laminated structures of the central complex (CX) in the Drosophila brain. Circumferential lamination of ring (R) neuron axons in the EB is regulated by the transmembrane proteins semaphorin-1a (Sema-1a) and Plexin A. In their absence, aberrant inhibitory synaptic connections are established across laminar boundaries, altering the electrical properties of a subclass or R neuron and emphasizing the importance of laminar segregation of distinct neuronal connections [47•]. Neuronal processes within the FB are organized in more classical laminae and columns [48•]. The secreted guidance cue Sema2b exhibits restricted expression in a small subset of FB laminae and serves as an attractive cue for small field P-Fr neuron-process lamination through signaling via the PlexB receptor [48•]. Loss of PlexB function in P-Fr neurons leads to disruption of laminar innervation such that P-Fr neuron processes now extended into non-target layers. This results in the formation of ectopic inhibitory connections between processes from a class of neuron called ExFl2 that normally targets a FB layer adjacent to laminae targeted by P-Fr neurons. ExF12 neurons are implicated in regulating adult fly sleep behavior [49,50], and these ectopic synaptic connections resulting from disruption of Sema2b/PlexB-mediated regulation of FB laminar organization give rise to increased wakefulness in adult flies. These results show that P-Fr neurons can modulate sleep behaviors and control arousal, and they reinforce the importance of lamination-mediated neural wiring for orchestrating normal behavioral output. This is underscored by detailed anatomical examination of laminar organization elsewhere in the CNS, where this mode of organizing connectivity is likely critical for normal behavior. For example, utilizing single cell labeling approaches, Tsubouchi and colleagues [51•] systematically mapped individual sensory neuron projections in Drosophila somatosensory circuits. They found that distinct sensory neuron sub-types exhibit modality-specific layered organization of their axon terminals, such that axons arising from different types of primary somatosensory neurons terminate in different layers in the ventral nerve cord (VNC) and also distinct brain subregions. This comprehensive assessment of sensory neuron central connectivity provides a rich experimental platform for discovering novel molecular determinants of neuronal laminar organization.
Advances in single cell RNAseq transcriptomic technologies coupled with single-cell anatomical analyses allow for a better understanding of cell type diversity within circuits and highlight the variety of cues necessary for wiring laminated structures. Sarin and colleagues [52•] investigated connectivity between rod and cone photoreceptors (PRs) and bipolar cells (BPs) in the outer plexiform layer (OPL) of the mouse retina, and they identified cell surface proteins and secreted ligands selectively expressed among these classes of retinal neurons. Using CRISPR to induce specific somatic mutations during the establishment of PR/BP connectivity in the OPL, a new role for Wnt5a/5b-dependent signaling via Ryk, Fzd4, and Fzd5 receptors, along with cytosolic disheveled (Dvl), was discovered that promotes rod PR/rod BP connectivity and OPL sublaminae formation. These approaches enable rapid evaluation of molecules that direct the formation of precise connections at the single cell level, and in the near future we can expect to have a much better understanding of the full molecular repertoire required for functional retinal circuit organization.
Synaptic targeting and circuit construction
The wide diversity of cell adhesion molecules (CAMs) that participate in heterophilic or homophilic interactions is instructive for understanding synaptic targeting and circuit construction [4]. For example, two subfamilies of the immunoglobulin superfamiliy (IgSF), the Dprs and Dpr-interacting proteins (DIPs), include 21 and 11 members, respectively, and initial observations supported the idea that specific binding between Dprs and DIPs mediates interactions between synaptic partners in Drosophila [53,54]. Biophysical and structural studies reveal that some DIPs and Dprs can undergo homophilic binding and define essential amino acids within these proteins that underlie these specific interactions [55–57]. Recent studies expand upon how members of these protein families regulate neuronal connectivity, including work showing that DIP-Dpr interactions are necessary for synapse formation in the Drosophila visual system lamina neuropil. Within the lamina, the synaptic terminals of photoreceptors (R) and neurites of lamina neurons (L) together form “cartridges” critical for normal visual system function. L cells send axons to the medulla neuropil where they form lamina-specific connections, enabling higher order connectivity [58,59•]. DIPs γ and β are required in L neurons for correct visual system function and are sufficient for localization of select L-L neuron synapses; mis-expression of DIPs γ and β in R neurons promotes ectopic synapse formation with Dpr-expressing L neurons. However, DIPs γ and β are not required for synapse formation per se since flies lacking both DIPs still form synapses in the lamina, though their location and distribution are abnormal [59•]. Similarly, interaction between DIP-y and Dpr11 is instructive for the development of color vision circuits in the optic lobe through regulation of the distal dendrite morphology of Dm8 amacrine cells and by defining precise presynaptic partners with R7 (long-wavelength) PRs [60•]. In the Drosophila visual system medulla neuropil, heterophilic interactions between DIP-α and its binding partners Dpr6 and 10 regulate multiple aspects layer-specific connectivity, including arborization within layers, synapse number, layer specificity and cell survival [61•]. Taken together, work in the fly visual system supports complex DIP/Drp hetero and homophilic interactions that direct select connectivity among distinct populations of neurons and also the subcellular localization of their synaptic connections.
Classical cadherins include ~20 related molecules, several of which direct the establishment of direction-selective (DS) circuitry in the mouse retina. Two type II cadherins, Cdh8 and Cdh9, function heterophilically to instruct On-BC2 and Off-BC5 BP cell types, respectively, to form connections with appropriate On-Off DS ganglion cells (ooDSGCs) [62]. Recent extension of this foundational work shows that though at least 15 different Cdhs are expressed by neurons that make up DS circuits, 6 of these function individually and in combinations to restrict the arbors of distinct neuronal types to select sublaminae within the inner plexiform layer (IPL) of the mouse retina [63•]. This work also shows that specific cadherin interactions are necessary for lamina-specific innervation by ooDSGC dendritic arbors and for ooDSGC-starburst amacrine cell (SAC) associations. For example, Cdh7 and Cdh18 function to regulate nasal-preferring ooDSGC dendritic targeting, whereas Cdh6, 9, and 10 are involved in dendritic targeting and DS responses of dorsal/ventral preferring ooDSGCs. These studies reveal the remarkable utilization of a single family of CAMs by the BPs, RGCs and amacrine cells that comprise DS circuits, and it will be interesting to determine whether other gene families are similarly devoted to wiring visual system circuits with distinct tuning properties.
These data show that combinatorial interactions among classes of cell surface receptors underlie synaptic specificity in the CNS. The vertebrate clustered protocadherins (Pcdh) are a subclass of the cadherin superfamily that includes proteins shown to be necessary for sorting olfactory neuron axons in the mouse into distinct glomeruli within the olfactory bulb by way of contact-mediated repulsion between axons that express identical combinations of Pcdh isoforms [64]. More recently, 20 cell-surface molecules were identified through profiling the cell-surface proteome of intact Drosophila olfactory tissue and shown to play roles in regulating neural circuit assembly [65•]. Many of these proteins had not previously been linked to neural development in flies. For example, LRP1 was found to cell-autonomously regulate olfactory projection neuron dendrite targeting within the antennal lobe. This powerful technological approach opens up the possibility of uncovering many additional cell-surface proteins required for correct neural connectivity and synapse formation in both invertebrates and vertebrates.
Subcellular control of synapse formation
Regulated axon and dendritic branching underlies lamina-specific innervation that ultimately sculpts neural circuits and specifies synaptic partners [42,66,67]. This synaptic specificity can often be resolved at the subcellular level to restricted regions of neural processes. Recent work uncovers mechanisms underlying subcellular regulation of synaptogenesis in Drosophila mechanosensory neuron axons within the CNS [68•]. This study identifies the phosphatase of regenerating liver-1 (Prl-1) as a key regulator of synaptogenesis in the contralaterally projecting axon collateral that branches out from dorsocental (DC) mechanosensory neuron axons and then projects across the CNS midline. The position of en passant synapses along the axon shaft and synapses in the other two main DC axon collaterals is regulated by a different mechanism. Interestingly, Prl-1 protein localization is spatially restricted within a single compartment of the DC axon midline crossing collateral, precisely where it is required for synapse formation. This requires the regulatory sequences in the long 5’ and 3’ untranslated regions (UTRs) of prl-1 mRNA, and this spatial restriction is required for localized synapse formation and elaboration of spatially restricted synapse-dense terminal arbors. Prl-1 does not appear to directly influence the initiation of terminal arborizations along the collateral since the early stages of arborization in the region of the CNS midline are not affected by loss of prl-1. In these DC mechanosensory neurons, Prl-1 modulates the insulin receptor (InR) pathway and likely acts upstream of Akt to regulate synapse formation and stabilization. Interestingly Prl-1 mutants at later pupal developmental stages show a reduction of terminal arbors, a consequence of elevated PI(4,5)P2 levels in these mutants. This suggests Prl-1 locally influences phosphoinositide-dependent PI3K-PTEN signaling (Figure 2a). Taken together, these data provide novel insight into the subcellular control of spatially restricted axon branching, protrusion and synapse formation. Whether local synapse enrichment is due to local translation or regulation of protein trafficking remains to be determined. These subcellular regulatory mechanisms will undoubtedly provide insight into the molecular mechanisms underlying collateral branching in other neuronal systems.
Figure 2. Subcellular regulation of axon guidance, branching and synapse formation.
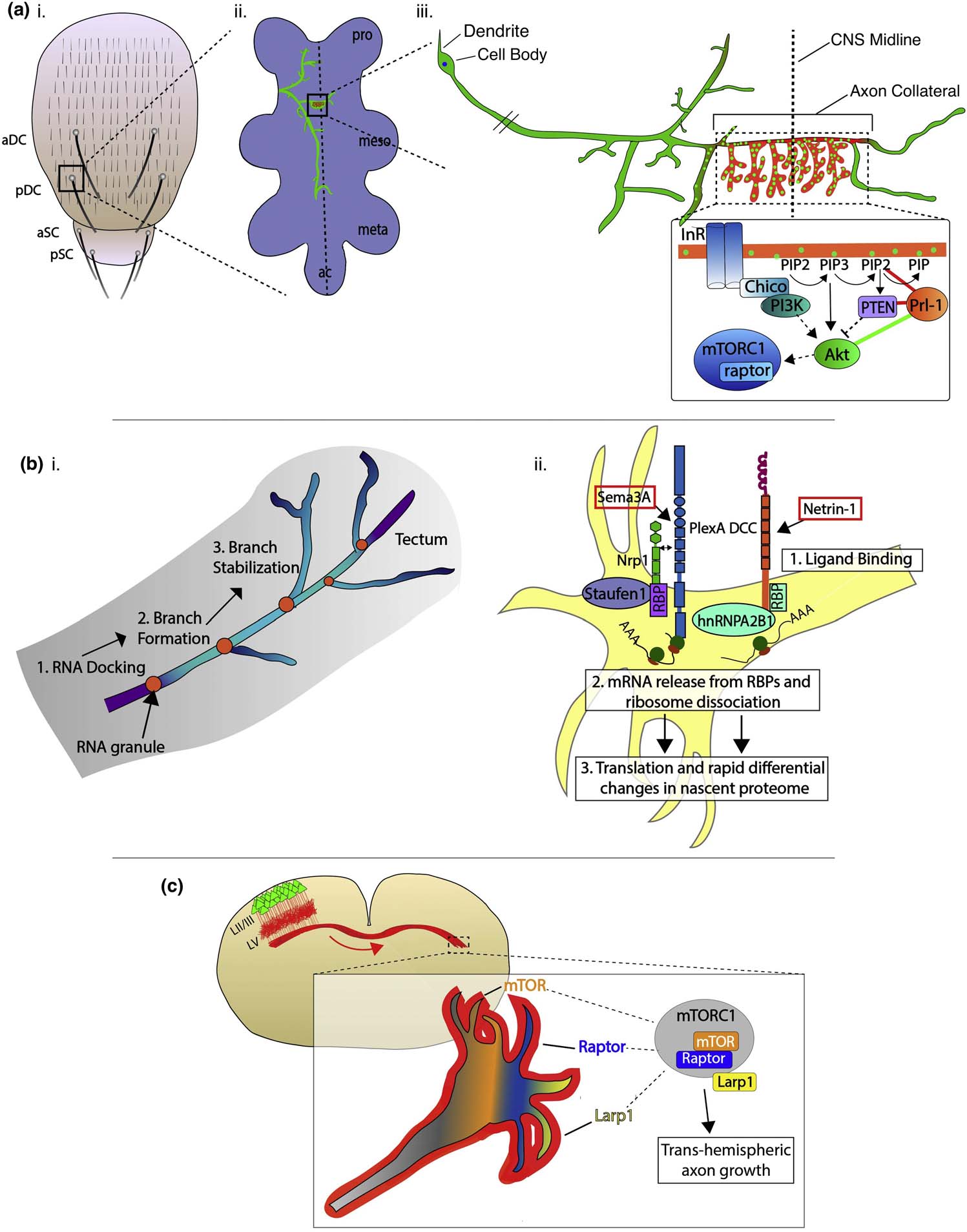
(a) Subcellular regulation of single axon collateral branches and synapses by Prl-1 signaling. (i) Depiction of the Drosophila dorsal thorax with central sensory bristles: anterior (a)/posterior (p) dorsocentral (aDC/pDC) and scutellar (aSC/pSC). (ii) pDC axon (green) targeting the ventral nerve cord (VNC) (light purple). (iii) Enlarged view of the pDC neuron. Subcellular localization of Prl-1 regulates terminal arborization (via regulation of phosphoinositide signaling) (red) and synapse formation (green) specifically in the contralaterally-projecting axon collateral (green bars in the signaling diagram, synergistic genetic interaction; red bars, antagonistic genetic interaction). (adapted from reference #68) (b) Local translation regulates axon branching and guidance. (i) RNA granule (orange) docking precedes protrusion formation and stable axon branching in Xenopus RGC axons within the optics tectum. (ii) Specific guidance cue signaling can elicit rapid changes in the nascent proteasome of RGC axons. Nrp1 and DCC associate with distinct RNA-binding proteins (RBPs) that bind specific subsets of mRNAs. Upon ligand binding, ribosomes rapidly dissociate from their receptors, enabling mRNA release and subsequent translation, ultimately resulting in rapid and distinct changes to the nascent axonal proteome. (c) Growth cones at the tips of layer II/III callosal projection neuron axons in the mouse have transcriptomes and proteomes distinct from their somatic compartments. mTORC1 complex components are selectively enriched at the tips of trans-hemispheric growth cones, and this signaling is necessary for normal callosal projections.
Similarly, subcellular regulation of synaptic targeting is observed in the Drosophila mushroom body. Specifically, the γ-lobe is comprised of γ-KC (Kenyon Cell) axons and is innervated by MB output neurons (MBONs) and dopaminergic neurons (DANs) in five distinct axonal zones (γ1-γ5). Very recent work identifies a cell-autonomous role for Dpr12 and a non-cell autonomous role for its binding partner, DIPδ, in regulating γ-KC projection into MB γ4/5 zones and interactions with protocerebral anterior medial DANs (PAM-DANs) [69•]. It will be interesting to define the co-receptors and downstream signaling mechanisms that regulate this DIP-Dpr interaction in the context of subcellular neuronal wiring.
Local translation also regulates synapse formation, and it has the potential for regulating subcellular control of wiring events [70,71]. Activation of guidance receptors, including DCC, neuropilin-1, and Robo2, can trigger local protein synthesis [71], and recent evidence for local translation within axonal compartments advances these findings. Tracking RNA granules in RGC axons in vivo reveals that RNA docking predicts branch points. RNA granules are only observed inside persistent branches and often are docked at sites where new protrusions or secondary branches form, which are necessary for synapse formation (Figure 2bi) [72].
Since local translation appears correlated with the formation of axon branches, what are the extrinsic cues that regulate these local translation events? In RGCs, classical guidance cues such as netrin-1, Sema3A, and BDNF can differentially control axonal translation of multiple proteins, including proteasomal subunits, proteins involved in metabolic pathways, histones and methyltransferases, demonstrating that regulation of the degradation and post-translational modification of proteins can occur in response to both repulsive and attractive guidance cues [73•]. Guidance cue receptors can interact with ribosomes and ribosome subunits within the cell, and the specificity of their RNA-binding partners is instructive for responses by growing axons to extrinsic guidance cues, suggesting cue-specific regulation of the local proteome (Figure 2bii) [74]. Interestingly, RGC axons display distinct proteomic signatures in response to changes in cue polarity (repulsion to attraction), suggesting subcellular regulation of axonal response to extrinsic cues. Similarly, local translation within growth cones of contralaterally-projecting cortical neurons regulates axon targeting [75•]. Specifically, growth cones at the tips of trans-hemispheric axons in the cerebral cortex are enriched in mTOR and proteins with mTOR binding motifs, and this signaling pathway is necessary for the formation of callosal projections (Figure 2c). Taken together these studies show that signal transduction during growth cone navigation can be controlled at the subcellular level by local translation events. Additionally, they also show that signaling through guidance receptors may control these events, raising interesting questions that relate to mRNA transport, regulation of local protein synthesis and transport of newly synthesized proteins to the cell surface.
Conclusions:
Recent progress in understanding neural guidance and circuit formation has greatly advanced the field. Many new roles for guidance cues have been uncovered with the advent of novel genetic, transcriptomic, and proteomic technologies. New tools that enable genetic access in the context of single cell analyses unveil numerous classes of neurons with diverse compositions of cell surface receptors that ultimately define their wiring with synaptic partners. The regulated distribution of cell-surface receptors, coupled with complex cytosolic signaling events that regulate cytoskeletal dynamics, local protein synthesis, and protein degradation, show how guidance molecules orchestrate circuit formation and fine tune synaptic connectivity. Subcellular control of guidance cue signaling is also crucial for regulating neural process guidance and synapse formation. Interestingly, several novel themes in neuronal guidance have recently emerged and identify additional cellular components not previously implicated in neural guidance or circuit formation. For example, recent observations show that cilia-specific optogenetic modulation of the PI3K/AKT signaling pathway can induce changes in axonal process dynamics and underlies axon tract defects associated with certain ciliopathies [76•]. Further, the ciliary small GTPase Arl13b regulates growth cone turning of commissural neurons mediated by Shh, but this does not occur in close proximity to the primary cilia [77]. These somewhat unexpected observations have important implications for understanding axon tract development and circuit formation, and they raise the possibility of extrinsic cues acting indirectly on growth cones to influence neurite growth and guidance.
The studies we have highlighted here demonstrate the continually emerging complexity of guidance cue regulation and function in neural circuit development and refinement. Future work will undoubtedly continue to uncover phylogenetically conserved cues underlying lamination and topographic organization of neuronal connections, providing the field with important answers to long-standing questions that address the molecular mechanisms dictating circuit assembly, synaptic function, and brain health.
Acknowledgements:
We thank Greg Bashaw, Roman Giger, Zhuhao Wu, anonymous reviewer, and members of the Kolodkin laboratory for helpful comments and critical reading of the manuscript. Work in the authors’ laboratory is supported by funding from the NIH to A.L.K., and by funding to J.M.D. from the Cellular and Molecular Medicine graduate training program NIH/GM T32 Training Grant at The Johns Hopkins School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Kolodkin AL, Tessier-Lavigne M: Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanes JR, Yamagata M: Many paths to synaptic specificity. Annu Rev Cell Dev Biol 2009, 25:161–195. [DOI] [PubMed] [Google Scholar]
- 3.Stoeckli ET: Understanding axon guidance: are we nearly there yet? Development 2018, 145. [DOI] [PubMed] [Google Scholar]
- 4.Sanes JR, Zipursky SL: Synaptic Specificity, Recognition Molecules, and Assembly of Neural Circuits. Cell 2020, 181:536–556. [DOI] [PubMed] [Google Scholar]
- 5.Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, Chedotal A: Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 2017, 545:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hand RA, Kolodkin AL: Netrin-Mediated Axon Guidance to the CNS Midline Revisited. Neuron 2017, 94:691–693. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M: Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994, 78:425–435. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M: Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci 2006, 26:8866–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, Butler SJ: Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 2017, 94:790–799 e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Bravo JA, Roig Puiggros S, Blockus H, Dominici C, Zelina P, Mehlen P, Chedotal A: Commissural neurons transgress the CNS/PNS boundary in absence of ventricular zone-derived netrin 1. Development 2018, 145. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Bravo JA, Roig Puiggros S, Mehlen P, Chedotal A: Synergistic Activity of Floor-Plate- and Ventricular-Zone-Derived Netrin-1 in Spinal Cord Commissural Axon Guidance. Neuron 2019, 101:625–634 e623. [DOI] [PubMed] [Google Scholar]; • Moreno-Bravo et al. revisit the role of netrin-1 secretion as both a local and long-range attractant and find distinct roles in hindbrain and spinal cord commisural axon guidance. Further, they establish a synergistic role between floor-plate and ventricular zone-derived netrin-1 in dictating proper commissural axon navigation to the floorplate in the spinal cord.
- 12.Wu Z, Makihara S, Yam PT, Teo S, Renier N, Balekoglu N, Moreno-Bravo JA, Olsen O, Chedotal A, Charron F, et al. : Long-Range Guidance of Spinal Commissural Axons by Netrin1 and Sonic Hedgehog from Midline Floor Plate Cells. Neuron 2019, 101:635–647 e634. [DOI] [PubMed] [Google Scholar]; • The authors here show netrin-1 acts as both a long-range and short-range cue for commissural axon growth cone navigation in the spinal cord. They also take a closer look at the roles of netrin-1 and Shh secreted from the floor plate on commissural axon guidance and find that these two cues collaborate to attract commissural axons at long range.
- 13.Kim M, Fontelonga TM, Lee CH, Barnum SJ, Mastick GS: Motor axons are guided to exit points in the spinal cord by Slit and Netrin signals. Dev Biol 2017, 432:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonanomi D, Valenza F, Chivatakarn O, Sternfeld MJ, Driscoll SP, Aslanian A, Lettieri K, Gullo M, Badaloni A, Lewcock JW, et al. : p190RhoGAP Filters Competing Signals to Resolve Axon Guidance Conflicts. Neuron 2019, 102:602–620 e609. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study uncovers a cell-intrinsic role for p190RhoGAP in regulating netrin-1 signaling through its receptor DCC. p190RhoGAP uses a non-canonical GAP-independent signaling mechanism to enable proper motor neuron exit from the spinal cord, showing that context-dependent intracellular signaling mechanisms can ensure that axons ignore inappropriate cues when navigating to their targets.
- 15.Jeong S, Juhaszova K, Kolodkin AL: The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron 2012, 76:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M: Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 2008, 58:325–332. [DOI] [PubMed] [Google Scholar]
- 17.Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. : A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev 2010, 24:396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET: RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev 2012, 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T, Huang H, Shao Q, Yee S, Majumder T, Liu G: miR-92 Suppresses Robo1 Translation to Modulate Slit Sensitivity in Commissural Axon Guidance. Cell Rep 2018, 24:2694–2708 e2696. [DOI] [PubMed] [Google Scholar]
- 20.Pignata A, Ducuing H, Boubakar L, Gardette T, Kindbeiter K, Bozon M, Tauszig-Delamasure S, Falk J, Thoumine O, Castellani V: A Spatiotemporal Sequence of Sensitization to Slits and Semaphorins Orchestrates Commissural Axon Navigation. Cell Rep 2019, 29:347–362 e345. [DOI] [PubMed] [Google Scholar]; • The authors perform an in-depth high resolution live imaging analysis of guidance cue receptor localization during commissural axon navigation in the spinal cord. Using pHLourin-tagged constructs, they visualize in real-time the precise dynamics and localization of receptors in the growth cone, including in specific sub-domains.
- 21.Jaworski A, Long H, Tessier-Lavigne M: Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci 2010, 30:9445–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorla M, Santiago C, Chaudhari K, Layman AAK, Oliver PM, Bashaw GJ: Ndfip Proteins Target Robo Receptors for Degradation and Allow Commissural Axons to Cross the Midline in the Developing Spinal Cord. Cell Rep 2019, 26:3298–3312 e3294. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Gorla et al. identify two Nedd-4-interacting proteins, Ndfip1 and Ndfip2, which facilitate Robo1 ubiquitination and degredation, avoiding premarture responsiveness to slit. Their results suggest that the overall Robo1 intracellular sorting mechanism is phyogeetically conserved.
- 23.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ: Comm sorts Robo to control axon guidance at the Drosophila midline. Cell 2002, 110:415–427. [DOI] [PubMed] [Google Scholar]
- 24.Ferent J, Giguere F, Jolicoeur C, Morin S, Michaud JF, Makihara S, Yam PT, Cayouette M, Charron F: Boc Acts via Numb as a Shh-Dependent Endocytic Platform for Ptch1 Internalization and Shh-Mediated Axon Guidance. Neuron 2019, 102:1157–1171 e1155. [DOI] [PubMed] [Google Scholar]; • This study defines an intracellular signaling mechanism that is necessary for Shh-mediated growth cone turning. The authors find that Shh induces Boc and Ptch1 internalization via the adaptor numb1 and also Shh-specific interactions with Boc.
- 25.Peng J, Fabre PJ, Dolique T, Swikert SM, Kermasson L, Shimogori T, Charron F: Sonic Hedgehog Is a Remotely Produced Cue that Controls Axon Guidance Trans-axonally at a Midline Choice Point. Neuron 2018, 97:326–340 e324. [DOI] [PubMed] [Google Scholar]
- 26.Fame RM, MacDonald JL, Macklis JD: Development, specification, and diversity of callosal projection neurons. Trends Neurosci 2011, 34:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clements R, Wright KM: Retinal ganglion cell axon sorting at the optic chiasm requires dystroglycan. Dev Biol 2018, 442:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry MD, Campbell KP: Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol 1996, 8:625–631. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RP, Kramer JM: Neural maintenance roles for the matrix receptor dystroglycan and the nuclear anchorage complex in Caenorhabditis elegans. Genetics 2012, 190:1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roumazeilles L, Dokalis N, Kaulich E, Lelievre V: It is all about the support - The role of the extracellular matrix in regenerating axon guidance. Cell Adh Migr 2018, 12:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yurchenco PD, Wadsworth WG: Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol 2004, 16:572–579. [DOI] [PubMed] [Google Scholar]
- 32.Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD: Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 2012, 76:931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindenmaier LB, Parmentier N, Guo C, Tissir F, Wright KM: Dystroglycan is a scaffold for extracellular axon guidance decisions. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This work describes a multifunctional role for the transmembrane glycoprotein, dystroglycan during axon guidance. The authors identified CELSR3 as a novel binding partner for dystroglycan important for specific guidance events, further demonstrating the importance of dystroglycan acting as a scaffold to coordinate interactions between the extracellular matrix and guidance cues.
- 34.Berns DS, DeNardo LA, Pederick DT, Luo L: Teneurin-3 controls topographic circuit assembly in the hippocampus. Nature 2018, 554:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study describes a role for the transmembrane receptor Teneurin-3 in mediating topographic targeting between proximal CA1 axons and target dendrites of the distal subiculum. The authors also show that Teneurin-3 acts pre- and post-synaptically to control this targeting.
- 35.Sudhof TC: Towards an Understanding of Synapse Formation. Neuron 2018, 100:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Toro D, Carrasquero-Ordaz MA, Chu A, Ruff T, Shahin M, Jackson VA, Chavent M, Berbeira-Santana M, Seyit-Bremer G, Brignani S, et al. : Structural Basis of Teneurin-Latrophilin Interaction in Repulsive Guidance of Migrating Neurons. Cell 2020, 180:323–339 e319. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors present a novel role for teneurin, latrophilin and FLRT protein-protein interactions in cortical neuron migration. This work defines these interactions using X-ray crystallography and shows how they regulate the migration of cortical neurons during neural development through contact-mediated repulsion.
- 37.Li J, Shalev-Benami M, Sando R, Jiang X, Kibrom A, Wang J, Leon K, Katanski C, Nazarko O, Lu YC, et al. : Structural Basis for Teneurin Function in Circuit-Wiring: A Toxin Motif at the Synapse. Cell 2018, 173:735–748 e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pederick DTL,JH; Gingrich EC; Xu C; Liu Y; He Z; Quake SR; Luo L: Latrophilin-2 repels Teneurin-3+ hippocampal axons during target selection. bioRxiv Preprint. [Google Scholar]
- 39.Jackson VA, del Toro D, Carrasquero M, Roversi P, Harlos K, Klein R, Seiradake E: Structural basis of latrophilin-FLRT interaction. Structure 2015, 23:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sando R, Jiang X, Sudhof TC: Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 2019, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraira VE, Ginty DD: The sensory neurons of touch. Neuron 2013, 79:618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson DA, Ma L: Developmental regulation of axon branching in the vertebrate nervous system. Development 2011, 138:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepherd GM, Rowe TB: Neocortical Lamination: Insights from Neuron Types and Evolutionary Precursors. Front Neuroanat 2017, 11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baier H: Synaptic laminae in the visual system: molecular mechanisms forming layers of perception. Annu Rev Cell Dev Biol 2013, 29:385–416. [DOI] [PubMed] [Google Scholar]
- 45.Di Donato V, De Santis F, Albadri S, Auer TO, Duroure K, Charpentier M, Concordet JP, Gebhardt C, Del Bene F: An Attractive Reelin Gradient Establishes Synaptic Lamination in the Vertebrate Visual System. Neuron 2018, 97:1049–1062 e1046. [DOI] [PubMed] [Google Scholar]; • The authors show that reelin signaling through the VLDLR receptor and the intracellular adaptor Dab1 regulates retinal ganglion cell lamination in the vertebrate visual system. Further, they find that reelin is present in a gradient in the tectal neuropil and serves as an attractive cue to direct RGCs to their proper syaptic laminae.
- 46.Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H: Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 2011, 146:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie X, Tabuchi M, Brown MP, Mitchell SP, Wu MN, Kolodkin AL: The laminar organization of the Drosophila ellipsoid body is semaphorin-dependent and prevents the formation of ectopic synaptic connections. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors show a role for Sema-1a and plexin A in regulating R axon lamination in the Drosophila ellipsoid body (EB). This provides evidence showing that EB lamination is critical for local pre-synaptic inhibitory circuit organization.
- 48.Xie X, Tabuchi M, Corver A, Duan G, Wu MN, Kolodkin AL: Semaphorin 2b Regulates Sleep-Circuit Formation in the Drosophila Central Brain. Neuron 2019, 104:322–337 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Xie et al. explore the role of Sema2b in regluating lamination in the fan-shaped body neuropil of the Drosophila brain central complex through Plexin B. They find that loss of of Sema2b/PlexB signnaling leads to ectopic synaptic connections across lamaine that n turn result in changes in fly sleep and arrousal, demonstrating the importance of lamination for proper neural circuit function.
- 49.Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN: Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol 2012, 22:2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian Y, Cao Y, Deng B, Yang G, Li J, Xu R, Zhang D, Huang J, Rao Y: Sleep homeostasis regulated by 5HT2b receptor in a small subset of neurons in the dorsal fan-shaped body of drosophila. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsubouchi A, Yano T, Yokoyama TK, Murtin C, Otsuna H, Ito K: Topological and modality-specific representation of somatosensory information in the fly brain. Science 2017, 358:615–623. [DOI] [PubMed] [Google Scholar]; • Tsubouchi et al. provide a systematic mapping of the Drosophila somatosensory system. They dissected preferential responses to wing and lenngth movements and from their analyses, they find that insect somatosensation organization is in some ways reminiscnent of that observed in vertebrates.
- 52.Sarin S, Zuniga-Sanchez E, Kurmangaliyev YZ, Cousins H, Patel M, Hernandez J, Zhang KX, Samuel MA, Morey M, Sanes JR, et al. : Role for Wnt Signaling in Retinal Neuropil Development: Analysis via RNA-Seq and In Vivo Somatic CRISPR Mutagenesis. Neuron 2018, 98:109–126 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Sarin et al. use a combination of RNAseq and CRISPR-Cas9 approaches to identify genes essential for circuit formation in the outer plexiform region of the mammalian retina. They discover a novel role for Wnt5, acting through a Ryk-dependent pathway, in outer plexiform layer development.
- 53.Carrillo RA, Ozkan E, Menon KP, Nagarkar-Jaiswal S, Lee PT, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K: Control of Synaptic Connectivity by a Network of Drosophila IgSF Cell Surface Proteins. Cell 2015, 163:1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan L, Zhang KX, Pecot MY, Nagarkar-Jaiswal S, Lee PT, Takemura SY, McEwen JM, Nern A, Xu S, Tadros W, et al. : Ig Superfamily Ligand and Receptor Pairs Expressed in Synaptic Partners in Drosophila. Cell 2015, 163:1756–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng S, Ashley J, Kurleto JD, Lobb-Rabe M, Park YJ, Carrillo RA, Ozkan E: Molecular basis of synaptic specificity by immunoglobulin superfamily receptors in Drosophila. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosmanescu F, Katsamba PS, Sergeeva AP, Ahlsen G, Patel SD, Brewer JJ, Tan L, Xu S, Xiao Q, Nagarkar-Jaiswal S, et al. : Neuron-Subtype-Specific Expression, Interaction Affinities, and Specificity Determinants of DIP/Dpr Cell Recognition Proteins. Neuron 2018, 100:1385–1400 e1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sergeeva AP, Katsamba PS, Cosmanescu F, Brewer JJ, Ahlsen G, Mannepalli S, Shapiro L, Honig B: DIP/Dpr interactions and the evolutionary design of specificity in protein families. Nat Commun 2020, 11:2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ting CY, Lee CH: Visual circuit development in Drosophila. Curr Opin Neurobiol 2007, 17:65–72. [DOI] [PubMed] [Google Scholar]
- 59.Xu C, Theisen E, Maloney R, Peng J, Santiago I, Yapp C, Werkhoven Z, Rumbaut E, Shum B, Tarnogorska D, et al. : Control of Synaptic Specificity by Establishing a Relative Preference for Synaptic Partners. Neuron 2019, 103:865–877 e867. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study investigates the role of DIP-Dpr signaling in regulating synaptic specificity in the lamina of the Drosophila optic lobe. The authors find that DIPs γ and β are not required for synapse formation but are required in L neurons for correct visual system function and for localization of select L-L neuron synapses.
- 60.Menon KP, Kulkarni V, Takemura SY, Anaya M, Zinn K: Interactions between Dpr11 and DIP-gamma control selection of amacrine neurons in Drosophila color vision circuits. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors here investigate the role of DIP-Dpr signaling in regulaing Drosophila color vision circuits. They show that Dpr11 and its binding partner DIP-γ regulate Dm8 amacrine cell morphology and synaptic targeting, and that this regulation defines ‘yellow’ and ‘pale’ color vision circuits.
- 61.Xu S, Xiao Q, Cosmanescu F, Sergeeva AP, Yoo J, Lin Y, Katsamba PS, Ahlsen G, Kaufman J, Linaval NT, et al. : Interactions between the Ig-Superfamily Proteins DIP-alpha and Dpr6/10 Regulate Assembly of Neural Circuits. Neuron 2018, 100:1369–1384 e1366. [DOI] [PMC free article] [PubMed] [Google Scholar]; • These results show that DIP-α and Dprs6 and 10 regulate the assembly of neural circuitry in the M3 layer of the medulla. Heterophilic interactions between DIP-α and Dpr6 and 10 regulate several aspects of lamination involving amacrine-like Dm4 and Dm12 neurons.
- 62.Duan X, Krishnaswamy A, De la Huerta I, Sanes JR: Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 2014, 158:793–807. [DOI] [PubMed] [Google Scholar]
- 63.Duan X, Krishnaswamy A, Laboulaye MA, Liu J, Peng YR, Yamagata M, Toma K, Sanes JR: Cadherin Combinations Recruit Dendrites of Distinct Retinal Neurons to a Shared Interneuronal Scaffold. Neuron 2018, 99:1145–1154 e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Duan et al. describe a role for 6 classical Cdhs in lamination of direction-selective circuits in the mouse retina. They find that while Cdhs 6, 9, and 10 regulate lamination of ventral motion-preferring DS cell dendrites, Cdhs 7 and 18 regulation lamination of nasal motion-prefering DS cell dendrites, exemplifying how cell-surface specific molecular intearctionas egulate select circuit formation.
- 64.Mountoufaris G, Chen WV, Hirabayashi Y, O’Keeffe S, Chevee M, Nwakeze CL, Polleux F, Maniatis T: Multicluster Pcdh diversity is required for mouse olfactory neural circuit assembly. Science 2017, 356:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Han S, Li H, Udeshi ND, Svinkina T, Mani DR, Xu C, Guajardo R, Xie Q, Li T, et al. : Cell-Surface Proteomic Profiling in the Fly Brain Uncovers Wiring Regulators. Cell 2020, 180:373–386 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study utilizes a novel approach to spatiotemporally resolve cell-surface proteomes in intact Drosophila olfactory bulb neuorns. The authors identify 20 cell-surface molecules that regulate olfactory neural circuit assembly. They also show that one of these molecules, LRP1, controls olfactory projection neuron dendrite targeting.
- 66.Hand RA, Khalid S, Tam E, Kolodkin AL: Axon Dynamics during Neocortical Laminar Innervation. Cell Rep 2015, 12:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalil K, Dent EW: Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci 2014, 15:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urwyler O, Izadifar A, Vandenbogaerde S, Sachse S, Misbaer A, Schmucker D: Branch-restricted localization of phosphatase Prl-1 specifies axonal synaptogenesis domains. Science 2019, 364. [DOI] [PubMed] [Google Scholar]; • Urwyler et al. describe a role for the Prl-1phosphatase in regulating subcellular localizaiton of synaptogenesis in a single target area of the Drosphila dorsoventral (DC) mechanosensory neuron. Additionally, they show that Prl-1 signaling likely acts downstream of the inositol receptor pathway through Akt.
- 69.Bornstein BA I; Berkun V; Meltzer H; Reh F; Keren-Shaul H; David E; Riemensperger T; Schuldiner O: Transneuronal interactions facilitate axonal compartment formation. bioRxiv Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors show subcellular regulation γ-KC projections into M γ4/5 zones of the Drosophila mushroom body, and also interactions with Protocerebral Anterior Medial DANs (PAM-DANs), occur through Dpr12 and its binding partner DIPδ.They find that Dpr12 acts cell-autonomously to enable γ-neuron projection into the γ4/5 compartment, which is the first report of a cell-autonomous role for Dpr proteins.
- 70.Cioni JM, Koppers M, Holt CE: Molecular control of local translation in axon development and maintenance. Curr Opin Neurobiol 2018, 51:86–94. [DOI] [PubMed] [Google Scholar]
- 71.Russell SA, Bashaw GJ: Axon guidance pathways and the control of gene expression. Dev Dyn 2018, 247:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong HH, Lin JQ, Strohl F, Roque CG, Cioni JM, Cagnetta R, Turner-Bridger B, Laine RF, Harris WA, Kaminski CF, et al. : RNA Docking and Local Translation Regulate Site-Specific Axon Remodeling In Vivo. Neuron 2017, 95:852–868 e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cagnetta R, Frese CK, Shigeoka T, Krijgsveld J, Holt CE: Rapid Cue-Specific Remodeling of the Nascent Axonal Proteome. Neuron 2018, 99:29–46 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrates how the nascent axonal proteome rapidly changes in response to attractive or repulsive guidance cues. The authors stimulate RGC axons with different guidance cues (Netrin-1, BDNF, Sem3a) and show how each cue elicits distinct proteomic signatures within the axon.
- 74.Koppers M, Cagnetta R, Shigeoka T, Wunderlich LC, Vallejo-Ramirez P, Qiaojin Lin J, Zhao S, Jakobs MA, Dwivedy A, Minett MS, et al. : Receptor-specific interactome as a hub for rapid cue-induced selective translation in axons. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poulopoulos A, Murphy AJ, Ozkan A, Davis P, Hatch J, Kirchner R, Macklis JD: Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 2019, 565:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Poulopoulos et al. present a novel technique for growth cone isolation from trans-hemispheric callosal projection neurons in the intact cortex. They perform transcriptomic and proteomic analyses on these growth cones and uncover a role for mTOR signaling in trans-hemispheric axon growth.
- 76.Guo J, Otis JM, Suciu SK, Catalano C, Xing L, Constable S, Wachten D, Gupton S, Lee J, Lee A, et al. : Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Dev Cell 2019, 51:759–774 e755. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study presents a role for Arl13b cilia signaling the modulation of axonal connectivity in the murine cortiex. The authors perform chemogenetic activation of ciliary GPCR signaling and cilia-specific optogenetic modulation of downstream signaling cascades, demonstrating cross-talk between cilia signaling and axon dynamics.
- 77.Ferent J, Constable S, Gigante ED, Yam PT, Mariani LE, Legue E, Liem KF Jr., Caspary T, Charron F: The Ciliary Protein Arl13b Functions Outside of the Primary Cilium in Shh-Mediated Axon Guidance. Cell Rep 2019, 29:3356–3366 e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]


