Abstract
Asherman’s syndrome is an endometrial regeneration disorder resulting from injury to the endometrial basal layer, causing the formation of scar tissue in the uterus and cervix. This usually leads to uterine infertility, menstrual disorders, and placental abnormalities. While stem cell therapy has shown extensive progress in repairing the damaged endometrium and preventing intrauterine adhesion, issues of low engraftment rates, rapid senescence, and the risk of tumorigenesis remain to be resolved for efficient and effective application of this technology in endometrial repair. This study addressed these challenges by developing a co-culture system to generate multi-lineage endometrial organoids (MLEOs) comprising endometrial epithelium organoids (EEOs) and endometrial mesenchymal stem cells (eMSCs). The efficacy of these MLEOs was investigated by seeding them on a biocompatible scaffold, the human acellular amniotic membrane (HAAM), to create a biological graft patch, which was subsequently transplanted into an injury model of the endometrium in rats. The results indicated that the MLEOs on the HAAM patch facilitated endometrial angiogenesis, regeneration, and improved pregnancy outcomes. The MLEOs on the HAAM patch could serve as a promising strategy for treating endometrial injury and preventing Asherman’s syndrome.
Keywords: Asherman’s syndrome, primary prevention, endometrial epithelium organoids, endometrial mesenchymal stem cell, human acellular amniotic membrane, regeneration
Background
The endometrium is a dynamically changing tissue that can undergo shedding and re-epithelialization every month without scar formation under the influence of hormones, such as estrogen and progesterone. Like skin repair, regeneration of the injured endometrium begins with the migration of epithelial cells from exposed endometrial glands and adjacent intact endometrium to the exposed stromal component1,2. Asherman’s syndrome, an acquired, gynecological disorder of the uterus results from injury to the endometrium basal layer primarily caused by hysteroscopic surgery and recurrent induced abortion, leading to loss of endometrial stem progenitor cells, and replacement of the injured area with pathological fibrotic tissue3,4. However, severe endometrial injuries are often irreparable, reducing the chance of subsequent pregnancy, and live birth rates 5 . Therefore, developing an approach for the primary prevention of uterine adhesion is urgent. In recent years, stem cell therapies have shown great potential in repairing injured endometrium, with some studies proving efficacy in clinical trials. As a new hope for endometrial regeneration, stem cell therapy might be more efficient than original prevention approaches for primary prevention of intrauterine adhesion (IUA). Therefore, developing an approach for the primary prevention of uterine adhesion is urgent.
Endometrial epithelial progenitor cells (EEPCs) have shown some promise in stem cell therapy for repairing the injured endometrium. However, the main limitation of the therapy is the low engraftment rate, which includes two factors: (1) EEPCs cannot expand long-term in vitro, resulting in an insufficient number of cells for transplantation and (2) deficient cell-cell interaction in the microenvironment causes a low survival rate of endometrial epithelial stem cells after transplantation to the injured area of the endometrium. The emergence of organoids has provided an alternative method to complement existing technologies to resolve this problem as they have high proliferation in vitro, recapitulate the microanatomy of the primary tissue, and can maintain genotype stability 6 . Current studies have indicated that endometrial epithelium organoids (EEOs) can regenerate injured endometrium7,8. However, tissue regeneration requires coordinated and dynamic remodeling of stem and progenitor cells within the surrounding niche. We proposed that combining organoids with mesenchymal cells could enhance the organoid-repairing efficiency of the injured endometrium.
Mesenchymal stem cells (MSCs) have become a potential cell for stem cell therapy due to their self-renewal, low immunogenicity, and immune regulatory function. It can be harvested from various tissues, including bone marrow9,10, umbilical cord blood 11 , amniotic membrane 12 , and endometrium 13 . Endometrial mesenchymal stem cells (eMSCs), one of the stem progenitor cells in the endometrium, may provide an appropriate microenvironment for repairing the endometrial cell niche.
In most mammals, the developing fetus is enclosed by an amniotic membrane or amnion. The human acellular amniotic membrane (HAAM) preserves extracellular matrix (ECM) components, including abundant collagen, laminin, fibronectin, elastin, hyaluronic acid, fibroblast growth, and transforming growth factors 14 . It provides low immunogenicity and good biocompatibility, making it an excellent biological scaffold. Over the last decades, researchers have focused on the biomedical applications of HAAM, such as the development of a simple sheet for skin or cornea repair, amnion nanocomposite, and hydrogels for high-throughput regeneration of muscle, cartilage, and tendon 15 .
In our study, we developed multi-lineage endometrial organoids (MLEOs) with EEOs and eMSCs to investigate their efficiency in repairing injured endometrium and decelerating apoptosis. We then seeded these organoids on HAAM, which was termed MLEOs on HAAM (MLEO-HAAM). Our results indicated that the MLEO-HAAM patch could serve as a promising approach to repair the injured endometrium, which had undergone mechanical abrasions to the basal layer, and could improve pregnancy outcomes.
Results
MLEOs Contribute to the Regeneration of Damaged Endometrium
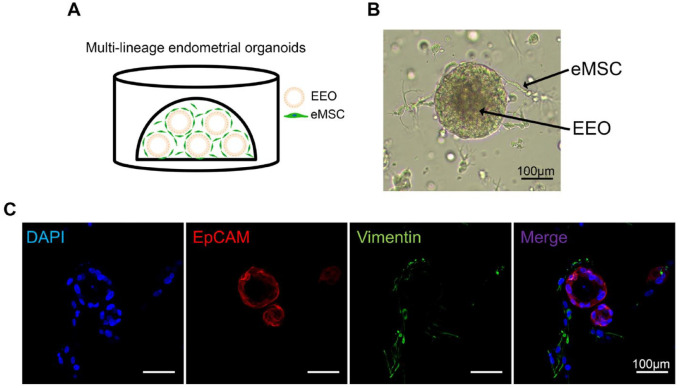
As endometrial mesenchymal cells supported the stem niche, we suspected that MLEOs could repair endometrial damage. To test this hypothesis, we established a MLEO system in which endometrial mesenchymal and epithelial cells were co-cultured in Matrigel as shown in the schematic diagram in Fig. 1A. Organoids and mesenchymal cells showed sufficient expansion, as illustrated in Fig. 1B. Confocal microscopy confirmed that these MLEOs were positive for the epithelial cell marker EpCAM, while the fibroblast-like cells were positive for the fibroblast cell marker Vimentin (Fig. 1C).
Figure 1.
The formation of multi-lineage endometrial organoids. (A) Schematic diagram of the MLEOs consisting of EEO and eMSCs embedded within the Matrigel dome. (B) A representative bright field image of EEO-eMSC MLEOs. Scale bar: 100 μm. (C) Immunofluorescence images of the MLEOs stained with antibodies against EpCAM (red), and Vimentin (mesenchymal cell marker, green). Scale bar: 100 μm. DAPI: 4',6-diamidino-2-phenylindole; MLEO: multi-lineage endometrial organoid; EEO: endometrial epithelium organoid; eMSCs: endometrial mesenchymal stem cells; EpCAM: endometrial epithelial cell marker.
Mesenchymal Cells Support the Growth and Self-Renew of EEOs
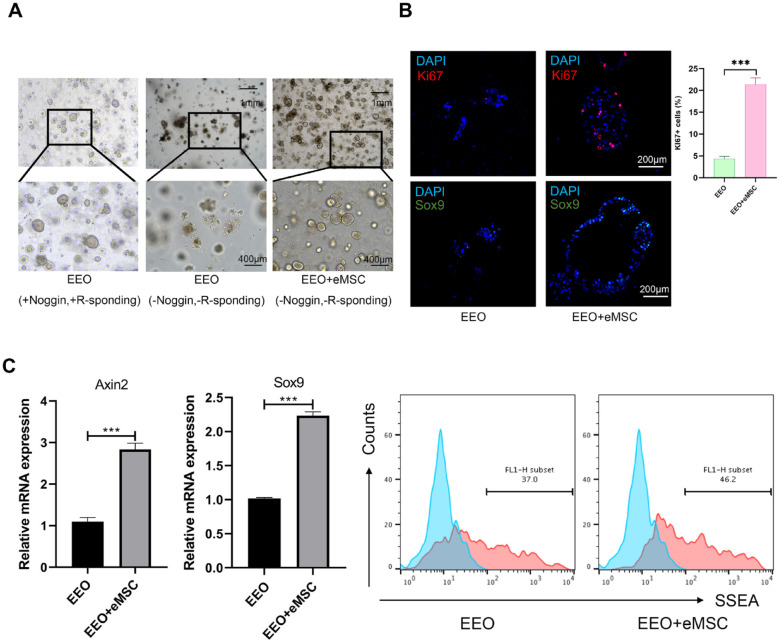
Mesenchymal cells could serve as a stem cell niche that supports the proper functioning and self-renewal of epithelial stem cells. To test this hypothesis in the endometrium, we utilized a 3D Matrigel culture system that allowed for the growth and self-renewal of EEO. Organoids were unable to proliferate or self-renew when Rspo1 and Noggin were withdrawn. However, when EEOs were co-cultured with endometrial mesenchymal cells, they spontaneously formed self-organizing organoids in the absence of Rspo1 and Noggin (Fig. 2A). Organoids in the co-culture group exhibited more Ki67+ cells (a marker associated with proliferation) and Sox9+ cells (a marker associated with stemness) as observed by immunofluorescence (Fig. 2B). Real-time polymerase chain reaction (RT-PCR) analysis demonstrated that organoids co-cultured with mesenchymal cells expressed higher levels of stemness-associated genes (Axin2 and Sox9) compared with the control group (Fig. 2C). The proportion of stage-specific embryonic antigen 1 (SSEA-1+) cells, considered as a marker for EEPCs, was measured using flow cytometry. The results showed that the proportion of SSEA-1+ cells increased in organoids co-cultured with mesenchymal cells (Fig. 2D). These findings suggest that endometrial mesenchymal cells provide a stem cell niche that enables the growth and self-renewal of EMOs.
Figure 2.
The eMSC maintains the stemness and proliferation of EEOs. (A) Representative bright field images of EEOs culture with Noggin and R-spondin1, EEOs and eMSCs co-culture system upon withdrawal of Noggin and R-spondin1. (B) Immunofluorescence staining of EEOs co-cultured with and without eMSCs. The expression of Ki67 (a marker of active cell proliferation, shown in red) and SOX9 (a marker of stemness, shown in green) was higher in the eMSCs+EEOs group compared with the EEOs-only group. Scale bar: 200 μm. Data are the mean ± SEM. Student’s t test; ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001. (C) Real-time PCR analyses of the endometrial progenitor stem cell markers SOX9 and Axin2. Data are the mean ± SEM. Student’s t test, ***P < 0.001. (D) Flow cytometry analysis of SSEA-1+ cells. DAPI: 4',6-diamidino-2-phenylindole; eMSC: endometrial mesenchymal stem cell; EEO: endometrial epithelium organoid; SEM: standard error of the mean; PCR: polymerase chain reaction.
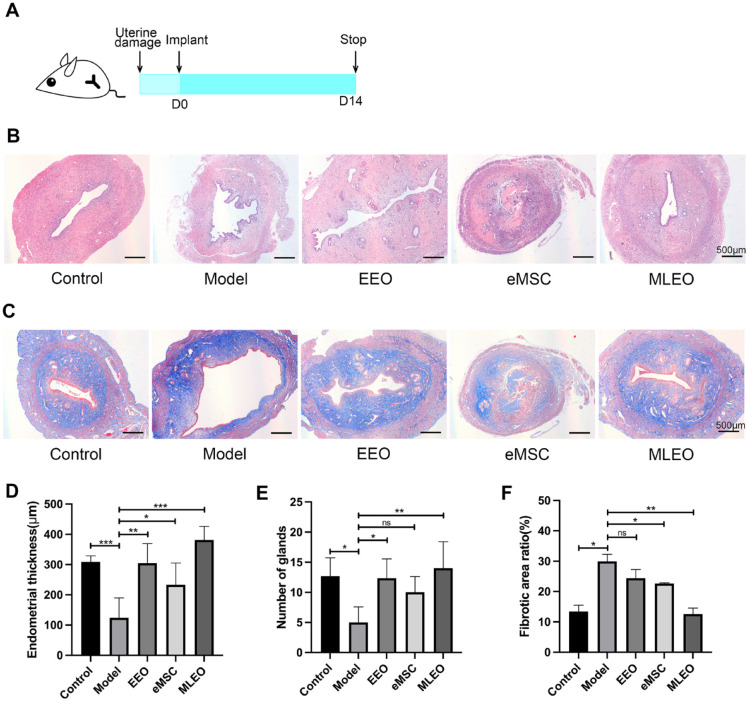
To evaluate the regenerative capabilities of MLEOs in injured endometrium, we established endometrial-injured rat models by performing endometrial abrasion curettage with a curette. Two weeks after surgery, we transplanted the MLEOs into the injured uterine and collected the uterine tissue (Fig. 3A). Histological analysis of uterine tissue stained with hematoxylin and eosin (H&E) showed that the endometrial thicknesses and the number of endometrial glands in all three cell-treatment groups had improved considerably as compared with the model group. In particular, the MLEO-treated group showed the most significant improvement (Fig. 3B–E). Masson’s trichrome staining was used to evaluate the fibrotic phenotype, and the fibrotic area ratio was significantly lower in the MLEO group than in the other groups (organoid group and mesenchymal cell group) (Fig. 3F).
Figure 3.
Efficacy of multi-lineage endometrial organoids in repairing injured endometrium. (A) Schematic diagram of intrauterine transplantation of MLEOs in endometrium-injured mice at different time points. (B) Representative H&E images of uterine sections at day 14 of injury treatment. (C) Masson trichrome-stained sections of the uterine at day 14 of injury treatment. (D) Statistical analysis of endometrial thickness after injury treatment. (E) Analysis of the number of glands. (F) Statistical analysis of fibrotic area measured in different groups. Data are the mean ± SEM. MLEO: multi-lineage endometrial organoid; H&E: hematoxylin and eosin; SEM: standard error of the mean; EEO: endometrial epithelium organoid; eMSC: endometrial mesenchymal stem cell. Student’s t test; ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001.
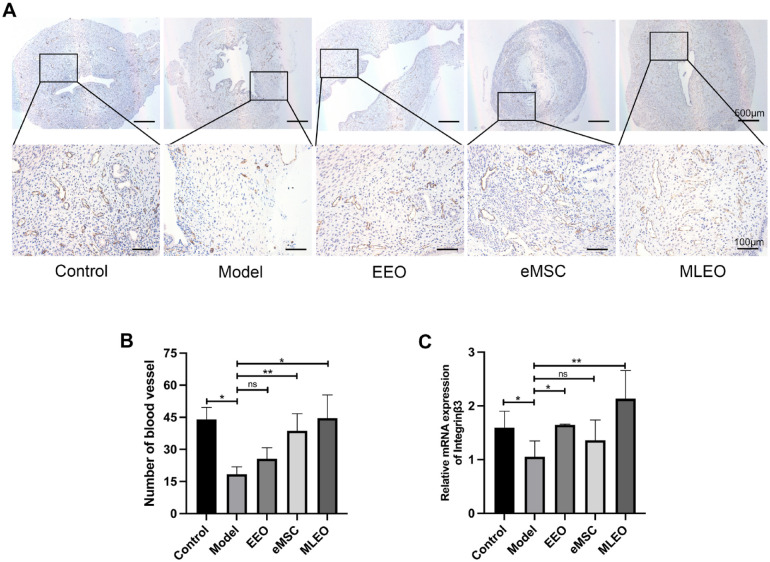
To assess the angiogenesis capacity of the different treatment groups, uterine tissue was stained with CD31 (an endothelial cell marker) (Fig. 4A). The expression of CD31 was significantly increased in the MLEO group as compared with the other cell-treatment groups (Fig. 4B). Moreover, to further investigate the uterine receptivity of the different groups, we analyzed the expression of integrinβ3, HOXA10, BCL6 16 , 17 (a marker of uterine receptivity) by quantitative real-time polymerase chain reaction (qRT-PCR) (Fig. 4C; Supplementary Fig. 2). The results demonstrated that the MLEO group showed the highest expression of integrinβ3, indicating that MLEOs might improve the pregnancy outcome of the injured uterus. Taken together, these results suggest that MLEO-HAAM treatment could potentially enhance the repairment of the injured endometrium, thereby improving pregnancy outcomes.
Figure 4.
Multi-lineage endometrial organoids improve the angiogenesis capacity and uterine receptivity of the injured endometrium. (A) Immunohistochemical staining of CD31 (an endothelial cell marker) in different groups. Scale bar: 100 μm. (B) Quantification of CD31 expression in different groups. (C) qPCR analysis integrinβ3 expression. Data are the mean ± SEM. Student’s t test. qPCR: quantitative polymerase chain reaction; SEM: standard error of the mean; EEO: endometrial epithelium organoid; eMSC: endometrial mesenchymal stem cell; MLEO: multi-lineage endometrial organoid; ns: non-significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Establishment of MLEOs on HAAM Patch
H&E staining showed that the resident cells of HAAM were removed entirely after the decellularization procedures (Supplementary Fig. 1a). We then seeded MLEOs on the HAAM to improve their survival after intrauterine transplantation (Supplementary Fig. 1b). H&E staining showed that the EEOs closely adhered to HAAM under the wrapping of eMSCs, displaying a flaky and cluster-like morphology (Supplementary Fig. 5c, d). Consistent with previous experimental results, EEOs maintained their proliferation state when co-cultured with eMSCs after seeding on the HAAM (Supplementary Fig. 5e, f).
MLEOs on HAAM Improve Pregnancy Outcome in Endometrium-Injured Rats
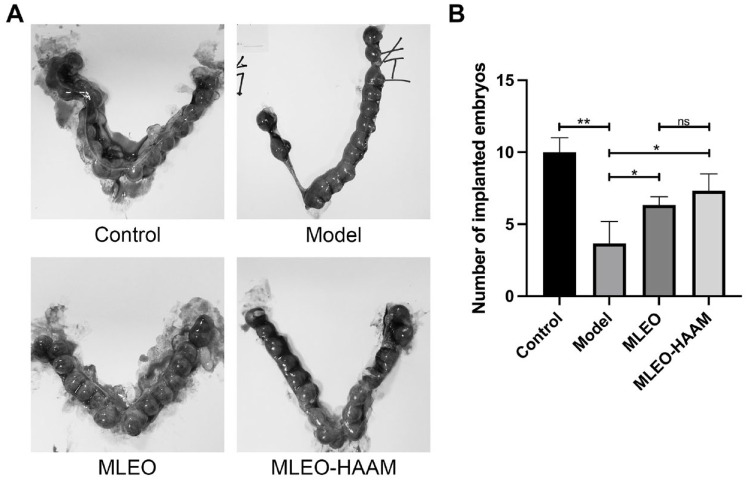
To verify the effectiveness of the MLEO-HAAM patch in repairing the injured endometrium in vivo, we divided the endometrial-injured rat models into three groups: model group, MLEO group, and MLEO-HAAM group. Compared with other groups, the number of implantation embryos significantly increased in the MLEOs in the HAAM-treated group (Fig. 5A, B), demonstrating that the MLEO-HAAM patch positively affects the repair of newly injured tissues, thus improving pregnancy outcomes.
Figure 5.
Multi-lineage endometrial organoids on HAAM improved pregnancy outcome in rats. (A) Representative of the number of implanted embryos following different treatments. (B) Comparison of embryo implantation in rats after treatment. HAAM: human acellular amniotic membrane; MLEO: multi-lineage endometrial organoid.
Discussion
Re-epithelialization of the endometrium is an efficient process that provides scar-free healing and repair. The extreme regenerative capacity of the endometrium is based on the presence of progenitors, located in the epithelial and stromal cell compartments of the basalis layer18,19,20. A previous study demonstrated that intrauterine transplantation of EEOs effectively repaired the basal layer of the endometrium in an IUA mice model and conducted the intact endometrium 7 . In our study, we generated the first MLEOs on HAAM patch and performed intrauterine transplantation to the endometrium injury in rat models. The follow-up results showed that the complex patch has the potential to repair the endometrium and improve the outcome of pregnancy.
It was suggested that the regenerative efficacy of stem cells depended on the stem cell niche provided by adjacent cells 21 . A study indicated that Bim1-expressing cells located in the crypt base of the small intestine could give rise to LGR5-expressing cells after ablating adjacent LGR5-expressing cells. This phenomenon suggested that a stem cell niche could transform reserve stem cells into functional stem cell populations to preserve the homeostasis of the small intestine epithelium 22 . Analogous to this study, the existence of the eMSCs imitated the microenvironment of the endometrial gland to provide the stem cell niche to EEOs, leading to increased expression of stem genes and maintained stemness of EEOs. In our study, EEOs co-cultured with eMSCs can spontaneously form self-organizing organoids without R-spondin1 and Noggin. To our knowledge, R-spondin1 and Noggin were the main factors in maintaining the stemness of the organoids in the culture medium. Complete withdrawal of R-spondin1 and Noggin could reduce the proliferation efficiency of the organoids or enhance apoptosis23,24. R-spondin1 has been implicated in the activation and regulation of the Wnt signaling pathway 25 . The paracrine effect of bone marrow MSCs could promote endometrial regeneration and proliferation via the Wnt/β-catenin signaling pathway 26 . A similar effect was described in the study on the paracrine effect of human umbilical cord MSCs on the endometrium 27 . Our finding suggests that even without R-spondin1, the eMSCs might generate Wnt niches to support organoid growth and maintain the stemness of organoids.
HAAM was used as a scaffold to load the MLEOs, which comprised EEOs and eMSCs. It provided a compatible scaffold with low immunogenicity and promoted cell proliferation, differentiation, and adhesion28,29. A recent report has indicated that rat hair follicle stem cells (rHFSCs) seeded on HAAM showed flaky and cluster-like morphology and could adhere and infiltrate more effectively into the wound 30 , like the morphology of the MLEOs observed in this study. Another study has shown that HAAM loaded with menstrual blood stem cells can regenerate fully injured skin and restore skin function.
HAAM could serve as a dependable potential scaffold with application in regeneration therapy. The China Food and Drug Administration has approved hyaluronic-acid gel (HA-GEL) for clinical intrauterine injection to prevent postoperative IUAs. HA-GEL has been shown to have good lubrication and compatibility. However, due to its liquid nature, it could be partially lost after intrauterine injection through the dilated cervix, and only a small portion remains in the uterine cavity. As a semi-solid film structure, HAAM is not easily lost as it can fit into the shape of the uterine endometrium. Moreover, HAAM provides a sufficient surface for the expansion of EEOs and eMSCs to achieve better repair of the injured endometrium.
There are some limitations in this study. First, organoids are cultured in Matrigel™31, which is a heterogeneous, complex mixture of ECM proteins, proteoglycans, and growth factors secreted by Engelbreth–Holm–Swarm mouse sarcoma cells. Thus, this tumor-derived matrix has limited clinical translational potential and a safer hydrogel is needed to replace Matrigel™ in future study. Second, further investigations would be required to confirm the applications of this novel repair system since we did not conduct long-term follow-ups on the endometrial-injured mouse model after treatment with the MLEOs on the HAAM patch to investigate spontaneous IUA rates. Further studies would be required to understand the histological changes in the endometrium during implantation.
Conclusion
Altogether, the MLEOs were shown to promote endometrial regeneration, inhibit fibrosis, and effectively improve pregnancy outcomes. The MLEO-HAAM patch has the potential to be a primary prevention method for IUA. Future studies could focus on the regenerative function of the MLEO-HAAM patches in other IUA animal models to further confirm in vivo endometrial regeneration.
Method
Organoids and Mesenchymal Cell Culture From Endometrial Biopsies
Endometrial biopsies were collected from premenopausal women undergoing routine hysterectomies for benign uterine conditions following the institutional review board-approved protocol of the Obstetrics and Gynecology Hospital of Fudan University. Endometrial biopsies were collected and transported from the operating room to the laboratory on ice. The tissue was then placed in a 6 cm sterile culture dish and extensively rinsed in Ca2+/Mg2+-free phosphate-buffered saline (PBS; Gibco). The endometrium was excised from the uterine tissues, minced into small pieces, and dissociated in a tissue dissociation medium (bioGenous Biotechnologies). The tissue was incubated at 37°C with gentle agitation for 40–50 min, and the extent of digestion was monitored every 15 min. The digestion was terminated by adding 10% fetal bovine serum (Wisent), and the supernatant was passed through a 100 µm strainer and then a 40 µm strainer to obtain the mesenchymal component. The pellet was resuspended and cultured in Dulbecco’s modified eagle medium (DMEM)/F12 (Gibco) with 10% fetal bovine and 1% penicillin/streptomycin. The 40 µm strainer was turned upside down and washed with DMEM/F12 supplemented with 1% penicillin/streptomycin to harvest the endometrial component. After centrifugation, the cells were then pelleted, resuspended in organoid culture ECM (bioGenous Biotechnologies), and seeded in 24-well plates as 40 µl droplets for organoid culture in the endometrial culture medium.
Separation of eMSCs by Flow Cytometry
Endometrial stromal cell suspensions (1 × 107 cells/ml) were first blocked in 3% bovine serum albumin (BSA) for 15 min at 4°C, washed with PBS, and then stained with PE-SUSD2 antibodies for 30 min. Flow cytometry analysis was performed on a BD FACSAria Fusion (BD Biosciences), and the data were analyzed using FlowJo 7.6.3. Cells were selected for fluorescence-activated cell sorting (FACS) by electronic gating according to their forward versus side scatter profile.
Preparation of HAAM Scaffolds
Fresh human amniotic membrane was rinsed with PBS and transferred to a 50 ml sterile centrifuge tube containing an equal volume of 1% Triton X-100 solution. The mixture was then incubated at 37°C at 120 rpm for 24 h. Following incubation, the amniotic membrane was removed from the centrifuge tube, placed in a 15 cm sterile culture dish, and washed five times with PBS. Subsequently, 0.25% trypsin (Gibco) and 0.02% ethylenediaminetetracetic acid disodium (EDTA-2Na) solution (Sigma) were added to the membrane and then incubated at 37°C with 120 rpm for 4 h. After incubation, the membrane was washed with PBS, and a prepared HAAM scaffold was laid flat on a new 15 cm sterile cell culture dish and subjected to ultraviolet radiation for 2 h. To further sterilize the scaffold, it was soaked in a mixture of penicillin/streptomycin for approximately 1 h. The scaffold was then placed into a vacuum freeze dryer for 4 h until it was completely dry. Finally, the HAAM scaffold was cut into 3 × 3 cm pieces and stored at 4°C for use in subsequent experiments.
Seeding eMSCs and EEOs on the HAAM Scaffolds
Before transplantation, the freeze-dried HAAM was soaked in DMEM/F12 with 1% penicillin/streptomycin for 30 min until it became soft. The soft HAAM was then transferred to a six-well plate and 2 ml of suspension mixture containing EEOs conducted by 2 × 105 epithelial cells and 1 × 105 eMSCs was added. The MLEO-HAAM patch was incubated in a 5% CO2 incubator at 37°C for 3 days. After 3 days, the complex was transplanted to the injured endometrium and allowed to strengthen the contact between the HAAM and organoid complex.
H&E Staining
The tissue or organoids slide was rehydrated by passing it through different gradients of alcohol solutions and finally with water. Hematoxylin solution (Servicebio) was added to stain the cell nuclei, followed by decolorization with 1% hydrogen chloride. Subsequently, the slide was stained with Eosin (Servicebio), followed by decolorization in 90% ethanol. The sections were mounted using a drop of mounting medium and a coverslip. Dehydration was achieved by dipping the slide in different gradients of alcohols and then xylene.
Immunofluorescence Staining
To prepare the tissue or organoid section for immunofluorescence staining, it was rehydrated by passing it through a series of alcohol solutions of different gradients and finally with water. The slide was then soaked in 0.01 M sodium citrate buffer and transferred to a pressure cooker for 30 min. Once the sodium citrate buffer had cooled down, the section was washed with PBS twice and blocked with 3% BSA for 1 h at room temperature. Next, the section was incubated with primary antibodies at 4°C overnight. After primary antibody incubation, the section was incubated with secondary donkey anti-rabbit or anti-mouse antibody for 1 h at room temperature. Finally, the cells were counterstained with 4',6-diamidino-2-phenylindole (DAPI; Beyotime) to identify the nuclei. Images were captured with a confocal microscope (Olympus). The following primary and secondary antibodies were used: anti-Axin2 (Abcam, 1:100), anti-Sox9 (Abcam, 1:200), anti-Ki-67 (Abcam, 1:250), anti-EpCAM (Protech, 1:100), anti-Vimentin (Abcam, 1:200).
Masson’s Trichrome Staining
The deparaffinized and hydrated section was transferred to distilled water and then soaked in Weigert’s iron hematoxylin (Sigma) for 5 min. The section was briefly washed in running water and rinsed in two changes of distilled water. Next, the section was placed in 1% hydrogen ethanol for 5 s, followed by washing in running tap water for 30 s. The section was then covered with ponceau acid fuchsin (Sigma) staining solution for 5 min. After staining, the glass slide was cleaned with 0.2% glacial acetic acid aqueous solution to remove any redundant red color. The slide was then covered with a phosphomolybdic-phosphotungstic acid solution for 5 min, spun until there was no redundant water, and then covered with an aniline blue solution for 10 min. After staining, the slide was rinsed in distilled water and soaked in an acetic acid solution for 10 s. Finally, the stained slides were dehydrated by dipping them in graded alcohols and then xylene.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from cells using the RNAprep pure Micro Kit (TIANGEN), and 5× FastKing-RT SuperMix (TIANGEN) was used to reverse-transcribe RNA into cDNA. Quantitative polymerase chain reaction (qPCR) was performed using 2× SYBR Green qPCR master mix (Bimake), and the expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated using the 2−ΔΔCt method. The following primers were used for RT-PCRs: Gapdh, forward: 5’-AACGGATTTGGTCGTATTG-3’, reverse: 5’-GGAAGATGGTGATGGGATT-3’; Axin2, forward: 5’-TGTTGACAGTGGATACAGGTCC-3’, reverse: 5’-TGTTTCTTACTGCCCACACG-3’; Sox9, forward: 5’-GGCAAGCTCTGGAGACTTCTG-3’, reverse: 5’-CCCGTTCTTCACCGACTTCC-3’; integrinβ3, forward: 5’-GAGTGCTCTGAGGAGGATTACCG-3’, reverse: 5’-TGCAGTAGTAGCCAGTCCAGTCC-3’; HOXA10, forward: 5’ -CTCCTACTCCTCCAACCTGC-3’, reverse: 5’-GTTCCTGCCCACCGTGCTAT-3’; BCL6, forward: ’-TAAGACTGGACCGAGGTTGT-3’, reverse:5’-CTCGCCTTCCCAGAAATGAA-3’.
Endometrial Injury Model and MLEOs on HAAM Complex Transplantation
Female Sprague–Dawley rats (6–8 weeks old) were obtained from the experimental animal center of Fudan University and were housed under controlled conditions, with a feeding temperature of 20–26°C, the humidity of 50%–60%, and a 12 h light/dark cycle. After administration of chloral hydrate, a small incision was made in each uterine horn at the utero-tubal junction and the horn was traumatized in a standardized fashion using a 27-bent gauge needle inserted two thirds of the way through the lumen and curettage for four times. After establishing the injury, different treatments were conducted in each group (n = 10) via direct injection or transplantation into the uterine cavity through the previous incision: (1) control group: untreated, (2) model group: right side of the untreated uterus after molding, (3) MLEOs group: the uterus received an eMSCs-EEOs-loaded Matrigel solution, and (4) MLEO-HAAM group: the organoid complex seeding surface of the patch was attached to the surface of the injured endometrium, and the whole patch was expanded with two small sizes gauge needle. The adherence of the whole patch to the injured endometrium was double-checked and then fixed by the absorbable suture on four corners.
Rats’ Mating
On the 14th day of following treatment, we carefully selected three of 10 rats from each group as successfully mated rats, maintaining a balanced proportion of female and male rats in a 1:1 ratio. Upon confirmation of pregnancy, we terminate the gestation in E13, to obtain uterine tissue for subsequent analysis and investigation.
Supplemental Material
Supplemental material, sj-tif-1-cll-10.1177_09636897231218408 for Multi-Lineage Human Endometrial Organoids on Acellular Amniotic Membrane for Endometrium Regeneration by Yuhui Xu, Shuyan Cai, Qian Wang, Minzhang Cheng, Xianrui Hui, Emmanuel Enoch Dzakah, Bing Zhao and Xiaojun Chen in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897231218408 for Multi-Lineage Human Endometrial Organoids on Acellular Amniotic Membrane for Endometrium Regeneration by Yuhui Xu, Shuyan Cai, Qian Wang, Minzhang Cheng, Xianrui Hui, Emmanuel Enoch Dzakah, Bing Zhao and Xiaojun Chen in Cell Transplantation
Footnotes
Author Contributions: BZ and XC designed and supervised the study; YX, SC, QW, MC, and XH performed the experiments and analyzed the data; SC, EED, BZ, and XC wrote the manuscript.
Availability of Data and Materials: The data sets used and/or analyzed during the current study are available from the corresponding author on request.
Ethics Approval: All animal breeding and experimental procedures were performed following the relevant guidelines and regulations of the Institutional Animal Care and Use Committee of the Obstetrics and Gynecology Hospital of Fudan University (No.2021-182).
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (31970761 and 31970761, B.Z), the Faculty Resources Project of the College of Life Sciences (Inner Mongolia University 2022-103, B.Z), Health Commission Emerging Interdisciplinary Research Project of Shanghai, China (2022JC007, X.C.), and Shenkang Clinical Science and Technology Innovation Project of China (SHDC22021219 2021, X.C.).
ORCID iD: Bing Zhao  https://orcid.org/0000-0001-9891-3569
https://orcid.org/0000-0001-9891-3569
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ludwig H, Spornitz UM. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann N Y Acad Sci. 1991;622:28–46. [DOI] [PubMed] [Google Scholar]
- 2. Ferenczy A. Studies on the cytodynamics of human endometrial regeneration. II. Transmission electron microscopy and histochemistry. Am J Obstet Gynecol. 1976;124(6):582–95. [DOI] [PubMed] [Google Scholar]
- 3. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, Yang X, Zhang P, Sheng W, Xu J, Zhou S. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. 2014;125(2):121–24. [DOI] [PubMed] [Google Scholar]
- 6. Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development (Cambridge, England). 2017;144(10):1775–86. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Xu D, Li Y, Lan J, Zhu Y, Cao J, Hu M, Yuan J, Jin H, Li G, Liu D. Organoid transplantation can improve reproductive prognosis by promoting endometrial repair in mice. Int J Biol Sci. 2022;18(6):2627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SR, Kim SR, Im JB, Park CH, Lee HY, Hong IS. 3D stem cell-laden artificial endometrium: successful endometrial regeneration and pregnancy. Biofabrication. 2021;13(4):045012. [DOI] [PubMed] [Google Scholar]
- 9. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 10. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–86. [DOI] [PubMed] [Google Scholar]
- 11. Fan D, Wu S, Ye S, Wang W, Guo X, Liu Z. Umbilical cord mesenchyme stem cell local intramuscular injection for treatment of uterine niche: protocol for a prospective, randomized, double-blinded, placebo-controlled clinical trial. Medicine (Baltimore). 2017;96(44):e8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu X, Yu F, Yan G, Hu Y, Sun H, Ding L. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation. Hum Reprod (Oxford, England). 2021;36(1):145–59. [DOI] [PubMed] [Google Scholar]
- 14. Wilshaw SP, Kearney J, Fisher J, Ingham E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A. 2008;14(4):463–72. [DOI] [PubMed] [Google Scholar]
- 15. Jahanafrooz Z, Bakhshandeh B, Behnam Abdollahi S, Seyedjafari E. Human amniotic membrane as a multifunctional biomaterial: recent advances and applications. J Biomater Appl. 2023;37(8):1341–54. [DOI] [PubMed] [Google Scholar]
- 16. Almquist LD, Likes CE, Stone B, Brown KR, Savaris R, Forstein DA, Miller PB, Lessey BA. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: a cohort study. Fertil Steril. 2017;108(6):1063–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godbole GB, Modi DN, Puri CP. Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction. 2007;134(3):513–23. [DOI] [PubMed] [Google Scholar]
- 18. Cousins FL, Filby CE, Gargett CE. Endometrial stem/progenitor cells-their role in endometrial repair and regeneration. Front Reprod Health. 2021;3:811537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–51. [DOI] [PubMed] [Google Scholar]
- 20. Liang J, Li X, Dong Y, Zhao B. Modeling human organ development and diseases with fetal tissue-derived organoids. Cell Transplant. 2022;31:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science (New York, NY). 2000;287(5457):1427–30. [DOI] [PubMed] [Google Scholar]
- 22. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lou L, Kong S, Sun Y, Zhang Z, Wang H. Human endometrial organoids: recent research progress and potential applications. Front Cell Dev Biol. 2022;10:844623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143(6):1518–29.e7. [DOI] [PubMed] [Google Scholar]
- 25. de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13(3):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan L, Cao J, Hu M, Xu D, Li Y, Zhao S, Yuan J, Zhang H, Huang Y, Jin H, Chen M, et al. Bone marrow mesenchymal stem cells combined with estrogen synergistically promote endometrial regeneration and reverse EMT via Wnt/β-catenin signaling pathway. Reprod Biol Endocrinol. 2022;20(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei X, Liu F, Zhang S, Xu X, Li J, Wang Q, Cai J, Wang S. Human umbilical cord mesenchymal stem cell-derived conditioned medium promotes human endometrial cell proliferation through Wnt/β-catenin signaling. Biomed Res Int. 2022;2022:8796093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gholipourmalekabadi M, Mozafari M, Salehi M, Seifalian A, Bandehpour M, Ghanbarian H, Urbanska AM, Sameni M, Samadikuchaksaraei A, Seifalian AM. Development of a cost-effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv Healthc Mater. 2015;4(6):918–26. [DOI] [PubMed] [Google Scholar]
- 29. Xue SL, Liu K, Parolini O, Wang Y, Deng L, Huang YC. Human acellular amniotic membrane implantation for lower third nasal reconstruction: a promising therapy to promote wound healing. Burns Trauma. 2018;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farzamfar S, Salehi M, Ehterami A, Naseri-Nosar M, Vaez A, Zarnani AH, Sahrapeyma H, Shokri MR, Aleahmad M. Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed Eng Lett. 2018;8(4):393–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-cll-10.1177_09636897231218408 for Multi-Lineage Human Endometrial Organoids on Acellular Amniotic Membrane for Endometrium Regeneration by Yuhui Xu, Shuyan Cai, Qian Wang, Minzhang Cheng, Xianrui Hui, Emmanuel Enoch Dzakah, Bing Zhao and Xiaojun Chen in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897231218408 for Multi-Lineage Human Endometrial Organoids on Acellular Amniotic Membrane for Endometrium Regeneration by Yuhui Xu, Shuyan Cai, Qian Wang, Minzhang Cheng, Xianrui Hui, Emmanuel Enoch Dzakah, Bing Zhao and Xiaojun Chen in Cell Transplantation