Abstract
Extracellular vesicles (EVs) from antler reserve mesenchymal (RM) cells play an important role in the paracrine regulation during rapid growth of antler without forming a tumor; therefore, RM-EVs become novel materials for anti-tumor studies, such as osteosarcoma treatment. However, the problem of low production of RM-EVs in traditional 2D culture limits its mechanism research and application. In this study, we established an optimal 3D culture system for antler RM cells to produce EVs (3D-RM-EVs). Morphology and property of harvested 3D-RM-EVs were normal compared with EVs from conventional 2D culture, and the miRNA profile in them was basically the same through transcriptome sequencing analysis. Based on the same number of RM cells, the volume of the culture medium collected by 3D cultural system concentrated nearly 30 times, making it more convenient for subsequent purification. In addition, EVs were harvested 30 times in 3D cultural system, greatly increasing the total amount of EVs (harvested a total of 2–3 times in 2D culture). Although 3D-RM-EVs had a limited inhibitory effect on the proliferation of K7M2 cells, the inhibition effect of 3D-RM-EVs loaded drugs (Ifosfamide + Etoposide) were more significant than that of positive drug group alone (P < 0.05). Furthermore, in vivo studies showed that 3D-RM-EVs loaded drugs (Ifosfamide + Etoposide) had the most significant tumor inhibition effect, with decreased tumor size, and could slow down body weight loss compared with Ifosfamide + Etoposide (IFO + ET) group. These results demonstrated that 3D-RM-EVs were efficiently prepared from antler RM cells and were effective as drug vehicles for the treatment of osteosarcoma.
Keywords: 3D cell culture, hollow fiber cell culture, extracellular vesicles, antler reserve mesenchymal cell, osteosarcoma
Introduction
Extracellular vesicles (EVs) are vesicular bodies secreted by cells and enveloped with a bilayer phospholipid membrane structure. EVs mediate information exchange between cells and participate in physiological and pathological processes of cells and tissues 1 . At present, EVs as a new tool for clinical diagnosis and treatment are becoming widely used2,3. However, the effects of EVs from different tissues and organ vary significantly. It was reported that EVs secreted from adipose-derived stem cells attenuates diabetic nephropathy 4 . EVs secreted from bone marrow mesenchymal stem cell played an important role in regulating osteoblast differentiation and osteogenic gene expression 5 . EVs secreted by human umbilical vein endothelial cells attenuated hypoxia/reoxygenation-induced apoptosis in neural cells 6 . Therefore, how to effectively use EVs from different issues and cell sources still requires extensive experimental verification and comprehensive consideration based on biological background.
Antler reserve mesenchymal (RM) cells locate in the antler growth center of deer and are responsible for phenomenal antler elongation rate 7 (up to 2 cm/day, a rate much higher than cancer8,9). Interestingly, it has been suggested that developing antlers are particularly resistant to tumor formation10,11, although gene expression profiles of antlers had a higher correlation with osteosarcoma 12 , we found many highly expressed anti-tumor miRNAs, such as miR-148a, miR-143, and miR-140, in the RM-EVs (data unpublished), which ensured that RM cells do not become cancerous themselves. However, there are also miRNAs in RM-EVs that promote cell proliferation, so how to use RM-EVs as anti-tumor drugs or drug vehicle still requires extensive experimental verification. On the contrary, human mesenchymal stem cell (MSC) or EVs administration contributed to tumor development in animal models by promoting angiogenesis, creating a niche to support cancer stem cell survival, or having immunosuppressive ability13,14. Thus, we envisage that RM-EVs, as a special tissue-derived EVs, would have future medical applications, such as anti-tumor drugs or drug vehicle in osteosarcoma treatment. However, RM cells, like the most other mesenchymal stem cells, have a low production of EVs secreted in 2D culture in vitro 15 , which thus would limit their future application 16 .
The preclinical and clinical development of EVs technology as a delivery platform requires large quantities of EVs. The proper production method for EVs must be established to support large-scale manufacturing3,16–18. Recent studies showed that 3D culture strategy was efficient to enhance the production of MSC-derived exosomes19–23. The hollow fiber cell culture system is one kind of 3D cultural system that simulates the physiological circulatory system in the body, providing cells with a more in vivo like growth mode, and can be used to concentrate cell secretion products 10 to 100 times than the 2D cultures, thus it is a better choice for EVs preparation24–27. However, the growth characteristics of different types of cells in this system and the optimal conditions for EVs production using this system still need to be continuously optimized.
To efficiently produce antler RM EVs and explore their application in osteosarcoma treatment, we used 3D cultural system (hollow fiber cell culture system) to produce 3D-RM-EVs, optimized the operating conditions, and established an efficient 3D culture production system for 3D-RM-EVs production. Subsequently, we conducted anti-tumor experiments using the produced 3D-RM-EVs. We demonstrated that 3D-RM-EVs were efficiently prepared from antler RM cells and were effective as drug vehicles for the treatment of osteosarcoma.
Materials and Methods
Isolation, Cultivation, and Characterization of RM Cells
The RM tissue of growing antlers was collected following the method reported preciously by us 7 , cut into small pieces, digested in collagenase, and then used for primary cell culture. RM cells with typical shape of mesenchymal stem cell were obtained after subculture. To characterize isolated RM cells, immunofluorescence staining of mesenchymal stem cell markers (CD73, CD90, and NESTIN) and embryonic stem cells marker (SOX2) were carried out, osteogenic/chondrogenic/adipogenic differentiation were induced for multipotency testing.
Cultivation of RM Cells in the 3D Cultural System
RM cells at logarithmic phase in the culture dishes were transferred into the hollow fiber cell culture system C2011, and cell adhesion and glucose consumption (cell state monitoring indicator) were compared between different seeding number of cells (107, 5 × 107, 108, 5 × 108, 109), between seeding time (once or twice), between different collection time (every 2, 3, or 4 days), and between different types of culture medium used in extracellular space of the system. Bicinchoninic Acid Assay (BCA) was used to quantify the total protein content of the collections, which indirectly reflects the content of EVs in the collections. The optimal cultivation conditions were determined through these multiple comparisons.
The cell aggregates attached to the hollow fiber were observed and detected histologically through frozen section and Alcian Blue staining.
Isolation and Characterization of 3D-RM-EVs
3D-RM-EVs were isolated through ultra-high-speed centrifugation (120,000 × g, 4°C for 70 min), the morphology of 3D-RM-EVs was observed by transmission electron microscopy. The vesicle size range and concentration of 3D-RM-EVs were measured using nanoflow cytometry. The yield of 3D-RM-EVs was compared with the production efficiency of RM-EVs obtained from the conventional 2D culture. The exosomal marker proteins of 3D-RM-EVs were detected through Western blot analysis.
BCA and Western Blot Analysis
Proteins of the EVs were extracted using Radio-immunoprecipitation assay buffer (RIPA) (Sigma, USA) containing protease inhibitors and phosphatase inhibitors, and protein concentrations were quantified using Omni-Easy Instant BCA Protein Assay Kit (ZJ102L, EpiZyme) according to the user manual. Quantified proteins were then analyzed recommended by the International Society for Extracellular Vesicles (ISEV) using Western blot with specific primary antibodies: Rabbit Anti-CD63 (ab134045, Abcam, US; 1:2,000); Rabbit Anti-CD81 (ab219209, Abcam, US; 1:2,000); Rabbit Anti-TSG101 (ab125011, Abcam, US; 1:2,000); and Rabbit Anti-CANX (ab133615, Abcam, US; 1:2,000).
Evaluation of Effects of 3D-RM-EVs as Drug Vehicle on Inhibiting Osteosarcoma Cells Proliferation In Vitro
Two concentrations (10 and 50 μg/ml) of 3D-RM-EVs were used to treat mouse osteosarcoma cells (K7M2 cells) in vitro. CCK8 was used to evaluate the effects on K7M2 cell proliferation, and EVs derived from human umbilical cord mesenchymal stem cells (U-MSCs) were taken as the control. Apoptotic rate of K7M2 cells treated with 3D-RM-EVs was detected through flow cytometry.
Drugs were loaded into 3D-RM-EVs through passive loading method 28 , 3D-RM-EVs were co-incubated with different concentrations of Ifosfamide + Etoposide (IFO + ET) for 18 h at room temperature, and then added to K7M2 cells (0–15 μg/ml). The positive drug combination IFO + ET in this study is a combination of positive drugs that have been identified by other studies, which have better therapeutic effects on osteosarcoma29,30. Proliferation rate of K7M2 cells was measured using CCK8 method. Ifosfamide + Etoposide (0–15 μg/ml) were added separately as the controls.
Evaluation of Effects of 3D-RM-EVs as Drug Vehicle on Inhibiting Osteosarcoma Progression In Vivo
Osteosarcoma orthotopic model was established using 4-week-old female Balb/c mice. In total, 28 mice were randomly divided into the following groups (7/group): Control, 3D-RM-EVs, IFO + ET, and 3D-RM-EVs + IFO + ET. RM-EVs/drugs were injected through tail vein every 2 days for 6 times: Control group, 200 μl saline; 3D-RM-EVs group, 106 vesicles in 200 μl saline; IFO + ET group, IFO 50 mg/kg, ET 10 mg/kg, dissolved in 200 μl saline; 3D-RM-EVs + IFO + ET group, 106 vesicles in 100 μl saline with 50 mg/kg IFO, and 10 mg/kg ET in 100 μl saline.
The treatment duration was 14 days in total. The volume of the tumor was measured every other day during treatment and calculated using the formula: V = 4 π/3 a × b2, where a and b (mm) represent the length of the tumor along the long axis of the tibia and half of the maximum length of the tumor perpendicular to the tibia, respectively. At the end of experiment, mice were anesthetized using Pentobarbital, tumor was cut off, photographed, weighed, and fixed in 4% paraformaldehyde for histological assessment.
Statistical Analysis
The data in this study are expressed as the mean ± standard deviation. Two-tailed Student’s t-text was used to compare the differences between two groups. Significant differences were considered when P < 0.05, extremely significant differences were considered when P < 0.01. Each experiment was repeated 3 times or more independently with similar results. The experimental data were drawn using Prism Graphpad software.
Results
Optimum Condition for Production of EVs by RM Cells Using 3D Cultural System
The RM cells were obtained from antler growth center (Fig. 1A); these cells had the characteristics of mesenchymal stem cells (CD73, CD90, and NESTIN positive), partial characteristics of embryonic stem cells (SOX2 positive) (Fig. 1B), and had the ability of adipogenesis, osteogenesis, and chondrogenesis (Fig. 1C). RM cells at logarithmic phase were detached from the culture dish and transferred into the 3D cultural system (Fig. 1D). The optimal number of cells for the first time transferring to the system was determined at 5 × 107, when less than 107, the glucose consumption of RM cells was too low, indicating poor cell status; however, when cell number was more than 108, a large number of cells cannot adhere to hollow fibers, resulting in waste. The time and method for changing the culture medium were determined as follows: circulating culture medium was changed every 2 days, and extracellular space medium was replaced with exosome-free medium after the third day of cell seeding. When RM cell aggregates in different sizes were observed on the hollow fiber (Fig. 1D), suggesting that there was still a lot of space on the fiber. During this period, the sugar consumption is unstable and then another batch of RM cells (5 × 107) were supplemented into the system. When glucose consumption was stable at about 70 mg/d, extracellular space medium containing EVs from RM cells were collected.
Figure 1.
Characteristics of RM cells and growth morphology in 3D cultural system. (A) Isolation of RM cells from antler growth center. (B) Immunofluorescence staining using CD73, CD90, NESTIN, and SOX2. (C) Chondrogenic, osteogenic, and adipogenic induction using OriCell Mesenchymal Stem Cells Differentiation Kit (GUXMX-90041, GUXMX-90021, and GUXMX-90031, respectively). (D) Transferring RM cells into 3D cultural system (hollow fiber cell culture system), cell mass with different sizes were observed on the hollow fiber, histological sections, and Alcian Blue staining indicated that the cell mass were aggregated RM cells. RM: reserve mesenchymal; DAPI: 4′,6-diamidino-2-phenylindole.
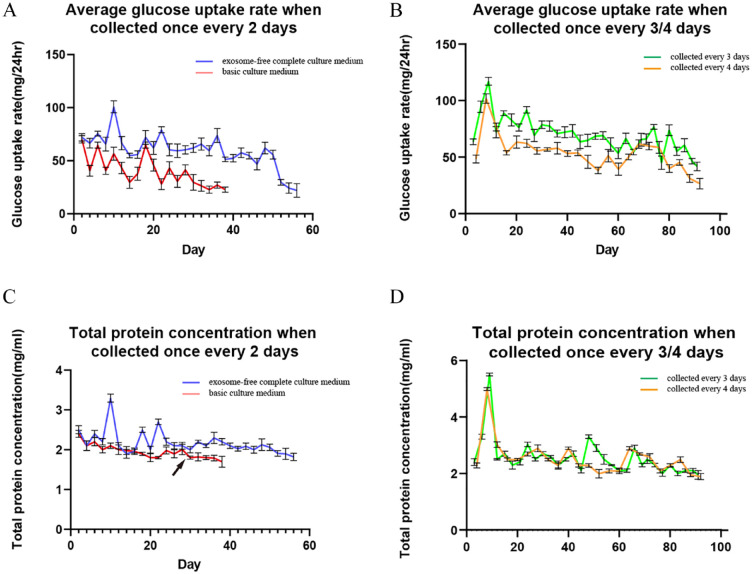
Glucose consumption was compared when basic culture medium or exosome-free complete culture medium was added to extracellular space of the 3D cultural system; the results showed that when using basic culture medium, RM cells did not adhere well to hollow fibers, resulting in a significant decrease in glucose consumption after 20 days of culture system operation (Fig. 2A). Therefore, providing an exosome-free complete culture medium was necessary to ensure normal cell adhesion to hollow fibers. Then, the effects of different collection intervals on glucose consumption were compared; it was found that collecting once every 3 days had the best effect (Fig. 2B). The collection interval of 2 days was less than 3 days in terms of duration of collection, and when collection interval was 4 days or more, RM cells no longer showed significant glucose consumption. The total protein contents of the products collected every 2, 3, and 4 days were analyzed, and the results showed that the trend of total protein change was basically consistent with the change in glucose consumption (Fig. 2C, D), which indirectly reflect the changes in the total content of EVs in the collection medium.
Figure 2.
Optimum condition for collecting EVs from RM cells using 3D cultural system. (A) Comparison of the glucose consumption between basic culture medium and exosome-free complete culture medium used in extracellular space, blue line represented exosome-free complete culture medium, and red line represented basic culture medium. (B) Effects of different collection intervals (every 3 and 4 days, respectively) on average glucose consumption, green line represented products were collected every 3 days, and yellow line represented products were collected every 4 days. (C) Comparison of the total protein concentration of the products between basic culture medium and exosome-free complete culture medium used in extracellular space, in the final stage of collecting using basic culture medium, total protein concentration decreased significantly (from Day 30 to 38), black arrows indicating this turning point. Blue line represented exosome-free complete culture medium, and red line represented basic culture medium. (D) Effects of different collection intervals (every 3 and 4 days, respectively) on total protein concentration of the products, green line represented products were collected every 3 days, and red line represented products were collected every 4 days. EV: extracellular vesicle; RM: reserve mesenchymal.
Biological Characteristics of 3D-RM-EVs
Extracellular space medium collected from 3D cultural system was centrifuged at 120,000 × g, 4°C for 70 min, and the precipitate was resuspended with phosphate-buffered saline (PBS), namely 3D-RM-EVs. Transmission electron microscopy examination showed that the morphology of the 3D-RM-EVs was uniform, presenting a typical saucer-shaped structure (Fig. 3A). Extracellular biomarker detection by Western blot showed that 3D-RM-EVs was CD63, CD81, and TSG101 positive, but did not express cellular protein Calnexin (Fig. 3B). Particle size analysis showed that the average diameter of 3D-RM-EVs was 66.37 nm, with a size distribution between 50 and 70 nm (Fig. 3C). These results indicated that morphology and property of 3D-RM-EVs were normal compared with EVs from conventional 2D culture.
Figure 3.
Biological characteristics of EVs from 3D cultured RM cells (3D-RM-EVs). (A) Transmission electron microscopy examination of 3D-RM-EVs and 2D-RM-EVs, bar 500 nm. (B) Western blot analysis of extracellular (CD63, CD81, and TSG101) and cellular (Calnexin) biomarkers of 3D-RM-EVs and 2D-RM-EVs. (C) Particle size analysis of 3D-RM-EVs and 2D-RM-EVs using nanoflow cytometry. (D) Comparison of the number of extracellular vesicles harvested from 3D cultural system with the amount harvested from conventional 2D culture. The error bars: standard errors of the mean from three independent experiments. (E) Comparison of the miRNA profiles of extracellular vesicles harvested from 3D cultural system with that from conventional 2D culture. (F) Correlation analysis of miRNA expression levels between 3D-RM-EVs and 2D-RM-EVs. (G) Shared miRNAs of 3D-RM-EVs and 2D-RM-EVs. EV: extracellular vesicle; RM: reserve mesenchymal. *P < 0.05; **P < 0.01.
We compared the amount of EVs harvested from 3D cultural system with the amount harvested from conventional 2D culture. The results showed that based on the same number of RM cells, when collecting one time, the total amount of EVs obtained was basically the same between 2D and 3D culture, but the volume of the culture medium collected by 3D cultural system was less, the medium was concentrated nearly 30 times (Fig. 3D), making it more convenient for subsequent purification. In addition, EVs were maximally harvested a total of 2 to 3 times in 2D culture, while in our 3D cultural system, EVs were harvested 30 times, greatly increasing the total amount of EVs (Fig. 3D).
Furthermore, miRNA profiles were compared between 3D-RM-EVs and 2D-RM-EVs, the results showed that compared with 2D-RM-EVs, 3D-RM-EVs has little difference in miRNAs expression. Three miRNAs, namely miR-148a, miR-21, and let-7i, accounted for more than 50% of the total miRNA expression of both 3D-RM-EVs and 2D-RM-EVs (Fig. 3E), and all the miRNA expression were closely correlated (Fig. 3F), 171 miRNAs were shared between 3D-RM-EVs and 2D-RM-EVs (Fig. 3G), indicating their functions were mainly the same. Therefore, 3D-RM-EVs was adopted in subsequent experiments.
3D-RM-EVs Equipped With Drugs Inhibited Mouse Osteosarcoma Cells Proliferation In Vitro
In order to evaluate the activity of the obtained 3D-RM-EVs in vitro, the 3D-RM-EVs were used to treat mouse osteosarcoma cells (K7M2 cells, Fig. 4A). The results showed that 3D-RM-EVs inhibited the proliferation of K7M2 cells, but had a limited inhibitory effect, with only 75% of normal cell viability level (Fig. 4B). In contrast, EVs derived from human umbilical cord mesenchymal stem cells promoted the proliferation of K7M2 cells (Fig. 4C). The results suggested that 3D-RM-EVs was a good drug vehicle for target treatment of osteosarcoma.
Figure 4.
3D-RM-EVs equipped with drugs inhibit mouse osteosarcoma cells proliferation in vitro. (A) Mouse osteosarcoma cells (K7M2 cells). (B) 3D-RM-EVs inhibited the proliferation of K7M2 cells, with cell viability decreased to 75% of normal level at 50 μg/ml of 3D-RM-EVs. The error bars: standard errors of the mean from three independent experiments. *P < 0.05; **P < 0.01. (C) Extracellular vesicles derived from human umbilical cord mesenchymal stem cells promote the proliferation of K7M2 cells. The error bars: standard errors of the mean from three independent experiments. *P < 0.05; **P < 0.01. (D) 3D-RM-EVs promoted the apoptosis of K7M2 cells, but the effect was not significant (P > 0.05). (E) Co-incubation mixture of 3D-RM-EVs and osteosarcoma therapeutic drugs (Ifosfamide + Etoposide) significantly inhibited K7M2 cells compared with positive drug group, *P < 0.05 at 1 μg/ml and/or more than 1 μg/ml. RM: reserve mesenchymal; EV: extracellular vesicle; UC-MSC: umbilical cord mesenchymal stem cell; IFO-ET: Ifosfamide + Etoposide; PE-A: Phycoerythrin A.
We further detected status of the K7M2 cell apoptosis after 3D-RM-EVs treatment; the results showed that 3D-RM-EVs promoted the apoptosis of K7M2 cells (Fig. 4D), but the effect was not significant (P > 0.05). Therefore, 3D-RM-EVs alone was not effective in the treatment of osteosarcoma. Subsequently, we co-incubated 3D-RM-EVs with osteosarcoma therapeutic drugs (Ifosfamide + Etoposide) and then the complex was used to treat K7M2 cells. The results showed that the inhibition effect of co-incubation group was more significant than that of positive drug group alone (P < 0.05) (Fig. 4E). The results further confirmed that 3D-RM-EVs had biological activity in vitro and served as a new drug vehicle in osteosarcoma treatment.
3D-RM-EVs Equipped With Drugs Inhibited Mouse Osteosarcoma Progression In Vivo
In order to evaluate the activity of the 3D-RM-EVs in vivo, the 3D-RM-EVs were used to treat osteosarcoma mice (K7M2 bearing mice, Fig. 5A). Histological studies showed that the K7M2 osteosarcoma was a cancerous tissue attached to the periosteum of the bone (Fig. 5A). We used 3D-RM-EVs alone or combined Ifosfamide + Etoposide (IFO + ET) to treat K7M2 bearing mice for 14 days (Fig. 5A), respectively. After treatment, the tumor morphology showed that the 3D-RM-EVs + IFO + ET group had the most significant tumor inhibition effect, while the control group had the largest tumor (Fig. 5B).
Figure 5.
3D-RM-EVs equipped with drugs inhibit mouse osteosarcoma progression in vivo. (A) osteosarcoma mice (K7M2 bearing mice), histological analysis showed that the K7M2 osteosarcoma was a cancerous tissue attached to the periosteum of the bone, control: means normal tissue. (B) Tumor morphology observation showed that the 3D-RM-EVs + IFO + ET group had the most significant tumor inhibition effect, while the control group had the largest tumor. (C) Tumor weight analysis indicated that the tumor weight in the 3D-RM-EVs + IFO + ET group (2.12 ± 0.265 g) was significantly lighter than that in the control group (3.86 ± 0.232 g), 3D-RM-EVs group (3.70 ± 0.286 g), and IFO + ET group (2.92 ± 0.317 g). (D) Tumor growth curve analysis indicated that co-incubation of 3D-RM-EVs with osteosarcoma therapeutic drugs (Ifosfamide + Etoposide) had the best effect. (E) Weight changes of mice during treatment, there was no difference in weight between the 3D-RM-EVs group and the control group. The IFO + ET group had the most significant weight loss, while the 3D-RM-EVs + IFO + ET group could slow down weight loss compared with IFO + ET group. The error bars: standard errors of the mean from three independent experiments. RM: reserve mesenchymal; EV: extracellular vesicle; IFO-ET: Ifosfamide + Etoposide. *P < 0.05; **P < 0.01.
Tumor weight analysis indicated that the tumor weight in the 3D-RM-EVs + IFO + ET group (2.12 ± 0.265 g) was significantly smaller than that in the control group (3.86 ± 0.232 g), 3D-RM-EVs group (3.70 ± 0.286 g), and IFO + ET group (2.92 ± 0.317 g) (Fig. 5C). The analysis of the tumor growth curve also indicated that co-incubation of 3D-RM-EVs with osteosarcoma therapeutic drugs (Ifosfamide + Etoposide) had the best effect (Fig. 5D).
In addition, we analyzed the body weight changes of mice during treatment (Fig. 5E), and the results showed that there was no difference in weight between the 3D-RM-EVs group and the control group. The IFO + ET group had the most significant weight loss, while the 3D-RM-EVs + IFO + ET group could slow down weight loss compared with IFO + ET group. These results demonstrated that 3D-RM-EVs had biological activity in vivo, in terms of loading clinical chemotherapy drugs, targeting osteosarcoma in vivo, and thus enhancing the effect of drugs.
Discussion
Since 2016, a commercial hollow fiber bioreactor system had been utilized effectively for large-scale EVs production 24 . In this bioreactor, cells were seeded into cylindrical fibers to simulate 3D culture24,25. It was reported that hollow fiber bioreactor system could obtain 5-fold more EVs compared with those obtained from the conventional 2D culture 24 . These effects benefited from the unique advantages of 3D culture. Our research also confirmed that by optimizing the 3D culture system reasonably and effectively, the production of EVs of RM cells was greatly improved, and the biological characteristics were comparable with 2D cultured EVs. Besides, the EVs prepared by 3D cultured cells not only had advantages in yield but also had improved functionality. It has been demonstrated that EVs derived from cells grown in a 3D environment showed better biological functions than those derived from cells in 2D culture 31 ; research also showed that cells in 3D culture secrete more interleukin-11 and proangiogenic cytokines than cells in 2D culture 32 . Therefore, using the 3D culture system to the production of EVs of RM cells and establishing the optimal culture conditions would be an excellent way to solve the problems of low EVs yield from RM cells. Although miRNA component of 3D-RM-EVs was mainly the same with 2D-RM-EVs, we believe that the RM cells were stable in vitro culture; however, small differences still need further studies.
The EVs of RM cells obtained from the 3D culture system in this study were not effective in inhibiting osteosarcoma when used alone, but the EVs of RM cells co-incubated with positive drugs showed a good inhibitory effect, which might also be due to the fact that the 3D-RM-EVs loaded certain drugs to target the tumor site more effectively. EVs have excellent drug carrier potential and can effectively transport small molecule substances and various drugs 33 . They provide the safe transfer of drugs during diffusion without deactivation or degradation 34 . At the same time, the targeting effect of EVs ensures that the drugs transported can reach the target site and have more efficient effects. In recent years, mesenchymal stem cells (MSCs)-derived EVs have been widely used as transport carriers for drugs or bioactive molecules in cell-free therapy for various diseases35–37. In addition, it was reported that siRNA could be loaded into EVs through electroporation for the treatment of Alzheimer’s disease, with a target protein knockout efficiency of 62% 38 . These reports demonstrated that EVs carried various small molecules and exerted their effects, which was better than using EVs or small molecules alone.
Among many diseases, tumor is still the biggest problem we need to overcome, especially for tumors with high malignancy, such as osteosarcoma39–41. Traditional chemotherapy drugs have shortcomings such as low targeting, drug resistance, and toxic and side effects with the increase in dose42,43. The application of nanomedicine carrier systems in cancer treatment has received attention from scholars. Nanomedicine carrier systems have many advantages such as structural stability, drug release, high delivery efficiency, biocompatibility, and targeting. They can not only improve drug treatment efficacy but also reduce the systemic toxicity of chemotherapy drugs44,45. However, the targeting and safety of nanoparticle drug delivery systems for tumors still need to be further improved 46 . EVs, especially EVs from mesenchymal stem cells 47 , as natural drug carriers, can be loaded with different drugs through different drug loading methods. They can transport the loaded drugs to the target tissue through natural tendency effects such as special affinity and homing effects with the source cells 48 . More and more studies are using EVs as natural sources of nanomaterials for drug delivery and cancer treatment49–53. Antler is a special organ with a very similar gene expression pattern to osteosarcoma but never cancerous; antler RM cells, as a kind of mesenchymal stem cells, have excellent characteristics as drug vehicle for osteosarcoma, and this inference had been confirmed in this study, and tumor size and weight were significantly decreased compared with positive drug group. Besides, the body weight is significantly higher than that of the positive drug group, indicating that 3D-RM-EVs, in addition to serving as a drug vehicle, can also alleviate the toxic effects of positive drugs on the body, which is benefit to clinical conditions.
In brief, we established the optimal 3D culture system of RM cells for 3D-RM-EVs production, the volume of the culture medium collected by 3D culture system concentrated nearly 30 times, making it more convenient for subsequent purification. In addition, EVs were harvested 30 times in 3D cultural system, greatly increasing the total amount of EVs. The harvested 3D-RM-EVs meet the criteria of standard EVs, and morphology and miRNA profile analysis showed that 3D-RM-EVs were comparable with EVs from conventional 2D culture, and the results of in vitro and in vivo evaluation demonstrated that 3D-RM-EVs were effective as drug vehicles for the treatment of osteosarcoma, with decreased tumor size and body weight loss compared with IFO + ET group.
Acknowledgments
The authors thank Eric Lord for English language editing.
Footnotes
Author Contributions: Conceptualization, P.H. and C.L.; methodology, P.H., J.Y. and Y.W.; validation, T.J. and Z.P.; formal analysis, P.H. and J.Y.; investigation, C.Z. and T.J.; resources, J.L.; data curation, P.H.; writing-original draft preparation, P.H.; writing-review and editing, C.L.; visualization, P.H. and J.Y.; supervision, C.L.; project administration, P.H.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Ethical Approval: The study was approved by the Institutional Animal Care and Use Committee of Institute of Antler Science and Product Technology, Changchun Sci-Tech University (CKARI202304, April 20, 2023).
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Not applicable.
Data Availability Statement: The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA020786) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Natural Science Foundation of Jilin Province (No. YDZJ202101ZYTS102), the National Natural Science Foundation of China (No. U20A20403), and the Scientific Research Starting Foundation of Changchun Sci-Tech University (202306).
ORCID iDs: Pengfei Hu  https://orcid.org/0000-0003-3816-1849
https://orcid.org/0000-0003-3816-1849
Chunyi Li  https://orcid.org/0000-0001-7275-4440
https://orcid.org/0000-0001-7275-4440
References
- 1. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. [DOI] [PubMed] [Google Scholar]
- 2. Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, Beheshtkhoo N, Kouhbanani M, Marofi F, Nikoo M, Jarahian M. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, Hsu MY. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–19. [DOI] [PubMed] [Google Scholar]
- 4. Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang Y, Xie H, Tu W, Fang H, Ji C, Yan T, Huang H, Yu C, Hu Q, Gao Z, Lv S. Exosomes secreted by HUVECs attenuate hypoxia/reoxygenation-induced apoptosis in neural cells by suppressing miR-21-3p. Am J Transl Res. 2018;10(11):3529–41. [PMC free article] [PubMed] [Google Scholar]
- 7. Li C, Clark DE, Lord EA, Stanton JA, Suttie JM. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat Rec. 2002;268(2):125–30. [DOI] [PubMed] [Google Scholar]
- 8. Landete-Castillejos T, Kierdorf H, Gomez S, Luna S, García AJ, Cappelli J, Pérez-Serrano M, Pérez-Barbería J, Gallego L, Kierdorf U. Antlers—evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone. 2019;128:115046. [DOI] [PubMed] [Google Scholar]
- 9. Gomez S, Garcia AJ, Luna S, Kierdorf U, Kierdorf H, Gallego L, Landete-Castillejos T. Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone. 2013;52(1):506–15. [DOI] [PubMed] [Google Scholar]
- 10. Goss RJ. Future directions in antler research. Anat Rec. 1995;241(3):291–302. [DOI] [PubMed] [Google Scholar]
- 11. Kierdorf U, Kierdorf H. Deer antlers—a model of mammalian appendage regeneration: an extensive review. Gerontology. 2011;57(1):53–65. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Zhang C, Wang N, Li Z, Heller R, Liu R, Zhao Y, Han J, Pan X, Zheng Z, Dai X, et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science. 2019;364(6446):eaav6335. [DOI] [PubMed] [Google Scholar]
- 13. Momin EN, Vela G, Zaidi HA, Quiñones-Hinojosa A. The oncogenic potential of mesenchymal stem cells in the treatment of cancer: directions for future research. Curr Immunol Rev. 2010;6(2):137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma AK, Fuller NJ, Sullivan RR, Fulton N, Hota PV, Harrington DA, Villano J, Hagerty JA, Cheng EY. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol. 2009;182(Suppl 4):1898–905. [DOI] [PubMed] [Google Scholar]
- 15. Feng ZY, Zhang QY, Tan J, Xie HQ. Techniques for increasing the yield of stem cell-derived exosomes: what factors may be involved. Sci China Life Sci. 2022;65(7):1325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martínez-Santillán A, González-Valdez J. Novel technologies for exosome and exosome-like nanovesicle procurement and enhancement. Biomedicines. 2023;11(5):1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiner AT, Witwer KW, van Balkom BWM, de Beer J, Brodie C, Corteling RL, Gabrielsson S, Gimona M, Ibrahim AG, de Kleijn D, Lai CP, et al. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med. 2017;6(8):1730–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–56. [DOI] [PubMed] [Google Scholar]
- 19. Yu W, Li S, Guan X, Zhang N, Xie X, Zhang K, Bai Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater Adv. 2022;133:112646. [DOI] [PubMed] [Google Scholar]
- 20. Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer’s disease therapy. Small. 2020;16(3):e1906273. [DOI] [PubMed] [Google Scholar]
- 21. Abdollahi S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol Bioeng. 2021;118(3):1029–49. [DOI] [PubMed] [Google Scholar]
- 22. Lee DH, Yun DW, Kim YH, Im GB, Hyun J, Park HS, Bhang SH, Choi SH. Various three-dimensional culture methods and cell types for exosome production. Tissue Eng Regen Med. 2023;20(4):621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15(4):427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan IK, Shukla N, Borrelli DA, Patel T. Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods Mol Biol. 2018;1740:35–41. [DOI] [PubMed] [Google Scholar]
- 26. Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Pavlakis GN. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7(1):1442088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo KW, Li N, Makani V, Singh RN, Atala A, Lu B. Large-scale preparation of extracellular vesicles enriched with specific microRNA. Tissue Eng Part C Methods. 2018;24(11):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donoso-Quezada J, Ayala-Mar S, González-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit Rev Biotechnol. 2020;40(6):804–20. [DOI] [PubMed] [Google Scholar]
- 29. Ferrari S, Ruggieri P, Cefalo G, Tamburini A, Capanna R, Fagioli F, Comandone A, Bertulli R, Bisogno G, Palmerini E, Alberghini M, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112–18. [DOI] [PubMed] [Google Scholar]
- 30. Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, Longhi A, Lervat C, Entz-Werle N, Casanova M, Aerts I, et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021;22(9):1312–21. [DOI] [PubMed] [Google Scholar]
- 31. Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17(7):932–39. [DOI] [PubMed] [Google Scholar]
- 32. Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tran PHL, Wang T, Yin W, Tran TTD, Nguyen TNG, Lee BJ, Duan W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int J Pharm. 2019;572:118786. [DOI] [PubMed] [Google Scholar]
- 34. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–58. [DOI] [PubMed] [Google Scholar]
- 36. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miranda KC, Bond DT, McKee M, Skog J, Păunescu TG, Da Silva N, Brown D, Russo LM. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78(2):191–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–45. [DOI] [PubMed] [Google Scholar]
- 39. Panez-Toro I, Muñoz-García J, Vargas-Franco JW, Renodon-Cornière A, Heymann MF, Lézot F, Heymann D. Advances in osteosarcoma. Curr Osteoporos Rep. 2023;21:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smrke A, Anderson PM, Gulia A, Gennatas S, Huang PH, Jones RL. Future directions in the treatment of osteosarcoma. Cells. 2021;10(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moukengue B, Lallier M, Marchandet L, Baud’huin M, Verrecchia F, Ory B, Lamoureux F. Origin and therapies of osteosarcoma. Cancers. 2022;14(14):3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Wu Q, Gong X, Liu J, Ma Y. Osteosarcoma: a review of current and future therapeutic approaches. Biomed Eng Online. 2021;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Li M, Guo P, He D. Survival benefits and challenges of adjuvant chemotherapy for high-grade osteosarcoma: a population-based study. J Orthop Surg Res. 2023;18(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu X, Liu C, Wang Y, Koivisto O, Zhou J, Shu Y, Zhang H. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. 2021;176:113891. [DOI] [PubMed] [Google Scholar]
- 45. Yu T, Cai Z, Chang X, Xing C, White S, Guo X, Jin J. Research progress of nanomaterials in chemotherapy of osteosarcoma. Orthop Surg. 2023;15(9):2244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de la Torre P, Perez-Lorenzo MJ, Alcazar-Garrido A, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: lessons from anti-angiogenesis treatments. Molecules. 2020;25(3):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu H, Deng S, Han L, Ren Y, Gu J, He L, Liu T, Yuan ZX. Mesenchymal stem cells, exosomes and exosome-mimics as smart drug carriers for targeted cancer therapy. Colloids Surf B Biointerfaces. 2022;209(Pt 1):112163. [DOI] [PubMed] [Google Scholar]
- 48. Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T, Ashery U, Popovtzer R, Offen D. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19(6):3422–31. [DOI] [PubMed] [Google Scholar]
- 49. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, Qiu Z, Wu Y, Wang L, Chen W. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang W, Lv S, Li W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, Hu C, Zhang L, Guo H, Gao S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14(7):1973–85. [DOI] [PubMed] [Google Scholar]
- 52. Carobolante G, Mantaj J, Ferrari E, Vllasaliu D. Cow milk and intestinal epithelial cell-derived extracellular vesicles as systems for enhancing oral drug delivery. Pharmaceutics. 2020;12(3):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]