Abstract
Purpose
Due to cancer survivors living longer and morbidity associated with cancer treatments, it is necessary to understand symptoms experienced by cancer survivors. This study will analyze the symptom burden among a large cohort of survivors across multiple cancer sites.
Methods
Data from the Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) study were used to examine the symptom burden of older cancer survivors. Poisson regression with robust standard errors was utilized to determine differences in symptoms by cancer site, treatment, and other covariates.
Results
The most frequently reported symptoms among cancer survivors were fatigue (15.8%) and feeling sad or depressed (14.1%). Multivariable analyses indicated that more symptoms were reported among survivors who were younger (p = 0.002), divorced or separated (p = 0.03), and had a combination of public and private insurance (p = 0.01). Survivors who received chemotherapy (p < 0.001), radiation (p = 0.01), or hormone therapy (p = 0.02) reported more symptoms than survivors who did not receive these treatments. Survivors diagnosed with cancer < 5 years ago reported fewer symptoms than longer-term survivors, particularly those diagnosed > 10 years ago (p = 0.02).
Conclusions
Results indicate that common physical and psychological symptoms are reported across cancer types. Cancer survivors diagnosed with cancer 10 or more years ago reported more symptoms than those recently diagnosed. This suggests that symptoms may remain a problem for some survivors decades after their diagnosis.
Implications for Cancer Survivors
Future research should focus on implementing active surveillance of cancer survivors. Healthcare providers and those who care for cancer survivors should understand that the symptom burden associated with cancer may persist even decades following diagnosis.
Keywords: Symptoms, Cancer survivors, Women’s Health Initiative, Late effects of cancer treatment
Due to earlier detection and vast improvements in cancer treatment over the past few decades, the number of cancer survivors in the USA has been steadily increasing. It is projected that the number of survivors will increase from 16.9 million in 2019 to an estimated 22.1 million cancer survivors by the year 2030 [1]. Among current cancer survivors, most were diagnosed five or more years ago, and almost two thirds are 65 years of age or older [1]. Despite these improvements, many cancer survivors report experiencing various cancer and/or treatment-related symptoms, sometimes for many years following cancer diagnosis [2, 3].
Physical and psychosocial symptoms experienced by cancer survivors can cause significant morbidity and distress [2, 3]. Side effects or symptoms associated with treatments may persist for many years after the therapy has ended. Some of these physical symptoms include gastrointestinal distress, difficulty breathing, difficulty sleeping, aches and pains, fatigue, and cognitive deficits [1, 2, 4]. Additional psychological symptoms include anxiety and depression [4]. Uncontrolled physical and psychological symptoms can cause significant distress, increased morbidity, and decreased quality of life for the cancer survivor [1–5].
Prior research has identified common symptoms consistent across 4 different types of cancer (breast, gynecologic, prostate, and colon) [4]. Data from the Women’s Health Initiative (WHI) Life and Longevity after Cancer (LILAC) offer a valuable opportunity to utilize a cohort of older, long-term cancer survivors to identify the symptom burden experienced across many cancer types, including cancers whose symptom burden remains understudied. This research could inform proactive strategies for reducing symptoms and morbidity, leading to improved quality of life. In order to explore the symptom burden of older cancer survivors we sought to (1) identify and enumerate commonly experienced symptoms by cancer type; (2) examine any differences in reported symptoms across cancer types; and (3) identify correlates of the total number of symptoms experienced by cancer survivors. The WHI LILAC cohort was used for these analyses.
Methods
Recruitment and inclusion
Data are from the LILAC (Life and Longevity after Cancer) ancillary study [6] of the Women’s Health Initiative (WHI). The WHI is a study of post-menopausal women’s health, which recruited over 160,000 women in the USA, aged 50–79 at baseline, between 1993 and 1998, and is still ongoing. The WHI included four overlapping clinical trials (two hormone therapy trials, a low-fat diet, and a calcium/vitamin D trial), as well as an observational study. The WHI clinical trials ended between 2002 and 2005, but all willing participants were re-consented for long-term follow-up for heart disease endpoints, cancer, and other health outcomes.
Within WHI, over 30,000 participants were diagnosed with cancer after recruitment, making the WHI cohort an important resource for studying cancer survivorship in women. The LILAC study began in 2013, and women who were diagnosed with one of eight cancers (breast, colorectal, endometrial, lung, ovarian, melanoma, non-Hodgkin lymphoma, and leukemia) any time after WHI enrollment were eligible for participation in LILAC. In LILAC, ovarian, fallopian tube, and primary peritoneal cancers were grouped together in a category hereafter referred to as epithelial/ovarian cancer (EOC). These cancers were combined because of their close biological relationship, as well as the need for larger numbers in this grouping [6]. All participants eligible for LILAC, who were still in active follow-up in WHI, were mailed a packet inviting them to participate in LILAC. Women who did not respond to the first mailing were sent a second mailing after 8 weeks. Additional information regarding the LILAC study can be found elsewhere [6].
Measures
Demographic items
Age (years), race/ethnicity, education, and height were obtained from the WHI baseline dataset. Options for race included American Indian or Alaskan Native, Asian or Pacific Islander, Black or African American, Hispanic/Latino, White, and other. Both race and ethnicity were asked as one question on the baseline WHI survey, rather than separate questions. Therefore, race and ethnicity cannot be analyzed as separate variables. Other demographic variables, including marital status and insurance status, were obtained from the LILAC baseline survey.
Health and comorbid conditions
Body mass index (BMI) was calculated using a participant’s self-reported weight from the LILAC baseline and their last measured height during WHI follow-up. Cardiovascular disease, diabetes, and hypertension were determined from WHI medical history assessments. Cardiovascular disease (CVD) was defined as a self-reported previous diagnosis of CVD at WHI baseline or self-report of one of the following conditions at follow-up: angina, atrial fibrillation, congestive heart failure, deep vein thrombosis, or pulmonary embolism. Diabetes was defined as a self-reported previous diagnosis of diabetes on the WHI eligibility screener or a self-report of diabetes requiring pill or insulin treatment at follow-up. Hypertension was defined as a self-reported previous diagnosis of hypertension at WHI baseline or a self-report of hypertension requiring pills at follow-up.
Cancer
Women enrolled in WHI provide an annual medical update, which includes whether they were diagnosed with any cancer since their last medical history update. Any self-reported cancer diagnoses are adjudicated by medical record view by trained physician adjudicators. Cancer stage was determined from the adjudicated data. In situ and localized stages at diagnosis were combined into one category due to a small number of participants being diagnosed with in-situ cancer. More information on this process can be found at https://www.whi.org/outcomes.
LILAC baseline questionnaire
The LILAC baseline survey included questions about cancer treatment (chemotherapy, radiation, hormone therapy, other: [stem cell or bone marrow transplant]; trastuzumab), cancer recurrence, symptoms occurring after initial cancer treatment not due to any other known condition, self-reported weight, insurance status, marital status, and social support. More information regarding the items and questions assessed on the LILAC baseline questionnaire can be found here (https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/F340-v3.pdf).
Symptoms occurring after cancer diagnosis
The LILAC baseline questionnaire also asked the participants to report new medical problems or symptoms experienced after their cancer treatment, and their persistence. These symptoms included low blood counts (anemia, neutropenia), high blood pressure, kidney problems, liver problems, blood clots (venous thromboembolisms), nerve problems/tingling sensations, hearing changes, skin rash/other skin disorders, memory problems, aching joints, hot flashes, radiation burns, shortness of breath, mouth sores/dry mouth, insomnia/sleep problems, heart disease, bleeding too easily, and weight gain of > 10 lb.
Psychosocial concerns and pain
On the LILAC questionnaire, participants completed a one-item measure about whether they often felt sad or depressed. Response categories were “yes” or “no.” Participants also self-reported their feelings of anxiety, fatigue, and distress during the past week, measured on a scale of 0 (none) to 10 (worst). Lastly, pain was self-reported on a scale from 0 (no pain) to 10 (pain as bad as you can imagine) in the last 24 h.
Statistical analyses
The total number of symptoms was computed as the number of symptoms that first appeared after initial cancer treatment and were still present at LILAC enrollment, plus the presence of depression (i.e., answer of “yes”), and scores > 5 on the pain, anxiety, fatigue, and distress items. These variables were dichotomized using the midpoint as recommended by Singh and colleagues [7], to indicate symptom levels that were mild, compared to moderate or higher, in order to not over-report symptoms that were mild and less bothersome [7]. The total number of symptoms reported could range from 0 (no symptoms reported) to 23 (every possible symptom reported). If a response to a symptom question was missing, we treated it as an unreported symptom and thus it contributed a “0” to the reported symptom count for the participant. Subjects who did not answer any of the symptom survey questions (n = 461) were excluded from all analyses.
Differences by cancer diagnosis, type of treatment, and other covariates were determined using Poisson regression with robust standard errors. Covariates (race, education, marital status, insurance type, BMI, cancer site and stage, treatment, time since diagnosis, and co-morbidities) were selected based on research that has shown an association with these cancer symptoms in published literature [3, 4, 8–12]. Multivariable regression models were run first using data on subjects with complete covariate information, and then on 30 imputed data sets created using a fully conditional specification procedure [13]. All analyses were performed using SAS Version 9.4 (SAS Inc., Cary, NC).
Results
Participants characteristics
Table 1 presents descriptive characteristics of the LILAC participants. Overall, there were 7928 women in LILAC, and 7467 women (94.2%) had symptom data available. The median number of years since cancer diagnosis was 6.7, ranging from 0.4 to 20.1 years. Most women were White (91.7%), college educated (84.9%), and married/living as married (46.7%) or widowed (35.0%). The most commonly diagnosed cancer was breast (54.3%), followed by melanoma (11.1%) and colorectal (10.1%) cancer. In terms of cancer treatment, 43.7% received radiation therapy, 36.3% reported receiving hormone therapy, and 28.6% received chemotherapy. Most participants (59.4%) were five or more years post-cancer diagnosis, and 71% were diagnosed with cancer at an in-situ (n = 361) or localized stage (n = 4951).
Table 1.
Number of symptoms present by demographic and clinical factors (n = 7467)
| n (%)+ | Mean # of symptoms | SD of symptoms | P | ||
|---|---|---|---|---|---|
| Race | White | 6849 (91.7) | 1.3 | 2.0 | < 0.001 |
| Black | 269 (3.6) | 1.8 | 2.1 | ||
| Other* | 336 (4.5) | 1.7 | 2.4 | ||
| Education | High school or less | 1083 (14.5) | 1.5 | 2.2 | 0.008 |
| College or higher | 6338 (84.9) | 1.3 | 2.0 | ||
| Marital status | Married/living as married | 3488 (46.7) | 1.3 | 2.0 | 0.007 |
| Widowed | 2617 (35.0) | 1.3 | 2.0 | ||
| Divorced/separated | 951 (12.7) | 1.6 | 2.2 | ||
| Never married | 338 (4.5) | 1.3 | 1.8 | ||
| Insurance | No insurance | 60 (0.01) | 1.9 | 2.6 | 0.004 |
| Public | 2501 (33.5) | 1.4 | 1.9 | ||
| Private | 631 (8.5) | 1.3 | 1.9 | ||
| Public + private | 3173 (42.5) | 1.5 | 2.1 | ||
| BMI (kg/m2) | Under/normal weight (< 25 kg/m2) | 3296 (44.1) | 1.2 | 1.8 | < 0.001 |
| Overweight (25–30 kg/m2) | 2293 (30.7) | 1.4 | 2.1 | ||
| Obese (≥ 30 kg/m2) | 1495 (20.0) | 1.8 | 2.3 | ||
| Cancer | Breast | 4054 (54.3) | 1.5 | 2.1 | < 0.001 |
| Colorectal | 752 (10.1) | 1.3 | 2.0 | ||
| Endometrial | 628 (8.4) | 1.1 | 1.8 | ||
| Lung | 401 (5.4) | 1.5 | 1.9 | ||
| Leukemia | 171 (2.3) | 1.3 | 1.7 | ||
| Lymphoma | 424 (5.7) | 1.6 | 2.1 | ||
| Melanoma | 829 (11.1) | 0.5 | 1.2 | ||
| EOC | 208 (2.8) | 2.2 | 2.4 | ||
| Chemotherapy | No/do not know | 5271 (70.6) | 1.1 | 1.8 | < 0.001 |
| Yes | 2138 (28.6) | 2.1 | 2.4 | ||
| Radiation therapy | No/do not know | 4130 (55.3) | 1.2 | 1.9 | < 0.001 |
| Yes | 3266 (43.7) | 1.6 | 2.1 | ||
| Hormone therapy | No/do not know | 4691 (62.8) | 1.2 | 1.9 | < 0.001 |
| Yes | 2711 (36.3) | 1.6 | 2.2 | ||
| Time since diagnosis | < 5 years | 3034 (40.6) | 1.3 | 1.8 | 0.003 |
| 5–10 years | 1916 (25.7) | 1.4 | 2.1 | ||
| > 10 years | 2517 (33.7) | 1.5 | 2.1 | ||
| Stage | In situ/localized | 5312 (71.1) | 1.2 | 1.9 | < 0.001 |
| Regional | 1460 (19.6) | 1.7 | 2.2 | ||
| Distant | 596 (8.0) | 1.9 | 2.2 | ||
| Diabetes ever | No | 6245 (83.6) | 1.3 | 1.9 | < 0.001 |
| Yes | 1222 (16.4) | 1.7 | 2.3 | ||
| Hypertension ever | No | 2583 (34.6) | 1.2 | 1.8 | < 0.001 |
| Yes | 4884 (65.4) | 1.5 | 2.1 | ||
| CVD ever | No | 5744 (76.9) | 1.3 | 1.9 | < 0.001 |
| Yes | 1723 (23.1) | 1.7 | 2.4 |
n = 15 American Indian/Alaskan Native, 123 Asian or Pacific Islander, 128 Hispanic/Latino, and 70 other
Percentage may not sum to 100 due to missing values
Symptom prevalence
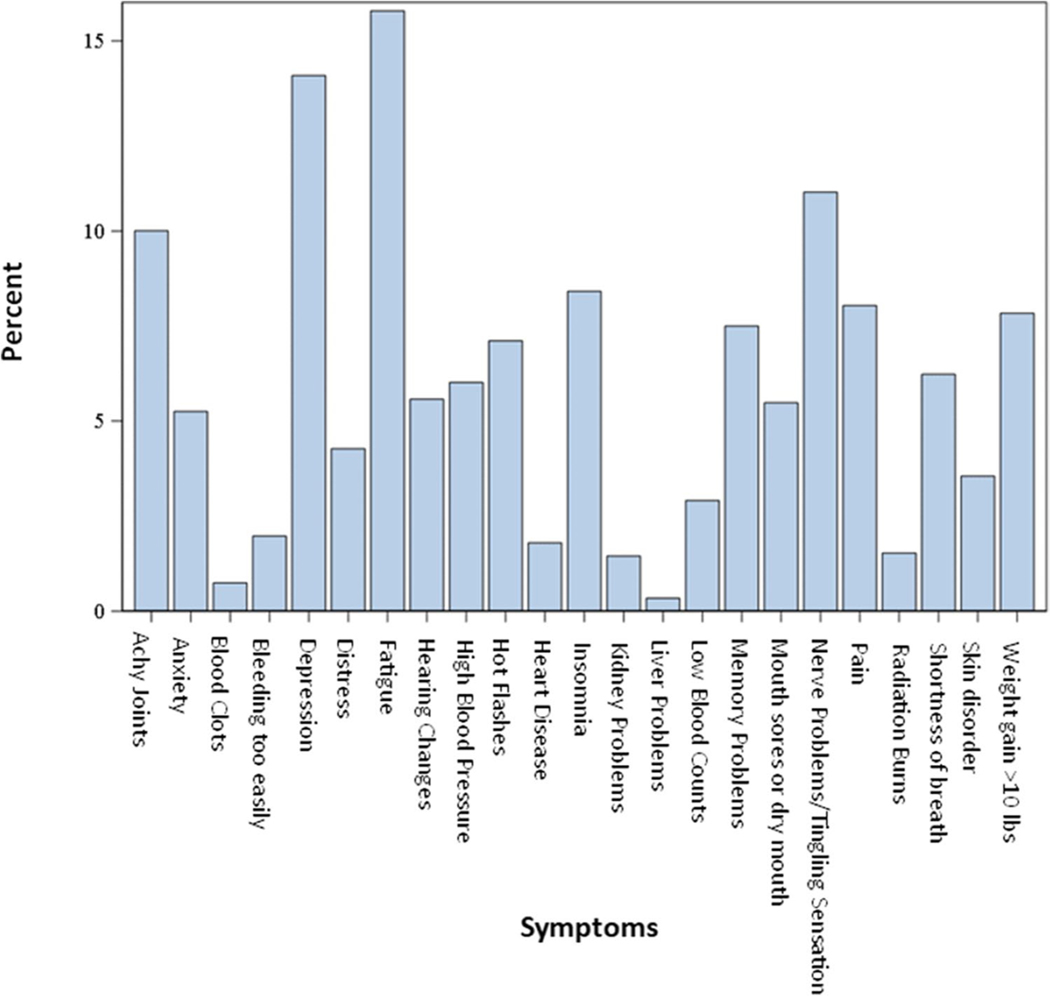
The frequency of each reported symptom is presented in Fig. 1. The symptoms of pain, anxiety, fatigue, and distress were dichotomized into moderate or less and more than moderate. A relatively small percentage of participants reported greater than moderate levels for each symptom: 16% for fatigue, 5% for anxiety, 8% for pain, and 4% for distress. Overall, the most commonly reported symptoms were fatigue (15.8%) and feeling sad or depressed (14.1%). However, reported symptoms differed by cancer type (Figures S1–S8 in the Supplementary Materials). Fatigue was the most commonly reported symptom among leukemia (25.1%), lung (22.4%), lymphoma (19.6%), breast (16.2%), colorectal (15.7%), and endometrial (14.8%) cancer survivors, while feeling sad or depressed was highest among melanoma survivors (8.8%). Nerve problems/tingling sensations were the most common symptom among EOC survivors (40.4%).
Fig. 1.
Relative frequency of symptoms
Descriptive and unadjusted results
The number of reported symptoms by key demographic and clinical characteristics are summarized in Table 1. At LILAC baseline, participants averaged 1.4 symptoms (SD = 2.0) with a range of 0 (n = 3617, 48.4%) to 15 (n = 1, 0.01%) symptoms. In these unadjusted analyses, the number of reported symptoms was higher for Black (mean = 1.8) and other non-White (mean = 1.7) participants compared to non-Hispanic White participants (mean = 1.3) (p < 0.001). Participants with no health insurance reported more symptoms (mean = 1.9) than participants who had some type of health insurance (p = 0.004). When considering comorbidities, obese participants reported more symptoms (mean = 1.8) than overweight (mean = 1.4) or under/normal weight (mean = 1.2) participants (p < 0.001). For factors related to cancer, participants who reported receiving chemotherapy reported more symptoms (mean = 2.1) than participants who did not receive chemotherapy (mean = 1.1) (p < 0.001). This was also true for radiation therapy (p < 0.001) and hormone therapy (p < 0.001). Participants whose cancer was diagnosed at a distant stage reported more symptoms (mean = 1.9) than participants whose cancer was diagnosed at regional (mean = 1.7) or in-situ/localized (mean = 1.2) stages (p < 0.001).
Multivariable regression analyses
Results for the multivariable regression model are presented in Table 2. These analyses indicate that more symptoms were reported among participants who were younger (p = 0.002), divorced or separated (p = 0.03), and with a combination of public and private insurance (p = 0.01). Participants who were overweight or obese also reported significantly more symptoms than underweight or normal weight participants (p < 0.001). For factors related to cancer, participants who reported chemotherapy (p < 0.001), radiation therapy (p = 0.01), or hormone therapy (p = 0.02) reported significantly more symptoms than participants who did not receive these treatments. Additionally, participants whose cancers were diagnosed at distant stages reported more symptoms than those diagnosed at regional or in situ/localized stages (p = 0.004). Survivors diagnosed with cancer < 5 years ago reported fewer symptoms than longer term survivors, particularly those diagnosed > 10 years ago (p = 0.02). Lastly, participants diagnosed with melanoma reported significantly fewer symptoms than every other cancer type. Similar results were observed when the imputed data were analyzed (Table S1 in the Supplementary Materials).
Table 2.
Multivariable regression model of covariables associated with the number of symptoms (n = 5820 participants with complete covariate information)
| Mean* ratio | 95% CI | p | |||
|---|---|---|---|---|---|
| Current age (years) | 0.990 | 0.983 | 0.996 | 0.002 | |
| Race | Black | 0.90 | 0.76 | 1.06 | 0.13 |
| Other | 1.13 | 0.96 | 1.32 | ||
| White | ref | ||||
| Education | College or higher | 0.94 | 0.85 | 1.05 | 0.29 |
| High school or less | ref | ||||
| Marital status | Married/living as married | 1.09 | 0.92 | 1.28 | 0.03 |
| Widowed | 1.12 | 0.95 | 1.33 | ||
| Divorced/separated | 1.25 | 1.04 | 1.49 | ||
| Never married | ref | ||||
| Insurance | No insurance | 1.07 | 0.75 | 1.54 | 0.01 |
| Private | 0.86 | 0.76 | 0.98 | ||
| Public | 0.90 | 0.84 | 0.97 | ||
| Public and private | ref | ||||
| BMI | Obese | 1.30 | 1.19 | 1.43 | < 0.001 |
| Overweight | 1.12 | 1.03 | 1.21 | ||
| Under/normal weight | ref | ||||
| Cancer | Breast cancer | 2.26 | 1.84 | 2.78 | < 0.001 |
| Colorectal cancer | 2.13 | 1.72 | 2.65 | ||
| EOC | 2.64 | 2.04 | 3.42 | ||
| Endometrial cancer | 2.04 | 1.63 | 2.54 | ||
| Leukemia | 1.67 | 1.20 | 2.31 | ||
| Lung cancer | 2.41 | 1.92 | 3.04 | ||
| Lymphoma | 2.21 | 1.73 | 2.82 | ||
| Melanoma | ref | ||||
| Chemotherapy | 1.61 | 1.48 | 1.75 | < 0.001 | |
| Radiation therapy | 1.11 | 1.02 | 1.21 | 0.01 | |
| Hormone therapy | 1.12 | 1.02 | 1.23 | 0.02 | |
| Time since diagnosis | < 5 years | 0.89 | 0.82 | 0.96 | 0.02 |
| 5–10 years | 0.96 | 0.88 | 1.05 | ||
| > 10 years | ref | ||||
| Stage | Distant | 1.32 | 1.13 | 1.55 | 0.004 |
| In situ/localized | 1.04 | 0.94 | 1.14 | ||
| Regional | ref | ||||
| Diabetes ever | 1.19 | 1.08 | 1.30 | < 0.001 | |
| Hypertension ever | 1.20 | 1.11 | 1.30 | < 0.001 | |
| CVD ever | 1.30 | 1.19 | 1.41 | < 0.001 |
Mean ratio is the ratio of the mean number of symptoms per person. For age, it is the ratio corresponding to a 1-year increase. Models are fully adjusted for all variables included in Table 2
Discussion
The goal of this study was to determine the symptom burden experienced by survivors of eight cancers in the WHI LILAC cohort. Results of this study suggest that despite differences in cancer sites and subsequent treatments, symptoms such as fatigue and depression are commonly reported across cancer types. These common symptoms have been reported in other studies of cancer survivorship, but our study is unique with the inclusion of additional understudied cancers [4]. Participants who received chemotherapy, radiation, or hormone therapy reported significantly more symptoms than those who did not report receiving these treatments. This was expected, as many of these symptoms likely occurred as a result of the treatment [4]. Additionally, results from this study suggest that those without health insurance reported more symptoms than those with insurance. Lack of health insurance for cancer care is related to financial strain, which can also be associated with increased symptom burden and decreased quality of life among cancer survivors [14].
A novel finding of this study is that participants who were diagnosed with cancer more than 10 years ago experienced significantly more symptoms than those who were diagnosed within the last 5 years, even after adjusting for other factors. This suggests that the symptom burden associated with cancer treatment may remain a problem even decades after diagnosis, similar to results reported in other studies colleagues [4]. However, most previous literature investigating symptoms in cancer survivors has predominantly focused on one specific symptom, such as pain, depression, and neuropathy [8, 11, 15–19]. Some studies did report multiple symptoms experienced by cancer survivors, but most of these studies report these symptoms for one cancer site, typically breast cancer [20–24]. Of the publications that include multiple symptoms reported among multiple cancer sites, most are review articles that cannot make comparisons between the populations that experience symptoms [3–5]. Most research articles analyzing symptoms reported among cancer survivors are limited by small sample sizes and by number of cancer types/sites included [10, 12]. Therefore, this current study fills a valuable gap in the literature by using a large cohort of cancer survivors to compare the symptom experience of survivors across multiple cancer types.
Strengths of this study include the large sample size, and eight different cancer types. Most cancer survivorship literature focuses on the most commonly diagnosed cancers, including breast, lung, or colorectal cancers. By including other cancer sites, we are able to make comparisons across multiple cancers and understand the long-term symptom experience among women diagnosed with one of these understudied cancers. Moreover, this study utilized a large cohort of female cancer survivors, some of whom were diagnosed with cancer 20 or more years ago. This adds to the growing body of survivorship literature. As the number of cancer survivors continues to increase, understanding the symptom burden experienced by long-term survivors is an increasingly important issue.
Despite these strengths, there are some limitations to this study. Participants were provided with a fixed checklist of symptoms from which to choose and were not given the option of including additional symptoms. Therefore, it is possible that there are symptoms the survivors experienced that were not included in the checklist, and therefore not recorded. Additionally, reliability and validity of the symptom scales for fatigue, anxiety, and depression were not assessed. However, these symptoms are commonly included in the Edmonton symptom assessment scale for individuals who have had cancer and have been utilized in numerous studies assessing cancer symptoms [25]. Further, although participants in this study indicated symptoms they experienced after a cancer diagnosis, we do not know if these symptoms are a result of cancer treatment. Since this is an aging cohort, they could possibly have some of these symptoms as a result of aging or another health condition. Additionally, survivorship bias is a limitation of the LILAC cohort study. Although all women in LILAC are cancer survivors, not all eligible survivors enrolled in the study. It is possible that the LILAC participants are healthier than those not included in LILAC, leading to a potential under-reporting of symptoms. Lastly, LILAC participants were predominantly non-Hispanic White, limiting the generalizability of these findings to other populations.
Results from this study expand the knowledge of symptoms experienced by cancer survivors, including those diagnosed with several understudied cancers, and reaffirms that fatigue and depression are commonly reported symptoms across many cancer types. Importantly, results of this study suggest that even survivors many years removed from their cancer diagnosis may experience symptoms as a result of their treatment. Future research should focus on implementing active surveillance of cancer survivors, particularly among those who may be at increased risk for high symptom burden. Clinical implications of these results indicate that it is important that primary care providers and others who care for long-term survivors understand that the symptom burden associated with cancer may persist even decades after diagnosis.
Supplementary Material
Funding
This work and the WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005. The WHI Life and Longevity after Cancer (LILAC) study is funded by the National Cancer Institute (UM1 CA173642). This paper is also supported by the Breast Cancer Research Foundation (BCRF).
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11764-022-01200-4.
Declarations
Ethics approval This study was performed in line with the Declaration of Helsinki and approval of this study was granted by each institution’s Institutional Review Board (IRB). Informed consent was obtained from all participants included in the study.
Conflict of interest Dr. Paskett is an MPI on grants from Pfizer and Merck Foundation. Dr. Pennell is a Co-I on grants sponsored by Pfizer and Sanofi.
References
- 1.Cancer treatment & survivorship facts & figures 2019–2021. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 2.Shahrokni A, Wu AJ, Carter J, Lichtman SM. Long-term toxicity of cancer treatment in older patients. Clin Geriatr Med. 2016;32(1):63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gegechkori N, Haines L, Lin JJ. Long-term and latent side effects of specific cancer types. Med Clin North Am. 2017;101(6):1053–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40(2):163–81. [DOI] [PubMed] [Google Scholar]
- 5.Gosain R, Miller K. Symptoms and symptom management in long-term cancer survivors. Cancer J. 2013;19(5):405–9. [DOI] [PubMed] [Google Scholar]
- 6.Paskett ED, Caan BJ, Johnson L, et al. The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) study: description and baseline characteristics of participants. Cancer Epidemiol Biomarkers Prev. 2018;27(2):125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JA, Satele D, Pattabasavaiah S, Buckner JC, Sloan JA. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haisfield-Wolfe ME, McGuire DB, Soeken K, Geiger-Brown J, De Forge BR. Prevalence and correlates of depression among patients with head and neck cancer: a systematic review of implications for research. Oncol Nurs Forum. 2009;36(3):E107–125. [DOI] [PubMed] [Google Scholar]
- 9.Halpern MT, Brawley OW. Insurance status, health equity, and the cancer care continuum. Cancer. 2016;122(20):3106–9. [DOI] [PubMed] [Google Scholar]
- 10.Horick NK, Muzikansky A, Gutierrez HL, Boyd KL, Finkelstein DM. Physical symptoms in long-term survivors of rare cancer. J Cancer Surviv. 2018;12(6):835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemp JR, Myers JS, Fabian CJ, et al. Cognitive functioning and quality of life following chemotherapy in pre- and perimenopausal women with breast cancer. Support Care Cancer. 2018;26(2):575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. Journal of the American Board of Family Medicine : JABFM. 2007;20(5):434–43. [DOI] [PubMed] [Google Scholar]
- 13.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42. [DOI] [PubMed] [Google Scholar]
- 14.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broeckel JA, Thors CL, Jacobsen PB, Small M, Cox CE. Sexual functioning in long-term breast cancer survivors treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2002;75(3):241–8. [DOI] [PubMed] [Google Scholar]
- 16.Clark CJ, Fino NF, Liang JH, Hiller D, Bohl J. Depressive symptoms in older long-term colorectal cancer survivors: a population-based analysis using the SEER-Medicare healthcare outcomes survey. Support Care Cancer. 2016;24(9):3907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallaway MS, Townsend JS, Shelby D, Puckett MC. Pain among cancer survivors. Prev Chronic Dis. 2020;17:E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlson CW, Alberts NM, Liu W, et al. Longitudinal pain and pain interference in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2020;126(12):2915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. 2017;166(2):519–26. [DOI] [PubMed] [Google Scholar]
- 20.Kenyon M, Mayer DK, Owens AK. Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN. 2014;43(3):382–98. [DOI] [PubMed] [Google Scholar]
- 21.Lovelace DL, McDaniel LR, Golden D. Long-term effects of breast cancer surgery, treatment, and survivor care. J Midwifery Womens Health. 2019;64(6):713–24. [DOI] [PubMed] [Google Scholar]
- 22.Maass S, Boerman LM, Brandenbarg D, et al. Symptoms in long-term breast cancer survivors: a cross-sectional study in primary care. Breast (Edinburgh, Scotland). 2020;54:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews H, Semper H. “Dropped from the system”: the experiences and challenges of long-term breast cancer survivors. J Adv Nurs. 2017;73(6):1355–65. [DOI] [PubMed] [Google Scholar]
- 24.Schultz PN, Klein MJ, Beck ML, Stava C, Sellin RV. Breast cancer: relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nurs. 2005;14(2):204–11. [DOI] [PubMed] [Google Scholar]
- 25.Oldenmenger WH, Raaf PJ, de Klerk C, van der Rijt C. Cut points on 0–10 numeric rating scales included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45(6):1083–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.