Abstract
Purpose
SUPREME, a phase IIIb study conducted in Italy, demonstrated safety and high efficacy of secukinumab for up to 72 weeks in patients with moderate-to-severe plaque-type psoriasis. SUPREME 2.0 study aimed to provide real-world data on the long-term drug survival and effectiveness of secukinumab beyond 72 weeks.
Patients and Methods
SUPREME 2.0 is a retrospective observational chart review study conducted in patients previously enrolled in SUPREME study. After the end of the SUPREME study, eligible patients continued treatment as per clinical practice, and their effectiveness and drug survival data were retrieved from medical charts.
Results
Of the 415 patients enrolled in the SUPREME study, 297 were included in SUPREME 2.0; of which, 210 (70.7%) continued secukinumab treatment throughout the 42-month observation period. Patients in the biologic-naïve cohort had higher drug survival than those in the biologic-experienced cohort (74.9% vs 61.7%), while HLA-Cw6–positive and HLA-Cw6–negative patients showed similar drug survival (69.3% and 71.9%). After 42 months, Psoriasis Area and Severity Index (PASI) 90 was achieved by 79.6% of patients overall; with a similar proportion of biologic-naïve and biologic-experienced patients achieving PASI90 (79.8% and 79.1%). The mean absolute PASI score reduced from 21.94 to 1.38 in the overall population, 21.90 to 1.24 in biologic-naïve and 22.03 to 1.77 in biologic-experienced patients after 42 months. The decrease in the absolute PASI score was comparable between HLA-Cw6–positive and HLA–Cw6-negative patients. The baseline Dermatology Life Quality Index scores also decreased in the overall patients (10.5 to 2.32) and across all study sub-groups after 42 months. Safety was consistent with the known profile of secukinumab, with no new findings.
Conclusion
In this real-world cohort study, secukinumab showed consistently high long-term drug survival and effectiveness with a favourable safety profile.
Keywords: psoriasis, secukinumab, real-world evidence, drug survival, SUPREME
Introduction
Psoriasis (PsO) is a chronic immune-mediated inflammatory multifactorial disease with a genetic predisposition and environmental triggers. The most prevalent and well-recognised morphological presentation of PsO is the chronic plaque-type, which is characterised by accelerated keratinocyte proliferation and hyperplasia of the epidermis resulting in the development of well-demarcated, erythematous, infiltrated and scaly plaques.1–3
With the advent of biologics, the therapeutic outcome of patients with moderate to severe psoriasis has substantially improved owing to their effectiveness and favourable safety profile. Depending on the phenotype, clinical features, immunological pathways involved and hereditary variables, patients with PsO may respond differently to various biologics. The human leucocyte antigen (HLA)-Cw6 is one of the most strongly associated psoriasis susceptibility alleles, and HLA-Cw6–positive Caucasian patients tend to have an early onset of the disease, more severe PsO and comorbidities.3–5 HLA-Cw6 positivity is associated with 10–20 fold increased risk of developing PsO.5 There is conflicting evidence or a lack of long-term clinical data regarding the association between HLA-Cw6 status and the effectiveness of various biologic therapies, including tumour necrosis factor (TNF-α), anti−interleukin-12/23, anti−interleukin-23 and anti−interleukin-17.6–10
Secukinumab is a recombinant human monoclonal antibody against interleukin-17A, a key cytokine involved in the pathogenesis of PsO, and has been proven to be highly efficacious in the treatment of PsO in adults.11–14 In the SUPREME study (NCT02394561),15,16 secukinumab demonstrated high and sustained efficacy and a favourable safety profile for up to 72 weeks of treatment in both HLA-Cw6–positive and HLA-Cw6–negative patients. However, real-world data on the long-term use of secukinumab are lacking, and outcomes from the SUPREME 2.0 study will contribute to a better understanding of the long-term management of patients with moderate to severe PsO.
This study aimed to provide information on the long-term drug survival and effectiveness of secukinumab by combining data from an initial interventional phase IIIb study (SUPREME study)15,16 conducted in Italy with data from a real-world clinical context for a minimum observation period of 42 months.
Materials and Methods
Study Design and Patients
SUPREME 2.0 was a real-world, retrospective, multicentre, observational cohort study that included data from two sources: the SUPREME study15,16 database and hospital chart records (Figure 1). SUPREME was a phase IIIb, 24-week randomised study (CAIN457AIT01; NCT02394561) conducted across 50 centres in Italy and included an extension period of up to 72 weeks.15,16 Patients in the SUPREME study were treated with subcutaneous secukinumab 300 mg (two 150 mg injections) per week for the first 5 weeks starting at week 0, followed by a maintenance period of 300 mg (two 150 mg injections) every 4 weeks for up to 24 weeks. Patients achieving PASI 75 at week 24 were eligible to enter the 48‐week extension phase.
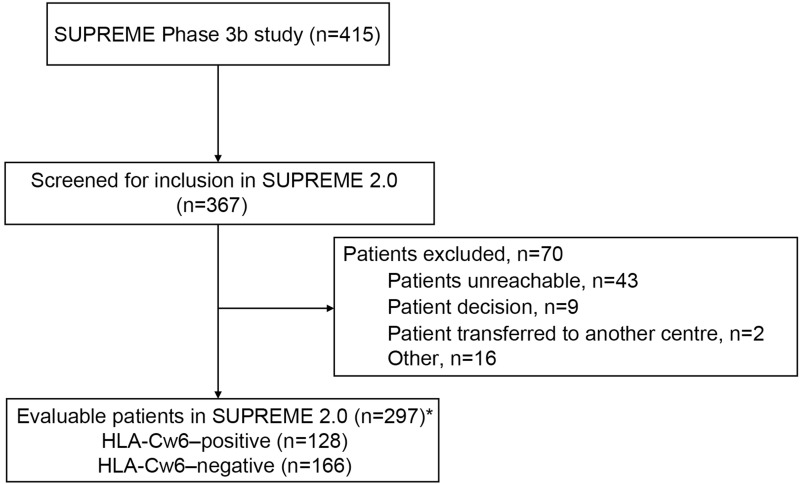
Figure 1.
Patient disposition: (A) Cumulative proportion of patients (B) Biologic-naïve and biologic-experienced patients. (C) HLA-Cw6–negative and HLA-Cw6–positive patients. *Data from patients deceased during the SUPREME study (n=8) were included.
Abbreviations: HLA, human leucocyte antigen; n, number of patients.
Patients with moderate to severe chronic plaque PsO who were enrolled in the SUPREME study were eligible to enter the SUPREME 2.0 study with a minimum observation period of 42 months. After the end of the SUPREME study, these patients continued treatment as per clinical practice, and their data were retrieved from medical charts to obtain information on the long-term drug survival and effectiveness of secukinumab in a mixed setting (randomised controlled plus real-world). Previously collected data of patients who deceased after the end of the SUPREME study were also used in the current study.
Patient’s baseline characteristics data and prior treatment information, before secukinumab treatment, were retrieved from the SUPREME database. Routine clinical data (eg Psoriasis Area and Severity Index [PASI] score, patient safety and Dermatology Life Quality Index [DLQI]) and PsO treatment information occurred after the end of the SUPREME study were retrieved directly from hospital charts (starting from the last study visit in the SUPREME study until the extraction date). Data from both the sources were linked to the patient ID, sex and date of birth. Patients (≥18 years) from the SUPREME study with moderate to severe chronic plaque-type PsO, a PASI score of ≥10 or >5 but <10 and a DLQI score of ≥10 at 6 months, who had received ≥1 dose of secukinumab 300 mg and had given written informed consent for SUPREME 2.0 were included.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines and in compliance with all federal, local or regional requirements. The institutional review board/ethics committee of each participating centre approved the study protocol (Supplementary Table 1). All patients provided consent to participate in the SUPREME and SUPREME 2.0 studies.
Endpoints and Assessments
The primary endpoint of this study was long-term drug survival after 42 months of treatment with secukinumab. Patients were considered to be “on secukinumab treatment” if at 42 months (±1 months) the physician declared that the patient was still on secukinumab treatment. Patients dropping out for any reason during the SUPREME study were considered “not on treatment”. The patients who were lost to follow-up at the time of data collection were considered as censored.
The secondary endpoints included long-term drug survival after 42 months of treatment with secukinumab in patients stratified by prior biologic experience and by HLA-Cw6 status, effectiveness of secukinumab based on PASI75/90/100 scores at 6 months and 42 months, and patient’s DLQI score change was assessed after 6 and 42 months.
The safety of secukinumab was also assessed at 42 months based on adverse events (AEs) and serious AEs (SAEs).
Statistical Analysis
The patient population considered for the statistical analyses comprised overall patients, HLA-Cw6–positive and HLA-Cw6–negative cohorts, and biologic-naïve and biologic-experienced cohorts. The biologic-naïve patients were those who had no history of biologic use for the treatment of PsO before secukinumab initiation, whereas the biologic-experienced included those patients who were exposed to at least one biologic therapy for PsO before secukinumab initiation.
Kaplan-Meier estimates and Log rank tests for group comparisons were used to evaluate the long-term drug survival at 42 months of potential treatment by biologic exposure status and HLA-Cw6 status. The changes in absolute PASI score and DLQI total score were evaluated every 6 months from the index date (ie first secukinumab dose administration) to 42 months and were provided as summary statistics. A linear Mixed Models for Repeated Measures (MMRM) was used to estimate differences between HLA-Cw6–positive and HLA-Cw6–negative patients in terms of changes in the PASI or DLQI scores from baseline.
The biologic-naïve versus biologic-experienced cohorts and HLA-Cw6-positive vs biologic-experienced cohorts were compared at each time point using point estimates, P-values and 95% confidence intervals (CIs) for the treatment difference based on least square means. The mean values of the absolute PASI scores were also computed at weeks 60 and 72 in patients who completed or did not complete their follow-up visits at weeks 60 and 72 during the SUPREME study. A longitudinal analysis and comparison of changes from baseline were performed using a linear MMRM, as described above, to evaluate differences between the two different study settings (randomised controlled trial [RCT] vs real world evidence [RWE]). The association between baseline covariates and the time to reach PASI75/90/100 was estimated using Cox proportional hazard models. Summary descriptive statistics are provided for AEs, SAEs, drug-related AEs and AEs leading to study drug discontinuation.
Results
Patients
Of the 415 patients included in the SUPREME study, 367 were screened for inclusion in the SUPREME 2.0 study. Of these, 297 (71.6%) patients were eligible for the study (Figure 1).
Due to COVID-19 pandemic related problems, the enrolment was closed on 31 March 2021 and 297 eligible patients from 38 centers (out of 415 patients from 43 centers in the SUPREME study) who had a minimum follow-up period of up to 42 months from index date, were enrolled in the SUPREME 2.0 study.
Overall, the average age of the patients, before secukinumab treatment initiation, was 45.9 years; 90.2% of patients were in the <65-year age group; 98.7% were Caucasians, and 70.7% were men (Table 1). The average age at which PsO was diagnosed was 27.8 years, and most patients (77.8%) had scalp PsO. Before secukinumab treatment initiation, the time since the first diagnosis of PsO was 18.7 years. A total of 20.9% of patients had psoriatic arthritis (PsA), of which 17.9% had polyarticular-type PsA which was the most predominant variant. Of 297 patients, 128 (43.1%) were HLA-Cw6–positive and 166 (55.9%) were HLA-Cw6–negative; by prior biologic experience status, 203 (68.4%) patients were biologic-naïve, and 94 (31.6%) patients were biologic-experienced. The baseline characteristics of HLA-Cw6–positive and HLA-Cw6–negative patients are presented in Table 1.
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | HLA-Cw6 Positive (N=128) | HLA-Cw6 Negative (N=166) | Overall Patient Cohort† (N=297) |
|---|---|---|---|
| Age (years) | 42.8±13.7 | 48.5±13.0 | P < 0.001 |
| 45.9±13.5 | |||
| Age categories (years), n (%) | P = 0.521 | ||
| <65 | 117 (91.4) | 148 (89.2) | 268 (90.2) |
| ≥65 | 11 (8.6) | 18 (10.8) | 29 (9.8) |
| Sex, n (%) | P = 0.358 | ||
| Male | 87 (68.0) | 121 (72.9) | 210 (70.7) |
| Female | 41 (32.0) | 45 (27.1) | 87 (29.3) |
| Race, n (%) | P = 1.000 | ||
| Caucasian | 128 (100.0) | 162 (97.6) | 293 (98.7) |
| Weight (kg) | 77.5±15.1 | 83.9±17.8 | P < 0.001 |
| 81.2±17.0 | |||
| Height (cm) | 171.9±9.1 | 171.6±9.4 | P = 0.791 |
| 171.6±9.2 | |||
| BMI categories (kg/m2), n (%) | P = 0.013 | ||
| <25 | 54 (42.2) | 47 (28.3) | 101 (34.0) |
| ≥25 | 74 (57.8) | 119 (71.7) | 196 (66.0) |
| Waist circumference (cm), n | 116 | 158 | 277 (P < 0.001) |
| 92.7±13.9 | 99.7±16.0 | 96.9±15.6 | |
| Smoking status, n (%) | P = 0.060 | ||
| Never | 42 (32.8) | 74 (44.6) | 118 (39.7) |
| Current | 68 (53.1) | 65 (39.2) | 133 (44.8) |
| Former | 18 (14.1) | 27 (16.3) | 46 (15.5) |
| Absolute PASI | 21.0±9.1 | 22.5±10.9 | 21.9±10.2 |
| Type of psoriasis history at diagnosis, n (%) | |||
| Nail psoriasis | 46 (35.9) | 65 (39.2) | 113 (38.1) |
| Scalp psoriasis | 95 (74.2) | 133 (80.1) | 231 (77.8) |
| Age at diagnosis of psoriasis (years) | 23.6±13.1 | 31.1±14.5 | 27.8±14.4 |
| Time since first diagnosis of psoriasis (years), n | 128 | 166 | 297 |
| 19.7±12.4 | 17.9±11.5 | 18.7±11.9 | |
| Number of patients with psoriatic arthritis, n (%) | 21 (16.4) | 40 (24.1) | 62 (20.9) |
| Type of psoriatic arthritis, n (%) | |||
| Polyarticular | 19 (14.8) | 33 (19.9) | 53 (17.9) |
| Monoarticular | 1 (0.8) | 4 (2.4) | 5 (1.7) |
| Axial | 4 (3.1) | 7 (4.2) | 11 (3.7) |
| Enthesitis | 4 (3.1) | 6 (3.6) | 10 (3.4) |
| Dactylitis | 7 (5.5) | 8 (4.8) | 15 (5.1) |
| Time since first diagnosis of psoriatic arthritis (years), n | 20 | 39 | 60 |
| 8.4±8.3 | 10.8±9.4 | 9.8±9.1 |
Notes: P-values for categorical variables were calculated using the chi-square test or Fisher exact test and those for continuous variables were calculated using the t-test. The data are mean±SD unless specified otherwise. †Three patients with missing HLA-Cw6 assessments are included in the “overall patient cohort” column.
Abbreviations: BMI, body mass index; HLA, human leucocyte antigen; N, number of patients in the population; n, number of patients; PASI, Psoriasis Area and Severity Index; SD, standard deviation.
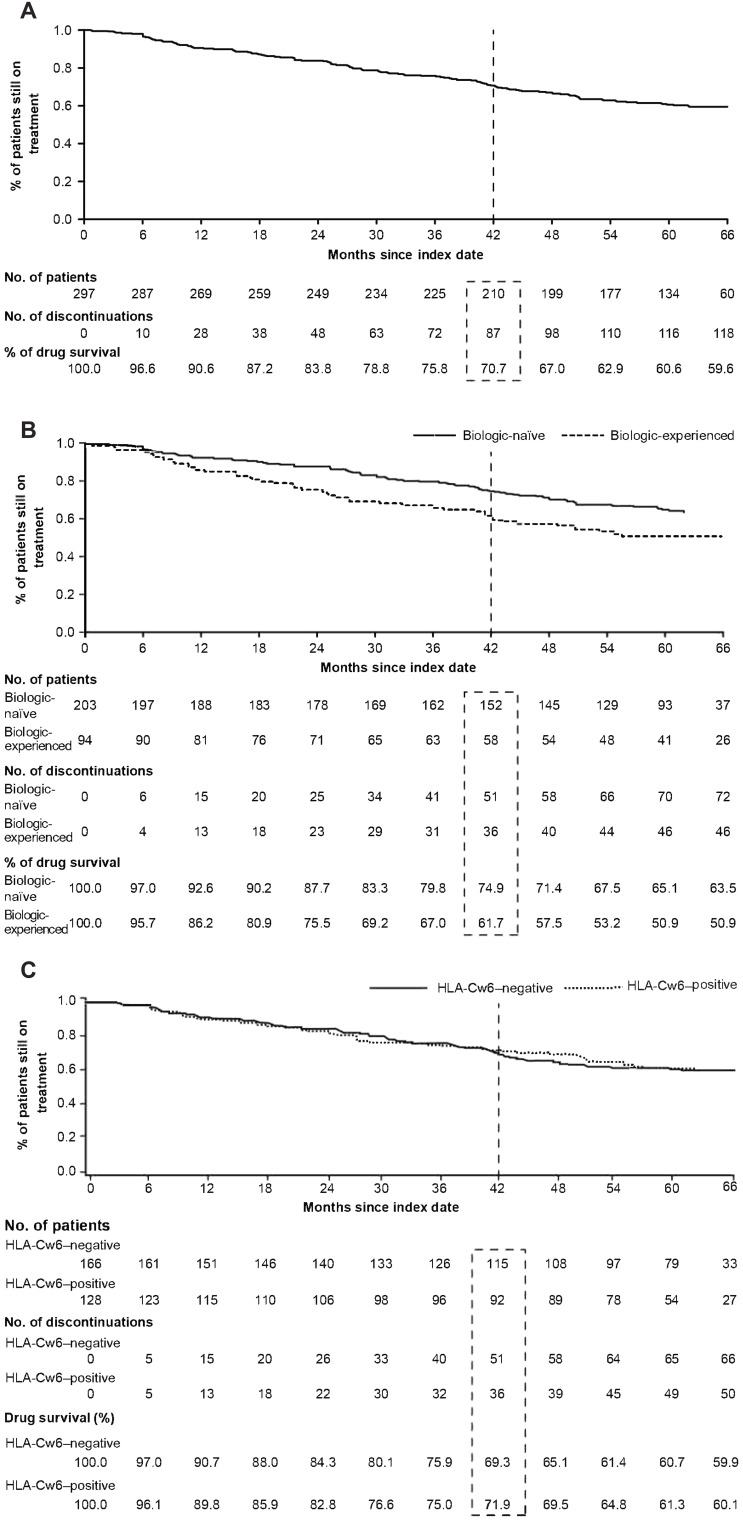
Drug Survival and Efficacy
After a minimum observation period of 42 months from the index date, 70.7% (n=210) of the patients were on secukinumab treatment (Figure 2A). The proportion of patients who were still on secukinumab treatment was significantly higher in the biologic-naïve cohort than in the biologic-experienced cohort (Figure 2B), while no significant difference was observed between the HLA-Cw6–negative and HLA-Cw6–positive patients (Figure 2C). Six months after the index date, 94.8% of the overall patients achieved PASI75, which was maintained until month 42 (Supplementary Figure 1a). A similar trend was seen in the biologic-naïve and biologic-experienced patients (Supplementary Figure 1b).
Figure 2.
Proportion of patients still on treatment with secukinumab after 42 months: (A) Cumulative proportion of patients. (B) Biologic-naïve and biologic-experienced patients. (C) HLA-Cw6–positive and HLA-Cw6–negative patients.
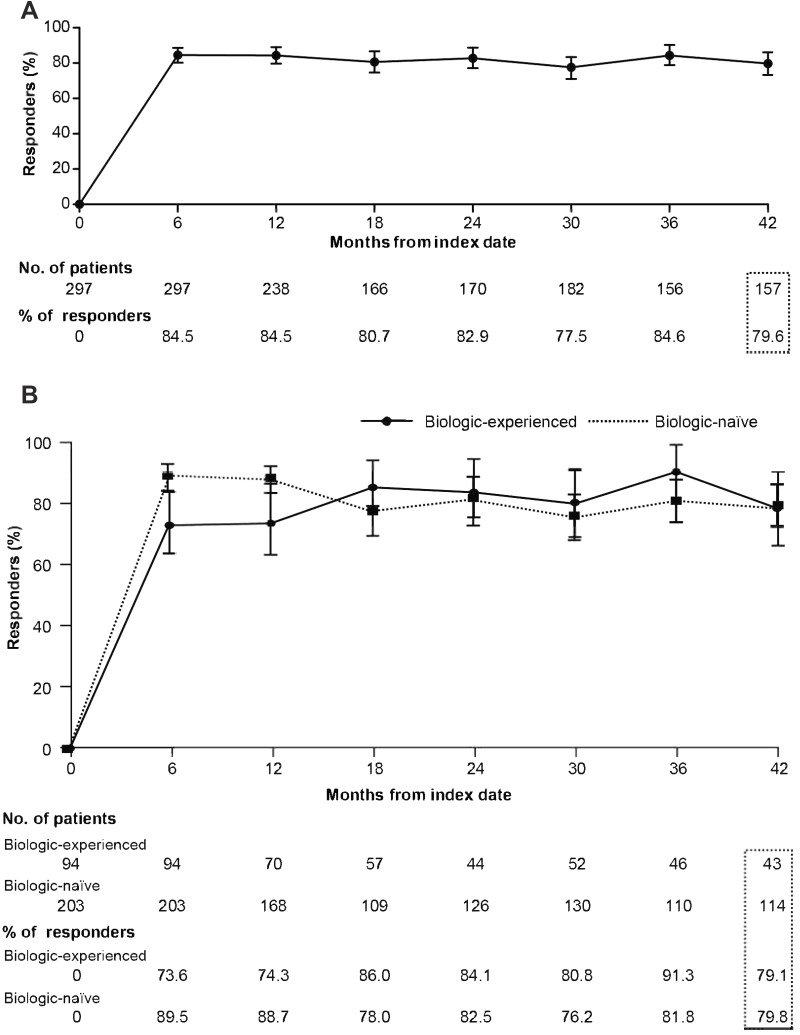
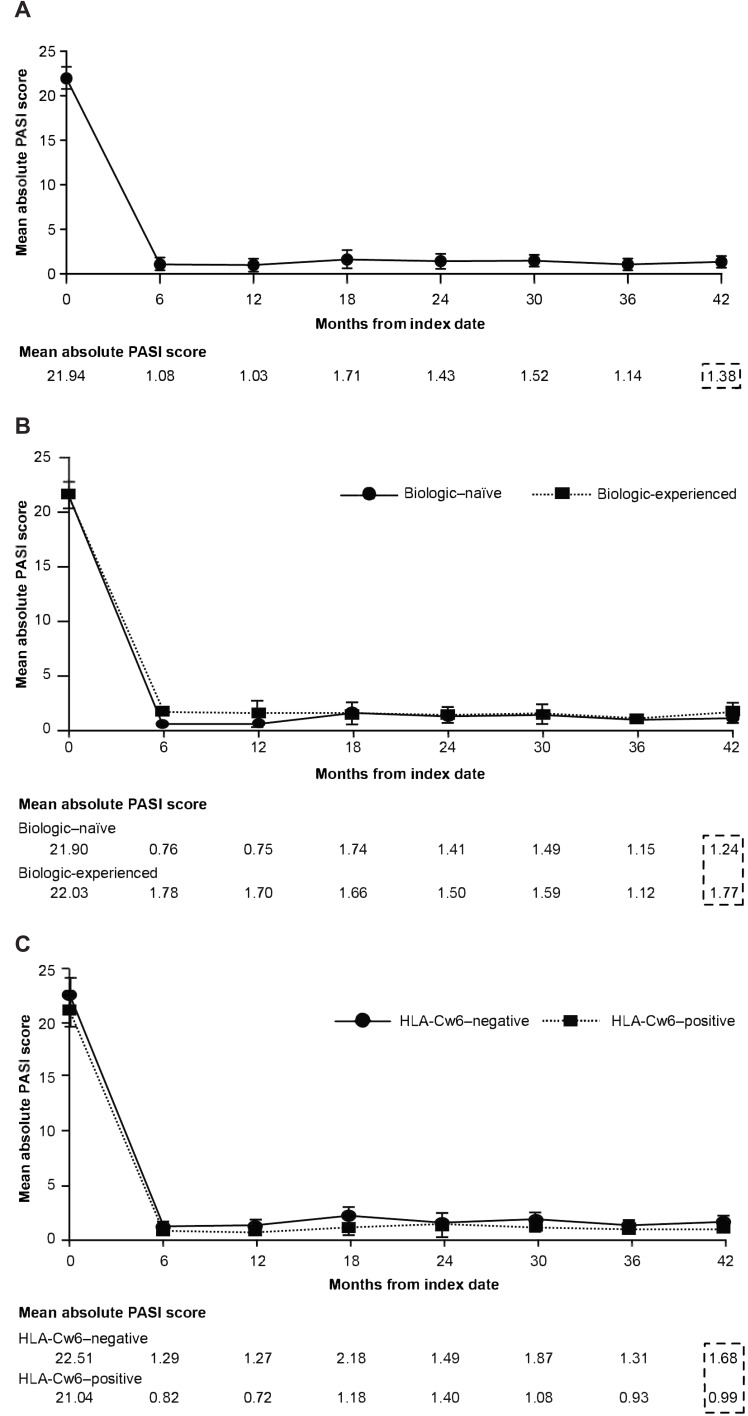
Similarly, improvements in PASI 90 and 100 responses with secukinumab treatment were observed at 6 months and 42 months after the index date in the overall patients as well as in the biologic-naive and biologic-experienced patients (Figure 3 and Supplementary Figure 2). The mean baseline absolute PASI score decreased from 21.94 to 1.38 after 42 months in the overall patients (Figure 4A), with similar decrease in the biologic-naïve and biologic-experienced patients (Figure 4B and C).
Figure 3.
Proportion of patients with a PASI90 response over time: (A) Cumulative proportion of patients. (B) Biologic-naïve and biologic-experienced patients.
Abbreviation: PASI, Psoriasis Area and Severity Index.
Figure 4.
Mean values of the absolute PASI scores over time: (A) Cumulative proportion of patients. (B) Biologic-naïve and biologic-experienced patients. (C) HLA-Cw6–negative and HLA-Cw6–positive patients.
Abbreviation: PASI, Psoriasis Area and Severity Index.
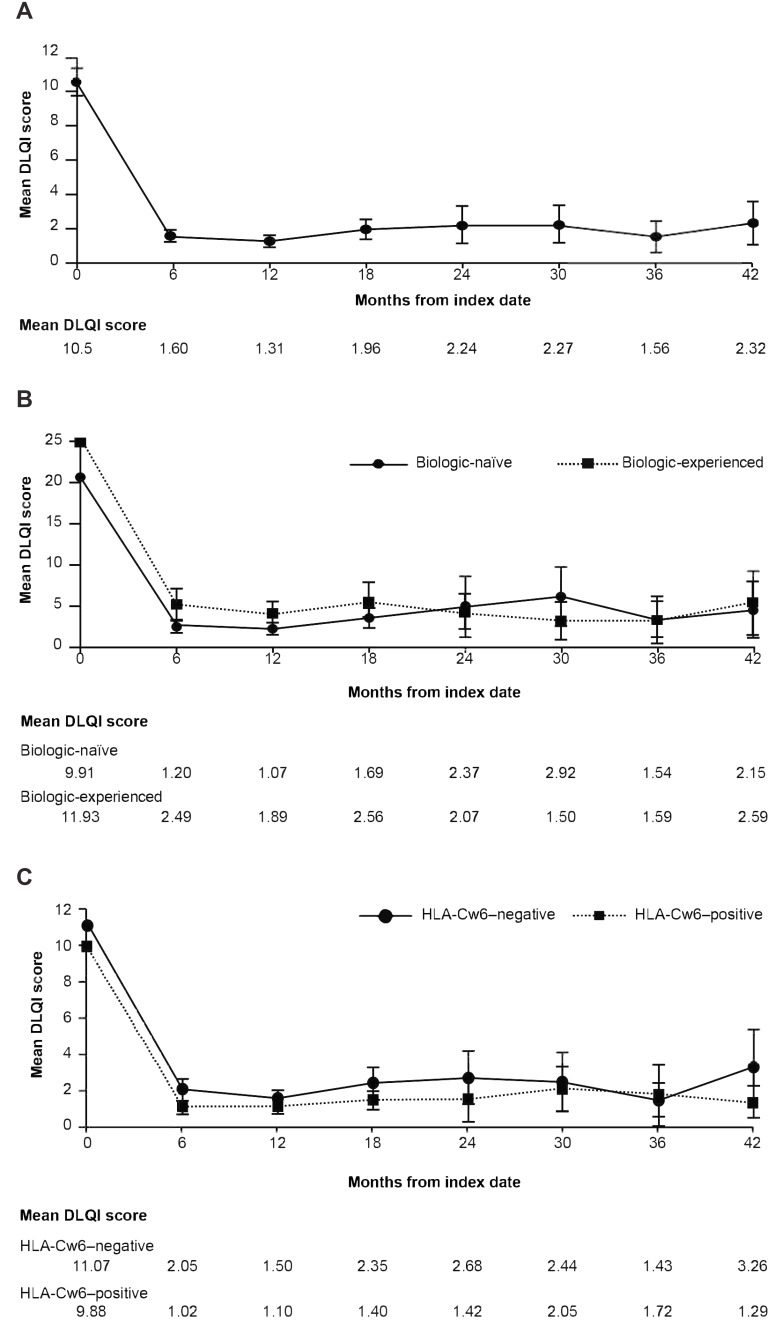
The improvement (ie reduction) in the mean baseline DLQI with secukinumab treatment was also seen after 42 months from the index date in the overall patients as well as across study cohorts (Figure 5).
Figure 5.
Mean values of DLQI over time: (A) Cumulative proportion of patients. (B) Biologic-naïve and biologic-experienced patients. (C) HLA-Cw6–negative and HLA-Cw6–positive patients.
Abbreviation: DLQI, Dermatology Life Quality Index.
The change from baseline in absolute PASI score at week 60/72 within the RCT settings (ie patients completed week 60/72 during the SUPREME study) was comparable to that assessed in the real-world settings (ie patients who completed the SUPREME study at any time before week 60/72 visit and enrolled in SUPREME 2.0) (Supplementary Table 2).
Safety
In total, 226 (76.1%) patients experienced at least one AE after the index date. The overall summary of AEs and SAEs is presented in Table 2. Overall, 72 (24.2%) patients experienced an AE related to the study drug, 17 patients (5.7%) discontinued the study drug because of an AE, and five patients (1.7%) suffered from coronavirus 2019 (COVID-19) during this study. In the entire study cohort, 48 patients (16.2%) experienced SAEs. Five patients experienced an SAE that led to discontinuation of the study drug: one each had ulcerative colitis, lung cancer, breast cancer, major depression and pulmonary mass related to respiratory, thoracic and mediastinal disorders. The patients who experienced SAE of ulcerative colitis and major depression had a medical history of conditions associated with the event, whereas other patients had a new onset of the event.
Table 2.
Patients with AEs and SAEs Leading to Discontinuation of the Study Drug
| n (%) | Overall Patient Cohort N=297 |
|---|---|
| AEs | 226 (76.1) |
| SAEs | 48 (16.2) |
| AE related to the study drug | 72 (24.2) |
| AE leading to study drug discontinuation | 17 (5.7) |
| Stomatitis | 1 (0.3) |
| Large intestine polyp | 1 (0.3) |
| Colitis ulcerative | 1 (0.3) |
| Rash pustular | 2 (0.7) |
| Upper respiratory tract infection | 1 (0.3) |
| Respiratory tract infection | 1 (0.3) |
| Infusion-related reaction | 1 (0.3) |
| Psoriatic arthropathy | 1 (0.3) |
| Lung neoplasm malignant | 1 (0.3) |
| Breast cancer | 1 (0.3) |
| Major depression | 1 (0.3) |
| Pulmonary mass | 1 (0.3) |
| Psoriasis | 2 (0.7) |
| Papule | 1 (0.3) |
| Dermatitis exfoliative | 1 (0.3) |
| Dermatitis atopic | 1 (0.3) |
| Skin exfoliation | 1 (0.3) |
| Hypertension | 1 (0.3) |
| SAE leading to study drug discontinuation | 5 (1.7) |
| Colitis ulcerative | 1 (0.3) |
| Lung cancer | 1 (0.3) |
| Breast cancer | 1 (0.3) |
| Major depression | 1 (0.3) |
| Pulmonary mass | 1 (0.3) |
Abbreviations: AE, adverse event; N, number of patients in the study cohort; n, number of patients with at least one AE in the category; SAE, serious AE.
Discussion
This observational, chart-review cohort study aimed to evaluate the patients in a real-world clinical setting after their participation in a RCT. Moreover, this real-world study provides long-term follow-up data on drug survival among the Italian population with psoriasis receiving secukinumab.
In this study, after an observation period of 42 months, the majority of the population (70.7%) was still on secukinumab treatment, demonstrating high long-term drug survival with secukinumab in real-world clinical practice. These results were in line with those reported in a real-world retrospective study (78.8%),17 although the follow-up period in this report (18 months) was shorter than that in the SUPREME 2.0 study. In yet another study with secukinumab, the patients demonstrated relatively lower drug persistence (41.7%) as compared to the current study; the follow-up period in SUPREME 2.0 was longer than the reported study.18 A greater proportion of biologic-naïve patients who demonstrated long-term drug survival with secukinumab continued secukinumab after 42 months than those who received biologics previously, which is in line with the previous RCTs, real-world, observational studies.18–20
The long-term drug survival with secukinumab at 42 months was nonetheless similar between HLA-Cw6–positive and HLA-Cw6–negative patients. Although HLA-Cw6 status is known to influence the treatment response, secukinumab, unlike ustekinumab20,21 and adalimumab,22,23 exhibited efficacy among patients with PsO irrespective of their HLA-Cw6 status. Reports on HLA-Cw6 status and drug efficacy are divergent, with most studies indicating that HLA-Cw6–positive patients showed a faster and increased response to ustekinumab20,21 and adalimumab22 compared to HLA-Cw6–negative patients. Conversely, in another study, adalimumab demonstrated better response in the HLA-Cw6–negative patients and that there was no noteworthy benefit in response to adalimumab over ustekinumab in the HLA-Cw6–positive patients.23 However, reports on the correlation between drug survival and HLA-Cw6 status are sparse.24
The drug survival rate reported in the SUPREME 2.0 real-world study was comparatively higher than that in previous real-world reports.17,19 This could be partly attributed to the smaller proportion of biologic-experienced patients who are more resistant to the treatment. The previous real-world reports suggested that secukinumab had similar sustained drug survival at 1 and 2 years as ustekinumab,25 and superior pooled drug survival at 1 year compared with ixekizumab.26 However, the results of the SUPREME 2.0 real-world study indicated that the real-world drug survival of secukinumab is higher than that reported previously.25,27 In this study, the proportion of biologic-naïve patients who achieved PASI75/90/100 responses was higher versus biologic-experienced patients. Previous studies suggested that PASI90 response rates were sustained from year 1 to year 5 in patients treated with secukinumab,28 and the response rates were comparatively higher than those reported with ustekinumab and etanercept treatment, although the design and time points of evaluation were different., The safety profile of secukinumab in the SUPREME 2.0 study was consistent with its known safety and tolerability profile. No new or unexpected safety concerns were identified during the long-term observation period. There was no change in the known frequency of AEs that would alter the current benefit-risk profile of secukinumab. This mixed design study provided additional information on treatment response in a real-world clinical setting. To date, no studies have used such a mixed design. Using this mixed design, our analysis demonstrated no difference in effectiveness between the controlled and RWE treatment settings.
The study was subject to the limitations inherent in retrospective chart reviews. Information collected using the electronic case report form was limited to what was available in the medical records at the participating centres. Patients in SUPREME 2.0 were previously enrolled in the randomized controlled trial SUPREME, which might be a source of selection bias. The SUPREME study used inclusion/exclusion criteria that were comparable to those used in real-world clinical practice; patients enrolled in the study were similar to the general PsO patient population in terms of severity and comorbidities. In addition, the COVID-19 pandemic influenced this investigation and hindered the recruitment of potential patients as per the initial study plan. Furthermore, five research centres were unable to engage actively in the research owing to administrative issues during the health emergency. The current study investigated the patients enrolled in an interventional clinical trial to assess long-term drug survival with secukinumab and may not necessarily be representative of all patients with moderate to severe chronic plaque-type PsO.
Conclusion
After a minimum observation period of 42 months of treatment, secukinumab demonstrated a high drug survival rate with a low incidence of permanent treatment discontinuation; survival rates were higher in the biologic-naïve than in the biologic-experienced patients and were similar between HLA-Cw6–positive and HLA-Cw6–negative patients. Furthermore, secukinumab demonstrated long-term effectiveness with no difference in percentage responders in biologic-naïve and biologic-experienced cohorts, coupled with an increase in the patients’ quality of life across biologic-naïve/biologic-experienced and HLA-Cw6–positive/HLA-Cw6–negative cohorts. No difference in drug effectiveness was observed between the clinical trial (SUPREME study) and real-world study (SUPREME 2.0). The safety profile of secukinumab was consistent with its established safety profile without any new safety signals.
Acknowledgments
The authors thank Esha Chakraborty, Jitendriya Mishra, Debashree De, Ramji Narayanan and Mansi Deshwal of Novartis for providing medical writing and editorial support, which was funded by Novartis Farma SpA, Milan Italy, in accordance with the Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Funding Statement
The study was funded by Novartis Farma SpA, Italy.
Abbreviations
AE, adverse event; CI, confidence intervals; COVID-19, coronavirus 2019; GCP, Good Clinical Practice; DLQI, Dermatology Life Quality Index; HLA, human leucocyte antigen; MMRM, mixed model for repeated measures; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; RCT, randomised controlled trial; RWE, real world evidence; SAE, serious AE; TNF, tumour necrosis factor.
Data Sharing Statement
The data sets generated and/or analysed during the current study are not publicly available. Novartis is committed to sharing access to patient-level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved based on scientific merit. All data provided are anonymised to respect the privacy of the patients who participated in the trial, in line with applicable laws and regulations. Data may be requested from the corresponding author of the manuscript.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines and in compliance with all federal, local or regional requirements. The institutional review board/ethics committee of each participating centre approved the study protocol. All patients provided consent to participate in the studies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
F. Russo has acted as a speaker/consultant for AbbVie, Novartis and Sanofi; M. Galluzzo has acted as a speaker and/or consultant for AbbVie, Almirall, Eli Lilly, Janssen-Cilag, LeoPharma, Novartis and Sanofi; L. Stingeni has acted as a speaker and board member for AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LeoPharma, Novartis and Sanofi; A. Conti has acted as a consultant for AbbVie, Abbott, Amgen, Celgene, Eli Lilly, Janssen Cilag, Leo Pharma, MSD, Novartis, Sandoz, Schering Plough, UCB Pharma and Wyeth; F. Bardazzi has acted as a speaker and/or consultant for Almirall, AbbVie, Celgene, Eli Lilly, Janssen, Leo Pharma and UCB Pharma; G. Girolomoni has received personal fees from AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli-Lilly, Fresenius Kabi, Galderma, Genzyme, Leo Pharma, Novartis, Pfizer, Regeneron, Samsung bioepis, Sanofi and UCB; M. Venturini served as a speaker or advisory board member for AbbVie, Almirall, Amgen, Eli Lilly, Galderma, LeoPharma, Novartis, Pierre Fabre and UCB Pharma; M.C. Fargnoli has served on advisory boards, received honoraria for lectures and received research grants from AbbVie, Almirall, Amgen, BMS, Galderma, Janssen, Kyowa Kyrin, Leo Pharma, Lilly, MSD, Novartis, Pfizer, Pierre Fabre, Sanofi-Regeneron, Sunpharma and UCB; M.L. Musumeci has served as a consultant/investigator for AbbVie, Biogen, Eli Lilly, Janssen Cilag and Novartis; P. Malleoli has acted as a consultant or had advisory board agreements for AbbVie, Almirall, Amgen, Eli Lilly, Janssen, Leo Pharma, Novartis and UCB Pharma; F. Loconsole reports no conflicts of interest; A. Offidani has received honoraria as speaker, scientific board member and consultant from AbbVie, Eli Lilly, Galderma, Leo Pharma, Novartis, Pfizer, Sanofi and UCB Pharma; V. Di Lernia has received honoraria as speaker, consultant or advisory board member from AbbVie, Amgen, Janssen and Novartis; Iris Zalaudek has received honoraria for lectures, advisory boards from Almirall, Novartis, MSD, BMS, Philogen, Sunpharma, Sanofi, Celgene, Kyowa Kyrin, Mallinckrodt, Janssen and Eli Lilly; G. Gigante, M. Bartezaghi, P. Ursoleo and E. Aloisi are employees of Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(2):ii30–6. doi: 10.1136/ard.2004.031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin DA, Towne JE, Kricorian G, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133(1):17–26. doi: 10.1038/jid.2012.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S2–6. [PubMed] [Google Scholar]
- 4.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32(1):227–255. doi: 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with Psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118(2):362–365. doi: 10.1046/j.0022-202x.2001.01656.x [DOI] [PubMed] [Google Scholar]
- 6.Burlando M, Russo R, Clapasson A, et al. The HLA-Cw6 Dilemma: is it really an outcome predictor in psoriasis patients under biologic therapy? A monocentric retrospective analysis. J Clin Med. 2020;9(10):3140. doi: 10.3390/jcm9103140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, DePrimo S, Chen Y, Li S, Munoz-Elias E. 451 Association between HLA-Cw6 status and response to guselkumab in patients with moderate to severe plaque psoriasis. Int Invest Dermatol. 2018;138(5):S76. [Google Scholar]
- 8.Li K, Huang CC, Randazzo B, et al. HLA-C*06:02 allele and response to il-12/23 inhibition: results from the ustekinumab phase 3 psoriasis program. J Invest Dermatol. 2016;136(12):2364–2371. doi: 10.1016/j.jid.2016.06.631 [DOI] [PubMed] [Google Scholar]
- 9.Temel B, Adisen E, Gonen S. HLA-Cw6 status and treatment responses between psoriasis patients. Indian J Dermatol. 2021;66(6):632–637. doi: 10.4103/ijd.IJD_282_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vugt LJ, van den Reek J, Meulewaeter E, et al. Response to IL-17A inhibitors secukinumab and ixekizumab cannot be explained by genetic variation in the protein-coding and untranslated regions of the IL-17A gene: results from a multicentre study of four European psoriasis cohorts. J Eur Acad Dermatol Venereol. 2020;34(1):112–118. doi: 10.1111/jdv.15787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 12.Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 13.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 14.Sigurgeirsson B, Schakel K, Hong CH, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. J Dermatol Treat. 2021;32(1):1–9. doi: 10.1080/09546634.2021.1902925 [DOI] [PubMed] [Google Scholar]
- 15.Costanzo A, Bianchi L, Flori ML, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: SUPREME study. Br J Dermatol. 2018;179(5):1072–1080. doi: 10.1111/bjd.16705 [DOI] [PubMed] [Google Scholar]
- 16.Papini M, Cusano F, Romanelli M, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: results from extension phase of the SUPREME study. Br J Dermatol. 2019;181(2):413–414. doi: 10.1111/bjd.18013 [DOI] [PubMed] [Google Scholar]
- 17.Torres T, Balato A, Conrad C, et al. Secukinumab drug survival in patients with psoriasis: a multicenter, real-world, retrospective study. J Am Acad Dermatol. 2019;81(1):273–275. doi: 10.1016/j.jaad.2019.02.031 [DOI] [PubMed] [Google Scholar]
- 18.Lee EB, Amin M, Egeberg A, Wu JJ. Drug survival of secukinumab for psoriasis in a real-world setting. J Dermatol Treat. 2019;30(2):150–151. doi: 10.1080/09546634.2018.1473838 [DOI] [PubMed] [Google Scholar]
- 19.Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–519. doi: 10.1111/bjd.16102 [DOI] [PubMed] [Google Scholar]
- 20.Talamonti M, Botti E, Galluzzo M, et al. Pharmacogenetics of psoriasis: HLA-Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br J Dermatol. 2013;169(2):458–463. doi: 10.1111/bjd.12331 [DOI] [PubMed] [Google Scholar]
- 21.Talamonti M, Galluzzo M, Chimenti S, Costanzo A. HLA-C*06 and response to ustekinumab in Caucasian patients with psoriasis: outcome and long-term follow-up. J Am Acad Dermatol. 2016;74(2):374–375. doi: 10.1016/j.jaad.2015.08.055 [DOI] [PubMed] [Google Scholar]
- 22.Coto-Segura P, Gonzalez-Lara L, Batalla A, Eiris N, Queiro R, Coto E. NFKBIZ and CW6 in adalimumab response among psoriasis patients: genetic association and alternative transcript analysis. Mol Diagn Ther. 2019;23(5):627–633. doi: 10.1007/s40291-019-00409-x [DOI] [PubMed] [Google Scholar]
- 23.Dand N, Duckworth M, Baudry D, et al. HLA-C*06:02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol. 2019;143(6):2120–2130. doi: 10.1016/j.jaci.2018.11.038 [DOI] [PubMed] [Google Scholar]
- 24.Zorlu O, Bulbul Baskan E, Yazici S, et al. Predictors of drug survival of biologic therapies in psoriasis patients. J Dermatol Treat. 2022;33(1):437–442. doi: 10.1080/09546634.2020.1763240 [DOI] [PubMed] [Google Scholar]
- 25.Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British association of dermatologists biologics and immunomodulators register (BADBIR). Br J Dermatol. 2020;183(2):294–302. doi: 10.1111/bjd.18981 [DOI] [PubMed] [Google Scholar]
- 26.Mourad AI, Gniadecki R. Biologic drug survival in psoriasis: a systematic review & comparative meta-analysis. Front Med Lausanne. 2020;7:625755. doi: 10.3389/fmed.2020.625755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzimichail G, Gunther J, Stander S, Thaci D. Drug survival of secukinumab, ustekinumab, and certolizumab pegol in psoriasis: a 2-year, monocentric, retrospective study. J Dermatol Treat. 2022;33(3):1749–1753. doi: 10.1080/09546634.2020.1854428 [DOI] [PubMed] [Google Scholar]
- 28.Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514. doi: 10.1111/jdv.14878 [DOI] [PMC free article] [PubMed] [Google Scholar]