Abstract
Escherichia coli WP2 bacteria with an ochre amino acid auxotrophy show no evidence of growth during the first few days after plating at densities above 108 on plates lacking the required amino acid. They lose viability for some days, and then a subpopulation recovers and there is cell turnover. At very low plating densities (around 102 per plate), almost every cell will eventually form a small but visible colony. At intermediate plating densities (106 to 107 per plate), there is an immediate increase in the number of viable bacteria. The results are consistent with a model that assumes that growth is dependent on trace amounts of tryptophan or a tryptophan-complementing substance and that death is due to extracellular toxic species in the medium, including active oxygen species. Mutations in mutT bacteria under these conditions result from incorporation of 7,8-dihydro-8-oxo-dGTP into DNA and thus largely reflect DNA synthesis associated with the increase in the number of viable cells at the initial density used (107 per plate). We show that the increase in cell number and much of this DNA synthesis can be eliminated by the presence of 108 scavenger bacteria and by removal of early-arising mutant colonies that release the required amino acid. The synthesis that remains is equivalent to less than a quarter of a genome per day and is marginally reduced, if at all, in a polA derivative. We cannot exclude the possibility that this residual DNA synthesis is peculiar to mutT bacteria due to transcriptional leakiness, although there is no evidence that this is a major problem in this strain. If such DNA synthesis also occurs in wild-type bacteria, it may well be important for adaptive mutation since use of a more refined agar in selective plates both eliminated the initial increase in cell number seen at low density (107 per plate) and reduced the rate of appearance of mutants at plating densities above 108 per plate.
The ability of bacteria to undergo spontaneous mutation under selection conditions, e.g., when deprived of a required amino acid or when presented with an energy source that they cannot metabolize, has received attention recently because of the suggestion that the mutations that arise are in some way directed or adaptive (9, 13, 14). Whether or not the latter suggestion is justified, it is certainly true that mutation processes under starvation conditions are different in many respects from those in growing cells (12). Foster (12) has also pointed out that the rate of mutation under such conditions is too high to be accounted for by the amount of DNA synthesis that is believed to take place (the equivalent of between 0.005 and 0.05 genomes per cell per day) if it is assumed that DNA synthesis in resting cells involves the whole genome and has an overall error rate similar to that of DNA replication in growing cells. This implies that some type of hypermutability operates in resting cells under selection conditions. Estimates of DNA synthesis under starvation conditions using labelled precursor are, however, subject to a potential error of underestimation if synthesis preferentially utilizes products of DNA and RNA breakdown in preference to the exogenous precursor.
Escherichia coli bacteria normally contain MutT protein that hydrolyzes an oxidation product of GTP, 7,8-dihydro-8-oxo-dGTP (8-oxo-dGTP), present in the nucleotide triphosphate pool. If not removed, this molecule may be incorporated into DNA instead of cytosine and so generate transversion mutations from A:T to C:G (10, 15, 19). In mutT bacteria, 8-oxo-dGTP is not removed from the pool and the rate of appearance of such mutations in growing cultures directly reflects the amount of DNA synthesized. When spontaneous mutation experiments were carried out under conditions of amino acid starvation with mutT strains, the rate of mutation was found to be high, implying that there was considerably more DNA synthesis under the starvation conditions used than might have been assumed (5). The present paper reports further examination of this phenomenon and its implications for mutation experiments under starvation conditions.
MATERIALS AND METHODS
The strains of bacteria used are described in Table 1.
TABLE 1.
Strains of E. coli used
| Strain | Relevant genotype | Source |
|---|---|---|
| WP2 | trpE65 | E. M. Witkin |
| WP6 | As WP2 but polA1 | E. M. Witkin |
| WU3610 | tyrA14 leu308 | E. M. Witkin |
| CC101T | mutT::Kmr | H. Maki |
| CM1339 | As WP2 but mutT::Kmr | P1 (CC101T) × WP2 |
| CM1377 | As WP6 but mutT::Kmr | P1 (CC101T) × WP6 |
For measurement of mutation under starvation conditions, bacteria from an overnight broth culture were centrifuged, washed, and resuspended in phage buffer (1), and aliquots containing in excess of 108 bacteria were plated on the surfaces of at least four minimal agar plates (11) supplemented with 0.4% glucose. Unless otherwise stated, plates were solidified with 1.5% conventional Difco Bacto or Lucas-Meyer agar. The plates were incubated at 37°C, and colonies were counted daily. The same method was used with mutT bacteria, where the appearance of mutants was taken as reflecting incorporation of 8-oxo-dGTP into DNA. In this case, the initial plating density was around 107 per plate. Where stated, 108 scavenger bacteria were also plated at the same time, and any colonies from preexisting mutants that had formed by the second day were removed with a cork borer. Control plates with scavenger bacteria alone were also plated.
Spontaneous mutation rates were determined by the method of Newcombe (16), in which the mutation rate is estimated from the number of mutants arising during growth on plates containing a small amount of the auxotrophic requirement. Around 106 bacteria grown overnight with shaking in Oxoid nutrient broth were plated on minimal agar plates containing either no tryptophan or a very low level. In the first experiment this was 0.1 μg per ml, and in two subsequent experiments it was 0.01 μg per ml. The number of viable auxotrophs resulting from growth on the tryptophan supplement was determined by washing off additional parallel plates with 10 ml of buffer after 10 h of incubation at 37°C, diluting the samples, and plating them on L-agar plates. The mutation rate α is given by M = Mo + α(N − No)/ln2, where Mo and M are the numbers of prototrophic colonies per unsupplemented plate and per tryptophan-supplemented plate, respectively, α is the mutation rate per bacterium per division cycle, and No and N are the average numbers of viable auxotrophs at the beginning and end of growth, respectively. Mutant counts were based on five plates in the first experiment and four plates in the two subsequent experiments.
To follow the fate of bacteria on starvation plates, any mutant colonies were removed with a cork borer and bacteria were washed off with 10 ml of phage buffer. Total bacterial counts were determined in a Thoma counting chamber, and viable counts were determined by diluting and plating on L-agar plates. Viable counts of mutT bacteria in the presence of scavenger bacteria were determined on plates containing kanamycin (50 μg/ml). Preliminary experiments established that recovery of a known number of bacteria from plates in this way was not significantly different from 100%.
RESULTS
Experiments with mutT+ bacteria.
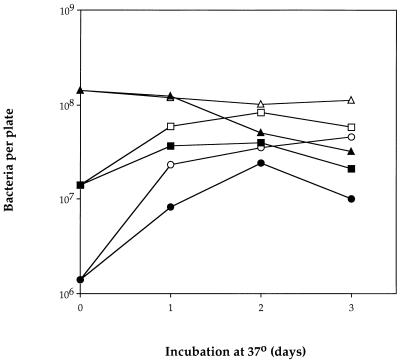
The experiments previously reported to show a high mutation rate for mutT bacteria under starvation conditions (5) were carried out with derivatives of strains WU3610, which carries an ochre mutation in tyrA, and IC3126, which carries a missense mutation in trpA. Similar results (unpublished) had been obtained with strain WP2, which has an ochre mutation in trpE. All of these strains are derivatives of E. coli B. Ochre auxotrophies are generally regarded as “tight,” although when around 100 bacteria of any of the above strains are plated in the absence of the required amino acid, small colonies can be seen with the naked eye after a week or so (unpublished observation). When WU3610 is plated at a cell density of around 3 × 108 per plate, its viability generally declines over the first 2 or 3 days, although after a period of 2 to 3 weeks there is evidence of some cell turnover and regrowth (2). Because of the high frequency of spontaneous mutants in overnight cultures of mutT bacteria, it was necessary to use a lower plating density (around 107 per plate) for the previously reported starvation mutation experiments. The assumption was made that the decline in viability at 107 per plate would not be significantly different from that observed at 3 × 108 per plate. This assumption has proved to be unjustified. We have measured total and viable counts of WU3610 and WP2 at different plating densities on glucose minimal plates made with Difco Bacto or Lucas-Meyer agar. In general, when WP2 was plated at around 108 cells per plate in the absence of tryptophan, the total count remained constant for 3 days; the viable count was unchanged for 24 h but then began to decline. At around 107 cells per plate, the total and viable counts increased over the first 48 h. The total count peaked at around 8 × 107 per plate, and the viable count peaked at 4 × 107 per plate and then declined. At around 106 per plate, the initial phase of cell division was even more apparent, although viability again declined after 48 h. These features were apparent in numerous experiments (not all of which included all plating densities). A representative experiment with all three densities is shown in Fig. 1.
FIG. 1.
Viable (solid symbols) and total (open symbols) counts of E. coli WP2 plated at different densities on tryptophan starvation plates made with conventional (Lucas-Meyer) agar and washed off after 1, 2, and 3 days of incubation at 37°C (representative experiment).
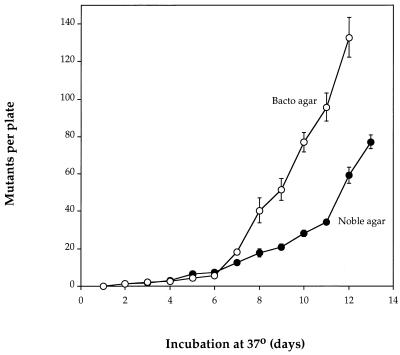
Similar results were obtained with WU3610 starved for tyrosine, where the increase in cell number at intermediate cell density was found to depend on a new gene, tas, that is able partially to complement the tyrA defect (21). We believe the increase in WP2 is due to traces of tryptophan or some other impurity in conventional agar (Difco Bacto or Lucas-Meyer) since on Difco Noble agar there was no evidence of any cell division at low cell density over the first 3 days. Moreover, viability began to decline from the moment of plating at all densities, although at the lowest density there was evidence of an increase in the number of viable bacteria on the third day (data not shown). When starvation plates made with Difco Noble agar were loaded with 2 × 108 WP2 bacteria and incubated and scored for the appearance of starvation-associated (stationary-phase) mutations, it was noted that despite the initial loss of viability, by 8 days there was a clear lawn of growth. Clearly a subpopulation had survived and increased in numbers. This subpopulation was able to undergo starvation-associated mutation, but the appearance of mutants was delayed by some days compared with the same culture on plates made with conventional agar (Fig. 2).
FIG. 2.
Appearance of starvation-associated mutants of E. coli WP2 as a function of time of incubation at 37°C on tryptophan starvation plates made with either Difco Noble agar or conventional Bacto agar (2 × 108 bacteria per plate). Points represent means and standard errors of three experiments.
Experiments with mutT bacteria.
We have thus established that at a density of around 107 WP2 bacteria per plate, there is substantial cell division over the first 48 h on minimal plates made with conventional Difco Bacto or Lucas-Meyer agar. The chromosomal replication associated with this must contribute significantly to the DNA synthesis responsible for the generation of A:T-to-C:G transversion mutations in the previously reported experiments with the mutT strains. In contrast, the results for WP2 show that at 108 bacteria per plate there is no discernible increase in either total or viable count, presumably because the tryptophan-complementing impurity is insufficient to allow detectable growth and is rapidly metabolized.
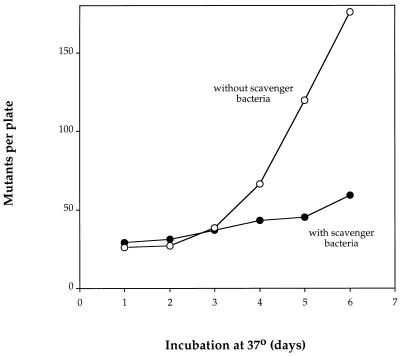
We therefore used a population of 108 WP2 bacteria to scavenge the impurity and followed the generation of transversion mutations in a population of CM1339 (a mutT derivative of WP2) mixed with them. The spontaneous mutation rate of WP2 is such that less than one preexisting prototrophic mutant is normally present in a plating of 108 bacteria, and mutants arising postplating are slow growing and have not appeared by 5 days. In these experiments, CM1339 bacteria were plated at around 107 per plate together with 108 WP2 cells. The number of mutants per plate was monitored up to 5 days of incubation at 37°C, and the bacteria on parallel plates were washed off for estimation of total and viable counts. The number of viable CM1339 cells was measured on kanamycin plates since the scavenger bacteria are sensitive to this antibiotic and only CM1339 forms colonies. It was immediately apparent from this first experiment that the presence of scavenger bacteria dramatically reduced the number of mutants arising in CM1339 (Fig. 3). As with WP2, there was no increase in viable count in the presence of scavengers and a large (in this experiment, 10-fold) increase in viable count in the absence of scavengers over the first 3 days in this experiment (data not shown).
FIG. 3.
Appearance of mutants in 107 cells of the mutT strain CM1339 as a function of time of incubation on tryptophan starvation plates at 37°C in the presence of 108 cells of strain WP2.
A set of experiments was therefore carried out to quantify the rate of mutation against the number of viable bacteria on the plate. Viability of CM1339 was found to decline slightly to around 40 to 50% after 2 days rather than increase as was observed in the absence of scavengers. The number of mutants counted on day 2 was subtracted from the number on day 5 and taken to be the number of transversion mutants arising on the plate between day 0 and day 3, allowing 2 days for the mutants to grow up and be counted. We have normalized this against the number of viable bacteria on the plate on day 2. The results are shown as set A in Table 2.
TABLE 2.
Appearance of tryptophan-independent mutants of CM1339 (mutT) and CM1377 (mutT polA) over 5 days on glucose minimal plates reflecting DNA synthesisa
| Strain | Mean mutants/plate on day:
|
Mean Y − X | Mean viable bacteria/plate on day 2 ± SE (106) | Mean Y − X/viable cell ± SE (10−6) [no. of expts] | |
|---|---|---|---|---|---|
| 2 (X) | 5 (Y) | ||||
| CM1339 (set A) | 19.6 | 59.9 | 40.3 | 5.78 ± 1.34 | 6.82 ± 1.22 [4] |
| CM1339 (set B) | 13.9 | 24.1 | 9.7 | 8.34 ± 2.56 | 1.37 ± 0.48 [6] |
| CM1377 (set B) | 12.0 | 17.9 | 5.34 | 6.75 ± 2.3 | 0.91 ± 0.38 [4] |
The mean initial plating density was close to 107 plus 108 scavenger bacteria of the kanamycin-sensitive strain WP2. Colonies existing on day 2 were those existing in the culture at the time of plating. In set B, these were removed with a cork borer. The number of viable cells of CM1339 or CM1377 was determined for a parallel set of plates by removing any mutant colonies, washing the bacteria off, and plating them on kanamycin plates. In some experiments, one or two mutants were seen on control plates with scavenger bacteria only. These were subtracted from the experimental plate numbers to give the values presented. The means in columns 2 to 5 are of individual experiments carried out on different days with different batches of plates. To obtain meaningful standard errors for the mutation rate, the values in column 6 are the means of the ratios for the individual experiments and not the ratios of the means which are marginally different.
After the first four such experiments, a further refinement was introduced. We observed that there was a tendency for some mutants appearing on days 4 and 5 to be situated close to mutant colonies that had appeared by day 2 (satellite colonies), suggesting that there might be some cross-feeding of the lawn by the early-arising colonies. In a second series of experiments (set B in Table 2), we therefore removed colonies that appeared on days 1 and 2 with a cork borer as soon as they appeared. This refinement eliminated satellite colonies, and the rate of mutation per viable cell was reduced from 6.82 × 10−6 to 1.37 × 10−6 (Table 2). We suggest that this latter figure is a close approximation to the number of misincorporation mutants arising over 3 days due to DNA turnover when 107 nongrowing bacteria of the mutT strain CM1339 are incubated in the absence of tryptophan. How much DNA replication is implied by this figure? The mutation rate α of CM1339 (the mutT derivative of WP2) growing on plates was estimated in three independent experiments using the method of Newcombe (16) and was found to be 6.83 × 10−7 (standard error = 0.48 × 10−7).
If 1.37 mutations arise per 106 bacteria over 3 days under starvation conditions, then 1.37/0.683 or 2.01 generation-equivalents of synthesis occur during this time, or 0.669 per day. This estimate depends, however, on the assumption that the proportions of 8-oxo-dGTP in the dGTP pool are similar in growing and in starved bacteria. Indirect evidence suggests that there may be three times as much in starved cells (6). We may therefore reduce our estimate by a factor of 3 to take account of this. Our best estimate, therefore, is that at total cell densities of greater than 108 per plate, there may be 0.223 genomes (say, around one-quarter of a genome) of strain CM1339 replicated per day over the first 3 days at 37°C on plates lacking tryptophan despite the fact that there is no increase in total count and the viable count is declining. It can also be seen from Table 2 that the effect of a polA mutation was minimal. The number of mutations arising in the polA mutT strain CM1377 was reduced by a third compared with that arising in CM1339, but the difference was not statistically significant.
DISCUSSION
The results presented above show that in these derivatives of E. coli B plated on media lacking a required amino acid, analysis of DNA and cell turnover is a complex business. On plates made with conventional agar at plating densities of around 107 bacteria per plate and below, the total and viable counts increase to nearly 108 per plate over the first 20 to 30 h, after which the total count remains constant and the viable count gently declines. In the case of the tryptophan requirer WP2, the increase in count over the first 3 days seems to be the result of impurities in the agar since it is not observed at higher plating densities or at lower densities when Difco Noble agar is used. Instead, an immediate decline in viability is apparent. By the third day this has stopped, and the viable count increases again so that by 7 days a thin but visible lawn has appeared. A simultaneous plating with 108 scavenger bacteria both prevents the initial increase in viable count and drastically reduces the rate of mutation in mutT bacteria.
The results over the first 3 days can be explained by a model which postulates (i) that growth on starvation plates is due to the presence of tryptophan (or a tryptophan-complementing contaminant) in conventional agar that can support growth to a level approaching 108 cells per plate, (ii) that loss of viability (as defined by the ability to form a colony on L agar) is due to membrane damage caused by components of the agar (these may include active oxygen species since the loss of viability in related strains WU3610, IC3126, and IC3742 is much reduced by the presence of catalase in the agar [3, 8]), and (iii) that the kinetics of growth and death reflect the balance between these two processes. Thus, at very low numbers of cells per plate, growth on the contaminating tryptophan exceeds the rate of cell death and the number of bacteria increases. At higher densities, the number of bacteria increases but more slowly as the tryptophan approaches exhaustion and the viable count falls below the total count until eventually death is more likely to happen than division. Above 108 per plate, the contaminating tryptophan is insufficient to give rise to detectable growth and only death is seen.
Beyond about 3 days there is a further complexity as a small subpopulation increases in number and begins to form a significant fraction of the total population as the original majority population loses viability. The emergence in stationary cultures of variants with an enhanced capacity for scavenging has been documented by Zambrano et al. (22).
We conclude that the initial increase in the number of viable bacteria accounts for much of the DNA synthesis that was reflected as mutation in mutT bacteria in a previous report. Of the remaining mutants appearing in mutT bacteria, a proportion is attributable to growth caused by nutrients leaching out of mature prototrophic colonies arising from established mutants that existed in the culture at the time of plating. This can be prevented by removing the colonies as soon as they appear. When this is done, the amount of DNA synthesis in mutT bacteria is estimated to be around one-quarter of a genome per day, although this figure depends on assumptions made concerning the extent of 8-oxo-dGTP contamination of the nucleotide triphosphate pool. It must be emphasized, however, that this residual rate of mutation occurs in a population in which there is no increase in cell number and where viability is gently declining. It may reflect DNA turnover in nondividing bacteria, but we cannot exclude the presence of a minority subpopulation in which growth is occurring.
This figure is around 10-fold lower than our first estimate (6) but is still around five times higher than the highest figure in the review by Foster (12). Most studies have shown little or no DNA synthesis in nondividing cells (reviewed in reference 6). Perhaps the most significant experiments were those of Tang and colleagues (20) in which growing cells were resuspended in buffer for 2 h to allow rounds of replication to be completed. After overnight refrigeration, they were then incubated in buffer at 37°C for a further 24 h. During this time there was no net change in cellular DNA content, but at least 20% of the genome was broken down and resynthesized. The synthesis was reduced by 90% in a polA mutant and was assumed to be repair synthesis. In the present work, the polA mutation had little effect. It should also be noted that the time scale of the present experiments was considerably longer than that in the experiments of Tang et al. (20).
The extrapolation of DNA turnover results from mutT to wild-type bacteria may be questioned in the light of new data showing that the transcriptional leakiness of an amber mutation in lacZ is greatly increased in a mutT background, an effect ascribed to the incorporation of 8-oxo-rGTP opposite adenine during transcription of the amber triplet (18). We have two reservations about the relevance of this observation to our system. First, the degree of leakiness in their study appears to be much greater than would be expected from the intracellular concentration of oxidized guanine residues (7). Second, whereas the leakiness of the lacZ allele in cells with a mutT background leads to a heavy lawn of growth after a few days on lactose plates even in the presence of scavenger bacteria, we have seen no visible difference in the lawns of strain WP2 and its mutT derivative after 1 to 2 weeks on minimal agar. Indeed, mutT bacteria lose viability in a similar manner to mut+ bacteria during the first few days under these conditions. It may well be that the lacZ allele is atypical and deserves further study. Nevertheless, we cannot exclude the possibility that transcriptional leakiness can lead to more DNA synthesis in mutT than in mut+ bacteria.
The implications of these results for stationary-phase mutation in mutT+ bacteria are not straightforward. The spectrum of mutants in starvation-associated mutation is different from that in growth-phase mutation, and so there can be no simple relation between the amount of DNA replicated and number of mutants produced. Evidence that many of the chromosomal mutations in these strains under starvation conditions are due to oxidized guanine residues in DNA has been presented (4, 8). In principle, residues such as 8-oxoguanine in the transcribed strand of DNA could cause miscoding errors during transcription and give rise to a transient prototrophic phenotype which could in turn trigger a round of DNA replication during which a miscoding could occur in newly synthesized DNA (2). 8-Oxoguanines at sites where miscoding was incapable of conferring a transient prototrophic phenotype would tend to be removed by MutM (Fapy) glycosylase before the next round of DNA turnover, and mutations at such sites would tend not to be detected. However, if there were indeed DNA turnover to the extent suggested by the present data, there would be adenines in the transcribed strands of the newly synthesized DNA opposite some of the 8-oxoguanine moieties in the parental nontranscribed strands. These adenines would result in transcripts conferring a transient prototrophic phenotype, which could trigger the further replication cycle needed to fix the mutation. Adenines not conferring a prototrophic phenotype would tend to be removed by MutY glycosylase, and mutations at these sites would also fail to be detected. This is, of course, a variant of the general slow-repair model for adaptive mutation originally put forward by Stahl (17).
ACKNOWLEDGMENTS
We thank H. Maki and E. M. Witkin for bacterial strains and A. Timms for discussion.
REFERENCES
- 1.Boyle J M, Symonds N. Radiation sensitive mutants of T4D. 1. T4y: a new radiation sensitive mutant, effect of the mutation on radiation survival growth and recombination. Mutat Res. 1969;8:431–459. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- 2.Bridges B A. Starvation-associated mutation in Escherichia coli: a spontaneous lesion hypothesis for “directed” mutation. Mutat Res. 1994;307:149–156. doi: 10.1016/0027-5107(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 3.Bridges B A. Starvation-associated mutation in Escherichia coli strains defective in transcription repair coupling factor. Mutat Res. 1995;329:49–56. doi: 10.1016/0027-5107(95)00016-c. [DOI] [PubMed] [Google Scholar]
- 4.Bridges B A. mutY ‘directs’ mutation? Nature. 1995;375:741–741. doi: 10.1038/375741a0. [DOI] [PubMed] [Google Scholar]
- 5.Bridges B A. Elevated mutation rate in mutT bacteria during starvation: evidence of DNA turnover? J Bacteriol. 1996;178:2709–2711. doi: 10.1128/jb.178.9.2709-2711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges B A. DNA turnover and mutation in resting cells. Bioessays. 1997;19:347–352. doi: 10.1002/bies.950190412. [DOI] [PubMed] [Google Scholar]
- 7.Bridges B A. RNA synthesis—MutT prevents leakiness. Science. 1997;278:78–79. doi: 10.1126/science.278.5335.78. [DOI] [PubMed] [Google Scholar]
- 8.Bridges B A, Sekiguchi M, Tajiri T. Effect of mutY and mutM/fpg-1 mutations on starvation-associated mutation in Escherichia coli: implications for the role of 7,8- dihydro-8-oxoguanine. Mol Gen Genet. 1996;251:352–357. doi: 10.1007/BF02172526. [DOI] [PubMed] [Google Scholar]
- 9.Cairns J, Overbaugh J, Miller S. The origin of mutants. Nature. 1988;335:142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng K C, Cahill D S, Kasai H, Loeb L A, Nishimura S. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G to T and A to C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 11.Davis B D, Mingioli E S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster P L. Adaptive mutation: the uses of adversity. Annu Rev Microbiol. 1993;47:467–504. doi: 10.1146/annurev.mi.47.100193.002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall B G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988;120:887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall B G. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics. 1990;126:5–16. doi: 10.1093/genetics/126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki H, Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 16.Newcombe H B. Delayed phenotypic expression of spontaneous mutations in Escherichia coli. Genetics. 1948;33:447–476. doi: 10.1093/genetics/33.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl F W. A unicorn in the garden. Nature. 1988;335:112–113. doi: 10.1038/335112a0. [DOI] [PubMed] [Google Scholar]
- 18.Taddei F, Hayakawa H, Bouton M-F, Cirinesi A-M, Matic I, Sekiguchi M, Radman M. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130. doi: 10.1126/science.278.5335.128. [DOI] [PubMed] [Google Scholar]
- 19.Tajiri T, Maki H, Sekiguchi M. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat Res. 1995;336:257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 20.Tang M-S, Wang T-C V, Patrick M H. DNA turnover in buffer-held Escherichia coli and its effect on repair of UV damage. Photochem Photobiol. 1979;29:511–520. doi: 10.1111/j.1751-1097.1979.tb07083.x. [DOI] [PubMed] [Google Scholar]
- 21.Timms, A. R., and B. A. Bridges. Reversion of the tyrosine ochre strain Escherichia coli WU3610 under starvation conditions depends on a new gene tas. Genetics, in press. [DOI] [PMC free article] [PubMed]
- 22.Zambrano M M, Siegele D A, Almiron M, Jormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]