Abstract
Research in preclinical models indicates that estrogens are neuroprotective and positively impact cognitive aging. However, clinical data are equivocal as to the benefits of menopausal estrogen therapy to the brain and cognition. Pre-existing cardiometabolic disease may modulate mechanisms by which estrogens act, potentially reducing or reversing protections they provide against cognitive decline. In the current review we propose mechanisms by which cardiometabolic disease may alter estrogen effects, including both alterations in actions directly on brain memory systems and actions on cardiometabolic systems, which in turn impact brain memory systems. Consideration of mechanisms by which estrogen administration can exert differential effects dependent upon health phenotype is consistent with the move towards precision or personalized medicine, which aims to determine which treatment interventions will work for which individuals. Understanding effects of estrogens in both healthy and unhealthy models of aging is critical to optimizing the translational link between preclinical and clinical research.
Keywords: Estrogen, Estradiol, Memory, Cognition, Menopause, Cardiovascular, Metabolism, Hippocampus, Cortex
1. Introduction
Results of basic and preclinical research conducted over the last several decades provide convincing evidence that estrogens are neuroprotective and exert positive effects on memory in aging females (Luine and Frankfurt, 2020). Estrogens have been shown to enhance synaptic plasticity (Stelly et al., 2012, McEwen et al., 1995), cholinergic neuro-transmission (Bohacek et al., 2008, Luine, 1985, Gibbs, 2003, Gabor et al., 2003), adult neurogenesis (Duarte-Guterman et al., 2015), and neuronal mitochondria bioenergetics (Rettberg et al., 2014). Effects are evident in brain areas important for cognition, including the hippocampus and cerebral cortex (Luine and Frankfurt, 2020). As would be expected, these changes translate into enhanced cognition in model systems of aging (Bimonte-Nelson et al., 2021, Daniel et al., 2015). Considering the strong evidence of the neuroprotective effects of estrogens in model systems, expectations were that menopausal hormone therapy would be neuroprotective and decrease the risk of Alzheimer’s disease and related dementias in women. However, clinical data have been unclear as to the advantages of hormone therapy on cognition, with reported results varying from beneficial to harmful (Pines, 2016). Here we propose that discrepancies in findings as to the effects of estrogens on the brain and cognition in research involving preclinical models and clinical studies involving postmenopausal women may be explained by differences in health status. Preclinical research typically uses healthy models of aging, whereas clinical research often involves subjects with a wide range of health conditions.
A goal of preclinical research is to accurately model the biological effects of an intervention to predict treatment outcomes. To reach that goal, appropriate model systems must be used. Thus, only studying potential strategies for successful aging in healthy models of aging will result in inaccurate predictions of health outcomes for populations with diverse health conditions. The use of preclinical models to determine mechanisms by which estrogens administered in midlife can affect the brain and cognitive aging must include an assessment of effects across individual health phenotypes. This approach to research is consistent with the move towards precision medicine, which aims to determine which treatment interventions will work for specific groups of people.
Although some early observational and small clinical studies supported a role for hormone therapy (HT) in the prevention of age-related cognitive decline (Sherwin, 2003), results of the large Women’s Health Initiative Memory Study (WHIMS) conducted by the National Institutes of Health indicated that HT regimens consisting of chronic conjugated equine estrogens (CEE) (Espeland et al., 2004, Shumaker et al., 2004a) or chronic CEE plus medroxyprogesterone (Rapp et al., 2003, Shumaker et al., 2003) do not improve cognition and may actually increase risk of dementia. Attempts to reconcile the unexpected results of the WHIMS with previous research results have focused on many methodological discrepancies across studies, including forms of hormones used, regimens, and routes of administration. In addition, much attention has focused on the importance of the timing of HT initiation. In contrast to most other clinical studies, HT in the WHIMS was administered to women aged 65 years and older (mean age of 73 years), which is on average more than two decades after ovarian hormone levels have declined at menopause. The critical period hypothesis, which proposes that cognitive benefits of estrogen may only be apparent if administered near the time of menopause, has been suggested as a possible explanation for the discrepant results across studies (Craig et al., 2005, Maki, 2013). Although existing clinical data provide some support for the critical period hypothesis of estrogen effects (Maki, 2013), recent results of the Kronos Early Estrogen Prevention Study (KEEPS) indicate no harm or benefit of either oral CEE or transdermal 17β-estradiol in healthy recently postmenopausal women (Miller et al., 2019). Ongoing trials such as KEEPS should provide more insight as enrolled women reach ages at which cognitive decline is more prevalent.
In support of the possibility that effects of estrogens on the brain and memory may diverge under conditions of health and disease are results of two follow-up studies to WHIMS in which estrogen effects were viewed in the context of type 2 diabetes. In the WHIMS, older women who had type 2 diabetes and were assigned to receive hormone therapy had decreased brain volume compared to those receiving placebo treatment (Espeland et al., 2015b). Hormone therapy did not affect brain volume in women without diabetes. Further, in women from the WHIMS, estrogen therapy exacerbated diabetes-associated risk of dementia and cognitive impairment (Espeland et al., 2015a). Together, results suggest that health status rather than timing may be a critical factor in determining efficacy of estrogen effects.
Open questions that must be answered to reconcile the evidence gap in our understanding of estrogen effects on cognitive aging include the following: What are mechanisms by which a healthy brain responds differently to estrogens than an unhealthy one? Do healthy and unhealthy cardiovascular and metabolic systems respond differently to estrogens, and if so, what are the downstream implications for the brain and cognition? Do the direct effects of estrogens on the brain under conditions of health and disease occur independently or do they interact with the potential indirect effects resulting from dysfunction of the cardiometabolic systems? The authors are actively investigating these questions as part of a National Institute on Aging-supported Program Project Grant, Estrogens, Cardiometabolic Health, and Female Cognitive Aging. Although we do not as yet have answers, the current review posits mechanisms by which estrogens effects on cognition and associated brain areas diverge under conditions of health and disease (See Fig. 1). In doing so, we highlight what is in our view, an optimal approach to the study of aging. We propose that consideration of the ability of a potential treatment (i.e., estrogen therapy) to impact cognitive trajectory in both healthy and unhealthy models of aging represents a paradigm shift in the use of model systems that could lead to optimization of the translational link between preclinical and clinical research.
Fig. 1. Mechanisms by which impacts of estrogens on the brain and cognition may diverge under conditions of cardiometabolic health and disease.
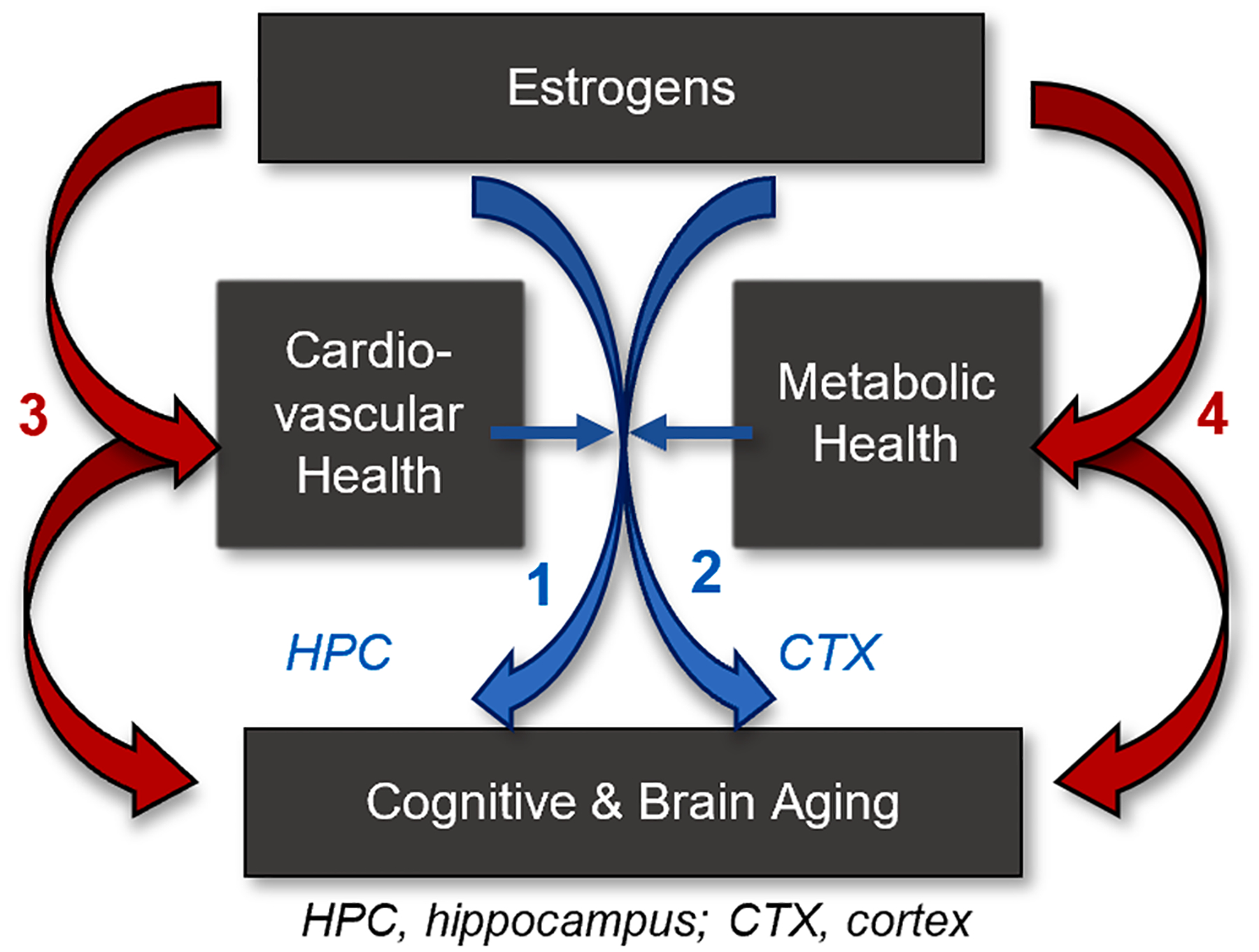
First, status of cardiovascular and metabolic health alters mechanisms by which estrogens act directly on memory systems of the brain (blue arrows) including the hippocampus (1) and cortex (2). Second, cardiovascular and metabolic health impact mechanisms by which estrogens act directly on cardiometabolic systems (red arrows), which subsequently impact the brain (3, 4).
2. Mechanisms by which direct actions of estradiol in the brain may diverge under conditions of health and disease
An unhealthy brain may respond differently to estrogens than a healthy brain if the molecular pathways by which estrogens typically act are not available or have been perturbed. Here we review potential mechanisms by which direct actions of estrogens in the hippocampus and cortex may be altered in healthy and unhealthy systems, alterations that could ultimately lead to individual differences in the response of postmenopausal estrogen therapy on cognitive aging.
2.1. Potential impact of cardiometabolic disease on mechanisms by which estradiol affects the hippocampal memory system
Much of the preclinical research since the results of the WHIMS were published, including our own, aimed to understand how the timing of the initiation of estrogen treatment impacted its ability to exert positive effects on the brain and memory. However, these data may be considered in a new light if viewed in terms of health and disease. Estrogen-induced change in body weight was often used as a measure of efficacy of the estrogen treatments with little consideration as to the broader implications for estrogen effects on cognition of these weight changes. As detailed below, we have taken a new look at our data into the effects of estradiol on the hippocampus and associated cognitive function in the context of its effects on body weight and the implications of those effects in the context of the relationship between cardiometabolic health, estrogens, and hippocampal mediated cognitive function.
2.1.1. Effects of timing of estradiol treatment on the hippocampal memory system
In the Daniel lab, we tested the critical period hypothesis as related to memory by determining if the timing of initiation of estradiol treatment in a preclinical rat surgical model of menopause impacts its ability to affect spatial memory (Daniel et al., 2006). Middle-aged rats were ovariectomized and received implants of capsules containing vehicle or estradiol. The control group received a vehicle implant, modeling women who do not receive hormone therapy following loss of ovarian function. A second group received an estradiol implant at the time of ovariectomy, modeling women who begin hormone therapy at the time of the loss of ovarian function. A third group began receiving estradiol treatment that was delayed until 5 months after ovariectomy, modeling women in the WHIMS that on average were more than a decade post-menopause. Spatial memory was assessed in a radial-arm maze task. Results revealed that continuous estradiol treatment initiated at the time of ovariectomy resulted in significantly enhanced memory as compared to both the control group that never received estradiol treatment as well as females who received delayed estradiol treatment. In subsequent work, we showed that short-term estradiol treatment initiated in the critical window following cessation of ovarian function exerted long-term enhancing effects on memory in aging rodents, effects that persisted long-after the termination of estradiol treatment (Witty et al., 2013, Rodgers et al., 2010, Black et al., 2018, Baumgartner et al., 2022).
We next looked to brain mechanisms that could underlie the critical period hypothesis. Changes in levels or responsiveness of estrogen receptors, particularly ERα, has been offered as an explanation for the existence of a “critical period” following the cessation of ovarian function during which estrogens must be administered to exert effects on the brain and cognition (Daniel, 2013). ERα, perhaps due to its increased ability to induce transcription linked to the estrogen response element (ERE) as compared to ERβ (Tremblay et al., 1997), is particularly important for maintaining hippocampal function under low estradiol levels (Foster, 2012). A decrease in estradiol responsiveness in the hippocampus following the loss of ovarian function may be due to age-related decline in levels of ERα, disrupting the ratio of ERα relative to ERβ and interfering with the transcriptional processes important for cognition (Bean et al., 2014).
We determined the ability of estradiol to impact levels of estrogen receptors in the hippocampus of aging female rats (Bohacek and Daniel, 2009) using the same paradigm as we used to assessed impacts on memory as described above. Estradiol that was initiated at the time of ovariectomy, but not when delayed for five months, resulted in significantly increased levels of ERα in the hippocampus as compared to vehicle treatment. Additionally, a period of short-term estradiol initiated during the critical window resulted in sustained levels of ERα that persisted well beyond the period of estradiol exposure (Rodgers et al., 2010, Witty et al., 2013, Baumgartner et al., 2022). We also showed a similar pattern of effects on hippocampal levels of the ERα-regulated protein choline acetyltransferase, the synthesizing enzyme for acetylcholine (Bohacek et al., 2008, Rodgers et al., 2010). In parallel to our interpretation of our memory data, our interpretation of these data was that there is a critical time window following the cessation of ovarian function during which estradiol must be initiated in order for it to positively impact the hippocampus.
2.1.2. The critical period hypothesis of estrogen effects, health status, and the hippocampus
In the past, we have used body weight as a measure of efficacy of our estradiol treatments with little consideration of the impact that those estradiol-induced changes in body weight can have for memory and the brain. Changes in the hormonal milieu at menopause are associated with increases in total body fat and weight in women, effects that can be attenuated by estrogen administration (Davis et al., 2012). In our preclinical model, we see similar results such that ovariectomy induces significant weight gain and estradiol treatment at the time of ovariectomy prevents weight gain. Thus, when estradiol treatment was initiated 5 months after ovariectomy in the groups with the delayed estradiol treatment in our studies that assessed the impact of estradiol timing on memory (see Daniel et al., 2006) and the hippocampus (see Bohacek and Daniel, 2009), they had already experienced significant ovariectomy-induced weight gain as compared to rats that received immediate estradiol treatment. Subsequent initiation of estradiol treatment 5 months after ovariectomy for a duration of 9 weeks did not significantly reduce body weight compared to the vehicle group (Daniel et al., 2006). Thus, estradiol can counteract ovariectomy-induced weight gain when initiated at the time of loss of ovarian function, but not after a long period of hormone deprivation. Interestingly, in our studies in which we terminated estradiol treatment after a short-term period (i.e. 40 days), rats subsequently gained weight to the level of ovariectomized controls. Results indicate that in contrast to the lasting effects on memory and hippocampal ERα levels, 40 days of previous midlife estradiol treatment does not exert lasting impact on body weight. These data support the hypothesis that when estradiol is initiated in what are likely healthy subjects and prior to ovariectomy-induced weight gain, it can exert lasting impacts on the brain and cognition that will persist even after subsequent weight gain and potential associated adverse health effects.
Our results indicate that estradiol, when initiated at the time of ovariectomy but not when initiated 5 months after, enhances memory, increases hippocampal levels of ERα and ERα-target proteins, and sup-presses ovariectomy-induced weight gain. Results could have at least two, not mutually exclusive interpretations. First, the data provide support for the critical period hypothesis, which posits that the timing of initiation is the important factor in determining efficacy of estradiol effects. Second, it could be that health status and not necessarily timing of initiation is the critical factor in determining efficacy of estradiol effects. In our results, the group in which estradiol was initiated 5 months post-ovariectomy had already experienced significant ovariectomy-induced weight gain prior to the estradiol administration. Such weight gain may have been accompanied by compromises to the cardiometabolic systems. Furthermore, a short-term period of estradiol treatment initiated at the time of ovariectomy results in persistent benefits to cognitive aging and the hippocampus beyond the period of estradiol exposure. Thus, when estradiol is initiated in what are likely healthy subjects and prior to ovariectomy-induced weight gain, it can exert lasting impacts on the brain and cognition that will persist even after subsequent weight gain and potential associated adverse health effects. In support of the second interpretation that health status and not necessarily timing of initiation are reports examining effects of hormone therapy in women with and without type 2 diabetes. In the Women’s Health Initiative Study, older women who had type 2 diabetes and assigned to receive hormone therapy had decreased brain volume as compared to those who received placebo treatment (Espeland et al., 2015b). Hormone therapy did not affect brain volume in women without diabetes. Further, in women from the WHIMS, estrogen only, but not estrogens combined with a progestin, exacerbated diabetes-associated risk of dementia and cognitive impairment (Espeland et al., 2015a). A direct test of the hypothesis that health status rather than timing is the critical factor in determining impact of estradiol on the brain and cognition is warranted.
The “healthy cell bias hypothesis” of estrogen action proposes that divergent effects of estrogens on the brain can be viewed in terms of differential effects of estrogen on neural mitochondria bioenergetics in healthy and unhealthy cells (Brinton, 2008, Yao and Brinton, 2012). The hypothesis proposes that in healthy neurons, estrogens enhance mitochondrial function by enhancing aerobic glycolysis coupled to the citric acid cycle, mitochondrial respiration, and ATP generation. However, in unhealthy cells, estrogens worsen neurodegeneration through increased load on dysregulated calcium homeostasis, on which mitochondria respiration and ATP generation depend. Thus, as brain health moves from healthy to unhealthy, so do effects of estrogens on the brain and cognition. Although ERβ is localized to mitochondria (Yang et al., 2004), estradiol-induced changes in ERα as we see in our model may affect function, as age-related shifts of the ERα/ERβ ratio in the hippocampus are hypothesized to disturb the signaling of each (Bean et al., 2014).
2.1.3. Potential mechanisms by which estradiol impacts on the hippocampus and memory diverge under healthy and unhealthy conditions
The importance ERα to human cognitive aging is highlighted by data revealing a relationship between levels of wild-type and polymorphisms of ERα with cognition (for review see Baumgartner and Daniel, 2021, Tecalco-Cruz et al., 2023). For example, a positive relationship was found between cognition and levels of wild-type ERα in the prefrontal cortex of Alzheimer’s patients (Kelly et al., 2008). Levels of ERα gene polymorphisms are associated with increased risk for Alzheimer’s disease (Brandi et al., 1999) and may act as effect modifiers for risk of Alzheimer’s disease in carriers of the APOE ε4 allele (Ryan et al., 2014). In community-dwelling women and men, ERα polymorphisms are associated with increased risk of age-related cognitive decline (Yaffe et al., 2002, Yaffe et al., 2009). In our own work (Rodgers et al., 2010, Witty et al., 2013), elevated levels of ERα are associated with enhanced memory in both the presence and absence of exogenously administered estradiol. Furthermore, in our rodent model of menopause we demonstrated a causal relationship between hippocampal ERα and memory in that increasing levels of ERα in the hippocampus via viral vector delivery enhanced memory (Witty et al., 2012) and chronically antagonizing brain ERα levels impaired memory (Black et al., 2016). Estrogen receptors remain transcriptionally active in the absence of circulating estrogens (Pollard et al., 2018, Baumgartner et al., 2019) to impact the hippocampus and memory via interactive actions of locally synthesized brain-derived estrogens and growth factor signaling (Pollard and Daniel, 2019, Baumgartner et al., 2022).
To determine mechanisms by which midlife estradiol treatment impacts levels of ERα in the hippocampus, we assessed treatment impacts on transcription of ERα. We found no effect of chronic estradiol treatment initiated immediately following ovariectomy on Esr1, the gene encoding ERα (Baumgartner et al., 2021). However, besides modification of ERα gene transcription, ERα levels can be impacted by changes in receptor degradation rate. ERα is degraded via the ubiquitin-proteasomal pathway (Tateishi et al., 2004). When the receptor is unliganded, the E3 ubiquitin ligase, C terminus of Hsc70-interacting protein (CHIP), binds ERα and targets it for ubiquitination and proteasomal degradation (Fan et al., 2005). Long-term estrogen deprivation following ovariectomy increases interaction between CHIP and ERα and thus subsequent ubiquitination of ERα (Zhang et al., 2011). Estradiol protects ERα from CHIP-mediated degradation likely due to its phosphorylation of ERα at serine 118 (S118) (Valley et al., 2008). Midlife estradiol treatment results in lasting increased phosphorylated levels ERα at S118 in the hippocampus (Grissom and Daniel, 2016) and decreased association between ERα and CHIP that occur in parallel to its increased levels of ERα protein levels in the hippocampus (Black et al., 2016). Collectively these data indicate the midlife estradiol treatment leads to sustained increase in hippocampus ERα due to its impact on the degradation rate of the protein.
Obesity is associated with dysfunction of the ubiquitin-proteasomal pathway. For example, in obese mice, the ubiquitin/proteasome system in the hypothalamus fails to maintain an adequate rate of protein recycling, leading to the accumulation of ubiquitinated proteins (Ignacio-Souza et al., 2014). Further, alterations in the ubiquitin-proteasomal pathway are present in the cortex of both middle-age spontaneously as well as diet-induced obese rats (Reddy et al., 2014). Of particular relevance to the current discussion is a report in which 6 weeks of a high-fat diet in rats resulted in dysregulation of the ubiquitin-proteasomal pathway across cellular components in the hippocampus (McFadden et al., 2020). Specifically, high-fat diet altered proteasome activity and protein polyubiquitination in the hippocampus, effects that correlated with impaired hippocampal dependent memory as measured by object location memory. Thus, the mechanism by which estrogens regulate ERα is impaired under conditions of metabolic disease.
2.1.4. Estrogens, cardiometabolic health, and the hippocampal memory system: Summary and conclusions
Lasting changes in levels of ERα resulting from midlife estradiol treatment are primarily due to lasting changes in protein degradation. Estradiol protects ERα from degradation via the ubiquitin-proteasomal pathway, a pathway that is dysregulated under obese conditions. Obesity can result in a variety of health conditions, including metabolic disease and hypertension. As illustrated in our hypothesized model (Fig. 2), alterations in the ubiquitin-proteasomal pathway under conditions of cardiometabolic health and disease may perturb the ability of estradiol to affect levels of ERα in the hippocampus of aging female rats and ultimately its ability to positively impact hippocampal dependent memory. Our model represents one of likely many potential mechanisms by which estrogen actions in the hippocampus and on related functions may be altered under conditions of health and disease. Future research using preclinical model systems to understand estrogens, aging, and the hippocampus should consider how health status impacts those mechanisms.
Fig. 2. Hypothesized model by which effects of estrogens on the hippocampus and memory diverge in healthy and unhealthy females.
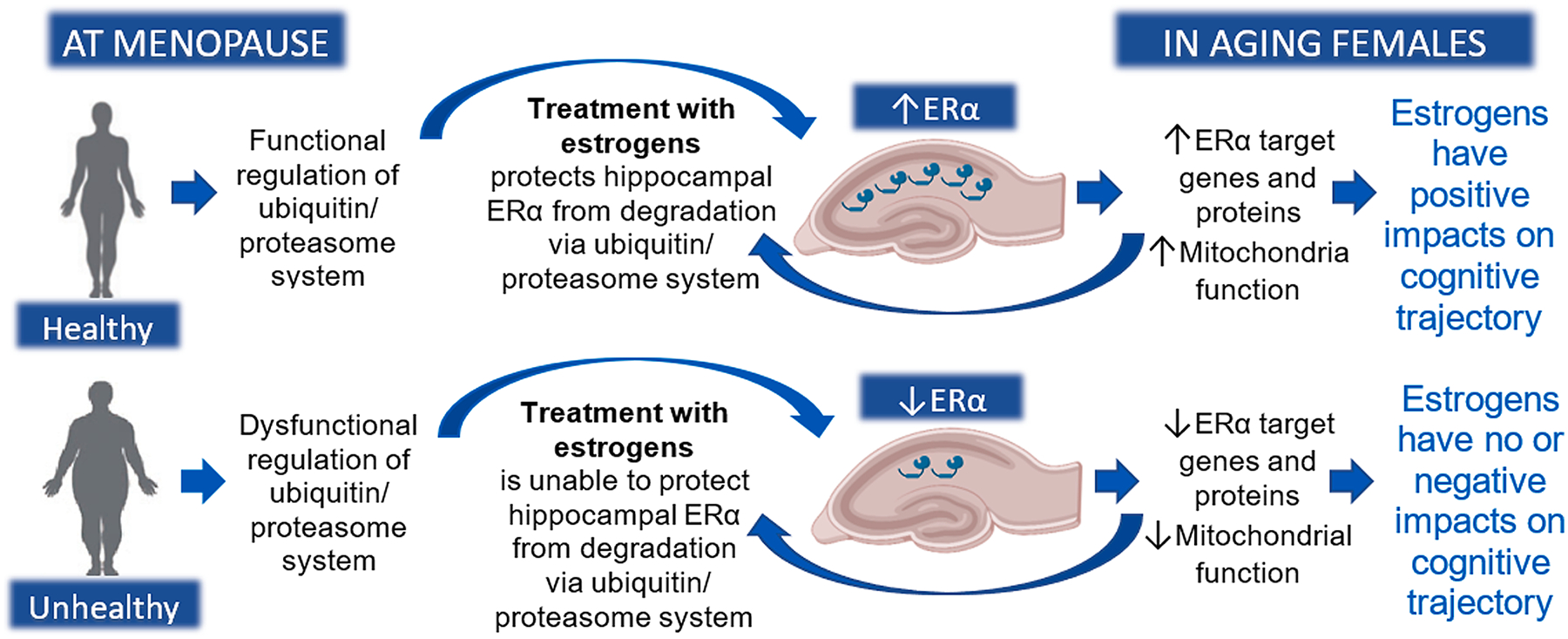
If individuals are healthy when treatment with estrogens is initiated, estrogens can act through a functional ubiquitin / proteasome system to protect hippocampal ERα from degradation. Once elevated levels are achieved, they will persist, creating a positive feedback loop and prolonged enhanced cognition. If individuals are unhealthy when treatment with estrogens is initiated, estrogens are unable to act to protect ERα in the hippocampus from degradation and has no effect on levels of ERα – a critical factor in the ability of estrogens to impact memory. Created with BioRender.com.
2.2. Potential impact of cardiometabolic disease on mechanisms by which estradiol affects cortical synaptic plasticity
It has been established that cardiovascular complications are directly associated with sharper cognitive decline (Gottesman et al., 2014) and contribute to Alzheimer’s disease and vascular dementia (Iadecola, 2010) by disrupting the neurovascular unit. Similarly, numerous studies have determined that individuals with diabetes, particularly type 2 diabetes, have a steeper decline of cognitive function (Mallorquí-Bagué et al., 2018, Moran et al., 2019) and a higher risk of developing Alzheimer’s disease and other related dementias than individuals without diabetes (Biessels et al., 2006, Kopf and Frölich, 2009, Moreno-Gonzalez et al., 2017). Therefore, to attain the fullest benefits of hormone therapy after menopause it is paramount to determine the comorbid conditions in which such approach may have a positive or negative impact on the patient, the reasons underlying those positive and negative effects, and the interventions suitable to revert or prevent the negative outcomes. In this sense, neurovascular coupling, i.e., the change in local perfusion occurring in response to neuronal activity, is altered in hypertension and metabolic disease (Kazama et al., 2003, Iadecola, 2017), which can lead to cognitive decline (de Montgolfier et al., 2020) by affecting neuronal activity and synaptic plasticity, as well as to vascular dementia and Alzheimer’s disease (Shabir et al., 2018). This intrinsically complex system, coupling brain activity with local cerebral blood flow, is tightly regulated by endothelial nitric oxide synthase (eNOS) activity (Hariharan et al., 2019), therefore providing a prospective impaired mechanism responsible for the lack of beneficial effect of hormone therapy after menopause on cognitive function. The presence of hypertension and metabolic disease prior to menopause may be impeding the beneficial effects of hormone therapy in preventing aging-related cognitive decline by blunting neurovascular coupling. This may lead to impaired local network activity and, consequently, impaired synaptic plasticity, which is essential for the formation and stabilization of the synapses required to create functional cortical circuits and therefore for cognition. In this scenario, interventions aimed to increase eNOS activity would be able to reverse the neurovascular deficits.
2.2.1. Impact of hypertension, metabolic disease, and reduction of gonadal hormone levels on cortical networks
Intrinsic membrane properties control the ability of synaptic inputs to trigger action potential firing, hence regulating neuronal communication and therefore, brain function. Being such a crucial mechanism in the control of neuronal activity, the lack of data on the impact of ovarian hormone loss on principal neurons of the neocortex is surprising. Similarly, there are no data on the effects of estrogen treatment, nor the impact of hypertension and metabolic disease, on intrinsic properties of these neurons, studies that could provide a mechanistic description of the changes occurring at the cellular level, and that affect neuronal function, under those circumstances. There are, however, studies showing that ovariectomy in rodents reduces intrinsic excitability in hippocampal pyramidal neurons (Carrer et al., 2003, Wu et al., 2011), although the timings of ovariectomy in these studies (2 days after birth and 7 months old) are not relevant for preclinical models of aging. Multiple lines of evidence suggest that inhibitory function is impaired in the aged brain (Schmolesky et al., 2000, Leventhal et al., 2003, Wong et al., 2006, Lenz et al., 2012, Haberman et al., 2013, Hickmott and Dinse, 2013, Ouellet and Villers-Sidani, 2014), including reports from the from our lab (Popescu et al., 2021) indicating a reduction in synaptic inhibition and an increase in excitability of layer 5 pyramidal neurons. The impaired inhibitory function has also been linked to various pathologies including Alzheimer’s disease (Verret et al., 2012). Overall, these findings suggest a shift in the excitation/inhibition (E/I) balance towards a more inhibited output of the circuit. Along these lines, it has been recently described that acute estradiol treatment of cortical neurons from ovariectomized mice returns the frequency of miniature excitatory postsynaptic currents (mEPSCs) to normal rates, indicating the restoration of excitatory synaptic sites (Ye et al., 2019) and of the E/I balance. The important role that inhibitory interneurons play in the production of gamma oscillations, which enhance cortical information processing and emerge from precise synaptic interactions between pyramidal neurons and parvalbumin-expressing fast-spiking interneurons (Sohal et al., 2009), makes these changes in neuronal excitability in the context of the absence of endogenous gonadal hormones a mechanism deemed of further investigations. In addition, and because parvalbumin fast-spiking interneurons utilize significantly more energy than other cortical neurons (Kann, 2016, Kann et al., 2014), it is possible that these neurons are more susceptible to mitochondria dysfunction due to heightened metabolic stress with aging, with cardiometabolic comorbidities, and after menopause. Dysfunction in parvalbumin interneurons may render fast network oscillations more susceptible for impairment affecting higher brain functions. In fact, reductions in gamma power have been found with normal aging and appear to precede and contribute to development of dementia and Alzheimer’s disease, while restoration of gamma oscillations ameliorate physiological and cognitive symptoms of Alzheimer’s disease (Iaccarino et al., 2016, Martorell et al., 2019).
When combining the deleterious effects of cardiovascular and metabolic disease with the effects of endogenous hormone secretion cessation after menopause and with biological aging, the questions that arise are whether excitatory and inhibitory cortical neurons show accelerating aging after menopause with the preexistence of hypertension and metabolic disease; whether energy efficiency of pyramidal neurons and parvalbumin fast-spiking interneurons is maintained after menopause; whether parvalbumin interneurons are more vulnerable under comorbid conditions of additional metabolic stress like hypertension and obesity; and whether midlife estrogen treatment is able to overcome these deficits.
2.2.2. Impact of circulating endogenous estrogens, and its cessation, on synaptic plasticity of principal cortical neurons
Due to the critical regulatory role that inhibitory neurons play on pyramidal neurons, alterations in the E/I balance affect the ability of excitatory neurons to experience synaptic plasticity (Froemke, 2015). The majority of excitatory cortical synapses occur at dendritic spines (Nimchinsky et al., 2001), small protrusions formed at the dendrite shaft containing neurotransmitter receptors and the cellular machinery to transduce chemical signals emitted by axonal boutons. As paramount components of cortical circuits, dendritic spines are responsible for the maintenance, storage, and recall of information and are the anatomical substrate for learning and memory (Yuste and Bonhoeffer, 2001). In vivo imaging studies of pyramidal neurons from different areas of the cortex have shown that learning of a motor task (Xu et al., 2009), a whisker-dependent localization task (Kuhlman et al., 2014), and associative learning (Muñoz-Cuevas et al., 2013) require creation and stabilization of new spines and elimination of weakened dendritic spines no longer needed. This indicates that learning is associated with an increase, with respect to baseline conditions, in the turnover rate of dendritic spines, followed by stabilization of newly formed dendritic spines. Using two-photon excitation microscopy, the Mostany laboratory recently identified age-related deficits in sensory-induced plasticity of layer 5 pyramidal neurons in the primary somatosensory cortex, barrel field (Voglewede et al., 2019) as well as altered synaptic dynamics of layer 5 pyramidal neurons in the motor cortex (Davidson et al., 2020). Using a whisker stimulation paradigm it was possible to detect deficits in sensory-evoked synaptic plasticity in aged mice (Voglewede et al., 2019) as well as different levels of plasticity across the estrous cycle (Alexander et al., 2018). Our data from aged (>18-month-old) mice show: (1) elevated dendritic spine turnover rate in layer 5 pyramidal neurons under baseline, unstimulated, conditions; (2) impaired sensory-evoked synaptic plasticity; and (3) decreased dendritic spine stability when compared with young adult (3- to 5-month-old) mice. We have also described the lack of significant changes in density, turnover ratio, and survival of dendritic spines across the estrous cycle in normal-cycling young virgin mice. However, our data suggest that the different levels of ovarian hormones at different stages of the cycle impact the degree of sensory-evoked synaptic plasticity (Alexander et al., 2018). Previous studies agree that ovariectomy leads to reduced spine density in pyramidal neurons of the neocortex and that estradiol treatment is able to correct it in both rodents (Muñoz-Cueto et al., 1990, Wallace et al., 2006, Chen et al., 2009, Ye et al., 2019) and non-human primates (Dumitriu et al., 2010). In the study by Ye et al. (Ye et al., 2019), longitudinal in vivo imaging data revealed that the decrease in spine density following ovariectomy was due to a reduction in the rate of spine gain combined with an increase in the rate of spine loss, and that these dynamic processes were restored after estradiol treatment. However, 2- to 3-month-old virgin mice were used in the study, and there was no interrogation of activity-dependent synaptic plasticity. Investigating the positive effects of estradiol after ovariectomy on sensory-evoked plasticity and how hypertension and metabolic disease abrogate those beneficial effects would shed light on the ability of estradiol to restore pre-ovariectomy synaptic dynamics crucial for the maintenance of neuronal circuits, and therefore for learning and memory, as well as on the impact of cardiometabolic comorbidities on the protective and restoring properties of estradiol in synaptogenesis and synaptic stability.
2.2.3. Impact of hypertension and metabolic disease on neurovascular coupling: Potential benefits of hormone therapy
Neurovascular coupling, i.e., the change in local perfusion occurring in response to neuronal activity, is crucial to maintain homeostasis of the brain parenchyma. A dual responsibility has been assigned to this functional mechanism: providing the fuel to sustain neuronal activity and clearing up the toxic by-products of neuronal metabolism (Iadecola, 2017). Neuronal activity leads to production of the vasodilator nitric oxide by neuronal nitric oxide synthase (nNOS), which is complemented by the tightly regulated activity of eNOS, promoting vasodilation (Attwell et al., 2010). In the context of circulating gonadal hormones, it is well established that estrogen increases NO availability by inducing the activation of its synthetizing enzyme, eNOS (Chambliss and Shaul, 2002, McNeill et al., 2002). On the other hand, dysregulation of the interaction between neural activity and blood vessels is linked to brain dysfunction and damage such as vascular dementia and Alzheimer’s disease (Shabir et al., 2018). In particular, it has been proposed that failure to clear up amyloid-β (Aβ) and tau due to impaired cerebral blood flow may have important implications for Alzheimer’s disease (Tarasoff-Conway et al., 2015). Evidence indicates that neurovascular coupling is altered in hypertension and metabolic disease (Girouard and Iadecola, 2006, Hu et al., 2019, Iadecola and Gottesman, 2019); however, the interaction between these comorbidities, menopause, estrogen therapy and neurovascular coupling is unresolved as the only study available was done in young (2- to 3-month-old), virgin ovariectomized mice subjected to acute intravenous administration of angiotensin II (Girouard et al., 2008). The authors did not observe significant differences between sham, ovariectomized, and ovariectomized mice treated with estradiol. They also treated intact female mice with angiotensin II for 7 days but failed to induce high blood pressure or to affect neurovascular coupling, which was assessed in animals under anesthesia, a variable that may have blunted the hyperemic response. In terms of metabolic disease, diabetes is associated with an impairment of cerebral functional hyperemia (Duarte et al., 2015, Hu et al., 2019, Yu et al., 2019). Furthermore, reduced neurovascular coupling has been reported in male rats after high fat diet (Li et al., 2013) and in young female rats after streptozotocin injection (Vetri et al., 2012).
Regarding the potential molecular mechanisms involved in neurovascular coupling dysfunction, the laboratory of Prasad Katakam and collaborators, as well as other labs, have implicated decreases in nitric oxide bioavailability, which could result from either reduced eNOS activity or increased reactive oxygen species generation from NADPH oxidase or the mitochondrial electron transport chain, in hypertension and high-fat diet-induced vascular dysfunction (Busija and Katakam, 2014, Busija et al., 2005, Busija et al., 2004, Erdös et al., 2006, Katakam et al., 1998, Katakam et al., 2009, Katakam et al., 2012, Katakam et al., 2005, Sakamuri et al., 2020). Furthermore, mice with genetic deletion of eNOS have been shown to exhibit hypertension and insulin resistance (Huang and Lo, 1998, Barua et al., 2010). Additionally, mitochondria play a critical role in activating nitric oxide synthase isoforms in endothelial cells, vascular smooth muscle cells, and neurons (Katakam et al., 2016, Katakam et al., 2014, Katakam et al., 2013). In addition, studies have shown that nitric oxide synthase-derived nitric oxide regulates mitochondrial electron transport chain and respiration (Brown and Borutaite, 2007, Pacher et al., 2007, Singh et al., 2007, Ignarro, 2010). Thus, nitric oxide synthase and mitochondria exhibit cross-regulation in vascular and neuronal cells.
A potential therapeutic target to improve eNOS activity/nitric oxide bioavailability and reduce reactive oxygen species is peroxynitrite. Peroxynitrite is generated from the reaction of nitric oxide and superoxide and has been shown to mediate the pathogenesis of vascular and neuronal injuries (Forman et al., 1998, Torreilles et al., 1999, Gürsoy-Ozdemir et al., 2000, Maruyama et al., 2001). Peroxynitrite produced in vascular cell microdomains has been recently implicated in hypertension accompanying obesity (Ottolini et al., 2020). Consistent with previous reports (Singh et al., 2007, Xiong et al., 2009), recently published data from the Katakam and Mostany laboratories show that peroxynitrite negatively regulates mitochondrial respiration (Albuck et al., 2020). Notably, our study demonstrated for the first time that eNOS knockout mice generate excessive peroxynitrite leading to impairment of respiration in isolated brain mitochondria indicating that eNOS deficiency leads to increased generation of peroxynitrite. This has implications for hypertension- and high fat diet-induced vascular and neuronal injury, which are characterized by decreased eNOS activity/NO availability. Thus, a combined approach, in which hormone therapy is complemented by manipulations aimed to reduce peroxynitrite generation, may revert the dysfunctional neurovascular coupling described in cardiometabolic disease.
2.2.4. Estrogens, cardiometabolic health, and cortical circuits and neurovascular coupling: Summary and conclusions
Both cardiometabolic disease and menopause have detrimental effects on synaptic plasticity of cortical circuits that may lead to cognitive deficits. While estrogen therapy seems to be able to revert some of these deficits in healthy aging conditions, the preexistence of comorbidities like hypertension and diabetes may impede the beneficial effects of estrogens (See Fig. 3). The effects of unhealthy aging may have a direct impact on the neurons being part of the cortical circuits and a defective, not complying neurovascular unit may be a possible contributor to dysfunctional neuronal activity. A tight neurovascular coupling regulation heavily relies on the bioavailability of nitric oxide in the endothelial cells covering the lining of brain blood vessels for effectively signaling for vasodilation. The availability of nitric oxide depends not only on the activity of its synthetizing enzyme but also on its less desirable conversion to oxidizing agents like peroxynitrite. Therefore, and as illustrated in our hypothesized model (Fig. 3), cardiometabolic disease prior to menopause hampers the beneficial effects of hormone therapy in preventing aging-related cognitive decline by disrupting neurovascular coupling. Disruption of neurovascular coupling may lead to impaired local network activity, possibly due by dysfunctional activity of highly energy demanding inhibitory neurons, and, consequently, impaired synaptic plasticity, which is essential for the formation and stabilization of the synapses required to create functional cortical circuits and therefore for cognition. Our model also hypothesizes that preventing peroxynitrite-related damage of endothelial cells by increasing eNOS activity and NO bioavailability, which is achieved with estrogens, may revert the neurovascular coupling deficits and improve the supply of oxygen and nutrients to the neurons, and therefore, improving mechanisms of synaptic plasticity ultimately leading to better memory and learning.
Fig. 3. Hypothesized model by which effects of estrogens on cortical synaptic plasticity and neurovascular coupling diverge in healthy and unhealthy females.
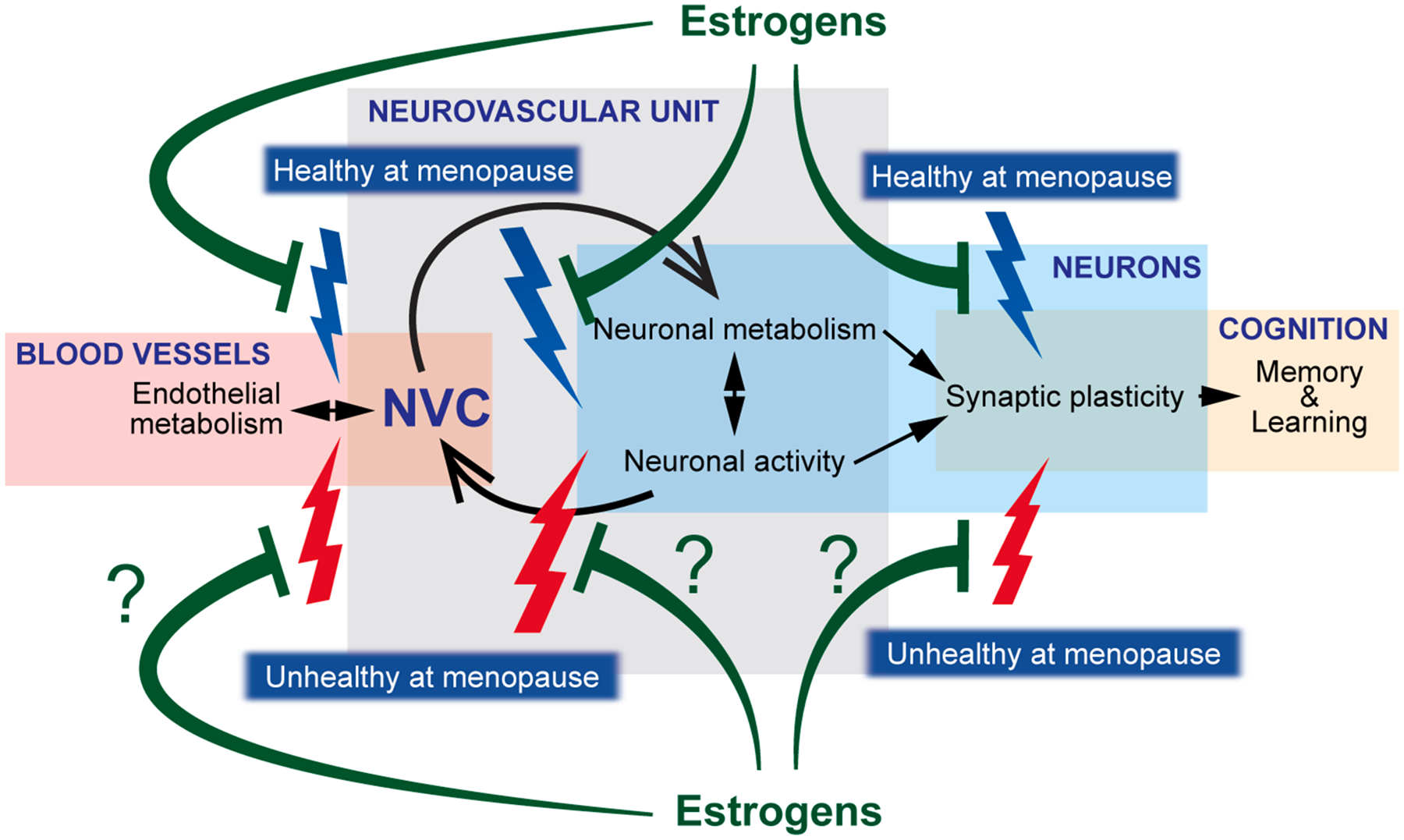
Learning and memory are dependent on proper synaptic plasticity mechanisms, which are directly and indirectly determined, among other mechanisms, by neuronal activity and metabolism, mechanisms mutually dependent on each other too. In turn, neurovascular coupling (NVC) depends and has effects on neuronal activity and metabolism and relies on the proper function of endothelial cells of the brain vasculature. It is known that cessation of endogenous circulating estrogens, even in healthy conditions, has detrimental effects on NVC, neuronal activity, and synaptic plasticity (blue thunderbolts) that are ultimately responsible for overall impaired cognition. While hormone therapy early after menopause prevents or reverts many of those deficits (green blunt arrows), it is unknown whether the neuroprotective effect of estrogens therapy is able to overcome the presence of prior history of cardiovascular or metabolic disease in unhealthy aging conditions at menopause (red thunderbolts).
3. Mechanisms by which actions of estradiol on cardiometabolic systems may diverge under conditions of health and disease and downstream consequences for the brain and memory
In addition to direct actions in the brain, estrogens act on cardiometabolic systems to impact their function. Here we review putative mechanisms by which cardiovascular and metabolic systems may respond differently to estrogens under conditions of health and disease, responses that would have downstream implications for the brain and cognitive aging.
3.1. Potential impact of estrogens on cardiovascular health and subsequent implications for cognitive aging
In addition to cognitive changes, the hormonal fluctuations of menopause are associated with a significant increase in cardiovascular risk. These two changes may not occur separately, as there is a strong link between cardiovascular diseases such as hypertension and hyper-cholesterolemia and the risk for dementia and Alzheimer’s disease. While vascular dementia was once considered a different pathological process from Alzheimer’s disease, growing evidence indicates that cardiovascular disease contributes to both forms of dementia. Currently, no therapeutic strategies target the cardiovascular system to protect the aging brain from this damage.
3.1.1. Impact of menopause on cardiovascular health
Menopause is accompanied by a loss of endogenous estrogens, cognitive impairment, and increased cardiovascular disease in both humans and animal models (Luine, 2008, Maranon and Reckelhoff, 2013). Preliminary data suggests that women who receive hormone therapy in the early menopausal years may have a lower risk for dementia and cardiovascular mortality later in life (Bagger et al., 2005, Alexandersen et al., 2006, Bagger et al., 2004). Similarly, work from the Lindsey and Daniel labs using rodent models shows that exposure to estradiol during a “critical window” after the loss of ovarian function provides benefits for both cognitive and cardiovascular function (Rodgers et al., 2010, Zimmerman et al., 2017). In contrast to this strong evidence of estrogen’s protective actions, the Women’s Health Initiative failed to find cognitive or cardiovascular benefits of menopausal hormone therapy, opposing previous findings from observational trials such as the Nurses’ Health Study (Manson et al., 2003, Grodstein et al., 1996, Shumaker et al., 2004b, Craig et al., 2005). One glaring difference is that the observational studies were conducted in younger, healthier subjects while the WHI enrolled older women that were many years past the menopausal transition. Hence, the response to MHT may have been altered by biological aging, the absence of estrogens for an extended time period, or subclinical disease progression (Manson et al., 2006). In line with this theory, current clinical recommendations emphasize that hormone therapy provides the most benefit for patients within 10 years of menopause (North American Menopause Society, 2017).
3.1.2. Estrogen receptors in cardiovascular tissues and the brain
Estrogen receptors α (ERα) and β (ERβ) are nuclear receptors that bind estrogen and translocate to the nucleus to produce genomic actions. About 20 years ago, a third membrane-bound and G protein-coupled estrogen receptor (GPER) was identified. Our laboratory and others show that selective activation of GPER induces many protective cardiovascular effects including decreased blood pressure (Lindsey et al., 2009), protection from renal damage (Lindsey et al., 2011), and beneficial effects on arterial remodeling (Liu et al., 2016, Ogola et al., 2019). In contrast to the profound effects of GPER in cardiovascular function, our work on the protective cognitive effects of estrogen indicates a critical role for ERα in the brain (Rodgers et al., 2010, Black et al., 2016). Using the novel technique of droplet digital PCR to quantify receptor transcript in different tissues, we determined that ERα and GPER are the dominant estrogen receptors in most tissues, with ERα highest in cardiovascular tissues and GPER highest in the brain (Hutson et al., 2019). What is unknown is whether the specific ratio of estrogen receptors in different tissues modulates the response to this hormone and whether disease states upset this balance and attenuate the protective effects of estrogens.
3.1.3. Impact of blood pressure and antihypertensives on cognitive health
A Finnish study found that increased systolic blood pressure in midlife is associated with a 3-fold higher Alzheimer’s risk ~ 20 years later (Kivipelto et al., 2001). The Honolulu-Asia aging study found that when left untreated, this same level of systolic blood pressure (>160 mmHg) increases the risk for dementia by 4.8-fold (Launer et al., 2000). Mechanisms that strongly link cardiovascular disease to cognitive dysfunction include neuronal loss and disruption of neurovascular coupling (Iadecola, 2010, Tublin et al., 2019). Additional characteristics associated with cardiovascular-induced brain damage include cerebral artery remodeling and fibrosis (Pires et al., 2013, Wegiel et al., 2002), microglial activation (Singh-Bains et al., 2019, Li et al., 2017), and white matter injury (Maillard et al., 2012, Wang et al., 2019).
Literature is conflicting over the level of protection provided by antihypertensives. The Honolulu-Asia aging study found that the association between increased systolic blood pressure and dementia is absent in men treated with antihypertensives (Launer et al., 2000). Further analysis of this cohort shows that even pre-hypertensive levels of blood pressure (120–140 mmHg) increase the risk for dementia, and the risk is exacerbated by the lack of antihypertensive treatment (Launer et al., 2010). In contrast to these studies connecting midlife hypertension to events much later, the Hypertension in the Very Elderly Trial (HYVET) found that one year of antihypertensive treatment in patients > 80 years did not significantly reduce dementia (Peters et al., 2008). The SPRINT MIND trial showed that standard (<140 mmHg) versus intensive (<120 mmHg) blood pressure lowering does not protect against dementia but reduces incident mild cognitive impairment (Williamson et al., 2019). While these were preliminary and underpowered results, one factor contributing to disparate results may be the age at treatment, as one study found that higher systolic blood pressure after 60 years of age is counterintuitively associated with a reduced risk for dementia (van Dalen et al., 2022). In addition, specific classes of antihypertensives may be better at protecting the brain from high blood pressure. Analysis of SPRINT data divided into two groups based on angiotensin II type 2 receptor activity found that antihypertensives stimulating this receptor provide greater protection (Marcum et al., 2022). However, while the Ginkgo Evaluation of Memory (GEM) Study from Johns Hopkins also showed that different classes of antihypertensives produced varying effects on dementia, this was independent of their ability to lower blood pressure, raising the question of whether some antihypertensives may have direct effects on the brain (Yasar et al., 2013).
3.1.4. Impact of arterial stiffness on cognitive health
The impact of hypertension on the brain may also depend on the level of associated tissue damage. A recent report from the SPRINT MIND trial shows that in hypertensive patients, higher levels of arterial stiffness correlate with worse cognitive function (Zang et al., 2021). Although arterial stiffness is a protective adaptation to higher wall stress due to hypertension, decreased arterial elasticity transmits higher pulsatile pressure to smaller arteries and target organs including the brain (Lemarie et al., 2010). Hypertension and arterial stiffness are independent risk factors for cognitive decline (Launer et al., 2000, Waldstein et al., 2008), and arterial stiffness impacts multiple aspects of cognitive health including global cognition, executive function, and memory (Alvarez-Bueno et al., 2020). The relationship between arterial stiffness and dementia has been established in many cross-sectional and longitudinal studies (Hazzouri and Yaffe, 2014). The Baltimore Longitudinal Study of Aging found in a cohort of stroke- and dementia-free persons that arterial stiffness is associated with cognitive decline before overt signs of dementia (Waldstein et al., 2008). The correlation between arterial stiffness and cognitive function is enhanced during aging (Elias et al., 2009), which may explain why studies that include patients from a wide age range fail to find this relationship (Poels et al., 2007). Moreover, because arterial stiffness is assumed to accompany changes in blood pressure, stiffening without hypertension goes undetected and has the potential to promote cognitive decline. Therefore, therapies that reduce arterial stiffness have the potential to provide greater protection to the brain during aging.
Clinical studies have established that aging and loss of circulating estrogens both contribute to increased arterial stiffness independent of blood pressure (Mitchell, 2014, Samargandy et al., 2020). Since aging and menopause normally accompany one another, many clinical studies are unable to distinguish the impact of each variable alone on arterial stiffness. Young women have less vascular stiffness in comparison with men, but this sex difference reverses during aging (Laogun and Gosling, 1982, Skurnick et al., 2010, Smulyan et al., 2001). Arterial stiffness in women, and especially postmenopausal women, is a significantly greater risk factor for cardiovascular mortality in comparison with age-matched men (Regnault et al., 2012). Arterial stiffness dramatically increases within a year of menopause, even after adjusting for blood pressure (Samargandy et al., 2020). However, the Baltimore Longitudinal Study of Aging found that postmenopausal hormone users did not experience the same increase in carotid stiffness as non-users, indicating that estrogens can provide protection (Nagai et al., 1999). While the impact of arterial stiffness on cardiovascular and cognitive outcomes is known, as well as the importance of estrogens in the disease process, little is known about the mechanisms by which estrogens protect from arterial stiffness and how this signaling may be altered during aging or the progression of cardiovascular disease.
3.1.5. Estrogen, cardiovascular health, and cognition: Summary and conclusions
Many critical gaps remain in our knowledge about the relationships between estrogen loss, cardiovascular disease, and their impact on Alzheimer’s disease and vascular dementia. Post-hoc analysis of the Women’s Health Initiative indicates that the negative results of the trial may have been influenced by aging, an extended period of ovarian hormone loss, or subclinical cardiovascular disease (Manson et al., 2006). However, the relative contribution of each of these factors on the response to estrogen has not been systematically tested and there is no proposed underlying mechanism for how these factors switch the impact of estrogens from protective to detrimental. As illustrated in our hypothesized model (Fig. 4), cardiovascular disease may downregulate estrogen receptor expression or function and reduce the ability of estradiol to induce vasodilation and decrease oxidative stress, blood pressure, arterial stiffness, and vascular remodeling. Thus, there is a critical need to establish whether the presence of cardiovascular disease attenuates the effects of estrogens, identify mechanisms that underlie the loss of estrogen’s protective effects in postmenopausal women, and determine the specific cardiovascular disease processes that propagate the development of Alzheimer’s disease and vascular dementia in aging women. Addressing these knowledge gaps will enable the development of novel methods for early detection and prevention of cardiovascular disease-induced cognitive decline as well as provide innovative strategies for improving menopausal hormone pharmacology to protect from Alzheimer’s disease and vascular dementia.
Fig. 4. Hypothesized model by which effects of estrogens in the vasculature diverge in healthy versus unhealthy arteries.

If arteries are healthy when treatment with estrogens is initiated, estrogens activate both the G protein-coupled receptor (GPER) and estrogen receptor alpha (ERα) to induce vasodilation and decrease oxidative stress, blood pressure, arterial stiffness, and vascular remodeling. These protective effects in the vasculature positively impact the brain and cognition. If arteries are unhealthy due to hypertension or other cardiovascular diseases when treatment with estrogens is initiated, GPER and ERα expression and/or signaling are downregulated so the vascular effects as well as downstream impact on the brain are reduced. Created with BioRender.com.
3.2. Potential impact of estradiol on the central regulation of glucose homeostasis and subsequent implications for hippocampal function
In addition to its role in cognitive and cardiovascular function, estrogens play critical roles in the regulation of metabolic factors, and its involvement in insulin sensitivity and glucose homeostasis has major clinical relevance. The loss of ovarian hormones during menopause itself is associated with a greater risk of development of diabetes, impaired glucose tolerance, elevated blood pressure and elevated triglyceride levels (Christakis et al 2020). In addition, sex differences in metabolic phenotype exist, and in general, young females are protected from obesity-related metabolic and cardiovascular complications before menopause in both animal models and clinical subjects (Lovejoy et al., 2009, Griffin et al., 2016, Mauvais-Jarvis, 2015). High fat diet-fed animals are used as an animal model of obesity, as high fat diet increases body mass and adiposity in both sexes (White et al., 2019). Both male and female preclinical obesity models developed mild hyperglycemia with less marked hyperinsulinemia in females, insulin resistance, and glucose intolerance (White et al., 2019). In contrast, young female rats on high fat diet maintained weight and glucose homeostasis, while male rats became obese with impaired glucose homeostasis (Underwood and Thompson, 2016). These sex differences no longer exist in ovariectomized animals (Stubbins et al., 2012), suggesting circulating ovarian hormones may provide metabolic protection to females. Furthermore, metabolic dysregulation occurs in ovariectomized mice on normal (Alonso et al., 2010, Zhu et al., 2013, Kim et al., 2014) and high fat diets (Riant et al., 2009). Ovariectomy alone caused a significant increase of body weight in female mice and treatment with estradiol, or a selective estrogen receptor modulator prevented body weight increase, visceral adipose tissue accumulation, adipocyte hypertrophy, and decreased serum leptin levels. Hormone treatment improved hepatic and muscle lipid homeostasis, increased energy expenditure, lowered fed and fasting glucose and insulin levels, and improved glucose and insulin tolerance (Kim et al., 2014). Further investigation of the impact of estrogens on liver glucose metabolism suggested reduced hepatic glucose output presumably via suppression of gluconeogenesis (Kim et al., 2014). Ovarian hormones impact glucose metabolism throughout the body as levels of several key enzymes involved in glycolysis and glucose metabolism in the brain are affected both by ovariectomy and treatment with 4-vinylcyclohexene diepoxide (VCD), which produces ovarian failure and provides for a transitional model of menopause (Kirshner et al., 2020). These effects in the brain are complex and vary across brain area and model of menopause.
Similarly, data from clinical trials suggest that menopausal hormone therapy in women without diabetes is associated with a reduction in fasting glucose and insulin levels, improved insulin sensitivity, and reduction of new-onset type 2 diabetes (Matthews et al., 1985, Espeland et al., 1998, Kanaya et al., 2003, Manson et al., 2013, Margolis et al., 2004, Salpeter et al., 2006). On the other hand, the effects of hormone therapy on metabolic measures in individuals with pre-existing metabolic disorders are less clear. Importantly, a previous study showed that the beneficial effects of estradiol on brain insulin sensitivity and brain oxidative stress were observed only in ovariectomized control diet-fed rats, but not in ovariectomized high fat-fed rats (Pratchayasakul et al., 2014). In this study, estradiol administration did not recover insulin stimulation of AKT phosphorylation in whole brain of high fat diet-fed rats, and estradiol administration decreased the level of oxidative stress only in animals kept on control diet, but not on high fat diet. In this section we review the link between estradiol, metabolic disorders, cognition and glycemia.
3.2.1. Obesity, diabetes mellitus and cognitive function: clinical studies
Both types 1 and 2 diabetes are associated with impaired cognitive function and increased risk for all dementias (Rawlings et al., 2017, Zilliox et al., 2016, Rawlings et al., 2014). Midlife obesity and diabetes mellitus are significant risk factors for Alzheimer’s disease (Ott et al., 1999, Gudala et al., 2013, Leibson et al., 1997, MacKnight et al., 2002, McCrimmon et al., 2012, Moreira et al., 2013, Reijmer et al., 2010, Whitmer, 2007a, Whitmer, 2007b) and vascular cognitive impairment and dementia (Biessels and Despa, 2018). Individuals with higher glucose levels, but without diabetes (Crane et al., 2013) also have an increased risk for dementia, and fluctuations in glucose levels and repeated hypoglycemic events are also linked to increased risk of dementia (Haroon et al., 2015, Exalto et al., 2013, Feinkohl et al., 2015, Geijselaers et al., 2015, Rawlings et al., 2017). Moreover, obese women with type 2 diabetes have a higher occurrence of cognitive decline than men (Yaffe et al., 2004) and are twice as likely to have dementia compared to women with normal weight (Whitmer et al., 2005). Importantly, as described above, cognitive impairments and risk for dementia was exacerbated by hormone therapy in women with pre-existing type 2 diabetes (Espeland et al., 2015a). While the factors contributing to the link between diabetes, hormone therapy and cognitive impairments in females are not completely understood, older women who had pre-existing type 2 diabetes and received hormone therapy had decreased brain volume compared to those receiving placebo treatment (Espeland et al., 2015b). Hormone therapy did not affect brain volume in women without diabetes.
3.2.2. Obesity, diabetes mellitus and cognitive function: Preclinical studies
The contribution of type 2 diabetes and obesity to increased risk of cognitive decline and dementias (Kanoski and Davidson, 2011) has been evidenced in animals on high fat diet and other models of obesity/diabetes including db/db mice and Zucker rats (Boitard et al., 2012, Greenwood and Winocur, 1990, Greenwood and Winocur, 1996, Greenwood and Winocur, 2005, Kanoski and Davidson, 2010, Kanoski et al., 2010, Li et al., 2002, Stranahan et al., 2008). Adverse effects of metabolic stress on cognition are mediated by hippocampal insulin resistance (McNay et al., 2010, Winocur and Greenwood, 2005, Winocur et al., 2005) and reduced structural (Arnold et al., 2014, Stranahan et al., 2008) and functional synaptic plasticity within the hippocampus (Grillo et al., 2011, Liu et al., 2015b). Specific impairments in long-term potentiation (LTP), the cellular correlate for learning and memory, have been reported in the hippocampus of high fat diet rodents (Karimi et al., 2013, Stranahan et al., 2008), obese Zucker rats (Alzoubi et al., 2005, Gerges et al., 2003), and db/db mice (Li et al., 2002). Moreover, several studies have demonstrated sex differences in the effects of high fat diet on plasticity and learning and memory (Salinero et al., 2020, Hwang et al., 2010) and interestingly, while young high fat diet-fed males exhibited aberrant glucose regulation, intact females did not, although both males and females exhibited impairments in spatial object recognition (Underwood and Thompson, 2016). These results suggest that obesity can dramatically impair hippocampal function directly in both males and females, and that improved glucose homeostasis does not correlate with spatial memory in females.
Pathophysiological changes that commonly correlate with cognitive impairment and dementia include disruptions in insulin signaling, autonomic function, mitochondrial metabolism, and neuroinflammation (Gaspar et al., 2016). It has been suggested that brain-specific insulin resistance and impaired glucose regulation contribute to the development of Alzheimer’s disease and its related dementias (de la Monte, 2012a, Akter et al., 2011, Ott et al., 1999), but the specific mechanisms of these contributions are unknown. Insulin signaling regulates several neurotransmitters in the brain (Boyd et al., 1985),(Wan et al., 1997), and has been shown to enhance learning and memory (Zhao and Alkon, 2001, Stern et al., 2014a, Stern et al., 2014b). Intriguingly, studies have suggested that exposure to anti-diabetic drugs (e.g., metformin), which control blood glucose levels, lowers risk for neurodegenerative diseases (Hsu et al., 2011, Ng et al., 2014), and analogues of glucagon-like peptide 1, which facilitate insulin signaling, also appear effective in preventing the development of memory impairment in animal models of diabetes and Alzheimer’s disease (Liu et al., 2015a, McClean and Holscher, 2014, McClean et al., 2011). In contrast, increasing the amount of circulating insulin had no effect on memory function in cognitively impaired patients (Marks et al., 2009, Shemesh et al., 2012), and recent intervention studies found no evidence of slower progression to Alzheimer’s disease in diabetic patients treated with anti-diabetic agents (Geijselaers et al., 2015, Areosa Sastre et al., 2017, de Galan et al., 2009), suggesting pre-existing conditions impair metabolic target (glucose homeostasis and insulin signaling) treatment effectiveness for cognition improvement. In spite of these disparate findings, there is general agreement that insulin deficiency (relative or absolute) contributes to the development of Alzheimer’s disease (de la Monte, 2012a, de la Monte, 2012b, Craft et al., 2012), and it is undoubtedly clear that better management of glucose homeostasis will improve cognitive function in patients with diabetes mellitus.
3.2.3. Regulation of glucose homeostasis by the hypothalamus
Homeostatic functions including metabolism are influenced by central pathways and mechanisms. The liver plays a crucial role in the maintenance of glucose homeostasis and is largely governed by the autonomic nervous system (Bruinstroop et al., 2014, Puschel, 2004). Activation of hepatic sympathetic innervation increases gluconeogenesis and glycogenolysis, whereas activation of parasympathetic nerves decreases glucose production and increases glucose storage (Nonogaki, 2000, Shimazu, 1996, Uyama et al., 2004, Shimazu and Fukuda, 1965). In type 2 diabetes mellitus excessive hepatic glucose production, in part due to inappropriately increased glycogenolysis and overactive gluconeogenic pathways, is a major contributing factor to hyperglycemia (Bogardus et al., 1984, DeFronzo et al., 1982, Shao et al., 2005). Intriguingly, increased activity of the sympathetic nervous system plays a role in the development of metabolic disorders, including obesity and type 2 diabetes mellitus (Carnethon et al., 2003, Schlaich et al., 2015, Thorp and Schlaich, 2015), and interestingly autonomic dysfunction is common in patients with several forms of cognitive impairment, including mild cognitive impairment, Alzheimer’s disease, and dementia with Lewy bodies. Patients with mild cognitive impairment exhibit sympathetic dysfunction compared to age-matched controls (Nicolini et al., 2014). Reduced heart rate variability, a sign of impaired cardiovagal autonomic function, is associated with lower cognitive performance (Frewen et al., 2013). Even in the early stages of Alzheimer’s disease, the central regulation of autonomic functions appears already affected (Parvizi et al., 1998, Parvizi et al., 2000, Rub et al., 2001). Specific measures of autonomic functions in patients with mild to moderate Alzheimer’s disease demonstrate suppressed parasympathetic tone accompanied by sympathetic predominance (Jensen-Dahm et al., 2015). Increased sympathetic nerve activity leads to elevated glucose levels through enhanced hepatic glucose production, glucagon, and adrenalin release and facilitates the development of insulin resistance through reduced muscle glucose uptake (Schlaich et al., 2015, Thorp and Schlaich, 2015).
Previous studies demonstrated that while ERα and ERβ mRNA expressions overlap in a variety of brain areas, there are distinct brain nuclei including the paraventricular nucleus of the hypothalamus (PVN), a key autonomic center, that exclusively expresses high levels of ERβ (Shughrue et al., 1997). ERβ was found in magnocellular and parvocellular neurons including oxytocin, vasopressin-expressing neuroendocrine neurons and preautonomic neurons (Kuiper et al., 1996, Isgor et al., 2003, Lee et al., 2013, Stern and Zhang, 2003). Intriguingly, ERβ is present on a large portion of PVN neurons projecting to the rostroventrolateral medulla, known to be crucial for the control of sympathetic activity (Stern and Zhang, 2003). Consistent with these findings, the involvement of PVN ERβ in the regulation of autonomic functions mainly in blood pressure regulation via the sympathetic nervous system was shown (Xue et al., 2013) (Gingerich and Krukoff, 2006). At the cellular level, estrogen modulates voltage-gated potassium channels in pre-sympathetic rostroventrolateral medulla-projecting PVN neurons (Lee et al., 2013). Moreover, ERβ regulates glutamatergic currents through relocation of GluN1 subunit of NMDA receptors as shown in the PVN of Angiotensin II hypertensive female mice (Marques-Lopes et al., 2017). Estradiol affects PI3K signaling and PI3K pathway regulates feeding and energy metabolism (Borges et al., 2016). In addition, in the Zsombok lab, we demonstrated that in liver-related PVN neurons insulin modulates neurotransmitter release in a PI3K-dependent manner (Gao et al., 2012); therefore, it is likely that estradiol and insulin interact to modulate neuronal activity in preautonomic PVN neurons, and thus contribute to the governance of the glucose homeostasis.
Energy status promotes memory function, and meal-associated memory function also regulates feeding behaviors. The hippocampus plays a critical role in the integration of energy balance-related signals with memory processes, including increased food intake and obesity (Kanoski and Grill, 2017). Importantly reduction of insulin receptors in the hippocampus disrupts glucose homeostasis, likely through insulin resistance and decreased insulin secretion, and impairs object and spatial memory (Soto et al., 2019). Thus, although the neurons of the PVN can directly modulate glucose homeostasis, hippocampal insulin resistance can also ultimately impair glucose homeostasis, possibly through feedback mechanisms. Despite the importance of the PVN in the central regulation of glucose homeostasis, the common intracellular PI3K pathway, and the potential influence of the hypothalamic and hippocampal areas, comprehensive studies are lacking.
3.2.4. Effects of estrogens on cognition and mechanisms of action
Estrogens are known to have neuroprotective effects in the hippocampus (Brinton, 2001), and supportive of cognitive function in healthy females (Hara et al., 2015), and thus loss of circulating estrogens during menopause or due to ovariectomy in our mouse model of menopause can have deleterious effects on hippocampal function and health. Indeed, the loss of normal estrous cycling in middle-age is associated with the onset of spatial memory decline studies in female rats (Markowska, 1999) and mice (Frick and Berger-Sweeney, 2001, Frick et al., 2000, Frick et al., 2002a, Frick et al., 2002b). Previous studies have shown that estradiol exerts beneficial effects on cognitive function in young and middle aged animals (Daniel, 2006), although the effects of estradiol are dependent on age, duration, route of administration and dose by age effect was observed (Foster et al., 2003, Heikkinen et al., 2002, Savonenko and Markowska, 2003, Williams et al., 2006). The beneficial effects of estradiol on memory are most likely mediated, at least in part, through estradiol’s actions on the structural and physiological properties of hippocampal neurons (Rudick and Woolley, 2001, Woolley and McEwen, 1992), and its ability to influence the induction of LTP, and memory formation is well-documented. In female rats, the induction of LTP in vivo is most likely to occur during proestrus, a point in the estrous cycle where endogenous estrogen levels are highest (Warren et al., 1995). A similar effect on LTP induction is observed in slices from the cycling rat (Bi et al., 2001). This effect appears to be mediated by estradiol, as LTP is enhanced in ovariectomized rats treated with estradiol compared to those left untreated (Cordoba Montoya and Carrer, 1997). While the impact of estradiol is clear in the young, healthy hippocampus, comprehensive studies investigating the effects of midlife estradiol treatment on hippocampal function during high fat diet in parallel to changes in glucose homeostasis and cellular changes in hypothalamic neurons involved the central regulation of glucose homeostasis in the context of obesity are lacking.
Estradiol exerts its actions by binding to two classical intracellular estrogen receptors, ERα and ERβ, which can bind directly to DNA act to directly influence gene expression. In addition, estradiol can also act through the membrane-bound G-protein-coupled estrogen receptor (GPER). The ERs and GPER can act rapidly at the membrane to exert rapid effects on several aspects of cell function, by acting at cell signaling pathways to regulate other neurotransmitter receptors and glutamate release (Boulware et al., 2005, Kim et al., 2006, Oberlander and Woolley, 2016), synaptic plasticity (Bi et al., 2001), spine morphology and actin dynamics (Avila et al., 2017, Kramar et al., 2009). Estradiol can rapidly activate the PI3K pathway in hippocampal and neocortical neurons (Mannella and Brinton, 2006, Singh, 2001, Yokomaku et al., 2003), increases hippocampal AKT phosphorylation in vitro (Akama and McEwen, 2003, Yokomaku et al., 2003), and Akt phosphorylation is increased during the high-estradiol phase of the rodent estrous cycle (Spencer et al., 2008, Znamensky et al., 2003). In the hippocampus, estradiol infusion into the hippocampus or lateral ventricles in middle-aged females activates the PI3K pathway and is necessary for estradiol-enhanced object recognition memory (Fan et al., 2010, Fortress et al., 2013). Interestingly, this pathway fails to be activated in aged females. Specifically, a single post-training estradiol injection in aged females failed to enhance object recognition memory and activate the PI3K cascade (Fan et al., 2010).
3.2.5. Effects of insulin on cognition and mechanisms of action
As discussed above, insulin and insulin signaling are potential targets for improving cognitive functions in addition to properly maintain overall glucose metabolism. The brain is a target of insulin signaling, and insulin is able to cross the blood–brain barrier; however, this process is impaired by high fat diet (Gray et al., 2017, Konishi et al., 2017) as insulin resistance develops. Furthermore, estrogen deficiency can increase insulin resistance (Alonso et al., 2010, Vogel et al., 2013), indicating an interaction between the hormone functions. Importantly, proper insulin signaling is crucial for normal hippocampal functioning (Fernandez and Torres-Aleman, 2012, Kleinridders et al., 2014). Insulin receptors are highly expressed in the hippocampus (Dore et al., 1997, Marks et al., 1991, Zhao et al., 1999, Hill et al., 1986), mostly in neuronal soma and synapses, and specifically the dendritic field of CA1 cells (Marks et al., 1988, Schwartz et al., 1992). Evidence suggests that insulin resistance reduces LTP induced by high frequency stimulation (Grillo et al., 2011), suggesting a possible mechanism for insulin resistance to affect hippocampus function. Indeed, ovariectomy and insulin resistance can have compounding negative metabolic effects in a pre-existing diabetic rodent model (Tawfik et al., 2015). In this scenario, estradiol treatment is not beneficial but deleterious, and we can speculate that insulin resistance and ovariectomy interact to impair signaling pathways important for both cognitive functions and central regulation of glucose homeostasis.
3.2.6. Convergence of actions of estrogens and insulin at the PI3K pathway
Insulin and estrogen receptor signaling both converge onto the PI3K cascade (Biessels and Reagan, 2015, Flak and Myers, 2016), which has many roles in cellular function (Katso et al., 2001, Franke et al., 1997). Disruption of this pathway through insulin resistance likely leads to an impaired ability of estradiol to exert positive effects on metabolic function within the hypothalamus and cognitive function within the hippocampus. Destabilization of actin filaments by PI3K activation is critical for both cell motility, maintenance of morphology (Cain and Ridley, 2009), and spine formation (Man et al., 2003), and likely contributes to the structural effects of E2 on dendritic spines. PI3K is robustly activated and necessary for both LTP and LTD induction (Man et al., 2003) in CA1 (Hou and Klann, 2004). Therefore, given its role in insulin and estrogen signaling, impairment of the PI3K cascade, mediated by pre-existing insulin resistance may disrupt the ability of estrogens to mediate neuroprotective effects.
3.2.7. Estrogens, glucose homeostasis, and cognition: Summary and conclusions
Together, these findings provide convincing evidence that obesity and diabetes mellitus are associated with cognitive impairments. Hormone therapy has considerable beneficial impacts on glucose homeostasis and cognition in healthy individuals, but equivocal impacts in individuals with metabolic disorders. Detailed investigation to better understand the effects and mechanisms of estrogens’ actions on cardiometabolic and cognitive functions in healthy and unhealthy individuals is crucial to develop specific individualized therapies that will contribute to cardiometabolic and cognitive health in women and men is necessary. We propose that impaired function of preautonomic PVN neurons and lack of response to estradiol leads to impaired glucose homeostasis, and hypothalamic and hippocampal neuronal properties and functions are affected by estradiol treatment likely via modulation of a common signaling mechanism. It is also likely that hypothalamic and hippocampal neurons interact and dysfunction in one will affect the other, although determining the level and magnitude of interaction requires further detailed studies. Specifically, we propose that insulin resistance caused by high-fat diet impairs downstream signaling pathways necessary for estradiol’s beneficial influence on central regulation of glucose homeostasis, hippocampal LTP, and hippocampus-dependent cognitive function. It is likely that high fat diet impairs glucose regulation through modulation of the hypothalamic neurons, and that high fat diet will impair the PI3K pathway in both these neurons in the hypothalamus and neurons in the hippocampus to affect sensitivity to estradiol. See Fig. 5 for schematic illustration of these hypothesized mechanisms.
Fig. 5. Hypothesized model by which effects of estrogens on the central regulation of glucose homeostasis and subsequent implications for hippocampal function diverge under conditions of healthy and unhealthy aging.
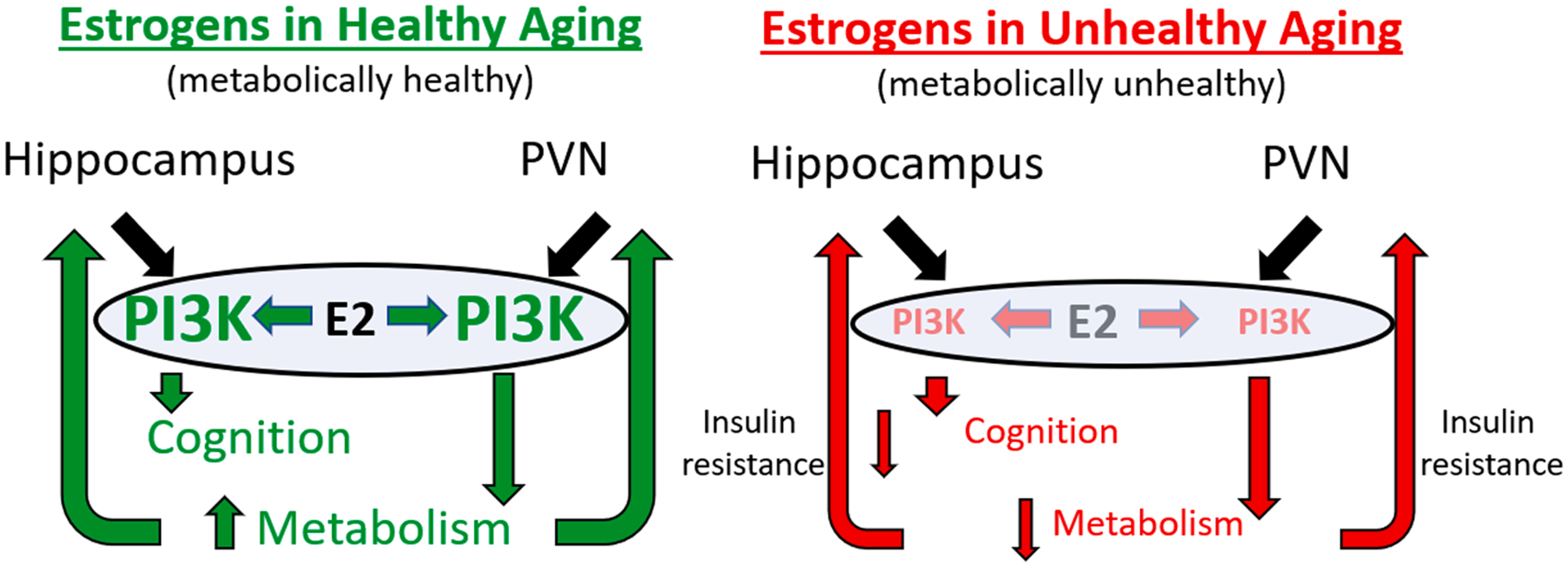
Insulin resistance caused by high fat diet impairs downstream signaling pathways necessary for beneficial influence of estrogens on central regulation of glucose homeostasis, hippocampal LTP, and hippocampus-dependent cognitive function. High fat diet impairs glucose regulation through modulation of the hypothalamic neurons, and high fat diet will impair the PI3K pathway in both these neurons in the hypothalamus and neurons in the hippocampus to affect sensitivity to estrogens.
4. General conclusions
In spite of decades of basic and preclinical research indicating that estrogens enhance cognitive and brain aging, the impact of menopausal hormone therapy on the aging cognitive trajectory remains unclear. The discrepancy between preclinical and clinical results may be explained, at least in part, by the mismatch between the primarily healthy model systems used in preclinical research and the wide range of health phenotypes exhibited by menopausal women. Here we posited four of likely many potential mechanisms by which the ability of estrogens to impact the brain and cognition can diverge under conditions of cardiometabolic health and disease. We suggest that these mechanisms by which divergent effects of estrogens occur involve two broad processes. First, we proposed hypothesized mechanisms by which the status of cardiovascular and metabolic health alters mechanisms by which estrogens act directly on memory systems of the brain including the hippocampus (Fig. 2) and cortex (Fig. 3). Second, we proposed mechanisms by which cardiovascular (Fig. 4) and metabolic health (Fig. 5) impact mechanisms by which estrogens act directly on cardiometabolic systems, which subsequently impact the brain. Our work continues to determine the validity of these hypothesized models. But more broadly, we propose that understanding when and how effects of estrogen administration on cognitive aging varies with health status is critical to understanding which individuals will or will not benefit from menopausal hormone therapy. Further, we suggest that to appropriately model treatment outcomes in populations with diverse health conditions, preclinical research must consider the ability of a potential treatment to impact the cognitive trajectory in both healthy and unhealthy models of aging.
Acknowledgements
This work is supported by the National Institutes of Health Grant P01AG071746 to JMD, SHL, RM, LAS, and AZ.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akama KT, McEwen BS, 2003. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J. Neurosci 23, 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB, 2011. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br. J. Clin. Pharmacol 71, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuck AL, Sakamuri SSVP, Sperling JA, Evans WR, Kolli L, Sure VNLR, Mostany R, Katakam PVG, 2020. Peroxynitrite Decomposition Catalyst Enhances Respiratory Function in Isolated Brain Mitochondria. Am. J. Phys. Heart Circ. Phys [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BH, Barnes HM, Trimmer E, Davidson AM, Ogola BO, Lindsey SH, Mostany R, 2018. Stable density and dynamics of dendritic spines of cortical neurons across the estrous cycle while expressing differential levels of sensory-evoked plasticity. Front. Mol. Neurosci 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen P, Tanko LB, Bagger YZ, Qin G, Christiansen C, 2006. The long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric 9, 108–118. [DOI] [PubMed] [Google Scholar]
- Alonso A, Gonzalez-Pardo H, Garrido P, Conejo NM, Llaneza P, Diaz F, del Rey CG, Gonzalez C, 2010. Acute effects of 17 beta-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age (Dordr.) 32, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Bueno C, Cunha PG, Martinez-Vizcaino V, Pozuelo-Carrascosa DP, Visier-Alfonso ME, Jimenez-Lopez E, Cavero-Redondo I, 2020. Arterial stiffness and cognition among adults: a systematic review and meta-analysis of observational and longitudinal studies. J. Am. Heart Assoc 9, e014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Alkadhi KA, 2005. Impairment of long-term potentiation in the CA1, but not dentate gyrus, of the hippocampus in Obese Zucker rats: role of calcineurin and phosphorylated CaMKII. J. Mol. Neurosci 27, 337–346. [DOI] [PubMed] [Google Scholar]
- Areosa Sastre A, Vernooij RW, Gonzalez-Colaco Harmand M, Martinez G, 2017. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev 6, CD003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF, 2014. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol. Dis 67, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA, 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila JA, Alliger AA, Carvajal B, Zanca RM, Serrano PA, Luine VN, 2017. Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27, 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Hansen HB, Mollgaard A, Ravn P, Qvist P, Kanis JA, Christiansen C, 2004. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone 34, 728–735. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C, Group PS, 2005. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 12, 12–17. [DOI] [PubMed] [Google Scholar]
- Barua A, Standen NB, Galiñanes M, 2010. Dual role of nNOS in ischemic injury and preconditioning. BMC Physiol 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner NE, Black KL, McQuillen SM, Daniel JM, 2021. Previous estradiol treatment during midlife maintains transcriptional regulation of memory-related proteins by ERα in the hippocampus in a rat model of menopause. Neurobiol. Aging 105, 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner NE, Daniel JM, 2021. Estrogen receptor α: a critical role in successful female cognitive aging. Climacteric 24, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner NE, Grissom EM, Pollard KJ, McQuillen SM, Daniel JM, 2019. Neuroestrogen-dependent transcriptional activity in the brains of ERE-Luciferase reporter mice following short- and long-term ovariectomy. eNeuro, 6(5) ENEURO.0275–19: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner NE, McQuillen SM, Perry SF, Miller S, Maroteaux MJ, Gibbs RB, Daniel JM, 2022. History of previous midlife estradiol treatment permanently alters interactions of brain insulin-like growth factor-1 signaling and hippocampal estrogen synthesis to enhance cognitive aging in a rat model of menopause. J. Neurosci 42, 7969–7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC, 2014. Estrogen receptors, the hippocampus, and memory. Neuroscientist 20, 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M, 2001. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. PNAS 98, 13391–13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Despa F, 2018. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol 14, 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Reagan LP, 2015. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci 16, 660–671. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P, 2006. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5, 64–74. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Bernaud VE, Koebele SV, 2021. Menopause, hormone therapy and cognition: maximizing translation from preclinical research. Climacteric 24, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KL, Witty CF, Daniel JM, 2016. Previous midlife oestradiol treatment results in long-term maintenance of hippocampal oestrogen receptor alpha levels in ovariectomised rats: mechanisms and implications for memory. J. Neuroendocrinol 28, 10.111/jne.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KL, Baumgartner NE, Daniel JM, 2018. Lasting impact on memory of midlife exposure to exogenous and endogenous estrogens. Behav. Neurosci 132, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D, 1984. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J. Clin. Invest 74, 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM, 2008. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J. Neuroendocrinol 20, 1023–1027. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM, 2009. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J. Neuroendocrinol 21, 640–647. [DOI] [PubMed] [Google Scholar]
- Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Laye S, Ferreira G, 2012. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 22, 2095–2100. [DOI] [PubMed] [Google Scholar]
- Borges BC, Elias CF, Elias LL, 2016. PI3K signaling: a molecular pathway associated with acute hypophagic response during inflammatory challenges. Mol. Cell. Endocrinol 438, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG, 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci 25, 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd FT Jr., Clarke DW, Muther TF, Raizada MK, 1985. Insulin receptors and insulin modulation of norepinephrine uptake in neuronal cultures from rat brain. J. Biol. Chem 260, 15880–15884. [PubMed] [Google Scholar]
- Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, Sorbi S, Mecocci P, Senin U, Govoni S, 1999. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochem. Biophys. Res. Commun 265, 335–338. [DOI] [PubMed] [Google Scholar]
- Brinton RD, 2001. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: recent insights and remaining challenges. Learn Mem 8, 121–133. [DOI] [PubMed] [Google Scholar]
- Brinton RD, 2008. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci 31, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Borutaite V, 2007. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc. Res 75, 283–290. [DOI] [PubMed] [Google Scholar]
- Bruinstroop E, Fliers E, Kalsbeek A, 2014. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract. Res. Clin. Endocrinol. Metab 28, 673–684. [DOI] [PubMed] [Google Scholar]
- Busija DW, Miller AW, Katakam P, Simandle S, Erdös B, 2004. Mechanisms of vascular dysfunctionin insulin resistance. Curr. Opin. Invest. Drugs 5, 929–935. [PubMed] [Google Scholar]
- Busija DW, Katakam PV, 2014. Mitochondrial mechanisms in cerebral vascular control: shared signaling pathways with preconditioning. J. Vasc. Res 51, 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Miller AW, Katakam P, Erdös B, 2005. Insulin resistance and associated dysfunction of resistance vessels and arterial hypertension. Minerva Med 96, 223–232. [PubMed] [Google Scholar]
- Cain RJ, Ridley AJ, 2009. Phosphoinositide 3-kinases in cell migration. Biol. Cell 101, 13–29. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D, 2003. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation 107, 2190–2195. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W, 2003. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J. Neurosci 23, 6338–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW, 2002. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev 23, 665–686. [DOI] [PubMed] [Google Scholar]
- Chen J-R, Yan Y-T, Wang T-J, Chen L-J, Wang Y-J, Tseng G-F, 2009. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex 19, 2719–2727. [DOI] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF, 1997. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res 778, 430–438. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B, 2012. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol 69, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG, 2005. The Women’s health initiative memory study: findings and implications for treatment. Lancet Neurol 4, 190–194. [DOI] [PubMed] [Google Scholar]
- Crane PK, Walker R, Larson EB, 2013. Glucose levels and risk of dementia. N. Engl. J. Med 369, 1863–1864. [DOI] [PubMed] [Google Scholar]
- Daniel JM, 2006. Effects of oestrogen on cognition: what have we learned from basic research? J. Neuroendocrinol 18, 787–795. [DOI] [PubMed] [Google Scholar]
- Daniel JM, 2013. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav 63, 231–237. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL, 2006. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147, 607–614. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP, 2015. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm. Behav 74, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AM, Mejía-Gómez H, Jacobowitz M, Mostany R, 2020. Dendritic spine density and dynamics of layer 5 pyramidal neurons of the primary motor cortex are elevated with aging. Cerebral Cortex 30, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P, 2012. Understanding weight gain at menopause. Climacteric 15, 419–429. [DOI] [PubMed] [Google Scholar]
- De Galan BE, Zoungas S, Chalmers J, Anderson C, Dufouil C, Pillai A, Cooper M, Grobbee DE, Hackett M, Hamet P, Heller SR, Lisheng L, Macmahon S, Mancia G, Neal B, Pan CY, Patel A, Poulter N, Travert F, Woodward M, Group DE, 2009. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 52, 2328–2336. [DOI] [PubMed] [Google Scholar]
- De la Monte SM, 2012a. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res 9, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Monte SM, 2012b. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 72, 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montgolfier O, Thorin-Trescases N, Thorin E, 2020. Pathological continuum from the rise in pulse pressure to impaired neurovascular coupling and cognitive decline. Am. J. Hypertens 33, 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defronzo RA, Simonson D, Ferrannini E, 1982. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23, 313–319. [DOI] [PubMed] [Google Scholar]
- Dore S, Kar S, Rowe W, Quirion R, 1997. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience 80, 1033–1040. [DOI] [PubMed] [Google Scholar]
- Duarte JV, Pereira JMS, Quendera B, Raimundo M, Moreno C, Gomes L, Carrilho F, Castelo-Branco M, 2015. Early disrupted neurovascular coupling and changed event level hemodynamic response function in Type 2 diabetes: an fMRI study. J. Cereb. Blood Flow Metab 35, 1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LA, 2015. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm. Behav 74, 37–52. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH, 2010. Estrogen and the aging brain: an elixir for the weary cortical network. Ann. N. Y. Acad. Sci 1204, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK, 2009. Arterial pulse wave velocity and cognition with advancing age. Hypertension 53, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW, 2006. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am. J. Physiol. Heart Circulat. Physiol 290, H1264–H1270. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, Bush TL, 1998. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI investigators postmenopausal estrogen/progestin interventions. Diabetes Care 21, 1589–1595. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J, 2004. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. J. Am. Med. Assoc 291, 2959–2968. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Brinton RD, Hugenschmidt C, Manson JE, Craft S, Yaffe K, Weitlauf J, Vaughan L, Johnson KC, Padula CB, Jackson RD, Resnick SM, 2015a. Impact of type 2 diabetes and postmenopausal hormone therapy on incidence of cognitive impairment in older women. Diabetes Care 38, 2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Brinton RD, Manson JE, Yaffe K, Hugenschmidt C, Vaughan L, Craft S, Edwards BJ, Casanova R, Masaki K, Resnick SM, 2015b. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology 85, 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exalto LG, Biessels GJ, Karter AJ, Huang ES, Katon WJ, Minkoff JR, Whitmer RA, 2013. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol 1, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Park A, Nephew KP, 2005. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol. Endocrinol 19, 2901–2914. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci 30, 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinkohl I, Price JF, Strachan MW, Frier BM, 2015. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res. Ther 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Aleman I, 2012. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci 13, 225–239. [DOI] [PubMed] [Google Scholar]
- Flak JN, Myers MG Jr., 2016. Minireview: CNS mechanisms of leptin action. Mol. Endocrinol 30, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman LJ, Liu P, Nagele RG, Yin K, Wong PY, 1998. Augmentation of nitric oxide, superoxide, and peroxynitrite production during cerebral ischemia and reperfusion in the rat. Neurochem. Res 23, 141–148. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, 2012. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22, 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J, 2003. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging 24, 839–852. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88, 435–437. [DOI] [PubMed] [Google Scholar]
- Frewen J, Finucane C, Savva GM, Boyle G, Coen RF, Kenny RA, 2013. Cognitive function is associated with impaired heart rate variability in ageing adults: the Irish longitudinal study on ageing wave one results. Clin. Auton. Res 23, 313–323. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J, 2001. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav. Neurosci 115, 229–237. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J, 2000. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience 95, 293–307. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Delaney SS, Berger-Sweeney J, 2002a. Sex differences in neurochemical markers that correlate with behavior in aging mice. Neurobiol. Aging 23, 145–158. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC, 2002b. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115, 547–558. [DOI] [PubMed] [Google Scholar]
- Froemke RC, 2015. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci 38, 195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB, 2003. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res 962, 244–247. [DOI] [PubMed] [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A, 2012. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar JM, Baptista FI, Macedo MP, Ambrosio AF, 2016. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem. Nerosci 7, 131–142. [DOI] [PubMed] [Google Scholar]
- Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ, 2015. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 3, 75–89. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Aleisa AM, Alkadhi KA, 2003. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience 120, 535–539. [DOI] [PubMed] [Google Scholar]
- Gibbs r. b., 2003. Effects of ageing and long-term hormone replacement on cholinergic neurones in the medial septum and nucleus basalis magnocellularis of ovariectomized rats. J. Neuroendocrinol 15, 477–485. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Krukoff TL, 2006. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 48, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C, 2006. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol 100, 328–335. [DOI] [PubMed] [Google Scholar]
- Girouard H, Lessard A, Capone C, Milner TA, Iadecola C, 2008. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am. J. Physiol.-Heart Circulat. Physiol 294, H156–H163. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH, 2014. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 71, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SM, Aylor KW, Barrett EJ, 2017. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 60, 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G, 1990. Learning and memory impairment in rats fed a high saturated fat diet. Behav. Neural Biol 53, 74–87. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G, 1996. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav. Neurosci 110, 451–459. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G, 2005. High-fat diets, insulin resistance and declining cognitive function. Neurobiol. Aging 26 (Suppl 1), 42–45. [DOI] [PubMed] [Google Scholar]
- Griffin C, Lanzetta N, Eter L, Singer K, 2016. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R211–R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Junor L, Wilson SP, Mott DD, Wilson MA, Reagan LP, 2011. Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol. Behav 105, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom EM, Daniel JM, 2016. Evidence for ligand-independent activation of hippocampal estrogen receptor-alpha by IGF-1 in hippocampus of ovariectomized rats. Endocrinology 157, 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH, 1996. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N. Engl. J. Med 335, 453–461. [DOI] [PubMed] [Google Scholar]
- Gudala K, Bansal D, Schifano F, Bhansali A, 2013. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig 4, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürsoy-Ozdemir Y, Bolay H, Saribaş O, Dalkara T, 2000. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke 31, 1974–1980 discussion 1981. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Koh MT, Gallagher M, 2013. Behaviorally activated mRNA expression profiles produce signatures of learning and enhanced inhibition in aged rats with preserved memory. PLoS One 8, e83674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, Morrison JH, 2015. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev 95, 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan A, Jing Y, Collie ND, Zhang H, Liu P, 2019. Altered neurovascular coupling and brain arginine metabolism in endothelial nitric oxide synthase deficient mice. Nitric Oxide 87, 60–72. [DOI] [PubMed] [Google Scholar]
- Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, Booth GL, 2015. Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care 38, 1868–1875. [DOI] [PubMed] [Google Scholar]
- Hazzouri AZA, Yaffe K, 2014. Arterial stiffness and cognitive function in the elderly. J. Alzheimers Dis 42, S503–S514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H, 2002. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm. Behav 41, 22–32. [DOI] [PubMed] [Google Scholar]
- Hickmott P, Dinse H, 2013. Effects of aging on properties of the local circuit in rat primary somatosensory cortex (S1) in vitro. Cerebral cortex 23, 2500–2513. [DOI] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J, 1986. Autoradiographic localization of insnulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17, 1127–1138. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E, 2004. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci 24, 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Wahlqvist ML, Lee MS, Tsai HN, 2011. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis 24, 485–493. [DOI] [PubMed] [Google Scholar]
- Hu B, Yan L-F, Sun Q, Yu Y, Zhang J, Dai Y-J, Yang Y, Hu Y-C, Nan H-Y, Zhang X, Heng C-N, Hou J-F, Liu Q-Q, Shao C-H, Li F, Zhou K-X, Guo H, Cui G-B, Wang W, 2019. Disturbed neurovascular coupling in type 2 diabetes mellitus patients: Evidence from a comprehensive fMRI analysis. NeuroImage: Clinical 22, 101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Lo EH, 1998. Genetic analysis of NOS isoforms using nNOS and eNOS knockout animals. Prog. Brain Res 118, 13–25. [DOI] [PubMed] [Google Scholar]
- Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH, 2019. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol. Sex Differ 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC, 2010. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 18, 463–469. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H, 2016. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola, 2017. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Gottesman RF, 2019. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ. Res 124, 1025–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola c., 2010. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio-Souza LM, Bombassaro B, Pascoal LB, Portovedo MA, Razolli DS, Coope A, Victorio SC, de Moura RF, Nascimento LF, Arruda AP, Anhe GF, Milanski M, Velloso LA, 2014. Defective regulation of the ubiquitin/proteasome system in the hypothalamus of obese male mice. Endocrinology 155, 2831–2844. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, 2010. Nitric oxide: biology and pathobiology Academic, London. [Google Scholar]
- Isgor C, Shieh KR, Akil H, Watson SJ, 2003. Colocalization of estrogen beta-receptor messenger RNA with orphanin FQ, vasopressin and oxytocin in the rat hypothalamic paraventricular and supraoptic nuclei. Anat Embryol (Berl) 206, 461–469. [DOI] [PubMed] [Google Scholar]
- Jensen-Dahm C, Waldemar G, Staehelin Jensen T, Malmqvist L, Moeller MM, Andersen BB, Hogh P, Ballegaard M, 2015. Autonomic dysfunction in patients with mild to moderate Alzheimer’s disease. J. Alzheimers Dis 47, 681–689. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E, Heart & estrogen, progestin replacement, s., 2003. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med 138, 1–9. [DOI] [PubMed] [Google Scholar]
- Kann, 2016. The interneuron energy hypothesis: Implications for brain disease. Neurobiol. Dis 90, 75–85. [DOI] [PubMed] [Google Scholar]
- Kann O, Papageorgiou IE, Draguhn A, 2014. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab 34, 1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL, 2010. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J. Exp. Psychol. Anim. Behav. Process 36, 313–319. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL, 2011. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol. Behav 103, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Grill HJ, 2017. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL, 2010. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J. Alzheimers Dis 21, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S, 2013. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res 1539, 1–6. [DOI] [PubMed] [Google Scholar]
- Katakam PVG, Tulbert CD, Snipes JA, Erdös B, Miller AW, Busija DW, 2005. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am. J. Physiol. Heart Circulat. Physiol 288, H854–H860. [DOI] [PubMed] [Google Scholar]
- Katakam PVG, Domoki F, Snipes JA, Busija AR, Jarajapu YPR, Busija DW, 2009. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am. J. Physiol. Regulat., Integrat. Comparat. Physiol 296, R289–R298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PVG, Snipes JA, Steed MM, Busija DW, 2012. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J. Cereb. Blood Flow Metab 32, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Ujhelyi MR, Hoenig ME, Miller AW, 1998. Endothelial dysfunction precedes hypertension in diet-induced insulin resistance. Am. J. Phys. Anthropol 275, R788–R792. [DOI] [PubMed] [Google Scholar]
- Katakam PVG, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gáspár T, Grovenburg SM, Snipes JA, Busija DW, 2013. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol 33, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PVG, Gordon AO, Sure VNLR, Rutkai I, Busija DW, 2014. Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am. J. Physiol. Heart Circulat. Physiol 307, H493–H503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PVG, Dutta S, Sure VN, Grovenburg SM, Gordon AO, Peterson NR, Rutkai I, Busija DW, 2016. Depolarization of mitochondria in neurons promotes activation of nitric oxide synthase and generation of nitric oxide. Am. J. Physiol. Heart Circulat. Physiol 310, H1097–H1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD, 2001. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol 17, 615–675. [DOI] [PubMed] [Google Scholar]
- Kazama K, Wang G, Frys K, Anrather J, Iadecola C, 2003. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol.-Heart Circulat. Physiol 285, H1890–H1899. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Bienias JL, Shah A, Meeke KA, Schneider JA, Soriano E, Bennett DA, 2008. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer’s disease: relationship to Mini-Mental State Examination scores. Curr. Alzheimer Res 5, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F, 2014. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab 3, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD, 2006. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience 141, 391–406. [DOI] [PubMed] [Google Scholar]
- Kirshner ZZ, Yao JK, Li J, Long T, Nelson D, Gibbs RB, 2020. Impact of estrogen receptor agonists and model of menopause on enzymes involved in brain metabolism, acetyl-CoA production and cholinergic function. Life Sci 256, 117975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A, 2001. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 322, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Ferris HA, Cai W, Kahn CR, 2014. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63, 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR, 2017. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. PNAS 114, E8478–E8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf D, Frölich L, 2009. Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J. Alzheimers Dis 16, 677–685. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G, 2009. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. Neurosci 29, 12982–12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, O’Connor DH, Fox K, Svoboda K, 2014. Structural Plasticity within the Barrel Cortex during Initial Phases of Whisker-Dependent Learning. J. Neurosci 34, 6078–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA, 1996. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS 93, 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laogun AA, Gosling RG, 1982. In vivo arterial compliance in man. Clin. Phys. Physiol. Meas 3, 201–212. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ, 2000. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol. Aging 21, 49–55. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Hughes T, Yu B, Masaki K, Petrovitch H, Ross GW, White LR, 2010. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension 55, 1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Ryu PD, Lee SY, 2013. Estrogen replacement modulates voltage-gated potassium channels in rat presympathetic paraventricular nucleus neurons. BMC Neurosci 14, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ, 1997. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann. N. Y. Acad. Sci 826, 422–427. [DOI] [PubMed] [Google Scholar]
- Lemarie CA, Tharaux PL, Lehoux S, 2010. Extracellular matrix alterations in hypertensive vascular remodeling. J. Mol. Cell. Cardiol 48, 433–439. [DOI] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Höffken O, Tossi MA, Kalisch T, Kowalewski R, Dinse HR, 2012. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J. Neurosci 32, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y, 2003. GABA and its agonists improved visual cortical function in senescent monkeys. Science (New York, N.Y.) 300, 812–815. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T, 2002. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 113, 607–615. [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, Zhang Q, Brann DW, Lima VV, Tostes RC, Ergul A, 2013. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am. J. Physiol. Regulat., Integrat. Comparat. Physiol 304, R1001–R1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen XZ, Li L, Zhao TV, Bernstein KE, Johnson AK, Lyden P, Fang J, Shi P, 2017. Brain transforming growth factor-beta resists hypertension via regulating microglial activation. Stroke 48, 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC, 2009. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 150, 3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC, 2011. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, Lindsey SH, 2016. GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am. J. Phys. Heart Circ. Phys 310, H953–H961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li G, Holscher C, Li L, 2015a. Neuroprotective effects of geniposide on Alzheimer’s disease pathology. Rev. Neurosci 26, 371–383. [DOI] [PubMed] [Google Scholar]
- Liu Z, Patil IY, Jiang T, Sancheti H, Walsh JP, Stiles BL, Yin F, Cadenas E, 2015b. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One 10, e0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy JC, Sainsbury A, Stock conference working, g., 2009. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev 10, 154–167. [DOI] [PubMed] [Google Scholar]
- Luine VN, 1985. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol 89, 484–490. [DOI] [PubMed] [Google Scholar]
- Luine VN, 2008. Sex steroids and cognitive function. J. Neuroendocrinol 20, 866–872. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M, 2020. Estrogenic regulation of memory: The first 50 years. Horm. Behav 121, 104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight C, Rockwood K, Awalt E, McDowell I, 2002. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement. Geriatr. Cogn. Disord 14, 77–83. [DOI] [PubMed] [Google Scholar]
- Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, Decarli C, 2012. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 11, 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, 2013. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallorquí-Bagué N, Lozano-Madrid M, Toledo E, Corella D, Salas-Salvadó J, Cuenca-Royo A, Vioque J, Romaguera D, Martínez JA, Wärnberg J, López-Miranda J, Estruch R, Bueno-Cavanillas A, Alonso-Gómez Á, Tur JA, Tinahones FJ, Serra-Majem L, Martín V, Lapetra J, Vázquez C, Pintó X, Vidal J, Daimiel L, Gaforio JJ, Matía P, Ros E, Granero R, Buil-Cosiales P, Barragán R, Bulló M, Castañer O, García-De-la-hera M, Yáñez AM, Abete I, García-Ríós A, Ruiz-Canela M, Díaz-López A, Jiménez-Murcia S, Martínez-González MA, de la Torre R, Fernández-Aranda F, 2018. Type 2 diabetes and cognitive impairment in an older population with overweight or obesity and metabolic syndrome: baseline cross-sectional analysis of the PREDIMED-plus study. Sci. Rep 8, 16128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, Macdonald JF, Wang YT, 2003. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38, 611–624. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD, 2006. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J. Neurosci 26, 9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M, Women’s health initiative, i., 2003. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med 349, 523–534. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Harman SM, Brinton EA, Cedars MI, Lobo R, Merriam GR, Miller VM, Naftolin F, Santoro N, 2006. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause 13, 139–147. [DOI] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, Lacroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, van Horn L, Vitolins MZ, Wallace RB, 2013. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. J. Am. Med. Assoc 310, 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranon R, Reckelhoff JF, 2013. Sex and gender differences in control of blood pressure. Clin. Sci. (Lond.) 125, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum ZA, Cohen JB, Zhang C, Derington CG, Greene TH, Ghazi L, Herrick JS, King JB, Cheung AK, Bryan N, Supiano MA, Sonnen JA, Weintraub WS, Williamson J, Pajewski NM, Bress AP, Systolic blood pressure intervention trial research, g., 2022. Association of antihypertensives that stimulate vs inhibit types 2 and 4 angiotensin II receptors with cognitive impairment. JAMA Netw. Open 5, e2145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV, Women’s health initiative, i., 2004. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47, 1175–1187. [DOI] [PubMed] [Google Scholar]
- Markowska, 1999. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J. Neurosci 19, 8122–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Maddison J, Eastman CJ, 1988. Subcellular localization of rat brain insulin binding sites. J. Neurochem 50, 774–781. [DOI] [PubMed] [Google Scholar]
- Marks JL, King MG, Baskin DG, 1991. Localization of insulin and type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. Adv. Exp. Med. Biol 293, 459–470. [DOI] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA, 2009. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci 29, 6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Tesfaye E, Israilov S, van Kempen TA, Wang G, Glass MJ, Pickel VM, Iadecola C, Waters EM, Milner TA, 2017. Redistribution of NMDA receptors in estrogen-receptor-beta-containing paraventricular hypothalamic neurons following slow-pressor angiotensin II hypertension in female mice with accelerated ovarian failure. Neuroendocrinology 104, 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell AJ, Paulson AL, Suk H-J, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim D-N-W, Kritskiy O, Barker SJ, Mangena V, Prince SM, Brown EN, Chung K, Boyden ES, Singer AC, Tsai L-H, 2019. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama W, Kato Y, Yamamoto T, Oh-Hashi K, Hashizume Y, Naoi M, 2001. Peroxynitrite induces neuronal cell death in aging and age-associated disorders: a review. J. Am. Aging Assoc 24, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- Mauvais-jarvis, 2015. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean PL, Holscher C, 2014. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology 86, 241–258. [DOI] [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E, Holscher C, 2011. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci 31, 6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM, 2012. Diabetes and cognitive dysfunction. Lancet 379, 2291–2299. [DOI] [PubMed] [Google Scholar]
- Mcewen BS, Gould E, Orchinik M, Weiland NG, Woolley CS, 1995. Oestrogens and the structural and functional plasticity of neurons: implications for memory, ageing and neurodegenerative processes. Ciba Found Symp 191, 52–66 discussion 66–73. [DOI] [PubMed] [Google Scholar]
- McFadden T, Musaus M, Nelsen JL, Martin K, Jones N, Smith P, Kugler H, Jarome TJ, 2020. Dysregulation of protein degradation in the hippocampus is associated with impaired spatial memory during the development of obesity. Behav. Brain Res 393, 112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS, 2010. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem 93, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN, 2002. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke 33, 1685–1691. [DOI] [PubMed] [Google Scholar]
- Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, Cedars MI, Dowling NM, Gleason CE, Hodis HN, Jayachandran M, Kantarci K, Lobo RA, Manson JE, Pal L, Santoro NF, Taylor HS, Harman SM, 2019. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 26, 1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, 2014. Arterial stiffness and hypertension. Hypertension 64, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Beare R, Wang W, Callisaya M, Srikanth V, For the alzheimer’s disease neuroimaging, i., 2019. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 92, e823–e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira RO, Campos SC, Soldera AL, 2013. Type 2 Diabetes Mellitus and Alzheimer’s Disease: from physiopathology to treatment implications. Diabetes Metab. Res. Rev [DOI] [PubMed] [Google Scholar]
- Moreno-Gonzalez I, Edwards III G, Salvadores N, Shahnawaz M, Diaz-Espinoza R, Soto C, 2017. Molecular Interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 22, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cueto JA, García-Segura LM, Ruiz-Marcos A, 1990. Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res 515, 64–68. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas F, Athilingam J, Piscopo D, Wilbrecht L, 2013. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat. Neurosci 16, 1367–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Earley CJ, Kemper MK, Bacal CS, Metter EJ, 1999. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc. Res 41, 307–311. [DOI] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B, 2014. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimers Dis 41, 61–68. [DOI] [PubMed] [Google Scholar]
- Nicolini P, Ciulla MM, Malfatto G, Abbate C, Mari D, Rossi PD, Pettenuzzo E, Magrini F, Consonni D, Lombardi F, 2014. Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One 9, e96656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K, 2001. Structure and function of dendritic spines. Annu. Rev. Physiol 64, 313–353. [DOI] [PubMed] [Google Scholar]
- Nonogaki, 2000. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43, 533–549. [DOI] [PubMed] [Google Scholar]
- North American Menopause Society 2017. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017/06/27 ed. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2016. 17beta-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. J. Neurosci 36, 2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogola BO, Zimmerman MA, Sure VN, Gentry KM, Duong JL, Clark GL, Miller KS, Katakam PVG, Lindsey SH, 2019. G protein-coupled estrogen receptor protects from angiotensin II-induced increases in pulse pressure and oxidative stress. Front Endocrinol (Lausanne) 10, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM, 1999. Diabetes mellitus and the risk of dementia: The Rotterdam study. Neurology 53, 1937–1942. [DOI] [PubMed] [Google Scholar]
- Ottolini M, Hong K, Cope EL, Daneva Z, Delalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE, Sonkusare SK, 2020. Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation 141, 1318–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet L, Villers-Sidani ED, 2014. Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex. Front. Neuroanat 8, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L, 2007. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 87, 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, van Hoesen GW, Damasio A, 1998. Severe pathological changes of parabrachial nucleus in Alzheimer’s disease. Neuroreport 9, 4151–4154. [DOI] [PubMed] [Google Scholar]
- Parvizi J, van Hoesen GW, Damasio A, 2000. Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann. Neurol 48, 344–353. [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C, Investigators H, 2008. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 7, 683–689. [DOI] [PubMed] [Google Scholar]
- Pines A, 2016. Alzheimer’s disease, menopause and the impact of the estrogenic environment. Climacteric 19, 430–432. [DOI] [PubMed] [Google Scholar]
- Pires PW, Dams Ramos CM, Matin N, Dorrance AM, 2013. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304, H1598–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM, 2007. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke 38, 888–892. [DOI] [PubMed] [Google Scholar]
- Pollard KJ, Daniel JM, 2019. Nuclear estrogen receptor activation by insulin-like growth factor-1 in Neuro-2A neuroblastoma cells requires endogenous estrogen synthesis and is mediated by mutually repressive MAPK and PI3K cascades. Mol. Cell. Endocrinol 490, 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KJ, Wartman HD, Daniel JM, 2018. Previous estradiol treatment in ovariectomized mice provides lasting enhancement of memory and brain estrogen receptor activity. Horm. Behav 102, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu IR, Le KQ, Ducote AL, Li JE, Leland AE, Mostany R, 2021. Increased intrinsic excitability and decreased synaptic inhibition in aged somatosensory cortex pyramidal neurons. Neurobiol. Aging 98, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchayasakul W, Chattipakorn N, Chattipakorn SC, 2014. Estrogen restores brain insulin sensitivity in ovariectomized non-obese rats, but not in ovariectomized obese rats. Metabolism 63, 851–859. [DOI] [PubMed] [Google Scholar]
- Puschel GP, 2004. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat. Rec. A Discov. Mol. Cell Evol. Biol 280, 854–867. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D, 2003. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. J. Am. Med. Assoc 289, 2663–2672. [DOI] [PubMed] [Google Scholar]
- Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E, 2014. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann. Intern. Med 161, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin E, 2017. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care 40, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SS, Shruthi K, Reddy VS, Raghu G, Suryanarayana P, Giridharan NV, Reddy GB, 2014. Altered ubiquitin-proteasome system leads to neuronal cell death in a spontaneous obese rat model. Biochim. Biophys. Acta 1840, 2924–2934. [DOI] [PubMed] [Google Scholar]
- Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P, 2012. Sex difference in cardiovascular risk: role of pulse pressure amplification. J. Am. Coll. Cardiol 59, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ, 2010. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab. Res. Rev 26, 507–519. [DOI] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD, 2014. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol 35, 8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P, 2009. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150, 2109–2117. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM, 2010. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology 151, 1194–1203. [DOI] [PubMed] [Google Scholar]
- Rub U, del Tredici K, Schultz C, Thal DR, Braak E, Braak H, 2001. The autonomic higher order processing nuclei of the lower brain stem are among the early targets of the Alzheimer’s disease-related cytoskeletal pathology. Acta Neuropathol 101, 555–564. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS, 2001. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci 21, 6532–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Carriere I, Carcaillon L, Dartigues JF, Auriacombe S, Rouaud O, Berr C, Ritchie K, Scarabin PY, Ancelin ML, 2014. Estrogen receptor polymorphisms and incident dementia: the prospective 3C study. Alzheimers Dement 10, 27–35. [DOI] [PubMed] [Google Scholar]
- Sakamuri SSVP, Sperling JA, Evans WR, Dholakia MH, Albuck AL, Sure VN, Satou R, Mostany R, Katakam PVG, 2020. Nitric oxide synthase inhibitors negatively regulate respiration in isolated rodent cardiac and brain mitochondria. Am. J. Physiol.-Heart Circulat. Physiol 318, H295–H300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinero AE, Robison LS, Gannon OJ, Riccio D, Mansour F, Abi-Ghanem C, Zuloaga KL, 2020. Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID. FASEB J 34, 15108–15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE, 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab 8, 538–554. [DOI] [PubMed] [Google Scholar]
- Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Hollenberg SM, el Khoudary SR, 2020. Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN heart study. Arterioscler. Thromb. Vasc. Biol 40, 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL, 2003. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 119, 821–830. [DOI] [PubMed] [Google Scholar]
- Schlaich M, Straznicky N, Lambert E, Lambert G, 2015. Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol 3, 148–157. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG, 2000. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci 3, 384–390. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr., 1992. Insulin in the brain: a hormonal regulator of energy balance. Endocr. Rev 13, 387–414. [DOI] [PubMed] [Google Scholar]
- Shabir O, Berwick J, Francis SE, 2018. Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci 19, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Qiao L, Janssen RC, Pagliassotti M, Friedman JE, 2005. Chronic hyperglycemia enhances PEPCK gene expression and hepatocellular glucose production via elevated liver activating protein/liver inhibitory protein ratio. Diabetes 54, 976–984. [DOI] [PubMed] [Google Scholar]
- Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T, 2012. Effect of intranasal insulin on cognitive function: a systematic review. J. Clin. Endocrinol. Metab 97, 366–376. [DOI] [PubMed] [Google Scholar]
- Sherwin, 2003. Estrogen and cognitive functioning in women. Endocr. Rev 24, 133–151. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Fukuda A, 1965. Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science 150, 1607–1608. [DOI] [PubMed] [Google Scholar]
- Shimazu t., 1996. Innervation of the liver and glucoregulation: roles of the hypothalamus and autonomic nerves. Nutrition 12, 65–66. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I, 1997. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388, 507–525. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-SMOLLER S, Wactawski-Wende J, 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama 289, 2651–2662. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH, Study WSHI, 2004b. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291, 2947–2958. [DOI] [PubMed] [Google Scholar]
- Shumaker S, Legault C, Kuller L, Rapp S, Thal L, Lane D, Fillit H, Stefanick M, Hendrix S, Lewis C, Masaki K, Coker L, 2004. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. J. Am. Med. Assoc 291, 2947–2958. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Hall ED, 2007. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res 85, 2216–2223. [DOI] [PubMed] [Google Scholar]
- Singh m., 2001. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine 14, 407–415. [DOI] [PubMed] [Google Scholar]
- Singh-Bains MK, Linke V, Austria MDR, Tan AYS, Scotter EL, Mehrabi NF, Faull RLM, Dragunow M, 2019. Altered microglia and neurovasculature in the Alzheimer’s disease cerebellum. Neurobiol. Dis 132, 104589. [DOI] [PubMed] [Google Scholar]
- Skurnick JH, Aladjem M, Aviv A, 2010. Sex differences in pulse pressure trends with age are cross-cultural. Hypertension 55, 40–47. [DOI] [PubMed] [Google Scholar]
- Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME, 2001. Comparative effects of aging in men and women on the properties of the arterial tree. J. Am. Coll. Cardiol 37, 1374–1380. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K, 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, Cai W, Konishi M, Kahn CR, 2019. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. PNAS 116, 6379–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS, 2008. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience 155, 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelly CE, Cronin J, Daniel JM, Schrader LA, 2012. Long-term oestradiol treatment enhances hippocampal synaptic plasticity that is dependent on muscarinic acetylcholine receptors in ovariectomised female rats. J. Neuroendocrinol 24, 887–896. [DOI] [PubMed] [Google Scholar]
- Stern SA, Chen DY, Alberini CM, 2014a. The effect of insulin and insulin-like growth factors on hippocampus- and amygdala-dependent long-term memory formation. Learn. Mem 21, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Kohtz AS, Pollonini G, Alberini CM, 2014b. Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacology 39, 2179–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Zhang W, 2003. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res 975, 99–109. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP, 2008. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18, 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J, Nunez NP, 2012. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr 51, 861–870. [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ, 2015. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol 11, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi Y, Kawabe Y, Chiba T, Murata S, Ichikawa K, Murayama A, Tanaka K, Baba T, Kato S, Yanagisawa J, 2004. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. EMBO J 23, 4813–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik SH, Mahmoud BF, Saad MI, Shehata M, Kamel MA, Helmy MH, 2015. Similar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem. Res. Int 2015, 567945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecalco-Cruz AC, López-Canovas L, Azuara-Liceaga E, 2023. Estrogen signaling via estrogen receptor alpha and its implications for neurodegeneration associated with Alzheimer’s disease in aging women. Metab. Brain Dis [DOI] [PubMed] [Google Scholar]
- Thorp AA, Schlaich MP, 2015. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res 2015, 341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torreilles F, Salman-Tabcheh S, Gúerin M, Torreilles J, 1999. Neurodegenerative disorders: the role of peroxynitrite. Brain Res. Brain Res. Rev 30, 153–163. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V, 1997. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol 11, 353–365. [DOI] [PubMed] [Google Scholar]
- Tublin JM, Adelstein JM, del Monte F, Combs CK, Wold LE, 2019. Getting to the heart of alzheimer disease. Circ. Res 124, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood EL, Thompson LT, 2016. A high-fat diet causes impairment in hippocampal memory and sex-dependent alterations in peripheral metabolism. Neural Plast 2016, 7385314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama N, Geerts A, Reynaert H, 2004. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol 280, 808–820. [DOI] [PubMed] [Google Scholar]
- Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET, 2008. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J. Mol. Endocrinol 40, 23–34. [DOI] [PubMed] [Google Scholar]
- Van Dalen JW, Brayne C, Crane PK, Fratiglioni L, Larson EB, Lobo A, Lobo E, Marcum ZA, Moll Van Charante EP, Qiu C, Riedel-Heller SG, Rohr S, Ryden L, Skoog I, Van Gool WA, Richard E, 2022. Association of systolic blood pressure with dementia risk and the role of age, u-shaped associations, and mortality. JAMA Intern Med 182, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ, 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetri F, Xu H, Paisansathan C, Pelligrino DA, 2012. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK Ca and Kir channels. Am. J. Physiol.-Heart Circulat. Physiol 302, H1274–H1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Mirhashemi F, Liehl B, Taugner F, Kluth O, Kluge R, Joost HG, Schurmann A, 2013. Estrogen deficiency aggravates insulin resistance and induces beta-cell loss and diabetes in female New Zealand obese mice. Horm. Metab. Res 45, 430–435. [DOI] [PubMed] [Google Scholar]
- Voglewede RL, Vandemark KM, Davidson AM, Dewitt AR, Heffler MD, Trimmer EH, Mostany R, 2019. Reduced sensory-evoked structural plasticity in the aging barrel cortex. Neurobiol. Aging 81, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB, 2008. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51, 99–104. [DOI] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M, 2006. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126, 176–182. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, Macdonald JF, Wang YT, 1997. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388, 686–690. [DOI] [PubMed] [Google Scholar]
- Wang YL, Chen W, Cai WJ, Hu H, Xu W, Wang ZT, Cao XP, Tan L, Yu JT, 2019. Associations of white matter hyperintensities with cognitive decline: a longitudinal study. J. Alzheimers Dis [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT, 1995. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703, 26–30. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Wisniewski T, de Leon MJ, Reisberg B, Pirttila T, Kivimaki T, Lehtimaki T, 2002. Vascular fibrosis and calcification in the hippocampus in aging, Alzheimer disease, and down syndrome. Acta Neuropathol 103, 333–343. [DOI] [PubMed] [Google Scholar]
- White MC, Miller AJ, Loloi J, Bingaman SS, Shen B, Wang M, Silberman Y, Lindsey SH, Arnold AC, 2019. Sex differences in metabolic effects of angiotensin-(1–7) treatment in obese mice. Biol. Sex Differ 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, 2007a. The epidemiology of adiposity and dementia. Curr. Alzheimer Res 4, 117–122. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, 2007b. Type 2 diabetes and risk of cognitive impairment and dementia. Curr. Neurol. Neurosci. Rep 7, 373–380. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr., Yaffe K, 2005. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC, 2006. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl) 188, 605–618. [DOI] [PubMed] [Google Scholar]
- Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr., 2019. Effect of Intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. J. Am. Med. Assoc 321, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, 2005. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging 26 (Suppl 1), 46–49. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS, 2005. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci 119, 1389–1395. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM, 2012. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One 7, e51385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM, 2013. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology 154, 842–852. [DOI] [PubMed] [Google Scholar]
- Wong TP, Marchese G, Casu MA, Ribeiro-Da-silva A, Cuello AC, Koninck Y, 2006. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci. Lett 397, 64–68. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci 12, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Adelman JP, Maylie J, 2011. Ovarian hormone deficiency reduces intrinsic excitability and abolishes acute estrogen sensitivity in hippocampal CA1 pyramidal neurons. J. Neurosci 31, 2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Singh IN, Hall ED, 2009. Tempol protection of spinal cord mitochondria from peroxynitrite-induced oxidative damage. Free Radic. Res 43, 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y, 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK, 2013. Estrogen receptor-beta in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P, 2002. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol. Psychiatry 51, 677–682. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K, 2004. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63, 658–663. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR, 2009. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol. Aging 30, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr., Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW, 2004. Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. USA 101, 4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Brinton RD, 2012. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer’s disease. Adv. Pharmacol 64, 327–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, Dekosky ST, Carlson MC, Ginkgo evaluation of memory study, i., 2013. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology 81, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Cudmore RH, Linden DJ, 2019. Estrogen-dependent functional spine dynamics in neocortical pyramidal neurons of the mouse. J. Neurosci 39, 4874–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H, 2003. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol. Endocrinol 17, 831–844. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yan L-F, Sun Q, Hu B, Zhang J, Yang Y, Dai Y-J, Cui W-X, Xiu S-J, Hu Y-C, Heng C-N, Liu Q-Q, Hou J-F, Pan Y-Y, Zhai L-H, Han T-H, Cui G-B, Wang W, 2019. Neurovascular decoupling in type 2 diabetes mellitus without mild cognitive impairment: potential biomarker for early cognitive impairment. Neuroimage 200, 644–658. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T, 2001. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci 24, 1071–1089. [DOI] [PubMed] [Google Scholar]
- Zang J, Shi J, Liang J, Zhang X, Wei W, Yao C, Zhuang X, Wu G, 2021. Pulse pressure, cognition, and white matter lesions: a mediation analysis. Front Cardiovasc Med 8, 654522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW, 2011. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. PNAS 108, E617–E624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL, 2001. Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol 177, 125–134. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL, 1999. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J. Biol. Chem 274, 34893–34902. [DOI] [PubMed] [Google Scholar]
- Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM, 2013. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW, 2016. Diabetes and Cognitive Impairment. Curr. Diab. Rep 16, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MA, Hutson DD, Trimmer EH, Kashyap SN, Duong JL, Murphy B, Grissom EM, Daniel JM, Lindsey SH, 2017. Long- but not short-term estradiol treatment induces renal damage in midlife ovariectomized Long-Evans rats. Am. J. Physiol. Renal Physiol 312, F305–F311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA, 2003. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J. Neurosci 23, 2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]


