Abstract
We have identified, cloned, and sequenced the hca cluster encoding the dioxygenolytic pathway for initial catabolism of 3-phenylpropionic acid (PP) in Escherichia coli K-12. This cluster maps at min 57.5 of the chromosome and is composed of five catabolic genes arranged as a putative operon (hcaA1A2CBD) and two additional genes transcribed in the opposite direction that encode a potential permease (hcaT) and a regulator (hcaR). Sequence comparisons revealed that while hcaA1A2CD genes encode the four subunits of the 3-phenylpropionate dioxygenase, the hcaB gene codes for the corresponding cis-dihydrodiol dehydrogenase. This type of catabolic module is homologous to those encoding class IIB dioxygenases and becomes the first example of such a catabolic cluster in E. coli. The inducible expression of the hca genes requires the presence of the hcaR gene product, which acts as a transcriptional activator and shows significant sequence similarity to members of the LysR family of regulators. Interestingly, the HcaA1A2CD and HcaB enzymes are able to oxidize not only PP to 3-(2,3-dihydroxyphenyl)propionate (DHPP) but also cinnamic acid (CI) to its corresponding 2,3-dihydroxy derivative. Further catabolism of DHPP requires the mhp-encoded meta fission pathway for the mineralization of 3-hydroxyphenylpropionate (3HPP) (A. Ferrández, J. L. García, and E. Díaz, J. Bacteriol. 179:2573–2581, 1997). Expression in Salmonella typhimurium of the mhp genes alone or in combination with the hca cluster allowed the growth of the recombinant bacteria in 3-hydroxycinnamic acid (3HCI) and CI, respectively. Thus, the convergent mhp- and hca-encoded pathways are also functional in S. typhimurium, and they are responsible for the catabolism of different phenylpropanoid compounds (3HPP, 3HCI, PP, and CI) widely available in nature.
Phenylpropanoid compounds are widely available in natural environments, and they can originate from putrefaction of proteins in soil or as breakdown products of several constituents of plants, such as lignin, various oils, and resins (2, 6, 14, 20). Microbial catabolism of phenylpropanoid compounds plays an important role not only in the natural degradative cycle of these aromatic molecules but also in their industrial applications such as wine making, aging, and storage (13). In particular, degradation of cinnamic acid (CI), 3-phenylpropionic acid (PP), and their hydroxylated derivatives has been reported in several bacteria, including Acinetobacter sp. (14), Pseudomonas sp. (2, 51), Arthrobacter sp. (51), Escherichia coli (10), and Rhodococcus globerulus (6). Although most of the intermediates of these pathways are known, there has been little genetic characterization of these degradative routes, with the exception of the 3-(3-hydroxyphenyl)propionate (3HPP) catabolic pathways of E. coli K-12 (20) and R. globerulus PWD1 (6).
Biochemical studies and the isolation and characterization of mutants defective in the catabolism of PP and 3HPP (compounds I and IV in Fig. 1B, respectively) revealed that in E. coli the aerobic degradation of these compounds proceeds by two initially separate routes that converge into 3-(2,3-dihydroxyphenyl)propionate (DHPP) (compound III), which suffers an extradiol ring cleavage and is ultimately degraded to Krebs cycle intermediates (9–11) (Fig. 1B). The cloning, sequencing, and transcriptional regulation of the meta fission cluster for the catabolism of 3HPP in E. coli K-12 have been recently reported (20).
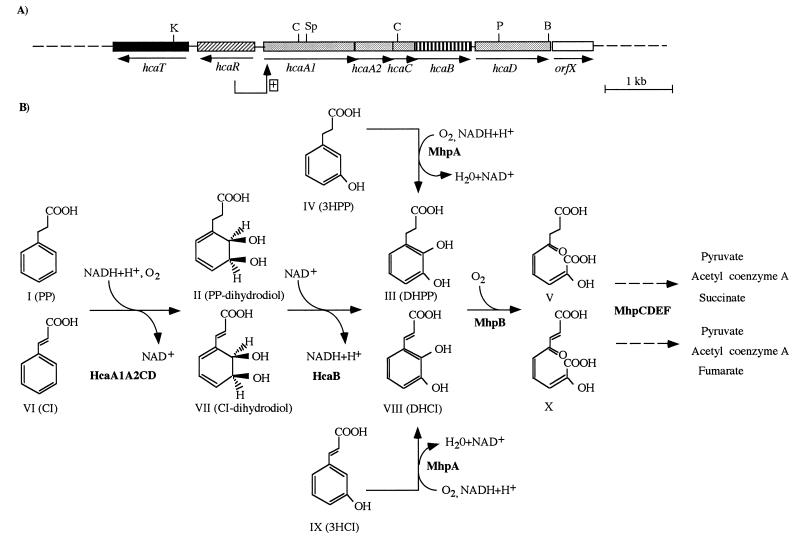
FIG. 1.
Convergent pathways for the catabolism of PP (CI) and 3HPP (3HCI) in E. coli. (A) Physical and genetic map of the chromosomal hca region. The locations of the genes are shown relative to those of some relevant restriction endonuclease sites, i.e., BamHI (B), ClaI (C), KpnI (K), PstI (P), and SphI (Sp). Arrows indicate the directions of gene transcription. The boxed plus sign indicates stimulation of gene expression by the hcaR gene product in the presence of PP. Genes with similar shadings encode subunits of the same protein. (B) Proposed biochemistry of the PP (3HPP) and CI (3HCI) catabolic pathways. HcaA1A2CD and HcaB are the enzymes encoded by the corresponding hca structural genes. MhpA to MhpF are the enzymes for the catabolism of 3HPP (3HCI) and DHPP (DHCI). The metabolites are PP (compound I), cis-3-(3-carboxyethyl)-3,5-cyclohexadiene-1,2-diol (compound II), DHPP (compound III), 3HPP (compound IV), 2-hydroxy-6-ketononadienedioate (compound V), CI (compound VI), cis-3-(3-carboxyethenyl)-3,5-cyclohexadiene-1,2-diol (compound VII), DHCI (compound VIII), 3HCI (compound IX), and 2-hydroxy-6-ketononatrienedioate (compound X). Enzymes: HcaA1A2CD, 3-phenylpropionate dioxygenase; HcaB, 3-phenylpropionate-dihydrodiol dehydrogenase; MhpA, 3-(3-hydroxyphenyl)propionate hydroxylase; MhpB, 3-(2,3-dihydroxyphenyl)propionate 1,2-dioxygenase; MhpC, 2-hydroxy-6-ketonona-2,4-dienedioate hydrolase; MhpD, 2-keto-4-pentenoate hydratase; MhpE, 4-hydroxy-2-ketovalerate aldolase; MhpF, acetaldehyde dehydrogenase (acylating).
The catabolism of PP in E. coli is initiated by a dioxygenolytic pathway (10, 11) (Fig. 1B). The first step is catalyzed by a 3-phenylpropionate dioxygenase, which inserts both atoms of molecular oxygen into positions 2 and 3 of the phenyl ring of PP, yielding cis-3-(3-carboxyethyl)-3,5-cyclohexadiene-1,2-diol (PP-dihydrodiol; compound II), which is subsequently oxidized by the 3-phenylpropionate-dihydrodiol dehydrogenase to give DHPP (compound III) (10, 11) (Fig. 1B). Enzyme assays and respirometry showed that the syntheses of enzymes required to convert the two initial growth substrates, PP and 3HPP, into DHPP are inducible and under separate control (10, 11). Very recently, it has been shown that in batch cultures the utilization of PP was immediately repressed by glucose (30).
Here we present the cloning, genetic characterization, and mechanism of regulation of the hca genes encoding the complete dioxygenolytic pathway for the catabolism of PP in E. coli K-12. This work constitutes the first genetic characterization of such a pathway and represents the first report of a gene cluster encoding a phenyl ring hydroxylating dioxygenase from E. coli. Moreover, we provide experimental evidence that 3HPP and PP catabolic pathways are also responsible for the catabolism of 3-hydroxycinnamic acid (3HCI) and CI, respectively.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli K-12 strains used were MC1061 [F− hsdR mcrB araD139 Δ(araABC-leu)7679 ΔlacX74 galU galK rpsL thi] (46), DH5α [F− endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA relA1 Δ(argF-lac)U169 deoR φ80lacZΔM15] (46), and MG1655 (F− λ−) (4). E. coli ED1061 is an hcaA1 mutant of E. coli MC1061 (this study). The other strain used in this study was Salmonella typhimurium LT-2 (20). For cloning and expression purposes we have used two chloramphenicol (CM) resistance low-copy-number cloning vectors, plasmids pCK01 (20) and pVTR-B (41), as well as the pUC18, pUC19 (46), and pUC18Not (20) vectors. Plasmid pPADR2 is an RSF1010-based promiscuous plasmid containing the complete mhp pathway (20). Plasmids pUC4K (Pharmacia) and pMAK700 (24) were used for insertional inactivation of the hcaA1 gene (former orfA) and construction of the strain E. coli ED1061, respectively. Unless otherwise stated, bacteria were grown in Luria-Bertani (LB) medium (46) at 37°C. When used as carbon sources, aromatic acids were supplied at 1 mM (CI) or 5 mM (PP, 3HPP, and 3HCI) to M63 minimal medium (36), and the cultures were incubated at 30°C (E. coli) or 37°C (S. typhimurium). To perform the red color formation assay, cells were incubated at 30°C (E. coli) or 37°C (S. typhimurium) on LB medium containing 5 mM PP. Where appropriate, antibiotics were added at the following concentrations: ampicillin (AP), 100 μg/ml; CM, 35 μg/ml; kanamycin (KM), 50 μg/ml.
DNA manipulations and sequencing.
Plasmid DNA was prepared by the rapid alkaline lysis method (46). Transformation of E. coli was carried out by the RbCl method (46). Electroporation (Gene Pulser, Bio-Rad) was used for S. typhimurium. DNA manipulations and other molecular biology techniques were essentially as described elsewhere (46). DNA fragments were purified by using low-melting-point agarose. Oligonucleotides were synthesized on an Oligo-1000M nucleotide synthesizer (Beckman Instruments, Inc.). Nucleotide sequences were determined directly from plasmids by using the dideoxy chain termination method (47). Standard protocols of the manufacturer for Taq DNA polymerase-initiated cycle sequencing reactions with fluorescently labeled dideoxynucleotide terminators (Applied Biosystems Inc.) were used. The sequencing reactions were analyzed with a 377 automated DNA sequencer (Applied Biosystems Inc.). Sequences were extended by designing primers based on the already-determined sequence.
Sequence data analyses.
Nucleotide sequence analyses were done with the DNA-Strider 1.2 program. Amino acid sequences were analyzed with Protein Analysis Tools on the ExPASy World Wide Web molecular biology server of the Geneva University Hospital and the University of Geneva. Nucleotide and protein sequence similarity searches were made by using the BLASTP, BLASTN, and BLASTX programs (1) via the National Institute for Biotechnology Information server. Pairwise and multiple protein sequence alignments were made with the ALIGN (59) and CLUSTAL W (56) programs, respectively, on the Baylor College of Medicine Human Genome Center server. The E. coli database collection ECDC (31) was accessed via the Internet.
Insertional inactivation of the hcaA1 gene (orfA) and construction of E. coli ED1061.
The 3′-end-truncated hcaA1 gene (former orfA) was PCR amplified from the chromosome of E. coli MC1061 by using oligonucleotides HCA5 (5′-CCGAATTCACATATTAGCAACCAACCAGC-3′ [the sequence corresponds to nucleotides 2384 to 2407 in Fig. 3; the engineered EcoRI site is underlined]) and HCA3 (5′-CCCTGCAGGTAAGCGGCGGTTTTATC-3′ [the sequence corresponds to nucleotides 3790 to 3815 in Fig. 3; the engineered PstI site is underlined]) as primers. The 1.4-kb amplified fragment was digested with EcoRI-PstI and cloned into the EcoRI-PstI double-digested pUC19 vector to form pHCA. The orfA in pHCA was inactivated by the insertion at its ClaI restriction site of a 1.3-kb AccI KM resistance cassette from plasmid pUC4K. The disrupted orfA was then subcloned as a 1.9-kb EcoRI-SphI blunt-ended fragment into the blunt-ended-SphI-digested pMAK700 plasmid, a pSC101-derived temperature-sensitive replicon (24), to form pHCA700. Because this plasmid replicates at 30°C but not at 44°C, it was possible to identify its integration through homologous recombination into the chromosome of E. coli MC1061 by selecting for KM resistance at 44°C. Since replication from the plasmid origin is deleterious to the host cell, when the cointegrates were subsequently grown at 30°C a second recombination event occurred, regenerating free plasmid in the cell and a disrupted orfA in the chromosome. The resident plasmid was then cured from the mutant strain by growing the cells at 44°C in LB medium without the antibiotic resistance marker of the vector, i.e., CM. A KM-resistant and CM-sensitive mutant strain was selected and named E. coli ED1061.
FIG. 3.
Nucleotide and derived amino acid sequences of the PP dioxygenolytic pathway. The sequence data in uppercase letters appear in the GenBank/EMBL data bank under accession numbers Y11071 (nucleotides 1 to 1433) and Y11070 (nucleotides 3948 to 7259). The sequence from nucleotide 1434 to 3947 (lowercase letters) was taken from the GenBank/EMBL data bank (accession number AE000340). Only the sequences of the 5′- and 3′-end-coding regions of the hca genes and orfX are shown. The 3′ end of the csiE gene is also shown. Short arrows, direction of gene transcription; asterisks, stop codons. Potential Shine-Dalgarno sequences are boldfaced. Inverted repeats are marked with facing arrows underneath the sequence. The putative binding motif of LTTRs (49) is doubly underlined, and the characteristic T and A residues are boxed. The nucleotide sequence present in oligonucleotide HCAR, used for PCR amplification of hcaA1, is italicized.
Construction of plasmids.
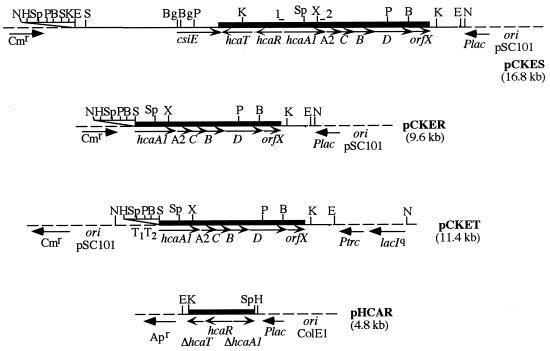
To isolate the hca cluster, we constructed an EcoRI DNA library of E. coli MC1061 into pUC18 using as host the mutant strain ED1061, and then the transformants were screened for their ability to recover the dark red phenotype of E. coli MC1061 due to DHPP accumulation when growing on PP-containing LB medium. All red colonies harbored the plasmid pHCAES containing a 13.2-kb EcoRI insert. However, since plasmid pHCAES was shown to be highly unstable after several rounds of cultivation, the 13.2-kb EcoRI fragment was subcloned into the low-copy-number vector pCK01. The resulting plasmid pCKES (Fig. 2) also conferred on E. coli ED1061 the ability to produce the dark red color when growing on PP-containing LB medium, and this phenotype was stably maintained when the cells were grown in the presence of CM.
FIG. 2.
Schematic representation of the subcloning and expression of the regulatory and catabolic hca genes. The subcloning strategies are described in detail in Materials and Methods. The relevant elements and restriction sites are indicated. The thick line represents the DNA fragment whose sequence is shown in Fig. 3. Vector-derived sequences are indicated by dashed lines. The Plac and the Ptrc promoters and direction of transcription are indicated (arrows). Δ, truncated gene. T1 and T2 are the transcriptional terminators of the E. coli rrnB operon (41). 1 and 2, oligonucleotides HCAR and HCA3, which were used as primers for the PCR to construct plasmid pCKER. The region encoding the replication (ori) function is also indicated. B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; K, KpnI; N, NotI; P, PstI; S, SmaI; Sp, SphI; X, XhoI. Apr and Cmr, genes conferring resistance to AP and CM, respectively.
The hcaR gene was isolated from plasmid pCKES as a KpnI-SphI 2.1-kb fragment and subcloned into the KpnI-SphI double-digested pUC19 cloning vector to form pHCAR (Fig. 2). To delete genes hcaRT from the hca cluster, an hcaA1 gene truncated at its 3′ end was PCR amplified from plasmid pCKES by using primers HCAR (5′-CCCCCGGGCCGTAGTTCCATCACCTTC-3′ [the sequence corresponds to nucleotides 2319 to 2338 in Fig. 3; the engineered SmaI restriction site is underlined]) and HCA3 (5′-CCCTGCAGGTAAGCGGCGGTTTTATC-3′ [the sequence corresponds to nucleotides 3790 to 3815 in Fig. 3). The 1.5-kb PCR product was digested with SmaI and XhoI, gel purified, and ligated to the SmaI-XhoI double-digested pCKES plasmid to form pCKER (Fig. 2). Since we have observed constitutive expression of the hca catabolic genes in E. coli ED1061(pCKER) cells, to avoid the possible transcriptional readthrough from the promoter of the CM resistance gene of the vector (Fig. 2), we subcloned the 6.0-kb EcoRI-HindIII fragment carrying the hcaA1A2CBDorfX genes into the EcoRI-HindIII double-digested low-copy-number pVTR-B vector. The resulting plasmid pCKET contained the catabolic hca genes together with its potential promoter region, downstream of the strong T1T2 transcriptional terminators of the E. coli rrnB operon (41) (Fig. 2), thus excluding additional expression signals from the vector.
Resting-cell reactions.
Phenylpropionate dioxygenase and phenylpropionate-dihydrodiol dehydrogenase activities were checked by analyzing the formation of DHPP in resting-cell assays. Thus, cultures of E. coli or S. typhimurium were grown overnight in LB medium and then diluted into fresh medium in the presence or absence of 1 mM aromatic inducer (PP or CI) to an optical density at 600 nm of about 0.08. Growth was resumed at 30°C (E. coli) or 37°C (S. typhimurium) until the cultures reached an optical density at 600 nm of about 0.8. The cell cultures were then centrifuged at 3,000 × g for 10 min at 20°C, and cells were washed and resuspended in a 0.05 volume of M63 minimal medium. The resting-cell reaction was performed in a final volume of 5 ml containing 4.5 ml of M63 minimal medium supplemented with 1 mM glucose and 0.5 ml of the cell suspension. The reaction was started by the addition of 0.5 mM PP or CI, and the tubes were incubated on a rotary shaking platform at a temperature of 30°C. Samples of 0.5 ml were taken at different times and centrifuged for 5 min at 10,000 × g to remove the cells. Products accumulated in the supernatant were analyzed with Gilson high-pressure liquid chromatography (HPLC) equipment using a Lichrosphere 5 RP-8 column (150 by 4.6 mm) and an isocratic flow of a 40% methanol-H2O mobile phase pumped at a flow rate of 1 ml/min. Peaks with retention times of 22.3, 15.9, 6.20, and 4.63 min, corresponding to those of authentic standard CI, PP, 2,3-dihydroxicinnamic acid (DHCI), and DHPP, respectively, were monitored at 210 nm.
To confirm the formation of DHCI, 1H nuclear magnetic resonance (NMR) spectra were recorded in CD3OD at 30°C on a Varian Unity 500 spectrometer. 1H chemical shifts were referenced to internal residual CHD2OD.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this study have been submitted to the GenBank/EMBL data bank under accession numbers Y11070 and Y11071.
RESULTS AND DISCUSSION
Cloning of the hca catabolic cluster of E. coli K-12.
Although during the course of this work the complete genome sequence of E. coli K-12 was reported (8), at the beginning of this research the analysis of the current E. coli database collection (ECDC release 27) (31) revealed the existence of an unmapped 4.6-kb sequence (accession no. Z37966), containing a 1.3-kb open reading frame (ORF) (orfA) that coded for a product showing significant similarity to the large terminal subunit of some multicomponent aromatic-ring initial dioxygenases (12). Since it was reported that the catabolism of PP in E. coli proceeds via dioxygenolytic attack of the ring (10, 11) (Fig. 1B), we assumed that orfA could encode a component of the 3-phenylpropionic acid (hydrocinnamic acid) initial dioxygenase (Hca dioxygenase). To test this assumption, we constructed E. coli MC1061 mutants by the insertion of a KM resistance cassette within orfA (see Materials and Methods). The selection of the mutant strains was based on the previous observation that accumulation of DHPP on rich medium generates a reddish-brown color due to autooxidation of this aromatic compound to the corresponding quinones and semiquinones (11). Thus, an E. coli strain such as MC1061, which contains a chromosomal deletion (ΔlacX74) spanning the initial genes of the mhp cluster responsible for the catabolism of DHPP (20), formed a dark red color when grown on PP-containing LB medium due to DHPP accumulation. Interestingly, the MC1061 KM-resistant mutants did not show the PP-dependent color reaction, indicating that the disrupted orfA was involved in the initial dioxygenation of PP. One of these mutants was selected, and it is referred to hereafter as E. coli ED1061.
To genetically characterize the complete Hca dioxygenase and the following enzyme of the dioxygenolytic pathway, i.e., the 3-phenylpropionate-dihydrodiol dehydrogenase (HcaB) (Fig. 1B), we constructed plasmid pCKES (Fig. 2) (see Materials and Methods), which contains a 13.2-kb EcoRI fragment from E. coli MC1061 that confers on E. coli ED1061 the ability to produce the dark red color during growth on PP-containing LB medium. Interestingly, S. typhimurium LT-2, which does not attack PP (see below) and lacks the mhp genes (20), showed the typical red color when transformed with pCKES on PP-containing LB medium. To directly assay the oxidation of PP to DHPP, resting-cell reactions were performed in the presence of PP, and the resultant products accumulated in the supernatants were analyzed by HPLC. E. coli mutant ED1061 and S. typhimurium LT-2 cells grown in LB medium in the presence of 1 mM PP were used as control bacteria since they did not attack this aromatic compound (Table 1). In contrast, resting cells of S. typhimurium LT-2(pCKES) and E. coli ED1061(pCKES) bacteria grown in LB medium in the presence of 1 mM PP rapidly consumed this aromatic compound and produced a metabolite that was eluted by HPLC as standard DHPP (Table 1). Therefore, all these data indicated that the 13.2-kb EcoRI DNA fragment encoded the complete dioxygenolytic pathway for the conversion of PP into DHPP; this pathway is also functional in Salmonella.
TABLE 1.
hca expression in S. typhimurium LT-2 and E. coli ED1061
| Straina | Inducer | Conversion of PP to DHPP (mol%)b |
|---|---|---|
| LT-2 | PP | BD |
| LT-2(pCKET, pHCAR) | None | 2 |
| LT-2(pCKET, pHCAR) | PP | 10 |
| ED1061 | PP | BD |
| ED1061(pCKES) | None | 2 |
| ED1061(pCKES) | PP | 80 |
Plasmids pCKET, pHCAR, and pCKES are diagrammed in Fig. 2.
Expression of the hca catabolic genes was monitored in resting-cell assays by measuring PP consumption and DHPP formation in HPLC. Cells were grown in LB medium in the presence or absence of 1 mM PP (inducer), and the resting-cell assays were performed for 60 min (S. typhimurium LT-2 cells) or 10 min (E. coli ED1061 cells) as described in Materials and Methods with 0.5 mM PP as substrate. BD, below detection limits.
Structural analysis of the hca genes.
The nucleotide sequence of a 7,259-bp DNA fragment that carries the hca cluster was determined (Fig. 3). Analyses of the ORFs and sequence comparisons (see below) suggested the existence of seven genes arranged as follows: (i) five genes encoding the 3-phenylpropionate dioxygenase (hcaA1A2CD) and 3-phenylpropionate-dihydrodiol dehydrogenase (hcaB), (ii) a regulatory gene (hcaR), and (iii) a gene (hcaT) that might encode a transporter. The hca genes are located on the chromosome in the order hcaTRA1A2CBD (Fig. 1A and 2). Downstream of the hcaD gene, the closely linked orfX (Fig. 1A and 3) could also be a member of the hca cluster. Interestingly, all five catabolic genes and orfX appear to be transcribed in the same direction. The Shine-Dalgarno sequences of hcaA1, hcaA2, hcaC, hcaB, and hcaD overlap the preceding ORFs (Fig. 3), suggesting that translational coupling occurs (23). Furthermore, immediately downstream of orfX there is an inverted-repeat sequence (Fig. 3) predicted to form a hairpin loop (ΔG, −23.9 kcal/mol) that could act as a transcriptional terminator of a potential operon. Genes hcaR and hcaT are located upstream of hcaA1A2CBD-orfX, but they are transcribed in the opposite direction (Fig. 1A and 3). Although the intergenic spacing between genes hcaR and hcaT was 159 bp, we could not detect in this DNA fragment typical transcriptional terminator and promoter sequences. The G+C content of the hca-coding regions averaged 53.8%, a value close to the mean G+C content of the E. coli genomic DNA (51.5%) (37).
The hca cluster maps immediately downstream of gene csiE, which encodes the stationary-phase inducible protein CsiE, at min 57.5 of the E. coli chromosome (8) and therefore far from min 8, where the mhp cluster responsible for DHPP degradation is located (20). Interestingly, the third aromatic catabolic pathway characterized so far in E. coli at the molecular level, i.e., the hpa cluster for 4-hydroxyphenylacetate degradation, was shown to map at min 98 (43). Thus, while in some Pseudomonas and Acinetobacter species a supraoperonic clustering of the aromatic catabolic genes has been observed in a limited region of the chromosome (15, 16, 60), in E. coli the aromatic catabolic clusters are dispersed throughout the genome.
The deduced amino acid sequences of the hca gene products were compared with entries in the databases, and the ones showing the highest similarities were then retrieved and analyzed (Table 2).
TABLE 2.
PP pathway genes, gene products, and identities with other proteins
| Gene | % G+C content | Gene product | Deduced no. of residues (kDa) | % Identity with other gene products (no. of residues)a |
|---|---|---|---|---|
| hcaA1 | 52.9 | Large terminal subunit of phenylpropionate dioxygenase | 453 (51.1) | 47.8, TcbAa (450); 47.6, IpbA1_BD2 (460); 47.4, BphA1_A1 (460); 47.3, TodC1 (450); 47.0, TecA1 (449); 46.8, BedC1 (450); 44.3, BphA1_P6 (461); 44.2, BphA1_KF (458); 44.2, BphA_B (457); 44.1, BpdC1 (461); 43.9, BphA_LB (459); 42.9, BnzA (448); 42.9, CumA1 (459); 42.9, IpbA1_JR (459); 42.9, BphA1_KKS (458); 27.5, CmtAb (434) |
| hcaA2 | 52.2 | Small terminal subunit of phenylpropionate dioxygenase | 172 (20.5) | 37.7, BphE_B (186); 34.4, CumA2 (186); 34.4, IpbA2_JR (186); 34.2, BphE_LB (188); 32.8, BphA2_A1 (187); 32.3, IpbA2_BD2 (187); 32.0, BphA2_KKS (193); 31.2, BedC2 (187); 30.7, TecA2 (187); 30.5, BnzB (187); 30.5, TodC2 (187); 30.5, TcbAb (187); 30.2, BphA2_KF (213); 30.2, CmtAc (180); 29.6, BpdC2 (186); 29.6, BphA2_P6 (186) |
| hcaC | 55.4 | Ferredoxin subunit of phenylpropionate dioxygenase | 106 (11.3) | 48.1, BphA3_A1 (107); 47.2, IpbA3_BD2 (107); 44.5, CumA3 (109); 43.6, IpbA3_JR1 (109); 43.5, TcbAc (107); 43.1, BphA3_KKS (109); 42.6, TecA3 (107); 41.8, BphF_LB (109); 41.3, BedB (107); 40.0, BphA3_P6 (108); 40.0, BphA3_KF (108); 40.0, BpdB (108); 39.8, TodB (107); 39.0, CmtAd (118); 37.4, BnzC (91); 36.7, BphF_B (109) |
| hcaD | 54.0 | Ferredoxin reductase subunit of phenylpropionate dioxygenase | 400 (43.9) | 34.6, CmtAa (402); 31.5, IpbA4_JR (411); 31.5, CumA4 (411); 30.9, BphG_B (406); 30.8, BphG_LB (408); 30.8, BphA4_KF (408); 29.7, BpdA (412); 29.7, BphA4_P6 (412); 29.5, TecA4 (410); 29.5, TcbAd (410); 28.8, TodA (410); 28.7, BphA4_KKS (410); 28.5, IpbA4_BD2 (412); 27.5, BedA (410); 27.3, BphA4_A1 (413); 27.2, BnzD (409) |
| hcaB | 55.9 | 2,3-Dihydroxy-2,3-dihydrophenylpropionate dehydrogenase | 270 (28.5) | 48.9, BphB_A1 (263); 46.2, BphB_KKS (276); 45.7, BphB_LB (277); 45.7, BphB_KF (277); 45.1, CumB (276); 44.3, BphB_B (281); 43.7, IpbB_JR (276); 43.1, TodD (275); 43.1, BnzE (275); 42.7, BpdD (280); 42.7, BphB_P6 (280); 41.7, TcbB (275); 22.2, CmtB (259); 17.4, BedD (365) |
| hcaR | 49.8 | Activator of hca cluster | 296 (32.8) | 35.6, CatR (289); 35.2, AlsR_E (297); 33.4, AlsR_B (302); 28.9, CatM (303); 28.9, BphR (314); 28.6, TfdR (295); 27.9, TcbR (294); 26.3, ClcR (294); 25.0, TfdT (228); 24.1, PcaQ (311); 19.0, NahR (374) |
| hcaT | 56.3 | Potential transporter | 379 (41.6) | 35.7, HI0308 (388); 23.6, MalA (394); 23.4, LacY (417); 21.6, CscB (415); 21.4, MhpT (403); 20.6, HppK (453); 20.4, HpaX (458); 20.1, MucK (413); 19.5, TfdK (460); 19.3, Pht1 (451); 19.3, MopB (449); 18.9, PcaT (429); 18.5, PcaK (448); 18.0, BenK (466); 17.7, PcaK_A (421) |
Sequences included in this analysis, with their accession numbers in parentheses, are as follows. Bed, benzene degradation of P. putida ML2 (L04642, U08463); Bnz, benzene degradation of P. putida 136-R3 (M17904); Bpd, biphenyl/chlorobiphenyl (PCB) degradation of Rhodococcus sp. strain M5 (U27591); Bph_A1, PCB degradation of Rhodococcus sp. strain RHA1 (D32142); Bph_B, PCB degradation of C. testosteroni B-356 (U47637, U47638); Bph_KF, PCB degradation of P. pseudoalcaligenes KF707 (M83673); Bph_KKS, PCB degradation of Pseudomonas sp. strain KKS102 (D17319); Bph_LB, PCB degradation of Pseudomonas sp. strain LB400 (M86348, X66122); Bph_P6, PCB degradation of R. globerulus P6 (X80041, X75633); Cmt, p-cumate degradation of P. putida F1 (U24215); Cum, cumene degradation of P. fluorescens IP01 (D37828); Ipb_BD2, isopropylbenzene degradation of R. erythropolis BD2 (U24277); Ipb_JR1, isopropylbenzene degradation of Pseudomonas sp. strain JR1 (U53507); Tcb, chlorobenzene degradation of Pseudomonas sp. strain P51 (U15298); Tec, tetrachlorobenzene degradation of Burkholderia sp. strain PS12 (U78099); Tod, toluene degradation of P. putida F1 (J04996); AlsR_B (L04470) and AlsR_E (D90801), regulators of the acetoin synthesis in Bacillus subtilis and E. coli, respectively; BphR, putative regulatory protein of biphenyl catabolism in Pseudomonas sp. strain KKS102 (D38633); CatR (M33817) and CatM (M76991), activators of the catechol degradation pathway of P. putida and Acinetobacter calcoaceticus, respectively; ClcR, activator of the 3-chlorocatechol degradation pathway of P. putida (L06464); NahR, activator of the naphthalene degradation pathway of P. putida (J04233); PcaQ, activator of the protocatechuate degradation pathway of Agrobacterium tumefaciens (U32867); TcbR, activator of the chlorobenzene degradation pathway of Pseudomonas sp. strain P51 (M80212); TfdT (AE16782) and TfdR (M98445), nonfunctional and functional activators of the chlorocatechol degradation pathway of Ralstonia eutrophus JMP134; HI0308, hypothetical protein from Haemophilus influenzae (U32716); MalA, maltose permease from B. stearothermophilus (L13418); LacY, lactose permease from E. coli (P02920); CscB, sucrose permease from E. coli (X63740); MhpT (D64043, X97543) and HppK (U89712), putative 3HPP transporters from E. coli and R. globerulus PWD1, respectively; HpaX, 4-hydroxyphenylacetic acid transporter from E. coli (Z37980); MucK (U87258) and BenK (AF009224), cis,cis-muconate and benzoate transporters, respectively, from A. calcoaceticus ADP1; TfdK, putative 2,4-dichlorophenoxyacetate transporter from R. eutrophus (U16782); Pht1, putative phthalate transporter from P. putida (D13229); MopB, 4-methylphthalate transporter from Burkholderia cepacia (U29532); PcaT, putative protocatechuate transporter from P. putida PRS2000 (U48776); PcaK, 4-hydroxybenzoate transporter from P. putida PRS2000 (U10895); PcaK_A, putative 4-hydroxybenzoate transporter from A. calcoaceticus ADP1 (L05770).
hca catabolic genes.
Sequence comparison analyses of the hcaA1, hcaA2, hcaC, and hcaD gene products revealed significant similarities with the corresponding four protein subunits of the three-component class IIB ring-activating dioxygenases (12), mainly with the analogous ipb, cum, and bph gene products (Table 2). The hcaA1A2CD genes are suggested, therefore, to encode the HcaA1A2CD initial dioxygenase of the PP catabolic pathway (Fig. 1).
The hcaA1 gene encodes a protein of 51,109 Da (453 amino acids) that shows significant similarity with the large (α) subunit of the terminal oxygenase component of multicomponent dioxygenases (Table 2). It is worth noting that although residues 85-CRHRAMRVSYADCGNTRAFTCPYH-108 in the HcaA1 protein match the binding site of a [2Fe-2S] Rieske-type iron-sulfur cluster (12) (the putative iron-sulfur ligands are underlined), the highly conserved G and S/T residues (12, 48) are replaced in HcaA1 by A and P (italicized), respectively. Residues 205-EQFASDQYHALFSH-218 in the HcaA1 primary structure match perfectly the mononuclear Fe(II) ligand at the site of oxygen activation (26). It should be noted that the C terminus of HcaA1 differs from that deduced from the reported sequence of orfA (accession no. Z37966), since orfA lacks a nucleotide leading to a change in the reading frame.
The hcaA2 and hcaC genes encode proteins of 20,579 Da (172 amino acids) and 11,328 Da (106 amino acids) whose deduced amino acid sequences show significant identity to those of the small (β) subunit of the terminal oxygenase and the ferredoxin component of multicomponent dioxygenases, respectively (Table 2) (3). Residues 42-CSHGNASMSEGYLEDDATVECPLH-65 in HcaC are likely to be involved in the coordination of a Rieske-type [2Fe-2S] cluster (the putative iron-sulfur ligands are underlined; the unusual proline residue which is also present in the Rieske-type cluster of HcaA1 is italicized).
The next gene of the hca cluster, hcaB, encodes a protein of 28,498 Da (270 amino acids) that shows significant identity with cis-dihydrodiol dehydrogenases that participate in pathways involving class IIB dioxygenases (Table 2) and convert the stable cis-dihydrodiols formed by the initial dioxygenases into the corresponding dihydroxy derivatives with regeneration of NADH (12). Therefore, HcaB is postulated to be the 3-phenylpropionate-dihydrodiol dehydrogenase (Fig. 1). The length of HcaB falls within the average of 270 amino acids for members of the short-chain alcohol dehydrogenase (type II) superfamily, which includes all dihydrodiol dehydrogenases in Table 2 with the exception of BedD (21). Residues 13-GGGSGLG-19 and 156-YTASKHAATGL-166 in HcaB fit the NAD+-binding domain (21) and the consensus pattern for short-chain alcohol dehydrogenases (18, 53), respectively. The highly conserved aspartate 92 of HcaB has been also implicated in the catalytic activity of analogous enzymes (38).
The hcaD gene encodes a 43,978-Da protein (400 amino acids) that is homologous to the ferredoxin reductase subunit of other dioxygenases (Table 2). Multiple sequence alignments revealed the three conserved motifs in the same relative locations found in other reductase components of class IIB dioxygenases (12). Thus, residues 10-GGGQAAAMAAASLRQQG-26 and 151-GAGTIGLELAASATQRRCKVTVIE-174 of HcaD match the consensus sequence postulated to be involved in binding of the ADP moiety of flavin adenine dinucleotide and NAD+ (amino acids in italics indicate a replacement of a consensus residue) (12), and the sequence 265-TCDPAIFAGGD-275 fits with the consensus motif that has been postulated to bind the O-3 group of the ribityl chain of the flavin moiety of flavin adenine dinucleotide (12).
The gene organization within the hca catabolic cluster is similar to that of the analogous ipb, cum, bph, tod, bed, tcb, bnz, and tec clusters encoding class IIB dioxygenases and consisting of the large subunit of the terminal oxygenase, small subunit, ferredoxin, and reductase (57). However, the hcaD gene, although physically linked to the other three genes encoding the HcaA1A2CD dioxygenase, is separated from them by the hcaB gene (Fig. 1A and 3). An unusual location of the gene encoding the reductase component has been also observed for bphA4 in Pseudomonas sp. strain KKS102 (28), bphG in Comamonas testosteroni B-356 (54), and cmtAa in Pseudomonas putida F1 (15). It has been reported that reductases have diverged more than the other components of the dioxygenases (57), and indeed the degrees of identity between HcaD and its orthologs in other clusters are lower than those observed for the other three HcaA1A2C subunits (Table 2). HcaD showed the highest level of identity to the CmtAa reductase component of the p-cumate dioxygenase (Table 2); HcaA1A2CD and CmtAabcd are the only class IIB dioxygenases described so far that attack carboxylated aryls.
At the 3′ end of the hcaD gene is located orfX (Fig. 1A and 3), which has two potential translational start codons at positions 6710 (ATG) and 6737 (GTG), although only the latter shows a putative Shine-Dalgarno sequence (GAGGT) at a reasonable distance (Fig. 3), and codes for a 155-amino-acid product of unknown function. It is worth noting that an additional ORF of unknown function has also been found in other gene clusters encoding biphenyl (19, 22, 55), isopropylbenzene (42), and benzoate (accession no. M76990) dioxygenases.
Regulation of the hca cluster.
The 5′ end of the hca region contains two genes, hcaR and hcaT, that are oriented in the direction opposite to that of the other hca genes (Fig. 1A and 3). The hcaR gene encodes a protein of 32,838 Da (296 amino acids) that shows a size and an amino acid sequence similar to those of LysR-type transcriptional regulators (LTTRs) (49) (Table 2). The majority of the genes encoding LTTRs are transcribed divergently from the genes that they regulate (49). In this sense, hcaR is transcribed divergently from the catabolic genes hcaA1A2CBD (Fig. 1A and 3). LTTRs show a high degree of similarity in the N-terminal domain, where the helix-turn-helix DNA-binding region is located (49). Thus, HcaR possesses a sequence (18-FTRAAEKLHTSQPSLSSQIRDLENCV-43) that matches the LTTR helix-turn-helix motif (Prosite signature PS00044) (5). The C-terminal domain of LTTRs seems to be involved in multimerization, and its consensus motif (V/L)X2GXG(V/I)XV(L/V)P (49) fits with the sequence (232-VGMGLGVTLIP-242) found in HcaR. Within the LysR family, HcaR shows the highest degrees of identity with the AlsR regulator of acetoin synthesis and with a select group of regulators from other biodegradative operons (Table 2). This group constitutes the Cat subfamily and includes the CatR, CatM, TfdR, TcbR, ClcR, and TfdT regulators that activate the genes encoding muconate- or chloromuconate-lactonizing enzymes and/or genes encoding oxygenases that act on catechol or chlorinated aromatic compounds from Acinetobacter, Pseudomonas, and Ralstonia species (33). Additionally, HcaR shows significant identity with the putative bphR gene product, which supposedly would be involved in regulation of biphenyl catabolism (27). Therefore, all of these observations strongly suggest that HcaR is the transcriptional regulator of the hca cluster of E. coli.
To study the regulation of the hca cluster, we performed complementation studies of strains lacking Hca activity. While resting-cell assays of E. coli ED1061(pCKES) bacteria grown in LB medium in the absence of PP did not show significant removal of this aromatic compound, resting cells of these bacteria grown in the presence of 1 mM PP revealed that removal of PP was concomitant with the appearance of a product which cochromatographed with authentic DHPP in HPLC (Table 1). These data, therefore, confirmed that the hca-encoded pathway was inducible. To demonstrate that HcaR was required for hca expression and to determine its mechanism of action, the hcaR and the hcaA1A2CBD-orfX genes were independently expressed from the compatible plasmids pHCAR and pCKET (Fig. 2), respectively. Since S. typhimurium LT-2 is unable to attack PP (Table 1), we used this strain as host for studying the regulation of the hca genes. Resting cells of S. typhimurium LT-2(pCKET) bacteria grown in the presence of 1 mM PP did not reveal the formation of DHPP, and PP remained unaltered. However, when the gene hcaR was provided in trans from plasmid pHCAR, the resulting strain S. typhimurium LT-2(pCKET, pHCAR) showed a significant conversion of PP into DHPP in a resting-cell assay when the bacteria were grown in the presence of 1 mM PP (Table 1). Furthermore, while S. typhimurium LT-2 harboring simultaneously the compatible plasmids pPADR2 (containing the mhp genes for the catabolism of 3HPP) (20) and pCKES grew efficiently (doubling time, 5 h) on minimal medium containing 5 mM PP as the sole carbon and energy source, S. typhimurium LT-2(pPADR2, pCKET) cells did not grow in this aromatic compound. Thus, all these data indicated that HcaR fostered inducible expression of the hca catabolic genes, behaving as a transcriptional activator. It should be mentioned that although the formation of PP-dihydrodiol (compound II in Fig. 1B) is assumed by previous work and by analogy to other systems, the HcaB activity per se has not been demonstrated.
A 135-bp intergenic region is located between the potential translational start sites of the divergently transcribed hcaR and hcaA1 genes (Fig. 3), suggesting that the hcaR promoter is located near or overlaps the regulated promoter of the putative hca catabolic operon. As it has been noted with other LysR-type regulatory targets (40), the A+T content (66%) of the hcaR-hcaA1 intergenic region is higher than that of the hca genes (47%). LTTRs characteristically bind to a consensus T-N11-A DNA binding motif, with the T and A being part of a short inverted repeat, positionally conserved upstream of the regulated promoter (49). In this sense, within the hca intergenic region and located 85 nucleotides upstream of the putative hcaA1 translation start site, we have found the sequence TAG-N7-CTA that matches the binding motif of LysR-type regulators (Fig. 3). Underlined are the guanine and cytosine of the dyad, which have been shown to be involved in the binding of some LTTRs (45). Demonstration of these assumptions and elucidation of the mechanisms of hca repression by glucose (30) and hcaR expression will require further research.
The hcaT gene encodes a protein of 41,619 Da (379 amino acids) that shows significant identity with several members of the major facilitator superfamily (MFS) of transport proteins (34) (Table 2). Interestingly, HcaT was smaller than other MFS members (about 400 amino acids), and some common amino acid sequences that characterize this superfamily (20) were not found in the primary structure of the hcaT gene product. However, analysis of the predicted secondary structure of HcaT revealed the characteristic 12 membrane-spanning α helices which are found in other MFS permeases and are believed to form a channel for transport through the membrane (34). Moreover, the hydrophilicity profile of HcaT showed that the protein could be divided by a central hydrophilic region into two halves, each containing six transmembrane domains (data not shown). Therefore, the putative HcaT protein might be involved in the uptake of PP in E. coli and could be another member of the rapidly expanding family of transporters for the catabolism of aromatic compounds (6).
It has been proposed that permeases for aromatic compounds are indirectly involved in the regulation of the catabolic pathways by bringing these substrates (inducers) inside the cell, leading to the induction of their respective regulatory proteins (43). This idea would agree with the close association between the hcaR and hcaT genes. Similar gene arrangements have been found for the permeases HpaX (4-hydroxyphenylacetate), PcaK (4-hydroxybenzoate), and HppK (3HPP) and the corresponding transcriptional regulators HpaA, PcaR, and HppR, respectively (6, 25, 39, 43, 44). Interestingly, the catabolism of 3HPP in E. coli also involves a gene encoding a putative 3HPP permease (MhpT) that shows similarity with HcaT (Table 2) and a gene encoding a transcriptional activator (MhpR) which belongs to a family of regulators different from that of HcaR; these two genes are not transcriptionally coupled in the mhp operon (20).
Catabolism of CI and 3HCI.
It is known that E. coli K-12 is also able to grow with 3HCI (compound IX in Fig. 1B) as the sole carbon and energy source (10). Since growth with 3HCI induces the synthesis of enzymes MhpA and MhpB, responsible for initial attack upon 3HPP, it was suggested that the same enzymes are used for catabolizing these two compounds (10). To demonstrate this hypothesis, plasmid pPADR2 was introduced into S. typhimurium LT-2, a strain unable to grow on 3HPP (20) and 3HCI. The recombinant strain S. typhimurium LT-2(pPADR2) acquired the ability to grow not only on 3HPP (20) but also on minimal medium containing 3HCI as the sole carbon and energy source. Hence, these data provided experimental demonstration that the mhp genes are also responsible for the mineralization of 3HCI.
Although E. coli cannot grow on CI (compound VI in Fig. 1B) as the sole carbon source, whole cells grown on PP rapidly oxidized CI, suggesting that Hca enzymes are also able to attack this aromatic compound (10). To confirm this assumption, E. coli ED1061(pCKES) bacteria were grown in LB medium containing 1 mM PP for the induction of the hca genes, and then they were used in a resting-cell assay with 0.5 mM CI as substrate. After 30 min of incubation at 30°C, CI was enzymatically converted to a product which cochromatographed in HPLC with authentic DHCI (compound VIII in Fig. 1B). NMR spectroscopy of the purified product confirmed it as DHCI (data not shown). Interestingly, the mutant strain E. coli ED1061 (control cells) grown in PP-containing LB medium did not attack CI in a resting-cell assay. The conversion of CI to DHCI was also observed with S. typhimurium LT-2(pCKES) cells. Hence, we concluded that the HcaA1A2CD dioxygenase and HcaB dihydrodiol dehydrogenase were responsible for CI oxidation and that DHCI was the final product of these reactions.
To analyze whether CI is an inducer of the hca genes, we performed a resting-cell assay using PP as substrate and E. coli ED1061(pCKES) bacteria grown in CI-containing LB medium. Since conversion of PP into DHPP was observed, we concluded that CI can also induce the hca genes. Moreover, we were able to demonstrate that the hca and mhp genes are responsible for CI mineralization by showing that S. typhimurium LT-2(pCKES, pPADR2) cells grew on minimal medium containing 1 mM CI as the sole carbon and energy source. It is worth noting that while in some soil Pseudomonas species and in Lactobacillus pastorianus the catabolism of CI could be accomplished by an initial reduction of the double bond of the side chain with the formation of PP (2, 7, 58), the catabolism of CI by the Hca enzymes produces DHCI, which, via the mhp-encoded pathway (20), will be finally mineralized to pyruvate, acetyl coenzyme A, and fumarate (Fig. 1B).
Since CI can induce the hca genes and is converted to DHCI by E. coli cells, it is difficult to explain the lack of growth of this bacterium on this aromatic compound. A possible explanation for this behavior could be that the DHCI generated in the reactions catalyzed by the Hca enzymes or other intermediates further down the mhp-encoded pathway accumulate to a toxic level that prevents the normal metabolic flux of the cell. When E. coli MG1655(pCKES, pPADR2) cells were grown on minimal medium containing 20 mM glycerol plus 1 mM CI and the culture supernatants were analyzed by HPLC, we observed that CI depletion was not accompanied by the accumulation of DHCI (data not shown). However, these supernatants acquired a yellow coloration that disappeared after acidification with HCl, thus suggesting accumulation of the ring fission product of DHCI. These data are in agreement with previous observations showing that whole cells of E. coli grown in PP or 3HPP were able to oxidize DHCI with the transient formation of a yellow compound having the typical characteristics of a ring fission product but differing from those of compound V derived from ring cleavage of DHPP (Fig. 1B) (10). Furthermore, it has been shown recently that although DHCI is a good substrate for the MhpB dioxygenase (50), the ring fission product of this compound is hydrolyzed by the MhpC enzyme 36-fold less efficiently than the ring fission product of DHPP (32). All these data taken together may suggest that CI cannot support the growth of E. coli because its oxidation to DHCI generates toxic levels of the corresponding ring fission product. Although the toxicity of the ring fission products has been reported (10), the possibility that CI can cause on E. coli toxic effects that are not directly related to its catabolism cannot be ruled out. Why this toxicity is not observed in S. typhimurium is still an open question.
In conclusion, the results presented here constitute the first genetic characterization of a dioxygenolytic pathway for the initial catabolism of PP and CI. It has been suggested that pathways for the catabolism of aromatic compounds widely available in nature, such as PP, HPP, phenylacetate, and hydroxyphenylacetate, are among the most ubiquitous aromatic-compound catabolic systems, and they are closer to central metabolism than those involved in the degradation of xenobiotic compounds (6). These pathways, which occupy central positions within secondary metabolism, may have been one of the most common sources for the initial recruitment of genes for many of the routes involved in the degradation of anthropogenic compounds which are more peripheral to the natural carbon cycle (6). Since the whole structure and organization of the hca cluster resemble those of clusters responsible for initial dioxygenation of the highly recalcitrant polychlorinated biphenyls (PCBs) (bph) and chlorinated benzenes (tcb and tec), it seems likely that these peripheral pathways have evolved from a central one, such as hca, through mutation, recombination, and gene transfer events. Interestingly, PCB degraders have been found to be associated with plant lignin degraders (29), and the breakdown of lignin is one of the major natural sources of phenylpropanoid compounds (2). Moreover, it has been postulated that in the PCB degrader Rhodococcus sp. strain RHA1, the meta cleavage pathway genes could have evolved from the same ancestor as hydroxyphenylacetate meta cleavage pathway genes (hpa and hpc) of E. coli (35). Thus, it is tempting to speculate that the catabolic pathways for the mineralization of PCBs may derive from aromatic-compound central pathways through the assembling of hca-like clusters with mhp- or hpa-like clusters. On the other hand, the characterization of the hca-encoded dioxygenolytic pathway of E. coli confirms that this bacterium is endowed with genetic systems (hca, mhp, and hpa) highly similar to those in environmentally relevant bacteria such as those of the genus Pseudomonas, and this fact should be taken into consideration when aromatic catabolic clusters are cloned and expressed in this enterobacterium. There are several reports on the cloning and expression of aromatic dioxygenases in E. coli claiming that equivalent enzymes from the host could explain partial activities observed when some of the subunits of the cloned dioxygenase were missing in the recombinant bacteria (17, 52). The expression of the hca genes in E. coli might explain these reported observations.
ACKNOWLEDGMENTS
We thank T. Bugg for providing DHPP and DHCI, M. Vicente and M. Aldea for the plasmid pMAK700 and strain MC1061, M. K. B. Berlyn for strain MG1655, and A. Díaz and G. Porras for assistance with the sequencing. The help of J. F. Espinosa with the NMR studies is gratefully acknowledged.
This work was supported by grants from CICYT (AMB94-1038-C02-02 and AMB97-063-C02-02). A. Ferrández was the recipient of a predoctoral fellowship from the Plan Nacional de Formación de Personal Investigador-MEC. E. Díaz was the recipient of a Contrato Temporal de Investigadores from the CSIC.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Andreoni V, Bestetti G. Comparative analysis of different Pseudomonas strains that degrade cinnamic acid. Appl Environ Microbiol. 1986;52:930–934. doi: 10.1128/aem.52.4.930-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asturias J A, Díaz E, Timmis K N. The evolutionary relationships of biphenyl dioxygenase from Gram-positive Rhodococcus globerulus P6 to multicomponent dioxygenases from Gram-negative bacteria. Gene. 1995;156:11–18. doi: 10.1016/0378-1119(94)00530-6. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann B J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella thyphimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1190–1219. [Google Scholar]
- 5.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1995. Nucleic Acids Res. 1995;24:189–196. doi: 10.1093/nar/24.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes M R, Duetz W A, Williams P A. A 3-(3-hydroxyphenyl)propionic acid catabolic pathway in Rhodococcus globerulus PWD1: cloning and characterization of the hpp operon. J Bacteriol. 1997;179:6145–6153. doi: 10.1128/jb.179.19.6145-6153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakley E R, Simpson F J. The microbial metabolism of cinnamic acid. Can J Microbiol. 1964;10:175–185. doi: 10.1139/m64-025. [DOI] [PubMed] [Google Scholar]
- 8.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 9.Bugg T D H. Overproduction, purification and properties of 2,3-dihydroxyphenylpropionate 1,2-dioxygenase from Escherichia coli. Biochim Biophys Acta. 1993;1202:258–264. doi: 10.1016/0167-4838(93)90013-h. [DOI] [PubMed] [Google Scholar]
- 10.Burlingame R, Chapman P J. Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J Bacteriol. 1983;155:113–121. doi: 10.1128/jb.155.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burlingame R P, Wyman L, Chapman P J. Isolation and characterization of Escherichia coli mutants defective for phenylpropionate degradation. J Bacteriol. 1986;168:55–64. doi: 10.1128/jb.168.1.55-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler C S, Mason J R. Structure-function analysis of the bacterial aromatic ring-hydroxylating dioxygenases. Adv Microb Physiol. 1997;38:47–84. doi: 10.1016/s0065-2911(08)60155-1. [DOI] [PubMed] [Google Scholar]
- 13.Cavin J-F, Barthelmebs L, Diviès C. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl Environ Microbiol. 1997;63:1939–1944. doi: 10.1128/aem.63.5.1939-1944.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagley S, Chapman P J, Gibson D T. The metabolism of β-phenylpropionic acid by an Achromobacter. Biochem J. 1965;97:643–650. doi: 10.1042/bj0970643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton R W. p-Cumate catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA carrying the cmt operon. J Bacteriol. 1996;178:1351–1362. doi: 10.1128/jb.178.5.1351-1362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton R W. p-Cymene catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA encoding conversion of p-cymene to p-cumate. J Bacteriol. 1997;179:3171–3180. doi: 10.1128/jb.179.10.3171-3180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton R W, Timmis K N. Characterization of a plasmid-specified pathway for catabolism of isopropylbenzene in Pseudomonas putida RE204. J Bacteriol. 1986;168:123–131. doi: 10.1128/jb.168.1.123-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensor C M, Tai H H. Site-directed mutagenesis of the conserved tyrosine 151 of human placental NAD+-dependent 15-hydroxyprostaglandin dehydrogenase yields a catalytically inactive enzyme. Biochem Biophys Res Commun. 1991;176:840–845. doi: 10.1016/s0006-291x(05)80262-1. [DOI] [PubMed] [Google Scholar]
- 19.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrández A, García J L, Díaz E. Genetic characterization and expression in heterologous hosts of the 3(3-hydroxyphenyl)propionate catabolic pathway of Escherichia coli K-12. J Bacteriol. 1997;179:2573–2581. doi: 10.1128/jb.179.8.2573-2581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong K P Y, Goh C B H, Tan H-M. Characterization and expression of the plasmid-borne bedD gene from Pseudomonas putida ML2, which codes for a NAD+-dependent cis-benzene dihydrodiol dehydrogenase. J Bacteriol. 1996;178:5592–5601. doi: 10.1128/jb.178.19.5592-5601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda M, Yasukouchi Y, Kikuchi Y, Nagata Y, Kimbara K, Horiuchi H, Takagi M, Yano K. Identification of the bphA and bphB genes of Pseudomonas sp. strain KKS102 involved in degradation of biphenyl and polychlorinated biphenyls. Biochem Biophys Res Commun. 1994;202:850–856. doi: 10.1006/bbrc.1994.2008. [DOI] [PubMed] [Google Scholar]
- 23.Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwood C S, Nichols N N, Kim M-K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikuchi, Y. GenBank accession no. D38633.
- 28.Kikuchi Y, Nagata Y, Hinata M, Kimbara K, Fukuda M, Yano K, Takagi M. Identification of the bphA4 gene encoding ferredoxin reductase involved in biphenyl and polychlorinated biphenyl degradation in Pseudomonas sp. strain KKS102. J Bacteriol. 1994;176:1689–1694. doi: 10.1128/jb.176.6.1689-1694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura N, Nishi A, Goto M, Furukawa K. Functional analyses of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovàrovà K, Käch A, Zehnder A J B, Egli T. Cultivation of Escherichia coli with mixtures of 3-phenylpropionic acid and glucose: steady-state growth kinetics. Appl Environ Microbiol. 1997;63:2619–2624. doi: 10.1128/aem.63.7.2619-2624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kröger M, Wahl R. Compilation of DNA sequences of Escherichia coli K12 (ECD and ECDC; update 1995) Nucleic Acids Res. 1996;24:29–31. doi: 10.1093/nar/24.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam W W Y, Bugg T D H. Purification, characterization, and stereochemical analysis of a C-C hydrolase: 2-hydroxy-6-keto-nona-2,4-diene-1,9-dioic acid 5,6-hydrolase. Biochemistry. 1997;36:12242–12251. doi: 10.1021/bi971115r. [DOI] [PubMed] [Google Scholar]
- 33.Leveau J H J, van der Meer J R. The tfdR gene product can successfully take over the role of the insertion element-inactivated TfdT protein as a transcriptional activator of the tfdCDEF gene cluster, which encodes chlorocatechol degradation in Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1996;178:6824–6832. doi: 10.1128/jb.178.23.6824-6832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marger M D, Saier M H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 35.Masai E, Sugiyama K, Iwashita N, Shimizu S, Hauschild J E, Hatta T, Kimbara K, Yano K, Fukuda M. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA 1. Gene. 1997;187:141–149. doi: 10.1016/s0378-1119(96)00748-2. [DOI] [PubMed] [Google Scholar]
- 36.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 37.Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from the international DNA sequence databases. Nucleic Acids Res. 1997;25:244–245. doi: 10.1093/nar/25.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakatsu C H, Providenti M, Wyndham R C. The cis-diol dehydrogenase cbaC gene of Tn5271 is required for growth on 3-chlorobenzoate but not 3,4-dichlorobenzoate. Gene. 1997;196:209–218. doi: 10.1016/s0378-1119(97)00229-1. [DOI] [PubMed] [Google Scholar]
- 39.Nichols N N, Harwood C S. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol. 1997;179:5056–5061. doi: 10.1128/jb.179.16.5056-5061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parke D. Characterization of PcaQ, a LysR-type transcriptional activator required for catabolism of phenolic compounds, from Agrobacterium tumefaciens. J Bacteriol. 1996;178:266–272. doi: 10.1128/jb.178.1.266-272.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Martin J, de Lorenzo V. VTR expression cassettes for engineering conditional phenotypes in Pseudomonas: activity of the Pu promoter of the TOL plasmid under limiting concentrations of the XylR activator protein. Gene. 1996;172:81–86. doi: 10.1016/0378-1119(96)00193-x. [DOI] [PubMed] [Google Scholar]
- 42.Pflugmacher U, Averhoff B, Gottschalk G. Cloning, sequencing, and expression of isopropylbenzene degradation genes from Pseudomonas sp. strain JR1: identification of isopropylbenzene dioxygenase that mediates trichloroethene oxidation. Appl Environ Microbiol. 1996;62:3967–3977. doi: 10.1128/aem.62.11.3967-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prieto M A, Díaz E, García J L. Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J Bacteriol. 1996;178:111–120. doi: 10.1128/jb.178.1.111-120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prieto M A, García J L. Identification of the 4-hydroxyphenylacetate transport gene of Escherichia coli W: construction of a highly sensitive cellular biosensor. FEBS Lett. 1997;414:293–297. doi: 10.1016/s0014-5793(97)01012-0. [DOI] [PubMed] [Google Scholar]
- 45.Romero-Arroyo C E, Schell M A, Gaines III G L, Neidle E L. catM encodes a LysR-type transcriptional activator regulating catechol degradation in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:5891–5898. doi: 10.1128/jb.177.20.5891-5898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato S-I, Nam J-W, Kasuga K, Nojiri H, Yamane H, Omori T. Identification and characterization of genes encoding carbazole 1,9a-dioxygenase in Pseudomonas sp. strain CA10. J Bacteriol. 1997;179:4850–4858. doi: 10.1128/jb.179.15.4850-4858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 50.Spence E L, Kawamukai M, Sanvoisin J, Braven H, Bugg T D H. Catechol dioxygenases from Escherichia coli (MhpB) and Alcaligenes eutrophus (MpcI): sequence analysis and biochemical properties of a third family of extradiol dioxygenases. J Bacteriol. 1996;178:5249–5256. doi: 10.1128/jb.178.17.5249-5256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strickland S, Massey V. The purification and properties of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973;248:2944–2952. [PubMed] [Google Scholar]
- 52.Suen W-C, Haigler B E, Spain J C. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol. 1996;178:4926–4934. doi: 10.1128/jb.178.16.4926-4934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sylvestre M, Hurtubise Y, Barriault D, Bergeron J, Ahmad D. Characterization of active recombinant 2,3-dihydro-2,3-dihydroxybiphenyl dehydrogenase from Comamonas testosteroni B-356 and sequence of the encoding gene (bphB) Appl Environ Microbiol. 1996;62:2710–2715. doi: 10.1128/aem.62.8.2710-2715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sylvestre M, Sirois M, Hurtubise Y, Bergeron J, Ahmad D, Shareck F, Barriault D, Guillemette I, Juteau J M. Sequencing of Comamonas testosteroni strain B-356-biphenyl/chlorobiphenyl dioxygenase genes: evolutionary relationships among Gram-negative bacterial biphenyl dioxygenases. Gene. 1996;174:195–202. doi: 10.1016/0378-1119(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 55.Taira K, Hirose J, Hayashida S, Furukawa K. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J Biol Chem. 1992;267:4844–4853. [PubMed] [Google Scholar]
- 56.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werlen C, Kohler H-P E, van der Meer J R. The broad substrate chlorobenzene dioxygenase and cis-chlorobenzene dihydrodiol dehydrogenase of Pseudomonas sp. strain P51 are linked evolutionarily to the enzymes for benzene and toluene degradation. J Biol Chem. 1996;271:4009–4016. doi: 10.1074/jbc.271.8.4009. [DOI] [PubMed] [Google Scholar]
- 58.Whiting G C, Carr J G. Metabolism of cinnamic acid and hydroxycinnamic acids by Lactobacillus pastorianus var. quinicus. Nature (London) 1959;184:1427–1428. doi: 10.1038/1841427a0. [DOI] [PubMed] [Google Scholar]
- 59.Wilbur W J, Lipman D J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci USA. 1983;80:726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams P A, Shaw L E. mucK, a gene in Acinetobacter calcoaceticus ADP1 (BD413), encodes the ability to grow on exogenous cis,cis-muconate as the sole carbon source. J Bacteriol. 1997;179:5935–5942. doi: 10.1128/jb.179.18.5935-5942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]