Abstract
Under anaerobic-dark growth conditions, in the presence of the alternative electron acceptor dimethyl sulfoxide (DMSO) or trimethylamine N-oxide (TMAO), Rhodobacter sphaeroides 2.4.1T respires anaerobically using the molybdoenzyme DMSO reductase (DMSOR). Genes encoding DMSOR and associated proteins are encoded by genes of the dor locus. Previously, we demonstrated that the expression of DMSOR is regulated by both the oxygen status of the cell via the FnrL protein and by the presence of DMSO or TMAO, presumably through the DorS-DorR two-component sensor-regulator system. Here we further investigate expression of the dor genes through the use of transcriptional lacZ fusions to the dorS, dorR, and dorC promoters. The expression of dorC::lacZ was strongly induced by the absence of oxygen and presence of DMSO. In accordance with our previous findings of DMSOR activity, dorC::lacZ expression was reduced by up to one-third when cells were grown photosynthetically in the presence of DMSO with medium or high light, compared to the expression observed after anaerobic-dark growth. The induction of dorC::lacZ expression in the presence of DMSO was dependent on the DorS and DorR proteins. Expression of the dorS and dorR genes was also induced in the absence of oxygen. In an FnrL mutant, dorS::lacZ expression was not induced when oxygen tensions in the media were lowered, in contrast to what occurred in the wild-type strain. The expression of dorS::lacZ and dorR::lacZ was dependent on the DorS and DorR proteins themselves, suggesting the importance of autoregulation. These results demonstrate a cascade regulation of dor gene expression, where the expression of the regulatory proteins DorS and DorR governs the downstream regulation of the dorCBA operon encoding the structural proteins of DMSOR.
Rhodobacter sphaeroides 2.4.1T is a facultative phototrophic bacterium which is capable of a wide range of metabolic lifestyles including aerobic, anaerobic, photosynthetic, and diazotrophic growth modes. Under anoxygenic growth conditions, in the absence of light, R. sphaeroides 2.4.1T respires anaerobically using dimethyl sulfoxide (DMSO) or trimethylamine N-oxide (TMAO) as the terminal electron acceptor (for a review see reference 14). Reduction of both compounds is achieved by a single enzyme, DMSO reductase (DMSOR), which is a monomeric periplasmic protein containing a molybdopterin cofactor and whose structure has recently been determined (12, 20).
As part of our low-redundancy sequencing strategy for chromosome II of R. sphaeroides 2.4.1T, we sequenced a 13-kb region containing genes homologous to the previously sequenced dmsCBA genes of R. sphaeroides f. sp. denitrificans and to the tor genes of Escherichia coli, which encode components of TMAO reductase (2, 9, 15, 16, 22, 25, 28). The dorC, dorB, and dorA genes form a single operon, and, respectively, encode a soluble c-type cytochrome, a membrane protein of unknown function, and DMSOR (16). Upstream of the dorCBA operon are two adjacent genes, dorS and dorR, that are transcribed divergently inward toward each other and which, respectively, encode putative sensor kinase and response regulator proteins of the two-component signal transduction family of proteins (16, 23). Strains with mutations in any of the dorS, dorR, or dorCBA genes are unable to grow anaerobically in the dark when DMSO or TMAO is supplied as the terminal electron acceptor and show negligible amounts of DMSOR-specific activity (16). These results indicate that the dor genes encode the sole system responsible for the reduction of both DMSO and TMAO in this bacterium.
We showed that the expression of the DorA protein was regulated by both the oxygen status of the cell and by the presence of DMSO or TMAO in the growth medium (16). We also demonstrated that the FnrL protein has a positive role in the regulation of DorA expression, suggesting that the expression of the dorCBA operon is responsive to redox control (31). Further, DorS and DorR mutants failed to accumulate DorA under any growth condition, demonstrating a positive role for these proteins in dorCBA expression (16). In a separate study, it was shown that the DmsR protein of R. sphaeroides f. sp. denitrificans induced the dmsCBA operon in response to DMSO, by binding to specific sites in the dmsC promoter (26). The DmsR protein of R. sphaeroides f. sp. denitrificans and the DorR protein of R. sphaeroides 2.4.1T are almost identical at the amino acid level, and thus it seems likely that the DorR protein plays a similar role in R. sphaeroides 2.4.1T. However, the authors did not report a corresponding DorS homolog and the cognate sensor protein for DmsR has not been identified.
We wished to further investigate how the dor genes of R. sphaeroides 2.4.1T are regulated by both oxygen and DMSO and at what level these two signals interact. Here we examine transcriptional regulation of the dorS, dorR, and dorC promoters and present data which demonstrate the requirement for DMSO and anaerobiosis for the regulation of these promoters. We also show that dorC expression is governed either directly or indirectly by light intensity. We further demonstrate that this system is under autoregulation and propose a cascade model for the regulation of DMSOR expression in R. sphaeroides 2.4.1T.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this work are listed in Table 1. E. coli strains were grown at 37°C in Luria-Bertani medium, and R. sphaeroides strains were grown at 30°C in Sistrom’s minimal medium A containing succinate as the carbon source (3, 19). Where appropriate, DMSO was added at a final concentration of 60 mM and TMAO was added at a final concentration of 30 mM. Cells were grown anaerobically in sealed glass tubes, which were first sparged with nitrogen gas, and were incubated in the dark for chemoheterotrophic growth or in front of a 10-W m−2 light source for photoheterotrophic growth, except in the experiments involving different light intensities. Aerobic cultures were grown by continuous sparging with a mixture of 30% O2–69% N2–1% CO2. For oxygen shift assays, cultures were grown aerobically for five to six culture doublings and then shifted to 2% O2–97% N2–1% CO2. One culture sample was removed prior to the oxygen shift, and further samples were assayed at appropriate intervals after the shift.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype and/or characteristics | Reference or source |
|---|---|---|
| R. sphaeroides | ||
| 2.4.1T | Wild type | 27 |
| NM15 | dorS::ΩStr/Spr | 16 |
| NM16 | dorR::ΩStr/Spr | 16 |
| JZ1678 | ΔfnrL::ΩKmr | 32 |
| E. coli | ||
| DH5αphe | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 λ−thi-1 gyrA relA1 phe::Tn10dCm | 6 |
| HB101 | F− Δ(gpt-proA)62 leuB6 supE44 ara-14 glaK2 lacYI Δ(mcrC-mrr) rpsL20 (Str) xyl-5 mtl-1 recA13 | 1 |
| Plasmids | ||
| pUI1087 | Cloning vector | 33 |
| pBS II | Cloning vector, Ampr, with T3 and T7 promoters | Stratagene |
| pRK2013 | Conjugative helper plasmid | 7 |
| pML5 | Promoterless lacZ transcriptional fusion vector; Tcr | 13 |
| pNMT68 | pBS II containing 659-bp dorC URS PCR product | This study |
| pNMT69 | pBS II containing 662-bp dorS URS PCR product | This study |
| pNMT77 | pML5 containing dorS::lacZ | This study |
| pNMT78 | pML5 containing dorC::lacZ | This study |
| pNMT92 | pBS II containing 525-bp dorR URS PCR product | This study |
| pNMT94 | pML5 containing dorR::lacZ | This study |
The media were supplemented with antibiotics, where appropriate, to maintain selection for plasmids or to select for recombinant strains. The final concentrations were as follows: ampicillin, 100 μg ml−1 (E. coli); kanamycin, 25 μg ml−1 (R. sphaeroides); spectinomycin, 25 μg ml−1 (R. sphaeroides); streptomycin, 25 μg ml−1 (R. sphaeroides); and tetracycline, 1 μg ml−1 (R. sphaeroides) and 10 μg ml−1 (E. coli).
Materials and reagents.
All reagents and materials used were of analytical grade and, except where noted, were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Construction of lacZ reporter fusion plasmids.
Standard recombinant DNA techniques were used throughout (19). Enzymes were purchased from New England Biolabs, Inc. (Beverly, Mass.), Stratagene (La Jolla, Calif.), Promega Corp. (Madison, Wis.), and Boehringer Mannheim Biochemicals, Bethesda Research Laboratories Life Technologies Inc. (Gaithersburg, Md.).
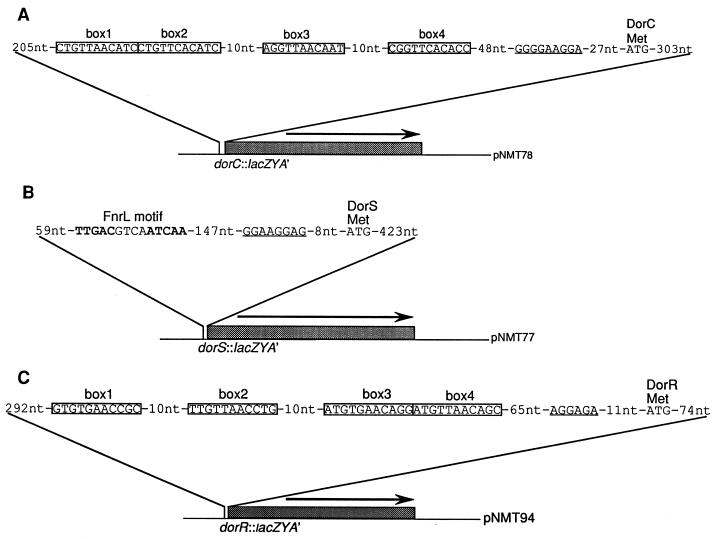
The upstream regulatory sequences (URS) of the dorS, dorR, and dorC genes were amplified by the PCR using Vent DNA polymerase (New England Biolabs) and oligonucleotides purchased from Bethesda Research Laboratories Life Technologies. The reaction conditions for each of the PCR amplifications were identical, consisting of 25 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min. PCR amplification of the dorS promoter region was performed using the primers DORSP1 (5′-CGCCTAGGACCTCGCGGATCGG-3′) and DORSP2 (5′-GCGTTCGAACCCGCGCCTCGGCG-3′), generating a 662-bp product. The PCR product was purified by using the Wizard PCR purification kit (Promega Corp.), and the ends were filled in by using Pfu polymerase (Stratagene). The blunt-ended PCR product was cloned into SmaI-digested pBS II, resulting in plasmid pNMT69. The dorR promoter region was amplified by using the primers DORRPBAM (5′-CGGCGCGGATCCCGCATCGAGTGGC-3′) and DORRPHIND (5′-CGCGGCAAGCTTGCGCAGATACATCG-3′) to generate a 525-bp product. The PCR product was cloned into pBS II, as above, to give plasmid pNMT92. The dorC promoter region was amplified by using the primers DORCP1 (5′-GCGCGACGTCGCGCGCTTCGCTGACTTCG-3′) and DORCP2 (5′-GCGCGACGTCCCGCATCGAGTGG-3′) to generate a 659-bp product. The PCR product was cloned into pBS II, as above, to give plasmid pNMT68. The sequence and orientation of the cloned products were confirmed before proceeding with additional cloning steps. All of the cloned products were subcloned into the promoterless lacZ vector pML5, using BamHI-HindIII double digestions. This resulted in the following plasmids: dorS::lacZ, pNMT77; dorR::lacZ, pNMT94; and dorC::lacZ, pNMT78 (Fig. 1). Each of the lacZ fusion plasmids was conjugated into R. sphaeroides 2.4.1T by triparental matings with pRK2013, as described previously (4).
FIG. 1.
Physical map of plasmids containing transcriptional lacZ fusions to the dorC (A), dorS (B), and dorR (C) URS of R. sphaeroides 2.4.1T. Putative DorR binding sites in the dorC and dorR URS are boxed (boxes 1 to 4). The putative FnrL binding motif in the dorS URS is shown in bold type. Putative Shine-Dalgarno sequences are underlined. The arrows indicate the direction of transcription. See the text for further details.
DNA sequencing.
Automated DNA sequencing was performed using an ABI 373A automatic DNA sequencer (Applied Biosystems Inc., Foster City, Calif.) at the DNA Core Facility of the Department of Microbiology and Molecular Genetics, The University of Texas Health Science Center, Houston. Oligonucleotides used for priming the sequencing reactions were purchased from Bethesda Research Laboratories Life Technologies. Sequences were analyzed by using the Genetics Computer Group programs and the BLAST server at the National Center for Biotechnology Information (5).
Cell extract preparation and assays of β-galactosidase activity.
Preparation of crude cell extracts and determination of β-galactosidase activities were performed as described previously (18, 24). Cell extract protein concentrations were determined by using the Pierce BCA Protein Assay Reagent (Pierce, Rockford, Ill.) with bovine serum albumin as a reference standard.
RESULTS
Regulation of dorC::lacZ expression.
Previous experiments revealed that the DorA protein was produced only in the absence of oxygen and in the presence of DMSO or TMAO (16). Since the dorA gene is in an operon downstream of the dorB and dorC genes, with a putative promoter region upstream of dorC, we constructed a dorC::lacZ fusion in order to measure expression from the dorC URS. After introduction of this fusion plasmid (pNMT78) (Fig. 1) into wild-type or various mutant strains of R. sphaeroides 2.4.1T, we measured β-galactosidase activities after growth under a number of different conditions.
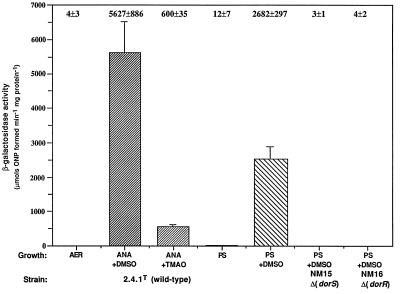
dorC::lacZ expression was maximal after anaerobic-dark growth in the presence of DMSO (Fig. 2). In contrast, very little or no β-galactosidase activity was evident after aerobic growth or photosynthetic growth in the absence of DMSO. Intriguingly, the expression of dorC::lacZ was approximately ninefold lower after anaerobic growth in the dark with TMAO than after growth with DMSO under similar conditions. Most interesting is the lower activity observed during photosynthetic growth in the presence of DMSO when compared to the activity after growth under anaerobic-dark conditions (Fig. 2). We previously observed that the specific activity of DMSOR after photosynthetic growth in the presence of DMSO was approximately 50% of that for anaerobic-dark conditions (16). Therefore, it appears that there is a good correlation between dorC transcription and DMSOR activity. We grew wild-type cells containing the various dor::lacZ fusions photosynthetically in the presence of DMSO at different light intensities and assayed β-galactosidase activities of extracts from these cultures in order to determine if there was an effect of light intensity on dor gene expression.
FIG. 2.
β-Galactosidase activities from cell extracts of R. sphaeroides strains containing the dorC::lacZ transcriptional fusion plasmid pNMT78. Growth conditions are as follows: AER (░⃞), aerobically with 60 mM DMSO; ANA+DMSO (▨), anaerobically in the dark with 60 mM DMSO; ANA+TMAO (▨), anaerobically in the dark with 30 mM TMAO; PS (▩), photosynthetically; PS+DMSO (▧), photosynthetically with 60 mM DMSO. Photosynthetic cultures were grown with a light intensity of 10 W m−2. Results are the mean values from triplicate assays of at least three independent cultures and are corrected for activity from the vector alone under the same conditions (pML5, <35 μmol of o-nitrophenol formed min−1 mg of protein−1). Vertical bars represent the standard deviation from the mean.
For the dorC::lacZ fusion, decreased β-galactosidase activities were observed after cells were grown under low (3 W m−2), medium (10 W m−2), or high (100 W m−2) light intensity compared to the activity after anaerobic-dark growth (Table 2). Growth under high light intensity resulted in the maximal decrease in activity, resulting in a level approximately threefold lower than that observed under anaerobic-dark conditions (Table 2). In contrast, expression of dorS::lacZ did not vary significantly with differing light intensity, although a small decrease in β-galactosidase activity was observed when cells were grown under low or medium light intensity (Table 2). Only when cells were grown under high light, did dorR::lacZ expression decrease, to a level approximately 50% of that observed in the dark or under low or medium light intensity (Table 2).
TABLE 2.
Effects of light intensity on dorC, dorS, and dorR expression
| Growth conditiona | β-Galactosidase
activityb
|
||
|---|---|---|---|
| dorC::lacZ | dorS::lacZ | dorR::lacZ | |
| Anaerobic-dark | 5,627 ± 886 | 182 ± 23 | 31 ± 12 |
| Photoheterotrophic | |||
| 3 W m−2 | 4,446 ± 289 | 165 ± 12 | 33 ± 5 |
| 10 W m−2 | 2,682 ± 297 | 125 ± 22 | 38 ± 5 |
| 100 W m−2 | 1,946 ± 375 | 175 ± 17 | 14 ± 3 |
Cultures were grown to mid-log phase in the presence of 60 mM DMSO under the conditions indicated and assayed for β-galactosidase activity.
Units of activity are micromoles of o-nitrophenol (ONP) formed minute−1 milligram of protein−1. Values represent the mean values ± the standard deviation of triplicate assays of at least three independent cultures and are corrected for activity from the vector alone under the same conditions (pML5, <35 μmol of ONP formed min−1 mg of protein−1).
It is clear that maximum expression of dorC::lacZ is observed after growth in the dark, in the absence of oxygen and in the presence of DMSO. We previously observed that the DorA protein was absent in DorR and DorS mutants, and it is believed that DorS and DorR form a two-component sensor-regulator system (16). To determine whether the DorSR system acts at the dorC URS, we examined the expression of dorC::lacZ in DorR and DorS mutant backgrounds after photosynthetic growth in the presence of DMSO, since the mutants are unable to grow anaerobically in the dark with DMSO or TMAO. The β-galactosidase activities in both mutants were very low when compared to activities in the wild-type background (Fig. 2). Similar low levels of activity were observed after photosynthetic growth in the absence of DMSO (data not shown). These results demonstrate a positive role for the DorSR system in the control of the dorCBA operon.
dorS::lacZ and dorR::lacZ expression.
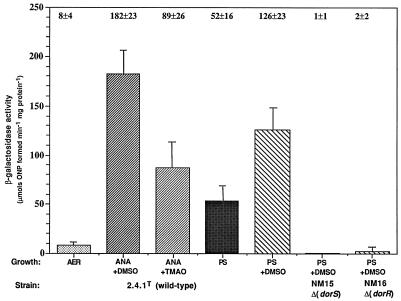
To further establish the roles of the DorS and DorR proteins in the regulation of dor expression, we were interested to learn how the genes encoding these proteins are themselves regulated. We constructed dorS::lacZ and dorR::lacZ fusions and measured the resulting β-galactosidase activities from these fusions in wild-type and mutant backgrounds after growth under different conditions. For both fusions, only very low levels of activity were observed after aerobic growth (Fig. 3 and 4). An approximately 20-fold induction of dorS::lacZ expression was observed after anaerobic-dark growth in the presence of DMSO (Fig. 3). The activity after anaerobic-dark growth with TMAO was approximately 45% of that when cells were grown with DMSO, in contrast to the approximate 90% decrease in dorC::lacZ expression observed between TMAO- and DMSO-grown cultures. In further contrast to dorC::lacZ expression, dorS::lacZ expression decreased only slightly after photosynthetic growth in the presence of DMSO, compared to the expression observed after anaerobic-dark growth. The expression of dorS::lacZ also appeared to be under DMSO-dependent control, as an approximately twofold increase in activity was observed after photosynthetic growth in the presence of DMSO compared to that in the absence of DMSO.
FIG. 3.
β-Galactosidase activities from cell extracts of R. sphaeroides strains containing the dorS::lacZ transcriptional fusion plasmid pNMT77. Growth conditions are as follows: AER (░⃞), aerobically with 60 mM DMSO; ANA+DMSO (▨), anaerobically in the dark with 60 mM DMSO; ANA+TMAO (▨), anaerobically in the dark with 30 mM TMAO; PS (▩), photosynthetically; PS+DMSO (▧), photosynthetically with 60 mM DMSO. Photosynthetic cultures were grown with a light intensity of 10 W m−2. Results are the mean values from triplicate assays of at least three independent cultures and are corrected for activity from the vector alone under the same conditions (pML5, <35 μmol of o-nitrophenol formed min−1 mg of protein−1). Vertical bars represent the standard deviation from the mean.
FIG. 4.
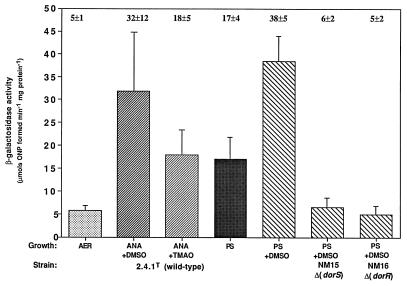
β-Galactosidase activities from cell extracts of R. sphaeroides strains containing the dorR::lacZ transcriptional fusion plasmid pNMT94. Growth conditions are as follows: AER (░⃞), aerobically with 60 mM DMSO; ANA+DMSO (▨), anaerobically in the dark with 60 mM DMSO; ANA+TMAO (▨), anaerobically in the dark with 30 mM TMAO; PS (▩), photosynthetically; PS+DMSO (▧), photosynthetically with 60 mM DMSO. Photosynthetic cultures were grown with a light intensity of 10 W m−2. Results are the mean values from triplicate assays of at least three independent cultures and are corrected for activity from the vector alone under the same conditions (pML5, <35 μmol of o-nitrophenol formed min−1 mg of protein−1). Vertical bars represent the standard deviation from the mean.
The expression of dorR::lacZ was much lower relative to that of the dorS::lacZ and dorC::lacZ fusions, but since the values presented are the results of three independent growths, each performed in triplicate, we believe that these represent the true values. Measurement of β-galactosidase activities from the dorR::lacZ fusion revealed that the expression of dorR, like that of dorS, is also induced by anaerobiosis, although only an approximately fivefold induction is observed in this case (Fig. 4). Similar to the reduction in dorS::lacZ expression, anaerobic-dark growth with TMAO resulted in an approximately 40% reduction in dorR::lacZ expression when compared to the levels observed when cells were grown with DMSO. When cells were grown photosynthetically, dorR::lacZ expression was stimulated twofold by the presence of DMSO. No significant differences were observed in the levels of dorR::lacZ expression between anaerobic-dark- and photosynthetically grown cultures in the presence of DMSO, in contrast to levels of both dorC::lacZ and dorS::lacZ expression (Fig. 4). When the light intensity was increased to 100 W m−2, dorR::lacZ expression was reduced by approximately 50% relative to that seen under low or medium light intensity (Table 2). This demonstrates that dorR expression is also under light-responsive regulation, but not as stringently as dorC expression.
Since the DorSR system positively regulated dorC::lacZ expression, we wondered if the expression of the dorS and dorR genes themselves was under autoregulation by the DorS and DorR proteins. Both the dorS::lacZ and dorR::lacZ fusions were introduced into the respective DorS and DorR mutant strains, NM15 and NM16, and the β-galactosidase activities were measured after photosynthetic growth in the presence of DMSO. The activities from both fusions were much lower than those for the wild-type strain under similar growth conditions and resembled the levels observed under aerobic conditions for each fusion, indicating a positive role for the DorS and DorR proteins in the autoregulation of dorS and dorR gene expression (Fig. 3 and 4). Further, it should also be noted that we assayed dorS::lacZ and dorR::lacZ expression in the mutant strains after photosynthetic growth in the absence of DMSO and found the levels of expression to be very similar irrespective of whether DMSO was present or not (data not shown).
FnrL positively regulates dorS::lacZ.
It was previously demonstrated than an FnrL mutant was unable to synthesize the DorC c-type cytochrome or the DorA protein, even when grown in low oxygen in the presence of DMSO (31). This suggested that at least one component of the dor cluster was under FnrL-mediated regulation. Upon inspection of the dor sequences, it was observed that within the dorS URS, there is a putative FnrL binding site, TTGAC-N4-ATCAA, differing from the consensus Fnr motif by only one nucleotide change (Fig. 1) (32). To determine whether the expression of dorS is regulated by FnrL, the dorS::lacZ fusion plasmid pNMT77 was introduced into the FnrL mutant strain JZ1678 and an oxygen shift experiment was performed where high-oxygen (30%) cultures were shifted to low-oxygen (2%) conditions in the presence of DMSO.
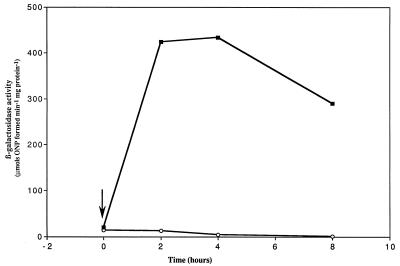
Again, an extremely low level of β-galactosidase activity was observed for both the wild-type and FnrL mutant strains under high-oxygen (30%) conditions (Fig. 5). After the cultures were shifted to 2% oxygen, a rapid increase in dorS::lacZ expression was observed in the wild-type strain which attained a plateau level 2 to 4 h postshift. After this time, dorS::lacZ expression decreased toward the steady-state level previously observed for anaerobic-dark cultures (Fig. 3). In contrast, no such increase was observed for dorS::lacZ expression in the FnrL mutant strain. In fact, dorS::lacZ expression actually decreased after the oxygen shift.
FIG. 5.
Kinetics of induction of dorS::lacZ transcriptional fusion in the wild-type strain 2.4.1T (▪) and FnrL mutant strain JZ1678 (○) following a shift from 30 to 2% oxygen, indicated by the vertical arrow. Cultures were sampled at the times indicated, and extracts from 15-ml samples were assayed for β-galactosidase activity. The values represent the means of triplicate assays from two independent growth experiments. The standard error in each case did not exceed 20% of the mean value.
DISCUSSION
Our previous results indicated that the expression of the dorCBA operon, encoding the structural components of DMSO reductase, is dually regulated by oxygen and DMSO (16). In order to further investigate the regulation of the dor genes in R. sphaeroides 2.4.1T, we constructed transcriptional lacZ fusions to the upstream regulatory sequences of the dorS, dorR, and dorC genes for use as reporters of promoter activity.
As predicted, expression of dorC::lacZ was induced by both the presence of DMSO and the absence of oxygen. After aerobic growth or photosynthetic growth in the absence of DMSO, negligible levels of β-galactosidase activity are observed. Further, it would appear that TMAO is less efficient as an inducer of dorC::lacZ expression than DMSO. We previously demonstrated that the dorCBA-encoded DMSOR is also responsible for TMAO reductase activity and that Dor mutants are unable to utilize TMAO as the terminal electron acceptor (16). The weaker induction by TMAO may be related to the possibility that the DorSR system is responsible for dorCBA induction in response to the presence of either DMSO or TMAO (17). A twofold decrease in dorS::lacZ and dorR::lacZ expression was observed after growth with TMAO compared with expression after growth with DMSO. Further, in DorS and DorR mutants dorC::lacZ was expressed at a similar low level when the cells were grown photosynthetically with either DMSO or TMAO (data not shown). These data suggest that the DorSR system is responsible for both DMSO- and TMAO-dependent sensing and regulation.
We also showed that DorS and DorR mutants are unable to synthesize the DorA protein (16). The expression of dorC::lacZ in the DorS and DorR mutant backgrounds was negligible after cells were grown photosynthetically in the presence of DMSO or TMAO. Since the DorS and DorR proteins are required for the induction of dorS::lacZ, dorR::lacZ, and dorC::lacZ expression in response to DMSO (or TMAO), we propose that the DorS and DorR proteins form a DMSO (and TMAO)-responsive regulatory system, homologous to the TorS and TorR proteins of E. coli (9, 22). However, since dorS::lacZ and dorR::lacZ expression in the absence of either DMSO or TMAO is still dependent on the DorSR system, we believe that this system is responsive to an additional signal. The fact that expression of dorC::lacZ is not induced in the absence of DMSO, in contrast to that of dorS::lacZ or dorR::lacZ, even when DorS and DorR are present, is explained by our recent finding that an additional regulatory system, which we term DorXY, is positively required for dorC::lacZ expression but not for dorS::lacZ or dorR::lacZ expression (17). We are currently investigating whether this system is an additional DMSO-dependent regulatory system.
Presumably, DorR is able to activate transcription by binding to conserved motifs in the dorC URS (Dor box, consensus A/CG/TGTTA/CACANC), which were previously identified as DmsR binding sites in R. sphaeroides f. sp. denitrificans (Fig. 1) (26). These motifs are very similar to the TorR binding sites identified in the torCDA URS in E. coli (21). This suggests that DorR is able to activate transcription in a manner similar to that by DmsR and TorR. In this respect, it is of importance to note the absence of a homolog of the TorT protein in R. sphaeroides 2.4.1T. In E. coli the TorT protein is required for torCDA expression, acting upstream of the TorS sensor protein (9, 10). The authors conclude that TorT is probably not a TMAO-binding protein, and its exact role remains unclear. Presumably, the absence of a TorT homolog in R. sphaeroides 2.4.1T reflects a functional difference in the signal transduction pathway between the Tor and Dor systems.
The reduction in dorC::lacZ expression when cells were grown photosynthetically with DMSO compared to anaerobic-dark growth was in accordance with the 50% decrease in DMSOR specific activity that we previously observed (16). Further, we found a correlation between the decrease in expression of dorC::lacZ and the increase in light intensity (Table 2). The observation that dorCBA expression is possibly light responsive is exciting since mechanisms for light-responsive gene regulation in R. sphaeroides 2.4.1T are poorly characterized. It has previously been demonstrated that several genes required for photosynthetic growth, namely crtA, crtI, puc, and bchF, are under light-dependent transcriptional regulation (30). In addition, increases in light intensity have been shown to affect the accumulation of bacteriochlorophyll in the cell, levels of puc (which encodes the B800-850 components of the light-harvesting complex) mRNA, and carotenoid accumulation (11, 29). It has been demonstrated that the PpsR protein functions in the light-responsive regulation of B800-850 abundance (8). Recently, it was shown that the activity of PpsR depends on the AppA protein, which may serve as a redox-dependent modulator of PpsR activity (8). Since dorS::lacZ and dorR::lacZ expression are much less affected than dorC::lacZ expression, we believe that the major target for light-dependent regulation is at the dorC URS. We are currently investigating whether the recently identified DorXY regulatory system is affected by light intensity or redox and whether dorC::lacZ expression is dependent on any of the previously identified redox-dependent regulatory systems of R. sphaeroides 2.4.1T (17).
In addition to examining the regulation of dorCBA expression, we were interested in how the genes encoding the DorS and DorR regulatory proteins themselves were regulated. dorS::lacZ expression was shown to be dependent on the absence of oxygen and the presence of DMSO. By performing an oxygen shift experiment using an FnrL mutant, we demonstrated the positive role for this protein in the induction of dorS::lacZ expression in response to lowering the oxygen concentration (Fig. 5). The presence of an Fnr binding motif in the dorS URS suggests that this FnrL-dependent regulation occurs directly at the dorS promoter. dorS::lacZ expression was also shown to be increased by the presence of DMSO after photosynthetic growth (Fig. 3). As for dorC::lacZ expression, we believe that this induction is due to the activities of the DorS and DorR proteins, since in the DorS and DorR mutant strains dorS::lacZ expression was extremely low. At present it is unclear as to how the DorS and DorR proteins affect dorS expression, since there are no putative DorR binding motifs present in the dorS URS. Experiments are currently under way to examine this further.
The expression of dorR::lacZ was also shown to be dependent on the absence of oxygen and the presence of DMSO. Further, dorR::lacZ expression was also dependent on the presence of the DorSR system. Since there are putative DorR binding motifs present in the dorR URS, it is believed that the positive regulation by DMSO occurs via DorR binding and activation through these sites. Since dorR::lacZ expression returned to aerobic levels in the DorS mutant, even when cells were grown photosynthetically in the presence of DMSO, we believe that the anaerobic induction of dorR::lacZ expression observed is dependent on the induction and activity of the DorS protein. It was therefore surprising to us to find that the DorS- and DorR-mediated effects on dorS and dorR expression were manifested even in the absence of DMSO. This suggests that the DorSR system may be responsive to an additional signal other than DMSO (or TMAO). It was previously demonstrated that the DmsR protein of R. sphaeroides f. sp. denitrificans was able to bind to and retard DNA on a gel of the dmsCBA URS in the absence of DMSO (26). This would suggest that DMSO is not the sole signal for the DorSR system.
Taking these results together, we would like to propose the following model for the regulation of DMSO reductase (dor) gene expression in R. sphaeroides 2.4.1T. Under oxygen-limited conditions the FnrL protein is able to induce the transcription of the dorS gene, encoding the DorS sensor-kinase protein (Fig. 6). DorS is able to phosphorylate its cognate regulator, DorR, in response to the presence of DMSO (or TMAO) and to an additional uncharacterized signal. Phosphorylated DorR is then able to activate transcription from both the dorR and dorCBA promoters. This leads to an increase in the synthesis of the DorR protein itself and production of functional DMSOR. DorR also appears to affect transcription of the dorS gene, by an as yet uncharacterized mechanism. An additional regulatory system, encoded by the dorX and dorY genes, is also required for dorCBA expression. The dorCBA and dorR promoters are also subject to light-responsive control. Therefore, it appears that a regulatory cascade is involved, whereby the regulation of the dorS and dorR genes, encoding regulatory proteins, controls the downstream expression of the dorCBA operon, encoding the structural components of the DMSOR enzyme. Further, we have demonstrated that this regulation is complex, requiring multiple signals and multiple regulatory proteins.
FIG. 6.
Model for the regulation of DMSO reductase (dor) gene expression in R. sphaeroides 2.4.1T. See the text for further details.
ACKNOWLEDGMENTS
We thank Tony Shaw (University of Queensland, Brisbane, Australia) and Silke Leimkühler (University of Bielefeld, Bielefeld, Germany) for generously providing plasmid pML5. We also thank Jill Zeilstra-Ryalls, Jesus Eraso, and Tracy Palmer for helpful comments and suggestions.
This work was supported by U.S. Public Health Service grant GM15590 to S.K.
REFERENCES
- 1.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 2.Choudhary M, MacKenzie C, Nereng K, Sodergren E, Weinstock G M, Kaplan S. Multiple chromosomes in bacteria: structure and function of chromosome II of Rhodobacter sphaeroides2.4.1. J Bacteriol. 1994;176:7694–7702. doi: 10.1128/jb.176.24.7694-7702.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1956;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 4.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a puf mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figurski D H, Helsinki D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid fuction provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomelsky M, Kaplan S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides2.4.1. J Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jourlin C, Bengrine A, Chippaux M, Méjean V. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol Microbiol. 1996;20:1297–1306. doi: 10.1111/j.1365-2958.1996.tb02648.x. [DOI] [PubMed] [Google Scholar]
- 10.Jourlin C, Simon G, Pommier J, Chippaux M, Méjean V. The periplasmic TorT protein is required for trimethylamine N-oxide reductase gene induction in Escherichia coli. J Bacteriol. 1996;178:1219–1223. doi: 10.1128/jb.178.4.1219-1223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiley P J, Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroideslight-harvesting B800-850-α and B800-850-β genes. J Bacteriol. 1987;169:3268–3275. doi: 10.1128/jb.169.7.3268-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisker C, Schindelin H, Rees D C. Molybdenum-cofactor-containing enzymes: structure and mechanism. Annu Rev Biochem. 1997;66:233–267. doi: 10.1146/annurev.biochem.66.1.233. [DOI] [PubMed] [Google Scholar]
- 13.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 14.McEwan A G. Photosynthetic electron transport and anaerobic metabolism in purple non-sulfur bacteria. Antonie van Leeuwenhoek. 1994;66:151–164. doi: 10.1007/BF00871637. [DOI] [PubMed] [Google Scholar]
- 15.Méjean V, Iobbi-Nivol C, Lepelletier M, Giordano G, Chippaux M, Pascal M-C. TMAO anaerobic respiration in Escherichia coli: involvement of the toroperon. Mol Microbiol. 1994;11:1169–1179. doi: 10.1111/j.1365-2958.1994.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 16.Mouncey N J, Choudhary M, Kaplan S. Characterization of genes encoding dimethyl sulfoxide reductase of Rhodobacter sphaeroides 2.4.1T: an essential metabolic gene function encoded on chromosome II. J Bacteriol. 1997;179:7617–7624. doi: 10.1128/jb.179.24.7617-7624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouncey, N. J., and S. Kaplan. Unpublished observations.
- 18.Mouncey N J, Kaplan S. Oxygen regulation of the ccoN gene encoding a component of the cbb3 oxidase in Rhodobacter sphaeroides 2.4.1T: involvement of the FnrL protein. J Bacteriol. 1998;180:2228–2231. doi: 10.1128/jb.180.8.2228-2231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Satoh T, Kurihara F N. Purification and properties of dimethylsulfoxide reductase containing a molybdenum cofactor from a photodenitrifier, Rhodopseudomonas sphaeroides f. sp. denitrificans. J Biochem. 1987;102:191–197. doi: 10.1093/oxfordjournals.jbchem.a122032. [DOI] [PubMed] [Google Scholar]
- 21.Simon G, Jourlin C, Ansaldi M, Pascal M-C, Chippaux M, Méjean V. Binding of the TorR regulator to cis-acting direct repeats activates toroperon expression. Mol Microbiol. 1995;17:971–980. doi: 10.1111/j.1365-2958.1995.mmi_17050971.x. [DOI] [PubMed] [Google Scholar]
- 22.Simon G, Méjean V, Jourlin C, Chippaux M, Pascal M-C. The torR gene of Escherichia coli encodes a response regulator protein involved in the expression of the trimethylamine N-oxide reductase genes. J Bacteriol. 1994;176:5601–5606. doi: 10.1128/jb.176.18.5601-5606.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock J, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai T-N, Havelka W A, Kaplan S. A broad host-range vector system for cloning and translational lacZfusion analysis. Plasmid. 1988;19:175–188. doi: 10.1016/0147-619x(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 25.Ujiiye T, Nakama H, Okubo A, Yamazaki S, Satoh T. Nucleotide sequence of the genes, encoding the pentaheme cytochrome (dmsC) and the transmembrane protein (dmsB), involved in dimethyl sulfoxide respiration from Rhodobacter sphaeroides f. sp. denitrificans. Biochim Biophys Acta. 1996;1277:1–5. doi: 10.1016/s0005-2728(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 26.Ujiiye T, Yamamoto I, Satoh T. The dmsR gene encoding a dimethyl sulfoxide-responsive regulator for expression of dmsCBA (dimethyl sulfoxide respiration genes) in Rhodobacter sphaeroides f. sp. denitrificans. Biochim Biophys Acta. 1997;1353:84–92. doi: 10.1016/s0167-4781(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 27.van Neil C B. The culture, general physiology, morphology, and classification of the non-sulfur purple and brown bacteria. Bacteriol Rev. 1944;8:1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto I, Wada N, Ujiiye T, Tachibana M, Matsuzaki M, Kajiwara H, Watanabe Y, Hirano H, Okubo A, Satoh T, Yamazaki S. Cloning and nucleotide sequence of the gene encoding dimethyl sulfoxide reductase from Rhodobacter sphaeroides f. sp. denitrificans. Biochim Biotechnol Biochem. 1995;59:1850–1855. doi: 10.1271/bbb.59.1850. [DOI] [PubMed] [Google Scholar]
- 29.Yeliseev A A, Eraso J M, Kaplan S. Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides2.4.1: involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J Bacteriol. 1996;178:5877–5883. doi: 10.1128/jb.178.20.5877-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeliseev A A, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides2.4.1. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 31.Zeilstra-Ryalls J H, Gabbert K, Mouncey N J, Kaplan S, Kranz R G. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J Bacteriol. 1997;179:7264–7273. doi: 10.1128/jb.179.23.7264-7273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrLgene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeilstra-Ryalls J H, Kaplan S. Regulation of 5-aminolevulinic acid synthesis in Rhodobacter sphaeroides2.4.1: the genetic basis of mutant H-5 auxotrophy. J Bacteriol. 1995;177:2760–2768. doi: 10.1128/jb.177.10.2760-2768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]