Abstract
Immune cells have emerged as powerful regulators of regenerative as well as pathological processes. The vast majority of regenerative immunoengineering efforts have focused on macrophages; however, growing evidence suggests that other cells of both the innate and adaptive immune system are as important for successful revascularization and tissue repair. Moreover, spatiotemporal regulation of immune cells and their signaling have a significant impact on the regeneration speed and the extent of functional recovery. In this review, we summarize the contribution of different types of immune cells to the healing process and discuss ways to manipulate and control immune cells in favor of vascularization and tissue regeneration. In addition to cell delivery and cell-free therapies using extracellular vesicles, we discuss in situ strategies and engineering approaches to attract specific types of immune cells and modulate their phenotypes. This field is making advances to uncover the extraordinary potential of immune cells and their secretome in the regulation of vascularization and tissue remodeling. Understanding the principles of immunoregulation will help us design advanced immunoengineering platforms to harness their power for tissue regeneration.
Keywords: Neutrophils, Macrophages, T cells, Immunomodulation, Cell delivery, Extracellular vesicles, Biomaterial, Immune cell metabolism, Stiffness, Patterning
1. Introduction
Tissue regeneration is a complex and dynamic process. Tissue injury initiates a sequence of fine-tuned processes that include recruitment of immune cells, removal of damaged cells, migration of fibroblasts, endothelial cells (ECs) and potentially progenitors to the affected area, cell proliferation and differentiation, and deposition and remodeling of extracellular matrix (ECM). Since tissue remodeling and cell survival rely heavily on the supply of oxygen, nutrients, and other factors from the vasculature, delayed or insufficient revascularization within damaged or implanted tissues impairs wound healing processes and may result in poor integration of transplanted tissues.
New blood vessels are formed via three mechanisms: vasculogenesis, angiogenesis, and arteriogenesis. Vasculogenesis is the process of de novo formation of blood vessels during embryonic development. Angiogenesis refers to the sprouting of capillaries from pre-existing blood vessels, while arteriogenesis describes remodeling and enlargement of collateral vessels when a major blood vessel is occluded and unable to provide adequate tissue perfusion [1]. Neovascularization in adults is most commonly stimulated by tissue hypoxia, inflammation, and changes in fluid shear stress. Hypoxia induces the expression of hypoxia-inducible factor 1a (HIF-1α) in local cells, which activates the transcription of several angiogenic factors such as vascular endothelial growth factor (VEGF), neuropilin-1, and angiopoietin-2 [2]. When quiescent endothelial cells (ECs) sense angiogenic signals, they promote vasodilatation and increase vascular permeability thereby allowing plasma proteins to leak out and create a provisional ECM. Local cells release proteases to degrade ECM and liberate ECM-bound angiogenic factors; ECs proliferate and collectively migrate into the surrounding tissue as multicellular sprouts, which consequently become lumenized. Growth factors such as platelet-derived growth factor B (PDGF-B) and heparin-binding epidermal growth factor-like growth factor (HB-EGF) [3] secreted by ECs control pericyte recruitment. Pericytes decrease the vascular tube diameter [4]. The extent of pericyte coverage varies among different vascular beds. Both ECs and pericytes then contribute to the deposition of a basement membrane [5]. The newly formed network requires further optimization, which is driven by blood flow forces. The poorly perfused branches are eliminated, and the remaining blood vessels are stabilized by mural cells and become more mature while ECs resume their quiescent phenotype.
Local inflammation can have proangiogenic effects as well. For example, proinflammatory cytokine TNF-α can induce the upregulation of Notch ligand Jagged1 in stalk cells that follow the leading tip cell in the vascular sprout [6]. This signaling helps to sustain elevated VEGF receptor expression in endothelial tip cells, which promotes vessel growth. On the other hand, high levels of TNF-α can loosen cell junctions [7] and inhibit endothelial sprouting. The overall effect of TNF-α on angiogenesis is, however, also affected by pericyte coverage of the blood vessel. Intermediate pericyte coverage can enhance the specification of tip and stalk cells and promote nascent spout growth and branching at high TNF-α levels, while high pericyte coverage can suppress the effect of the inflammatory environmental stimuli [8].
Arteriogenesis is stimulated by changes in vascular wall shear stress, which lead to the increase in vascular diameter and the vessel wall thickness. Activation of ECs leads to the upregulation of cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) and the secretion of chemokines like CCL2, which recruit monocytes and other immune cells that then facilitate vascular remodeling [9,10].
Strategies for therapeutic revascularization utilizing growth factors such as VEGF have been widely explored to promote angiogenesis, although some clinical trials failed to demonstrate significant benefits [11–13], which might be due to their low stability and short half-life in the body or the fact that VEGF also increase the permeability of blood vessels which might not be beneficial mainly in the later stages of vessel maturation. On the other hand, immune cells play an important role in the regulation of angiogenesis; harnessing immune cells to promote angiogenesis and tissue regeneration emerge as a promising therapeutic approach. Immune cells not only protect the body against invading pathogens but they have also recently been recognized as important regulators of tissue repair. Following tissue damage, cells within the wounded area release signals to attract cells of the innate immune system (e.g., neutrophils and macrophages). If the injury process persists for a longer period of time, cells of the adaptive immune system, such as B and T cells, will also participate in the restoration of tissue homeostasis. Immune cell phenotype and the duration of the particular immune response can, however, dramatically influence the healing process.
Therefore, in this review, we provide an overview of different types and phenotypes of immune cells and their respective effects on angiogenesis and tissue repair. We focus on strategies that utilize immune cells or their extracellular vesicles (EVs) to promote vascularization for tissue regeneration and approaches to recruit and induce pro-regenerative immune cell phenotypes in situ.
2. Roles of immune cells in angiogenesis
Tissue injury typically leads to vascular compromise, platelet activation, and coagulation. The recruitment of innate and adaptive immune cells from circulation and local tissues plays important roles in inflammatory responses, angiogenesis, and tissue remodeling. In this section, we discuss the roles of the immune cells in wound healing, with a focus on angiogenesis (Table 1).
Table 1.
Roles of immune cells in angiogenesis.
| Cells | Characteristics and effect on angiogenesis | Reference |
|---|---|---|
| Platelets | Pro-angiogenic growth factors released from α-granules | [15,16] |
| Microvesicles derived from activated platelets promote EC proliferation and migration | [18] | |
| Direct interaction with other immune cell types (P-selectin; CD40L) | [19,20,22] | |
| Paracrine immunomodulatory, pro-angiogenic effects | [23] | |
| Neutrophils | ||
| N1-like | Cytotoxic, predominant in the early phase of tissue repair | [26] |
| Secretion of inflammatory cytokines: TNFα, IL-1β or IL-6 | [26] | |
| N2-like | N2 neutrophils secrete IL-10 and chitinase Ym1, which promote angiogenesis | [31–33] |
| Expression of signals that increase neutrophil clearance (CD206, ‘eat-me’ signals) and macrophage polarization into M2-like phenotype | [35,37] | |
| Macrophages | ||
| M1-like | Proinflammatory, phagocytize cellular debris or apoptotic cells | [38,39] |
| Markers: CD86, iNOS, CD38 | [39] | |
| Cytokine secretion: TNF-β, IL-1β, or IL-6 | [39] | |
| Critical at the beginning of angiogenesis | [45] | |
| Secrete high levels of VEGF | [46] | |
| M2-like | Anti-inflammatory, reparatory phenotype | [38] |
| Markers: CD206 | [40] | |
| Secrete IL-10 and arginase-1, TGF-β or PDGF | [40,41] | |
| Promote pericyte recruitment and blood vessel stabilization and remodeling | [42,43] | |
| Dendritic cells | Depending on stimulation secrete pro-angiogenic (VEGF, bFGF) or anti-angiogenic factors (IL-12, IL-18) | [50] |
| Direct effect on neovascularization through the stimulation of ECs | [50] | |
| Indirect effect: via the recruitment and polarization of other immune cells (effector T cells) | [53] | |
| T cells | ||
| Th1 cells | Secretion of high levels of IL-2, TNF-α, and IFN-γ | [57] |
| No benefit to revascularization or induction of vascular regression | [57] | |
| Th2 cells | Secretion of cytokines like IL-4 | [58] |
| Macrophage polarization into M2 phenotype | [57,59] | |
| Secretion of angiogenic factors that enhance EC migration and sprouting | [58] | |
| Tregs | Secretion of anti-inflammatory cytokines like IL-4 | [61] |
| M2 macrophage polarization | [62] | |
| Secretion of IL-10, promote EC proliferation | [63] | |
| Secretion of amphiregulin, which can induce VEGF synthesis | [64,65] | |
| B cells | Stimulation of macrophage pro-angiogenic activity via Fcγ receptor engagement | [69] |
| mature naïve B cells can increase angiogenesis by secreting pro-angiogenic mediators such as VEGF or TGF-β | [70,71] | |
| Indirectly enhancement of the pro-angiogenic properties of other cell types | [54] | |
2.1. Platelets
Platelets are among the first cells of the innate immune system to be recruited to the site of injury. Following endothelial damage, these small anucleated cellular fragments derived from megakaryocytes adhere to suddenly exposed subendothelial extracellular matrix (ECM) proteins, such as collagen, which trigger platelet activation and aggregation [14]. Platelets contain three types of granules that are released to the extracellular space upon activation: α-granules, dense granules and lysosomes; a-granules are the most abundant, comprising around 10% of platelet volume, and contain hundreds of compounds involved in coagulation, inflammation, wound healing, and angiogenesis. These compounds include not only pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF) and insulin-like growth factors (IGF) but also angiogenesis inhibitors such as thrombospondin (TSP)-1 and angiostatin [15]. Despite the presence of both pro- and anti-angiogenic modulators, activated platelets have an overall stimulatory effect on angiogenesis [16]. Platelet dense granules and lysosomes seem not be essential for the formation of new blood vessels [17]. Additionally, microvesicles (0.1–1 μm in diameter) derived from activated platelets have been shown to promote endothelial proliferation, survival, migration and tube formation in vitro presumably caused by phospholipids, such as sphingosine 1-phosphate and growth factors such as VEGF and bFGF [18]. Besides their function as important regulators of hemostasis, activated platelets also recruit other immune cells to the site of injury and modulate their functions either by directly interacting with them or by releasing different paracrine factors in their soluble forms or enclosed within microvesicles. The first physical interaction between platelets and leukocytes, in particular neutrophils, is formed between P-selectin on activated platelets and P-selectin glycoprotein ligand-1 (PSGL-1) on neutrophils. This interaction recruits free-floating neutrophils to the affected site and also activates their immunological functions [19]. Activated platelets also express CD40 ligand (CD40L) on their surface that interacts with cell surface receptors of macrophages, B cells, or T cells [20]. CD40-CD40L interactions can trigger the release of superoxide and reactive oxygen species (ROS) by leukocytes or promote activation and maturation of dendritic cells (DCs) [21]. CD40L can stimulate VEGF secretion by macrophages, which then promotes angiogenesis [22]. However, platelet surface expression of CD40L is temporary. Soon after activation, CD40L is cleaved by extracellular proteases and released either in a soluble form or carried on the surface of platelet microvesicles, which can stimulate EC proliferation and promote sprouting [23].
2.2. Neutrophils
Neutrophils, the most abundant white blood cells in humans (5–10 × 1010 cells produced daily) [24], are among the first cells to be recruited to the site of injury (Fig. 1A). They are well known for their bactericidal functions including phagocytosis, release of cytotoxic granular content, production of ROS, and formation of neutrophil extracellular traps (NETs) [25]. Additionally, recent discoveries demonstrate that they also play important roles in tissue repair and angiogenesis [26]. In the absence of neutrophils, macrophages alone are not able to sufficiently revascularize implanted biomaterials. Neutrophil recruitment to the implant, therefore, seems to be crucial for the successful engraftment of bioengineered capillaries. It was also observed that implanted unassembled vascular cells or vascular cell-derived conditioned medium attract more neutrophils than implants with fully assembled vascular networks. This effect was ascribed to the inhibition of Notch signaling by factors released from the unassembled vascular cells [27].
Fig. 1. Engagement and polarization of immune cells in different phases of re-vascularization and tissue regeneration.
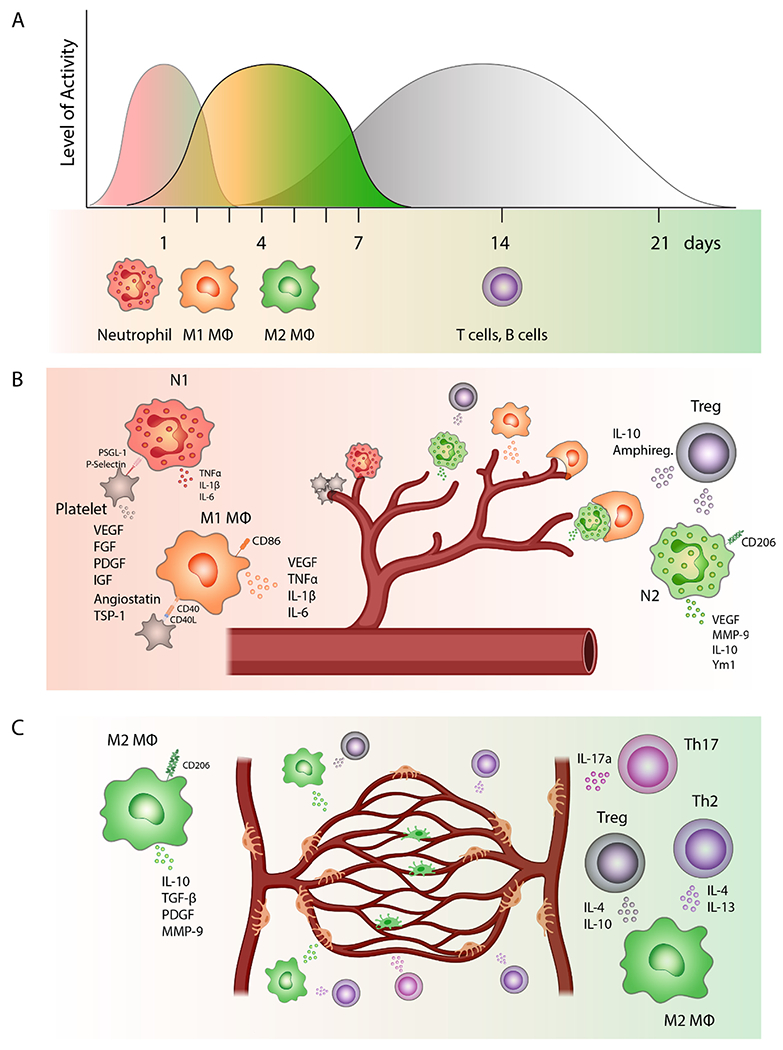
(A) Physiological timeline of sequential recruitment of different types of immune cells to the damaged tissue and an average duration of their involvement in revascularization and tissue repair. Neutrophils are among the first immune cells to be recruited to the wound bed followed by macrophages, which are first polarized into pro-inflammatory M1-like phenotypes. Gradually over the course of the healing process, the balance is shifted to M2-like anti-inflammatory macrophages. Finally, after approximately 3–5 days, the activated effector T cells and B cells travel to the site of injury to modulate tissue regeneration. (B) The beginning of the healing process. P-selectin on the surface of activated platelets helps to recruit free-floating neutrophils to the affected site. Neutrophils are first polarized into pro-inflammatory N1 phenotype and secrete cytokines like TNFα, IL-1β or IL-6. Activated platelets also express CD40 ligand (CD40L) on their surface that interacts with the receptor on the surface of macrophages. This interaction can stimulate VEGF secretion by macrophages, which promotes angiogenesis. M1-like macrophages are important at the beginning of the angiogenic process when they are in a close contact with tip cells of sprouting capillaries. Neutrophils, which are polarized in the presence of TGF-β into an anti-inflammatory N2-like phenotype, also secrete VEGF and metalloproteinases, which release growth factors bound to ECM. Apoptotic N2-like neutrophils are phagocytosed by macrophages, which induce their polarization into M2-like anti-inflammatory state. Regulatory T cells (Tregs) represent a subset of CD4 + T cells that are involved in the regulation of the healing process by suppressing proinflammatory immune responses. Tregs also secrete amphiregulin, which can promote angiogenesis by inducing VEGF synthesis in other cell types. (C) Blood vessel stabilization and tissue remodeling. M2-like anti-inflammatory macrophages, which express high levels of CD206, dominate in later stages of the healing process where they secrete cytokines like IL-10 or growth factors such as TGF-β or PDGF. M2-like macrophages also promote anastomoses, stabilize growing blood vessels, and consequently are involved in vascular network remodeling. CD4 + T helper 2 (Th2) cells, Tregs, and Th17 cells can promote regeneration and angiogenesis either directly, by secreting angiogenic factors that enhance EC proliferation and migration, or indirectly by secreting cytokines like IL-4 which can induce macrophage polarization into M2-like phenotype.
Depending on the signals received from the microenvironment, neutrophils can be polarized to different phenotypes, ranging from the proinflammatory N1 to the anti-inflammatory N2 state. This differential activation can influence the recruitment and function of other immune cells e.g. monocytes, macrophages, B cells, or T cells [28]. Transforming growth factor-μ (TGF-μ) has been shown to play a major role in neutrophil polarization state [29]. TGF-β inhibition induces cytotoxic N1-like phenotype; characterized by secretion of inflammatory cytokines like TNF-α, IL-1β or IL-6 (Fig. 1B) [26]. On the contrary, sustained presence of TGF-β results in N2-like neutrophils secreting anti-inflammatory factors and proangiogenic mediators such as VEGF and matrix metalloproteinases (e.g., MMP-9) [30], which are responsible for the degradation of ECM components and the release of VEGF and other growth factors bound to the ECM. These neutrophils usually express IL-10 [31] and chitinase Ym1 [32–34], which promote angiogenesis. It is suggested that the polarization state of neutrophils changes temporarily over the course of tissue repair [35] and is dramatically dependent on the signals received from the microenvironment. N1-like neutrophils are predominant in the early phase of tissue repair, whereas the N2-like subsets appear later and contribute to the healing process [36], not only through the secretion of the above mentioned factors, but also expression of signals that increase their clearance by macrophages. For example, N2-like neutrophils express mannose receptor CD206 [35] and apoptotic neutrophils upregulate so called ‘eat-me’ signals [37] (such as phosphatidylserine) that promote their phagocytosis by macrophages, which consequently induces macrophage polarization into an M2 anti-inflammatory state. If this sequence is disturbed and neutrophils remain in the proinflammatory N1-like state or are not properly cleared from the injured tissue, they might significantly contribute to the excessive inflammation [26].
2.3. Macrophages
Macrophages are innate immune cells that arrive to the damaged site usually within 1 day post-wounding [38] and similarly to neutrophils, can be polarized into different phenotypes depending on the microenvironment. M1 proinflammatory macrophages can be induced in vitro by cytokines such as IFN-γ or bacterial components like lipopolysaccharide (LPS), whereas polarization into M2 anti-inflammatory macrophages can be achieved through stimulation with IL-4 and IL-13 [38].
However, M1 and M2 macrophages are just the two extremes of the whole spectrum of macrophage phenotypic states, which can be found during the regeneration process. Generally, at the beginning of the healing process, macrophages are polarized into an M1-like proinflammatory phenotype that phagocytize cellular debris or apoptotic cells, express markers such as CD80, CD86, and nitric oxide synthase (iNOS); and secrete proinflammatory cytokines [39], e.g., tumor necrosis factor (TNF-α,) IL-1β, or IL-6. However, the number of these proinflammatory M1-like macrophages decrease over time and by day 4 post-injury, the M2-like reparative phenotype prevails. M2-like macrophages express high levels of CD206 and suppress inflammation by secretion of IL-10 or arginase-1 [40]. Both M1 iNOS and M2 arginase-1 metabolize the substrate L-arginine, however, M2-like macrophage metabolism results in the production of ornithine that promotes cell proliferation and tissue repair. M2-like macrophages also secret growth factors like TGF-β or PDGF [41], which are important for pericyte recruitment [42] and consequent blood vessel stabilization. Moreover, M2-like macrophages produce MMPs (especially MMP-9) to remodel ECM and release VEGF [43,44].
The importance of macrophages in the revascularization process was documented in previous studies, where the ablation of macrophages during the early phases of wound healing significantly impaired neoangiogenesis [45]. In the same way macrophage function changes over time during tissue regeneration, different macrophage phenotypes are involved in regulating distinct parts of angiogenic processes. M1-like phenotype seems to be critical at the beginning of angiogenesis around day 3 post-injury, when highly disorganized and leaky blood vessels appear. At this time, proinflammatory macrophages secrete high levels of VEGF [46] and are in a close contact with tip cells of sprouting capillaries (Fig. 1B) [47]. However, long-term presence of M1-like macrophages causes vessel regression [48]. M2-like anti-inflammatory macrophages dominate in later stages to promote anastomoses, stabilize growing blood vessels, and subsequently participate in vascular network remodeling (Fig. 1C). It was also shown that conditioned medium from M2-like macrophages was sufficient to induce anastomoses, which indicates that, at this stage, the cell-cell contact is not necessary [46]. Moreover, there is a possibility that macrophages also directly contribute to angiogenesis by transdifferentiating into ECs, pericytes or smooth muscle cells [49].
2.4. Dendritic cells
DCs are professional antigen-presenting cells that reside in an immature state in peripheral tissues. Upon recognition of a foreign antigen, they undergo maturation and migrate to the secondary lymphoid organs where they present the processed antigen to naive T cells and thus govern T cell activation and polarization. In their immature state, DCs might promote tolerance by inducing regulatory T cell formation through TGF-β production, whereas mature DCs stimulate immunological responses by controlling effector T cell polarization [50]. Depending on the signals from the microenvironment, DCs express various pro-angiogenic (VEGF, bFGF) or anti-angiogenic factors (IL-12, IL-18) [50] that can modulate neovascularization directly, through the stimulation of ECs, or indirectly via the recruitment and polarization of other immune cells. For example, DCs might produce TSP-1, which binds to VEGF or other growth factors and impairs their angiogenic effects [51]. TSP-1 also binds to MMP-9 and suppresses its activation [52]. While under hypoxic conditions, DCs promote CD4 + T helper cell polarization into a Th2 pro-angiogenic phenotype [53].
2.5. T Cells
T cells differentiate into the CD4 + or CD8 + subsets in the thymus and afterwards migrate to the secondary lymphoid tissues (e.g., spleen or lymph nodes). Here T cells encounter their cognate antigen presented by an antigen presenting cell (APC) that induces their proliferation and terminal differentiation into either effector or memory T cells. This interaction between T cells and APCs is modulated by the T cell receptors (TCRs) present on the cell surface that bind to an antigen coupled to MHC on the surface of the APC. This binding leads to T cell activation and initiates a cascade of events that includes cytoskeletal reorganization, Ca2+ influx, and cytokine production. After approximately 3–5 days, the activated effector T cells leave the lymph nodes and travel to the site of injury [54]. Cytotoxic CD8 + T cells facilitate removal of necrotic tissue and increase macrophage activation, but their effect on tissue regeneration is generally detrimental [55,56]. CD4 + T cells might limit or promote regeneration, depending on the specific subtype. CD4 + T helper 1 (Th1) cells secrete high levels of IL-2, TNF-α, and IFN-γ. This phenotype has been reported to either provide no benefit to revascularization or even inhibit EC proliferation and migration, which induces vascular regression [57]. On the contrary, CD4 + T helper 2 (Th2) cells and Th17 cells [58] can promote regeneration and angiogenesis either directly, by secreting angiogenic factors that enhance EC migration and sprouting, or indirectly by secreting cytokines like IL-4 or IL-13 which can induce macrophage polarization into M2-like phenotype [57,59].
Regulatory T cells (Tregs) represent another subset of CD4 + T cells that are involved in the regulation of the healing process by suppressing proinflammatory immune responses. Tregs modulate tissue regeneration by controlling neutrophil infiltration and behavior. For instance, Tregs promote neutrophil secretion of anti-inflammatory molecules including IL-10, TGF-β, heme oxygenase-1, and indoleamine 2,3-dioxygenase (IDO) [60]. Similar to Th2 cells, Tregs secrete anti-inflammatory cytokines like IL-4 to induce M2-like macrophage polarization [61,62]. Besides their effect on macrophages, Tregs might also secrete IL-10 by themselves and promote EC proliferation [63]. Tregs were also reported to secrete amphiregulin (ligand of epidermal growth factor receptor), which can promote regeneration [64] and angiogenesis by inducing VEGF synthesis in other cell types [65], or they can facilitate regeneration by affecting local stem and progenitor cells [66,67].
2.6. B cells
Besides having the potential to differentiate into plasma cells producing antibodies, B cells can also present antigens to T cells and modulate local immune responses through the production of various cytokines. It was shown that IgG immunoglobulins produced by B cells at the site of injury facilitate the macrophage phagocytosis of dead cells [68]. Production of IgG can also stimulate macrophage pro-angiogenic activity via Fcμ receptor engagement [69]. Moreover, mature naïve B cells can increase angiogenesis and accelerate tissue regeneration by either secreting pro-angiogenic mediators such as VEGF [70] or TGF-β [71], or indirectly enhancing the pro-angiogenic properties of fibroblasts and other cells in the wound microenvironment [54].
3. Revascularization and regeneration in aging and disease
The body’s regenerative ability declines with age, which is accompanied with altered immune responses, impaired angiogenesis, and increased risk of developing chronic wounds or autoimmune diseases. Aging is characterized by decreased oxygen supply to tissues, reduced response to hypoxia and delayed neovascularization [72]. Moreover, aging organisms show signs of the low-grade systemic inflammation also known as “inflammageing”, which is associated with an increase of circulating myeloid cell populations relative to lymphoid cell populations in part caused by the decline in thymic activity necessary for the development of T cells and also by changes in the bone marrow, where myelopoiesis and lymphopoiesis take place [73]. In addition to these alterations, aging is also marked by increased levels of circulating proinflammatory mediators such as IL-1, IL-6, or TNF-α [74], which may decrease the sensitivity of immune cells. Despite the same levels of chemokines at the wound site, the recruitment of neutrophils and macrophages to the affected tissue is diminished in aged compared to young animals. Moreover, some studies also report disturbed phagocytic abilities of neutrophils and macrophages in aged or diseased tissues, which leads to further alterations in the normal dynamics of the healing process. T cell infiltration into the injured tissue in aged animals is delayed but T cells ultimately reach higher numbers in the affected tissue. Age-associated T cells also exhibit T cell exhaustion phenotype, which is associated with an incomplete differentiation of aged CD4+ T cells into effector cells. IFN-inducible CXCR3 ligand, CXCL9, is one of the chemokines that was found to be involved in aging, adverse tissue remodeling and poor vascular function [75]. Aged ECs and certain immune cells secrete increased levels of the angio-static factor CXCL9 [76], which inhibits blood vessel growth by interacting with VEGF and preventing its binding to ECs [77].
Altered HIF-signaling can be also found in autoimmune diseases such as diabetes mellitus, where insufficient angiogenesis contributes to impaired tissue regeneration and development of chronic wounds [78]. Chronic wounds fail to heal and remain in the early inflammatory state of the healing process even after 12 weeks. Macrophages in chronic wounds have reduced ability to phagocytose apoptotic cells and neutrophils, which accumulate in the wound and create strong inflammatory environment [38]. Macrophages are not able to efficiently transfer to anti-inflammatory phenotype but they release increased levels of metalloproteinases that degrade ECM.
Chronic tissue injuries may also lead to normal tissue architecture destruction, excessive matrix deposition, scar formation, tissue hypoxia, and abnormal neovascularization. Most newly formed blood vessels in fibrotic tissue are immature and fail to mitigate the ischemic state of the tissue. VEGF secreted in the affected tissue also increase vessel permeability and stimulate myofibroblast proliferation and collagen synthesis [79]. M2-like macrophages that remain in the fibrotic tissue for a prolonged time release factors such as TGF-μ, PDGF and galectin-3, which activate myofibroblasts and promote their survival [80].
Together, these observations suggest that any alterations in the normal regeneration process even though they are caused by increased expression of otherwise pro-regenerative growth factors or prolonged presence of immune cells that have normally reparative function might disrupt the balanced signaling that lead to the reestablishment of tissue homeostasis.
4. In vitro and in vivo models for evaluating angiogenesis
As described above, regeneration is dependent on a proper tissue revascularization, which is deeply affected by the signaling from immune cells and other cells in the microenvironment. To dissect the complexity, several in vitro assays have been developed over the years to investigate specific stages of the angiogenic process. In contrast to in vivo experiments, in vitro assays are relatively simple and enable high-throughput screening of different pro-or anti-angiogenic compounds or conditions [81]. Besides standard proliferation tests that are used to compare the growth rate of vascular cells treated with different agents, other in vitro models such as wound closure model or trans-well cell migration assay (also known as Boyden chamber assay) are used to evaluate EC migration [82]. In the wound closure assay, cells are grown to confluency and then scraped to create a cell-free area into which cells migrate to. The cell migration ability is generally evaluated as a percentage of the wound closure after several hours to days. The trans-well cell migration assay is based on a chamber separated by a microporous membrane into two compartments with different media composition. This assay can be used to evaluate chemotactic properties of different cells or agents. The microporous membrane can be also coated with different ECM proteins to study cell invasion. Moreover, ECs can be seeded on specific substratum or embedded into a hydrogel as single cells or spheroids and their migration can be observed by time-lapse microscopy. These types of experiments can be done also with co-cultures of two or more cell types that can be labeled with different fluorescent dyes to evaluate the cell-cell interactions and contribution of different cell types to the neovascularization [48]. Furthermore, more physiologically relevant conditions can be simulated by applying fluid flow in the system, for example, by utilizing different organ-on-a-chip platforms.
One of the most widely used in vivo angiogenic assays is the plug assay that is versatile, least costly, and easy to perform [81]. A hydrogel containing the studied agent or cells is injected subcutaneously and the blood vessel sprouting into the plug can be evaluated after a week. Neovascular quantification is based on the vascular ingrowth in the plug, blood vessel density, or the expression of EC genes evaluated by quantitative polymerase chain reaction. Permeability and maturation of newly formed blood vessels can be evaluated after intravenous injection of fluorescently-labeled dextran of different molecular weights. Cutaneous wound assay is another very frequently used in vivo angiogenesis assay [83,84]. During the healing process, vascular density increases by several folds generally within a week after wounding and returns to the initial level after 3 weeks. Therefore, it is advisable to evaluate the blood vessel density at several time points and after the revascularization peak use this model to assess vessel maturation and remodeling. Most commonly, the total number of blood vessels per area (vessels/mm2) is evaluated together with the number of vascular branches and a vessel diameter. The quantification of blood vessel maturation can be done by staining pericytes and other mural cells and by evaluating their association with blood vessels. Parameters such as the metabolic status of the tissue and the oxygenation level can be also used to determine the efficacy of the vascular supply. Myocardial ischemia and hind-limb ischemia models are also widely used in vivo models to study angiogenesis and arteriogenesis. Prolonged myocardial ischemia caused by inadequate blood supply can lead to myocardial infarction (MI) and even heart failure. MI is typically induced by the ligation of the left anterior descending coronary artery in small animal models (typically mice or rats) and the treatment can be initiated immediately after the infarction or a few days later. In addition to the standard histology and immunohistochemistry, heart function and structure can be monitored by echocardiography. In larger animal models, blood vessels can be visualized for example by computed tomography angiography [85]. The extent of neovascularization and tissue regeneration in hind-limb ischemia model is dependent not only on the animal age but also on the species and even on the choice of mouse or rat strain. For example, C57Bl6 mice can often fully recover without any therapeutic interference while other mouse strains such as Balb/C display a poor regenerative capacity [86]. Another limiting factor is that majority of the in vivo experiments are done on young animals that might not reflect the systemic changes observed in older patients. Thus, the limitations and specifics of different models need to be taken into account when designing the experiments.
5. Delivery of immune cells and EVs
Immune cells significantly contribute to the healing process and revascularization. However, a disturbance in the physiological healing process can have detrimental effects on tissue regeneration. Immune cells that erroneously persist as a specific phenotype, such as M1-like macrophages during chronic inflammation or M2-like macrophages during the fibrotic process, prevent proper immune signaling and may extend the duration of injury. Therefore, different approaches have been developed in order to manipulate immune cells to promote tissue repair or revascularization. This section will focus on the delivery of immune cells and their derivatives such as EVs.
5.1. Immune cell delivery
Delivery of ex vivo expanded immune cells, mainly macrophages, was tested as a treatment option for multiple different diseases with generally positive effects on regeneration and revascularization (Table 2). Monocytes or macrophages from healthy donors transplanted into the wounds of normal or diabetic mice accelerated wound healing by increasing angiogenesis as demonstrated by CD31 staining. The same positive results were also obtained after the transplantation of monocytes or macrophages from diabetic patients into diabetic mice. It thus appears that increasing the number of macrophages at the site of injury above the physiological level can promote revascularization and significantly accelerate wound healing [87] and that the transplantation of autologous macrophages can be beneficial even for patients with autoimmune diseases. Although the pro-regenerative properties are generally ascribed to the M2-like macrophages [88–90], there are also studies showing that M1-like macrophages are helpful in promoting tissue repair. However, these macrophages do not persist in the pro-inflammatory phenotype but evolve into the M2 state over time [91]. Incorporation of macrophages into engineered tissues also significantly enhanced vascular ingrowth and supported anastomosis between host and graft vessels, which was verified by video recordings of red blood cell flow in the newly formed vasculature [48,90].
Table 2.
Clinical trials and in vivo studies, in which immunoengineering strategies were used to promote regeneration and angiogenesis.
| Approach | Therapeutic agent | Disease/ model | Major therapeutic effect | Reference |
|---|---|---|---|---|
| Cell delivery | Macrophages | Rat ischemic hind limb model | Stimulate capillary formation and arteriogenesis by producing angiogenic growth factors |
[88] |
| Macrophages incorporated into engineered skeletal muscle | Dorsal skinfold window-chamber model in nude mice | Stimulate muscle satellite cell-mediated myogenesis, limiting apoptosis Augment blood vessel ingrowth |
[90] | |
| CD90 + mesenchymal stem cells and M2-like macrophages | Phase II clinical trial Ischemic heart failure |
Reduction in clinical cardiac events | [89] | |
| CD90 + mesenchymal stem cells and M2-like macrophages | Phase II clinical trial Critical limb ischemia |
Amputation-free survival improved wound healing Possible mechanisms: increased tissue vascularity, remodeling of fibrotic tissue, modulation of the inflammatory response |
[228,229,230] | |
| EV delivery | M2 Mϕ-derived exosomes | Mouse excisional wound healing model | Accelerates wound healing, Enhanced angiogenesis Enhanced re-epithelialization Increased collagen deposition | [107] |
| EV mimetics delivery | Leukosomes from proteins derived from the leukocyte plasma membrane and synthetic phospholipids | Mouse model of localized inflammation induced by lipopolysaccharide (LPS) | Leukosomes target inflamed vasculature Improve tissue healing by preserving tissue architecture and reducing neutrophil infiltration. |
[116] |
| In situ immune cell recruitment | Co-delivery of CSF1 with VEGF for macrophage recruitment | Hydrogel discs containing growth factors implanted into the mouse cornea | Robust angiogenic response Significant increase in vessel density and total vessel length Increased pericyte coverage |
[131] |
| D-peptide crosslinked microporous annealed particle scaffold for the recruitment of myeloid cells |
Mouse excisional wound healing model | Enhanced hydrogel degradation Reduced scar formation Neogenesis of hair follicles Higher vessel ingrowth Significantly higher numbers of neutrophils infiltrating the scaffold after 1 day |
[231,141] | |
| Antigen-releasing scaffold for the recruitment of CD4 + Th2 T cells | Mouse hindlimb ischemia model | Enhance angiogenesis, vessel density, increased perfusion in ischemic limbs Reduced necrosis and enhanced regenerating myofibers in the muscle |
[143] | |
| In situ manipulation of immune cell phenotypes | Decellularized bone scaffold with fast initial release of IFN-γ followed by sustained release of IL-4 | Mouse subcutaneous implantation model | Increased blood vessel density in scaffolds containing IFN-γ, which polarize macrophages into M1-like phenotype | [42] |
| Lipoxin A4 encapsulated LXA4 in poly-lactic-co-glycolic acid (PLGA) microparticles | Rat excisional wound healing model | Reduced neutrophil chemotaxis Accelerated wound closure Increased matrix remodeling, Increased number of blood vessels. More macrophages and IL-4 |
[168] | |
| Hydrogels of different stiffnesses | Subcutaneous implantation of the hydrogels in mice: Mouse excisional wound healing model |
M1-like macrophages were more abundantly distributed on stiff hydrogels, while M2-like macrophages were found more on soft hydrogels Thinner fibrotic capsule formation around softer hydrogels Softer hydrogels reduce the final scar size |
[177,179,178] |
Moreover, the therapeutic efficacy of delivered immune cells can be further potentiated by utilizing the cells themselves as drug carriers [92]. Immune cells can be equipped with backpacks filled with bioactive molecules, which are released upon stimulation in a highly localized fashion (Fig. 2A). The geometry of the cellular backpacks was shown to be an important parameter affecting the stability of the cargo attachment to the cell surface; non-spherical particles are internalized to a lesser extent by macrophages and retain the capacity for drug release longer compared to their spherical counterparts [93]. The attachment of the backpacks to the immune cell surface can be achieved by covalent coupling to cell surface proteins [94], covering the backpack with a cell-adhesive layer, such as hyaluronic acid modified with aldehyde and poly(allylamine) hydrochloride, or coupling to cell-specific antibodies, e.g., anti-CD45 antibodies for T cells. Backpacks then gradually release compounds, which continuously guide the polarization of immune cells. An interesting system utilizes stimuli-responsive cargo release via a crosslinker system that responds to the local changes in redox environment. Upon antigen recognition, T cells increase their surface reduction potential which leads to the release of cytokines from nanogel backpacks and enhanced T cell expansion [95].
Fig. 2. Delivery or recruitment of immune cells to enhance angiogenesis and regeneration.
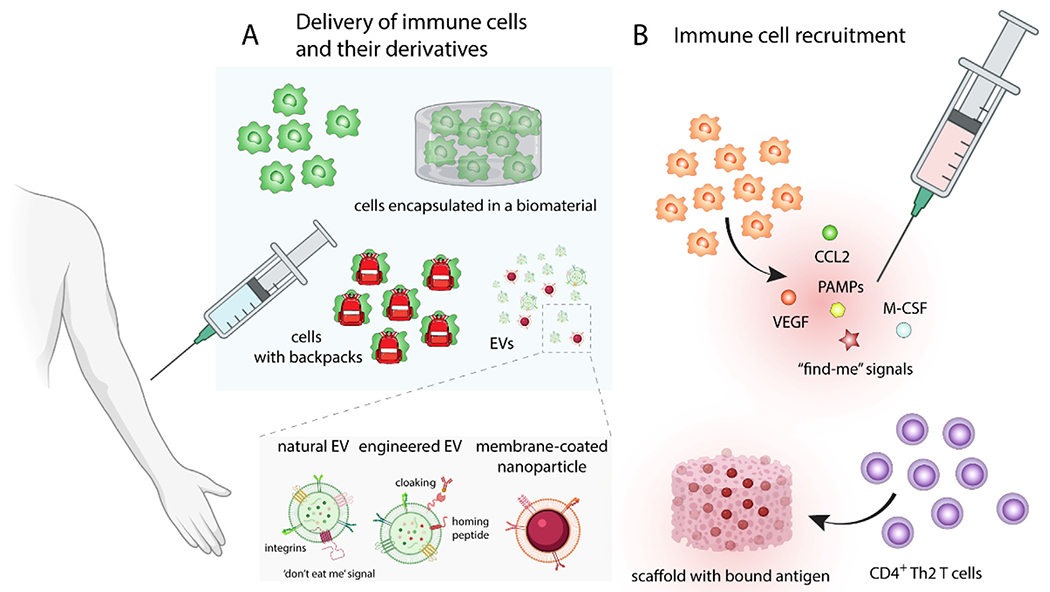
(A) Delivery of ex vivo expanded immune cells, mainly M2-like pro-regenerative macrophages or immune cells equipped with backpacks bound to their surface, which gradually release compounds that continuously guide the polarization of immune cells. In order to improve immune cell retention at the site of the injury and increase cell viability, cells can be encapsulated various types of implantable or injectable biomaterials. Moreover, extracellular vesicles (EVs) secreted by cells can replicate the therapeutic effect of delivered cells. EVs can be furthermore modified by various techniques such as cloaking, loaded with drugs, or conjugated with homing peptides to further increase their pro-healing effects and homing specificity. (B) Macrophage recruitment can be promoted by cytokines like macrophage colony-stimulating factor (M-CSF), growth factors such as VEGF, chemokines like CCL2, or “find-me” signals, which are normally secreted by apoptotic cells. Biomaterials can provide not only the sustained and prolonged presentation of therapeutics but they can also enhance their stability and localization. Biomaterial scaffold with bound antigen can increase the local concentration of antigen-specific CD4 + Th2 T cells and promote angiogenesis and regeneration.
Intravascular or localized administration of macrophages or other immune cells through needles has adverse effects on cell viability. This consequence, along with poor retention of the administered cells in the site of injury [96], requires more attention and may explain the discrepancies in the reported therapeutic effects of immune cells delivered by injections. These issues might be overcome through cell encapsulation in various types of implantable or injectable biomaterials that can protect cells during the administration process, shield against stresses from the new microenvironment, and improve targeted localization and function. The biomaterial scaffolds used for these purposes can have porous structures, with pore sizes in a range of tens to hundreds of micrometers, to allow for delivered cell migration out of the scaffold [97]. For example, cryogels are injectable porous materials [98] that are typically formed by freezing the gel mixture and consequently removing ice crystals by thawing. This process leads to the formation of a macroporous sponge-like structure that is able to squeeze within the needle and then to rapidly restore its original shape and size following injection.
Additionally, biodegradable materials with smaller pore sizes can be used for cell encapsulation [99]. Injectable hydrogels for these applications can be formed by in situ chemical crosslinking, which is most commonly photo-initiated, redox-initiated, or polymerized by Michael-type addition. Alternatively, hydrogels can be cross-linked by external stimuli such as temperature, pH, ion concentration, or hydrophobic interactions. Some of these physically cross-linked hydrogels have shear-thinning properties [100], which means that they liquefy under shear stress and may solidify upon relaxation. This improves the retention and spatial control of encapsulated cells following injection, as the rapid solidification post-injection can limit leakage to the neighboring tissues. The degradation of such hydrogels and the release of encapsulated cells can be realized by reversing the gelation mechanism or, in many cases, by incorporating degradable sequences in the biomaterial. The addition of in situ degradable peptides, such as MMP-sensitive sequences or stimuli responsive peptides, enables better control of the degradation rate compared to hydrolysis or by other enzyme-mediated processes. Materials for these purposes might be composed of natural or synthetic polymers, which can be further modified, for example, with ECM-derived cell adhesion peptides, such as RGD or REDV derived from fibronectin, GFOGER from collagen, or IKVAV from laminin, to support cell adhesion to the scaffold and prevent cell death from anoikis [101]. Alternatively, cells can be covered with a thin layer of a non-biodegradable material, e.g. agarose, that permits the secretion of paracrine factors from the caged cells to modulate the microenvironment [102].
5.2. EV delivery
The outcomes of cell therapies can vary greatly as the exposure to microenvironmental cues can polarize transplanted cells into different phenotypes and influence their secretome. Moreover, cell viability and survival play a significant role. Therefore, based on observations that the therapeutic effect of cells might be, to a great extent, replicated by the factors secreted by these cells, cell-free therapies are under development. EVs have received much attention recently as mediators of tissue regeneration. These small membrane-enclosed vesicles, 30 nm – 1 μm in diameter, contain bioactive molecules and genetic information derived from the cell of origin and are able to shuttle their EV content into the target cells and influence their behavior. Platelet EVs, for example, can accelerate the restoration of endothelial integrity after vascular injury [103] and enhance healing of chronic diabetic wounds by increasing the proliferation and migration of ECs [104]. This prohealing effect of EVs may be mediated by proangiogenic growth factors contained in EVs and their effect on YAP activation, which increases fibroblast migration, proliferation, and collagen synthesis to enhance wound closure [104]. Moreover, platelet EVs were reported to influence CD4+ T cell activation by increasing TGF-β production, decreasing IFN-γ, and inducing differentiation towards immunosuppressive Tregs [105], which facilitate tissue regeneration.
Like platelet EVs, macrophage EVs have also been reported to influence the tissue regeneration process. Macrophage EVs were shown to contain proteins such as VEGF and Wnt3a, and miRNAs such as miR-130a, which regulate angiogenesis. Once transplanted in a hydrogel plug in mouse model, the presence of EVs increased the blood vessel ingrowth into the hydrogel and the diameter of newly formed vessels was larger in comparison to the control group [106]. However, the content of EVs and their effects might be also dependent on the macrophage phenotype from which they are derived. EVs isolated from M2 anti-inflammatory macrophages contain bioactive molecules generally connected with this phenotype, which include chemokine CCL24 and glycoprotein lactadherin (MFG-E8) that promote M2-like differentiation, and chemokines SDF-1 and eotaxin-1 that promote angiogenesis. The addition of M2 EVs to proinflammatory M1-like macrophages was shown to convert their phenotype to an M2 pro-healing state [107]. This strategy might be particularly beneficial for the treatment of non-healing wounds or other diseases where the persistence of M1-like macrophages and their inability to transition to an anti-inflammatory M2 state hinders the healing process. Favorable effects on tissue regeneration were also reported for neutrophil EVs [108] and T cell EVs [109], but one can expect that the EV composition will be significantly dependent on the parental cell phenotype.
EVs secreted by different cells have specific receptors and adhesion molecules on their surface, which intrinsically enable preferential targeting to particular tissues [110]. This property of EVs can be exploited to target their delivery to specific cell types or injury sites. The homing specificity of EVs can be further improved by displaying a particular protein or tissue-homing peptide on their surface. One example approach fuses the protein sequence with the C1C2 domain of lactadherin, which localizes to EV membranes [111]. Alternatively, EV decoration with tissue-specific antibodies or homing peptides might be ensured by so called “cloaking”, which utilizes a phospholipid membrane anchor coupled with PEG and streptavidin to which any biotinylated molecule can be bound [112]. Moreover, peptides that bind to EV surface markers such as CD63 were recently discovered and offer further possibilities for EV functionalization and cargo loading [113].
Besides natural EVs, bioinspired vesicles can be generated by cell extrusion through microchannels or filters with pores smaller than 1 μm [114]. Alternatively, cell membranes might be isolated and used for nanoparticle coating. This approach was studied, for example, with platelet membrane-cloaked polymeric nanoparticles, which selectively adhered to damaged vasculature [115]. Moreover, integration of cell surface molecules such as leukocyte-derived adhesion molecules (LFA-1 and PSGL-1) into synthetic phospholipid vesicles also demonstrated an improvement in EV homing specificity to inflamed endothelium [116].
To further increase the therapeutic potential of EVs or their artificial alternatives, vesicles might be loaded with drugs or other therapeutic agents through different procedures. Example encapsulation techniques include passive loading by co-incubation with a therapeutic agent or active loading by sonication, electroporation, repeated freeze-thawing, saponin permeabilization, or coextrusion [117]. The choice of the optimal drug loading methodology is dependent on drug hydrophobicity and EV lipid composition. Hydrophobic drugs can generally penetrate the lipid bilayer of EVs, and their encapsulation efficiency is therefore similar for both passive and active loading methods, while the encapsulation level of intermediate or hydrophilic drugs can be significantly improved by a suitable active loading method. EVs can also be attached to or loaded with metal-based nanoparticles (like gold or paramagnetic iron oxide nanoparticles) to enable high precision imaging and spatial control under a magnetic field [118,119].
The circulation kinetics and biodistribution of exogenously administered EVs significantly affect their therapeutic efficacy. Besides the composition, EV route of administration and spatiotemporal release rate can undoubtedly impact their overall effect. The majority of therapeutic EVs have so far been administered in solution by injection. However, subcutaneous injections may not provide a prolonged and localized effect due to the leakage of EVs into blood or neighboring tissues [120,121]. Also, EVs administered intravenously are cleared from circulation within minutes [122], most commonly taken up by cells of the innate immune system [123]. Nevertheless, EV circulation half-life might be significantly affected by the EV surface composition. It was observed that EVs containing CD47 on their surface, which is a ‘don’t eat me’ signal that inhibits phagocytosis, showed enhanced retention in systemic circulation [124]. Furthermore, biomaterials can provide spatial and temporal control over EV release [125,126], which may help to overcome these hurdles and advance clinical translation of therapeutic EVs.
Thus, delivery of autologous immune cells, mainly macrophages, seems to have a positive effect on the regeneration of various tissues. One of the therapeutics effects that were observed is accelerated tissue revascularization, which is indispensable for cell survival and successful tissue repair. However, the therapeutic efficacy of such approach will be most likely dependent on many factors, including the patient disease stage, which might significantly affect the function of immune cells to be transplanted. Moreover, transplanted immune cells might not be retained at the site of the defect and might migrate to undesired locations, where they can aggravate other co-existing diseases such as cancer that might not yet be diagnosed. In this regard, EVs seem to be a safer option as they can be better controlled and might have similar therapeutic effect. Moreover, EVs can be potentially derived from genetically modified cells with HLA knockout antigens that might enable the use of universal allogenic EVs with much well characterized content for different therapeutic applications. In comparison to cell therapy, this approach would be also much more cost effective. However, the field has yet to see if one dose of EVs can replicate the long term therapeutic effect of cell delivery.
6. Strategies for immune cell recruitment
Instead of delivering ex vivo expanded immune cells, tissue regeneration might also be supported by recruiting and modulating endogenous immune cells at the site of injury [127,128]. Besides a variety of biomolecules for immune cell recruitment, drug delivery and biomaterials engineering have shown great potential for immune cell modulation.
Macrophage recruitment has been tested with cytokines like macrophage colony-stimulating factor (M-CSF) (Fig. 2B), which promoted macrophage infiltration to the damaged tissue and accelerated wound healing and neovascularization by regulating macrophage migration, proliferation, and polarization into an M2-like phenotype [129,130]. Robust macrophage recruitment was also observed after co-delivery of two proangiogenic growth factors PDGF-BB and FGF2; this strategy was comparably efficient to M-CSF and VEGF modification in promoting rapid and stable vascularization of artificial scaffolds [131]. Interestingly, a significant delay in macrophage infiltration was observed in diabetic wounds, where macrophage numbers started to increase after 10 days, while in normal wounds macrophages already begin to retreat at that time point. The treatment with proinflammatory chemokine (C-C motif) ligand 2 (CCL2) helped to restore macrophage kinetics and significantly improved wound healing [132].
Besides cytokines and growth factors, macrophages can be attracted by so called “find-me” signals secreted by apoptotic cells. Sphingosin-1-phosphate (S1P) is one of these cues secreted by cells when tissue homeostasis is disturbed [133]. However, S1P can interact with multiple receptors and depending on the binding site differentially stimulate macrophages. S1P binding to S1PR1 promotes macrophage migration, whereas binding to S1PR2 suppresses macrophage migration. Therefore, S1PR1-specific agonist SEW2871, that does not act on S1PR2, is used to increase macrophage infiltration in some tissue engineering applications. Incorporation of micelles containing SEW2871 into gelatin hydrogels increased macrophage migration [134], but this strategy did not lead to enhanced regeneration unless SEW2871 was co-delivered with stromal cell-derived factor 1 (SDF-1), which promoted the recruitment of mesenchymal stem cells [135] that polarized infiltrated macrophages towards pro-regenerative M2-like phenotypes.
Moreover, delivery of pro-inflammatory molecules such as pathogen-associated molecular patterns, which are recognized by Toll-like receptors (TLRs), can be used to attract immune cells [136]. For instance, an LPS-modified scaffold attracted M1-like macrophages, which are important for the initiation of the angiogenic process. Similarly, wound treatment with CpG oligonucleotides that activate TLR9 promoted the migration of macrophages to the damaged site and increased the production of VEGF, which accelerated re-vascularization and regeneration [137].
Biomaterial delivery platforms not only provide sustained and prolonged presentation of therapeutics but also enhance their stability and localization [138]. However, the choice of biomaterial can significantly affect the immune cell behavior. For instance, fibrin hydrogel scaffolds can attract a higher number of macrophages compared to gelatin hydrogels and polarize them into an M2-like phenotype [139]. The morphology of macrophages cultured on fibrin gels was reported to be round. Therefore, the anti-inflammatory phenotype might potentially be explained by observations of another study, which showed that preventing macrophage spreading reduced actin polymerization and consequently suppressed their pro-inflammatory phenotype [140].
Apart from macrophage recruitment, there is also evidence that regeneration and angiogenesis can be enhanced through adaptive immune activation. Administration of hydrogel with incorporated D-enantiomeric peptides into a skin wound induced T-helper cell dependent antibody responses, which enhanced recruitment of macrophages to the wound. Increased myeloid cell infiltration boosted tissue regeneration and even promoted hair neogenesis [141]. Extracellular matrix scaffolds derived from decellularized tissues can also promote tissue regeneration by creating a microenvironment that support T cell polarization to Th2 cells, which release anti-inflammatory cytokines and modulate local macrophages toward an M2-like phenotype [142]. Moreover, reintroduction of scaffold-bound antigen, in vaccinated animals can concentrate antigen-specific CD4+ Th2 T cells within the scaffold and adjacent tissues. These cells consequently enhance vascularization and regeneration of an ischemic tissue by suppressing the secretion of pro-inflammatory cytokines such as IFN-γ and by increasing the production of pro-regenerative cytokines like IL-10 [143].
In contrast to immune cell delivery, in situ immune cell recruitment represent an attractive, scalable and cost-effective approach to boost revascularization and tissue regeneration by harnessing patient’s immune cells. Many problems with tissue repair seem to originate from delayed or insufficient immune cell recruitment and angiogenesis, which hamper the progression from proinflammatory to anti-inflammatory phase of the healing process. This approach is based on the idea that once the appropriate types and numbers of immune cells are recruited to the local tissue, the desirable regenerative signaling can be restored. To gain further control of the immune cell polarization, additional strategies can be explored as needed.
7. Strategies for in situ manipulation of immune cell phenotypes
7.1. Delivery of immunomodulatory factors
Pro-inflammatory as well as anti-inflammatory immune cells are important players in tissue regeneration. However, the gradual progression of the regeneration process relies on both the strength of the immune cell response and the specific timeline of distinct immune cell contributions to re-establish tissue homeostasis. Various immunomodulatory strategies have been developed to manipulate immune cell phenotypes and ratios to promote revascularization and regeneration. For example, intravenous administration of negatively charged nanoparticles at an early phase of regeneration can bind to the circulating immune cells [144]. Immune cells that internalize these particles traffic to the spleen rather than to the site of injury, which reduces the numbers of M1-like macrophages in the damaged tissue and shifts the balance towards M2-like macrophages. Alternative to indirect strategies that prevent the influx of immune cells, biomaterial delivery platforms can be used for the localized delivery of immunomodulatory factors to the site of injury. This approach can limit side effects often associated with systemic administration of the therapeutics and may also increase their half-life in the body. The local retention and the release profiles of immunomodulators can be controlled by adjusting material properties. For example, modulation of the swelling rate, degradation rate, or material chemistry, which can influence the affinity of the immunomodulatory agent to the biomaterial, can be tailored to achieve the desired type and intensity of immune response. Immune-instructive biomaterials releasing cytokines or growth factors (e.g., IL-4 [145,146]) to promote phenotypic switch, most often towards M2-like macrophages, were shown to accelerate regeneration. However, the sequential release of multiple immunomodulatory agents inspired by the natural healing process might provide even better control over the dynamic actions of immune cells. For instance, a decellularized scaffold with initial fast release of M1-promoting IFN-γ followed by sustained release of IL-4 (Fig. 3A) was developed to stimulate angiogenesis and regeneration [42].
Fig. 3. Modulation of immune cell phenotype and behavior in situ.
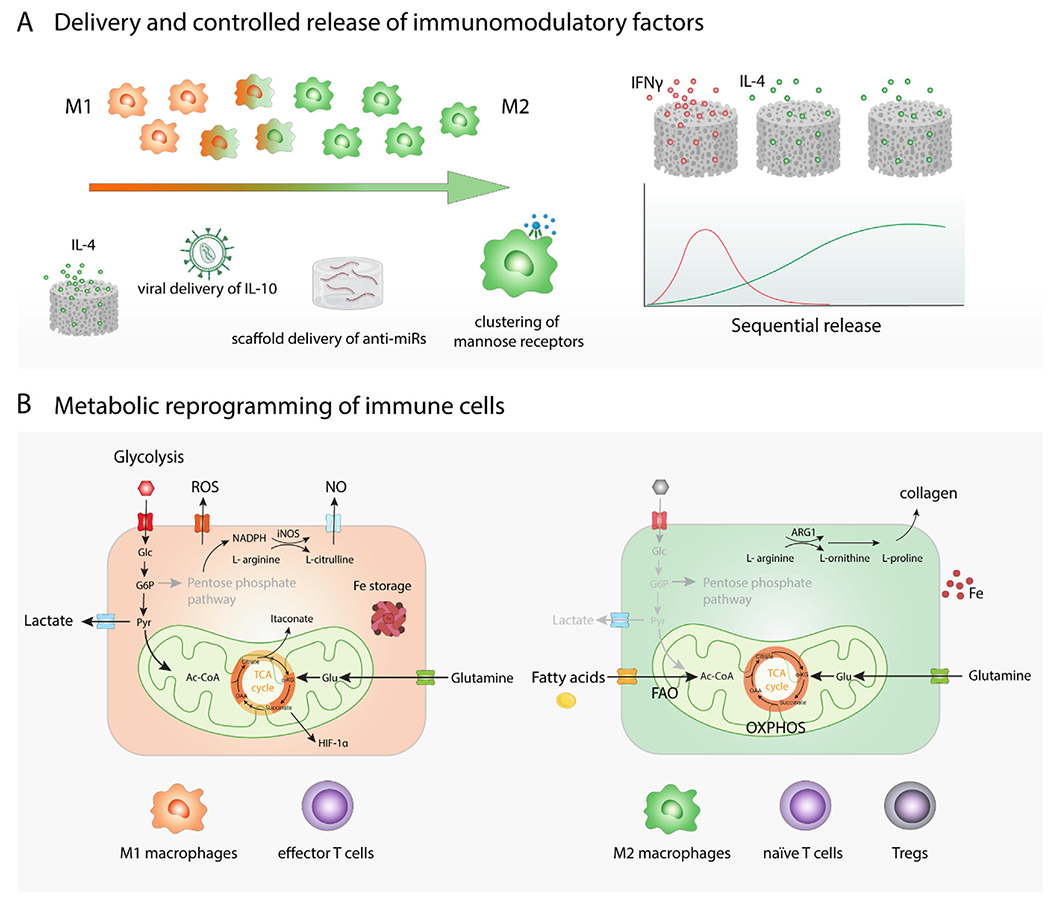
(A) Delivery and controlled release of immunomodulatory factors. To promote phenotypic switch towards M2-like macrophages, various immune-instructive biomaterials releasing growth factors or cytokines like IL-4 can be used. Sequential release of multiple immunomodulatory agents from a biomaterial, such as initial fast release of M1-promoting IFN-γ followed by sustained release of IL-4, was developed to better control dynamic phenotypic switch of macrophages. Genetic material delivery using different types of viruses or biomaterials has been employed to provide instructive signaling for immune cells. Nanoparticle-triggered clustering of mannose receptors on the surface of macrophages promote their polarization into M2-like phenotype. (B) Metabolic reprograming of immune cells. Glycolysis-based energy metabolism is generally connected with inflammatory reactions while metabolism based on oxidative phosphorylation is associated with anti-inflammatory processes.
Similar to protein delivery, genetic material delivery using different types of viruses or biomaterials has been employed to provide instructive signaling for immune cells. For example, lentiviral delivery of IL-10, which plays important role in scarless healing [147], induces sustained macrophage polarization towards an anti-inflammatory phenotype even in the presence of inflammatory stimuli [148]. Furthermore, the delivery of other small nucleic acids involved in gene regulation has been investigated to modulate immune cell polarization. For instance, miR-15b/16, which is able to induce Treg formation [149], might have vast therapeutic potential. The non-viral scaffold-mediated antagomiR-133a delivery platform is an example of a therapeutic inhibition of miRNA, which consequently induces M2-like macrophages and accelerates tissue repair [150]. Alternatively, clustering of the mannose receptors on the surface of macrophages by nanoparticles decorated with glucomannan can also induce macrophage polarization into M2-like phenotype [151].
Cathelicidins, short cationic antimicrobial peptides, produced by neutrophils, monocytes, and mast cells are another interesting immunomodulatory agents with pro-angiogenic properties. In addition to their antimicrobial activities, these peptides are also chemotactic for neutrophils, monocytes, and T cells [152] and can stimulate macrophage differentiation toward a proinflammatory phenotype [153]. Nevertheless, these peptides can also inhibit apoptosis of hypoxic ECs [154], increase VEGF expression and induce angiogenesis by inhibiting degradation of HIF-1α [155]. Delivery of human LL-37 peptide in nanoparticles accelerated neovascularization and promoted wound healing [156] and the topical treatment of large chronic leg ulcers with LL-37 peptide showed promising results also in clinical trials [157,158].
7.2. Metabolic reprograming of immune cells
Depending on the signals received from the microenvironment, immune cells can change their metabolism which induces reprogramming into different phenotypes. Generally, immune cell energy metabolism based on glycolysis is connected with inflammatory reactions, while metabolism based on oxidative phosphorylation is associated with anti-inflammatory processes (Fig. 3B). Pro-inflammatory M1-like macrophages rely on glycolysis even in aerobic conditions, which enables them to rapidly provide energy to maintain redox homeostasis and to produce substances like ROS necessary for the clearance of pathogens. M1-like macrophages are also characterized by their high inducible nitric oxide synthase (iNOS) activity [159]. The consequent NO production can inactivate iron-sulfur-containing complexes of the mitochondrial electron transport chain and cause mitochondrial dysfunction, which then hampers the repolarization of M1-like macrophages into M2-like phenotype [160]. The tricarboxylic acid (TCA) cycle of M1 macrophages is disrupted at two points [161], which leads to the accumulation of citrate that is utilized for the synthesis of the anti-bacterial compound itaconate. Another breakdown of TCA is observed at succinate dehydrogenase, which leads to succinate accumulation. Succinate acts as a pro-inflammatory molecule and stabilizes hypoxia-inducible factor (HIF) [162], which in turn induces the expression of the glucose receptor GLUT1 and promotes aerobic glycolysis by increasing the expression of lactate dehydrogenase essential for regenerating NAD +. M1-like macrophages also favor iron storage over its transport, which decreases iron availability and contributes to the growth restriction of extracellular microbes. In contrast, M2-like macrophages increase iron secretion with ferroportin and utilize mainly oxidative phosphorylation to meet their ATP requirements [163]. M2-like macrophages also display increased fatty acid uptake and synthesize lipids with anti-inflammatory properties like resolvins and lipoxins, while M1-like macrophages produce inflammatory leukotrienes and prostanoids [164].
The differences in the macrophage metabolism are also leveraged by ECs to promote revascularization. ECs generate majority of their energy via glycolytic conversion of glucose to lactate. During muscle regeneration after ischemia, ECs instruct metabolic reprogramming of macrophages towards M2-like phenotype by providing macrophages with lactate, which can serve as a substrate for oxidative phosphorylation. Lactate-polarized macrophages in return upregulate the VEGF expression to stimulate angiogenesis [165].
Metabolic reprogramming of immune cells thus offers new ways to shape immune responses in order to support tissue regeneration. For example, sirtuins, the NAD-dependent deacetylases, favor the M2-like macrophage phenotype by inhibiting glycolysis and stimulating mitochondrial oxidation of fatty acids [166]. Administration of sirtuin activators can promote regeneration by suppressing inflammation and decreasing the levels of proinflammatory cytokines [167]. Metabolic reprogramming of macrophages can be also induced by lipids. For instance, incubation of macrophages with saturated fatty acids, such as palmitate, induces their polarization into M1-like phenotype, while unsaturated fatty acids, such as eicosapentaenoic acid, promote M2-like polarization characterized by an increased secretion of IL-10. Similarly, delivery of lipoxin A4 encapsulated into poly-lactic-co-glycolic acid (PLGA) microparticles improves stability of this lipid derivative and accelerates wound healing by promoting M2-like macrophage expansion and angiogenesis [168].
naïve T cells have low metabolic requirements and rely mostly on fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS) for their energy production. Upon activation, T cells increase in size and start to rapidly divide. To cover the increased amounts of energy, activated T cells upregulate aerobic glycolysis, which although inefficient, is a faster bioenergetic pathway compared to OXPHOS when there is a steady glucose supply [169]. Cell metabolism also plays a significant role in T cell differentiation. For example, CD4 + Th1, Th2, and Th17 subsets upregulate glycolysis for anabolic purposes, whereas CD4 + Treg cells mainly prefer FAO-fueled OXPHOS for energy generation. This phenomenon might be potentially explained by the growth kinetics of the different T cell subsets. Unlike effector T cells that exhibit a sudden proliferation burst, Tregs grow continuously at moderate levels [170]. These distinct metabolic programs can be manipulated by controlling the mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) pathways, which play opposing roles in the control of these processes. mTOR increases glycolysis and diminishes FAO, while AMPK suppresses mTOR signaling and promotes OXPHOS. Thus, Treg numbers can be significantly increased after administration of metformin, an inducer of AMPK [171], or after treatment with rapamycin, an inhibitor of mTOR [172].
7.3. Tuning mechanical cues of the microenvironment
When designing immunomodulatory biomaterials, it is also important to take mechanical properties into account. Biophysical cues, such as stiffness of the microenvironment and the way these materials react toward applied forces (like stress relaxation), have a great impact on immune cell behavior; documented by several studies dealing with different types of immune cells [173]. For example, platelet adhesion and spreading are dependent on the stiffness of the substrate. When comparing substrates with stiffnesses from 0.25 to 100 kPa, an increase in number of adherent platelets can be observed on stiffer substrates that reaches a plateau on 50 kPa gels. Importantly, stiffer substrates also cause higher levels of platelet activation as determined by increases in integrin αIIbβ3 activation, P-selectin secretion, and phosphatidylserine exposure [174].
Neutrophils also spread less on softer gels (5 kPa) compared to stiffer gels (50 kPa). Moreover, stiffness of the material also affects neutrophil migration. On softer surfaces, cells move faster but migrate a shorter distance because they often change the direction of movement. While on stiffer gels neutrophils move more slowly but more persistently, resulting in greater traveled distance despite their slower movement [175].
Macrophage polarization is dependent on the microenvironmental rigidity as well. Stiff collagen-coated polyacrylamide gels (323 kPa) prime macrophages towards M1-like phenotype, while medium (88 kPa) and soft (11 kPa) gels direct macrophages into anti-inflammatory state, even in the absence of chemical induction factors like LPS or IL-4. After IFN-γ and LPS treatment, the secretion of proinflammatory factors further increases mainly on stiff gels, while after chemical induction into M2, the highest levels of anti-inflammatory cytokines (e.g., IL-10) peak on softer substrates and this behavior doesn’t seem to be dependent on the surface chemical composition [176]. Similar behavior was observed on gelatin methacrylamide (GelMA) hydrogels with stiffness of 2, 10, and 29 kPa. Softer hydrogels increased macrophage secretion of TGF-β, and subcutaneously implanted hydrogels showed increased M2-like macrophage infiltration but decreased fibrotic tissue deposition around the material [177]. Macrophage response to the stiffness of the microenvironment is dependent on the activity of transcriptional coactivators Yes-associated protein (YAP) and the sensing by non-specific cation channel Piezo1, which mediates Ca2+ influx into the cells [178]. Upon macrophage adhesion to stiff surfaces, YAP translocates to the nucleus and this increase in active YAP is associated with enhanced macrophage inflammatory activation. YAP entry into the nucleus is dependent on the cytoskeletal polymerization while active YAP inhibits actin polymerization and macrophage contractility [179].
Different subsets of macrophages also differ in their migratory ability. M0 and M2 macrophages can migrate faster than M1 macrophages (Fig. 4Ai). Macrophages can generally adopt two types of migratory behavior: amoeboid migration, which is adhesion independent movement used mainly by neutrophils and lymphocytes, and mesenchymal migration that is characterized by the formation of actin-rich protrusions and adhesive interactions with the substrate that are followed by detachment and retraction of the cell rear. Macrophages cultured on the softer substrates display fast amoeboid migration, while macrophages on stiff gels adopt slower mesenchymal migration. However, this behavior is probably not only governed by substrate stiffness, but it is also affected by the expression of cell adhesion molecules like integrins and availability of adhesion ligands. Usually, low cell-substrate adhesion does not support cell motility and high adhesion inhibits cell locomotion, whereas intermediate adhesion to the substrate generates optimal conditions for cell migration. Accordingly, M1-like macrophages were reported to express higher levels of integrin αDα2 compared to M2-like macrophages and therefore migrated more slower [180]. This can also shed light on their biological roles, as inflammatory macrophages are more dedicated to clearing pathogens or irregularities within the local environment while anti-inflammatory macrophages can travel and screen longer distances to regulate regeneration and inflammation.
Fig. 4. In situ modulation of immune cell function by changing biomaterial properties and mechanical stimulation.
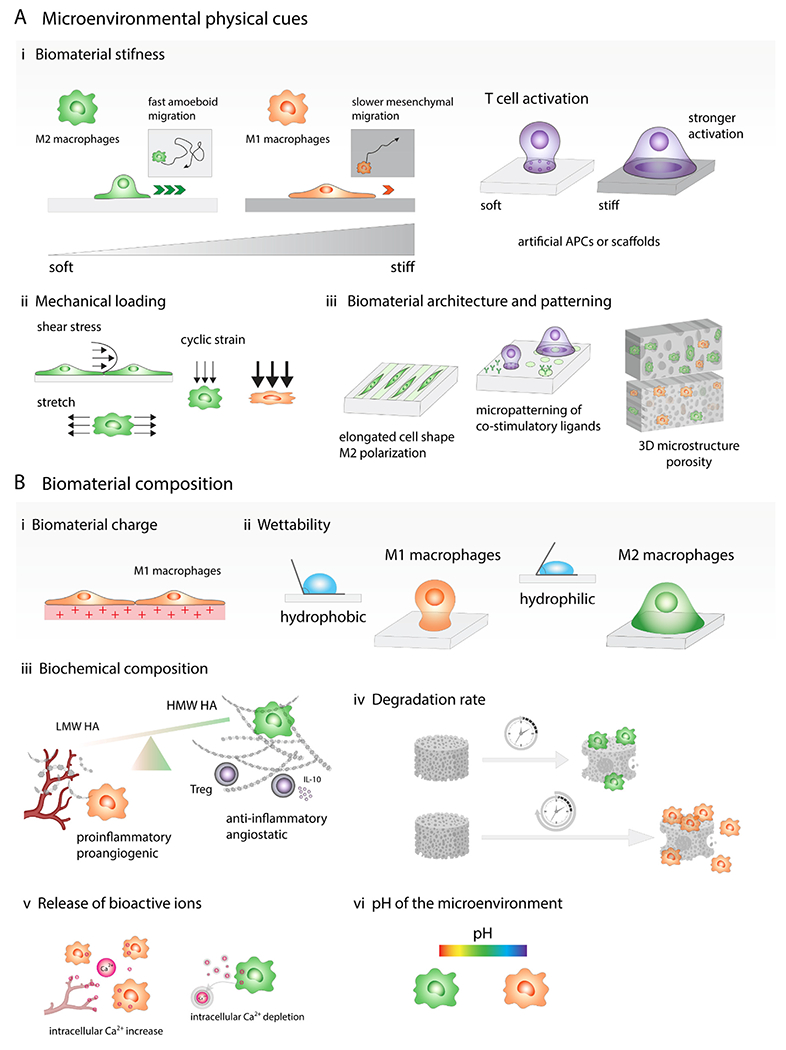
(A) Microenvironmental physical cues. (i) Stiff biomaterials prime macrophages towards M1-like phenotype, while softer ones direct macrophages into anti-inflammatory state. Macrophages cultured on the softer substrates display fast amoeboid migration, while macrophages on stiff gels adopt slower mesenchymal migration. T cell activation by interactions with antigen presenting cell (APC) can be replicated by biomaterials functionalized with ligands that bind to receptors on the T cell surface and biomaterial stiffness is one of the parameters that might significantly affect the level of T cell activation. Generally, stronger T cell activation is observed on stiffer materials. (ii) Immune cell phenotype can be also affected by mechanical loading such as tensile, compressive and shear forces. Moderate cyclic strain can promote M2-like phenotype while higher cyclic strain triggered induction of M1-like macrophages. (iii) Biomaterial architecture and patterning can modulate cell shape and cytoskeletal organization, which impact the immune cell behavior. Macrophage elongated morphology induces M2-like polarization and reduces secretion of proinflammatory cytokines by these cells. Micropatterning can also affect T cell activation, for example, focal presentation of antibodies against CD3 and CD28 increases T cell activation. Microstructure of 3D scaffolds such as pore size and geometry affect immune cell infiltration and polarization. (B) Biomaterial composition. (i) Positively charged materials are more likely to trigger proinflammatory immune cell responses. (ii) Hydrophobic biomaterials tend to induce proinflammatory M1-like macrophage activation while hydrophilic or neutral surfaces create more anti-inflammatory microenvironment. (iii) Low molecular weight hyaluronic acid (LMW HA) fragments promote angiogenesis, high molecular weight HA have angiostatic properties, suppress M1-like polarization and promote Treg formation. (iv) Slowly degrading biomaterials induce prolonged proinflammatory reactions while scaffolds that degrade faster result in constructive tissue remodeling. (v) Release of bioactive ions: calcium signaling plays important role in the proinflammatory activation of macrophages and intracellular Ca2+ oscillations also occur in migrating ECs during capillary sprouting, on the contrary, calcium ion depletion leads to M2-like polarization. (vi) Biomaterials can change the pH of the microenvironment, which can contribute to the immune cell phenotypic changes, alkaline microenvironment can promote M1-like macrophage polarization while acidic pH polarizes macrophages into M2-like phenotype.
To increase the numbers of specific T cell subsets that might affect tissue regeneration, T cells first need to be activated by interactions with antigen presenting cell (APC). T cells need two signals to become activated: binding of the T cell receptor (TCR) to an antigen presented on the MHC complex of APC followed by the co-stimulatory interaction of CD28 on the surface of T cell with CD80/CD86 on the surface of APC cell. An additional the third signal represents a cytokine exposure that determines further T cell specialization, such as Th1 type (cells exposed to IL-12), Th2 (IL-4), or Th-17 (IL-6, IL-23).
Such T cell activation processes might be, to some extent, replicated by biomaterials functionalized with ligands that bind to receptors on the immune cell surface. In these systems, biomaterial stiffness is one of the parameters that might significantly affect the level of T cell activation. Generally, stronger T cell activation is observed on stiffer materials (Fig. 4Ai). For example, the activation of CD4+ naïve T cells on polyacrylamide gels modified with anti-CD3 and anti-CD28 antibodies was stronger on gels with stiffness of 100 kPa than on the softer gels of 0.5–10 kPa [181,182]. However, it is hard to draw conclusions on the optimal biomaterial stiffness that would induce the highest T cell activation as different studies have used varying densities of T cell stimulating signals and adhesion molecules, which might have significant impact on the final cell response. This was supported by the observation that T cells adhering to substrates grafted with anti-CD3 and anti-CD28 via the TCR complex spread minimally on softer materials (0.5 kPa), maximally on substrates of 5 kPa, and decays to minimum again at 7 MPa. However, if the surface was functionalized with anti-CD3 and ICAM-1, a ligand of integrin LFA-1, T cell spreading did not decrease on substrates that were stiffer than 5 kPa [183]. It thus appears that TCR mediated binding to anti-CD3 coated surfaces is myosin-independent and that LFA-1 promotes the growth of an actin network at TCR activation sites, which enhances the cytoskeletal tension and changes the T cell sensitivity to the substrate stiffness [184]. Moreover, T cell sensing of stiffness might be also dependent on the ability of biomaterials to support TCR clustering on the T cell surface, which was shown to be necessary for efficient T cell activation [185]. It is also possible that different T cell subsets have different preferences for optimal stiffness-dependent activation. For example, the induction of Tregs from naïve CD4+ T cells is observed when the cells are cultured on anti-CD3 and anti-CD28 coated softer substrates (100 kPa) compared to that of higher rigidities (3 MPa) [186]. However, in this work, substrates softer than 100 kPa were not tested, so the efficiency of Treg induction on even softer materials remains to be elucidated.
Besides T cell activation and proliferation, rigidity of the microenvironment also affects T cell motility. Under pathological conditions, tissue stiffness increases and is accompanied by changes in ECM structure, density and composition. For example, the collagen structure of normal skin is composed of dense layers of interwoven collagen fibers, while in the inflamed skin, collagen fibers are transformed into loose networks of much thicker fibers with increased fibronectin deposition. During inflammation, T cell motility significantly increases as T cells upregulate αV integrins that help them adhere to fibronectin and facilitate their migration in inflamed tissues [187]. This effect was also replicated in vitro, where activated T cells migrated more in hard (40 kPa) threedimensional (3D) alginate scaffolds modified with RGD as opposed to soft (4 kPa) hydrogels [188]. The physiological context for using this range of stiffnesses comes from empirical measurements of mouse lymph nodes prior (~4 kPa) and after systemic viral infection with lymphocytic choriomeningitis virus (LCMV) (Armstrong strain) ~ 40 kPa [189].
Physical cues of ECM such as density, stiffness, and degradability also affect the behavior of ECs such as the invasion of endothelial sprouts. In softer less-crosslinked matrix, ECs migrated faster but easily lost intercellular connectivity and migrated as single cells, whereas in the matrix with intermediate crosslinking densities and slower degradation rate, ECs maintained cellular contacts and migrated collectively as multicellular sprouts [190,191].
The behavior of vascular cells and immune cells is also affected by mechanical stimuli in the microenvironment such as cyclic strain, compression and fluid shear stress (Fig. 4Aii). For example, it was reported that moderate cyclic strain (7%) promoted macrophage polarization into M2-like phenotype and ECM synthesis while higher cyclic strain (12%) triggered induction of M1-like macrophages [192]. Higher mechanical loading with early onset also decreased vascular network length and branching; however once a critical stage of vascular maturity was reached, the same mechanical stimulation did not have a negative effect on the vascular network [193]. In addition, intraluminal shear stress over the endothelium and transmural flow through the endothelium above 10 dyn/cm2 triggered endothelial cell sprouting by upregulating MMP-1 [194]. Whether the immune cells in microcirculation play a role in this process remains to be determined.
7.4. Engineering patterning, topography and porosity
As previously described, stiffness of the microenvironment can modulate cell shape and cytoskeletal organization to impact immune cell behavior. Therefore, it is not surprising that control of immune cell shape can also induce different phenotypes and functions, without changing the stiffness of the biomaterial. For instance, macrophages cultured on micropatterned substrates containing 20-μm wide lines of fibronectin separated by 20-μm wide lines of non-adhesive surface adopted elongated morphology, which induced their polarization toward an M2-like phenotype and reduced secretion of proinflammatory cytokines. Moreover, such restriction in cell spreading also enhanced M2-like polarization when induced by IL-4/IL-13 stimulation yet reduced the secretion of proinflammatory factors when induced by IFN-γ/LPS [195]. In addition, T cell activation can be increased by the micropatterning of co-stimulatory ligands, where the focal presentation of antibodies against CD3 and CD28 increases T cell activation [196] (Fig. 4Aiii).
Beside micropatterned substrates, cell shape can be also modulated by surface topography. Similar to the previously discussed stripes, micro and nano-patterned grooves were also shown to promote cell elongation. In the case of macrophages, cell elongation was the highest on substrates with 0.5–5 μm wide grooves and cells cultured on these surfaces were polarized towards an anti-inflammatory phenotype and secreted significantly higher levels of anti-inflammatory IL-10 [197]. Also, ECs cultured on microgrooves had elongated morphology and secreted EVs to promote anti-inflammatory macrophage phenotype, in comparison to ECs with cobblestone shape [198]. Moreover, hierarchical micro-nano topography was shown to better suppress pro-inflammatory signaling in macrophages than flat surfaces and nanostructures. Conditioned medium from macrophages grown on the surfaces with micro and nano-features also increased the expression of angiogenic genes in ECs [199]. In addition, differential T cell spreading on various topographies leads to different levels of T cell proliferation and activation; for example, 100- and 200 nm grid patterns improve T cell expansion comparing to the flat surfaces [200].
Furthermore, 3D scaffold microstructure is critical not only for immune cell infiltration and angiogenesis but also for immunomodulation. A variety of fabrication methods have been developed to engineer biomaterials of defined microarchitecture and pore sizes. These features can be tuned by adjusting microparticle/microfiber size and alignment, by laser ablation or by incorporating into a scaffold sacrificial microfibers/microparticles, which are consequently removed to increase the scaffold porosity and thus the recruitment of immune cells including macrophages and DCs [201–205]. In electrospun scaffolds, thicker electrospun microfibers (3 μm) with pores of 30 μm in diameter induce more M2-like macrophages and promote increased angiogenic factors secretion [206]. In porous scaffolds generated with the use of ice crystal/cryoprotectant-based porogen, scaffolds with smaller and softer pores (30 μm, 20 kPa) contain more pro-inflammatory macrophages, whereas scaffolds with larger and stiffer pores (90 μm, 190 kPa) induce more anti-inflammatory macrophages [207].
Macrophages seeded on collagen/chitosan scaffolds cross-linked with genipin with average pore sizes of 160 and 360 μm showed M1-like polarization after 1 day but macrophages in the scaffold with larger pores expressed higher levels of the M2-related genes and secreted more anti-inflammatory and proangiogenic cytokines in the later time points. Supernatants derived from the macrophages cultured on the scaffold with larger pores also promoted higher ECs migration and tube formation in vitro. After subcutaneous implantation, scaffolds with larger pores showed increased blood vessel infiltration into the scaffold and lower numbers of M1-like macrophages than scaffolds with smaller pore sizes [208]. Scaffolds with pore sizes in a range of 20–90 μm also promoted DC maturation [201]. Myeloid cells infiltrating scaffolds with 40-μm or 100-μm spherical pores secreted EVs to upregulate Treg transcriptional markers in T cells. However, EVs secreted by cells infiltrating scaffolds with 100-μm pores also increased Th1 inflammatory gene expression in T cells, which explains the reduced inflammation and foreign body reaction in the scaffolds with 40-μm pores [209]. These studies underscore the importance and complexity of the effects of 3D scaffolds on immune cells, where besides the pore geometry, the immune cell behavior is influenced by the chemical composition as well as stiffness of the biomaterial. More in-depth investigations are, therefore, needed to better understand how biomaterials can be engineered to direct immune cell behavior and signaling to promote regeneration.
7.5. Biomaterial composition
When designing immunomodulatory biomaterials and biointerfaces to direct immune cell phenotype and behavior, biomaterial properties such as surface charge, wettability, chemical composition, and degradability need to be taken into account as well. Generally, positively charged materials are more likely to trigger proinflammatory immune cell responses than neutral or negatively charged materials (Fig. 4Bi). Moreover, positively charged particles are quicky opsonized and more readily internalized by macrophages, therefore, they have much shorter half-life in the bloodstream (~2h) than anionic and neutral particles [210].
Besides surface charge, biomaterial wettability can affect protein adsorption and conformation and consequently also immune cell adhesion and polarization (Fig. 4Bii). Hydrophobic biomaterials tend to induce proinflammatory M1-like macrophage activation while hydrophilic or neutral surfaces create more anti-inflammatory microenvironment by polarizing macrophages and T cells into pro-healing phenotypes, which leads to a faster inflammation resolution and tissue repair [211,212].
Synthetic biomaterials can be manufactured with high precision, and their properties can be tailored to meet a large range of requirements. However, they are not able to simulate the complexity of the natural ECM signaling. Therefore, additional modifications and coatings with ECM components can be further applied. For example, addition of heparan sulfate to the formulation can enhance the biomaterial immunomodulatory properties because heparan sulfate binds various cytokines (IL-10, SDF-1, TNF-α) and growth factors (VEGF, FGF-2, PDGF, EGF), which can affect immune cell polarization and stimulate angiogenesis [213]. Hyaluronic acid (HA) is another glycosaminoglycan with broad immunomodulatory activities. Low molecular weight HA fragments are proangiogenic and promote EC migration, proliferation and collagen synthesis while they have also proinflammatory properties and stimulate activation of leukocytes through toll-like receptors (TLR) [214,215]. High molecular weight HA (over 1000 kDa) have, on the other hand, angiostatic properties, suppress M1-like polarization and promote the production of pro-resolving cytokine IL-10 and Treg formation [216] (Fig. 4Biii).
More complex ECM scaffolds can be obtained by tissue decellularization. Comparison of macrophage reactions to a decellularized ECM (urinary bladder matrix, UBM) and synthetic material (polycaprolactone, PCL) showed that UBM accelerated regeneration by inducing IL-4 and Th2 immune responses while PCL triggered IL-17-driven fibrosis. The decellularized ECM promoted macrophage polarization into two primary subsets. The proinflammatory macrophages (CD301b+ CD9+) were enriched in the scaffold from 1 to 3 weeks while the anti-inflammatory macrophages (CD301b+ CD9−) remained enriched in the scaffold for longer time. The anti-inflammatory macrophages were phagocytic and expressed high levels of eotaxin-2, a chemokine that attracts eosinophils [217]. In contrast, PCL showed low levels of both of these subsets at all time points, which highlights the complexity of immune signaling and dynamics of the microenvironment that can lead to different outcomes.
Moreover, decellularization of different tissues or just the usage of different decellularization agents can lead to different immune cell reactions that can be caused by cell remnants, ECM components or EVs [218] that remained in the scaffold [219]. Generally, slowly degrading or chemically crosslinked decellularized tissues induce prolonged proinflammatory reactions while scaffolds that degrade faster result in constructive tissue remodeling [220] (Fig. 4Biv). Biomaterial degradation rate and dynamic changes in biomaterial structure and composition as well as release of various bioactive degradation products and ions thus need to be taken into account when designing immunomodulatory biomaterials. For example, calcium signaling plays important role in the recruitment of macrophages to the wound and their proinflammatory activation [221]. Intracellular Ca2+ oscillations also occur in migrating ECs during capillary sprouting after VEGF binding to the receptor on EC membrane [215]. Biomaterials that can control calcium levels were developed to dynamically regulate polarization stages of macrophages. For instance, lanthanide-doped upconverting nanoparticles with mesoporous silica shell were developed as nanocarriers of calcium regulators. These particles enable temporal regulation of the intracellular calcium levels after near-infrared light excitation, which triggers intracellular release of calcium regulators. Using this system, it was observed that the elevation of intracellular calcium ions promoted macrophage polarization into proinflammatory M1-like phenotype while calcium ion depletion led to M2-like polarization [222] (Fig. 4Bv). This modulatory effect might be, however, strongly dependent on the concentration of bioactive ions. Hydrogels containing calcium silicate as a source of multiple bioactive ions were also shown to decrease apoptosis of ECs and to stimulate angiogenesis, which led to increased survival of ischemic tissue [223]. Other bioactive ions such as Cu2+ are able to stabilize HIF-1α, which leads to increased secretion of VEGF and enhanced angiogenesis [224,225]. However, ion release from the biomaterials can also change the pH of the microenvironment, which can contribute to the immune cell phenotypic changes [226]. Alkaline microenvironment (pH 8.2) can promote M1-like macrophage polarization while acidic pH (pH 6.6) tend to polarize macrophages into M2-like phenotype [227] (Fig. 4Bvi).
In situ manipulation of immune cell phenotypes represent a complex set of strategies that have been so far explored to repolarize immune cells and to control their function in favor of tissue revascularization and regeneration. Many times, immune cells persist in proinflammatory state or in M2-like state in a fibrotic tissue and do not follow the natural timeline of the healing process. The goal of regenerative immunoengineering is, therefore, to remove brakes that slow down the regeneration process. It is evident that it is not just the delivery of immunomodulatory factors that can affect the immune cell phenotypic change and behavior. Immune cell metabolism is closely connected with the epigenetic regulation of gene expression and thus targeting metabolism of immune cells can lead to significant changes in their secretion and function. Moreover, biomaterial properties such as stiffness, architecture, and composition for both immune cell delivery and for in situ immunomodulation need to be carefully considered in order to support the desired immune cell phenotype and function. Currently, our understanding of how these properties can regulate immune cells and how different types of immune cells modulate the revascularization and tissue regeneration is still limited. The complexity and interconnectivity of the in vivo system make it is hard to evaluate which biomaterial properties are perceived as essential by the immune cells. The advances in intravital microscopy and research in small model organisms such as zebrafish and organ-on-a-chip systems can extend our knowledge and give us more clues for designing the next generation immunomodulatory biomaterials.
8. Conclusion and perspectives
Immune cells are becoming increasingly recognized as important regulators of re-vascularization and tissue regeneration. So far, regenerative therapies have mainly focused on macrophages and their delivery, recruitment, and repolarization to promote tissue repair. However, there are indications that other immune cell types, such as neutrophils or T cells are just as important for successful tissue regeneration. Our view of the immune system and its capabilities is rather simplified in this regard. For instance, macrophages are simply divided into two groups of “good” (M2) and “bad” (M1) phenotypes, but such clear distinctions may not be appropriate in various context. There are multifaceted functions of M1 and M2 macrophages with many other transitional phenotypes. The underappreciated M1 macrophages are emerging as very valuable participants of the initial phase of the healing process for their role in cleaning the wound and secreting pro-angiogenic factors, while on the other hand M2-like macrophages can be detrimental for tissue repair if they persist in the site of injury for longer periods of time. It is evident that the timing of different immune cell involvement as well as sequential changes in the immune cell signaling are very important. A better understanding of these processes will be crucial for the design of more advanced regenerative strategies. To fully understand the complex interactions among immune cells and local cells, single cell RNA sequencing of cells in regenerating tissues, in combination with biocomputational analysis, will provide unprecedented insights into the mechanisms at the molecular level and the network of cell-cell communications with an unprecedent temporal and spatial resolution. Furthermore, this single-cell approach will allow us to gain an in-depth understanding of the effects of cell delivery and drug delivery and enable rational design of therapeutics with precise control of the immune cells and regeneration process. It is also important to understand the effects of the delivered immune cells on the function and phenotypes of the other immune cells present in the wound. To our knowledge, there are currently no definitive studies that characterize this process in details, which requires cell tracing and analysis. We believe that the advances in the intravital imaging, genetic manipulations and single cell sequencing analysis would enable to precisely distinguish delivered/recruited immune cells and tissue-resident immune cells.
Genetic engineering of immune cells, as represented by chimeric antigen receptor T-cell therapy, has been successfully developed to treat cancer. With the advances of synthetic biology and gene editing technologies such as CRISPR/Cas9 system, it is possible to engineer immune cells with desirable functions in targeting, secretion and immunoactivation or immunosuppression. One may also engineer cells that can secrete EVs or molecules with immunomodulatory effects. To realize these therapies, a key step is to understand the mechanisms of immunoregulation and identify the potential therapeutic targets.
Cell delivery is often complicated by issues of cell viability and uncontrollable/unpredictable cell behavior. These problems might be potentially overcome by delivering immune cells with other cell types such as mesenchymal stem cells, which will maintain the immune cells in anti-inflammatory phenotypes. This cell therapy, known as Ixmyelocel-T, is already showing promising results in phase 2 clinical trials for patients with ischemic heart failure and critical limb ischemia [89,228–230]. Moreover, further improvement in cell viability and function can be achieved by engineering cell spheroids or encapsulating cells in hydrogels/scaffolds, which can maintain delivered cells at the desired place and localize their immunomodulatory effect. This approach might also prevent side effects that can be caused by uncontrolled spreading of delivered cells to different organs and tissues.
Delivery of therapeutic EVs instead of their parental cells might be an interesting option to overcome the major disadvantage of cell therapy, which is the inability to precisely control cell behavior. EVs secreted by specific cell types that have immunomodulatory effects can be collected and delivered as immunomodulators to treat various types of diseases or to accelerate revascularization and tissue regeneration. The content of EVs can be further manipulated by specific culture conditions or genetic modifications of their cells of origin or drug loading into EVs. The barriers to translate this approach into clinical applications include batch-to-batch variations in EV preparations and the complex secretome yet to be characterized to identify the active components. It is likely that the therapeutic efficacy relies on the combined effects of multiple components, and thus the quality control of manufacturing process is critical to ensure the consistency of EV therapeutic effects. Moreover, this cell-free therapy might be further combined with biomaterial scaffolds to achieve better spatiotemporal control of release kinetics during the healing process.
The next-generation strategies to enhance tissue repair should be tailored to the needs of individual patients, which can be done by implementing new technologies that can provide higher design flexibility or through tissue-responsive biomaterials that can provide immunomodulation depending on the state of tissue damage. Biomaterial mechanical properties as well as chemical composition should be carefully considered as they can impact the immune cell polarization. For example, in addition to the stiffness and topography and microstructure of the biomaterials, there is evidence that viscoelastic properties of the scaffolds could have impact on cellular responses, which remain to be investigated for immune cell modulation. Moreover, differences in the metabolism between pro-inflammatory or anti-inflammatory cells might be utilized to guide the immune cell polarization.
Furthermore, to address the temporal regulation of immune cells during tissue regeneration, biomaterials that would consecutively release different immunomodulatory agents to attract and polarize various immune cell types during the course of tissue regeneration might be designed to more accurately mimic the natural healing process and offer higher level of control over the immune cell actions. Moreover, delivery systems that would be able to repeatedly engage pro-inflammatory and anti-inflammatory immune cells and basically reproduce the healing process several times might be an interesting way to deal with larger defects where it is not possible to stick to the normal healing timeline.
In conclusion, being able to control and manipulate the immune system appears as a very promising strategy to accelerate tissue regeneration. However, our understanding of the interplay between immune cells and the damaged tissue is complicated by our inability to accurately replicate the complexity of the immune system and the microenvironment with the use of in vitro models. Advancement in technology development in mechanistic investigations and biomaterial delivery systems, such as single cell profiling, computational biology, artificial intelligence, high-resolution time-lapse microscopy, non-invasive monitoring and actuation, and tunable and responsive biomaterials, can offer valuable insight into the spatiotemporal dynamics of immune cell actions and inspire future immuno-engineering efforts for the development of effective therapies.
Acknowledgements
This work is supported in part by grants from National Institute of Health (R56DE029157, R01HL149940, R01CA234343 and DK112939 to S.L. and R01HL148714 to R.A.), the California Institute for Regenerative Medicine (Grant Number DISC2COVID19-11838), UCLA David Geffen School of Medicine – Oversight COVID-19 Research Committee (OCRC) (Award Number: OCRC #45), and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Innovation Award. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH, CIRM or/and other agency of the State of California. Tyler Hoffman and Crystal Xiao are gratefully acknowledged for the language revision of the manuscript.
Abbreviations:
- APC
Antigen Presenting Cell
- CCL2
chemokine (C-C motif) ligand 2
- CD40L
CD40 Ligand
- DCs
Dendritic Cells
- ECs
Endothelial Cells
- ECM
Extracellular Matrix
- EVs
Extracellular Vesicles
- FGF
Fibroblast Growth Factor
- HA
Hyaluronic acid
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- HIF-1a
hypoxia-inducible factor-1a
- IDO
Indoleamine 2,3-Dioxygenase
- IGF
Insulin-like Growth Factor
- IFN-γ
Interferon Gamma
- IL
Interleukin
- LPS
bacterial Lipopolysaccharide
- M–CSF
Macrophage Colony-Stimulating Factor
- MHC
Major Histocompatibility Complex
- MMP
Matrix Metalloproteinases
- PCL
polycaprolactone
- PDGF
Platelet-derived Growth Factor
- PSGL-1
P-Selectin Glycoprotein Ligand-1
- ROS
Reactive Oxygen Species
- SDF-1
Stromal Cell-derived Factor 1
- S1P
Sphingosin-1-Phosphate
- TCR
T Cell Receptor
- TGF-β
Transforming Growth factor-β
- Th cells
CD4+ T helper cells
- TLR
Toll-like Receptor
- TNFα
Tumor Necrosis Factor Alpha
- Tregs
Regulatory T cells
- TSP-1
thrombospondin 1
- UBM
Urinary bladder matrix
- VEGF
Vascular Endothelial Growth Factor
- YAP
Yes-associated protein
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Faber JE, Chilian WM, Deindl E, van Royen N, Simons M, A brief etymology of the collateral circulation, Arterioscler. Thromb. Vasc. Biol 34 (2014) 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carmeliet P, Jain RK, Molecular mechanisms and clinical applications of angiogenesis, Nature 473 (2011) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stratman AN, Schwindt AE, Malotte KM, Davis GE, Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization, Blood 116 (2010) 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE, Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices, Blood 114 (2009) 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Davis GE, Stratman AN, Sacharidou A, Koh W, Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting, Int Rev Cell Mol Biol 288 (2011) 101–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH, The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis, Cell 137 (2009) 1124–1135. [DOI] [PubMed] [Google Scholar]
- [7].Aveleira CA, Lin C-M, Abcouwer SF, Ambrósio AF, Antonetti DA, TNF-α Signals Through PKCζ/NF-κB to Alter the Tight Junction Complex and Increase Retinal Endothelial Cell Permeability, Diabetes 59 (2010) 2872–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kang T-Y, Bocci F, Jolly MK, Levine H,Onuchic JN, Levchenko A, Pericytes enable effective angiogenesis in the presence of proinflammatory signals, Proc. Natl. Acad. Sci 116 (2019) 23551–23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W, Monocyte Chemotactic Protein-1 Increases Collateral and Peripheral Conductance After Femoral Artery Occlusion, Circ. Res 80 (1997) 829–837. [DOI] [PubMed] [Google Scholar]
- [10].Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S, Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice, Circulation 108 (2003) 205–210. [DOI] [PubMed] [Google Scholar]
- [11].Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK,Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, C Rundle A, Fine J, McCluskey ER, The VIVA Trial, Circulation 107 (2003) 1359–1365. [DOI] [PubMed] [Google Scholar]
- [12].Rust R, Gantner C, Schwab ME, Pro- and antiangiogenic therapies: current status and clinical implications, FASEB J. 33 (2019) 34–48. [DOI] [PubMed] [Google Scholar]
- [13].Stewart DJ, Kutryk MJB, Fitchett D, Freeman M, Camack N, Su Y, Siega AD, Bilodeau L, Burton JR, Proulx G, Radhakrishnan S, VEGF Gene Therapy Fails to Improve Perfusion of Ischemic Myocardium in Patients With Advanced Coronary Disease: Results of the NORTHERN Trial, Mol. Ther 17 (2009) 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R, Engineering vascularized skeletal muscle tissue, Nat. Biotechnol 23 (2005) 879–884. [DOI] [PubMed] [Google Scholar]
- [15].Walsh TG, Metharom P, Berndt MC, The functional role of platelets in the regulation of angiogenesis, Platelets 26 (2015) 199–211. [DOI] [PubMed] [Google Scholar]
- [16].Brill A, Elinav H, Varon D, Differential role of platelet granular mediators in angiogenesis, Cardiovasc. Res 63 (2004) 226–235. [DOI] [PubMed] [Google Scholar]
- [17].Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, Saffaripour S,Ware J, Ruggeri ZM, Jain RK, Folkman J, Wagner DD, Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage, PNAS 103 (2006) 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim HK, Song KS, Chung J-H, Lee KR, Lee S-N, Platelet microparticles induce angiogenesis in vitro, Br. J. Haematol 124 (2004) 376–384. [DOI] [PubMed] [Google Scholar]
- [19].Ribeiro LS, Migliari Branco L, Franklin BS, Regulation of Innate Immune Responses by Platelets, Front. Immunol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aloui C, Prigent A, Sut C, Tariket S, Hamzeh-Cognasse H, Pozzetto B, Richard Y, Cognasse F, Laradi S, Garraud O, The signaling role of CD40 ligand in platelet biology and in platelet component transfusion, Int. J. Mol. Sci 15 (2014) 22342–22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ali RA, Wuescher LM, Worth RG, Platelets: essential components of the immune system, Curr, Trends Immunol. 16 (2015) 65–78. [PMC free article] [PubMed] [Google Scholar]
- [22].Chakrabarti S, Rizvi M, Morin K, Garg R, Freedman JE, The role of CD40L and VEGF in the modulation of angiogenesis and inflammation, Vasc. Pharmacol 53 (2010) 130–137. [DOI] [PubMed] [Google Scholar]
- [23].Leroyer AS, Rautou P-E, Silvestre J-S, Castier Y, Lesèche G, Devue C, Duriez M, Brandes RP, Lutgens E, Tedgui A, Boulanger CM, CD40 Ligand+ Microparticles From Human Atherosclerotic Plaques Stimulate Endothelial Proliferation and Angiogenesis: A Potential Mechanism for Intraplaque Neovascularization, J. Am. Coll. Cardiol 52 (16) (2008) 1302–1311. [DOI] [PubMed] [Google Scholar]
- [24].Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER, Neutrophil kinetics in health and disease, Trends Immunol. 31 (2010) 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L, Update on Neutrophil Function in Severe Inflammation, Front. Immunol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Phillipson M, Kubes P, The Healing Power of Neutrophils, Trends Immunol. 40 (7) (2019) 635–647. [DOI] [PubMed] [Google Scholar]
- [27].Lin RZ, Lee CN, Moreno-Luna R, Neumeyer J, Piekarski B, Zhou P, Moses MA, Sachdev M, Pu WT, Emani S, Melero-Martin JM, Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks, Nat. Biomed. Eng 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Selders GS, Fetz AE, Radic MZ, Bowlin GL, An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration, Regen Biomater 4 (1) (2017) 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM, Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN, Cancer Cell 16 (2009) 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Christoffersson G, Vågesjö E,Vandooren J, Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M, VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue, Blood 120 (2012) 4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, D Losordo R Kishore, Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium, Circ. Res 109 (2011) 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shao R, YKL-40 acts as an angiogenic factor to promote tumor angiogenesis, Front. Physiol 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T, Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer, Oncogene 31 (2012) 3111–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbi AL, Lizasoain I, Moro MA, N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone, Stroke 44 (2013) 3498–3508. [DOI] [PubMed] [Google Scholar]
- [35].Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M,Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML, Temporal neutrophil polarization following myocardial infarction, Cardiovasc. Res 110 (2016) 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet M.j The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions, J. Exp. Med 204 (2007) 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Elliott MR, Koster KM, Murphy PS, Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses, J. Immunol 198 (4) (2017) 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Krzyszczyk P, Schloss R, Palmer A, Berthiaume F, The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Prowound Healing Phenotypes, Front. Physiol 9 (2018), 419–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Troidl C, Möllmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsässer A, Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction, J. Cell Mol. Med 13 (2009) 3485–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang Z, Ming X-F, Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders, Front. Immunol 5 (2014), 533–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Murray PJ, Wynn TA, Protective and pathogenic functions of macrophage subsets, Nat. Rev. Immunol 11 (2011) 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spiller KL, Nassiri S, Witherel CE, Anfang RR,Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, Asosingh K, Erzurum S, Anand-Apte B, Cross-Talk between Vascular Endothelial Growth Factor and Matrix Metalloproteinases in the Induction of Neovascularization in Vivo, Am. J. Pathol 176 (2010) 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kwee BJ, Mooney DJ, Manipulating the intersection of angiogenesis and inflammation, Ann. Biomed. Eng 43 (2015) 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN, Macrophages are required for neonatal heart regeneration, J. Clin. Invest 124 (2014) 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials 35 (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P, Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression, EMBO J. 37 (13) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Graney PL, Ben-Shaul S, Landau S, Bajpai A, Singh B, Eager J, Cohen A, Levenberg S, Spiller KL, Macrophages of diverse phenotypes drive vascularization of engineered tissues, Sci. Adv 6 (2020) eaay6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL, Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis, Microcirculation (New York, N.Y. 1994) 23 (2016) 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M, Dendritic cell–endothelial cell cross-talk in angiogenesis, Trends Immunol. 28 (2007) 385–392. [DOI] [PubMed] [Google Scholar]
- [51].Bosisio D, Ronca R, Salvi V, Presta M, Sozzani S, Dendritic cells in inflammatory angiogenesis and lymphangiogenesis, Curr. Opin. Immunol 53 (2018) 180–186. [DOI] [PubMed] [Google Scholar]
- [52].Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML, Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor, PNAS 98 (2001) 12485–12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X, HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia, Immunol. Cell Biol 88 (2010) 165–171. [DOI] [PubMed] [Google Scholar]
- [54].Sirbulescu RF, Boehm CK, Soon E, Wilks MQ, Ilies I, Yuan H, Maxner B, Chronos N, Kaittanis C, Normandin MD, El Fakhri G, Orgill DP, Sluder AE, C Poznansky M, Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions, Wound Repair Regen 25 (2017) 774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, Dahne M, Hartwig T, Akyüz L, Meisel C, Unterwalder N, Singh NB, Reinke P, Haas NP, Volk H-D, Duda GN, Terminally Differentiated CD8+ T Cells Negatively Affect Bone Regeneration in Humans, Sci. Transl. Med 5 (2013), 177ra136–177ra136. [DOI] [PubMed] [Google Scholar]
- [56].Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, DeLeon-Pennell KY, CD8+ T-cells negatively regulate inflammation post-myocardial infarction, American Journal of Physiology-Heart and Circulatory, Physiology 317 (3) (2019) H581–H596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kwee BJ, Budina E, Najibi AJ, Mooney DJ, CD4 T-cells regulate angiogenesis and myogenesis, Biomaterials 178 (2018) 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hata T, Takahashi M, Hida S, Kawaguchi M, Kashima Y, Usui F, Morimoto H, Nishiyama A, Izawa A, Koyama J, Iwakura Y, Taki S, Ikeda U, Critical role of Th17 cells in inflammation and neovascularization after ischaemia, Cardiovasc. Res 90 (2010) 364–372. [DOI] [PubMed] [Google Scholar]
- [59].De Palma M, Biziato D, Petrova TV, Microenvironmental regulation of tumour angiogenesis, Nat. Rev. Cancer 17 (2017) 457–474. [DOI] [PubMed] [Google Scholar]
- [60].Lewkowicz N, Klink M, Mycko MP, Lewkowicz P, Neutrophil – CD4+CD25+ T regulatory cell interactions: A possible new mechanism of infectious tolerance, Immunobiology 218 (2013) 455–464. [DOI] [PubMed] [Google Scholar]
- [61].Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S, Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation, Circ. Res 115 (2014) 55–67. [DOI] [PubMed] [Google Scholar]
- [62].Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS, CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages, Proc. Natl. Acad. Sci. U S A 104 (2007) 19446–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Leung OM, Li J, Li X, Chan VW, Yang KY, Ku M, Ji L, Sun H, Waldmann H, Tian XY, Huang Y, Lau J, Zhou B, Lui KO, Regulatory T Cells Promote Apelin-Mediated Sprouting Angiogenesis in Type 2 Diabetes, Cell Reports 24 (2018)1610–1626. [DOI] [PubMed] [Google Scholar]
- [64].D’Alessio FR, Kurzhagen JT, Rabb H, Reparative T lymphocytes in organ injury, J. Clin. Invest 129 (2019) 2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang C-Q, Huang Y-W, Wang S-W, Huang Y-L, Tsai C-H, Zhao Y-M, Huang BF, Xu G-H, Fong Y-C, Tang C-H, Amphiregulin enhances VEGF-A production in human chondrosarcoma cells and promotes angiogenesis by inhibiting miR-206 via FAK/c-Src/PKCδ pathway, Cancer Lett. 385 (2017) 261–270. [DOI] [PubMed] [Google Scholar]
- [66].Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, Almada AE, Mondino A, Wagers AJ, Manfredi AA, Rovere-Querini P, Kumar A, FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration, PLoS ONE 10(6) (2015) e0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, Rosenblum MD, Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation, Cell 169 (2017) 1119–1129.e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nishio N, Ito S, Suzuki H, Isobe K-I, Antibodies to wounded tissue enhance cutaneous wound healing, Immunology 128 (2009) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM, FcRγ Activation Regulates Inflammation-Associated Squamous Carcinogenesis, Cancer Cell 17 (2010) 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W, Hoon DS, Wakabayashi M, Forman S, Yu H, B cells promote tumor progression via STAT3 regulated-angiogenesis, PLoS One 8 (2013) e64159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Iwata Y, Yoshizaki A, Komura K, Shimizu K, Ogawa F, Hara T, Muroi E, Bae S, Takenaka M, Yukami T, Hasegawa M, Fujimoto M, Tomita Y, Tedder TF, Sato S, CD19, a Response Regulator of B Lymphocytes, Regulates Wound Healing through Hyaluronan-Induced TLR4 Signaling, Am. J. Pathol 175 (2) (2009) 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sgonc R, Gruber J, Age-Related Aspects of Cutaneous Wound Healing: A Mini-Review, Gerontology 59 (2) (2013) 159–164. [DOI] [PubMed] [Google Scholar]
- [73].Mogilenko DA, Shchukina I, Artyomov MN, Immune ageing at single-cell resolution, Nat. Rev. Immunol (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ferrucci L, Fabbri E, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nature reviews, Cardiology 15 (9) (2018) 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sayed N, Huang Y, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T, Tibshirani R, Hastie T, Alpert A, Cui L.u., Kuznetsova T, Rosenberg-Hasson Y, Ostan R, Monti D, Lehallier B, Shen-Orr SS, Maecker HT, Dekker CL, Wyss-Coray T, Franceschi C, Jojic V, Haddad F, Montoya JG, Wu JC, Davis MM, Furman D, An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging, Nature, Aging 1 (7) (2021) 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S, CXC chemokines: the regulatory link between inflammation and angiogenesis, Trends Immunol. 25 (4) (2004) 201–209. [DOI] [PubMed] [Google Scholar]
- [77].Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z, Wang L, Huang M, Jia C, Lu J, Liu S, Chen H, Li M, Cai D, Jiang Y, Jin D, Bai X, Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone, Nat. Commun 7 (2016), 13885–13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Okonkwo U, DiPietro L, Diabetes and Wound Angiogenesis, Int. J. Mol. Sci 18 (7) (2017) 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang J, Chu M, Differential roles of VEGF: Relevance to tissue fibrosis,J. Cell. Biochem 120 (7) (2019) 10945–10951. [DOI] [PubMed] [Google Scholar]
- [80].Braga TT, Agudelo JSH, Camara NOS, Macrophages During the Fibrotic Process: M2 as Friend and Foe, Front. Immunol 6 (2015), 602–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF, Bergers G, Bikfalvi A, Bischoff J, Böck BC, Brooks PC, Bussolino F, Cakir B, Carmeliet P, Castranova, Cimpean AM, Cleaver O, Coukos G, Davis GE, De Palma M, Dimberg A, Dings RPM, Djonov V, Dudley AC, Dufton NP, Fendt S-M, Ferrara N, Fruttiger M, Fukumura D, Ghesquière B, Gong Y, Griffin RJ, Harris AL, Hughes CCW, Hultgren NW, Iruela-Arispe ML, Irving M, Jain RK, Kalluri R, Kalucka J, Kerbel RS, Kitajewski J, Klaassen I, Kleinmann HK, Koolwijk P, Kuczynski E, Kwak BR, Marien K, Melero-Martin JM, Munn LL, Nicosia RF, Noel A, Nurro J, Olsson A-K, Petrova TV, Pietras K, Pili R, Pollard JW, Post MJ, Quax PHA, Rabinovich GA, Raica M, Randi AM, Ribatti D, Ruegg C, Schlingemann RO, Schulte-Merker S, Smith LEH, Song JW, Stacker SA, Stalin J, Stratman AN, Van de Velde M, van Hinsbergh VWM, Vermeulen PB, Waltenberger J, Weinstein BM, Xin H, Yetkin-Arik B, Yla-Herttuala S, Yoder MC, Griffioen AW, Consensus guidelines for the use and interpretation of angiogenesis assays, Angiogenesis 21 (3) (2018) 425–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boyden S, The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes, J. Exp. Med 115 (1962) 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S, State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization, Circ. Res 116 (2015) e99–e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang N, Fang Z, Contag PR, Purchio AF, West DB, Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse, Blood 103 (2004) 617–626. [DOI] [PubMed] [Google Scholar]
- [85].Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C, Zhu K, Ni C, Wang K, Kong M, Li X, Fan Y, Zhang F, Chen Q.i., Li Y.i., Li Q, Liu C, Zhu J, Zhong S, Wang J, Chen Y, Zhao J, Zhu D, Wu R, Chen J, Zhu W, Yu H, Ardehali R, Zhang J, Wang J, Hu X, Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates, Sci. Transl. Med 13 (584) (2021). [DOI] [PubMed] [Google Scholar]
- [86].Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W, Impact of mouse strain differences in innate hindlimb collateral vasculature, Arterioscler. Thromb. Vasc. Biol 26 (3) (2006) 520–526. [DOI] [PubMed] [Google Scholar]
- [87].Hu MS, Walmsley GG, Barnes LA, Weiskopf K, Rennert RC, Duscher D, Januszyk M, Maan ZN, Hong WX, Cheung ATM, Leavitt T, Marshall CD, Ransom RC, Malhotra S, Moore AL, Rajadas J, Lorenz HP, Weissman IL, Gurtner GC, Longaker MT, Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair, JCI Insight 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hirose N, Maeda H, Yamamoto M, Hayashi Y, Lee G-H, Chen L, Radhakrishnan G, Rao P, Sasaguri S, The local injection of peritoneal macrophages induces neovascularization in rat ischemic hind limb muscles, Cell Transplant. 17 (1-2) (2008) 211–222. [DOI] [PubMed] [Google Scholar]
- [89].Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, Recker D, DeMaria A, Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial, Lancet 387 (10036) (2016) 2412–2421. [DOI] [PubMed] [Google Scholar]
- [90].Juhas M, Abutaleb N, Wang JT, Ye J, Shaikh Z, Sriworarat C, Qian Y, Bursac N, Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration, Nature, Biomed. Eng 2 (12) (2018) 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP, Chazaud B, Butler-Browne G, Savino W, Mouly V, Riederer I, Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation, Mol. Ther 20 (2012) 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Visser JG, Van Staden ADP, Smith C, Harnessing macrophages for controlled-release drug delivery: lessons from microbes, Front. Pharmacol 10 (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Champion JA, Mitragotri S, Role of target geometry in phagocytosis, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang B, Abraham WD, Zheng Y, Bustamante López SC, Luo SS, Irvine DJ, Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells, Sci. Transl. Med 7 (2015), 291ra294–291ra294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tang L, Zheng Y, Melo MB, Mabardi L, Castaño AP, Xie Y-Q, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, Irvine DJ, Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery, Nat. Biotechnol 36 (2018) 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hou D, Youssef EA-S, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL, Radiolabeled Cell Distribution After Intramyocardial, Intracoronary, and Interstitial Retrograde Coronary Venous Delivery, Circulation 112 (2005), I-150–I-156. [DOI] [PubMed] [Google Scholar]
- [97].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT, Biopolymer implants enhance the efficacy of adoptive T-cell therapy, Nat. Biotechnol 33 (1) (2015) 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Eggermont LJ, Rogers ZJ, Colombani T, Memic A, Bencherif SA, Injectable Cryogels for Biomedical Applications, Trends Biotechnol. 38 (4) (2020) 418–431. [DOI] [PubMed] [Google Scholar]
- [99].Nicodemus GD, Bryant SJ, Cell encapsulation in biodegradable hydrogels for tissue engineering applications, Tissue engineering, Part B, Reviews 14 (2) (2008) 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Guvendiren M, Lu HD, Burdick JA, Shear-thinning hydrogels for biomedical applications, Soft Matter 8 (2) (2012) 260–272. [Google Scholar]
- [101].Dellacherie MO, Seo BR, Mooney DJ, Macroscale biomaterials strategies for local immunomodulation, Nat. Rev. Mater 4 (6) (2019) 379–397. [Google Scholar]
- [102].Wang C, Adrianus GN, Sheng N, Toh S, Gong Y, Wang D-A, In vitro performance of an injectable hydrogel/microsphere based immunocyte delivery system for localised anti-tumour activity, Biomaterials 30 (36) (2009) 6986–6995. [DOI] [PubMed] [Google Scholar]
- [103].Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Muller-Newen G, Soehnlein O, Weber C, Platelet Microparticles Enhance the Vasoregenerative Potential of Angiogenic Early Outgrowth Cells After Vascular Injury, Circulation 122 (5) (2010) 495–506. [DOI] [PubMed] [Google Scholar]
- [104].Guo S-C, Tao S-C, Yin W-J, Qi X, Yuan T, Zhang C-Q, Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model, Theranostics 7 (1) (2017) 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sadallah S, Amicarella F, Eken C, Iezzi G, Schifferli J, Ectosomes released by platelets induce differentiation of CD4+T cells into T regulatory cells, Thromb. Haemost 112 (12) (2014) 1219–1229. [DOI] [PubMed] [Google Scholar]
- [106].Gangadaran P, Rajendran RL, Oh JM, Hong CM, Jeong SY, Lee S-W,Lee J, Ahn B-C, Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo, Exp. Cell Res 394 (2020) 112146. [DOI] [PubMed] [Google Scholar]
- [107].Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH, Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing, Adv. Sci. (Weinheim, Baden-Wurttemberg, Germany) 6 (2019) 1900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell’Accio F, Pitzalis C, Oliani SM, Jan LY, Perretti M, Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis, Sci. Transl. Med 7 (2015), 315ra190–315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD, CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells, Matrix Biol. 37 (2014) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature 527 (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, SuSu Khine J-B Le Pecq, Exosome Display technology: Applications to the development of new diagnostics and therapeutics, Blood Cells Mol. Dis. 35 (2) (2005) 158–168. [DOI] [PubMed] [Google Scholar]
- [112].Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ,Valle J, Echavez AK, Marbán E, Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display, J. Nanobiotechnol 16 (2018) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q, Moulton HM, Seow Y, Yin H, Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy, Sci. Transl. Med 10 (2018) eaat0195. [DOI] [PubMed] [Google Scholar]
- [114].Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, Nilsson J, Lötvall J, Kim Y-K, Gho YS, Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors, ACS Nano 7 (9) (2013) 7698–7710. [DOI] [PubMed] [Google Scholar]
- [115].Hu C-M, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L, Nanoparticle biointerfacing by platelet membrane cloaking, Nature 526 (7571) (2015) 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E, Biomimetic proteolipid vesicles for targeting inflamed tissues, Nat. Mater 15 (2016) 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM, Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins, J. Control. Release 205 (2015) 35–44. [DOI] [PubMed] [Google Scholar]
- [118].Silva AK, Luciani N, Gazeau F, Aubertin K, Bonneau S, Chauvierre C, Letourneur D, Wilhelm C, Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting, Nanomedicine 11 (2015) 645–655. [DOI] [PubMed] [Google Scholar]
- [119].He C, Zheng S, Luo Y, Wang B, Exosome theranostics: biology and translational medicine, Theranostics 8 (2018) 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Phinney DG, Pittenger MF, Concise review: MSC-derived exosomes for cell-free therapy, Stem cells 35 (2017) 851–858. [DOI] [PubMed] [Google Scholar]
- [121].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, Di Paolo G, Homma S, Sims PA, Topkara VK, Vunjak-Novakovic G, Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells, Nat. Biomed. Eng 2 (5) (2018) 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO, Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter, ACS Nano 8 (1) (2014) 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, Imai T, Saji H, Takakura Y, Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice, J. Pharm. Sci 104 (2) (2015) 705–713. [DOI] [PubMed] [Google Scholar]
- [124].Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R, Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature 546 (7659) (2017) 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Brennan MÁ, Layrolle P, Mooney DJ, Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration, Adv. Funct. Mater 30 (37) (2020) 1909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mardpour S, Ghanian MH, Sadeghi-abandansari H, Mardpour S, Nazari A, Shekari F, Baharvand H, Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure, ACS Appl. Mater. Interfaces 11 (2019) 37421–37433. [DOI] [PubMed] [Google Scholar]
- [127].Vanden Berg-Foels WS, In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment, Tissue Eng. Part B: Rev 20 (1) (2014) 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Boehler RM, Graham JG, Shea LD, Tissue engineering tools for modulation of the immune response, Biotechniques 51 (2011) 239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Dumont NA, Frenette J, Macrophage Colony-Stimulating Factor-Induced Macrophage Differentiation Promotes Regrowth in Atrophied Skeletal Muscles and C2C12 Myotubes, Am. J. Pathol 182 (2013) 505–515. [DOI] [PubMed] [Google Scholar]
- [130].Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA, Wells CA, Little MH, A Hume D, Ricardo SD, Colony-Stimulating Factor-1 Promotes Kidney Growth and Repair via Alteration of Macrophage Responses, Am. J. Pathol 179 (2011) 1243–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hsu CW, Poche RA, Saik JE, Ali S, Wang S, Yosef N, Calderon GA, Scott L Jr., Vadakkan TJ, Larina IV, West JL, Dickinson ME, Improved Angiogenesis in Response to Localized Delivery of Macrophage-Recruiting Molecules, PLoS One 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Qin S, DiPietro LA, Zloza A, Zhang C, Shafikhani SH, Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response, PLoS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Weigert A, Olesch C, Brune B, Sphingosine-1-Phosphate and Macrophage Biology-How the Sphinx Tames the Big Eater, Front. Immunol 10 (2019), 1706–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Murakami M, Saito T, Tabata Y, Controlled release of sphingosine-1-phosphate agonist with gelatin hydrogels for macrophage recruitment, Acta Biomater. 10 (2014) 4723–4729. [DOI] [PubMed] [Google Scholar]
- [135].Kim YH, Tabata Y, Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell-derived factor-1 and a macrophage recruitment agent enhances wound closure, J Biomed Mater Res A 104 (2016) 942–956. [DOI] [PubMed] [Google Scholar]
- [136].Zarubova J, zhang X, Hoffman T, Hasani-Sadrabadi MM, Li S, Biomaterial-based immunoengineering to fight COVID-19 and infectious diseases, Matter 4 (5) (2021) 1528–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sato T, Yamamoto M, Shimosato T, Klinman DM, Accelerated wound healing mediated by activation of Toll-like receptor 9, Wound repair and regeneration : official publication of the Wound Healing Society [and] theEuropean Tissue Repair Society 18 (2010) 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Singh A, Peppas NA, Hydrogels and scaffolds for immunomodulation, Adv. Mater 26 (38) (2014) 6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Tanaka R, Saito Y, Fujiwara Y, Jo J-I, Tabata Y, Preparation of fibrin hydrogels to promote the recruitment of anti-inflammatory macrophages, Acta Biomater. 89 (2019) 152–165. [DOI] [PubMed] [Google Scholar]
- [140].Jain N, Vogel V, Spatial confinement downsizes the inflammatory response of macrophages, Nat. Mater 17 (12) (2018) 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Griffin DR, Archang MM, Kuan C-H, Weaver WM, Weinstein JS, Feng AC, Ruccia A, Sideris E, Ragkousis V, Koh J, Plikus MV, Di Carlo D, Segura T, Scumpia PO, Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing, Nat. Mater 20 (2021) 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH, Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells, Science 352 (2016) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih T-Y, White D, Mooney DJ, Treating ischemia via recruitment of antigen-specific T cells, Sci. Adv 5 (2019) eaav6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Park J, Zhang Y, Saito E, Gurczynski SJ, Moore BB, Cummings BJ, Anderson AJ, Shea LD, Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury, Proc. Natl. Acad. Sci 116 (2019) 14947–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Raimondo TM, Mooney DJ, Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice, Proc. Natl. Acad. Sci 115 (2018) 10648–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Shahbazi M-A, Sedighi M, Bauleth-Ramos T, Kant K, Correia A, Poursina N, Sarmento A, Hirvonen J, Santos HA, Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles, ACS Omega 3 (12) (2018) 18444–18455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].King A, Balaji S, Le LD, Crombleholme TM, Keswani SG, Regenerative Wound Healing: The Role of Interleukin-10, Adv Wound Care (New Rochelle) 3 (4) (2014) 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Leonard JN, Shea LD, Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype, Biotechnol. Bioeng 111 (6) (2014) 1210–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Singh Y, Garden OA, Lang F, Cobb BS, MicroRNA-15b/16 Enhances the Induction of Regulatory T Cells by Regulating the Expression of Rictor and mTOR, J. Immunol 195 (12) (2015) 5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Castaño IM, Raftery RM, Chen G, Cavanagh B, Quinn B, Duffy GP, O’Brien FJ, Curtin CM, Rapid Bone Repair with the Recruitment of CD206+ M2-like Macrophages Using Non-Viral Scaffold-Mediated miR-133a Inhibition of Host Cells, Acta Biomater. 109 (2020) 267–279. [DOI] [PubMed] [Google Scholar]
- [151].Gan J, Dou Y, Li Y, Wang Z, Wang L, Liu S, Li Q, Yu H, Liu C, Han C, Huang Z, Zhang J, Wang C, Dong L, Producing anti-inflammatory macrophages by nanoparticle-triggered clustering of mannose receptors, Biomaterials 178 (2018) 95–108. [DOI] [PubMed] [Google Scholar]
- [152].Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O, Ll-37, the Neutrophil Granule-And Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-Like 1 (Fprl1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells, J. Exp. Med 192 (2000) 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff THM, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH, LL-37 Directs Macrophage Differentiation toward Macrophages with a Proinflammatory Signature, J. Immunol 185 (3) (2010) 1442–1449. [DOI] [PubMed] [Google Scholar]
- [154].Wu J, Parungo C, Wu G, Kang PM, Laham RJ, Sellke FW, Simons M, Li J, PR39 Inhibits Apoptosis in Hypoxic Endothelial Cells, Circulation 109 (13) (2004) 1660–1667. [DOI] [PubMed] [Google Scholar]
- [155].Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J.o., Aird W, Rosenberg RD, Hampton TG, Li J, Sellke F, Carmeliet P, Simons M, PR39, a peptide regulator of angiogenesis, Nat. Med 6 (1) (2000) 49–55. [DOI] [PubMed] [Google Scholar]
- [156].Chereddy KK, Her C-H, Comune M, Moia C, Lopes A, Porporato PE, Vanacker J, Lam MC, Steinstraesser L, Sonveaux P, Zhu H, Ferreira LS, Vandermeulen G, Práat V, PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing, J. Control. Release 194 (2014) 138–147. [DOI] [PubMed] [Google Scholar]
- [157].Grönberg A, Mahlapuu M, Ståhle M, Whately-Smith C, Rollman O, Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial, Wound Repair Regenerat. 22 (2014) 613–621. [DOI] [PubMed] [Google Scholar]
- [158].Mahlapuu M, Sidorowicz A, Mikosinski J, Krzyżanowski M, Orleanski J, Twardowska-Saucha K, Nykaza A, Dyaczynski M, Belz-Lagoda B, Dziwiszek G, Kujawiak M, Karczewski M, Sjöberg F, Grzela T, Wegrzynowski A, Thunarf F, Björk J, Ekblom J, Jawien A, Apelqvist J, Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial, Wound Repair Regenerat. 29 (2021) 938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Galván-Peña S, O’Neill LAJ, Metabolic Reprograming in Macrophage Polarization, Front. Immunol 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Susan M, Berg R, Luque-Martin H-J, Boshuizen MCS, Ahmed M, Hoeksema MA, de Vos AF, de Winther MPJ, Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages, Cell Reports 17 (2016) 684–696. [DOI] [PubMed] [Google Scholar]
- [161].Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN, Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization, Immunity 42 (2015) 419–430. [DOI] [PubMed] [Google Scholar]
- [162].Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LAJ, Succinate is an inflammatory signal that induces IL-1β through HIF-1α, Nature 496 (2013) 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Juban G, Chazaud B, Metabolic regulation of macrophages during tissue repair: insights from skeletal muscle regeneration, FEBS Lett. 591 (2017) 3007–3021. [DOI] [PubMed] [Google Scholar]
- [164].De Santa F, Vitiello L, Torcinaro A, Ferraro E, The Role of Metabolic Remodeling in Macrophage Polarization and Its Effect on Skeletal Muscle Regeneration, Antioxid. Redox Signal 30 (12) (2019) 1553–1598. [DOI] [PubMed] [Google Scholar]
- [165].Zhang J,Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, D’Hulst G, Gilardoni P, Turiel G, Fan Z, Wang T, Planque M, Carmeliet P, Pellerin L, Wolfrum C, Fendt S-M, Banfi A, Stockmann C, Soro-Arnáiz I, Kopf M, De Bock K, Endothelial Lactate Controls Muscle Regeneration from Ischemia by Inducing M2-like Macrophage Polarization, Cell Metab. 31 (2020) 1136–1153.e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Houtkooper RH, Pirinen E, Auwerx J, Sirtuins as regulators of metabolism and healthspan, Nat. Rev. Mol. Cell Biol 13 (2012) 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chen H, Ji H, Zhang M, Liu Z, Lao L, Deng C,Chen J, Zhong G, An Agonist of the Protective Factor SIRT1 Improves Functional Recovery and Promotes Neuronal Survival by Attenuating Inflammation after Spinal Cord Injury, J. Neurosci 37 (2017) 2916–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Reis MB, Pereira PAT, Caetano GF, Leite MN, Galvão AF, Paula-Silva FWG, Frade MAC, Faccioli LH, Diaz BL, Lipoxin A4 encapsulated in PLGA microparticles accelerates wound healing of skin ulcers, PLoS ONE 12 (7) (2017) e0182381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Tang C-Y, Mauro C, Similarities in the Metabolic Reprogramming of Immune System and Endothelium, Front. Immunol 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ghesquière B, Wong BW, Kuchnio A, Carmeliet P, Metabolism of stromal and immune cells in health and disease, Nature 511 (2014) 167–176. [DOI] [PubMed] [Google Scholar]
- [171].Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC, Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets, J. Immunol 186 (2011) 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL, Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin, PLoS One 4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhang X, Kim T-H, Thauland TJ, Li H, Majedi FS, Ly C, Gu Z, Butte MJ, Rowat AC, Li S, Unraveling the mechanobiology of immune cells, Curr. Opin. Biotechnol 66 (2020) 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA, Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation, Proc. Natl. Acad. Sci 111 (2014) 14430–14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX, Neutrophil morphology and migration are affected by substrate elasticity, Blood 114 (2009) 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].He X-T, Wu R-X, Xu X-Y, Wang J, Yin Y, Chen F-M, Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions, Acta Biomater. 71 (2018) 132–147. [DOI] [PubMed] [Google Scholar]
- [177].Zhuang Z, Zhang Y, Sun S, Li Q, Chen K, An C, Wang L, van den Beucken JJJP, Wang H, Control of Matrix Stiffness Using Methacrylate-Gelatin Hydrogels for a Macrophage-Mediated Inflammatory Response, ACS Biomater. Sci. Eng 6 (2020) 3091–3102. [DOI] [PubMed] [Google Scholar]
- [178].Atcha H, Jairaman A,Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, Brumm KT, Lim HE, Othy S, Cahalan MD, Pathak MM, Liu WF, Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing, Nat. Commun 12 (2021) 3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Meli VS, Atcha H, Veerasubramanian PK, Nagalla RR, Luu TU, Chen EY, Guerrero-Juarez CF, Yamaga K, Pandori W, Hsieh JY, Downing TL, Fruman DA, Lodoen MB, Plikus MV, Wang W, Liu WF, YAP-mediated mechanotransduction tunes the macrophage inflammatory response, Sci. Adv 6 (2020) eabb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Cui K, Ardell CL, Podolnikova NP, Yakubenko VP, Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by alphaDbeta2 and alphaMbeta2 Integrin-Mediated Adhesion, Front. Immunol 9 (2018) 2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Judokusumo E, Tabdanov E, Kumari S, Dustin M, Kam L, Mechanosensing in T Lymphocyte Activation, Biophys. J 102 (2) (2012) L5–L7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Saitakis M, Dogniaux S, Goudot C, Bufi N, Asnacios S, Maurin M, Randriamampita C, Asnacios A, Hivroz C, Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity, eLife 6 (2017) e23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wahl A, Dinet C, Dillard P, Nassereddine A, Puech P-H, Limozin L, Sengupta K, Biphasic mechanosensitivity of T cell receptor-mediated spreading of lymphocytes, Proc. Natl. Acad. Sci 116 (13) (2019) 5908–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tabdanov E, Gondarenko S, Kumari S, Liapis A, Dustin ML, Sheetz MP, Kam LC, Iskratsch T, Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells, Integr. Biol 7 (10) (2015) 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Hickey JW, Vicente FP, Howard GP, Mao H-Q, Schneck JP, Biologically Inspired Design of Nanoparticle Artificial Antigen-Presenting Cells for Immunomodulation, Nano Lett. 17 (11) (2017) 7045–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Nataraj NM, Dang AP, Kam LC, Lee JH, Ex vivo induction of regulatory T cells from conventional CD4(+) T cells is sensitive to substrate rigidity, J. Biomed. Mater. Res. Part A 106 (2018) 3001–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun Y-M, Lambert K, Acharya M, Billroth-MacLurg AC, Rosenberg AF, Topham DJ, Yagita H, Kim M, Lacy-Hulbert A, Meier-Schellersheim M, Fowell DJ, Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV, Nat. Immunol 14 (2013) 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Majedi FS, Hasani-Sadrabadi MM, Thauland TJ, Li S, Bouchard LS, Butte MJ, T-cell activation is modulated by the 3D mechanical microenvironment, Biomaterials 252 (2020) 120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Meng KP, Majedi FS, Thauland TJ, Butte MJ, Tissue mechanics controls T-cell activation and metabolism, bioRxiv (2019) 581322. [Google Scholar]
- [190].Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA, Chen CS, Matrix degradability controls multicellularity of 3D cell migration, Nat. Commun 8 (2017) 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wang WY, Lin D, Jarman EH, Polacheck WJ, Baker BM, Functional angiogenesis requires microenvironmental cues balancing endothelial cell migration and proliferation, Lab Chip 20 (2020) 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ballotta V, Driessen-Mol A, Bouten CVC, Baaijens FPT, Strain-dependent modulation of macrophage polarization within scaffolds, Biomaterials 35 (2014) 4919–4928. [DOI] [PubMed] [Google Scholar]
- [193].Ruehle MA, Eastburn EA, LaBelle SA, Krishnan L, Weiss JA, Boerckel JD, Wood LB, Guldberg RE, Willett NJ, Extracellular matrix compression temporally regulates microvascular angiogenesis, Sci. Adv 6 (2020) eabb6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Galie PA, Nguyen D-HT, Choi CK, Cohen DM, Janmey PA, Chen CS, Fluid shear stress threshold regulates angiogenic sprouting, Proc. Natl. Acad. Sci. U.S.A 111 (2014) 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF, Modulation of macrophage phenotype by cell shape, Proc. Natl. Acad. Sci 110 (2013) 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Delcassian D, Sattler S, Dunlop IE, T cell immunoengineering with advanced biomaterials, Integr Biol (Camb) 9 (2017) 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Luu TU, Gott SC, Woo BWK, Rao MP, Liu WF, Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype, ACS Appl. Mater. Interfaces 7 (51) (2015) 28665–28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Liang J, Gu S, Mao X, Tan Y, Wang H, Li S, Zhou Y, Endothelial Cell Morphology Regulates Inflammatory Cells Through MicroRNA Transferred by Extracellular Vesicles, Front. Bioeng. Biotechnol 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Yang C, Zhao C, Wang X, Shi M, Zhu Y, Jing L, Wu C, Chang J, Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation, Nanoscale 11 (38) (2019) 17699–17708. [DOI] [PubMed] [Google Scholar]
- [200].Hu J, Gondarenko AA, Dang AP, Bashour KT, O’Connor RS, Lee S, Liapis A, Ghassemi S, Milone MC, Sheetz MP, Dustin ML, Kam LC, Hone JC, High-Throughput Mechanobiology Screening Platform Using Micro- and Nanotopography, Nano Lett. 16 (4) (2016) 2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Chen R, Ma H, Zhang L, Bryers JD, Precision-porous templated scaffolds of varying pore size drive dendritic cell activation, Biotechnol. Bioeng 115 (4) (2018) 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Kim J, Li WA, Sands W, Mooney DJ, Effect of pore structure of macroporous poly(lactide-co-glycolide) scaffolds on the in vivo enrichment of dendritic cells, ACS Appl. Mater. Interfaces 6 (11) (2014) 8505–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Timnak A, Gerstenhaber JA, Dong K, Har-el Y.-e., Lelkes PI, Gradient porous fibrous scaffolds: a novel approach to improving cell penetration in electrospun scaffolds, Biomed. Mater 13 (2018) 065010. [DOI] [PubMed] [Google Scholar]
- [204].Kurpinski KT, Stephenson JT, Janairo RRR, Lee H, Li S, The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds, Biomaterials 31 (13) (2010) 3536–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lee B-P, Jeon H, Wang A, Yan Z, Yu J, Grigoropoulos C, Li S, Femtosecond laser ablation enhances cell infiltration into three-dimensional electrospun scaffolds, Acta Biomater. 8 (7) (2012) 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL, Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds, Biomaterials 34 (2013) 4439–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Jiang S, Lyu C, Zhao P, Li W, Kong W, Huang C, Genin GM, Du Y, Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D, Nat. Commun 10 (2019) 3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Yin Y, He X-T, Wang J, Wu R-X, Xu X-Y, Hong Y-L, Tian B-M, Chen F-M, Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold, Appl. Mater. Today 18 (2020) 100466. [Google Scholar]
- [209].Hady TF, Hwang B, Pusic AD, Waworuntu RL, Mulligan M, Ratner B, Bryers JD, Uniform 40-μm-pore diameter precision templated scaffolds promote a pro-healing host response by extracellular vesicle immune communication, J Tissue Eng Regen Med 15 (2021) 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Albanese A, Tang PS, Chan WCW, The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems, Annu. Rev. Biomed. Eng 14 (2012) 1–16. [DOI] [PubMed] [Google Scholar]
- [211].Lv L, Xie Y, Li K, Hu T, Lu X, Cao Y, Zheng X, Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization, Adv. Healthcare Mater 7 (19) (2018) 1800675. [DOI] [PubMed] [Google Scholar]
- [212].Hotchkiss KM, Clark NM, Olivares-Navarrete R, Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations, Biomaterials 182 (2018) 202–215. [DOI] [PubMed] [Google Scholar]
- [213].Whitelock JM, Iozzo RV, Heparan Sulfate: A Complex Polymer Charged with Biological Activity, Chem. Rev 105 (2005) 2745–2764. [DOI] [PubMed] [Google Scholar]
- [214].Zamboni F, Vieira S, Reis RL, Miguel Oliveira J, Collins MN, The potential of hyaluronic acid in immunoprotection and immunomodulation: Chemistry, processing and function, Prog. Mater Sci 97 (2018) 97–122. [Google Scholar]
- [215].Rayahin JE, Buhrman JS, Zhang Y.u., Koh TJ, Gemeinhart RA, High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation, ACS Biomater. Sci. Eng. 1 (7) (2015) 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ruppert SM, Hawn TR, Arrigoni A, Wight TN, Bollyky PL, Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation, Immunol. Res 58 (2-3) (2014) 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Sommerfeld SD, Cherry C, Schwab RM, Chung L, Maestas DR, Laffont P, Stein JE, Tam A, Ganguly S, Housseau F, Taube JM, Pardoll DM, Cahan P, Elisseeff JH, Interleukin-36γ–producing macrophages drive IL-17–mediated fibrosis, Sci. Immunol 4 (40) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB, Badylak SF, Matrix-bound nanovesicles within ECM bioscaffolds, Sci. Adv 2 (2016) e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF, Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component, Biomaterials 30 (8) (2009) 1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Brown BN, Londono R, Tottey S, Zhang L.i., Kukla KA, Wolf MT, Daly KA, Reing JD, Badylak SF, Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials, Acta Biomater. 8 (3) (2012) 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Sipka T, Peroceschi R, Hassan-Abdi R, Groß M, Ellett F, Begon-Pescia C, Gonzalez C, Lutfalla G, Nguyen-Chi M, Damage-Induced Calcium Signaling and Reactive Oxygen Species Mediate Macrophage Activation in Zebrafish, Front. Immunol 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kang H, Zhang K, Wong DSH, Han F, Li B, Bian L, Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization, Biomaterials 178 (2018) 681–696. [DOI] [PubMed] [Google Scholar]
- [223].Xu P, Xing M, Huang H, Xue K, Chang J, Liu K, Calcium silicate-human serum albumin composite hydrogel decreases random pattern skin flap necrosis by attenuating vascular endothelial cell apoptosis and inflammation, Chem. Eng. J. 423 (2021) 130285. [Google Scholar]
- [224].Zhou Y, Han S, Xiao L, Han P, Wang S, He J, Chang J, Wu C, Xiao Y, Accelerated host angiogenesis and immune responses by ion release from mesoporous bioactive glass, J. Mater. Chem. B 6 (20) (2018) 3274–3284. [DOI] [PubMed] [Google Scholar]
- [225].Shen P, Chen Y, Luo S, Fan Z, Wang J, Chang J, Deng J, Applications of biomaterials for immunosuppression in tissue repair and regeneration, Acta Biomater. 126 (2021) 31–44. [DOI] [PubMed] [Google Scholar]
- [226].Li J, Jiang X, Li H, Gelinsky M, Gu Z, Tailoring Materials for Modulation of Macrophage Fate, Adv. Mater 33 (12) (2021) 2004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Wu H, Yin Y, Hu X, Peng C, Liu Y, Li Q, Huang W, Huang Q, Effects of Environmental pH on Macrophage Polarization and Osteoimmunomodulation, ACS Biomater. Sci. Eng 5 (2019) 5548–5557. [DOI] [PubMed] [Google Scholar]
- [228].Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E, Velazquez O, Marston WA, Bartel RL, Longcore A, Stern T, Watling S, Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia, J. Vasc. Surg 54 (4) (2011) 1032–1041. [DOI] [PubMed] [Google Scholar]
- [229].Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL, Cellular Therapy With Ixmyelocel-T to Treat Critical Limb Ischemia: The Randomized, Double-blind, Placebo-controlled RESTORE-CLI Trial, Mol. Ther 20 (6) (2012) 1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Ledford KJ, Zeigler F, Bartel RL, Ixmyelocel-T, an expanded multicellular therapy, contains a unique population of M2-like macrophages, Stem Cell Res. Ther 4 (2013) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T, Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks, Nat. Mater 14 (2015) 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]


