Abstract
Escherichia coli has only a single copy of a gene for tRNA6Leu (Y. Komine et al., J. Mol. Biol. 212:579–598, 1990). The anticodon of this tRNA is CAA (the wobble position C is modified to O2-methylcytidine), and it recognizes the codon UUG. Since UUG is also recognized by tRNA4Leu, which has UAA (the wobble position U is modified to 5-carboxymethylaminomethyl-O2-methyluridine) as its anticodon, tRNA6Leu is not essential for protein synthesis. The BT63 strain has a mutation in the anticodon of tRNA6Leu with a change from CAA to CUA, which results in the amber suppressor activity of this strain (supP, Su+6). We isolated 18 temperature-sensitive (ts) mutants of the BT63 strain whose temperature sensitivity was complemented by introduction of the wild-type gene for tRNA6Leu. These tRNA6Leu-requiring mutants were classified into two groups. The 10 group I mutants had a mutation in the miaA gene, whose product is involved in a modification of tRNAs that stabilizes codon-anticodon interactions. Overexpression of the gene for tRNA4Leu restored the growth of group I mutants at 42°C. Replacement of the CUG codon with UUG reduced the efficiency of translation in group I mutants. These results suggest that unmodified tRNA4Leu poorly recognizes the UUG codon at 42°C and that the wild-type tRNA6Leu is required for translation in order to maintain cell viability. The mutations in the six group II mutants were complemented by introduction of the gidA gene, which may be involved in cell division. The reduced efficiency of translation caused by replacement of the CUG codon with UUG was also observed in group II mutants. The mechanism of requirement for tRNA6Leu remains to be investigated.
In the universal genetic code, 61 sense codons correspond to 20 amino acids, and the various tRNA species mediate the flow of information from the genetic code to amino acid sequences. Since codon-anticodon interactions permit wobble pairing at the third position, 32 tRNAs, including tRNAfMet, should theoretically be sufficient for a complete translation system. Although some organisms have fewer tRNAs (1), most have abundant tRNA species and multiple copies of major tRNAs. For example, Escherichia coli has 86 genes for tRNA (79 genes identified in reference 14, 6 new ones reported in reference 3, and one fMet tRNA at positions 2945406 to 2945482) that encode 46 different amino acid acceptor species. Although abundant genes for tRNAs are probably required for efficient translation, the significance of the apparently nonessential tRNAs has not been examined.
E. coli has five isoaccepting species of tRNALeu. According to the wobble rule, tRNA1Leu recognizes only the CUG codon. The CUG codon is also recognized by tRNA3Leu (tRNA2Leu) and thus tRNA1Leu may not be essential for protein synthesis. Similarly, tRNA6Leu is supposed to recognize only the UUG codon, but tRNA4Leu can recognize both UUA and UUG codons. Thus, tRNA6Leu appears to be dispensable. The existence of an amber suppressor mutation of tRNA6Leu (supP, Su+6) supports this possibility. tRNA6Leu is encoded by a single-copy gene, leuX (supP), and Su+6 has a mutation in the anticodon, which suggests loss of the ability to recognize UUG (26). Why are so many species of tRNALeu required? Holmes et al. (12) examined the utilization of the isoaccepting species of tRNALeu in protein synthesis and showed that utilization differs depending on the growth medium; in minimal medium, isoacceptors tRNA2Leu (cited as tRNA3Leu; see Materials and Methods) and tRNA4Leu are the predominant species that are found bound to ribosomes, but an increased relative level of tRNA1Leu is found bound to ribosomes in rich medium. The existence of tRNA6Leu is puzzling. This isoaccepting tRNA accounts for approximately 10% of the tRNALeu in total-cell extracts. However, little if any tRNA6Leu is found on ribosomes in vivo, and it is also only weakly active in protein synthesis in vitro with mRNA from E. coli (12). It thus appears that tRNA6Leu is only minimally involved in protein synthesis in E. coli.
To investigate the role of tRNA6Leu in E. coli, we attempted to isolate tRNA6Leu-requiring mutants from an Su+6 strain. These mutants required wild-type tRNA6Leu for survival at a nonpermissive temperature. We report here the isolation and the characterization of these mutants.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used were derivatives of E. coli BT3 [F− lacZ(Am1000) trp(Am) met(Am) bfe(Am) tsx(Am) str], which was described by Yamao et al. (25). The Su+6 gene of BT63 was transduced by P1 phage from E. coli 2B6 [F− lacZ(Am) trp(Am) str Su+6] (26). Temperature-sensitive derivatives of strain BT63 were isolated in the present study.
Media and growth conditions.
Cells were cultured predominantly at 37°C in Luria-Bertani (LB) medium. For preparation of plasmids, we used terrific broth medium (23) that contained antibiotics.
Plasmids.
Plasmids p652-0 and p652-1 were derivatives of pUC19 that contained the 6-kb KpnI fragment and the 5.4-kb KpnI-SmaI fragment, respectively, of Kohara’s phage 652 (13). Plasmids p560-0, p560-1, p560-2, and p560-3 were derivatives of pMW119 that contained the 7-kb EcoRI fragment, the 4-kb EcoRI-HpaI fragment, the 1.5-kb EcoRI-SacI fragment, and the 2.5-kb SmaI-HpaI fragment, respectively, from Kohara’s phage 560. Plasmids p648-0 and p648-1 were derivatives of pHSG576 (21) that contained the 6-kb EcoRI fragment and the 5.5-kb EcoRI-BamHI fragment, respectively, of Kohara’s phage 648. pHSGlacZwt (pMWlacZwt) and pHSGlacZttg (pMWlacZttg) contained a wild-type lacZ gene and a point-mutated lacZ gene, respectively. The construction of these plasmids is described below. pUCleuX was a derivative of pUC19 that contained the 4-kb HindIII fragment of Kohara’s phage 660. The HincII site in the multiple cloning site of this plasmid was removed for construction of the tRNA6Leu deletion.
DNA manipulation and sequencing of DNA.
Plasmids were isolated by the alkaline lysis method (2). Methods for restriction digestion, agarose gel electrophoresis, and DNA ligation were those described by Sambrook et al. (19). DNA was sequenced by the chain termination method with materials and protocols from a Sequenase kit (version 2.0; U.S. Biochemicals, Cleveland, Ohio). The sequencing primer was 5′-GATTGAAGCAGAAGCCTGCG-3′, which corresponds to positions 963 to 982 of the sequence of lacZ.
Isolation of temperature-sensitive mutants.
Mutagenesis with N-methyl-N′-nitronitrosoguanidine (NTG) was performed essentially as described by Miller (16). Cells of E. coli BT63 were grown to exponential phase and washed twice in the original volume of 0.1 M citrate buffer at pH 5.5. NTG was added to cells at a final concentration of 100 μg/ml, and the mixture was incubated at 37°C in a water bath. After a 1-h incubation, cells were washed once to remove NTG, diluted 100-fold in LB broth (to approximately 2 × 106 cells/ml), divided into 100 small test tubes (50 μl per tube), and grown up overnight at 32°C without agitation. Penicillin screening was used to enrich cultures for temperature-sensitive (Ts) mutants. The overnight cultures were diluted with 2 ml of fresh medium, incubated for 1 h at 32°C, and then transferred to a water bath at 42°C. After a 30-min incubation at 42°C, ampicillin (75 μg/ml) was added to each culture. After incubation for 3 h at 42°C in the presence of ampicillin, 5 μl of each of the 100 independent cultures was spread on half of an LB plate, and then plates were incubated at 32°C. Ten colonies per plate were picked up and replicated on two LB plates to check for temperature sensitivity. In the case of plates on which no Ts mutant was found, 10 more colonies were checked for temperature sensitivity. A total of 100 independent Ts mutants were isolated.
Complementation test.
Using phage clones, we streaked lysates of λ phages (>109 PFU per ml) vertically on LB plates and then cross-streaked cultures of strains horizontally over lysates, and vice versa. Each plate was incubated for 1 day at 42°C. In such a system, if complementation occurs, colonies should appear after the cross. When Kohara’s phage library was used for screening, the first complementation test was performed with mixtures of lysates of 10 clones and a second test allowed identification of specific clones. In the case of plasmid complementation, transformants were grown at a permissive temperature, and then temperature sensitivity was checked.
Site-directed mutagenesis.
The 6.3-kb KpnI-XbaI DNA fragment containing the lacZ gene from λgt11 (27) was cloned into the pHSG399 vector (21), and then the 1.7-kb ApaI-SalI fragment was deleted. The resultant plasmid (pHSGlacZwt) was used as a template for PCR mutagenesis. DNA primers were synthesized by KURABO Co. (Osaka, Japan). The sequences of DNA primers for PCR were 5′-ACCATTTTCAATCCGCACC-3′ (complementary to positions 999 to 1017) and 5′-TTGTTGTTGTTGAACGGCAAGCCG-3′ (a point-mutated primer corresponding to positions 1018 to 1041 of the lacZ sequence). Primers were phosphorylated by polynucleotide kinase (TOYOBO, Osaka, Japan) before PCR. PCR was carried out for 30 cycles with the Taq PL PCR system (Stratagene, La Jolla, Calif.). Each cycle consisted of 94°C for 30 s, 55°C for 1 s, and 74°C for 6 min. The product of the PCR, a single band of DNA, was purified by electrophoresis in low-melting-point agarose (Bethesda Research Laboratories, Gaithersburg, Md.) and self-ligated. Point mutations were confirmed by DNA sequencing. KpnI-HindIII fragments containing wild-type lacZ and point-mutated lacZ were recloned into the low-copy-number plasmid pMW119 (Nippongene, Tokyo, Japan). The resultant plasmids were designated pMWlacZwt and pMWlacZttg, respectively.
Measurement of β-galactosidase activity.
Assays of β-galactosidase activity were performed as described by Miller (17).
Construction of deletion mutants.
For construction of the tRNA6Leu deletion, the 1.0-kb HincII fragment within pUCleuX was replaced with the kanamycin resistance (Kmr) marker from pUC4KAPA (Pharmacia, Uppsala, Sweden). The resultant plasmid, pUCΔleuX, was digested by HindIII, and the ΔleuX fragment was used to transform JC7623. Ampicillin-sensitive, kanamycin-resistant transformants were selected and used for P1 transduction. In the case of the miaA deletion, p652-0 was digested by NruI (one site within miaA) and ligated with the chloramphenicol resistance (Cmr) marker from pHSG399. The ΔmiaA fragment from the resultant plasmid, pΔmiaA, was used for transformation. For the gidA deletion, the Cmr marker was ligated into the NruI site (one site) within the gidA gene of p560-1. The rest of the procedure was the same as for construction of the tRNA6Leu deletion.
P1 transduction.
Plate lysates of P1vir (>109 PFU per ml) were prepared with the donor bacterium JC7623 derivatives (deletion mutants). A 100-μl overnight culture of strain W3110 and an equal volume of the lysate were incubated in the presence of 2.5 mM CaCl2 at 37°C for 30 min. After centrifugation, cells were resuspended in 200 μl of LB broth and further incubated at 37°C for 45 min to express drug resistance genes. The cells were transferred onto LB agar plates containing the appropriate antibiotics and incubated at 32°C. Transductants that appeared were purified once to remove P1-free phages.
Nomenclature of the tRNALeu isoaccepting species.
Since the nomenclature of the tRNALeu isoaccepting species is not established, we clarify the relationship between nomenclature and anticodon (in parentheses) which we use in this report: tRNA1Leu (CAG), tRNA2Leu (GAG), tRNA3Leu (UAG), tRNA4Leu (UAA), and tRNA6Leu (CAA). The anticodons were described by unmodified forms.
RESULTS
Isolation and classification of tRNA6Leu-requiring mutants.
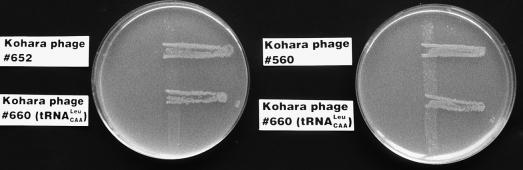
Among 100 independent Ts mutants derived from BT63, we selected mutants whose temperature sensitivity was suppressed by infection with Kohara’s phage clone 660 (13), which includes the wild-type gene for tRNA6Leu (leuX). Kohara’s phage clones are cI− phage. Therefore, wild-type λ phage was used to lysogenize the parental strain before selection of Ts mutants. Eighteen mutants were isolated as tRNA6Leu-requiring mutants. Since the BT63 strain grows at 42°C, Ts mutants obtained in this experiment were assumed to have secondary mutations in addition to supP (Su+6). We carried out complementation tests using Kohara’s library of phage clones (13). The complementation test revealed that tRNA6Leu-requiring mutants could be divided into two groups (Table 1). All mutants were complemented by Kohara’s phage clone 660. Most of them were weakly complemented by Kohara’s phage clones 648 and 649. Ten of them were also complemented by Kohara’s phage clone 652 (group I), and six were complemented by Kohara’s phage clone 560 (group II) (Fig. 1).
TABLE 1.
Classification of tRNA6Leu-requiring mutants
Numbers are those of Kohara’s clones (13).
±, weak complementation.
FIG. 1.
Complementation after incubation at 42°C for 24 h. We streaked cultures of strains ts29 (left) and ts39 (right) vertically on LB plates and then cross-streaked lysates of λ phages (>109 PFU per ml) horizontally over the cultures.
Identification of sites of mutations.
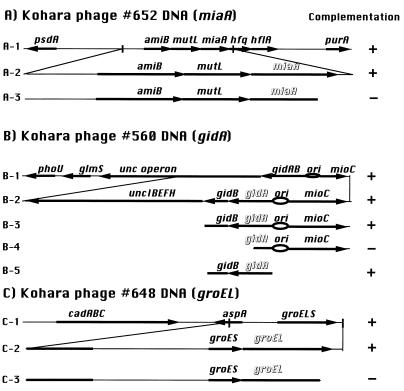
We subcloned DNA fragments from Kohara’s phage clones into a plasmid vector. Then we constructed several deletion clones and identified the gene that complemented the Ts phenotype of our mutants. For complementation tests, we used strains ts29 and ts39 as examples of group I and group II mutants, respectively. The 4-kb HindIII fragment of Kohara’s phage 660, containing the leuX gene, complemented both mutants, a result that suggested that they were indeed tRNA6Leu-requiring mutants. Figure 2 shows the results of complementation tests. The 6-kb KpnI fragment of Kohara’s phage clone 652 complemented the group I mutants, but deletion of the miaA gene eliminated the capacity for complementation (Fig. 2A). The miaA gene is involved in the modification of tRNAs. The product of the miaA gene catalyzes the first step in the biosynthesis of 2-methylthio-N6-(Δ2-isopentenyl)-adenosine (ms2i6A), which is found adjacent to the anticodon in several species of tRNA. This modification stabilizes codon-anticodon interactions (24) and thereby enhances rates of elongation and growth (6). Although ms2i6A deficiency decreases elongation rates, such a deficiency enhances proofreading during translation (4, 6, 7). In particular, ms2i6A deficiency in tRNA4Leu results in a decreased frequency of errors (7).
FIG. 2.
Complementation tests were carried out with group I mutant ts29 and group II mutant ts39. Symbols + and − refer to growth at 42°C. The phage clones and plasmids used were as follows: A-1, Kohara phage 652; A-2, pUC19 containing the 6-kb KpnI fragment (p652-0); A-3, pUC19 containing the 5.4-kb KpnI-SmaI fragment (p652-1); B-1, Kohara phage 560; B-2, pMW119 containing the 7-kb EcoRI fragment (p560-0); B-3, pMW119 containing the 4-kb EcoRI-HpaI fragment (p560-1); B-4, pMW119 containing the 1.5-kb EcoRI-SacI fragment (p560-2); B-5, pMW119 containing the 2.5-kb SmaI-HpaI fragment (p560-3); C-1, Kohara phage 648; C-2, pHSG576 containing the 6-kb EcoRI fragment (p648-0); and C-3, pHSG576 containing the 5.5-kb EcoRI-BamHI fragment (p648-1).
As shown in Fig. 2B, the group II mutant was complemented by a plasmid that carried only the gidA gene. The gidA (glucose-inhibited cell division) gene is located next to the ori region in the E. coli genome and appears to be involved in cell division. The precise function of the product of the gidA gene is unknown.
Weak complementation by Kohara’s phage 648 appeared to be due to overexpression of chaperonin, groELS (11) (Fig. 2C). This hypothesis was supported by the fact that complementation by plasmid clones was more effective than that by Kohara’s phage.
Efficiency of translation using the UUG codon.
Since it was reported previously that ms2i6A deficiency in tRNA4Leu results in a decreased frequency of error (7), we postulated that the requirement for wild-type tRNA6Leu of group I mutants might have been caused by a shortage of tRNAs that can interact with the UUG codon due to the decreased ability of tRNA4Leu to translate the UUG codon.
First, we examined the effects of overexpression of tRNA4Leu. The 1.5-kb EcoRI fragment of Kohara’s phage 340, which includes the gene for tRNA4Leu, was cloned into the EcoRI site of pMW119, and the resultant plasmid was used to transform mutants ts29 and ts39. Only the group I mutant ts29 recovered temperature resistance upon overexpression of tRNA4Leu (data not shown).
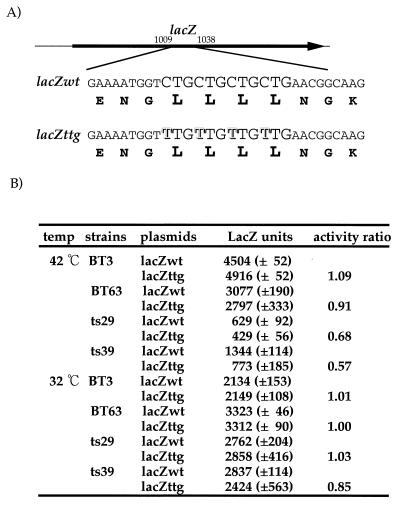
Second, we performed a codon substitution experiment. β-Galactosidase contains four leucine residues from amino acids 340 to 343 (Fig. 3A). All four leucine codons are the major codon CUG. We replaced all four CUG codons with UUG codons and then examined the effects of such replacement in both wild-type and mutant strains. As shown in Fig. 3B, a decrease in β-galactosidase activity at 42°C was observed in both group I mutant ts29 and group II mutant ts39. No significant effect was observed in BT63 cells, suggesting that tRNA4Leu can normally recognize the UUG codon in wild-type strains.
FIG. 3.
Sites of point mutations. Letters outlined in black indicate sites of mutations. The encoded amino acids were unchanged by these mutations. lacZwt, wild-type lacZ. (B) β-Galactosidase activities in Miller units. Cultures were preincubated at 37°C for 1 h and then shifted to 42 or 32°C after addition of 1 mM isopropyl-β-d-thiogalactopyranoside. After 3 h, β-galactosidase activities were measured. Values presented are averages of results from three samples.
Mutations in an unmutagenized background.
Since NTG treatment induces several mutations, we attempted to move these mutations in the unmutagenized parental background to verify that the tRNA6Leu-dependent phenotype is due solely to mutations at these loci. At first, we tried to construct deletion mutants in a W3110 background. The deletion strains were prepared by using strain JC7623, and deletion markers (ΔtRNA6Leu::Kmr, ΔmiaA::Cmr, and ΔgidA::Cmr) were transferred to strain W3110 by P1 transduction. As shown in Table 2, the double-deletion mutant W3110 ΔtRNA6LeuΔmiaA could not grow at 42°C but was viable at 32°C. This result indicates that tRNA4Leu without ms2i6A modification can recognize the UUG codon efficiently enough to maintain cell survival at 32°C but not at 42°C. Temperature sensitivity was suppressed by introduction of either the miaA gene or the gene for tRNA6Leu, which is the same phenotype as the group I mutants.
TABLE 2.
Temperature sensitivity of mutant strains
| Strain | Growth at:
|
|
|---|---|---|
| 42°C | 32°C | |
| W3110 ΔtRNA6Leu | + | + |
| W3110 ΔmiaA | + | + |
| W3110 ΔtRNA6LeuΔmiaA | − | + |
| W3110 gidA*a | + | + |
| W3110 ΔtRNA6LeugidA* | − | + |
gidA* indicates the gidA gene of strain ts39.
However, the construction of a gidA deletion mutant was unsuccessful. We selected six independent Cmr transformants of JC7623 and checked replacement of the ΔgidA gene by PCR. All transformants carried the original gidA gene in addition to the ΔgidA gene. This result indicates that an insertion event instead of recombination occurred in this case for some unknown reason. The cotransduction efficiency between gidA and ΔgidA (Cmr) was about 80%. Taking advantage of this high cotransduction efficiency, we moved the gidA mutation of strain ts39 (gidA*) into W3110 ΔtRNA6Leu. The resultant W3110 ΔtRNA6LeugidA* strain showed a Ts phenotype, and the temperature sensitivity was suppressed by introduction of either the gidA gene or the gene for tRNA6Leu, which is the same phenotype as the group II mutants (Table 2).
DISCUSSION
The diversification of tRNA species might have occurred by amplification of their genes and changes in anticodon sequences. In the course of diversification, it appears that nonessential species of tRNA were also generated. The fact that such tRNA species have been maintained throughout evolution implies that they confer some selective advantage under certain circumstances. In this study, we adopted a new approach to investigate the importance of one such tRNA, tRNA6Leu. In a uropathogenic strain, E. coli 536, tRNA6Leu is known to be necessary for virulence (cited as tRNA5Leu; see Materials and Methods) (18, 20). Although the gene for tRNA6Leu is not essential for E. coli K-12, we succeeded in this study in isolating tRNA6Leu-requiring mutants.
Our group I mutants were complemented by introduction of the wild-type miaA gene carried by a phage or a plasmid. The product of the miaA gene catalyzes the modification of the adenosine moiety adjacent to the anticodon in several species of tRNA. The modification (ms2i6A) stabilizes codon-anticodon interactions (24) but increases translational error via misreading of the third position in the codon (4, 6, 7). The results of overexpression of the gene for tRNA4Leu and of our codon substitution experiment indicated that the temperature sensitivity of group I mutants might be caused by a shortage of tRNAs that can read the UUG codon. In the Su+6 strain, the UUG codon is recognized only by tRNA4Leu. tRNA4Leu recognizes UUA and UUG codons, with a slight preference for the UUA codon (10). Moreover, ms2i6A deficiency in tRNA4Leu is known to decrease the frequency of errors during translation (7). Considering these facts, we propose that ms2i6A deficiency in tRNA4Leu enhances the preference for the UUA codon and the efficiency of recognition of the UUG codon is reduced. Therefore, group I mutants require the wild-type tRNA6Leu at 42°C. Our data suggested that tRNA4Leu without ms2i6A modification can recognize the UUG codon efficiently enough to maintain cell survival at 32°C, since a strain with deletions of both the miaA gene and the gene for tRNA6Leu required introduction of either the miaA gene or the gene for tRNA6Leu for survival at 42°C but could survive on an LB plate at 32°C. Temperature may strongly affect the stability of codon-anticodon interactions.
Group II mutants were not suppressed by overexpression of tRNA4Leu, but a decrease in the efficiency of recognition of the UUG codon was observed. Since the gidA gene, which complemented the mutation in group II mutants, seems to be involved in cell division, the mechanism for the dependence on wild-type tRNA6Leu may involve some aspect of cell division. Although we did not mention it, the Su+6 strain BT63 formed filamentous cells especially at high temperatures. The suppressor mutation in tRNA2Ser (supH) is also known to cause filamentation. A mutant with such a mutation was first isolated as a mutant with a defect in cell division, ftsM (8), and later the ftsM gene was shown to be identical to serU, a gene for tRNA2Ser (15). Several other mutations in tRNAs that affect cell division or DNA replication, such as mutations in tRNA1Ser (divE) (22), tRNA4Arg (dnaY) (9), and tRNA3Leu (5), have been reported. It is still unclear how mutations in tRNAs disturb cell division and how mutations in the gidA gene cause the requirement for tRNA6Leu at high temperatures.
ACKNOWLEDGMENT
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (09278219) from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Andachi Y, Yamao F, Muto A, Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. J Mol Biol. 1989;209:37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bouadloun F, Srichaiyo T, Isaksson L A, Björk G R. Influence of modification next to the anticodon in tRNA on codon context sensitivity of translational suppression and accuracy. J Bacteriol. 1986;166:1022–1027. doi: 10.1128/jb.166.3.1022-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M X, Bouquin N, Norris V, Casaregola S, Seror S J, Holland I B. A single base change in the acceptor stem of tRNA3Leu confers resistance upon Escherichia coli to the calmodulin inhibitor, 40/80. EMBO J. 1991;10:3113–3122. doi: 10.1002/j.1460-2075.1991.tb07865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz I, Pedersen S, Kurland C G. Effects of miaA on translation and growth rates. Mol Gen Genet. 1987;208:373–376. doi: 10.1007/BF00328126. [DOI] [PubMed] [Google Scholar]
- 7.Diaz I, Ehrenberg M. ms2i6A deficiency enhances proofreading in translation. J Mol Biol. 1991;222:1161–1171. doi: 10.1016/0022-2836(91)90599-2. [DOI] [PubMed] [Google Scholar]
- 8.Drapeau G R, Chausseau J P, Gariepy F. Unusual properties of a new division mutant of Escherichia coli. Can J Microbiol. 1983;29:694–699. doi: 10.1139/m83-113. [DOI] [PubMed] [Google Scholar]
- 9.Garcia G M, Mar P K, Mullin D A, Walker J R, Prather N E. The E. coli dnaY gene encodes an arginine transfer RNA. Cell. 1986;45:453–459. doi: 10.1016/0092-8674(86)90331-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldman E, Holmes W M, Hatfield G W. Specificity of codon recognition by Escherichia coli tRNALeu isoaccepting species determined by protein synthesis in vitro directed by phage RNA. J Mol Biol. 1979;129:567–585. doi: 10.1016/0022-2836(79)90469-8. [DOI] [PubMed] [Google Scholar]
- 11.Hartl F U, Hlodan R, Langer T. Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci. 1994;19:20–25. doi: 10.1016/0968-0004(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 12.Holmes W M, Goldman E, Miner T A, Hatfield G W. Differential utilization of leucyl-tRNAs by Escherichia coli. Proc Natl Acad Sci USA. 1977;74:1393–1399. doi: 10.1073/pnas.74.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 14.Komine Y, Adachi T, Inokuchi H, Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K-12. J Mol Biol. 1990;212:579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- 15.Leclerc G, Sirard C, Drapeau G R. The Escherichia coli cell division mutation ftsM1 is in serU. J Bacteriol. 1989;171:2090–2095. doi: 10.1128/jb.171.4.2090-2095.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 125–129. [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 18.Ritter A, Blum G, Emody L, Kerenyi M, Bock A, Neuhierl B, Rabsch W, Scheutz F, Hacker J. tRNA genes and pathogenicity islands: influence on virulence and metabolic properties of uropathogenic Escherichia coli. Mol Microbiol. 1995;17:109–121. doi: 10.1111/j.1365-2958.1995.mmi_17010109.x. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Susa M, Kreft B, Wasenauer G, Ritter A, Hacker J, Marre R. Influence of cloned tRNA genes from a uropathogenic Escherichia coli strain on adherence to primary human renal tubular epithelial cells and nephropathogenicity in rat. Infect Immun. 1996;64:5390–5394. doi: 10.1128/iai.64.12.5390-5394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 22.Tamura F, Nishimura S, Ohki M. The E. coli divE mutation, which differentially inhibits synthesis of certain proteins, is in tRNA1Ser. EMBO J. 1984;3:1103–1107. doi: 10.1002/j.1460-2075.1984.tb01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartof K D, Hobbs C A. Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus. 1987;9:12. [Google Scholar]
- 24.Vacher J, Grosjean H, Houssier C, Buckingham R H. The effect of point mutations affecting Escherichia coli tryptophan tRNA on codon-anticodon interactions and on UGA suppression. J Mol Biol. 1984;177:329–342. doi: 10.1016/0022-2836(84)90460-1. [DOI] [PubMed] [Google Scholar]
- 25.Yamao F, Inokuchi H, Ozeki H. Mischarging mutants of Su+2 glutamine tRNA in E. coli. I. Mutations near the anticodon cause mischarging. Jpn J Genet. 1988;63:237–249. doi: 10.1266/jjg.63.237. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura M, Inokuchi H, Ozeki H. Identification of tRNA suppressors in Escherichia coli. IV. Amber suppressor Su+6 a double mutant of a new species of leucine tRNA. J Mol Biol. 1984;177:627–644. doi: 10.1016/0022-2836(84)90041-x. [DOI] [PubMed] [Google Scholar]
- 27.Young R A, Davis R W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci USA. 1983;80:1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]