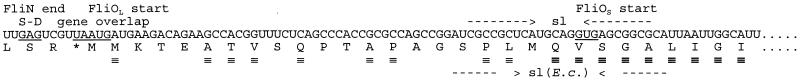Abstract
The flagellar gene fliO of Salmonella typhimurium can be translated from an AUG codon that overlaps the termination codon of fliN (K. Ohnishi et al., J. Bacteriol. 179:6092–6099, 1997). However, it had been concluded on the basis of complementation analysis that in Escherichia coli a second start codon 60 bp downstream was the authentic one (J. Malakooti et al., J. Bacteriol. 176:189–197, 1994). This raised the possibility of tandem translational starts, such as occur for the chemotaxis gene cheA; this possibility was increased by the existence of a stem-loop sequence covering the second start, a feature also found with cheA. Protein translated from the first start codon was detected regardless of whether the second start codon was present; it was also detected when the stem-loop structure was disrupted or deleted. Translation from the second start codon, either as the natural one (GUG) or as AUG, was not detected when the first start and intervening sequence were intact. Nor was it detected when the first codon was attenuated (by conversion of AUGAUG to AUAAUA; in S. typhimurium there is a second, adjacent, AUG) or eliminated (by conversion to CGCCGC); disruption of the stem-loop structure still did not yield detectable translation from the second start. When the entire sequence up to the second start was deleted, translation from the second start was detected provided the natural codon GUG had been converted to AUG. A fliO null mutant could be fully complemented in swarm assays whenever the first start and intervening sequence were present, regardless of the state of the second start. Reasonably good complementation occurred when the first start and intervening sequence were absent provided the second start was intact, either as AUG or as GUG; thus translation from the GUG codon must have been occurring even though protein levels were too low to be detected. The translated intervening sequence is rather divergent between S. typhimurium and E. coli and corresponds to a substantial cytoplasmic domain prior to the sole transmembrane segment, which is highly conserved; the sequence following the second start begins immediately prior to that transmembrane segment. The significance of the data for FliO is discussed and compared to the equivalent data for CheA. Attention is also drawn to the fact that given an optimal ribosome binding site, AUA can serve as a fairly efficient start codon even though it seldom if ever appears to be used in nature.
The fliO gene of Salmonella typhimurium (and of several other bacterial species) is a gene of poorly understood function. It is essential for flagellar assembly, yet its product, a membrane protein, has not been detected within flagellar structure, nor is there any evidence that it plays a regulatory role. The genes immediately upstream of fliO (fliM and fliN) are structural, encoding two of the three components of the flagellar switch, the structure that determines the direction of flagellar rotation (11). The exact functions of the products of the genes downstream of fliO (fliP, fliQ, and fliR) are not known, but they are believed to be involved in the export and assembly of external flagellar components such as the filament protein, flagellin. This belief is based in part on the absence of evidence for a structural or regulatory role, in part on the fact that (at least in the case of FliP and FliR [2]) they are associated with the flagellar basal body, and in part because they have homologs in systems (collectively called type III secretory pathways) which are believed to be responsible for the export of virulence factors in a wide range of pathogenic bacteria (4). In the case of FliO, there is as yet no obvious homolog in these systems. Nonetheless, it seems plausible that FliO, too, is involved in flagellar protein export.
We recently described the cloning of the fliO, fliP, fliQ, and fliR genes of S. typhimurium and the characterization of their products (14). In that study, we briefly noted the complexities surrounding the question of translation of the fliO gene. The purpose of the present study is to address these complexities and how they are reflected in the ability of FliO to support flagellar function.
Based on the sequence, it seemed likely to us that translation would start at an AUG codon that was in UAAUG overlap with the UAA stop codon of fliN (Fig. 1). However, in a study of this region in Escherichia coli, Malakooti et al. (12) had concluded that translation started at an in-frame GUG codon 60 bp downstream (the equivalent codon in S. typhimurium is 63 bp downstream). For convenience, we refer to these two sites as the first and second starts, respectively, while recognizing that the validity of these terms remains to be established.
FIG. 1.
Sequence of the 5′ region of the flagellar gene fliO of S. typhimurium, extending from the 3′ end of the upstream gene, fliN, where there is a UAAUG overlap (underlined) between the fliN termination codon UAA and a potential AUG start codon for fliO, and continuing through a possible second start site that is in frame with the first. S-D, Shine-Dalgarno sequence for the first start; sl and arrowed lines, stem-loop sequence with the second start (GUG, underlined) lying within the loop region; sl(E.c.) and arrowed lines, position and extent of the corresponding stem-loop sequence in E. coli (note that the RNA sequences are not identical in the two species). The deduced amino acid sequence of the C terminus of FliN and the N terminus of FliO of S. typhimurium is shown below the RNA sequence. FliOL, N terminus from first start; FliOS, N terminus from second start; ≡, position of identity between the S. typhimurium and E. coli FliO sequences; bold ≡, position of identity following the loop region, i.e., essentially following the second start.
There were several reasons why we thought that the first start would be the correct one or at least the principal one. (i) The start/stop overlap seen between fliN and the first start is a common feature among adjacent flagellar genes in an operon (10). (ii) Gaps of as large as 60 to 63 bp within an operon are unusual, at least in the flagellar gene system; indeed, there is only one known example of a comparable gap (54 bp, between the last of the rod genes, flgG, and the basal-body L-ring gene, flgH [7]). (iii) A 100-bp window starting with the AUG codon scores as a coding region at the 99% confidence level (3). (iv) The deduced amino acid sequences for the region between the two potential starts in S. typhimurium and E. coli, though not as highly conserved as some other parts of the sequence, show significant similarity, have a basic residue near the terminus, and are of essentially the same length. (v) The apparent molecular mass for FliO (ca. 16 kDa) is closer to the deduced one from the upstream start (13 kDa) than that from the downstream start (11 kDa).
The conclusion that the second start was the correct one—i.e., that the 60 bp in between the first and second starts was noncoding rather than part of the fliO gene—was based on the fact that a plasmid in which the insert began at the second start complemented a fliO mutant (12). However, that result is open to another interpretation: (i) that the fliO gene normally begins at the first start, (ii) that under some circumstances translation can begin at the second start, and (iii) that the amino acid sequence between the two starts is not absolutely required for function.
The fliO sequences of both E. coli and S. typhimurium have a stem-loop sequence covering the second start (Fig. 1). This is a feature that is also present in one of the chemotaxis genes, cheA, whose product is an autokinase which is part of the chemotaxis sensory transduction cascade. cheA is a well-documented example of an unusual phenomenon in bacteria, a gene with tandem in-frame translational starts (8). This led us to consider whether fliO might be another example of the same phenomenon. In this study we examine the consequences—in terms of both protein products and complementation in motility tests—of various changes to the first start, the second start, and the stem-loop sequence of fliO.
MATERIALS AND METHODS
Strains and plasmids.
E. coli XL-1 Blue (Stratagene, La Jolla, Calif.) was used as the recipient for cloning experiments. UH869 is a minicell-producing strain of E. coli (5). YK4458 is an E. coli fliO mutant and was used for complementation (18). All plasmids used are pUC19 based. Plasmids pGS16, pGS17, pGS18, and pGS34 were constructed in a previous study (14). FLAG-tagged fliO-containing plasmids are listed in Table 1 and shown schematically in Fig. 2.
TABLE 1.
Consequences of changes to fliO in terms of protein productsa and complementation
| fliO version | Plasmid | Detection by anti-FLAG antibodyb
|
Complementationc | ||
|---|---|---|---|---|---|
| FliOL | FliOL′ | FliOS | |||
| Wild type | pGS34 | y | y | n | ++++ |
| Wild typed | pGS73 | y | nd | n | ++++ |
| Second start eliminated (GUG→GUA) | pGS47 | y | y | n | ++++ |
| Second starts eliminated (GUG→GUA, AUG→AUA) | pGS48 | y | y | n | +++ |
| Stem-loop disrupted | pGS55 | y | y | n | ++++ |
| Second starts eliminated and stem-loop disrupted | pGS52 | y | y | n | ++++ |
| 21-bp deletion in stem-loop | pGS60 | ye | n | n | ++ |
| Second start enhanced (GUG→AUG) | pGS68 | y | y | n | +++ |
| First start attenuated (AUGAUG→AUAAUA) | pGS51 | (y) | (y) | n | +++ |
| First start eliminated (AUGAUG→CGCCGC) | pGS67 | n | n | n | − |
| First start eliminated (AUGAUG→CGCCGC); stem-loop disrupted | pMK75 | n | n | n | − |
| First start eliminated (AUGAUG→CGCCGC); stem-loop disrupted and second start enhanced (GUG→AUG) | pMK76 | n | n | n | − |
| First start and intervening sequence eliminated | pGS74 | n | n | n | ++ |
| First start and intervening sequence eliminated; second start enhanced (GUG→AUG) | pGS50 | n | n | y | +++ |
| Wild type (E. coli) | pGS71 | y | y | n | +++ |
| First start and intervening sequence eliminated (E. coli) | pGS72 | n | n | n | + |
All versions are from S. typhimurium unless otherwise stated. They all have a FLAG tag, which is at the C terminus except for pGS73, where it is at the N terminus. The tags did not affect complementation properties.
FliOL, FliO translated from the first start; FliOL′, modified form of FliOL; FliOS, FliO translated from the second start. y, yes; n, no; (y), intensity lower than wild-type intensity.
Swarm diameter after 11 h at 30°C. ++++, >40 mm; +++, 31 to 40 mm; ++, 21 to 30 mm; +, 11 to 20 mm; −, <11 mm.
N-terminal FLAG tag. Although FliOL′ was not detected in immunoblots (Fig. 7), indicating it had lost its N-terminal FLAG tag, it was detected in autoradiograms.
Except for the shortening caused by the 7-amino-acid deletion.
FIG. 2.
Schematic representations of the plasmids listed in Table 1 and used for product identification and complementation analysis. Wild-type sequence at the two starts is shown in normal type, and altered sequence is shown in bold type. The stem-loop structure surrounding the second start is indicated by gray arrows, which are absent if the structure has been disrupted or deleted. Ter, termination site. Black boxes indicate FLAG tags: N terminal in the case of pGS73 and C terminal in all other cases.
PCR and cloning.
Synthetic primers containing restriction sites and mutagenic primers were synthesized by using a model 393 DNA/RNA synthesizer (Applied Biosystems, Foster City, Calif.). PCR was carried out by using an MJ Minicycler (MJ Research, Watertown, Mass.) and Taq polymerase (Boehringer Mannheim Corp., Indianapolis, Ind.). PCR products were purified by using a Qiagen gel extraction kit (Qiagen Inc., Chatsworth, Calif.). Plasmids were purified by using a QIAprep Spin plasmid kit. All plasmid constructions were verified by sequencing using the Sequenase protocol (U.S. Biochemicals, Cleveland, Ohio).
35S labeling of proteins in minicells.
Minicell radiolabeling experiments were carried out as described by Homma et al. (5).
Immunological methods.
To detect FLAG-tagged proteins, samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% acrylamide), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.), probed with anti-FLAG M2 monoclonal antibody (Scientific Imaging Systems, Eastman Kodak Co., New Haven, Conn.), and detected by using the enhanced chemiluminescence assay (Amersham International, Little Chalfont, United Kingdom) according to the manufacturer’s instructions.
Swarm plate complementation assay.
Soft tryptone motility plates were made as described previously (14) and supplemented with ampicillin (50 μg/ml) as needed. Freshly transformed YK4458 cells were spotted onto the plates in duplicate and incubated at 30°C, and the swarm diameter was measured at various times.
The fliO sequence of E. coli.
While checking the sequences of our plasmids with E. coli fliO inserts, we noted three positions that differed from the sequence reported in reference 12 (GenBank accession no. P22586). Our sequence agrees with the sequences from the E. coli genome project, University of Wisconsin, Madison (accession no. ECAE000287), and the Japan E. coli genome sequencing project (accession no. D90835). At the amino acid level, the changes are R15→A and V18→L; at the nucleotide level, these changes also cause minor changes in the stem-loop structure (see Results).
RESULTS
Translation of fliO can initiate at the first start.
In our analysis of the fliO, fliP, fliQ, and fliR genes of S. typhimurium (14), we had used a pET-based plasmid, pGS45, to overproduce and purify FliO; its insert begins at the first start of fliO and continues through to a His-tag fusion at the 3′ end of fliO. This enabled us to establish two things. (i) Since a major protein (apparent molecular mass of about 17.5 kDa) encoded by the insert could be purified by Ni-nitrilotriacetic acid chromatography, translation must have continued all the way to the C terminus of FliO. (ii) Since the purified protein had an N-terminal amino acid sequence (determined for the first 13 amino acids) that perfectly matched that of the deduced sequence from the first start, translation must have initiated at that start. Thus, the entire fliO sequence can be translated from the first start (which on the chromosome overlaps with fliN) to the terminus (which on the chromosome overlaps with fliP).
In minicell experiments with the untagged full-length protein (pGS17), two major bands had been seen, one with an apparent molecular mass of about 16.5 kDa and another at about 15 kDa (14). From now on we refer to the upper of the two bands, which we identify as the product from the first start, as FliOL (meaning the long form of FliO) and refer to the lower of the two bands as FliOL′. The shift in mobility of FliOL′ with respect to FliOL seemed too small for it to be the product of translation from the second start (the short form of FliO, or FliOS). Its identity will be addressed later. No band was observed at the position expected for FliOS.
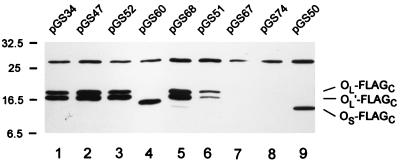
In similar minicell experiments with plasmid pGS34, which encodes C-terminally FLAG-tagged FliOL, two major bands were again seen in radiolabeling experiments, which we identify as FliOL-FLAGC and FliOL′-FLAGC, respectively (Fig. 3, lane 1). Their apparent molecular masses were shifted upward by about 1 kDa compared with those of the untagged protein. Immunoblotting using monoclonal anti-FLAG antibody established that translation had proceeded through to the tagged C terminus for both forms (Fig. 4, lane 1). Thus FliOL′ cannot be a C-terminal truncation of FliOL.
FIG. 3.
Autoradiogram of products from various FLAG-tagged fliO-containing plasmids (cf. Fig. 2 and Table 1) in minicell expression experiments. The positions of bands corresponding to FliOL-FLAGC, FliOL′-FLAGC, and FliOS-FLAGC are indicated to the right. The bands in the 25-kDa range are nonspecific and were also seen with the vector. Positions of molecular mass markers (in kilodaltons) are shown to the left.
FIG. 4.
Immunoblots, using anti-FLAG antibody, of products from various FLAG-tagged fliO-containing plasmids (cf. Fig. 2 and Table 1). The bands corresponding to FliOL-FLAGC, FliOL′-FLAGC, and FliOS-FLAGC are indicated to the right. The band in the 25-kDa range is nonspecific and was also seen with the vector. Positions of molecular mass markers (in kilodaltons) are shown to the left.
No band was detected either in the autoradiograph or in the immunoblot at the position expected for FliOS-FLAGC.
Eliminating the second start has no effect on translation from the first start.
Plasmid pGS18 has the GUG second start of plasmid pGS17 changed to GUA (a Val codon). A C-terminal FLAG tag was introduced to pGS18 to give plasmid pGS47. This plasmid encodes FliOL-FLAGC with no amino acid change at the second start position if translation is occurring from the first start.
Autoradiograms from minicell expression experiments gave a pattern for pGS47 that was indistinguishable from that for pGS34: FliOL-FLAGC and FliOL′-FLAGC were clearly present, at exactly the same positions as before, and (as expected because of elimination of the second start) there was no evidence that FliOS-FLAGC was being produced. Immunoblotting (Fig. 4, lane 2) confirmed the identities of FliOL-FLAGC and FliOL′-FLAGC.
In the S. typhimurium sequence, there is another potential second start (an AUG) within the stem-loop structure (Fig. 1). It is not present at the equivalent position in E. coli, making it a less likely start than the GUG second start that both species share. Nevertheless, we eliminated it by converting it to AUA (an Ile codon) while retaining the GUG→GUA change. The resulting plasmid, pGS48, gave the same results in minicell expression and immunoblotting experiments as pGS34 and pGS47 (data not shown).
Eliminating the stem-loop has no effect on translation from the first start.
Having established that translation from the first start is unaffected by the presence or absence of the second start or starts, we next addressed the stem-loop sequence. In S. typhimurium, the stem consists of nine uninterrupted base pairs. Modifying the 5′ arm of the stem from 5′-CGCCGCUCA-3′ to 5′-CUCCAUUAA-3′ resulted in the loss of four base pairs in the stem while leaving the amino acid sequence unchanged; the change in nucleotide sequence is predicted to cause a lowering of the melting temperature by 16°C (20). We made these changes alone (plasmid pGS55) and in combination with the changes to the second starts (plasmid pGS52). In the latter case, although the second base in the 3′ arm of the stem was altered from G to A, the corresponding base pair had already been disrupted by the changes to the 5′ arm. In minicell radiolabeling experiments, pGS52 resulted in the presence of both FliOL-FLAGC and FliOL′-FLAGC, and immunoblotting confirmed that both forms retained their C terminus (Fig. 4, lane 3). Again, there was no evidence for FliOS-FLAGC. The results with plasmid pGS55 were identical. Thus, the absence of the stem-loop feature does not interfere with translation from the first start, nor does it give detectable translation from the second start.
Effect of an internal deletion in the region of the stem-loop.
In our first attempt to alter the 5′ arm of the stem (see above), a PCR error occurred whose effect was an in-frame deletion of 21 bp (seven codons) that included about half of the 5′ arm of the stem, the entire loop, and the entire 3′ arm of the stem (Fig. 2). The resulting plasmid, pGS60, although unintended, proved useful. In minicell radiolabeling experiments, only a single major band was seen, with an apparent molecular mass of around 15.5 kDa, in reasonable agreement with the value expected after the loss of seven amino acids. We identify this band as FliOLΔ7-FLAGC, since it was seen in immunoblots (Fig. 4, lane 4) as well as in the autoradiograph. The absence of a second band corresponding to FliOL′Δ7-FLAGC indicates that the event responsible for the FliOL′ form is prevented by the deletion in the region of the stem-loop.
Translation from the second start is not observed when the first start is intact and the second start is converted from GUG to the preferred codon AUG.
We noted above that when the first and second starts were left in their wild-type state, no FliOS-FLAGC was detected (plasmid pGS34). We next strengthened the second start by converting the GUG codon to the preferred codon, AUG, yielding plasmid pGS68. In minicell radiolabeling experiments and immunoblots (Fig. 4, lane 5), the FliOL-FLAGC and FliOL′-FLAGC forms were seen as before, but FliOS-FLAGC could still not be seen.
Translation from the second start when the first start is attenuated or eliminated but the intervening sequence is retained.
In the wild-type cell, both the first start and the intervening sequence are present upstream of the second start. If the second start is a real one, it should not make any difference to translation from that start if the first start is eliminated. Furthermore, retention of the intervening region should provide a good approximation of the chromosomal arrangement.
In S. typhimurium there are two adjacent AUG codons (AUGAUG) at the first start site. To ensure that the second codon could not be used as an alternative start, we converted the sequence to AUAAUA (coding for Ile-Ile), yielding plasmid pGS51. To our surprise, the pattern in minicell expression experiments was almost unchanged compared with plasmids like pGS34 where the first start was intact (autoradiograph, Fig. 3, cf. lanes 1 and 2; immunoblot, Fig. 4, cf. lanes 1 and 6), with both FliOL-FLAGC and FliOL′-FLAGC still present in their original positions. The only difference was a moderate reduction in intensity of the two bands. FliOS-FLAGC was still not detected.
These observations implied that AUA was being recognized with fairly high efficiency as a start codon. We tested this hypothesis by changing the AUGAUG sequence to CGCCGC (corresponding to Arg-Arg), where CGC seemed as distant as possible from any of the natural start codons. This yielded plasmid pGS67 and resulted in complete disappearance of the bands corresponding to both FliOL-FLAGC and FliOL′-FLAGC in both autoradiographs and immunoblots (Fig. 4, lane 7). There was still no evidence for FliOS-FLAGC.
Translation can initiate from the second start when the first start and intervening sequence are unavailable.
The insert of plasmid pGS16 places the second start in an optimal arrangement with an optimized ribosome binding site and continues through to the 3′ end of the gene. Thus, it lacks not only the first start but also the intervening sequence between the two starts and the stem-loop structure (since the 5′ arm of the stem and most of the loop are absent). (The same was true of the E. coli construction used in reference 12.) When pGS16 was modified by addition of a C-terminal FLAG tag, to give plasmid pGS74, and minicell expression experiments were carried out, there was again no evidence of FliOS-FLAGC either in autoradiographs or in immunoblots (Fig. 4, lane 8).
However, when the second start was optimized by conversion to AUG, to give plasmid pGS50, a substantial band was observed at about 14 kDa in minicell expression experiments (Fig. 3, lane 3) and immunoblots (Fig. 4, lane 9), which we identify as FliOS-FLAGC. It has an apparent molecular mass of 14 kDa, substantially less than the values for FliOL-FLAGC (17.5 kDa) and FliOL′-FLAGC (16.5 kDa), in reasonable agreement with the difference of 21 amino acids between FliOL and FliOS. The value of 14 kDa is also in good agreement with the value of 13 kDa for the corresponding untagged protein in E. coli (12). As expected, there was no evidence for FliOL-FLAGC; also, there was no evidence for FliOL′-FLAGC, indicating that the first start or intervening sequence is at least partly involved in its generation.
Translation from the second start when the first start is eliminated, the intervening sequence is retained, and the stem-loop sequence is disrupted.
The success in detecting FliOS-FLAGC with pGS50 might have been in part be due to the loss of the stem-loop structure. We therefore constructed a hybrid of plasmid pGS67 (where the first start had been eliminated by the AUGAUG→CGCCGC mutation) and pGS55 (where the stem loop had been disrupted) to see whether this disruption would facilitate translation from the second start in the absence of translation from the first start. The resulting plasmid, pMK75, as expected did not give the bands corresponding to FliOL-FLAGC or FliOL′-FLAGC, but it also gave no evidence for FliOS-FLAGC (data not shown); this was still true when the second start codon was optimized by replacement of GUG by AUG to give plasmid pMK76. Thus, the presence or absence of the stem-loop does not seem to be a contributing factor to translation (or lack thereof) from the second start site.
Complementation properties associated with the different versions of FliO.
We next examined how well the various fliO constructions could complement an E. coli fliO null mutant (YK4458) in swarm tests on semisolid tryptone agar. The results are summarized in Table 1, and selected examples are shown in Fig. 5. Tagging of the C terminus with a FLAG tag did not affect the complementation properties of any of the FliO versions that we tested; the same was true of the only N-terminally FLAG-tagged version used (plasmid pGS73).
FIG. 5.
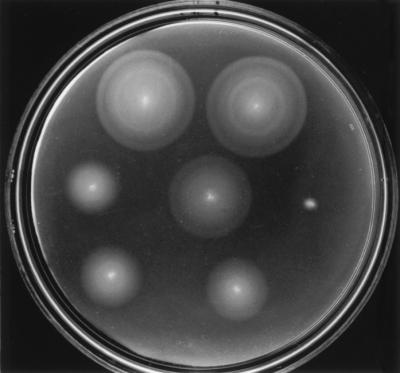
Selected examples of complementation by swarm assay. Top row left, pGS34 (wild type); top row right, pGS52 (second starts eliminated and stem-loop disrupted); middle row left, pGS60 (21-bp deletion in stem-loop); middle row center, pGS51 (first start attenuated, AUGAUG→AUAAUA); middle row right, pGS67 (first start eliminated, AUGAUG→CGCCGC); bottom row left, pGS74 (first start and intervening sequence eliminated); bottom row right, pGS50 (first start and intervening sequence eliminated; second start enhanced, GUG→AUG). Plates were incubated for 7 h at 30°C.
All plasmids that had the entire fliO gene intact, from the first start at the interface with fliN through to the terminus at the interface with fliP, complemented fully, producing essentially wild-type swarming. This was true when the second start(s) had been altered to noninitiation codons and when the stem loop had been disrupted without altering the amino acid sequence. Where a 21-bp deletion had been made in the vicinity of the stem-loop (plasmid pGS60), however, complementation was poor. This result is not surprising since the deletion causes the loss of the first four amino acids of the predicted transmembrane helix, shortening it from 19 amino acids to 15; extension of the helix in the C-terminal direction is highly unlikely since the next two amino acids are Lys and Arg.
Where the first start had been changed from AUGAUG to AUAAUA (plasmid pGS51), complementation occurred at essentially wild-type levels. Where it had been changed to CGCCGC, the plasmid (pGS67) did not complement at all.
Where the possibility of using the first start and the intervening region between the two starts had been totally eliminated, by placing the second start at the beginning of the plasmid insert, well positioned to a strong ribosome binding consensus, complementation was fair if the second start had been left as GUG (plasmid pGS74) and slightly better if it had been changed to AUG (pGS50).
Is there a species difference between S. typhimurium and E. coli in terms of fliO expression and complementation?
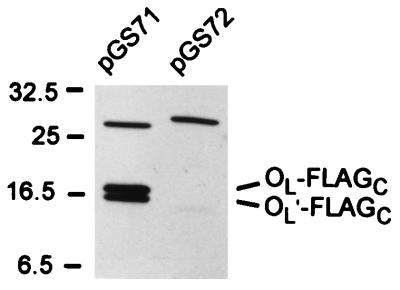
The previous study of E. coli fliO (12) did not contain data on protein production or complementation when the plasmid insert began at the first start. We therefore generated plasmid pGS71, whose insert is the E. coli equivalent of pGS34 (i.e., it encodes the entire fliO gene from the first start), and plasmid pGS72, whose insert is the E. coli equivalent of pGS74 (i.e., it encodes the fliO gene from the second start). In immunoblots (Fig. 6), plasmid pGS71 gave strong bands at the positions expected for FliOL-FLAGC and FliOL′-FLAGC but no band at the position expected for FliOS-FLAGC. As expected, plasmid pGS72 failed to give bands corresponding to FliOL-FLAGC and FliOL′-FLAGC; it also failed to give a band corresponding to FliOS-FLAGC. Complementation with pGS71 was comparable to that of pGS34; complementation with pGS72, where only the second start was present, was considerably poorer. The success of Malakooti et al. (12) in detecting FliOS and our failure to do so unless we optimized the start codon are probably reflections of different expression levels as a result of the different vectors used rather than a fundamental difference between the two species. It is clear that both pGS74 (S. typhimurium) and pGS72 (E. coli) sustained production of FliOS-FLAGC at levels sufficient to provide some function, even though these levels were too low to be detected biochemically.
FIG. 6.
Immunoblotting with anti-FLAG antibody in minicell expression experiments with FLAG-tagged plasmids containing E. coli fliO inserts. pGS71 and pGS72 are the E. coli equivalents of pGS34 and pGS74, respectively. The bands corresponding to FliOL-FLAGC and FliOL′-FLAGC are indicated to the right. The band in the 25-kDa range is nonspecific (cf. Fig. 4) and was also seen with the vector. The slight shift in its apparent molecular mass between the two lanes is a gel artifact, seen also with a fainter nonspecific band at a higher apparent molecular mass (not shown). Positions of molecular mass markers (in kilodaltons) are shown to the left.
What is the identity of FliOL′?
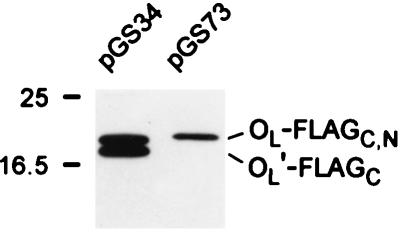
We described above some constraints on the identity of FliOL′. It has an intact FliO C terminus (since it could be detected with anti-FLAG antibody) and was still seen when the stem-loop structure has been disrupted but not when seven amino acids in the stem loop had been deleted. This would seem to leave only a few possibilities: N-terminal cleavage, proteolysis, or modification; or internal deletion or modification. To distinguish among these possibilities, we attached a FLAG tag to the N terminus of FliOL, to give plasmid pGS73. A comparison between immunoblots of pGS34 (with the FLAG tag at the C terminus) and pGS73 revealed that in the latter the band corresponding to FliOL-FLAGN could be clearly seen but there was no evidence for a band corresponding to FliOL′-FLAGN (Fig. 7). We conclude that FliOL′ is the result of N-terminal proteolytic cleavage but have no reason to believe that it has any physiological significance.
FIG. 7.
Immunoblotting with anti-FLAG antibody in minicell expression experiments with C-terminally and N-terminally FLAG-tagged versions of FliO (plasmids pGS34 and pGS73, respectively). The bands corresponding to FliOL-FLAGC and FliOL-FLAGN, and to FliOL′-FLAGC are indicated to the right; note the absence of a band corresponding to FliOL′-FLAGN in the pGS73 lane, indicating that the N-terminal FLAG tag has been lost in the modification process. Positions of molecular mass markers (in kilodaltons) are shown to the left.
DISCUSSION
What does the N-terminal sequence of FliO contribute to function?
In complementation tests, full-length FliO was clearly superior to the N-terminally truncated version, and therefore we feel justified in viewing the first translational start as being the primary one. Nonetheless, the short form of FliO did support swarming to a considerable degree (and did so even when the cellular levels were too low to be detected, even by immunoblotting [Fig. 4, lane 8, pGS74]).
What permits the short form to be functional? Or, put another way, why is a sequence of 21 amino acids at the N terminus not absolutely essential for function? FliO is predicted (1) to have a single membrane span, with the window of maximum hydrophobicity starting around residue 22. Thus N-terminal truncation by 21 amino acids would not be expected to interfere severely with membrane insertion; if the N-terminal sequence functions primarily as a cytoplasmic anchor and does not have any additional role, it may be that the short N-terminal cytoplasmic sequence of the second-start version of the protein suffices, but that the full sequence is superior. Specifically, the full-length version of the cytoplasmic anchor contains charged residues (one in the case of E. coli and two in the case of S. typhimurium), whereas the truncated version has none. It is interesting that the amino acid sequence of the N-terminal sequence of FliO in the two species is much less well conserved (9 of 21 amino acid identities) than the sequence that constitutes the predicted transmembrane helix (16 of 17 identities plus a conservative substitution).
Thus, the N-terminal cytoplasmic domain may play a general structural role, whereas the membrane span and the periplasmic domain are more critical. It is striking how precisely the position of the second start corresponds to the boundary between the N-terminal aqueous domain and the transmembrane span, and how sharp the transition is between poorly conserved and highly conserved amino acid sequence.
Does translation from the second start play a physiologically significant role in the wild-type cell?
If translation from the first start site results in a superior product, are the second start site and its accompanying stem-loop feature sequence simply accidents? We do not think so, for two reasons.
The first is that E. coli and S. typhimurium both possess the second start and the stem-loop sequence, yet the stem-loop feature is not conserved in detail. Specifically, the stem-loop structures (Fig. 1) differ with respect to (i) their detailed sequences, (ii) the length of the stem, (iii) the size of the loop, and (iv) the precise position of the start codon with respect to the loop. It is hard to imagine how this would arise if the second start site and the stem loop themselves were not important.
The second is that there is a well-studied case of tandem translation starts, in the cheA gene (which encodes an essential component in the chemotaxis signal transduction system). The situation with cheA closely resembles the present one in that the second start exists at the 3′ end of the loop of a stem-loop structure (8), the second start and the stem-loop structure are both present in both E. coli and S. typhimurium, and the stem-loops are divergent in their details.
For the above reasons, we conclude that there is likely to be a real physiological role associated with the tandem starts of fliO, of the stem-loop structure, and perhaps also of the short form of FliO, even though we do not have any clear idea of what these functions are.
A comparison with the tandem start system of CheA.
Tandem translational start systems are not common in prokaryotes. The best-studied example is that of CheA. It is instructive to compare its properties with those that we have found for FliO. What do they have in common, and in what ways do they differ?
(i) The CheA and FliO tandem start systems exist in both E. coli and S. typhimurium.
As with FliO, the distances between the two starts are large (ca. 21 codons for the FliO system; ca. 97 codons for the CheA system), but they are not identical between E. coli and S. typhimurium. The second start is covered by a stem-loop sequence, with the second start codon residing at the junction of the 3′ end of the loop and the 3′ arm of the stem. Again, the exact position is not conserved between species, nor are the details of the stem or of the loop. However, for both genes in both species the qualitative features are remarkably similar.
(ii) Relative amounts of the two forms.
CheAL and CheAS are synthesized in approximately a 10:1 ratio (8), although the ratio varies depending on such issues as the relative frames of cheA and the upstream gene motB. In contrast, we found that the FliOL/FliOS ratio is much higher than this. In fact, we were unable to detect FliOS in any construct where FliOL was being synthesized, and were only able to detect it, and to obtain complementation from it, when the entire intervening region had been eliminated. However, we have not extensively examined context issues, and our constructs for translating from the first start were optimized and may have been producing much more FliOL than is produced from the chromosome or is required for normal function.
(iii) The short forms of CheA and FliO are largely dispensable.
With both CheA and FliO, the second start can be eliminated with at most a marginal effect. Sanatinia et al. (17) replaced the second start codon (AUG) of cheA with a variety of other codons and examined the effect on swarming. Provided the substitutions were conservative (e.g., Met→Ile), swarming was about 80% of the wild-type level. We have obtained similar results with FliO.
(iv) The basis for function of the two forms of CheA and FliO.
CheA, an autokinase, is a complex multidomain protein and functions as a dimer. Because CheAS completely lacks the domain that contains the phosphorylation site, His48, it cannot function in the absence of CheAL. CheAL-CheAS heterodimers, however, function normally, with CheAS providing the catalytic (kinase) domain and transphosphorylating the acceptor domain of CheAL (22). There are also indications that CheAS has some distinctive properties of its own; for example, it inhibits the chemotaxis process by enhancing dephosphorylation of CheY by CheZ (21).
The situation with FliO is different. Both FliOL alone and FliOS alone can provide all of the essential elements for function, although FliOL is significantly better at doing so. Why then does the translational start for synthesizing FliOS (and the associated stem-loop structure) exist? Why does this form of the protein exist? As was stated above, we do not accept the hypothesis that it is an accident. Although in the laboratory it appears to be dispensable, we must assume that in the wild there are circumstances where either it is produced in larger amounts or it provides a functional advantage over FliOL. Alternatively, it may primarily represent a regulatory feature, as the stem-loop feature would indicate. Sanatinia et al. (17) have suggested, for example, that the short form of CheA may be deleterious if produced in too large amounts and that the stem-loop structure serves to down-regulate translation from the second start. This, however, seems an elaborate evolutionary strategy when a simple conservative mutation at the second start codon could probably achieve the same goal. It is clear that with FliO, as with CheA, there remains an unsolved mystery concerning these tandem starts.
There is another case in the flagellar regulon where translation from an internal start provides a substantial degree of function. This is the fliN gene, which lies immediately upstream of fliO. Irikura et al. (6) noted that several frameshifts early on in the S. typhimurium fliN gene all led to termination at the same codon yet did not give the expected nonflagellate phenotype; a potential second (in-frame) start lay just downstream of the termination site. Subsequently, Tang et al. (19) established that an N-terminally truncated version of E. coli FliN using that second start was substantially functional. The role of the N-terminal sequence remains unclear. There is, however, a distinction, between the FliN case and the cases of CheA and FliO, in that the former does not have a stem-loop sequence blanketing the second start.
AUA can be an effective codon for initiation of translation.
Although this study had no intention of exploring the issue of start codon usage, it did produce an unexpected result in this regard. When we replaced the AUGAUG double start at the first start by AUAAUA, we expected this would prevent translation from that start. Instead, FliOL and its related product FliOL′ were observed at the same positions as with the AUGAUG version, with at most a threefold reduction in intensity. (Substitution by CGCCGC, in contrast, resulted in no detectable FliO products.) These results implied that at least under our conditions, AUA was almost as effective a start codon as AUG. We subsequently uncovered several similar examples in the literature. Peijnenburg et al. (15) created a gene overlap AUAG between the penicillinase gene (penP) and the β-galactosidase gene (lacZ) in both E. coli and Bacillus subtilis and found that translation initiation of LacZ from the AUA codon proceeded with reasonable efficiency. Romero and Garcia (16) cloned a phage murein hydrolase gene and tested expression from all four AUN triplets; AUA was the best of the unusual start codons, functioning with about 7.5% of the efficiency of AUG. Köpke and Leggatt (9) showed that in an archaeal gene, a natural AUA codon downstream and in frame with an AUG codon was recognized by E. coli ribosomes. Finally, Mulero and Fox (13) noted a low but accurate translation from a mutant AUA codon in mitochondrial mRNA from Saccharomyces cerevisiae. From these and our data, it is clear that AUA can function as a reasonably efficient start codon. Why it is not used more commonly in nature is not clear. One reason may be that initiation of translation from a sense codon for isoleucine within a coding sequence could obviously have undesirable consequences.
ACKNOWLEDGMENTS
We thank Gabriele Miller for technical assistance.
This work was supported by USPHS grant AI12202.
REFERENCES
- 1.Engelman D M, Goldman A, Steitz T A. The identification of helical segments in the polypeptide chain of bacteriorhodopsin. Methods Enzymol. 1982;88:81–88. [Google Scholar]
- 2.Fan F, Ohnishi K, Francis N R, Macnab R M. The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol. 1997;26:1035–1046. doi: 10.1046/j.1365-2958.1997.6412010.x. [DOI] [PubMed] [Google Scholar]
- 3.Fickett J W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982;10:5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 5.Homma M, Kutsukake K, Iino T. Structural genes for flagellar hook-associated proteins in Salmonella typhimurium. J Bacteriol. 1985;163:464–471. doi: 10.1128/jb.163.2.464-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irikura V M, Kihara M, Yamaguchi S, Sockett H, Macnab R M. Salmonella typhimurium fliG and fliN mutations causing defects in assembly, rotation, and switching of the flagellar motor. J Bacteriol. 1993;175:802–810. doi: 10.1128/jb.175.3.802-810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones C J, Homma M, Macnab R M. L-, P-, and M-ring proteins of the flagellar basal body of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989;171:3890–3900. doi: 10.1128/jb.171.7.3890-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kofoid E C, Parkinson J S. Tandem translation starts in the cheA locus of Escherichia coli. J Bacteriol. 1991;173:2116–2119. doi: 10.1128/jb.173.6.2116-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köpke A K E, Leggatt P A. Initiation of translation at an AUA codon for an archaebacterial protein gene expressed in E. coli. Nucleic Acids Res. 1991;19:5169–5172. doi: 10.1093/nar/19.19.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macnab R M. The end of the line in bacterial sensing: the flagellar motor. Cold Spring Harbor Symp Quant Biol. 1988;53:67–75. doi: 10.1101/sqb.1988.053.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Macnab R M. Flagellar switch. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 181–199. [Google Scholar]
- 12.Malakooti J, Ely B, Matsumura P. Molecular characterization, nucleotide sequence, and expression of the fliO, fliP, fliQ, and fliR genes of Escherichia coli. J Bacteriol. 1994;176:189–197. doi: 10.1128/jb.176.1.189-197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulero J J, Fox T D. Reduced but accurate translation from a mutant AUA initiation codon in the mitochondrial COX2 mRNA of Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:383–390. doi: 10.1007/BF00281787. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi K, Fan F, Schoenhals G J, Kihara M, Macnab R M. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J Bacteriol. 1997;179:6092–6099. doi: 10.1128/jb.179.19.6092-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peijnenburg A A C M, Venema G, Bron S. Translational coupling in a penP-lacZ gene fusion in Bacillus subtilis and Escherichia coli. Use of AUA as a restart codon. Mol Gen Genet. 1990;221:267–272. doi: 10.1007/BF00261730. [DOI] [PubMed] [Google Scholar]
- 16.Romero A, Garcia P. Initiation of translation at AUC, AUA and AUU codons in Escherichia coli. FEMS Microbiol Lett. 1991;84:325–330. doi: 10.1016/0378-1097(91)90377-m. [DOI] [PubMed] [Google Scholar]
- 17.Sanatinia H, Kofoid E C, Morrison T B, Parkinson J S. The smaller of two overlapping cheA gene products is not essential for chemotaxis in Escherichia coli. J Bacteriol. 1995;177:2713–2720. doi: 10.1128/jb.177.10.2713-2720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki T, Komeda Y. Incomplete flagellar structures in Escherichia coli mutants. J Bacteriol. 1981;145:1036–1041. doi: 10.1128/jb.145.2.1036-1041.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H, Billings S, Wang X, Sharp L, Blair D F. Regulated underexpression and overexpression of the FliN protein of Escherichia coli and evidence for an interaction between FliN and FliM in the flagellar motor. J Bacteriol. 1995;177:3496–3503. doi: 10.1128/jb.177.12.3496-3503.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thein S L, Wallace R B. The use of synthetic oligonucleotides as specific hybridization probes in the diagnosis of genetic disorders. In: Davis K E, editor. Human genetic diseases: a practical approach. Herndon, Va: IRL Press; 1986. pp. 33–50. [Google Scholar]
- 21.Wolfe, A. J. 1997. Personal communication.
- 22.Wolfe A J, Stewart R C. The short form of the CheA protein restores kinase activity and chemotactic ability to kinase-deficient mutants. Proc Natl Acad Sci USA. 1993;90:1518–1522. doi: 10.1073/pnas.90.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]