Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Syngenta Crop Protection AG submitted a request to the competent national authority in Greece to set an import tolerance for the active substance lambda‐cyhalothrin in avocados. The data submitted in support of the request were found to be sufficient to derive maximum residue level (MRL) proposals for avocados. Since the general data gap related to toxicity of degradation products formed under sterilisation conditions and identified in the framework of the MRL review has not yet been addressed, a risk management decision is required as to whether it is appropriate to take over the proposed MRLs in the MRL legislation. Adequate analytical methods for enforcement are available to control the residues of lambda‐cyhalothrin in the commodity under consideration at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of lambda‐cyhalothrin according to the reported agricultural practice is unlikely to present a risk to consumer health. However, the consumer exposure calculation shall be considered provisional, pending the toxicological assessment of the compounds formed under sterilisation conditions.
Keywords: avocados, consumer risk assessment, lambda‐cyhalothrin, MRL, pesticide
SUMMARY
In accordance with Article 6 of Regulation (EC) No 396/2005, Syngenta Crop Protection AG submitted an application to the competent national authority in Greece (Rapporteur Member State, RMS) to set an import tolerance for the active substance lambda‐cyhalothrin in avocados.
The application, alongside the dossier containing the supporting data in IUCLID format, was submitted through the EFSA Central Submission System on 20 January 2022. The appointed RMS, Greece, assessed the dossier and declared its admissibility on 26 October 2022. Subsequently, following the implementation of the EFSA's confidentiality decision, the non‐confidential version of the dossier was published by EFSA and a public consultation was launched on the dossier. The consultation aimed to consult stakeholders and the public on the scientific data, studies and other information part of, or supporting, the submitted application, in order to identify whether other relevant scientific data or studies are available. The consultation run from 5 May 2023 to 26 May 2023. No additional data nor comments were submitted in the framework of the consultation.
At the end of the commenting period, the RMS proceeded to draft the evaluation report, in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 31 July 2023. The RMS proposed to establish maximum residue level (MRL) for avocados imported from Mexico at the level of 0.15 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified points that needed further clarification, and requested the RMS to address them. The additional information was duly considered by the RMS who submitted a revised evaluation report to EFSA on 1 September 2023, which replaced the previously submitted evaluation report.
Based on the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009 in accordance with Commission Regulation (EU) No 1141/2010, the data evaluated in previous MRL assessments and the additional data provided by the RMS in this application, the following conclusions are derived.
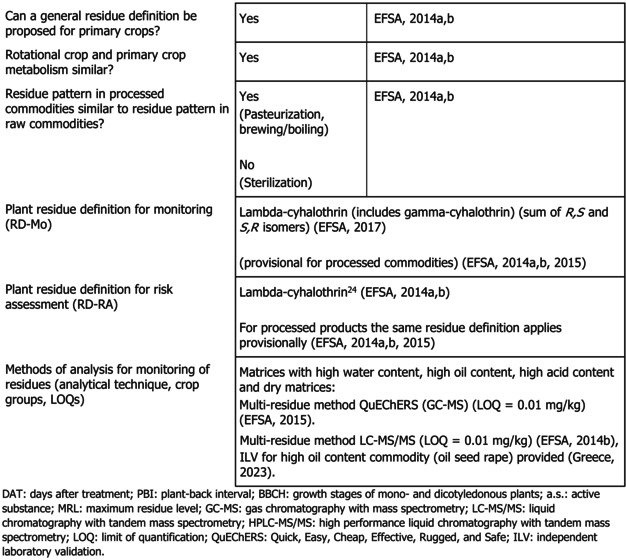
The metabolism of lambda‐cyhalothrin in primary and rotational crops was sufficiently investigated in four different crop category groups. Studies investigating the effect of processing on the nature of lambda‐cyhalothrin (hydrolysis studies) demonstrated that the active substance is stable under pasteurisation and baking, brewing and boiling but extensively degrades under sterilisation conditions, forming the degradation products Ia, IV and gamma‐lactone. Following the MRL review, European Commission set a general footnote in the MRL legislation to all commodities, highlighting that information on compounds Ia, IV and gamma‐lactone which are formed under sterilisation conditions was unavailable, and that it should be submitted by 6 July 2020 in order to be taken into account in an appropriate confirmatory data assessment. At the moment, this point has not been addressed yet.
As the use of lambda‐cyhalothrin under assessment is on a permanent crop and refers to a use authorised in a third country, investigations of residues in rotational crops are not required.
Based on the results of the metabolism studies, the hydrolysis studies and taking into account that analytical enforcement methods do not allow to discriminate between individual cyhalothrin isomers/mixture of isomers, the residue definition for enforcement for all plant commodities has been set as ‘lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers)’. The residue definition for risk assessment is lambda‐cyhalothrin as derived during the MRL review and peer review.
The residue definition was set on a tentative basis for processed products, pending the assessment of toxicological properties of compounds Ia, IV and gamma‐lactone, formed under conditions simulating sterilisation. EFSA concluded that for avocados, the metabolism of lambda‐cyhalothrin in primary and in rotational crops has been sufficiently addressed and that the previously derived residue definitions are applicable. The assessment of data to characterise the toxicological profile of degradation products formed after sterilisation has not been addressed yet. However, since avocadoes are mostly consumed raw and sterilisation of avocados is less common, the general data gap is of lower relevance for this commodity.
Sufficiently validated analytical multiresidue methods are available to quantify residues of lambda‐cyhalothrin in avocados according to the enforcement residue definition. The methods enable quantification of residues at or above 0.01 mg/kg (limit of quantification [LOQ]).
Extraction efficiency of enforcement and data generation method was not considered proven for high‐oil content commodities (relevant for avocados) according to the recent European Commission technical guideline, based on the information provided. However, it is reported by the EMS that a data package on extraction efficiency covering the information provided for this assessment should be provided for the renewal of approval assessment. Therefore, it is to be noted that the conclusions reported in this reasoned opinion might need to be reconsidered in the context of the outcome of the peer review assessment.
The available residue trials are sufficient to derive MRL proposal of 0.15 mg/kg for avocados.
Processing factor (PF) for avocados was derived from the submitted residue trials and is recommended to be included in Annex VI of Regulation (EC) No 396/2005 as follows:
-
•
Avocado whole fruit/pulp: 0.42
Additional studies investigating the magnitude of lambda‐cyhalothrin residues in processed commodities are not required considering the low individual contribution of avocados to the total dietary intake (< 0.07% of the ADI) for the use under assessment.
Residues of lambda‐cyhalothrin in commodities of animal origin were not assessed since avocados are normally not fed to livestock.
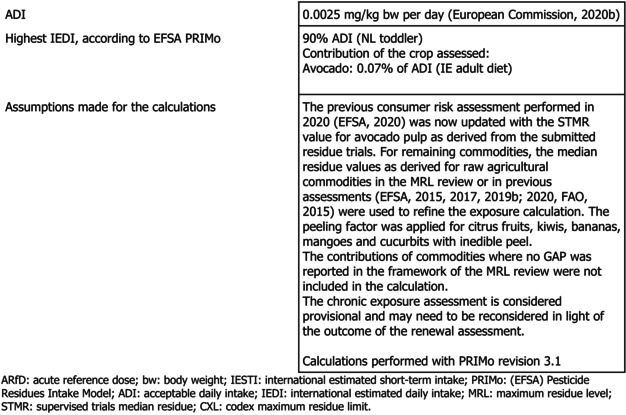
The toxicological profile of lambda‐cyhalothrin was assessed in the framework of the EU pesticides peer review for renewal of the approval under Regulation (EC) No 1107/2009 in accordance with Commission Regulation (EU) No 1141/2010. The data were sufficient to derive an acceptable daily intake (ADI) of 0.0025 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.005 mg/kg bw.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo) for the current residue definition for risk assessment. The estimated long‐term intake was 91% of the ADI (NL toddler diet). The contribution of residues in avocados to the overall consumer exposure was low (0.07% of the ADI [IE adult]). The estimated short‐term exposure conducted according to the currently agreed methodology did not exceed the ARfD for avocados (maximally 10.1% of the ARfD [DE child]).
EFSA concluded that the proposed import tolerance of lambda‐cyhalothrin on avocados will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers' health.
EFSA derived MRL proposals as reported in the summary table below.
The conclusions reported in this reasoned opinion and the consumer risk assessment might need to be reconsidered in light of the outcome of the renewal of the approval of lambda‐cyhalothrin.
EFSA proposes to amend the existing MRL as reported in the summary table below.
Full details of all end points and the consumer risk assessment can be found in Appendices B, C–D.
| Code a | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition:Lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers) F | ||||
| 0163010 | Avocados | 0.01* (ft) |
0.15 (ft) Further risk management considerations necessary |
The submitted data are sufficient to derive an import tolerance (Mexican GAP). Risk for consumers unlikely The tolerance in Mexico for lambda‐cyhalothrin in avocados is 0.2 mg/kg. A Codex MRL for lambda‐cyhalothrin in avocados is currently not set It is noted that a general data gap on toxicological data for degradation products observed in standard hydrolysis studies representative for sterilisation conditions has been introduced in the EU MRL legislation for all food commodities. This point has not yet been addressed. Considering that avocados are mostly consumed unprocessed, the data gap is considered of lower relevance. However, further risk managers discussions are recommended |
Abbreviations: GAP, Good Agricultural Practice; MRL, maximum residue level; NEU, northern Europe; SEU, southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
ft: The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
ASSESSMENT
The European Food Safety Authority (EFSA) received an application to set an import tolerance for the active substance lambda‐cyhalothrin in avocados. The detailed description of the existing use of lambda‐cyhalothrin authorised in Mexico in avocados, which is the basis for the current MRL application, is reported in Appendix A.
Lambda‐cyhalothrin is the ISO common name for the 1:1 mixture of (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxy‐benzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate (IUPAC). It represents a 1:1 mixture of two of the four components of the insecticide cyhalothrin: the R,S‐ and the S,R isomers. The isomer S,R alone is the active substance gamma‐cyhalothrin, which is also approved for use in plant protection products. The chemical structures of the active substance and its main metabolites are reported in Appendix E.
Lambda‐cyhalothrin 1 was included in Annex I to Directive 91/414/EEC 2 on 1 January 2002 by Commission Directive 2000/80/EC 3 and is deemed to be approved under Regulation (EC) No 1107/2009 in accordance with Commission Implementing Regulation (EU) No 540/2011. 4 The approval has been renewed by Commission Implementing Regulation (EU) 2016/146 5 which entered into force on 1 April 2016. The representative uses evaluated in the peer review for renewal were foliar spraying applications on wheat, potato, plum, peach and tomato. The renewal assessment report (RAR) has been peer reviewed by EFSA (EFSA, 2014b). Sweden acted as rapporteur Member State (RMS) in both the original and renewal approval procedures. Lambda‐cyhalothrin was approved for the use as an insecticide, but the applicant was requested to submit confirmatory information to the Commission, the Member States and EFSA by 1 April 2018. 6 The assessment of confirmatory data assessment has been performed (EFSA, 2020b). Lambda‐cyhalothrin has been included in the list of candidates for substitution.
EU MRLs for lambda‐cyhalothrin are established in Annex II of Regulation (EC) No 396/2005. 7 The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed (EFSA, 2014a) and revised in 2015 (EFSA, 2015) and the proposed modifications have been implemented in the MRL legislation. In 2017, EFSA issued a focused review on the assessment of the existing maximum residue levels (MRLs) for lambda‐cyhalothrin which might lead to consumers intake concerns based on the new toxicological reference values for gamma‐cyhalothrin and the data currently available to EFSA for lambda‐cyhalothrin (EFSA, 2017). After completion of the MRL review, EFSA has issued two reasoned opinions on the modification of MRLs for lambda‐cyhalothrin (EFSA, 2019b, 2020a). The proposals from these reasoned opinions have been considered in recent MRL regulation(s). 8 Certain Codex maximum residue limits (CXLs) for cyhalothrins (sum of all isomers) have been taken over in the EU MRL legislation; however, no Codex MRL has been set for avocados. 9 , 10 , 11
In accordance with Article 6 of Regulation (EC) No 396/2005 and following the provisions set by the ‘Transparency Regulation’ (EU) 2019/1381, 12 the applicant Syngenta Crop Protection AG submitted on 20 January 2022 an application to the competent national authority in Greece, alongside the dossier containing the supporting data using the IUCLID format.
The appointed RMS, Greece, assessed the dossier and declared its admissibility on 26 October 2022. Subsequently, following the implementation of the EFSA's confidentiality decision, the non‐confidential version of the dossier was published by EFSA and a public consultation was launched on the dossier. The consultation aimed to consult stakeholders and the public on the scientific data, studies and other information part of, or supporting, the submitted application, in order to identify whether other relevant scientific data or studies are available. The consultation run from 5 May 2023 to 26 May 2023. No additional data nor comments were submitted in the framework of the consultation.
At the end of the commenting period, the RMS proceeded to draft the evaluation report, in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 31 July 2023. The RMS proposed to establish maximum residue level (MRL) for avocados imported from Mexico at the level of 0.15 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified points that needed further clarification, which were requested from the RMS. The additional information was duly considered by the RMS who submitted a revised evaluation report to EFSA on 1 September 2023 (Greece, 2023), which replaced the previously submitted evaluation report.
EFSA based its assessment on the evaluation report submitted by the RMS (Greece, 2023), the renewal assessment report (RAR) and its addendum (Sweden, 2013, 2014) prepared in the framework of Commission Regulation (EU) No 1141/2010, 13 the revised Commission review report on lambda‐cyhalothrin (European Commission, 2020b), the conclusion on the peer review of the pesticide risk assessment of the active substance (EFSA, 2014b), the reasoned opinions related to the review of the existing MRLs for lambda‐cyhalothrin (EFSA, 2014a, 2015, 2017, 2019b, 2020a) and the EFSA scientific report on scientific support for preparing an EU position in the 48th Session of the Codex Committee on Pesticide Residues (CCPR) (EFSA, 2016).
For this application, the data requirements established in Regulation (EU) No 544/2011 14 and the guidance documents applicable at the date of submission of the IUCLID application are applicable (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2010, 2017, 2020a, 2021; OECD, 2007, 2011). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/2011. 15
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application, including the end points of relevant studies assessed previously, is presented in Appendix B.
The evaluation report submitted by the RMS (Greece, 2023) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion. 16
1. RESIDUES IN PLANTS
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of radiolabelled lambda‐cyhalothrin in primary crops belonging to the group of fruit crops, leafy crops, cereals/grass, pulses/oilseeds has been investigated in the framework of the EU pesticides peer review and the MRL review (EFSA, 2014a, 2014b).
In the crops tested, lambda‐cyhalothrin was the predominant residue (37%–95% total radioactive residue [TRR]) while compound Ia was identified as a significant metabolite in soyabeans and cotton leaves only (17%–25% TRR). Based on the chiral analysis on residue trial samples assessed in the framework of the EU pesticides peer review renewal, EFSA concluded that the impact of the change in the ratio of the isomers of the active substance on the toxicological burden the consumer is exposed to, was of low concern (EFSA, 2014a, 2014b).
For the authorised use assessed in this application, the metabolic behaviour in primary crops is sufficiently addressed.
1.1.2. Nature of residues in rotational crops
Investigations of residues in rotational crops are not required for imported crops.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of lambda‐cyhalothrin was investigated in the framework of the EU pesticides peer review and the MRL review (EFSA, 2014a, 2014b).
Lambda‐cyhalothrin remained stable under hydrolytic conditions representative of pasteurisation and baking, brewing and boiling (82%–91% TRR), while a significant degradation occurred under conditions simulating sterilisation. Hydrolytic cleavage of the parent molecule to form compound Ia, compound IV and gamma‐lactone was noted. The MRL review and the EU pesticides peer review considered toxicity of these compounds as not sufficiently addressed (EFSA, 2014a, 2014b, 2015). Therefore, for all crops which may be consumed after processing that were assessed in the MRL review, including avocados, a general data gap 17 was implemented in the EU legislation by Regulation (EU) 2018/960 18 as confirmatory data to be submitted by 6 July 2020. For avocados under assessment in this application, it is to be noted that while the general data gap applies, avocados are mostly consumed raw and the general data gap is considered of lower relevance for this commodity than for the others.
Nevertheless, since new studies investigating the toxicity of degradation products Ia, IV and gamma‐lactone were not provided in the framework of the current assessment, the Article 12 confirmatory data gap identified by the MRL review remains open. It is to be noted that avocados may be subject to the heating processes including sterilisation, and therefore, this data gap remains relevant also for the use under assessment. Furthermore, processing studies with avocado have not been submitted under the present assessment and there is no information on the magnitude of degradation products in processed commodities of avocados (see also Section 1.2.3).
1.1.4. Analytical methods for enforcement purposes in plant commodities
During the peer review under Regulation (EC) No 1141/2010, the QuEChERS multiresidue method (using GC–MS) and a LC–MS/MS multiresidue method were considered sufficiently validated for monitoring lambda‐cyhalothrin in plant commodities with high water content, high oil content, high acid content and in dry commodities (EFSA, 2014b, 2015). The methods enable quantification of residues at or above the LOQ of 0.01 mg/kg, however do not allow to distinguish the different isomers of lambda‐cyhalothrin (EFSA, 2014a, 2014b, 2015).
Additional method validation data
In this application, a new independent laboratory validation (ILV) was provided for the above‐mentioned multiresidue LC–MS/MS method in high oil content matrices thus addressing the initial data gap from the MRL review for such study. Residues from rape seeds were extracted with acetonitrile following the addition of water and were analysed by gas chromatography with mass spectrometric detection (GC–MS/MS) and subsequently two transitions, a primary and confirmatory transition, were monitored (Greece, 2023).
Overall, EFSA concludes that the QuEChERS multiresidue method (GC‐MS) and the multiresidue LC‐MS/MS method have been sufficiently validated for the determination of residues of lambda‐cyhalothrin in avocado with a limit of quantification (LOQ) of 0.01 mg/kg.
Extraction efficiency
With regard to extraction efficiency, the LC‐MS/MS multiresidue method uses acetonitrile:water (1/1, v/v) as a solvent system and the multiresidue GC‐MS method uses acetone:hexane (1/1, v/v) for extraction, followed by washing of the organic extract with water to remove acetone (Sweden, 2013; EFSA, 2014b).
In the metabolism studies, high‐oil commodities including soyabeans were subjected to combustion of individual beans (study report RJ0438B, 1986), whereby cotton seeds (study report RJ0393B, 1985) were extracted with hexane. The hexane extract was partitioned with acetonitrile and the pellet was further extracted with acetonitrile and subsequently 1 M hydrochloric acid (Greece, 2023).
However, in soyabeans and cotton seeds metabolism studies, the residue levels were too low for further identification and characterisation (EFSA, 2014a). According to the EMS, it can therefore not be concluded as to whether the parent was present in the extracts of the metabolism study (Greece, 2023).
Notwithstanding differences in the extraction systems used in the metabolism studies and in the multiresidue methods, it cannot be concluded that extraction efficiency of enforcement methods was demonstrated according to the extraction efficiency Technical Guideline (European Commission, 2017) because residues in the samples from the metabolism studies were too low for the characterisation of TRR, and therefore, these data cannot be used to conclude on the extraction efficiency and the requirements of the guidance could therefore not be addressed.
Further investigation on this matter would in principle be required. It is noted that extraction efficiency of the methods for enforcement needs to be assessed in the framework of the peer review for the renewal of approval of lambda‐cyhalothrin. Therefore, the conclusions reported in this reasoned opinion might need to be reconsidered in the context of the outcome of the peer review.
1.1.5. Storage stability of residues in plants
The storage stability of lambda‐cyhalothrin in plants stored under frozen conditions was investigated in the framework of the MRL review and the pesticides peer review (EFSA, 2014a, 2014b). It was demonstrated that in high water content, high oil content (relevant for the crop under assessment) and in dry matrices residues were stable for at least 26 months when stored at −18°C. New storage stability data were not submitted in the context of this application and are not necessary since avocado samples from the residue trials were stored for a time period not exceeding the demonstrated storage stability interval.
1.1.6. Proposed residue definitions
In the framework of the MRL review and peer review, residue definitions for lambda‐cyhalothrin only were derived (EFSA, 2014a, 2014b). It was noted that the enforcement residue definition is not specific to lambda‐cyhalothrin and cover also residues arising from uses of gamma‐cyhalothrin (EFSA, 2017). The residue definition for enforcement and risk assessment in all plants was proposed as:
Residue definition for enforcement: lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers) (EFSA, 2017).
Residue for risk assessment: lambda‐cyhalothrin (EFSA, 2014a, 2014b).
The residue definitions are applicable to primary crops. A specific residue definition for rotational crops is not deemed necessary (EFSA, 2014a). For processed commodities, the same residue definitions were proposed on tentative basis, pending the assessment of the toxicological properties of the degradation products formed under sterilisation conditions, i.e. compounds Ia, IV and gamma‐lactone (EFSA, 2014a, 2014b, 2015).
The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned residue definition.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In support of the authorised use of lambda‐cyhalothrin in Mexico, the applicant submitted residue trials on avocados. The samples were analysed for the parent compound according to the residue definitions for enforcement and risk assessment.
According to the assessment of the RMS, the LC‐MS/MS method (GRM043.10B) used to analyse residue trial samples was sufficiently validated and fit for purpose. Regarding extraction efficiency, this method uses acetone/hexane (50/50 v/v) extraction solvent. The solvent system is different to hexane which was used in the metabolism study of cotton seeds where the residues were too low to be further characterised (see Section 1.1.4 above). It can therefore not be concluded that the parent was present in the extract of the metabolism study in cotton seeds (Greece, 2023).
EFSA notes that the extraction efficiency of the analytical methods applied for residue trials is not proven as indicated according to the requirements of the extraction efficiency Technical Guideline, SANTE 2017/10632 GD (European Commission, 2017), and the lack of these data introduces additional uncertainty of the present assessment.
Avocado samples before analysis were stored under conditions for which integrity of the samples is demonstrated (Greece, 2023).
Authorised Mexican good agricultural practices (GAPs) on avocados: 1 × 42.4 g a.s./ha, PHI 14 days.
In support of the MRL application for avocados imported in the European Union, the applicant submitted four GAP compliant residue trials on avocado performed in Mexico during the 2020–2021 growing period. An adjuvant was not added. Trials were conducted as decline trials (PHIs of 0, 7, 14, 21 and 28 days). On the day of sampling, avocados were peeled and the pit was removed. All fractions (pulp, peel and pit) were weighed, the pit was discarded assuming that no residue would occur in it and the pulp and peel fractions were placed in frozen storage. Residues in whole avocado ranged from 0.011 to 0.065 mg/kg and in pulp from < 0.01 to 0.01 mg/kg. No residues of lambda‐cyhalothrin were detected at or above the limit of quantification of 0.01 mg/kg in any of the untreated samples except in one avocado peel sample where it was close to the LOQ (0.0102 mg/kg) (Greece, 2023).
The current residue data set is sufficient to derive an MRL proposal of 0.15 mg/kg for avocado in support of the authorised GAPs of lambda‐cyhalothrin on avocado in Mexico.
The tolerance established in Mexico for lambda‐cyhalothrin in avocado is 0.2 19 mg/kg.
1.2.2. Magnitude of residues in rotational crops
Investigations of residues in rotational crops are not required for imported crops.
1.2.3. Magnitude of residues in processed commodities
Studies investigating the magnitude of lambda‐cyhalothrin residues in processed avocados were not submitted in this MRL application. The residue trial data in the raw agricultural commodity relating to avocado pulp and peel demonstrated that peeling significantly reduces residues, resulting in residue levels at or below the LOQ of 0.01 mg/kg in avocado pulp (Greece, 2023). These data allow to derive robust peeling factor which is recommended to be included in Annex VI of Regulation (EC) No 396/2005.
Although processing studies are not required considering the low individual contribution of avocados to the total dietary intake (< 0.07% of the ADI, see Section 4 below), noting the high toxicity of the active substance and the unknown toxicity of degradation products Ia, IV and gamma lactone which are formed under sterilisation conditions (see Section 1.1.3), processing studies investigating the effect of processing on the magnitude of these compounds in sterilised avocado products would allow to conclude on the relevance of these degradation products in processed avocado commodities.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive an MRL proposal and, noting the lack of studies investigating the toxicity of degradation products Ia, IV and gamma‐lactone which are formed under sterilisation conditions, risk assessment values for the commodity under evaluation (see Appendix B). In Section 3 below, EFSA assessed whether residues on the crop resulting from the authorised use are likely to pose a consumer health risk.
2. RESIDUES IN LIVESTOCK
Not relevant as avocados are not used for feed purposes.
3. CONSUMER RISK ASSESSMENT
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019a). This exposure assessment model contains food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
The toxicological reference values for lambda‐cyhalothrin used in the risk assessment (ADI of 0.0025 mg/kg bw per day and ARfD of 0.005 mg/kg bw) were derived in the framework of the EU pesticides peer review (European Commission, 2020b).
Short‐term (acute) dietary risk assessment
The highest residue derived for the pulp of avocados from supervised field trials was used as an input value for the calculation of the acute exposure. For the remaining commodities, the risk assessment values were available from the MRL review and the EFSA outputs issued afterwards. The list of input values can be found in Appendix D.1.
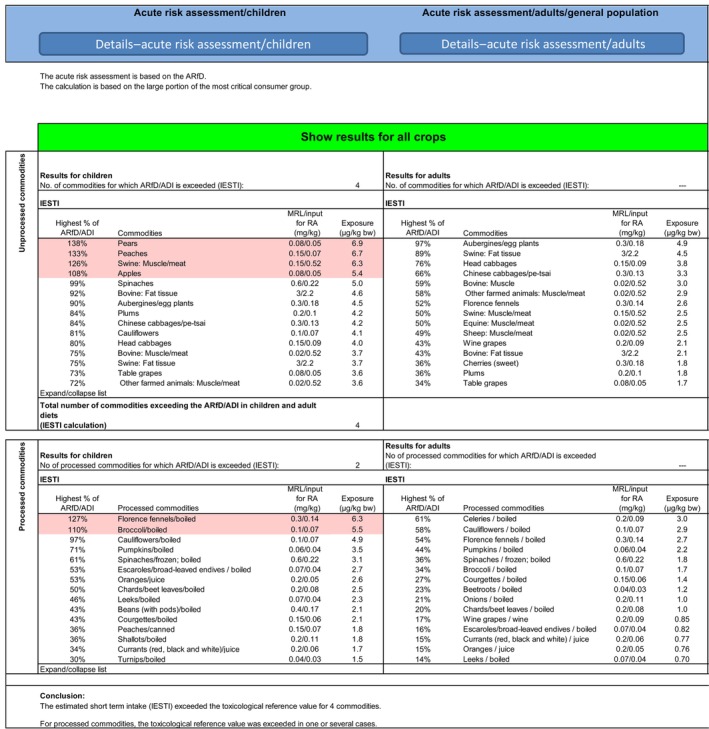
The acute exposure to residues of lambda‐cyhalothrin from the intake of avocados was 10.1% of the ARfD (DE child), not exceeding the ARfD (see Appendix B).
In a previous assessment (EFSA, 2020), EFSA identified possible short‐term intake concerns for table grapes, pears, peaches, apples and swine meat; in addition, exceedances of the ARfD were identified for boiled broccoli and fennel. 20
To address the acute intake concerns, EFSA made an attempt to refine the exposure calculations: For table grapes, 21 the current EU MRL is based on the critical SEU use assessed for lambda‐cyhalothrin in the MRL review (0.08 mg/kg with an HR of 0.05 mg/kg) (EFSA, 2015), and therefore, the use of a conversion factor of 2 to accommodate for the higher toxicity of gamma‐cyhalothrin as derived by a focused review (EFSA, 2017), which was also used in the previous EFSA assessments on lambda‐cyhalothrin (EFSA, 2019b, 2020), is not considered necessary. Hence, for this use, no acute exceedance of the reference dose is observed.
For swine muscle/meat, an exceedance of the acute reference dose is noted (126% ARfD). The current MRL is based on a CXL of 3 mg/kg expressed on fat basis with an HR of 2.2 mg/kg (referring to meat consisting of 20% fat and 80% muscle). In a previous assessment (EFSA, 2015), EFSA suggested a tentative EU MRL of 0.01* mg/kg for swine muscle, based on an European dietary burden calculation. With the related HR for swine meat (0.07 mg/kg), the expected exposure is below the ARfD.
For the remaining plant products for which the exposure calculation exceeds the ARfD using PRIMo 3.1, options for further refinement of the risk assessment shall be explored, e.g. in the context of the assessment of confirmatory data, 22 in the process of the renewal in the approval 23 or under a specific mandate.
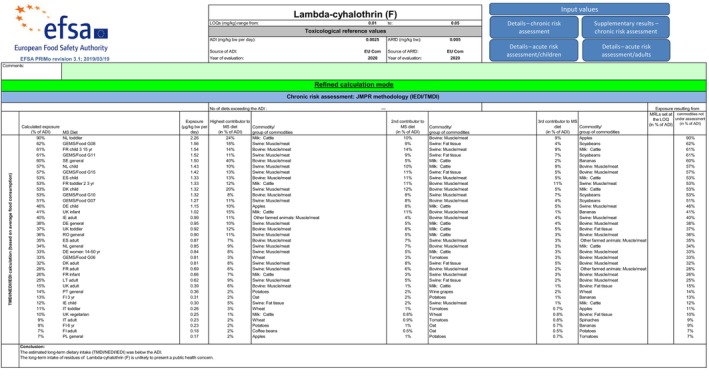
Long‐term (chronic) dietary risk assessment
In the framework of the MRL review, a comprehensive long‐term exposure assessment was performed using PRIMo rev 2.0, taking into account the existing uses at EU level and the acceptable CXLs (EFSA, 2014a, 2015). This exposure assessment has been updated several times with risk assessment values for the crops assessed in the EFSA outputs issued after the MRL review (EFSA, 2017, 2019b, 2020) and with the Codex MRLs implemented in the EU legislation (FAO, 2015). EFSA now updated the latest consumer risk assessment performed in 2020 with the supervised trials median residue (STMR) value for avocado pulp as derived from the submitted residue trials. Exposure calculations were performed with revision 3.1. of the EFSA PRIMo. The crops on which no uses were reported in the MRL review or in subsequent EFSA outputs were excluded from the exposure calculation.
The input values used in the exposure calculations are summarised in Appendix D.1.
The long‐term exposure did not exceed the ADI; the maximum calculated long‐term exposure accounted for 91% of the ADI (NL toddler) and the contribution of avocados was 0.07% ADI (IE adult) (see Appendix B.3).
For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
EFSA points out that the consumer risk assessment needs to be regarded as provisional pending the assessment of the confirmatory data related to the data gap on toxicological properties of the compounds Ia, IV and gamma‐lactone formed under sterilisation conditions, identified by the review of the existing MRLs for lambda‐cyhalothrin and taken over in EU legislation.
For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. CONCLUSION AND RECOMMENDATIONS
The data submitted in support of this MRL application were found to be sufficient to derive an MRL proposal for avocados in support of the authorised use of lambda‐cyhalothrin in Mexico.
A general data gap on toxicological data for degradation products observed in standard hydrolysis studies representative for sterilisation conditions has been introduced in the EU MRL legislation for all food commodities. This point has not yet been addressed. However, since sterilisation of avocados is not a common practice, the general data gap is considered to be of lower relevance for avocados. Further risk management discussions are recommended.
Possible short‐term intake concerns for some food commodities were identified in a previous EFSA assessment due to the use of a new version of PRIMo, which could not yet be addressed (i.e. for apples, pears, peaches, boiled fennel, boiled broccoli and swine meat). EFSA therefore recommends exploring the possible refinement of the exposure calculations in the context in the context of the assessment of confirmatory data, in the process of the renewal in the approval or under a specific mandate or to consider lowering the MRLs. To address the acute intake concern for swine muscle/meat, risk managers may consider as an option replacing the Codex MRL with the MRL of 0.01* mg/kg calculated for the European uses in feed items (EFSA, 2015).
EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of lambda‐cyhalothrin on avocados according to the authorised agricultural practice is unlikely to present a risk to consumer health. However, the consumer exposure calculation shall be considered provisional and might need to be reconsidered in light of the outcome renewal of the approval of lambda‐cyhalothrin.
The MRL recommendations are summarised in Appendix B.4.
ABBREVIATIONS
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CCPR
Codex Committee on Pesticide Residues
- CEN
European Committee for Standardisation (Comité Européen de Normalisation)
- CF
conversion factor for enforcement to risk assessment residue definition
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DP
dustable powder
- DS
powder for dry seed treatment
- DT90
period required for 90% dissipation (define method of estimation)
- dw
dry weight
- EC
emulsifiable concentrate
- ECD
electron capture detector
- EDI
estimated daily intake
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- ESI
electrospray ionisation
- EURL
EU Reference Laboratory (former Community Reference Laboratory (CRL))
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐ECD
gas chromatography with electron capture detector
- GC‐FID
gas chromatography with flame ionisation detector
- GC‐MS
gas chromatography with mass spectrometry
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- GLP
Good Laboratory Practice
- GR
granule
- GS
growth stage
- HPLC‐MS/MS
high performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- IPCS
International Programme of Chemical Safety
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint FAO/WHO Meeting on Pesticide Residues
- K oc
organic carbon adsorption coefficient
- LC
liquid chromatography
- LOD
limit of detection
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NPD
nitrogen/phosphorous detector
- OECD
Organisation for Economic Co‐operation and Development
- PAFF
Standing Committee on Plants, Animals, Food and Feed
- PBI
plant back interval
- PF
processing factor
- PHI
pre‐harvest interval
- Pow
partition coefficient between n‐octanol and water
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- Rber
statistical calculation of the MRL by using a non‐parametric method
- Rmax
statistical calculation of the MRL by using a parametric method
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- RPF
relative potency factor
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern Europe
- STMR
supervised trials median residue
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- WHO
World Health Organization
- ZC
mixed CS and SC formulation
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2022‐00758
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyrightholder and users should seek permission to reproduce the content from the original source.
ACKNOWLEDGEMENTS
EFSA wishes to thank Stathis Anagnos, Mavriou Galini, Matteo Lazzari and Elena Taglianini for the support provided to this scientific output.
APPENDIX A. Summary of intended GAP triggering the amendment of existing EU MRLs
A.1.
| Crop and/or situation | NEU, SEU, MS or country | F G or I a | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b | Conc. a.s. (g/kg) | Method kind | Range of growth stages and season c | Number min–max | Interval between application (days) min–max | g a.s./hL min–max | Water (L/ha) min–max | Rate min–max | Unit | ||||||
| Avocado | IT (Mexico) | F | Trips (Frankliniella occidentalis), leafhopper (Idona minuenda) | ZC | 106 | Foliar spray | At detection of first adults | 1 | – | 4.24 | 1000 | 42.4 | g a.s./ha | 14 | ZC: combined SC for lambda‐cyhalothrin and CS for thiamethox) |
Abbreviations: a.s., active substance; CS, capsule suspension; GAP, Good Agricultural Practice; MRL, maximum residue level; MS, Member State; NEU, northern European Union; SEU, southern European Union; SC, suspension concentrate; ZC, a mixed formulation of CS and SC.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum pre‐harvest interval.
APPENDIX B. List of end points
B.1. Residues in plants
B.1.1. Nature of residues and analytical methods for enforcement purposes in plant commodities
B.1.1.1. Metabolism studies, analytical methods and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/source |
|---|---|---|---|---|---|
| Fruit crops | Apple | Spotting onto fruit, 33 μg/fruit | 0, 7, 14, 28, 56 DAT | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Tomato | Foliar, 4 × 100 g/ha | 3 DALA | [cyclopropyl‐14C] and [phenoxy‐14C]‐Lambda‐cyhalothrin (EFSA, 2014b) | ||
| Leafy crops | Cabbage | Spotting onto crop, 26 μg/leaf | 2, 4, 5, 6, 7 weeks after application | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Foliar, 4–8 × 55 g/ha | 7 DALA | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a, 2014b) | |||
| Cereals/grass | Wheat | Foliar, 2 × 224 g/ha | 14, 85 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐Lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Foliar, 3 × 224 g/ha | 30 DALA | ||||
| Pulses/oilseeds | Soyabeans | Foliar, 2 × 20 g/ha | 39, 51 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐Lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Cotton | Foliar, 3 × 66 g/ha | 30, 50 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐Lambda‐cyhalothrin (EFSA, 2014a, 2014b) | ||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
| Root/tuber crops | Carrot | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |||
| Leafy crops | Lettuce | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |||
| Cereal (small grain) | Wheat | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |||
| Other | |||||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | [cyclopropyl‐14C]‐ and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a, 2014b) | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||||
| Sterilisation (20 min, 120°C, pH 6) | No | Extensive degradation of the parent to form compounds Ia (59% TRR), IV (63% TRR) and gamma‐lactone (15% TRR) (EFSA, 2014a, 2014b) | |||
| Other processing conditions | – | ||||
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Apple, peach, sugar beet root, cabbage, potato, green peas | −18 | 26 | Months | Parent | EFSA (2014a) | |
| High oil content | Cotton seed, rape seed | −18 | 26 | Months | Parent | EFSA (2014a) | |
| Hops | –18 | 8 | Months | Parent | EFSA (2014a) | ||
| Dry/high starch | Wheat grain | –18 | 26 | Months | Parent | EFSA (2014a) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region a | Residue levels observed in the supervised residue trials (mg/kg) | Comments/source | Calculated MRL (mg/kg) | HR b (mg/kg) | STMR c (mg/kg) | CF d |
|---|---|---|---|---|---|---|---|
| Avocado [0163010]Authorised use:1 × 42.4 g a.s./ha at detection of first adults of trips/leaf hopper, PHI: 14 days | Mexico | Residue trials on avocado compliant with the authorised GAP. Residues in whole fruits were calculated considering the weights of the whole fruits and individual fractions (peel, pulp and pit) whereby it was assumed that no residues occur in the pit (Greece, 2023) | 0.15 |
Mo: 0.065 RApulp: 0.01 |
Mo: 0.025 RApulp: 0.01 |
1 |
Abbreviations: GAP, Good Agricultural Practice; Mo, monitoring; MRL, maximum residue level; RA, risk assessment.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, northern Europe, EU or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
The residue values for whole fruits (mg/kg) were calculated by adding the residue of pulp (mg/kg) multiplied by the weight of the pulp fraction to the residues in peel (mg/kg) multiplied by the weight of the peel fraction and by dividing the sum through the weight of the whole fruits. It was assumed that no residue would occur in pits and pits were discarded after having been weighed. The following weights of whole fruits, peel and pulp were reported for the four trials: trial 1: whole fruits 3.155 kg, pulp: 2.274 kg, peel: 0.468 kg and pit: 0.376 kg; trial 2: whole fruits 2.600 kg, pulp: 1.724 kg, peel: 0.662 kg and pit: 0.325 kg; trial 3: whole fruits 3.540 kg, pulp: 2.387 kg, peel: 0.411 kg and pit: 0.671 kg; trial 4: whole fruits 2.660 kg, pulp: 1.741 kg, peel: 0.455 kg and pit: 0.455 kg. It is to be noted that in the first and third trial residues in the pulp were below the LOQ of 0.01 mg/kg and therefore for each of these two trials the residues in peel were multiplied by the weight of the peel fraction and divided by the weight of whole fruits, respectively to derive the residues in whole fruits.
Higher residue at longer PHI of 28 days.
Higher residue at longer PHI of 21 days.
B.1.2.2. Residues in rotational crops

B.1.2.3. Processing factors
| Processed commodity | Number of valid studies a | Processing factor (PF) | CFP b | Comment/source | |
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| Avocado whole fruit, pulp | 4 | < 0.14; < 0.33; < 0.5; 1 | < 0.42 | 1 | Greece (2023) |
Abbreviation: PF, processing factor.
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Conversion factor for risk assessment in the processed commodity; median of the individual conversion factors for each processing residues trial.
B.2. Residues in livestock
Not relevant because avocados are not a feed item.
B.3. Consumer risk assessment

B.4. Recommended MRLs
| Code a | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers) F | ||||
| 0163010 | Avocados | 0.01* (ft) |
0.15 (ft) Further risk management considerations necessary |
The submitted data are sufficient to derive an import tolerance (Mexican GAP). Risk for consumers unlikely The tolerance in Mexico for lambda‐cyhalothrin in avocados is 0.2 mg/kg. A Codex MRL for lambda‐cyhalothrin in avocados is currently not set A risk management decision is required as to whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
Abbreviations: GAP, Good Agricultural Practice; MRL, maximum residue level; NEU, northern Europe; SEU, southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
ft: The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
APPENDIX C. Pesticide Residue Intake Model (PRIMo)
C.1.
<The PRIMO image of the report sheet will be included here by the publisher before publication of the output>.
APPENDIX D. Input values for the exposure calculations
D.1. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | |||
|---|---|---|---|---|---|
| Existing/proposed MRL | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Avocados | 0.15 | 0.01 | STMR (pulp) | 0.01 | HR (pulp) |
| Citrus fruits | 0.2 | 0.003 | STMR × PeF (EFSA, 2015) | 0.0096 | HR × PeF (EFSA, 2015) |
| Tree nuts | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Apples | 0.08 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | |
| Pears | 0.08 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | HR (EFSA, 2015, 2017) |
| Medlar | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Loquat | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Quinces | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Apricots | 0.15 | 0.03 | STMR (EFSA, 2015) | 0.07 | HR (EFSA, 2015) |
| Cherries | 0.3 | 0.13 | STMR (EFSA, 2015) | 0.18 | HR (EFSA, 2015) |
| Peaches | 0.15 | 0.03 | STMR (EFSA, 2015, 2017) | 0.07 | HR (EFSA, 2015) |
| Plums | 0.2 | 0.02 | STMR (EFSA, 2015, 2017) | 0.1 | HR (EFSA, 2015) |
| Table grapes | 0.08 | 0.01 | STMR (EFSA, 2017) a | 0.05 | HR (EFSA, 2017) a |
| Wine grapes | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Strawberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Cane fruits | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Blueberries, Cranberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Currants | 0.2 | 0.06 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Gooseberries, Rose hips | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Mulberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Azaroles, Elderberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Table olives | 1 | 0.13 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Kaki/Japanese persimmons | 0.09 | 0.02 | STMR (EFSA, 2015) | 0.04 | HR (EFSA, 2015) |
| Kiwi | 0.05 | 0.0105 | STMR × PeF (EFSA, 2015) | 0.0273 | HR × PeF (EFSA, 2015) |
| Bananas | 0.15 | 0.0231 | STMR × PeF (EFSA, 2015) | 0.0264 | HR × PeF (EFSA, 2015) |
| Mangoes | 0.2 | 0.014 | STMR × PeF (EFSA, 2015) | 0.0196 | HR × PeF (EFSA, 2015) |
| Potatoes | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Tropical roots and tuber veg | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Beetroot | 0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Carrots | 0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Celeriac | 0.07 | 0.03 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
|
Horseradish Jerusalem artichokes Parsnips Parsley root Salsify Swedes Turnips |
0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Radishes | 0.15 | 0.02 | STMR (EFSA, 2015) | 0.05 | HR (EFSA, 2015) |
| Bulb vegetables | 0.2 | 0.05 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Tomatoes | 0.07 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | HR (EFSA, 2015, 2017) |
| Peppers | 0.1 | 0.02 | STMR (EFSA, 2015, 2017) | 0.06 | HR (EFSA, 2015, 2017) |
| Aubergines | 0.3 | 0.03 | STMR (EFSA, 2015, 2017) | 0.18 | HR (EFSA, 2015, 2017) |
| Okra | 0.3 | 0.03 | STMR (EFSA, 2015) | 0.18 | HR (EFSA, 2015) |
| Cucumbers | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Gherkins | 0.15 | 0.04 | STMR (EFSA, 2015) | 0.06 | HR (EFSA, 2015) |
| Courgettes | 0.15 | 0.04 | STMR (EFSA, 2015, 2017) | 0.06 | HR (EFSA, 2015, 2017) |
| Cucurbits with inedible peel | 0.06 | 0.005 | STMR × PeF (EFSA, 2015, 2017) | 0.02 | STMR × PeF (EFSA, 2015, 2017) |
| Sweet corn | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Flowering brassica | 0.1 | 0.02 | STMR (EFSA, 2015, 2017) | 0.07 | HR (EFSA, 2015, 2017) |
| Brussels sprouts | 0.04 | 0.02 | STMR (EFSA, 2015) | 0.02 | HR (EFSA, 2015) |
| Head cabbages | 0.15 | 0.03 | STMR (EFSA, 2015, 2017) | 0.09 | HR (EFSA, 2015, 2017) |
| Chinese cabbages | 0.3 | 0.08 | STMR (EFSA, 2015, 2017) | 0.13 | HR (EFSA, 2015, 2017) |
| Kohlrabi | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Lamb's lettuces | 1.5 | 0.34 | STMR (EFSA, 2015) | 0.63 | HR (EFSA, 2015) |
| Lettuces | 0.15 | 0.03 | STMR (EFSA, 2015) | 0.06 | HR (EFSA, 2015) |
| Escarole | 0.07 | 0.02 | STMR (EFSA, 2015, 2017) | 0.04 | HR (EFSA, 2015, 2017) |
| Cresses, Land cresses | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Roman rocket | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Baby leaf crops | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Spinach | 0.6 | 0.20 | STMR (EFSA, 2015, 2017) | 0.22 | HR (EFSA, 2015, 2017) |
| Chards/Beet leaves | 0.2 | 0.05 | STMR (EFSA, 2015) | 0.08 | HR (EFSA, 2015) |
| Herbs and edible flowers | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Beans with pods | 0.4 | 0.11 | STMR (EFSA, 2015) | 0.17 | HR (EFSA, 2015) |
| Beans without pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Peas with pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Peas without pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Lentils | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Asparagus | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Celeries | 0.2 | 0.05 | STMR (EFSA, 2019b) | 0.09 | HR (EFSA, 2019b) |
| Florence fennels | 0.3 | 0.11 | STMR (EFSA, 2019b) | 0.14 | HR (EFSA, 2019b) |
| Globe artichokes | 0.15 | 0.04 | STMR (EFSA, 2015) | 0.07 | HR (EFSA, 2015) |
| Leeks | 0.07 | 0.02 | STMR (EFSA, 2015) | 0.04 | HR (EFSA, 2015) |
| Wild fungi | 0.50 | 0.17 | STMR (EFSA, 2015, 2017) | 0.23 | HR (EFSA, 2015, 2017 |
| Pulses | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Oilseeds | 0.2 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Soya beans | 0.05 | 0.05 | EU MRL (EFSA, 2015) b | 0.05 | EU MRL EFSA, 2015) b |
| Olives for oil production | 0.5 | 0.11 | STMR (EFSA, 2015) | 0.11 | STMR (EFSA, 2015) |
| Barley | 0.5 | 0.09 | STMR (EFSA, 2015) | 0.09 | STMR (EFSA, 2015) |
| Maize/corn | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Oats | 0.3 | 0.09 | STMR (EFSA, 2015) | 0.09 | STMR (EFSA, 2015) |
| Rice | 0.2 | 0.04 | STMR (EFSA, 2019b) | 0.04 | STMR (EFSA, 2019b) |
| Sorghum | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Wheat, Rye | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Coffee | 0.01 * | 0.01 | STMR (FAO, 2015) | 0.01 | STMR (FAO, 2015) |
| Hops (dried) | 10 | 3.30 | STMR (EFSA, 2015) | 3.6 | HR (EFSA, 2015) |
| Seed spices | 0.3 | 0.02 | STMR (EFSA, 2020) | 0.13 | HR (EFSA, 2015) |
| Fruit spices, except cardamom | 0.3 | 0.02 | STMR (seed spices) (EFSA, 2020) | 0.13 | HR (EFSA, 2015) |
| Cardamom | 2 | 0.28 | STMR (FAO, 2015) | 3.06 | HR (EFSA, 2015) |
| Root and rhizome spices | 0.05 | 0.05 | STMR (EFSA, 2015) | 0.05 | HR (EFSA, 2015) |
| Sugar beet roots | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Sugar canes | 0.05 | 0.02 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Chicory roots | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Swine, meat | 0.15 | 0.23 | STMR (EFSA, 2015) c | 0.52 | HR (EFSA, 2015) c |
| Swine, fat | 3 | 1.00 | STMR (EFSA, 2015) | 2.2 | HR (EFSA, 2015) |
| Swine, liver | 0.05 | 0.008 | STMR (EFSA, 2015) | 0.2 | HR (EFSA, 2015) |
| Swine, kidney | 0.2 | 0.03 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Swine, edible offal | 3 | 0.03 | STMR (EFSA, 2015) d | 0.09 | HR (EFSA, 2015) d |
| Ruminant, meat | 0.15 | 0.23 | STMR (EFSA, 2015) c | 0.52 | HR (EFSA, 2015) c |
| Ruminant, fat | 3 | 1.00 | STMR (EFSA, 2015) | 2.2 | HR (EFSA, 2015) |
| Ruminant, liver | 0.05 | 0.008 | STMR (EFSA, 2015) | 0.2 | HR (EFSA, 2015) |
| Ruminant, kidney | 0.2 | 0.03 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Ruminant, edible offal | 3 | 0.03 | STMR (EFSA, 2015) d | 0.09 | HR (EFSA, 2015) d |
| Poultry meat | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Poultry fat | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Poultry liver | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Equine, other farmed, meat | 0.15 | 0.23 | STMR (EFSA, 2015) c | 0.52 | HR (EFSA, 2015) c |
| Equine, other farmed, fat | 3 | 1.00 | STMR (EFSA, 2015) | 2.2 | HR (EFSA, 2015) |
| Equine, other farmed, liver | 0.05 | 0.008 | STMR (EFSA, 2015) | 0.2 | HR (EFSA, 2015) |
| Equine, other farmed, kidney | 0.2 | 0.03 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Equine, other farmed edible offal | 3 | 0.03 | STMR (EFSA, 2015) d | 0.09 | HR (EFSA, 2015) d |
| Ruminant milk | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Bird's eggs | 0.01 * | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
Abbreviations: HR, highest residue; PeF, peeling factor; STMR, supervised trials median residue.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
STMR derived from the approved use of gamma‐cyhalothrin was multiplied by a potency factor of 2 to take into account the hazard contribution of gamma‐cyhalothrin to lambda‐cyhalothrin (EFSA, 2017). This was not considered relevant for lambda‐cyhalothrin and therefore this potency factor was not applied to the risk assessment of lambda‐cyhalothrin in line with the residue definition for risk assessment.
EU MRL implemented in MRL legislation and derived during the MRL review (EFSA, 2015).
Consumption figures in the EFSA PRIMo are expressed as meat. Input values for mammals are calculated considering a 80%/90% muscle/fat content (FAO, 2016).
For edible offal of mammals, the input values derived for liver were included in the calculation.
APPENDIX E. Used compound codes
E.1.
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
| Lambda‐cyhalothrin |
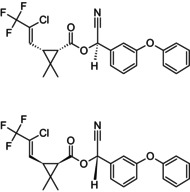
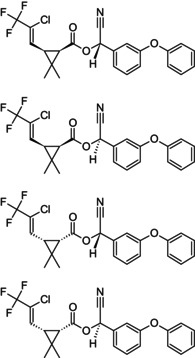
Reaction product comprising equal quantities of (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: reaction product comprising equal quantities of (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@@H]1[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl) = C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
|
| Gamma‐cyhalothrin |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F ZXQYGBMAQZUVMI‐GCMPRSNUSA‐N |
|
| Cyhalothrin |
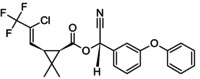
(RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: (RS)‐α‐cyano‐3‐phenoxybenzyl (1RS)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate CC1(C)[C@H]([C@H]1\C=C(/Cl)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1.FC(F)(F)C(/Cl) = C/[C@H]1[C@@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C.Cl\C(=C/[C@@H]1[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl) = C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C OOAOVGPMANECPJ‐RWEUCVCFSA‐N |
|
| Compound Ia |
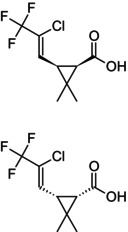
(1R,3R)‐3‐[(1Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐en‐1‐yl]‐2,2‐dimethylcyclopropane‐1‐carboxylic acid—(1S,3S)‐3‐[(1Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐en‐1‐yl]‐2,2‐dimethylcyclopropane‐1‐carboxylic acid (1/1) Cl\C(=C/[C@H]1[C@@H](C(=O)O)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl) = C/[C@@H]1[C@H](C(=O)O)C1(C)C DPUIEEBDWOJPHB‐OBDQHKNMSA‐N |
|
| Compound IV |
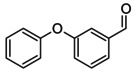
3‐phenoxybenzaldehyde O=Cc1cc(Oc2ccccc2)ccc1 MRLGCTNJRREZHZ‐UHFFFAOYSA‐N |
|
| Gamma‐lactone (R947650) |
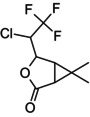
(1RS,4RS,5SR)‐4‐[(1RS)‐1‐chloro‐2,2,2‐trifluoroethyl]‐6,6‐dimethyl‐3‐oxabicyclo[3.1.0]hexan‐2‐one (Unstated stereochemistry) CC1(C)C2C(=O)OC(C(Cl)C(F)(F)F)C21 ZSSZFVGRINYCPY‐UHFFFAOYSA‐N |
|
Abbreviations: InChiKey, International Chemical Identifier Key; IUPAC, International Union of Pure and Applied Chemistry; SMILES, simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116,563, 15 Jun 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121,153, 22 Mar 2021).
EFSA (European Food Safety Authority) , Bellisai, G. , Bernasconi, G. , Cabrera, L. C. , Castellan, I. , del Aguila, M. , Ferreira, L. , Santonja, G. G. , Greco, L. , Jarrah, S. , Leuschner, R. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Ruocco, S. , Santos, M. , Scarlato, A. P. , Szot, M. … Verani, A. (2023). Setting of an import tolerance for lambda‐cyhalothrin in avocados. EFSA Journal, 21(12), e8464. 10.2903/j.efsa.2023.8464
Approved: 17 November 2023
Notes
It should be noted that Lambda‐cyhalothrin, Gamma‐cyhalothrin, Cyhalothrin, Compound Ia and Gamma‐lactone are identified as a pesticide active substance/metabolites that meet the definition of per‐ and polyfluoroalkyl substances (PFAS) based on its chemical structure (https://echa.europa.eu/hot‐topics/perfluoroalkyl‐chemicals‐pfas).
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Commission Directive 2000/80/EC of 4 December 2000 amending Annex I to Council Directive 91/414/EEC concerning the placing of plant protection products on the market, so as to consolidate that Annex and include a further active substance. OJ L 309, 9.12.2000, p. 14–23.
Commission Implementing Regulation (EU) No 540/2011 of 23 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) 2016/146 of 4 February 2016 renewing the approval of the active substance lambda‐cyhalothrin, as a candidate for substitution, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Implementing Regulation (EU) No 540/2011. OJ L 30, 5.2.2016, p. 7–11.
The applicants shall submit confirmatory information as regards: 1. A systematic review to assess the evidence available as regards potential sperm effects linked to exposure to lambda‐cyhalothrin using guidance available (e.g. EFSA GD on Systematic Review methodology, 2010); 2. Toxicological information to assess the toxicological profile of the metabolites V (PBA) and XXIII (PBA(OH)). The applicants shall submit that information to the Commission, the Member States and the Authority by 1 April 2018.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: http://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/public/?event=pesticide.residue.selection&language=EN
Commission Regulation (EU) No 459/2010 of 27 May 2010 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for certain pesticides in or on certain products. OJ L 129, 28.5.2010, p. 3–49.
Commission Regulation (EU) 2017/626 of 31 March 2017 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid, cyantraniliprole, cypermethrin, cyprodinil, difenoconazole, ethephon, fluopyram, flutriafol, fluxapyroxad, imazapic, imazapyr, lambda‐cyhalothrin, mesotrione, profenofos, propiconazole, pyrimethanil, spirotetramat, tebuconazole, triazophos and trifloxystrobin in or on certain products C/2017/2035. OJ L 96, 7.4.2017, p. 1–43.
Commission Regulation (EU) 2019/50 of 11 January 2019 amending Annexes II, III, IV and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for chlorantraniliprole, clomazone, cyclaniliprole, fenazaquin, fenpicoxamid, fluoxastrobin, lambda‐cyhalothrin, mepiquat, onion oil, thiacloprid and valifenalate in or on certain products C/2019/20 OJ L 10, 14.1.2019, p. 8–59.
Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC, PE/41/2019/REV/1. OJ L 231, 6.9.2019, p. 1–28.
Commission Regulation (EU) No 1141/2010 of 7 December 2010 laying down the procedure for the renewal of the inclusion of a second group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances.OJ L 322, 8.12.2010, p. 10–19.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Background documents to this reasoned opinion are published on OpenEFSA portal and are available at the following link: https://open.efsa.europa.eu/study‐inventory/EFSA‐Q‐EFSA‐Q‐2022‐00758
The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
Commission Regulation (EU) 2018/960 of 5 July 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for lambda‐cyhalothrin in or on certain products C/2018/4128. OJ L 169, 6.7.2018, p. 27–50.
The MRLs for these commodities were established when previous versions of EFSA PRIMo were used for risk assessment. The higher exposure results derived with PRIMo 3.1 compared to PRIMo 3/PRIMo 2 can be explained by the higher consumption data and/or different unit weight data which triggers the IESTI case implemented in PRIMo 3.1.
For table grapes a potency factor of two derived for gamma‐cyhalothrin (EFSA, 2017) was erroneously included for the risk assessment of lambda‐cyhalothrin (EFSA, 2019b, 2020).
Confirmatory data requested in the MRL review should have been submitted by 6 July 2020. The assessment of the requested information is still pending.
The process on the second renewal of the approval of lambda‐cyhalothrin assessment is ongoing giving an opportunity to consider identified data gaps including toxicological properties of the degradation products which can be considered in the ongoing renewal assessment.
It is noted that for gamma‐cyhalothrin uses a separate risk assessment shall be performed, comparing the exposure to gamma‐cyhalothrin with the respective toxicological reference values for gamma‐cyhalothrin.
REFERENCES
- EFSA (European Food Safety Authority) . (2014a). Reasoned opinion on the review of the existing maximum residue levels (MRLs) for lambda‐cyhalothrin according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 12(1), 3546. 10.2903/j.efsa.2014.3546 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014b). Conclusion on the peer review of the pesticide risk assessment of the active substance lambda‐cyhalothrin. EFSA Journal, 12(5), 3677. 10.2903/j.efsa.2014.3677 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015). Revision of the review of the existing maximum residue levels for the active substance lambda‐cyhalothrin. EFSA Journal, 13(12), 4324. 10.2903/j.efsa.2015.4324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2016). Scientific report of EFSA on scientific support for preparing an EU position in the 48th session of the codex committee on pesticide residues (CCPR). EFSA Journal, 14(8), 4571. 10.2903/j.efsa.2016.4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , De Lentdecker, C. , Erdos, Z. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nougadere, A. , Pedersen, R. , Reich, H. , Sacchi, A. , Santos, M. , … Villamar‐Bouza, L. (2017). Reasoned opinion on the focused review of the existing maximum residue levels for lambda‐cyhalothrin in light of the unspecific residue definition and the existing good agricultural practices for the substance gamma‐cyhalothrin. EFSA Journal, 15(7), 4930. 10.2903/j.efsa.2017.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , Ferreira, L. , Greco, L. , Jarrah, S. , Leuschner, R. , Medina, P. , Miron, I. , Nougadere, A. , Pedersen, R. , Reich, H. , Santos, M. , Stanek, A. , Tarazona, J. , Theobald, A. , & Villamar‐Bouza, L. (2018). Guidance on use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA Journal, 16(1), 5147. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Pedersen, R. , Raczyk, M. , Reich, H. , Ruocco, S. , Sacchi, A. , Santos, M. , Stanek, A. , Tarazona, J. , … Verani, A. (2019a). Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication, 16(3), EN‐1605. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Abdourahime, H. , Anastassiadou, M. , Brancato, A. , Brocca, D. , Carrasco Cabrera, L. , De Lentdecker, C. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lostia, A. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nave, S. , Pedersen, R. , … Villamar‐Bouza, L. (2019b). Reasoned opinion on the modification of the existing maximum residue levels for lambda‐cyhalothrin in celeries, fennel and rice. EFSA Journal, 17(1), 5546. 10.2903/j.efsa.2019.5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Bernasconi, G. , Brancato, A. , Carrasco Cabrera, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Rojas, A. , Sacchi, A. , Santos, M. , Stanek, A. , Theobald, A. , … Verani, A. (2020a). Reasoned opinion on the modification of the existing maximum residue levels for lambda‐cyhalothrin in seed and fruit spices. EFSA Journal, 18(6), 6110. 10.2903/j.efsa.2020.6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020b). Technical report on the outcome of the consultation with member states, the applicant and EFSA on the pesticide risk assessment for lambda‐cyhalothrin in light of confirmatory data. EFSA Supporting Publication, 17(6), EN−1883. 10.2903/sp.efsa.2020.EN-1883 [DOI] [Google Scholar]
- European Commission . (1997a). Appendix A. Metabolism and distribution in plants. 7028/VI/95‐rev.3, 22 July 1997 .
- European Commission . (1997b). Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997 .
- European Commission . (1997c). Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997 .
- European Commission . (1997d). Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997 .
- European Commission . (1997e). Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997 .
- European Commission . (1997f). Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997 .
- European Commission . (1997g). Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95 22 July 1997. As amended by the document: Classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, Finalised in the Standing Committee on the Food Chain and Animal Health at its Meeting of 23–24 March 2010 .
- European Commission . (2010). Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010 .
- European Commission . (2017). Technical guideline on the evaluation of extraction efficiency of residue analytical methods. SANTE 2017/10632, Rev. 5, 11 May 2023 .
- European Commission . (2020a). Technical guidelines on data requirements for setting maximum residue levels, comparability of residue trials and extrapolation on residue data on products from plant and animal origin. SANTE/2019/12752, 23 November 2020 .
- European Commission . (2020b). Final Review report for the active substance lambda‐cyhalothrin finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 11 December 2015 in view of the renewal of the approval of lambda‐cyhalothrin as active substance in accordance with Regulation (EC) No 1107/2009. SANCO/12282/2014 Rev 5, 17 July .
- European Commission . (2021). Guidance document on pesticide analytical methods for risk assessment and post‐approval control and monitoring purposes. SANTE/2020/12830, Rev.1 24. February 2021 .
- FAO (Food and Agriculture Organization of the United Nations) . (2015). Lambda‐cyhalothrin. In Pesticide residuesin food–2015. Evaluations, part I, residues FAO Plant Production and Protection Paper 226. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2016). Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. In Pesticide Residues (3rd ed.. FAO Plant Production and Protection Paper 225, p. 298). [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) . (2007). Guidance Document on Pesticide Residue Analytical Methods. In: Series on Pesticides No 39/Series on Testing and Assessment No 72. ENV/JM/ MONO(2007)17, 13 August 2007 .
- OECD (Organisation for Economic Co‐operation and Development) . (2011). OECD MRL calculator: Spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. https://www.oecd.org
- Greece . (2023). Evaluation report on the modification of MRL for lambda‐cyhalothrin in avocado. July 2023, revised in September 2023, 53 pp . www.efsa.europa.eu
- Sweden . (2013). Renewal Assessment Report (RAR) on the active substance lambda‐cyhalothrin prepared by the rapporteur Member State Sweden in the framework of Commission Regulation (EU) No 1141/2010. February 2013 . www.efsa.europa.eu
- Sweden . (2014). Final addendum to renewal assessment report on lambda‐cyhalothrin, compiled by EFSA, February 2014 . www.efsa.europa.eu


