Abstract
Context
Impairments in musculoskeletal and mental health are common in adults with Hypophosphatasia (HPP). Restricted phosphorus intake has been suggested to positively affect symptoms in HPP, but there is a lack of interventional evidence.
Objective
This work aimed to evaluate the effect of a phosphorus-restricted, calcium-adjusted diet on musculoskeletal and mental health in HPP.
Methods
A prospective, noncontrolled, single-center interventional study (NuSTEPS II) was conducted among outpatients at the Osteology Department, University of Wuerzburg, Germany. A total of 26 adults with an established HPP diagnosis received a standardized diet with a defined daily intake of phosphorus (1160-1240 mg/d) and calcium (870-930 mg/d) over 8 weeks. Main outcome measures were functional testing and patient-reported outcome measures.
Results
At 8 weeks, significant improvements were observed in usual gait speed (P = .028) and the chair-rise test (P = .019), while no significant changes were seen in the 6-minute walk test (P = .468) and the timed up-and-go test (P = .230). Pain was not significantly reduced according to the visual analog scale (VAS) (P = .061), pain subscale of the 36-Item Short-Form Health Survey (SF-36) (P = .346), and Pain Disability Index (P = .686). Further, there was a significant improvement in the SF-36 vitality subscale (P = .022) while all other subscales as well as the Lower Extremity Functional Scale (P = .670) and the Fatigue Assessment Scale (P = .392) did not change significantly. Adjustments of mineral intake were not associated with relevant alterations regarding the intake of energy and energy-supplying nutrients or body composition.
Conclusion
Adjusting phosphorus and calcium intake may positively affect individual symptoms in adults with HPP, but overall clinical effectiveness regarding major issues like pain and endurance appears limited.
Keywords: hypophosphatasia, dietary phosphorus, dietary calcium, fatigue, pain, physical function
The rare, inherited metabolic disorder Hypophosphatasia (HPP) is caused by loss-of-function variants in the ALPL gene, leading to impaired activity of the tissue-nonspecific alkaline phosphatase (TNAP) and subsequent accumulation of its natural phosphate metabolites, including pyridoxal-5-phosphate (PLP), inorganic pyrophosphate (PPi) as well as phosphoethanolamine (PEA) [1-3].The disease affects patients at all ages and is associated with a broad spectrum of clinical manifestations and a wide range of severity [4, 5]. Many common symptoms in adult patients involve the musculoskeletal system, including pain, muscle weakness, and in certain instances soft tissue calcifications and pseudofractures [6-8]. Many adult patients suffer from persistent fatigue and are limited in their physical functioning, including both gross and fine motor activities [9]. Further to that, dental issues, neurological, and psychological symptoms such as headaches, sleep disturbances, depression as well as anxiety are prevalent [7, 10, 11]. In summary, these manifestations cause a high burden of disease in adult patients, independent of the age of onset, with impairments of both, musculoskeletal and mental health [8, 12].
The results of a recent cross-sectional study indicate that part of these clinical symptoms might be affected by dietary phosphorus, calcium, and the calcium-phosphorus ratio [13]. Phosphorus and calcium are important minerals of the human body and their dietary intake is linked to a variety of health-related implications, including but not limited to bone and muscle [14-16]. While calcium is most abundant in milk, dairy products, green leafy vegetables, nuts, seeds, certain waters, and fortified products, phosphorus is present in nearly all foods, especially those rich in protein, partly owing to the usage of inorganic phosphate as a food additive [15, 17]. For the general adult population aged 19 years and older, a daily intake of 700 mg phosphorus is set as the recommended daily allowance (RDA) both in the United States and Germany, but actual intakes are considerably higher in many regions of the world [18-21]. Considering compromised phosphate metabolism in HPP, dietary phosphate restriction has been proposed for HPP [22] and appears to be a common recommendation among patient groups and caregivers.
Our own previous work showed that both very high and particularly low absolute intakes of phosphorus and calcium, as well as a dietary imbalance of these two minerals, are associated with an increased frequency of fatigue and musculoskeletal and neuropsychiatric symptoms [13]. Vice versa, a moderate phosphorus intake together with adequate dietary calcium and a calcium-phosphorus ratio slightly less than 1 seemed promising to positively affect musculoskeletal and mental health. Adjusting dietary phosphorus and calcium by specific selection of foods could thus be a practical approach to limit HPP-related symptoms. However, interventional scientific evidence confirming improvement or therapeutic benefit of dietary adjustments to support such recommendations is missing. Therefore, this study NuSTEPS II aimed to investigate the musculoskeletal and mental health in adult HPP patients following an 8-week restricted phosphorus, calcium-adjusted diet.
Materials and Methods
Study Design
NuSTEPS II was a single-center, noncontrolled interventional study conducted at the Orthopedic Institute, Koenig-Ludwig-Haus, University Wuerzburg, Germany, in cooperation with the Institute of Food Science and Human Nutrition, Leibniz University, Hanover, Germany. The study consisted of an 8-week intervention period during which participants followed a restricted phosphorus, calcium-adjusted diet with 2 examinations at the beginning and at the end of the study. The objectives of this exploratory study are 2-fold, covering clinical and biochemical outcomes. The analyses presented here focus on clinical and patient-reported parameters. All participants provided written informed consent before any study-related procedures. The study was approved by the ethics committee of the Medical Faculty of the University of Wuerzburg (N. 6/18) and prospectively registered with the World Health Organization–compliant German Clinical Trials Register (DRKS00015225).
Study Population
Inclusion criteria were a minimum age of 18 years and an established diagnosis of HPP, defined as reduced serum alkaline phosphatase (ALP) activity below age-/sex-specific reference range and genetically confirmed ALPL variant and/or elevated PLP (urine or serum), above the upper limit of normal, and/or symptoms of the disease. Individuals were not eligible if they had any accompanying illness or medical treatment that could interfere with mineral absorption or excretion, particularly gastrointestinal and renal disorders. Intake of any nutritional supplements had to be discontinued 4 weeks before the first examination except for vitamin D and magnesium. Participants were recruited via the Orthopedic Institute, University of Wuerzburg, and public notice placed by the German patient organization Hypophosphatasie Deutschland e.V.
Dietary Intervention and Assessments
Based on the results of the previously conducted cross-sectional study [13], intakes of 1160 to 1240 mg phosphorus and 870 to 930 mg calcium per day, corresponding to a calcium-phosphorus ratio of 0.7 to 0.8, were defined as the target intake for the restricted phosphorus, calcium-adjusted study diet. These provided lower and upper limits had to be followed daily whenever possible. Prior to nutritional intervention, habitual nutrient intake was assessed using 3-day dietary records, including all consumed foods and drinks with weighed or standard household sizes, fat content, brand name, and manufacturer to check ingredients or nutrients contained, including mineral content of bottled and tap water. For home-cooked food, recipes were collected.
To facilitate dietary adjustment in daily life, target intake values as well as the phosphorus and calcium content of common foods and drinks were converted into points as follows: 20 mg phosphorus = 1 phosphorus point and 15 mg calcium = 1 calcium point, so that the daily target levels were 58 to 62 phosphorus and calcium points each. Participants received food tables indicating calcium and phosphorus points per portion and recipes for main meals developed by a nutritionist to help maintain a balanced calcium-phosphorus ratio as part of nutritional counseling. Due to the varying calcium and phosphorus content of water from different sources, the specific mineral content of ingested water was researched individually and converted into points. The points of consumed foods and drinks were documented on a daily basis, so patients were continuously overviewing their intakes themselves. Additionally, participants indicated consumed foods and drinks with quantities in the food tables as a tally sheet to allow exact calculation of the nutrient intakes. At baseline, participants received nutritional counseling explaining the dietary specifications and providing general information about calcium and phosphorus in the diet, including the presence of phosphate-containing food additives that should be avoided during the intervention whenever possible. For any questions and ambiguities arising during the assessment period, patients could directly call a nutritionist for clarification.
Physical Examination
Physical examination was accomplished as per the sites established clinical routine at the beginning and at the end of the 8-week nutritional intervention. Body composition was examined using a bioelectrical impedance analyzer (BIA 101 Anniversary, Akern s.r.l.) according to established standards. Physical function was assessed using the Short Physical Performance Battery (SPPB) [23], 6-minute walk test (6MWT) [24], timed up-and-go (TUG) test [25], and measurement of the grip strength [26] with a handheld dynamometer (DynEx, Akern s.r.l.).
Patient-reported Outcome Measures
Self-perceived musculoskeletal and mental health were retrieved using patient-reported outcome measures (PROMs) at both visits, including the 36-Item Short-Form Health Survey (SF-36) version 1.3 [27-29], the Pain Disability Index (PDI) [30, 31], and the Lower Extremity Functional Scale (LEFS) [32-34]. Pain intensity over the past 7 days was assessed using a 10-cm visual analog scale (VAS) ranging from 0 (no pain) to 10 (intolerable pain). Further, the 10-item Fatigue Assessment Scale (FAS) allowing for distinction of physical and mental fatigue was included [35, 36]. For the FAS sum score, 10 to 21 points indicate no fatigue while 22 to 50 points are considered suggestive for fatigue, with a change of 4 points being considered the minimal clinically important difference (MCID) [37, 38].
Data Analysis and Statistical Methods
Dietary documentations were analyzed using PRODI 6.11 expert with database extension (Nutri Science GmbH), whereby data were checked for completeness, readability, and plausibility by a nutritionist and ambiguous documentation was clarified in personal communication. Constituents of consumed foods that were not included in the software were researched and added to the database manually. Data sets from participants not complying with the protocol requirements during the conduct of the study were excluded from the per-protocol analysis and only analyses with paired data from both visits were evaluated. The evaluation of adherence to the dietary specifications as well as statistical analyses were based on nutrient intakes from food table data. In that regard, the simplified point system to facilitate dietary adjustment in daily life is associated with inaccuracies, that is, the intakes of phosphorus, calcium, and the calcium-phosphorus ratio calculated from food table data differed on average by ± 7.0% from the intakes derived from the documented points. To correct for this inaccuracy, adherence to the dietary specifications was evaluated using ± 7.0% extended target ranges for phosphorus, calcium, and the calcium-phosphorus ratio. Subgroup analyses were performed considering groups with increases vs decreases for phosphorus intake, calcium intake, and the calcium-phosphorus ratio, to reveal potential implications of the dynamics of adjustment. IBM SPSS software (version 28, IBM) and R software (version 4.2.3, R Core Team) with the package ggplot2 [39] (version 3.4.1) were used for statistical analyses and illustrations, respectively. Owing to the small sample size and in line with the prespecified analysis plan, the nonparametric paired-samples Wilcoxon signed rank test (2-tailed) was selected to analyze changes after intervention. The Wilcoxon-Mann-Whitney test or Fisher exact test (nominal data) was used to test for differences between subgroups regarding baseline characteristics. The chi-square test of independence and Fisher exact test if any expected cell frequency was less than 5 were used to evaluate associations between the adjustment of the corresponding mineral and the test outcome (improvement vs worsening/no change). For all analyses, values of P less than .05 were considered statistically significant. No adjustment for multiple testing was performed. The data are reported with medians (ranges) and counts (percentages) for categorical variables.
Results
Study Population
Out of 35 individuals (28 female) enrolled, 4 participants discontinued prematurely for personal reasons, 3 were unable to attend the follow-up visit due to COVID-19, and data of 2 patients could not be included in the analysis for reasons of nonadherence, leaving 26 participants (21 female) with complete data that could be included in the final per-protocol analysis. Baseline characteristics are depicted in Table 1.
Table 1.
Baseline characteristics
| All patients | Female patients | Male patients | |
|---|---|---|---|
| (n = 26) | (n = 21) | (n = 5) | |
| Age, y | |||
| Median (range) | 56.0 (18.0-68.0) | 55.0 (18.0-68.0) | 56.0 (54.0-64.0) |
| Height, cm | |||
| Median (range) | 168 (152-195) | 166 (152-182) | 176 (173-195) |
| Weight, kg | |||
| Median (range) | 70.9 (47.6-113) | 70.2 (47.6-108) | 77.9 (67.0-113) |
| BMI | |||
| Median (range) | 23.8 (18.8-37.8) | 23.8 (18.8-37.8) | 23.9 (22.4-36.3) |
| Age at diagnosis, y | |||
| Median (range) | 51.5 (15.0-66.0) | 50.0 (15.0-66.0) | 52.0 (51.0-61.0) |
| Alkaline phosphatase, U/L | |||
| Median (range) | 23.5 (11.0-52.0) | 23.0 (11.0-52.0) | 24.0 (16.0-38.0) |
| Self-reported form of nutrition | |||
| Mixed diet n (%) | 20 (77) | 16 (76) | 4 (80) |
| Mixed diet with few meat n (%) | 5 (19) | 4 (19) | 1 (20) |
| Ovo-lacto-vegetarian diet n (%) | 1 (4) | 1 (5) | 0 (0) |
| Ovo-lacto-vegetarian diet with fish n (%) | 0 (0) | 0 (0) | 0 (0) |
| Vegan n (%) | 0 (0) | 0 (0) | 0 (0) |
| Smokers n (%) | 2 (8) | 1 (5) | 1 (20) |
Abbreviation: BMI, body mass index
Nutrient Intake and Body Composition
Participants followed the restricted phosphorus, calcium-adjusted diet for a median (range) of 55 (51-59) days. Overall, the adherence to the dietary specifications was very high. The target range for phosphorus extended by ± 7.0% was achieved on 73.3% (1040 out of 1419) of patient days. Further to that, calcium intakes and calcium-phosphorus ratios were within the ± 7.0% expanded target ranges on 72.7% (1032/1419) and 80.1% (1137/1419) of patient days, respectively. Participants attained all 3 ± 7.0% extended target ranges simultaneously on 60.8% (863/1419) of all days. Very low (<1000 mg/d) and high (>1400 mg/d) phosphorus intakes were rare and occurred at only 160 and 49 of 1419 patient days, respectively. Similarly, calcium intakes were less than 750 mg/d on 194 of 1419 days and greater than 1050 mg/d on 45 days.
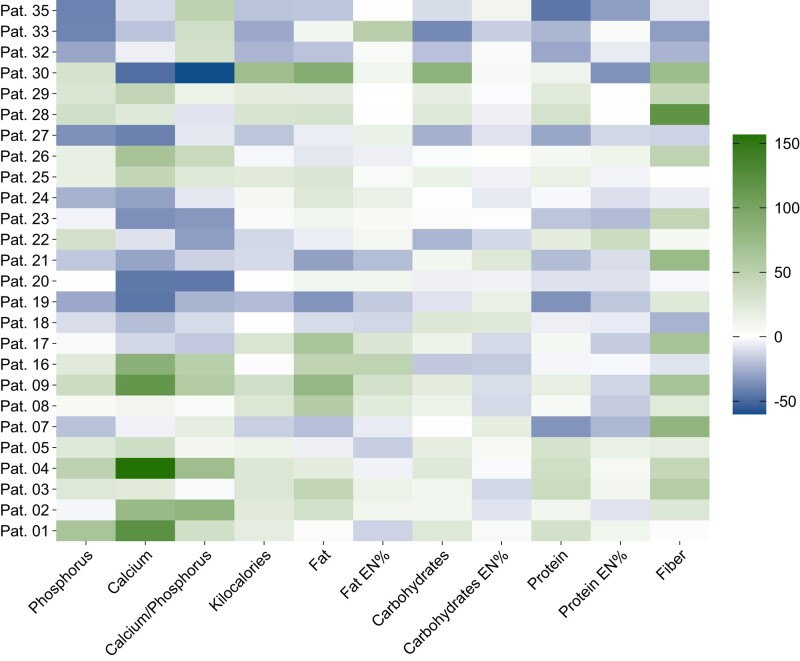
Details on nutrient intakes before and throughout the intervention are provided in Table 2. In general, there were no significant changes in calcium and phosphorus intakes as well as the calcium-phosphorus ratio. However, individual intakes altered substantially, with part of the participants increasing and others decreasing their respective consumption. Specifically, the range of those participants with particularly high or low intakes of calcium or phosphorus at baseline narrowed markedly. Overall, there were similar proportions of individuals who decreased or increased their dietary intake of phosphorus, calcium, and the calcium-phosphorus ratio, respectively. In those participants who augmented their phosphorus intake during the intervention (n = 14), baseline median (range) intake was 914 (650-1121) mg/d. During intervention, their median (range) phosphorus intake increased to 1150 (1000-1212) mg/d, corresponding to individual increases by 5.49% to 64.7% (median: 25.8%). Conversely, participants with high phosphorus consumption at baseline (n = 12) reduced their intake from a median (range) of 1469 (1195-2042) mg/d by −40.6% to −0.25% (median: −21.8%) to 1180 (1124-1387) mg/d. Similarly, there were 12 vs 14 participants who increased vs decreased dietary calcium. Baseline median (range) calcium intakes in these 2 groups were 543 (335-754) mg/d and 1162 (843-1675) mg/d, respectively. Individual changes ranged from −47.3% to −4.03% (median: −24.7%) in the reduction group and from 10.6% to 156% (median: 55.1%) in participants who had to increase their calcium intakes. Most patients adjusted their absolute phosphorus and calcium intakes in the same direction, that is, they decreased or increased both minerals concordantly. Only 3 participants with increasing and 1 patient with decreasing phosphorus intake adapted their dietary calcium in an opposite direction. However, the extent of change in each mineral varied and overall, the calcium-phosphorus ratio increased in 14 and decreased in 12 participants. Baseline median (range) calcium-phosphorus ratios in these patients were 0.53 (0.43-0.73) and 0.89 (0.75-1.90), respectively. Only 3 of the latter participants showed a baseline calcium-phosphorus ratio of 1 or greater. Individual changes in participants with increasing and decreasing calcium-phosphorus ratio varied from 4.35% to 80.8% (median: 35.0%) and from −59.9% to −1.86% (median: −15.5%), respectively. Alterations in the calcium-phosphorus ratio resulted predominantly from adjustments in calcium intake. The percentage changes of nutrient intakes per patient are depicted in Fig. 1.
Table 2.
Nutrient intake per day at baseline and during intervention
| At baseline | During intervention | Pa | |
|---|---|---|---|
| (n = 26) | (n = 26) | ||
| Median (range) | Median (range) | ||
| Phosphorus, mg | 1065 (650-2042) | 1157 (1000-1387) | .960 |
| Calcium, mg | 875 (335-1675) | 871 (741-972) | .803 |
| Calcium-phosphorus ratio | 0.71 (0.43-1.90) | 0.75 (0.64-0.79) | .635 |
| Energy, kcal | 1930 (1150-3122) | 2018 (1664-2410) | .499 |
| Fat, g | 73.9 (34.9-117) | 83.0 (62.3-113) | .150 |
| Fat, EN% | 37.3 (22.3-45.5) | 37.6 (31.5-45.4) | .136 |
| Carbohydrates, g | 212 (107-376) | 232 (145-293) | .483 |
| Carbohydrates, EN% | 45.0 (26.2-62.0) | 45.6 (32.0-52.1) | .367 |
| Protein, g | 61.2 (43.4-121) | 63.7 (55.9-82.8) | .779 |
| Protein, EN% | 14.7 (9.75-19.9) | 13.4 (10.3-15.6) | .031 |
| Fiber, g | 17.2 (8.77-57.6) | 24.8 (10.6-53.4) | .030 |
Abbreviation: EN%, energy percentage.
a Wilcoxon signed rank test.
Figure 1.
Heat map illustrating the percentage change of nutrient intakes per patient. Color intensity indicates the individual percentage change of specific nutritional constituents with green vs blue colors representing increased vs decreased intakes, respectively; Abbreviation: EN%, energy percent/percentage proportion of total energy intake.
Daily energy intake as well as the absolute and relative amounts of fat and carbohydrates remained unchanged at 8 weeks compared to baseline. The median (range) proportional protein intake decreased significantly from 14.7 (9.75-19.9) energy percentage (EN%) before the intervention to 13.4 (10.3-15.6) EN% at follow-up (P = .031), while the absolute amount of protein intake was not significantly different. Overall, there was actually a slight increase of absolute protein intake by 7.44%. The median (range) absolute intake of fiber increased significantly from 17.2 (8.77-57.6) g to 24.8 (10.6-53.4) g per day (P = .030), corresponding to a median percentage change of 23.5%. Intraindividual alterations of macronutrient intake are shown in Fig. 1.
In the overall cohort, there was a statistically significant decrease in bodyweight (P = .036) from a baseline median (range) of 70.9 (47.6-113) kg to 69.7 (47.9-110) kg. Correspondingly, the median body mass index (BMI) decreased significantly (P = .038) from 23.8 (18.8-37.8) kg/m2; to 23.1 (18.9-36.9) kg/m2;. However, the median individual changes were less than 1% and thus the absolute individual reductions were only minor. Moreover, constitutional assessment by bioelectrical impedance analysis (BIA) did not reveal any clinically meaningful changes along the 8 weeks of dietary intervention (data not shown).
Assessment of Physical Function
The outcomes of functional testing are depicted in Table 3.
Table 3.
Outcomes of functional testing and patient-reported outcome measures at baseline and at 8 weeks
| n | At baseline | At 8 wk | Pa | ||
|---|---|---|---|---|---|
| Median (range) | Median (range) | ||||
| Functional testing | Usual gait speed, m/s | 24 | 1.37 (0.82-2.11) | 1.51 (0.74-2.14) | .028 |
| Time to complete CRT, s | 25 | 12.5 (6.27-31.8) | 10.2 (5.71-27.3) | .019 | |
| Handgrip strength, kg | |||||
| Right hand | 26 | 23.3 (5.47-49.5) | 25.5 (7.37-48.2) | .006 | |
| Left hand | 26 | 21.1 (9.63-49.0) | 22.5 (5.27-47.7) | .419 | |
| Walking distance in 6MWT, m | 24 | 481 (300-698) | 484 (261-696) | .468 | |
| Time to complete TUG test, s | 25 | 8.93 (5.86-17.0) | 8.65 (5.82-15.3) | .230 | |
| PROMs | VAS score for pain intensity | 25 | 5.50 (0.80-8.50) | 4.60 (0.30-8.40) | .061 |
| PDI score | 26 | 24.5 (1.00-47.0) | 23.6 (0.00-49.5) | .686 | |
| SF-36 scores | |||||
| PCS | 24 | 36.6 (15.3-51.1) | 32.6 (20.7-59.1) | .663 | |
| MCS | 24 | 50.9 (30.9-68.7) | 50.4 (23.9-64.9) | ≥.999 | |
| Physical functioning | 25 | 70.0 (15.0-100) | 55.0 (20.0-100) | .133 | |
| Physical role function | 25 | 50.0 (0.00-100) | 50.0 (0.00-100) | .571 | |
| Pain | 25 | 41.0 (12.0-72.0) | 41.0 (0.00-100) | .346 | |
| General health perception | 24 | 44.4 (15.0-72.0) | 47.0 (10.0-87.0) | .437 | |
| Vitality | 24 | 30.0 (0.00-80.0) | 42.5 (0.00-80.0) | .022 | |
| Social functioning | 25 | 87.5 (37.5-100) | 75.0 (12.5-100) | .216 | |
| Emotional role function | 25 | 66.7 (0.00-100) | 100 (0.00-100) | .994 | |
| Mental well-being | 24 | 72.0 (40.0-92.0) | 66.0 (36.0-88.0) | .311 | |
| FAS scores | |||||
| Total | 26 | 32.0 (11.0-44.0) | 27.0 (11.0-43.0) | .392 | |
| Physical | 26 | 18.0 (6.00-24.0) | 15.0 (6.00-24.0) | .524 | |
| Mental | 26 | 14.0 (5.00-22.0) | 11.5 (5.00-19.0) | .267 | |
| LEFS score | 26 | 50.5 (23.5-80.0) | 50.5 (10.0-80.0) | .670 | |
Abbreviations: 6MWT, 6-minute walk test; CRT, chair-rise test; FAS, Fatigue Assessment Scale; LEFS, Lower Extremity Functional Scale; MCS, mental component summary; PCS, physical component summary; PDI, Pain Disability Index; PROMs, patient-reported outcome measures; SF-36, 36-Item Short-Form Health Survey; TUG, timed up-and-go; VAS, visual analog scale.
a Wilcoxon signed rank test.
Short physical performance battery
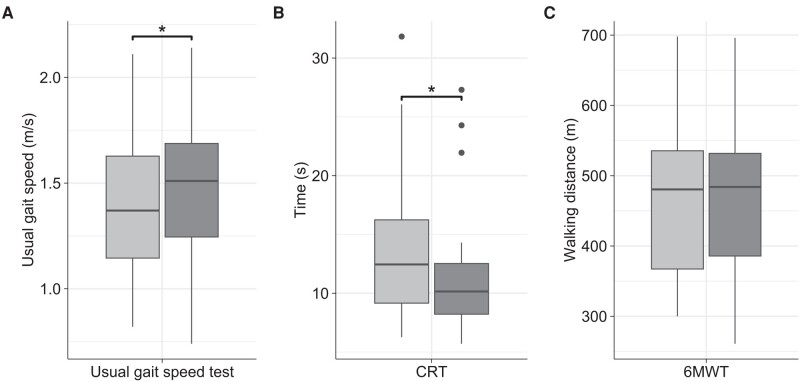
The assessment of usual gait speed and the CRT as part of the SPPB were completed at both visits by 24 and 25 participants, respectively. Median (range) gait speed increased significantly from 1.37 (0.82-2.11) m/s at baseline to 1.51 (0.74-2.14) m/s at 8 weeks (P = .028) (Fig. 2A). The time required to complete the CRT improved significantly (P = .019) from a median (range) value of 12.5 (6.27-31.8) s at baseline to 10.2 (5.71-27.3) s at follow-up (Fig. 2B). With regard to individual values, an improvement was observed in 66.7% (16/24) and 68.0% (17/25) of patients in usual gait speed and CRT, respectively. Balance test results were not significantly different across the intervention.
Figure 2.
Box plots depicting results for usual gait speed test, CRT and 6MWT at baseline and at 8 weeks. Light gray: at baseline; dark gray: at 8 weeks; A, Usual gait speed test (n = 24); B, CRT (n = 25); C, 6MWT (n = 24); CRT, chair-rise test; 6MWT, 6-minute walk test; *P less than .05.
Handgrip strength
All participants completed grip strength assessment at both examinations with results showing slight increases at both hands. On the right side, this increase from 23.3 (5.47-49.5) kg at baseline to 25.5 (7.37-48.2) kg at 8 weeks was statistically significant (P = .006) while the increase in the left hand from 21.1 (9.63-49.0) kg at baseline to 22.5 (5.27-47.7) kg at follow-up was not (P = .419).
Six-minute walk test and timed up-and-go test
The 6MWT and the TUG test were completed at both visits by 24 and 25 participants, respectively, none of whom used assistive devices. Median (range) walking distance covered in the 6MWT was 481 (300-698) m at start and 484 (261-696) m at the end of the intervention and thus not significantly different (P = .468) (Fig. 2C). In line with that, the proportion of participants with an improvement vs decline was almost balanced (13 vs 10/24) and individual results remained largely consistent with intraindividual differences exceeding ±10% in only 4 individuals. Likewise, the time required to accomplish the TUG test did not change significantly with 8.93 (5.86-17.0) s at baseline and 8.65 (5.82-15.3) s at follow-up (P = .230).
Patient-reported Outcome Measures
The outcomes of PROMs are shown in Table 3.
Pain
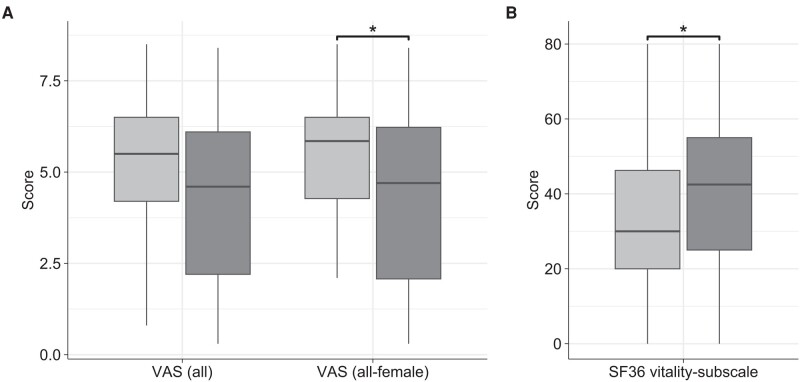
Median (range) pain intensity over the past 7 days on the VAS was indicated at 5.50 (0.80-8.50) at baseline and reduced to 4.60 (0.30-8.40) after 8 weeks of nutritional intervention (Fig. 3A). Still, this decrease was not statistically significant (P = .061). When considering only women to account for potential bias due to sex differences, there was a significant improvement in pain intensity (P = .047) from 5.85 (2.10-8.50) to 4.70 (0.30-8.40), corresponding to a median percentage change of −16.9% (Fig. 3A). The pain-subscale within the SF-36 reflecting pain intensity and the extent of pain-related limitations in daily life over the past 4 weeks did not change significantly with median (range) scores of 41.0 (12.0-72.0) at baseline and also 41.0 (0.00-100) at follow-up (P = .346). Similarly, the PDI did not change significantly (P = .686) due to the intervention with median (range) scores of 24.5 (1.00-47.0) at baseline and 23.6 (0.00-49.5) at the end of the study.
Figure 3.
Comparative delineation of patient-reported pain intensity on VAS and the SF-36 vitality subscale at baseline and at 8 weeks. Light gray: At baseline; dark gray: at 8 weeks; A, VAS for pain intensity of all patients (n = 25) and of all-female patients (n = 20); B, vitality subscale of the SF-36 (n = 24); VAS, visual analog scale; SF-36; 36-Item Short-Form Health Survey *P less than .05.
Fatigue and vitality
Mental and physical exhaustion were evaluated by the vitality subscale of the SF-36 and the FAS. At baseline, the median (range) score of the SF-36 vitality subscale was 30.0 (0.00-80.0) with only 25.0% (6/24) of participants achieving values greater than or equal to 50. After the intervention, this score improved significantly (P = .022) to 42.5 (0.00-80.0), corresponding to a median percentage change of 9.72% (Fig. 3B). Moreover, 11 out of 24 participants (45.8%) attained scores greater than or equal to 50 while the proportion of patients with values less than 25 decreased from 37.5% (9/24) to 20.8% (5/24). The baseline median (range) FAS total score was 32.0 (11.0-44.0) with physical and mental aspects of fatigue contributing 18.0 (6.00-24.0) and 14.0 (5.00-22.0) points and thus almost equally to that sum. At the end of the intervention, physical and mental fatigue decreased similarly to 15.0 (6.00-24.0) and 11.5 (5.00-19.0), respectively, yielding a slightly but not significantly diminished sum score of 27.0 (11.0-43.0) (P = .392). An improvement exceeding the MCID defined as a change greater than or equal to 4 points was observed in 7 out of the 14 patients who had declining FAS total score.
Physical and mental component summary scores of the 36-Item Short-Form Health Survey
At baseline, the median (range) physical component summary (PCS) score of the SF-36 was 36.6 (15.3-51.1) and thus well below the standard sample average of 50 (SD 10) while the baseline mental component summary (MCS) score was higher with a baseline median (range) of 50.9 (30.9-68.7). Both PCS (P = .663) and MCS (P = 1.000) scores did not change significantly during the nutritional intervention with median (range) values of 32.6 (20.7-59.1) and 50.4 (23.9-64.9) at follow-up, respectively. Except for vitality, no significant changes were observed in any of the other subscales of the SF-36.
Lower Extremity Functional Scale
The LEFS was completed by all participants at both visits but did not change significantly with median (range) scores of 50.5 (23.5-80.0) at baseline and 50.5 (10.0-80.0) at 8 weeks (P = .670).
Subgroup Analyses
Subgroup analyses to investigate whether a decrease or increase of dietary intake of phosphorus or calcium or the calcium-phosphorus ratio had a specific effect on certain outcomes did not yield consistent meaningful results. For completeness, results of these additional calculations are being reported in the supplementary materials [40].
Intervention-related Side Effects
There were no adverse events or untoward concomitant findings that could be causatively related to the nutritional intervention. No patient canceled participation due to nutrition-related adverse events or discomfort.
Discussion
To the best of our knowledge, this is the first study to investigate the effect of a restricted phosphorus, calcium-adjusted diet on musculoskeletal and mental health in adult HPP patients. Notwithstanding comprehensive documentation requirements and mandatory nutritional constraints, evaluation of the nutritional data confirmed both high adherence and the feasibility of adjusting diet and associated recording efforts. Out of 35 participants enrolled, 26 completed the entire interventional program and consistently achieved the nutritional goals. Specifically, the participants adhered to dietary targeted ranges for phosphorus, calcium, and the calcium-phosphorus ratio on the vast majority of days. Accordingly, days with very low or high calcium and/or phosphorus intakes, which are suspected to increase the frequency of HPP-related symptoms [13], were rare.
As expected, baseline assessment showed reduced physical performance, particularly limited walking distance in the 6MWT, slower completion of CRT, and lower hand grip strength in HPP patients as compared to the general population at similar age [41-43]. Only the results for TUG test and usual gait speed at baseline were within the range of values expected for the general population in the fifth decade [44, 45]. With that in mind, it appears unlikely that the observed improvement in gait speed by about 9.59% at the end of the intervention is primarily a result of adjusted diet but may rather be a consequence of procedural variations and patients getting familiar with the testing procedure. This same pattern may apply to the slight improvements seen in the TUG test, even though these were not statistically significant. Conversely, the significant improvement in the CRT from a median (range) of 12.5 (6.27-31.8) s to 10.2 (5.71-27.3) s may not be appropriately explained by mere familiarization with the test procedure, specifically when considering the extent of improvement and the fact that more than two-thirds (68.0%) of the patients improved. CRT results attained at the end of the study almost reached mean (SD) age- and sex-specific normative values [42].
The slight but statistically significant increase in handgrip strength by about 7.15% in the right hand with largely unaltered values on the left side suggests that this may again be a matter of participant familiarization and procedural variation rather than an intervention-associated effect. Accordingly, the median distance covered during the 6MWT remained unchanged at a low level of about 480 m from baseline to the end of the study, again suggesting no substantial effect of the intervention on exercise capacity.
The pain level assessed with the SF-36 at baseline was increased as compared to the population average and similar to that reported for other HPP cohorts [9, 12, 46], indicating persistent pain and pain-related limitations in daily life. However, this did not change significantly following nutritional adjustment. Similarly, the baseline median (range) PDI score where higher scores reflect more severe impairment in daily activities due to pain was elevated to 24.5 (1.00-47.0) out of 70 points. In 4 participants (15.4%) and 7 participants (26.9%), baseline PDI levels were even worse/higher than reported in a study [47] of patients with widespread pain (mean [SD]: 41.4 [10.9]) and chronic low back pain (mean [SD]: 36.5 [13.8]), respectively. However, these values remained by and large unchanged over the 8 weeks of nutritional modifications. Similarly, the median (range) pain intensity in the VAS at baseline was 5.50 (0.80-8.50), again confirming persistent moderate pain in this patient group [48]. While changes in pain intensity failed to reach statistical significance in the overall cohort, this value reduced significantly in female patients, with an individual median change of −16.9%. Sex-related variations in pain perception are well established [49] and may have led to different changes during the intervention, but these findings may also be just coincidental. More so since alterations in pain assessed with both the PDI and the SF-36 were not statistically significant, even among women. This discrepancy across the various questionnaires in terms of improvement may be explained by the different assessment periods (past 7 days for VAS, past 4 weeks for SF-36, not specified for PDI) or the fact that both the SF-36 and the PDI capture pain-related limitations, while the VAS merely focuses on pain itself. In that regard, this finding may suggest that the improvements in pain intensity were not sufficient to cause relevant effects on daily life. Further research is advisable to substantiate if the improved pain intensity specifically in female patients was only a coincidental finding or if dietary changes even over more than 8 weeks would result in a sustained decrease in pain intensity and subsequently measurable improvements in the SF-36 and PDI.
According to recent data from the Global HPP Registry, fatigue affects 23.4% to 46.5% of adult patients [46], and several studies consistently show impaired vitality in HPP as measured by the SF-36 [9, 8, 12]. In line with this, the categorized analysis of vitality turned out to be the lowest rated of all SF-36 subscales in the present cohort with a median (range) score of 30.0 (0.00-80.0) at baseline, thus further confirming substantial compromise of this aspect in HPP patients. The observed significant improvement in that regard even exceeded the suggested minimally important difference (MID) of 5 points [50], indicating a relevant improvement in vitality over the 8 weeks of dietary adjustment.
Regarding fatigue, the baseline median (range) FAS sum score was 32.0 (11.0-44.0) and thus clearly beyond the threshold of 22 points considered indicative of fatigue. Since to the best of our knowledge the FAS has not been used in HPP patients before, there are no disease-related comparative data. However, the median FAS sum score in the HPP patients assessed was even worse than what has been reported for patients with rheumatoid arthritis and osteoarthritis, having mean (SD) values of 29.9 (3.38) and 26.1 (3.67), respectively [51]. More detailed evaluations of the FAS in the present study revealed that both physical and mental aspects contributed equally to fatigue in our study population, reflecting the fact that HPP is associated with limitations of both bodily and mental health. Across the nutritional intervention, there was a noticeable but no statistically significant decrease in the FAS sum score. Since this potential effect is based on patient-perceived vitality and fatigue and thus highly subjective, further research is needed to better understand if this was just a matter of coincidence or whether dietary adjustment can actually have a positive and sustained effect on fatigue/reduced vitality in HPP patients.
The median PCS score of the SF-36 at baseline in this study was equivalent to what has been reported in other HPP groups before [8, 12, 46] and thus confirmed the high physical disease burden in these patients. Still, after 8 weeks of nutritional intervention, none of the physical scales showed a significant change, suggesting that the intervention did not have a relevant effect on self-perceived physical health despite the slight improvements observed in some of the functional assessments. This would further underscore the interpretation that alterations in physical performance are within the scope of physiological variation and do not indicate relevant functional improvements. Correspondingly, patient-perceived lower-extremity functioning assessed by the LEFS remained stable across the intervention, with a median score of 50.5 obtained at both visits, that is, persistently below the median (range) of 77 (4.5-80.0) described for healthy individuals of similar age [52].
Mental health as measured by the MCS score of the SF-36 did not change significantly at 8 weeks, either. However, it is important to note that in contrast to the physical component subscales, most of the MCS subscale scores at baseline were in line or even slightly below the US reference standard sample except for the vitality score. This fact may have contributed to the lack of significant changes in categories other than in the vitality subscale.
Overall, results in physical function and PROMs did not differ between patient groups with increasing vs those with decreasing intakes of phosphorus, calcium, and the calcium-phosphorus ratio, and we could not identify particular subgroups who experienced an exceptional benefit. Conversely, there was no decline in any of the PROMs or functional assessments in any of the subgroups and none of the participants experienced intervention-related adverse events. Accordingly, even though the clinical effectiveness of the dietary intervention over 8 weeks assessed here was limited, the results eventually support rather than oppose our previous work [13] and the idea of recommending a balanced intake of phosphorus and calcium in adults with HPP.
This becomes even more relevant when considering that almost half of the patients (42.3%) improved their calcium intake from a median (range) of 554 (335-754) mg/d to 861 (741-929) mg/d without having a substantially higher symptom burden despite the concomitant increase in phosphorus intake. Even though the evaluation of the supply with other nutrients was not within the scope of designated outcomes of this study, there was a significant increase in median (range) fiber intake from 17.2 (8.77-57.6) g/d at baseline to 24.8 (10.6-53.4) g/d during the intervention. Although this intake still did not meet the German recommended level of 30 g/d or more [20] during the 8 weeks, this suggests that the study diet allowed the selection of nutritionally valuable foods such as vegetables, whole grains, legumes, fruits, nuts, and seeds, many of which are rich in phosphorus but also in fiber.
Interpretation of the data should be made with respect to certain limitations. We did not perform adjustments for multiple testing in this exploratory, hypothesis-generating approach, so even statistically significant results have to be interpreted thoughtfully. The dietary adjustment was self-administered in the participants’ home setting and not conducted under clinical supervision. A nutritionist stayed in contact with participants to minimize implementation and documentation errors, but bias cannot be completely ruled out. Due to the rarity of HPP, the sample size was small and lacked a control group, which may reduce the power to establish causality and the transferability to other HPP patients. Furthermore, the proportion of participants who did not complete the entire intervention program was relatively large, but this was partly caused by COVID-19, and the complete data sets demonstrated the generally high adherence to the nutritional requirements and the feasibility of the study.
Conclusion
In summary, this study indicates that a restricted phosphorus, calcium-adjusted diet, defined as intakes of 1160 to 1240 mg phosphorus and 870 to 930 mg calcium per day, may positively affect certain aspects of musculoskeletal and mental health in adult patients with HPP. However, the overall clinical effectiveness on major issues like endurance and pain appears to be limited. Regarding specific aspects like vitality, further research is required to determine if the improvement was merely a random result or whether adjusting dietary phosphorus and calcium can actually contribute to beneficial and lasting effects. Notwithstanding this, improvements were noted in patients with both increasing and decreasing intakes of phosphorus, calcium, and the calcium-phosphorus ratio, supporting the idea of recommending a balanced intake of phosphorus and calcium in adults with HPP.
Acknowledgments
We thank all participants and all those involved in the conduct of the study.
Abbreviations
- 6MWT
6-minute walk test
- ALP
alkaline phosphatase
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- CRT
chair-rise test
- EN%
energy percentage
- FAS
Fatigue Assessment Scale
- HPP
Hypophosphatasia
- LEFS
Lower Extremity Functional Scale
- MCID
minimal clinically important difference
- MCS
mental component summary
- MID
minimally important difference
- PCS
physical component summary
- PDI
Pain Disability Index
- PEA
phosphoethanolamine
- PLP
pyridoxal-5-phosphate
- PPi
inorganic pyrophosphate
- PROMs
patient-reported outcome measures
- RDA
recommended daily allowance
- SF-36
36-Item Short-Form Health Survey
- SPPB
Short Physical Performance Battery
- TNAP
tissue-nonspecific alkaline phosphatase
- TUG
timed up-and-go
- VAS
visual analog scale
Contributor Information
Katinka Kuehn, Faculty of Natural Science, Institute of Food Science and Human Nutrition, Leibniz University Hannover, 30167 Hanover, Germany.
Andreas Hahn, Faculty of Natural Science, Institute of Food Science and Human Nutrition, Leibniz University Hannover, 30167 Hanover, Germany.
Lothar Seefried, Email: l-seefried.klh@uni-wuerzburg.de, Clinical Trial Unit, Orthopedic Institute, Koenig-Ludwig-Haus, University of Wuerzburg, 97074 Wuerzburg, Germany.
Author Contributions
Conception/idea: all authors; study design: all authors; study lead investigator: L.S. and A.H.; enrolled and studied patients: K.K. and L.S.; collection and assembly of data: K.K.; data analysis: all authors; data interpretation: all authors; manuscript preparation: K.K.; manuscript content review and revisions: all authors; approval of final manuscript: all authors.
Disclosures
Parts of this work have been supported by the “Studienstiftung des Deutschen Volkes.” L.S. has received honoraria for lectures and advice from AstraZeneca/Alexion, Amgen, AM-Pharma, Chiesi, Gedeon-Richter, GlaxoSmithKline, Inozyme, Ipsen, KyowaKirin, medi, Novartis, STADA, Theramex, and UCB and research grants to his institution (University of Wuerzburg) from AstraZeneca/Alexion, Chiesi, KyowaKirin, and Novartis. K.K. and A.H. report no conflict of interest during the course of the study.
Data Availability
All data and study materials are stored at the Department of Orthopedics, University of Wuerzburg, for 10 years. Restrictions apply to the availability of the data generated and analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Clinical Trial Information
Clinical trial registration number DRKS00015225 (registered August 14, 2018).
References
- 1. Bianchi ML. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int. 2015;26(12):2743‐2757. [DOI] [PubMed] [Google Scholar]
- 2. Linglart A, Biosse-Duplan M. Hypophosphatasia. Curr Osteoporos Rep. 2016;14(3):95‐105. [DOI] [PubMed] [Google Scholar]
- 3. Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Högler W, Langman C, Da Gomes Silva H, et al. Diagnostic delay is common among patients with hypophosphatasia: initial findings from a longitudinal, prospective, global registry. BMC Musculoskelet Disord. 2019;20(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whyte MP. Hypophosphatasia—aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233‐246. [DOI] [PubMed] [Google Scholar]
- 6. Berkseth KE, Tebben PJ, Drake MT, Hefferan TE, Jewison DE, Wermers RA. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone. 2013;54(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt T, Mussawy H, Rolvien T, et al. Clinical, radiographic and biochemical characteristics of adult hypophosphatasia. Osteoporos Int. 2017;28(9):2653‐2662. [DOI] [PubMed] [Google Scholar]
- 8. Weber TJ, Sawyer EK, Moseley S, Odrljin T, Kishnani PS. Burden of disease in adult patients with hypophosphatasia: results from two patient-reported surveys. Metab Clin Exp. 2016;65(10):1522‐1530. [DOI] [PubMed] [Google Scholar]
- 9. Durrough C, Colazo JM, Simmons J, et al. Characterization of physical, functional, and cognitive performance in 15 adults with hypophosphatasia. Bone. 2021;142:115695. [DOI] [PubMed] [Google Scholar]
- 10. Colazo JM, Hu JR, Dahir KM, Simmons JH. Neurological symptoms in Hypophosphatasia. Osteoporos Int. 2019;30(2):469‐480. [DOI] [PubMed] [Google Scholar]
- 11. Colazo JM, Hu JR, Dahir KM, Simmons JH. Correction to: neurological symptoms in Hypophosphatasia. Osteoporos Int. 2019;30(2):535. [DOI] [PubMed] [Google Scholar]
- 12. Seefried L, Dahir K, Petryk A, et al. Burden of illness in adults with Hypophosphatasia: data from the global hypophosphatasia patient registry. J Bone Miner Res. 2020;35(11):2171‐2178. [DOI] [PubMed] [Google Scholar]
- 13. Kuehn K, Hahn A, Seefried L. Mineral intake and clinical symptoms in adult patients with Hypophosphatasia. J Clin Endocrinol Metab. 2020;105(8):dgaa324. [DOI] [PubMed] [Google Scholar]
- 14. Serna J, Bergwitz C. Importance of dietary phosphorus for bone metabolism and healthy aging. Nutrients. 2020;12(10):3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cormick G, Belizán JM. Calcium intake and health. Nutrients. 2019;11(7):1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018;19(1):6‐11.e3. [DOI] [PubMed] [Google Scholar]
- 17. Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(3):519‐530. [DOI] [PubMed] [Google Scholar]
- 18. Welch AA, Fransen H, Jenab M, et al. Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 2009;63(S4):S101‐S121. [DOI] [PubMed] [Google Scholar]
- 19. McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001-2014. Nutrients. 2017;9(2):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährung, eds. Referenzwerte für die Nährstoffzufuhr. 2nd ed., 7th updated version. Bonn; 2021.
- 21. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; 1997. [PubMed] [Google Scholar]
- 22. Wenkert D, Podgornik MN, Coburn SP, Ryan LM, Mumm S, Whyte MP. Dietary phosphate restriction therapy for hypophosphatasia: preliminary observations. J Bone Miner Res. 2002;17:S384. [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85‐M94. [DOI] [PubMed] [Google Scholar]
- 24. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166(1):111‐117. [DOI] [PubMed] [Google Scholar]
- 25. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142‐148. [DOI] [PubMed] [Google Scholar]
- 26. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222‐226. [DOI] [PubMed] [Google Scholar]
- 27. Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41(10):1359‐1366. [DOI] [PubMed] [Google Scholar]
- 28. Morfeld M, Kirchberger I, Bullinger M. SF-36: Fragebogen zum Gesundheitszustand. 2nd supplemented and revised ed. Hogrefe; 2011.
- 29. Morfeld M, Kirchberger I, Bullinger M. SF-36: Fragebogen zum Gesundheitszustand: Deutsche Version des Short Form-36 Health Survey: Manual. 2nd supplemented and revised ed. Hogrefe; 2011.
- 30. Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59(3):974. [DOI] [PubMed] [Google Scholar]
- 31. Dillmann U, Nilges P, Saile H, Gerbershagen HU. Behinderungseinschätzung bei chronischen Schmerzpatienten. Schmerz. 1994;8(2):100‐110. [DOI] [PubMed] [Google Scholar]
- 32. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371‐383. [PubMed] [Google Scholar]
- 33. Naal FD, Impellizzeri FM, Torka S, Wellauer V, Leunig M, von Eisenhart-Rothe R. The German Lower Extremity Functional Scale (LEFS) is reliable, valid and responsive in patients undergoing hip or knee replacement. Qual Life Res. 2015;24(2):405‐410. [DOI] [PubMed] [Google Scholar]
- 34. Stratford PW, Hart DL, Binkley JM, Kennedy DM, Alcock GK, Hanna SE. Interpreting lower extremity functional Status scores. Physiother Can. 2005;57(02):154. [Google Scholar]
- 35. Hendriks C, Drent M, Elfferich M, de Vries J. The fatigue assessment scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018;24(5):495‐503. [DOI] [PubMed] [Google Scholar]
- 36. Michielsen HJ, de Vries J, van Heck GL, van de Vijver FJ, Sijtsma K. Examination of the dimensionality of fatigue. Eur J Psychol Assess. 2004;20(1):39‐48. [Google Scholar]
- 37. de Kleijn WPE, de Vries J, Wijnen PAHM, Drent M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med. 2011;105(9):1388‐1395. [DOI] [PubMed] [Google Scholar]
- 38. de Vries J, Michielsen H, van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br J Health Psychol. 2004; 9(3):279‐291. [DOI] [PubMed] [Google Scholar]
- 39. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Second edition Springer; 2016. [Google Scholar]
- 40. Kuehn K, Hahn A, Seefried L. Data from: Impact of restricted phosphorus, calcium-adjusted diet on musculoskeletal and mental health in Hypophosphatasia. Research Data Repository of the Leibniz University Hannover. Deposited 29 November 2023. 10.25835/cju4rpk8. [DOI] [PMC free article] [PubMed]
- 41. Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150‐156. [DOI] [PubMed] [Google Scholar]
- 42. Bergland A, Strand BH. Norwegian reference values for the Short Physical Performance Battery (SPPB): the Tromsø Study. BMC Geriatr. 2019;19(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69‐74. [PubMed] [Google Scholar]
- 44. Kear BM, Guck TP, McGaha AL. Timed Up and Go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182‐189. [DOI] [PubMed] [Google Scholar]
- 46. Dahir KM, Seefried L, Kishnani PS, et al. Clinical profiles of treated and untreated adults with hypophosphatasia in the Global HPP Registry. Orphanet J Rare Dis. 2022;17(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soer R, Köke AJA, Vroomen PCAJ, et al. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine (Phila Pa 1976). 2013;38(9):E562‐E568. [DOI] [PubMed] [Google Scholar]
- 48. Boonstra AM, Schiphorst Preuper HR, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155(12):2545‐2550. [DOI] [PubMed] [Google Scholar]
- 49. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23(4):731‐739. [DOI] [PubMed] [Google Scholar]
- 51. Alikari V, Sachlas A, Giatrakou S, et al. Fatigue in arthritis: a multidimensional phenomenon with impact on quality of life: fatigue and quality of life in arthritis. Adv Exp Med Biol. 2017;987:243‐256. [DOI] [PubMed] [Google Scholar]
- 52. Dingemans SA, Kleipool SC, Mulders MAM, et al. Normative data for the Lower Extremity Functional Scale (LEFS). Acta Orthop. 2017;88(4):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and study materials are stored at the Department of Orthopedics, University of Wuerzburg, for 10 years. Restrictions apply to the availability of the data generated and analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.