Abstract
The immunological sequelae of adeno-associated virus (AAV)-mediated gene transfer in vivo is quite complex. In murine models, most AAV capsids are associated with minimal or dysfunctional T cell responses to antigenic transgene products. In this study we compared T cell activation against AAV2/8 and AAV2/rh32.33 vectors expressing nuclear-targeted LacZ (nLacZ), GFP, or firefly luciferase in murine skeletal muscle. We show that, unlike AAV8, AAVrh32.33 yields qualitatively and quantitatively robust T cell responses to both the capsid and transgene product. AAV2/rh32.33.CB.nLacZ, but not AAV2/8, drives a high degree of cellular infiltration and a loss of detectable transgene expression in C57BL/6 mice. However, cellular immunity to AAVrh32.33 is ablated in the absence of CD4, CD40L, or CD28, permitting stable β-galactosidase expression. Treatment of CD40L−/− mice with the CD40 agonist, FGK45, failed to restore the CD8 response to AAV2/rh32.33.nLacZ, suggesting that additional factors are involved. Our results suggest that specific domains within the AAVrh32.33 capsid augment the adaptive response to both capsid and transgene Ags in a CD4-dependent pathway involving CD40L signaling and CD28 costimulation. Structural comparison of the AAV8 and rh32.33 capsids has identified key differences that may drive differential immunity by affecting tropism, Ag presentation or the activation of innate immunity. This murine model of AAV-mediated cytotoxicity allows us to delineate the mechanism of viral immune activation, which is relevant to the translation of AAV technology in higher order species.
Gene therapy holds great potential for the treatment of monogenetic disease (1). However, the safety and efficacy of gene transfer can be negatively affected by host immune responses to either the delivery vehicle or the encoded protein (2). Adeno-associated virus (AAV)3 is a promising gene delivery vector due to its ability to achieve sustained, high-level expression of a packaged gene within target tissues (3, 4). AAVs are nonpathogenic and capable of transducing both dividing and nondividing cells. They belong to the genus Dependovirus, requiring a helper virus for productive infection (5). In fact, the first AAV serotypes were discovered as contaminants of adenoviral preps (6, 7). Since that time, numerous serotypes and over 120 capsid variants composing six phylogenetic clades and two clonal isolates have been described (8-14). Although the majority of capsid sequences are highly conserved (~80% homology to AAV2) (11), a divergent group of variants, as represented by AAV4, maintain only ~60% homology to AAV2, the prototype serotype (13). AAVrh32.33, a novel engineered vector isolated from rhesus macaques and phylogenetically closest to AAV4, is evolutionarily and structurally divergent from other AAVs. Importantly, its seroprevalence in human populations is significantly reduced compared with AAV2, AAV7, and AAV8 making it attractive for broad potential applications (15).
As a member of the Parvoviridae family, AAV is characterized by a small, 4.7-kb, ssDNA genome housed in a nonenveloped, icosahedral capsid (16, 17). The genome consists of a nonstructural rep gene, and a structural cap gene, flanked by two inverted terminal repeats. The cap gene encodes three overlapping viral structural proteins, VP1, VP2, and VP3, expressed in a ratio of ~1:1:10 via alternative splicing and unconventional start codon usage (18). VP3 monomers compose ~90% of capsid quaternary structure and consist of a highly conserved eight-stranded β-barrel motif (βB-βI) (19, 20). Due to this conservation, capsid architecture is maintained for all structurally mapped AAVs, regardless of primary sequence homology (9, 19-22). The majority of sequence variation falls within the surface loops linking these β-strands. Surface loops compose three protrusions surrounding a depression at the 3-fold axis of symmetry and dictate the unique phenotypes of each capsid (23-25). Evaluation of capsid biology using cross-packaged vectors confirms that capsid structure can affect tissue tropism, antigenicity, transduction efficiency, and vector performance (26, 27).
Due to the stability of expression of foreign transgene products in numerous murine tissues, AAV was generally considered minimally immunogenic for many years (3, 4, 28). However, recent reports have demonstrated that it is possible to generate humoral or cell-mediated immune responses to vector encoded proteins (29-33). Remarkably, even in cases in which stable expression of a foreign transgene product is achieved in murine models, expression of the identical transgene product in non-human primates is typically transient, accompanied by a substantial IFN-γ-producing T cell response and a brief elevation in liver transaminases (G. Gao, Q. Wang, R. Calcedo, L. Mays, P. Bell, L. Wang, R. Grant, J. Sammiguel, B. Furth, and J. Wilson, manuscript in preparation).
In this study, we have modeled differential immune activation to AAV by identifying two capsid variants with distinct immune activation profiles in murine skeletal muscle: AAV8 and AAVrh32.33. Our data indicate that the structure of the AAV capsid can impact the threshold required to activate a CTL response to AAV, where certain capsid variants more readily activate cellular immunity. We show that, unlike AAV8, the AAVrh32.33 capsid drives qualitatively and quantitatively robust IFN-γ-producing T cell responses to both capsid and transgene Ags, which was consistent for all transgene products tested as well as in multiple strains of mice. Using this model, we aim to delineate mechanistic aspects of cellular immune activation to AAV vectors in mice, highlighting the role of CD4+ T cell help and costimulation.
Materials and Methods
Animals
Male C57BL/6, BALB/c, CD40L−/−, and CD28−/− mice (6- to 8-wk-old) were purchased from The Jackson Laboratory. CD40L- and CD28-deficient mice were on a C57BL/6 background. Mice were maintained in the Animal Facility of Translational Research Laboratories. All experimental procedures involving the use of mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Vector production and purification
Recombinant AAV vectors with viral capsids from AAV8 or AAVrh32.33 expressing nuclear-targeted LacZ (nLacZ), GFP, or luciferase were manufactured as previously described (28) by PennVector at the University of Pennsylvania (Philadelphia, PA). Briefly, an AAV cis-plasmid containing transgene cDNA driven by the chicken β-actin promoter and flanked by AAV2 inverted terminal repeats was packaged by triple transfection of human embryonic kidney 293 cells using an adenovirus helper plasmid (pAdΔF6), and a chimeric packaging construct containing the AAV2 rep gene and the AAV8 or AAVrh32.33 cap gene. Vectors were purified by three rounds of cesium chloride gradient centrifugation. The genome titer (genome copies (GC) per milliliter) of AAV vectors was determined by real-time PCR.
Animal treatments
Mice were anesthetized i.p. with ketamine (70 mg/kg of body weight) and xylazine (7 mg/kg of body weight). A total of 1011 GC of recombinant AAV vector was injected into the anterior tibialis muscle in a volume of 50 μl of sterile PBS. In vivo depletion of CD4+ T cells was performed by injecting mice i.p. with 0.1 mg of GK1.5 on days −2, 0, 2, and 4 relative to vector injection. In preliminary studies using this treatment protocol >99% of CD4+ T cells were depleted. To block CD40L (CD154)-CD40 interactions, C57BL/6 mice were treated i.p. with 0.25 mg of MR-1 on days 0, 3, 6, and 9 relative to vector administration. To provide exogenous ligation of CD40, CD40L+/+, and CD40L−/− mice were injected i.p. with 0.2 mg of FGK45 on days 0, 1, and 5 relative to vector administration. Where indicated, whole blood was extracted by retro-orbital bleeds using a heparinized capillary tube in the lateral canthus of the eye on lightly anesthetized animals (ketamine (35 mg/kg) and xylazine (5 mg/kg), i.p.). At various time points, vector postinjection mice were sacrificed by CO2 inhalation followed by cervical dislocation to harvest spleen and muscle.
Real-time in vivo imaging
In vivo bioluminescent imaging was performed with the Xenogen IVIS imaging system. The 150 mg/kg of D-luciferin substrate was i.p administered, and the luminescence was captured from ventral views. Mice received D-luciferin (Caliper) exactly 15 min before imaging and were anesthetized during this interval (10 min before imaging). Anesthetized mice were then placed in the IVIS Lumina Imaging System and imaged (exposed for 10–30 s). Two mice were imaged at each time. Regions of interest from displayed images were drawn on the sites of vector injection (left hind limb) and were quantified as total flux (photons per second) being released by luciferase activity using Living Image 2.5 software (Caliper-Xenogen).
Histology and transgene detection
To examine expression of nuclear β-galactosidase (β-gal), X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactopyranoside) staining on cryosections from snap frozen muscles was performed according to standard protocols (34). Sections were slightly counterstained with Fast Red to visualize nuclei. Muscles expressing GFP were fixed overnight in formalin, washed in PBS for several hours, and then snap frozen for sectioning. Cryosections were mounted in Vectashield containing DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories) to show nuclei.
Immunostaining
To analyze the cell types within infiltrates, immunostaining and confocal microscopy was performed. Frozen sections were fixed in acetone at −20°C for 5 min, blocked in PBS containing 1% donkey serum, and incubated with rat Abs against CD8 (1/20; BD Pharmingen) followed by TRITC-labeled donkey anti-rat Abs (Jackson ImmunoResearch Laboratories). Sections were refixed with 4% paraformaldehyde/PBS for 15 min, permeabilized with 0.2% Triton X-100 in 1% donkey serum/PBS for 30 min, and incubated with goat Abs against CD4 (1/20; R&D Systems) and rabbit Abs against Foxp3 (1/20; BioLegend). Sections were then stained with Cy5-labeled donkey anti-goat and FITC-labeled donkey anti-rabbit Abs (all from Jackson ImmunoResearch Laboratories) and finally mounted in Vectashield with DAPI. All Abs were diluted in PBS with 1% donkey serum and slides were washed several times in PBS after each fixation and incubation step. Images were acquired with a Zeiss LSM 510 confocal microscope and pseudocolored using the Zeiss LSM Image Browser.
Morphometric analysis
To quantify GFP expression, images were taken of the area with the strongest GFP fluorescence in each muscle section with a 10X objective at identical settings. The brightness of each image was measured with ImageJ software (W. Rasband, National Institutes of Health, http://rsb.info.nih.gov/ij/) on a scale from 0 (darkest) to 255 (brightest) and averaged for each group. To measure nuclear β-gal expression, images of muscle sections showing the area of strongest expression were taken with a 4X objective and nuclei that stained positive with X-Gal were counted using AnalySIS (Soft Imaging System) and ImageJ software. A macro was generated under AnalySIS that performed the following operations sequentially: 1) convert image into gray scale, 2) perform shade correction (corrects for uneven illumination caused by the microscope), 3) set threshold between 0 and 120 (selects the X-Gal-positive areas), 4) perform phase color-coding (shows the selected areas in the image), 5) binarize image, and 6) invert image. The resulting picture is a black and white image that shows the X-Gal-positive areas in black over a white background. This image was then again converted into gray scale and opened in ImageJ. Black dots with a minimum size of 15 pixels (corresponding to β-gal-positive nuclei) were counted with ImageJ and values averaged for each group.
MHC class I tetramer staining
PE-conjugated MHC class I H2-Kb-ICPMYARV tetramer complex was obtained from Beckman Coulter. At various time points after vector injection, tetramer staining was performed on heparinized whole blood cells isolated by retro-orbital bleeds. Cells were costained for 30 min at room temperature with PE-conjugated tetramer and FITC-conjugated anti-CD8α (Ly-2) Ab (BD Pharmingen). RBC were then lysed and cells were fixed with iTAg MHC tetramer lysing solution supplemented with fix solution (Beckman Coulter) for 15 min at room temperature. The cells were then washed three times in PBS and resuspended in 0.01% BD CytoFix (BD Biosciences). Data were gathered with an FC500 flow cytometer (Beckman Coulter) and were analyzed with FlowJo analysis software (Tree Star). In the analysis, lymphocytes were selected on the basis of forward and side scatter characteristics, followed by selection of CD8+ cells, and then the tetramer-positive CD8+ T cell population.
Intracellular cytokine staining
Spleens harvested from treated mice were transferred into Liebowitz’s-15 (L-15, Cellgro; Mediatech) at room temperature. The tissue was then homogenized and passed through a 70-μm nylon cell strainer (Fisher Scientific) to remove cell clumps and un-dissociated tissue. The cells were centrifuged for 5 min at 1600 rpm at room temperature, resuspended in fresh L-15 medium, and centrifuged again. The cell pellet was resuspended in T cell assay medium (DMEM, Cellgro; Mediatech), 10% heat-inactivated FBS (HyClone), 1% penicillin/streptomycin (Cellgro; Mediatech), 1% l-glutamine, 10 mM HEPES (Cellgro; Mediatech), 0.1 mM nonessential amino acids (Invitrogen), sodium pyruvate, and 10−6 M 2-ME (Cellgro; Mediatech).
After resuspending at a concentration of 107 cells/ml, splenocytes were plated at 106/well in triplicate on 96-well round-bottom plates. T cell assay medium was supplemented with 1 μg/ml brefeldin A (GolgiPlug; BD Pharmingen) and 20 ng/ml mouse IL-2 (BD Pharmingen). A total of 1 μg/ml β-gal CD8 H2-Kb T cell epitope (ICPMYARV; Mimotopes), 2 μg/ml GFP or luciferase peptide libraries, or 2 μg/ml AAV8 or AAVrh32.33 capsid peptide libraries were added to the corresponding experimental wells. Control cells were incubated without peptide, or in the presence of PMA (0.05 μg/ml) and ionomycin (1 μg/ml) (PMA/I; Sigma-Aldrich). Cells were incubated for 5 h at 37°C,in 10% CO2. Following the stimulation, cells were washed and stained with FITC-conjugated anti-mouse CD8α (Ly-2) Ab (BD Pharmingen) for 30 min at 4°C. Cells were washed with PBS/1% FBS, then permeabilized in Cytofix/Cytoperm solution at 4°C for 20 min. Cells were washed again thoroughly with 1X Perm/Wash Buffer and stained with anti-cytokine Abs including anti-mouse IFN-γ PE, TNF-α, PE-Cy7, and IL-2 allophycocyanin (BD Pharmingen) for 45 min at 4°C. After washing, cells were examined by flow cytometric analysis. Samples were acquired on an FC500 (Beckman Coulter) and analyzed using FlowJo software (Tree Star).
IFN-γ ELISPOT
Splenocytes from treated mice were harvested as described. Following isolation and washing steps, splenocytes were overlaid onto a Ficoll-Paque (Amersham Biosciences) gradient layer and centrifuged for 20 min at 2000 rpm at room temperature to remove RBC. The lymphocyte band was then recovered and further washed two times in PBS/1% FBS.
To determine the number of cells secreting IFN-γ in response to antigenic stimulation, an IFN-γ ELISPOT assay was performed according to the manufacturer’s instructions (BD Biosciences). Briefly, 96-well plates were coated with capture Ab overnight at 4°C, and blocked for 2 h at 25°C with RPMI 1640, 10% FBS, 1% penicillin-streptomycin-l-glutamine. Splenocytes were plated at two densities (105 and 2.5 × 105 cells/well) in T cell assay medium supplemented with 1 μg/ml nLacZ dominant peptide (ICPMYARV; Mimotopes), 2 μg/ml GFP or luciferase peptide libraries, or 2 μg/ml AAV8 or AAVrh32.33 capsid peptide libraries. PMA/I (Sigma-Aldrich) was used to stimulate a separate population of lymphocytes (plated at 2 × 104/well) as a nonspecific, positive control. Cells plated in medium alone (no peptide stimulation) served as a negative control. Plates were incubated for 18 h at 37°C, 5% CO2. Following incubation plates were washed vigorously in deionized water, PBS/0.05% Tween 20 (Sigma-Aldrich), and incubated for 2 h at room temperature with 2 μg/ml of biotinylated anti-mouse IFN-γ detection Ab. Following three washes with PBS/0.05% Tween 20, the plates were incubated with 5 μg/ml enzyme conjugate (streptavidin-HRP) for 1 h at room temperature. Following a series of washes with PBS/0.05% Tween 20 and PBS, spots were developed using the AEC Substrate Set (BD Pharmingen). Color development was stopped after 8 min by washing with distilled water. Plates were dried overnight at room temperature and read using the AID ELISPOT reader system (Cell Technology). Responses greater than 200 spot-forming units (SFU) or at least 2 logs over background were considered.
AAVrh32.33 model building
AAVrh32.33 shows highest sequence similarity to AAV4 among the AAV serotypes for which crystal structures are available. Thus the AAVrh32.33 VP3 monomer three-dimensional model was generated from its primary amino acid sequence (NCBI Accession no. ACB55318) (http://www.ncbi.nlm.nih.gov/) using the SWISS-MODEL model building program (35) with the structure of the AAV4 VP3 coordinates (PDB Accession No. 2G8G) (http://www.rcsb.org/pdb/home/home.do) (25) as a template. The model of AAVrh32.33 VP3 was then superimposed onto the AAV8 crystal structure (PDB Accession no. 2QA0) (http://www.rcsb.org/pdb/home/home.do) (22) using the secondary-structure matching option (36) of the COOT program (37). Stretches of two or more Cα positions that were greater than 1 Å apart between the two structures were identified as variable loop regions. To visualize where these variable regions are located within the predicted structure of the assembled AAVrh32.33 capsid, icosahedral symmetry operators were applied to the VP3 model coordinates by matrix multiplication using the program O (38) and the variable regions were highlighted in the context of the viral structural proteins within one viral asymmetric unit. The surface representation image was generated using the program PyMOL (39).
Statistical analysis
Statistical analysis was performed using the SigmaStat 3.5 program (SPSS). Statistical significance was set at p < 0.05 and statistical power at 0.80. Results are presented as the sample average ± SD. Two-group comparisons were made using the Mann-Whitney rank sum U test when data were not normally distributed, or the unpaired Student’s t test when data sets followed a normal distribution. ANOVA Student-Newman-Keuls test was used for multiple group comparisons.
Results
Functional comparison between AAV8 and AAVrh32.33 vectors
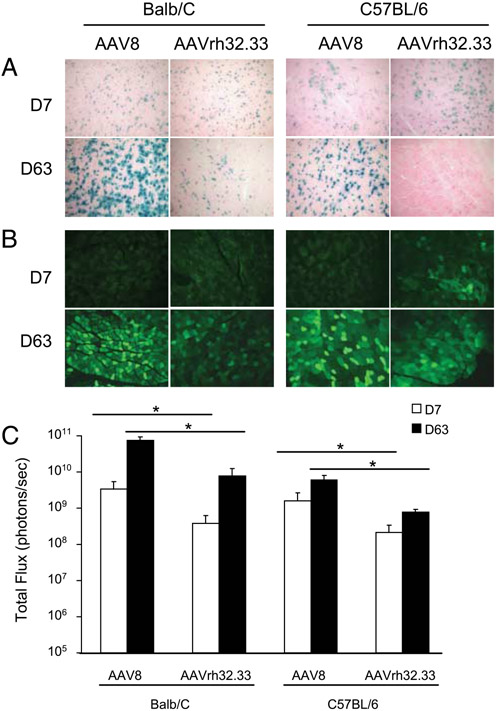
Our initial objective was to characterize the effect of the AAV capsid on vector performance and transgene stability in murine skeletal muscle. We compared AAV2/8 with AAV2/rh32.33 in vivo following an i.m. injection of 1011 GC in C57BL/6 and BALB/c mice. The onset and stability of expression was monitored using the classical reporter molecules nLacZ, enhanced GFP, and firefly luciferase (ffLuc). AAV2/8 was stable with all transgene products tested, but this result did not hold true for AAV2/rh32.33 (Fig. 1). AAV2/8.nLacZ expression was stable through day 63 with no visible signs of infiltration (Fig. 1A). AAV2/rh32.33.nLacZ showed a comparable degree of β-gal expression to AAV2/8 at day 7; however, by day 63 the number of β-gal-positive cells was substantially reduced. At day 63, few residual β-gal-positive fibers remained in the BALB/c strain, whereas expression in C57BL/6 mice was completely lost (Fig. 1A). There were no discernible differences in the onset of GFP expression from AAV2/8 and AAV2/rh32.33 by day 7 (Fig. 1B). In both strains, GFP expression from both AAV2/8 and AAV2/rh32.33 increased between days 7 and 63. The number of GFP-positive fibers at day 63 was slightly lower with AAV2/rh32.33 compared with AAV2/8. Luciferase expression was significantly higher with AAV2/8 vs AAV2/rh32.33 at all time points (Fig. 1C). Unlike the decline in transgene expression observed with AAV2/rh32.33.nLacZ and GFP, total flux of luminescence remained high for both AAV2/8.ffLuc and AAV2/rh32.33.ffLuc vectors through day 63.
FIGURE 1.
Stability of transgene expression in mouse skeletal muscle. C57BL/6 male mice were injected in the left tibialis anterior with 1011 GC of AAV2/8, and AAV2/rh32.33 vectors expressing the classical reporter molecules nLacZ (A), GFP (B), and ffLuc (C). At 7 and 63 days postinjection mice were sacrificed and skeletal muscle harvested for X-Gal histochemical stain (A) and immunohistochemistry (B). Live imaging was performed using a Xenogen imager to quantify luciferase expression in the skeletal muscle (C). Data are mean ± SD for four mice per group. Images represent regions of highest expression within an individual section. Statistical analysis compared AAV2/8 with AAV2/rh32.33 vectors for each time point. *, p < 0.001, by unpaired Student’s t test.
The effect of recombinant AAV vector capsid on T cell responses toward capsid and transgene Ags
The decline in transgene expression observed with AAV2/rh32.33.nLacZ and GFP, but not AAV2/8 vectors, led us to investigate whether AAV2/rh32.33 generated a greater degree of cellular immunity toward the transgene product. At day 28, lymphocytes were isolated from the spleen for IFN-γ ELISPOT assay (Fig. 2). Overall, compared with AAV2/8, the number of IFN-γ-secreting T cells toward the transgene product was consistently greater with AAV2/rh32.33. For example, nLacZ-specific IFN-γ-producing T cells were low following AAV2/8.CB.nLacZ injection (<200 SFU/106 splenocytes in both strains), whereas the response was significantly higher with AAV2/rh32.33 (averaging 638 SFU/106 splenocytes in BALB/c mice and 1264 SFU/106 splenocytes in their C57BL/6 counterparts) (Fig. 2A). The same trend was observed with GFP in C57BL/6 mice, where AAV2/8 vectors generated little to no IFN-γ-producing GFP-specific T cells (66 SFU/106 splenocytes), whereas AAV2/rh32.33 drove a significantly greater response (495 SFU/106 splenocytes). In BALB/c mice in which there is a dominant CD8+ T cell epitope to GFP, a less dramatic difference in transgene response is observed between AAV2/8 and AAV2/rh32.33, with both generating high cell numbers at 463 and 545 SFU/106 splenocytes, respectively (Fig. 2A). This suggests that highly immunodominant transgenes may overcome the threshold required to generate T cells even in the presence of the AAV8 capsid. The T cell response to luciferase was also high for both vectors, in both mouse strains (Fig. 2A). Although it is possible for AAV2/8 vectors to elicit transgene T cell responses in some cases, under no circumstances was AAV2/8 able to generate T cell responses greater than AAV2/rh32.33.
FIGURE 2.
Recombinant AAV vector capsid impacts the activation of IFN-γ-producing T cells following i.m. injection. C57BL/6 mice were injected with 1011 GC of AAV2/8 or AAV2/rh32.33 expressing the classical reporter molecules nLacZ, GFP, or ffLuc. At 28 days postinjection, mice were sacrificed and lymphocytes harvested from spleen. IFN-γ secretion in response to Ag stimulation was determined by IFN-γ ELISPOT. A, Lymphocytes were stimulated with the H-2Kb-ICPMYARV dominant epitope of nLacZ (C57BL/6 mice), the H-2Ld-TPHPARIGL dominant epitope of nLacZ (BALB/c mice), or the complete peptide libraries of GFP or luciferase to determine the transgene response. B, Cells were stimulated with the capsid peptide libraries of AAV8 and AAVrh32.33 to determine the capsid response. Data are mean ± SD for four mice per group. Statistical analysis compared AAV2/rh32.33 to AAV2/8 vectors for each transgene product. *, p < 0.001, by Mann-Whitney rank sum U test (A) or unpaired Student’s t test (B).
Splenocytes were also stimulated with the AAV8 and AAVrh32.33 capsid peptide libraries to measure capsid-specific IFN-γ-producing T cell responses in C57BL/6 and BALB/c mice. Fig. 2B shows that AAV2/8 vectors generated little to no capsid-specific T cells in either strain (<25 and <50 SFU/106 splenocytes in BALB/c and C57BL/6 mice, respectively), consistent with previous reports. The AAVrh32.33 capsid, in contrast, was highly immunogenic irrespective of transgene, generating strong AAVrh32.33-specific IFN-γ-producing T cells in both BALB/c (150–200 SFU/106 splenocytes) and C57BL/6 mice (1000–1400 SFU/106 splenocytes). It is important to note that capsid T cell responses were not cross-reactive. For example, cells isolated from mice injected with AAV2/rh32.33.nLacZ did not respond when stimulated with the AAV8 capsid peptide library and vice versa (data not shown).
Characterization of the T cell response to AAV8 and AAVrh32.33
For a more in depth comparison of the T cell responses to these vectors, we focused on AAV2/8 and AAV2/rh32.33 expressing nLacZ in C57BL/6 mice, as it provided the most definitive example of differential immune activation and the influence of AAV capsid on the generation of immunity. An evaluation of the kinetics of this response confirmed that AAV2/8.nLacZ generates minimal T cell activation to either the AAV8 capsid (Fig. 3A) or to the nLacZ transgene, by IFN-γ ELISPOT (Fig. 3B) or nLacZ-specific MHC class I tetramer stain (Fig. 3B). When compared with mice injected with sterile PBS alone, the T cell responses to AAV2/8.nLacZ capsid do not rise above background levels at any point during the response (Fig. 3A and data not shown). In contrast, T cells to the AAVrh32.33 capsid are barely above background at day 7, expand and peak at around 4 wk postinjection, then contract over time through day 63 (Fig. 3A). A similar kinetic response to the nLacZ transgene was observed from the AAV2/rh32.33 vector (Fig. 3B).
FIGURE 3.
Kinetics of T cell activation following i.m. injection of AAV2/8, and AAV2/rh32.33. C57BL/6 mice were injected with 1011 GC of AAV.nLacZ in the left tibialis anterior. At days 7, 28, and 63 postinjection, mice were sacrificed and lymphocytes harvested from spleen. IFN-γ-producing T cell responses to AAV capsid (A) and nLacZ transgene (B, primary axis) were determined by IFN-γ ELISPOT. At various time points postinjection, lymphocytes were isolated from whole blood and stained with a PE-conjugated H-2Kb-ICPMYARV tetramer together with a FITC-conjugated anti-CD8 Ab to determine the percentage of nLacZ-specific CD8+ T cells in the total CD8+ T cell population (B, secondary axis). Data are mean ± SD for four mice per group. Statistical analysis compared AAV2/8 with AAV2/rh32.33 at day 28. *, p < 0.001, by unpaired Student’s t test.
The kinetics of the T cell response to AAV2/rh32.33 and AAV2/8 were consistent with the degree of cellular infiltration present in the muscles of these mice 28 days postinjection with vector (Fig. 4). At the peak of the response, mice injected with AAV2/rh32.33 had a large number of cellular infiltrates detectable by X-Gal histochemical stain (Fig. 4A). The degree of cellular infiltration to AAV2/8.nLacZ was minimal, reflecting what is seen when mice are injected with PBS alone (Fig. 4A and data not shown). The difference in total cellular infiltration was confirmed by confocal microscopy (Fig. 4B), where AAV8 shows little infiltration of CD4+ or CD8+ T cells. In contrast, these cells are highly abundant in mice injected with AAV2/rh32.33.nLacZ (Fig. 4C). Sections were stained for Foxp3, a marker for regulatory T cells; very few Foxp3+ CD4 T cells were observed (Fig. 4B).
FIGURE 4.
Cellular infiltration in skeletal muscle following i.m. injection of AAV2/8 or AAV2/rh32.33 expressing nLacZ. Day 28 postinjection of 1011 GC into the right tibialis anterior of C57BL/6 mice, skeletal muscle was harvested for X-Gal histochemical stain (A) and confocal microscopy (B and C). Sections were stained with DAPI (B), and CD8-TRITC shown in blue, CD4-Cy5 in green, and Foxp3-FITC Abs in red (C). Representative sections are from four mice per group.
At 3 wk postinjection of AAV2/8 or AAV2/rh32.33 vectors, splenocytes were harvested and stained for expression of IFN-γ, TNF-α, and IL-2 by CD8+ T cells using intracellular cytokine staining. Before staining, cells were stimulated with either the AAV8 or AAVrh32.33 capsid peptide libraries (Fig. 5A) or the β-gal CD8-dominant epitope ICPMYARV (Fig. 5B) to determine the capsid- and transgene-specific T cell responses. The AAV8 capsid library was used to stimulate cells from AAV2/8-injected animals and the AAVrh32.33 capsid library was used to stimulate AAV2/rh32.33-injected animals. Data are expressed as the percentage of CD8+ T cells capable of secreting individual or multiple cytokines. CD8+ T cells to AAV2/rh32.33.nLacZ capsid were highly functional; substantial IFN-γ, TNF-α–producing single positive and double positive populations were observed. Single, double, and triple positive populations involving IL-2 production were present, although very few cells were able to express IL-2. The same trends were observed when the cells from these animals were stimulated with the β-gal CD8-dominant epitope (Fig. 5B). Cytokine production by CD8+ T cells from mice injected with AAV2/8.nLacZ was low following stimulation with the AAV8 capsid library or β-gal-dominant peptide, reflecting the levels seen in mice injected with a PBS vehicle control (Fig. 5 and data not shown).
FIGURE 5.
Characterization of the T cell response to AAV.nLacZ in the presence or absence of CD4+ T cell help. C57BL/6 mice were injected with 1011 GC of AAV2/8 or AAV2/rh32.33.nLacZ in the presence or absence of the CD4+ T cell-depleting Ab, GK1.5. Lymphocytes isolated from spleen were stimulated with AAV8 and AAVrh32.33 capsid libraries (A) or the nLacZ dominant peptide (B), then evaluated by intracellular cytokine staining to measure the production of IFN-γ, TNF-α, and IL-2 cytokines by CD8+ T cells. Data are expressed as the frequency of multiple cytokine-secreting Ag-specific CD8+ T cells present 3 wk after i.m. injection. Data are mean ± SD for four mice per group. Representative data from two independent experiments are shown. Statistical analysis compared AAV2/8, AAV2/rh32.33, and AAV2/rh32.33 in combination with GK1.5 for each cytokine profile. *, p < 0.001; **, p < 0.015, by ANOVA Student-Newman-Keuls test.
The role of CD4+ Th cells and costimulation in generating capsid and transgene responses
One potential mechanism of T cell activation to AAV2/rh32.33.nLacZ involves CD4+ T cells providing help to prime a functional CD8 response. To test this hypothesis C57BL/6 mice were injected with AAV2/rh32.33.nLacZ in the presence of the CD4-depleting Ab, GK1.5. The peak IFN-γ-producing T cell response to capsid and transgene was monitored by ELISPOT. The nLacZ-specific CD8+ T cell response was evaluated by MHC class I tetramer stain, and muscles were sectioned to analyze cellular infiltration and expression stability by X-Gal histochemical stain (Fig. 6). Intracellular cytokine staining was also performed to characterize the functionality of the CD8+ T cell responders (Fig. 5). In the absence of CD4+ T cell help, capsid- and transgene-specific T cell responses were completely ablated (Fig. 6). The remaining CD8+ T cell population was no longer able to produce IFN-γ, TNF-α, or IL-2 in any combination above background levels (Fig. 5). In the absence of a T cell response, β-gal expression in the skeletal muscle was stable at day 28 with no visible signs of cellular infiltration, in contrast to what is normally seen following AAV2/rh32.33.nLacZ (Fig. 6).
FIGURE 6.
Impairment of CD4, CD40L, or CD28 ablates the IFN-γ-producing T cell response to AAV2/rh32.33.nLacZ and restores β-gal expression in skeletal muscle. C57BL/6, CD40L−/−, and CD28−/− mice were injected with 1011 GC of vector in combination with either the CD4-depleting Ab GK1.5, CD40L blocking Ab MR-1, or the CD40 agonist FGK45. Mice were sacrificed 28 days postinjection with vector, and lymphocytes were isolated from the spleen. Following stimulation with the AAVrh32.33 capsid library or the nLacZ dominant peptide, T cell responses were determined by IFN-γ ELISPOT (primary axis). Lymphocytes isolated from whole blood were stained using the PE-conjugated H-2Kb-ICPMYARV tetramer together with a FITC-conjugated anti-CD8 Ab to determine the percentage of nLacZ-specific CD8+ T cells in the total CD8+ T cell population (secondary axis). X-Gal histochemical staining was performed on sectioned skeletal muscle. Representative sections from four mice per group are shown. Data are mean ± SD for four mice per group, and represent five independent experiments performed using common controls. Statistical analysis compared AAV2/rh32.33 alone in wild-type C57BL/6 mice with AAV2/rh32.33 given in combination with GK1.5, MR-1, FGK45 in CD40L−/− and CD28−/− mice. *, p < 0.001, by ANOVA Student-Newman-Keuls test.
One proposed mechanism of CD4+ T cell help involves CD40L (CD154) on the CD4+ T cell licensing the APC by signaling through CD40. The “licensed” APC then up-regulates expression of proinflammatory cytokines, molecules involved in Ag processing and presentation, as well as cell surface molecules including B7 (CD80/86). B7 is a costimulatory molecule that then interacts with CD28 on the CD8+ T cell to provide a necessary second signal during T cell priming. We examined whether blockade of these interactions would reduce the CD8+ T cell responses to AAV2/rh32.33.nLacZ. The importance of CD40L signaling in generating T cells to AAV2/rh32.33.nLacZ was confirmed in two ways: using the CD40L blocking Ab, MR-1, as well as CD40L−/− mice. In both cases, in the absence of CD40L, the T cell response to AAV2/rh32.33.nLacZ was ablated and β-gal expression was stable in the muscle through day 28 with no apparent infiltration (Fig. 6). The role of CD28 was confirmed using CD28−/− mice, where the capsid and transgene T cell responses were completely or partially ablated, respectively (Fig. 6). Residual nLacZ-specific CD8+ T cells may account for the very minor degree of cellular infiltration and the loss of β-gal expression still observed. In an attempt to restore the strong T cell response in our system, CD40L−/− mice were coinjected with AAV2/rh32.33.nLacZ in combination with the CD40 agonistic Ab, FGK45. To our surprise, exogenous activation of CD40 in the absence of its ligand was not sufficient to restore the functional T cell responses to either capsid or transgene Ags (Fig. 6). This suggests that the CD4-dependent CD8+ T cell responses to recombinant AAV vectors may not strictly follow the classical pathway of CD4 help.
Structural differences between the capsids of AAV8 and AAVrh32.33
Phylogenetically, AAV8 and AAVrh32.33 are two of the most divergent capsid variants. In comparison, the AAV2 capsid differs from AAV8 by 16.5% of VP1 amino acid composition, whereas AAVrh32.33 differs from both AAV2 and AAV8 capsids by over 32%. A superimposition of viral protein (VP) monomers of the AAVrh32.33 model and the AAV8 crystal structure shows that the core β-barrel is highly conserved as has been observed among all the known parvovirus structures. Structurally variable loops lie interspersed between the β-strands (Fig. 7A). Structural differences between the AAV8 and AAVrh32.33 capsids were identified in each of the nine variable regions (I to IX) defined when comparing AAV2 and AAV4 (25). This result is consistent with the fact that AAV8 is highly homologous to AAV2, and AAVrh32.33 is similar to AAV4, respectively. The most prominent differences, due to insertions or deletions of amino acids residues in AAVrh32.33 relative to AAV8, were observed in variable regions I and III-VII (Fig. 7A). These variable regions are spread throughout the VP monomer, but are clustered from symmetry related VP monomers on the capsid surface when displayed in the context of a viral asymmetric unit in a surface representation of nine VP monomers (Fig. 7B). For instance in Fig. 7B, regions IV (green) and VIII (purple) of a monomer are adjacent to regions V (cyan) and VI (blue) of a 3-fold-related monomer. The most variable regions, I (red), III (yellow), IV (green), V (cyan), VI (blue), and VII (magenta), are clustered around the icosahedral 2- and 3-fold symmetry axes.
FIGURE 7.
Structural comparison of the AAV8 and AAVrh32.33 capsids. A, Superimposition of the VP3 monomer of AAV8 (green) and the predicted model for AAVrh32.33 (orange). The major differences between AAV8 and AAVrh32.33 are located in variable regions I and III-VII. B, Predicted AAVrh32.33 VP3 structure (orange monomer) and the icosahedral 5- (5f), 3- (3f), and 2-fold (2f) symmetry VP monomers (all in light gray) inside gray surface density. Variable loops of AAVrh32.33 that differ from AAV8 appear as colored regions on the capsid surface: I-red, II-dark green, III-yellow, IV-green, V-cyan, VI-blue, VII-magenta, VIII-purple, IX-dark gray. The triangle defines a viral asymmetric unit bounded by 5-, 3-, and 2-fold icosahedral symmetry axes.
Discussion
Understanding the immune consequences of AAV gene therapy is pivotal in the translation of this vector platform to the clinic. Vectors based on AAV fail to elicit cellular immunity to antigenic transgene products and give rise to long-term transgene expression in most murine models, which is a desirable property for gene therapy (2). Recent studies have better characterized the nature of the T cell responses to vectors based on a number of commonly used AAV capsids showing the production of dysfunctional transgene-specific T cells (40, 41), which may reflect the induction of tolerance (42). However, AAV-mediated expression of a foreign transgene in non-human primates is transient in nature, as the transgenic Ag raises a destructive cellular immune response (Gao et al., manuscript in preparation). In addition, the generation of CD8 T cells to the AAV capsid in a clinical trial of liver-directed gene transfer, and the concurrent development of liver toxicity, is another illustration of the limitations of our current animal models (32).
A better understanding of the critical vector-cell interactions that drive or suppress effector T cell responses would be helpful in predicting and managing vector-host interactions in the clinic. One approach we have taken is to use natural variation in AAV capsid structure to map structural domains that may mediate these key steps in T cell activation. To this end, we identified two structurally related AAV capsids from natural variants AAV2/8 and AAV2/rh32.33 that produce qualitatively different effector T cell responses to the transgene product. The goal of this study was to better understand the key cellular steps in CD8+ T cell activation where these two vectors diverge.
By comparing AAV2/8 to AAV2/rh32.33 vectors expressing nLacZ in murine muscle, we were able to model differential immune activation to the vector capsid and vector encoded transgene. AAV8.nLacZ elicits virtually no immune response to either nLacZ or the capsid, consistent with previous reports of aberrant T cell responses in mice (40, 41, 43). AAVrh32.33.nLacZ, however, overcomes the threshold required for immune activation, to generate a strong T cell response to both the AAVrh32.33 capsid as well as the nLacZ transgene. T cell responses correlate with a high degree of cellular infiltration in the skeletal muscle and a loss of β-gal expression over time, reflecting what is normally seen with a more immunogenic vector, such as adenovirus-expressing LacZ (44). Our hypothesis would predict that capsid variation impacts on T cell activation through interactions with the host cell such as antigenicity, receptor binding, and transduction efficiency (24, 25).
The ability of AAVrh32.33 to drive capsid and transgene T cell responses that are equal to or greater than responses seen with AAV8 was consistent for nLacZ, GFP, and ffLuc in both C57BL/6 and BALB/c mice. Otherwise stated, the effect of capsid structure on the CTL response was independent of transgene product or MHC haplotype. Consistent with what others have shown, the magnitude of the transgene-specific T cell response to these vectors was influenced not only by the capsid, but also by the inherent antigenicity of the encoded transgene (45). For instance, in C57BL/6 mice, where GFP is minimally immunogenic, AAV8 was unable to generate GFP-specific T cells (46). However, in BALB/c mice where there is a strong immunodominant epitope to GFP, AAV8 elicited a substantial GFP-specific response, suggesting that the immunogenicity of the transgene overcame the threshold for generating T cells and bypassed the need for support from the AAV capsid (47). The AAV2/rh32.33 capsid was able to overcome this threshold and elicit a strong GFP-specific T cell response even in the C57BL/6 strain. It is also important to note that the presence of transgene-specific T cells did not always correlate with a loss of transgene expressing cells over time. Although AAV2/rh32.33.nLacZ and GFP resulted in a decline in transgene expression by day 63, expression from AAV2/rh32.33.ffLuc was stable despite a large number of CD8+ T cells to the luciferase transgene. The presence of substantial numbers of dysfunctional transgene-specific T cells at the peak of the response may be the result of persistent, high level Ag expression leading to T cell exhaustion and the loss of functionality (48).
The ability of capsid structure to influence not only the capsid-specific but also the transgene-specific CD8+ T cell response could be interpreted in two different ways: either the AAVrh32.33 capsid is augmenting a normally weak cellular response to AAV, or the AAV8 capsid is suppressing the standard CTL response seen with AAVrh32.33, or some combination of the two ideas. Several serotypes, such as AAV2, AAV7, and AAV8, are known to generate dysfunctional CD8+ T cell responses to an HIVgag transgene in mice (40, 41). Although HIVgag-specific CD8+ T cell responses are primed by these vectors, these cells are unable to secrete cytokines or undergo proliferation in response to Ag re-exposure. Moreover, in the current study, AAV8.nLacZ was unable to generate a substantial CD8+ T cell population to either capsid or transgene Ags. The aberrant T cell responses to AAV8 capsid-based vectors could result from anergy, functional exhaustion, or active suppression. Cao et al. (42) has provided evidence that hepatic gene transfer of AAV2 vectors results in the induction of CD4+CD25+Foxp3+ regulatory T cells. The authors suggest that regulatory T cells are capable of inducing tolerance, suppressing transgene-specific immune responses. Lin et al. (41), also noted an increase in CD4+CD25+ T cells following AAV2/7.HIVgag immunization; however, the depletion of CD25+ regulatory T cells did not improve the proliferative capacity of the transgene-specific CD8+ T cells in this case. Analysis of CD4 T cell responses to capsid and transgene Ags following AAV2 gene transfer to mice and nonhuman primates showed poor proliferative responses and little to no secretion of cytokines (49). The classic AAV serotype, AAV2, has been shown to elicit poor innate immune activation when compared with adenovirus, potentially explaining the weak activation of CD4 T cells (50). Delayed or incomplete activation of CD4+ T cells by AAV could prevent proper APC recruitment, licensing, and the expression of costimulatory molecules required to avoid functional anergy (45). However, studies attempting to restore the proliferative capacity of AAV7-induced HIVgag-specific CD8+ T cells by coadministration of adjuvants suggested that TLR-induced nonspecific inflammation alone failed to generate CTLs to AAV (41). In this same study, the authors demonstrate that AAV-induced transgene-specific T cells express markers of exhaustion such as PD-1; however, blockage of PD-1 is unable to rescue the proliferative capacity of these cells (41). Ultimately, the affect of capsid structure on cellular immunity is multifaceted and both AAV8-mediated suppression as well as AAVrh32.33-mediated enhancement may play a role. Future studies evaluating the role of AAV capsid structure in specific vector-host cell interactions are necessary to further delineate this mechanism.
Our next goal was to use this model of cellular immunity to characterize the mechanism of T cell activation to AAVrh32.33 in murine skeletal muscle. Sarukhan et al. (45) has demonstrated that AAV2 results in delayed CD4+ T cell kinetics when compared with the more immunogenic adenoviral vectors. The high degree of CD4 infiltration in skeletal muscle with AAVrh32.33, but not with AAV8, supports the hypothesis that CD4 T cells are necessary to promote a CD8+ T cell response. Indeed, the depletion of CD4+ T cells in this system completely ablates the generation of CD8+ T cells to AAVrh32.33, concurrently alleviating the associated cytotoxic sequelae. Numerous studies have demonstrated the requirement for CD4 help in priming CD8+ T cell responses. However, certain pathogens, including acute lymphocytic choriomeningitis virus and Listeria monocytogenes, are CD4-independent (51-54). Using L. monocytogenes as a model, Shedlock et al. (51) has shown that CD8+ T cell responses are likely to require CD4 help when the pathogen is not immunogenic enough to directly activate APCs. Thus, the dependence of AAV on CD4+ T cell help is not surprising given the noninflammatory nature of the vector (50).
One proposed mechanism of CD4+ T cell help involves CD40L+ CD4+ T cells “licensing” CD40-expressing APCs that are then capable of priming naive CD8+ T cells in a CD28-dependent fashion (55-57). Licensed APCs up-regulate the expression of costimulatory molecules (CD80/86), cytokines (namely, IL-12), and Ag processing and presentation machinery (58-60). Alternatively, CD40L-bearing CD4+ T cells may provide help directly by engaging CD40-expressing CD8+ lymphocytes (61). In the case of AAVrh32.33, ablation of CD8+ T cell responses to both capsid and transgene Ags in the absence of CD40L confirms that CD40-CD40L signaling is necessary to the CD4-dependent CD8+ T cell response. CD40-CD40L signaling is not always required to facilitate CD4+ T cell help: Lu et al. (62) demonstrated that dendritic cells from CD40−/− mice were equally capable of being licensed by CD4+ Th cells as their wild-type counterparts in vitro. In vivo, well-characterized viral models demonstrate that the requirement for CD40-CD40L costimulation is pathogen-dependent. Although CD40L deficiency does not affect the CD8+ T cell response to lymphocytic choriomeningitis virus or herpes simplex virus, the primary CD8+ T cell response to vesicular stomatitis virus is significantly impaired in the absence of CD40-CD40L signaling (63, 64).
Many studies using CD4− or CD40L−/− mice have shown that exogenous CD40 activation using the agonistic Ab FGK45 can bypass the need for CD4 help, restoring the CD8+ T cell response (55-57, 60). However, our data show that this bypass is not the case with AAVrh32.33, where the CD8+ T cell response was not restored in CD40L−/− mice supplemented with FGK45. These results indicate that CD40-CD40L signaling is necessary but not sufficient to support the generation of CD8+ T cells to both capsid and transgene Ags. This result may suggest a role for CD40L signaling beyond the activation of APCs or CD8s alone, as well as a role for additional molecules in priming CD8 cells. One additional molecule required for the induction of cellular immunity to AAVrh32.33 is CD28; the engagement of CD80/86 on the APC with CD28+CD8+ T cells is a necessary second signal in T cell priming, without which primed T cells are often functionally anergic and unable to proliferate and secrete IL-2 in response to subsequent Ag encounter (65).
The availability of high resolution structures of AAV8 and a close relative of AAVrh32.33 called AAV4 provided an opportunity to observe regions on the surface that may mediate differential receptor binding relevant to the divergent T cell responses. These two capsids retain a substantial amount of three-dimensional structural similarity, although differences were noted at the previously described variable regions. The most substantial structural differences were mapped to six regions (I and III-VII) spanning variable sequences. At least four of these regions colocalize within a single surface domain as visualized by the assembly of nine VP monomers. Current efforts are directed at using this structural data to guide the design of hybrids between AAV8 and AAVrh32.33. Studies comparing the performance of hybrids in the presence or absence of specific structural domains will be necessary to map the capsid domain responsible for differential T cell activation.
In summary, our findings demonstrate that the AAVrh32.33 capsid, unlike other traditionally studied AAV serotypes, generates robust polyfunctional CD8+ T cells to both capsid and transgene Ags. These T cells appear to be activated in a classical, CD4-dependent pathway involving CD40L signaling and CD28 costimulation. Further comparison of the AAVrh32.33 capsid to the structurally and functionally divergent AAV8 capsid in this murine model will allow us to assess differential vector-host cell interactions to understand the mechanism by which traditional AAV serotypes avoid the activation of cellular immunity.
Acknowledgments
We thank Arbans Sandhu and Julie Johnston at the PennVector Facility (University of Pennsylvania) for supplying the recombinant AAV vectors, and Deirdre McMenamin and Regina Munden (University of Pennsylvania) for assistance with animal studies.
This work was supported by Grants P01-HL059407, P30-DK47757 (to J.M.W.), T32-AR053461-03 (to L.E.M.), and R01-GM082946-01 (to M.A.M.) from the National Institutes of Health and by GlaxoSmithKline. Part of the information included in this paper was presented in The Eleventh Annual Meeting of the American Society of Gene Therapy held in Boston, MA, May 28 through June 1, 2008, as Oral Abstract no. 425, as well as The Twelfth Annual Parvovirus Workshop held in Cordoba, Spain, June 1 through 5, 2008, as Oral Abstract no. 3.9.
Footnotes
Abbreviations used in this paper: AAV, adeno-associated virus; β-gal, β-galactosidase; GC, genome copy; SFU, spot-forming unit; nLacZ, nuclear-targeted LacZ; VP, viral protein.
Disclosures
L. Vandenberghe and J. Wilson are inventors on patents licensed to various biopharmaceutical companies. J. Wilson holds equity in, consults for, and receives a grant from ReGenX Inc. The remaining authors have no financial conflict of interest.
References
- 1.Verma IM, and Weitzman MD. 2005. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem 74: 711–738. [DOI] [PubMed] [Google Scholar]
- 2.Zaiss AK, and Muruve DA. 2008. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 15: 808–816. [DOI] [PubMed] [Google Scholar]
- 3.Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM, et al. 1999. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med 5: 56–63. [DOI] [PubMed] [Google Scholar]
- 4.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, Stafford DW, Patel S, Thompson AR, Nichols T, et al. 1999. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med 5: 64–70. [DOI] [PubMed] [Google Scholar]
- 5.Geoffroy MC, and Salvetti A. 2005. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr. Gene Ther 5: 265–271. [DOI] [PubMed] [Google Scholar]
- 6.Melnick JL, Mayor HD, Smith KO, and Rapp F. 1965. Association of 20-millimicron particles with adenoviruses. J. Bacteriol 90: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison RW, Casto BC, and Hammon WM. 1965. Adenovirus-associated defective virus particles. Science 149: 754–756. [DOI] [PubMed] [Google Scholar]
- 8.Muramatsu S, Mizukami H, Young NS, and Brown KE. 1996. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 221: 208–217. [DOI] [PubMed] [Google Scholar]
- 9.Padron E, Bowman V, Kaludov N, Govindasamy L, Levy H, Nick P, McKenna R, Muzyczka N, Chiorini JA, Baker TS, and Agbandje-McKenna M. 2005. Structure of adeno-associated virus type 4. J. Virol 79: 5047–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutledge EA, Halbert CL, and Russell DW. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol 72: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, and Wilson JM. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99: 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, Calcedo R, Sanmiguel J, Abbas Z, and Wilson JM. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA 100: 6081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, and Wilson JM. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol 78: 6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Wang L, Takeuchi T, and Kanda T. 2004. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 330: 375–383. [DOI] [PubMed] [Google Scholar]
- 15.Calcedo R, Vandenberghe LH, Gao G, Lin J, and Wilson JM. 2009. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis 199: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayor HD, and Melnick JL. 1966. Small deoxyribonucleic acid-containing viruses (picodnavirus group). Nature 210: 331–332. [DOI] [PubMed] [Google Scholar]
- 17.Chapman MS, and Rossmann MG. 1993. Structure, sequence, and function correlations among parvoviruses. Virology 194: 491–508. [DOI] [PubMed] [Google Scholar]
- 18.Muralidhar S, Becerra SP, and Rose JA. 1994. Site-directed mutagenesis of adeno-associated virus type 2 structural protein initiation codons: effects on regulation of synthesis and biological activity. J. Virol 68: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kronenberg S, Kleinschmidt JA, and Bottcher B. 2001. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. EMBO Rep. 2: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, and Chapman MS. 2002. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 99: 10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters RW, Agbandje-McKenna M, Bowman VD, Moninger TO, Olson NH, Seiler M, Chiorini JA, Baker TS, and Zabner J. 2004. Structure of adeno-associated virus serotype 5. J. Virol 78: 3361–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam HJ, Lane MD, Padron E, Gurda B, McKenna R, Kohlbrenner E, Aslanidi G, Byrne B, Muzyczka N, Zolotukhin S, and Agbandje-McKenna M. 2007. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol 81: 12260–12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, Flotte T, and Muzyczka N. 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol 74: 8635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochrie MA, Tatsuno GP, Christie B, McDonnell JW, Zhou S, Surosky R, Pierce GF, and Colosi P. 2006. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J. Virol 80: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindasamy L, Padron E, McKenna R, Muzyczka N, Kaludov N, Chiorini JA, and Agbandje-McKenna M. 2006. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J. Virol 80: 11556–11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, and Walsh CE. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther 2: 619–623. [DOI] [PubMed] [Google Scholar]
- 27.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, and Muzyczka N. 2004. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther 10: 302–317. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, and Wilson JM. 2005. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 105: 3079–3086. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Chirmule N, Gao G, and Wilson J. 2000. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J. Virol 74: 8003–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, Pasi KJ, Ertl HC, Herzog RW, and High KA. 2000. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol. Ther 1: 225–235. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Dobrzynski E, Schlachterman A, Cao O, and Herzog RW. 2005. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 105: 4226–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. 2006. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med 12: 342–347. [DOI] [PubMed] [Google Scholar]
- 33.Zaiss AK, and Muruve DA. 2005. Immune responses to adeno-associated virus vectors. Curr. Gene Ther 5: 323–331. [DOI] [PubMed] [Google Scholar]
- 34.Bell P, Limberis M, Gao G, Wu D, Bove MS, Sanmiguel JC, and Wilson JM. 2005. An optimized protocol for detection of E. coli β-galactosidase in lung tissue following gene transfer. Histochem. Cell Biol 124: 77–85. [DOI] [PubMed] [Google Scholar]
- 35.Arnold K, Bordoli L, Kopp J, and Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201. [DOI] [PubMed] [Google Scholar]
- 36.Krissinel E, and Henrick K. 2004. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr 60: 2256–2268. [DOI] [PubMed] [Google Scholar]
- 37.Emsley P, and Cowtan K. 2004. COOT: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60: 2126–2132. [DOI] [PubMed] [Google Scholar]
- 38.Jones TA, Zou JY, Cowan SW, and Kjeldgaard M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47(Pt. 2): 110–119. [DOI] [PubMed] [Google Scholar]
- 39.DeLano WL 2002. The PyMOL Molecular Graphics System DeLano Scientific, San Carlos, CA. [Google Scholar]
- 40.Lin J, Zhi Y, Mays L, and Wilson JM. 2007. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J. Virol 81: 11840–11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SW, Hensley SE, Tatsis N, Lasaro MO, and Ertl HC. 2007. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Invest 117: 3958–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, and Herzog RW. 2007. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 110: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, Sanmiguel J, Desai RA, Chen CS, Johnston J, et al. 2006. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med 12: 967–971. [DOI] [PubMed] [Google Scholar]
- 44.Jooss K, Yang Y, Fisher KJ, and Wilson JM. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol 72: 4212–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O, and Jooss K. 2001. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J. Virol 75: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skelton D, Satake N, and Kohn DB. 2001. The enhanced green fluorescent protein (eGFP) is minimally immunogenic in C57BL/6 mice. Gene Ther. 8: 1813–1814. [DOI] [PubMed] [Google Scholar]
- 47.Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, and Kohn D. 1999. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 6: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 48.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, and Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol 77: 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, and Wilson JM. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol 74: 2420–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, and Muruve DA. 2001. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol 76: 4580–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, and Shen H. 2003. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol 170: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 52.Matloubian M, Concepcion RJ, and Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol 68: 8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manickan E, and Rouse BT. 1995. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol 69: 8178–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stohlman SA, Bergmann CC, Lin MT, Cua DJ, and Hinton DR. 1998. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol 160: 2896–2904. [PubMed] [Google Scholar]
- 55.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, and Heath WR. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393: 478–480. [DOI] [PubMed] [Google Scholar]
- 56.Ridge JP, Di Rosa F, and Matzinger P. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393: 474–478. [DOI] [PubMed] [Google Scholar]
- 57.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, and Melief CJ. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393: 480–483. [DOI] [PubMed] [Google Scholar]
- 58.Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ,van der Voort EI, Rea D, Offringa R, Geuze HJ, Melief CJ, and Ossendorp F. 2000. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med 192: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, and Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med 184: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, and Wilson JM. 1996. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 273: 1862–1864. [DOI] [PubMed] [Google Scholar]
- 61.Bourgeois C, Rocha B, and Tanchot C. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297: 2060–2063. [DOI] [PubMed] [Google Scholar]
- 62.Lu Z, Yuan L, Zhou X, Sotomayor E, Levitsky HI, and Pardoll DM. 2000. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med 191: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andreasen SO, Christensen JE, Marker O, and Thomsen AR. 2000. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J. Immunol 164: 3689–3697. [DOI] [PubMed] [Google Scholar]
- 64.Edelmann KH, and Wilson CB. 2001. Role of CD28/CD80–86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol 75: 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bluestone JA 1995. New perspectives of CD28–B7-mediated T cell costimulation. Immunity 2: 555–559. [DOI] [PubMed] [Google Scholar]